Abstract
Ganoderma triterpenes (GTs) are the major secondary metabolites of Ganoderma lucidum, which is a popularly used traditional Chinese medicine for complementary cancer therapy. The present study was to establish a fingerprint evaluation system based on Similarity Analysis (SA), Cluster Analysis (CA) and Principal Component Analysis (PCA) for the identification and quality control of G. lucidum. Fifteen samples from the Chinese provinces of Hainan, Neimeng, Shangdong, Jilin, Anhui, Henan, Yunnan, Guangxi and Fujian were analyzed by HPLC-PAD and HPLC-MSn. Forty-seven compounds were detected by HPLC, of which forty-two compounds were tentatively identified by comparing their retention times and mass spectrometry data with that of reference compounds and reviewing the literature. Ganoderic acid B, 3,7,15-trihydroxy-11,23-dioxolanost-8,16-dien-26-oic acid, lucidenic acid A, ganoderic acid G, and 3,7-oxo-12-acetylganoderic acid DM were deemed to be the marker compounds to distinguish the samples with different quality according to both CA and PCA. This study provides helpful chemical information for further research on the anti-tumor activity and mechanism of action of G. lucidum. The results proved that fingerprints combined with chemometrics are a simple, rapid and effective method for the quality control of G. lucidum.
Keywords: Ganoderma lucidum, triterpenes, HPLC-MSn, Similarity Analysis (SA), chemometrics
1. Introduction
Ganoderma lucidum (Leyss. ex Fr.) Karstis is one of the most highly used medicinal fungi in the world. Its fruiting body, called lingzhi or reishi, has been widely used in traditional Chinese medicine (TCM) as a dietary supplement and medicinal herb in China and other eastern countries. Modern medical research has indicated that G. lucidum has comprehensive biological activities, such as anti-cancer [1,2,3,4,5], immune-modulating [1,3,6], anti-oxidant [6,7,8], anti-microbial [9], anti-inflammatory [10], anti-HIV-1 [11], and so on, among which the most attractive is its anti-cancer activity.
To date, more than 400 compounds were isolated and identified from G. lucidum. Over 150 compounds such as ganoderic acid A (GA-A), GA-C2, GA-D, GA-DM, GA-lactone, ganoderiol F, ganodermanotriol and so on belong to the Ganoderma terpene (GT) class which are regarded as the main medicinal components [9,12,13,14,15]. Accumulating evidence has shown that GTs can inhibit the proliferation of hepatoma cells and HeLa cells, as well as human colon cancer cells HT-29 [16,17,18]. The type and content of triterpene acids reflects the quality of G. lucidum, so GTs could be used as marker components to evaluate the quality of G. lucidum.
The therapeutic effects of traditional Chinese medicines (TCMs) are based on the complex interactions of numerous complicated chemical constituents as a whole system, so methods are needed in order to control the quality of this complex system. In this case, HPLC fingerprints of key components provide a new approach for quality control of traditional Chinese medicines. There are many studies about fingerprints analysis combined with chemometrics for the quality control of traditional Chinese medicines and to find the bioactive components [19,20,21].
Some studies on the fingerprints of G. lucidum have been reported [22,23,24,25], but in these studies, only a few compounds were identified by HPLC-MSn. Yang [26] focused on chemical identification of the GTs, and identified thirty-two compounds, but no marker compounds were found from cluster analysis (CA) and principal component analysis (PCA).
In the present study, forty-seven peaks were detected in HPLC-PDA, of which thirty-seven were common peaks in the similarity analysis. Forty-two known triterpenoids were identified by high-resolution liquid mass spectrometry. To the best of our knowledge, this is the first time that so many compounds were identified. We also found for the first time that ganoderic acid B, 3,7,15-trihydroxy-11,23-dioxo-lanost-8,16-dien-26-oic acid, lucidenic acid A, ganoderic acid G, and 3,7-oxo-12-acetylganoderic acid DM might be suitable marker compounds to distinguish between G. lucidum samples of different quality, according to CA and PCA. This study provides helpful chemical information for further research on the anti-tumor activity and mechanism of action of G. lucidum. The method developed in our study also provides a scientific foundation for the quality control of G. lucidum.
2. Results and Discussion
2.1. Validation of the Method
The relative retention time, relative peak area and similarities were used to evaluate the quality of the fingerprints. Dehydrotumulosic acid (peak 15) which is a large single peak in the middle of the chromatogram, was assigned as the reference peak to calculate relative retention times and relative peak areas.
The precision was determined by repeated injection of the same sample solution six consecutive times. The RSDs of relative retention time and relative peak area of the common peaks were all below 0.94% and 2.88%, respectively; the similarities of different chromatograms were all above 0.995.
The repeatability was evaluated by the analysis of six prepared samples. The RSDs of relative retention time and relative retention time of the common peaks were all below 0.95% and 2.86%, respectively; the similarities of different chromatograms were all above 0.995.
Stability testing was performed with one sample over 24 h. The RSDs of relative retention time and relative retention time of the common peaks were all below 1.06% and 2.71%; the similarities of different chromatograms were all 1.000. All these results indicated that the samples remained stable during the testing period and the conditions were satisfactory for the fingerprint analysis.
2.2. Similarity Analysis (SA)
The chromatographic profile must be representative of all the samples and have the features of integrity and fuzziness. By analyzing the mutual pattern of chromatograms, the identification and authentication of the samples can be conducted well even if the amounts of some chemical constituents are different from the others.
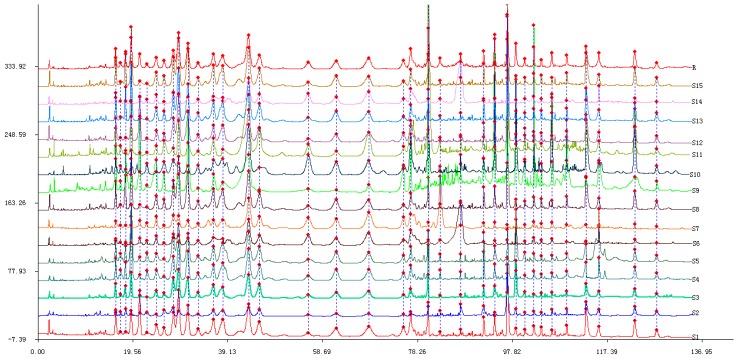
Fifteen batches of samples from different habitats were determined and the chromatograms were analyzed by SES to generate a common pattern R (Figure 1). The peak area of the common peaks was list in the supplementary materials. SES for Chromatographic Fingerprint was performed to calculate the similarities of different chromatograms compared to the common pattern. The results are shown in Table 1.
Figure 1.
Overlaid HPLC chromatograms of samples from No. S1 to S15. The common pattern (marked R) was obtained by using the Similarity Evaluation System (SES) for the Chromatographic Fingerprints of TCMs.
Table 1.
The results of similarities of the chromatograms from different origins.
| No. | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | R |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 1.000 | 0.820 | 0.925 | 0.848 | 0.799 | 0.723 | 0.701 | 0.921 | 0.699 | 0.692 | 0.748 | 0.714 | 0.774 | 0.708 | 0.723 | 0.935 |
| S2 | 0.820 | 1.000 | 0.831 | 0.733 | 0.707 | 0.673 | 0.636 | 0.803 | 0.670 | 0.803 | 0.797 | 0.777 | 0.624 | 0.642 | 0.687 | 0.864 |
| S3 | 0.925 | 0.831 | 1.000 | 0.914 | 0.853 | 0.795 | 0.768 | 0.961 | 0.663 | 0.735 | 0.813 | 0.833 | 0.728 | 0.785 | 0.838 | 0.965 |
| S4 | 0.848 | 0.733 | 0.914 | 1.000 | 0.877 | 0.711 | 0.676 | 0.911 | 0.597 | 0.672 | 0.744 | 0.674 | 0.604 | 0.694 | 0.509 | 0.907 |
| S5 | 0.799 | 0.707 | 0.853 | 0.877 | 1.000 | 0.659 | 0.622 | 0.853 | 0.562 | 0.618 | 0.680 | 0.651 | 0.671 | 0.636 | 0.689 | 0.857 |
| S6 | 0.723 | 0.673 | 0.795 | 0.711 | 0.659 | 1.000 | 0.843 | 0.728 | 0.509 | 0.739 | 0.744 | 0.653 | 0.481 | 0.984 | 0.648 | 0.825 |
| S7 | 0.701 | 0.636 | 0.768 | 0.676 | 0.622 | 0.843 | 1.000 | 0.706 | 0.512 | 0.669 | 0.695 | 0.665 | 0.705 | 0.862 | 0.642 | 0.791 |
| S8 | 0.921 | 0.803 | 0.961 | 0.911 | 0.853 | 0.728 | 0.706 | 1.000 | 0.664 | 0.697 | 0.784 | 0.913 | 0.695 | 0.719 | 0.733 | 0.956 |
| S9 | 0.699 | 0.670 | 0.663 | 0.597 | 0.562 | 0.509 | 0.512 | 0.664 | 1.000 | 0.675 | 0.665 | 0.714 | 0.774 | 0.500 | 0.723 | 0.772 |
| S10 | 0.692 | 0.803 | 0.735 | 0.672 | 0.618 | 0.739 | 0.669 | 0.697 | 0.675 | 1.000 | 0.799 | 0.650 | 0.711 | 0.720 | 0.686 | 0.826 |
| S11 | 0.748 | 0.797 | 0.813 | 0.744 | 0.680 | 0.744 | 0.695 | 0.784 | 0.665 | 0.799 | 1.000 | 0.651 | 0.671 | 0.708 | 0.689 | 0.874 |
| S12 | 0.714 | 0.777 | 0.833 | 0.674 | 0.651 | 0.653 | 0.665 | 0.913 | 0.714 | 0.650 | 0.651 | 1.000 | 0.695 | 0.505 | 0.733 | 0.867 |
| S13 | 0.774 | 0.624 | 0.728 | 0.604 | 0.671 | 0.481 | 0.705 | 0.695 | 0.774 | 0.711 | 0.671 | 0.695 | 1.000 | 0.681 | 0.742 | 0.854 |
| S14 | 0.708 | 0.642 | 0.785 | 0.694 | 0.636 | 0.984 | 0.862 | 0.719 | 0.500 | 0.720 | 0.708 | 0.505 | 0.681 | 1.000 | 0.554 | 0.810 |
| S15 | 0.723 | 0.687 | 0.838 | 0.509 | 0.689 | 0.648 | 0.642 | 0.733 | 0.723 | 0.686 | 0.689 | 0.733 | 0.742 | 0.554 | 1.000 | 0.863 |
| R | 0.935 | 0.864 | 0.965 | 0.907 | 0.857 | 0.825 | 0.791 | 0.956 | 0.772 | 0.826 | 0.874 | 0.867 | 0.854 | 0.810 | 0.863 | 1.000 |
The conclusion can be drawn from the results that the similarities of different chromatograms compared to the common pattern are all above 0.800, except for samples S7 (0.791) and S9 (0.772), which indicates that the chemical constituents of different samples are not highly influenced by their sources. The common pattern is a very positive identification for the samples of G. lucidum.
2.3. Identification of the Compounds Present
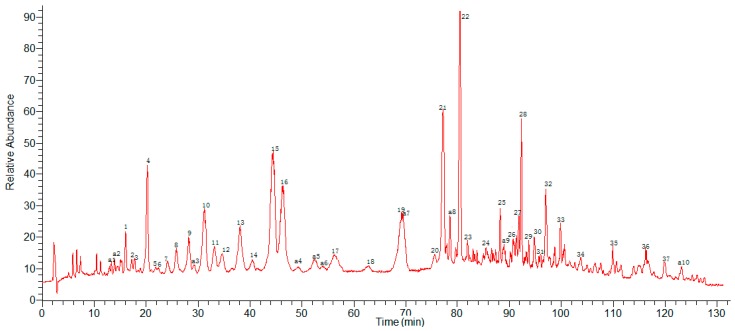
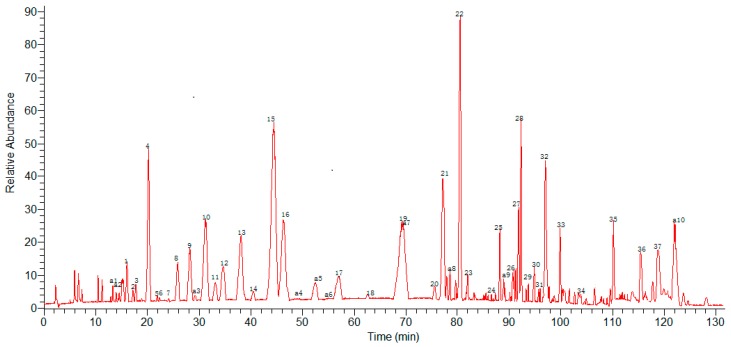
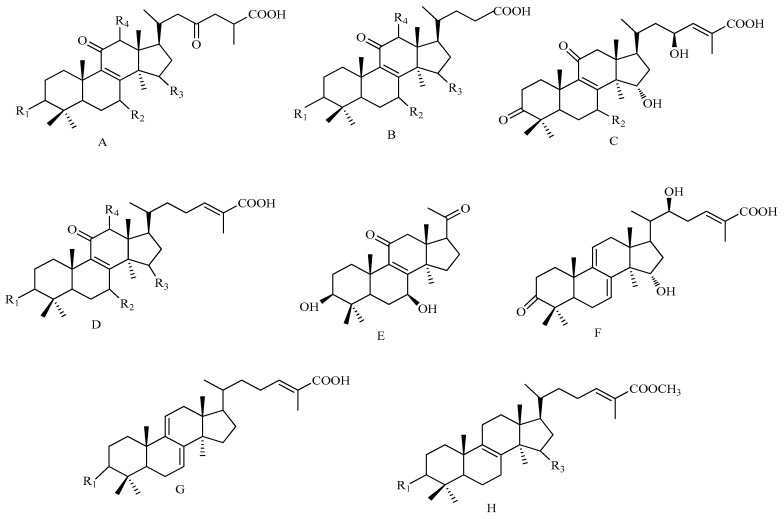
HPLC-ESI-MSn method was employed to identify the components in G. lucidum (Figure 2 and Figure 3) Molecular weights and fragmentation information (Table 2 and Table 3) were obtained. The possible structures of 37 common peaks and ten other peaks a1–a10 were deduced, as shown in Figure 4. Under the optimized MS conditions, the negative mode was used to identify the peaks.
Figure 2.
HPLC chromatograms of G. lucidum.
Figure 3.
Negative mode of the HPLC-MSn chromatograms of G. lucidum.
Table 2.
The HPLC-MSn data and compound names of the 47 peaks.
| Peak No. | tR (min) | [M− H]− | Negative Mode | Identification |
|---|---|---|---|---|
| 1 | 16.07 | 533.3109 | MS1:533.3109 [M − H]− MS2:533.3109→515.3029 [M − H − 18(H2O)]−, 485.2977 [M − H − 18(H2O) − 30(2CH3)]− MS3:515.3029→497.3448 [M − H − 18(H2O) − 18(H2O)]−, 303.1085 [M − H − 18(H2O) − 18(H2O) − 194(pyrolysis fragments of D ring)]− 485.2977→467.3855 [M − H − 18(H2O) − 30(2CH3) − 18(H2O)]− |
12-hydroxyganoderic C2 [26,27] |
| 2 | 17.39 | 515.3452 | MS1:515.3452 [M − H]− | Unknown |
| 3 | 17.79 | 613.2977 | MS1:613.2977 [M − H]− MS2:613.2977→595.3029 [M − H − 18(H2O)]−, 553.3198[M − H − 18(H2O) − 42(CH2=CO)]− MS3:553.3198→535.2648 [M − H − 18(H2O) − 42(CH2=CO) − 18(H2O)]− 343.1749 [M − H − 18(H2O) − 192(pyrolysis fragments of D ring)]− |
3-acetylganoderenic acid K [26] |
| 4 | 20.22 | 515.3011 | MS1:515.3011 [M − H]− MS2:515.3011→497.9281 [M − H − 18(H2O)]−, 453.2738[M − H − 18(H2O) − 44(CO2)]− MS3:453.2738→438.2719 [M − H − 18(H2O) − 44(CO2) − 15(CH3)]−, 423.2209[M − H − 18 (H2O) − 44(CO2) − 30(2CH3)]−, 497.9281→305.2222 [M − H − 18(H2O) − 192(pyrolysis fragments of D ring)]− |
3,7,15-trihydroxy-11,23-dioxo-lanost-8,16-dien-26-oic acid [28] |
| 5 | 21.84 | 517.3159 | MS1:517.3159 [M − H]− MS2:517.3159→499.3881 [M − H − 18(H2O)]−, 481.3099[M − H − 36(2H2O)]−, 455.4148[M − H − 18(H2O) − 44(CO2)]−, 437.4261[M − H − 36(2H2O) − 44(CO2)]− MS3:499.3881→481.3099 [M − H − 18(H2O) − 18(H2O)]−, 481.3099→287.2234 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring)]− |
Ganoderic acid C2 [26,29,30] |
| 6 | 22.83 | 501.3214 | MS1:501.3214 [M − H]− MS2:501.3214→483.3465 [M − H − 18(H2O)]−, 439.4045 [M − H − 18(H2O) − 44(CO2)]−, 421.3404[M − H − 36(2H2O) − 44(CO2)]−, 289.1908 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring)]− |
Ganolucidic acid B [26] |
| 7 | 24.10 | 457.2592 | MS1:457.2592 [M − H]− MS2:457.2592→ 438.9782 [M − H − 18(H2O) − H]−, 420.9395[M − H − 36(2H2O) − H]−, 413.1963[M − H − 44(CO2)]−, 397.1818 [M − H − 44(CO2) − 16(CH4)]−, 395.1743[M − H − 44(CO2) − 18H2O]−, 303.0224 [M − H − 138(pyrolysis fragments of D ring) − 16(CH4)]− |
3-hydroxy-4,4,14-trimethyl-7,11,15-trioxochol-8-en-24-oic-acid [26] |
| 8 | 25.83 | 529.2786 | MS1:529.2786 [M − H]−, 511.2697 [M − H − 18(H2O)]− MS2:511.2697→467.3350 [M − H − 18(H2O) − 44(CO2)]−, 437.3528[M − H − 18(H2O) − 44(CO2) − 30(2CH3)]−, 317.0999 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring)]− MS3:467.3350→423.3057 [M − H − 18(H2O) − 44(CO2) − 44(CO2)]− |
Ganoderic acid C6 [26] |
| 9 | 28.17 | 531.2941 | MS1:531.2941 [M − H]−, 513.2853 [M − H − 18(H2O)]− MS2:513.2853→469.3372 [M − H − 18(H2O) − 44(CO2)]−, 454.2572 [M − H − 18(H2O) − 44(CO2) − 15(CH3)]−, 436.2994 [M − H − 18(H2O) − 44(CO2) − 18(H2O) − 15(CH3)]−, 301.1445 [M − H − 18(H2O) − 18(H2O) − 194(pyrolysis fragments of D ring)]− MS3:469.3372→451.3330 [M − H − 18(H2O) − 44(CO2) − 18(H2O)]−, 265.0820 [M − H − 18(H2O) − 44(CO2) − 204 (pyrolysis fragments of C ring)]− |
Ganoderic acid G [26,31] |
| 10 | 31.25 | 516.2992 | MS1:516.2992 [M − H]−, 497.2901 [M − H − 18(H2O)]− MS2:497.2901→453.2937 [M − H − 18(H2O) − 44(CO2)]−, 303.2104 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring)]−, 287.2104 [M − H − 194(pyrolysis fragments of D ring) − 16(CH4)]− MS3:453.2937→435.2029 [M − H − 44(CO2) − 36(2H2O)]−, 409.3284 [M − H − 18(H2O) − 44(CO2) − 44(CO2)]−, 249.0864 [M − H − 18(H2O) − 44(CO2) − 204(pyrolysis fragments of C ring)]− |
Ganoderic acid B [26,30,31] |
| 11 | 33.14 | 511.2698 | MS1:511.2698 [M − H]− MS2:511.2698→493.3167 [M − H − 18(H2O)]−, 467.3325 [M − H − 44(CO2)]−, 449.3569 [M − H − 18(H2O) − 44(CO2)]−, 434.2375 [M − H − 18(H2O) − 59(Ac-)]− MS3:493.3167→245.1126 [M − H − 18(H2O) − 44(CO2) − 204 (pyrolysis fragments of C ring)]− 147.0566 [M − H − 18(H2O) − 44(CO2) − 204 (pyrolysis fragments of C ring) − 98(pyrolysis fragments of A ring)]− |
unknown |
| 12 | 34.63 | 513.2588 | MS1:513.2588 [M − H]− MS2:513.2588→495.2083 [M − H − 18(H2O)]−, 451.2515 [M − H − 18(H2O) − 44(CO2)]−, 436.2632 [M − H − 18(H2O) − 59(Ac-)]− MS3:495.2083→249.0978 [M − H − 18(H2O) − 36(2H2O) − 16(CH4) − 194(pyrolysis fragments of D ring)]− |
Ganoderic acid AM1 [26,32] |
| 13 | 38.02 | 573.3042 | MS1:573.3042 [M − H]−, 555.2953 [M − H − 18(H2O)]− MS2:555.2953→511.2890 [M − H − 18(H2O) − 44(CO2)]−, 496.3256 [M − H − 18(H2O) − 59(CH3COO-)]− MS3:511.2890→265.0914 [M − H − 18(H2O) − 44(CO2) − 42(CH2=CO) − 204(pyrolysis fragments of C ring)]− 496.3256→302.1797 [M − H − 18(H2O) − 59((CH3COO) − 194(pyrolysis fragments of D ring)]− |
Ganoderic acid K [26] |
| 14 | 40.45 | 457.2594 | MS1:457.2594 [M − H]− MS2:457.2594→442.4391 [M − H − 15(CH3)]−, 439.0501[M − H − 18(H2O)]−, 421.4436[M − H − 36(2H2O)]− 395.3611 [M − H − 18(H2O) − 44(CO2)]−, 301.3354 [M − H − 138(pyrolysis fragments of D ring) − 18(H2O)]− |
Lucidenic acid A [26] |
| 15 | 44.49 | 515.3004 | MS1:515.3004 [M − H]− MS2:515.3004→497.2571 [M − H − 18(H2O)]−, 479.3175 [M − H − 36(2H2O)]− MS3:497.2571→435.3353 [M − H − 18(H2O) − 18(H2O) − 44(CO2)]−, 303.1984 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring)]− |
Ganoderic acid A [26,30,31] |
| 16 | 46.25 | 571.2893 | MS1:571.2893 [M − H]−, 553.2797 [M − H − 18(H2O)]− MS2:553.2797→511.2424 [M − H − 18(H2O) − 42(CH2=CO)]−, 481.3605 [M − H − 18(H2O) − 42(CH2=CO) − 30(2CH3)]−, MS3:511.2424→467.3026 [M − H − 18(H2O) − 42(CH2=CO) − 44(CO2)]−, 437.3870 [M – H − 18(H2O) − 42(CH2=CO) − 44(CO2) -30(2CH3)]−, 303.1073[M − H − 18(H2O) − 42(CH2=CO) − 194(pyrolysis fragments of D ring) − 14(CH2)]−, 301.1706[M – H − 18(H2O) − 42(CH2=CO) − 194(pyrolysis fragments of D ring) − 16(CH4)]− |
Ganoderic acid H [26,33] |
| 17 | 52.47 | 527.2637 | MS1:527.2637 [M − H]−, 509.2544 [M − H − 18(H2O)]− MS2:509.2544→465.2312 [M − H − 18(H2O) − 44(CO2)]−, 435.2996 [M − H − 18(H2O) − 44(CO2) − 30(2CH3)]−, 301.2139 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring) − 14(CH2)]−, 299.1358 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring) − 16(CH4)]− |
12-hydroxy-3,7,11,15,23-pentaoxo-lanost-8-en-26-oic acid [26] |
| 18 | 62.71 | 615.2795 | MS1:615.2795 [M − H]−, 597.3021 [M − H − 18(H2O)]− MS2:597.3021→553.2849 [M − H − 18(H2O) − 44(CO2)]−, 511.2561 [M − H − 18(H2O) − 44(CO2) − 42(CH2=CO)]−, 493.2861 [M − H − 18(H2O) − 88(2CO2) − 16(CH4)]−, 467.4220[M − H − 18(H2O) − 88(2CO2) − 42(CH2=CO)]− MS3:553.2849→509.1722 [M − H − 18(H2O) − 44(CO2) − 44(CO2)]−, 479.1404 [M − H − 18(H2O) − 44(CO2) − 44(CO2) − 30(2CH3)]−,449.4641 [M − H − 18(H2O) − 44(CO2) − 44(CO2) − 42(CH2=CO) − 18(H2O)]− |
12,15-bis(acetyloxy)-3-hydroxy-7,11,23-trioxo-lanost-8-en-26-oic-acid [26] |
| 19 | 69.36 | 513.2836 | MS1:513.2836 [M − H]−, 495.2746 [M − H − 18(H2O)]− MS2:495.2746→451.3033 [M − H − 18(H2O) − 44(CO2)]−, 436.2344 [M − H − 18(H2O) − 44(CO2) − 15(CH3)]−, 301.1673 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring)]−, 285.1029 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring) − 14(CH2)]−, MS3:451.3033→433.3118 [M − H − 18(H2O) − 44(CO2) − 18(H2O)]−, 407.2886[M − H − 18(H2O) − 44(CO2) − 44(CO2)]−, 247.0793 [M − H − 18(H2O) − 44(CO2) − 204(pyrolysis fragments of C ring)]− |
Ganoderic acid D [26,30] |
| 20 | 75.66 | 511.2693 | MS1:511.2693 [M − H]− MS2:511.2693→493.2604 [M − H − 18(H2O)]−, 449.2799[M − H − 18(H2O) − 44(CO2)]− MS3:493.2604→299.2487 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring)]− 449.2799→434.2175 [M − H − 18(H2O) − 44(CO2) − 15(CH3)]−, 419.3584 [M − H − 18(H2O) − 44(CO2) − 30(2CH3)]− |
Ganoderic acid F [26] |
| 21 | 77.24 | 499.3067 | MS1:499.3067 [M − H]− MS2:499.3067→481.3056 [M − H − 18(H2O)]−, 437.3787 [M − H − 18(H2O) − 44(CO2)]−, MS3:481.3056→287.2167 [M − H − 18(H2O) − 194(pyrolysis fragments of D ring)]−, 437.3787→419.2850 [M − H − 18(H2O) − 44(CO2) − 18(H2O)]− |
Ganolucidic acid D [26] |
| 22 | 80.47 | 569.2731 | MS1:569.2731 [M − H]−, 551.0040 [M − H − 18(H2O)]− MS2:551.0040→509.2411 [M − H − 18(H2O) − 42(CH2=CO)]−, 479.2818[M − H − 18(H2O) − 42(CH2=CO) − 30(2CH3)]−, 317.2806 [M − H − 204 (pyrolysis fragments of C ring) − 30(2CH3)]− MS3:509.2411→465.2256 [M − H − 18(H2O) − 42(CH2=CO) − 44(CO2)]−, 435.3218 [M − H − 18(H2O) − 42(CH2=CO) − 44(CO2) − 30(2CH3)]−, 301.2180[M − H − 18(H2O) − 42(CH2=CO) − 194(pyrolysis fragments of D ring) − 14(CH2)]− |
12-acetoxyganoderic acid F [26,27] |
| 23 | 81.87 | 513.2857 | MS1:513.2857 [M − H]− MS2:513.2857→451.2750 [M − H − 18(H2O) − 44(CO2)]−, 436.3795 [M − H − 18(H2O) − 44(CO2) − 15(CH3) ]−, 305.2700 [M − H − 194(pyrolysis fragments of D ring) − 14(CH2)]−, 251.1266 [M − H − 44(CO2) − 204(pyrolysis fragments of C ring) − 14(CH2)]− MS3:451.2750→421.2310 [M − H − 18(H2O) − 44(CO2) − 30(2CH3)]−, 403.253 [M − H − 18(H2O) − 44(CO2) − 30(2CH3) − 18(H2O)]− |
Ganoderic acid J [26] |
| 24 | 86.30 | 497.2899 | MS1:497.2899 [M − H]− MS2:497.2899→479.2302 [M − H − 18(H2O)]−, 453.2728 [M − H − 44(CO2)]−, 435.2746 [M − H − 18(H2O) − 44(CO2)]−, 285.1586 [M − H − 18(H2O − 194(pyrolysis fragments of D ring)]− |
Ganoderic acid GS [32] |
| 25 | 88.22 | 483.3108 | MS1:483.3108 [M-H]− MS2:483.3108→467.2955 [M − H − 16(CH4)]−, 465.3409 [M − H − 18(H2O)]−, 439.3409 [M − H − 44(CO2)]−, 421.3387 [M − H − 18(H2O) − 44(CO2)]−, 385.1546 [M − H − 98(pyrolysis fragments of A ring)]−, 345.2003 [M − H − 138(pyrolysis fragments of B ring)]−, 315.1342 [M − H-178(pyrolysis fragments of D ring)], 287.1245 [M − H − 138(pyrolysis fragments of B ring) − 18(H2O)]− MS3:345.2003→301.2150 [M − H − 138(pyrolysis fragments of B ring) − 44(CO2)]−, 271.0611 [M − H − 138(pyrolysis fragments of B ring) − 44(CO2) − 30(2CH3)]−, 269.1784 [M − H − 138(pyrolysis fragments of B ring) − 44(CO2) − 32(2CH4)]− |
3,7-oxo-12-hydroxy-ganoderic acid DM [27,32] |
| 26 | 91.31 | 529.3177 | MS1:529.3177 [M − H]− MS2:529.3177→511.3445 [M − H − 18(H2O)]−, 493.3448 [M − H − 36(2H2O)]−, 467.3685 [M − H − 18(H2O) − 44(CO2)]−, 299.1341 [M − H − 36(2H2O) − 194(pyrolysis fragments of D ring)]− MS3:467.3685→449.3226 [M − H − 18(H2O) − 44(CO2) − 18(H2O)]−, 419.1971 [M − H − 18(H2O) − 44(CO2) − 18(H2O) − 30(2CH3)]−, 263.3528 [M − H − 18(H2O) − 44(CO2) − 204(pyrolysis fragments of C ring)]−, 247.0979 [M − H − 18(H2O) − 44(CO2) − 204(pyrolysis fragments of C ring) − 16(CH4)]− |
12-hydroxyganoderic acid D [26] |
| 27 | 91.83 | 613.3005 | MS1:613.3005 [M − H]−, 595.2902 [M − H − 18(H2O)]− MS2:595.2902→553.2996 [M − H − 18(H2O) − 42(CH2=CO)]−, 523.2399 [M − H − 18(H2O) − 44(CO2) − 28(2CH2))]−, 509.3708 [M − H − 18(H2O) − 44(CO2) − 42(CH2=CO)]− MS3:553.2996→479.2277 [M − H − 18(H2O) − 42(CH2=CO) − 44(CO2) − 30(2CH3)]−, 465.3148[M − H − 18(H2O) − 42(CH2=CO) − 88(2CO2)]−, 345.2563 [M − H − 18(H2O) − 42(CH2=CO) − 194(pyrolysis fragments of D ring) − 14(CH2)]−, 343.3474 [M − H − 18(H2O) − 42(CH2=CO) − 194(pyrolysis fragments of D ring) − 16(CH4)]− |
3-acetylganoderic acid H [26] |
| 28 | 91.30 | 570.0023 | MS1:570.0023 [M − H] | Unknown |
| 29 | 93.34 | 483.3266 | MS1:483.3266 [M − H]− MS2:483.3266→465.3160 [M − H − 18(H2O)]−, 447.2954 [M − H − 36(2H2O)]−, 439.4073453.2728 [M − H − 44(CO2)]−, 421.4003 [M − H − 18(H2O) − 44(CO2)]−, 361.1981 [M − H − 18(H2O) − 44(CO2) − 60(CH3COOH)]−, 255.1103 [M − H − 178(pyrolysis fragments of D ring) − 18(H2O) − 32(2CH4)]− |
15-hydroxyganoderic acid DM [32] |
| 30 | 95.05 | 525.3211 | MS1:525.3211 [M − H]− MS2:525.3211→483.2451 [M − H − 42(CH2=CO)]−, 439.4126 [M − H − 42(CH2=CO) − 44(CO2)]−, 421.4462 [M − H − 42(CH2=CO) − 44(CO2) − 18(H2O)]−,329.4416 [M − H − 18(H2O) − 178(pyrolysis fragments of D ring)]− MS3:483.2451→465.3002 [M − H − 42(CH2=CO) − 18(H2O)]−, 287.2225 [M − H − 42(CH2=CO) − 18(H2O) − 178(pyrolysis fragments of D ring)]−, 269.1860 [M − H − 42(CH2=CO) − 36(2 H2O) − 178(pyrolysis fragments of D ring)]- |
3,7-oxo-12-acetylganoderic acid DM [26] |
| 31 | 96.23 | 571.2204 | MS1:571.2204 [M − H]− | Unknown |
| 32 | 97.07 | 499.3419 | MS1:499.3419 [M − H]− MS2:499.3419→481.2946 [M − H − 18(H2O)]−, 455.0124 [M − H − 44(CO2)]− , 437.2764 [M − H − 18(H2O) − 44(CO2)]− 287.0924 [M − H − 194(pyrolysis fragments of D ring) − 18(H2O)]− |
Ganolucidic acid A [26] |
| 33 | 99.83 | 467.3156 | MS1:467.3156 [M − H]− MS2:467.3156→449.3837 [M − H − 18(H2O)]−, 423.3398 [M − H − 44(CO2)]−, 383.0190 [M − H − 84(2CH2=CO)]−, 257.1906 [M − H − 178 (pyrolysis fragments of D ring) − 32(2CH4)]− MS3:423.3398→407.2750 [M − H − 44(CO2) − 16(CH4)]−, 337.3115 [M − H − 44(CO2) − 44(CO2) − 42(CH2=CO)]−, 311.2945 [M − H − 44(CO2) − 98(pyrolysis fragments of A ring) − 14(CH2)]− |
Ganoderic acid DM [32] |
| 34 | 103.86 | 401.0025 | MS1:401.0025 [M - H]− MS2:401.0025→383.1729 [M − H − 18(H2O)]−, 344.2189 [M − H − 42(CH2=CO) − 15(CH3)]−, 303.2025 [M − H − 18(H2O) − 80(pyrolysis fragments of D ring)]− |
Lucidone A [32] |
| 35 | 111.95 | 453.3369 | MS1:453.3369 [M − H]− MS2:453.3369→435.2218 [M − H − 18(H2O)]−, 409.4311 [M − H − 44(CO2)]−, 393.2309 [M − H − 60 (CH3COOH)]−, 391.4413 [M − H − 18(H2O) − 44(CO2)]−, 207.1283[M − H − 42(CH2=CO) − 204(pyrolysis fragments of C ring)]− MS3:393.2309→375.2531 [M − H − 60 (CH3COOH) − 18(H2O)]−, 359.2667 [M − H − 60 (CH3COOH) − 18(H2O) − 16(CH4)]− |
Ganoderic acid TR or Ganoderic acid Y [32] |
| 36 | 116.41 | 495.2749 | MS1:495.2749 [M − H]− MS2:495.2749→477.4175 [M − H − 18(H2O)]−, 451.2777 [M − H − 44(CO2)]−, 436.2990 [M − H − 44(CO2) − 15(CH3)]−, 301.1088 [M − H − 194(pyrolysis fragments of D ring)]−, 285.1394 [M − H − 194(pyrolysis fragments of D ring) - 16(CH4)]−, 247.1259 [M − H − 44(CO2) − 204(pyrolysis fragments of C ring)]− |
3,11,15-trioxochol-8-en-24-oic acid [26,27] |
| 37 | 119.35 | 459.2901 | MS1:459.2901 [M − H]− MS2:459.2901→441.4392 [M − H − 18(H2O)]−, 423.2791 [M − H − 36(2H2O)]−, 397.6952 [M − H − 18(H2O) − 44(CO2)]−, 285.2697 [M − H − 36(2H2O) − 138(pyrolysis fragments of D ring)]−, 269.1612 [M − H − 36(2H2O) − 138(pyrolysis fragments of D ring) − 16(CH4)]− |
7,15-dihydroxy-4,4,14-trimethyl-3,11-dioxochol-8-en-24-oic acid [26] |
| a1 | 13.31 | 527.2641 | MS1:527.2641 [M − H]− MS2:527.2641→509.2797 [M − H − 18(H2O)]−, 483.2253 [M − H − 44(CO2)]−, 465.2714 [M − H − 18(H2O) − 44(CO2)]−, 317.1736 [M − H − 18(H2O) − 192(pyrolysis fragments of D ring)]−MS3:465.2714→447.2611 [M − H − 18(H2O) − 44(CO2) − 18(H2O)] −, 421.2402 [M − H − 18(H2O) − 44(CO2) − 44(CO2)]− |
3,12-dihydroxy-4,4,14-trimethyl-7,11,15- trioxo-lanost-8,9,20,22-en-26-oic acid [26,27] |
| a2 | 13.71 | 511.3550 | MS1:511.3550 [M − H]− MS2:511.3550→469.3110 [M − H − 42(CH2=CO)]−, 467.2477[M − H − 44(CO2)]−, 425.3692[M − H − 42(CH2=CO) − 44(CO2)]−, 303.1880 [M − H − 192(pyrolysis fragments of D ring) − 16(CH4)]− |
Ganoderic acid Mf [26,33] |
| a3 | 29.16 | 459.2763 | MS1:459.2763 [M − H]− MS2:459.2763→441.2818 [M − H − 18(H2O)]−, 423.3502 [M − H − 36(2H2O)]−, 397.4172 [M − H − 18(H2O) − 44(CO2)]− 303.2930 [M − H − 18(H2O) − 138(pyrolysis fragments of D ring)]−, 289.2338 [M − H − 18(H2O) − 138(pyrolysis fragments of D ring) − 14(CH2)]−, 288.4626 [M − H − 18(H2O) − 138(pyrolysis fragments of D ring) − 15(CH3)]− |
Lucidenic acid N [26] |
| a4 | 49.03 | 511.2703 | MS1:511.2703 [M − H]−, 493.2587 [M − H − 18(H2O)]− MS2:493.2587→478.3034 [M − H − 18(H2O) − 15(CH3)]−, 449.3233[M − H − 18(H2O) − 44(CO2)]−, 431.3262 [M − H − 18(H2O) − 44(CO2) − 18(H2O)]−, 301.0695 [M − H − 192(pyrolysis fragments of D ring)]−, 261.1931 [M − H − 204 (pyrolysis fragments of C ring) − 28 (CO)]−, 247.0212[M - H − 204 (pyrolysis fragments of C ring) − 42(CH2=CO)]− |
Ganoderenic acid D [26] |
| a5 | 52.47 | 515.3007 | MS1:515.3007 [M − H]− MS2:515.3007→497.3394 [M − H − 18(H2O)]−, 453.2672 [M − H − 18(H2O) − 44(CO2)]−, 435.3178[M − H − 36(2H2O) − 44(CO2)]− MS3:497.3394→435.3178 [M − H − 18(H2O) − 18(H2O) − 44(CO2)]−, 303.2353 [M − H − 18(H2O) − 194 (pyrolysis fragments of D ring)]− |
Ganoderic acid δ [31,33] |
| a6 | 54.24 | 527.2637 | MS1:527.2637 [M − H]−, 509.2544 [M - H - 18(H2O)]− MS2:509.2544→479.1830 [M − H − 18(H2O) − 30(2CH3)]−, 465.2850 [M − H − 18(H2O) − 44(CO2)]−, 435.2603 [M − H − 18(H2O) − 44(CO2) − 30(2CH3)]−, 317.2471 [M − H − 18(H2O) − 192 (pyrolysis fragments of D ring)]−, 301.1240 [M − H − 18(H2O) − 192 (pyrolysis fragments of D ring) − 16(CH4)]−, 299.1788 [M − H − 18(H2O) − 192 (pyrolysis fragments of D ring) − 18(H2O)]− |
Elfvingic acid A [26] |
| a7 | 69.32 | 513.2836 | MS1:513.2836 [M − H]−, 495.2746 [M − H − 18(H2O) − MS2:495.2746→451.3008 [M − H − 18(H2O) − 44(CO2)]−, 437.3971 [M − H − 18(H2O) − 44(CO2) − 14(CH2)]−, 303.1641 [M − H − 18(H2O) − 192 (pyrolysis fragments of D ring)]−, 287.1062 [M − H − 18(H2O) − 192 (pyrolysis fragments of D ring) − 16(CH4)]− MS3:451.3008→433.2937 [M − H − 18(H2O) − 44(CO2) − 18(H2O)]−, 407.3061 [M − H − 18(H2O)) − 44(CO2) − 44(CO2)]−, 247.0545 [M − H − 18(H2O) − 44(CO2) − 18(H2O) − 204 (pyrolysis fragments of C ring)]− |
Ganoderenic acid B [26] |
| a8 | 79.87 | 513.2494 | MS1:513.2836 [M - H]− MS2:513.2494→471.1854 [M − H − 42(CH2=CO)]−, 456.3038 [M − H − 42(CH2=CO) − 15(CH3)]−, 453.1012 [M − H − 42(CH2=CO) − 18(H2O)]−, 435.2854 [M − H − 42(CH2=CO) − 36(2H2O)]−, 301.2219 [M − H − 42(CH2=CO) − 138 (pyrolysis fragments of D ring) − 32(2CH4)]− |
Lucidenic acid D [26] |
| a9 | 88.41 | 555.2974 | MS1:555.2974 [M - H]− MS2:555.2974→537.0157 [M − H − 18(H2O)]−, 513.3628 [M − H − 42(CH2=CO)]−, 495.2735 [M − H − 18(H2O) − 42(CH2=CO)]−, 451.3274 [M − H − 18(H2O) − 42(CH2=CO) − 44(CO2)]− MS3:513.3628→263.1146 [M - H − 42(CH2=CO) − 56(2CO) − 194 (pyrolysis fragments of D ring)]−, 249.3468 [M − H − 42(CH2=CO) − 18(H2O) − 42(CH2=CO) − 204 (pyrolysis fragments of C ring)]−, 247.0499 [M − H − 42(CH2=CO) − 18(H2O) − 44(CO2) − 204 (pyrolysis fragments of C ring)]− |
Lucidenic acid GS-3 [32,33] |
| a10 | 124.88 | 471.3473 | MS1:471.3473 [M − H]− MS2:471.3473→435.4189 [M − H − 36 (2H2O)]−, 395.3422 [M − H − 32(2CH4) − 44(CO2)]−, 367.1648 [M − H − 44(CO2) - 60(CH3COOH)]−, 353.1996 [M − H − 44(CO2) − 60(CH3COOH) − 14(CH2)]− |
unknown |
Table 3.
The chemical structures of the identified compounds.
| No. | Chemical Name | Ty. | R1 | R2 | R3 | R4 | C=C | M |
|---|---|---|---|---|---|---|---|---|
| 1 | 12-Hydroxyganoderic acid C2 | A | β-OH | β-OH | α-OH | OH | - | 534.3109 |
| 3 | 3-Acetylganoderenic acid K | A | β-OAc | β-OH | =O | β-OAc | Δ20, 22 | 613.2977 |
| 4 | 3,7,15-Trihydroxy-11,23-dioxolanost-8,16-dien-26-oic acid | A | β-OH | β-OH | β-OH | - | Δ16, 17 | 516.3011 |
| 5 | Ganoderic acid C2 | A | β-OH | β-OH | α-OH | H | - | 518.3159 |
| 6 | Ganolucidic acid B | A | β-OH | H | α-OH | H | - | 502.3214 |
| 7 | 3-Hydroxy-4,4,14-trimethyl-7,11,15-trioxochol-8-en-24-oic-acid | B | β-OH | =O | =O | H | - | 458.2592 |
| 8 | Ganoderic acid C6 | A | β-OH | =O | =O | β-OH | - | 530.2786 |
| 9 | Ganoderic acid G | A | β-OH | β-OH | =O | β-OH | - | 532.2941 |
| 10 | Ganoderic acid B | A | β-OH | β-OH | =O | H | - | 516.2992 |
| 12 | Ganoderic acid AM1 | A | β-OH | =O | =O | H | - | 514.2588 |
| 13 | Ganoderic acid K | A | β-OH | β-OH | =O | β-OAc | - | 574.3042 |
| 14 | Lucidenic acid A | B | =O | β-OH | =O | H | 458.2594 | |
| 15 | Ganoderic acid A | A | =O | β-OH | α-OH | H | - | 516.3004 |
| 16 | Ganoderic acid H | A | β-OH | =O | =O | β-OAc | - | 572.2893 |
| 17 | 12-Hydroxy-3,7,11,15,23-pentaoxolanost-8-en-26-oic acid | A | =O | =O | =O | -OH | - | 528.2637 |
| 18 | 12,15-Bis(acetyloxy)-3-hydroxy-7,11,23-trioxo-lanost-8-en-26-oic-acid | A | OH | =O | OAc | OAc | - | 616.2795 |
| 19 | Ganoderic acid D | A | =O | β-OH | =O | H | - | 514.2836 |
| 20 | Ganoderic acid F | A | =O | =O | =O | H | - | 512.2693 |
| 21 | Ganolucidic acid D | C | - | - | - | - | - | 500.3067 |
| 22 | 12-Acetoxyganoderic acid F | A | =O | =O | =O | β-OAc | - | 570.2731 |
| 23 | Ganoderic acid J | A | =O | =O | α-OH | H | - | 514.2857 |
| 24 | Ganoderic acid GS | A | =O | =OH | =O | =O | - | 498.2899 |
| 25 | 3,7-Oxo-12-hydroxy-ganoderic acid DM | D | =O | =O | H | OH | - | 484.3108 |
| 26 | 12-Hydroxyganoderic acid D | A | =O | β-OH | =O | OH | - | 530.3177 |
| 27 | 3-Acetylganoderic acid H | A | β-OAc | =O | =O | β-OAc | - | 614.3005 |
| 29 | 15-Hydroxyganoderic acid DM | D | =O | H | -OH | H | - | 484.3266 |
| 30 | 3,7-Oxo-12-acetylganoderic acid DM | D | =O | =O | - | β-OAc | - | 526.3211 |
| 32 | Ganolucidic acid A | A | =O | H | α-OH | H | - | 500.3419 |
| 33 | Ganoderic acid DM | D | =O | H | H | H | - | 468.3156 |
| 34 | Lucidone A | E | - | - | - | - | - | 402.0025 |
| 35 | Ganoderic acid TR | F | - | - | - | - | - | 454.3369 |
| Ganoderic acid Y | G | β-OH | - | - | - | - | ||
| 36 | 3,11,15-Trioxochol-8-en-24-oic acid | A | =O | H | =O | H | - | 496.2749 |
| 37 | 7,15-Dihydroxy-4,4,14-trimethyl-3,11-dioxochol-8-en-24-oic acid | B | =O | OH | OH | H | - | 460.2901 |
| a1 | 3,12-Dihydroxy-4,4,14-trimethyl-7,11,15-trioxolanost-8,9,20,22-en-26-oic acid | A | β-OH | =O | =O | β-OH | Δ20, 22 | 528.2641 |
| a2 | Ganoderic acid Mf | H | β-OAc | - | - | - | - | 512.3550 |
| a3 | Lucidenic acid N | B | β-OH | β-OH | =O | H | - | 460.2763 |
| a4 | Ganoderenic acid D | A | =O | β-OH | =O | H | △20, 22 | 512.2703 |
| a5 | Ganoderic acid δ | C | - | -OH | - | H | - | 516.3007 |
| a6 | Elfvingic acid A | A | =O | =O | β-OH | α-OH | Δ20, 22 | 528.2637 |
| a7 | Ganoderenic acid B | A | β-OH | β-OH | =O | H | Δ20, 22 | 514.2836 |
| a8 | Lucidenic acid D | B | =O | =O | =O | β-OAc | - | 514.2494 |
| a9 | Lucidenic acid GS-3 | A | β-OH | β-OH | =O | β-OAc | - | 556.2974 |
Figure 4.
The chemical structures of the identified compounds.
As shown in Table 2, in the negative mode ESI-MS spectra, the [M − H]− and [M − H2O − H]− ions were found for all 47 compounds. The [M − CO2 − H]− ion was seen for most of the compounds. In type A and C, the molecular weight of pyrolysis fragments of D ring was 194, while there is aΔ20, 22 or Δ16, 17, the molecular weight of pyrolysis fragments of D ring was 192. In type B, the molecular weight of pyrolysis fragments of D ring was 138. In type D, the molecular weight of pyrolysis fragments of D ring was 178. In type E, the molecular weight of pyrolysis fragments of D ring was 80, only for compound 34. In type F, the molecular weight of pyrolysis fragments of D ring was also 194, without R1, R2, R3, and R4, only for compound 35. In type G, the molecular weight of pyrolysis fragments of D ring was also 178, without R2, R3, and R4, only for compound 35. In type H, the molecular weight of pyrolysis fragments of D ring was also 192, without C=C, only for compound a2.
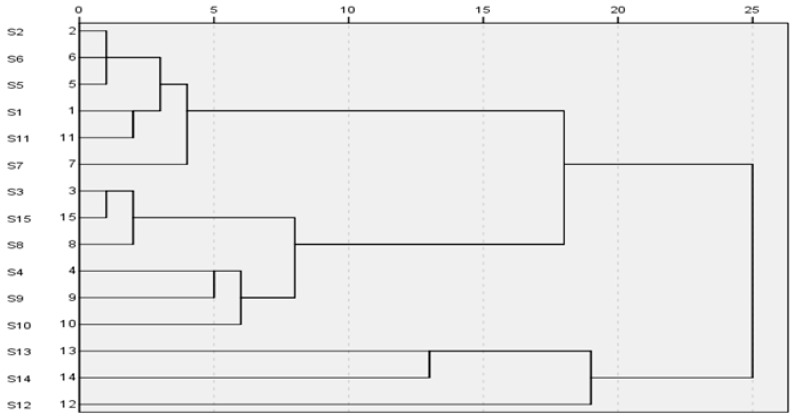
2.4. Cluster Analysis (CA)
Cluster analysis is a multivariate analysis technique that is used to sort samples into groups. It is widely applied for fingerprint analysis, because it is a nonparametric data interpretation method and simple to use. CA provides a visual representation of complex data. Average linkage between groups was applied, and Pearson correlation was selected as a measurement. The method can classify different herbs by measuring the peak areas from their corresponding HPLC fingerprints. The common characteristic peaks, which were calculated by the Similarity Evaluation System, were selected for the CA. Cluster analysis of G. lucidum samples was performed based on the relative peak areas of all 37 common peaks.
The CA results are shown in Figure 5, where the quality characteristics are revealed more clearly. The cluster analysis results show that the samples could be divided into three quality clusters. Among them, Cluster I includes the samples S2, S6, S5, S1, S11 and S7, Cluster III includes S13 S14 and S12, the others are in Cluster II. All the compounds in Cluster II had much lower concentrations than the other two clusters.
Figure 5.
Results of cluster analysis of 15 samples.
Cluster I was distinguished as it contains more 3-acetylganoderenic acid K (F3), ganoderic acid G (F9), ganoderic acid B (F10), unknown F11, lucidenic acid A (F14), and 3,7-oxo-12-acetylganoderic acid DM (F30) than Clusters II and III. The higher concentration of these compounds in Cluster I may be due to the good quality of G. lucidum herb. This indicated that these compounds could be used as marker compounds to distinguish the G. lucidum samples with different quality. The results of CA could be validated against each other and provided more references for the quality evaluation of G. lucidum.
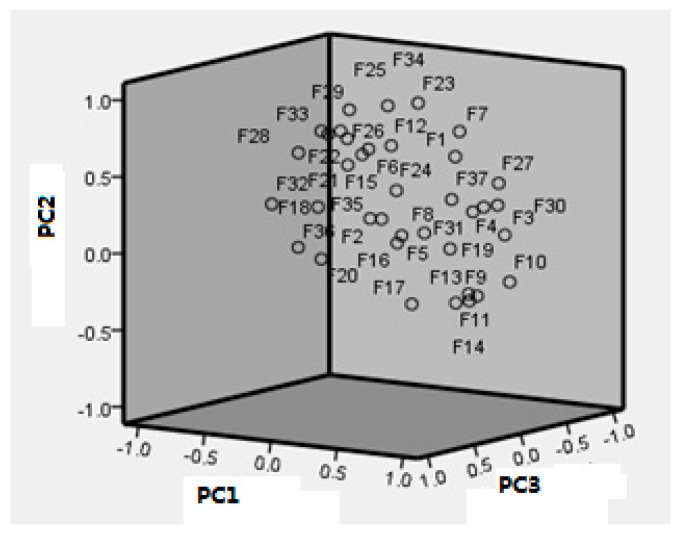
2.5. Principal Components Analysis (PCA)
To evaluate the variations in quality of the 15 samples, PCA was carried out with the relative amounts of each identified component. The contents of 37 fingerprint peaks were applied to evaluate the sample variations. Figure 6 shows the score plots obtained by PCA. The first six principal components accounted for 93.69% of the total variance. Examination of the score plots indicates that the main components responsible for the separation were ganoderic acid B (F10), 3-acetylganoderenic acid K (F3), 3,7-oxo-12-acetylganoderic acid DM (F30), ganoderic acid G (F9), 3,7,15-trihydroxy-11,23-dioxolanost-8,16-dien-26-oic acid (F4), lucidenic acid A (F14), 3-acetyl-ganoderic acid H (F27) and unknown F11, as shown in Figure 6 and Table 4.
Figure 6.
PCA scores plots of the sample from different regions.
Table 4.
Factor loading matrix of the testing samples.
| Peak No. | Principal Component Values | |||||
|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | |
| 1 | 0.058 | 0.077 | −0.014 | −0.087 | −0.025 | 0.007 |
| 2 | −0.018 | −0.012 | −0.087 | 0.074 | 0.407 | 0.008 |
| 3 | 0.092 | 0.006 | −0.059 | 0.024 | −0.079 | 0.058 |
| 4 | 0.078 | 0.040 | −0.018 | −0.077 | 0.037 | 0.053 |
| 5 | 0.019 | −0.050 | −0.043 | 0.307 | −0.017 | 0.117 |
| 6 | −0.010 | 0.096 | 0.062 | 0.048 | −0.095 | 0.242 |
| 7 | 0.041 | 0.079 | −0.157 | 0.057 | 0.035 | 0.121 |
| 8 | 0.057 | −0.033 | 0.051 | 0.046 | −0.051 | 0.147 |
| 9 | 0.079 | −0.067 | −0.024 | 0.077 | 0.085 | 0.048 |
| 10 | 0.096 | −0.025 | −0.044 | 0.025 | −0.023 | 0.019 |
| 11 | 0.077 | −0.050 | 0.078 | −0.080 | 0.040 | 0.046 |
| 12 | 0.015 | 0.090 | 0.070 | −0.074 | 0.006 | 0.162 |
| 13 | 0.057 | 0.011 | 0.032 | 0.004 | 0.019 | 0.386 |
| 14 | 0.078 | −0.047 | 0.037 | 0.023 | −0.008 | 0.072 |
| 15 | −0.003 | 0.060 | 0.064 | −0.033 | 0.076 | 0.075 |
| 16 | 0.042 | −0.054 | 0.034 | −0.089 | 0.164 | 0.259 |
| 17 | 0.049 | −0.062 | 0.115 | −0.069 | 0.117 | 0.068 |
| 18 | −0.054 | −0.005 | −0.049 | 0.290 | 0.054 | 0.013 |
| 19 | 0.043 | −0.006 | −0.025 | 0.167 | 0.064 | 0.177 |
| 20 | −0.017 | −0.026 | 0.115 | −0.069 | 0.117 | 0.068 |
| 21 | −0.021 | 0.039 | 0.019 | 0.101 | 0.077 | 0.049 |
| 22 | −0.015 | 0.050 | 0.015 | 0.099 | −0.056 | 0.093 |
| 23 | 0.000 | 0.128 | −0.153 | 0.023 | 0.095 | 0.043 |
| 24 | 0.032 | 0.002 | 0.016 | −0.086 | 0.139 | 0.182 |
| 25 | −0.018 | 0.106 | 0.025 | −0.008 | −0.100 | 0.012 |
| 26 | −0.031 | 0.058 | −0.061 | −0.011 | 0.206 | 0.130 |
| 27 | 0.078 | 0.069 | −0.070 | −0.029 | −0.048 | 0.054 |
| 28 | −0.035 | 0.055 | 0.123 | −0.071 | −0.051 | 0.021 |
| 29 | −0.029 | 0.065 | 0.031 | 0.103 | −0.135 | 0.052 |
| 30 | 0.085 | 0.048 | −0.050 | −0.082 | −0.027 | 0.007 |
| 31 | 0.075 | 0.012 | −0.020 | 0.025 | −0.052 | 0.062 |
| 32 | −0.049 | 0.042 | 0.239 | −0.059 | −0.126 | 0.241 |
| 33 | −0.040 | 0.069 | −0.029 | −0.098 | 0.186 | 0.118 |
| 34 | −0.007 | 0.176 | 0.028 | −0.131 | −0.076 | 0.343 |
| 35 | 0.029 | −0.041 | 0.059 | 0.159 | −0.182 | 0.239 |
| 36 | −0.020 | −0.039 | 0.203 | 0.040 | −0.111 | 0.004 |
| 37 | 0.068 | −0.003 | −0.016 | 0.056 | −0.109 | 0.220 |
These components were deemed to be the marker compounds of sample variation. This result is in accord with the one obtained from the cluster analysis (CA). The combination of PCA and CA was thus a useful tool for quality control and evaluation of G. lucidum.
3. Materials and Methods
3.1. Samples and Reagents
Fifteen G. lucidum samples were purchased from different regions of China and authenticated by Professor Chun-Sheng Liu (School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China). Each sample (three replicates) was placed in a dark and dry environment. The regions where the 15 samples were obtained are listed in Table 5. HPLC grade acetonitrile and acetic acid were obtained from Fisher (Waltham, MA, USA); distilled water was bought from Watsons (Beijing, China) and was filtered through a 0.22 µm membrane (Dikma, Beijing, China) prior to use. All other reagents were of analytical grade.
Table 5.
The regions of origin of the 15 samples.
| No. | Region | No. | Region |
|---|---|---|---|
| S1 | Haikou, Hainan | S9 | Huangshan, Anhui |
| S2 | Baotou, Neimemg | S10 | Jinzhai, Anhui |
| S3 | Taishan, Shandong | S11 | Xinyang, Henan |
| S4 | Jiaxing, Shandong | S12 | Dali, Yunnan |
| S5 | Jilin, Jilin | S13 | Tianlin, Guangxi |
| S6 | Changbaishan, Jilin | S14 | Shanghai |
| S7 | Changchun, Jilin | S15 | Fuzhou, Fujian |
| S8 | Jingzhou, Hunan |
3.2. Sample Preparation
Dried powder of G. lucidum from different regions (1 g) was accurately weighed out and transferred into a 100 mL conical flask. Chloroform (50 mL) was added to the flask and the flask with the chloroform and powder was placed in an ultrasonic extraction device and extracted for 30 min twice. The solution was cooled and filtered through filter paper, and then the solvent was recovered using a rotary evaporator. The residue was dissolved in a 10 mL volumetric flask using methanol. The solution was filtered through a 0.22 µm membrane filter for fingerprint analysis.
3.3. Apparatus and Parameters
A Waters Alliance HPLC 2695 series instrument (Waters, Manchester, UK) was used to perform the high performance liquid chromatography (HPLC) analysis. Mobile phase: A (acetonitrile); B (H2O:CH3COOH, 100:0.2, v/v). Column: Agilent C18 (250 mm × 4.6 mm, 5 μm), maintained at 30 °C with flow rate of 1.0 mL·min−1. The detection wavelength was set at 254 nm for acquiring chromatograms. The injection volume was 20 µL. Gradient elution procedure: 0 min (20 % A) → 8 min (29% A) → 25 min (29% A) → 55 min (30% A) → 65 min (30% A) → 70 min (31% A) → 90 min (65% A) → 110 min (90% A) → 135 min (90% A).
The LCMS-IT-TOF instrument (Shimadzu, Kyoto, Japan) was equipped with an ESI source used in negative ionization mode. The interface and MS parameters were as follows: nebulizer pressure, 100 kPa; dry gas, N2 (1.5 L/min); drying gas temperature, 200 °C; spray capillary voltage, 4000 V; scan range, m/z 100–1000. Mobile phase: A (acetonitrile); B (H2O:CH3COOH, 100:0.2, v/v). Column: Agilent C18 (250 mm × 4.6 mm, 5 μm), maintained at 30 °C with flow rate of 1.0 mL·min−1. The injection volume was 20 µL. Gradient elution procedure: 0 min (20 % A) → 8 min (29% A) → 25 min (29% A) → 55 min (30% A) → 65 min (30% A) → 70 min (31% A) → 90 min (65% A) → 110 min (90% A) → 135 min (90% A).
3.4. Statistical Analyses
The HPLC data were used for fingerprint analysis and chemometrics. HPLC-MSn was used for identification of the 47 peaks. Cluster analysis (CA) and principal components analysis (PCA) were performed by SPSS (SPSS statistical software package, version 20.0, SPSS Inc., Chicago, IL, USA).
4. Conclusions
The therapeutic effects of traditional Chinese medicines (TCM) are based on the complex interactions of complicated chemical constituents as a whole system. HPLC and HPLC-MSn fingerprint analysis combined with chemometrics were employed to study the complex G. lucidum system. According to previous extensive phytochemical and pharmacological studies, triterpenoid acids were the most important chemical components in the samples, which had a variety of potential biological activities. The qualitative analysis and quantification of triterpenoid acids can better reflect the therapeutic effects and quality of G. lucidum. The chromatographic method is predominant to control the quality and stability of the complex system. This study provided a systematic method for the quality control of G. lucidum by HPLC fingerprinting and the HPLC-MSn evaluation system based on Similarity Analysis (SA), Cluster Analysis (CA) and Principal Component Analysis (PCA). As a result, a common mutual pattern was established by determining and comparing the fingerprints of 15 samples of G. lucidum from different regions. Forty-seven compounds were detected by HPLC-MSn, of which forty-two compounds were tentatively identified by comparing their retention times, and mass spectrometry data with that of reference compounds and literature data. Ganoderic acid B (10), 3,7,15-trihydroxy-11,23-dioxo-lanost-8,16-dien-26-oic acid (F4), Lucidenic acid A (F14), Ganoderic acid G (F9), unknown (F11), 3,7-oxo-12-acetylganoderic acid DM (F30) were deemed to be the markers to distinguish G. lucidum samples of different quality. The proposed method can be used to improve the quality control of G. lucidum, thus ensuring the effectiveness of G. lucidum herbs. There are still five peaks—2, 11, 28, 31 and a10—which were not identified by HPLC-MSn, of which compound 11 were used as marker compound to distinguish the G. lucidum of different quality. These components require further study.
Acknowledgments
The authors gratefully acknowledge the financial support from the Ministry of Science and Technology support project (No. 2012BAI29B01) and National Natural Science Foundation of China (No. 81274187).
Supplementary Materials
The supplementary materials are available online.
Author Contributions
Conceived and designed the experiments: Lanzhen Zhang, Lingfang Wu. Performed the experiments: Lingfang Wu, Wenjing Chen, Wenyi Liang, Shi Li, Qi Qi, Yaping Cui. Analyzed the data: Lingfang Wu. Wrote the paper: Ling-Fang Wu, Lanzhen Zhang.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples are available from the authors.
References
- 1.Bojana B. Ganoderma lucidum: A potential for biotechnological production of anti-cancer and immunomodulatory drugs. Recent Pat. Anticancer Drug Discov. 2013;8:255–287. doi: 10.2174/1574891x113089990036. [DOI] [PubMed] [Google Scholar]
- 2.Xia Q., Zhang H.Z., Sun X.F., Zhao H.J., Wu L.F., Zhu D., Yang G.H., Shao Y.Y., Zhang X.X., Mao X., et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules. 2014;19:17478–17535. doi: 10.3390/molecules191117478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng L., Yuan L., Du Y., Chen Y., Zhang M.H., Gu J.F., He J.J., Wang Y., Cao W. Anti-Lung cancer activity through enhancement of immunomodulation and induction of cell apoptosis of total triterpenes extracted from Ganoderma luncidum (Leyss. ex Fr.) Karst. Molecules. 2013;18:9966–9981. doi: 10.3390/molecules18089966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RadwanFaisal F.F., Hossain A., God J.M., Leaphart N., Elvington M., Nagarkatti M., Tomlinson S., Haque A. Reduction of myeloid-derived suppressor cells and lymphoma growth by a natural triterpenoid. J. Cell. Biochem. 2015;116:102–114. doi: 10.1002/jcb.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang S.M., Yang X.L., Wang B.W., Zhu H.S., Xu J.L. Antitumor activity of ethanol-soluble and acidic components from Ganoderma lucidum. Nat. Prod. Res. 2004;16:146–148. [Google Scholar]
- 6.Smina T.P., Mathew J., Janardhanan K.K., Devasagayam T.P. Antioxidant activity and toxicity profile of total triterpenes isolated from Ganoderma lucidum (Fr.) P. Karst occurring in South India. Environ. Toxicol. Pharmacol. 2011;32:438–446. doi: 10.1016/j.etap.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Kan Y.J., Chen T.Q., Wu Y.B., Wu J.G., Wu J.Z. Antioxidant activity of polysaccharide extracted from Ganoderma lucidum using response surface methodology. Int. J. Biol. Macromol. 2015;10:943–955. doi: 10.1016/j.ijbiomac.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 8.Shi M., Zhang Z.Y., Yang Y.N. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide (GLP) Carbohydr. Polym. 2013;1:200–206. doi: 10.1016/j.carbpol.2013.02.081. [DOI] [PubMed] [Google Scholar]
- 9.Li P.Z., Zhang K.C. Isolation, purification, and antimicrobial activity of ganoderic acids M1 from the fermented mycelia of Ganoderma lucidum. Nat. Prod. Res. 1999;11:67–70. [Google Scholar]
- 10.Liu Y.J., Du J.L., Cao L.P., Jia R., Shen Y.J., Zhao C.Y., Xu P., Yin G.J. Anti-inflammatory and hepatoprotective effects of Ganoderma lucidum polysaccharides on carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio L.) Int. Immunopharmacol. 2015;1:112–120. doi: 10.1016/j.intimp.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Min B.S., Nakamura N., Miyashiro H., Bae K.W., Hattori M. Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998;10:1607–1612. doi: 10.1248/cpb.46.1607. [DOI] [PubMed] [Google Scholar]
- 12.Mizushina Y., Takahashi N., Hanashima L., Koshinom H., Esumi Y., Uzawa J., Sugawara F., Sakaguchi K. Lucidenic acid O and lactone, new terpene inhibitors of eukaryotic DNA polymerases from a basidiomycete, Ganoderma lucidum. Bioorg. Med. Chem. 1999;7:2047–2052. doi: 10.1016/S0968-0896(99)00121-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Z.Z., Yin R.H., Chen H.P., Feng T., Li Z.H., Dong Z.J., Cui B.K., Liu J.K. Two new triterpenoids from fruiting bodies of fungus Ganoderma lucidum. J. Asian Nat. Prod. Res. 2015;17:1–6. doi: 10.1080/10286020.2014.996139. [DOI] [PubMed] [Google Scholar]
- 14.Liu D.Z., Zhu Y.Q., Li X.F., Shan W.G., Gao P.F. New Triterpenoids from the Fruiting Bodies of Ganoderma lucidum and Their Bioactivities. Chem. Biodivers. 2014;6:982–986. doi: 10.1002/cbdv.201400004. [DOI] [PubMed] [Google Scholar]
- 15.Li Y.B., Liu R.M., Zhong J.J. A new ganoderic acid from Ganoderma lucidum mycelia and its stability. Fitoterapia. 2013;83:115–122. doi: 10.1016/j.fitote.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Weng C.J., Chau C.F., Yen G.C., Liao J.W., Chen D.H., Chen K.D. Inhibitory effects of Ganoderma Lucidum on tumorigenesis and metastasis of human hepatoma cells in cells and animal models. J. Agric. Food Chem. 2009;55:5049–5057. doi: 10.1021/jf900828k. [DOI] [PubMed] [Google Scholar]
- 17.Yue Q.X., Song X.Y., Ma C., Feng L.X., Guan S.H., Wu W.Y., Yang M., Jiang B.H., Liu X., Cui Y.J., et al. Effects of triterpenes from Ganoderma lucidum on protein expression profile of HeLa cells. Phytomedicine. 2010;17:606–613. doi: 10.1016/j.phymed.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Thyagarajan A., Jedinak A., Nquyen H., Terry C., Baldridge L.A., Jiang J., Sliva D. Triterpenes from Ganoderma lucidum induce autophagy in colon cancer through the inhibition of p38 mitogen-activated kinase (p38 MAPK) Nutr. Cancer. 2010;62:630–640. doi: 10.1080/01635580903532390. [DOI] [PubMed] [Google Scholar]
- 19.Sun H., Chen X., Zhang A., Sakurai T., Jiang J., Wang X. Chromatographic fingerprinting analysis of Zhizhu Wan preparation by high-performance liquid chromatography coupled with photodiode array detector. Pharmacogn. Mag. 2014;10:470–476. doi: 10.4103/0973-1296.141819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donno D., Boggia R., Zunin P., Cerutti A.K., Guido M., Mellano M.G., Prgomet Z., Beccaro G.L. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. J. Food Sci. Technol. 2015 doi: 10.1007/s13197-015-2115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J.Q., Fan X.H., Cheng Y.Y., Agarwal R., Moore C.M.V., Chen S.T., Tong W.D. Chemometric analysis for identification of botanical raw materials for pharmaceutical use: A case study using Panax notoginseng. PLoS ONE. 2014;9:e87462. doi: 10.1371/journal.pone.0087462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y., Yan Y., Xie M.Y., Nie S.P., Liu W., Gong X.F., Wang Y.X. Development of a chromatographic fingerprint for the chloroform extracts of Ganoderma lucidum by HPLC and LC-MS. J. Pharm. Biomed. Anal. 2008;3:469–477. doi: 10.1016/j.jpba.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Zhu S.B., Xie M.Y., Nie S.P., Liu W., Li C., Gong X.F., Wang Y.X. Quality control and original discrimination of Ganoderma lucidum based on high-performance liquid chromatographic fingerprints and combined chemometrics methods. Anal. Chim. Acta. 2008;2:146–156. doi: 10.1016/j.aca.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Shi X.M., Zhang J.S., Tang Q.J., Yang Y., Hao R.X., Pan Y.J. Fingerprint analysis of Lingzhi (Ganoderma) strains by high-performance liquid chromatography coupled with chemometric methods. World J. Microbiol. Biotechnol. 2008;11:2443–2450. doi: 10.1007/s11274-008-9766-7. [DOI] [Google Scholar]
- 25.Zhang J., Luo X., Zheng L., Xu X., Ye L. Discrimination of Ganoderma based on high performance liquid chromatographic fingerprints combined with chemometrics methods. Ann. Assoc. Can.-Fr. Pour l'Avancement Sci. 2009;6:776. [PubMed] [Google Scholar]
- 26.Yang M., Wang X.M., Guan S.H., Xia J.M., Sun J.H., Guo H., Guo D.A. Analysis of Triterpenoids in Ganoderma lucidum Using Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2007;18:927–939. doi: 10.1016/j.jasms.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Cheng C.R., Yang M., Wu Z.Y. Fragmentation pathways of oxygenated tetracyclic triterpenoids and their application in the qualitative analysis of Ganoderma lucidum by multistage tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011;25:1323–1335. doi: 10.1002/rcm.4989. [DOI] [PubMed] [Google Scholar]
- 28.Hu L.L., Ma Q.Y., Huang S.Z. Three new lanostanoid triterpenes from the fruiting bodies of Ganoderma tropicum. J. Asian Nat. Prod. Res. 2013;4:357–362. doi: 10.1080/10286020.2013.764869. [DOI] [PubMed] [Google Scholar]
- 29.Kohda H., Tokumoto W., Sakamoto K. The biologically active constituents of Ganoderma lucidum (FR.) Karst. histamine release-inhibitory triterpenes. Chem. Pharm. Bull. 1985;4:1367–1374. doi: 10.1248/cpb.33.1367. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y.L., Liu Y.P., Qiu F. Sensitive and selective liquid chromatography–tandem mass spectrometry method for the determination of five ganoderic acids in Ganoderma lucidum and its related species. J. Parm. Biomed. Anal. 2011;54:717–721. doi: 10.1016/j.jpba.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Qian Z.M., Jing Z., Li D.P. Analysis of global components in Ganoderma using liquid chromatography system with multiple columns and detectors. J. Sep. Sci. 2012;35:2725–2734. doi: 10.1002/jssc.201200441. [DOI] [PubMed] [Google Scholar]
- 32.Yan Z., Xia B., Qiu M.H. Fast analysis of triterpenoids in Ganoderma lucidum spores by ultra-performance liquid chromatography coupled with triple quadrupole mass spectrometry. Biomed. Chromatogr. 2013;11:1560–1567. doi: 10.1002/bmc.2960. [DOI] [PubMed] [Google Scholar]
- 33.Min B.S., Gao J.J., Nakamura N. Triterpenes from the spores of Ganoderma Lucidum and their cytotoxicity against meth-A and LLC tumor cells. Chem. Pharm. Bull. 1998;7:1026–1033. doi: 10.1248/cpb.48.1026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.