Abstract
Human papillomavirus (HPV) has long been recognized as the causative agent of cervical cancer. High-risk HPV types 16 and 18 alone are responsible for over 70% of all cases of cervical cancers. More recently, HPV has been identified as an etiological factor for several other forms of cancers, including oropharyngeal, anogenital, and skin. Thus, the association of HPV with these malignancies creates an opportunity to control these HPV lesions and HPV-associated malignancies through immunization. Strategies to prevent or to therapeutically treat HPV infections have been developed and are still pushing innovative boundaries. Currently, commercial prophylactic HPV vaccines are widely available, but they are not able to control established infections or lesions. As a result, there is an urgent need for the development of therapeutic HPV vaccines, to treat existing infections, and to prevent the development of HPV-associated cancers. In particular, DNA vaccination has emerged as a promising form of therapeutic HPV vaccine. DNA vaccines have great potential for the treatment of HPV infections and HPV-associated cancers due to their safety, stability, simplicity of manufacturability, and ability to induce antigen-specific immunity. This review focuses on the current state of therapeutic HPV DNA vaccines, including results from recent and ongoing clinical trials, and outlines different strategies that have been employed to improve their potencies. The continued progress and improvements made in therapeutic HPV DNA vaccine development holds great potential for innovative ways to effectively treat HPV infections and HPV-associated diseases.
Keywords: : HPV, DNA vaccine, therapeutic vaccine, HPV E6, HPV E7, cervical cancer, dendritic cells
Introduction
Human papillomavirus and associated diseases
Human papillomavirus (HPV) is a known etiological factor of cervical cancer—the fourth most common female cancer worldwide.1–4 It has been identified as the causative agent of cervical intraepithelial neoplasia (CIN) and squamous intraepithelial lesions, as well as cervical cancer precursor lesions, as it has a propensity to infect the genital tract.5 HPV infects mucosal and cutaneous membranes and is present in more than 95% of all cervical cancers.1,6 However, HPVs have also been implicated in various different clinical lesions such as warts, cysts, and keratosis as well as associated with other cancers, including penile, vaginal, vulvar, anogenital, oropharyngeal, and skin.7–9
Molecular biology and pathogenesis of HPV
HPV is a small (∼8000 base pairs), non-enveloped, double-stranded, circular DNA virus that has a tendency to manifest through an epitheliotropic fashion.10 More than 200 types of HPV exist, classified into low- and high-risk types depending on their ability to cause cancer.11 Low-risk HPVs cause genital and skin warts, whereas high-risk HPVs are responsible for the malignant transformation of infected cells.12–14 Among the high-risk types, HPV-16 and HPV-18 are responsible for over 70% of cervical cancers, which is why they have been the main focus for many developing HPV therapies.15 The HPV genome encodes for two classes of proteins: early proteins (E1, E2, E4, E5, E6, and E7), which regulate the viral life cycle, and late proteins (major and minor capsid proteins L1 and L2), which are structural components of the viral capsid responsible for virion assembly and lytic release. While E1 is a replication factor, E2 is a transcription regulator of all HPV viral proteins, capable of regulating viral DNA replication and viral RNA transcription. E4 regulates the cytoskeleton structure of infected epithelial cells. E5, E6, and E7 mediate cellular transformation. E6 and E7 are especially important, as they are oncoproteins that repress tumor suppressors p53 and pRb, respectively, preventing the activation of apoptotic pathways, further promoting cell proliferation, ultimately leading to the progression of HPV-associated malignancies.12,14 During the manifestation of HPV-associated cervical cancer, viral integration into the host genome often leads to the deletion of E2, E4, E5, L1, and L2 viral proteins and the constitutive upregulation of E6 and E7 oncogenes.
The clinical stages of cervical premalignancies stem from increasing severities of dysplasia: CIN grades 1, 2, and 3 (CIN1, CIN2, and CIN3, respectively). CIN1 is also called low-grade squamous intraepithelial lesions, while CIN2/3 are also called high-grade squamous intraepithelial lesions. The abnormal cell growth of CIN1/2 lesions frequently spontaneously regress, having been cleared by the immune system after an HPV infection. However, progression to CIN2/3 puts individuals at high risk for developing cervical cancer.16
Preventive HPV vaccines and the need for therapeutic vaccines
Identifying HPV as an etiological factor of cervical cancer and other HPV-associated malignancies helped give rise to the development of immunization strategies to prevent HPV infection and associated diseases caused by HPV. These preventive vaccines target L1 and/or L2 in order to generate neutralizing antibodies against HPV and establish protective immunity through the use of virus-like particles.17,18 Commercially available efficacious prophylactic vaccines include the bivalent Cervarix® (GlaxoSmithKline)19 as well as multivalent Gardasil® and Gardasil-9® (Merck).20,21 The recent development of Gardasil-9® has increased preventive coverage from HPV types 6, 11, 16, and 18 to 6, 11, 16, 18, 31, 33, 45, 52, and 58.20,22 Prophylactic HPV vaccines have been shown to effectively prevent vaccinated, individuals from contracting HPV infections;23 however, these preventive vaccines have not been successful in treating established HPV infections.24,25
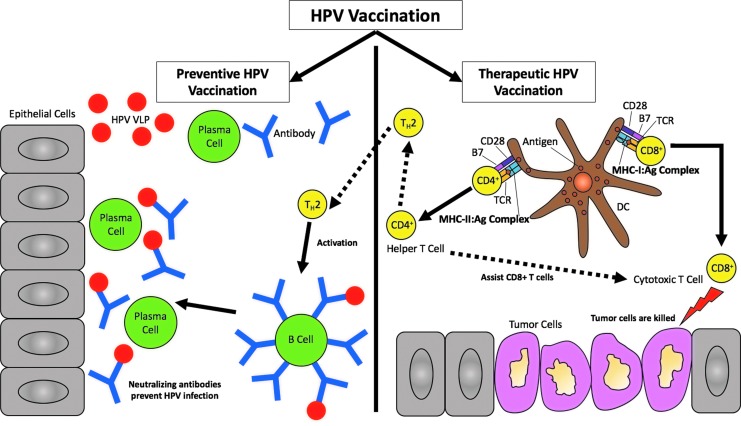
For unvaccinated individuals or those already infected, there remains a chance that infection with high-risk HPV types such as HPV-16 and/or HPV-18 could form precancerous lesions, eventually leading to malignant transformation.5 Therefore, there remains an urgent need for the development of therapeutic HPV vaccines to tackle existing HPV infections, prevent the development of cancer, and act as immunotherapies for HPV-associated malignancies. DNA vaccines are an emerging field of therapeutics that provide great potential for the treatment of HPV infections and HPV-associated cancers. A schematic of how preventive and therapeutic vaccines work can be found in Fig. 1.
Figure 1.
Human papilloma virus (HPV) vaccination. HPV vaccination is achieved from either preventive or therapeutic vaccines. The goal of preventive HPV vaccines is to prevent HPV infections by targeting humoral immunity through delivering virus-like particles (VLPs) encoding late HPV viral capsid proteins L1 and/or L2. Once VLPs are delivered, T-helper 2 (TH2) cells activate B-cells to bind to the particles to become plasma cells, allowing antibodies to be generated. The neutralizing antibodies produced via preventive vaccines will block the HPV infection and induce protection from HPV. On the other hand, therapeutic HPV vaccines target cell-mediated immunity through professional antigen-presenting cells (APCs). APCs such as dendritic cells (DCs) present major histocompatibility complex (MHC)-peptide complexes to T-cells to allow for T-cell priming into effector cells. Helper CD4+ T-cells are presented via MHC class II molecules, whereas cytotoxic CD8+ T-cells, or cytotoxic T lymphocytes (CTLs), are presented via MHC class I molecules. CD4+ T-cells further differentiate into TH cells to amplify CTL responses as well as activating humoral B-cells to produce more neutralizing antibodies. CTLs mediate the antigen-specific killing of tumor cells.
The development of therapeutic vaccines
When HPV integrates into the host genome of infected high-grade lesions or HPV-associated malignancies, HPV late genes L1 and L2 are lost.26 As a result, L1- or L2-specific neutralizing antibodies generated by prophylactic vaccines are no longer effective against these HPV-infected cells. In an attempt to clear HPV infections or preexisting HPV-associated lesions, therapeutic HPV vaccines are being developed. In order to eliminate established infection or lesions, therapeutic vaccines generate T-cell-mediated immunity by specifically targeting HPV early antigens that are constitutively expressed across both infected and cancerous cells.27,28 As a result, early genes are targeted as they are expressed throughout the virus's life cycle and help regulate the progression of HPV-associated precancerous and cancer lesions. Specifically, the E6 and E7 proteins represent two ideal therapeutic HPV vaccine targets because they are constantly expressed and involved in the malignant transformation of HPV-associated cancers.29,30 Thus, the current development of therapeutic HPV vaccines focuses on activating T-cells specific for the HPV E6 and E7 antigens.
Current therapeutic approaches against HPV include live vector–based, peptide-/protein-based, whole cell–based, and nucleic acid–based vaccines. Each type of therapeutic vaccine has its own advantages and disadvantages, which we have summarized in Table 1 (also see reviews from Yang and colleagues31,32). Since this review focuses specifically on therapeutic HPV DNA vaccines, they are not included in Table 1 and instead are described in great detail in subsequent sections of this review.
Table 1.
Summary of the advantages and disadvantages of therapeutic vaccines
| Therapeutic vaccine | Description | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Live vector–based | Highly immunogenic attenuated bacterial or viral vectors that carry recombinant DNA encoding the antigen of interest into the host to elicit an immune response. Bacterial vectors such as Listeria monocytogenes and viral vectors such as the vaccinia virus have been shown to generate potent humoral and cellular immune responses in pre-clinical models | • Highly immunogenic • Mimics natural course of infection |
• Preexisting immunity against vector • No repeated administration • Safety risks for immunocompromized individuals |
15, 33–42 |
| Peptide-/protein-based | Peptide-based vaccines are peptide segments containing epitopes of tumor-associated antigens or other antigenic proteins of interest while protein-based vaccines are the antigenic proteins themselves. | Peptide-based: • Safe • Stable • Easy to produce Protein-based: • Safe • Stable • Easy to produce • No HLA restriction |
Peptide-based: • HLA restriction • Low immunogenicity • Requires co-administration of adjuvants Protein-based: • Low immunogenicity • Requires co-administration of adjuvants • Generates antibodies instead of cytotoxic T lymphocytes |
15, 43–46 |
| Whole cell–based | Whole cell vaccines involve the adoptive transfer of autologous dendritic or tumor cells that have been prepared to express the target antigens and other costimulatory molecules. In dendritic cell (DC)-based vaccines, DCs are loaded with HPV antigens ex vivo and delivered into the patient. Tumor cell-based vaccines aim to improve the immunogenicity of tumor cells by increasing the expression of immune modulatory proteins, such as IL-2, IL-12, and GM-CSF. | DC-based: • Highly immunogenic Tumor cell-based: • Likely to express tumor antigens |
• Expensive • Labor intensive • Safety concerns |
47–50 |
| Nucleic acid–based (RNA-based only) | RNA replicon vaccines involve the insertion of an RNA sequence encoding the target antigens into disabled viral vectors derived from RNA viruses such as the Sindbis virus, Venezuelan Equine Encephalitis virus, and Simian Foamy virus. | • Transient infections • Multiple administrations • Sustained antigen expression • No risk of chromosomal integration and cellular transformation |
• Difficult to produce and store • Unstable • Labor intensive • No intercellular spreading • Dose-limiting toxicities • Multiple mRNAs cannot be combined in the same formulation • Human safety data for RNA has not been well developed |
51–54 |
GM-CSF, granulocyte-macrophage colony-stimulating factor; HLA, human leukocyte antigen; HPV, human papilloma virus; IL, interleukin.
Therapeutic HPV DNA Vaccines
DNA vaccines are safe, stable, easy to prepare and produce at high purity, and allow for repeated administration without loss of efficacy.55 Because of these advantages and the potential for the control of HPV infections and HPV-associated lesions, DNA vaccines are actively being developed. In comparison with RNA vaccines, they are stable and inexpensive to store and transport. They do not have the same dose-limiting toxicity problems as RNA vaccines, and multiple DNA plasmids may be combined into a single formulation to allow delivery of multiple antigens.54 DNA vaccines also sustain the expression of antigens within cells for longer periods of time when compared with RNA or protein-based vaccines.56 However, there are some limitations to DNA vaccines. DNA has a limited ability to spread or amplify between cells in vivo and does not hold intrinsic specificity for targeting antigen-presenting cells (APCs).57,58 Additionally, there are theoretical risks of having body reactions, although no evidence of antivaccine DNA immune responses or genomic integration has been observed to date in the thousands of individuals vaccinated with DNA vaccines.59
As a result of these limitations, the potency of therapeutic HPV DNA vaccines may be affected. Several strategies to enhance the potency of these vaccines have emerged in order to overcome such obstacles; we discuss these in the next section. Because adaptive immune responses must be generated for a vaccine to be effective, targeting DNA vaccines to professional APCs, particularly dendritic cells (DCs), plays a key role because they serve as the bridge between innate and adaptive immune responses. Therefore, many of the strategies to enhance therapeutic HPV DNA vaccine potency focus on targeting DCs.15 A schematic of how therapeutic HPV DNA vaccines work can be found in Fig. 2. Additionally, a list of advantages and disadvantages of therapeutic HPV DNA vaccines can be found in Table 2.
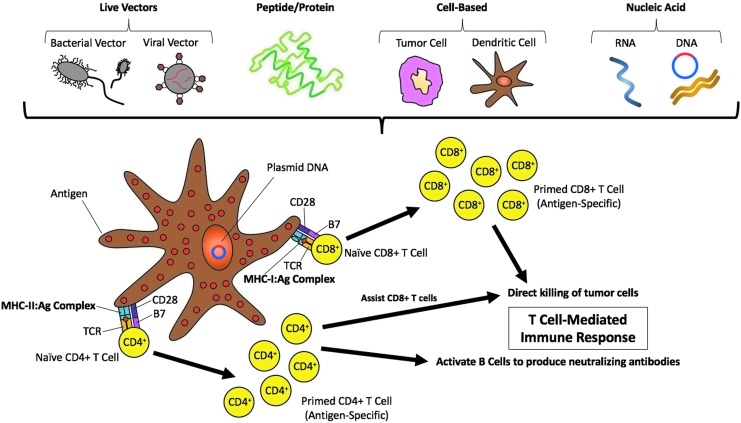
Figure 2.
Therapeutic HPV DNA vaccination. Several methods of therapeutic vaccinations have been developed. Specifically, for HPV, therapeutic vaccines activate the adaptive immune system by targeting the E6 and/or E7 antigen(s). These methods include live vector–based (bacterial or viral vector) vaccines, peptide-/protein-based vaccines, cell-based (tumor cell or dendritic cell) vaccines, and nucleic acid–based (RNA or DNA) vaccines. While preventive vaccines elicit a humoral immune response through neutralizing antibodies, therapeutic vaccines utilize the cell-mediated immune system to control HPV infections. This schematic outlines therapeutic HPV DNA vaccination. DNA plasmids that encode for HPV antigens, such as early proteins 6 and 7 (E6, E7), are transfected into antigen presenting cells (APCs) such as dendritic cells (DCs). APCs are activated upon direct transfection of these HPV antigens or through indirect transfer of the antigens by way of cross-presentation. DCs home to draining lymph nodes where they can prime naïve T-cells upon presentation of antigenic peptides to T-cells via major histocompatibility complexes (MHCs) The interaction between the MHC molecules and the antigens (i.e., MHC:antigen [Ag] complex) with the T-cell receptor (TCR) is assisted by co-stimulatory compounds, namely B7 present on DCs and CD28 present on T-cells. MHC-I molecules present to CD8+ T-cells and MHC II molecules present to CD4+ T-cells to initiate a cell-mediated immune response. Activated CD8+ T-cells directly kill tumor cells by inducing apoptosis. This immune response is further supported by CD4+ T-cells, which assist in the killing of tumors.
Table 2.
Summary of the advantages and disadvantages of therapeutic HPV DNA vaccines
| Advantage | Attribute of therapeutic HPV DNA vaccines |
|---|---|
| Design | • Generates effective CTL and antibody responses |
| • Can express tumor antigenic peptides | |
| • Prolonged antigen expression increases the chances of developing immunologic memory | |
| • Expressed antigens are subjected to the same glycosylation and post-translational modifications as natural viral infections | |
| Safety | • Will not revert to virulent form |
| • Does not contain actual infectious agents | |
| • Repeated administration is safe and effective | |
| • Clinical trials have not shown adverse events | |
| Stability | • Temperature-stable |
| • Long shelf life | |
| Manufacture | • High purity large scale production |
| • Rapid production and formulation | |
| • Easy to store and transport |
| Disadvantage | Attribute |
|---|---|
| Specificity | • Low transfection efficiency |
| • Low immunogenicity | |
| • DNA is unable to amplify or spread intercellularly | |
| • Not specifically targeted to APCs | |
| Body reaction | • Possibility for immune responses generated against DNA |
| • Risk of genomic integration |
APCs, antigen-presenting cells; CTL, cytotoxic T lymphocyte.
Strategies to Enhance Therapeutic Hpv Dna Vaccine Potency
Potential strategies to enhance the potency of therapeutic HPV DNA vaccines have focused on (1) increasing DC uptake of HPV antigens, (2) improving DC processing and presentation of HPV antigens, and (3) enhancing DC and T-cell interactions (Table 3). Here, we discuss the various ways to enhance the potency of therapeutic HPV DNA vaccines through preclinical studies and the latest completed or ongoing clinical trials (Table 4).
Table 3.
Different strategies to enhance the potency of therapeutic HPV DNA vaccines
| Strategy | Approach | References | Relevant clinical trials (NCT numbers) |
|---|---|---|---|
| [1] Increasing DC uptake of HPV antigens | |||
| [I] Routes of administration | [a] ID and subdermal administration via gene gun [b] Electroporation-mediated IM or mucosal administration [c] ID administration followed by laser treatment [d] Microspheres and nanoparticle-based delivery systems [e] Other novel delivery systems |
60–66 65, 67–70, 72–74 75, 76 77–81 82–84 |
02139267; 02411019; 03185013; 02241369 |
| [II] Intercellular spreading of HPV antigens to DCs | Fuse HPV antigens with [a] VP22 [b] MVP22 |
85–91, 93, 144 94 |
|
| [III] Targeting antigens directly to DCs | Fuse HPV antigens with [a] HSP70 [b] CTLA4 |
95–98 100–105 |
00788164 |
| [IV] Enhancing the release of HPV antigens into DC Surroundings | [a] Chemotherapy using • DMXAA • EGCG • Apigenin • Cisplatin • Bortezomib [b] Radiation |
106, 111 107 108 109 109, 110 112 |
|
| [2] Improving HPV antigen expression, processing, and presentation in DCs | |||
| [I] Enhancing antigen expression in transfected DCs | [a] Enhancing transcription of HPV DNA by co-administering demethylating agents [b] Enhancing translation of HPV mRNA: • Codon optimization • Kozak sequence addition |
113–117 73, 115, 119, 120 121, 122 |
03185013; 03180684 |
| [II] Enhancing MHC I and MHC II expression in DCs | Co-administer: [a] CIITA [b] HDACi |
123–125 126–130 |
|
| [III] Enhancing antigen processing through the MHC I pathway cross-presentation | [a] Targeting antigens for proteosomal degradation by way of fusion with • PVX-CP • γ-tubulin or ubiquitin • Destabilizing mutations [b] Targeting antigens to the ER by way of fusion with • Signal peptides • ER chaperone proteins (CRT) [c] Targeting antigens to the cytoplasm by way of fusion with • ETA • Flt3L |
132, 133 132–136 137 138–141 66, 142–149 150, 151 72, 152–155 |
02411019; 02139267; 02596243 |
| [IV] Enhancing antigen processing through the MHC II pathway | Fuse HPV antigens with LAMP-1 | 156–160 | |
| [V] Bypassing antigen processing by the MHC I pathway | Fuse HPV antigens with SCT | 161–163 | |
| [3] Enhancing DC function and survival, DC and T-cell interactions, and T-cell function survival | |||
| [I] Enhancing DC function and survival | [a] Enhance DC cross-priming abilities by: • Co-administering TLR agonists and DC-activating cytokines (GM-CSF) • Fusing HPV antigens with HSV-1 gD [b] Prolonging the survival of antigen-expressing DCs by way of co-administering • BCL-xL • CTGF • Bax and Bak RNAi |
167–177 172, 178 180 181 182 |
03206138; 03180684 |
| [II] Boosting DC and T-cell interactions | Generate CD4+ T-cell help by way of co-administering • Ii-PADRE • CD4+ T-cell epitopes (TTFC and BPV-1 L1 or L2) • Xenogeneic MHC I |
184–187 171, 188 190 |
|
| [III] Promoting T-cell function and survival | [a] Enhance T-cell development and maintenance by way of co-administering • IL-2 • Anti-4-1BB • IL-7 • IL-12 [b] Target effector T-cells to tumor sites by co-administering IP-10 [c] Reducing apoptosis of T-cells • Anti-PD-L1 blockage • FasL blockage |
191, 192 193, 194 195, 196 197 203–205 208 209–212 |
02860715; 0320613 02172911; 03162224; 02241369 03162224 |
| [IV] Enhancing immune responses through elimination of Tregs | [a] CTX [b] Monoclonal anti-CD25 |
216, 217 216, 217 |
|
BCL-xL, B-cell lymphoma-extra large; BPV-1, bovine papillomavirus type 1; CIITA, major histocompatibility complex (MHC) class II transactivator; CRT, calreticulin; CTGF, connective tissue growth factor; CTLA4, cytotoxic T-lymphocyte antigen 4; CTX, cyclophosphamide; DMXAA, 5,6-dimethylxanthenon-4-acetic acid; EGCG, epigallocathechin-3-gallate; ER, endoplasmic reticulum; ETA, exotoxin A; HDACi, histone deacetylase inhibitor; HSP70, heat shock protein 70; HSV-1, herpes simplex virus type 1; ID, intradermal; Ii-PADRE, invariant Pan HLA-DR reactive epitope; IM, intramuscular; LAMP-1, lysosomal-associated membrane protein type 1; MHC, major histocompatibility complex; NCT, National Clinical Trial; PD-L1, programmed death-ligand 1; PVX-CP, potato virus X coat protein; SCT, single-chain trimers; TLR, toll-like receptor; Tregs, regulatory T-cells; TTFC, tetanus toxin fragment C.
Table 4.
Latest clinical trials
| DNA vaccine construct | Additional treatment(s) | Encoded antigen | Plasmid construct | Route of administration | Enhancement method | Sponsor | Trial design | Clinical outcomes, side effects, and date of completion | Reference/clinical trial number |
|---|---|---|---|---|---|---|---|---|---|
| pNGVL4a-CRT/E7(detox) | NA | HPV-16 E7 | pNGVL4a plasmid encoding HPV-16 E7(detox) fused to CRT | ID, IM, or intralesional | CRT enhances processing of Ag through MHC class I pathway. | Sidney Kimmel Comprehensive Cancer Center | Phase 1 trial in HPV 16+ CIN2/3 patients (32 patients) | Clinical outcome: 8 of 27 (30%) patients who received all vaccinations and underwent loop electrosurgical excision procedure showed histologic regression to CIN1 or less. Side effects: injection site reactions. |
66 |
| pNGVL4a-Sig/E7(detox)/HSP70 | NA | HPV-16 E7 | pNGVL4a plasmid encoding Sig-E7(detox) fused to HSP70 | IM | HSP70 targets Ag to DCs. A signal sequence (Sig) promotes Ag secretion. |
Sidney Kimmel Comprehensive Cancer Center | Phase 1/II trial in HPV 16+ CIN2/3 patients (15 patients) | Clinical outcome: 3 of 9 (33%) patients in the highest-dose cohort showed complete histologic regression. Side effects: transient injection site discomfort. |
98 |
| pNGVL4a/E7(detox)/HSP70 | TA-HPV vaccine and imiquimod | HPV-16 E7 | pNGVL4a plasmid encoding HPV-16 E7(detox) fused to CRT and HSP70 | DNA vaccine: IM TA-HPV and imiquimod: local |
CRT enhances processing of Ag through MHC class I pathway. HSP70 targets Ag to DCs. TA-HPV is a recombinant vaccinia virus expressing HPV-16/18 E6 and E7. Imiquimod is a topical cream that activates DCs. |
Sidney Kimmel Comprehensive Cancer Center | Phase 1 trial in HPV16+ CIN2/3 patients (48 estimated patients) | July 2017 | NCT00788164 |
| GX-188E | NA | HPV-16/18 E6/E7 | pGX27 plasmid encoding HPV-16/18 E6/E7 fused to extracellular domain of Flt3L and tpa signal sequence | IM/EP | Flt3L targets Ag to DC and enhances Ag presentation tpa targets the fusion protein to the secretory pathway. Tpa signal sequence targets the fusion protein to the secretory pathway. |
Genexine, Inc | Phase 1 trial in HPV16/18+ CIN3 patients (9 patients) | Clinical outcome: 7 out of 9 patients showed complete histologic regression and viral clearance within 36 weeks of follow-up. Side effects: no serious vaccine-associated adverse events at all administered doses. |
72 |
| Phase 2 trial in HPV16/18+ CIN3 patients in South Korea (72 estimated patients) | March 2016 | NCT02139267 | |||||||
| Phase 2 trial in HPV16/18+ CIN2/3 patients in Eastern Europe (134 estimated patients) | August 2018 | NCT02596243 | |||||||
| Follow-up of immune response in CIN3 patients who participated in phase 2 trial | November 2018 | NCT02411019 | |||||||
| GX-188E | GX-17 or imiquimod | HPV-16/18 E6/E7 | GX-188E: pGX27 plasmid encoding HPV-16/18 E6/E7 fused to extracellular domain of Flt3L and tpa signal sequence GX-17: plasmid encoding Fc-fused IL-7 |
DNA vaccine: IM/EP GX-17 or imiquimod: local |
Flt3L targets Ag to DC and enhances Ag presentation tpa targets the fusion protein to the secretory pathway. Tpa signal sequence targets the fusion protein to the secretory pathway. IL-7 promotes T-cell development and proliferation. Imiquimod is a topical cream that activates DCs. |
Seoul St. Mary's Hospital | Phase 1 trial in HPV-16/18+ CIN3 patients (50 estimated patients) | October 2018 | NCT03206138 |
| VGX-3100 | NA | HPV-16/18 E6/E7 | Two plasmids encoding codon optimized HPV-16/18 E6 and E7 | IM/EP | Codon optimization enhances antigen expression in DCs. | Inovio Pharmaceuticals | Phase 1 trial in HPV-16/18+ CIN2/3 patients (18 patients) | Side effects: mild injection site reactions. | 73 |
| Follow-up on previous Phase 1 trial: fourth dose of VGX-3100 in patients treated with three doses | 120 | ||||||||
| Phase 2b trial in HPV-16/18+ CIN2/3 patients (167 patients) | Clinical outcome: 55 (48.2%) of 114 patients treated with VGX-3100 and 12 (30.0%) of 40 patients in placebo group showed histopathological regression (p = 0.034). Side effects: Injection site reactions, erythema. |
74 | |||||||
| Phase 3 trial in HPV-16/18+ CIN2/3 patients (198 estimated patients) | August 2020 | NCT03185013 | |||||||
| VGX-3100 | Imiquimod | HPV-16/18 E6/E7 | Two plasmids encoding codon optimized HPV-16/18 E6 and E7 | Imiquimod: local | Codon optimization enhances antigen expression in DCs. Imiquimod is a topical cream that activates DCs. |
Inovio Pharmaceuticals | Phase 2 trial in HPV-16/18+ patients with HSIL of the vulva (36 estimated patients) | August 2020 | NCT03180684 |
| INO-3112 (VGX-3100 + INO-9012) | NA | HPV-16/18 E6/E7 | VGX-3100: two plasmids encoding codon optimized HPV-16/18 E6 and E7 INO-9012: plasmid encoding IL-12 |
IM/EP | Codon optimization enhances antigen expression in DCs. IL-12 promotes T-cell function. |
Inovio Pharmaceuticals | Phase 1/2a trial in HPV+ patients with head and neck squamous cell carcinoma (22 estimated patients) | Side effects: injection site pain, local erythema, and hematoma/swelling | 218 |
| Phase 1/2a trial in HPV-16/18+ patients with cervical cancer (10 estimated patients) | March 2018 | NCT02172911 | |||||||
| INO-3112 (VGX-3100 + INO-9012) | Durvalumab | HPV-16/18 E6/E7 | VGX-3100: two plasmids encoding codon optimized HPV-16/18 E6 and E7 INO-9012: plasmid encoding IL-12 |
IM/EP | Codon optimization enhances antigen expression in DCs. IL-12 promotes T-cell function. Durvalumab is a human monoclonal antibody against PD-L1 that promotes T-cell survival. |
MedImmune LLC | Phase 1b/2a trial in HPV-16/18+ patients with recurrent or metastatic head and neck squamous cell carcinoma (40 estimated patients) | July 2019 | NCT03162224 |
| INO-3106 + INO-9012 | NA | HPV-6 E6/E7 | INO-3106: plasmid encoding HPV-6 E6 and E7 INO-9012: plasmid encoding IL-12 |
IM/EP | IL-12 promotes T-cell function. | Inovio Pharmaceuticals | Phase 1 trial in HPV6+ patients with aerodigestive precancerous lesions and malignancies (6 estimated patients) | December 2018 | NCT02241369 |
| VB10.16 | NA | HPV-16 E6/E7 | Plasmid encoding HPV-16 E6/E7 fusion protein dimerized to a protein that targets APC receptors | IM | An encoded protein targets Ag to APCs. | Vaccibody AS | Phase 1/2 trial in HPV16+ CIN2/3 patients (36 estimated patients) | September 2017 | NCT02529930 |
Ag, antigen; CIN, cervical intraepithelial neoplasia (types 1–3); E6/7, early protein 6/7; EP, electroporation; HSIL, high-grade squamous intraepithelial lesions; NA, not applicable.
Increasing the number of HPV antigen-loaded DCs
For DCs to be loaded with HPV antigens, DNA plasmids encoding HPV antigens can directly transfect DCs or the antigens can spread from the transfected cells to DCs. Such antigen spreading may occur intercellularly, in which the transfected cells release antigens that are then taken up by DCs.
Routes of administration
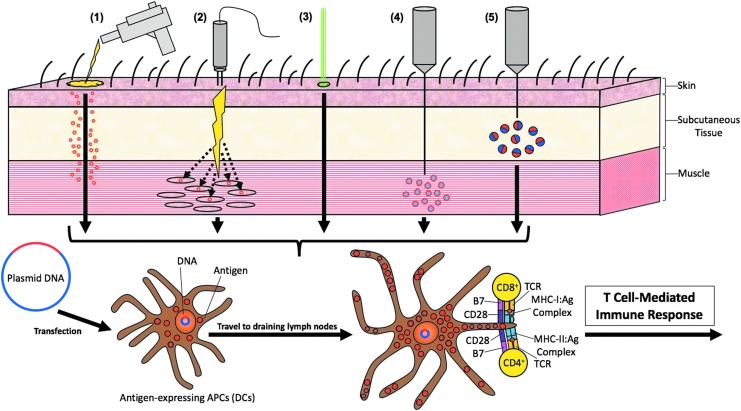
Transfection efficiency is a crucial determinant of the efficacy of DNA vaccines. Different routes of administration have been explored in order to maximize transfection efficiency and enhance therapeutic vaccination potency. These strategies (outlined in Fig. 3) include intradermal (ID) and subdermal gene gun delivery, electroporation-mediated intramuscular (IM) or mucosal administration, intradermal administration followed by laser treatment, and microsphere-/nanoparticle-based delivery systems.
Figure 3.
Routes of administration. Different routes of administering DNA vaccines have been established to enhance antigen uptake by DCs in order to enhance T-cell-mediated immune response by both CD4+ and CD8+ T-cells. (1) DNA vaccines can be delivered intradermally and subdermally through a gene gun. In this process, the biological and ballistic instrument delivers HPV DNA-coated gold particles directly into immature DCs located under the skin (i.e., Langerhans cells). (2) HPV DNA vaccines can also be injected intramuscularly followed by electroporation in order to enhance antigen expression in muscle cells. This will allow for inflammatory responses in local myocytes, leading to the antigens to be taken up by DCs. (3) HPV DNA uptake by DCs can also be enhanced through intradermal administration followed by laser treatment. (4) HPV DNA can also be delivered via intramuscular injection encapsulated in microspheres and nanoparticles in order to enhance the uptake of the vaccine by DCs. (5) There are other novel delivery systems to enhance DNA vaccine potency such as subcutaneously injecting HPV DNA mixed with PEI600-Tat.
Intradermal and subdermal administration via gene gun
The gene gun is a high-velocity biological and ballistic transfection device in which DNA-coated subcellular-sized particles (such as gold beads) deliver DNA directly to cells at the site of administration.60 ID administration relies on keratinocytes and epidermal DCs being directly transfected. DCs eventually mature and migrate to local lymphoid tissues to present HPV antigens and prime HPV antigen-specific T-cells. It was believed that Langerhans cells are crucial for this process, but it has been shown that at least three functionally distinct skin DC subsets serve important roles.61 While ID gene gun delivery of HPV-16 E7 gene can induce antitumor immunity,62 a subdermal tissue approach is also possible.63 Gene gun delivery has been shown to be more dose-efficient than biojector and syringe IM administrations.64 However, gene guns do not necessarily generate stronger immune responses or antitumor effects.65 A clinical trial exploring different routes of administration of a National Gene Vector Laboratory (NGVL) plasmid vaccine, pNGVL4a-CRT/E7(detox), showed that gene gun administration of this vaccine was well tolerated but did not provide a better route of administration in generating therapeutic antilesional effects compared with intralesional or IM administration of naked DNA vaccines.66
Electroporation-mediated IM injection or mucosal administration
Compared with conventional IM injection, IM injection followed by electroporation improves transfection efficiency through low voltage currents, which create small ruptures in the cellular membrane for plasmids to enter through.67 In this process, some cells may be damaged or killed. Subsequently, the release of pro-inflammatory danger signals recruits more immune cells to the site of antigen production, further enhancing the presentation of antigens and establishment of effective immune responses.68 It was shown that even a small amount of DNA can increase both humoral and cellular immune responses of a DNA vaccine in vivo when administered with electroporation.69 Electroporation-mediated administration of the therapeutic HPV pNGVL4a-CRT/E7(detox) DNA vaccine generated the highest number of antigen-specific CD8+ T-cells and the strongest antitumor responses compared with conventional IM injection and epidermal gene gun–mediated administrations in a preclinical model.65 More recently, electroporation has also been targeted to mucosal surfaces with possible clinical implications for mucosal site–related diseases such as human immunodeficiency virus (HIV) and HPV.70,71 Electroporation has been used for delivery of GX-188E (a plasmid encoding fusion proteins of HPV-16/18 E6/E7 linked to the FMS-like tyrosine kinase 3 ligand, or Flt3L) in phase 1 and 2 trials72 [National Clinical Trial numbers NCT02139267, NCT02411019], delivery of VGX-3100 (mixture of two plasmids encoding optimized HPV-16/18 E6/E7 consensus) in phase 1, 2, and 3 trials73,74 [NCT03185013], and delivery of INO-3106 (a plasmid encoding HPV-6 E6/E7) in a phase 1 trial [NCT02241369], and did not elicit study-related serious adverse events in the completed GX-188E and VGX-3100 phase 1 trials.72,73 The adverse events surrounding this strategy relate to injection side pain and are generally transient in nature and of minimal severity. These DNA electroporation-related side effects, however, are largely limited to transient pain of mild to moderate severity without any systemic effects.
Intradermal administration followed by laser treatment
Femtosecond laser poration is an administration technique that uses a low-energy laser beam source to modify cell membrane permeability without the risk of permanent tissue damage, and has been used to enhance the transfection efficiency of naked DNA constructs.75 ID administration of antigens followed by second-generation femtosecond laser treatment has been shown to generate potent antigen-specific cellular immune responses in a preclinical model through improved precision and efficiency in targeting the vaccination site.76 In the context of HPV-related diseases, however, this technique has not been extensively explored but it may hold great potential for future therapeutic HPV DNA vaccine development.
Microspheres and nanoparticle-based delivery systems
Microspheres and nanoparticles composed of biodegradable and biocompatible polymers encapsulate DNA to protect them from nucleases, while serving as suitable targets to be phagocytosed by APCs.77,78 A phase 1 trial of ZYC101, a plasmid encoding for multiple human leukocyte antigen (HLA)-A2restricted HPV-16 E7 epitopes to patients with high-grade cervical intraepithelial lesions of the uterine cervix, showed that the agent is well tolerated. In addition, interferon gamma (IFN-γ) production to the encapsulated peptide epitopes was observed continuously 6 months after initial administration.79 A newer version of this drug, amolimogene (ZYC101a), containing plasmid DNA encoding HPV-16/18 E6/E7 proteins, was tested in a phase 2 and 3 clinical trial for CIN2/3 patients; there was significantly higher resolution of the disease in women younger than 25 years (70%) than an age-controlled placebo group (23%).80,81
Other novel delivery systems
PEI600-Tat, a conjugate of the basic domain of the HIV type 1 (HIV-1) Tat protein and polyethylenimine (PEI), a low molecular weight polymer, have been shown to be biocompatible as an effective nonviral gene delivery vector that increases transfection efficiency.82,83 HPV-16 E7 DNA mixed with PEI600-Tat at the ratio of 50:10 and injected subcutaneously into mice generated significantly stronger humoral and cellular immune responses compared with registering HPV-16 E7 DNA alone.84
Intercellular spreading of HPV antigens to DCs
It is important that HPV antigens enter APCs so they can be processed and presented to prime T-cells to generate adaptive immune responses. If therapeutic HPV DNA vaccines do not directly transfect APCs, the spreading of HPV antigens to APCs such as DCs becomes crucial. One way this occurs is through intercellular spreading of HPV antigens to DCs. Since encoded antigens do not easily spread between cells in vivo, it is possible to link antigens of interest to proteins capable of intercellular transport; antigens are transported into different cells alongside such proteins.
One such example is the herpes simplex virus type 1 (HSV-1) tegument protein VP22, which is highly effective at intercellular transport. When VP22 is linked to other proteins such as GFP,85,86 p53,87 and EGFP88 as chimeric polypeptides, the chimeric polypeptides spread between cells and accumulate in recipient cell nuclei. Although there have been reports that the results were due to fixation artifacts89 and studies suggesting that proteins fused to VP22 are not intercellularly trafficked,90 other studies that explored vaccination of DNA constructs of VP22 fused to HPV antigens in murine models still showed generation of more HPV antigen-specific CD8+ T-cells and enhanced antitumor effects against E7-expressing tumors in in vivo tumor protection or treatment experiments.91,92 Michel et al. demonstrated that when the HPV-16 E7 gene was fused with the VP22 sequence and applied as a DNA vaccine in murine models, it generated enhanced E7-specific cytotoxic T-lymphocyte (CTL) responses compared with vaccination with the E7 gene alone, although the authors point out that other factors such as increased protein quantities possibly due to increased de novo synthesis or increased protein stability may also play a role in this phenomenon.93 Exhibiting some homology to the HSV-1 VP22 protein, the Marek's disease virus type 1 (MVP-1) VP22 protein (MVP22) also showed properties of intercellular transport.94 When linked to the HPV-16 E7 protein and administered to mice as a DNA vaccine, MVP22 induced significantly more IFN-γ-secreting E7-specific CD8+ T-cell precursors than wild-type E7 DNA alone, while also generating stronger CD8-dependent antitumor effects.94
Targeting antigens directly to DCs
Apart from intercellular spreading of HPV antigens, exogenous antigens released by transfected cells can be targeted to DCs by fusing the antigens with DC-binding molecules. These molecules are often DC receptor ligands, such as heat shock protein 70 (HSP70) and CTL antigen 4 (CTLA4).
HSP70
Heat shock protein 70 is a protein that acts as both a chaperone and a danger-associated molecular pattern (DAMP). As a chaperone, HSP70 binds to peptides; HSP70-peptide complexes bind to CD91 and are subsequently presented by major histocompatibility complex (MHC) class I molecules of APCs.95 Therefore, linking HPV antigens to HSP70 targets these antigens to APCs, particularly DCs, and enhances presentation through the MHC class I pathway. When HSP70 acts as a DAMP, it interacts with toll-like receptor 2 (TLR2) and TLR4 to promote DC activation and better cross-priming.96 Both chaperone and DAMP properties exhibit great potential for enhanced therapeutic effects when the target antigen sequence is fused to the HSP70 gene. Indeed, administration of a DNA vaccine encoding HPV-16 E7 linked to HSP70 (E7/HSP70) in a murine model was immunologically more effective than administering a DNA vaccine encoding HPV-16 E7 alone.97 Furthermore, pNGVL4a-Sig/E7(detox)/HSP70, a plasmid expressing a detoxified form of HPV-16 E7 fused to HSP70, has undergone phase 1 and 2 clinical trials in patients with HPV-16+ CIN2/3 lesions. Results confirmed the vaccine's safety and therapeutic potential: three out of nine individuals in the highest-dose cohort demonstrated complete histologic regression.98 pNGVL4a-Sig/E7(detox)/HSP70 in combination with TA-HPV, a recombinant vaccinia virus encoding HPV-16/18 E6/E7 fusion protein, has shown 57% success rate in developing a cellular immune response99 and is currently undergoing a phase 1 clinical trial in CIN3 patients [NCT00788164].
CTLA4
The ligand of B7 molecules on APCs, CTLA4 is expressed on the surfaces of T-cells and has negative regulatory effects on T-cell activation; it is important for T-cell homeostasis.100,101 Linking HPV antigens to CTLA4 possibly targets the antigens to the surface of DCs. Previous studies have shown that DNA vaccines encoding antigen-CTLA4 fusion proteins generate more potent immune responses than DNA vaccines encoding the antigen alone.102–104 The DNA vaccine pCTLA4-E7E6 encodes CTLA4 fused to HPV-16 E7 and E6. Significantly higher anti-E7/E6 antibodies, relatively stronger antigen-specific CTL responses, and relatively stronger antitumor effects were observed when introduced as therapeutic vaccination into TC-1 tumor-bearing mice, compared with those of DNA encoding E6/E7 proteins (pE7E6) alone.105
Enhancing the release of HPV antigens into DC surroundings
Another approach to increase uptake of HPV antigens by DCs is to enhance the release of HPV antigens into DC surroundings. Common cancer treatments such as chemotherapy and radiation induce apoptosis of tumor cells, leading to the release of HPV antigens into circulation, where it would be easier for DCs to encounter and uptake them.
Chemotherapeutic agents such as 5,6-dimethylxanthenon-4-acetic acid (DMXAA),106 epigallocathechin-3-gallate (EGCG),107 4′,5,7-trihydroxyflavone (apigenin),108 cis-diamminedichloroplatinum (cisplatin),109 and bortezomib110 have been thoroughly explored. DMXAA is a vascular disrupting agent that can prevent adequate blood supply to tumors, leading to tumor necrosis.111 E7 DNA vaccination combined with DMXAA treatment generated E7-specific CD8+ T-cell responses and potent antitumor effects in TC-1 tumor-bearing mice.106 Similarly, the flavonoid EGCG induced tumor cell apoptosis in a dose-dependent manner, and DNA vaccination of pcDNA3-Sig/E7/LAMP-1 via gene gun with co-administration of EGCG through drinking water in murine models generated stronger antigen-specific antitumor responses than either DNA vaccination or EGCG treatment alone.107 Apigenin is another flavonoid, which generated potent E7-specific CD8+ T-cell responses in combination with pcDNA-E7-HSP70 vaccination, and demonstrated better antitumor effects against TC-1 tumor in murine models compared with either DNA vaccination or apigenin administration alone.108 Combining chemotherapeutic agents cisplatin and bortezomib with CRT/E7 DNA vaccine also generated strong E7-specific CD8+ T-cell responses and antitumor effects against murine E6/E7-expressing TC-1 models relative to either DNA vaccination or chemotherapy alone.109,110
Another popular method to treat tumors in a clinical setting is radiation. Low-dose radiation therapy combined with DNA vaccination of CRT/E7(detox) generated a higher titer of E7-specific CD8+ T-cells and displayed the greatest antitumor effects against TC-1 tumors in murine models, compared with DNA vaccination or radiotherapy alone.112 In addition, TC-1 tumor cells were more susceptible to lysis by E7-specific CTLs after radiotherapy.112
Improving HPV antigen expression, processing, and presentation in DCs
After DNA plasmids encoding HPV antigens are directed to and uptaken by DCs, they must be expressed, processed, and presented by the transfected DCs. Antigen expression involves the transcription and translation of HPV DNA. The translated antigens are then presented through the MHC I or MHC II pathway. Various strategies to enhance DNA vaccine potency include upregulating the expression of target antigens and MHC I and II molecules, as well as through enhancing the processing of antigens through the MHC I or MHC II pathways.
Enhancing antigen expression in transfected DCs
Enhancing transcription of HPV DNA
Low levels of HPV antigen expression have been reported in cell lines transfected with wild-type HPV-16 E6 or E7 DNA.113–115 Various strategies have been developed to increase antigen expression levels by enhancing the transcription and translation of HPV DNA. Demethylating agents can potentially enhance transcription of genes encoding for HPV antigens by inhibiting methylation of the cytomegalovirus promoter region commonly used in DNA vaccine expression vectors. Methylation of CpG islands in cytomegalovirus promoter regions have been shown to silence gene expression.116 When combined with a DNA vaccine encoding calreticulin linked to HPV-16 E7 (CRT/E7), treatment with 5-aza-2′-deoxycytidine (DAC), a demethylating agent, upregulated CRT/E7 expression and enhanced E7-specific CD8+ T-cell immune responses in vaccinated mice.117
Enhancing translation of HPV mRNA
Posttranscriptional mechanisms may also play a role in low HPV antigen expression. In one study, Cos-1 cells transfected with wild-type HPV-16 E7 DNA was shown to express high levels of E7 mRNA and low levels of the E7 protein.113 It has been hypothesized that selection pressure imposed on HPV resulted in the viral codon usage to differ from human codon usage.118 Due to viral codon bias, the E6 and E7 gene sequences are poorly recognized by the cellular translational machinery, thereby preventing high expressions of the early viral proteins and enabling the virus to evade immune responses.119
One potential strategy to overcome viral codon bias and thus enhance the translation of genes encoding for HPV antigens is codon optimization, in which viral genes are synthetically engineered to contain only codons preferred by highly expressed human genes.115 Codon-optimized E6 or E7 DNA enhanced antigen expression in transfected cell lines and antigen-specific CD8+ T-cell immune responses in vaccinated mice.115,119 Clinically, VGX-3100 consists of two DNA plasmids encoding optimized consensus HPV-16 and HPV-18 E6 and E7 genes. A phase 1 trial demonstrated that VGX-3100 delivered by electroporation was safe and well-tolerated and induced the proliferation of HPV-specific CD8+ T-cells in patients with CIN2/3.73 A follow-up study administered an additional dose of VGX-3100 to patients who had completed the previous phase 1 trial and demonstrated that a single boost dose of VGX-3100 could augment cellular and humoral immune responses above preboost levels.120 In a phase 2b trial, histological regression was observed in 53 of 107 VGX-3100 recipients.73 Additionally, there is currently a phase 3 trial recruiting patients to continue studying the efficacy, safety, and tolerability of VGX-3100 [NCT03185013]. A phase 2 trial investigating the efficacy of VGX-3100 combined with imiquimod is also currently being conducted [NCT03180684].
Another potential strategy to enhance translation of HPV DNA is the addition of a Kozak sequence and/or a leader sequence. The Kozak sequence is a highly conserved sequence in eukaryotic mRNA, whereas the leader sequence is the mRNA region directly upstream of the initiation codon. Both the Kozak sequence and the leader sequence are important in regulating mRNA translation. One study demonstrated that modifications to DNA vaccines encoding HPV-6 and HPV-11 consensus E6/E7 fusion proteins, including codon optimization and the addition of a Kozak sequence and a highly efficient leader sequence, enhanced E6- and E7-specific CD8+ T-cell immune responses in vaccinated mice.121 Another study compared the effects of codon optimization (opt) and the addition of a Kozak sequence (K) by studying four different plasmids encoding for HPV-16 E7, including E7opt+K, E7opt–K, E7wt+K, and E7wt–K. Comparable E7-specific CD8+ T-cell immune responses and tumor protection were observed in mice vaccinated with the E7opt+K and E7opt–K plasmids. However, the E7opt+K plasmid generated the highest level of E7 expression and was the only plasmid to induce regression of preexisting tumors in vaccinated mice, suggesting a correlation between the level of protein expression and the efficiency of CTL responses.122
Enhancing MHC I and MHC II expression in DCs
MHC I/II expressions in DCs can be enhanced by administering an MHC class II transactivator (CIITA) or a histone deacetylase inhibitor (HDACi). CIITA is a master regulator of MHC II expression and also upregulates MHC I expression on the surface of DCs.123,124 Kim et al. used CIITA DNA to upregulate MHC I/II expression and enhance antigen presentation through MHC class I/II pathways in transfected DCs.125 They also showed that co-administration of CIITA with a DNA vaccine encoding CRT/E6 enhanced E6-specific CD8+ T-cell immune responses, provided long-term protection against TC-1 tumors, and prolonged survival in vaccinated mice.125
Histone deacetylase inhibitors cause hyperacetylation of core histones and upregulate expression of suppressed genes.126 Previous studies have shown that HDACi can enhance DNA vaccines by increasing the expression of the encoded antigens and MHC I/II molecules.127–129 Treatment with AR-42, a novel HDACi, increased surface expressions of MHC I molecules in transfected TC-1 cells while increasing the susceptibility of the tumor cells to E7-specific CD8+ T-cells.130 In addition, co-administration of AR-42 with a HPV DNA vaccine encoding for CRT/E7 enhanced E7-specific immune response, decreased E6/E7-expressing tumor growth, and promoted longer survival in vaccinated mice.130
Enhancing antigen processing through the MHC I cross-presentation pathway
MHC class I molecules present endogenous antigens to CD8+ T-cells to stimulate antigen-specific killing but may also utilize cross-presentation mechanisms of exogenous peptides.131 After exogenous antigens are phagocytosed, they are processed by proteasomes. In one specific cross-presentation route, these processed antigens will be loaded on MHC class I molecules that are present in the endosplasmic reticulum (ER), thus allowing for a cytosolic pathway through ER loading. Similarly, processed antigens can undergo another cytosolic pathway in which they are reimported directly into the phagosome and loaded onto MHC I molecules present. Alternatively, processed antigens can be processed in a third route through direct degradation into smaller peptides within the phagosome and subsequently loaded onto MHC class I molecules through a vacuolar pathway. Various strategies have been developed to enhance the HPV antigen processing and presentation through the MHC I pathways in order to optimize the antitumor effects of CD8+ T-cells by targeting the antigens for (1) proteasomal degradation, (2) entry into ER, or (3) targeting the antigen in the cytoplasm.
Targeting antigens for proteasomal degradation
MHC I molecules bind to peptides generated from proteasomal degradation of cytosolic proteins. The rate of antigen degradation by the ubiquitin-proteasome pathway effects antigen presentation through MHC I pathway.132 Therefore, to enhance the MHC I pathway by targeting the antigens to the proteasome, HPV antigens have been linked to various molecules, such as potato virus X coat protein (PVX-CP), γ-tubulin, and ubiquitin. Fusion of HPV-16 E7 to PVX-CP increased the rate of proteasomal degradation in transfected cells.133 A DNA vaccine encoding the fusion protein inhibited E6/E7-expressing tumor growth and enhanced both E7-specific CD4+ and CD8+ immune responses in vaccinated mice.133 Likewise, γ-tubulin can target proteins to the centrosome, a perinuclear organelle that contains a high density of proteasomes. Fusion of HPV-16 E7 to γ-tubulin enhanced E7 presentation through MHC I pathway, increased the number of E7-specific CD8+ T-cell precursors, and provoked a potent antitumor effect against TC-1 in vaccinated mice.134 Fusion of HPV-16 E6 and/or E7 proteins to ubiquitin induced long-term humoral and cell-mediated immunities and generated both protective and therapeutic antitumor effects in vaccinated mice.135,136
An alternative strategy to enhance proteasomal degradation of HPV antigens involves destabilizing mutations in HPV genes. One study demonstrated that a DNA vaccine containing two mutations in zinc-binding motifs of the HPV-16 E7 gene generated stronger E7-specific CD8+ T-cell responses and greater tumor protection in vaccinated mice when compared with the wild-type DNA vaccine.137
Targeting antigens to the ER
MHC I molecules bind to their respective antigens in the ER. HPV antigens can be targeted to the ER through linkage to various molecules, such as the signal peptide of the adenoviral E3 protein, Pseudomonas aeruginosa exotoxin A domain II [ETA(dII)], and calreticulin. Signal peptides are short peptides at the N-terminus of newly synthesized proteins that determine the localization of the proteins within the cell. DNA vaccines encoding the HPV-16 E7 gene linked to an adenoviral E3 signal sequence, IFN-γ-induced protein 10 (IP-10), or B-cell activating factor promoted E7 accumulation in the endoplasmic reticulum and enhanced E7-specific CD8+ T-cell response in vaccinated mice.138–140 Fusion of E7 to ER chaperone molecules, such as ER-60, tapasin, and calnexin also enhanced E7-specific T-cell responses and generated protective and therapeutic antitumor effects in vaccinated mice.141
CRT is a polypeptide chaperone molecule within the ER that aids in antigen presentation through MHC I pathways.142 Vaccination with HPV-16 E7 fused to CRT increased E7-specific CD8+ T-cell precursors and enhanced antitumor effects against E7-expressing tumors in vaccinated mice when compared with wild-type E7 DNA or CRT DNA.143 In addition, a DNA vaccine encoding CRT/E7 generated the greatest E7-specific CD8+ T-cell immune responses and antitumor effects in vaccinated mice, when compared with other intracellular targeting strategies, including lysosomal-associated membrane protein type 1 (LAMP-1), HSP70, and ETA.144 CRT linkage to other HPV antigens, such as E6 and E6/E7/L2 fusion proteins, has also been shown to enhance antigen-specific CD8+ T-cell immune response and provoke potent antitumor effects in vaccinated mice.145,146 A recent phase 1 clinical trial demonstrated that the DNA vaccine, pNGVL4a-CRT/E7(detox), was both well-tolerated and capable of increasing the level of intraepithelial CD8+ T-cell infiltrates in patients with HPV-16+ CIN2/3. Furthermore, 8 of 27 (30%) patients who received all vaccinations and underwent loop electrosurgical excision procedures showed histologic regression to CIN1 or less.66
However, CRT overexpression has been associated with the development of pancreatic and breast cancers.147,148 To overcome CRT overexpression, only the import and retention signals of CRT in the ER can be inserted, rather than the full-length CRT gene. A DNA vaccine encoding HPV-16 E6 and E7 flanked by ER import and retention signals (SP-E6E7-KDEL) generated comparable antigen-specific CD8+ T-cell responses and therapeutic antitumor effects in vaccinated mice when compared with mice vaccinated with a DNA vaccine encoding E6 and E7 fused to full-length CRT.149 Thus, import and retention signal sequences provide a safe and equally potent alternative.
Targeting antigen to the cytoplasm
Pseudomonas aeruginosa exotoxin A (ETA) is a bacterial toxin with three functional domains. In particular, domain II facilitates the translocation of proteins from the endosomal/lysosomal compartments to the cytoplasm.150 It has been hypothesized that ETA(dII) can enhance antigen presentation through the cross-presentation pathway: when antigens fused to ETA(dII) are released from transfected cells and subsequently uptaken by APCs, ETA(dII) can direct the antigens to the cytoplasm, escape the MHC II pathway, and enter the MHC I pathway instead. In vitro assays demonstrated that ETA(dII)/E7 was presented through the MHC class I pathway more efficiently in transfected cells when compared with wild-type E7.151 In addition, higher percentages of specific lysis were observed in DCs pulsed with cell lysates of 293T cells transfected with ETA(dII)/E7 DNA, indicating that the fusion of ETA(dII) may enhance MHC class I presentation of E7 via a cross-priming mechanism. Furthermore, vaccination with DNA encoding ETA(dII)/E7 increased the number of E7-specific CD8+ T-cell precursors and was able to control E7-expressing metastatic lung tumors in vaccinated mice.151
Flt3 is a tyrosine kinase receptor expressed by DCs. Its ligand, Flt3L, has been shown to expand distinct populations of DCs in vivo.152 Tissue plasma activator (tpa) signal sequences have been used to drive a target protein into the secretory pathway.153 Flt3L, along with tpa, enhances HPV antigen targeting to DCs and antigen cross-presentation. It was shown that Flt3L-E7 fusion proteins are more readily uptaken and presented by DCs through MHC I pathway, when compared with wild-type E7 protein.154 Vaccination of mice with tFE67(Co), a codon-optimized construct including a tpa signal sequence, Flt3L, and fusion of E6 and E7 (tFE67(Co)), induced stronger E6- and E7-specific CD8+ T-cell responses and led to complete TC-1 tumor regression.155 A phase 1 clinical trial demonstrated the safety of the GX-188E DNA vaccine encoding for HPV-16/18 E6/E7 fusion protein linked to Flt3L and tpa administered IM with electroporation.72 E6/E7-specific T-cell response was observed in all nine patients with CIN3, and seven patients displayed complete lesion regression and viral clearance within 36 weeks of follow-up. A follow-up is currently being conducted to monitor changes of immune response in the phase 1 subjects [NCT02411019]. Furthermore, two additional phase 2 trials studying the safety and efficacy of GX-188E are also currently ongoing [NCT02139267, NCT02596243].
Enhancing antigen processing through the MHC II pathway
CD4+ T helper cells produce cytokines and are required to initiate CD8+ T-cell immune responses and maintain long-term memory.156 MHC II molecules present exogenous antigens that have been phagocytosed or endocytosed by APCs to CD4+ T-cells. Linkage of antigens to the sorting signal of LAMP-1 is a potential strategy to enhance HPV antigen processing through MHC II pathway and thus antigen presentation to CD4+ T helper cells. LAMP-1 is a transmembrane protein predominantly localized in lysosomes and late endosomes.157 Fusion of HPV-16 E7 to the sorting signal of LAMP-1 directed E7 antigen toward endosomal/lysosomal compartments and enhanced E7 presentation through MHC class II pathway in transfected cells to activate CD4+ T-cells.158 Mice vaccinated with DNA encoding the chimeric E7/LAMP-1 protein also demonstrated greater numbers of E7-specific CD4+ T helper cells and CD8+ T-cell precursors, stronger E7-specific antibody response, and greater antitumor activity.158,159 Alternatively, antigens can be linked to MHC class II–associated invariant chain (Ii) to target the antigens to the MHC class II pathway. Fusion of HPV-16 E7 to Ii was shown to shift the localization of E7 from the nucleus to the endosomes. In addition, a DNA vaccine encoding Ii/E7 fusion protein enhanced E7-specific CD4+ and CD8+ T-cell responses and offered greater protective antitumor effects in vaccinated mice when compared with E7 fused to ubiquitin or E7 alone.160 A schematic diagraming antigen processing and presentation through MHC class I and II pathways can be found in Fig. 4.
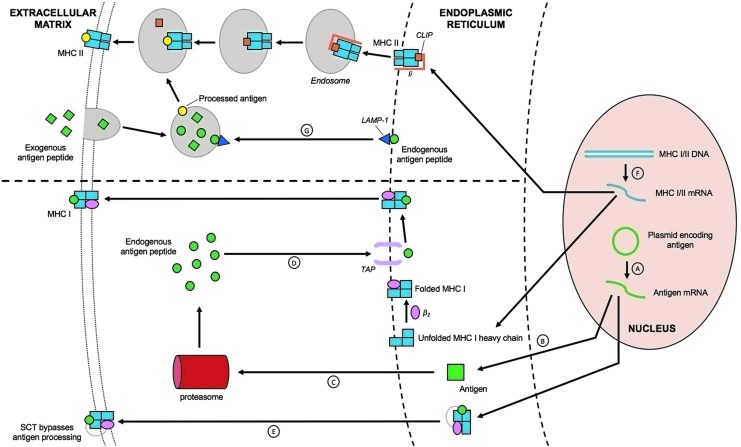
Figure 4.
MHC class I/II pathways enhance antigen processing and presentation. There are several ways in which to improve HPV antigen expression, processing, and presentation in DCs. Of these strategies, enhancing MHC I/II expressions in DCs helps increase antigen processing and presentation. (A) For MHC class I pathways, antigen transcription can be enhanced by diacylglycerols. (B) Plasmid design (i.e., codon optimization, Kozak/leader sequences) also enhance antigen (Ag) translation. (C) Fusion to potato virus X coat protein, γ-tubulin, or ubiquitin and destabilizing mutations target Ags for proteasomal degradation. (D) Subsequent fusion to signal peptides (i.e., adenoviral E3, IP-10, BAFF) or endoplasmic reticulum (ER) chaperone proteins (i.e., GRT, Tap, C21) target endogenous Ags to be presented onto MHC class I molecules in the ER by way of tapasin. These MHC I molecules are later transported from the ER to the extracellular matrix. (E) Single-chain trimers (SCTs) are also involved in bypassing proteasomal antigen processing. (F) Alternatively, co-administering class II transactivator and histone deacetylase inhibitor can enhance MHC I/II expressions through transcription. Class 2-associated Ii chain peptide (CLIP) is a part of the invariant chain (Ii) that binds MHC class II molecules prior to full assembly of the MHC receptor. They help prevent self-peptide fragments from binding until localization of MHC class II molecules into endosomes and lysosomes takes place. (G) Fusion to lysosomal-associated membrane protein type 1 (LAMP-1) targets Ags to the endosomes and lysosomes for export to the extracellular matrix by presentation to MHC class II molecules.
Bypassing antigen processing and presentation of the MHC I pathway
Antigen processing through MHC I pathway involves the loading of antigenic peptides onto newly assembled MHC I heavy chain and β2 microglobulin (β2m). A DNA vaccine encoding single-chain trimers (SCT) consisting of MHC I heavy chain linked β2 microglobulin and peptide has been shown to enhance antigen presentation through the MHC class I pathway by bypassing antigen processing and presentation.161 SCT technology has many advantages compared with vaccines employing conventional antigen processing. Normal antigen processing through MHC class I presentation may be limited by the various steps involved in antigen processing and presentation pathway. In contrast, SCT technology allows the direct presentation of the most immunogenic epitope through MHC class I molecules directly on the surface of transfected cells. Furthermore, the covalent bonding between the three molecules in the trimer increases the stability of the MHC:peptide complex. The stable single-chain construct has been shown to induce stable cell surface expression of the chimeric protein in transfected cells, leading to enhanced presentation of the linked peptide through MHC class I molecules.162 A DNA vaccine encoding for HPV-16 E6 CTL epitope in SCT produced a greater E6-specific CD8+ T-cell immune response and enhanced tumor protection in vaccinated mice when compared with wild-type HPV-16 E6 DNA.163
Enhancing DC function and survival, DC and T-cell interactions, and T-cell function survival
After HPV antigen uptake and presentation, DCs need to interact with T-cells in order to generate effector CD8+ T-cells that have cytotoxic abilities, or effector CD4+ T-cells that help prime CD8+ T-cells.164,165 The generation of potent cellular immune responses, including CTL responses to kill tumor cells, is crucial for antitumor effects.166 Thus, other ways to enhance therapeutic HPV DNA vaccine potency include: (1) enhancing DC function and survival, (2) boosting DC and T-cell interactions, (3) promoting T-cell function and survival, and (4) enhancing immune responses through elimination of regulatory T-cells (Tregs).
Enhancing DC function and survival
DC activation is necessary for T-cell priming. Studies have also shown that certain forms of DC activation enhance cross-priming, which then induces CTL response.167,168 Strategies to activate DCs and enhance the ability of DCs to cross-prime CD8+ T-cells include co-administration of TLR agonists [NCT03206138, NCT03180684]169,170 and DC-activating cytokines,171 and fusion of HPV antigen gene sequences with the human HSV-1 gD protein.172
Activation of the TLRs present on macrophages and DCs has been shown to induce pro-inflammatory cytokine secretion and enhance presentation of antigens to naïve T-cells.173,174 In one murine model, the TLR3 agonist poly(I:C) and the TLR7 agonist resiquimod were co-administered with HPV-16 E7 DNA vaccine, enhancing antigen-specific lymphocyte proliferation and cytolytic activity and antitumor effects against the TC-1 tumor model, compared with effects of the DNA vaccine alone.169 In another murine model, co-administration of the TLR7 agonist imiquimod and the CRT/E7 DNA vaccine also generated enhanced antitumor immunity and antitumor effects.170 Clinical trials combining HPV-16/18 E6/E7 DNA vaccination with local administration of imiquimod at the lesion site are currently being conducted [NCT03206138, NCT03180684].
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine that has been shown to stimulate DC proliferation and is important for DC development.175–177 In mice bearing cervicovaginal TC-1 tumors in the cervicovaginal tract, IM vaccination with CRT/E7 DNA vaccine followed by intravaginal administration of GM-CSF caused an accumulation of E7-specific CD8+ T-cells and DCs in cervicovaginal tumors, and demonstrated antitumor effects, possibly mediated by enhanced cross-presentation of antigens by DCs.171
HSV-1gD protein has been reported to induce activation of myeloid dendritic cells and to induce type 1 IFN secretion.178 A DNA vaccine construct of fusion of HPV-16 E6 and/or E7 protein sequences to the gD protein generated HPV antigen–specific CD8+ T-cell immune responses and partial therapeutic effect (40%) against TC-1 tumor in murine models.172
DCs undergo a fairly rapid rate of turnover, with a half-life of around 2 days. Once activated, they generate maximal immune responses that consequently lead to cell death through multiple apoptotic pathways.179 Strategies to prolong the survival of antigen-expressing DCs have thus been employed. Gene gun co-administration of DNA encoding apoptosis inhibitors such as BCL-xL specifically prolonged the survival of antigen-expressing DCs. This generated enhanced antigen-specific CD8+ T-cell immune responses and antitumor effects.180 Administration of another DNA vaccine construct of HPV-16 E7 sequence fused to the apoptosis inhibitory protein connective tissue growth factor prolonged the survival of transfected DCs in vivo and generated a higher number of E7-specific CD8+ T-cells than administration of the E7 DNA vaccine alone.181 However, administration of anti-apoptotic proteins raises safety concerns for cellular transformation, and thus co-administration of siRNA that targets key pro-apoptotic proteins Bak and Bax is deemed a more plausible strategy,182 as RNA is susceptible to degradation and will only generate transient effects.
Boosting DC and T-cell interactions
CD4+ T-cells help mediate DC and T-cell interactions. They are important in promoting CD8+ T-cell survival and generating CD8+ T-cell memory function.165,183 Thus, CD4+ T-cell activation enhances the antigen-specific immune response generated by CD8+ T-cells.
The invariant chain (Ii) is a critical molecule in the MHC class II presentation pathway. It binds to newly synthesized MHC class II α and β chains, and is later proteolytically degraded in the endosomal-lysosomal pathway, leaving only the class II–associated Ii chain peptide (CLIP) fragment. CLIP is later replaced by peptides that bind to the antigen-binding groove of MHC class ii2 molecules.184 The invariant Pan HLA-DR reactive epitope (Ii-PADRE) is an Ii in which CLIP is replaced by PADRE. Instead of being eventually replaced by peptides like CLIP, PADRE is strongly bound to and presented on MHC class II molecules to induce PADRE-specific CD4+ T-cell immune responses.185 The interleukin-2 (IL-2) cytokines generated by the PADRE-specific CD4+ T-cells can then enhance CD8+ T-cell responses.186 Co-administration of DNA encoding Ii-PADRE with antigen-encoding DNA vaccines elicited stronger antigen-specific CD8+ T-cell immune responses and generated antitumor effects against antigen-expressing tumor cells in mice.185 Furthermore, mice vaccinated with DNA vaccine construct that directly fused Ii-PADRE to HPV-16 E6 (Ii-PADRE-E6) generated significantly stronger E6-specific CD8+ T-cell immune responses and antitumor effects against E6-expressing tumors, compared with mice vaccinated with Ii-E6 DNA.187 This suggests that CD4+ T helper cells, through boosting DC and CD8+ T-cell interactions, can play an important role in enhancing the potency of therapeutic HPV DNA vaccines.
Similar methods to enhance CD4+ T-cell help include co-administration of DNA encoding other CD4+ T-cell epitopes, such as tetanus toxin fragment C (TTFC) and bovine papillomavirus type 1 (BPV-1) L1 or L2 antigens.171,188 TTFC includes a promiscuous CD4+ helper epitope that binds to numerous histocompatibility alleles, activating a large amount of TTFC-specific CD4+ T-cells and subsequently inducing CD8+ T-cell responses.188 BPV-1 L1 or L2 antigens also contain CD4+ T-cell epitopes that would stimulate generation of CD4+ T-cell help.171 Indeed, administration of DNA vaccines encoding HPV-16 antigens fused with TTFC, or co-administration of vectors expressing BPV-1 L1 and/ or L2 DNA linked with CRT/E7 DNA both generated enhanced antigen-specific CD8+ T-cell immune responses and antitumor effects, compared with administration of DNA vaccine encoding for HPV antigen alone.171,188 More recently, fusing BPV-1 L1 with the E7 epitope has also shown immunogenicity in eliciting effective E7-specific antitumor T-cell- and B-cell-mediated responses.189
Another way to enhance DC and T-cell interactions is through co-administration of xenogeneic MHC class I molecules. The generated local inflammation state by the xenogenic MHC class I molecules attracts immune cells to antigen sites and causes increased release of antigen from muscle cells, leading to enhanced antigen uptake by APCs and stronger APC and T-cell interactions at the local inflammatory site. Further, it has been shown that co-administration of xenogeneic MHC class I DNA with the CRT-E7 DNA vaccine recruited APCs and CD8+ T-cells to the injection site.190 This suggests that cross-priming happened in the local muscle tissue in addition to the draining lymph nodes.
Promoting T-cell function and survival
T-cell function and survival can be promoted by co-administration of cytokines and co-stimulatory molecules important for T-cell development and maintenance, such as IL-2, anti-4-1BB, IL-7, IL-12, B7, and IL-33. Chemokines that target effector T-cells to tumor sites such as IP-10 also generate synergistic effects. In addition, anti-programmed death-ligand 1 (anti-PD-L1) and Fas ligand (FasL) blockage reduce apoptosis of T-cells.
IL-2 is a cytokine produced by activated T-cells and helps induce T-cell proliferation. Although IL-2 is important for the development and maintenance of Tregs, which exert negative regulatory effects on the immune response, it is still a key cytokine in cytotoxic and helper T-cell activation.191 DNA vaccines encoding fusion of IL-2 to HPV-16 E7 generated the highest frequency of E7-specific CD8+ T-cells and demonstrated the strongest antitumor effects against E7-expressing tumors relative to control DNA vaccine constructs.192 4-1BB receptors play a role in the preferential induction of CD8+ T-cell proliferation and co-administration of IL-2 cDNA, and anti-4-1BB antibodies with the HPV E7 construct generated enhanced IFNγ production from E7-specific CD8+ T-cells and increased E7-specific CTL activity.193,194
IL-7 is an important cytokine in T- and B-cell development, specifically, naïve T-cells require IL-7 for homeostatic proliferation and survival.195 In mice, intravaginal co-administration of Fc-fused IL-7 recruited several types of immune cells to the genital tract and generated a higher number of HPV antigen-specific CD8+ T-cells in the genital mucosa, leading to stronger antitumor effects.196 A clinical trial examining the safety and efficacy of the HPV therapeutic DNA vaccine GX-188E co-administered with GX-17 (a protein drug recombining human IL-7 and hybrid Fc) [NCT02860715] or imiquimod is currently being conducted [NCT03206138].
IL-12 produced by DCs is involved in the differentiation of naïve T-cells into T helper 1 cells, leading to increased CD8+ activity and the production of IFNγ by T-cells.197,198 Multiple phase 1/2 clinical trials are currently exploring the effects of co-administration of plasmids encoding IL-12 [NCT02172911, NCT03162224, NCT02241369].
B7.1/B7.2 (CD80/CD86) molecules on APCs bind to CD28 on T-cells to provide co-stimulatory signals for T-cell activation.199 Thus, DNA vaccine vectors that co-express B7.1 and/or B7.2 may enhance T-cell activation. One study has shown that the PVAX1/HPV-58 mE6E7FcGB recombinant DNA vaccine, which co-expresses GM-CSF and B7.1, was able to enhance antigen-specific T-cell immune responses while delaying tumor development in HPV-58(+) B16/HPV-58 E6/E7 tumor-challenged mice.200
IL-33 is a cytokine expressed by epithelial and endothelial cells. Its release during cell injuries promotes pro-inflammatory responses, activates and recruits APCs, and enhances adaptive immunity.201 Co-administration of a plasmid encoding IL-33 and a plasmid encoding HPV-16/ConE6E7 was been shown to enhance antigen-specific CD4+ and CD8+ T-cell immunity while inducing regression in established TC-1 tumor-bearing mice.202
IP-10 (CXCL10) is a chemokine that can recruit effector T-cells and may play a role in effector T-cell generation. It has also been found to activate APCs.203,204 It is possible that co-administration of IP-10 would enhance antigen-specific effector T-cell functions to generate stronger antitumor effects. Indeed, co-administration of plasmids encoding IP-10 with plasmids encoding E7-NT-gp96 at TC-1 tumor cell inoculation site was able to significantly suppress TC-1 tumor growth.205
Immune checkpoints negatively regulate the immune system to maintain self-tolerance and prevent the development of autoimmune diseases. However, immune checkpoint pathways are also exploited by tumor cells to inhibit T-cell immune responses. Such T-cell immune checkpoint pathways involve checkpoint proteins such as CTLA4 and PD1. Checkpoint blockades have therefore emerged as a promising method to enhance T-cell function (for review, see Pardoll, 2012206). In mice, co-administration of soluble PD-1 DNA with the HPV-16 E7 DNA vaccine was shown to enhance E7-specific CD8+ T-cell responses and to induce antitumor effects.207
PD-L1 on tumor cells promotes T-cell apoptosis.208 Anti-PD-L1 therapy can block PD-L1 on tumors to prevent induction of T-cell death and enhance antitumor immune responses. A phase 1b/IIa clinical trial to evaluate the safety and tolerability, antitumor activity, and immunogenicity of HPV DNA vaccine MEDI0457 (INO 3112) [a mixture of three plasmids encoding HPV-16/-18 E6/E7 antigens and IL-12] co-administered with durvalumab (MEDI4736) [a human monoclonal anti-PD-L1] in patients with HPV-associated recurrent or metastatic head and neck cancer is currently being conducted [NCT03162224].
FasL expressed by DCs can induce apoptosis of both CD4+ and CD8+ T-cells by binding to Fas, a death receptor expressed by T-cells.209–211 Through co-administration of DNA encoding short hairpin RNA that targets FasL with a therapeutic DNA vaccine encoding for HPV-16 E7 intradermally, the successfully transfected DCs that present HPV-16 E7 may lead to the reduction of FasL expression. Apoptosis of E7-specific CD8+ T-cells is therefore reduced. In line with this, significantly stronger E7-specific CD8+ T-cell responses and stronger CTL responses against E7-expressing tumors were observed.212
Eliminating immunosuppressive regulatory T-cells improves immune responses
Tregs have been shown to accumulate in the tumor microenvironment and reduce tumor-associated antigen-specific T-cell immunity.213 Treg frequency was not only higher in total peripheral blood in CIN and cervical patients, but it was also higher in the subpopulation of CD4+ T-cells.214 In addition, increased infiltration of Foxp3+ Tregs is often associated with the suppression of immune responses in HPV(+) tongue squamous cell carcinoma samples while promoting the progression of tumors.215 Therefore, depletion of Tregs serves as a potential method to enhance antitumor immune responses in patients treated with therapeutic HPV DNA vaccination. Agents that have been used in combination with HPV DNA vaccines to deplete Tregs include cyclophosphamide and anti-CD25 monoclonal antibody (PC61). More potent antigen-specific immune responses including higher levels of HPV antigen–specific CD8+ T-cells and stronger antitumor effects were observed in both studies, compared with DNA vaccination or Treg-suppressing agent treatment alone.216,217
Conclusion
The development of preventive HPV vaccines has made considerable progress over the past decade. With increasing coverage of various HPV strains, Gardasil®/Gardasil-9® and Cervarix® have provided the public with a means of thwarting HPV infections. However, there remains a large number of individuals with existing HPV infection or HPV-associated diseases who are at risk of developing HPV-associated malignancies and cancers. Therefore, the need for therapeutic HPV vaccines is more important than ever. The main focus of this review was to discuss the current therapeutic HPV DNA vaccines available and being developed for the treatment of HPV infections and HPV-associated diseases. Therapeutic HPV DNA vaccines express tumor antigenic peptides and utilize APCs such as DCs to provide effective CTL and antibody responses, allowing for the development of an adaptive immune response and immunologic memory. DNA vaccines are safe, stable, and easy to produce, demonstrating potential for large-scale clinical distribution. Many therapeutic HPV DNA vaccines are undergoing clinical trials to assess safety and efficacy in humans, with the hopes of providing a possible alternative or addition to current treatment methods for HPV-associated malignancies, such as chemotherapy and radiotherapy. There are many strategies to improve the potency of therapeutic HPV DNA vaccines, including increasing DC uptake of HPV antigens, improving DC processing and presentation of HPV antigens, and enhancing DC and T-cell interactions. Expanding our knowledge of APC biology and CTL response generation will ultimately help advance approaches to optimize vaccine-based T-cell immunity and further develop innovative strategies to maximize the efficacy of therapeutic HPV DNA vaccines. A deeper knowledge of the tumor microenvironments also holds great potential for improving therapeutic efficacies.
The future of the development of therapeutic HPV DNA vaccines relies heavily on the vaccine's administration in combination with other treatments to produce synergistic effects. When combined with therapeutic HPV DNA vaccines, conventional treatments, such as chemotherapy and radiotherapy, result in enhanced CD8+ T-cell responses and antitumor effects. As a result, the prospect of combining therapeutic HPV DNA vaccines with chemotherapy and radiation therapy holds great potential in optimizing therapeutic efficacies.
In addition, further improvements in adjuvant therapy, prime-boost regimen, and other combinatorial methods may provide better therapeutic HPV DNA vaccine strategies to enhance T-cell immune responses. For example, a heterologous DNA prime-recombinant vaccinia vector–based boost vaccination that targets HPV-16 and HPV-18 E6/E7 was found to be safe and tolerable. This vaccination strategy was found to generate a more potent immune response than DNA alone, thus providing impetus for future strategies to enhance CTL immune responses.99
Co-administering drugs that activate DCs and plasmids encoding cytokines that promote T-cell function also holds potential for eliciting stronger immune responses. The various methods of vaccine administration discussed in this review also hold potential to boost the immunotherapeutic responses elicited by therapeutic HPV DNA vaccines. For example, the aforementioned femtosecond laser treatment method has been previously shown to enhance DNA transfection efficiency administered intradermally and intratumorally in vivo.219 As a result, this technology represents a potentially safe and novel technique to enhance DNA vaccine potency through better DNA transfection efficiency. Further optimization of this delivery strategy may provide improvements in DNA vaccination for the therapeutic treatment of HPV.
Currently, more research on the effects of combination approaches and their underlying mechanisms is needed to possibly optimize different synergistic approaches individualized for each patient. A previous study has shown that an HPV DNA vaccine using human CRT linked to HPV-16 E6, E7, and L2 (hCRTE6E7L2) successfully generated both potent E6/E7-specific CD8+ T-cell immune responses and L2-specific neutralizing antibody responses.145 The addition of L2 is especially imperative as it has been shown to generate specific humoral immune responses, leading to a pan-protective effect against different types of HPV infections.220,221 Thus, hCRTE6E7L2 has the potential to protect against HPV infection and generate potent T-cell-based immune responses. More recently, a study investigating a therapeutic vaccine employing E7 and L2 demonstrated that the vaccination was also able to elicit strong L2-specific immunoglobulin G humoral responses while still providing modest T-cell-mediated immunity.222 This provides excellent prospect for the future of DNA vaccine research capable of generating both preventive and therapeutic effects against HPV infections. With the well-established effects of prophylactic HPV vaccines, the development of future DNA vaccines may combine the effects of both preventive and therapeutic measures into a single administration to healthy patients. These continuous efforts to develop therapeutic vaccines will help control HPV-associated malignancies alongside conventional HPV treatment methods.
Acknowledgments
The authors thank Andrew Yang for his helpful discussion during the preparation of this review.
This work was supported by the United States National Institutes of Health Cervical Cancer Specialized Program of Research Excellence (P50 CA098252) and Research Project Grant (R01 CA114425).
Author Disclosure
No competing financial interests exist.
References
- 1.Small W, Jr., Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer 2017;123:2404–2412 [DOI] [PubMed] [Google Scholar]
- 2.Khallouf H, Grabowska AK, Riemer AB. Therapeutic vaccine strategies against human papillomavirus. Vaccines (Basel) 2014;2:422–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30 Suppl 5:F12–23 [DOI] [PubMed] [Google Scholar]
- 4.Wakeham K, Kavanagh K. The burden of HPV-associated anogenital cancers. Curr Oncol Rep 2014;16:402. [DOI] [PubMed] [Google Scholar]
- 5.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev 2003;16:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol 2017;40:80–85 [PubMed] [Google Scholar]
- 7.Ljubojevic S, Skerlev M. HPV-associated diseases. Clin Dermatol 2014;32:227–234 [DOI] [PubMed] [Google Scholar]
- 8.Bansal A, Singh MP, Rai B. Human papillomavirus-associated cancers: A growing global problem. Int J Appl Basic Med Res 2016;6:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbyn M, de Sanjose S, Saraiya M, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer 2012;131:1969–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham SV. Human papillomavirus: Gene expression, regulation and prospects for novel diagnostic methods and antiviral therapies. Future Microbiol 2010;5:1493–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egawa N, Egawa K, Griffin H, et al. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015;7:3863–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doorbar J, Egawa N, Griffin H, et al. Human papillomavirus molecular biology and disease association. Rev Med Virol 2015;25 Suppl 1:2–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibeanu OA. Molecular pathogenesis of cervical cancer. Cancer Biol Ther 2011;11:295–306 [DOI] [PubMed] [Google Scholar]
- 14.Sanclemente G, Gill DK. Human papillomavirus molecular biology and pathogenesis. J Eur Acad Dermatol Venereol 2002;16:231–240 [DOI] [PubMed] [Google Scholar]
- 15.Lin K, Roosinovich E, Ma B, et al. Therapeutic HPV DNA vaccines. Immunol Res 2010;47:86–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frazer IH, Leggatt GR, Mattarollo SR. Prevention and treatment of papillomavirus-related cancers through immunization. Annu Rev Immunol 2011;29:111–138 [DOI] [PubMed] [Google Scholar]
- 17.Kash N, Lee MA, Kollipara R, et al. Safety and Efficacy Data on Vaccines and Immunization to Human Papillomavirus. J Clin Med 2015;4:614–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol 2010;11:1048–1056 [DOI] [PubMed] [Google Scholar]
- 19.Schiller JT, Muller M. Next generation prophylactic human papillomavirus vaccines. Lancet Oncol 2015;16:e217–225 [DOI] [PubMed] [Google Scholar]
- 20.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015;372:711–723 [DOI] [PubMed] [Google Scholar]
- 21.Cuzick J. Gardasil 9 joins the fight against cervix cancer. Expert Rev Vaccines 2015;14:1047–1049 [DOI] [PubMed] [Google Scholar]
- 22.Signorelli C, Odone A, Ciorba V, et al. Human papillomavirus 9-valent vaccine for cancer prevention: A systematic review of the available evidence. Epidemiol Infect 2017;145:1962–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma B, Maraj B, Tran NP, et al. Emerging human papillomavirus vaccines. Expert Opin Emerg Drugs 2012;17:469–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang A, Jeang J, Cheng K, et al. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev Vaccines 2016;15:989–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper DM, Williams KB. Prophylactic HPV vaccines: Current knowledge of impact on gynecologic premalignancies. Discov Med 2010;10:7–17 [PubMed] [Google Scholar]
- 26.Klaes R, Woerner SM, Ridder R, et al. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res 1999;59:6132–6136 [PubMed] [Google Scholar]
- 27.van der Burg SH, Melief CJ. Therapeutic vaccination against human papilloma virus induced malignancies. Curr Opin Immunol 2011;23:252–257 [DOI] [PubMed] [Google Scholar]
- 28.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008;8:351–360 [DOI] [PubMed] [Google Scholar]
- 29.Hung CF, Monie A, Alvarez RD, et al. DNA vaccines for cervical cancer: From bench to bedside. Exp Mol Med 2007;39:679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vici P, Pizzuti L, Mariani L, et al. Targeting immune response with therapeutic vaccines in premalignant lesions and cervical cancer: Hope or reality from clinical studies. Expert Rev Vaccines 2016;15:1327–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang A, Farmer E, Wu TC, et al. Perspectives for therapeutic HPV vaccine development. J Biomed Sci 2016;23:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang A, Farmer E, Lin J, et al. The current state of therapeutic and T cell-based vaccines against human papillomaviruses. Virus Res 2017;231:148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith GL, Mackett M, Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature 1983;302:490–495 [DOI] [PubMed] [Google Scholar]
- 34.Moss B, Smith GL, Gerin JL, et al. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature 1984;311:67–69 [DOI] [PubMed] [Google Scholar]
- 35.Detmer A, Glenting J. Live bacterial vaccines–a review and identification of potential hazards. Microb Cell Fact 2006;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miles B, Safran HP, Monk BJ. Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11-001. Gynecol Oncol Res Pract 2017;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Hernandez E, Gonzalez-Sanchez JL, Andrade-Manzano A, et al. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Ther 2006;13:592–597 [DOI] [PubMed] [Google Scholar]
- 38.Brun JL, Dalstein V, Leveque J, et al. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. Am J Obstet Gynecol 2011;204:169 e161–168 [DOI] [PubMed] [Google Scholar]
- 39.Gunn GR, Zubair A, Peters C, et al. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol 2001;167:6471–6479 [DOI] [PubMed] [Google Scholar]
- 40.Hagensee ME, Carter JJ, Wipf GC, et al. Immunization of mice with HPV vaccinia virus recombinants generates serum IgG, IgM, and mucosal IgA antibodies. Virology 1995;206:174–182 [DOI] [PubMed] [Google Scholar]
- 41.Chang CL, Ma B, Pang X, et al. Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Mol Ther 2009;17:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marzi A, Kercher L, Marceau J, et al. Stat1-deficient mice are not an appropriate model for efficacy testing of recombinant vesicular stomatitis virus-based filovirus vaccines. J Infect Dis 2015;212 Suppl 2:S404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartkowiak T, Singh S, Yang G, et al. Unique potential of 4–1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proc Natl Acad Sci U S A 2015;112:E5290–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidenreich R, Jasny E, Kowalczyk A, et al. A novel RNA-based adjuvant combines strong immunostimulatory capacities with a favorable safety profile. Int J Cancer 2015;137:372–384 [DOI] [PubMed] [Google Scholar]
- 45.Shen KY, Chang LS, Leng CH, et al. Self-adjuvanting lipoimmunogens for therapeutic HPV vaccine development: Potential clinical impact. Expert Rev Vaccines 2015;14:383–394 [DOI] [PubMed] [Google Scholar]
- 46.Neefjes J, Jongsma ML, Paul P, et al. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol 2011;11:823–836 [DOI] [PubMed] [Google Scholar]
- 47.Chang EY, Chen CH, Ji H, et al. Antigen-specific cancer immunotherapy using a GM-CSF secreting allogeneic tumor cell-based vaccine. Int J Cancer 2000;86:725–730 [DOI] [PubMed] [Google Scholar]
- 48.Mikyskova R, Indrova M, Simova J, et al. Treatment of minimal residual disease after surgery or chemotherapy in mice carrying HPV16-associated tumours: Cytokine and gene therapy with IL-2 and GM-CSF. Int J Oncol 2004;24:161–167 [PubMed] [Google Scholar]
- 49.Lesterhuis WJ, Aarntzen EH, De Vries IJ, et al. Dendritic cell vaccines in melanoma: From promise to proof? Crit Rev Oncol Hematol 2008;66:118–134 [DOI] [PubMed] [Google Scholar]
- 50.Keenan BP, Jaffee EM. Whole cell vaccines–Past progress and future strategies. Semin Oncol 2012;39:276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng WF, Hung CF, Hsu KF, et al. Cancer immunotherapy using Sindbis virus replicon particles encoding a VP22-antigen fusion. Hum Gene Ther 2002;13:553–568 [DOI] [PubMed] [Google Scholar]
- 52.Cassetti MC, McElhiney SP, Shahabi V, et al. Antitumor efficacy of Venezuelan equine encephalitis virus replicon particles encoding mutated HPV16 E6 and E7 genes. Vaccine 2004;22:520–527 [DOI] [PubMed] [Google Scholar]
- 53.Berglund P, Quesada-Rolander M, Putkonen P, et al. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res Hum Retroviruses 1997;13:1487–1495 [DOI] [PubMed] [Google Scholar]
- 54.Kubler H, Scheel B, Gnad-Vogt U, et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: A first-in-man phase I/IIa study. J Immunother Cancer 2015;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gurunathan S, Klinman DM, Seder RA. DNA vaccines: Immunology, application, and optimization*. Annu Rev Immunol 2000;18:927–974 [DOI] [PubMed] [Google Scholar]
- 56.Donnelly JJ, Ulmer JB, Shiver JW, et al. DNA vaccines. Annu Rev Immunol 1997;15:617–648 [DOI] [PubMed] [Google Scholar]
- 57.Lin K, Doolan K, Hung CF, et al. Perspectives for preventive and therapeutic HPV vaccines. J Formos Med Assoc 2010;109:4–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowe DB, Shearer MH, Kennedy RC. DNA vaccines: Successes and limitations in cancer and infectious disease. J Cell Biochem 2006;98:235–242 [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Troilo PJ, Wang X, et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther 2004;11:711–721 [DOI] [PubMed] [Google Scholar]
- 60.O'Brien JA, Lummis SC. Biolistic transfection of neuronal cultures using a hand-held gene gun. Nat Protoc 2006;1:977–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagao K, Ginhoux F, Leitner WW, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A 2009;106:3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen CH, Ji H, Suh KW, et al. Gene gun-mediated DNA vaccination induces antitumor immunity against human papillomavirus type 16 E7-expressing murine tumor metastases in the liver and lungs. Gene Ther 1999;6:1972–1981 [DOI] [PubMed] [Google Scholar]
- 63.Dileo J, Miller TE, Jr, Chesnoy S, et al. Gene transfer to subdermal tissues via a new gene gun design. Hum Gene Ther 2003;14:79–87 [DOI] [PubMed] [Google Scholar]
- 64.Trimble C, Lin CT, Hung CF, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine 2003;21:4036–4042 [DOI] [PubMed] [Google Scholar]
- 65.Best SR, Peng S, Juang CM, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine 2009;27:5450–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alvarez RD, Huh WK, Bae S, et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3). Gynecol Oncol 2016;140:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neumann E, Rosenheck K. Permeability changes induced by electric impulses in vesicular membranes. J Membr Biol 1972;10:279–290 [DOI] [PubMed] [Google Scholar]
- 68.Keane-Myers AM, Bell M. Evolution of electroporated DNA vaccines. Methods Mol Biol 2014;1121:269–278 [DOI] [PubMed] [Google Scholar]
- 69.Widera G, Austin M, Rabussay D, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol 2000;164:4635–4640 [DOI] [PubMed] [Google Scholar]
- 70.Kichaev G, Mendoza JM, Amante D, et al. Electroporation mediated DNA vaccination directly to a mucosal surface results in improved immune responses. Hum Vaccin Immunother 2013;9:2041–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun Y, Peng S, Qiu J, et al. Intravaginal HPV DNA vaccination with electroporation induces local CD8+ T-cell immune responses and antitumor effects against cervicovaginal tumors. Gene Ther 2015;22:528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim TJ, Jin HT, Hur SY, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun 2014;5:5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bagarazzi ML, Yan J, Morrow MP, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 2012;4:155ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015;386:2078–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeira E, Manevitch A, Khatchatouriants A, et al. Femtosecond infrared laser-an efficient and safe in vivo gene delivery system for prolonged expression. Mol Ther 2003;8:342–350 [DOI] [PubMed] [Google Scholar]
- 76.Zeira E, Manevitch A, Manevitch Z, et al. Femtosecond laser: A new intradermal DNA delivery method for efficient, long-term gene expression and genetic immunization. FASEB J 2007;21:3522–3533 [DOI] [PubMed] [Google Scholar]
- 77.Hedley ML, Curley J, Urban R. Microspheres containing plasmid-encoded antigens elicit cytotoxic T-cell responses. Nat Med 1998;4:365–368 [DOI] [PubMed] [Google Scholar]
- 78.Tahamtan A, Ghaemi A, Gorji A, et al. Antitumor effect of therapeutic HPV DNA vaccines with chitosan-based nanodelivery systems. J Biomed Sci 2014;21:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klencke B, Matijevic M, Urban RG, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: A phase I study of ZYC101. Clin Cancer Res 2002;8:1028–1037 [PubMed] [Google Scholar]
- 80.Alvarez-Salas LM. Amolimogene bepiplasmid, a DNA-based therapeutic encoding the E6 and E7 epitopes from HPV, for cervical and anal dysplasia. Curr Opin Mol Ther 2008;10:622–628 [PubMed] [Google Scholar]
- 81.Garcia F, Petry KU, Muderspach L, et al. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: A randomized controlled trial. Obstet Gynecol 2004;103:317–326 [DOI] [PubMed] [Google Scholar]
- 82.Wang S. Tat peptide conjugates of low molecular weight polyethylenimine as effective non-viral gene delivery vectors. Mol Ther 2006;13:S76 [Google Scholar]
- 83.Alexis FL S.-L.; Wang S. Covalent attachment of low molecular weight poly(ethylene imine) improves tat peptide mediated gene delivery. Adv Mater 2006;18:2174–2178 [Google Scholar]
- 84.Bolhassani A, Ghasemi N, Servis C, et al. The efficiency of a novel delivery system (PEI600-Tat) in development of potent DNA vaccine using HPV16 E7 as a model antigen. Drug Deliv 2009;16:196–204 [DOI] [PubMed] [Google Scholar]
- 85.Elliott G, O'Hare P. Intercellular trafficking of VP22-GFP fusion proteins. Gene Ther 1999;6:149–151 [DOI] [PubMed] [Google Scholar]
- 86.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell 1997;88:223–233 [DOI] [PubMed] [Google Scholar]
- 87.Phelan A, Elliott G, O'Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat Biotechnol 1998;16:440–443 [DOI] [PubMed] [Google Scholar]
- 88.Lai Z, Han I, Zirzow G, et al. Intercellular delivery of a herpes simplex virus VP22 fusion protein from cells infected with lentiviral vectors. Proc Natl Acad Sci U S A 2000;97:11297–11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lundberg M, Johansson M. Is VP22 nuclear homing an artifact? Nat Biotechnol 2001;19:713–714 [DOI] [PubMed] [Google Scholar]
- 90.Beerens AM, Rots MG, de Vries EF, et al. Fusion of herpes simplex virus thymidine kinase to VP22 does not result in intercellular trafficking of the protein. Int J Mol Med 2007;19:841–849 [DOI] [PubMed] [Google Scholar]
- 91.Hung CF, Cheng WF, Chai CY, et al. Improving vaccine potency through intercellular spreading and enhanced MHC class I presentation of antigen. J Immunol 2001;166:5733–5740 [DOI] [PubMed] [Google Scholar]
- 92.Kim TW, Hung CF, Kim JW, et al. Vaccination with a DNA vaccine encoding herpes simplex virus type 1 VP22 linked to antigen generates long-term antigen-specific CD8-positive memory T cells and protective immunity. Hum Gene Ther 2004;15:167–177 [DOI] [PubMed] [Google Scholar]
- 93.Michel N, Osen W, Gissmann L, et al. Enhanced immunogenicity of HPV 16 E7 fusion proteins in DNA vaccination. Virology 2002;294:47–59 [DOI] [PubMed] [Google Scholar]
- 94.Hung CF, He L, Juang J, et al. Improving DNA vaccine potency by linking Marek's disease virus type 1 VP22 to an antigen. J Virol 2002;76:2676–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Basu S, Binder RJ, Ramalingam T, et al. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001;14:303–313 [DOI] [PubMed] [Google Scholar]
- 96.Garrod T, Grubor-Bauk B, Yu S, et al. Encoded novel forms of HSP70 or a cytolytic protein increase DNA vaccine potency. Hum Vaccin Immunother 2014;10:2679–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Farzanehpour M, Soleimanjahi H, Hassan ZM, et al. HSP70 modified response against HPV based tumor. Eur Rev Med Pharmacol Sci 2013;17:228–234 [PubMed] [Google Scholar]
- 98.Trimble CL, Peng S, Kos F, et al. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res 2009;15:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maldonado L, Teague JE, Morrow MP, et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med 2014;6:221ra213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCoy KD, Le Gros G. The role of CTLA-4 in the regulation of T cell immune responses. Immunol Cell Biol 1999;77:1–10 [DOI] [PubMed] [Google Scholar]
- 101.Tai X, Van Laethem F, Pobezinsky L, et al. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood 2012;119:5155–5163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boyle JS, Brady JL, Lew AM. Enhanced responses to a DNA vaccine encoding a fusion antigen that is directed to sites of immune induction. Nature 1998;392:408–411 [DOI] [PubMed] [Google Scholar]
- 103.Deliyannis G, Boyle JS, Brady JL, et al. A fusion DNA vaccine that targets antigen-presenting cells increases protection from viral challenge. Proc Natl Acad Sci U S A 2000;97:6676–6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yin Y, Wu C, Song J, et al. DNA immunization with fusion of CTLA-4 to hepatitis B virus (HBV) core protein enhanced Th2 type responses and cleared HBV with an accelerated kinetic. PLoS One 2011;6:e22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gan L, Jia R, Zhou L, et al. Fusion of CTLA-4 with HPV16 E7 and E6 enhanced the potency of therapeutic HPV DNA vaccine. PLoS One 2014;9:e108892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng S, Monie A, Pang X, et al. Vascular disrupting agent DMXAA enhances the antitumor effects generated by therapeutic HPV DNA vaccines. J Biomed Sci 2011;18:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kang TH, Lee JH, Song CK, et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res 2007;67:802–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chuang CM, Monie A, Wu A, et al. Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. J Biomed Sci 2009;16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tseng CW, Hung CF, Alvarez RD, et al. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res 2008;14:3185–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tseng CW, Monie A, Wu CY, et al. Treatment with proteasome inhibitor bortezomib enhances antigen-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. J Mol Med (Berl) 2008;86:899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ching LM, Cao Z, Kieda C, et al. Induction of endothelial cell apoptosis by the antivascular agent 5,6-Dimethylxanthenone-4-acetic acid. Br J Cancer 2002;86:1937–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tseng CW, Trimble C, Zeng Q, et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer Immunol Immunother 2009;58:737–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu W, Gao F, Zhao KN, et al. Codon modified human papillomavirus type 16 E7 DNA vaccine enhances cytotoxic T-lymphocyte induction and anti-tumour activity. Virology 2002;301:43–52 [DOI] [PubMed] [Google Scholar]
- 114.Stacey SN, Jordan D, Williamson AJ, et al. Leaky scanning is the predominant mechanism for translation of human papillomavirus type 16 E7 oncoprotein from E6/E7 bicistronic mRNA. J Virol 2000;74:7284–7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin CT, Tsai YC, He L, et al. A DNA vaccine encoding a codon-optimized human papillomavirus type 16 E6 gene enhances CTL response and anti-tumor activity. J Biomed Sci 2006;13:481–488 [DOI] [PubMed] [Google Scholar]
- 116.Hirasawa Y, Arai M, Imazeki F, et al. Methylation status of genes upregulated by demethylating agent 5-aza-2'-deoxycytidine in hepatocellular carcinoma. Oncology 2006;71:77–85 [DOI] [PubMed] [Google Scholar]
- 117.Lu D, Hoory T, Monie A, et al. Treatment with demethylating agent, 5-aza-2'-deoxycytidine enhances therapeutic HPV DNA vaccine potency. Vaccine 2009;27:4363–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002;2:59–65 [DOI] [PubMed] [Google Scholar]
- 119.Cid-Arregui A, Juarez V, zur Hausen H. A synthetic E7 gene of human papillomavirus type 16 that yields enhanced expression of the protein in mammalian cells and is useful for DNA immunization studies. J Virol 2003;77:4928–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Morrow MP, Kraynyak KA, Sylvester AJ, et al. Augmentation of cellular and humoral immune responses to HPV16 and HPV18 E6 and E7 antigens by VGX-3100. Mol Ther Oncolytics 2016;3:16025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shin TH, Pankhong P, Yan J, et al. Induction of robust cellular immunity against HPV6 and HPV11 in mice by DNA vaccine encoding for E6/E7 antigen. Hum Vaccin Immunother 2012;8:470–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steinberg T, Ohlschlager P, Sehr P, et al. Modification of HPV 16 E7 genes: Correlation between the level of protein expression and CTL response after immunization of C57BL/6 mice. Vaccine 2005;23:1149–1157 [DOI] [PubMed] [Google Scholar]
- 123.Martin BK, Chin KC, Olsen JC, et al. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity 1997;6:591–600 [DOI] [PubMed] [Google Scholar]
- 124.Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell 2002;109 Suppl:S21–33 [DOI] [PubMed] [Google Scholar]
- 125.Kim D, Hoory T, Monie A, et al. Enhancement of DNA vaccine potency through coadministration of CIITA DNA with DNA vaccines via gene gun. J Immunol 2008;180:7019–7027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wade PA. Transcriptional control at regulatory checkpoints by histone deacetylases: Molecular connections between cancer and chromatin. Hum Mol Genet 2001;10:693–698 [DOI] [PubMed] [Google Scholar]
- 127.Lai MD, Chen CS, Yang CR, et al. An HDAC inhibitor enhances the antitumor activity of a CMV promoter-driven DNA vaccine. Cancer Gene Ther 2010;17:203–211 [DOI] [PubMed] [Google Scholar]
- 128.Magner WJ, Kazim AL, Stewart C, et al. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol 2000;165:7017–7024 [DOI] [PubMed] [Google Scholar]
- 129.Vanniasinkam T, Ertl H, Tang Q. Trichostatin-A enhances adaptive immune responses to DNA vaccination. J Clin Virol 2006;36:292–297 [DOI] [PubMed] [Google Scholar]
- 130.Lee SY, Huang Z, Kang TH, et al. Histone deacetylase inhibitor AR-42 enhances E7-specific CD8(+) T cell-mediated antitumor immunity induced by therapeutic HPV DNA vaccination. J Mol Med (Berl) 2013;91:1221–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Joffre OP, Segura E, Savina A, et al. Cross-presentation by dendritic cells. Nat Rev Immunol 2012;12:557–569 [DOI] [PubMed] [Google Scholar]
- 132.Grant EP, Michalek MT, Goldberg AL, et al. Rate of antigen degradation by the ubiquitin-proteasome pathway influences MHC class I presentation. J Immunol 1995;155:3750–3758 [PubMed] [Google Scholar]
- 133.Massa S, Simeone P, Muller A, et al. Antitumor activity of DNA vaccines based on the human papillomavirus-16 E7 protein genetically fused to a plant virus coat protein. Hum Gene Ther 2008;19:354–364 [DOI] [PubMed] [Google Scholar]
- 134.Hung CF, Cheng WF, He L, et al. Enhancing major histocompatibility complex class I antigen presentation by targeting antigen to centrosomes. Cancer Res 2003;63:2393–2398 [PubMed] [Google Scholar]
- 135.Liu WJ, Zhao KN, Gao FG, et al. Polynucleotide viral vaccines: Codon optimisation and ubiquitin conjugation enhances prophylactic and therapeutic efficacy. Vaccine 2001;20:862–869 [DOI] [PubMed] [Google Scholar]
- 136.Chandra J, Dutton JL, Li B, et al. DNA vaccine encoding HPV16 oncogenes E6 and E7 induces potent cell-mediated and humoral immunity which protects in tumor challenge and drives E7-expressing skin graft rejection. J Immunother 2017;40:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi W, Bu P, Liu J, et al. Human papillomavirus type 16 E7 DNA vaccine: Mutation in the open reading frame of E7 enhances specific cytotoxic T-lymphocyte induction and antitumor activity. J Virol 1999;73:7877–7881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kang TH, Kim KW, Bae HC, et al. Enhancement of DNA vaccine potency by antigen linkage to IFN-gamma-inducible protein-10. Int J Cancer 2011;128:702–714 [DOI] [PubMed] [Google Scholar]
- 139.Wu CC, Wu FC, Hsu YT, et al. Enhanced anti-tumor therapeutic efficacy of DNA vaccine by fusing the E7 gene to BAFF in treating human papillomavirus-associated cancer. Oncotarget 2017;8:33024–33036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smahel M, Polakova I, Pokorna D, et al. Enhancement of T cell-mediated and humoral immunity of beta-glucuronidase-based DNA vaccines against HPV16 E7 oncoprotein. Int J Oncol 2008;33:93–101 [PubMed] [Google Scholar]
- 141.Lin CT, Chang TC, Chao A, et al. Enhancement of DNA vaccine potency through linkage of antigen gene to ER chaperone molecules, ER-60, tapasin, and calnexin. J Biomed Sci 2005;12:279–287 [DOI] [PubMed] [Google Scholar]
- 142.Sadasivan B, Lehner PJ, Ortmann B, et al. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity 1996;5:103–114 [DOI] [PubMed] [Google Scholar]
- 143.Cheng WF, Hung CF, Chai CY, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest 2001;108:669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim JW, Hung CF, Juang J, et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene Ther 2004;11:1011–1018 [DOI] [PubMed] [Google Scholar]
- 145.Kim D, Gambhira R, Karanam B, et al. Generation and characterization of a preventive and therapeutic HPV DNA vaccine. Vaccine 2008;26:351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Peng S, Trimble C, Ji H, et al. Characterization of HPV-16 E6 DNA vaccines employing intracellular targeting and intercellular spreading strategies. J Biomed Sci 2005;12:689–700 [DOI] [PubMed] [Google Scholar]
- 147.Eric-Nikolic A, Milovanovic Z, Sanchez D, et al. Overexpression of calreticulin in malignant and benign breast tumors: Relationship with humoral immunity. Oncology 2012;82:48–55 [DOI] [PubMed] [Google Scholar]
- 148.Sheng W, Chen C, Dong M, et al. Overexpression of calreticulin contributes to the development and progression of pancreatic cancer. J Cell Physiol 2014;229:887–897 [DOI] [PubMed] [Google Scholar]
- 149.Perez-Trujillo JJ, Garza-Morales R, Barron-Cantu JA, et al. DNA vaccine encoding human papillomavirus antigens flanked by a signal peptide and a KDEL sequence induces a potent therapeutic antitumor effect. Oncol Lett 2017;13:1569–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Prior TI, FitzGerald DJ, Pastan I. Translocation mediated by domain II of Pseudomonas exotoxin A: Transport of barnase into the cytosol. Biochemistry 1992;31:3555–3559 [DOI] [PubMed] [Google Scholar]
- 151.Hung CF, Cheng WF, Hsu KF, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res 2001;61:3698–3703 [PubMed] [Google Scholar]
- 152.Miller G, Pillarisetty VG, Shah AB, et al. Murine Flt3 ligand expands distinct dendritic cells with both tolerogenic and immunogenic properties. J Immunol 2003;170:3554–3564 [DOI] [PubMed] [Google Scholar]
- 153.Costa SM, Paes MV, Barreto DF, et al. Protection against dengue type 2 virus induced in mice immunized with a DNA plasmid encoding the non-structural 1 (NS1) gene fused to the tissue plasminogen activator signal sequence. Vaccine 2006;24:195–205 [DOI] [PubMed] [Google Scholar]
- 154.Dimitrov I, Zakhariev T. [Effect of some natural and social factors on the physical development of newborn infants]. Akush Ginekol (Sofiia) 1975;14:119–126 [PubMed] [Google Scholar]
- 155.Seo SH, Jin HT, Park SH, et al. Optimal induction of HPV DNA vaccine-induced CD8+ T cell responses and therapeutic antitumor effect by antigen engineering and electroporation. Vaccine 2009;27:5906–5912 [DOI] [PubMed] [Google Scholar]
- 156.Lin CT, Chang TC, Shaw SW, et al. Maintenance of CD8 effector T cells by CD4 helper T cells eradicates growing tumors and promotes long-term tumor immunity. Vaccine 2006;24:6199–6207 [DOI] [PubMed] [Google Scholar]
- 157.Chen JW, Murphy TL, Willingham MC, et al. Identification of two lysosomal membrane glycoproteins. J Cell Biol 1985;101:85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu TC, Guarnieri FG, Staveley-O'Carroll KF, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci U S A 1995;92:11671–11675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ji H, Wang TL, Chen CH, et al. Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-expressing tumors. Hum Gene Ther 1999;10:2727–2740 [DOI] [PubMed] [Google Scholar]
- 160.Brulet JM, Maudoux F, Thomas S, et al. DNA vaccine encoding endosome-targeted human papillomavirus type 16 E7 protein generates CD4+ T cell-dependent protection. Eur J Immunol 2007;37:376–384 [DOI] [PubMed] [Google Scholar]
- 161.Mottez E, Langlade-Demoyen P, Gournier H, et al. Cells expressing a major histocompatibility complex class I molecule with a single covalently bound peptide are highly immunogenic. J Exp Med 1995;181:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yu YY, Netuschil N, Lybarger L, et al. Cutting edge: Single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J Immunol 2002;168:3145–3149 [DOI] [PubMed] [Google Scholar]
- 163.Huang CH, Peng S, He L, et al. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther 2005;12:1180–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ni K, O'Neill HC. The role of dendritic cells in T cell activation. Immunol Cell Biol 1997;75:223–230 [DOI] [PubMed] [Google Scholar]
- 165.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 2003;300:337–339 [DOI] [PubMed] [Google Scholar]
- 166.Maher J, Davies ET. Targeting cytotoxic T lymphocytes for cancer immunotherapy. Br J Cancer 2004;91:817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Langenkamp A, Messi M, Lanzavecchia A, et al. Kinetics of dendritic cell activation: Impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol 2000;1:311–316 [DOI] [PubMed] [Google Scholar]
- 168.Schulz O, Diebold SS, Chen M, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 2005;433:887–892 [DOI] [PubMed] [Google Scholar]
- 169.Sajadian A, Tabarraei A, Soleimanjahi H, et al. Comparing the effect of Toll-like receptor agonist adjuvants on the efficiency of a DNA vaccine. Arch Virol 2014;159:1951–1960 [DOI] [PubMed] [Google Scholar]
- 170.Chuang CM, Monie A, Hung CF, et al. Treatment with imiquimod enhances antitumor immunity induced by therapeutic HPV DNA vaccination. J Biomed Sci 2010;17:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee SJ, Song L, Yang MC, et al. Local administration of granulocyte macrophage colony-stimulating factor induces local accumulation of dendritic cells and antigen-specific CD8+ T cells and enhances dendritic cell cross-presentation. Vaccine 2015;33:1549–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lasaro MO, Diniz MO, Reyes-Sandoval A, et al. Anti-tumor DNA vaccines based on the expression of human papillomavirus-16 E6/E7 oncoproteins genetically fused with the glycoprotein D from herpes simplex virus-1. Microbes Infect 2005;7:1541–1550 [DOI] [PubMed] [Google Scholar]
- 173.Datta SK, Redecke V, Prilliman KR, et al. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J Immunol 2003;170:4102–4110 [DOI] [PubMed] [Google Scholar]
- 174.Oh JZ, Kurche JS, Burchill MA, et al. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood 2011;118:3028–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992;176:1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: Molecular control and implications for immune homeostasis and therapy. Blood 2012;119:3383–3393 [DOI] [PubMed] [Google Scholar]
- 177.Xu Y, Zhan Y, Lew AM, et al. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol 2007;179:7577–7584 [DOI] [PubMed] [Google Scholar]
- 178.Pollara G, Jones M, Handley ME, et al. Herpes simplex virus type-1-induced activation of myeloid dendritic cells: The roles of virus cell interaction and paracrine type I IFN secretion. J Immunol 2004;173:4108–4119 [DOI] [PubMed] [Google Scholar]
- 179.Kushwah R, Hu J. Dendritic cell apoptosis: Regulation of tolerance versus immunity. J Immunol 2010;185:795–802 [DOI] [PubMed] [Google Scholar]
- 180.Kim TW, Hung CF, Ling M, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest 2003;112:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cheng WF, Chang MC, Sun WZ, et al. Connective tissue growth factor linked to the E7 tumor antigen generates potent antitumor immune responses mediated by an antiapoptotic mechanism. Gene Ther 2008;15:1007–1016 [DOI] [PubMed] [Google Scholar]
- 182.Kim TW, Lee JH, He L, et al. Modification of professional antigen-presenting cells with small interfering RNA in vivo to enhance cancer vaccine potency. Cancer Res 2005;65:309–316 [PubMed] [Google Scholar]
- 183.Laidlaw BJ, Craft JE, Kaech SM. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat Rev Immunol 2016;16:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cresswell P. Invariant chain structure and MHC class II function. Cell 1996;84:505–507 [DOI] [PubMed] [Google Scholar]
- 185.Hung CF, Tsai YC, He L, et al. DNA vaccines encoding Ii-PADRE generates potent PADRE-specific CD4+ T-cell immune responses and enhances vaccine potency. Mol Ther 2007;15:1211–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim D, Monie A, He L, et al. Role of IL-2 secreted by PADRE-specific CD4+ T cells in enhancing E7-specific CD8+ T-cell immune responses. Gene Ther 2008;15:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wu A, Zeng Q, Kang TH, et al. Innovative DNA vaccine for human papillomavirus (HPV)-associated head and neck cancer. Gene Ther 2011;18:304–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Oosterhuis K, Ohlschlager P, van den Berg JH, et al. Preclinical development of highly effective and safe DNA vaccines directed against HPV 16 E6 and E7. Int J Cancer 2011;129:397–406 [DOI] [PubMed] [Google Scholar]
- 189.Yang A, Peng S, Farmer E, et al. Enhancing antitumor immunogenicity of HPV16-E7 DNA vaccine by fusing DNA encoding E7-antigenic peptide to DNA encoding capsid protein L1 of Bovine papillomavirus. Cell Biosci 2017;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kang TH, Chung JY, Monie A, et al. Enhancing DNA vaccine potency by co-administration of xenogenic MHC class-I DNA. Gene Ther 2010;17:531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol 2004;172:3983–3988 [DOI] [PubMed] [Google Scholar]
- 192.Lin CT, Tsai YC, He L, et al. DNA vaccines encoding IL-2 linked to HPV-16 E7 antigen generate enhanced E7-specific CTL responses and antitumor activity. Immunol Lett 2007;114:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim H, Kwon B, Sin JI. Combined stimulation of IL-2 and 4-1BB receptors augments the antitumor activity of E7 DNA vaccines by increasing Ag-specific CTL responses. PLoS One 2013;8:e83765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shuford WW, Klussman K, Tritchler DD, et al. 4–1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med 1997;186:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ralston E, Hall ZW. Restricted distribution of mRNA produced from a single nucleus in hybrid myotubes. J Cell Biol 1992;119:1063–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Choi YW, Kang MC, Seo YB, et al. Intravaginal administration of Fc-fused IL7 suppresses the cervicovaginal tumor by recruiting HPV DNA vaccine-induced CD8 T cells. Clin Cancer Res 2016;22:5898–5908 [DOI] [PubMed] [Google Scholar]
- 197.Heufler C, Koch F, Stanzl U, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol 1996;26:659–668 [DOI] [PubMed] [Google Scholar]
- 198.Flingai S, Czerwonko M, Goodman J, et al. Synthetic DNA vaccines: Improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front Immunol 2013;4:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Esensten JH, Helou YA, Chopra G, et al. CD28 Costimulation: From mechanism to therapy. Immunity 2016;44:973–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang H, Yu J, Li L. A DNA vaccine encoding mutated HPV58 mE6E7-Fc-GPI fusion antigen and GM-CSF and B7.1. Onco Targets Ther 2015;8:3067–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Haraldsen G, Balogh J, Pollheimer J, et al. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol 2009;30:227–233 [DOI] [PubMed] [Google Scholar]
- 202.Villarreal DO, Wise MC, Walters JN, et al. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res 2014;74:1789–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dufour JH, Dziejman M, Liu MT, et al. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol 2002;168:3195–3204 [DOI] [PubMed] [Google Scholar]
- 204.Grell S, Christensen JD, Fjalland B. The influence of morphine and naloxone on plasma oxytocin concentration in the rat. Pharmacol Toxicol 1988;63:274–276 [DOI] [PubMed] [Google Scholar]
- 205.Mohit E, Bolhassani A, Zahedifard F, et al. Immunomodulatory effects of IP-10 chemokine along with PEI600-Tat delivery system in DNA vaccination against HPV infections. Mol Immunol 2013;53:149–160 [DOI] [PubMed] [Google Scholar]
- 206.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Song MY, Park SH, Nam HJ, et al. Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1. J Immunother 2011;34:297–306 [DOI] [PubMed] [Google Scholar]
- 208.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med 2002;8:793–800 [DOI] [PubMed] [Google Scholar]
- 209.Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity 2005;23:649–659 [DOI] [PubMed] [Google Scholar]
- 210.Lu L, Qian S, Hershberger PA, et al. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J Immunol 1997;158:5676–5684 [PubMed] [Google Scholar]
- 211.Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med 1996;183:1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang B, Mao CP, Peng S, et al. RNA interference-mediated in vivo silencing of fas ligand as a strategy for the enhancement of DNA vaccine potency. Hum Gene Ther 2008;19:763–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006;6:295–307 [DOI] [PubMed] [Google Scholar]
- 214.Visser J, Nijman HW, Hoogenboom BN, et al. Frequencies and role of regulatory T cells in patients with (pre)malignant cervical neoplasia. Clin Exp Immunol 2007;150:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liang D, Xiao-Feng H, Guan-Jun D, et al. Activated STING enhances Tregs infiltration in the HPV-related carcinogenesis of tongue squamous cells via the c-jun/CCL22 signal. Biochim Biophys Acta 2015;1852:2494–2503 [DOI] [PubMed] [Google Scholar]
- 216.Chuang CM, Hoory T, Monie A, et al. Enhancing therapeutic HPV DNA vaccine potency through depletion of CD4+CD25+ T regulatory cells. Vaccine 2009;27:684–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Peng S, Lyford-Pike S, Akpeng B, et al. Low-dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic HPV vaccine. Cancer Immunol Immunother 2013;62:171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Aggarwal CC R. B.; Morrow, M. P.; Kraynyak, K.; Bauml, J.; Weinstein, G. S. Immunogenicity results using human papillomavirus (HPV) specific DNA vaccine, INO-3112 (HPV16/HPV18 plasmids + IL-12) in HPV+ head and neck squamous cell carcinoma (HNSCCa). Journal of Clinical Oncology 2017;35:6073–6073 [Google Scholar]
- 219.Tsen SW, Wu CY, Meneshian A, et al. Femtosecond laser treatment enhances DNA transfection efficiency in vivo. J Biomed Sci 2009;16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang JW, Roden RB. L2, the minor capsid protein of papillomavirus. Virology 2013;445:175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang JW, Roden RB. Virus-like particles for the prevention of human papillomavirus-associated malignancies. Expert Rev Vaccines 2013;12:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Massa S, Paolini F, Curzio G, et al. A plant protein signal sequence improved humoral immune response to HPV prophylactic and therapeutic DNA vaccines. Hum Vaccin Immunother 2017;13:271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]