Abstract
The shortage of human organs for transplantation is a devastating medical problem. One way to expand organ supply is to derive functional organs from patient-specific stem cells. Due to their capacity to grow indefinitely in the laboratory and differentiate into any cell type of the human body, patient-specific pluripotent stem (PS) cells harbor the potential to provide an inexhaustible supply of donor cells for transplantation. However, current efforts to generate functional organs from PS cells have so far been unsuccessful. An alternative and promising strategy is to generate human organs inside large animal species through a technique called interspecies blastocyst complementation. In this method, animals comprised of cells from human and animal species are generated by injecting donor human PS cells into animal host embryos. Critical genes for organ development are knocked out by genome editing, allowing donor human PS cells to populate the vacated niche. In principle, this experimental approach will produce a desired organ of human origin inside a host animal. In this mini-review, we focus on recent advances that may bring the promise of blastocyst complementation to clinical practice. While CRISPR/Cas9 has accelerated the creation of transgenic large animals such as pigs and sheep, we propose that further advances in the generation of chimera-competent human PS cells are needed to achieve interspecies blastocyst complementation. It will also be necessary to define the constituents of the species barrier, which inhibits efficient colonization of host animal embryos with human cells. Interspecies blastocyst complementation is a promising approach to help overcome the organ shortage facing the practice of clinical medicine today.
Keywords: pluripotent stem cells, interspecies chimeras, chimeras, interspecies blastocyst complementation, blastocyst complementation, organ shortage, organ generation, organ transplantation, Pdx1, pluripotency, reprogramming, mouse pluripotent stem cells, human pluripotent stem cells, naive pluripotent stem cells, naive pluripotency, primed pluripotent stem cells, primed pluripotency, CRISPR, Cas9
Introduction
The shortage of viable organs for transplantation impedes the treatment of organ failure. Despite considerable efforts, thousands of patients continue to die while awaiting organ transplant each year [1]. The increase of organ failure in aging societies has worsened the problem of organ shortage. To address this issue, various strategies are being pursued. Transplantation of organs formerly deemed undesirable is being considered. Attempts to increase organ awareness aim to target the potential of willing but unregistered donors [2]. Porcine organs are also considered a potentially favorable source [3,4]. Despite different approaches to resolve this devastating medical problem, increasing the number of allografts available for transplant remains the central challenge.
One strategy to expand the number of organs available for transplantation is to generate functional organs from patient-specific induced pluripotent stem (iPS) cells [5]. The defining features of human iPS cells – indefinite self-renewal in culture as well as capacity to differentiate into any cell type – in principle, allows for access to an endless supply of therapeutically relevant cells [6,7]. Further, iPSC-derived organs would be genetically identical to their intended patients and recipients. Autologous or allogeneic transplantation of iPSC-derived organs is anticipated to avoid immune rejection or complications of immunosuppression regimens [8-10].
Classical strategies for obtaining desired cells from human PS cells involve differentiation in a dish. However, in vitro differentiation possesses key disadvantages, including: the danger of remnant undifferentiated human PS cells developing into teratomas post-transplantation [11] and failure to achieve functional maturation of in vitro generated human PS cell derivatives which typically manifest immature (typically fetal-like) features. Current methodologies are not compatible with producing complex three-dimensional tissues, such as transplantable organs. Consequently, new approaches to cell differentiation are needed to overcome these barriers.
Natural selection has produced intricate developmental programs within organisms. Rather than attempting to replicate this complexity in vitro, it may be possible to exploit such developmental programs to generate human organs inside animal hosts directly [12-14]. This would entail the production of interspecies chimeras -- animals comprised of cells from two different species (i.e., human patient and a pig or sheep host). Overcoming the limitations posed by in vitro differentiation, development of human PS cells in interspecies chimeras with the animal host would, if successful, enable the generation of functionally mature, complex three-dimensional transplantable organs. A tractable method for establishing the development of human cells inside animal hosts would lay the foundation for producing transplantable organs from patient-specific stem cells.
In this mini-review, we highlight recent findings that advance the goal of generating human organs inside large animal hosts such as pigs and sheep. Interspecies blastocyst complementation requires the generation of genetically edited animals that can be chimerized by human donor PS cells. While much success has been achieved in creating transgenic pig or sheep, we suggest that interspecies chimera generation will require addressing two major challenges: first, resolving the lack of chimera-competent human PS cells; and second, understanding the species barrier that causes poor chimeric contribution of human donor PS cells. Surmounting these challenges will be necessary for chimeric contribution of human PS cells to distantly related large animal hosts, such as pigs and sheep.
Blastocyst Complementation: An Introduction
Perturbing the genetic programs underlying organogenesis can produce organisms lacking entire organs [15-17]. When organ generation is disrupted through genetic intervention, the remaining host cells and tissue will still persist, continuing to provide extrinsic factors and inductive interactions necessary for instructing organ formation [15-17]. A vacant developmental niche forms; donor wild-type PS cells are introduced into host blastocysts. The resulting chimeric embryos are transferred into a pseudopregnant foster mother for subsequent development. Meanwhile, the introduced chimera-competent PS cells developmentally compensate and colonize the empty niche, generating a donor cell-derived organ [15-17]. This complementation of organogenesis-disabled host blastocysts with wild-type donor cells is termed blastocyst complementation (Figure 1) [12,15-19].
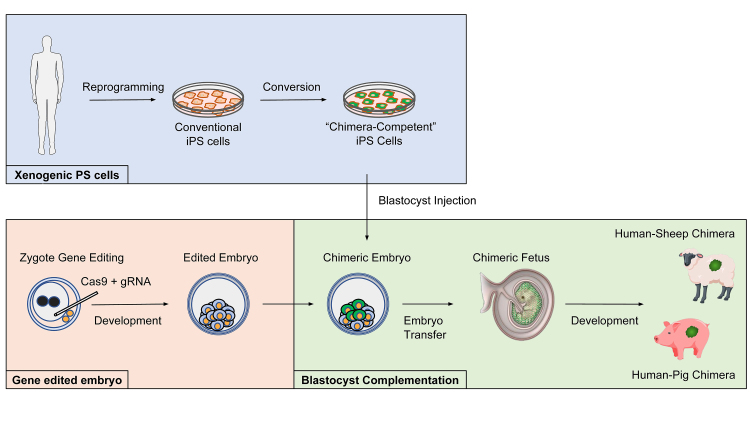
Figure 1.
Interspecies blastocyst complementation. Organ generation via interspecies blastocyst complementation could help to solve the severe shortage of organ donors worldwide. The genetic modification of host animals to disable organ development may enable donor human PS cells or progenitors to populate the targeted organ with minimal competition from the host. First, embryos of large animal hosts such as pigs or sheep are edited using CRISPR/Cas9 to disable formation of a target organ. Second, human xenogenic chimera-competent pluripotent stem cells are generated – first by: 1) reprogramming somatic cells to generate conventional human induced pluripotent stem cells (iPSCs) followed by 2) converting conventional human iPSCs to a chimera-competent state. Human xenogenic PS cells are then introduced into host animal embryos by blastocyst injection and the resulting chimeric embryo is transferred into a pseudopregnant foster mother. The chimeric embryo is allowed to develop in utero and if the method is successful, human-pig or human-sheep chimeras are born.
Interspecies Blastocyst Complementation in Rodents
The first report of interspecies blastocyst complementation for creating organs involved a study where Pdx1-deficient mouse blastocysts were complemented with rat PS cells [16]. The resulting rat-mouse interspecies chimeras possessed an entirely rat pancreas [16,17,19]. It is important to note that interspecies chimeras generated between rats and mice possessed vessels, nerves, and some interstitial elements that were blends of mouse and rat cells that may pose a problem for clinical translation [16]. Despite this caveat, this study was the first to report generation of a PS cell-derived functional organ from one species inside the animal of another species.
Large Animal Hosts
Adapting blastocyst complementation for human organ production will require the use of sufficiently sized animals. Pigs and sheep may represent particularly suitable large animal hosts because of their similarity to humans with regard to organ size as well as other advantages, such as breeding potential, period to reproductive maturity, and number of offspring unlike other animals such as non-human primates (NHPs) like baboons. Additionally, pigs have a lower cost of maintenance compared with NHPs such as baboon [20]. It is worth noting that the amenability of sheep for interspecies chimera formation has been demonstrated and extensively investigated through studies of sheep-goat interspecies chimeras [21,22].
The possibility of interspecies blastocyst complementation for organ generation in large animal hosts was illustrated in a study where pancreatogenesis-disabled pigs were complemented with allogeneic blastomeres [23]. The resulting adult pig chimeras possessed entirely donor-derived pancrea. Moreover, gene-edited Pdx1-knockout sheep that can potentially serve as a host for interspecies organ generation have also been generated [24]. As noted above, xeno-generation of human organs inside animal hosts will require production of human organs without animal host-derived nerves and vasculature. In this regard, additional blastocyst complementation experiments are needed to identify the correct strategy for removal of host-derived vasculature and nerves. As interspecies blastocyst complementation approaches continue to improve, it will be desirable to create customized pig or sheep hosts with appropriately complemented nerves and vasculature.
The above experiments used pre-existing cell lines to create animal hosts that can be complemented with wild-type cells. It will be ideal to employ optimized genome editing strategies to create customized animal hosts with target lineages disrupted, which will permit colonization of the devoid niche with donor cells. Applying CRISPR/Cas9 genome editing in zygotes may achieve this end [25,26]. The use of CRISPR/Cas9 in zygotes has enabled production of knockout animals in various species, including large animals such as pigs and sheep [24]. Furthermore, targeting of multiple lineages will be needed to achieve the production of human organs without animal host-derived nerves and vasculature. In these regards, highly multiplexed genetic engineering by CRISPR/Cas9 may prove useful [3,4].
Identifying Human Chimera-Competent Pluripotent Stem Cells
The studies above suggest that developing an interspecies blastocyst complementation platform will require creating genetically modified animal hosts and human chimera-competent PS cells. Hence, an essential technology needed for successful complementation of pig or sheep embryos is human chimera-competent PS cells. As it remains unclear whether such cells exist in humans, scientists have gleaned insight from rodent chimera-competent PS cells to generate human analogs [36].
Conventional Mouse and Human PS cells
While both manifest potential to form all three germ layers – ectoderm, mesoderm, and endoderm – conventional human PS cells show strikingly distinct characteristics when compared to mouse PS cells. Although both mouse and human PS cells can be derived from the inner cell mass and by direct reprogramming of somatic cells, human ES and iPS cells require radically distinct conditions from mouse ES and iPS cells for their continuous propagation in vitro [5-7,27]. Human PS cells are typically derived and cultured in FGF-containing medium [28]. In contrast, the standard culture conditions for derivation and maintenance of mouse iPS cells involve an optimal combination of leukemia inhibitory factor (LIF) and two kinase inhibitors (2i) – PD0325901 and CHIR99021, small molecule inhibitors of the MEK and GSK3 kinases, respectively – that sustain mouse PS cells in a pre-implantation inner cell mass-like state with high-grade chimera-competency [29,30]. In striking contrast, LIF and inhibitors of MEK and GSK3 kinases induce differentiation of human PS cells [31,32]. Another obvious difference between conventional human PS cells and mouse PS cells is their appearance – mouse ES cells grow as three-dimensional domed colonies whereas human PS cells grow as two-dimensional flat colonies.
Perhaps the most significant difference between mouse and human PS cells relates to whether each cell type corresponds to a cellular state competent to form chimeras. While mouse ES cells reliably form chimeras, it is unlikely conventional human PS cells correspond to a chimera-competent cellular state. Mouse and rat ES cells propagated in 2i exhibit high-grade chimeric contribution and germline transmission [33,34]. Although evaluating the chimera competency of human ES cells is ethically constrained, primate ES cells grown in FGF-containing human ES cell culture conditions also have a flat morphology and notably fail to generate chimeras after blastocyst injection [35]. Given the chimera-competency of rodent PS cells, it has been speculated that the application of 2i to PS cells from other species may lead to the generation of chimera-competent ES cells from other species, including primates and humans [32-36].
Naive and Primed Pluripotent States
Current evidence suggests these differences between mouse and human stem cells may be ascribed to discrete developmental identities. The generation of a novel type of PS cell from post-implantation rodent embryos, termed epiblast stem cells (EpiS cells), using FGF-containing human ES cell culture conditions suggested that human ES cell pluripotency likely reflects a later stage of development [37,38]. Like human ES cells, EpiS cells also grow as flat colonies and depend on FGF signaling. Notably, the most significant difference between mouse ES and EpiS cells are their chimera-forming properties. EpiS cells, unlike mouse ES cells, fail to give rise to chimeras when introduced into preimplantation embryos [38,39]. Conversely, EpiS cells, unlike mouse ES cells, efficiently contribute to all three germ layers when transferred into post-implantation epiblasts [40,41]. Mouse ES cells, in contrast, form teratomas when grafted onto post-implantation epiblasts [42]. These observations lent support for the idea that matching developmental stage is critical for chimera-competency [42]. Moreover, it became evident that the culture conditions (signaling environment) dictates the PS cell state – i.e., whether a stem cell manifests features of chimera-competent mouse ES cells or chimera-incompetent EpiS cells. Today, it is commonly accepted that PS cells exhibit features associated with different stages of embryonic development. The terms “naive” and “primed” were introduced by Nichols and Smith to designate PS cells with “pre-implantation” or “post-implantation” character (Figure 2) [36,43].
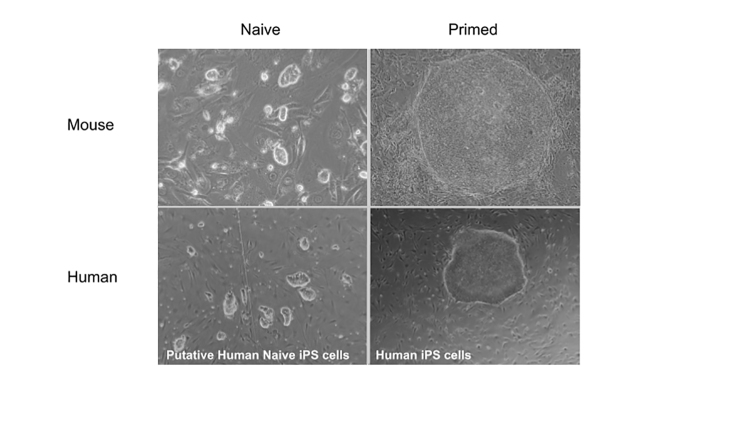
Figure 2.
Mouse and human naive and primed pluripotent stem cells. (Top left) Mouse naive embryonic stem (ES) cells; (top right) Mouse primed epiblast stem (EpiS) cells; (bottom left) putative human naive induced pluripotent stem (iPS) cells; (bottom right) human primed iPS cells. Mouse ES cells were grown in N2B27-2i/LIF conditions. Mouse EpiS cells and human iPS cells were grown in FGF-containing medium. Human naive iPS cells were grown in a modified 2i/LIF medium (ADLA, data unpublished).
Naive-like Human PS Cells
The existence of distinct murine PS cell states stimulated interest in generating human naive PS cells (Figure 2) [36]. Various groups have claimed generation of human naive PS cells by combining 2i with different experimental methods (Table 1) [32,44-52]. Human naive-like cells in 2i-containing culture conditions exhibit some mouse naive features, such as domed colony morphology and self-renewal in 2i [32,44-53]. However, such cells remained FGF-dependent, reflecting species differences or possibly indicating failure to reach a fully naive state [45,48,49,51,53-56]. While still unclear, it is possible that the difficulties in achieving a rodent-like naive pluripotent state may be linked to differences in the signaling mechanisms governing rodent and primate embryogenesis [57], as well as the absence of diapause in humans, a phenomenon thought to provide the basis for rodent naive pluripotency propagation in vitro [58, 59]. At the same time, while signaling mechanisms may diverge across species, it appears that core aspects of the transcription factor network governing rodent naive pluripotency are manifest in primate embryos and some reported human naive cells [46,47,51,57,60-63]. Whether capture of human PS cells with such transcription factor governance actually results in chimera-competent human PS cells is a very interesting question that has not been resolved [17].
Table 1. Culture conditions for distinct pluripotent states in mice and humans.
| Species | Pluripotent State | Culture Condition | Reference |
| Mouse | Naive | Serum; N2B27-LIF/BMP4 (serum-free medium with LIF and BMP4) | [80,81,82,83] |
| N2B27-2i | [29] | ||
| Primed | FGF/Activin-A | [37,38] | |
| N2B27-FGF2/IWR1 | [41] | ||
| Human | Naive-Like | N2B27-2i/LIF + DOX; N2B27-2i/LIF + Forskolin | [32] |
| KSR-2i/LIF (+ leaky transgene support?) | [44] | ||
| KSR-NHSM | [45] | ||
| mTESR1-3iL (PD/BIO/DOR/LIF) | [54] | ||
| KSR-PD/CH/FGF2 or KSR-PD/CH/SU/LIF | [48] | ||
| FMM: KSR, ROCKi, GSK3i, MEKi, bFGF, LIF | [55] | ||
| N2B27-5iLA | [46] | ||
| KSR-PD/CH/LIF/FGF2 | [53] | ||
| N2B27-t2iL+Go | [47,62,63] | ||
| KSR-4i | [56] | ||
| KSR-FGF/LIF/2i/Forskolin/Ascorbic Acid | [49] | ||
| STAT3-ER + KSR-2i/LIF | [50] | ||
| N2B27-5iLAF | [51,84] | ||
| N2B27-LCDM | [52] | ||
| Primed | FGF2/KSR; mTESR1 | [27,85] | |
| FGF2/IWR1 in mTESR1 base | [41] | ||
| “Intermediate” | N2B27-FAC, 0.05% BSA, 1% KSR | [17,69] |
Various culture regimens have been designed to capture and maintain naive and primed pluripotent states in mice and humans. In mice, the regimen of “2i” is used to propagate naive PS cells, whereas FGF and ACTIVIN-containing conditions are employed to propagate primed PS cells. In humans, primed PS cells generally contain FGF and ACTIVIN, but various culture regimes have been designed for human naive-like cells. Generally human naive-like cells are comprised of knockout serum replacement (KSR)-containing medium supplemented with 2i, FGF and LIF; or serum-free medium supplemented with 2i and transgene support. In three instances, t2iL + Go, 5i/L/A, and LCDM are human naive-like cells propagated without KSR, FGF2, or transgene support. Finally, putative “intermediate” FAC human PS cells are propagated in N2B27, FGF, ACTIVIN, and CHIR99021.
Abbreviations: 2i: CHIR99021 and PD0325901; NHSM: FGF2, TGF-beta1, LIF, PD0325901, CHIR99021, SP600125, SB203580, Go6983, Y-27632, 5iLA: LIF, ACTIVIN A, PD0325901, SB590885, IM-12, WH-4-023, Y-27632, t2iL + Go: LIF, PD0325901, CHIR99021, Go6983, 5iLAF: LIF, ACTIVIN A, FGF2, PD0325901, SB590885, IM-12, WH-4-023, Y-27632, LCDM: LIF, CHIR99021, (S)-(+)-dimethindene maleate, minocycline. FAC: FGF2, ACTIVIN A, CHIR99021. BSA: Bovine serum albumin.
Efforts to Generate Human-animal Interspecies Chimeras
Human chimera-competent PS cells, if they exist, may be able to contribute to the embryos of other species. Indeed, experiments involving human primed PS cells and mouse post-implantation embryos suggest feasibility. Consistent with the generation of chimeras by grafting murine primed PS cells onto egg-cylinder stage embryos, transplantation of conventional human PS cells into mouse egg-cylinder embryos results in differentiation into multiple fates, although it is unclear whether cooperative morphogenesis between donor and host cells occurred [41,64]. For generation of blastocyst stage chimeras, various groups have introduced alternative naive-like PS cells into mouse pre-implantation embryos [17,45,46,52,65]. However, in all cases the degree of chimeric contribution was essentially non-existent, especially when compared with higher-grade interspecies chimerism observed in mouse-rat interspecies chimeras [46,66]. Limited but detectable contribution of human cells to pig embryos has also been observed [17]. It is worth noting that the Belmonte study, unlike other studies evaluating interspecies chimerism, detected expression of lineage-affiliated markers in chimeric embryos, suggesting that differentiation of human PS cells may have occurred in vivo [17].
Synchronization of developmental stage between transplanted PS cells with host embryos seems to be needed for efficient chimera formation [41,42,64]. However, the introduction of human naive-like PS cells into murine and porcine pre-implantation embryos still fails to generate interspecies chimeras [17,46,66]. It is possible that “truly naive” human PS cells have not yet been generated, and this may account for the failure to generate blastocyst-stage chimeras [60,67]. Another possibility is that human naive pluripotent stem cells, classified as naive based on rodent molecular criteria, may not be developmentally equivalent to or synchronized with pig host embryos at the time of blastocyst injection [68]. It has been proposed that alternative human PS cells into late pig blastocysts may favor their development into chimeras [68]. Indeed, while introduction of certain naive-like human PS cells such as 4i and NHSM cells generated higher percentages of pig blastocysts containing human cells, introduction of an alternative human PS cell type cultured in FAC medium rather than other reported naive-like cells resulted in the formation of human-pig interspecies chimeras in the few instances it was observed [17,69].
Whether or not a human PS cell type satisfies molecular criteria for naive pluripotency, it still remains imperative to explore all culture parameters to maximize the interspecies chimera-competency of donor human PS cells [17,70]. Alternative strategies such as disabling apoptosis may help human stem cells overcome stage-related compatibility barriers to interspecies chimera formation. Indeed, overexpression of BCL2 endows chimera-incompetent rat EpiS cells with capacity to contribute to mouse blastocysts [71]. More generally, it is clear that the generation of naive PS cells may not be sufficient for achieving efficient interspecies chimera formation. Yet-to-be defined impediments may play a significant role.
The Species Barrier
An alternative explanation for the inefficient chimeric contribution of existing human naive PS cells to the embryos of other species is that additional unidentified parameters impede interspecies chimera formation [42] (Figure 3).
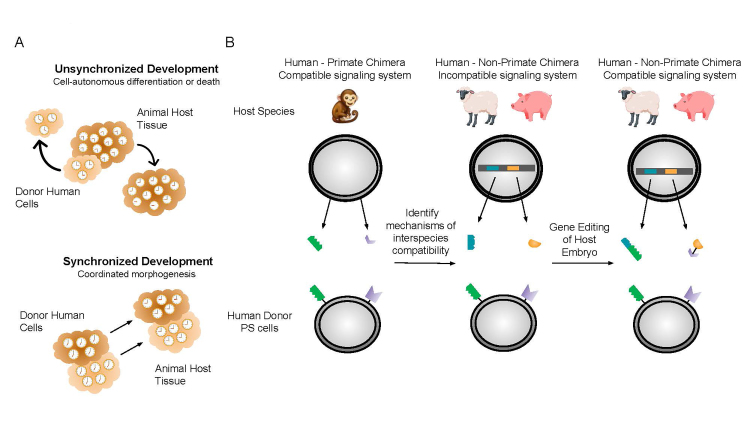
Figure 3.
Species barrier that impedes interspecies chimerism. A. Understanding the species barrier: synchronizing developmental speed. It is unclear why the efficiency of interspecies chimerism between humans and large animal species is low. The undefined parameters that impede interspecies chimerism are referred to as the species barrier. One possible component of the species barrier is the difference in developmental speed between species. How species-specific developmental timing is controlled is largely unknown. Experiments have shown that developmental speed may be species-specific and cell-autonomous (top). Some reports have suggested that developmental timing can be at least modestly modulated. In order for interspecies chimerism to occur, it will be necessary to achieve coordinated morphogenesis between human cells and animal host tissue (bottom). B. Engineering developmental compatibility across species. The existence of viable adult interspecies chimeras between mice and rats suggests the feasibility of generating interspecies chimeras using human cells. Choosing a host that is evolutionarily closer to humans, such as non-human primates (NHP), may help increase the degree of chimaerism by donor human PS cells. It may be possible to use human-primate chimeras to gain insight into the mechanisms underlying interspecies chimeric compatibility (compatible signaling environment). Using these insights, one can genetically “humanize” compatible large animal hosts (incompatible signaling environment) using multiplexed CRISPR/Cas9 gene editing. If successful, appropriately targeted genetic interventions will result in a more compatible signaling environment for higher efficiency interspecies chimerism.
Developmental Speed
One possible component of the species barrier is differences in the developmental speed between species [72]. Our understanding of how species-specific developmental timing is regulated remains primitive and poor. It has been observed that when mouse EpiS cells and human PS cells are subjected to the same neural differentiation protocol, human PS cell differentiation is comparatively prolonged with differences in the rates of differentiation mirroring differences in development timing in vivo. As a further corroboration, generation of teratomas from human PS cells in immunodeficient mice showed that human cells maintained a human rather than the mouse host developmental timing. These in vitro and in vivo data suggest that developmental timing involves a meaningful degree of cell autonomy, at least in these experimental contexts.
It may seem intuitive that synchronized developmental timing between donor cells of one species with host cells of another species may be necessary for achieving interspecies chimerism in vivo (Figure 3A). It may be possible to modulate developmental timing in vitro, albeit relatively incrementally. For example, modification of culture conditions reduces the time needed to derive different neural cells from human PS cells. For interspecies chimera production, it may be possible to synchronize human donor cells with pig development by “humanizing” the pig host embryo. Multiplexed gene editing to “humanize” pig embryos may prove useful in this regard [3,4].
Divergent Embryology
Additional evolutionary differences may also play a role. Primates and large animals such as pigs and sheep have undergone evolutionary divergence with regard to peri-implantation development. Unlike primates that possess a short pre-attachment period, both pigs and sheep exhibit a long pre-attachment period. During this pre-attachment period, both pigs and sheep embryos undergo a process in which the blastocyst evolves into a filamentous structure, extending up to 1 meter long in pigs [73]. Primates, in contrast, do not undergo such a process [67]. Such differences may prove pivotal for achieving efficient interspecies chimerism in post-implantation conceptuses.
A hypothetical strategy to bypass divergent embryology between humans and large animals is to identify more permissive stages for engraftment of human cells into animal hosts. One such candidate embryonic stage would be a mid-embryonic stage called “the phylotypic period,” a timepoint in development (i.e., gastrulation) of an animal that resembles other species [74]. This stage may also exhibit increased interspecies conservation of gene expression and signaling milieu. Future comparative embryology studies between human and pigs and sheep may prove fruitful for identifying more permissive stages for cross-species chimera generation.
The existence of viable adult rat-mouse chimeras suggests that the results obtained when introducing human PS cells into mouse and pig host embryos may differ if host embryos from more closely related species such as primates are used. To develop effective strategies to lower species barriers, it may prove informative to study chimerism in early-stage human-monkey embryos cultured to post-implantation stages to identify impediments to human-non-primate interspecies chimera formation (Figure 3B). Such experiments could inform strategies to improve human chimerism in a distantly related animal host.
Future Directions
Advances in the generation of interspecies chimeras and blastocyst complementation methodology will lay a foundation for generating transplantable patient-specific organs inside large animal hosts such as pigs or sheep. While CRISPR/Cas9 will enable the generation of appropriately customized animal hosts, there remain many barriers to regular adoption of this practice. Whether extensive chimerism can be obtained between humans and more closely related species such as primates is likely but still remains undemonstrated. It is not known whether it will be possible to engineer human organs without contaminating animal host-derived nerves and vasculature, which is clinically problematic. Fundamental problems remain, such as how best to resolve differences in developmental speed and to overcome distinct developmental processes that have arisen from evolution. Moreover, as the xenogenic immune response is one of the most robust, it will be instructive to understand the mechanism of xenogenic immune tolerance in interspecies chimeras such as those between mouse and rat or sheep and goats [16,75]. Strategies such as “humanization” of host animals may be needed for translation into the clinic. Humanization of host animals raises several contentious ethical questions and guidelines from the International Society for Stem Cell Research (ISSCR) and other regulatory authorities indicate there are ethical issues involved in interspecies chimera research [76]. We will refer readers to various commentary articles that address these issues [77,78]. The community must carefully consider future challenges and proceed forward within ethical, legal, and social guidelines [79].
Glossary
- PS
pluripotent stem cells
- iPS
induced pluripotent stem cells
- NHPs
non-human primates
- LIF
leukemia inhibitory factor
- 2i
kinase inhibitors
- EpiS cells
epiblast stem cells
Author Contributions
ADLA drafted the mini-review and all other authors helped editing and writing the review. ADLA and NP generated figures. DER supervised ADLA.
References
- Girlanda R. Deceased organ donation for transplantation: challenges and opportunities. World J Transplant. 2016;6(3):451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AM, Muir KW. Awareness and attitudes toward corneal donation: challenges and opportunities. Clin Ophthalmol. 2018;12:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Guell M, Niu D, George H, Lesha E, Grishin D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science. 2015;350:1101–4. [DOI] [PubMed] [Google Scholar]
- Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR/Cas9. Science. 2017;357:1303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe S, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cells derived from human somatic cells. Science. 2007;318:1917–20. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–5. [DOI] [PubMed] [Google Scholar]
- Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–4. [DOI] [PubMed] [Google Scholar]
- Morizane A, Kikuchi T, Hayashi T, Mizuma H, Takara S, Doi H, et al. MHC matching improves engrafting of iPSC-derived neurons in non-human primates. Nat Commun. 2017;8:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–77. [DOI] [PubMed] [Google Scholar]
- Wu J, Platero Luengo A, Gil MA, Suzuki K, Cuello C, Morales Valencia M, et al. MArtinez EA, Izpisua Belmonte JC. Generation of human organs in pigs via interspecies blastocyst complementation. Reprod Domest Anim. 2016;51 Suppl 2:18–24. [DOI] [PubMed] [Google Scholar]
- Masaki H, Nakauchi H. Interspecies chimeras for human stem cell research. Development. 2017;144:2544–7. [DOI] [PubMed] [Google Scholar]
- Suchy F, Yamaguchi T, Nakauchi H. iPSC-derived organs in vivo: challenges and promise. Cell Stem Cell. 2018;22:21–4. [DOI] [PubMed] [Google Scholar]
- Stanger BZ. Tanaka, AJ, Melton DM. Organ Size Is Limited by the Number of Embryonic Progenitor Cells in the Pancreas but Not the Liver. Nature. 2007;445:886–91. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–99. [DOI] [PubMed] [Google Scholar]
- Wu J, Platero-Leungo A, Sakurai M, Sugawara A, Gil MA, Yamauchi T, et al. MArtinez EA, Ross PJ, Izpisua Belmonte JC. Interspecies chimerism with mammalian pluripotent stem cells. Cell. 2017;168:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R, Nakauchi H. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol. 2012;180:2417–26. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sato H, Kato-Itoh M, Goto T, Hara H, Sanbo M, et al. Interspecies organogenesis generates autologous functional islets. Nature. 2017;542:191–6. [DOI] [PubMed] [Google Scholar]
- Cooper DK. A brief history of cross-species organ transplantation [Bayl Univ Med Centr] Proc. 2012;25:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehilly CB, Willadsen SM, Tucker EM. Interspecific chimerism between sheep and goat. Nature. 1984;307:634–6. [DOI] [PubMed] [Google Scholar]
- Polzin VJ, Anderson DL, Anderson GB. BonDurant RH, Butler JE, Pashen RL, Penedo MC, Rowe JD. Production of sheep-goat chimeras by inner cell mass transplantation. J Am Sci. 1987;65:325–30. [DOI] [PubMed] [Google Scholar]
- Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci USA. 2013;110:4557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino M, Rashid ST, Suchy FP, McNabb BR, van der Meulen T, Fine EJ, et al. CRISPR/Cas9 microinjection in oocytes disables pancreas development in sheep. Sci Rep. 2017;7:17472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. [DOI] [PubMed] [Google Scholar]
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–8. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroviak T, Loos R, Bertone P, Smith A, Nichols J. The ability of inner cell mass cells to self-renew as embryonic stem cells is acquired upon epbilast specification. Nat Cell Biol. 2014;16:516–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J, Smith A. Capturing pluripotency. Cell. 2008;132:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, et al. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA. 2010;107:9222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–98. [DOI] [PubMed] [Google Scholar]
- Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Ramsey C, Ma H, Lee HS, Penedo MC, et al. Generation of chimeric rhesus monkeys. Cell. 2012;148:285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–92. [DOI] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–5. [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–9. [DOI] [PubMed] [Google Scholar]
- Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi T, Oki S, Kitajima K, Meno C. Epiblast ground state is controlled by canonical Wnt/Beta-catenin signaling in the postimplantation embryo and epiblast stem cells. PLoS One. 2013;8:e63378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Okamura D, Li M, Suzuki K, Luo C, Ma L, et al. An alternative pluripotent state confers interspecies chimeric competency. Nature. 2015;521:316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MA, Markoulaki S, Jaenisch R. Matched developmental timing of donor cells with the host is crucial for chimera formation. Stem Cell Reports. 2018;10:1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Smith A. Pluripotency in the embryo and in culture. Cold Spring Harb Perspect Biol. 2012;4:a008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang J, Liu H, Lu D, Chen X, Zenonos Z, et al. Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc Natl Acad Sci USA. 2011;108:18283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, et al. MAssarwa R, Novershtern N, Hanna JH. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–6. [DOI] [PubMed] [Google Scholar]
- Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014;15:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell. 2014;158:1254–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, et al. Ruohola-Baker. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci USA. 2014;111:4484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal G, Warrier S, Ghimire S, Broekaert D, Van der Jeught M, Lierman S, et al. Alternative routes to induce naive pluripotency in human embryonic stem cells. Stem Cells. 2015;33:2686–98. [DOI] [PubMed] [Google Scholar]
- Chen H, Aksoy I, Gonnot F, Osteil P, Aubry M, Hamela C, et al. Reinforcement of Stat3 activity reprogrammes human embryonic stem cells to naive-like pluripotency. Nat Commun. 2015;6:7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor WA, Chen D, Liu W, Kim R, Sahakyan A, Lukianchikov A, et al. Naive human pluripotent cells feature a methylation landscape devoid of blastocyst or germline memory. Cell Stem Cell. 2016;18:323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu B, Xu J, Wang J, Wu J, Shi C, et al. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell. 2017;169:243–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie G, Singh M, Ghanbarian AT, Rasko T, Szvetnik A, et al. Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature. 2014;516:405–9. [DOI] [PubMed] [Google Scholar]
- Chan YS, Goke J, Ng JH, Lu X, Gonzales KA, Tan CP, et al. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell. 2013;13:663–75. [DOI] [PubMed] [Google Scholar]
- Valamehr B, Robinson M, Abujarour R, Rezner B, Vranceanu F, Le T, et al. Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Reports. 2014;2:366–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroviak T, Loos R, Lombard P, Okahara J, Behr R, Sasaki E, et al. Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev Cell. 2015;35:366–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Chambers I, Taga T, Smith A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development. 2001;128:2333–9. [DOI] [PubMed] [Google Scholar]
- Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, et al. Cell. 2009;138:722–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Angeles A, Ferrari F, Xi R, Fujiwara Y, Benvenisty N, Deng H, et al. Hallmarks of pluripotency. Nature. 2015;525:469–78. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Okamoto I, Sasaki K, Yabuta Y, Iwatani C, Tsuchiya H, et al. A developmental coordinate of pluripotency among mice, monkeys, and humans. Nature. 2016;537:57–62. [DOI] [PubMed] [Google Scholar]
- Guo G, von Meyenn F, Rostovskaya M, Clarke J, Dietmann S, Baker D, et al. Epigenetic resetting of human pluripotency. Development. 2017;144:2748–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, von Meyenn F, Santos F, Chen Y, Reik W, Bertone P, et al. Naïve pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem Cell Reports. 2016;6:437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascetti VL, Pedersen RA. Human-mouse chimerism validates human stem cell pluripotency. Cell Stem Cell. 2016;18:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki H, Kato-Itoh M, Umino A, Sato H, Hamanaka S, Kobayashi T, et al. Interspecific in vitro assay for the chimera-forming ability of human pluripotent stem cells. Development. 2015;142:3222–30. [DOI] [PubMed] [Google Scholar]
- Theunissen TW, Friedli M, He Y, Planet E, O’Neil RC, Markoulaki S, et al. Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell. 2016;19:502–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroviak T, Nichols J. Primate embryogenesis predicts the hallmarks of human naive pluripotency. Development. 2017;144:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Ibeas P, Sang F, Zhu Q, Tang WWC, Withey S, Klisch D, Loose M, Surani MA, Alberio R. Lineage segregation, pluripotency and X-chromosome inactivation in the pig pre-gastrulation embryo. bioRxiv. 2018. [Google Scholar]
- Tsukiyama T, Ohinata Y. A modified EpiSC culture condition containing a GSK3 inhibitor can support germline-competent pluripotency in mice. PLoS One. 2014;9:e95329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Izpisua Belmonte JC. Dynamic pluripotent stem cell states and their applications. Cell Stem Cell. 2015;17:509–25. [DOI] [PubMed] [Google Scholar]
- Masaki H, Kato-Itoh M, Takahashi Y, Umino A, Sato H, Ito K, et al. Inhibition of apoptosis overcomes stage-related compatibility barriers to chimera formation in mouse embryos. Cell Stem Cell. 2016;19:587–92. [DOI] [PubMed] [Google Scholar]
- Barry C, Schmitz MT, Jiang P, Schwartz MP, Duffin BM, Swanson SS, et al. Species-Specific Developmental Timing Is Maintained by Pluripotent Stem Cells Ex Utero. Dev Biol. 2017;423:101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod. 2010;16:135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie N, Kuratani S. Comparative transcriptome analysis reveals vertebrate phylotypic period during organogenesis. Nat Commun. 2011;2:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffing NA, Anderson GB, Bondurant RH, Pashen RL, Bernoco D. Antibody response to ewes and does to chimeric sheep-goat pregnancy. Biol Reprod. 1963;49:1260–9. [DOI] [PubMed] [Google Scholar]
- Daley GQ, Hyun I, Apperley JF, Barker RA, Benvenisty N, Bredenoord AL, et al. Setting global standards for stem cell research and clinical translation: the 2016 ISSCR guidelines. Stem Cell Reports. 2016;6:787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret R, Martinez E, Vialla F, Giquel C, Thonnat-Marin A, De Vos J. Human-animal chimeras: ethical issues about farming chimeric animals bearing human organs. Stem Cell Res Ther. 2016;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Greely HT, Jaenisch R, Nakauchi H, Rossant J, Belmonte JC. Stem cells and interspecies chimeras. Nature. 2016;540:51–9. [DOI] [PubMed] [Google Scholar]
- Sharma A, Sebastiano V, Scott CT, Magnus D, Koyano-Nakagawa N, Garry DJ, et al. Lift NIH restrictions on chimera research. Science. 2015;350:640. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1998;336:688–90. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–92. [DOI] [PubMed] [Google Scholar]
- Pastor WA, Liu W, Chen D, Ho J, Kim R, Hunt TJ, et al. TFAP2C regulates transcription in human naive pluripotency by opening enhancers. Nat Cell Biol. 2018;20:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Nat Methods. 2006;3:637–46. [DOI] [PubMed] [Google Scholar]