Abstract
Microscopic and molecular studies suggest that lichen symbioses contain a plethora of associated fungi. These are potential producers of novel bioactive compounds, but strains isolated on standard media usually represent only a minor subset of these fungi. By using various in vitro growth conditions we are able to modulate and extend the fraction of culturable lichen-associated fungi. We observed that the presence of iron, glucose, magnesium and potassium in growth media is essential for the successful isolation of members from different taxonomic groups. According to sequence data, most isolates besides the lichen mycobionts belong to the classes Dothideomycetes and Eurotiomycetes. With our approach we can further explore the hidden fungal diversity in lichens to assist in the search of novel compounds.
Keywords: Dothideomycetes, Eurotiomycetes, Leotiomycetes, nuclear ribosomal subunits DNA, nutrients, Sordariomycetes
1. Introduction
Lichens are self-sustaining symbiotic associations of specialized fungi (the mycobionts), and green algae or cyanobacteria (the photobionts), which are located extracellularly within a matrix of fungal hyphae and from which the fungi derive carbon nutrition [1]. Lichens are characterized by a specific structure, the lichen thallus, which is typically determined by the mycobiont (hence, lichens are classified according to the mycobiont). The classic concept of lichens as a dual partnership has been emended recently, since other microorganisms such as bacteria and additional fungi are regularly present in the thalli [2,3,4,5,6,7,8,9,10]. There is evidence from culture-independent methods that associated bacterial communities potentially influence the fitness of the lichen thallus [11,12].
Such work still needs to be accomplished with the fungal associates of lichens, but it is known that numerous lichenicolous fungi can modify the morphology of their hosts [13,14]. The biological effects of lichen-inhabiting (lichenicolous) fungi range from degradation to hypertrophication (formation of fungal galls) of their hosts. Unfortunately, nothing is yet known about the regulatory processes and effective molecules that mediate these fungal interactions. In a pioneering attempt, Hawksworth et al. [15] used lichen thalli and thin-layer chromatography to directly detect compounds possibly originating from lichen-invading fungi. This approach provided a first insight into compound patterns involved in fungal interactions, but these are restricted to bulk compound in the thallus, and may overlook regulatory molecules acting at low concentration. We think that improvement and standardization of culturing conditions are needed to promote discovery of compounds produced by lichen-inhabiting fungi and the further study of their bioactive potential. The search for novel compounds from lichenicolous fungi is not just a matter of mere academic curiosity, but can be of broader pharmaceutical interest. A recent review by Kellogg and Raja [16] lists already 140 novel secondary metabolites from cultured lichenicolous fungi that have been found recently, including information about bioactivity as far as it is known today. For the complexity and diversity of lichenicolous life strategies, lichens are a particularly rich source of yet to be discovered fungi and compounds.
However, some technical issues remain to be solved with complex symbiotic systems, such as lichens. Externally visible fungi, or those which produce phenotypic symptoms on their hosts, are not necessarily the same fungi that can be retrieved easily in culture. In fact, symptomless fungi residing or growing in lichens are very common [3]. The biological roles and the abundances of these cryptically occurring (=endolichenic) fungi are still unclear. Although numerous fungi have been retrieved by culture-dependent approaches so far [9,17,18], only a few studies reliably assign isolates to visible phenotypes of lichenicolous fungi [19,20,21,22].
Culturing protocols are available since long time for the mycobiont of lichens and these were improved over the past decades [23,24,25,26,27,28,29]. Different media compositions may modulate the growth of the mycobiont and may also lead to different chemical spectra, which quite often differ from those of the native lichens [30,31,32,33,34]. While culturing the mycobionts is thus well established, the isolation of the lichen-associated fungi is not properly explored. Handling of the material prior to isolation of fungi can significantly influence the results. For example, inappropriate handling and post-harvest moulding may introduce fungi not present originally, or limited surface sterilization methods could favor fungi loosely attached to the surface [7,8,9,10,11,12,13,14,15,16,17]. Hardly any study so far has compared media composition for the influence on the growth of the lichen-associated fungi in axenic culture and whether isolates representing different fungal classes could be specifically retrieved using different media. In this study we focused on the cultivation of fungi associated with crustose, epilithic lichens (Appendix A). We argued that these lichens and their inhabitants [9] might have more stable (mineral-dependent) substrate parameters than lichens on organic substrates.
2. Results
2.1. Molecular Identification of the Isolated Fungal Strains
We report here the isolation of 92 lichen-associated fungal strains: 67 Eurotiomycetes, 14 Dothideomycetes, eight Leotiomycetes and three Sordariomycetes (Appendix B and Appendix C). These isolates are genetically identified in each of the four fungal classes within the same lineages previously recognized by Muggia et al. [9]. In Eurotiomycetes most of the new isolates represent melanized fungi and are recovered in one major lineage (clade VI, Appendix B)—confirming the recurrent occurrence of this fungus in crustose lichens—found in different lichen species and in multiple thalli of the same lichen species. Additional three, minor lineages (clade IV, V, and VII, Appendix B) also group melanized strains. In Dothideomycetes the new isolates are recovered within Pleosporales in the Phoma lineage and in Myriangiales. Leotiomycetes and Sordariomycetes are the least represented among the retrieved strains: only 11 isolates have been identified in addition to those reported by Muggia et al. [9]. The recovered isolates in Leotiomycetes represent two unnamed lineages, the first within the core of the class and the second unresolved at its base (Appendix B). Within the Sordariomycetes the isolated strains mainly belong to the orders Xylariales and Coniochaetales. Only two isolates were identified as Agaricomycetes (not shown). In total 29 isolates of Lecanoromycetes, corresponding to the lichen mycobionts, have also been obtained. Only 10% of the total isolates were green algae (not included in any further analyses). Although we considered an expanded range of culture media, we did not recover any Basidiomycetes isolate.
2.2. Correlation of Fungal Strains with Type of Growth Media
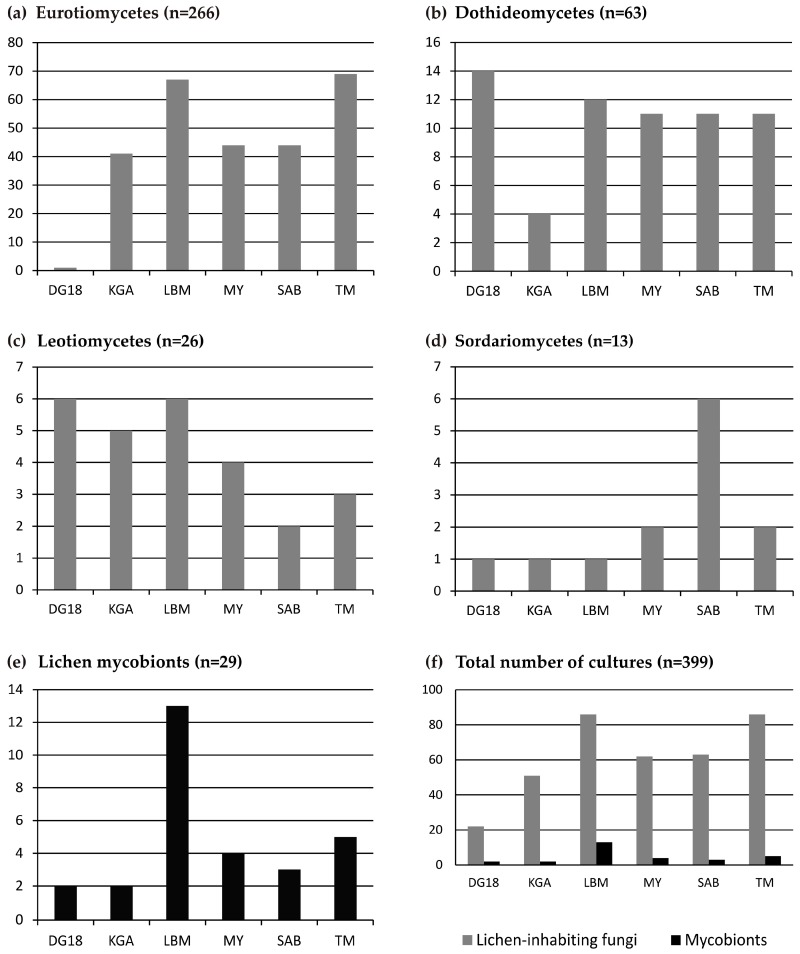
The successful isolation and growth of strains representing the different fungal groups was correlated with the type of medium (Figure 1 and Figure 2). The media differed in the presence of inorganic and organic compounds and were differently enriched by nutrients, such as sugars, metal compounds, amino acids and vitamins (Appendix D). Our analyses include the new isolates here presented and those previously published by Muggia et al. [9] for a total of 399 strains. Eurotiomycetes were mainly isolated on Trebouxia Medium (TM) and Lilly & Barnet Medium (LBM), whereas Dichloran/Glycerol agar medium DG18 turned out to be ineffective for their growth (Figure 1a). Dothideomycetes mainly grew on DG18 and LBM (Figure 1b), Leotiomycetes on LBM and DG18 (Figure 1c) and Sordariomycetes on Sabouraud Medium (SAB) (Figure 1d). Lichen mycobionts were isolated mostly on LBM (13 out of 29 isolates) and TM (Figure 1e). In general LBM and TM proved to be the most suitable media for the isolation of a maximum number of fungal taxa, as 52% of the isolates (comprising Eurotiomycetes, Dothideomycetes, Leotiomycetes and lichen mycobionts) grew well on them (Figure 1f).
Figure 1.
Abundance plots showing the growth preference of the fungal isolates on the six growth media: (a) Eurotiomycetes; (b) Dothideomycetes; (c) Leotiomycetes; (d) Sordariomycetes; (e) lichen mycobionts; (f) total number of lichen-inhabiting fungal isolates compared with isolates of the lichen mycobionts. DG18: Dichloran/Glycerol agar, KGA: PDA Potato/Dextrose agar, LBM: Lilly and Barnet medium, MY: Malt Yeast-extract, SAB: Sabouraud, TM: Trebouxia medium.
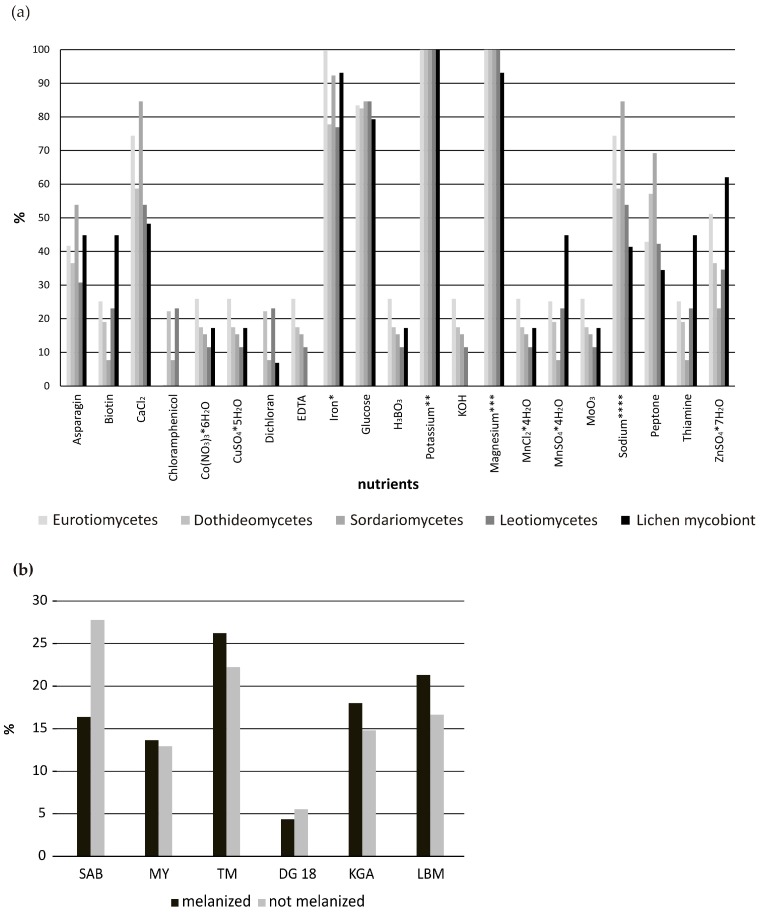
Figure 2.
(a) Abundance plot showing the growth preference of the five groups of fungal isolates (non-lichenized Eurotiomycetes, Dothideomycetes, Leotiomycetes, Sordariomycetes and lichen mycobionts, mostly Lecanoromycetes) according to the micronutrients present in the culture media. Abundance values are expressed in percentage. The presence of iron, potassium, magnesium and sodium in the medium compounds is summed up as follows: * iron: Fe(NO3)3·9H2O, FeSO4··7H2O or iron (from yeast extract); ** potassium: K2HPO4, KH2PO4 or potassium (from yeast and malt extract); *** magnesium: MgSO4·7H2O or magnesium (from yeast and malt extract); **** sodium: NaCl, NaNO3; (b) Abundance plot comparing the growth preference of melanized and non-melanized fungi on the six culture media (total number of individuals compared n = 243). DG18: Dichloran/Glycerol agar, KGA: PDA Potato/Dextrose agar, LBM: Lilly and Barnet medium, MY: Malt Yeast-extract, SAB: Sabouraud, TM: Trebouxia medium.
We observe that the presence of magnesium, potassium, iron and glucose in growth media is pivotal for the successful isolation of all the fungal classes (Figure 2a). Alternatively, the presence of chloramphenicol in DG18, ethylendiamintetraacetic acid (EDTA) and potassium hydroxide in TM highly reduces the successful isolation of Eurotiomycetes and lichen mycobionts. Zinc and manganese compounds, alternatively, seem to be important for the growth of the lichen mycobionts, whereas asparagine, CaCl2, sodium and peptone (majorly in SAB and TM) seem to be important for the growth of Sordariomycetes. Fungal growth in our experiments was not dependent on the pH of the media, as this was kept within a narrow range in all media with values of (5.2–) 5.6 (–6.0).
The great majority of the isolated strains represent melanized fungi belonging to the classes Eurotiomycetes and Dothideomycetes. They were isolated mostly on TM, and this seems to correlate with the abundant presence of metal ions in the medium. Non-melanized fungi, alternatively, have been mostly isolated on SAB. The percentages of melanized and non-melanized fungi isolated on the other four media differ only slightly (Figure 2b).
The environmental samples used as source of fungal isolation are lichens belonging to the class Lecanoromycetes, to which also certain lichenicolous fungi belong. In the environmental samples the morphological determination of lichenicolous fungi revealed the presence of Arthoniomycetes, Dothideomycetes and Eurotiomycetes. Lecanoromycetes are, however, the least obtained isolates (29, genetically identified only as the lichen mycobionts) and the highest number of isolates is represented by Eurotiomycetes (266) and to a lesser extent by Dothideomycetes (representing the lichenicolous and the endolichenic fungi). Arthoniomycetes did not grow on any medium used. The presence of Agaricomycetes, Leotiomycetes and Sordariomycetes in the thalli could be assessed only when the corresponding isolates were genetically identified.
Isolates belonging to Eurotiomycetes, Dothideomycetes, Leotiomycetes and Sordariomycetes developed a mycelium of 5–10 mm in diameter within three months. Isolates corresponding to lichen mycobionts were characterized by a slower growth rate and mycelia of up to 5 mm in diameter could be recovered only one year after the original inoculation.
3. Discussion
To our knowledge, only Vinayaka et al. [35] have compared the influence of three different media on the success rate and systematic bias of endolichenic fungal isolation. The authors recovered 30 taxa, of which the highest number of isolates grew on malt yeast extract (MYA) medium [35]. We have analyzed here six growth media and have recovered the highest number of isolates on LBM. The presence of biotin and thiamin (vitamins B1, B12) in LBM is likely to facilitate the development of the mycelia on the artificial substrate. The highest numbers of melanized isolates are recovered on TM and LBM. It remains nevertheless difficult to compare precise nutrition requirements of the fungal groups because some media ingredients are only sold as extracts, such as potato, malt and yeast extract. The approximate composition of these condiments had to be retrieved from literature or chemical studies of their components. These media are both rich in metal ions, which may enhance the isolation of those fungi which are able to metabolize these compounds for faster growth.
The diversity of lichen–associated fungi recovered in culture may depend on the type of surface sterilization applied on the thallus and the growth medium used in the initial isolation step. Several protocols for thallus sterilization have been reported in the literature [3,36,37], which apply either washing steps with sodium hypochlorite solutions or ethanol dilutions. In this study thallus fragments were washed with Tween 80 to remove a great part of loosely attached bacteria, with the aim to increase the isolation success of lichen-inhabiting fungi, and cleaning the thalli as much as possible from other spurious particles. The thallus pieces were then smashed in sterile conditions and fragments of less than 0.5 mm in size were inoculated. It should, however, be noticed that lichens do not have a clear separation of external and internal colonization by microorganisms as found in plants, where the cuticula forms a clear border between external and internal microbiota. As microorganisms invade the layers of lichens at variable depths the duration of surface sterilization procedures decreases the number of fungi that can be retrieved. Moreover, the access of sterilization liquids to lichen surfaces is dependent on the microarchitecture and hydrophobicity of lichens. The swelling of hydrated lichens fragments may limit the access of sterilization solutions to thallus fissures (such as cracks between the areoles of crustose lichens). Thus, fungi attached on the surface of these thallus fissures would not be degraded unequivocally by sterilization. In addition, if lichens possess hydrophobic surface structures, this may also prevent access of polar sterilization.
A recent review highlights the potential of lichenicolous fungi as bioresources of novel bioactive compounds [16]. Due to the limited morphological characters and frequent lack of reproductive structures in the cultured mycelia, these strains are hardly identifiable without DNA sequence data. Up to now, in the majority of the cases, the new metabolites are reported from unidentified fungi, which are characterized by strain numbers or morphotypes assignable to hyphomycete genera that lack phylogenetic background [31,38]. The antibacterial compounds lichenicolin A and B, active against Gram-positive bacteria, were isolated from the strain labelled as LL-RB0668 [31]. New heptaketides were isolated from an endolichenic Corynespora sp. from Usnea cavernosa with cytotoxic activities against cancer cell lines [39]. Furthermore, eight novel metabolites have been characterized from an endolichenic pleosporalean fungus [40]. Some of these fungi were also tested for the production of diverse metabolic patterns on different media or against human pathogenic bacteria for pharmaceutical purposes [38,41].
The fungal strains tested so far for their metabolite production mostly derived from epiphytic and terricolous macrolichens, the thalli of which are leaf-like or fruticose (highly three-dimensional), and represent very widespread genera—such as Aspergillus, Chaetomium, Penicillium, Sporomiella, Trichoderma—of endophytes commonly found as plant pathogens and saprotrophs [41]. In many cases the host lichens are unknown either, which hampers reproducibility of the results. Exceptions are Aspergillus versicolor, producing three new anthraquinones derivatives, isolated form the lung lichen Lobaria retigera [42], and Sporomiella irregularis producing the new xanthone glycoside sporormielloside, isolated from Usnea mutabilis [43].
Interestingly, many of the isolates obtained here or previously characterized by Muggia et al. [9] from epilithic lichens do not correspond to the genera mentioned above. It seems, therefore, that the growth form of the lichen hosts influences the diversity of the associated fungi: while Eurotiomycetidae, Leotiomycetes and Sordariomycetes are mainly recovered from foliose and fruticose macrolichens [3,7], taxa belonging to Chaetothyriomycetidae and Dothideomycetes are mainly isolated from crustose thalli on rocks [9,44]. Many of these fungi represent new monophyletic lineages, which occur in diverse lichen hosts or share the same host species. The different growth rates that these taxa present in axenic culture might also be correlated with their specificity towards the lichen hosts. Slow growing fungi may be more adapted to the lichens than the ubiquitous strains, which present faster growth rates and may be nutrient-deprived in lichens but proliferate on culture media. Further, it might be speculated that, beside the three dimensionally structure, the air-filled medulla of foliose and fruticose lichens increases the frequency and the duration of condition with limited gas diffusions due to the lack of hydrophobicity. Therefore a longer air-filled medulla in (some but not all) crustose lichens might play a role in the composition of associated fungi.
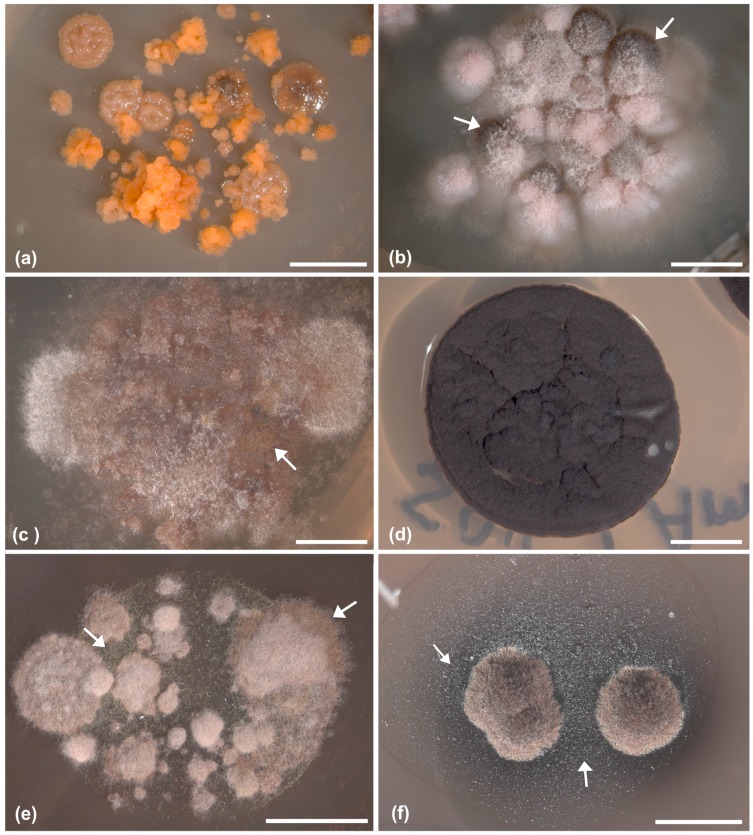
So far, crustose lichens have been seldom considered as sources of lichen-associated fungi, but our results highlight that they harbor a great diversity of lichen-inhabiting fungi, which excrete pigments into the medium (Figure 3) and deserve further chemical characterizations for the exploitation of their metabolic potential. Many of these organisms remain uncultivable [10] because they require the biological context in the lichen species host, which can hardly be provided by in vitro conditions. This may lead to an under-representation of the true organismal diversity in lichens.
Figure 3.
Habit of representative fungal strains which develop peculiar phenotypes of secondary metabolites on diverse culture media (the acronym of medium name and the number of the strain are reported in parentheses): (a,b,d) Eurotiomycetes (Chaetothyriales, A1109, A1165 and A527) fungi (on MY and SAB); (c,e) Dothideomycetes fungi (on DG18; A1086 and A1168); (f) lichen mycobiont Tephromela atra (on LBM, A1180). (a) Two different strains with diverse phenotypes have grown out from a single inoculum. (d) Chaetothyrialean black fungi (A527) have been the most commonly isolated strains from rock inhabiting lichens. Arrows indicates the areas of the mycelia (b,c,e) or of the medium (f) where secondary metabolites accumulate and deserve further analyses. Scale bars = (a–d,f) 4 mm, (e) 5 mm.
With our work we intended to expand the spectrum of cultivable lichenicolous fungi by using a wider range of media conditions. Certainly, this will not lead to a complete inventory of these interesting fungi, but provide means for selective isolation and for better growth of biotechnologically or pharmaceutically interesting fungi. We think that lichen-specific fungi are worth of further investigation for bioactive principles, as these fungi are adapted to live in symbioses with their hosts. As a further step towards uncovering the metabolic potential of lichen-inhabiting fungi, we envision the efficiency of co-culturing of symbionts, which may also involve bacteria as a common component of lichens. Lichen-associated bacteria have already been shown to represent chemically interesting bioresources [45], but their potential effect on a wider range of lichen-derived fungi in co-cultures remains an exciting endeavor for future studies.
4. Materials and Methods
4.1. Sampling and Culture Isolation
Lichen thalli were sampled in May–July 2012 (Appendix E) on the Koralpe mountain range (Styria, Austria), as described in Fleischhacker et al. [46]) and Muggia et al. [9]. Subsets of thalli visibly infected by 34 species of lichenicolous fungi (Appendix A)—at least 15 samples from each plot—were selected for culture isolation so that we had a total of 190 thalli representing 21 lichen species. We considered only lichens from rocks, majorly with a crust-like growth type. The ratio of crustose to foliose lichens was 9:1.
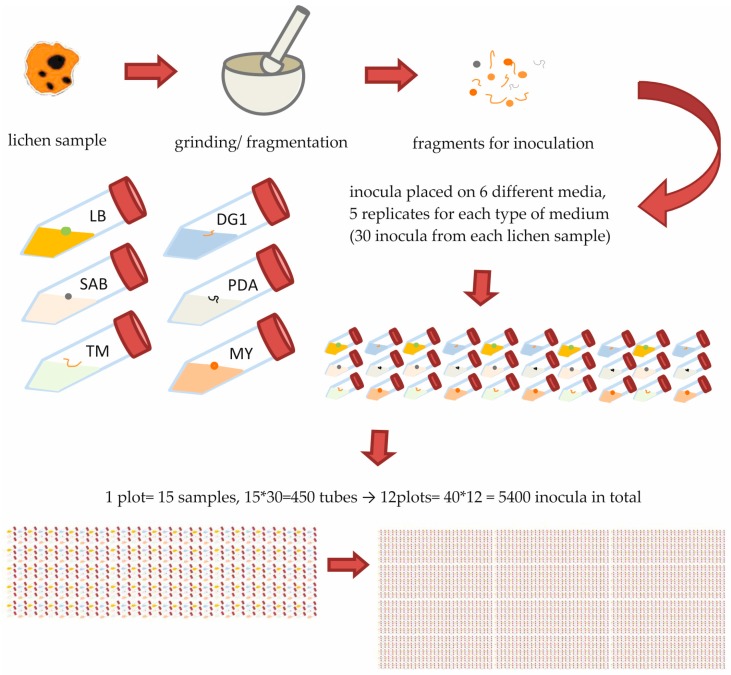
The fungal isolation followed the protocol of Yamamoto et al. [47] and is schematically reported in Appendix F. Briefly, approximately 2 mm2 fragments of infected lichen thalli were dissected with a sterile razor blade. The fragments were washed three times for 15 min with distilled sterile water, 30 min with 500 μL Tween 80 (diluted 1:10) and finally twice for 15 min with sterile water. As the aim of the study was to isolate lichenicolous fungi and any other fungus residing within the lichen thallus [9,46], the washing steps were performed to remove any spurious organism or particles (bacteria, spores, fragments of fungal hyphae, yeast) loosely attached on the thallus external surface. The washed samples were then grinded in sterile water and tiny thallus fragments were picked and transferred individually into slanted agar tubes. In order to promote the growth of as many different lichen-inhabiting fungi as possible, covering a broad spectrum of fungal growth requirements, we inoculated the dissected fragments on six different media (Appendix D): Lilly & Barnett (LB [48]); Trebouxia medium (TM [49]), Malt Yeast-extract (MY [48]), Sabouraud (SAB [50]), Potato Dextrose agar (PDA=KGA, ApplChem A5828), Dichloran/Glycerol agar (DG18 [51]). MY, SAB and PDA are full, organic media containing several micronutrients and are therefore used to characterize a wide variety of fungi and yeasts [52]. LB medium is principally used for the isolation of lichenized fungi, while TM is mainly used for the growth of photobionts, but as it is also rich in carbohydrates it works well also for lichenized and non-lichenized fungi [49]. To cover a broader spectrum in fungal growth requirements we also used DG18, which is a special medium for xerophilic fungi [51]. In total we inoculated 5400 tubes (Appendix F). Five tubes of each medium were inoculated for each sample, resulting in a total of 30 inocula. The tubes were incubated in a growing chamber at 20 °C, with a light-dark regime of 14:10 h, light intensity of 60–100 μmol photons m−2s−1 and 60% humidity. After three to five months, the inocula reached about 1–3 mm in diameter and it was possible to gain subcultures which were necessary for the isolation of DNA for genetic identification and morphological analyses [9]. The subcultures were set on agar Petri plates using the same growth medium where the inoculum grew successfully; ampicillin was further added to avoid eventual post bacteria contamination. The cultured strains are stored at the University of Graz in the culture collection of the first author LM and are preserved as cryostocks, as cited in Muggia et al. [9].
4.2. Morphological Analyses
Morphological and anatomical characters of the cultured strains were analyzed using light microscopy and documented with digital photographs as in Muggia et al. [9]. Analyses and photographs were performed on 10 month to one year old subcultures considering the following characters: form of growth, branching of the hyphae and melanization. Small fragments of the mycelia were taken; squashed sections were mounted in water and studied by light microscopy. All images were acquired with a ZEISSAxioCam MRc5 (Zeiss, Jena, Germany) digital camera fitted to the microscope. Both images of growth habit and hyphae structure [9] were digitally processed using the CombineZM software [53]. The photos were slightly refined in sharpness and color tone with Adobe Photoshop 7.0 (© Adobe System Incorporated, San Jose, CA, USA) and the figures were prepared with CorelDRAW X7 (© Corel Corporation, Ottawa, Canada).
4.3. DNA Extraction, Amplification and Sequencing
The identity of each grown isolate was checked with sequences of at least two genetic markers. Small parts of the sub-cultured fungi were taken, transferred into 1.5 mL reaction tubes containing sterile tungsten beads (Qiagen, Vienna, Austria) for homogenization, frozen and ground using a TissueLyserII (Retsch, Haan, Germany). The DNA was extracted following either the cetyltrimethyl ammonium bromide CTAB protocol of Cubero et al. [54] or using the DNeasy Plant Mini Kit (Qiagen). The industrial kit was used for those most melanized isolates for which the CTAB protocol failed in extracting amplifiable DNA.
The identity of the cultured fungal strains was studied with sequences of the nuclear large and partial nuclear small ribosomal subunits (nucLSU and nucSSU) and the mitochondrial small ribosomal subunit (mtSSU). Primers and PCR conditions, sequencing and sequence analysis were performed as in Muggia et al. [9].
4.4. Phylogenetic Analyses for Isolate Identification
We checked the identity of the newly generated sequences with sequences available in the GenBank database by BLAST similarity search [55] and with those generated previously by Muggia et al. [9]. Taxa which most closely matched our sequences for a value not lower than 95% identity and the further most closely related ones (up to 90% identity) were selected for the phylogenetic analyses. As our sequences (Appendix C) showed closest matches with representatives of the classes Eurotiomycetes (particularly in the subclasses Chaetothyriomycetidae), Dothideomycetes, Leotiomycetes and Sordariomycetes, we prepared four different datasets representing each fungal group. The here newly obtained sequences were added to the datasets previously constructed by Muggia et al. [9], which were carefully selected on the base of previous phylogenetic analyses considering the aforementioned classes [56,57,58,59,60,61,62,63,64,65,66,67]. Each dataset represents the widest possible spectrum of taxon diversity, including at least three representative taxa for each different family or order of the four classes (Appendix B). Sequence alignments for each locus (nucLSU, nucSSU and mtSSU) and for each fungal class (Eurotiomycetes, Dothideomycetes, Leotiomycetes and Sordariomycetes) were prepared manually in BioEdit [68]. Introns and ambiguous SNPs were removed from the alignment. For a number of specimens we were unable to generate sequences for all of the selected loci and for other taxa sequences were not available in GenBank. The analyses included only samples with at least two sequenced loci.
As the single locus analyses were congruent for the four individual classes, the final sequence analyses were performed on combined 3-locus datasets (nuLSU, nuSSU and mtSSU) for Dothideomycetes and Eurotiomycetes and 2-locus datasets (nuLSU and nuSSU) for Leotiomycetes and Sordariomycetes as in previous studies [9,69,70,71]. The multilocus datasets were generated with the SequenceMatrix program [72] and the phylogenetic analyses were performed using the maximum likelihood (ML) approach. In the multilocus datasets the loci were treated in partitions by genes nucLSU, nucSSU and mtSSU. The ML analyses were performed using the program RAxML v.7.1.3 [73], by applying the GTRMIX model. The phylogenetic trees were visualized in TreeView [74].
4.5. Statistical Analyses
Analyses and figures of media composition and growth preferences of fungal groups were performed in Microsoft Excel 2010 (Microsoft, Redmond, WA, USA) and CorelDrawX7.
Acknowledgments
The authors are grateful to the Austrian Science Fund for financial support (FWF project P24114-B16). We thank Gerhard Gößler for constructive discussion and Antonia Fleischhacker for helping in preparing the fungal cultures.
Appendix A
Table A1.
Lichens and lichenicolous fungal species and the number of thalli used in the culture experiments.
| Lichen Species | N. of Thalli | Lichenicolous Fungus | N. of Thalli |
|---|---|---|---|
| Acarospora fuscata | 1 | Polycoccum microsticticum | 1 |
| Aspicilia sinensis | 1 | Endococcus verrucosus | 1 |
| Aspicilia sp. | 8 | Cercidospora solearispora | 1 |
| Endococcus rugulosus | 7 | ||
| Aspilidea myrinii | 13 | Sagediopsis fissurisedens | 13 |
| Immersaria athroocarpa | 1 | Muellerella pygmaea-Ia * | 1 |
| Lecanora bicincta | 9 | Arthonia varians | 7 |
| Sphaerellothecium atryneae | 2 | ||
| Lecanora intricata | 5 | Muellerella pygmaea-Li * | 5 |
| Lecanora polytropa | 47 | Arthonia sp. | 3 |
| Carbonea supersparsa | 3 | ||
| Cercidospora epipolytropa | 14 | ||
| Lichenoconium lecanorae | 11 | ||
| Muellerella pygmaea-Lp * | 16 | ||
| Lecanora rupicola | 5 | Arthonia varans | 5 |
| Lecanora swartzii | 1 | Sphaerellothecium atryneae | 1 |
| Lecidea lapicida | 4 | Muellerella pygmaea | 4 |
| Lecidea sp. | 22 | Cecidonia umbonella | 2 |
| Muellerella pygmaea var. pygmaea | 20 | ||
| Pertusaria corallina | 5 | Sclerococcum sphaerale | 5 |
| Pertusaria lactea | 4 | Stigmidium eucline | 4 |
| Protoparmelia badia | 1 | Phacographa protoparmeliae | 1 |
| Ophioparma ventosa | 1 | Muellerella pygm. var. ventosicola | 1 |
| Rhizocarpon geographicum s.l. | 36 | Endococcus macrosporus | 17 |
| Endococcus propinquus | 1 | ||
| Muellerella pygmaea-Rg * | 17 | ||
| Opegrapha geographicola | 1 | ||
| Schaereria fuscocinerea | 2 | Endococcus perpusillus | 1 |
| Muellerella pygmaea | 1 | ||
| Sporastatia polyspora | 1 | Polycoccum sporastatiae | 1 |
| Tephromela atra | 21 | Muellerella atricola | 3 |
| Skyttea tephromelarum | 2 | ||
| Taeniolella atricerebrina | 16 | ||
| Umbilicaria cylindrica | 2 | Stigmidium gyrophorarum | 2 |
(*) The lichenicolous fungus Muellerella pygmaea is reported with the initial of the species name of its host to differentiate the recognized varieties as not yet formally described [46].
Appendix B
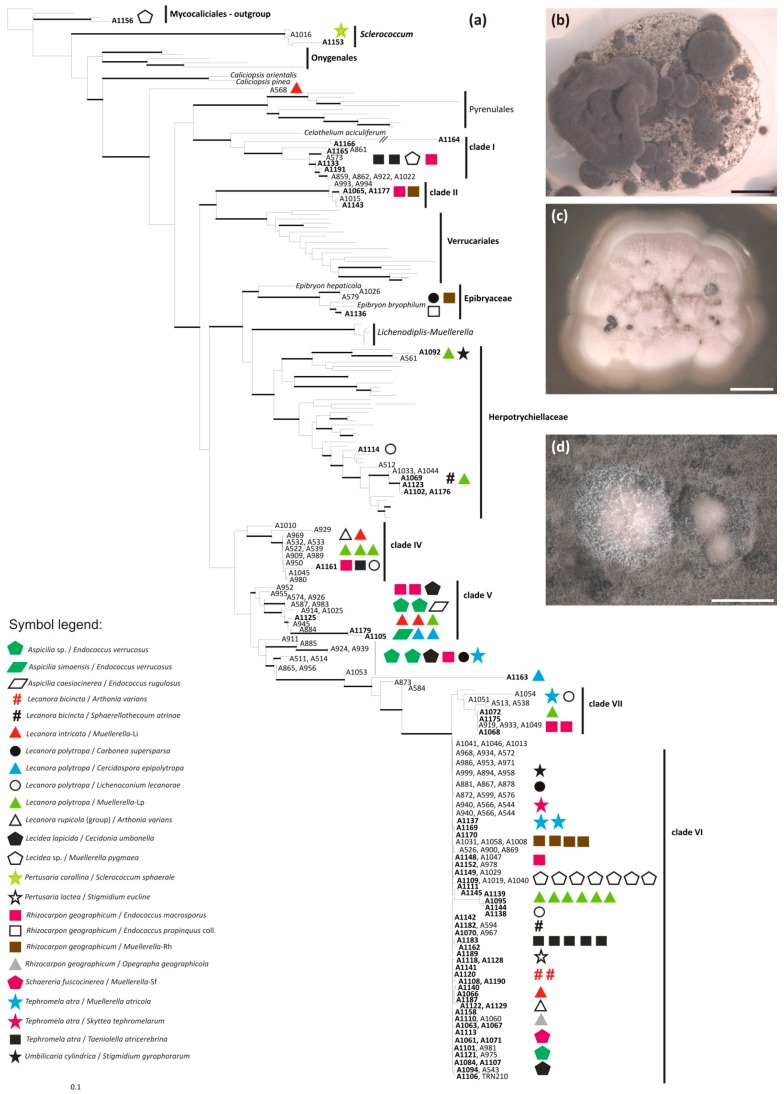
Figure A1.
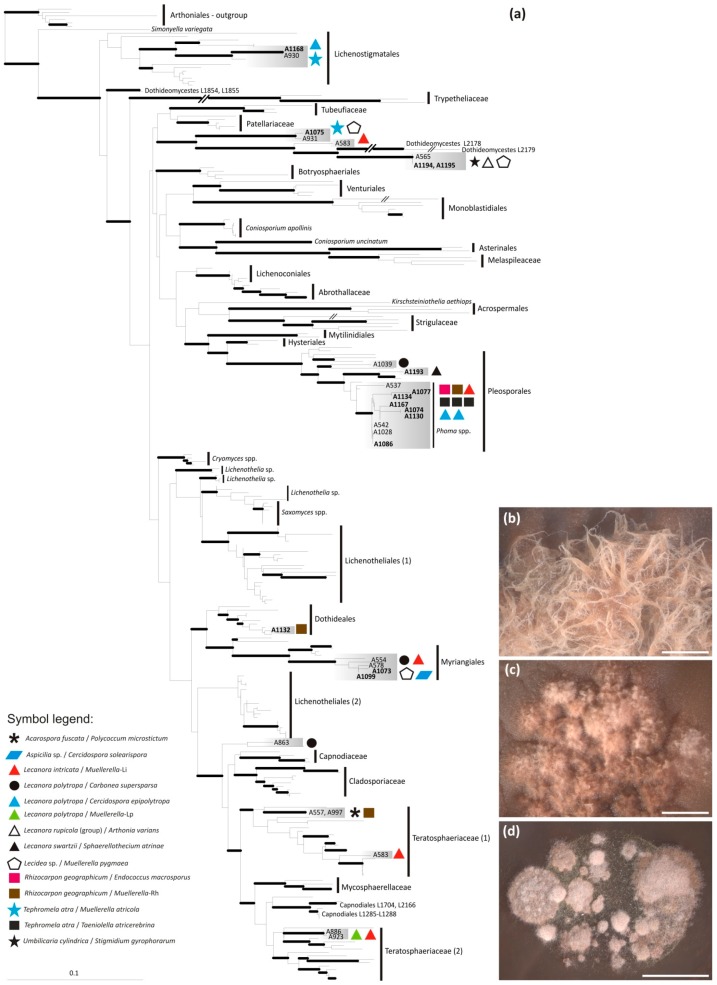
Phylogenetic position of fungal strains belonging to the class Eurotiomycetes (a) and habit of representative cultured strains: (b) A522 (clade IV); (c) A1133 (clade I); (d) A1175 (clade VII). The symbol legend describes the identity of the original samples from which the fungi were isolated: lichen species name/lichenicolous fungus species name infecting that lichen thallus. The isolated strains are reported with their DNA ID number; newly characterized strains are in bold; strains already characterized in Muggia et al. [9] and included in the analyses of growth media are not in bold. The nomenclature of the clades follows Muggia et al. [9]. Fully supported branches (bootstrap support = 100%) are in bold. Scale bar = (b,c) 4 mm, (d) 2 mm.
Figure A2.
Phylogenetic position of fungal strains belonging to the class Dothideomycetes (a) and habit of representative cultured strains: (b) A1077 (Pleosporales, Phoma clade); (c) A1086 (Pleosporales, Phoma clade); (d) A1168 (Lichenostigmatales). The symbol legend reported describes the identity of the original samples from which the fungi were isolated: lichen species name/lichenicolous fungus species name infecting that lichen thallus. The isolated strains are reported with their DNA ID number; newly characterized strains are in bold; strains already characterized in Muggia et al. [9] and included in the analyses of growth media are not in bold. The nomenclature of the clades follows Muggia et al. [9]. Fully supported branches (bootstrap support = 100%) are in bold. Scale bar = (b) 2 mm, (c) 4 mm, (d) 5 mm.
Figure A3.
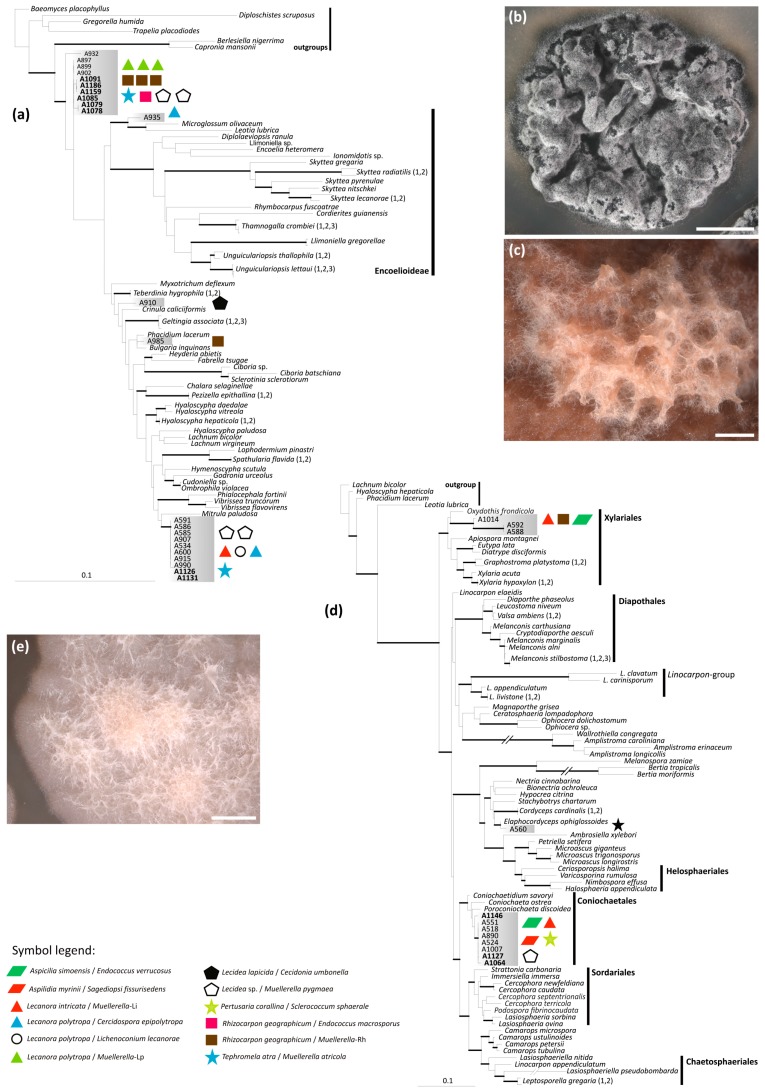
Phylogenetic position of fungal strains belonging to the class Leotiomycetes (a) and habit of representative cultured strains: (b) A1085 (Leotiomycetes) and (c) A1019 (Leotiomycetes). Phylogenetic position of fungal strains belonging to the class Sordariomycetes (d) and habit of representative cultured strain (e) A518 (Sordariomycetes, Coniochaetales). The symbol legend reported describes the identity of the original samples from which the fungi were isolated: lichen species name/lichenicolous fungus species name infecting that lichen thallus. The isolated strains are reported with their DNA ID number; newly characterized strains are in bold; strains already characterized in Muggia et al. [9] and included in the analyses of growth media are not in bold. The nomenclature of the clades follows Muggia et al. [9]. Fully supported branches (bootstrap support = 100%) are in bold. Scale bar = (b) 4 mm, (c) 1 mm, (e) 2 mm.
Appendix C
Table A2.
NCBI accession numbers for the newly characterized isolates of lichen-inhabiting fungi included in the phylogenetic analyses of Appendix A.
Appendix D
Composition of the growth media used to isolate and culture lichen-associated fungi and lichen mycobionts.
Table A3.
Lilly & Barnett medium (LB, Lilly and Barnett, 1951).
| Nutrients | Total Amount of Nutrient in 1 L Medium |
|---|---|
| Glucose | 10 g |
| Asparagine | 2 g |
| K2HPO4 | 1 g |
| MgSO4·7H2O | 0.5 g |
| Fe(NO3)3·9H2O | 0.2 g |
| ZnSO4·7H2O | 0.2 g |
| MnSO4·4H2O | 0.1 g |
| Thiamine | 100 µg (stock solution: 100 mg/L), 1 mL from the stock solution |
| Biotin | 5 µg (stock solution: 25 mg/L) 200 µL from the stock solution |
| Agar | 15 g |
| Distilled H2O | 986 mL |
Table A4.
Trebouxia medium (TM, Ahmadjian, 1987).
| Nutrients | Total Amount of Nutrient in 1 L Medium |
|---|---|
| BBM | 970 mL |
| Peptone | 10 g |
| Glucose | 20 g |
| Agar | 20 g |
Table A5.
Bold’s Basal medium (BBM, Nichols and Bold, 1965) as part of the TM components.
| Solutions A1 a | 400 mL | Solutions A2 | 1000 mL | ||
|---|---|---|---|---|---|
| A1/1 | NaNO3 | 10 g | A2/1 | H3BO3 | 11.42 g/L |
| A1/2 | KH2PO4 | 7 g | A2/2 | FeSO4·7H2O ZnSO4·7H2O MnCl2·4H2O |
4.98 g/L 8.82 g/L 1.44 g/L |
| A1/3 | K2HPO4 | 3 g | A2/3 | MoO3
CuSO4·5H2O Co(NO3)3·6H2O |
0.7 1g/L 1.57 g/L 0.49 g/L |
| A1/4 | MgSO4·7H2O | 3 g | A2/4 | EDTA KOH |
50 g/L 31 g/L |
| A1/5 | CaCl | 1 g | |||
| A1/6 | NaCl | 1 g |
a 10 mL of each solutions A1/1–A1/6 and 1 mL of each solution A2/1–A2/4 are added into 1000 mL distilled water.
Table A6.
Malt Yeast-extract (MY, Lilly and Barnett, 1951).
| Nutrients | Total Amount of Nutrient in 1 L Medium |
|---|---|
| Malt extract | 20 g |
| Yeast extract | 2 g |
| Agar | 20 g |
Table A7.
Sabouraud (SAB, Pagano, Levin and Trejo, 1958).
| Nutrients | Total Amount of Nutrient in 1 L Medium |
|---|---|
| Glucose | 20 g |
| Peptone | 10 g |
| Yeast extract | 5 g |
| Agar | 20 g |
Table A8.
Potato Dextrose agar (PDA, ApplChem, A5828, pH 5.6 (20 °C).
| Nutrients | Total Amount of Nutrient in 1 L Medium |
|---|---|
| Potato extract (solid) | 4 g |
| Glucose | 20 g |
| Agar | 15 g |
Table A9.
Dichloran/Glycerol agar (DG18, Hocking and Pitt, 1980 pH: 5.6 ± 0.2 at 25 °C).
| Nutrients | Total Amount of Nutrient in 1 L Medium |
|---|---|
| Peptone | 5 g |
| Glucose | 10 g |
| Potassium dihydrogen phosphate | 1 g |
| Dichloran 0.2% in EtOH 1 mL | 0.002 g |
| Magnesium sulfate | 0.5 g |
| Chloramphenicol | 0.1 g |
| Agar | 15.0 g |
Appendix E
Table A10.
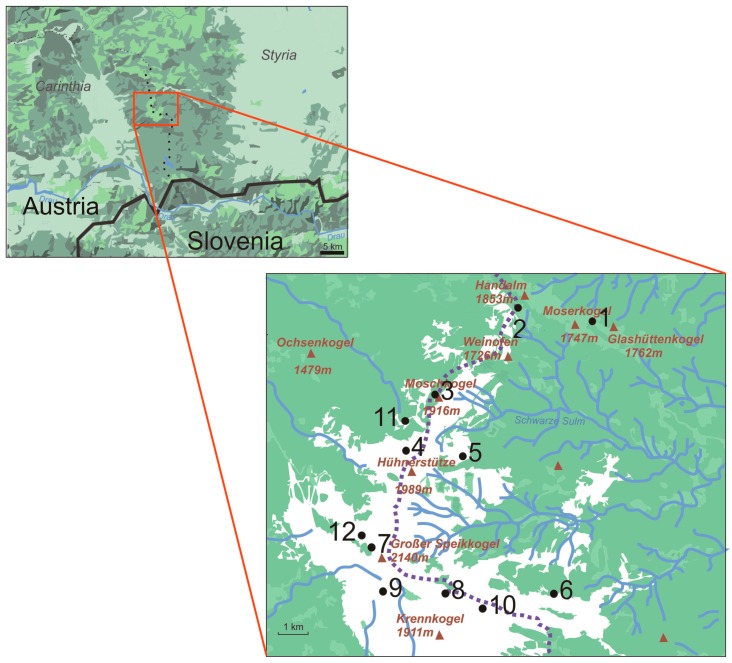
Geographic information of the collecting sites is reported: ID number of the site, name of the site, geographical coordinates and altitude. All sites are located in subalpine to alpine habitat above the timberline, ranging between 1800 and 2100 m above sea level (a.s.l.), on the Koralpe Mountain range, in the region Styria (ST) and Carinthia (K) in Austria. Landscape characterized by big boulders and cliffs of homogeneous size of siliceous-schist/gneissic rocks separated by wide areas of pastures or dwarf shrub formations.
| Site N. | Mountain Top Name | Latitude | Longitude | Altitude a.s.l. |
|---|---|---|---|---|
| 1 | Glashüttenkogel (ST) | 46°50′12′′ N–46°50′′20′′ N | 15°02′35′′ E–15°03′00′′ E | 1750 m |
| 2 | Handalm (ST) | 46°50′38′′ N | 15°01′10′′ E | 1800 m |
| 3 | Moschkogel (ST) | 46°49′21′′ N–46°49′22′′ N | 14°59′29′′ E–14°59′34′′ E | 1900 m |
| 4 | Hühnerstütze (K) | 46°48′23′′ N–46°48′31′′ N | 14°58′57′′ E–14°59′08′′ E | 1970 m |
| 5 | Loskogel (ST) | 46°48′23′′ N–46°48′25′′ N | 15°00′31′′ E–15°00′33′′ E | 1780 m |
| 6 | Glitzfelsen (ST) | 46°46′50′′ N–46°46′53′′ N | 15°01′24′′ E–15°01′35′′ E | 1810 m |
| 7 | Großer Speikkogel (K) | 46°47′21′′ N–46°47′23′′ N | 14°57′58′′ E–14°58′06′′ E | 2088 m |
| 8 | Ochsenstein (K) | 46°46′51′′ N–46°46′54′′ N | 14°59′23′′ E–14°59′26′′ E | 1980 m |
| 9 | Krakaberg (K) | 46°46′43′′ N–46°46′47′′ N | 14°58′13′′ E–14°58′14′′ E | 2050 m |
| 10 | Ochsenofen (K) | 46°46′29′′ N–46°46′31′′ N | 15°00′45′′ E–15°00′52′′ E | 1760 m |
| 11 | Großer Speikkogel (K) | 46°47′39′′ N | 14°57′42′′ E | 2000 m |
| 12 | Sprungkogel (K) | 46°48′54′′ N | 14°58′14′′ E | 1860 m |
Figure A4.
Schematic map of the geographic location of the collecting sites: the enlarged rectangle shows the detailed locations of the sites on the Koralpe mountain range.
Appendix F
Figure A5.
Workflow of the procedure used to set fungal isolates and replicates in the present research.
Author Contributions
L.M. conceived and designed the study, L.M. and T.K. performed the experiments and analyzed the data; L.M., T.K. and M.G. wrote the paper. All authors approved the final version.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Footnotes
Sample Availability: Not available.
References
- 1.Hawksworth D.L., Honegger R. The lichen thallus: A symbiotic phenotype of nutritionally specialized fungi and its response to gall producers. In: Williams M.A.J., editor. Plant Galls. Organisms, Interactions, Populations. Clarendon Press; Oxford, UK: 1994. pp. 77–98. The Systematics Association, Special Volume. [Google Scholar]
- 2.Cardinale M., Vieira de Castro J., Müller H., Jr., Berg G., Grube M. In situ analysis of the bacterial community associated with the reindeer lichen Cladonia arbuscula reveals predominance of Alphaproteobacteria. FEMS Microbiol. Ecol. 2008;66:63–71. doi: 10.1111/j.1574-6941.2008.00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Arnold A.E., Miadlikowska J., Higgins K.L., Sarvate S.D., Gugger P., Way A., Hofstetter V., Kauff F., Lutzoni F. Hyperdiverse fungal endophytes and endolichenic fungi elucidate the evolution of major ecological modes in the Ascomycota. Syst. Biol. 2009;58:283–297. doi: 10.1093/sysbio/syp001. [DOI] [PubMed] [Google Scholar]
- 4.Hodkinson B.P., Gottel N.R., Schadt C.W., Lutzoni F. Photoautotrophic symbiont and geography are major factors affecting highly structured and diverse bacterial communities in the lichen microbiome. Environ. Microbiol. 2012;14:147–161. doi: 10.1111/j.1462-2920.2011.02560.x. [DOI] [PubMed] [Google Scholar]
- 5.Bates S.T., Cropsey G.W.G., Caporaso J.R., Knight R., Fierer N. Bacterial communities associated with the lichen symbiosis. Appl. Environ. Microbiol. 2011;77:1309–1314. doi: 10.1128/AEM.02257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U’Ren J.M., Lutzoni F., Miadlikowska J., Arnold A.E. Intensive sampling reveals ecological distinctiveness and continua among culturable symbiotrophic and saprotrophic Ascomycota in a montane forest. Microb. Ecol. 2010;60:340–353. doi: 10.1007/s00248-010-9698-2. [DOI] [PubMed] [Google Scholar]
- 7.U’Ren J.M., Lutzoni F., Miadlikowska J., Laetsch A., Arnold A.E. Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am. J. Bot. 2012;99:898–914. doi: 10.3732/ajb.1100459. [DOI] [PubMed] [Google Scholar]
- 8.U’Ren J.M., Riddle J.M., Monacell J.T., Carbone I., Miadlikowska J., Arnold A.E. Tissue storage and primer selection influence pyrosequencing-based inferences of diversity and community composition of endolichenic and endophytic fungi. Mol. Ecol. Resour. 2014;14:1032–1048. doi: 10.1111/1755-0998.12252. [DOI] [PubMed] [Google Scholar]
- 9.Muggia L., Fleischhacker A., Kopun T., Grube M. Extremotolerant fungi from alpine rock lichens and their phylogenetic relationships. Fungal Divers. 2016;76:119–142. doi: 10.1007/s13225-015-0343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spribille T., Tuovinen V., Resl P., Vanderpool D., Wolinski H., Aime M.C., Schneider K., Stabentheiner E., Toome-Heller M., Thor G., et al. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science. 2016:aaf8287. doi: 10.1126/science.aaf8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grube M., Cardinale M., Vieira de Castro J. Jr., Müller H., Berg G. Species-specific structural and functional diversity of bacterial communities in lichen symbioses. ISME J. 2009;3:1105–1115. doi: 10.1038/ismej.2009.63. [DOI] [PubMed] [Google Scholar]
- 12.Grube M., Cernava T., Soh J., Fuchs S., Aschenbrenner I., Lassek C., Wegner U., Becher D., Riedel K., Sensen C.W., et al. Exploring functional contexts of symbiotic sustain within lichen-associated bacteria by comparative omics. ISME J. 2015;9:412–424. doi: 10.1038/ismej.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawksworth D.L. The lichenicolous hyphomycetes. Bull. Br. Mus. Nat. Hist. Bot. Ser. 1979;9:1–98. [Google Scholar]
- 14.Lawrey J.D., Diederich P. Lichenicolous fungi: Interactions, evolution, and biodiversity. Bryologist. 2003;106:80–120. doi: 10.1639/0007-2745(2003)106[0080:LFIEAB]2.0.CO;2. [DOI] [Google Scholar]
- 15.Hawksworth D.L., Paterson R.R.M., Vote N. An investigation into the occurrence of metabolites in obligately lichenicolous fungi from thirty genera. In: Feige G.B., Lumbsch H.T., Huneck S., editors. Phytochemistry and Chemotaxonomy of Lichenized Ascomycetes—A Festschrift in Honour of Siegfried Huneck. J. Cramer; Berlin, Germany: Stuttgart, Germany: 1993. pp. 101–108. Bibliotheca Lichenologica. [Google Scholar]
- 16.Kellogg J., Raja H.A. Endolichenic fungi: A new source of rich bioactive secondary metabolites on the horizon. Phytochem. Rev. 2016 doi: 10.1007/s11101-016-9473-1. [DOI] [Google Scholar]
- 17.Crittenden P.D., David J.C., Hawksworth D.L., Campbell F.S. Attempted isolation and success in the culturing of a broad spectrum of lichen-forming and lichenicolous fungi. New Phytol. 1995;130:267–297. doi: 10.1111/j.1469-8137.1995.tb03048.x. [DOI] [Google Scholar]
- 18.Prillinger H., Kraepelin G., Lopandic K., Schweigkofler W., Molnar O., Weigang F., Dreyfuss M.M. New species of Fellomyces isolated from epiphytic lichen species. Syst. Appl. Microbiol. 1997;20:572–584. doi: 10.1016/S0723-2020(97)80029-X. [DOI] [Google Scholar]
- 19.Diederich P., Lawrey J.D., Sikaroodi M., van den Boom P.P., Ertz D. Briancoppinsia, a new coelomycetous genus of Arthoniaceae (Arthoniales) for the lichenicolous Phoma cytospora, with a key to this and similar taxa. Fungal Divers. 2012;52:1–12. doi: 10.1007/s13225-011-0105-1. [DOI] [Google Scholar]
- 20.Lawrey J.D., Diederich P., Nelsen M.P., Freebury C., Van den Broeck D., Sikaroodi M., Ertz D. Phylogenetic placement of lichenicolous Phoma species in the Phaeosphaeriaceae (Pleosporales, Dothideomycetes) Fungal Divers. 2012;55:195–213. doi: 10.1007/s13225-012-0166-9. [DOI] [Google Scholar]
- 21.Ertz D., Lawrey J.D., Common R.S., Diederich P. Molecular data resolve a new order of Arthoniomycetes sister to the primarily lichenized Arthoniales and composed of black yeasts, lichenicolous and rock-inhabiting species. Fungal Divers. 2014;66:113–137. doi: 10.1007/s13225-013-0250-9. [DOI] [Google Scholar]
- 22.Muggia L., Kopun T., Ertz D. Phylogenetic placement of the lichenicolous, anamorphic genus Lichenodiplis and its connection to Muellerella-like teleomorphs. Fungal Biol. 2015;119:1115–1128. doi: 10.1016/j.funbio.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Ahmadjian V. A Guide to the algae occurring as lichen symbionts: Isolation, culture, cultural physiology, and identification. Phycologia. 1967;6:127–160. doi: 10.2216/i0031-8884-6-2-127.1. [DOI] [Google Scholar]
- 24.Yamamoto Y., Mizuguchi R., Yamada Y. Tissue cultures of Usnea rubescens and Ramalina yasudae and production of usnic acid in their cultures. Agric. Biol. Chem. 1985;49:3347–3348. doi: 10.1271/bbb1961.49.3347. [DOI] [Google Scholar]
- 25.Yamamoto Y., Hamade R., Kinoshita Y., Higuchi M., Yoshimura I., Sekiya J., Yamada Y. Biological approaches using lichen-derived cultures: Growth and primary metabolism. Symbiosis. 1994;16:203–217. [Google Scholar]
- 26.Stocker-Wörgötter E. Experimental cultivation of lichens and lichen symbionts. Can. J. Bot. 1995;73:579–589. doi: 10.1139/b95-298. [DOI] [Google Scholar]
- 27.Stocker-Wörgötter E. Investigating the production of secondary compounds in cultured lichen mycobiont. In: Kranner I., Beckett R.P., Varma A.K., editors. Protocols in Lichenology. Culturing, Biochemistry, Ecophysiology and Use in Biomonitoring. Springer; Berlin/Heidelberg, Germany: 2002. pp. 296–306. [Google Scholar]
- 28.Stocker-Wörgötter E., Hager A. Culture methods for lichens and lichen symbionts. In: Nash T.H. III, editor. Lichen Biology. 2nd ed. Cambridge University Press; Cambridge, UK: 2008. pp. 353–363. [Google Scholar]
- 29.McDonald T., Gaya E., Lutzoni F. Twenty-five cultures of lichenizing fungi available for experimental studies on symbiotic systems. Symbiosis. 2013;59:165–171. doi: 10.1007/s13199-013-0228-0. [DOI] [Google Scholar]
- 30.Behera B.C., Verma N., Sonone A., Makhija U. Antioxidant and antibacterial activities of lichen Usnea ghattensis in vitro. Biotechnol. Lett. 2005;27:991–995. doi: 10.1007/s10529-005-7847-3. [DOI] [PubMed] [Google Scholar]
- 31.He H., Bigelis R., Yang H.Y., Chang L.-P., Singh M.P. Lichenicolins A and B, new bisnaphthopyrones from an unidentified lichenicolous fungus Strain LL-RB0668. J. Antibiot. 2005;58:731–736. doi: 10.1038/ja.2005.99. [DOI] [PubMed] [Google Scholar]
- 32.Brunauer G., Hager A., Grube M., Türk R., Stocker-Wörgötter E. Alterations in secondary metabolism of aposymbiotically grown mycobionts of Xanthoria elegans and cultured resynthesis stages. Plant Physiol. Biochem. 2007;45:146–151. doi: 10.1016/j.plaphy.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Stocker-Wörgötter E., Elix J.A., Grube M. Secondary chemistry of lichen-forming fungi: Chemosyndromic variation and DNA-analyses of cultures and chemotypes in the Ramalina farinacea complex. Bryologist. 2004;107:152–162. doi: 10.1639/0007-2745(2004)107[0152:SCOLFC]2.0.CO;2. [DOI] [Google Scholar]
- 34.Stocker-Wörgötter E., Elix J.A., Schumm F., Hametner C. Bushfire and lichen communities: Ecophysiology, culturing and secondary chemistry of two Australasian lichen species, Thysanothecium scutellum and T. hookeri (Cladoniaceae, lichenized Ascomycetes) Bibl. Lichenol. 2012;108:241–256. [Google Scholar]
- 35.Vinayaka K.S., Krishnamurthy Y.L., Banakar S., Kekuda T.R.P. Association and variation of endophytic fungi among some macrolichens in central Western Ghats, Southern India. Int. J. Curr. Microbiol. Appl. Sci. 2016;5:115–124. doi: 10.20546/ijcmas.2016.506.014. [DOI] [Google Scholar]
- 36.Guo L.D., Huang G.R., Wang Y., He W.H., Zheng W.H., Hyde K.D. Molecular identification of white morphotype strains of endophytic fungi from Pinus tabulaeformis. Mycol. Res. 2003;107:680–688. doi: 10.1017/S0953756203007834. [DOI] [PubMed] [Google Scholar]
- 37.Li W.C., Zhou J., Guo S.Y., Guo L.D. Endophytic fungi associated with lichens in Baihua mountain of Beijing, China. Fungal Divers. 2007;25:69–80. [Google Scholar]
- 38.Padhi S., Tayung K. In vitro antimicrobial potentials of endolichenic fungi isolated from thalli of Parmelia lichen against some human pathogens. Beni-Suef Univ. J. Basic Appl. Sci. 2015;4:299–306. doi: 10.1016/j.bjbas.2015.11.006. [DOI] [Google Scholar]
- 39.Paranagama P.A., Wijeratne E.M., Burns A.M., Marron M.T., Gunatilaka M.K., Arnold A.E., Gunatilaka A.A. Heptaketides from Corynespora sp. inhabiting the cavern beard lichen, Usnea cavernosa: First report of metabolites of an endolichenic fungus. J. Nat. Prod. 2007;70:1700–1705. doi: 10.1021/np070466w. [DOI] [PubMed] [Google Scholar]
- 40.Jiao Y., Li G., Wang H.Y., Liu J., Li X.B., Zhang L.L., Zhao Z.T., Lou H.X. New metabolites from endolichenic fungus Pleosporales sp. Chem. Biodivers. 2015;12:1095–1104. doi: 10.1002/cbdv.201400279. [DOI] [PubMed] [Google Scholar]
- 41.Wijeratne E.M.K., Bashyal B.P., Gunatilaka M.K., Arnold A.E., Gunatilaka A.A.L. Maximizing chemical diversity of fungal metabolites: Biogenetically related heptaketides of the endolichenic fungus Corynespora sp. J. Nat. Prod. 2010;73:1156–1159. doi: 10.1021/np900684v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dou Y., Wang X., Jiang D., Wang H., Jiao Y., Lou H., Wang X. Metabolites from Aspergillus versicolor, an endolichenic fungus from the lichen Lobaria retigera. Drug Discov. Ther. 2014;8:84–88. doi: 10.5582/ddt.8.84. [DOI] [PubMed] [Google Scholar]
- 43.Yang B.J., Chen G.D., Li Y.J., Hu D., Guo L.D., Xiong P., Gao H. A new xanthone glycoside from the endolichenin fungus Sporormiella irregularis. Molecules. 2016;21:764. doi: 10.3390/molecules21060764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harutyunyan S., Muggia L., Grube M. Black fungi in lichens from seasonally arid habitats. Stud. Mycol. 2008;61:83–90. doi: 10.3114/sim.2008.61.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki M.T., Parrot D., Berg G., Grube M., Tomasi S. Lichens as natural sources of biotechnologically relevant bacteria. Appl. Microbiol. Biotechnol. 2016;100:583–595. doi: 10.1007/s00253-015-7114-z. [DOI] [PubMed] [Google Scholar]
- 46.Fleischhacker A., Grube M., Kopun T., Hafellner J., Muggia L. Community analyses uncover high diversity of lichenicolous fungi in Alpine habitats. Microb. Ecol. 2015;70:348–360. doi: 10.1007/s00248-015-0579-6. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto Y., Kinoshita Y., Yoshimura I. Protocols in Lichenology. Springer; Berlin/Heidelberg, Germany: 2002. Chapter 2. Culture of thallus fragments and redifferentiation of lichens; pp. 34–46. [Google Scholar]
- 48.Lilly H.L., Barnett V.G. Physiology of the Fungi. 1st ed. McGraw Hill Book Co.; New York, NY, USA: 1951. p. 464. [Google Scholar]
- 49.Ahmadjian V. Laboratory culture of lichens and lichen symbionts; Proceedings of the Symposium on Tissue Culture of Lichen and Bryophyte; Kyoto, Japan. 23 April 1987; Neyagawa, Japan: Nippon Paint Co., Ltd.; 1987. pp. 1–13. [Google Scholar]
- 50.Pagano J., Levin J.D., Trejo W. Diagnostic medium for the differentiation of species of Candida. Antibiot. Annu. 1958;5:137–143. [PubMed] [Google Scholar]
- 51.Hocking A.D., Pitt J.I. Dichloran-glycerol medium for enumeration of xerophilic fungi from low moisture foods. Appl. Environ. Microbiol. 1980;39:488–492. doi: 10.1128/aem.39.3.488-492.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bills G.F., Foster M.S., II . Biodiversity of Fungi Inventory and Monitoring Methods. Academic Press; Burlington, MA, USA: 2004. Formulae for selected materials used to isolate and study fungi and fungal allies; pp. 595–618. [Google Scholar]
- 53.Hadley A. CombineZM 1.0.0. [(accessed on 14 May 2015)]; Available online: http://combinezm.en.lo4d.com/
- 54.Cubero O.F., Crespo A., Fatehi J., Bridge P.D. 1999. DNA extraction and PCR amplification method suitable for fresh, herbarium stored and lichenized fungi. Plant Syst. Evol. 1999;217:243–249. doi: 10.1007/BF01084401. [DOI] [Google Scholar]
- 55.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 56.Zhang N., Castlebury L.A., Miller A.N., Huhndorf S.M., Schoch C.L., Seifert K.A., Rossman A.Y., Rogers J.D., Kohlmeyer J., Volkmann-Kohlmeyer B., et al. An overview of the systematic of Sordariomycetes based on four-gene phylogeny. Mycologia. 2006;98:1076–1087. doi: 10.1080/15572536.2006.11832635. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z., Johnston P.R., Takamatsu S., Spatafora J.W., Hibbett D.S. Toward a phylogenetic classification of Leotiomycetes based on rDNA data. Mycologia. 2006;98:1065–1075. doi: 10.1080/15572536.2006.11832634. [DOI] [PubMed] [Google Scholar]
- 58.Gueidan C., Ruibal C., de Hoog G.S., Gorbushina A., Untereiner W.A., Lutzoni F. A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineage. Stud. Mycol. 2008;61:111–119. doi: 10.3114/sim.2008.61.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gueidan C., Ruibal C., de Hoog G.S., Schneider H. Rock-inhabiting fungi originated during periods of dry climate in the late Devonian and middle Triassic. Fungal Biol. 2011;115:987–996. doi: 10.1016/j.funbio.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Ruibal T., Gueidan C., Selbmann L., Gorbushina A.A., Crous P.W., Groenewald J.Z., Muggia L., Grube M., Isola D., Schoch C.L., et al. Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Stud. Mycol. 2009;64:123–133. doi: 10.3114/sim.2009.64.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schoch C.L., Crous P.W., Groenewald J.Z., Boehm E.W.A., Burgess T.I., de Gruyter J., de Hoog G.S., Dixon L.J., Grube M., Gueidan C., et al. A class-wide phylogenetic assessment of Dothideomycetes. Stud. Mycol. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huhndorf S.M., Miller A.N. A molecular re-appreisal of taxa in the Sordariomycetidae and a new species of Rimaconus from New Zealand. Stud. Mycol. 2011;68:203–210. doi: 10.3114/sim.2011.68.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Untereiner W.A., Gueidan C., Orr M.J., Diederic P. The phylogenetic position of the lichenicolus ascomycete Capronia peltigerae. Fungal Divers. 2011;49:225–233. doi: 10.1007/s13225-011-0097-x. [DOI] [Google Scholar]
- 64.Muggia L., Gueidan C., Knudsen K., Perlmutter G., Grube M. The lichen connections of black fungi. Mycopathologia. 2013;175:523–535. doi: 10.1007/s11046-012-9598-8. [DOI] [PubMed] [Google Scholar]
- 65.Hyde K.D., Gareth Jones E.B., Liu J.K., Ariyawansa H., Boehm E., Boonmee S., Braun U., Chomnunti P., Crous P.W., Dai D.Q., et al. Families of Dothideomycetes. Fungal Divers. 2013;63:1–313. doi: 10.1007/s13225-013-0263-4. [DOI] [Google Scholar]
- 66.Maharachchikumbura S.S.N., Hyde K.D., Gareth Jones E.B., Xu J.C. Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers. 2015;72:199–301. doi: 10.1007/s13225-015-0331-z. [DOI] [Google Scholar]
- 67.Suija A., Ertz D., Lawrey J.D., Diedetrich P. Multiple origin of the lichenicolous life habit in Helotiales, based on nuclear ribosomal sequences. Fungal Divers. 2015;70:55–72. doi: 10.1007/s13225-014-0287-4. [DOI] [Google Scholar]
- 68.Hall T.A. BioEdit: A user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- 69.Dettman J.R., Jacobs D.J., Taylor J.W. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution. 2003;57:2703–2720. doi: 10.1111/j.0014-3820.2003.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 70.Kauff F., Lutzoni F. Phylogeny of the Gyalectales and Ostropales (Ascomycota, Fungi): Among and within order relationships based on nuclear ribosomal RNA small and large subunits. Mol. Phylogenetics Evol. 2002;25:138–156. doi: 10.1016/S1055-7903(02)00214-2. [DOI] [PubMed] [Google Scholar]
- 71.Miadlikowska J., Kauff F., Hofstetter V., Fraker E., Grube M., Hafellner J., Reeb V., Hodkinson B.P., Kukwa M., Lücking R., et al. New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA- and two protein-coding genes. Mycologia. 2006;98:1088–1103. doi: 10.1080/15572536.2006.11832636. [DOI] [PubMed] [Google Scholar]
- 72.Vaidya G., Lohman D.J., Meier R. SequenceMatrix: Concatenation software for the fast assembly of multigene datasets with character set and codon information. Cladistics. 2010;27:171–180. doi: 10.1111/j.1096-0031.2010.00329.x. [DOI] [PubMed] [Google Scholar]
- 73.Stamatakis A., Ludwig T., Meier H. RAxML-iii: A fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics. 2005;21:456–463. doi: 10.1093/bioinformatics/bti191. [DOI] [PubMed] [Google Scholar]
- 74.Page R.D.M. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]