Abstract
Tobacco exposure during development leads to neurobehavioral dysfunction in children, even when exposure is limited to secondhand smoke. We have previously shown in rats that developmental exposure to tobacco smoke extract (TSE), at levels mimicking secondhand smoke, starting preconception and extending throughout gestation, evoked subsequent locomotor hyperactivity and cognitive impairment. These effects were greater than those caused by equivalent exposures to nicotine alone, implying that other agents in tobacco smoke contributed to the adverse behavioral effects. In the present study, we examined the critical developmental windows of vulnerability for these effects, restricting TSE administration (0.2 mg/kg/day nicotine equivalent, or DMSO vehicle, delivered by subcutaneously-implanted pumps) to three distinct 10 day periods: the 10 days preceding mating, the first 10 days of gestation (early gestation), or the second 10 days of gestation (late gestation). The principal behavioral effects revealed a critical developmental window of vulnerability, as well as sex selectivity. Late gestational TSE exposure significantly increased errors in the initial training on the radial-arm maze in female offspring, whereas no effects were seen in males exposed during late gestation, or with either sex in the other exposure windows. In attentional testing with the visual signal detection test, male offspring exposed to TSE during early or late gestation showed hypervigilance during low-motivating conditions. These results demonstrate that gestational TSE exposure causes persistent behavioral effects that are dependent on the developmental window in which exposure occurs. The fact that effects were seen at TSE levels modeling secondhand smoke, emphasizes the need for decreasing involuntary tobacco smoke exposure, particularly during pregnancy.
Keywords: Behavioral Teratogenesis, Nicotine, Second-hand Smoke, Tobacco, Development
Introduction
Active maternal smoking during pregnancy is a clear contributor to increased risk of neurodevelopmental disorders [1, 2]. Indeed, even lower-level exposures associated with secondhand tobacco smoke can cause persistent neurobehavioral effects [3-5]. These include increased externalizing behavior [6], emotional dysfunction [7] and impaired neuromotor development [8, 9]. In previous studies, we showed that tobacco smoke extract (TSE) administered to rat dams at levels simulating secondhand smoke exposure, commenced in the premating period and continued throughout gestation, leads to persistent anomalies of both synaptic and behavioral function [10, 11]. Importantly, the effects of TSE were greater than those elicited by equivalent exposures to nicotine alone, indicating that other components of tobacco smoke including polycyclic aromatic hydrocarbons such as benzo[a]pyrene or heavy metals such as cadmium contribute significantly to the adverse outcomes.
Subsequently, we developed models to explore the impact of TSE exposure during restricted periods during the reproductive cycle: for ten days before mating; ten days during early gestation or ten days during late gestation. Rats are born at an immature state relative to humans. The first and second halves of the rat gestational period correspond to the first two trimesters of human gestation. In a neurochemical study that was the companion of the current neurobehavioral study, we found that late gestational TSE exposure caused the most pronounced effects on synaptic development in cholinergic and serotonergic systems [12]. More modest neural effects were seen with early gestational exposure. There were also some significant neurochemical anomalies evoked by exposure prior to mating, which may be due to a persistence of TSE chemical components well past the end of direct exposure, or alternatively, epigenetic changes in the ovum that ultimately affect brain development even when exposure terminates prior to brain formation. In the current study, we assessed the long-term behavioral effects of low-level TSE exposure during the same critical periods, focusing on tests of locomotor activity, emotional behaviors and cognitive function, and distinguishing between males and females.
Methods
Tobacco Smoke Extract
Tobacco Smoke Extract (TSE) was prepared by Arista Laboratories (Richmond, VA, USA) from Kentucky Reference cigarettes (KY3R4F) on a Rotary Smoke Machine under International Organization for Standardization (ISO) mechanical smoking conditions. The smoke condensate was collected on 92 mm filter pads, which were then extracted by shaking with undiluted dimethyl sulfoxide (DMSO) for 20 min, to obtain a solution extract of 20 mg of TSE per ml. Condensate aliquots were stored in amber vials at −80°C until used. Two cigarettes were smoked to produce each ml of extract and the final product contained 0.8 mg/ml nicotine as determined by Arista Laboratories (Richmond, VA, USA).
Animal Exposure
The study was conducted humanely with the protocols approved by the Duke University Animal Care and Use Committee and were in accordance with the federal and state guidelines. Sprague-Dawley rats were purchased from Charles River Laboratories (Raleigh, NC, USA) and were shipped by climate-controlled truck (transportation time < 1 hr). They were allowed to adjust to the laboratory housing facility for at least two weeks before the study commenced and were kept on a reverse 12:12 hr light/dark schedule. The rats had free access to water and food except when food was restricted for food-motivated tests (see below). Behavioral testing took place during the dark (active) part of the cycle.
Treatments were given via iPrecio® microinfusion pumps (Primetech, Inc., Tokyo, Japan), implanted subcutaneously. These pumps are refillable via a percutaneous septum, so that a single surgery and pump implant could be used for delivery of different treatments in sequence. Animals were anesthetized (60 mg/kg ketamine + 0.15-0.50 mg/kg dexmedetomidine given i.p; followed post-implant by 0.15 mg/kg atipamezole + 5 mg/kg ketoprofen given s.c. and topical bupivacaine). There were four treatment groups, each comprising 12-13 dams: (1) controls received DMSO vehicle throughout the entire 30-day treatment period; (2) the TSE premating group received TSE for the first 10 days, followed by DMSO for the remaining 20 days; (3) the TSE early gestation group received DMSO for the first 10 days, then TSE for the next 10 days, followed by DMSO for the last 10 days; and (4) the TSE late gestation group received DMSO for the first 20 days, then TSE for 10 days. To maintain a constant dose level equivalent to 0.2 mg/kg/day of nicotine, the pump flow rate was adjusted upward to compensate for weight gain during pregnancy. At the end of the first 10-day infusion period, mating was initiated by including a male rat in the cage for 4 days, after which the pregnant dams were placed in breeding cages. Because conception could have taken place at any point during the mating period, the actual gestational exposure periods have an uncertainty of a few days; using parturition dates as a benchmark, the values ranged as follows: TSE premating group, begun 10-13 days prior to fertilization and terminating between 0-3 days pre-fertilization; TSE early gestation group, begun between 1-4 days pre-fertilization and terminating between gestational days 6-9; TSE late gestation group, begun between 7-9 days of gestation and terminating between days 17-19 of gestation. We previously established the bioequivalence of nicotine delivered in TSE as compared to nicotine alone [10].
The birth date was designated postnatal day (PN) 0, at which point litters were culled to 8-10 pups to ensure standard nutrition. The culled litters were as nearly sex balanced as possible. Weaning occurred on PN21. Animals underwent behavioral testing, in seven separate cohorts, with each treatment group represented within each cohort.
Behavioral Test Battery
At weaning (PN21), one male and one female were chosen from each litter in each exposure group to undergo behavioral tests. There were 11-13 litters in each condition with one male and one female tested from each litter. Males and females were housed in same-sex cages with three to four animals in each cage and had free access to food until testing began for the radial-arm maze and the operant visual signal detection task. The rats were also briefly food-deprived for 24 h before novelty suppressed feeding tests. Behavioral testing began when the rats were four weeks of age and continued on a week-to-week basis into full adulthood as described below.
The behavioral test battery included tests of locomotor activity, emotional function and cognition. This is a similar test battery to the one we used in our previous study of TSE and nicotine exposure throughout gestation [11]. Tests were administered with at least three days between successive tests to minimize carryover effects. All rats from all treatment groups went through the same battery in the same test order.
Week 4: Elevated Plus Maze
Animals were tested on the elevated plus maze (Med Associates, St Albans, VT, USA) to assess their anxiety-like behavior vs. risk-taking behavior. The maze measured 142 cm × 104 cm × 76 cm high and consisted of two arms with 15 cm high enclosed walls and two open arms with low 2 cm railings. Each rat was assessed individually on the elevated plus maze for a single five-min session. The percent time the rat spent in the open vs. enclosed arms of the maze was calculated, as well as the number of crossings across the center. Arm entries were defined as all four paws crossing the arm threshold of the maze. The dependent measures were percent of time in the open arms to index anxiety-like behavior and the number of center crossings to measure locomotion in this five-min test.
Week 5: Figure-8 Apparatus Test of Locomotor Activity
Locomotor activity was assessed in an enclosed maze in the shape of a figure-8. The Figure-8 apparatus consisted of a continuous alley that measured 10 cm × 10 cm, with the entire maze measuring 70 cm × 42 cm. Animals were allowed to freely explore the apparatus, and locomotor activity was assessed by the crossing of eight photo-beams located at equal points in the alley. Each locomotor test session lasted 1 h, and photo-beam breaks were tallied in 5 min blocks across the one-hour test session. The mean number of photobeam breaks per five-minute time block within the session indexed locomotor activity, while the linear trend of decreasing beam breaks over the twelve sequential time blocks within the session indexed the habituation of activity with experience in the apparatus over the one-hour test session.
Week 6: Novelty Suppressed Feeding
To assess fear responsivity, the offspring rats were tested for suppression of feeding in a novel environment. The rats had food restricted for 24 h prior to the test session. The novel environment consisted of a plastic rectangular cage (different from the home cage) placed in the middle of a brightly lit testing room, with no cage top or bedding in the cage. Twelve standard rat-chow pellets were weighed before testing and were spread across the cage floor in 4 rows of 3 pellets each. The sessions lasted 10 min and the latency for the rat to begin eating was recorded. Eating was defined as the act of chewing the food and not merely sniffing, holding, or carrying the food around in the mouth. The food pellets which remained after the test session were weighed to determine the amount of food eaten. The dependent measures were: amount of food eaten, latency to begin eating, the number of eating bouts and the duration of eating.
Week 7: Novel Object Recognition
Recognition of a novel vs. familiar object was used to test attention and memory in a low-motivational state. Tests were conducted in opaque plastic enclosures measuring 70 cm × 41 cm × 33 cm. Objects consisted of plastic, glass, or ceramic material and were randomized for each animal. Animals were first habituated to the apparatus in two 10 min sessions over the course of two days. Testing began on day 3 with a 10 min familiarization session in which two identical objects (A/A) were placed in the cage for the animal to explore. The A/A session was then followed by a 15 min, 1 h, or 6 h delay period spent in the animal’s home cage. The animal was then placed back in the enclosure with one object from the A/A session and with another, dissimilar, “novel” object (A/B session). Between sessions, the objects were wiped clean with a solution of 10% acetic acid in order to avoid odor recognition cues by the rats. The test session lasted for ten min. Analysis considered the preference in the first and second halves of the sessions. The behavior during the first five-min block within the session was with a more clearly differential novelty of the two objects compared with the second five min of the test session. The time in seconds spent actively exploring each object was recorded during each five-min block during the ten-min session and used for analysis.
Week 8-11: Radial-Arm Maze
Spatial learning and memory were tested in the 16-arm radial maze. The maze was black painted wood with a central platform (50 cm diameter) and 16 radiating arms, each 10 cm wide × 60 cm long. A food cup was placed 2 cm from the end of each arm. Visual cues (cardboard shapes) were placed on the walls of the testing room to facilitate spatial orientation. Each rat was habituated in the maze for two 10 min sessions in which they were placed on the central platform inside a large, round, opaque cylinder, with half-pieces of sugar coated cereal (Froot Loops®; Kellogg’s Inc, Battle Creek MI, USA). Twelve of the arms were baited at the beginning of each session to test working memory performance and the remaining four arms were always left unbaited to test reference memory as we have used previously to evaluate developmental TSE effects [11]. The baited arms of the maze for each rat remained constant throughout the series of testing sessions, but which arms were baited differed randomly from rat to rat. Each trial began by placing the rat on the central platform inside the opaque cylinder for 10 s, after which the cylinder was lifted and the rat was allowed to move freely. Each session lasted 10 min or until the rat had entered all twelve baited arms, whichever came first. Each rat was assessed for working and reference memory errors over 24 sessions. Working memory errors were defined as repeat entries into baited arms, and reference memory errors were defined as entry into one of the arms that was never baited. Latency was calculated as the total session time divided by the number of arm entries. There was one session per day. After each session, the maze was cleaned with a damp cloth using 10% acetic acid.
The initial 6 sessions were run with the standard food restriction condition. Over the ensuing 18 sessions (7-24), we introduced an additional factor, alternating sessions in which the rats were tested under food-restricted or fed conditions to measure the choice accuracy under higher and lower motivation states. The rats received their daily meal either before or after the test session to determine whether there were differential effects under lower- and higher-motivating states. The dependent measures were the number of working and reference memory errors and response latency (seconds per arm entry).
Weeks 12-40: Operant Visual Signal Detection Task for Attention
The task was conducted as described in detail previously [11]. Briefly, each rat was placed in an operant chamber and was trained to press one of two retractable levers in response to a visual cue-light that was illuminated for a duration of 500 ms. If the cue-light became illuminated (“signal” trial), the animal needed to press the lever designated as the "signal" lever to receive a 20 mg food pellet reward. If the cue-light did not illuminate (“blank” trial) the animal needed to press the opposite lever in the chamber to receive the reward. The choice of “signal” and “blank” levers was randomized among the rats. If the rat made no response within 5 s of insertion of the response levers into the chamber, both levers retracted and a response “failure” was recorded. There were equal numbers of “signal” and “blank” trials in each test session with a total of 240 trials. “Hit” responses were correct choices on the signal trials while “correct rejection” responses were correct choices on blank trials. Percent correct hit and percent correct rejection per session were the dependent measures for response accuracy on this attention task.
The first six sessions were conducted under the standard, restricted feeding paradigm to motivate the subjects for the appetitively reinforced task. Over the following 12 sessions, as with the radial-arm maze, we alternated between testing the rats on the standard restricted feeding paradigm vs. being freely fed. For Sessions 7-12 the lever designations for "signal" and "blank" remained the same but we altered feeding each day between testing on the standard restricted feeding paradigm vs. being freely fed, so that there were 3 days under each feeding paradigm. For the final six sessions (13-18), we introduced an additional challenge, reversal of the response contingency. The hit lever for a light stimulus was switched to the opposite side for the remaining six sessions. For either the pre-reversal or post-reversal phases, we tested the animals for six sessions with three alternating sessions for each feeding condition. The rats received their daily meal either before or after the test session to determine whether there were differential effects under lower- and higher-motivating states. Analysis was conducted of the choice accuracy data including these factors as well as TSE exposure and sex.
Data Analysis
For each behavioral test, the data were evaluated by analysis of variance (ANOVA). Litter was the unit of variance. The between litters factor was timing of TSE treatmen-. The within litter factor was sex. Within subjects repeated factors were sessions and time block within session. Because each litter contributed one male and one female, sex was treated as a repeated measure within litter. Post-hoc tests for differences between TSE groups and controls or among the TSE groups themselves, were conducted with Fisher’s Least Significant Difference Test. Significance was assumed at the level of p<0.05 (two-tailed). For interactions at p<0.1, we also examined whether lower-order main effects were detectable after subdivision of the interactive variables [13]. The p<0.1 criterion for interaction terms was not used to assign significance to the effects but rather to identify interactive variables requiring subdivision for lower-order tests of the main effects of TSE, the variable of chief interest.
Results
Maternal, Litter and Growth Effects
As described previously [12] in an article describing the neurochemical results of these exposures, these TSE treatment regimens had little or no effect on maternal weight gain, the proportion of dams giving birth, litter size or sex ratio. The particular littermates used for the behavioral studies displayed no statistically significant treatment effects on body weight, although over a larger cohort there was transiently decreased body weight of 3-5% for the early gestational exposure group at PND 30 [12].
Elevated Plus Maze
There were no significant effects of TSE treatment on percent time spent in the open vs. closed arms or center crosses in the elevated plus maze (data not shown).
Figure-8 Apparatus Test of Locomotor Activity
TSE exposure during the three different developmental windows did not produce any significant effects on locomotor activity or habituation. Regardless of exposure condition the rats showed a significant reduction in activity over the course of the one-hour test session (F(11,495)=127.69, p<0.0005), indicating the characteristic habituation of locomotor activity associated with this test.
Novelty Suppressed Feeding
We did not observe statistically significant TSE treatment effects on any of the four measures of novelty suppressed feeding: amount eaten, latency to begin eating, the number of eating bouts or the duration of eating (data not shown).
Novel Object Recognition
No significant effects of TSE during the ten-day windows of exposure were detected with the novel object recognition test. Rats investigated the novel object more than the familiar object (F(1,21)=30.10, p<0.0005), and there was also a significant main effect of time in the test with significantly less investigation during minutes 6-10 than 1-5 (F(1,21)=58.36, p<0.0005). With this habituation to the novel object over time in the test there was a significantly lower difference between investigation of the novel and familiar object as evidenced by the significant interaction of novel vs. familiar investigation × the first vs. second five min time block within the session (F(1,21)=17.68, p<0.0005). Both of these differences indicate that the test worked as designed. Time interval between the familiarization session and the recognition session was not found to be significant.
Radial-Arm Maze
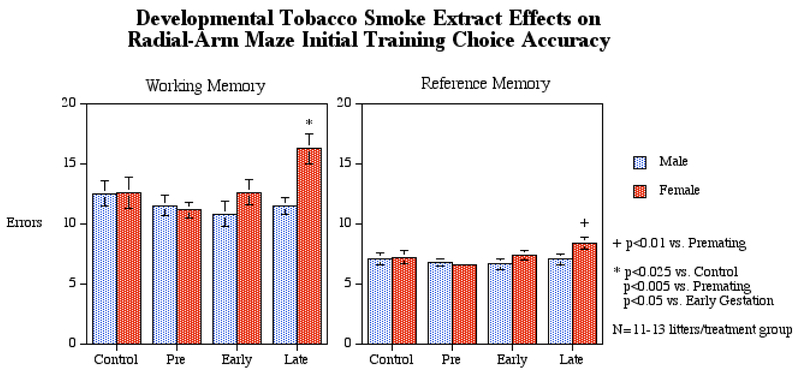
In the first six sessions of radial-arm maze acquisition training, the main effect of session (F(5,220)=2.42, p<0.05) was significant reflecting the overall improvement in choice accuracy with training (Fig. 1). During the first six sessions there were interactions of TSE treatment × sex (F(3,44)=2.45, p<0.08) and TSE × sex × error type (F(3,44)=2.22, p<0.1) that prompted follow-up tests of the simple main effects of TSE treatment in males and females as well as in males and females separated into working and reference memory types. Males did not show significant TSE effects either with total errors or working or reference memory errors individually. In contrasts, females showed significant TSE effects on overall errors (F(3,44)=4.49, p<0.01), as well as specifically on working (F(3,44)=3.69, p<0.025) and reference memories (F(3,44)=2.87, p<0.05). Post-hoc comparisons among treatment groups showed that the late gestational TSE exposure had significantly more overall errors than any of the other treatment groups (p<0.025 vs. control, p<0.005 vs. premating, p<0.025 vs. early gestation). This pattern was repeated with working memory errors (p<0.025 for late gestation vs. control, p<0.005 vs. premating, p<0.05 vs. early gestation). With reference memory errors, the intergroup comparisons showed a significant difference only between the late gestational exposure and premating exposure groups (p<0.01). Thus, the TSE effects of increasing working memory errors in the female offspring of the late gestational exposure appear to be the principal driver behind the overall increased errors of females in the radial arm maze.
Figure 1.
Working and reference memory errors in the radial-arm maze by male and female rats during the initial 6 sessions of training (mean ± sem) with N=11-13 litters per group. Late gestational TSE exposure significantly increased working and reference memory errors in female offspring. Abbreviations: Con = control, Pre = premating exposure, Early = early gestational exposure, Late = late gestational exposure.
When we introduced the additional factor of alternating food-restricted and fed conditions (sessions 7-24), we found a significant effect of TSE exposure × sex × session × food condition (F(6,88)=11.95, p<0.025; data not shown). Follow-up tests of the simple main effects of TSE exposure on choice accuracy across food restricted and fed conditions in each sex during each group of six sessions showed only one incidence of a significant TSE effect, for males in the fed condition during the 7-12 session group. Post hoc comparisons among the treatment groups showed that the male rats whose mothers had been treated with TSE during the premating period had significantly fewer errors than each of the three other treatment groups including controls (p<0.05).
Visual Signal Detection Task
Initial Training:
Similar to the radial arm maze tests, the initial 6 sessions of training of the visual signal detection task were under the standard restricted feeding schedule. TSE exposure elicited a significant effect that depended on trial type (treatment × error type, (F(3,44)=3.99, p<0.025). However, the follow-up tests of the simple main effects of TSE with each error type did not detect significant TSE effects for either trial type. There were several significant effects that supported the validity of the test. There was a significant (F(5,220)=109.53, p<0.0005) effect of consecutive session number, reflecting the continuing improvement in accuracy across successive training sessions. There was also a significant difference (F(1,44)=134.49, p<0.0005) in accuracy for hit vs. correct rejection trials, with more accurate performance for correct rejection trials.
Pre and Post Reversal:
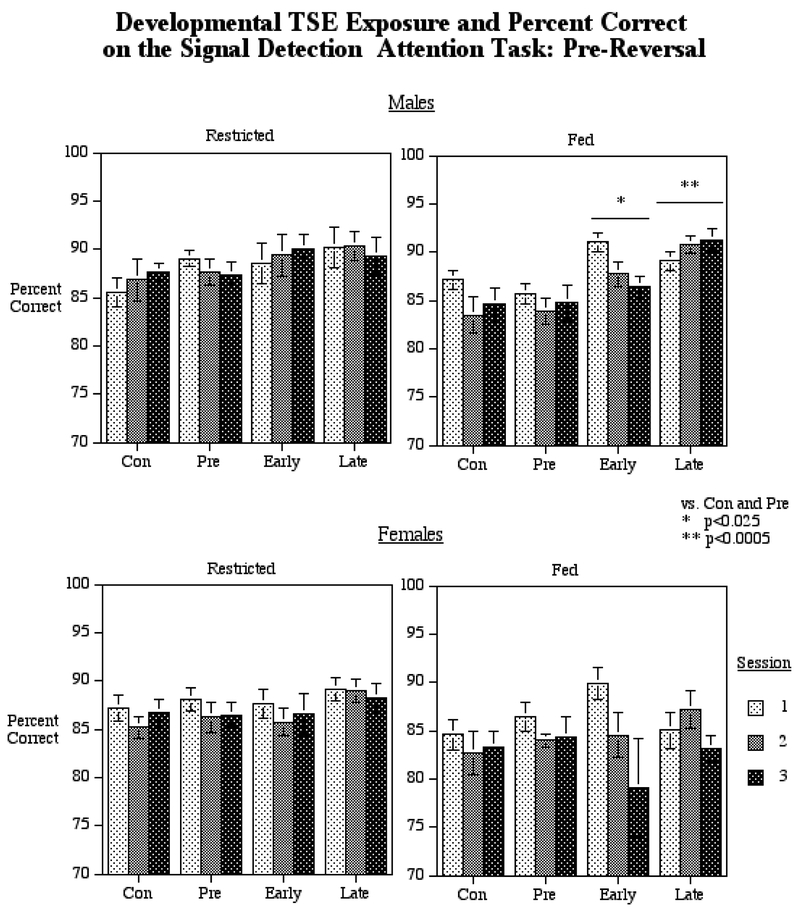
For the tests of feeding status and pre- vs. post-reversal, we found a significant interaction of TSE treatment × sex × feeding status × pre- vs. post-reversal (F(3,44)=3.04, p<0.05). Since trial type (Hit vs. Correct Rejection) was not differentially affected we combined the two trial types into a single index of response accuracy for presentation. Accordingly, we separated the data for pre- vs. post-reversal, for males and females and for feeding status, and performed follow-up tests of the simple main effects of TSE treatment for each sex under restricted and fed conditions before and after the reversal (Fig. 2). For the pre-reversal phase under fed conditions, we found a significant main effect of TSE exposure for males (F(3,44)=7.93, p<0.0005). Posthoc comparisons showed that the early (p<0.025) or late (p<0.0005) gestation exposure groups had higher percent correct performance than either the control or premating exposure groups as shown in the upper right panel of figure 2.
Figure 2.
Visual attention performance: Effects of TSE exposure on percent correct under feeding restricted and fed conditions (A) before and (A) after the reversal of contingencies (mean ± sem). There were three sessions run with in each of the two feeding conditions (restricted and fed). Early and late gestational TSE exposure caused a significantly higher percent correct performance under the fed condition in male but not female offspring averaged across the three sessions . Abbreviations: Con = control, Pre = premating exposure, Early = early gestational exposure, Late = late gestational exposure.
The veracity of the testing was supported by the expected, robust effects on factors such as training stage, sex and error type [14]. The analysis showed a significant main effect of sex (p<0.05) with females having lower percent correct performance than males. There was also a significant main effect of reversal (F(1,44)=1773.30, p<0.0005) with a dramatic decrease in accuracy with the reversal. The main effect of session was also significant (F(2,88)=124.00, p<0.0005), with continual improvement in accuracy across successive sessions. As seen in the first six sessions there was a significant main effect of hit vs. correct rejection performance (F(1,44)=23.10, p<0.0005) with better accuracy on correct rejection than hit trials. This is commonly seen with this signal detection task [15].
Discussion
Our principal finding is that the late gestational period represents a critical window in which TSE exposure elicits cognitive deficits, corresponding to the hierarchy of synaptic defects in acetylcholine and serotonin systems, which likewise show a progressive increase in sensitivity as gestation proceeds [12]. In our earlier work, we showed that nicotine exposure throughout gestation, at levels mimicking secondhand smoke and paralleling our TSE exposure model, also evoked long-term impairment of cognitive performance [11]. Nicotine given throughout gestation caused impaired working memory performance in female but not male offspring, interestingly TSE given throughout gestation did have this same effect [11]. The similarity of these findings is further supported by their similar selectivity for sex (greater effect in females) and for memory type (greater effect on working memory).
The visual signal detection task, a test of attention, also demonstrated a critical period of sensitivity as well as sex selectivity. In this case, we found that either early or late gestational TSE exposure, but not premating exposure, evoked hypervigilance under low-motivational (fed) conditions, with the effect seen in males and not detected in females. When motivation was enhanced (fasted conditions), the TSE effects were no longer detected, paralleling our earlier findings for comparable tests with continuous gestational TSE exposure [11]. In the previous study [11] we did not test the animals in the lower motivation, pre-fed condition as we did in the current study. This is where the significant effect of TSE exposure was seen. This pattern of results could also be interpreted that the groups exposed to TSE either during early or late gestation did not show control-like changes in choice accuracy with variations of motivational state. This points out the importance of performing behavioral tests under conditions that mimic different motivational states. The two error types were analyzed as factors in the same design. We combined the two trial types into a single output just for graphical presentation since the TSE exposure effects were not found to vary with trial type.
Other behavioral tests were essentially negative for TSE effects. This is not surprising for the elevated plus maze, which likewise showed no significant effects in our previous work with longer periods of TSE exposure [11]. However, we had found that continuous gestational TSE treatment had significant effects on performance in the figure-8 maze, on novelty suppressed feeding and on novel object recognition [11], whereas no significant effects were found with the shorter windows of treatment used in the present study. These effects may require a prolonged period of gestational exposure, so that no single, shorter window is effective.
The same issue needs to be emphasized in considering the apparent absence of effects of premating TSE exposure on behavioral performance in the present study. We did find premating effects on acetylcholine synaptic function, albeit to a lesser extent than those of early or late gestational TSE exposures [12]. The synaptic effects may be of insufficient magnitude to affect cognitive performance in the radial-arm maze, or alternatively this specific task may be insufficiently sensitive to pick up the differences. Indeed, we have previously shown that pharmacologic challenge can reveal cognitive impairment that is masked by compensatory changes [16], and it would be worthwhile to perform such tests on the premating exposure group.
In conclusion, the present findings confirm that TSE is a neurobehavioral teratogen even at exposures extending down to levels commensurate with second-hand smoke. Additionally, we found that the late gestational period represents a critical window of vulnerability for adverse effects of TSE on cognitive performance; likewise, early and late gestational TSE exposure targeted produced hypervigilance under low-motivational conditions. Sex-selectivity was a particular hallmark of both effects, as was the case with our earlier behavioral studies with continuous gestational TSE exposure [11] or for neurochemical effects of TSE either given throughout gestation or in discrete windows during gestation [10, 12]. These results add to the growing body of information showing that secondhand smoke exposure during pregnancy is injurious to neurodevelopment, with adverse effects on both synaptic and behavioral function. Future research with further exposure during the first postnatal week, which in the rat corresponds to development during the third trimester of human pregnancy, would provide additional information concerning TSE effects on later developing brain systems. Avoiding involuntary smoke exposure over the course of pregnancy, and particularly in later stages of gestation, can help minimize adverse neurobehavioral effects on the offspring.
Highlights.
Late gestational TSE exposure increased errors in the initial training on the radial-arm maze in female offspring
In attentional testing, male offspring exposed to TSE during gestation showed hypervigilance during low-motivating conditions.
Gestational TSE exposure causes persistent behavioral effects that are dependent on the developmental window in which exposure occurs.
Effects were seen at TSE levels modeling secondhand smoke, emphasizes the need to decrease involuntary tobacco smoke exposure during pregnancy.
Acknowledgement
This work was supported by the National Institutes of Health (ES022831) and by the U.S. Environmental Protection Agency (83543701). EPA support does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Abbreviations:
- ANOVA
analysis of variance
- PND
postnatal day
- TSE
tobacco smoke extract
Footnotes
Disclosure
TAS has received consultant income in the past three years from Pardieck Law (Seymour, IN, USA) and Walgreens Co. (Deerfield, IL, USA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pauly JR, Slotkin TA, Maternal tobacco smoking, nicotine replacement and neurobehavioural development, Acta Paediatr, 97 (2008) 1331–1337. [DOI] [PubMed] [Google Scholar]
- [2].Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, Elam KK, Natsuaki MN, Neiderhiser JM, Harold GT, Maternal smoking during pregnancy and offspring conduct problems: evidence from three independent genetically-sensitive research designs, JAMA Psychiat, 70 (2013) 956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].DiFranza JR, Aligne CA, Weitzman M, Prenatal and postnatal environmental tobacco smoke exposure and children's health, Pediatrics, 113 (2004) 1007–1015. [PubMed] [Google Scholar]
- [4].Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R, Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents, Environ. Health Perspect, 113 (2005) 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Herrmann M, King K, Weitzman M, Prenatal tobacco smoke and postnatal secondhand smoke exposure and child neurodevelopment, Current Opinion in Pediatrics, 20 (2008) 184–190. [DOI] [PubMed] [Google Scholar]
- [6].Liu J, Leung PWL, McCauley L, Ai Y, Pinto-Martin J, Mother's environmental tobacco smoke exposure during pregnancy and externalizing behavior problems in children, Neurotoxicology, 34 (2013) 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bandiera FC, Richardson AK, Lee DJ, He JP, Merikangas KR, Secondhand smoke exposure and mental health among children and adolescents, Arch. Pediatr. Adolesc. Med, 165 (2011) 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Evlampidou I, Bagkeris M, Vardavas C, Koutra K, Patelarou E, Koutis A, Chatzi L, Kogevinas M, Prenatal second-hand smoke exposure measured with urine cotinine may reduce gross motor development at 18 months of age, Journal of Pediatrics, 167 (2015) 246–252. [DOI] [PubMed] [Google Scholar]
- [9].Yeramaneni S, Dietrich KN, Yolton K, Parsons P, Aldous KM, Haynes EN, Secondhand tobacco smoke exposure and neuromotor function in rural children, Journal of Pediatrics, 167 (2015) 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Slotkin TA, Skavicus S, Card J, Stadler A, Levin ED, Seidler FJ, Developmental neurotoxicity of tobacco smoke extract toward cholinergic and serotonergic systems: More than just nicotine, Toxicol Sci, 147 (2015) 178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hall BJ, Cauley M, Burke D, Kiany A, Slotkin TA, Levin ED, Cognitive and behavioral impairments evoked by low level exposure to tobacco smoke constituents: Comparison with nicotine alone, Toxicologial Sciences, 15 (2016) 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Slotkin TA, Stadler A, Skavicus S, Card J, Ruff J, Levin ED, Seidler FJ, Is there a critical period for the developmental neurotoxicity of low-level tobacco smoke exposure?, Toxicol Sci, 155 (2016) 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Snedecor GW, Cochran WG, Statistical Methods, Iowa State University Press, Ames, Iowa, 1967. [Google Scholar]
- [14].Levin ED, Timofeeva OA, Yang L, Petro A, Ryde IT, Wrench N, Seidler FJ, Slotkin TA, Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging, Behavioural Brain Research, 208 (2010) 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Levin ED, Cauley M, Rezvani AH, Improvement of attentional function with antagonism of nicotinic receptors in female rats, Eur J Pharmacol, 702 (2013) 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Aldridge JE, Levin ED, Seidler FJ, Slotkin TA, Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression, Environ Health Perspect, 113 (2005) 527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]