Abstract
Chronic exposure to seizures in patients with left hemisphere (LH) epileptic focus could favor higher activation in the contralateral hemisphere during language processing, but the cognitive effects of this remain unclear. This study assesses the relationship between asymmetry in hemispheric activation during language fMRI and performance in verbal and non-verbal tasks. Whereas prior studies primarily used fMRI paradigms that favor frontal lobe activation and less prominent activation of the medial or superior temporal lobes, we used a verbal comprehension paradigm previously demonstrated to activate reliably receptive language areas. Forty-seven patients with drug-resistant epilepsy candidates for surgery underwent a multidisciplinary assessment, including a comprehensive neuropsychological evaluation and an fMRI verbal comprehension paradigm. Patients were distributed in two groups depending on laterality indexes (LI): typical hemispheric asymmetry (unilateral left activation preponderance; n = 23) and atypical hemispheric asymmetry (bilateral or unilateral right preponderance; n = 24). Right-handedness and right hemisphere (RH) focus were significant predictors of typical asymmetry. Patients with typical activation pattern presented better performance intelligence quotient and verbal learning than patients with atypical hemispheric asymmetry (for all, p < 0.014). Patients with LH focus had more frequently atypical hemispheric asymmetry than patients with RH focus (p = 0.05). Specifically, they showed lower LI and this was related to worse performance in verbal and non-verbal tasks. In conclusion, an increased activation of homologous RH areas for verbal comprehension processing could imply a competition of cognitive resources in the performance of the same task, disrupting cognitive performance.
Keywords: Typical asymmetry, Language, fMRI, Epilepsy, Cognitive performance
Highlights
-
•
Patients with LH focus more frequently have atypical hemispheric asymmetry.
-
•
In patients with TLE, those with HS have lower LI and poor verbal memory.
-
•
Lower LIs are related to worse performance in verbal and non-verbal tasks.
-
•
Right-handedness and RH focus significantly predict typical hemispheric asymmetry.
-
•
LI, side of seizure focus and gender significantly predict cognitive performance.
1. Introduction
Some 30% of patients with epilepsy have drug-resistant epilepsy (Barr and Morrison, 2014), in which brain injury around eloquent language areas can induce inter-hemispheric language reorganization (Tzourio-Mazoyer et al., 2017). As a result, patients with epilepsy have bilateral or right-hemispheric language lateralization more frequently than the general population (Hamberger and Cole, 2011). This pattern of language lateralization was defined as atypical in classic studies based on the Wada test, considering left-hemispheric lateralization as the typical pattern (Mateer and Dodrill, 1983).
Several factors have been proposed as possible mediators of this atypical pattern, such as age at epilepsy onset (Brázdil et al., 2003; Miró et al., 2014), gender (Adcock et al., 2003; Helmstaedter et al., 1997), handedness (Corballis et al., 2012), and location of seizure focus (Duke et al., 2012). The explicative mechanism has not yet been fully explained (Piervincenzi et al., 2016).
Although a certain consensus exists about language reorganization in patients with left hemisphere (LH) focus, the cognitive implications of this remain unclear. Using the Wada test, atypical language lateralization in patients with LH focus was related to decreases in non-verbal functions, but preserved verbal abilities (Loring et al., 1999; Strauss et al., 1990). Using an fMRI language paradigm, Berl et al. (2005) showed that those with atypical hemispheric asymmetry had lower scores in performance intelligence quotient (IQ) than those with typical asymmetry, although performances in verbal tasks are similar. However, Thivard et al. (2005) found better performance in verbal fluency and delayed verbal memory in those with atypical patterns of activation (considering that as a compensatory mechanism).
fMRI allows us to approach this problem in a non-invasive way (Benjamin et al., 2017), although results are far from homogeneous due to the high variability in the paradigms used (Tzourio-Mazoyer et al., 2017). Among them, a single phonemic verbal fluency paradigm has been one of the most frequently used. However, this paradigm could favor left frontal lobe activation corresponding to Broca's area, and less prominent activation of the medial or superior temporal lobes (Bonelli et al., 2012). Verbal comprehension paradigms would provide better information about semantic and syntactic processing (Ni et al., 2000), so it is likely to show higher sensitivity in temporal lobe epilepsy (TLE) – the most frequent type of drug-resistant epilepsy (Téllez-Zenteno and Hernández-Ronquillo, 2012). Additionally, verbal comprehension paradigms are a little less dependent on the active performance of the patient, thus the patient only has to listen and understand a short story and the task is carried out covertly. The non-excessive complexity of this paradigm could avoid poor activation patterns as a result of underperformance (Miró et al., 2014).
The current study assesses the relationship between asymmetry in the hemispheric activation during an fMRI verbal comprehension paradigm and performance in verbal and non-verbal tasks when considering possible mediator factors. According to the ‘crowding’ phenomenon, higher right hemisphere (RH) activation during language processing could imply a competition of cognitive resources in the performance of the same task, disrupting cognitive performance (Jokeit and Ebner, 2002). Considering that chronic epilepsy could imply a progressive cognitive deterioration, and that previous studies have found that cognitive variables are interrelated (Cano-López et al., 2017) and depend on a functional brain network (Dinkelacker et al., 2015), we hypothesize that higher RH activation during verbal comprehension could disrupt performance in various cognitive domains. For that, firstly, a positive relationship is expected between typical hemispheric asymmetry and performance in verbal tasks, but also in non-verbal tasks for which the RH is typically dominant, in the total sample. Secondly, in patients with LH focus, more frequent atypical asymmetry than in patients with RH focus is hypothesized, and this will be related to lower performance in verbal and non-verbal tasks. Finally, the role of side of seizure focus, location of seizure focus, age at epilepsy onset, seizure frequency, number of antiepileptic drugs (AEDs), gender, and handedness on fMRI activation patterns, and the impact of these factors and fMRI activation patterns on performance in verbal and non-verbal tasks are examined.
This study contributes to understanding the relationship between the asymmetry in fMRI activation and the cognitive profile of patients who are candidates for epilepsy surgery, which could be useful in the clinical management of such patients. Whereas prior studies primarily used fMRI paradigms that favor frontal lobe activation and less prominent activation of the medial or superior temporal lobes, we used a verbal comprehension paradigm previously demonstrated to reliably activate receptive language areas. Additionally, we include more cognitive domains than previous studies to more comprehensively analyse this issue.
2. Material and methods
2.1. Sample
The sample was composed of 47 adult patients with drug-resistant epilepsy that were candidates for epilepsy surgery (mean age ± SD: 33.72 ± 12.15, range: 18–61).
2.2. Procedure
This study was conducted in the Epilepsy Unit at the Hospital Clínic of Barcelona between 2008 and 2017 in accordance with the Declaration of Helsinki. Informed consent was obtained from participants in the study.
Medical history provided characteristics of the patients such as gender, age, level of education, handedness, age at epilepsy onset, duration of epilepsy (years), frequency of seizures (seizures per month), pre-surgical number of AEDs and type of AEDs.
Pre-surgical assessment included the diagnosis of drug-resistant epilepsy, as well as the lateralization and location of the epileptogenic area. Assessment was made by the multidisciplinary team staff members based on an evaluation that included: seizure history and semiology; neurologic examination; long-term video-EEG monitoring; 3-Tesla magnetic resonance imaging (MRI); fluorodeoxyglucose (FDG)-positron emission tomography (PET); single photon emission computed tomography (SPECT); psychiatric assessment; language fMRI assessment; and neuropsychological evaluation.
Considering this assessment, patients were divided into groups based on the side of seizure focus –LH focus (80.9%) and RH focus (19.1%), and the location of seizure focus –temporal (72.3%) and extratemporal (27.7%).
2.3. Neuropsychological assessment
2.3.1. IQ outcome
This was estimated by means the verbal subtests (vocabulary and digit span) and performance subtests (block design and digit symbol) of the Wechsler Adult Intelligence Scale-3rd Edition (Wechsler et al., 1997). Z-scores of each subtest were computed.
2.3.2. Executive functions
The Trail Making Test (TMT; Reitan and Wolfson, 1985) was used to measure various executive functions (working memory, attention, planning and set shifting) that require motor skills and visual-spatial processing. In part A, participants were requested to draw a line to connect 25 circles with successive numbers and in the correct order, while in part B letters and numbers had to be linked. Z-scores for both parts were computed.
2.3.3. Logical memory
Evaluated by means Logical Memory I and II subtests of the Wechsler Memory Scale-3rd Edition (Wechsler, 1997), consisting of immediate and delayed recall of two short stories. Z-scores of immediate and delayed logical memory were computed.
2.3.4. Verbal learning and memory
The Rey Auditory Verbal Learning Test (Rey, 1964) was used to evaluate the patient's ability to encode, consolidate, and retrieve verbal information. It consisted of five trials of learning a list of 15 words. Verbal learning was computed by summing the total number of correctly reproduced words over the five learning trials, and long-term verbal memory was computed as the total number of correctly recalled words 30 min after the list presentation. These scores were transformed into z-scores following the normative data.
2.3.5. Visual memory
Evaluated using the Visual Reproduction I and II subtests of the Wechsler Memory Scale-Revised (Wechsler, 1987). Participants were instructed to draw geometric designs from memory after seeing them for a brief period of time. Z-scores of immediate and delayed visual memory were computed.
2.3.6. Naming functions
The Boston Naming Test (Kaplan et al., 2001) was used to assess visual confrontation naming. Semantic and phonemic cues were provided in the case of no response or incorrect response. The total score was computed as the number of cards correctly named without phonemic cues and with 60 being the maximum score, and it was transformed into z-scores following the normative data of Aranciva et al. (2012).
2.3.7. Phonemic fluency
The total number of words generated in one minute for the letters F, A, and S was obtained (Spreen and Benton, 1977). The total score was computed as the sum of all admissible words for the three letters, and it was transformed into z-scores following the normative data of Tombaugh et al. (1999).
2.3.8. Semantic fluency
Participants were asked to think of the names of as many animals that they could in one minute (Rosen, 1980). The total score was computed as the sum of admissible words for this semantic category, and it was transformed into z-scores following the normative data of Tombaugh et al. (1999).
2.4. Language fMRI acquisition and analyses
The study was performed at the Magnetic Resonance Image Core Facility at IDIBAPS located in the Diagnostic Imaging Centre at Hospital Clinic (CDIC) using the blood-oxygen level-dependent (BOLD) fMRI signal. All scans were performed on a 3 T Siemens MAGNETOM TIM Trio scanner (Siemens Medical Systems, Germany), using an 8-channel head coil for radio frequency transmission and signal reception. Each subject underwent a 3D structural scan high-resolution T1-weighted MPRAGE sequence. Acquisition consisted of a set of 240 adjacent sagittal images with a slice thickness of voxel size 1x1x1 mm, using a spoiled gradient echo sequence (TR:2300 ms, TE:298 ms, NEX:1, flip angle: 90°, FOV: 256 × 256). fMRI images were acquired in the axial plane with an EPI sequence (TR:3000 ms, TE: 30 ms, flip angle: 90°, pixel matrix: 3.75 × 3.75 mm, slice thickness: 3 mm).
We used an fMRI adaptation of the logical memory test of the Wechsler Memory Scale-3rd Edition (Wechsler, 1997) to analyse brain activation during verbal comprehension. The experimental design was an AB ‘boxcar’ with the 30-s verbal comprehension task (A) alternating with 30 s of control task (B) for a total of six cycles (over 3 min). In the verbal comprehension task, participants were instructed to listen and understand a short story, while the control task consisted of listening to the language content of the story digitally backwards. After the acquisition, participants were asked about the story to control their performance in the task.
Data were analysed using statistical parametric mapping (SPM 12; Wellcome Trust Centre for Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm/). Scans from each subject were realigned using the mean image as a reference to remove any minor motion-related signal change, and coregistered with the anatomical image. The resulting data set was segmented, and scans were spatially normalised into standard space (3 × 3 × 3 mm; Montreal Neurology Institute, MNI coordinates) and spatially smoothed with a Gaussian kernel of 8 mm FWHM.
After data pre-processing, statistical analyses were performed at the single-subject level using the general linear model implemented in SPM (Friston et al., 1994). Condition-specific effects were estimated by creating boxcar functions of task against control. Statistical parametrical maps were created and thresholded using a p-value of 0.001, uncorrected for multiple comparisons (T value >3.26). The contrast image (verbal comprehension relative to baseline) for each subject was then entered into a second level one-sample t-test to analyse activations in the ROIs. The ROIs included superior temporal gyrus (Brodmann's area (BA) 22), middle temporal gyrus (BA 21), temporal pole (BA 38), angular gyrus (BA 39) and auditory cortex (BA 41) in both hemispheres. Selection of ROIs was based on lesion and imaging data implicating them in language processing (Ahmad et al., 2003), and was supported by SPM maps, which are not based on a priori assumptions, using Wake Forest University (WFU) PickAtlas Tool Version 2.4 (http://fmri.wfubmc.edu/software/PickAtlas) software (Maldjian et al., 2003). We report all ROIs activations corrected for multiple comparisons [family wise error (FWE)], p < 0.05. Parametrical maps were inspected for their validity and all participants activated ROIs. Using these parametrical maps, we computed the sum of the activated voxels in the ROIs volumes.
Laterality indexes (LIs) that reflect the interhemispheric difference between voxel counts in left and right homologous ROIs were calculated for each ROI using the bootstrap method of the SPM toolbox (Wilke and Lidzba, 2007). Activation was considered ‘left-sided’ if the LI was >0.2; ‘right-sided’ if the LI was smaller than −0.2; and bilateral if the LI ranged between 0.2 and −0.2 (Ahmad et al., 2003; Lee et al., 2010). Asymmetry in the hemispheric activation was categorised as: ‘typical’ -unilateral left preponderance (LI > 0.2) and ‘atypical’-bilateral (LI between −0.2 and 0.2) or unilateral right preponderance (LI < −0.2), as in previous studies that include the same paradigm (Chaudhary et al., 2017; Gaillard et al., 2007) or other auditory paradigms that require verbal comprehension (Berl et al., 2005). Thus, the higher LI, the higher degree of LH activation.
2.5. Statistical analyses
The Kolmogorov-Smirnov test was carried out to examine the normality of the data. t-tests for independent samples were then performed for between-group comparisons based on the asymmetry in the hemispheric activation (typical or atypical) on demographic (age) and epilepsy-related variables (age at epilepsy onset, epilepsy duration, number of AEDs and frequency of seizures per month), according to Levene's test for equality of variance. The chi-square test was used to study the differences between these groups in categorical variables such as gender, educational level, handedness, epilepsy type (comparing the frequency of frontal lobe epilepsy (FLE), TLE, parietal lobe epilepsy, occipital lobe epilepsy and multifocal epilepsy), location of seizure focus (temporal or extratemporal), side of seizure focus, etiology of the pathology (comparing the frequency of hippocampal sclerosis (HS), focal cortical dysplasia, tumor, gliosis, heterotopia, general atrophy, encephalocele, encephalomalacia, subcortical lesions and non-specific pathology), presence of HS versus all other pathologies, frequency of use of each type of AED, seizure type, and presence of psychiatric disorders.
To check the impact of the side of seizure focus and the location of seizure focus on LI, univariate ANOVAs were carried out in the total sample. Univariate ANOVAs were also performed in the subgroup of TLE, including the presence of HS as between-subject factor. Secondary analyses were carried out to evaluate the differences in age at epilepsy onset, gender, and handedness depending on the side of seizure focus, the location of seizure focus or the presence of HS (in TLE subgroup), using chi-square test or t-test for independent samples where appropriate. When significant differences in these variables were found, they were included as covariates in the previous ANOVAs.
To evaluate the possible predictors of LI, hierarchical regressions were carried out, including LI as a dependent variable, and two separate blocks of independent variables (block 1: gender and handedness; and block 2: side of seizure focus, age at epilepsy onset, seizure frequency and number of AEDs).
To analyse the impact of the asymmetry in the hemispheric activation (typical or atypical) and the side of seizure focus (LH or RH focus) on cognitive performance, univariate ANOVAs were carried out in the total sample, including the hemispheric activation and the side of seizure focus as between-subject factors, and cognitive scores as dependent variables. These ANOVAs were repeated in the subgroup of TLE, including also the presence of HS as a between-subject factor.
Spearman correlations were performed to establish the association between LI and cognitive performance in the total sample, in groups based on the location of seizure focus, and in groups based on the side of seizure focus. Multiple testing correction controlling the False Discovery Rate (FDR) was applied in these correlations (Benjamini and Yekutieli, 2001). The FDR was set to 0.10, which implies that the proportion of significant associations which are actually false discoveries is limited no >10%, as in other neuropsychological studies (Gallagher et al., 2014).
To evaluate the role of possible predictors of cognitive performance, hierarchical regressions were carried out on the total sample, including cognitive scores as dependent variables and three separate blocks of independent variables (block 1: gender and handedness; block 2: side of seizure focus, location of seizure focus, and age at epilepsy onset; and block 3: LI).
Statistical analysis was carried out using SPSS 22.0 and two-tailed tests with p set to 0.05 considered as significant.
3. Results
3.1. fMRI results
Significant activation was demonstrated in the bilateral superior temporal gyrus, middle temporal gyrus and angular gyrus, as well as in the right temporal pole and auditory cortex during the verbal comprehension fMRI paradigm (Fig. 1 and Table 2).
Fig. 1.
Structural axial images fused with the activation maps in ROIs during an fMRI verbal comprehension paradigm in two patients. Patient A had a RH epileptic focus and presented typical hemispheric asymmetry (LI = 0.80). Patient B had a LH epileptic focus and presented atypical hemispheric asymmetry (LI = −0.65).
Table 2.
fMRI activation peaks for the main effects of verbal comprehension in the total sample in ROIs shown corrected for multiple comparisons (FWE) p < 0.05.
| Z-score | Corrected p-value (FWE) | Coordinates (x, y, z) in MNI space | Region |
|---|---|---|---|
| 4.68 | 0.001 | −54, −1, −13 | Left superior temporal gyrus (BA 22) |
| 4.49 | 0.002 | −54, −10, −7 | Left superior temporal gyrus (BA 22) |
| 5.46 | 0.0001 | 48, −19, −7 | Right superior temporal gyrus (BA 22) |
| 4.75 | 0.001 | 54, −4, −13 | Right superior temporal gyrus (BA 22) |
| 3.79 | 0.031 | 57, −19, −7 | Right superior temporal gyrus (BA 22) |
| 4.18 | 0.007 | −54, −46, 2 | Left middle temporal gyrus (BA 21) |
| 3.95 | 0.018 | 60, −37, −1 | Right middle temporal gyrus (BA 21) |
| 3.92 | 0.020 | 54, −4, −19 | Right middle temporal gyrus (BA 21) |
| 4.62 | 0.001 | 54, 8, −19 | Right temporal pole (BA 38) |
| 4.56 | 0.002 | −57, −49, 11 | Left angular gyrus (BA 39) |
| 4.29 | 0.005 | −54, −58, 14 | Left angular gyrus (BA 39) |
| 3.80 | 0.030 | −39, −55, 20 | Left angular gyrus (BA 39) |
| 4.96 | 0.0001 | 63, −49, 14 | Right angular gyrus (BA 39) |
| 4.22 | 0.006 | 42, −34, 5 | Right auditory cortex (BA 41) |
MNI space, coordinates related to a standard brain defined by the Montreal Neurological Institute (MNI); BA, Brodmann's area.
3.2. Characteristics of patients based on LI and LI predictors
Using LI classification criteria, 23 patients had left-sided activation (48.9%), 19 patients had right-sided activation (40.4%) and 5 patients had bilateral activation (10.6%). Thus, patients were distributed into two groups of hemispheric asymmetry (Table 1): typical (48.9%) and atypical (51.1%).
Table 1.
Characteristics of the total sample (mean ± SD or %) and groups with typical and atypical hemispheric asymmetry.
| Characteristics | Total | Typical asymmetry | Atypical asymmetry | p |
|---|---|---|---|---|
| Age | 33.72 ± 12.15 | 35.26 ± 13.57 | 32.25 ± 10.69 | 0.40 |
| Gender | 0.65 | |||
| Female | 22 (46.8%) | 10 (43.5%) | 12 (50.0%) | |
| Male | 25 (53.2%) | 13 (56.5%) | 12 (50.0%) | |
| Educational level | 0.11 | |||
| Primary education | 23 (48.9%) | 9 (39.1%) | 14 (58.3%) | |
| Secondary education | 7 (14.9%) | 2 (8.7%) | 5 (20.8%) | |
| Lower-university education | 10 (21.3%) | 8 (34.8%) | 2 (8.3%) | |
| University education | 7 (14.9%) | 4 (17.4%) | 3 (12.5%) | |
| Handedness | 0.24 | |||
| Right | 25 (53.2%) | 13 (56.5%) | 12 (50.0%) | |
| Left | 20 (42.6%) | 8 (34.8%) | 12 (50.0%) | |
| Ambidextrous | 2 (4.3%) | 2 (8.7%) | 0 (0.0%) | |
| Epilepsy type | 0.24 | |||
| FLE | 8 (17.0%) | 1 (4.3%) | 7 (29.2%) | |
| TLE | 34 (72.3%) | 20 (87.0%) | 14 (58.3%) | |
| PLE | 1 (2.1%) | 0 (0.0%) | 1 (4.2%) | |
| OLE | 1 (2.1%) | 1 (4.3%) | 0 (0.0%) | |
| Multifocal | 3 (6.4%) | 1 (4.3%) | 2 (8.3%) | |
| Location of seizure focus | 0.03 | |||
| Temporal | 34 (72.3%) | 20 (87.0%) | 14 (58.3%) | |
| Extratemporal | 13 (27.7%) | 3 (13.0%) | 10 (41.7%) | |
| Side of seizure focus | 0.05 | |||
| RH | 9 (19.1%) | 7 (30.4%) | 2 (8.3%) | |
| LH | 38 (80.9%) | 16 (69.6%) | 22 (91.7%) | |
| Age at epilepsy onset | 11.91 ± 11.02 | 9.70 ± 8.82 | 14.13 ± 12.66 | 0.18 |
| Epilepsy duration | 21.83 ± 13.86 | 25.57 ± 16.19 | 18.09 ± 10.07 | 0.07 |
| Etiology of pathology | 0.20 | |||
| HS | 17 (36.2%) | 8 (34.8%) | 9 (37.5%) | |
| Focal cortical dysplasia | 10 (21.3%) | 6 (26.1%) | 4 (16.7%) | |
| Tumor | 2 (4.2%) | 1 (4.3%) | 1 (4.2%) | |
| Gliosis | 3 (6.4%) | 0 (0.0%) | 3 (12.5%) | |
| Heterotopia | 3 (6.4%) | 3 (13.0%) | 0 (0.0%) | |
| General atrophy | 4 (8.5%) | 1 (4.3%) | 3 (12.5%) | |
| Encephalocele | 1 (2.1%) | 0 (0.0%) | 1 (4.2%) | |
| Encephalomalacia | 1 (2.1%) | 0 (0.0%) | 1 (4.2%) | |
| Subcortical lesions | 1 (2.1%) | 0 (0.0%) | 1 (4.2%) | |
| Non-specific pathology | 5 (10.6%) | 4 (17.4%) | 1 (4.2%) | |
| Presence of HS | 0.85 | |||
| Yes | 17 (36.2%) | 8 (34.8%) | 9 (37.5%) | |
| No | 30 (63.8%) | 15 (65.2%) | 15 (62.5%) | |
| Number of AEDs | 2.75 ± 0.84 | 2.64 ± 0.66 | 2.86 ± 0.99 | 0.38 |
| Type of AEDs | ||||
| Levetiracetam | 20 (42.6%) | 12 (52.2%) | 8 (33.3%) | 0.19 |
| Lacosamide | 19 (40.4%) | 8 (34.8%) | 11 (45.8%) | 0.44 |
| Carbamazepine | 14 (29.8%) | 6 (26.1%) | 8 (33.3%) | 0.59 |
| Eslicarbazepine | 6 (12.8%) | 4 (17.4%) | 2 (8.3%) | 0.35 |
| Sodium valproate | 5 (10.6%) | 3 (13.0%) | 2 (8.3%) | 0.60 |
| Lamotrigine | 3 (6.4%) | 1 (4.3%) | 2 (8.3%) | 0.58 |
| Perampanel | 1 (2.1%) | 0 (0.0%) | 1 (4.2%) | 0.32 |
| Clobazam | 14 (29.8%) | 9 (39.1%) | 5 (20.8%) | 0.17 |
| Zonisamide | 3 (6.4%) | 1 (4.3%) | 2 (8.3%) | 0.58 |
| Clonazepam | 3 (6.4%) | 2 (8.7%) | 1 (4.2%) | 0.53 |
| Oxcarbazepine | 11 (23.4%) | 6 (26.1%) | 5 (20.8%) | 0.67 |
| Phenobarbital | 5 (10.6%) | 1 (4.3%) | 4 (16.7%) | 0.16 |
| Topiramat | 7 (14.9%) | 3 (13.0%) | 4 (16.7%) | 0.73 |
| Phenytoin | 3 (6.4%) | 0 (0.0%) | 3 (12.5%) | 0.08 |
| Pregabalin | 5 (10.6%) | 2 (8.7%) | 3 (12.5%) | 0.67 |
| Retigabine | 1 (2.1%) | 0 (0.0%) | 1 (4.2%) | 0.32 |
| Seizures per month | 15.68 ± 16.67 | 12.45 ± 14.40 | 18.63 ± 18.39 | 0.22 |
| Seizure type | 0.36 | |||
| SPS | 4 (8.5%) | 2 (8.7%) | 2 (8.3%) | |
| CPS | 18 (38.3%) | 9 (39.1%) | 9 (37.5%) | |
| GTCS | 4 (8.5%) | 1 (4.3%) | 3 (12.5%) | |
| SPS + CPS | 6 (12.8%) | 5 (21.7%) | 1 (4.2%) | |
| SPS + GTCS | 1 (2.1%) | 0 (0.0%) | 1 (4.2%) | |
| CPS + GTCS | 9 (19.1%) | 5 (21.7%) | 4 (16.7%) | |
| SPS + CPS + GTCS | 5 (10.6%) | 1 (4.3%) | 4 (16.7%) | |
| Psychiatric disorder | 0.85 | |||
| Yes | 17 (36.2%) | 8 (34.8%) | 9 (37.5%) | |
| No | 30 (63.8%) | 15 (65.2%) | 15 (62.5%) |
Note. CPS: complex partial seizure, FLE: frontal lobe epilepsy, GTCS: secondary generalized seizures, HS: hippocampal sclerosis, ILE: insular epilepsy, LH: left hemisphere, OLE: occipital lobe epilepsy, PLE: parietal lobe epilepsy, RH: right hemisphere, SPS: simple partial seizure, TLE: temporal lobe epilepsy.
No significant differences between these groups were found in age, age at epilepsy onset, epilepsy duration, number of AEDs, and frequency of seizures. Additionally, there were no significant differences between these groups in gender, educational level, handedness, epilepsy type, etiology of the pathology, presence of HS versus all other pathologies, frequency of use of each type of AED, seizure type and presence of psychiatric disorders. However, a significant difference was found for the side of seizure focus, with a higher proportion of patients with LH focus having atypical hemispheric asymmetry. Accordingly, patients with LH focus have lower LI than patients with RH focus (F(1,46) = 6.31, p = .016, n2p = 0.12; LI = −0.06 ± 0.08 versus LI = 0.42 ± 0.17, respectively). Groups with LH and RH focus did not differ in age at epilepsy onset, gender, nor handedness.
A significant difference was also found for the location of seizure focus, with a higher proportion of patients with an extratemporal focus having atypical hemispheric asymmetry. These groups did not differ in age at epilepsy onset and gender, but in the group of patients with an extratemporal focus, there was a higher proportion of left-handedness patients (76.9%) than in the group with temporal focus (29.4%) (χ2 = 8.68, p = 0.003). For that, to analyse the effect of the location of seizure focus on LI, handedness was included as covariate, and no significant effects were found (F(1,46) = 2.40, p = 0.13, n2p = 0.05), so the location of seizure focus was not included as possible predictor of LI in the regression analysis. Additionally, in the subgroup of 34 patients with TLE, patients without HS (n = 18) tended to have higher LI than those with HS (n = 16) (F(1,33) = 3.40, p = 0.07, n2p = 0.10; LI = 0.28 ± 0.51 versus LI = −0.05 ± 0.53, respectively). These patients did not differ in age at epilepsy onset, gender, nor handedness.
In the total sample, higher LI was predicted by right-handedness and RH focus, while age at epilepsy onset, seizure frequency, number of AEDs and gender were not significant predictors (Table 3).
Table 3.
Hierarchical regression analyses investigating the effect of gender, handedness, side of seizure focus and age at epilepsy onset on LI.
| LI (indicating typical hemispheric asymmetry) |
|||||||
|---|---|---|---|---|---|---|---|
| Std β | p | ∆ R2 | p | Adj. R2 | F | p | |
| Block 1 | 0.06 | 0.28 | 0.02 | 1.44 | 0.25 | ||
| Gender | −0.11 | 0.49 | |||||
| Handedness | 0.40 | 0.02⁎ | |||||
| Block 2 | 0.21 | 0.01⁎ | 0.21 | 2.78 | 0.03⁎ | ||
| Side of seizure focus | 0.38 | 0.02⁎ | |||||
| Age at epilepsy onset | −0.26 | 0.12 | |||||
| Seizure frequency | 0.08 | 0.63 | |||||
| Number of AEDs | −0.14 | 0.39 | |||||
p < 0.05.
3.3. Cognitive performance depending on asymmetry in hemispheric activation and side of seizure focus
Significant effects of ‘asymmetry in the hemispheric activation’ were found in the digit symbol task and verbal learning in the total sample, patients with typical hemispheric asymmetry having better performance in both tasks (F(1,41) = 7.03, p = 0.01, n2p = 0.16, and F(1,42) = 6.56, p = 0.014, n2p = 0.14, respectively) (Fig. 2).
Fig. 2.
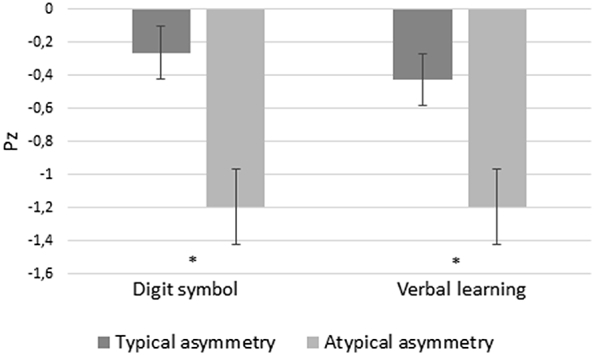
Performance in Digit symbol task and verbal learning (z-scores) depending asymmetry in hemispheric activation during verbal comprehension.
These significant effects remained in the subgroup of patients with TLE (n = 34) (for all, p < 0.032). Additionally, in this subgroup, patients without HS (n = 18) had higher scores in delayed logical memory and long-term verbal memory than those with HS (n = 16) (F(1,33) = 4.46, p = 0.04, n2p = 0.16 and F(1,33) = 4.98, p = 0.034, n2p = 0.15, respectively). Given the limited patients in our study with an extratemporal focus (n = 13), we cannot analyse the effect of the asymmetry in the hemispheric activation on cognitive performance in this subgroup.
No significant effects of the side of seizure focus on any cognitive task were found.
3.4. LI, cognitive performance, and predictor factors
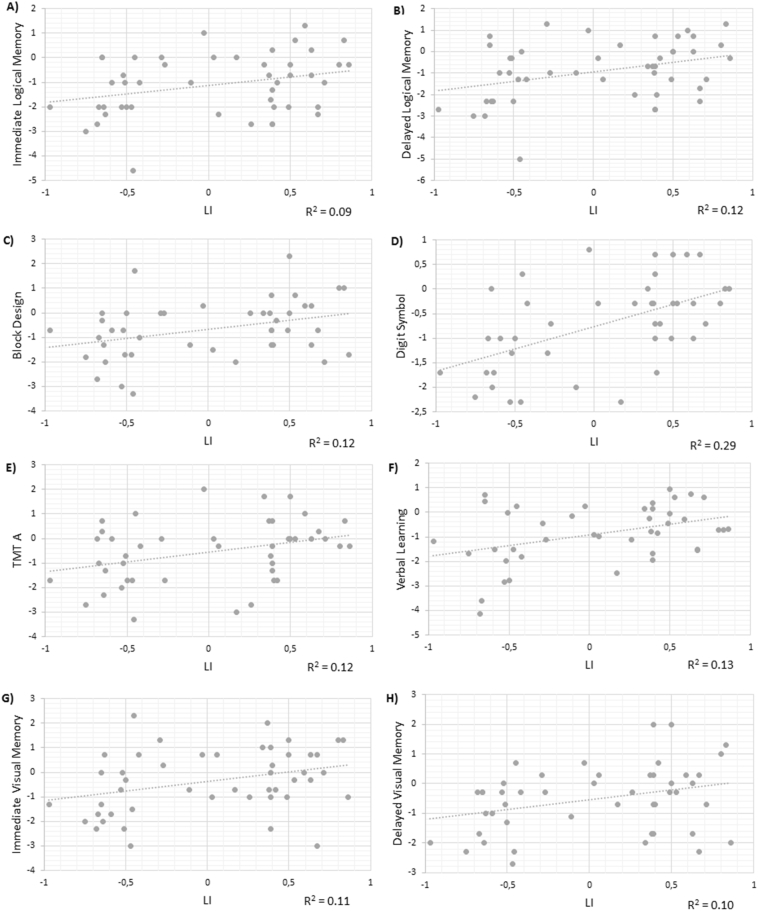
Positive associations were found between LI and immediate logical memory (r = 0.30, p = 0.04), delayed logical memory (r = 0.36, p = 0.01), block design (r = 0.36, p = 0.018), digit symbol (r = 0.53, p < 0.0001), TMT A (r = 0.34, p = 0.026), immediate visual memory (r = 0.37, p = 0.01), delayed visual memory (r = 0.32, p = 0.028), and verbal learning (r = 0.36, p = 0.02) (Fig. 3). All correlations passed FDR multiple testing correction.
Fig. 3.
Associations between LI and cognitive performance (z-scores) in the total sample.
In the subgroup of patients with TLE (n = 34), LI was also significantly related to immediate logical memory (r = 0.32, p = 0.05), delayed logical memory (r = 0.37, p = 0.03), digit symbol (r = 0.48, p = 0.005), immediate visual memory (r = 0.33, p = 0.05), and delayed visual memory (r = 0.34, p = 0.05). Only the association between LI and digit symbol remained significant with FDR < 0.10 in this subgroup (Fig. S1). In patients with extratemporal focus (n = 13), LI was not related to performance in any cognitive task.
Fig. S1.

Associations between LI and performance in the digit symbol task (z-scores) in patients with TLE.
In the subgroup of patients with LH focus (n = 38), higher LI was related to better performance in immediate logical memory (r = 0.32, p = 0.05), delayed logical memory (r = 0.36, p = 0.027), digit symbol task (r = 0.45, p = 0.009), verbal learning (r = 0.43, p = 0.01), long-term verbal memory (r = 0.36, p = 0.03), and immediate visual memory (r = 0.38, p = 0.02). Only correlations between LI and digit symbol task, verbal learning and immediate visual memory passed FDR multiple testing correction in this subgroup (Fig. S2). In patients with RH focus (n = 9), LI was positively related to performance in TMT A (r = 0.70, p = 0.04), but this correlation did not survive at FDR < 0.10.
Fig. S2.
Associations between LI and cognitive performance (z-scores) in patients with LH focus
Hierarchical regressions (Table 4) revealed that female gender and higher LI were significant predictors of better performance in delayed logical memory and in TMT A. RH focus and higher LI significantly predicted higher long-term verbal memory scores. Additionally, female gender was a significant predictor of better performance in the block design task, and higher LI was a significant predictor of better performance in digit symbol task. Handedness, location of seizure focus and age at epilepsy onset did not predict cognitive performance. No predictors were found for other cognitive tasks.
Table 4.
Hierarchical regression analyses investigating the effect of gender, handedness, side of seizure focus, location of seizure focus, age at epilepsy onset and LI on cognitive performance.
| Block design |
|||||||
|---|---|---|---|---|---|---|---|
| Std β | p | ∆ R2 | p | Adj. R2 | F | p | |
| Block 1 | 0.13 | 0.07 | 0.08 | 2.85 | 0.07 | ||
| Gender | 0.33 | 0.03⁎ | |||||
| Handedness | 0.03 | 0.85 | |||||
| Block 2 | 0.15 | 0.07 | 0.18 | 2.79 | 0.03⁎ | ||
| Side of seizure focus | 0.18 | 0.25 | |||||
| Location of seizure focus | −0.20 | 0.21 | |||||
| Age at epilepsy onset | 0.15 | 0.34 | |||||
| Block 3 | 0.04 | 0.15 | 0.20 | 2.75 | 0.03⁎ | ||
| LI | 0.24 | 0.15 | |||||
| Digit symbol |
|||||||
|---|---|---|---|---|---|---|---|
| Std β | p | ∆ R2 | p | Adj. R2 | F | p | |
| Block 1 | 0.04 | 0.49 | −0.01 | 0.73 | 0.49 | ||
| Gender | 0.22 | 0.14 | |||||
| Handedness | −0.15 | 0.37 | |||||
| Block 2 | 0.19 | 0.05⁎ | 0.12 | 2.05 | 0.10 | ||
| Side of seizure focus | 0.10 | 0.51 | |||||
| Location of seizure focus | −0.25 | 0.12 | |||||
| Age at epilepsy onset | 0.10 | 0.47 | |||||
| Block 3 | 0.16 | 0.01⁎ | 0.28 | 3.64 | 0.01⁎ | ||
| LI | 0.48 | 0.01⁎ | |||||
| TMT A |
|||||||
|---|---|---|---|---|---|---|---|
| Std β | p | ∆ R2 | p | Adj. R2 | F | p | |
| Block 1 | 0.09 | 0.16 | 0.04 | 1.91 | 0.16 | ||
| Gender | 0.35 | 0.03⁎ | |||||
| Handedness | −0.22 | 0.26 | |||||
| Block 2 | 0.09 | 0.26 | 0.07 | 1.63 | 0.18 | ||
| Side of seizure focus | −0.10 | 0.54 | |||||
| Location of seizure focus | −0.27 | 0.11 | |||||
| Age at epilepsy onset | 0.12 | 0.45 | |||||
| Block 3 | 0.10 | 0.03⁎ | 0.16 | 2.36 | 0.05⁎ | ||
| LI | 0.39 | 0.03⁎ | |||||
| Delayed logical memory |
|||||||
|---|---|---|---|---|---|---|---|
| Std β | p | ∆ R2 | p | Adj. R2 | F | p | |
| Block 1 | 0.13 | 0.05⁎ | 0.09 | 3.26 | 0.05⁎ | ||
| Gender | 0.36 | 0.02⁎ | |||||
| Handedness | −0.04 | 0.81 | |||||
| Block 2 | 0.08 | 0.26 | 0.12 | 2.18 | 0.08 | ||
| Side of seizure focus | 0.04 | 0.81 | |||||
| Location of seizure focus | −0.14 | 0.37 | |||||
| Age at epilepsy onset | 0.26 | 0.09 | |||||
| Block 3 | 0.12 | 0.02⁎ | 0.21 | 3.01 | 0.02⁎ | ||
| LI | 0.37 | 0.02⁎ | |||||
| Long-term verbal memory |
|||||||
|---|---|---|---|---|---|---|---|
| Std β | p | ∆ R2 | p | Adj. R2 | F | p | |
| Block 1 | 0.07 | 0.25 | 0.02 | 1.43 | 0.25 | ||
| Gender | 0.29 | 0.06 | |||||
| Handedness | −0.20 | 0.29 | |||||
| Block 2 | 0.13 | 0.17 | 0.08 | 1.68 | 0.17 | ||
| Side of seizure focus | −0.39 | 0.02⁎ | |||||
| Location of seizure focus | −0.25 | 0.15 | |||||
| Age at epilepsy onset | 0.28 | 0.10 | |||||
| Block 3 | 0.13 | 0.02⁎ | 0.20 | 2.66 | 0.03⁎ | ||
| LI | 0.43 | 0.02⁎ | |||||
p < .05.
4. Discussion
The current study indicates that typical asymmetry in the hemispheric activation in a verbal comprehension paradigm is related to better cognitive performance in patients with epilepsy. Patients with LH focus have more frequently atypical hemispheric asymmetry, showing lower LI than patients with RH focus, and this is related to worse cognitive performance. Right-handedness and RH focus are significant predictors of typical hemispheric asymmetry, and LI, side of seizure focus and gender significantly predict cognitive performance.
fMRI verbal comprehension paradigm was effective to activate reliably receptive language areas, according to previous studies (Thivard et al., 2005; Ives-Deliperi et al., 2013). Patients with typical and atypical asymmetry in the hemispheric activation did not differ in gender and age at epilepsy onset. Previous studies are inconsistent, some of them found that women (Helmstaedter et al., 1997) and patients with early age at epilepsy onset (Brázdil et al., 2003) were more likely to show atypical language lateralization in Wada tests. Other fMRI studies have found no differences based on gender (Adcock et al., 2003) or age at epilepsy onset (Thivard et al., 2005; Janszky et al., 2006). It has even been suggested that shifts in language lateralization can occur in adolescence or adulthood (Hertz-Pannier et al., 2002). Although this reasoning remains speculative, our results suggest that the adult brain may be more plastic than commonly thought, and repeated seizures could imply slowly progressive structural disturbances, contributing to greater RH activation during language processing due to the damage in the LH. According to previous studies, patients with LH focus had significantly lower LI (Adcock et al., 2003; Janszky et al., 2003), independently of the age at epilepsy onset, gender, and handedness.
Patients with an extratemporal focus had more frequently atypical hemispheric asymmetry, but also more frequently left-handedness than patients with a temporal focus. When handedness was controlled, no differences in LI were found depending on the location of seizure focus. Previous studies have found that left TLE may have more wide-ranging consequences on distributed language processing areas than left FLE (Duke et al., 2012). Given the limited patients in our study with FLE, we cannot exclude a specific frontal seizure focus effect on LI. Consequently, our group of patients with extratemporal focus included different epilepsy types, which could explain the lack of differences found. In fact, Berl et al. (2005) also found no differences in LI between patients with temporal and extratemporal focus. Additionally, patients with TLE with HS tended to have lower LI than those without HS and, consequently, more atypical asymmetry during verbal comprehension processing, according to previous studies (Duke et al., 2012; Weber et al., 2006). Mesial TLE with HS is a particularly severe focal epilepsy syndrome associated with high degree of intractability with AEDs (Wieser and ILAE Commision on Neurosurgery of Epilepsy, 2004), and may be more closely associated with disturbances of cortical function than other forms of focal epilepsy (Weber et al., 2006). In this sense, a mesial temporal focus may have an indirect effect on consolidation of language networks trough altered verbal memory processing (Duke et al., 2012).
Right-handedness and RH focus significantly predicted higher LI (and, consequently, higher LH activation), according to previous studies examining the role of handedness (Corballis et al., 2012), side of seizure focus (Adcock et al., 2003; Janszky et al., 2003) or both (Stewart et al., 2014). Surprisingly, seizure frequency was not related to LI. A possible explanation could be that it is the chronic exposure to seizures, rather than their frequency, that is related to LI.
fMRI activation pattern had implications on cognitive performance: patients with typical hemispheric asymmetry perform better in the digit symbol task and in verbal learning in the total sample and in the subgroup of patients with TLE. As expected, in this subgroup, patients with HS had worse performance in verbal memory than those without HS (Miller et al., 1993).
Despite these meaningful distinctions when classifying asymmetry patterns, the degree of asymmetry may have clinical implications (Bonelli et al., 2012), and so we analysed the relationships between LI and cognitive scores and found that higher LI was associated with better scores in a wide variety of verbal and non-verbal tasks, such as immediate and delayed logical memory, block design, digit symbol, TMT A, immediate and delayed visual memory, and verbal learning in the total sample. The relationship between LI and performance in digit symbol remained in the subgroup of patients with TLE, while no significant relationships were found in the subgroup of patients with an extratemporal focus. In healthy participants, atypical fMRI activation patterns have also been related to poor performances in verbal memory and spatial domains (Mellet et al., 2014). Contrarily, Thivard et al. (2005) found higher verbal memory in patients with epilepsy with atypical hemispheric asymmetry than in those with typical asymmetry, although the first group was composed of only seven patients and this limits any conclusions. As far as we know, no studies have analysed the potential role of the location of seizure focus in the relationships between LI and cognitive performance. Our results suggest that atypical hemispheric asymmetry in receptive language areas during verbal comprehension may be related to cognitive dysfunction mainly in patients with TLE. It should be noted that this group includes patients with HS, and the hippocampus is integrated into different cognitive systems by multiple reciprocal connections (Eichenbaum et al., 1996), so functional disturbances originating in the hippocampus could affect language networks, and this could be related to a variety of cognitive impairments not directly related to the temporal lobe. However, due to the small number of patients with an extratemporal focus, our findings should be interpreted with caution.
The fact that, in our study, LI was related to verbal functions and non-verbal functions emphasises that cognitive variables are interrelated (Cano-López et al., 2017), depending on a functional brain network (Dinkelacker et al., 2015). Chronic epilepsy could imply an additional indirect impairment of functional compensation in non-epileptic areas, being the explicative mechanism of progressive cognitive deterioration in these patients (Elger et al., 2004). In fact, chronic epilepsy lasting more than two decades is related to a worsening in cognitive functions (Jokeit and Ebner, 2002).
Considering that patients with LH focus presented lower LI than those with RH focus, we analysed these groups separately. In patients with RH focus, no significant relationships between LI and cognitive performance were found, although this group was composed of only nine patients and this limited the establishment of any firm conclusions. In patients with LH focus, higher LI was related to better scores in digit symbol, verbal learning, and immediate visual memory. Using the Wada test, Strauss et al. (1990) found that patients with atypical language dominance and early age at epilepsy onset performed more poorly than those with typical dominance on a wide variety of non-verbal tasks, despite the preservation of verbal functions. In contrast, we found worse performances in non-verbal tasks, as well as in verbal functions in patients with LH focus and atypical hemispheric asymmetry. The later age at epilepsy onset of our sample could imply that greater RH activation during language processing was less adaptive in terms of cognitive performance.
In line with our results, Berl et al. (2005) found that adults and children with LH focus and typical hemispheric asymmetry had higher performance IQ than those with atypical asymmetry. However, our sample only included adult patients in order to reduce variability, and we assessed visual memory and executive functions apart from IQ and verbal domains. Our findings suggest that atypical hemispheric asymmetry during verbal comprehension, which is more frequent in patients with LH focus, could imply a competition of cognitive resources in the performance of the same task, disrupting cognitive performance, even in non-verbal tasks, for which the RH is typically dominant. This phenomenon was described as ‘crowding’, and occurs when ‘one hemisphere tries to do more than it had originally been meant to do’ (Teuber, 1974). This effect implicitly assumes two separate lesions in patients with LH focus and atypical hemispheric asymmetry: one structural (in the LH) and the other functional (in the RH) (Satz et al., 1985). Although this remains speculative, our results suggest that the atypical hemispheric asymmetry during verbal comprehension in these patients could reflect variations in the global organization of the brain instead of exclusively in the organization of language.
It should be noted that patients with drug-resistant epilepsy may be impaired on multiple cognitive domains, which can be driven by important factors unrelated to hemispheric asymmetry during verbal comprehension. RH focus was also a significant predictor of better long-term verbal memory performance, according to previous studies that consistently have associated verbal memory deficits with left TLE (for review, see Tramoni-Negre et al., 2017). Additionally, gender was a significant predictor of performance in delayed logical memory, in the TMT A, and in the block design task (with higher scores in women than men, independently of fMRI activation). Berger et al. (2017) found similar gender differences in verbal tasks in patients with LH focus. However, block design and TMT A requires visual and motor abilities, and in studies with healthy participants, men presented higher scores than women in these type of tasks (Boghi et al., 2006), in contrast with our results. As far as we know, no studies have examined gender differences in performance of this task in patients with epilepsy. Even after controlling for gender, handedness, side of seizure focus, location of seizure focus and age at epilepsy onset, higher LI was a significant predictor of performance in logical memory, long-term verbal memory, TMT A and digit symbol task.
Our study has limitations. Firstly, although all the patients presented drug-resistant epilepsy, the group was quite diverse in terms of the exact localisation of epileptic focus. Secondly, large sample sizes could provide more information about groups, thereby ensuring statistical power. Thirdly, the fMRI paradigm was carried out covertly, and, although participants were asked about the story after the acquisition, no records on this performance were available, so performance in the neuropsychological assessment was used as an approximate measure of task adherence and general motivation. Fourthly, there are important challenges associated with concluding language lateralization based on a single paradigm (Seghier, 2008), since some patients may have typical asymmetry on one language paradigm, but atypical asymmetry on another paradigm that involves a different language network depending on the location of the pathology. In this sense, the lack of healthy controls to determine LI in this study should be noted as a limitation. Finally, our results should be taken with caution, since cognitive performance could be influenced by important factors such as the amount of inter-ictal discharge activity on the day of testing (for review, see Drane et al., 2016).
5. Conclusions
Using a verbal comprehension paradigm that reliably activates receptive areas, typical hemispheric asymmetry is related to better cognitive performance, not only in language-related cognitive functions but also in non-verbal functions in patients with drug-resistant epilepsy. Additionally, we found that patients with LH focus can have more preponderant RH activation during verbal comprehension, and that could imply a competition of cognitive resources in the performance of the same task and a disruption in cognitive performance. These findings emphasise the need to consider cognitive functions as related processes and network dependent, and could be useful in the clinical management of these patients.
The following are the supplementary data related to this article.
Z-scores in neuropsychological tasks in the total sample (mean ± SD) and groups with typical and atypical hemispheric asymmetry.
Acknowledgments
Acknowledgements
We are indebted to the Magnetic Resonance Imaging Core Facility of the IDIBAPS for the technical help. This work was partially developed at the building Centro Esther Koplowitz. The authors are grateful to John Rawlins for the revision of English style and grammar.
Funding
This work was supported by the Ministry of Economy and Competitiveness and the European Regional Development Fund (MINECO/FEDER) [PSI2015-66600-P]; the CERCA Programme/Generalitat de Catalunya; the Generalitat Valenciana (Valencian Government) [PROMETEOII/2015/020]; and the Spanish Ministry of Education, Culture and Sport [FPU14/00471].
Declarations of interest
None.
References
- Adcock J.E., Wise R.G., Oxbury J.M., Oxbury S.M., Matthews P.M. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. NeuroImage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Ahmad Z., Balsamo L.M., Sachs B.C., Xu B., Gaillard W.D. Auditory comprehension of language in young children neural networks identified with fMRI. Neurology. 2003;60:1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Aranciva F., Casals-Coll M., Sánchez-Benavides G., Quintana M., Manero R.M., Rognoni T., Calvo L., Palomo R., Tamayo F., Peña-Casanova J. Estudios normativos españoles en población adulta joven (Proyecto NEURONORMA jóvenes): normas para el Boston Naming Test y el Token Test. Neurologia. 2012;27:394–399. doi: 10.1016/j.nrl.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Barr W.B., Morrison C. Springer; New York: 2014. Handbook on the Neuropsychology of Epilepsy. [Google Scholar]
- Benjamin C.F., Walshaw P.D., Hale K., Gaillard W.D., Baxter L.C., Berl M.M., Polczynska M., Noble S., Alkawadri R., Hirsch L.J., Constable R.T., Bookheimer S.Y. Presurgical language fMRI: mapping of six critical regions. Hum. Brain Mapp. 2017;38:4239–4255. doi: 10.1002/hbm.23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001;29:1165–1188. [Google Scholar]
- Berger J., Oltmanns F., Holtkamp M., Bengner T. Sex differences in verbal and nonverbal learning before and after temporal lobe epilepsy surgery. Epilepsy Behav. 2017;66:57–63. doi: 10.1016/j.yebeh.2016.11.037. [DOI] [PubMed] [Google Scholar]
- Berl M.M., Balsamo L.M., Xu B., Moore E.N., Weinstein S.L., Conry J.A., Pearl P.L., Sachs B.C., Grandin C.B., Frattali C., Ritter F.J., Sato S., Theodore W.H., Gaillard W.D. Seizure focus affects regional language networks assessed by fMRI. Neurology. 2005;65:1604–1611. doi: 10.1212/01.wnl.0000184502.06647.28. [DOI] [PubMed] [Google Scholar]
- Boghi A., Rasetti R., Avidano F., Manzone C., Orsi L., D'Agata F., Caroppo P., Bergui M., Rocca P., Pulverenti L., Bradac G.B., Bogetto F., Mutani R., Mortara P. The effect of gender on planning: an fMRI study using the Tower of London task. NeuroImage. 2006;33:999–1010. doi: 10.1016/j.neuroimage.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Bonelli S.B., Thompson P.J., Yogarajah M., Vollmar C., Powell R.H., Symms M.R., McEvoy A.W., Micallef C., Koepp M.J., Duncan J.S. Imaging language networks before and after anterior temporal lobe resection: results of a longitudinal fMRI study. Epilepsia. 2012;53:639–650. doi: 10.1111/j.1528-1167.2012.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brázdil M., Zákopčan J., Kuba R., Fanfrdlová Z., Rektor I. Atypical hemispheric language dominance in left temporal lobe epilepsy as a result of the reorganization of language functions. Epilepsy Behav. 2003;4:414–419. doi: 10.1016/s1525-5050(03)00119-7. [DOI] [PubMed] [Google Scholar]
- Cano-López I., Vázquez J.F., Campos A., Gutiérrez A., Garcés M., Gómez-Ibáñez A., Conde R., González-Bono E., Villanueva V. Age at surgery as a predictor of cognitive improvements in patients with drug-resistant temporal epilepsy. Epilepsy Behav. 2017;70:10–17. doi: 10.1016/j.yebeh.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Chaudhary K., Ramanujam B., Kumaran S.S., Chandra P.S., Wadhawan A.N., Garg A., Tripathi M. Does education play a role in language reorganization after surgery in drug refractory temporal lobe epilepsy: an fMRI based study? Epilepsy Res. 2017;136:88–96. doi: 10.1016/j.eplepsyres.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Corballis M.C., Badzakova-Trajkov G., Häberling I.S. Right hand, left brain: genetic and evolutionary bases of cerebral asymmetries for language and manual action. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3:1–7. doi: 10.1002/wcs.158. [DOI] [PubMed] [Google Scholar]
- Dinkelacker V., Valabregue R., Thivard L., Lehéricy S., Baulac M., Samson S., Dupont S. Hippocampal-thalamic wiring in medial temporal lobe epilepsy: enhanced connectivity per hippocampal voxel. Epilepsia. 2015;56:1217–1226. doi: 10.1111/epi.13051. [DOI] [PubMed] [Google Scholar]
- Drane D.L., Ojemann J.G., Kim M.S., Gross R.E., Miller J.W., Faught R.E., Loring D.W. Interictal epileptiform discharge effects on neuropsychological assessment and epilepsy surgical planning. Epilepsy Behav. 2016;56:131–138. doi: 10.1016/j.yebeh.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke E.S., Tesfaye M., Berl M.M., Walker J.E., Ritzl E.K., Fasano R.E., Conry J.A., Pearl P.L., Sato S., Theodore W.H., Gaillard W.D. The effect of seizure focus on regional language processing areas. Epilepsia. 2012;53:1044–1050. doi: 10.1111/j.1528-1167.2012.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H., Schoenbaum G., Young B., Bunsey M. Functional organization of the hippocampal memory system. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13500–13507. doi: 10.1073/pnas.93.24.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elger C.E., Helmstaedter C., Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.P., Frith C.D., Frackowiak R.S. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1994;2:189–210. [Google Scholar]
- Gaillard W.D., Berl M.M., Moore E.N., Ritzl E.K., Rosenberger L.R., Weinstein S.L., Conry J.A., Pearl P.L., Ritter F.F., Sato S., Vezina L.G. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Gallagher D.T., Hadjiefthyvoulou F., Fisk J.E., Montgomery C., Robinson S.J., Judge J. Prospective memory deficits in illicit polydrug users are associated with the average long-term typical dose of ecstasy typically consumed in a single session. Neuropsychology. 2014;28:43–54. doi: 10.1037/neu0000004. [DOI] [PubMed] [Google Scholar]
- Hamberger M.J., Cole J. Language organization and reorganization in epilepsy. Neuropsychol. Rev. 2011;21:240–251. doi: 10.1007/s11065-011-9180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C., Kurthen M., Linke D.B., Elger C.E. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings, EEG, sex, and age at onset of epilepsy. Brain Cogn. 1997;33:135–150. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L., Chiron C., Jambaqué I., Renaux-Kieffer V., Van de Moortele P., Delalande O., Fohlen M., Brunelle F., Le Bihane D. Late plasticity for language in a child's non-dominant hemisphere: a pre-and post-surgery fMRI study. Brain. 2002;125:361–372. doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- Ives-Deliperi V.L., Butler J.T., Meintjes E.M. Functional MRI language mapping in pre-surgical epilepsy patients: findings from a series of patients in the Epilepsy Unit at Mediclinic Constantiaberg. S. Afr. Med. J. 2013;103:363–368. doi: 10.7196/samj.6336. [DOI] [PubMed] [Google Scholar]
- Janszky J., Jokeit H., Heinemann D., Schulz R., Woermann F.G., Ebner A. Epileptic activity influences the speech organization in medial temporal lobe epilepsy. Brain. 2003;126:2043–2051. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- Janszky J., Mertens M., Janszky I., Ebner A., Woermann F.G. Left-sided interictal epileptic activity induces shift of language lateralization in temporal lobe epilepsy: an fMRI study. Epilepsia. 2006;47:921–927. doi: 10.1111/j.1528-1167.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Jokeit H., Ebner A. Effects of chronic epilepsy on intellectual functions. Prog. Brain Res. 2002;135:455–463. doi: 10.1016/S0079-6123(02)35042-8. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., Weintraub S. Lippincott Williams & Wilkins; Philadelphia: 2001. Boston Naming Test. [Google Scholar]
- Lee D.J., Pouratian N., Bookheimer S.Y., Martin N.A. Factors predicting language lateralization in patients with perisylvian vascular malformations. J. Neurosurg. 2010;113:723–730. doi: 10.3171/2010.2.JNS091595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring D.W., Strauss E., Hermann B.P., Perrine K., Trenerry M.R., Barr W.B., Westerveld M., Chelune G.J., Lee G.P., Meador K.J. Effects of anomalous language representation on neuropsychological performance in temporal lobe epilepsy. Neurology. 1999;53:260–277. doi: 10.1212/wnl.53.2.260. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mateer C.A., Dodrill C.B. Neuropsychological and linguistic correlates of atypical language lateralization: evidence from sodium amytal studies. Hum. Neurobiol. 1983;2:135–142. [PubMed] [Google Scholar]
- Mellet E., Zago L., Jobard G., Crivello F., Petit L., Joliot M., Mazoyer B., Tzourio-Mazoyer N. Weak language lateralization affects both verbal and spatial skills: an fMRI study in 297 subjects. Neuropsychologia. 2014;65:56–62. doi: 10.1016/j.neuropsychologia.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Miller L.A., Muñoz D.G., Finmore M. Hippocampal sclerosis and human memory. Arch. Neurol. 1993;50:391–394. doi: 10.1001/archneur.1993.00540040051014. [DOI] [PubMed] [Google Scholar]
- Miró J., Ripollés P., López-Barroso D., Vilà-Balló A., Juncadella M., de Diego-Balaguer R., Marco-Pallares J., Rodríguez-Fornés A., Falip M. Atypical language organization in temporal lobe epilepsy revealed by a passive semantic paradigm. BMC Neurol. 2014;14:98. doi: 10.1186/1471-2377-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W., Constable R.T., Mencl W.E., Pugh K.R., Fulbright R.K., Shaywitz S.E., Gore J.C., Shankweiler D. An event-related neuroimaging study distinguishing form and content in sentence processing. J. Cogn. Neurosci. 2000;12:120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Piervincenzi C., Petrilli A., Marini A., Caulo M., Committeri G., Sestieri C. Multimodal assessment of hemispheric lateralization for language and its relevance for behavior. NeuroImage. 2016;142:351–370. doi: 10.1016/j.neuroimage.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Reitan R., Wolfson D. Neuropsychological Press; Tucson, AZ: 1985. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Assessment. [Google Scholar]
- Rey A. Presse Universitaire de France; Paris: 1964. L'examen clinique en psychologie. [Google Scholar]
- Rosen W.G. Verbal fluency in aging and dementia. J. Clin. Exp. Neuropsychol. 1980;2:135–146. [Google Scholar]
- Satz P., Orsini D.L., Saslow E., Henry R. The pathological left-handedness syndrome. Brain Cogn. 1985;4:27–46. doi: 10.1016/0278-2626(85)90052-1. [DOI] [PubMed] [Google Scholar]
- Seghier M.L. Laterality index in functional MRI: methodological issues. Magn. Reson. Imaging. 2008;26:594–601. doi: 10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O., Benton A.L. Neuropsychology Laboratory. University of Victoria; Victoria, B.C.: 1977. Neurosensory center comprehensive examination for aphasia (NCCEA), 1977 revision: manual of instructions. [Google Scholar]
- Stewart C.C., Swanson S.J., Sabsevitz D.S., Rozman M.E., Janecek J.K., Binder J.R. Predictors of language lateralization in temporal lobe epilepsy. Neuropsychologia. 2014;60:93–102. doi: 10.1016/j.neuropsychologia.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E., Satz P., Wada J. An examination of the crowding hypothesis in epileptic patients who have undergone the carotid amytal test. Neuropsychologia. 1990;28:1221–1227. doi: 10.1016/0028-3932(90)90057-u. [DOI] [PubMed] [Google Scholar]
- Téllez-Zenteno J.F., Hernández-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res. Treat. 2012;2012 doi: 10.1155/2012/630853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber H.L. Why two brains? In: Schmitt F.O., Worden F.G., editors. The Neurosciences. Third Study Program. MIT Press; Cambridge, MA: 1974. pp. 71–74. [Google Scholar]
- Thivard L., Hombrouck J., du Montcel S.T., Delmaire C., Cohen L., Samson S., Dupont S., Chiras J., Baulac M., Lehéricy S. Productive and perceptive language reorganization in temporal lobe epilepsy. NeuroImage. 2005;24:841–851. doi: 10.1016/j.neuroimage.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Tombaugh T.N., Kozak J., Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch. Clin. Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- Tramoni-Negre E., Lambert I., Bartolomei F., Felician O. Long-term memory deficits in temporal lobe epilepsy. Rev. Neurol. 2017;173:490–497. doi: 10.1016/j.neurol.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Perrone-Bertolotti M., Jobard G., Mazoyer B., Baciu M. Multi-factorial modulation of hemispheric specialization and plasticity for language in healthy and pathological conditions: a review. Cortex. 2017;86:314–339. doi: 10.1016/j.cortex.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Weber B., Wellmer J., Reuber M., Mormann F., Weis S., Urbach H., Ruhlmann J., Elger C.E., Fernández G. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;129:346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation. TX; San Antonio: 1987. Wechsler memory scale-revised manual. [Google Scholar]
- Wechsler D. Psychological Corporation. TX; San Antonio: 1997. Wechsler memory scale (WMS-III) [Google Scholar]
- Wechsler D., Coalson D.L., Raiford S.E. Psychological Corporation. TX; San Antonio: 1997. WAIS-III: Wechsler adult intelligence scale. [Google Scholar]
- Wieser H.G., ILAE Commission on Neurosurgery of Epilepsy Mesial temporal lobe epilepsy with hippocampal sclerosis. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
- Wilke M., Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J. Neurosci. Methods. 2007;163:128–136. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Z-scores in neuropsychological tasks in the total sample (mean ± SD) and groups with typical and atypical hemispheric asymmetry.