Phages and their derivatives are increasingly being reconsidered for use in the treatment of bacterial infections due to the rising rates of antibiotic resistance. We assessed the antistaphylococcal effect of the endolysin SAL200 in combination with standard-of-care (SOC) antibiotics.
KEYWORDS: Staphylococcus aureus, phage, endolysin, synergism
ABSTRACT
Phages and their derivatives are increasingly being reconsidered for use in the treatment of bacterial infections due to the rising rates of antibiotic resistance. We assessed the antistaphylococcal effect of the endolysin SAL200 in combination with standard-of-care (SOC) antibiotics. The activity of SAL200 when it was combined with SOC antibiotics was assessed in vitro by checkerboard and time-kill assays and in vivo with murine bacteremia and Galleria mellonella infection models. SAL200 reduced the SOC antibiotic MICs and showed a ≥3-log10-CFU/ml reduction of Staphylococcus aureus counts within 30 min in time-kill assays. Combinations of SAL200 and SOC antibiotics achieved a sustained decrease of >2 log10 CFU/ml. SAL200 significantly lowered the blood bacterial density within 1 h by >1 log10 CFU/ml in bacteremic mice (P < 0.05 versus untreated mice), and SAL200 and SOC antibiotic combinations achieved the lowest levels of bacteremia. The bacterial density in splenic tissue at 72 h postinfection was the lowest in mice treated with SAL200 and SOC antibiotic combinations. SAL200 combined with SOC antibiotics also improved Galleria mellonella larva survival at 96 h postinfection. The combination of the phage endolysin SAL200 with SOC antistaphylococcal antibiotics showed synergistic effects in vitro and in vivo. The combination of SAL200 with SOC antibiotics could help in the treatment of difficult-to-treat S. aureus infections.
INTRODUCTION
Staphylococcus aureus is a virulent opportunistic pathogen that commonly causes invasive infections, including bacteremia (1). S. aureus bacteremia is frequently complicated by metastatic infections that can occur in various organ systems and that can lead to prolonged antibiotic therapy and a poor prognosis due to serious sequelae (2). Methicillin-resistant S. aureus (MRSA) has spread since its initial description in 1961 and remains a major worldwide health problem (3). The rate of mortality from S. aureus bacteremia reaches 20 to 30%, while the rate of mortality from MRSA bacteremia is even higher, with clinical cure rates estimated to be 50 to 60% (4–6). Vancomycin remains the first-line agent for the treatment of serious MRSA infections (2, 7). However, global surveillance studies have indicated a gradual rise in the vancomycin MIC, and there is evidence that the increased numbers of treatment failures are related to an elevated vancomycin MIC, raising concerns about its clinical efficacy and prompting the need for alternative therapeutic options (7–9).
Therapy using phages and their derivatives for treating bacterial infections has been reconsidered due to the increasing rates of resistance to standard-of-care (SOC) antibiotics (10). Phage endolysins (lysins) are bacteriophage-encoded peptidoglycan hydrolases that lyse Gram-positive bacterial cell walls from within the bacterial cells in order to release progeny phages after replication in the bacteriophage life cycle (11, 12). In previous studies, exogenously applied endolysins have shown similar rapid lysis of Gram-positive bacteria in vitro and also in various animal models of infection (11, 13–18). Endolysins differ from SOC antibiotics, in that they are generally genus/species specific and therefore are likely to cause minimal insult to the normal flora. Endolysins act on contact and thus are able to rapidly lyse bacteria, regardless of their growth phase or antibiotic resistance profile (11, 13, 14).
SAL200 is a new phage endolysin-based candidate antistaphylococcal drug formulated for injection (19, 20). Its active pharmaceutical ingredient is the recombinant phage endolysin SAL-1, derived from the staphylococcus-specific bacteriophage SAP-1 (19, 20). In previous in vitro and in vivo studies, SAL200 has shown rapid and effective bactericidal activity against various S. aureus strains, including MRSA strains (19, 20).
In this study, we show that SAL200 acts synergistically when combined with SOC antibiotics in vitro. We also show that SAL200 enhances the antistaphylococcal activities of SOC antibiotics in a murine bacteremia model as well as an invertebrate model using larvae of Galleria mellonella.
(This study was presented in part as a mini-oral presentation at the 27th Annual Meeting of the European Congress of Clinical Microbiology and Infectious Diseases, 2017, Vienna, Austria.)
RESULTS
SAL200 is rapidly bactericidal and synergizes with SOC antibiotics in vitro.
The MICs of the tested S. aureus isolates are summarized in Table 1. SAL200 MICs were between 0.8 and 1.6 μg/ml, regardless of methicillin resistance. MICs for the combination of SAL200 with SOC antibiotics are summarized in Tables 2 and 3. No SAL200 and SOC antibiotic combinations resulted in antagonism. Synergy was seen for the combination of SAL200 and nafcillin in the S. aureus strains ATCC B1707, LAC, and Newman. Synergy was also seen for the combination of SAL200 and vancomycin in the S. aureus strains ATCC B1707, Newman, and ATCC 29213.
TABLE 1.
MICs of SAL200 and SOC antibiotics against Staphylococcus aureus isolates
| Straina | Median (range) MIC (μg/ml) |
||
|---|---|---|---|
| SAL200 | Nafcillin | Vancomycin | |
| ATCC 33591 (MRSA) | 1.6 (0.8–1.6) | >64 (>64) | 1.5 (1.0–2.0) |
| ATCC B1707 (MRSA) | 1.2 (0.8–1.6) | 32 (32) | 1.5 (1.0–2.0) |
| LAC (MRSA) | 1.6 (1.6) | 32 (32–64) | 1.0 (1.0) |
| Newman (MSSA) | 0.8 (0.8–1.6) | 0.5 (0.5) | 1.0 (1.0–2.0) |
| ATCC 29213 (MSSA) | 1.2 (0.8–1.6) | 0.5 (0.5) | 1.0 (1.0) |
ATCC, American Type Culture Collection; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus.
TABLE 2.
MICs for combination of SAL200 and nafcillin
| Straina | MIC (μg/ml) |
Lowest FIC index | Interpretation | ||
|---|---|---|---|---|---|
| SAL200 | Nafcillin | SAL200-nafcillin | |||
| ATCC 33591 | 0.781 | 256 | 0.391/4 | 0.516 | Indifferent |
| ATCC B1707 | 0.781 | 16 | 0.195/2 | 0.375 | Synergistic |
| LAC | 0.781 | 16 | 0.195/4 | 0.5 | Synergistic |
| Newman | 1.563 | 0.5 | 0.391/0.125 | 0.5 | Synergistic |
| ATCC 29213 | 0.781 | 0.5 | 0.391/0.016 | 0.533 | Indifferent |
ATCC, American Type Culture Collection.
TABLE 3.
MICs for combination of SAL200 and vancomycin
| Straina | MIC (μg/ml) |
Lowest FIC index | Interpretation | ||
|---|---|---|---|---|---|
| SAL200 | Vancomycin | SAL200-vancomycin | |||
| ATCC 33591 | 1.563 | 2 | 0.781/0.031 | 0.515 | Indifferent |
| ATCC B1707 | 1.563 | 2 | 0.391/0.5 | 0.5 | Synergistic |
| LAC | 1.563 | 1 | 0.781/0.063 | 0.563 | Indifferent |
| Newman | 1.563 | 2 | 0.391/0.5 | 0.5 | Synergistic |
| ATCC 29213 | 0.781 | 2 | 0.195/0.25 | 0.375 | Synergistic |
ATCC, American Type Culture Collection.
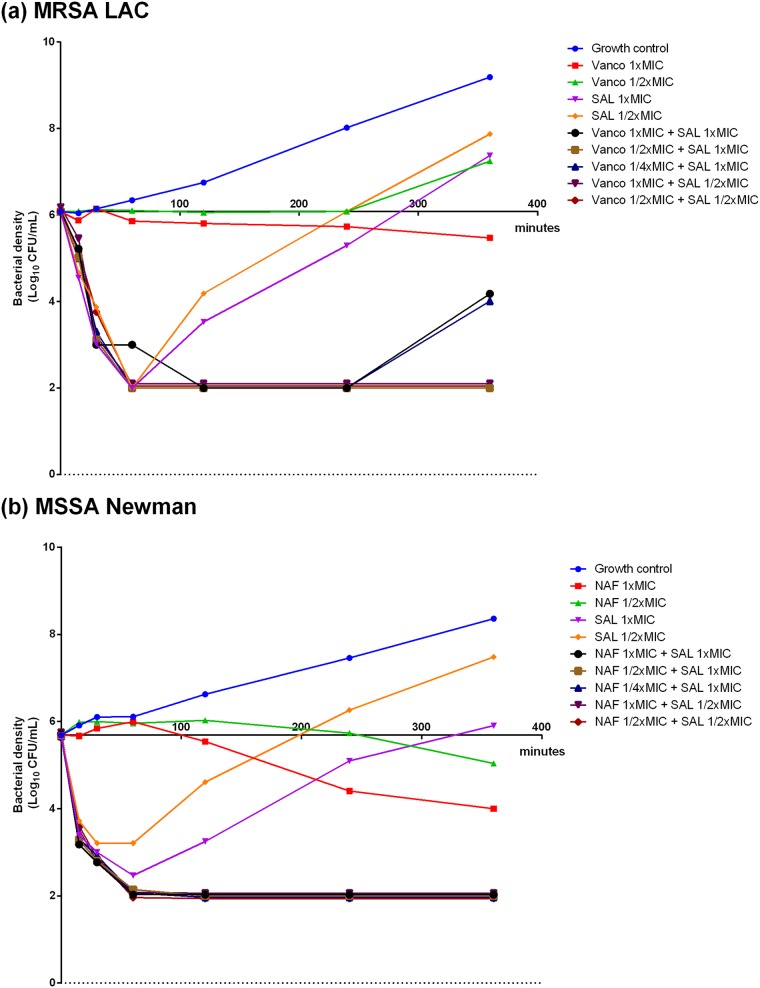
A bactericidal effect was seen with exposure to SAL200 within 30 min in all S. aureus strains, while minimal bactericidal activity was observed for SOC antibiotics alone (Fig. 1; see also Fig. S2 in the supplemental material). Although SAL200 was rapidly bactericidal alone even at sub-MIC doses, the effect did not last long and bacterial regrowth was observed in all S. aureus isolates tested. However, SAL200 synergistically inhibited bacterial growth when combined with sub-MIC doses of SOC antibiotics, leading to a sustained reduction of the viable bacterial count of ≥3 log10 CFU/ml.
FIG 1.
Time-kill curves for MRSA LAC (a) and MSSA Newman (b) treated with buffer and MICs and sub-MICs of either SAL200, SOC antibiotics, or combinations of SAL200 and SOC antibiotics. The curves were jittered vertically by adding random numbers within the range of −0.1 to 0.1 unit to the bacterial densities at times of 60, 120, 240, and 360 min after culture. Vanco, vancomycin; NAF, nafcillin; SAL, SAL200.
SAL200 with SOC antibiotics synergistically reduces bacteria in the bloodstream and in metastatic foci in mice.
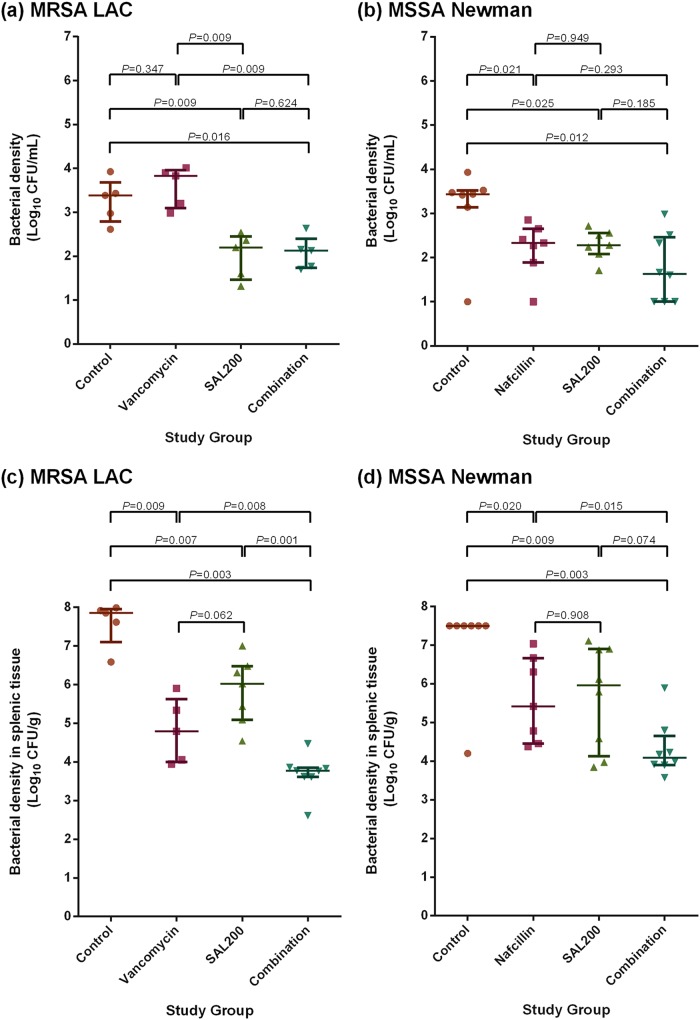
Mice with intraperitoneally induced S. aureus bacteremia received either SAL200 alone, SOC antibiotics alone, SAL200 and SOC antibiotics in combination, or phosphate-buffered saline (PBS) as a negative control. Mice treated with SAL200 alone showed a significantly lower bacterial density in blood at 1 h after treatment compared to the negative controls (Fig. 2a and b; 2.202 versus 3.385 log10 CFU/ml [P = 0.009], respectively, for LAC-infected mice and 2.284 versus 3.437 log10 CFU/ml [P = 0.025], respectively, for Newman-infected mice), and mice receiving the combination of SAL200 and SOC antibiotics showed the lowest median blood bacterial density at 1 h after treatment (2.125 and 1.635 log10 CFU/ml for LAC- and Newman-infected mice, respectively).
FIG 2.
Bacterial density in blood from MRSA LAC-infected mice at 1 h after treatment (a), blood from MSSA Newman-infected mice at 1 h after treatment (b), the spleen at 72 h after MRSA LAC infection (c), and the spleen at 72 h after MSSA Newman infection (d). As only 1 mouse in the negative-control group survived beyond 72 h after MSSA Newman infection, a direct comparison with the control group was not possible. For comparison, a hypothetical splenic bacterial density was presumed to exceed 7.5 log10 CFU/g in the dead mice.
The extent of metastatic infections, as assessed by the splenic bacterial density at 72 h after infection, was similar to the acute posttreatment bacterial densities observed in blood; the median number of log10 CFU per gram in the combination treatment group was significantly lower than that in the group treated with the SOC alone (Fig. 2c and d; 3.771 versus 4.794 log10 CFU/g [P = 0.008], respectively, for LAC-infected mice and 4.087 versus 5.416 log10 CFU/g [P = 0.015], respectively, for Newman-infected mice) and was the lowest among all active treatment groups.
Treatment with SAL200 with SOC antibiotics improves the survival of Galleria mellonella larvae.
Treatment with SOC antibiotics or the combination of SAL200 and SOC antibiotics showed significantly improved the survival rates for infected larvae at 96 h postinfection compared to those for the negative controls (6.7% [P = 0.0002] and 33.3% [P < 0.0001] survival, respectively, for LAC-infected larvae and 46.7% [P < 0.0001] and 73.3% [P < 0.0001] survival, respectively, for Newman-infected larvae) (Fig. 3).
FIG 3.
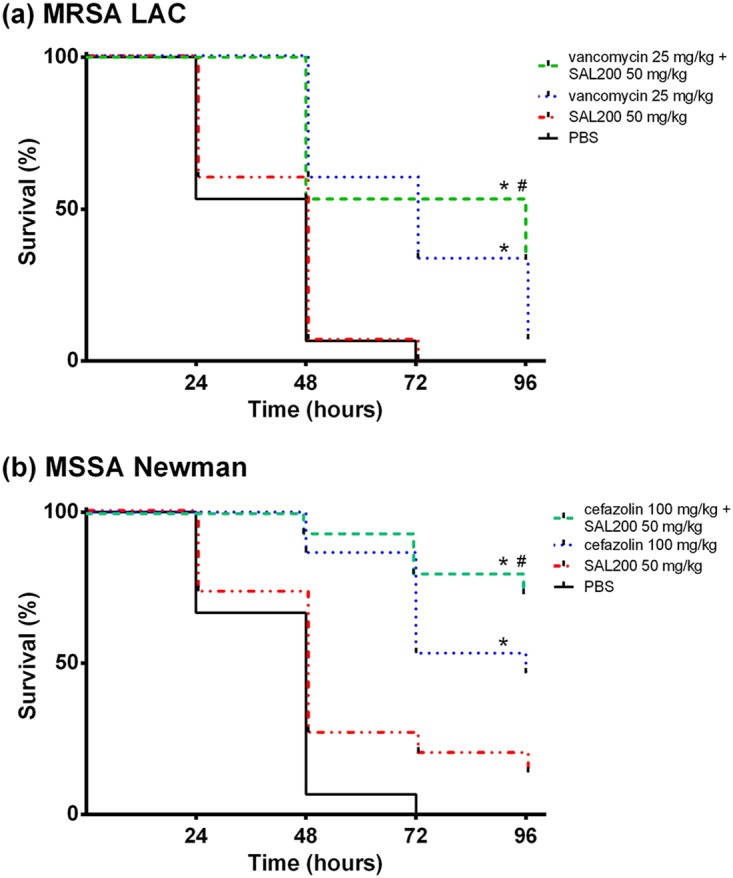
Effect of SAL200 combined with SOC antibiotics on survival of Galleria mellonella larvae infected with MRSA LAC (a) and MSSA Newman (b). The survival lines were jittered to prevent overplotting. *, P < 0.01 for the tested antibiotic compared with the PBS-treated control; #, P values for the combination compared to SOC antibiotics (P = 0.153 for vancomycin-SAL200 versus vancomycin and P = 0.140 for cefazolin-SAL200 versus cefazolin).
DISCUSSION
Our findings show that the endolysin SAL200 enhances the antibacterial activity of SOC antibiotics against S. aureus both in vitro and in vivo. Regardless of their antibacterial resistance profile, well-characterized S. aureus strains have consistently shown low SAL200 MICs, which are even lower when SAL200 is combined with SOC antibiotics in a checkerboard assay. The rapid bactericidal effect of SAL200 was demonstrated by a time-kill assay, which further showed the synergistic activity of SAL200 when combined with SOC antibiotics. This synergistic activity was also demonstrated in vivo with both a murine bacteremia model and a Galleria mellonella larva infection model.
All combinations of SAL200 with SOC antibiotics led to a consistent decrease in the MICs of SOC antibiotics in all S. aureus strains tested in the checkerboard assays, showing that the presence of SAL200 increases the susceptibility of S. aureus strains to SOC antibiotics. Vancomycin MICs decreased by 4 to 64 times in individual strains, and nafcillin MICs decreased to 2 to 4 μg/ml in MRSA strains, suggesting that SAL200 can facilitate antibiotic therapy in nonsusceptible S. aureus strains with SOC antibiotic MICs close to the breakpoint for resistance. Although some S. aureus strains failed to show definite synergism (defined when the lowest fractional inhibitory concentration index [∑FIC] was ≤0.5), an ∑FIC of <1 was noted in all strains tested. Therefore, we believe that the addition of SAL200 to SOC antibiotics can potentiate SOC antibiotic activity.
SAL200 was rapidly bactericidal in time-kill assays, in which a ≥3-log10 reduction in the number of bacterial CFU per milliliter was observed within 30 min even when it was used at sub-MIC doses, in contrast to the gradual growth inhibition by SOC antibiotics, which took hours to achieve a 1-log10 reduction. The rapid bactericidal effect of SAL200 appeared to be dose dependent, as bacterial regrowth occurred faster with lower doses of SAL200 in most strains. A higher bacterial inoculum or a lower endolysin dose is likely to result in an incomplete bactericidal effect and leave a higher residual bacterial burden, thereby resulting in regrowth following the initial rapid decrease in the number of viable bacteria. The regrown S. aureus strains were still susceptible to SAL200 with unchanged MIC values (data not shown). Effective growth inhibition was observed with the combination of SAL200 and SOC antibiotics, in which the reduction in bacterial growth was sustained in most strains even with sub-MIC doses.
The rapid bactericidal effect of SAL200 was also observed in vivo. Within 1 h of treatment, LAC- and Newman-infected mice treated with SAL200 or the combination of SAL200 plus SOC antibiotics showed a significantly lower level of bacteremia than untreated mice. LAC-infected mice treated with SAL200 showed a significantly lower bacteremia level than mice treated with vancomycin, a possible consequence of the different killing rates of SAL200 and vancomycin. Mice treated with the combination of SAL200 and SOC antibiotics had the lowest posttreatment bacterial levels of the groups tested, suggestive of synergy between SAL200 and SOC antibiotics. We note that the synergistic interaction between SAL200 and SOC antibiotics persisted over time and effectively decreased metastatic infections. Specifically, at 72 h after infection, mice receiving the combination of SAL200 plus SOC antibiotics showed a significantly lower splenic bacterial density than mice treated with SOC antibiotics alone.
Although all of the described experiments suggested synergism between SAL200 and SOC antibiotics, the effect size differed among assays. The rapid action time and short half-life described in previous studies of SAL200 (19–21) are likely to be responsible for the different results seen in our assays. The checkerboard assays and the overall survival of G. mellonella larvae were assessed at 18 h and 96 h postinfection, respectively. Therefore, rapid bacteriolytic effects are possibly masked by the regrowth of residual bacteria over time, minimizing the overall effect of SAL200. However, in time-kill assays, the killing activity of each drug and combination is quantitated in real time, reflecting the killing rates and extent during the measurement time. Similarly, the bacterial density in blood was measured 1 h after treatment in S. aureus-infected mice, reflecting the early bacteriolytic effects of SAL200.
These results are consistent with those of previous studies reporting synergistic interactions between other endolysins and antibiotics (22–25), but a definite mechanism of synergism is still not well defined (11). Daniel et al. used a checkerboard assay and a murine MRSA septicemia model to show that the chimeric endolysin ClyS interacted synergistically with oxacillin (22). They hypothesized that oxacillin increases internal peptidoglycan hydrolases and autolysins by inhibiting cell wall assembly enzymes and that the addition of an endolysin further shifts the balance of the cell wall toward increased degradation, causing bacteriolysis (22). Schuch et al. demonstrated synergism between the endolysin CF-301 and SOC antibiotics (vancomycin and daptomycin), and the authors hypothesized that endolysins increase cell wall permeability by cleaving a sufficient number of peptidoglycan bonds, which further enhances antibiotic penetration (25). Our results may indicate that the initial reduction of the inoculum by SAL200 could have further enhanced the subsequent antibacterial effect of vancomycin and nafcillin. As suggested in other studies, mechanisms beyond an inoculum effect are also likely to take part in and increase antibiotic potency.
Given their distinctive mechanisms of action, endolysins are an attractive new therapeutic option to overcome antibacterial resistance (11, 14). However, their mechanism of bacteriolysis also raises several concerns. Intracellular bacterial materials, toxins, and peptidoglycans are likely to be secreted into the host environment, elevating cytokines and causing inflammatory reactions. Entenza et al. showed that rats receiving higher doses of endolysins induced a higher level of circulating cytokines as a result of rapid bacterial lysis in a rat endocarditis model (26). Although our mouse study was not designed to assess cytokine release after endolysin treatment, we did observe that mice treated with SAL200 appeared to be more ill immediately after treatment than their negative controls treated with buffer alone. This could be a result of increased cytokine release induced by the rapid and extensive bacteriolysis by SAL200, but further studies are needed.
Although some differences in effect size were noted among the strains tested, SAL200 demonstrated several properties related to overcoming antibacterial resistance and therapeutic failure, suggesting its usefulness as a therapeutic option for difficult-to-treat S. aureus infections. SAL200 is rapidly bactericidal, is effective against S. aureus strains regardless of antibacterial resistance, shows low levels of resistance, interacts synergistically with and potentiates the activity of SOC antibiotics, and effectively reduces metastatic infections. In previous studies, SAL200 rapidly and effectively lysed planktonic S. aureus and proved effective against biofilms (20).
This study has some limitations. First, SAL200 was given only in single doses in the in vivo studies. As SAL200 is a protein of microbial origin, there is a possibility that it might be immunogenic in humans, inducing antibody formation, which would limit its use. Further studies regarding its immunogenicity are necessary, and a phase I clinical trial to evaluate multiple dosing of SAL200 is currently in preparation. Second, we used mice and G. mellonella larvae to assess SAL200's in vivo antibacterial activity; therefore, its effectiveness against human infections has not been assessed. As indicated by Pincus et al., it is possible that the observed bactericidal effect may not be reproduced in human blood (27), and lysin activity might be influenced by host immunity and the infection site. As the human body and immune system are very complex and different from those in in vivo animal models, it is necessary to investigate SAL200's effect against human infections in a clinical trial. A phase II clinical trial to assess the efficacy and safety of SAL200 in S. aureus bacteremia is under way (ClinicalTrials.gov identifier NCT03089697). Finally, the proinflammatory potential of SAL200 was not assessed, and further studies regarding SAL200-induced cytokine production are required.
In conclusion, the combination of the phage endolysin SAL200 with SOC antistaphylococcal antibiotics showed synergistic effects in vitro and in vivo. The combination produced an enhanced reduction of bacteremia as well as metastatic foci, suggesting that SAL200 could be useful as an adjunctive therapy in the treatment of difficult-to-treat S. aureus infections, including persistent bacteremia or metastatic S. aureus infection.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and antibiotic preparation.
Five S. aureus strains, two methicillin-susceptible strains (Newman and ATCC 29213) and three methicillin-resistant strains (LAC, ATCC 33591, and ATCC B1707), were used in this study. The bacterial strains were cultured in cation-adjusted Mueller-Hinton broth (CAMHB; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) supplemented with NaCl (Sigma-Aldrich, Saint Louis, MO, USA). All strains were stored at −70°C in cryobeads, and fresh cultures were used for each experiment.
The standard-of-care antistaphylococcal antibiotics were nafcillin or cefazolin for methicillin-susceptible S. aureus (MSSA) strains and vancomycin for MRSA strains. The SAL200 formulation contained 18 mg/ml SAL-1, 1.56 g/liter l-histidine (pH 6.0), 50 g/liter d-sorbitol, 1.47 g/liter CaCl2·2H2O, and 1 g/liter poloxamer 188, as previously described (19), and was provided by iNtRON Biotechnology, Inc. (Seongnam, Republic of Korea).
In vitro antibacterial combination assays.
MIC values for SAL200 and the SOC antibiotics were independently determined three times, using the broth microdilution method with an inoculum dose of 5 × 105 CFU/ml, according to Clinical and Laboratory Standards Institute (CLSI) guidelines (28).
Checkerboard assays were performed to test for synergism between SOC antibiotics and SAL200 using the broth microdilution method with a final inoculum dose of 5 × 105 CFU/ml. The final concentration ranges were 0.016 to 16 mg/liter nafcillin (for MSSA) or 1 to 512 mg/liter nafcillin (for MRSA), 0.031 to 32 mg/liter vancomycin, and 0.098 to 6.25 mg/liter SAL200. After 18 h of incubation at 37°C in air, we determined the MICs for each antibiotic alone and in combination and calculated the fractional inhibitory concentration (FIC) as the MIC of drug A in combination with drug B divided by the MIC of drug A alone. The ∑FIC was calculated by adding the FIC values for each drug in the combination, and the lowest ∑FIC for each combination was used to interpret the interaction between the two drugs: an ∑FIC of ≤0.5 was defined as synergistic, an ∑FIC of >0.5 but ≤4 was defined as indifferent, and an ∑FIC of >4 was defined as antagonistic (29, 30).
Time-kill assays were performed by the macrodilution method according to CLSI guidelines (31) in CAMHB inoculated with each isolate to a final concentration of 5 × 105 CFU/ml. The isolates were tested with nafcillin (MSSA) or vancomycin (MRSA) and SAL200 alone and in combination at concentrations of 1/4×, 1/2×, 1×, and 2× MIC. The tubes were cultured at 37°C with continuous shaking at 200 rpm, and subcultures for inoculum quantitation were taken at 0, 15, 30, 60, 120, 240, and 360 min after culture. Time-kill curves were plotted as time against the logarithm of the inoculum (in number of CFU per milliliter). Bactericidal activity was defined as a ≥3-log10-CFU/ml reduction of viable bacteria. Synergism was defined as a ≥2-log10-CFU/ml reduction when the bacteria were treated with the combination compared to that when they were treated with the most active constituent alone at 360 min, and antagonism was defined as an ≥2-log10-CFU/ml increase (31, 32). Each assay was performed in duplicate.
Murine bacteremia model.
Specific-pathogen-free female BALB/c mice that were 5 to 6 weeks of age and that weighed 16 to 19 g (Orient Bio, Seongnam, Republic of Korea) were used. Bacteremia was induced by intraperitoneal injection of 500 μl of an exponential-phase bacterial inoculum with a concentration of 2 × 109 CFU/ml. The mice were divided into four treatment groups, as follows (5 to 8 mice per group): (i) an inactive control group (which received 200 μl intravenous phosphate-buffered saline), (ii) an SOC antibiotic treatment group (which received either 25 mg/kg of body weight subcutaneous vancomycin or 200 mg/kg intramuscular nafcillin), (iii) an SAL200 treatment group (which received 25 mg/kg intravenous SAL200 plus 25 mg/kg intraperitoneal SAL200), and (iv) a combination treatment group (which received SOC [either 25 mg/kg subcutaneous vancomycin or 200 mg/kg intramuscular nafcillin] plus SAL200 [25 mg/kg intravenous SAL200 plus 25 mg/kg intraperitoneal SAL200]). The mice were treated according to study group 1 h after infection, and blood was collected from the tail vein for culture 1 h after treatment. Postinfection survival was assessed every 24 h until 72 h following infection. All mice were sacrificed, and their spleens were harvested for culture 72 h after infection (see Fig. S1 in the supplemental material). The experiment was performed with MRSA LAC and MSSA Newman and repeated 2 to 3 times for each isolate. All in vivo studies were performed in accordance with the guidelines of the Seoul National University Hospital Institutional Animal Care and Use Committee.
Galleria mellonella infection model.
Healthy Galleria mellonella larvae (Ecowin, Daegu, Republic of Korea) that weighed 200 to 250 mg and that were in their final instar stage were selected, food deprived, and stored at 15°C for 24 h before infection (33). The larvae were injected via the last left proleg with 10 μl of a bacterial suspension containing 5 × 108 CFU/ml of the MRSA LAC strain or the MSSA Newman strain. This dose was previously determined to yield 80% mortality at 72 h postinfection. All injections were delivered using 2-in., 26-gauge needles (Hamilton syringe needle; catalog number 7779-04; Hamilton Company, Reno, NV, USA) attached to a 500-μl Hamilton syringe (1750 RN; catalog number 81230) and mounted on a dispenser (Hamilton PB600 repeating dispenser; catalog number 83700). Infected larvae were split into four experimental groups (15 larvae per group): (i) an inactive control group (which received phosphate-buffered saline), (ii) an SOC antibiotic treatment group (which received either 25 mg/kg vancomycin for MRSA LAC-infected larvae or 100 mg/kg cefazolin for MSSA Newman-infected larvae), (iii) an SAL200 treatment group (which received 50 mg/kg SAL200), and (iv) a combination treatment group (which received SOC plus SAL200 treatment). All antimicrobial drugs were delivered into the hemocoel via injections of 10 μl into the last right proleg at 1 h following infection. Larvae were incubated in vented 12-well culture plates (SPL Life Sciences, Pocheon, Republic of Korea) at 37°C in air for 96 h and inspected and scored every 24 h for death, failure to move in response to touch, and melanization. All experiments were independently performed 2 to 3 times.
Statistical methods.
Statistical analyses were performed using the IBM SPSS Statistics (version 22.0) software package (SPSS Inc., Chicago, IL, USA). Survival data were plotted using the Kaplan-Meier method, and a log-rank test was used for comparisons between groups. Bacterial density data were analyzed using the Mann-Whitney U test and are presented as the median and interquartile range. A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported by grants from the SNUH Research Fund (grant no. 04-2015-0210), by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education (grant no. NRF-2016R1D1A1A02936919), by the Korean Health Technology R&D Projects, funded by the Ministry for Health & Welfare, Republic of Korea (HI17C1395), and by a research fund from iNtRON Biotechnology, Inc.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
S. Y. Jun is employed by the commercial company iNtRON Biotechnology, Inc. The phage endolysin SAL200 used in this study is produced by iNtRON and is currently in use in a human clinical trial (NCT03089697). None of the other authors has a conflict to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00731-18.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF, Infectious Diseases Society of America. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 3.Moellering RC., Jr 2012. MRSA: the first half century. J Antimicrob Chemother 67:4–11. doi: 10.1093/jac/dkr437. [DOI] [PubMed] [Google Scholar]
- 4.Bayer AS, Lam K, Ginzton L, Norman DC, Chiu CY, Ward JI. 1987. Staphylococcus aureus bacteremia. Clinical, serologic, and echocardiographic findings in patients with and without endocarditis. Arch Intern Med 147:457–462. [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 6.Song KH, Kim ES, Sin HY, Park KH, Jung SI, Yoon N, Kim DM, Lee CS, Jang HC, Park Y, Lee KS, Kwak YG, Lee JH, Park SY, Song M, Park SK, Lee YS, Kim HB, Korea Infectious Diseases (KIND) Study Group. 2013. Characteristics of invasive Staphylococcus aureus infections in three regions of Korea, 2009-2011: a multi-center cohort study. BMC Infect Dis 13:581. doi: 10.1186/1471-2334-13-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodvold KA, McConeghy KW. 2014. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 58(Suppl 1):S20–S27. doi: 10.1093/cid/cit614. [DOI] [PubMed] [Google Scholar]
- 8.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Gao W, Christiansen KJ, Coombs GW, Johnson PD, Howden BP. 2011. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis 204:340–347. doi: 10.1093/infdis/jir270. [DOI] [PubMed] [Google Scholar]
- 9.Aguado JM, San-Juan R, Lalueza A, Sanz F, Rodriguez-Otero J, Gomez-Gonzalez C, Chaves F. 2011. High vancomycin MIC and complicated methicillin-susceptible Staphylococcus aureus bacteremia. Emerg Infect Dis 17:1099–1102. doi: 10.3201/eid/1706.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reardon S. 2014. Phage therapy gets revitalized. Nature 510:15–16. doi: 10.1038/510015a. [DOI] [PubMed] [Google Scholar]
- 11.Pastagia M, Schuch R, Fischetti VA, Huang DB. 2013. Lysins: the arrival of pathogen-directed anti-infectives. J Med Microbiol 62:1506–1516. doi: 10.1099/jmm.0.061028-0. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki S, Rashel M, Uchiyama J, Sakurai S, Ujihara T, Kuroda M, Ikeuchi M, Tani T, Fujieda M, Wakiguchi H, Imai S. 2005. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J Infect Chemother 11:211–219. doi: 10.1007/s10156-005-0408-9. [DOI] [PubMed] [Google Scholar]
- 13.Loessner MJ. 2005. Bacteriophage endolysins—current state of research and applications. Curr Opin Microbiol 8:480–487. doi: 10.1016/j.mib.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Fischetti VA. 2008. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol 11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson D, Loomis L, Fischetti VA. 2001. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci U S A 98:4107–4112. doi: 10.1073/pnas.061038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCullers JA, Karlstrom A, Iverson AR, Loeffler JM, Fischetti VA. 2007. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog 3:e28. doi: 10.1371/journal.ppat.0030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Q, Nelson D, Zhu S, Fischetti VA. 2005. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother 49:111–117. doi: 10.1128/AAC.49.1.111-117.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashel M, Uchiyama J, Ujihara T, Uehara Y, Kuramoto S, Sugihara S, Yagyu K, Muraoka A, Sugai M, Hiramatsu K, Honke K, Matsuzaki S. 2007. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J Infect Dis 196:1237–1247. doi: 10.1086/521305. [DOI] [PubMed] [Google Scholar]
- 19.Jun SY, Jung GM, Son JS, Yoon SJ, Choi YJ, Kang SH. 2011. Comparison of the antibacterial properties of phage endolysins SAL-1 and LysK. Antimicrob Agents Chemother 55:1764–1767. doi: 10.1128/AAC.01097-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jun SY, Jung GM, Yoon SJ, Oh MD, Choi YJ, Lee WJ, Kong JC, Seol JG, Kang SH. 2013. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int J Antimicrob Agents 41:156–161. doi: 10.1016/j.ijantimicag.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Jun SY, Jang IJ, Yoon S, Jang K, Yu KS, Cho JY, Seong MW, Jung GM, Yoon SJ, Kang SH. 2017. Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob Agents Chemother 61:e02629-. doi: 10.1128/AAC.02629-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA. 2010. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 54:1603–1612. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loeffler JM, Fischetti VA. 2003. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob Agents Chemother 47:375–377. doi: 10.1128/AAC.47.1.375-377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chhibber S, Kaur T, Sandeep K. 2013. Co-therapy using lytic bacteriophage and linezolid: effective treatment in eliminating methicillin resistant Staphylococcus aureus (MRSA) from diabetic foot infections. PLoS One 8:e56022. doi: 10.1371/journal.pone.0056022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, Fischetti VA, Huang DB, Nowinski RC, Wittekind M. 2014. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis 209:1469–1478. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Entenza JM, Loeffler JM, Grandgirard D, Fischetti VA, Moreillon P. 2005. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob Agents Chemother 49:4789–4792. doi: 10.1128/AAC.49.11.4789-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pincus NB, Reckhow JD, Saleem D, Jammeh ML, Datta SK, Myles IA. 2015. Strain specific phage treatment for Staphylococcus aureus infection is influenced by host immunity and site of infection. PLoS One 10:e0124280. doi: 10.1371/journal.pone.0124280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. CLSI document M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 30.Garcia LS. (ed). 2010. Synergism testing: broth microdilution checkerboard and broth macrodilution methods. In Clinical microbiology procedures handbook, 3rd ed and 2007 update. ASM Press, Washington, DC. doi: 10.1128/9781555817435. [DOI] [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline. CLSI document M26-A. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.Eliopoulos GM, Eliopoulos CT. 1988. Antibiotic combinations: should they be tested? Clin Microbiol Rev 1:139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai CJ, Loh JM, Proft T. 2016. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 7:214–229. doi: 10.1080/21505594.2015.1135289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.