Abstract
Background: Differentiated thyroid cancer typically has an indolent clinical course but can cause significant morbidity by local progression. Oncologic surgical resection can be technically difficult due to the proximity to critical normal structures in the neck. Our objective was to review the safety, feasibility, and outcomes of definitive-intent intensity-modulated radiation therapy (IMRT) and to analyze whether patients receiving concurrent chemotherapy (CC-IMRT) had higher rates of disease control and survival over IMRT alone in patients with unresectable or gross residual disease (GRD).
Methods: Eighty-eight patients with GRD or unresectable nonanaplastic, nonmedullary thyroid cancer treated with definitive-intent IMRT between 2000 and 2015 were identified. Local progression-free survival (LPFS), distant metastasis-free survival (DMFS), and overall survival (OS) were evaluated using the Kaplan–Meier method. Univariate and multivariate analyses using cox regression were used to determine the impact of clinical conditions and treatment on LPFS, DMFS, and OS.
Results: Of the 88 patients identified, 45 (51.1%) were treated CC-IMRT and 43 (48.9%) were treated with IMRT alone. All patients treated with CC-IMRT received weekly doxorubicin (10 mg/m2). The median follow-up among surviving patients was 40.3 months and 29.2 months for all patients. The LPFS at 4 years was 77.3%. Patients receiving CC-IMRT had higher LPFS compared with IMRT alone (CC-IMRT 85.8% vs. IMRT 68.8%, p = 0.036). The 4-year OS was 56.3% for all patients. Patients treated with CC-IMRT had higher OS compared to patients treated with IMRT alone (CC-IMRT 68.0% vs. IMRT 47.0%, p = 0.043). On multivariate analysis, receipt of concurrent chemotherapy was associated with a lower risk of death (HR 0.395, p = 0.019) and lower risk of local failure (HR 0.306, p = 0.042). Grade 3+ acute toxicities occurred in 23.9% of patients, the most frequent being dermatitis (18.2%) and mucositis (9.1%). 17.1% of patients required a percutaneous endoscopic gastrostomy (PEG) tube during or shortly after completion of RT, with 10.1% of patients needing a PEG more than 12 months after therapy. The rates of acute and late toxicities were not statistically higher in the CC-IMRT cohort, although trends towards higher toxicity in the CC-IMRT were present for dermatitis and PEG requirement.
Conclusions: IMRT is a safe and effective means to achieve local control in patients with unresectable or incompletely resected nonanaplastic, nonmedullary thyroid cancer. Concurrent doxorubicin was not associated with worse toxicity and should be considered in these patients given its potential to improve local control and overall survival.
Keywords: : IMRT, concurrent chemoradiation therapy, thyroid cancer
Introduction
Thyroid cancer is increasing in incidence and is now the fifth leading cause of cancer in women the United States. A total of 56,870 cases are expected to be diagnosed in 2017 (1). The majority of thyroid cancers are differentiated with papillary and follicular histologies accounting for over 90% of new diagnoses (2). The majority of differentiated thyroid cancers are indolent and survival is excellent due to effective local therapy for many early stage lesions and increasing detection of lesions at a smaller size (3). Despite excellent survival, approximately one third of patients recur, with two thirds recurring locally (4). Surgery is the mainstay of therapy for differentiated thyroid cancer; however, locally advanced thyroid cancers are frequently incompletely excised due to the critical anatomic structures that may be involved with tumor in oncologic resection in the neck. Moreover, the anatomic site of primary thyroid carcinoma makes locoregional recurrence a significant contributor to morbidity and mortality, making locoregional disease control an important measure in these patients.
Due to the potential morbidity of repeat resection in patients with incompletely resected disease, adjuvant local therapy with external beam radiation therapy (EBRT) is sometimes considered. The National Comprehensive Cancer Network recommends consideration of EBRT in patients with gross residual disease (GRD) or unresectable disease not amenable to radioactive iodine therapy (RAI), and in patients with locoregional recurrence after surgery not amenable to additional resection (5). The American Thyroid Association recommends consideration of EBRT in patients requiring frequent surgeries for recurrent local disease and in patients with locally advanced disease making complete resection a threat to preservation of function of the upper aerodigestive tract (6).
The optimal adjuvant regimen for unresectable or incompletely resected differentiated thyroid cancer has not been established prospectively. Retrospective series have demonstrated improved locoregional control with EBRT in patients treated at high risk for local recurrence such as microscopically positive margins, GRD, or in unresectable disease (7–9). The role of concurrent chemoradiation with EBRT (CC-EBRT) in improving locoregional control has not been well studied. In a prior retrospective analysis of patients treated at Memorial Sloan Kettering Cancer Center, CC-EBRT was well tolerated and associated with improved local progression free survival (LPFS) in patients with poorly differentiated histologies (8). Particularly, there is a paucity of literature on the use of modern radiotherapy techniques, such as intensity-modulated radiation therapy (IMRT), in treating thyroid cancer. We report on our experience treating patients with definitive-intent radiation therapy uniformly treated using the same radiation therapy technique, IMRT, with or without concurrent radiosensitizing doxorubicin.
Methods and Materials
The institutional review and privacy boards approved this retrospective study with a waiver of informed consent. Patient confidentiality was maintained as required by the Health Insurance Portability and Accountability Act. Between 2000 and September 2015, all patients who completed definitive-intent IMRT to the primary site for pathologically confirmed GRD or unresectable non-anaplastic non-medullary thyroid cancer at a large tertiary cancer center were studied. Eighty-eight patients were identified. The T (tumor stage) and N (nodal stage) categories were determined from the disease extent at the time of initial presentation according to the Joint Committee on Cancer guidelines (seventh edition). Unresectable disease includes patients with upfront unresectable disease and those with unresectable recurrent disease. The reported M (distant metastasis) category was recorded at the time of IMRT start. Gross tumor volume (GTV) was measured using the volume of tumor as contoured during radiation treatment planning. Karnofsky Performance Status (KPS) (10) and Charlson Comorbidity Index (CCI) (11) were determined at the initial consultation for radiation therapy. KPS was available for all patients. CCI was generated using the patient's disease status and past medical history as recorded in the consultation note.
Pathologic findings
Nonanaplastic thyroid carcinoma was histologically confirmed by internal pathological review. Poorly differentiated thyroid carcinomas were defined on the basis of ≥5 mitoses per 10 high-power microscopic fields and/or the presence of tumor necrosis. As previously reported, this definition identifies patients with an intermediate prognosis between those with well differentiated and anaplastic carcinoma (12–14). High-risk histology was defined as any component of poorly differentiated histology, tall-cell variant of papillary carcinoma, or Hürthle cell histology.
Radiotherapy technique
IMRT was used in all patients. Our radiation technique has been previously described (8). Briefly, we treated various clinical target volumes as follows: (1) low-risk areas including elective lymph node volumes to 54 Gy (2) high-risk areas including the operative or tumor bed, operative thyroid gland volume, tracheoesophageal grooves and central nodal compartment to 60 Gy, (3) close or microscopically positive margins to 66 Gy, and (4) areas of gross disease to 70 Gy. Inclusion of elective lateral neck nodal volumes was at the discretion of the treating physician. The gross tumor volume was defined as the gross extent of tumor visible by imaging studies and clinical examination. The clinical target volume was expanded to a planning target volume typically 3 mm to account for intrafraction patient motion and interfraction setup error. All patients were immobilized in the treatment position using a three or five point Aquaplast mask.
Organs at risk (OAR) such as parotid glands, larynx, lungs, esophagus, brachial plexus, and spinal cord were contoured. A dose volume histogram was constructed to evaluate target coverage and the doses to surrounding OARs. OAR dose constraints included <26 Gy mean parotid dose but this is highly variable depending on the location of the target volumes, <45 Gy mean larynx dose, <20 Gy mean lung dose, <65 Gy maximum brachial plexus point dose, and <45 Gy maximal spinal cord point dose. In addition, the volume of lung receiving 20 Gy or more was limited to 37%. Esophagus dose is as low as possible without compromising target. In some instances, the target volume will be compromised in its coverage with attempt to protect the esophagus. This is done at the discretion of the treating physician.
Chemotherapy
The chemotherapeutic agent in all patients receiving CC-IMRT was doxorubicin 10 mg/m2 delivered weekly starting during the first week of CC-IMRT, typically after one to three fractions of IMRT. Low dose doxorubicin is our institutional standard for radiosensitization in nonanaplastic thyroid cancer patients being treated with RT, however the decision to add doxorubicin is made by the treating physicians.
Toxicity and response assessment
Patients were assessed jointly by radiation oncology, medical oncology, endocrinology, and/or head and neck surgery weekly during radiation and at approximate intervals of 4, 8, and 12 weeks after completion of treatment, then every 3 months for 2 years followed by every 6–12 months thereafter. In the early 2000s, a standardized toxicity form was implemented to help improve the accuracy and reproducibility in recording treatment toxicities for dermatitis, nausea, vomiting, mucositis, xerostomia, dysphagia, hoarseness, fatigue, and requirement of a feeding tube. The incidence of the worst-grade toxicity sustained by a patient up to 90 days after the start of radiation therapy was recorded as an acute toxicity event based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (15). All late toxicity (>90 days post completion of treatment) was scored with the Radiation Therapy Oncology Group late radiation morbidity scoring system (16).
Statistical analysis
Locoregional progression, distant metastasis, and death were recorded from the start of radiotherapy. Locoregional progression was defined as local (i.e., thyroid bed) or nodal (i.e., central compartment or cervical and superior mediastinal lymph nodes) disease progression (i.e., new or enlarging disease in the thyroid bed, central compartment or lymph nodes on ultrasound, diagnostic RAI, positron-emission tomography/computed tomography, computed tomography, and/or magnetic resonance imaging). Biopsy confirmation was not required. Stable disease with no evidence of progression was not classified as progression. If systemic therapy or adjuvant RAI was initiated after IMRT for clinical suspicion of progressive disease in the neck, these patients were classified as having locoregional progression. Patients without evidence of metastatic disease at the time of IMRT were followed for the development of distant metastases; patients with M1 disease prior to IMRT were excluded from this subset analysis.
The Kaplan–Meier method was used to analyze LPFS, distant metastasis-free survival (DMFS) and overall survival (OS). The log-rank test was used to compare survival curves when indicated. Cox hazard ratios were performed to conduct univariate analysis (UVA) and multivariate analyses (MVA). Any category with a P value less than 0.200 on UVA was included in the MVA. Comparisons between cohorts were performed using the Chi-square test, medians were compared using Mann-Whitney U test. Comparisons between toxicities between CC-IMRT and IMRT were performed using Fisher's Exact Test. A probability value of <0.05 was considered statistically significant. Analyses were performed in SPSS statistics version 24 (IBM, Armonk, NY) and/or Prism version 7 (GraphPad Software Inc, La Jolla, CA).
Results
Patient and tumor characteristics
The median age was 64.7 years (range 30.0–87.7 years) with a median overall follow-up of 40.3 months (range 1.8–133.0 months) among surviving patients and 29.2 (range 1.8–133.0 months) among all patients (Table 1). The dominant histology was papillary, occurring in 57 (64.8%) patients. Twenty-one (23.9%) patients had poorly differentiated histology. The remaining 10 (11.4%) patients had Hürthle cell carcinoma. Overall, 41 (46.6%) patients had high-risk pathology. The median number of surgeries prior to radiation therapy was 2 (range 0–5). Three patients had no surgery. Twenty patients had recurrent disease of which resection was not attempted. Seventy-seven patients had received radioactive iodine, 60 prior to IMRT, 3 after IMRT, and 14 both before and after IMRT. Amongst those receiving radioactive iodine, the median dose for both pre- and post-IMRT was 1 with a range of 1 to 5 doses pre-IMRT and 1 to 3 doses post-IMRT. The median dose prior to IMRT was 252 mCi (range 61 to 1555 mCi) and 194 mCi (range 51 to 599 mCi) post IMRT. Forty percent of patients were treated with a tyrosine kinase inhibitor (TKI) most frequently sorafenib and/or pazopanib (65.7%), although no patient received TKI concurrently with IMRT.
Table 1.
Patient and Treatment Characteristics
| Overall (N = 88) | IMRT (N = 43) | CC-IMRT (N = 45) | p-Value | |
|---|---|---|---|---|
| Female | 44 | 23 | 21 | 0.466 |
| Male | 44 | 20 | 24 | |
| Age | ||||
| ≤45 years | 8 | 6 | 2 | 0.121 |
| >45 years | 80 | 37 | 43 | |
| Median KPS (range) | 90% (70–100) | 90% (70–100) | 90% (70–100) | 0.686 |
| KPS ≥80 | 26 | 11 (25.6%) | 15 (33.3%) | 0.107 |
| Median CCI score (range) | 6.5 (2–7) | 7 (2–7) | 5 (2–7) | 0.295 |
| CCI ≥6 | 46 | 26 (60.5%) | 20 (44.4%) | 0.133 |
| Number of operations (range) | 2 (0–5) | 2 (1–5) | 2 (0–5) | 0.417 |
| RT >3 months post op or unresected. | 48 | 23 (53.5%) | 25 (55.6%) | 0.456 |
| Median follow-up in months | 29.2 (1.8–133.0) | 29.6 (2.2–133.0) | 26.8 (1.8–82.4) | 0.449 |
| Median tumor volume in cc (range) | 30.4 (1.2–867.8) | 26.9 (1.2–315.0) | 39.3 (2.27–867.8) | 0.446 |
| Tumor stage | ||||
| T1 | 4 | 1 | 3 | 0.442 |
| T2 | 7 | 2 | 5 | |
| T3 | 8 | 5 | 3 | |
| T4 | 68 | 34 | 34 | |
| Unknown | 1 | 1 | 0 | |
| Nodal Stage | ||||
| N0 | 19 | 8 | 11 | 0.506 |
| N1 | 39 | 35 | 34 | |
| DM at IMRT | ||||
| No | 47 | 23 | 24 | 0.988 |
| Yes | 41 | 20 | 21 |
CCI, Charlson Comorbidity Index; CC-IMRT, concurrent chemoradiation with intensity-modulated radiation therapy; DM, distant metastasis; KPS, Karnofsky Performance Status; PEG, percutaneous endoscopic gastrostomy; RT, radiation therapy.
Treatment characteristics
The median IMRT dose was 70.0 Gy (range 69.96–72.08 Gy) in a median 33 fractions (range 33–35 fractions). Concurrent chemotherapy was administered in 45 (51.1%) patients. There was no difference in patients with N1 disease who received CC-IMRT (75.6% CC-IMRT vs. 81.4% IMRT, p = 0.506), or in distant metastases at the time of IMRT between these two groups (46.7% CC-IMRT vs. 46.5% IMRT, p = 0.988). The median number of surgeries prior to IMRT was 2 and did not differ between IMRT and CC-IMRT (p = 0.417). KPS and CCI did not differ between the IMRT and CC-IMRT groups.
Toxicity
Acute and late toxicities are summarized in Table 2. Acute grade 3 dermatitis (18.2%) and mucositis (9.1%) were most frequently observed. There was a trend towards worse dermatitis with CC-IMRT (26.7% CC-IMRT vs. 9.3% IMRT, p = 0.052). No other differences in acute toxicity were observed. Overall, 15 patients (17.1%) required a percutaneous endoscopic gastrostomy (PEG) tube placed after the start of radiation therapy. One PEG was placed due to a combination of treatment toxicity and local progression. The remaining 14 were placed due to toxicity alone. Ten CC-IMRT patients required a PEG tube during or within 60 days of treatment compared to 3 in the IMRT group (p = 0.070). An additional 2 patients in the IMRT group had a PEG placed >60 days after completion of therapy. The rate of late PEG dependence (PEG in place for >12 months or not removed) amongst patients with PEG tubes placed during or after IMRT or CC-IMRT was 10.1%. The overall tracheostomy rate after initiation of RT was 9.1%, and was not statistically different between IMRT and CC-IMRT. Sixteen late grade 2+ toxicities were observed including xerostomia in 7 (8.0%) patients and dysphagia in 8 (9.1%). The rates of late toxicities were not statistically higher in the CC-IMRT cohort.
Table 2.
Acute and Late Toxicities of Treatment
| Overall | IMRT | CC-IMRT | p-Value | |
|---|---|---|---|---|
| Acute (grade 3+), n (%) | (N = 88) | (N = 43) | (N = 45) | |
| Dermatitis | 16 (18.2%) | 4 (9.3%) | 12 (26.7%) | 0.052 |
| Nausea | 1 (1.1%) | 1 (2.3%) | 0 (0%) | 0.489 |
| Vomiting | 2 (2.3%) | 2 (4.7%) | 0 (0%) | 0.236 |
| Mucositis | 8 (9.1%) | 6 (14.0%) | 2 (4.4%) | 0.152 |
| Xerostomia | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| Dysphagia | 6 (6.8%) | 3 (7.0%) | 3 (6.7%) | 1.000 |
| Hoarseness | 3 (3.4%) | 1 (2.3%) | 2 (4.4%) | 1.000 |
| Fatigue | 3 (3.4%) | 1 (2.3%) | 2 (4.4%) | 1.000 |
| Late (grade 2+), n (%) | (N = 78) | (N = 39) | (N = 39) | |
| Dermatitis | 1 (1.3%) | 1 (2.6%) | 0 (0%) | 1.000 |
| Xerostomia | 7 (9.0%) | 6 (15.4%) | 1 (2.6%) | 0.108 |
| Dysphagia | 8 (10.3%) | 7 (17.9%) | 1 (2.6%) | 0.056 |
| Hoarseness | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| (N = 88) | (N = 43) | (N = 45) | ||
| PEG During or within 60 days of RT, n (%) | 13 (14.8%) | 3 (7.0%) | 10 (22.2%) | 0.070 |
| PEG >60 days post RT, n (%) | 2 (2.3%) | 2 (4.7%) | 0 (0%) | 0.236 |
| Trach during or within 60 days of RT, n (%) | 3 (3.4%) | 1 (2.3%) | 2 (4.4%) | 1.000 |
| Trach >60 days post RT, n (%) | 5 (5.7%) | 2 (4.7%) | 3 (6.7%) | 1.000 |
Locoregional progression-free survival
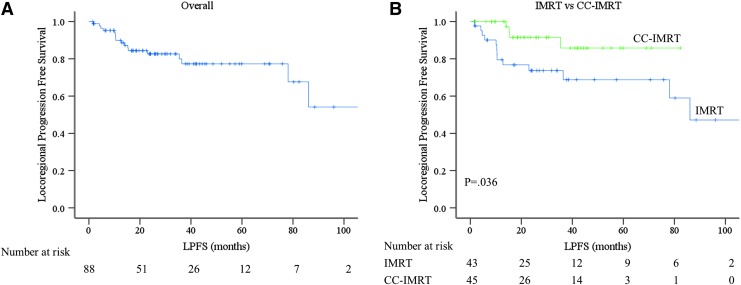
The 4-year actuarial LPFS was 77.3% (Fig. 1A). Seventeen (19.3%) patients failed locally at a median of 12.8 months (range 1.6–86.1 months). Thirteen (30.2%) patients failed locally in the IMRT group compared with 4 (8.9%) in the CC-IMRT group. The CC-IMRT group had a significantly higher 4-year LPFS (85.8% CC-IMRT vs. 68.8% IMRT, p = 0.036) (Fig. 1B). There was no difference in LPFS according to tumor stage, T4 vs. T1–3, p = 0.908 (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/thy), nodal status, N1 vs. N0, p = 0.954 (Supplementary Fig. S1B), or histology, high risk vs. low risk, p = 0.101 (Supplementary Fig. S1C), or RT <3 months after surgery compared with >3 months after surgery, p = 0.860 (Supplementary Fig. S1D).
FIG. 1.
Kaplan-Meier estimates of locoregional progression free survival. (A) Kaplan–Meier estimate of locoregional progression free survival. (B) Kaplan-Meier estimate of locoregional progression free survival based on IMRT versus CC-IMRT. Blue line represents IMRT and green line CC-IMRT (p = 0.036). CC-IMRT, concurrent chemoradiation with intensity-modulated radiation therapy; IMRT, intensity-modulated radiation therapy. Color images are available online at www.liebertpub.com/thy
The median survival following local failure was 15.3 months, with 6 patients alive at the time of analysis. Among the 17 local failures, 11 patients failed in the thyroid bed or tracheoesophageal groove, 4 of whom concomitantly failed in the cervical or mediastinal lymph nodes. The remaining 6 patients failed in the cervical, paratracheal, or supraclavicular lymph nodes. Twelve failures (70.6%) occurred within the high dose region (≥60 Gy); 3 (17.6%) failed within the low dose elective neck (54 Gy). Only one failure occurred completely out of field in a level II lymph node. Five patients underwent additional local therapy after radiation. One patient underwent definitive re-irradiation to 70 Gy, which was well tolerated with grade 1 dysgeusia, dysphagia, and xerostomia as the only documented late effects. One patient underwent cryoablation which was well tolerated but ineffective in controlling local progression. Three patients underwent salvage surgery, including 1 patient with a neck dissection, 1 patient who underwent two further resections and tracheostomy placement, and 1 patient who underwent a salvage laryngectomy and is alive 3 years after laryngectomy without evidence of local disease.
Distant metastasis-free survival
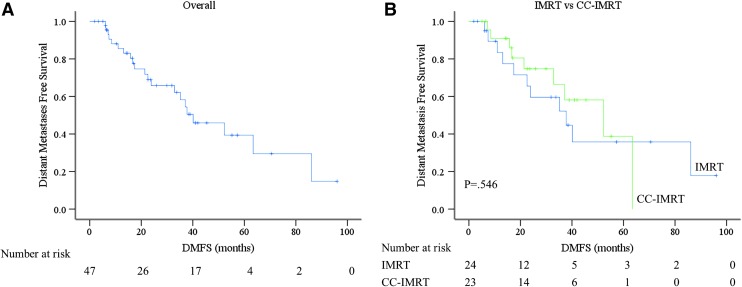
Of the 47 patients without metastases at the start of radiation treatment, 21 subsequently developed metastases at a median 21.4 months after starting RT (Fig. 2A). There were no differences in DMFS between the other examined groups, including 58.1% CC-IMRT versus 34.1% IMRT, p = 0.546 (Fig. 2B), T4 versus T1-3, p = 0.487 (Supplementary Fig. S2A), N1 versus N0, p = 0.608 (Supplementary Fig. S2B), low risk versus high risk histology, p = 0.238) (Supplementary Fig. S2C), or RT <3 months after surgery compared with >3 months after surgery, p = 0.246 (Supplementary Fig. S2D). The primary sites of metastatic failure were the lung (61.5%), nonregional lymph nodes (15.4%), bone and brain (both 11.5%).
FIG. 2.
Kaplan-Meier estimates of distant metastasis free survival. (A) Kaplan-Meier estimate of distant metastasis free survival. (B) Kaplan–Meier estimate of distant metastasis free survival based on IMRT versus CC-IMRT. Blue line represents IMRT and green line CC-IMRT (p = 0.546). Color images are available online at www.liebertpub.com/thy
Overall survival
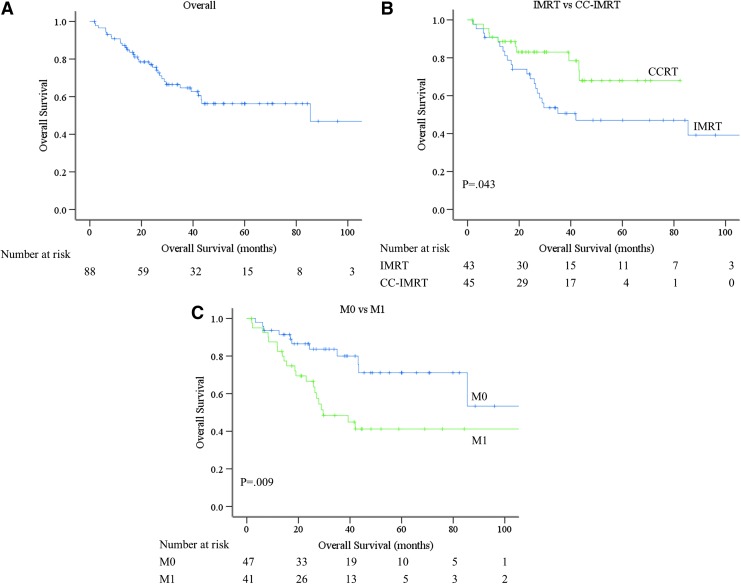
The median 4-year OS was 56.3% for all patients (Fig. 3A). Thirty-eight patients died at a median 21.0 months (range 1.9–85.5 months). The CC-IMRT cohort had longer 4-year OS compared with the IMRT cohort (CC-IMRT 68.0% vs. IMRT 47.0%, p = 0.043) (Fig. 3B). The presence of metastases at the time of RT was associated with decreased OS (41.2% M1 vs. 71.1% M0, p = 0.009) (Fig. 3C). There was no difference in OS according to T4 vs. T1-3, p = 0.653 (Supplementary Fig. S3A), N1 vs. N0, p = 0.913 (Supplementary Fig. S3B), low risk versus high risk histology, p = 0.955 (Supplementary Fig. S3C), or RT <3 months after surgery compared with >3 months after surgery, p = 0.889 (Supplementary Fig. S3D).
FIG. 3.
Kaplan-Meier estimates of overall survival. (A) Kaplan–Meier estimate of overall survival. (B) Kaplan–Meier estimate of distant overall survival based on IMRT versus CC-IMRT. Blue line represents IMRT and green line CC-IMRT (p = 0.043). (C) Kaplan–Meier estimate of overall survival based on metastases (M) status. Blue line represents M0 status and green line M1 status (p = 0.009). Color images are available online at www.liebertpub.com/thy
Gross tumor volume
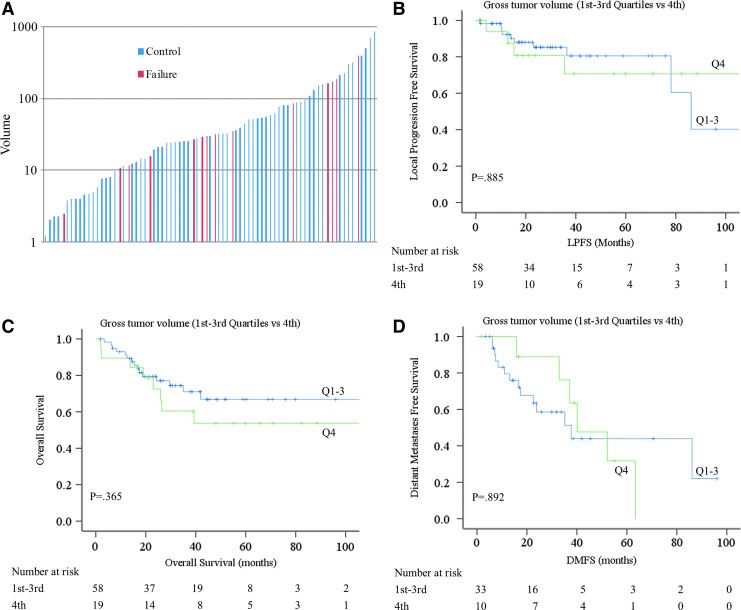
The median GTV was 30.4 cc with a range of 1.2–867.8 cc. There was no difference in the median tumor volume between IMRT versus CC-IMRT patients (Table 1). Patients with local progression were present throughout the spectrum of GTV (Fig. 4A). When comparing the first through third quartile of GTV versus the fourth quartile, no differences in LPFS (80.5% 1st–3rd vs. 70.7% 4th, p = 0.885) (Fig. 4B), OS (66.8% 1st–3rd vs. 53.7% 4th), p = 0.365 (Fig. 4C), and DMFS (43.9% 1st–3rd vs. 47.6% 4th, p = 0.892) (Fig. 4D) were seen.
FIG. 4.
Gross tumor volume and local failures. (A) Column plot of the GTV (y axis) with each column representing a single patient. Blue bars depict patients without local progression, red bars depict the patients with local progression. (B) Kaplan–Meier estimate of locoregional progression free survival based on GTV. Blue line represents the 1st–3rd quartiles and green line represents 4th quartile (p = 0.885). (C) Kaplan—Meier estimate of overall survival based on GTV. Blue line, 1st–3rd quartile; green line, 4th quartile (p = 0.365). (D) Kaplan–Meier estimate of distant metastasis free survival on GTV. Blue line, 1st–3rd quartile; green line, 4th quartile (p = 0.892). GTV, gross tumor volume. Color images are available online at www.liebertpub.com/thy
Univariate and multivariate analyses
CC-IMRT was associated with a lower rate of local progression on UVA (hazard ratio [HR] 0.314; p = 0.047) and on MVA (HR 0.306; p = 0.042) (Table 3). Furthermore, high risk pathology was associated with a lower rate of local progression on UVA, but not on MVA. Age ≤45 years and CCI score <6 were associated with distant metastasis on UVA, but not on MVA. CC-IMRT was associated with a lower risk of death on UVA (HR 0.468, p = 0.048) and MVA (HR 0.395, p = 0.019). M1 status at the time of RT, CCI score ≥6, KPS >80 and age ≤45 years were associated with risk of death on UVA but not MVA (Table 3).
Table 3.
Univariate and Multivariate Analysis, Cox Model
| Univariate analysis | Hazard ratio | CI | p-Value |
|---|---|---|---|
| Local progression-free survival | |||
| Age | |||
| ≤45 years | 1 | ||
| >45 years | 0.951 | 0.216–4.192 | 0.948 |
| KPS | |||
| ≤80 | 1 | ||
| >80 | 0.383 | 0.088–1.680 | 0.203 |
| CCI score | |||
| <6 | 1 | ||
| ≥6 | 1.271 | 0.489–3.303 | 0.622 |
| T-stage | |||
| No | 1 | ||
| Yes | 0.936 | 0.302–2.901 | 0.908 |
| N-status | |||
| N0 | 1 | ||
| N1 | 1.034 | 0.336–3.185 | 0.954 |
| M-status at RT | |||
| M0 | 1 | ||
| M1 | 0.957 | 0.64–2.517 | 0.929 |
| Gross tumor volume | |||
| Quartile 1–3 | 1 | ||
| Quartile 4 | 1.262 | 0.671–2.371 | 0.470 |
| Pathology | |||
| Low risk | 1 | ||
| High risk | 0.426 | 0.149–1.217 | 0.111 |
| RT after surgery | |||
| ≤3 months | 1 | ||
| >3 months | 1.092 | 0.413–2.888 | 0.860 |
| Concurrent chemo with IMRT | |||
| No | 1 | ||
| Yes | 0.314 | 0.100–0.984 | 0.047 |
| Multivariate analysis | |||
| Pathology | |||
| Low risk | 1 | ||
| High risk | 0.414 | 0.145–1.181 | 0.099 |
| Concurrent chemo with IMRT | |||
| No | 1 | ||
| Yes | 0.306 | 0.097–0.959 | 0.042 |
| Distant metastasis-free survival | |||
| Univariate analysis | |||
| Age | |||
| ≤45 years | 1 | ||
| >45 years | 3.361 | 0.765–14.759 | 0.108 |
| KPS | |||
| ≤80 | 1 | ||
| >80 | 1.792 | 0.514–6.251 | 0.360 |
| CCI score | |||
| <6 | 1 | ||
| ≥6 | 4.137 | 1.144–14.957 | 0.030 |
| T-Stage | |||
| No | 1 | ||
| Yes | 0.672 | 0.217–2.079 | 0.490 |
| N-Status | |||
| N0 | 1 | ||
| N1 | 1.336 | 0.440–4.056 | 0.609 |
| Gross tumor volume | |||
| Quartile 1–3 | 1 | ||
| Quartile 4 | 0.934 | 0.349–2.498 | 0.892 |
| Pathology | |||
| Low risk | 1 | ||
| High risk | 1.698 | 0.697–4.134 | 0.244 |
| RT after surgery | |||
| ≤3 months | 1 | ||
| >3 months | 0.577 | 0.226–1.476 | 0.251 |
| Concurrent chemo with IMRT | |||
| No | 1 | ||
| Yes | 0.761 | 0.313–1.852 | 0.547 |
| Multivariate analysis | |||
| Age | |||
| ≤45 years | 1 | ||
| >45 years | 3.078 | 0.692–13.701 | 0.140 |
| CCI score | |||
| <6 | 1 | ||
| ≥6 | 3.545 | 0.976–12.875 | 0.055 |
| Overall survival | |||
| Univariate analysis | |||
| Age | |||
| ≤45 years | 1 | ||
| >45 years | 4.384 | 0.595–32.318 | 0.147 |
| KPS | |||
| ≤80 | 1 | ||
| >80 | 2.130 | 1.047–4.334 | 0.037 |
| CCI score | |||
| <6 | 1 | ||
| ≥6 | 2.426 | 1.147–5.133 | 0.020 |
| T-Stage | |||
| No | 1 | ||
| Yes | 1.226 | 0.503–2.989 | 0.654 |
| N-Status | |||
| N0 | 1 | ||
| N1 | 1.048 | 0.453–2.427 | 0.913 |
| M-Status at RT | |||
| M0 | 1 | ||
| M1 | 2.568 | 1.236–5.335 | 0.011 |
| Gross tumor volume | |||
| Quartile 1–3 | 1 | ||
| Quartile 4 | 1.484 | 0.628–3.506 | 0.368 |
| Pathology | |||
| Low risk | 1 | ||
| High risk | 0.980 | 0.488–1.969 | 0.955 |
| RT after surgery | |||
| ≤3 months | 1 | ||
| >3 months | 0.951 | 0.471–1.920 | 0.889 |
| Concurrent chemo with IMRT | |||
| No | 1 | ||
| Yes | 0.468 | 0.220–0.994 | 0.048 |
| Multivariate analysis | |||
| Age | |||
| ≤45 years | 1 | ||
| >45 years | 4.220 | 0.538–33.111 | 0.171 |
| KPS | |||
| ≤80 | 1 | ||
| >80 | 1.581 | 0.749–3.339 | 0.230 |
| CCI score | |||
| <6 | 1 | ||
| ≥6 | 0.610 | 0.084–4.415 | 0.625 |
| M-status at RT | |||
| M0 | 1 | ||
| M1 | 2.864 | 0.415–19.754 | 0.286 |
| Concurrent chemo with IMRT | |||
| No | 1 | ||
| Yes | 0.395 | 0.182–0.859 | 0.019 |
CI, 95% confidence interval.
Mutational profiling
Available BRAF and TERT mutational analysis was evaluated for our patients (Table 4). BRAF was tested in in 44 patients, of whom 23 were found to have a mutation. TERT mutation data were available for 19 patients, of whom 15 were found to have a mutation. No associations between BRAF or TERT mutations and LPFS, DMFS, or OS were observed.
Table 4.
Mutational Analysis by Event
| Local failure | Post RT distant metastasis | Death | |
|---|---|---|---|
| BRAF | |||
| Negative (n = 21) | 4 | 8 | 6 |
| Positive (n = 23) | 4 | 8 | 2 |
| TERT | |||
| Negative (n = 4) | 1 | 1 | 0 |
| Positive (n = 15) | 3 | 6 | 3 |
Discussion
The standard of care for differentiated thyroid cancers is surgical resection with or without adjuvant therapies such as thyrotropin suppression or RAI in high-risk patients or those with residual or metastatic disease. These therapies help provide a good expectation of survival due to the frequent indolent nature of differentiated thyroid cancer even in the metastatic setting. The present study seeks to help define the role of IMRT and CC-IMRT in patients with unresectable disease or in those with especially high likelihood of local progression after grossly incomplete resection.
A number of retrospective reports have demonstrated the efficacy of radiation therapy in the adjuvant setting for differentiated thyroid cancer. We previously evaluated our experience treating patients with GRD or unresectable disease and reported a 77.3% overall LPFS at 3 years (8). Chow et al. found a significantly improved rate of locoregional control (63.4% vs. 24%; p < 0.0001) and cancer specific survival (74.1% vs. 49.7%) with the use of EBRT (with or without RAI) in patients with papillary thyroid cancer (17). Patients were treated with a median dose of 60 Gy using conventional EBRT. A report from MD Anderson Cancer Center (MDACC) demonstrated the efficacy of 3D conformal EBRT (3D-CRT) in differentiated thyroid cancer (7). These authors reported a 79.0% rate of LPFS at 4 years, with no difference in local control between IMRT, used in 44% of patients, and 3D-CRT (56% of patients). They did, however, observe a reduction in the rate of late toxicity with IMRT (2% vs. 12% with 3D-CRT). Median radiation dose was 60 Gy and ranged from 38 to 72 Gy. Additionally, the MDACC study excluded patients with unresectable disease, and only 11% of patients had GRD, and thus represents a different population than the present study in which we report on 30 (33.7%) unresectable tumors and 59 (66.3%) with GRD after resection.
To our knowledge, this report is the most uniform series in the literature in terms of radiation technique and dose. All patients in our study were treated with IMRT to definitive dosing consistent with our institutional standard for definitive-intent treatment of GRD or unresectable disease. Gross disease received a median of 70 Gy, with a range of 69.96 to 72.08 Gy. This uniformity reduces influences on the data by patient inhomogeneity such as those receiving more palliative courses and allows more confident interpretation of disease control and toxicities.
Over half of the patients in this study were treated with concurrent weekly doxorubicin as a radiosensitizer. We previously reported that patients receiving concurrent chemotherapy with RT had a small trend towards improved LPFS (90.0% vs. 73.0% at 3 years, p = 0.347) (8). With maturation of our institutional cohort, we now observe an improvement in LPFS and OS in patients treated with CC-IMRT compared with those treated with IMRT alone. Importantly, the higher rate of OS in the CC-IMRT cohort was strengthened in the MVA including patient age, M-status, patient KPS, and CCI. We were interested to determine whether local control was influenced by the volume of tumor treated with IMRT. Interestingly, there was no identifiable relationship between the GTV and LPFS, suggesting that even patients with bulky tumors or large volume GRD can expect high rates of local control. Because all but 3 patients had at least one surgery prior to IMRT, we attempted to stratify patients who received immediate adjuvant IMRT for GRD with those who progressed subsequently and underwent IMRT for intact recurrent disease. The median time from last surgery to IMRT among patients who had surgery within 3 months was 1.9 months (range 0.98 to 2.90 months) compared with a median of 12.3 months for patients whose surgery was >3 months prior to IMRT. There were no differences in LPFS, DMFS, or OS between these groups. Taken together, these findings suggest that in appropriately selected patients even bulky tumors may have a high rate of local control with IMRT. This is important to consider in patients for whom a maximal resection would lead to unacceptable morbidity and provides evidence that local control can still be achieved with multidisciplinary care of these patients.
Our institutional practice regarding elective nodal volumes both central and lateral necks that are routinely covered for thyroid cancer has evolved over the years. Radiation target volumes were available for 73 patients in this cohort. All received coverage of the central compartment, generally extending from just below the true vocal cords to the carina encompassing the esophagus and trachea with the minimal lateral boundary as the internal carotid arteries or lateral mediastinum depending on the level. The remaining cervical lymph node stations in the lateral necks were covered as follows: level IB in 8 patients (electively in 5); level II in 41 patients (electively in 32); level III in 49 patients (electively in 38); level IV in 53 patients (electively in 40); and level V in 25 patients (electively in 21). Only 1 patient failed completely out of field in a level II lymph node. The remaining failures occurred within the thyroid bed, tracheoesophageal groove or in lymph node levels containing or closely adjacent to levels containing gross disease at the time of radiation. Given the increased toxicity associated with elective nodal coverage and the rarity of failures outside of areas of gross disease, we no longer routinely cover the elective nodal volumes in the lateral necks. The central compartment and involved nodal levels should be included in all cases. We also do not cover the retropharyngeal nodal regions. Lastly, every case is carefully discussed with the treating surgeon and in situations where a salvage lateral neck dissection can be performed, we do not cover the lateral neck in the target volume despite having pathologic nodal involvement.
IMRT was well tolerated in these patients with a 17.9% rate of grade 3 dermatitis and 12.4% rate of grade 3 mucositis. No grade 4+ toxicities were noted. Our toxicity rates compare favorably to other historical series. MDACC reported a low rate of severe late treatment-related toxicity with IMRT (2%) (7). They did not report acute toxicities. In a 13 patient phase I study of IMRT (50 Gy in 28 fractions to the elective nodal regions with simultaneous integrated boot to 58.8 Gy to gross disease) in advanced thyroid cancer, a 31% rate of grade 3 dysphagia and 38.5% grade 3 dermatitis were observed (18). Our rates of toxicity compare favorably especially considering the administration of concurrent chemotherapy in half of our patients, however we acknowledge the benefits of prospective data gathering, which the present study lacks. With the exception of nearly significantly higher rates of grade 3 dermatitis and PEG placement with CC-IMRT, other treatment related toxicities were similar between CC-IMRT and IMRT supporting the safety of combined chemotherapy and radiation in patients with unresectable or GRD differentiated thyroid cancer. The overall rate of PEG utilization during or following RT was 17% with a clear trend towards increased PEG requirement in CC-IMRT patients. When considering IMRT or CC-IMRT in these patients, it is important to understand such risks and their attendant complications against the benefit of potentially increased locoregional control. It is also important to note that ten percent of these patients required a PEG for greater than 12 months, reflecting the importance of early and aggressive swallow rehabilitation in this population.
It is important to note the limitations of the present study. Primarily, this is a retrospective analysis comparing outcomes after receipt of full course IMRT with or without concurrent use of a chemotherapeutic agent. It is therefore not possible to compare the outcomes of these patients with those with gross residual or unresectable disease who received alternative therapies such as maintenance on TKI, conventional chemotherapy, lower dose radiotherapy, or observation. Additionally, we cannot comment on the completion rate of treatment, as some patients intended for full course IMRT may have not received the entire planned therapy. Many considerations go into whether or not to offer chemotherapy to these patients, and while we have attempted to account for as much bias as possible, elimination of confounders is not entirely achievable. Additionally, subjective evaluations such as toxicity grading vary between practitioners. These limitations highlight the need for prospective studies in this group of patients.
Conclusion
IMRT is well tolerated in patients with unresectable or incompletely resected non-medullary differentiated thyroid cancer and should be considered in appropriate patients who may benefit from long-term locoregional disease control. Concurrent chemotherapy with radiosensitizing doxorubicin was associated with higher rates of OS and LPFS compared to IMRT alone. Prospective trials are warranted to evaluate the value of CC-IMRT in this patient population.
Supplementary Material
Acknowledgments
This study was supported in part by MSK Cancer Center Support Grant/Core Grant (P30 CA008748). Paul B. Romesser is supported in part by a K12 Paul Calebresi Career Development Award for Clinical Oncology (K12 CA184746).
Author Disclosure Statement
No competing financial interests exist.
Refereneces
- 1.Siegel RL, Miller KD, Jemal A. 2017. Cancer statistics, 2017. CA Cancer J Clin 67:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Sherman SI. 2003. Thyroid carcinoma. Lancet 361:501–511 [DOI] [PubMed] [Google Scholar]
- 3.Haugen BR. 2017. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: what is new and what has changed? Cancer 123:372–381 [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferri EL, Kloos RT. 2001. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 86:1447–1463 [DOI] [PubMed] [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Thyroid Carcinoma. Available at: www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accesed March21, 2018)
- 6.Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM, Mandel SJ, Morris JC, Nassar A, Pacini F, Schlumberger M, Schuff K, Sherman SI, Somerset H, Sosa JA, Steward DL, Wartofsky L, Williams MD. 2017. American Thyroid Association Guidelines on the Management of Thyroid Nodules and Differentiated Thyroid Cancer Task Force Review and Recommendation on the Proposed Renaming of Encapsulated Follicular Variant Papillary Thyroid Carcinoma Without Invasion to Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 27:481–483 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz DL, Lobo MJ, Ang KK, Morrison WH, Rosenthal DI, Ahamad A, Evans DB, Clayman G, Sherman SI, Garden AS. 2009. Postoperative external beam radiotherapy for differentiated thyroid cancer: outcomes and morbidity with conformal treatment. Int J Radiat Oncol Biol Phys 74:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romesser PB, Sherman EJ, Shaha AR, Lian M, Wong RJ, Sabra M, Rao SS, Fagin JA, Tuttle RM, Lee NY. 2014. External beam radiotherapy with or without concurrent chemotherapy in advanced or recurrent non-anaplastic non-medullary thyroid cancer. J Surg Oncol 110:375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terezakis SA, Lee KS, Ghossein RA, Rivera M, Tuttle RM, Wolden SL, Zelefsky MJ, Wong RJ, Patel SG, Pfister DG, Shaha AR, Lee NY. 2009. Role of external beam radiotherapy in patients with advanced or recurrent nonanaplastic thyroid cancer: Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys 73:795–801 [DOI] [PubMed] [Google Scholar]
- 10.Yates JW, Chalmer B, McKegney FP. 1980. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 45:2220–2224 [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383 [DOI] [PubMed] [Google Scholar]
- 12.Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, Shah JP, Singh B, Ghossein RA. 2006. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer 106:1286–1295 [DOI] [PubMed] [Google Scholar]
- 13.Terezakis SA, Lee NY. 2010. The role of radiation therapy in the treatment of medullary thyroid cancer. J Natl Compr Canc Netw 8:532–540; quiz 541 [DOI] [PubMed] [Google Scholar]
- 14.Volante M, Landolfi S, Chiusa L, Palestini N, Motta M, Codegone A, Torchio B, Papotti MG. 2004. Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: a clinicopathologic study of 183 patients. Cancer 100:950–957 [DOI] [PubMed] [Google Scholar]
- 15.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. 2003. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181 [DOI] [PubMed] [Google Scholar]
- 16.Cox JD, Stetz J, Pajak TF. 1995. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31:1341–1346 [DOI] [PubMed] [Google Scholar]
- 17.Chow SM, Yau S, Kwan CK, Poon PC, Law SC. 2006. Local and regional control in patients with papillary thyroid carcinoma: specific indications of external radiotherapy and radioactive iodine according to T and N categories in AJCC 6th edition. Endocr Relat Cancer 13:1159–1172 [DOI] [PubMed] [Google Scholar]
- 18.Urbano TG, Clark CH, Hansen VN, Adams EJ, Miles EA, Mc Nair H, Bidmead AM, Warrington J, Dearnaley DP, Harmer C, Harrington KJ, Nutting CM. 2007. Intensity modulated radiotherapy (IMRT) in locally advanced thyroid cancer: acute toxicity results of a phase I study. Radiother Oncol 85:58–63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.