Abstract
Marine sponges are known as a rich source for novel bioactive compounds with valuable pharmacological potential. One of the most predominant sponge genera is Hyrtios, reported to have various species such as Hyrtios erectus, Hyrtios reticulatus, Hyrtios gumminae, Hyrtios communis, and Hyrtios tubulatus and a number of undescribed species. Members of the genus Hyrtios are a rich source of natural products with diverse and valuable biological activities, represented by different chemical classes including alkaloids, sesterterpenes and sesquiterpenes. This review covers the literature until June 2016, providing a complete survey of all compounds isolated from the genus Hyrtios with their corresponding biological activities whenever applicable.
Keywords: bioactive, marine natural products, marine sponges, Hyrtios, alkaloids
1. Introduction
Marine ecosystems contain enormous, still unexplored and taxonomically diverse macro- and microorganisms. These marine organisms are able to produce novel molecules with large structural diversity and various interesting pharmacological activities [1,2,3,4]. Since 1985, more than 4000 marine natural products possessing a wide range of biological activities have been isolated and characterized [5]. About twenty-four marine natural products are currently in phase I–III clinical trials [6,7]. Moreover, there are currently eight marine natural products on the market, possessing different pharmacological activities [8]. Sponges (phylum Porifera) are among the oldest multicellular animals with a fossil record dating back to Precambrian times [9]. Sponges are widespread in tropical reefs in a great abundance, but can also be found in polar latitudes and the deep, sea as well as in fresh water lakes and rivers [10]. Marine sponges continue to attract attention as rich sources of structurally novel secondary metabolites that are potential lead compounds for the development of new drugs [11]. With more than 280 new isolated compounds from sponges reported during 2014, sponges have returned again to be a superior source of new biologically active marine natural products [11].
Sponges belonging to the genus Hyrtios (Kingdom: Animalia, phylum: Porifera, class: Demospongiae, order: Dictyoceratida, family: Thorectidae) are reported to be rich sources of bioactive secondary metabolites. Among marine sponges of the genus Hyrtios, the sponge H. erectus is the most frequently investigated source of bioactive natural products. For example, several indole alkaloids [12,13], β-carboline alkaloids [14,15] and sesterterpenes [16,17] were isolated from this Hyrtios species. Biological investigations of alkaloids and sesterterpenes isolated from H. erectus revealed that some of these compounds possess noteworthy anticancer [18,19] and antimicrobial [20,21] activities. The marine sponge H. erectus has been collected from different marine environments, including the Red Sea in Egypt [13,16] and Okinawa in Japan [18,22]. The Indonesian marine sponge H. reticulatus is another frequently studied marine sponge of this genus reported as a good source of novel β-carboline alkaloids [23,24,25].
In addition, natural product discovery projects from the three sponges H. gumminae, H. communis and H. tubulatus (which has been allocated to the new genus Dysidea tubulata) have been reported in the literature. H. gumminae was collected from the Andaman Sea in Thailand and was found to be a source of novel sesterterpenoids [26]. Moreover, biological and chemical investigations of the extracts of several undescribed species of the genus Hyrtios can be found in the literature. Novel derivatives of puupehenone, a sesquiterpene-methylene quinone, [27,28,29] and alkaloids [30,31,32,33,34,35,36] were the most predominant isolated compounds from the undescribed species of the genus Hyrtios. Here, we are reporting an overview on the chemical structures of marine natural products isolated from diverse marine sponges of the genus Hyrtios, together with their isolation sources as well as their biological activities whenever applicable.
2. H. erectus
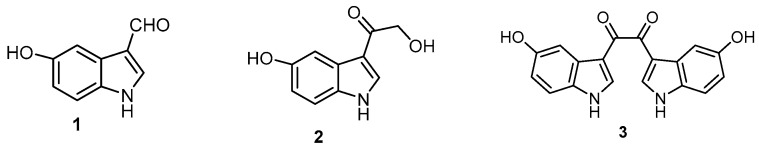
5-Hydroxyindole-3-aldehyde (1) together with the related compounds hyrtiosins A (2) and B (3) were isolated from the Okinawan marine sponge H. erectus collected near Ishigaki Island (Figure 1). Compound 1 showed in vitro cytotoxic activity against human epidermoid carcinoma KB cells with IC50 (half maximal inhibitory concentration, concentration causing 50% of the desired activity) value of 4.3 µg/mL, while hyrtiosins A (2) and B (3) were less cytotoxic than 1 [18].
Figure 1.
Chemical structures of 5-hydroxyindole-3-aldehyde (1), hyrtiosin A (2) and hyrtiosin B (3).
Hyrtiomanzamine 4, a β-carboline alkaloid, was isolated from the marine sponge H. erectus collected in the Red Sea (Figure 2). Compound 4 exhibited immunosuppressive activity with an EC50 (half maximal effective concentration, concentration causing 50% of the desired activity) of 2 µg/mL in the B lymphocytes reaction assay and no cytotoxic activity on KB cells was observed [37].
Figure 2.
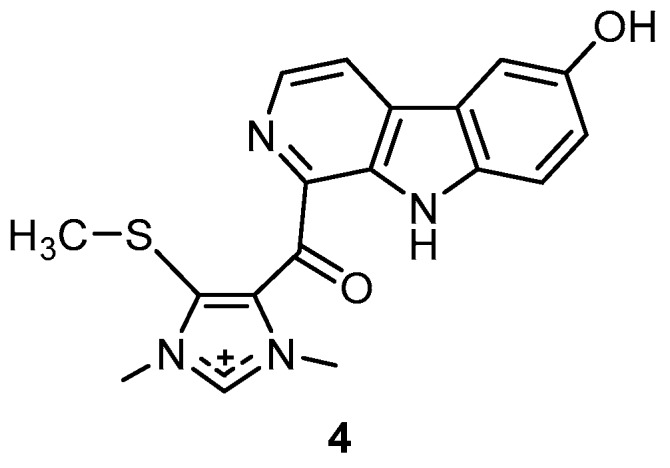
Chemical structure of hyrtiomanzamine (4).
Two pentacyclic sesterstatins, sesterstatins 4 (5) and 5 (6), were isolated from the marine sponge H. erectus collected in the Republic of Maldives (Figure 3). Compounds 5 and 6 inhibited the growth of a number of human cancer cell lines, including P388 leukemia, BXPC-3 pancreas, RPMI-7951 melanoma, U251 CNS, KAT-4 thyroid, NCI-H460 lung NSC, FADU pharynx and DU-145 prostate, with GI50 values ranging from 1.6 to 4.9 µg/mL. In addition, disc diffusion assays showed the ability of compound 6 to inhibit the growth of the Gram-positive bacterium Micrococcus luteus, with minimum inhibitory concentration (MIC) 25–50 µg/disk [19].
Figure 3.
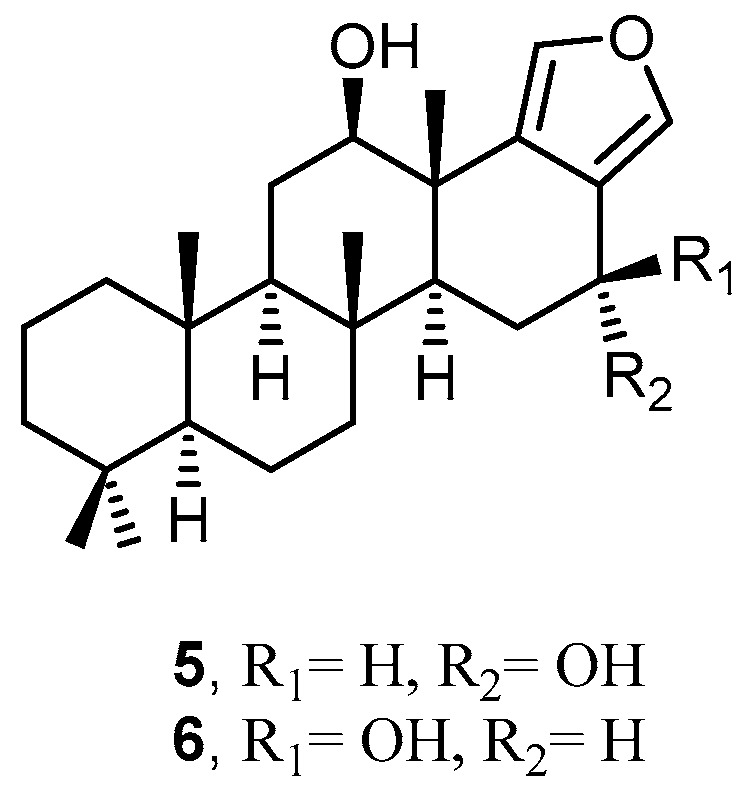
Chemical structures of sesterstatin 4 (5) and sesterstatin 5 (6).
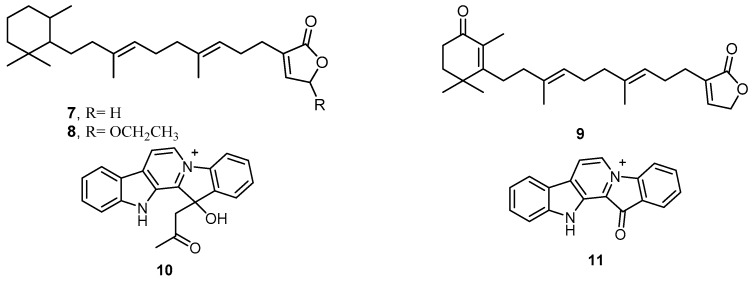
Fractionation of the dichloromethane extract of the marine sponge H. erectus, collected from Fiji, led to the isolation of the two sesterterpenes 7 and 8, isodehydroluffariellolide (9), homofascaplysin A (10), and fascaplysin (11) (Figure 4). Biological evaluation of these isolated compounds revealed activities for compounds 10 and 11, both being potently active in vitro against the causative agent of tropical malaria Plasmodium falciparum strain K1 with IC50 values of 14 and 50 ng/mL, respectively, and against P. falciparum strain NF54 with IC50 values of 24 and 34 ng/mL, respectively [20]. Thus, homofascaplysin A (10), and fascaplysin (11) could serve as promising antimalarial agents for future work.
Figure 4.
Chemical structures of compound 7, compound 8, isodehydroluffariellolide (9), homofascaplysin A (10) and fascaplysin (11).
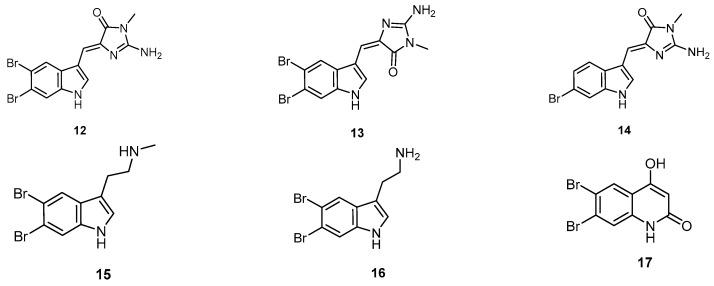
Five indole alkaloids 12–16 were isolated from the marine sponge H. erectus, which has been collected at Iriomote-Island, Okinawa Prefecture, Japan. Furthermore, the quinolone 17 was also isolated from the same marine sponge (Figure 5). The indole alkaloids 12–16 showed 100% selective inhibitory activity against the neuronal isozyme of nitric oxide synthase at a concentration of 125 µg/mL [12].
Figure 5.
Chemical structures of the indole alkaloids (12–16) and compound 17.
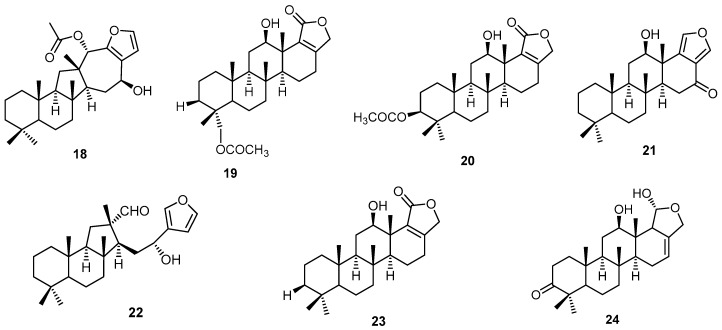
A pentacyclic sesterterpene ester salmahyrtisol A (18), three scalarane-type sesterterpenes, 3-acetyl sesterstatin (19), 19-acetyl sesterstatin 3 (20), and salmahyrtisol B (21), together with sesterterpenes hyrtiosal (22), scalarolide (23), and salmahyrtisol C (24) were obtained from the methanol extract of the Red Sea sponge H. erectus collected from a depth of 15–20 m from El Quseir, 120 km south of Hurghada, Egypt (Figure 6). Compounds 18–21 showed significant in vitro cytotoxicity against murine leukemia, human lung carcinoma, and human colon carcinoma, with IC50 values ≥1 µg/mL [16].
Figure 6.
Chemical structures of salmahyrtisol A (18), 3-acetyl sesterstatin (19), 19-acetyl sesterstatin 3 (20), salmahyrtisol B (21), hyrtiosal (22), scalarolide (23) and salmahyrtisol C (24).
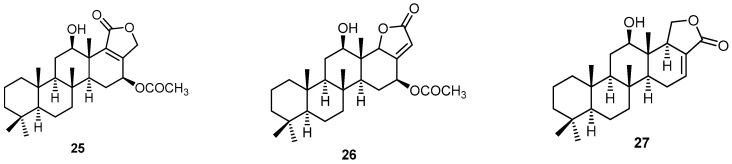
The scalarane-type pentacyclic sesterterpene sesterstatin 7 (25) has been isolated from the marine sponge H. erectus, collected by hand using scuba at a depth of 15 m off Safaga at the Egyptian Red Sea coast, along with 16-epi-scalarolbutenolide (26), 25-dehydroxy-12-epi-deacetylscalarin (27) and 3-acetylsesterstatin 1 (20) (Figure 7). Compound 25 displayed 63% growth inhibition of Mycobacterium tuberculosis (H37Rv) (ATCC 27294) at a concentration of 6.25 µg/mL. Compound 26 showed moderate antimycobacterial activity (40% inhibition at 6.25 µg/mL), while compounds 27 and 20 exhibited weak activities at the same concentration [38].
Figure 7.
Chemical structures of sesterstatin 7 (25), 16-epi-scalarolbutenolide (26) and 25-dehydroxy-12-epi-deacetylscalarin (27).
Sesterstatin 6 (28), another scalarane-type pentacyclic sesterterpene, was isolated from the Republic of Maldives marine sponge H. erectus (Figure 8). Compound 28 exhibited significant anticancer activity against murine P388 lymphocytic leukemia (ED50, effective dose causing 50% of the desired activity, 0.17 µg/mL) and a series of human tumor cell lines (GI50, concentration causing 50% of growth inhibition of cell proliferation, 0.18–89 µg/mL) [39].
Figure 8.
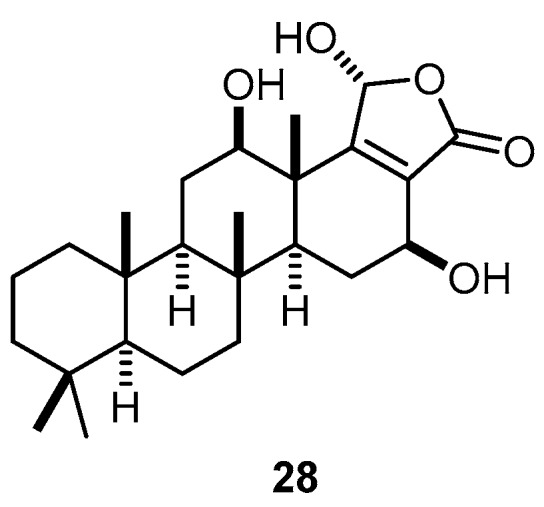
Chemical structure of sesterstatin 6 (28).
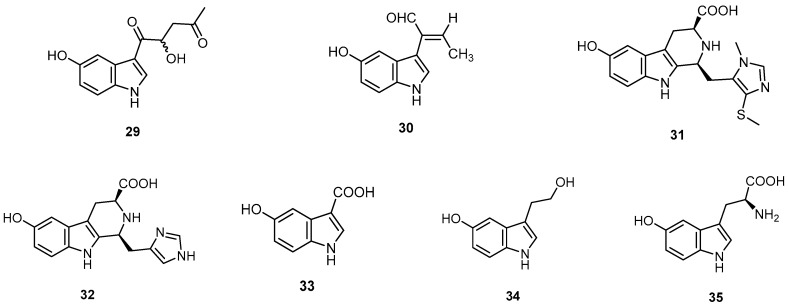
Hainanerectamines A (29) and B (30), two new indole alkaloids, and hainanerectamine C (31), a new β-carboline alkaloid, together with five known alkaloids (1, 32–35), were isolated from the Hainan marine sponge H. erectus collected off Lingshui Bay, Hainan Province, China (Figure 9). Compounds 30–32 showed moderate inhibitory activity against Aurora A, a member of the serine/threonine kinase family involving in the regulation of cell division and a new target in cancer treatment, with IC50 values of 24.5, 13.6, and 18.6 μg/mL, respectively [14].
Figure 9.
Chemical structures of hainanerectamine A (29), hainanerectamine B (30), hainanerectamine C (31) and the alkaloids 32–35.
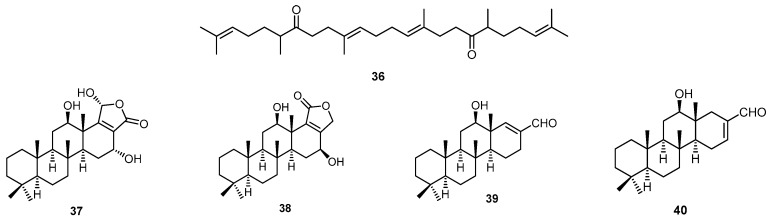
An acyclic diketotriterpenoid (36) was isolated from the marine sponge H. erectus collected in Indonesia [40]. Three scalarane sesterterpenoids, hyrtiolide (37), 16-hydroxyscalarolide (38), and 12-deacetyl-∆17-hyrtial (39), along with scalarolide (23) and 12-deacetylhyrtial (40) were isolated from the marine sponge H. erectus collected from the coral reef of Ishigaki Island, Okinawa, Japan (Figure 10). Compounds 39 and 40 showed antiproliferative activity towards KB cells with IC50 values of 2.82 and 10 µg/mL, respectively [17].
Figure 10.
Chemical structures of the diketotriterpenoid 36, hyrtiolide (37), 16-hydroxyscalarolide (38), 12-deacetyl-∆17-hyrtial (39) and 12-deacetylhyrtial (40).
Hyrtiosulawesine (41), a β-carboline alkaloid, together with 5-hydroxyindole-3-carbaldehyde (1), hyrtiosin B (3), and 5-hydroxy-3-(2-hydroxyethyl)indole (34) were isolated from the Indonesian specimens of a H. erectus collected from the northwest side of Lankai Island, off Makassar, South West Sulawesi (Figure 11) [15].
Figure 11.
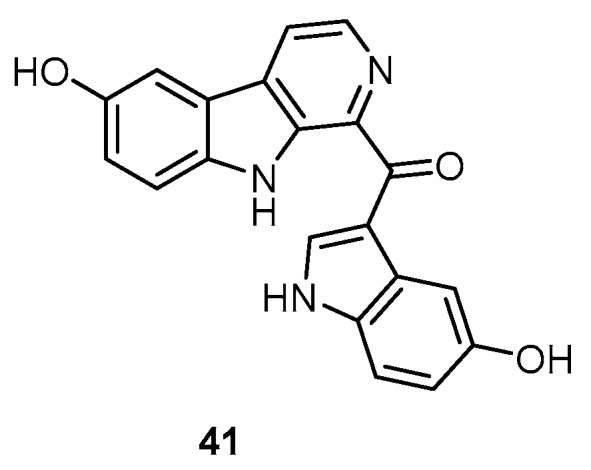
Chemical structure of hyrtiosulawesine (41).
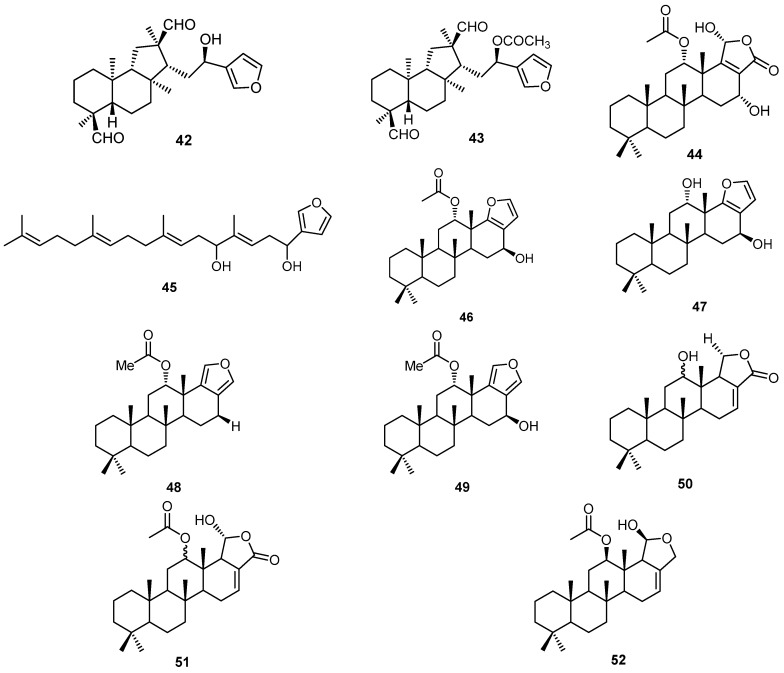
Eleven sesterterpenes, 20-formylhyrtiosal (42), 16-O-acetyl-20-formylhyrtiosal (43), 12-α-O-acetylhyrtiolide (44), 5,10-dihydroxyfurospinulosine-1 (45), and compounds 46–52 have been isolated from the marine sponge H. erectus collected at Hainan, China (Figure 12) [41].
Figure 12.
Chemical structures of 20-formylhyrtiosal (42), 16-O-acetyl-20-formylhyrtiosal (43), 12-α-O-acetylhyrtiolide (44), 5,10-dihydroxyfurospinulosine-1 (45) and compounds 46–52.
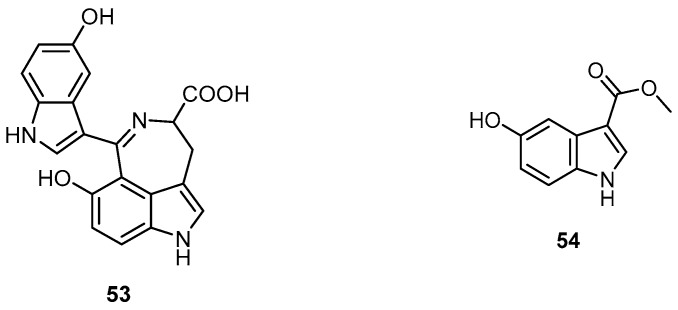
Fractionation of the methanolic extract of the marine sponge H. erectus, collected from Safaga at the Egyptian Red Sea coast, led to the isolation of the azepino-indole-type alkaloid hyrtiazepine (53) and 5-hydroxy-1H-indole-3-carboxylic acid methyl ester (54) (Figure 13), together with the known compounds hyrtiosulawesine (41), 5-hydroxyindole-3-carbaldehyde (1), hyrtiosin A (2), and hyrtiosin B (3). Hyrtiosulawesine (41) exhibited antiphospholipase A2 activity with an IC50 value of 14 µM in a fluorometric assay using Crotalus adamanteus venom phospholipase A2 [13].
Figure 13.
Chemical structures of hyrtiazepine (53) and compound 54.
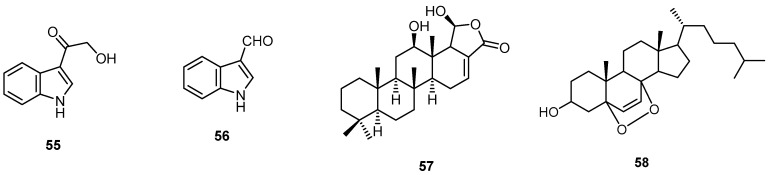
Deoxyhyrtiosine A (55) and indole-3-carbaldehyde (56) were isolated for the first time from the marine sponge H. erectus collected from the Red Sea in Egypt. In addition, the four known indoles; 5-hydroxy-1H-indole-3-carboxylic acid methyl ester (54), 5-hydroxy-1H-indole-3-carbaldehyde (1) and hyrtiosine A (2) were obtained. Three scalarane sesterterpenes, 16-hydroxyscalarolide (38), scalarolide (23) and 12-O-deacetyl-12-epi-scalarine (57), as well as 5α,8α-epidioxy-cholesta-6-en-3β-ol (58) were also isolated (Figure 14). Compounds 38, 54 and 56 exhibited growth inhibition activity against the L5178Y mouse lymphoma cell line, while compounds 1, 38 and 55 showed mild antimicrobial activities against the Gram-positive bacterium Bacillus subtilis and the fungus Saccharomyces cerevisiae [21].
Figure 14.
Chemical structures of deoxyhyrtiosine A (55), indole-3-carbaldehyde (56), 12-O-deacetyl-12-epi-scalarine (57) and 5α,8α-epidioxy-cholesta-6-en-3β-ol (58).
Hyrtiosal (59), isolated from the marine sponge H. erectus collected from Kerama Islands, Okinawa, inhibited HIV-1 integrase binding to viral DNA with an IC50 of 9.60 ± 0.86 µM (Figure 15) [22].
Figure 15.
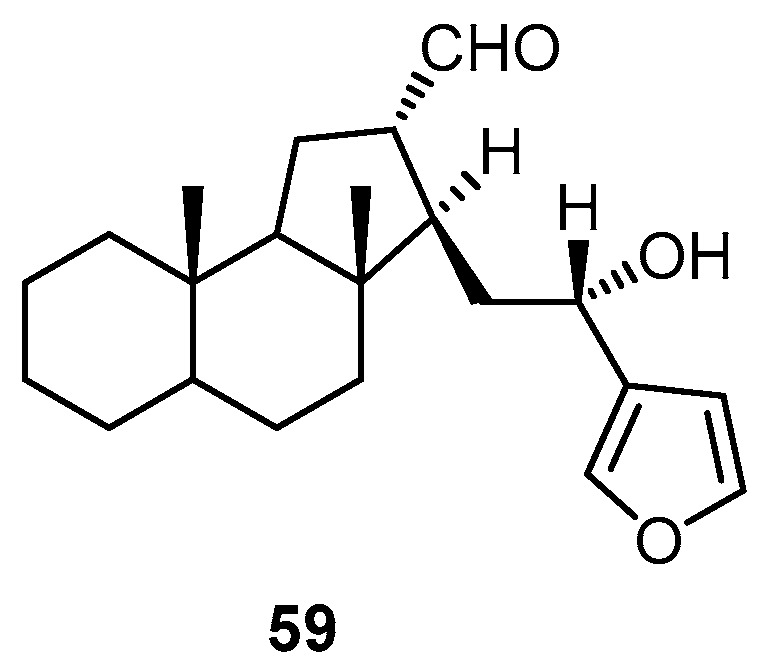
Chemical structure of hyrtiosal (59).
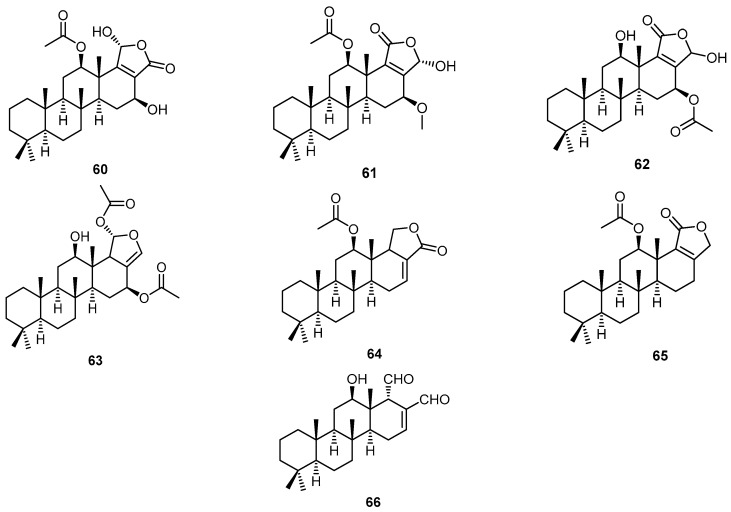
Two new sesterterpene analogs, 12-acetoxy,16-epi-hyrtiolide (60) and 12β-acetoxy,16β-methoxy,20α-hydroxy-17-scalaren-19,20-olide (61), together with seven previously reported scalarane-type sesterterpenes, 23, 25, and 62–66, were isolated from the sponge H. erectus collected from the Red Sea, Egypt (Figure 16). The isolated compounds 25 and 60–66 showed considerable in vitro anticancer activity against breast adenocarcinoma (MCF-7), colorectal carcinoma (HCT-116) and hepatocellular carcinoma cells (HepG2), with IC50 values of 0.7–57.5 µM [42].
Figure 16.
Chemical structures of 12-acetoxy,16-epi-hyrtiolide (60), 12β-acetoxy,16β-methoxy,20α-hydroxy-17-scalaren-19,20-olide (61) and the sesterterpenes 62–66.
3. H. reticulatus
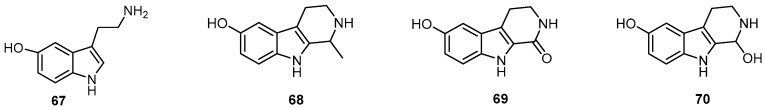
Serotonin (67), 6-hydroxy-1-methyl-1,2,3,4-tetrahydro-β-carboline (68), 6-hydroxy-3,4dihydro-1-oxo-β-carboline (69) and 1,6-dihydroxy-1,2,3,4-tetrahydro-β-carboline (70) were isolated from the Indonesian specimens of the marine sponges H. reticulatus, collected from the west side of Bone Lola Reef, off Makassar, South West Sulawesi, Indonesia (Figure 17) [15].
Figure 17.
Chemical structures of serotonin (67) and compounds 68–70.
Hyrtiocarboline (71), 1-imidazoyl-3-carboxy-6-hydroxy-β-carboline alkaloid, were derived from a Papua New Guinea marine sponge, H. reticulatus (Figure 18). Compound 71 exhibited selective anticancer activity against H522-T1 non-small cell lung, MDA-MB-435 melanoma, and U937 lymphoma cancer cell lines, with IC50 values of 1.2, 3.0 and 1.5 µg/mL, respectively [23].
Figure 18.
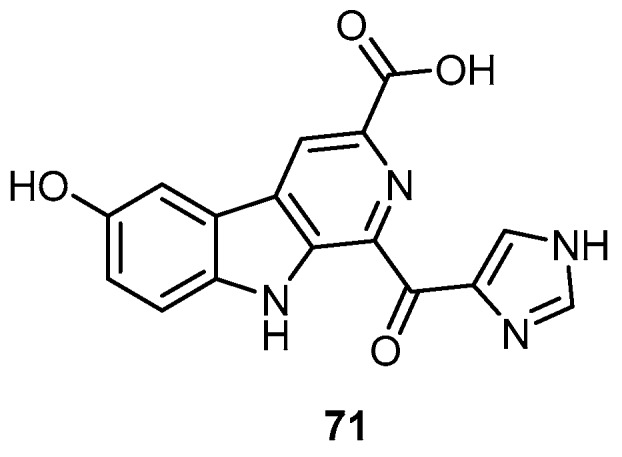
Chemical structure of hyrtiocarboline (71).
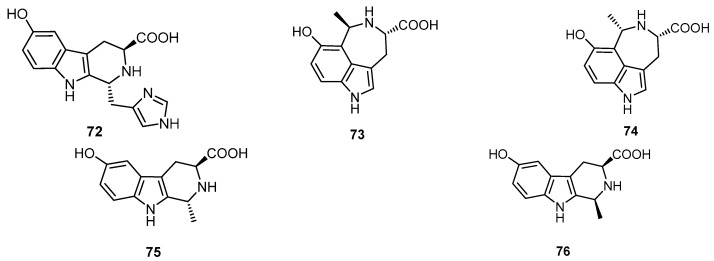
Hyrtioreticulin A (72), hyrtioreticulin B (32) and hyrtioreticulins C-E (73–75) were identified in the marine sponge H. reticulatus, collected at a depth of 10 m in North Sulawesi, Indonesia, along with a known alkaloid, hyrtioerectine B (76) (Figure 19). The tetrahydro-β-carboline alkaloids hyrtioreticulins A (72) and B (32) inhibited ubiquitin-activating enzyme (E1) with IC50 values of 0.75 and 11 µg/mL, respectively. Interestingly, only five E1 inhibitors, panapophenanthrine, himeic acid A, largazole, and hyrtioreticulins A and B (72 and 32), were isolated from natural sources and, among them, compound 72 is the most potent E1 inhibitor [24].
Figure 19.
Chemical structures of hyrtioreticulin A (72), hyrtioreticulins C-E (73–75) and hyrtioerectine B (76).
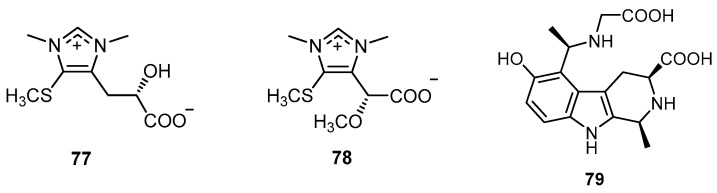
Two new 1,3-dimethyl-5-(methylthio)imidazolium alkaloids, reticulatins A (77) and B (78), and a new indole alkaloid hyrtioreticulin F (79) were isolated from the water-soluble fraction of the ethanol extract of the Indonesian marine sponge H. reticulatus (Figure 20) [25].
Figure 20.
Chemical structures of reticulatin A (77), reticulatin B (78) and hyrtioreticulin F (79).
4. H. gumminae
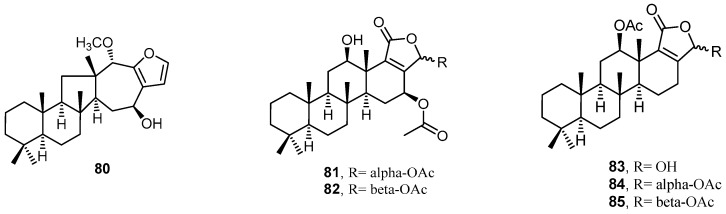
Chemical investigation on the ethylacetate-soluble fraction of the methanol extract of the marine sponge H. gumminae collected from Similan Island in the Andaman Sea, Thailand, yielded four sesterterpenoids, 12β,20-dihydroxy-16β-acetoxy-17-scalaren-19,20-olide (62), similan A (80), 12β-acetoxy20-hydroxy-17-scalaren-19,20-olide (83), and 12β,16α,20-trihydroxy-17-scalaren-19,20-olide (86), along with hyrtiosal (22), 12-epi-O-deacetyl-19-deoxyscalarin (27), hyrtiolide (37), and compounds 81, 82, 84, 85, and 87–89 (Figure 21). Some of these isolated compounds were tested for their in vitro anticancer activity against HuCCA-1 (human cholangiocarcinoma), KB (human epidermoid carcinoma of the mouth), HeLa (human cervical carcinoma), MDA-MB-231 (hormone-independent breast cancer), T47D (hormone-dependent breast cancer), and H69AR (multidrug-resistant small-cell lung cancer). Compounds 22 and 27 showed moderate anticancer activities (IC50 values of 5.2–57 µM) [26].
Figure 21.
Chemical structures of similan A (80) and compounds 81–89.
5. H. communis
The extract of marine sponge H. communis, collected from a depth of 18–21 m from the northern reefs region off the coast of Palau, was found to inhibit transcription factor hypoxia-inducible factor-1 (HIF1) activation in T47D human breast tumor cells. Bioassay-guided fractionation of the H. communis extract led to the isolation and identification of the sesterterpenes 90–102 (Figure 22). Thorectidaeolide A (90), 4-acetoxythorectidaeolide A (91) and luffariellolide (100) showed potent inhibition activities of HIF-1 activation, with IC50 values of 3.2, 3.5, and 3.6 µM, respectively. Compound 100 exhibited a significant cytotoxic activity, which can be explained by its HIF-1 inhibitory activity [43].
Figure 22.
Chemical structures of the sesterterpenes 90–102.
6. H. tubulatus
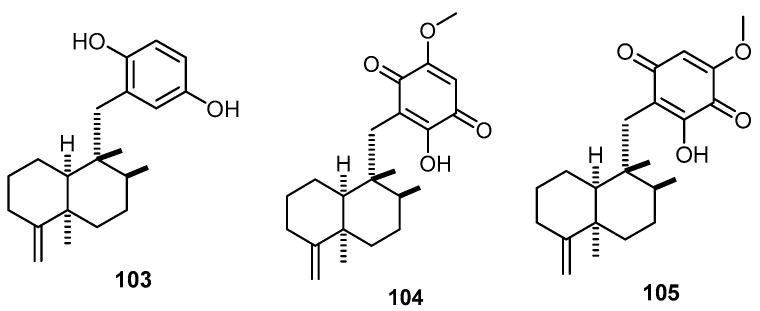
Arenarol (103) together with 5-epiilimaquinone (104) and 21-hydroxy-19-methoxyarenarone (105), which bear the 4,9-friedodrim-4(15)-ene skeleton, were isolated from the marine sponge H. tubulatus (which is currently identified as Dysidea tubulata) collected at a depth of 34.9 m by scuba diving off the South coast of Curaçao, Netherlands Antilles (Figure 23) [44].
Figure 23.
Chemical structures of arenarol (103), 5-epiilimaquinone (104) and 21-hydroxy-19-methoxyarenarone (105).
7. Undescribed Marine Sponges of the Genus Hyrtios
Dipuupehedione (106) has been isolated from the dichloromethane extract of a Caledonian marine sponge Hyrtios sp. (Figure 24), collected by SCUBA diving in New Caledonia (East Coast). Compound 106 showed significant cytotoxic activity on KB cells with IC50 value of 3 µg/mL [27].
Figure 24.
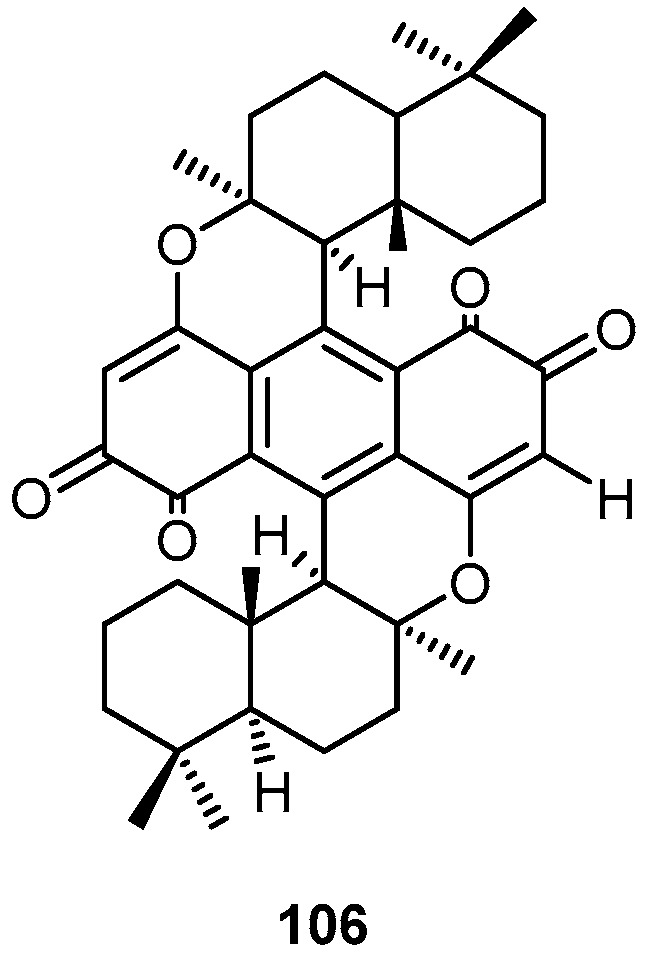
Chemical structure of dipuupehedione (106).
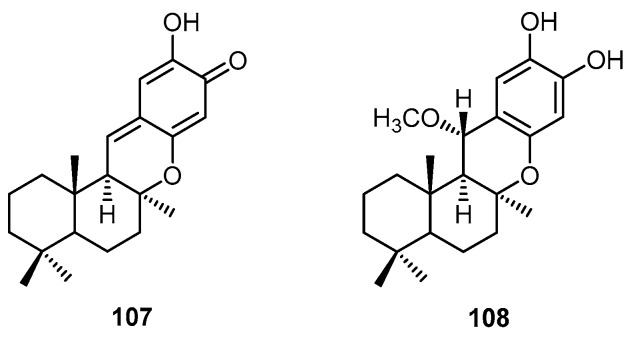
Fractionation and chemical investigation of the dichloromethane extract of the marine sponge Hyrtios sp. collected from the East Coast of New Caledonia afforded dipuupehedione (106), puupehenone (107) and 15α-methoxypuupehenol (108) (Figure 25). Compound 108 exhibited similar antimicrobial and antifungal activity as puupehenone (107) and a lower cytotoxity towards KB cells with ED50 values of 6 and 0.5 µg/mL, respectively. Compound 108 exhibited slightly higher in vitro antimalarial activity than puupehenone 107, against three strains of Plasmodium falciparum [28].
Figure 25.
Chemical structures of puupehenone (107) and 15α-methoxypuupehenol (108).
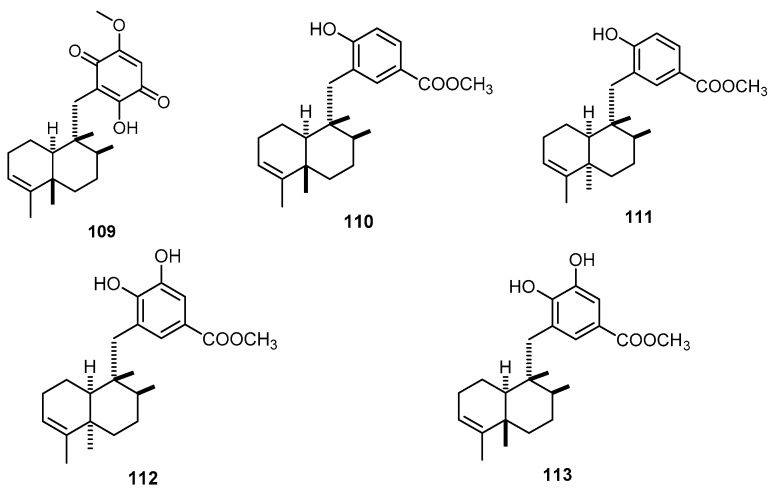
The methanolic extract of a marine sponge of the genus Hyrtios, collected at a depth of 35–45 m by dredging off Mahé (Seychelles Islands), was found to contain isospongiaquinone (109) together with four compounds with a 4,9-friedodrim-3-ene skeleton, hyrtiophenol (110), 5-epihyrtiophenol (111), 18-hydroxy-5-epihyrtiophenol (112) and 18-hydroxyhyrtiophenol (113) (Figure 26) [44].
Figure 26.
Chemical structures of isospongiaquinone (109), hyrtiophenol (110), 5-epihyrtiophenol (111), 18-hydroxy-5-epihyrtiophenol (112) and 18-hydroxyhyrtiophenol (113).
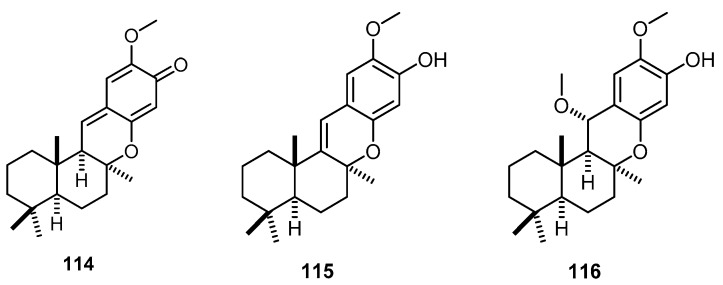
Chemical Investigations on an Indonesian Hyrtios sp. collected from Togian Island in Tomini Bay, north Sulawesi, Indonesia, led to the isolation and identification of three compounds, (+)-(5S,8S,9R,10S)-20-methoxypuupehenone (114), (+)-(5S,8S,10S)-20-methoxy-9,15-ene-puupehenol (115) and (+)-(5S,8S,9R,10S)-15,20-dimethoxypuupehenol (116) (Figure 27) [29].
Figure 27.
Chemical structures of compounds 114–116.
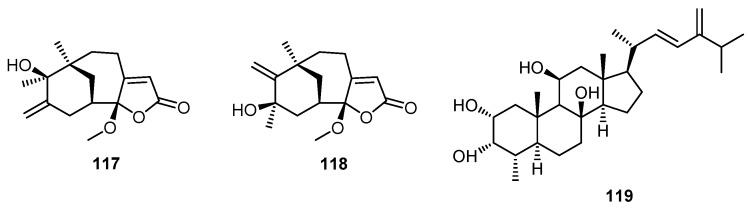
Two sesquiterpene γ-methoxybutenolides, hyrtiosenolide A (117) and hyrtiosenolide B (118), along with a 4α-methyl polyoxygenated steroid, hyrtiosterol (119), were obtained from a marine sponge of the genus Hyrtios collected from the Red Sea, Hurghada, Egypt (Figure 28). Hyrtiosenolides A (117) and B (118) displayed weak in vitro antibacterial activity against Escherichia coli. An inhibition zone of 7 mm was observed when 100 µg of 117 or 118 was applied to a 6 mm diameter paper disk on an agar plate inoculated with E. coli [45].
Figure 28.
Chemical structures of hyrtiosenolide A (117), hyrtiosenolide B (118) and hyrtiosterol (119).
A trichlorinated metabolite, poipuol (120), was isolated from an undescribed marine sponge Hyrtios sp. collected in Kauai Island, Hawaii (Figure 29) [46].
Figure 29.
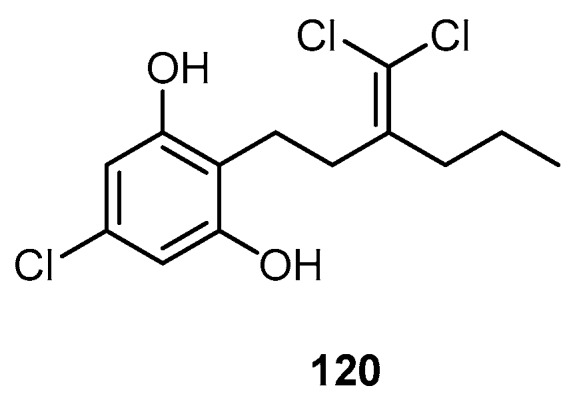
Chemical structure of poipuol (120).
Hyrtinadine A (121), a cytotoxic bis-indole alkaloid with a pyrimidine moiety, was isolated from a marine sponge Hyrtios sp. collected off Unten-Port, Okinawa (Figure 30). Compound 121 was the first example of a bis-indole alkaloid with a 2,5-disubstituted pyrimidine ring between two indole rings. Hyrtinadine A (121) showed in vitro cytotoxic activity against murine leukemia L1210 cells with IC50 value of 1 µg/mL and against human epidermoid carcinoma KB cells with IC50 value of 3 µg/mL [47].
Figure 30.
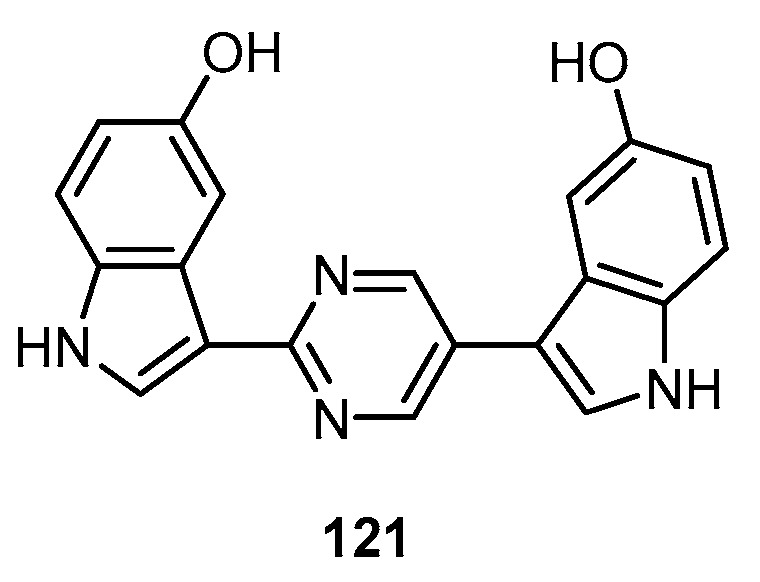
Chemical structure of hyrtinadine A (121).
Biological and chemical investigations on the crude extract of the Micronesian marine sponge Hyrtios sp. resulted in the isolation of a new alkaloid, 1-carboxy-6-hydroxy-3,4-dihydro-β-carboline (122) (Figure 31), together with the known metabolites, 5-hydroxyindole-3-carbaldehyde (1), hyrtiosin A (2), hyrtiosin B (3), 5-hydroxy-1H-indole-3-carboxylic acid methyl ester (54), serotonin (67) and 6-hydroxy-3,4-dihydro-1-oxo-beta-carboline (69). Among these isolated compounds, hyrtiosin B (3) showed a potent inhibitory activity against isocitrate lyase (ICL) of Candida albicans with an IC50 value of 89.0 µM [48].
Figure 31.
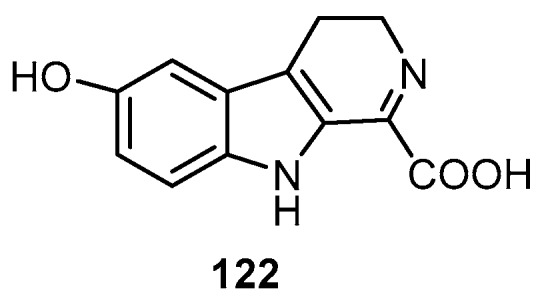
Chemical structure of compound 122.
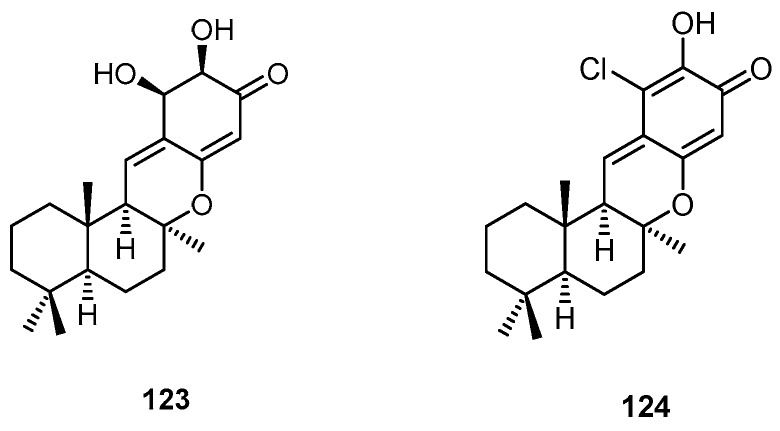
The sesquiterpene-dihydroquinone derivative puupehanol (123) and chloropuupehenone (124) were isolated from a marine sponge of the genus Hyrtios collected in Papua New Guinea, together with the known compound puupehenone (107) (Figure 32). Compound 107 showed potent antifungal activity against Cryptococcus neoformans and Candida krusei with minimum fungicidal concentration (MFC) values of 1.25 and 2.50 µg/mL, respectively [49].
Figure 32.
Chemical structures of puupehanol (123) and chloropuupehenone (124).
Two structurally unique bisindole alkaloids possessing the canthin-6-one skeleton with a hydroxyindole and an imidazolium unit, namely hyrtimomine D (125) and hyrtimomine E (126), have been isolated from an Okinawan marine sponge Hyrtios sp. collected off Kerama Islands (Figure 33). Compounds 125 and 126 exhibited antifungal activity against C. albicans (IC50, 4 and 8 μg/mL, respectively) and C. neoformans (IC50, 4 and 8 μg/mL, respectively). Furthermore, hyrtimomine D (125) displayed inhibitory activity against the Gram-positive bacterium Staphylococcus aureus with MIC value of 4 μg/mL and against Trichophyton mentagrophytes with MIC value of 16 μg/mL [30].
Figure 33.
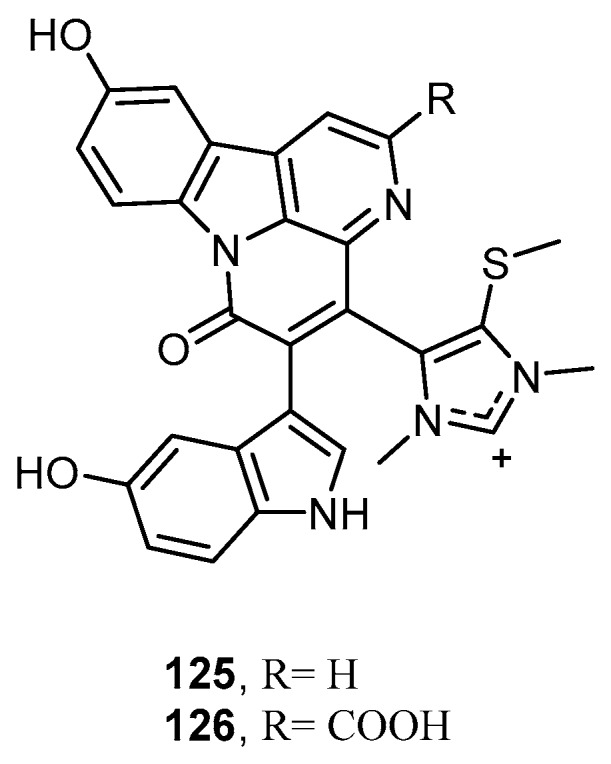
Chemical structures of hyrtimomine D (125) and hyrtimomine E (126).
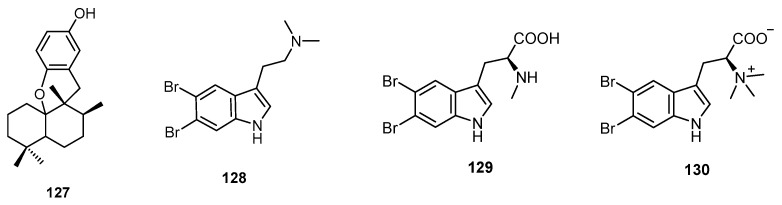
Chromatographic fractionation of the extracts of Hyrtios sp., collected from Fiji Islands, afforded aureol (127) together with five dibromoalkaloids (15, 16, 128–130). The structures of compounds 15, 16, and 128–130 were identified as N-methyl-5,6-dibromotryptamine (15), 5,6-dibromotryptamine (16), N,N-dimethyl-5,6-dibromotryptamine (128), 5,6-dibromoabrine (129) and 5,6-dibromo-l-hypaphorine (130) (Figure 34). The sesquiterpene aureol (127) exhibited potent antioxidant activity with an oxygen radical absorbance capacity (ORAC) value of 0.29, while compound 130 displayed a weak bee venom PLA2 inhibition (IC50 0.2 mM) and an antioxidant activity with an ORAC value of 0.22 [31].
Figure 34.
Chemical structures of aureol (127), N,N-dimethyl-5,6-dibromotryptamine (128), 5,6-dibromoabrine (129) and 5,6-dibromo-L-hypaphorine (130).
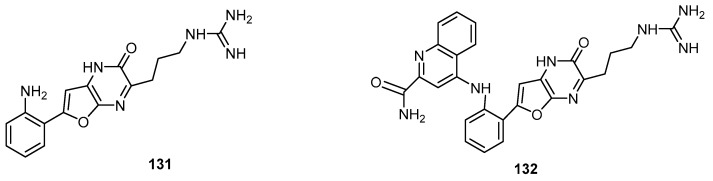
The two new alkaloids hyrtioseragamine A (131) and hyrtioseragamine B (132), the first natural products possessing a furo[2,3-b]pyrazin-2(1H)-one moiety and a guanidino group, were isolated from an marine sponge Hyrtios sp. collected off Seragaki, Okinawa (Figure 35). Compounds 131 and 132 exhibited antifungal activities against Aspergillus niger with MIC values of 8.33 and 16.6 μg/mL, respectively, and against Cryptococcus neoformans with MIC values of 33.3 and 16.6 μg/mL, respectively. However, compounds 131 and 132 did not show in vitro cytotoxic activity against murine lymphoma L1210 and human epidermoid carcinoma KB cells (IC50 > 10 μg/mL) [32].
Figure 35.
Chemical structures of hyrtioseragamine A (131) and hyrtioseragamine B (132).
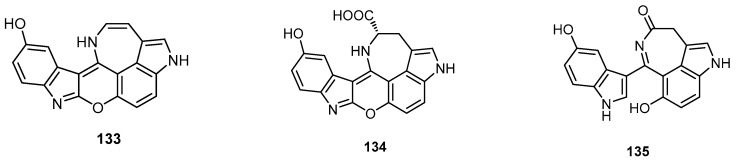
Hyrtimomine A (133) and hyrtimomine B (134), new heteroaromatic alkaloids possessing a fused hexacyclic 6/5/6/6/7/5 ring system, and hyrtimomine C (135), a new alkaloid consisting of hydroxyindole and azepino-hydroxyindole moieties, were discovered from an Okinawan marine sponge Hyrtios sp. collected off Kerama Islands, Okinawa (Figure 36). Hyrtimomine A (133) showed in vitro cytotoxic activity against human epidermoid carcinoma KB cells (IC50 = 3.1 μg/mL) and murine leukemia L1210 cells (IC50 = 4.2 μg/mL), while compounds 134 and 135 did not show cytotoxic activity (IC50 > 10 μg/mL) [33].
Figure 36.
Chemical structures of hyrtimomine A (133), hyrtimomine B (134) and hyrtimomine C (135).
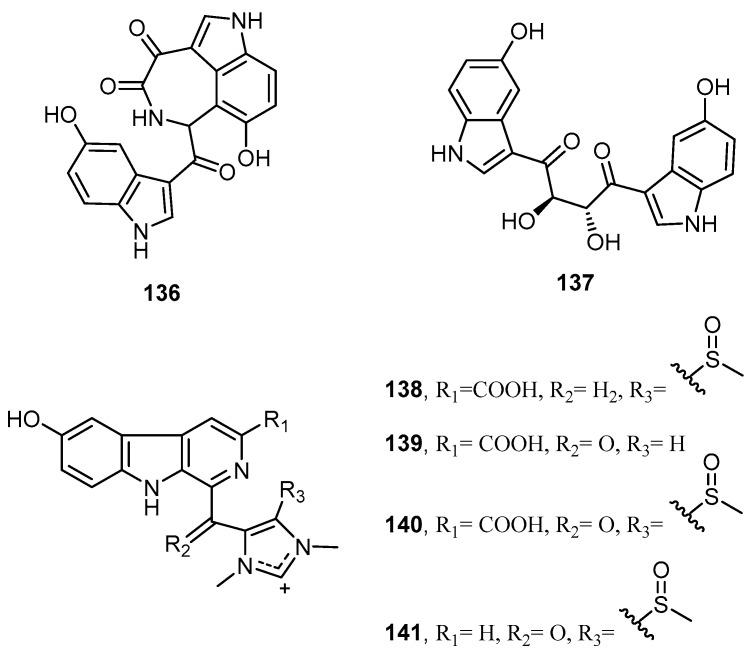
A new structurally unique bisindole alkaloid possessing an α-keto-ɛ-caprolactam ring, hyrtimomine F (136), a new symmetrical bisindole alkaloid, hyrtimomine G (137), and four new indole alkaloids possessing β-carboline skeleton with an imidazolium unit, hyrtimomines H–K (139–141), were isolated from Okinawan marine sponges Hyrtios spp. collected at Kerama Islands (Figure 37). Compounds 136, 137, and 139 showed inhibitory effects against A. niger with IC50 value of 8 µg/mL, while 139 displayed inhibitory effect against C. neoformans with IC50 of 4 µg/mL [34].
Figure 37.
Chemical structures of hyrtimomines F–K (136–141).
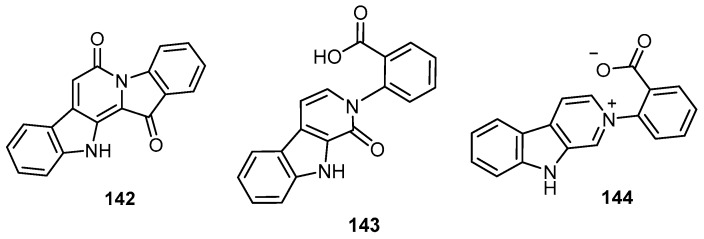
Mass-guided fractionation of the methanolic extract from a specimen of the Australian marine sponge Hyrtios sp. led to the isolation of two tryptophan alkaloids, 6-oxofascaplysin (142), and secofascaplysic acid (143), in addition to the two metabolites fascaplysin (11) and reticulatate (144) (Figure 38). Compounds 11 and 142–144 displayed in vitro cytotoxic activity against a prostate cancer cell line (LNCaP) with IC50 values ranging from 0.54 to 44.9 μM [35].
Figure 38.
Chemical structures of 6-oxofascaplysin (142), secofascaplysic acid (143) and reticulatate (144).
Two new relatively rare bisindole alkaloids possessing a 3,4-fused azepinoindole skeleton, hyrtinadine C (145) and hyrtinadine D (146), were isolated from an Okinawan marine sponge Hyrtios sp. collected off Unten Port (Figure 39). Compound 145 showed antifungal activity against A. niger with IC50 value of 32 µg/mL, while compound 146 showed antibacterial activity against the Gram-negative bacterium E. coli with MIC value of 16 µg/mL and against the Gram-positive bacterium B. subtilis with MIC value of 16 µg/mL [36].
Figure 39.
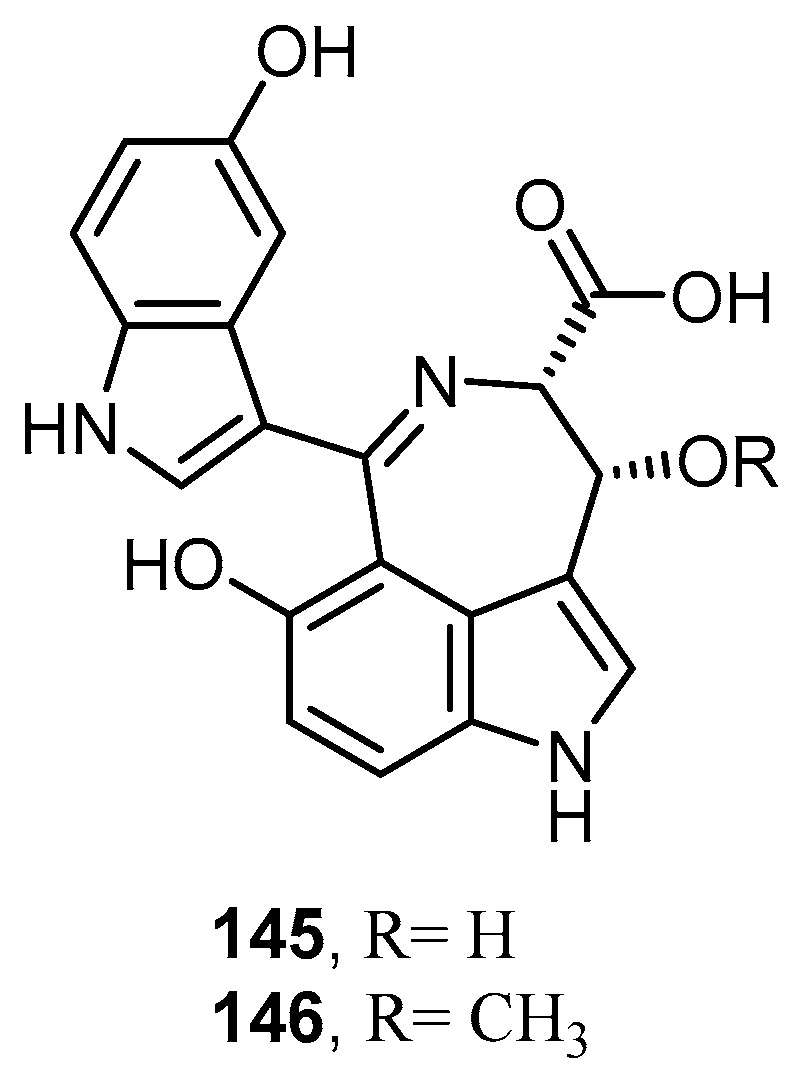
Chemical structures of hyrtinadine C (145) and hyrtinadine D (146).
Genus Hyrtios attracted scientists’ attention and sparked high synthetic efforts for the synthesis of the isolated compounds from its members [37,38]. Several compounds have been synthesized such as Salmahyrtisol A from Hyrtios erecta [39,40], Similan A from the Thai sponge Hyrtios gumininae [41,42], sesterstatin 1 from Hyrtios erecta [43,44], Spongistatins 1 (Altohyrtin A ) from Hyrtios erecta [45,46], (−)-Hyrtiosal and its C-16 epimer have been synthetized from sclareol [47] which was previously isolated from the sponge Hyrtios erectus [48].
8. Conclusions
Marine sponges harbor a huge repertoire of yet undiscovered natural products possessing a broad-spectrum of pharmacological applications. Among the several Hyrtios species discovered, H. erectus, H. reticulatus, H. gumminae, H. communis, and H. tubulatus were the most prolific producers of secondary metabolites with various pharmaceutically and medically relevant bioactivities (Table 1). A total of 146 natural products from various marine sponges belonging to the genus Hyrtios were reported in MarinLit database until 2016 as well as in the literature. H. erectus represents the most frequently investigated source of bioactive natural products from Hyrtios sp. in terms of number of natural products isolated. The discovery of new species from the genus Hyrtios indicates that there is room for new natural products discovery.
Table 1.
Secondary metabolites isolated from marine sponges from the genus Hyrtios.
| Name of Hyrtios sp. | Number of Secondary Metabolites | Bioactivity | Reference |
|---|---|---|---|
| Hyrtios erectus | 66 | In vitro cytotoxic activity against human epidermoid carcinoma KB cells. Immunosuppressive activity in the B-lymphocytes reaction assay. Inhibited the growth of a number of human cancer cell lines, including P388 leukemia, BXPC-3 pancreas, RPMI-7951 melanoma, U251 CNS, KAT-4 thyroid, NCI-H460 lung NSC, FADU pharynx and DU-145 prostate. Inhibit the growth of the Gram-positive bacterium Micrococcus luteus. Antimalarial agents. Showed 100% selective inhibitory activity against the neuronal isozyme of nitric oxide synthase. Showed significant in vitro cytotoxicity against murine leukemia, human lung carcinoma, and human colon carcinoma. Antimycobacterial activity. Exhibited significant anticancer activity against murine P388 lymphocytic leukemia and a series of human tumor cell lines. Inhibitory activity against Aurora A Antiproliferative activity towards KB cells Antiphospholipase A2 activity. Growth inhibition activity against the L5178Y mouse lymphoma cell line, Antimicrobial activities against the Gram-positive bacterium Bacillus subtilis and the fungus Saccharomyces cerevisiae. In vitro anticancer activity against breast adenocarcinoma (MCF-7), colorectal carcinoma (HCT-116) and hepatocellular carcinoma cells (HepG2). |
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] |
| Hyrtios reticulatus | 13 | Selective anticancer activity against H522-T1 non-small cell lung, MDA-MB-435 melanoma, and U937 lymphoma cancer cell lines. Inhibited ubiquitin-activating enzyme (E1). |
[12] [18] [19] [20] |
| Hyrtios gummina | 10 | In vitro anticancer activity against HuCCA-1 (human cholangiocarcinoma), KB (human epidermoid carcinoma of the mouth), HeLa (human cervical carcinoma), MDA-MB-231 (hormone-independent breast cancer), T47D (hormone-dependent breast cancer), and H69AR (multidrug-resistant small-cell lung cancer). | [21] |
| Hyrtios communis | 13 | Inhibit transcription factor hypoxia-inducible factor-1 (HIF1) activation in T47D human breast tumor cells. Significant cytotoxic activity. |
[22] |
| Hyrtios tubulatus currently identified as Dysidea tubulata) | 3 | No biological activities have been reported | [23] |
| Undescribed marine sponges of the genus Hyrtios | 41 | Antimicrobial and antifungal activity. In vitro antimalarial activity. Antibacterial activity against Escherichia coli. In vitro cytotoxic activity against murine leukemia L1210 cells and against human epidermoid carcinoma KB cells. A potent inhibitory activity against isocitrate lyase (ICL) of Candida albicans. Potent antifungal activity against Cryptococcus neoformans and Candida krusei with minimum fungicidal concentration (MFC). Antifungal activity against C. albicans and C. neoformans and against Trichophyton mentagrophytes. Potent antioxidant activity. Antifungal activities against Aspergillus niger and against Cryptococcus neoformans. In vitro cytotoxic activity against human epidermoid carcinoma KB cells and murine leukemia L1210 cells. Inhibitory effects against A. niger and inhibitory effect against C. neoformans. In vitro cytotoxic activity against a prostate cancer cell line. Antifungal activity against A. niger. |
[24] [25] [23] [26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36] [37] |
With the currently available data a correlation between geographical area where the sponges were collected and the type of metabolites found for this particular species can be concluded. Sponges collected off Okinawa (Japan) were richer in alkaloids, especially indole alkaloids (indole alkaloids possessing β-carboline skeleton with an imidazolium unit, azepino-hydroxyindole moieties) and bisindole alkaloids. In addition, sponges collected off Fiji were rich with brominated alkaloids and sesterterpenes. Sponges collected from the Republic of Maldives were very rich in scalarane-type pentacyclic sesterterpene and sesterstatins. Furthermore, sponges collected off Indonesia are rich in β-carboline alkaloids. On the other hand, sponges collected off the Red sea were rich in terpenoids, especially sesterterpenes, sesterstatins as well as indole alkaloids and azepino alkaloids, with the majority of the isolated compounds being terpenoids. The different geographical chemotypes might be explained by variations in the microbial community associated with the respective sponges. Sponges have developed intimate association with a huge diversity of microorganisms, such as viruses, bacteria, archaea, fungi, protozoa and single-celled algae. It is often unclear whether the compounds of interest are biosynthesized by the sponges or their associated microbes [50,51]. Many bioactive natural products from marine invertebrates have striking similarities to metabolites of their associated microorganisms, especially bacteria [52,53,54,55,56,57]. In most cases, the development of sponge-derived drugs is challenged by environmental concerns and technical problems associated with harvesting large amounts of sponges. Sponge-associated microorganisms may represent a sustainable source of sponge-derived natural products that could be established through a symbiont culture or by transferring its biosynthetic genes into culturable microorganisms [58]. Based on available scientific literature, it is evident that marine sponges within genus Hyrtios represent a rich source of natural products with various biological activities.
Acknowledgments
This publication was funded by the German Research Foundation (DFG) and the University of Wuerzburg in the funding programme Open Access Publishing
Author Contributions
N.H.S. collected a complete survey of all compounds isolated from the genus Hyrtios; E.M.E.-H. classified the compounds according to their isolation sources and wrote the manuscript; U.R.A. interpreted and revised the results, and wrote the manuscript; M.A.F., T.A.M.G. and M.S.K. discussed the results scientifically and contributed to editing of the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Lane N. Marine microbiology: Origins of death. Nature. 2008;453:583–585. doi: 10.1038/453583a. [DOI] [PubMed] [Google Scholar]
- 2.Rateb M.E., Ebel R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011;28:290–344. doi: 10.1039/c0np00061b. [DOI] [PubMed] [Google Scholar]
- 3.Li J.W., Vederas J.C. Drug discovery and natural products: End of an era or an endless frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 4.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2016;33:382–431. doi: 10.1039/C5NP00156K. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y., Chen J., Hu G., Yu J., Zhu X., Lin Y., Chen S., Yuan J. Statistical research on the bioactivity of new marine natural products discovered during the 28 years from 1985 to 2012. Mar. Drugs. 2015;13:202–221. doi: 10.3390/md13010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman D.J., Cragg G.M. Advanced preclinical and clinical trials of natural products and related compounds from marine sources. Curr. Med. Chem. 2004;11:1693–1713. doi: 10.2174/0929867043364982. [DOI] [PubMed] [Google Scholar]
- 7.Mehbub M.F., Lei J., Franco C., Zhang W. Marine Sponge Derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs. 2014;12:4539–4577. doi: 10.3390/md12084539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins A., Vieira H., Gaspar H., Santos S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs. 2014;12:1066–1101. doi: 10.3390/md12021066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hentschel U., Piel J., Degnan S.M., Taylor M.W. Genomic insights into the marine sponge microbiome. Nature. 2012;10:641–654. doi: 10.1038/nrmicro2839. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt S., Tsai P., Bell J., Fromont J., Ilan M., Lindquist N., Perez T., Rodrigo A., Schupp P.J., Vacelet J., et al. Assessing the complex sponge microbiota: Core, variable and species-specific bacterial communities in marine sponges. Isme J. 2012;6:564–576. doi: 10.1038/ismej.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2015;32:116–211. doi: 10.1039/C4NP00144C. [DOI] [PubMed] [Google Scholar]
- 12.Aoki S., Ye Y., Higuchi K., Takashima A., Tanaka Y., Kitagawa I., Kobayashi M. Novel neuronal nitric oxide synthase (nNOS) selective inhibitor, aplysinopsin-type indole alkaloid, from marine sponge Hyrtios erecta. Chem. Pharm. Bull. 2001;49:1372–1374. doi: 10.1248/cpb.49.1372. [DOI] [PubMed] [Google Scholar]
- 13.Sauleau P., Martin M.T., Dau M.E., Youssef D.T., Bourguet-Kondracki M.L. Hyrtiazepine, an azepino-indole-type alkaloid from the Red Sea marine sponge Hyrtios erectus. J. Nat. Prod. 2006;69:1676–1679. doi: 10.1021/np060132r. [DOI] [PubMed] [Google Scholar]
- 14.He W.F., Xue D.Q., Yao L.G., Li J.Y., Li J., Guo Y.W. Hainanerectamines A–C, alkaloids from the Hainan sponge Hyrtios erecta. Mar. Drugs. 2014;12:3982–3993. doi: 10.3390/md12073982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmoun M., Devijver C., Daloze D., Braekman J.C., van Soest R.W. 5-hydroxytryptamine-derived alkaloids from two marine sponges of the genus Hyrtios. J. Nat. Prod. 2002;65:1173–1176. doi: 10.1021/np020009+. [DOI] [PubMed] [Google Scholar]
- 16.Youssef D.T., Yamaki R.K., Kelly M., Scheuer P.J. Salmahyrtisol A, a novel cytotoxic sesterterpene from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2002;65:2–6. doi: 10.1021/np0101853. [DOI] [PubMed] [Google Scholar]
- 17.Miyaoka H., Nishijima S., Mitome H., Yamada Y. Three new scalarane sesterterpenoids from the Okinawan sponge Hyrtios erectus. J. Nat. Prod. 2000;63:1369–1372. doi: 10.1021/np000115g. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi J., Murayama T., Ishibashi M., Kosuge S., Takamatsu M., Ohizumi Y., Kobayashi H., Ohta T., Nozoe S., Takuma S. Hyrtiosins A and B, new indole alkaloids from the Okinawan marine sponge Hyrtios erecta. Tetrahedron. 1990;46:7699–7702. doi: 10.1016/S0040-4020(01)90065-1. [DOI] [Google Scholar]
- 19.Pettit G.R., Tan R., Melody N., Cichacz Z.A., Herald D.L., Hoard M.S., Pettit R.K., Chapuis J.C. Antineoplastic agents. 397: Isolation and structure of sesterstatins 4 and 5 from Hyrtios erecta (the Republic of Maldives) Bioorg. Med. Chem. Lett. 1998;8:2093–2098. doi: 10.1016/S0960-894X(98)00373-4. [DOI] [PubMed] [Google Scholar]
- 20.Kirsch G., Kong G.M., Wright A.D., Kaminsky R. A new bioactive sesterterpene and antiplasmodial alkaloids from the marine sponge hyrtios cf. erecta. J. Nat. Prod. 2000;63:825–829. doi: 10.1021/np990555b. [DOI] [PubMed] [Google Scholar]
- 21.Ashour M.A., Elkhayat E.S., Ebel R., Edrada R., Proksch P. Indole alkaloid from the Red Sea sponge Hyrtios erectus. Arkivoc. 2007;15:225–231. [Google Scholar]
- 22.Du L., Shen L., Yu Z., Chen J., Guo Y., Tang Y., Shen X., Jiang H. Hyrtiosal, from the marine sponge Hyrtios erectus, inhibits HIV-1 integrase binding to viral DNA by a new inhibitor binding site. Chem. Med. Chem. 2008;3:173–180. doi: 10.1002/cmdc.200700223. [DOI] [PubMed] [Google Scholar]
- 23.Inman W.D., Bray W.M., Gassner N.C., Lokey R.S., Tenney K., Shen Y.Y., Tendyke K., Suh T., Crews P. A beta-carboline alkaloid from the Papua New Guinea marine sponge Hyrtios reticulatus. J. Nat. Prod. 2010;73:255–257. doi: 10.1021/np9005426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanokuchi R., Imada K., Miyazaki M., Kato H., Watanabe T., Fujimuro M., Saeki Y., Yoshinaga S., Terasawa H., Iwasaki N., et al. Hyrtioreticulins A–E, indole alkaloids inhibiting the ubiquitin-activating enzyme, from the marine sponge Hyrtios reticulatus. Bioorg. Med. Chem. 2012;20:4437–4442. doi: 10.1016/j.bmc.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 25.Imada K., Sakai E., Kato H., Kawabata T., Yoshinaga S., Nehira T., Terasawa H., Tsukamoto S. Reticulatins A and B and hyrtioreticulin F from the marine sponge Hyrtios reticulatus. Tetrahedron. 2013;69:7051–7055. doi: 10.1016/j.tet.2013.06.043. [DOI] [Google Scholar]
- 26.Mahidol C., Prawat H., Sangpetsiripan S., Ruchirawat S. Bioactive scalaranes from the Thai sponge Hyrtios gumminae. J. Nat. Prod. 2009;72:1870–1874. doi: 10.1021/np900267v. [DOI] [PubMed] [Google Scholar]
- 27.Bourguet-Kondracki M.-L., Debitus C., Guyot M. Dipuupehedione, a cytotoxic new red dimer from a new caledonian marine sponge hyrtios sp. Tetrahedron Lett. 1996;37:3861–3864. doi: 10.1016/0040-4039(96)00700-9. [DOI] [Google Scholar]
- 28.Bourguet-Kondracki M.L., Lacombe F., Guyot M. Methanol adduct of puupehenone, a biologically active derivative from the marine sponge Hyrtios species. J. Nat. Prod. 1999;62:1304–1305. doi: 10.1021/np9900829. [DOI] [PubMed] [Google Scholar]
- 29.Pina I.C., Sanders M.L., Crews P. Puupehenone congeners from an indo-pacific hyrtios sponge. J. Nat. Prod. 2003;66:2–6. doi: 10.1021/np020279s. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka N., Momose R., Takahashi Y., Kubota T., Takahashi-Nakaguchi A., Gonoi T., Fromont J., Kobayashi J. Hyrtimomines D and E, bisindole alkaloids from a marine sponge Hyrtios sp. Tetrahedron Lett. 2013;54:4038–4040. doi: 10.1016/j.tetlet.2013.05.073. [DOI] [Google Scholar]
- 31.Longeon A., Copp B.R., Quevrain E., Roue M., Kientz B., Cresteil T., Petek S., Debitus C., Bourguet-Kondracki M.L. Bioactive indole derivatives from the South Pacific marine sponges Rhopaloeides odorabile and Hyrtios sp. Mar. Drugs. 2011;9:879–888. doi: 10.3390/md9050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi Y., Iinuma Y., Kubota T., Tsuda M., Sekiguchi M., Mikami Y., Fromont J., Kobayashi J. Hyrtioseragamines A and B, new alkaloids from the sponge Hyrtios species. Org. Lett. 2011;13:628–631. doi: 10.1021/ol102867x. [DOI] [PubMed] [Google Scholar]
- 33.Momose R., Tanaka N., Fromont J., Kobayashi J. Hyrtimomines A–C, new heteroaromatic alkaloids from a sponge Hyrtios sp. Org. Lett. 2013;15:2010–2013. doi: 10.1021/ol400687b. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka N., Momose R., Takahashi-Nakaguchi A., Gonoi T., Fromont J., Kobayashi J. Hyrtimomines, indole alkaloids from Okinawan marine sponges Hyrtios spp. Tetrahedron. 2014;70:832–837. doi: 10.1016/j.tet.2013.12.032. [DOI] [Google Scholar]
- 35.Khokhar S., Feng Y., Campitelli M.R., Ekins M.G., Hooper J.N., Beattie K.D., Sadowski M.C., Nelson C.C., Davis R.A. Isolation, structure determination and cytotoxicity studies of tryptophan alkaloids from an Australian marine sponge Hyrtios sp. Bioorg. Med. Chem. Lett. 2014;24:3329–3332. doi: 10.1016/j.bmcl.2014.05.104. [DOI] [PubMed] [Google Scholar]
- 36.Kubota T., Nakamura K., Sakai K., Fromont J., Gonoi T., Kobayashi J. Hyrtinadines C and D, New Azepinoindole-Type Alkaloids from a Marine Sponge Hyrtios sp. Che. Pharm. Bull. 2016;64:975–978. doi: 10.1248/cpb.c16-00201. [DOI] [PubMed] [Google Scholar]
- 37.Bourguet-Kondracki M.L., Martin M.T., Guyot M. A new β-carboline alkaloid isolated from the marine sponge Hyrtios erecta. Tetrahedron Lett. 1996;37:3457–3460. doi: 10.1016/0040-4039(96)00573-4. [DOI] [Google Scholar]
- 38.Youssef D.T., Shaala L.A., Emara S. Antimycobacterial scalarane-based sesterterpenes from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2005;68:1782–1784. doi: 10.1021/np0502645. [DOI] [PubMed] [Google Scholar]
- 39.Pettit G.R., Tan R., Cichacz Z.A. Antineoplastic agents. 542. Isolation and structure of sesterstatin 6 from the Indian Ocean sponge Hyrtios erecta. J. Nat. Prod. 2005;68:1253–1255. doi: 10.1021/np0402221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams D.E., Tahir A., Andersen R.J. A new acyclic diketotriterpenoid isolated from the indonesian marine sponge Hyrtios erectus. J. Nat. Prod. 1999;62:653–654. doi: 10.1021/np980526l. [DOI] [PubMed] [Google Scholar]
- 41.Qiu Y., Deng Z., Pei Y., Fu H., Li J., Proksch P., Lin W. Sesterterpenoids from the marine sponge Hyrtios erectus. J. Nat. Prod. 2004;67:921–924. doi: 10.1021/np030457x. [DOI] [PubMed] [Google Scholar]
- 42.Elhady S.S., Al-Abd A.M., El-Halawany A.M., Alahdal A.M., Hassanean H.A., Ahmed S.A. Antiproliferative Scalarane-Based Metabolites from the Red Sea Sponge Hyrtios erectus. Mar. Drugs. 2016;14:130. doi: 10.3390/md14070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J., Du L., Kelly M., Zhou Y.D., Nagle D.G. Structures and potential antitumor activity of sesterterpenes from the marine sponge Hyrtios communis. J. Nat. Prod. 2013;76:1492–1497. doi: 10.1021/np400350k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salmoun M., Devijver C., Daloze D., Braekman J.C., Gomez R., de Kluijver M., Van Soest R.W. New sesquiterpene/quinones from two sponges of the genus Hyrtios. J. Nat. Prod. 2000;63:452–456. doi: 10.1021/np9903346. [DOI] [PubMed] [Google Scholar]
- 45.Youssef D.T., Singab A.N., van Soest R.W., Fusetani N. Hyrtiosenolides A and B, two new sesquiterpene gamma-methoxybutenolides and a new sterol from a Red Sea sponge Hyrtios species. J. Nat. Prod. 2004;67:1736–1739. doi: 10.1021/np049853l. [DOI] [PubMed] [Google Scholar]
- 46.Sata N., Galario M.A., Sitachitta N., Scheuer P.J., Kelly M. Poipuol, a new metabolite from a Hawaiian sponge of the genus Hyrtios. J. Nat. Prod. 2005;68:262–263. doi: 10.1021/np0496789. [DOI] [PubMed] [Google Scholar]
- 47.Endo T., Tsuda M., Fromont J., Kobayashi J. Hyrtinadine A, a bis-indole alkaloid from a marine sponge. J. Nat. Prod. 2007;70:423–424. doi: 10.1021/np060420n. [DOI] [PubMed] [Google Scholar]
- 48.Lee H.S., Yoon K.M., Han Y.R., Lee K.J., Chung S.C., Kim T.I., Lee S.H., Shin J., Oh K.B. 5-Hydroxyindole-type alkaloids, as Candida albicans isocitrate lyase inhibitors, from the tropical sponge Hyrtios sp. Bioorg. Med. Chem. Lett. 2009;19:1051–1053. doi: 10.1016/j.bmcl.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Xu W.H., Ding Y., Jacob M.R., Agarwal A.K., Clark A.M., Ferreira D., Liang Z.S., Li X.C. Puupehanol, a sesquiterpene-dihydroquinone derivative from the marine sponge Hyrtios sp. Bioorg. Med. Chem. Lett. 2009;19:6140–6143. doi: 10.1016/j.bmcl.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osinga R., Armstrong E., Burgess J.G., Hoffmann F., Reitner J., Schumann-Kindel G. Sponge–microbe associations and their importance for sponge bioprocess engineering. Hydrobiologia. 2001;461:55–62. doi: 10.1023/A:1012717200362. [DOI] [Google Scholar]
- 51.Wilson M.C., Mori T., Ruckert C., Uria A.R., Helf M.J., Takada K., Gernert C., Steffens U.A., Heycke N., Schmitt S., et al. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature. 2014;6506:58–62. doi: 10.1038/nature12959. [DOI] [PubMed] [Google Scholar]
- 52.Proksch P., Edrada R.A., Ebel R. Drugs from the seas-current status and microbiological implications. Appl. Microbiol. Biotechnol. 2002;59:125–134. doi: 10.1007/s00253-002-1006-8. [DOI] [PubMed] [Google Scholar]
- 53.Unson M.D., Holland N.D., Faulkner D.J. A brominated secondary metabolite synthesized by the cyanobacterial symbiont of a marine sponge and accumulation of the crystalline metabolite in the sponge tissue. Mar. Biol. 1994;119:1–11. doi: 10.1007/BF00350100. [DOI] [Google Scholar]
- 54.Piel J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 2004;21:519–538. doi: 10.1039/b310175b. [DOI] [PubMed] [Google Scholar]
- 55.Flowers A.E., Garson M.J., Webb R.I., Dumdei E.J., Charan R.D. Cellular origin of chlorinated diketopiperazines in the dictyoceratid sponge Dysidea herbacea (Keller) Cell Tissue Res. 1998;292:597–607. doi: 10.1007/s004410051089. [DOI] [PubMed] [Google Scholar]
- 56.Thiel V., Imhoff J.F. Phylogenetic identification of bacteria with antimicrobial activities isolated from Mediterranean sponges. Biomol. Eng. 2003;20:421–423. doi: 10.1016/S1389-0344(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 57.Kubota T., Kamijyo Y., Takahashi-Nakaguchi A., Fromont J., Gonoi T., Kobayashi J. Zamamiphidin A, a New Manzamine Related Alkaloid from an Okinawan Marine Sponge Amphimedon sp. Org. Lett. 2013;15:610–612. doi: 10.1021/ol3034274. [DOI] [PubMed] [Google Scholar]
- 58.Anjum K., Abbas S.Q., Shah S.A., Akhter N., Batool S., ul Hassan S.S. Marine Sponges as a Drug Treasure. Biomol. Ther. 2016;24:559. doi: 10.4062/biomolther.2016.559. [DOI] [PMC free article] [PubMed] [Google Scholar]