Sulfide production has been proposed to be a universal defense mechanism against antibiotics in bacteria (K. Shatalin, E.
KEYWORDS: Staphylococcus aureus, aminoglycosides, resistance mechanisms
ABSTRACT
Sulfide production has been proposed to be a universal defense mechanism against antibiotics in bacteria (K. Shatalin, E. Shatalina, A. Mironov, and E. Nudler, Science 334:986–990, 2011, doi:10.1126/science.1209855). To gain insight into the mechanism underlying sulfide protection, we systematically and comparatively addressed the interference of sulfide with antibiotic activity against Staphylococcus aureus, as a model organism. The impact of sulfide and sulfide precursors on the antibiotic susceptibility of S. aureus to the most important classes of antibiotics was analyzed using modified disk diffusion assays, killing kinetic assays, and drug uptake studies. In addition, sulfide production and the impact of exogenously added sulfide on the physiology of S. aureus were analyzed. Sulfide protection was found to be limited to aminoglycoside antibiotics, which are known to be taken up by bacterial cells in an energy-dependent process. The protective mechanism was found to rely on an inhibitory effect of sulfide on the bacterial respiratory chain, leading to reduced drug uptake. S. aureus was found to be incapable of producing substantial amounts of sulfide. We propose that bacterial sulfide production should not be regarded as a general defense mechanism against antibiotics, since (i) it is limited to aminoglycosides and (ii) production levels vary considerably among species and, as for S. aureus, may be too low for protection.
INTRODUCTION
The rapid emergence of antibiotic-resistant bacteria has become a global threat. Therefore, studies on antibiotics and bacterial resistance modes have become a central topic for research, in hopes of finding alternative therapies (1–3). The formation of reactive oxygen species (ROS) is considered to occur as a general downstream effect in bacteria challenged with bactericidal antibiotics (4). In 2011, a novel antibiotic resistance mechanism mediated by hydrogen sulfide (H2S) was proposed for several pathogenic bacteria, including Staphylococcus aureus (5). It was proposed that sulfide reduces the cellular formation of ROS by interfering with the Fenton reaction and by stimulating the ROS-scavenging enzymes superoxide dismutase and catalase (5).
Sulfide is a weak acid and the equilibrium between its protonated and deprotonated states depends greatly on the pH. Here, we use the term sulfide to describe the sum of the different protonated forms present in solution (H2S, HS−, and S2−). Sulfide is a major part of the microbial sulfur cycle, as several bacteria can use sulfide as their sole electron source (6). It is produced during dissimilatory sulfate reduction, where it results from the stepwise reduction of sulfate via sulfite and an unusual protein trisulfide (7), and can also be formed as the product of cysteine degradation either via cystathionine-β-synthase (CBS) and cystathionine-γ-lyase (CSE) or via 3-mercaptopyruvate sulfurtransferase (3MST) (5). These enzymes have also been shown to produce sulfide in mammals. Here, sulfide has been identified as a third gasotransmitter, in addition to nitric oxide (NO) and carbon monoxide (CO), and various physiological and pathophysiological functions have been attributed to sulfide (8–10). It is well known for its toxicity, which has been associated with its ability to interact with heme proteins, most notably cytochromes in the respiratory chain (11–14).
Aminoglycosides target the 30S subunit of bacterial ribosomes, leading to a cascade of pleiotropic effects that account for the bactericidal activity (15). They are among the antibiotics that are commonly used to treat infections caused by Gram-positive cocci such as S. aureus. Before they can interact with their intracellular targets, aminoglycosides need to cross the cytoplasmic membrane. This process is energy dependent, requiring a threshold electrochemical potential across the cytoplasmic membrane. In line with that, compounds that inhibit the respiratory chain, such as cyanide, or uncouple the electrochemical gradient, such as carbonyl cyanide m-chlorophenyl hydrazone (CCCP), have been shown to protect bacteria from aminoglycoside toxicity (reviewed by Taber et al. [16]). Furthermore, anaerobes are usually not susceptible to aminoglycosides. Given the known inhibitory effect of sulfide on the respiratory chain and the dependence of aminoglycoside uptake on respiration, we hypothesized that sulfide impairs the S. aureus respiratory chain and consequently reduces uptake of the drug.
RESULTS
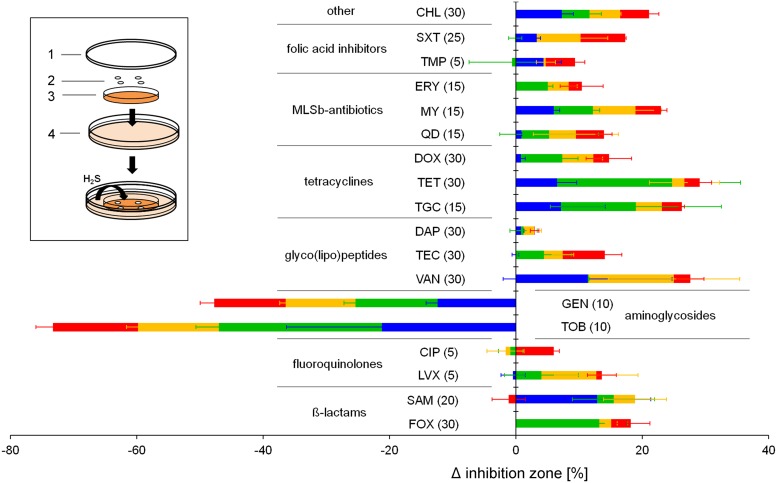
Sulfide production has been proposed to be a universal defense mechanism against antibiotics in various bacteria, including S. aureus (5), such that interference with sulfide formation may become a useful strategy to restore the antibiotic susceptibility of pathogenic bacteria. In search of novel antistaphylococcal targets, we aimed to systematically address the impact of sulfide on antibiotic susceptibility of different S. aureus strains. To this end, we established a modified disk diffusion assay that allows continuous incubation with gaseous sulfide (Fig. 1), thereby diminishing effects that occur due to the oxidation of sulfide in liquid medium. The system is based on the continuous production of gaseous sulfide by Escherichia coli, which can have a direct impact on a regular disk diffusion assay in which S. aureus is plated on Mueller-Hinton (MH) agar plates. In E. coli, sulfide is produced mainly from cysteine by 3MST, encoded by sseA (5). Therefore, the ΔsseA strain served as a control to exclude the impact of other volatile compounds produced by E. coli. To verify the system, sulfide quantification was performed by replacing the inner MH agar plate with a petri dish filled with a solution of zinc acetate (2% in H2O) and using the methylene blue assay method the next day. An intense blue color, indicative of sulfide, was observed when the E. coli wild-type (WT) strain was used, while no color change was observed with the control E. coli ΔsseA strain (data not shown).
FIG 1.
Impact of externally added sulfide on the susceptibility of S. aureus to several antibiotics. The susceptibility of S. aureus HG003 (red), RN4220 (orange), Newman (green), and USA300 (blue) to various classes of antibiotics was assessed. Cells were plated on MH agar plates and incubated with or without the external addition of sulfide. FOX, cefoxitin; SAM, ampicillin-sulbactam; LVX, levofloxacin; CIP, ciprofloxacin; TOB, tobramycin; GEN, gentamicin; VAN, vancomycin; TEC, teicoplanin; DAP, daptomycin; TGC, tigecycline; TET, tetracycline; DOX, doxycycline; QD, quinupristin-dalfopristin; MY, lincomycin; ERY, erythromycin; TMP, trimethoprim; SXT, trimethoprim-sulfamethoxazole; CHL, chloramphenicol. Numbers in parentheses indicate antibiotic contents (in micrograms). Shown are the mean differences (± standard deviations) in the inhibition zones between sulfide-treated and nontreated plates. Experiments were performed in triplicate. The inset shows a schematic illustration of the assay. 1, lid of the petri dish; 2, antimicrobial susceptibility disks; 3, S. aureus plated on MH agar; 4, 145-mm-diameter petri dish containing 35 ml of LB inoculated with the E. coli ΔsseA strain or W3110 supplemented with cysteine. Sulfide produced by E. coli affects the S. aureus disk diffusion assay.
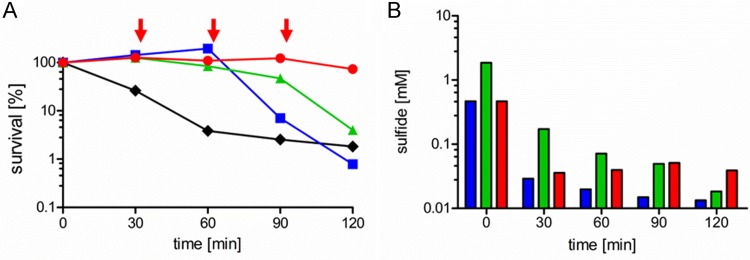
Surprisingly, we observed sulfide-mediated protection of all S. aureus strains tested only against the class of aminoglycoside antibiotics. In contrast, the strains tended to be more sensitive to the other classes of antibiotics in the presence of sulfide (Fig. 1). We obtained highly similar values for S. aureus RN4220, the methicillin-sensitive S. aureus (MSSA) strain used by Shatalin et al. (5), and the closely related strain HG003, which we then chose as a model organism for the following studies. Killing kinetic assays were performed to verify the observations from the agar diffusion assay. Therefore, a culture in the exponential growth phase was treated with the aminoglycoside gentamicin and sulfide; the latter was added either at the beginning of the experiment, to a final concentration of 1 or 4 mM, or by periodic addition of 1 mM sulfide every 30 min. A protective effect was detectable in the presence of all sulfide concentrations, in comparison to the untreated sample (Fig. 2A). However, only periodic addition of sulfide led to constant protection. This corresponds to the sulfide concentrations measured simultaneously with the killing kinetics (Fig. 2B). Under oxic conditions, sulfide is known to quickly oxidize to a mixture of various compounds, such as polysulfide, thiosulfate, and sulfate (17, 18). In line with that, the sulfide concentrations at time zero were significantly lower than the theoretically calculated values and, during the experiment, sulfide levels declined quickly within the first minutes after addition in all samples. In the case of periodic addition, however, the sulfide concentration never dropped below a certain level, and constant protection was observed over the entire time course. This finding indicates that a certain minimal sulfide concentration is necessary for permanent protection against aminoglycoside toxicity. By comparing the measured sulfide concentrations with the degree of protection, we estimated this minimal concentration to be 30 to 50 μM. Furthermore, it was verified using cyanide that the inhibition of the respiratory chain protects S. aureus against gentamicin toxicity like sulfide (data not shown), as reported previously (19–21).
FIG 2.
Correlation of protection of S. aureus HG003 from gentamicin toxicity with sulfide stability. (A) Survival of S. aureus grown to an OD600 of 0.5 in LB after treatment with 25 mg/liter gentamicin and different concentrations of sulfide (1 mM [blue line], 4 mM [green line], or periodic addition of 1 mM sulfide at 30-min intervals, as indicated by arrows [red line]) or without addition of sulfide (black line). (B) Sulfide concentrations measured during the killing experiment shown in panel A. Colors correspond to the different sulfide concentrations added, as described for the experiment in panel A. The data show a representative experiment of three independent experiments with comparable results.
The correlation of the protective effect with sulfide stability shows that indeed sulfide is the protective agent and not the emerging oxidation products. This is in line with results from killing assays performed in lysogeny broth (LB) that had been preincubated with sulfide for 2 h. No sulfide was detected in this medium, showing that the sulfide was fully oxidized, and no protection against gentamicin was observed (data not shown). The observations described above indicated a protective mechanism in addition to those proposed by Shatalin et al. (5), which we aimed to reveal next.
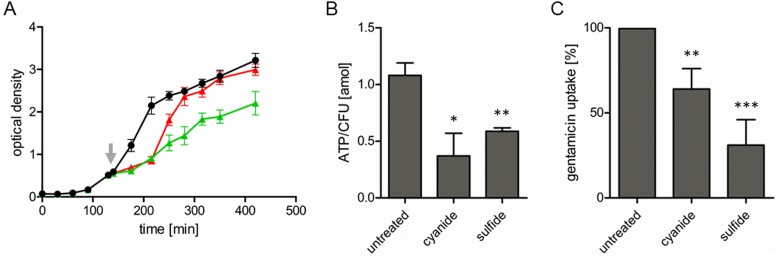
Sulfide is well known to be cytotoxic due to its interactions with heme groups (22, 23), leading to inhibition of cytochromes, which are essential parts of the respiratory chain. It is noteworthy that the uptake of aminoglycosides is dependent on the membrane potential of the bacterial cell (16, 24, 25), which in turn is directly dependent on respiration. Therefore, we hypothesized that the additional protective mechanism of sulfide is based on inhibition of the respiratory chain, ultimately leading to reduced drug uptake. In line with the cytotoxic character of sulfide, reduced growth of S. aureus was observed in the presence of sulfide and in the presence of cyanide, which is another known inhibitor of the respiratory chain. Similar growth delays were observed upon addition of sulfide or cyanide (Fig. 3A). After approximately 2 h, however, the growth rate in the presence of sulfide was restored to levels similar to those in the untreated sample, presumably due to the aforementioned oxidation of sulfide.
FIG 3.
Effects of sulfide and cyanide on S. aureus growth, ATP concentrations, and gentamicin uptake. (A) Cell growth was monitored by measuring the OD600. In the exponential growth phase (OD600 of ∼0.5), the culture was split (indicated by the arrow) and 1 mM sulfide (red line) or 0.1 mM cyanide (green line) was added or the cells were left untreated (black line). The data are means ± standard deviations of three independent experiments. (B) Effects of sulfide and cyanide on ATP levels in S. aureus. Cells were grown to an OD600 of 0.5 and then incubated with 0.1 mM cyanide or 1 mM sulfide for 10 min or left untreated. ATP levels are expressed in attomoles of ATP per CFU, which was determined simultaneously. (C) Impact of sulfide and cyanide on gentamicin uptake by S. aureus. Uptake of bodipy FL-labeled gentamicin was determined under conditions as in panel B. Values determined for the untreated control were set as 100%, and the treated samples are shown in relation to the control. Data are means ± standard deviations of three independent experiments. Statistical analysis was analyzed by t test: *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
We next measured the impact of sulfide on the aerobic respiration of S. aureus, and we found the oxygen consumption to be 181 ± 19 nmol/min in the presence of sulfide, compared to 217 ± 7 nmol/min in the untreated sample. Cellular ATP concentrations, determined as a measure of the energy status of the cells, were found to be significantly reduced upon addition of cyanide or sulfide (Fig. 3B). To confirm that the reduced ATP concentrations were not due to decreased bacterial cell numbers, CFU in the presence of sulfide or cyanide were determined, and similar values (6.1 × 107 ± 0.4 × 107 and 5.8 × 107 ± 0.8 × 107 CFU/ml, respectively) were obtained, in comparison to the untreated sample (6.5 × 107 ± 0.4 × 107 CFU/ml). Therefore, sulfide impairs aerobic respiration, leading to reduced energy levels in S. aureus, without strongly influencing cell viability.
To confirm that the protection mechanism is based on reduced drug uptake, aminoglycoside uptake by S. aureus was directly studied by fluorescence microscopy using bodipy FL-labeled gentamicin. Therefore, we first used cyanide to inhibit the S. aureus respiratory chain and found significantly reduced drug uptake (Fig. 3C). This showed that, for the labeled version of gentamicin also, a functional respiratory chain is needed for efficient drug uptake. When cells were pretreated with sulfide, we observed drastically reduced uptake of labeled gentamicin, in comparison to the untreated sample (Fig. 3C). In conclusion, our results show that there is specific protection of S. aureus against aminoglycoside antibiotics that is mediated by inhibiting respiration and consequently reducing the energy status of the cell, ultimately affecting drug uptake.
Shatalin and colleagues, in all of their experiments with S. aureus, used dl-propargylglycine (PAG) and aminooxyacetate (AOAA), which are known inhibitors of the sulfide-producing enzymes CBS and CSE, respectively (5, 26). Therefore, we studied the impact of these inhibitors on the vitality of S. aureus. While no toxic effect of PAG was found at concentrations up to 128 mg/liter, the MIC of AOAA was found to be 16 mg/liter. Checkerboard dilution assays carried out with AOAA and gentamicin or ampicillin revealed no synergistic effects of the inhibitor and the antibiotic, with fractional inhibitory concentration (FIC) index values of 2.15 ± 0.09 and 1.125 ± 0.13, respectively.
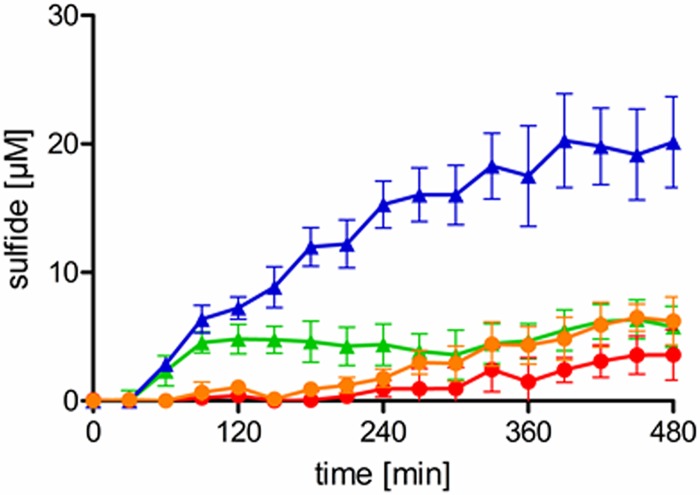
While sulfide was exogenously added in the previous experiments, we finally addressed the question of whether S. aureus is capable of producing enough sulfide to protect itself against antibiotics. In line with previous observations (5), sulfide production was observed, using lead acetate paper strips, when S. aureus was grown with proper supplementation with cysteine or cystine (data not shown). However, this method displays several drawbacks, i.e., it measures only gaseous sulfide, it is only semiquantitative, and, most importantly, it sums up sulfide production over several hours. Therefore, we measured sulfide production of S. aureus HG003 using the methylene blue method and using E. coli as the control for sulfide production (Fig. 4). Of note, the sulfide concentration in the S. aureus culture never exceeded 6 μM, regardless of whether cysteine or cystine was added. In contrast, up to 20 μM sulfide was detected in the E. coli culture grown in LB supplemented with cysteine. Addition of sublethal concentrations of gentamicin did not induce sulfide production (data not shown), as would be expected if sulfide production were an active resistance mechanism against aminoglycosides. These results clearly show that, under the conditions used, S. aureus is not capable of producing sulfide at a concentration that leads to protection against aminoglycosides. In line with that finding, the addition of neither cysteine nor cystine to the growth medium protected S. aureus from gentamicin-mediated killing (data not shown).
FIG 4.
Sulfide production by S. aureus and E. coli. Sulfide was quantified in the supernatant of S. aureus cultures grown in LB supplemented with cysteine (orange line) or cystine (red line) and in E. coli cultures supplemented with cysteine (blue line) or cystine (green line). The data are means ± standard deviations of two independent experiments.
DISCUSSION
Sulfide has gained considerable scientific attention since it has been identified as a third gasotransmitter in mammalian physiology (27–29). Like the other two gasotransmitters, CO and NO, sulfide was shown to affect diverse physiological functions (28). Besides sharing a role in signal transduction, the three gasses are all well known for their toxicity, which in the case of sulfide is mainly based on its tendency to bind to cytochromes and other metalloenzymes and to reduce protein disulfide bridges (13, 30). Sulfide was proposed to be a universal defense against antibiotics in bacteria (5). While different bacteria and representative antibiotics were used in that study, here we used the Gram-positive pathogen S. aureus, to systematically and comparatively address the sulfide-mediated protection against the most important classes of antibiotics. We found sulfide-mediated protection against aminoglycosides and observed a clear correlation of sulfide stability and protection, which showed that sulfide is the protective agent and not its oxidation products. This is of importance because it was recently shown that the role of sulfide oxidation has been largely underestimated and some characteristics associated with sulfide should rather be linked to polysulfides (31, 32). The fact that we found protection only against aminoglycosides suggested a mechanism different from the ones postulated previously (5). Those authors proposed universal protection against antibiotics mediated by inhibition of the Fenton reaction via decreases in the intracellular levels of cysteine, iron, and H2O2 and the activation of ROS-detoxifying enzymes (5). However, it is well known that a threshold membrane energization is needed for the uptake of gentamicin (24, 25), which was shown here to be impaired by sulfide. This mechanism reflects the situation in S. aureus small-colony variants (SCVs), which are resistant to aminoglycosides. SCVs are subpopulations of S. aureus that grow slowly because of, for example, a defect in menadione or heme biosynthesis. Therefore, essential components of the respiratory chain are missing, leading to reduced membrane potential and ultimately reduced drug uptake (33, 34).
The different observations made in this study and by Shatalin and colleagues (5) with regard to S. aureus may be explained by the fact that all of their findings for S. aureus were based on the use of PAG and AOAA. These compounds are known inhibitors of the sulfide-producing enzymes CBS and CSE. Despite being widely used to inhibit CBS, AOAA is well known to be a nonspecific inhibitor of aminotransferases and other pyridoxal 5′-phosphate-dependent enzymes, as it blocks enzyme activity by covalently binding to the cofactor (35, 36); therefore, it is not specific for CBS. Most importantly, we observed that S. aureus does not produce sulfide at substantial levels, which was already shown earlier (37). Therefore, the physiological effects of PAG and AOAA in S. aureus cells cannot be attributed to a defect in sulfide production. In addition, checkerboard dilution assays carried out with inhibitors and antibiotics showed that there were no synergistic effects of these compounds. Therefore, it can be assumed that the increased antibiotic susceptibility of S. aureus in the presence of the inhibitors (5) is due to the addition of the individual toxic effects.
Despite the fact that S. aureus is not capable of producing substantial amounts of sulfide endogenously, the sulfide-mediated protection against aminoglycosides, which are often used as adjunctive therapy to treat staphylococcal infections, may be of clinical importance. This is because we have estimated that a minimal concentration of 30 to 50 μM is needed to fully protect S. aureus. Most importantly, the sulfide concentration in the blood of healthy humans was found to be in the range of 30 to 60 μM (38), although levels as high as 100 μM have been reported (39). It is thought that sulfide plays an important role in inflammation, and it was shown in a mouse model that the administration of bacterial lipopolysaccharides increased plasma sulfide concentrations significantly (40). In line with that finding, increased sulfide levels (150 μM) have been measured in the blood of patients suffering from septic shock (40). Moreover, a recent study reported high concentrations of sulfide in the sputum of children suffering from cystic fibrosis (CF) (41). Those authors reported sulfide production rates of 0.3 μM/min and final concentrations of up to 300 μM in vitro. Importantly, S. aureus is the primary respiratory pathogen in young CF patients, and aminoglycosides are routinely used to treat bacterial infections of CF lungs. Of note, aminoglycosides are usually not used as monotherapy to treat infections caused by S. aureus. Instead, they are often used in combination with antibiotics targeting cell wall biosynthesis (e.g., β-lactams or vancomycin). Although the precise mechanism of synergy is not fully understood, it has been shown that, for enterococci, a damaged cell wall leads to increased uptake of streptomycin (42). In such a scenario, sulfide-mediated protection is likely abrogated.
Sulfide can freely diffuse across membranes only in its fully protonated form (43). At the physiological pH of blood (pH of ∼7.36), ∼30% of sulfide is present in its fully protonated form, as H2S (44). Because the intracellular pH of S. aureus is ∼7.8, it is expected that more sulfide would be deprotonated, leading to accumulation of sulfide within the cell. It must be noted, however, that all S. aureus strains encode an enzyme system thought to be responsible for sulfide detoxification (45).
In addition to the effect on aminoglycoside uptake, sulfide might affect bacterial susceptibility to other cationic molecules, such as lantibiotics and in particular host defense peptides, which have also been shown to require a certain threshold membrane potential (46–48). Moreover, the mechanism presented here is most likely not limited to S. aureus but also may protect other bacteria from aminoglycoside antibiotics. An identical mechanism has been shown to be the reason for NO-mediated protection of bacteria from aminoglycosides (20). It has been shown that nitrosylation of cytochromes in the respiratory chain of Salmonella by NO is responsible for reduced drug uptake and an NO donor protects S. aureus from aminoglycoside killing (20). Besides sulfide and NO, the third mammalian gasotransmitter, CO, is known to specifically inhibit cytochromes (13). Therefore, it can be speculated that CO can protect bacteria from aminoglycosides as well.
Together, our results show that the sulfide-mediated protection mechanisms postulated earlier (5) need to be extended by the mechanism described in this study. The fact that we observed protection only against aminoglycoside antibiotics clearly shows that, at least in S. aureus, the resistance mechanisms proposed by Shatalin and colleagues (5) can be neglected. Together with the fact that S. aureus is not capable of producing substantial amounts of sulfide endogenously, we propose that sulfide production should not be regarded as a universal protection mechanism against antibiotics.
MATERIALS AND METHODS
Bacterial strains.
Staphylococcus aureus HG003 (49), RN4220 (50), Newman (51), USA300 (52), and SG511 (Robert Koch Institute, Berlin, Germany) and the Escherichia coli strains W3110 (53) and ΔsseA (54) were used in this study. Bacteria were cultivated in LB at 37°C unless otherwise stated. Liquid cultures were shaken at 160 rpm.
Chemicals.
ATP, PAG, AOAA, sodium sulfide nonahydrate, and sodium hydrosulfide hydrate were purchased from Sigma-Aldrich. Sulfide solutions were freshly prepared before each experiment. Bodipy FL succinimidyl ester and antimicrobial susceptibility disks were purchased from Thermo Fisher. For daptomycin, sterile cellulose disks (Carl Roth) were loaded with 30 μg of daptomycin (Cubist Pharmaceuticals). Gentamicin and all other chemicals were purchased from Carl Roth.
Susceptibility testing.
MICs were determined by standard broth microdilution, according to the Clinical and Laboratory Standards Institute guidelines (55). To check the relationships between AOAA and antibiotics, checkerboard microdilution assays were performed. FIC indices were calculated as described previously (56), and interactions were defined as synergistic for FIC index values of ≤0.5 and nonsynergistic for values between 0.5 and 4.
For modified disk diffusion assays, S. aureus strains were grown in MH broth to an optical density at 600 nm (OD600) of 0.5. The cell suspension was diluted 10-fold, and 100 μl of the suspension was spread on MH agar plates before antimicrobial susceptibility disks were applied. The plates were placed in polypropylene petri dishes (diameter, 145 mm) containing 35 ml of a cell suspension of either E. coli W3110 (providing continuous sulfide production) or the ΔsseA strain (control). For these suspensions, E. coli strains were grown in LB to an OD600 of 0.5 and diluted 100-fold in fresh LB. In the case of E. coli W3110, LB was supplemented with 10 mM l-cysteine as a substrate for sulfide production, which was omitted for the control ΔsseA strain because we found that small amounts of sulfide are formed nonenzymatically in sterile LB supplemented with cysteine. Plates were incubated for 15 h at 37°C, and the inhibition zones were measured.
For killing kinetic assays, S. aureus was grown until an OD600 of 0.5 was reached. Cells were treated with 1 mM sodium hydrosulfide hydrate, 0.1 mM sodium cyanide, 1 mM cystine, or 2 mM cysteine, and gentamicin sulfate was added to a final concentration of 25 mg/liter. At different time points, samples were taken, serially diluted in 0.9% NaCl solution, and streaked on LB agar plates. Colony counts were determined after overnight incubation at 37°C. Counts at time zero were set as 100%.
Sulfide quantification.
Sulfide levels were quantified with a modified methylene blue assay (57). Samples were taken simultaneously with the killing kinetic assays and centrifuged. To 250 μl of 2% zinc acetate solution, 395 μl of the supernatant and 100 μl of dimethyl-p-phenylenediamine chloride (0.2% solution in 20% H2SO4) were added. Five microliters of ammonium iron(III) sulfate (10% solution in 2% H2SO4) was added, and the solution was incubated for 20 min at room temperature. To determine the sulfide concentration, absorption at 670 nm was measured. Detection of sulfide via lead acetate paper strips was performed as described previously (5).
Labeling of gentamicin with bodipy FL.
Labeling of gentamicin with bodipy FL was performed as described previously (58). The sample was mixed with chloroform in a 1:1 ratio and incubated for 1 h. The upper layer (hydrophilic phase), in which gentamicin and fluorescently labeled gentamicin were present, was used for future experiments. Successful labeling of gentamicin was verified by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry. The preparations containing fluorescently labeled gentamicin and control preparations (lacking bodipy FL succinimidyl ester) displayed identical MIC values.
Uptake of bodipy FL-labeled gentamicin.
S. aureus cells were grown to an OD600 of 0.5. Bacterial cultures (500 μl) were incubated for 10 min with either 1 mM freshly prepared sodium hydrosulfide hydrate or 0.1 mM sodium cyanide. The cells were then incubated for 15 min with 20 μl fluorescently labeled gentamicin. Cells were washed twice with fresh LB, and microscopy was performed as described previously (59). For each experiment, at least 2.5 × 104 cells were analyzed. Photographs were analyzed using ImageJ software, calculating the ratio between the total area of cells (phase-contrast channel) and the integrated density of fluorescent cells (bodipy FL channel).
Determination of cellular ATP concentrations.
Cellular ATP concentrations were measured with the BacTiter-Glo microbial cell viability assay (Promega), according to the manufacturer's protocol. S. aureus HG003 was grown to an OD600 of 0.5, and 1 ml of culture was incubated for 10 min with either 1 mM sodium hydrosulfide hydrate or 0.1 mM sodium cyanide. One hundred microliters of cell suspension was mixed with 100 μl of BacTiter-Glo reagent, the mixture was incubated for 5 min, and luminescence was measured using a Tecan Spark microplate reader.
Oxygen consumption.
Oxygen consumption of S. aureus was determined by measuring the oxygen partial pressure using an oxygen electrode connected to an oxygen measurement controller (digital model 20; Rank Brothers). Five milliliters of bacterial culture (OD600 of 0.5) was incubated for 1 min at 37°C in the presence or absence of 1 mM sodium hydrosulfide hydrate. The culture was then transferred to the incubation chamber, which was set at 37°C and rinsed with air. The chamber was closed, and oxygen consumption was measured, with constant stirring. Values were plotted, and the slope of the linear part of the graph was used for the calculation of oxygen consumption. Values were normalized to the OD600 of the cell suspension.
ACKNOWLEDGMENTS
This work was supported by the German Center for Infection Research and the Fonds der Chemischen Industrie.
We declare that no conflicts of interest exist.
REFERENCES
- 1.Butler MS, Blaskovich MA, Cooper MA. 2017. Antibiotics in the clinical pipeline at the end of 2015. J Antibiot (Tokyo) 70:3–24. doi: 10.1038/ja.2016.72. [DOI] [PubMed] [Google Scholar]
- 2.Vuong C, Yeh AJ, Cheung GY, Otto M. 2016. Investigational drugs to treat methicillin-resistant Staphylococcus aureus. Expert Opin Invest Drugs 25:73–93. doi: 10.1517/13543784.2016.1109077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurlenda J, Grinholc M. 2012. Alternative therapies in Staphylococcus aureus diseases. Acta Biochim Pol 59:171–184. [PubMed] [Google Scholar]
- 4.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Shatalin K, Shatalina E, Mironov A, Nudler E. 2011. H2S: a universal defense against antibiotics in bacteria. Science 334:986–990. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 6.Brune DC. 1995. Isolation and characterization of sulfur globule proteins from Chromatium vinosum and Thiocapsa roseopersicina. Arch Microbiol 163:391–399. doi: 10.1007/BF00272127. [DOI] [PubMed] [Google Scholar]
- 7.Santos AA, Venceslau SS, Grein F, Leavitt WD, Dahl C, Johnston DT, Pereira IAC. 2015. A protein trisulfide couples dissimilatory sulfate reduction to energy conservation. Science 350:1541–1545. doi: 10.1126/science.aad3558. [DOI] [PubMed] [Google Scholar]
- 8.Wallace JL, Blackler RW, Chan MV, Da Silva GJ, Elsheikh W, Flannigan KL, Gamaniek I, Manko A, Wang L, Motta J-P, Buret AG. 2015. Anti-inflammatory and cytoprotective actions of hydrogen sulfide: translation to therapeutics. Antioxid Redox Signal 22:398–410. doi: 10.1089/ars.2014.5901. [DOI] [PubMed] [Google Scholar]
- 9.Predmore BL, Lefer DJ, Gojon G. 2012. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal 17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura H. 2012. Metabolic turnover of hydrogen sulfide. Front Physiol 3:101. doi: 10.3389/fphys.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ríos-González BB, Román-Morales EM, Pietri R, López-Garriga J. 2014. Hydrogen sulfide activation in hemeproteins: the sulfheme scenario. J Inorg Biochem 133:78–86. doi: 10.1016/j.jinorgbio.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietri R, Román-Morales E, López-Garriga J. 2011. Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid Redox Signal 15:393–404. doi: 10.1089/ars.2010.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper CE, Brown GC. 2008. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr 40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 14.Khan AA, Schuler MM, Prior MG, Yong S, Coppock RW, Florence LZ, Lillie LE. 1990. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol Appl Pharmacol 103:482–490. doi: 10.1016/0041-008X(90)90321-K. [DOI] [PubMed] [Google Scholar]
- 15.Davis BD. 1987. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev 51:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taber HW, Mueller JP, Miller PF, Arrow AS. 1987. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev 51:439–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen KY, Morris JC. 1972. Kinetics of oxidation of aqueous sulfide by O2. Environ Sci Technol 6:529–537. doi: 10.1021/es60065a008. [DOI] [Google Scholar]
- 18.Chen KY, Gupta SK. 1973. Formation of polysulfides in aqueous solution. Environ Lett 4:187–200. doi: 10.1080/00139307309436596. [DOI] [PubMed] [Google Scholar]
- 19.Lebeaux D, Chauhan A, Létoffé S, Fischer F, De Reuse H, Beloin C, Ghigo JM. 2014. pH-mediated potentiation of aminoglycosides kills bacterial persisters and eradicates in vivo biofilms. J Infect Dis 210:1357–1366. doi: 10.1093/infdis/jiu286. [DOI] [PubMed] [Google Scholar]
- 20.McCollister BD, Hoffman M, Husain M, Vázquez-Torres A. 2011. Nitric oxide protects bacteria from aminoglycosides by blocking the energy-dependent phases of drug uptake. Antimicrob Agents Chemother 55:2189–2196. doi: 10.1128/AAC.01203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller MH, Edberg SC, Mandel LJ, Behar CF, Steigbigel NH. 1980. Gentamicin uptake in wild-type and aminoglycoside-resistant small-colony mutants of Staphylococcus aureus. Antimicrob Agents Chemother 18:722–729. doi: 10.1128/AAC.18.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brittain T, Yosaatmadja Y, Henty K. 2008. The interaction of human neuroglobin with hydrogen sulphide. IUBMB Life 60:135–138. doi: 10.1002/iub.16. [DOI] [PubMed] [Google Scholar]
- 23.Pálinkás Z, Furtmüller PG, Nagy A, Jakopitsch C, Pirker KF, Magierowski M, Jasnos K, Wallace JL, Obinger C, Nagy P. 2015. Interactions of hydrogen sulfide with myeloperoxidase. Br J Pharmacol 172:1516–1532. doi: 10.1111/bph.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mates SM, Eisenberg ES, Mandel LJ, Patel L, Kaback HR, Miller MH. 1982. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc Natl Acad Sci U S A 79:6693–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mates SM, Patel L, Kaback HR, Miller MH. 1983. Membrane potential in anaerobically growing Staphylococcus aureus and its relationship to gentamicin uptake. Antimicrob Agents Chemother 23:526–530. doi: 10.1128/AAC.23.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. 2013. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br J Pharmacol 169:922–932. doi: 10.1111/bph.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R. 2002. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 28.Kimura H. 2015. Signaling molecules: hydrogen sulfide and polysulfide. Antioxid Redox Signal 22:362–376. doi: 10.1089/ars.2014.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo C. 2018. A timeline of hydrogen sulfide (H2S) research: from environmental toxin to biological mediator. Biochem Pharmacol 149:5–19. doi: 10.1016/j.bcp.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beauchamp RO, Bus JS, Popp JA, Boreiko CJ, Andjelkovich DA. 1984. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol 13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 31.Toohey JI, Cooper AJL. 2014. Thiosulfoxide (sulfane) sulfur: new chemistry and new regulatory roles in biology. Molecules 19:12789–12813. doi: 10.3390/molecules190812789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greiner R, Pálinkás Z, Bäsell K, Becher D, Antelmann H, Nagy P, Dick TP. 2013. Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal 19:1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumert N, von Eiff C, Schaaff F, Peters G, Proctor RA, Sahl HG. 2002. Physiology and antibiotic susceptibility of Staphylococcus aureus small colony variants. Microb Drug Resist 8:253–260. doi: 10.1089/10766290260469507. [DOI] [PubMed] [Google Scholar]
- 34.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 35.Rej R. 1977. Aminooxyacetate is not an adequate differential inhibitor of aspartate aminotransferase isoenzymes. Clin Chem 23:1508–1509. [PubMed] [Google Scholar]
- 36.McMaster OG, Du F, French ED, Schwarcz R. 1991. Focal injection of aminooxyacetic acid produces seizures and lesions in rat hippocampus: evidence for mediation by NMDA receptors. Exp Neurol 113:378–385. doi: 10.1016/0014-4886(91)90029-C. [DOI] [PubMed] [Google Scholar]
- 37.Soutourina O, Dubrac S, Poupel O, Msadek T, Martin-Verstraete I. 2010. The pleiotropic CymR regulator of Staphylococcus aureus plays an important role in virulence and stress response. PLoS Pathog 6:e1000894. doi: 10.1371/journal.ppat.1000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whiteman M, Haigh R, Tarr JM, Gooding KM, Shore AC, Winyard PG. 2010. Detection of hydrogen sulfide in plasma and knee-joint synovial fluid from rheumatoid arthritis patients: relation to clinical and laboratory measures of inflammation. Ann N Y Acad Sci 1203:146–150. doi: 10.1111/j.1749-6632.2010.05556.x. [DOI] [PubMed] [Google Scholar]
- 39.Richardson CJ, Magee EAM, Cummings JH. 2000. A new method for the determination of sulphide in gastrointestinal contents and whole blood by microdistillation and ion chromatography. Clin Chim Acta 293:115–125. doi: 10.1016/S0009-8981(99)00245-4. [DOI] [PubMed] [Google Scholar]
- 40.Li L. 2005. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 41.Cowley ES, Kopf SH, Lariviere A, Ziebis W, Newman DK. 2015. Pediatric cystic fibrosis sputum can be chemically dynamic, anoxic, and extremely reduced due to hydrogen sulfide formation. mBio 6:e00767-15. doi: 10.1128/mBio.00767-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moellering RC, Weinberg AN. 1971. Studies on antibiotic synergism against enterococci. II. Effect of various antibiotics on the uptake of 14C-labeled streptomycin by enterococci. J Clin Invest 50:2580–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathai JC, Missner A, Kugler P, Saparov SM, Zeidel ML, Lee JK, Pohl P. 2009. No facilitator required for membrane transport of hydrogen sulfide. Proc Natl Acad Sci U S A 106:16633–16638. doi: 10.1073/pnas.0902952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagy P, Pálinkás Z, Nagy A, Budai B, Tóth I, Vasas A. 2014. Chemical aspects of hydrogen sulfide measurements in physiological samples. Biochim Biophys Acta 1840:876–891. doi: 10.1016/j.bbagen.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 45.Shen J, Keithly ME, Armstrong RN, Higgins KA, Edmonds KA, Giedroc DP. 2015. Staphylococcus aureus CstB is a novel multidomain persulfide dioxygenase-sulfurtransferase involved in hydrogen sulfide detoxification. Biochemistry 54:4542–4554. doi: 10.1021/acs.biochem.5b00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kagan BL, Selsted ME, Ganz T, Lehrer RI. 1990. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A 87:210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahl HG, Kordel M, Benz R. 1987. Voltage-dependent depolarization of bacterial membranes and artificial lipid bilayers by the peptide antibiotic nisin. Arch Microbiol 149:120–124. doi: 10.1007/BF00425076. [DOI] [PubMed] [Google Scholar]
- 48.Lehrer RI, Barton A, Daher KA, Harwig SSL, Ganz T, Selsted ME. 1989. Interaction of human defensins with Escherichia coli: mechanism of bactericidal activity. J Clin Invest 84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbert S, Ziebandt AK, Ohlsen K, Schäfer T, Hecker M, Albrecht D, Novick R, Götz F. 2010. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect Immun 78:2877–2889. doi: 10.1128/IAI.00088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kreiswirth BN, Löfdahl S, Betley MJ, O'Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 51.Duthie ES, Lorenz LL. 1952. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol 6:95–107. [DOI] [PubMed] [Google Scholar]
- 52.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 53.Bachmann BJ. 1972. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev 36:525–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 56.Hsieh MH, Yu CM, Yu VL, Chow JW. 1993. Synergy assessed by checkerboard: a critical analysis. Diagn Microbiol Infect Dis 16:343–349. doi: 10.1016/0732-8893(93)90087-N. [DOI] [PubMed] [Google Scholar]
- 57.Trüper HG, Schlegel HG. 1964. Sulphur metabolism in Thiorhodaceae. I. Quantitative measurements on growing cells of Chromatium okenii. Antonie Van Leeuwenhoek 30:225–238. [DOI] [PubMed] [Google Scholar]
- 58.Henry-Stanley MJ, Hess DJ, Wells CL. 2014. Aminoglycoside inhibition of Staphylococcus aureus biofilm formation is nutrient dependent. J Med Microbiol 63:861–869. doi: 10.1099/jmm.0.068130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardt P, Engels I, Rausch M, Gajdiss M, Ulm H, Sass P, Ohlsen K, Sahl HG, Bierbaum G, Schneider T, Grein F. 2017. The cell wall precursor lipid II acts as a molecular signal for the Ser/Thr kinase PknB of Staphylococcus aureus. Int J Med Microbiol 307:1–10. doi: 10.1016/j.ijmm.2016.12.001. [DOI] [PubMed] [Google Scholar]