Abstract
Two new thymol derivatives, 7,9-diisobutyryloxy-8-ethoxythymol (1) and 7-acetoxy-8-methoxy-9-isobutyryloxythymol (2), were isolated from fresh roots of Ageratina adenophora, together with four known compounds, 7,9-di-isobutyryloxy-8-methoxythymol (3), 9-oxoageraphorone (4), (−)-isochaminic acid (5) and (1α,6α)-10-hydroxycar-3-ene-2-one (6). Their structures were established on the basis of detailed spectroscopic analysis, and they were all isolated from the roots of A. adenophora for the first time. All the compounds were tested for their in vitro antibacterial activity toward three Gram-positive and two Gram-negative bacterial strains. Thymol derivatives 1–3 only selectively showed slight in vitro bacteriostatic activity toward three Gram-positive bacteria. The two known carene-type monoterpenes 5 and 6 were found to show moderate in vitro antibacterial activity against all five tested bacterial strains, with MIC values from 15.6 to 62.5 μg/mL. In addition, compounds 5 and 6 were further revealed to show in vitro cytotoxicity against human tumor A549, HeLa and HepG2 cell lines, with IC50 values ranging from 18.36 to 41.87 μM. However, their cytotoxic activities were inferior to those of reference compound adriamycin.
Keywords: Ageratina adenophora, thymol derivatives, monoterpenes, antibacterial, cytotoxicity
1. Introduction
Ageratina adenophora (Spreng.) King and Robinson is a perennial, herbaceous invasive plant, native to Mexico and Costa Rica, which has invaded around 30 countries in tropical and subtropical zones of the world [1,2]. This plant was first introduced to Yunnan Province of China in the 1940s, and by now it has rapidly spread across a large area of southwest China, including Yunnan, Guizhou, Guangxi, Sichuan, Chongqing and Xizang provinces [3]. The rapid spread of A. adenophora in China has caused serious economic losses to agriculture, forestry and livestock, and damaged the ecology and environment of China’s native habitat [4,5].
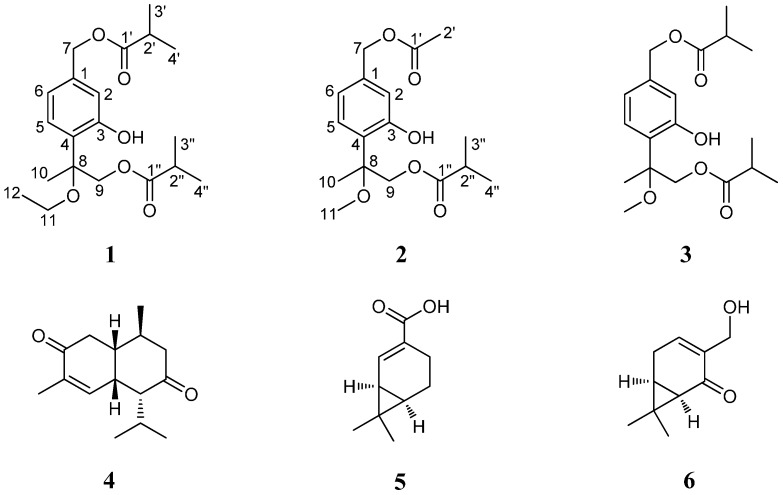
A. adenophora is seldom attacked by microorganisms (including bacteria and fungi) and insects, suggesting that rich bioactive secondary metabolites that might be defense-related, would exist in this plant. Previous phytochemical studies have revealed structurally diverse chemicals including (mono-, sesqui-, di-, and tri-) terpenoids, phenylpropanoids, flavonoids, coumarins, sterols and alkaloids were reported from this species [6,7,8], some of which were shown to possess allelopathic [9,10], phytotoxic [11] and antifeedant [12] activities. Our recent study also revealed some bioactive natural products, including bioactive quinic acid derivatives and monoterpenes from the aerial parts of A. adenophora and some phenolic compounds with allelopathic potential from the roots of this species [13,14,15]. In continuation of our work on searching for bioactive natural compounds of A. adenophora, a further study on the roots of this invasive plant was conducted, which led to the isolation of two new thymol derivatives (1 and 2) along with four known compounds (3–6) (Figure 1). Herein, we report the isolation and structural elucidation of these compounds, as well as their antibacterial and cytotoxic activities.
Figure 1.
Chemical structures of compounds 1−6.
2. Results and Discussion
The EtOH extract of the fresh roots of A. adenophora was initially partitioned between water and EtOAc. The EtOAc soluble fraction was then subjected to silica gel column chromatography (CC) followed by CCs of silica gel, reverse phase silica gel and Sephadex LH-20, to yield new thymols 1 and 2 along with other four known metabolites, 7,9-di-isobutyryloxy-8-methoxythymol (3) [16], 9-oxoageraphorone (4) [17], (−)-isochaminic acid (5) [18] and (1α,6α)-10-hydroxy-3-carene-2-one (6) [14]. The structures of the known compounds were determined by interpretation of their spectroscopic data, as well as by comparison with reported values.
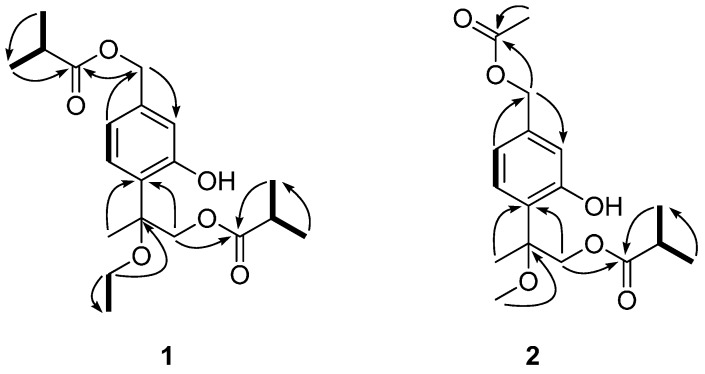
Compound 1 was isolated as a yellowish oil. Its molecular formula C20H30O6 was determined by HR-ESI-MS at m/z 389.1942 [M + Na]+ (calcd. for C20H30O6Na, 389.1940) (see the supplementary), corresponding to 6 degrees of unsaturation. The IR spectrum displayed absorptions at 3434 and 1737 cm−1 indicative of the existence of hydroxyl and carbonyl groups. In the 1H-NMR spectrum, signals for an oxymethylene at δH 5.05 (s, 2H), one tertiary methyl at δH 1.67 (s, 3H), four secondary methyls at δH 1.20 (d, 6H) and 1.13 (d, 6H), a primary methyl at δH 1.25 (t, 3H), and a 1,3,4-trisubstituted phenyl group were readily recognized. In the 13C-NMR and DEPT spectra (Table 1), 20 carbons including six methyls, three oxygenated methylenes, two methines, one quaternary carbon, two carboxyl carbons and six aromatic carbons (3 × C and 3 × CH) were displayed. These above data and literature precedents supported 1 to be a thymol derivative with one ethoxy group and two isobutyryloxy groups in the molecule [16,19]. Careful comparison showed that the 1H- and 13C-NMR spectroscopic data (see Table 1) of 1 were very close to those of 7,9-di-isobutyryloxy-8-methoxythymol [16], a literature reported thymol derivative which was also obtained in the present study as compound 3, except that the resonances for the methoxy group in 3 were replaced by signals (δH 3.54 (H-11a), 3.39 (H-11b), 1.25 (H3-12); δC 59.1 (C-11), 15.4 (C-12)) for an ethoxy group in 1. These findings suggested 1 to be a thymol derivative close to 3, with only difference of the methoxy group at C-8 in 3 being replaced by an ethoxy group (Figure 1). This proposed structure was well supported by 2D NMR analyses including 1H-1H COSY and HMBC experiments (Figure 2). In the 1H-1H COSY spectrum, signals correlated to the four H-atom coupling systems, i.e., C-5 through C-6, C-11 through C-12, C-2′ through C-4′ and C-2″ through C-4″ were all exhibited. The observation of 1H-13C long-range correlation signals in the HMBC spectrum of H-9 and H-3″(4″) with C-1″ (δC 176.9) (Figure 2) evidenced the location of an isobutyryloxy group at C-9 (δC 68.3). The location of the ethoxy group at C-8 was assigned by significant HMBC correlations of H-11 with C-8 (δC 80.8). The ester bond linkage of C-7 with the other isobutyryloxy group at C-1′ was supported by the observation of HMBC correlations of H-7 (δH 5.04) and H-3′(4′) (δH 1.20) with C-1′ (δC 176.6). Therefore, compound 1 was determined as 7,9-diisobutyryloxy-8-ethoxythymol.
Table 1.
The 1H- and 13C-NMR data of compounds 1, 2 and 3.
| H/C | 1 a | 2 a | 3 b | |||
|---|---|---|---|---|---|---|
| δH (Mult., J in Hz) | δC (DEPT) | δH (Mult., J in Hz) | δC (DEPT) | δH (Mult., J in Hz) | δC (DEPT) | |
| 1 | 138.2 (s) | 137.9 (s) | 138.3 (s) | |||
| 2 | 6.84 (d, 1.2) | 116.3 (d) | 6.85 (s) | 116.6 (d) | 6.86 (s) | 116.4 (d) |
| 3 | 156.5 (s) | 156.4 (s) | 156.4 (s) | |||
| 4 | 124.4 (s) | 123.8 (s) | 123.6 (s) | |||
| 5 | 7.01 (d, 8.0) | 127.6 (d) | 7.01 (d, 8.4) | 127.8 (d) | 7.02 (d, 8.4) | 127.9 (d) |
| 6 | 6.82 (dd, 8.0, 1.2) | 118.8 (d) | 6.82 (d, 8.4) | 119.2 (d) | 6.83 (d, 8.4) | 119.0 (d) |
| 7 | 5.04 (s) | 65.3 (t) | 5.03 (s) | 65.5 (t) | 5.05 (s) | 65.3 (t) |
| 8 | 80.8 (s) | 81.3 (s) | 81.4 (s) | |||
| 9 | 4.36 (d, 11.6) | 68.3 (t) | 4.33 (d, 12.0) | 68.2 (t) | 4.34 (d, 11.6) | 68.3 (t) |
| 4.23 (d, 11.6) | 4.22 (d, 12.0) | 4.23 (d, 11.6) | ||||
| 10 | 1.66 (s) | 21.0 (q) | 1.64 (s) | 20.2 (q) | 1.65 (s) | 20.2 (q) |
| 11 | 3.54 (m), 3.39 (m) | 59.1 (t) | 3.28 (s) | 50.9 (q) | 3.29 (s) | 51.0 (q) |
| 12 | 1.25 (t, 7.2) | 15.4 (q) | ||||
| 1′ | 176.6 (s) | 170.8 (s) | 176.6 (s) | |||
| 2′ | 2.56 (m) | 34.0 (d) | 2.10 (s) | 20.9 (q) | 2.60 (m) | 33.9 (d) |
| 3′ | 1.20 (d, 6.8) | 19.0 (q) | 1.20 (d, 6.8) | 18.8 (q) | ||
| 4′ | 1.20 (d, 6.8) | 19.0 (q) | 1.20 (d, 6.8) | 18.9 (q) | ||
| 1“ | 176.9 (s) | 176.5 (s) | 176.9 (s) | |||
| 2“ | 2.62 (m) | 34.0 (d) | 2.55 (m) | 33.9 (d) | 2.60 (m) | 34.0 (d) |
| 3“ | 1.13 (d, 6.8) | 18.9 (q) | 1.12 (d, 6.6) | 18.8 (q) | 1.12 (d, 6.8) | 18.9 (q) |
| 4“ | 1.13 (d, 6.8) | 18.9 (q) | 1.13 (d, 6.6) | 18.8 (q) | 1.13 (d, 6.8) | 20.2 (q) |
a Recorded in CDCl3; b Recorded in CD3OD.
Figure 2.
Selected HMBC ( ) and COSY (
) and COSY ( ) correlations of compounds 1 and 2.
) correlations of compounds 1 and 2.
Compound 2 was also obtained as a yellowish oil. Its molecular formula C17H24O6 was deduced from the HR-ESI-MS m/z 347.1479 [M + Na]+ (calcd. for C17H24O6Na, 347.1471). Its spectral features and physicochemical properties suggested 2 to be also a thymol derivative. Careful comparison indicated that the 1H and 13C (DEPT) NMR data (Table 1) of 2 were very similar to those of 3, except that the resonances for the isobutyryloxy group that located at C-7 in 3 were replaced by the signals (δH 2.10 (3H, s, H3-2′); δC 170.8 (C-1′), 20.9 (C-2′)) for an acetoxy group in 2 (Figure 1). This deduction was supported by its molecular formula and the observation of significant 1H-13C long-range correlation signals in the HMBC spectrum of H-7 (δH 5.03) and H-2′ (δH 2.10) with C-1′ (δC 170.8) (Figure 2). By further detailed analysis of 2D NMR (1H-1H COSY, HSQC, and HMBC) spectra (Figure 2), the 1H- and 13C spectroscopic NMR data were unambiguously assigned as shown in Table 1, and they were fully supported by the structure of 2 as shown in Figure 1. Accordingly, the structure of 2 was thus determined as 7-acetoxy-8-methoxy-9-isobutyryloxythymol.
Among these isolated compounds, 1–3 were thymol derivatives. Thymols, first found in Thymus plants [20], were recently discovered to be in rich supply in Eupatorium species [19,21,22]. Due to the thymol derivatives usually being oily like compounds which were difficult to prepare single crystals for X-ray analysis, it is generally difficult to determine the stereochemistry of thymols at the stereogenic center C-8 [19,23]. Just like the thymol precedents in the literature, the stereochemistry of thymols 1–3 at the stereogenic center C-8 were also still an open question. Compounds 5 and 6 were two carene-type monoterpenes with potential anti-fungal activities that we have recently obtained from the aerial parts of A. adenophora [14]. To the best of our knowledge, this is the first time these compounds have been obtained from the roots of this plant.
These six compounds were tested for their in vitro antibacterial activities against five bacterial strains, including three Gram-positive bacteria of Staphylococcus aureus, Bacillus thuringiensis and Bacillus subtilis, and two Gram-negative bacteria of Escherichia coli and Shigella dysenteria. The experimental results obtained from the bioassay (Table 2) showed that the two carene-type monoterpenes 5 and 6 were moderately active toward the five bacterial strains with MIC values from 15.6 to 62.5 μg/mL. The thymol compounds 1–3 only selectively showed weak bacteriastatic activity toward the three Gram-positive bacterial strains, and no antibacterial activity was detected for compound 4 in this bioactive assay.
Table 2.
MIC values of compounds 1–6 in μg/mL against five bacterial strains.
| Compounds | Staphylococcus aureus | Bacillus thuringiensis | Bacillus subtilis | Escherichia coli | Shigella dysenteriae |
|---|---|---|---|---|---|
| 1 | >200 | >200 | 125 | >200 | >200 |
| 2 | 125 | 62.5 | 62.5 | >200 | >200 |
| 3 | >200 | 125 | 125 | >200 | >200 |
| 4 | >200 | >200 | >200 | >200 | >200 |
| 5 | 31.3 | 31.3 | 15.6 | 62.5 | 62.5 |
| 6 | 15.6 | 31.3 | 15.6 | 62.5 | 62.5 |
| KS | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 |
| C | >200 | >200 | >200 | 7.8 | 7.8 |
KS = Kanamycin sulfate, C = Cefradine.
Compounds 1–6 were further evaluated for their in vitro cytotoxicity against three human cancer cell lines (A549, HeLa and HepG2) using a MTT method as described. The resulting IC50 values are displayed in Table 3, as compared to adriamycin as positive control. Compounds 5 and 6 showed moderate cytotoxicity against all the three tested cancer cell lines, with IC50 values ranging from 18.36 to 41.87 μM. No obvious activity was detected for the other four isolates (1–4).
Table 3.
Cytotoxicity of compounds 1–6 (IC50, µM).
| Compounds | A549 | HeLa | HepG2 |
|---|---|---|---|
| 1–4 | >100 | >100 | >100 |
| 5 | 32.37 ± 3.75 | 25.64 ± 2.34 | 41.87 ± 6.53 |
| 6 | 30.65 ± 3.87 | 18.36 ± 1.72 | 39.44 ± 3.61 |
| Adriamycin | 0.68 ± 0.06 | 0.46 ± 0.05 | 1.23 ± 0.02 |
Values represent mean ± SD (n = 3) based on three individual experiments.
A. adenophora is a well-known invasive plant which has spread rapidly in the southwest part of China. To date, phytochemical investigations have revealed diverse chemicals from this plant species. However, those studies were mainly focused on the aerial parts, and seldom concentrated on the roots. Our recent study revealed a series of phenolic compounds with allelopathic potential from the roots of this plant [15]. The present study further indicated that the roots of A. adenophora are rich in potential bioactive compounds worthy of further investigation.
3. Materials and Methods
3.1. General Information
Optical rotations were obtained on a Perkin-Elmer 341 polarimeter (Perkin-Elmer, Waltham, MA, USA) with MeOH as solvent. UV spectra were recorded in MeOH on a Perkin-Elmer Lambda 35 UV-vis spectrophotometer. IR spectra (KBr) were recorded on a Bruker Tensor 27 spectrophotometer in cm−1. 1H (600 MHz and 400 MHz), 13C (150 MHz and 100 MHz), and 2D NMR spectra were recorded in CDCl3 and CD3OD on a Bruker DRX-400 instrument and a Bruker AVANCE 600 instrument with TMS as an internal standard. HR-ESI-MS data were obtained on a Water Q-TOF Premier mass spectrometer and HR-EI-MS data were obtained on a Finigan MAT 95XP mass spectrometer. ESIMS were collected on an MDS SCIEX API 2000 LC/MS/MS instrument. Preparative HPLC was conducted using a CXTH P3000 HPLC pump and a UV3000 UV-Vis Detector with a Fuji-C18 column (10 µm–100A, ChuangXinTongHeng Science And Technology Co., Ltd., Beijing, China). For column chromatography (CC), silica gel (200–300 mesh, Qingdao Marine Chemical Inc.), YMC ODS-A (50 μm, YMC Co. Ltd., Kyoto, Japan) were used, and Sephadex LH-20 (Pharmacia Fine Chemical Co. Ltd., Uppsala, Sweden) were used. Analytical grade petroleum ether (b.p. 60–90 °C), methanol, ethyl acetate, chloroform, acetone were purchased from Tianjin Fuyu Fine Chemical Industry Co. (Tianjin, China); HPLC grade methanol was purchased from J&K Chemical Ltd. (Beijing, China); Fractions were monitored by pre-coated HSGF254 TLC Yantai Jiangyou Silica Gel Co. Ltd. (Yantai, China), and spot detection was performed under fluorescent light (λ = 254 nm), and then spraying 10% H2SO4 in ethanol, followed by heating. RPMI-1640 medium and fetal calf serum were purchased from Gibco BRL (Gaithersburg, MD, USA). Kanamycin sulfate, Resazurin, Cefradine, CDCl3, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Chemical Co. (Sigma-Aldrich, St. Louis, MO, USA). Adriamycin was obtained from Pfizer Italia SRL (Roma, Italy). p-Nitrophenyl-α-d-glucopyranoside (PNPG) were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
3.2. Plant Materials
The root material of A. adenophora was collected in a suburb of Kunming, Yunnan province, P. R. China, in September 2010, identified by one of the authors (J.-W.T.). A voucher specimen (No. 20100902) was deposited at the Laboratory of Phytochemistry at the South China Botanical Garden, Chinese Academy of Sciences.
3.3. Extraction and Isolation
The fresh root material (14.5 kg) of A. adenophora were cut into pieces and extracted three times with 95% EtOH (3 × 20 L) at room temperature. After removal of the EtOH in vacuo, the viscous concentrate was suspended in 10% ethanol in H2O (2.5 L) and extracted with ethyl acetate (EtOAc) (3 × 2.5 L). The oily EtOAc extract (76 g) was then applied on silica gel (500 g) column chromatography and eluted with 2L petroleum ether/CHCl3 (1:1, v/v) to yield an olily fraction (22.1 g) which was further subjected to silica gel column chromatography using a gradient of petroleum ether/CHCl3 (90:10–0:100, v/v) and CHCl3–MeOH (100:0–80:20, v/v) to give sub-fractions F1–F7. The fraction F2 (4.0 g), obtained from the elution of petroleum ether/CHCl3 of 1:1, was separated by RP-C18 MPLC eluted with MeOH/H2O (from 4:6 to 7:3, v/v) and further purified by Sephadex LH-20 column chromatography with elution solvent of MeOH to give compound 5 (2.6 mg). The fraction F4 (4.7 g), obtained from the elution of CHCl3/MeOH of 95:5, was applied on silica gel (200–300 mesh) column chromatography and eluted with a gradient of n-hexane/ethyl acetate to afford fractions F4-1–F4-8 on the basis of thin layer chromatography (TLC) profiles. Fraction F4-3 (1.3 g), obtained from the elution of n-hexane/ethyl acetate of 8.5:1.5, was separated by silica gel column chromatography eluted with petroleum ether/Me2CO (from 9:1 to 7:3, v/v) to give subfractions F4-3-1–F4-3-5, of which subfraction F4-3-4 (0.4 g) was further purified by RP-C18 HPLC eluted with 70% MeOH in H2O to afford compound 1 (tR = 37.2 min, 2.8 mg). Fraction F4-4 (1.1 g), obtained from the elution of n-hexane/ethyl acetate of 7:3, was separated by RP-C18 HPLC eluted with a MeOH/H2O gradient (from 50:50 to 80:20, v/v) to provide pure compound 4 (tR = 68.1 min, 5.6 mg). Fraction F4-6 (0.7 g) was divided into subfractions F4-6-1–F4-6-4, of which subfraction F4-6-4 (39.2 mg) was purified repeatedly by Sephadex LH-20 columns eluted with CHCl3/MeOH (20:80, v/v) to afford compound 2 (3.0 mg). Fraction F4-6-3 was separated by RP-C18 MPLC eluted with MeOH/H2O (from 50:50–100:0, v/v) to attain subfractions F4-6-3-1–F4-6-3-4. Subfraction F4-6-3-1 was then further purified by RP-C18 MPLC eluted with MeOH/H2O (from 50:50–100:0, v/v) to attain pure compound 6 (tR = 13.5 min, 2.4 mg), and subfraction F4-6-3-3 was purified by Sephadex LH-20 column eluted with acetone to afford compound 3 (4.7 mg).
7,9-Diisobutyryloxy-8-ethoxythymol (1). yellowish oil; [α] −5.0 (c 0.06, CHCl3); IR (KBr) νmax 3434, 1737, 1265, 1187, 1155 cm−1; UV (MeOH) λmax (log ε) nm: 205 (4.16), 279 (3.31); ESI-MS m/z 755 [2M + Na]+, 389 [M + Na]+; HR-ESI-MS m/z 389.1942 [M + Na]+ (calcd. for C20H30O6Na, 389.1940); For 1H (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) data, see Table 1.
7-Acetoxy-8-methoxy-9-isobutyryloxythymol (2). yellowish oil; [α] +3.8 (c 0.87, CHCl3); IR (KBr) νmax 3318, 1739, 1629, 1577 cm−1; UV (MeOH) λmax (log ε) nm: 204 (4.27), 219 (3.80), 279 (3.31); ESI-MS m/z 687 [2M + K]+, 671 [2M + Na]+, 363 [M + K]+, 347 [M + Na]+, 683 [2M + Cl]−, 359 [M + Cl]−, 323 [M–H]−; HR-ESI-MS m/z 347.1479 [M + Na]+ (calcd. for C17H24O6Na, 347.1471). For 1H (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) data, see Table 1.
3.4. Antibacterial Assay
The antibacterial activities of 1–6 were tested by using a microdilution method as we described previously [24]. Three Gram-positive bacteria strains, Staphylococcus aureus, Bacillus thuringiensis and Bacillus subtilis, and two Gram-negative bacterial species, Escherichia coli and Shigella dysenteria, were used in the bioassay. In the test, indicator solution of resazurin (100 μg/mL, 100 μL) was first placed into each control wells (11th column) in 96-well microplates for the assay. Subsequently, indicator solution (100 μg/mL, 7.5 mL) was mixed with test organism (106 cfu/mL, 5 mL) followed by transferring (100 μL, each) to growth control wells (12th column) and all test wells (1–10th column) in the 96-well microplates. Then, each of the sample solutions (1.0 mg/mL of test compounds in methanol, 100 μL) and positive control solution (1.0 mg/mL of kanamycin sulfate or cefradine in methanol) as well as negative control sample (pure MeOH) were applied to the wells in the 1st column of the plates. In each test microplate, the six compound samples along with positive control and negative control samples were applied. Once all controls and samples were properly applied to the 1st column of wells in the microplates, half of the homogenized content (100 μL) from these wells was then transferred parallel to the 2nd column of wells, and each subsequent column of wells was treated similarly (doubling dilution) up to the 10th column, followed by discarding the last 100 μL aliquot. Finally, the plates were incubated at 37 °C for 5–6 h until the color of growth control wells change to pink. The lowest concentration for each test compound at which color change occurred was recorded as its primary MIC value. The averages of the primary values from three individual tests were calculated and these were taken as the final MIC values for the test compounds. MIC values for test compounds were displayed in Table 2.
3.5. Cytotoxic Assay
Compounds 1–6 were evaluated for their cytotoxity against human lung adenocarcinoma (A549), human cervical carcinoma (HeLa) and human liver hepatocellular carcinoma (HepG2) cell lines. The three tumor cell lines were obtained from Kunming Institute of Zoology, Chinese Academy of Sciences. The cytotoxic activities of the tested compounds were assayed according to an MTT method by using 96 well plates [25]. In brief, the cells were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum in a humidified atmosphere with 5% CO2 at 37 °C. 100 μL adherent cells at the density of 5 × 104 cell/mL was seeded into each well of 96-well cell culture plates and incubated in 5% CO2 at 37 °C for 24 h to form a monolayer on the flat bottoms. Then, the supernatant per well was removed and subsequently added with 100 μL fresh medium and 100 μL medium containing a test compound. The plate was then incubated in 5% CO2 at 37 °C. After 72 h, 20 μL of 5 mg/mL MTT in DMSO was added into each well and incubated for 4 h. The supernatant per well was carefully removed and 150 μL DMSO was added. The plate was then vortex shaken for 15 min to dissolve blue formazan crystals. The optical density (OD) of each well was measured on a Genois microplate reader (Tecan GENios, Männedorf, Switzerland) at the wavelength of 570 nm. All assays were performed in triplicate and adriamycin was used as a positive control. In each experiment, each of the tumor cell lines was exposed to the test compound at concentrations 50, 25, 12.5, 6.25, 3.125, 1.5625 µg/mL. The inhibitory rate of cell growth was calculated according to the following formula: Inhibition rate (%) = (ODcontrol − ODtreated)/ODcontrol × 100%. IC50 values were calculated by SPSS 16.0 statistic software. The values were based on three individual experiments and expressed as means ± standard deviation (SD).
4. Conclusions
Six natural products, including two new thymol derivatives 1 and 2, were isolated from the roots of A. adenophora. Their structures were identified by extensive spectroscopic analysis, including NMR and HR-ESI-MS techniques. All the compounds were isolated from the roots of A. adenophora for the first time. Thymol compounds 1−3, and monoterpenes 5 and 6 selectively showed in vitro antibacterial activity against five assayed bacterial strains. Compounds 5 and 6 were further revealed to show moderate in vitro cytotoxicity against human tumor A549, HeLa and HepG2 cell lines. The present study indicates that the roots of A. adenophora are also rich in potential bioactive compounds worthy of further investigation.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (30970453 and 31270406), the Natural Science Foundation of Guangdong Province (2014A030313742), and the Science and Technology Project of Guangdong Province (2014B020206003).
Supplementary Materials
The following are available online, HR-ESI-MS and NMR spectra data of compounds 1 and 2 as supporting information.
Author Contributions
L.-M. Dong, M. Zhang and Q.-L. Xu performed the isolation, structural elucidation of the chemicals and participated in the bioassay experiments. Q. Zhang, B. Luo, Q.-W. Luo and W.-B. Liu partially contributed the isolation of the chemicals and the bioactive assay. J.-W. Tan designed and organized the whole research of this study. All authors approved the final version manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: not available.
References
- 1.Qiang S. The history and status of the study on crofton weed (Eupatorium adenophorum Spreng.), a worst worldwide weed. J. Wuhan Bot. Res. 1998;16:366–372. [Google Scholar]
- 2.Wang J.J. The crofton weed, Ageratina adenophora. In: Wan F.H., Zheng X.B., Guo J.Y., editors. Biology and Management of Invasive Alien Species in Agriculture and Forestry. Science Press; Beijing, China: 2005. pp. 650–661. [Google Scholar]
- 3.Sun X.Y., Lu Z.H., Sang W.G. Review on studies of Eupatorium adenophorum—An important invasive species in China. J. For. Res. 2004;15:319–322. [Google Scholar]
- 4.Wan F.H., Guo J.Y., Wang D.H. Alien invasive species in China: Their damages and management strategies. Biodivers. Sci. 2002;10:119–125. [Google Scholar]
- 5.Wan F.H., Liu W.X., Guo J.Y., Qiang S., Li B.P., Wang J.J., Yang G.Q., Niu H.B., Gui F.R., Huang W.K., et al. Invasive mechanism and control strategy of Ageratina adenophora (Sprengel) Sci. China Life Sci. 2010;53:1291–1298. doi: 10.1007/s11427-010-4080-7. [DOI] [PubMed] [Google Scholar]
- 6.Yan Q.S., Yang J., Li H.M., Cao A.C., Chen Q.H., Wen Y.Q., He L. Advances in the studies on the chemical components and bioactivity of Eupatorium adenophorum Spreng as a intruding species. J. Beijing Norm. Univ. 2006;42:70–73. [Google Scholar]
- 7.Li Y.M., Li Z.Y., Ye M. The chemical compositions and their bioactivities in the different parts of Eupatorium adenophorum Spreng. J. Yunnan Agric. Univ. 2008;23:42–46. [Google Scholar]
- 8.He L., Hou J., Gan M.L., Shi J.G., Chantrapromma S., Fun H.K., Williams I.D., Sung H.H.Y. Cadinane sesquiterpenes from the leaves of Eupatorium adenophorum. J. Nat. Prod. 2008;71:1485–1488. doi: 10.1021/np800242w. [DOI] [PubMed] [Google Scholar]
- 9.Yang G.Q., Wan F.H., Liu W.X., Zhang X.W. Physiological effects of allelochemicals from leachates of Ageratina adenophora Spreng on rice seedlings. Allelopathy J. 2006;18:237–245. [Google Scholar]
- 10.Zhao X., Zheng G.W., Niu X.M., Li W.Q., Wang F.S., Li S.H. Terpenes from Eupatorium adenophorum and their allelopathic effects on Arabidopsis seeds germination. J. Agric. Food Chem. 2009;57:478–482. doi: 10.1021/jf803023x. [DOI] [PubMed] [Google Scholar]
- 11.Zheng G.W., Jia Y.X., Zhao X., Zhang F.J., Luo S.H., Li S.H., Li W.Q. o-Coumaric acid from invasive Eupatorium adenophorum is a potent phytotoxin. Chemoecology. 2012;22:131–138. doi: 10.1007/s00049-012-0105-y. [DOI] [Google Scholar]
- 12.Shi W., Luo S.H., Li S.H. Defensive sesquiterpenoids from leaves of Eupatorium adenophorum. Chin. J. Chem. 2012;30:1331–1334. doi: 10.1002/cjoc.201200279. [DOI] [Google Scholar]
- 13.Zhang M., Liu W.X., Zheng M.F., Xu Q.L., Wan F.H., Wang J., Lei T., Zhou Z.Y., Tan J.W. Bioactive quinic acid derivatives from Ageratina adenophora. Molecules. 2013;18:14096–14104. doi: 10.3390/molecules181114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Q.L., Zhang M., Zhou Z.Y., Liu W.X., Wan F.H., Wang H.F., Tan J.W. Two new carene-type monoterpenes from aerial parts of Ageratina adenophora. Phytochem. Lett. 2014;9:123–126. doi: 10.1016/j.phytol.2014.05.004. [DOI] [Google Scholar]
- 15.Zhou Z.Y., Liu W.X., Pei G., Ren H., Wang J., Xu Q.L., Xie H.H., Wan F.H., Tan J.W. Phenolic compounds from Ageratina adenophora roots and their allelopathic effects on Arabidopsis seeds germination and seedling growth. J. Agric. Food Chem. 2013;61:111792–111799. doi: 10.1021/jf400876j. [DOI] [PubMed] [Google Scholar]
- 16.Maldonado E., Marquez C.L., Ortega A. A thymol derivative from Calea nelsonii. Phytochemistry. 1992;31:2527–2528. doi: 10.1016/0031-9422(92)83316-Q. [DOI] [Google Scholar]
- 17.Ding Z.H., Guo Y.B., Ding J.K. Chemical constituents from the flowers of Eupatorium adenophorum. Acta Bot. Yunnanica. 1999;21:505–511. [Google Scholar]
- 18.Jayasinghe L., Kumarihamy B., Jayarathna K., Udishani N., Bandara B., Hara N., Fujimoto Y. Antifungal constituents of the stem bark of Bridelia retusa. Phytochemistry. 2003;62:637–641. doi: 10.1016/S0031-9422(02)00623-4. [DOI] [PubMed] [Google Scholar]
- 19.Tori M., Ohara Y., Nakashima K., Sono M. Thymol derivatives from Eupatorium fortunei. J. Nat. Prod. 2001;64:1048–1051. doi: 10.1021/np0101191. [DOI] [PubMed] [Google Scholar]
- 20.Vernet P. Composition of essential oil of Thymus vulgaris L. [Labiee]: Mode of hereditary transmission of three terpenes [thymol, carvacrol and linalol] C. R. Seances. Acad. Sci. Ser. D. 1977;284:1289–1292. [Google Scholar]
- 21.Zhang K.M., Shi L., Jiang C.D., Li Z.Y. Inhibition of Ageratina adenophora on spore germination and gametophyte development of Macrothelypteris torresiana. J. Integr. Plant. Biol. 2008;50:559–564. doi: 10.1111/j.1744-7909.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen J.J., Tsai Y.C., Hwang T.L., Wang T.C. Thymol, benzofuranoid, and phenylpropanoid derivatives: anti-inflammatory constituents from Eupatorium cannabinum. J. Nat. Prod. 2011;74:1021–1027. doi: 10.1021/np100923z. [DOI] [PubMed] [Google Scholar]
- 23.Talavera-Alemán A., Rodríguez-García G., López Y., García-Gutiérrez H.A., Torres-Valencia J.M., Río R.E.D., et al. Systematic evaluation of thymol derivatives possing stereogenic or prostereogenic centers. Phytochem. Rev. 2016;15:251–277. doi: 10.1007/s11101-015-9412-6. [DOI] [Google Scholar]
- 24.Wang J., Ren H., Xu Q.L., Zhou Z.Y., Wu P., Wei X.Y., Cao Y., Chen X.X., Tan J.W. Antibacterial oleanane-type triterpenoids from pericarps of Akebia trifoliata. Food Chem. 2015;168:623–629. doi: 10.1016/j.foodchem.2014.07.105. [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Xu Q.L., Zheng M.F., Ren H., Lei T., Wu P., Zhou Z.Y., Wei X.Y., Tan J.W. Bioactive 30-oroleanane riterpenes from the ericarps of Akebia trifoliata. Molecules. 2014;19:4301–4312. doi: 10.3390/molecules19044301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.