Abstract
Carbapenem-resistant Acinetobacter baumannii (CRAB) infections are an increasing concern in intensive care units (ICUs) worldwide. The combination of carbapenemases and 16S rRNA-methyltransferases (16S-RMTases) further reduces the therapeutic options. OXA-carbapenemase/A. baumannii clone tandems in Latin America have already been described; however, no information exists in this region regarding the occurrence of 16S-RMTases in this microorganism. In addition, the epidemiology of A. baumannii in ICUs and its associated resistance profiles are poorly understood. Our objectives were as follows: to study the clonal relationship and antibiotic resistance profiles of clinical and digestive colonizing A. baumannii isolates in an ICU, to characterize the circulating carbapenemases, and to detect 16S-RMTases. Patients admitted between August 2010 and July 2011 with a clinically predicted hospital stay > 48 hr were included. Pharyngeal and rectal swabs were obtained during the first fortnight after hospitalization. Resistance profiles were determined with MicroScan® and VITEK2 system. Carbapenemases and 16S-RMTases were identified by PCR and sequencing, and clonality was assessed by pulsed-field gel electrophoresis and multilocus sequence typing. Sixty-nine patients were studied and 63 were diagnosed with bacterial infections. Among these, 29 were CRAB isolates; 49 A. baumannii were isolated as digestive colonizers. These 78 isolates were clustered in 7 pulsetypes, mostly belonging to ST79. The only carbapenemase genes detected were blaOXA-51 (n = 78), blaOXA-23 (n = 62), and blaOXA-58 (n = 3). Interestingly, two clinical isolates harbored the rmtC 16S-RMTase gene. To the best of our knowledge, this is the first description of the presence of rmtC in A. baumannii.
Keywords: : Acinetobacter baumannii; carbapenem resistance; blaOXA,rmtC
Introduction
Carbapenem-resistant Acinetobacter baumannii (CRAB) infections in critically ill patients are an increasing source of concern in intensive care units (ICUs) worldwide. In 2013, the Centers for Disease Control and Prevention (CDC) categorized multidrug-resistant A. baumannii as a serious threat, responsible in the United States only, for nearly 7,000 infections and 500 deaths per year.1 In addition, in 2017 the World Health Organization (WHO) submitted a list of priority bacteria that require urgent development of new antimicrobials; topping that list was CRAB.2 In this regard, aminoglycosides are considered a high-priority group in the critically important antimicrobials list issued by WHO3; due to their synergistic effect, they are often used in combination with β-lactams to treat MDR gram-negative bacterial infections in healthcare settings, thus constituting one of the last therapeutic options.
Concerning 16S rRNA methyltransferases (16S-RMTases), only a few have been detected in A. baumannii (ArmA, RmtA-RmtH, and NpmA). ArmA is highly prevalent among CRAB strains worldwide, and is mostly associated with the carbapenemase gene blaOXA-23.4 Conversely, RmtB has also been detected in A. baumannii, but only sporadically.5 On the other hand, due to the intrinsic broad antibiotic resistance of A. baumannii, carbapenems often constitute the first line of treatment for infections caused by this microorganism.6,7 Therefore, 16S-RMTases, which confer resistance to all clinically relevant aminoglycosides, together with carbapenemases, constitute a grave concern for public health worldwide.8
Although carbapenemases belonging to each Ambler class have been described in A. baumannii, the most frequently detected are those corresponding to class D β-lactamases, such as OXA-23 like, OXA-40 like, OXA-58 like, and OXA-143 like.9,10 OXA carbapenemases tend to show low levels of identity between different groups; carbapenem resistance conferred by such enzymes is more related to their expression levels than to their hydrolytic profiles, or enzyme-related kinetic properties.11 Accordingly, the insertion of different mobile genetic elements, such as ISAba1 or ISAba2, upstream of blaOXA genes provides strong promoters that enhance the expression levels of the latter.12,13
Traditionally, sequence types belonging to clonal complexes CC1, CC2, and CC3 have been acknowledged as the main lineages harboring oxa carbapenemases, and have been implicated in nosocomial outbreaks in Europe, Asia, and North America.14 Nevertheless, the most frequently detected OXA carbapenemase/ST clone tandem in Latin America is OXA-23 in A. baumannii ST79, ST15, or ST25 (none of which belong to any of the aforementioned clonal complexes).15–17
We have previously reported that our ICU ventilator-associated pneumonia (VAP) caused by A. baumannii was preceded by digestive and/or respiratory colonization (DRC) events, and that some of the detected clones were endemic to the ICU.18
The aims of this work were to study the clonal relationship of clinical and DRC A. baumannii isolates in the ICU, to analyze the resistance profiles, and to determine the circulating carbapenemases and 16S-RMTases, as well as the clones harboring such enzymes.
Materials and Methods
Definitions
Patients were considered community derived if admission to the ICU occurred within 24 hr of being hospitalized, whereas patients were regarded as hospital derived whenever admission to the ICU occurred >24 hr after being hospitalized.
DRC was considered positive if cultures of pharyngeal or rectal swabs yielded A. baumannii colonies.
Colonization upon admission or ICU-acquired infections were defined according to Medina-Presentado et al.18 Briefly, colonization upon admission was considered if A. baumannii was isolated from admission samples (pharyngeal and/or rectal); conversely, colonization events were considered ICU acquired if admission samples yielded negative results, but A. baumannii was later recovered from subsequent samples.
Sample processing
We studied clinical and digestive colonizing A. baumannii isolates obtained from adult ICU inpatients in Uruguay's University Hospital (Hospital de Clínicas Dr. Manuel Quintela). All patients admitted between August 2010 and July 2011 with a clinically predicted hospital stay >48 hr were included in a successive manner. Clinical samples were submitted to the Clinical Laboratory Department of Hospital de Clínicas and processed routinely (see below). On the other hand, surveillance samples (i.e., DRC samples) were submitted to the Bacteriology and Virology Department for further processing. To avoid redundant patient data, only strains featuring different antibiotic resistance profiles per patient were included in this study.
Bacterial identification and antibiotic susceptibility testing of microorganisms obtained from clinical samples were performed with the VITEK2 system (BioMérieux, Marcy-l'Étoile, France); the microdilution method was performed with the MicroScan® system (Beckman Coulter, Pasadena) following the manufacturer's instructions. We tested susceptibility to the following antibiotics: gentamicin (N), amikacin (A), tobramycin (T), ciprofloxacin (P), ceftazidime (Z), cefepime (F), imipenem (I), meropenem (M), trimethoprim-sulfamethoxazole (X), and colistin (C). In addition, the occurrence of class A and B carbapenemases was sought phenotypically by double-disc synergy tests.19
Clinical isolates showing resistance to both gentamicin and amikacin were screened for high-level aminoglycoside-resistance, in accordance to Hidalgo et al., by their ability to grow on brain heart infusion agar containing both 200 mg/L amikacin and 200 mg/L gentamicin.20
Susceptibility results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST; www.eucast.org). All nonduplicate clinical isolates for each patient were analyzed.
DRC samples were streaked on MacConkey agar plates (Oxoid Ltd., Basingstoke, UK) supplemented with 1 mg/L cefotaxime (Libra, Montevideo, Uruguay) and 0.125 mg/L ciprofloxacin (ION, Montevideo, Uruguay). Up to five putative Acinetobacter spp. colonies per plate were identified by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Bruker, MA). Antibiotic susceptibility testing of DRC isolates was performed with the MicroScan system.
Genes coding for Class A, B, and D carbapenemases were identified by PCR (Table 1).21–23 Clinical isolates showing high resistance levels to aminoglycosides were screened by PCR for 16S-RMTase genes (armA, rmtA-rmtH, and npmA) (Table 1).24–27
Table 1.
Primers Used to Detect Resistance Genes
| Primer | Sequence (5′-3′) | Product size (bp) | mT | Reference |
|---|---|---|---|---|
| blaIMP | 5′-AGTCAGGTTTGGCAGATCCGT-3′ | 684 | 52 | 18 |
| 5′-GGTTTAACAAAACAACCACC-3′ | ||||
| blaSPM | 5′-ATGAACTCACCTAAATCGAGAGCC-3′ | 633 | 60 | 18 |
| 5′-AAACAGCAGTTTCTTCTTGGCC-3′ | ||||
| blaSIM | 5′-TATTCGGCACTTTAAATACCGCG-3′ | 635 | 62 | 18 |
| 5′-GCCACAGTGAAATCGGAGACG-3′ | ||||
| blaGIM | 5′-CTTGTAGCGTTGCCAGCTTT-3′ | 722 | 56 | 18 |
| 5′-TTAATCAGCCGACGCTTCAG-3′ | ||||
| blaVIM | 5′-TAGGAATTCACCATGTTCAAACTTTTGAGTAAGT-3′ | 800 | 55 | 18 |
| 5′-ATAAAGCTTAGCTACTCAACGACTGAGCGA-3′ | ||||
| blaNDM | 5′-GGTTTGGCGATCTGGTTTTC-3′ | 621 | 56 | 18 |
| 5′-CGGAATGGCTCATCACGATC-3′ | ||||
| blaKPC-2 | 5′-AACAAGGAATATCGTTGATG-3′ | 915 | 50 | 18 |
| 5′-AGATGATTTTCAGAGCCTTA-3′ | ||||
| blaOXA-48 | 5′-TTGGTGGCATCGATTATCGG-3′ | 743 | 50 | 18 |
| 5′-GAGCACTTCTTTTGTGATGGC-3′ | ||||
| blaOXA-51 like | 5′-TAATGCTTTGATCGGCCTTG-3′ | 353 | 52 | 17 |
| 5′-TGGATTGCACTTCATCTTGG-3′ | ||||
| blaOXA-23 like | 5′-GATCGGATTGGAGAACCAGA-3′ | 501 | 52 | 17 |
| 5′-ATTTCTGACCGCATTTCCAT-3′ | ||||
| blaOXA-24 like | 5′-GGTTAGTTGGCCCCCTTAAA-3′ | 246 | 52 | 17 |
| 5′-AGTTGAGCGAAAAGGGGATT-3′ | ||||
| blaOXA-58 like | 5′-AAGTATTGGGGCTTGTGCTG-3′ | 599 | 52 | 17 |
| 5′-CCCCTCTGCGCTCTACATAC-3′ | ||||
| blaOXA-143 like | 5′-TGGCACTTTCAGCAGTTCCT-3′ | 149 | 52 | 19 |
| 5′-TAATCTTGAGGGGGCCAACC-3′ | ||||
| armA | 5′-ATTCTGCCTATCCTAATTGG-3′ | 315 | 55 | 20 |
| 5′-ACCTATACTTTATCGTCGTC-3′ | ||||
| rmtA | 5′-CTAGCGTCCATCCTTTCCTC-3′ | 635 | 55 | 20 |
| 5′-TTGCTTCCATGCCCTTGCC-3′ | ||||
| rmtB | 5′-GCTTTCTGCGGGCGATGTAA-3′ | 173 | 55 | 20 |
| 5′-ATGCAATGCCGCGCTCGTAT-3′ | ||||
| rmtC | 5′-CGAAGAAGTAACAGCCAAA-3′ | 711 | 55 | 20 |
| 5′-ATCCCAACATCTCTCCCACT-3′ | ||||
| rmtD | 5′-CGGCACGCGATTGGGAAGC-3′ | 401 | 55 | 20 |
| 5′-CGGAAACGATGCGACGAT-3′ | ||||
| rmtE | 5′-ATGAATATTGATGAAATGGTT-3′ | 818 | 55 | 21 |
| 5′-TGATTGATTTCCTCCGTTTTT-3′ | ||||
| rmtF | 5′-ACGCATCTGCACCAGATCACC-3′ | 414 | 61 | This work |
| 5′-GGGCAGGAGCTTCATCAGAA-3′ | ||||
| rmtG | 5′-AAATACCGCGATGTGTGTCC-3′ | 251 | 55 | 22 |
| 5′-ACACGGCATCTGTTTCTTCC-3′ | ||||
| rmtH | 5′-AGGTGGAAAAGCAGGCAAG-3′ | 490 | 55 | This work |
| 5′-CTCAAACCAGGTGGCGTAGT-3′ | ||||
| npmA | 5′-CTCAAAGGAACAAAGACGG-3′ | 641 | 58 | 23 |
| 5′-GAAACATGGCCAGAAACTC-3′ |
mT, melting temperature.
Clonality was assessed by pulsed-field gel electrophoresis (PFGE) following digestion with restriction enzyme ApaI (Thermo Scientific, Waltham, MA). Band patterns were analyzed with BioNumerics v.6.6 software (Applied Maths, Sint-Martens-Latem, Belgium) with 2% tolerance and 0% optimization. Strains were grouped in clusters on the basis of an 80% cutoff in accordance with Rafei et al.28
Multilocus sequence typing (MLST) was performed on at least one clinical isolate per PFGE profile, following the Pasteur scheme guidelines stated by Diancourt et al.29
Numerical variables were analyzed using Fisher's exact test and expressed with their standard deviation; p-values <0.05 were considered statistically significant. Relative risks (RR) and 95% confidence intervals (95% CIs) were calculated using standard methods. Statistics analyses were performed with the SPSS 17.0 software (SPSS, Inc, Chicago, IL).
Results
Sixty-nine patients (40 men and 29 women) were admitted to the ICU between August 1, 2010, and August 31, 2011. The mean age (±standard deviation) was 52.7 ± 18.8 years. The median APACHE II score upon admission was 23.1 ± 6, the length of stay in the ICU was 14.7 ± 12.1 days, and crude mortality was 33.3%.
Diagnoses upon admission to the ICU were as follows: VAP and respiratory sepsis (20.3%), sepsis responding to other causes (24.6%), severe trauma or central nervous system (CNS) trauma (24.6%), CNS infections and acute bacterial meningitis (8.7%), stroke (8.7%), and other causes (13%) (Table 2).
Table 2.
Demographic, Clinical, and Microbiological Data of the Studied Patients
| Gender (male) | 40 (58%) |
| Age (SD) | 52.7 ± 18.8 (range 17–85) |
| ICU length of stay (SD) | 14.7 ± 12.1 |
| APACHE II score (SD) | 23.1 ± 6.0 |
| Mortality | 23 (33.3%) |
| Diagnosis upon admission in the ICU (%) | |
| VAP and respiratory sepsis | 14 (20.3) |
| Severe trauma | 12 (17.4) |
| CNS infections and acute bacterial meningitis | 6 (8.7) |
| Peritoneal sepsis | 6 (8.7) |
| Stroke | 6 (8.7) |
| Soft tissue-related sepsis | 5 (7.2) |
| CNS trauma | 5 (7.2) |
| Other causes for sepsis | 6 (8.7) |
| Cardiac/pulmonary insufficiency | 2 (2.9) |
| Reanimation cardiorespiratory arrest | 2 (2.9) |
| Other | 5 (7.2) |
| ICU-acquired infections (%) | 65 |
| VAP | 23 (35.4) |
| Purulent tracheobronchitis | 11 (16.9) |
| Bacteremia | 14 (21.5) |
| Urinary tract infections | 3 (4.6) |
| Catheter-related infections | 3 (4.6) |
| Neurosurgical infections | 4 (6.2) |
| Tertiary peritonitis | 2 (3.1) |
| Skin and soft tissues infections | 2 (3.1) |
| Other foci | 3 (4.6) |
| Microorganisms detected (%) | 82 |
| Gram-negative rods | 61 (74.4) |
| Acinetobacter baumannii | 29 (35.4) |
| Pseudomonas aeruginosa | 10 (12.2) |
| Klebsiella pneumoniae | 9 (11.0) |
| Escherichia coli | 4 (4.9) |
| Other enterobacteria | 7 (8.5) |
| H. influenzae | 2 (2.4) |
| Gram-positive cocci | 21 (25.6) |
| Methicillin-susceptible Staphylococcus aureus | 9 (11.0) |
| Staphylococcus pneumoniae | 6 (7.3) |
| Methicillin-resistant S. aureus | 3 (3.7) |
| Enterococcus spp. | 1 (1.2) |
| Coagulase-negative Staphylococcus | 2 (2.4) |
APACHE II, Acute Physiology and Chronic Health Evaluation II; CNS, central nervous system; ICU, intensive care unit; SD, standard deviation; VAP, ventilator-associated pneumonia.
Sixty-five ICU-acquired infections were diagnosed: 35.4% corresponded to VAP, 21.5% to bacteremia, 16.9% to purulent tracheobronchitis, 6.2% to neurosurgical infections, 4.6% to urinary tract infections, 4.6% to catheter-related infections, and 10.1% to other causes (Table 2).
Eighty-two bacterial agents were recovered from the aforementioned infections; 74.4% were gram-negative rods and the remaining 25.6% corresponded to gram-positive bacteria. Among the former, the most frequently detected were as follows: A. baumannii 35.4%, Pseudomonas aeruginosa 12.2%, and Klebsiella pneumoniae 10.6%; among gram-positive bacteria, 42.8% were methicillin-susceptible Staphylococcus aureus (MSSA), 28.6% were Staphylococcus pneumoniae, 14.3% were methicillin-resistant S. aureus (MRSA), and 14.3% corresponded to other species (Table 2).
A. baumannii colonization and infection
DRC by A. baumannii was detected in 49 patients (71%), 13 of which (26.5%) were already colonized upon admission to the ICU. Regarding the latter, only 1 case of DRC was community acquired, whereas the remaining 12 cases corresponded to acquisitions in different hospital wards.
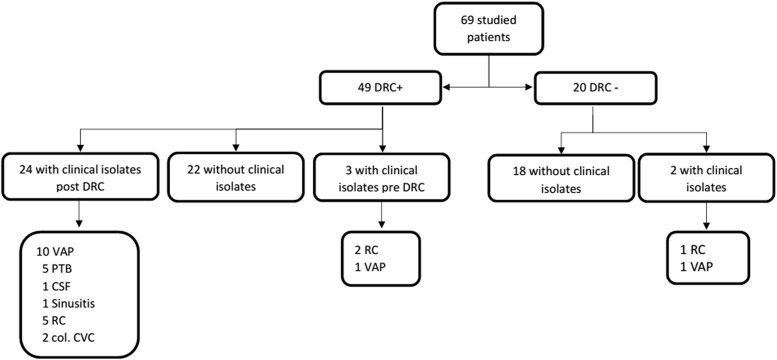
A. baumannii was recovered from clinical samples in 29 patients, 27 of which were also colonized (p < 0.001, RR: 11.04, 95% CI: 2.31–52.9); 25 of the former corresponded to respiratory samples (14 respiratory secretions, 10 tracheal aspirates, 1 and bronchoalveolar lavage), 2 to central venous catheter samples, 1 to a facial sinus drainage, and 1 to cerebrospinal fluid (Fig. 1). All 29 clinical isolates showed resistance to meropenem (28 of these were also imipenem resistant), cefepime, and ciprofloxacin, whereas 6 isolates displayed resistance to amikacin, gentamicin, and tobramycin, and 3 isolates showed resistance to all the studied antibiotics (except colistin) (Fig. 2). Conversely, all 29 isolates were susceptible to colistin, 69% (n = 20) were susceptible to tobramycin, and 58.6% (n = 17) to gentamicin.
FIG. 1.
Distribution of clinical isolates in relationship to the patients' status (i.e., presence or absence of DRC); col. CVC, colonized central venous catheter; CSF, cerebrospinal fluid; DRC, digestive and/or respiratory colonization; PTB, purulent tracheobronchitis; RC, respiratory-colonization (RC refers to clinical isolates from respiratory samples [i.e., respiratory secretions or tracheal aspirates], which nevertheless were interpreted as colonization events by the medical staff); VAP, ventilator-associated pneumonia.
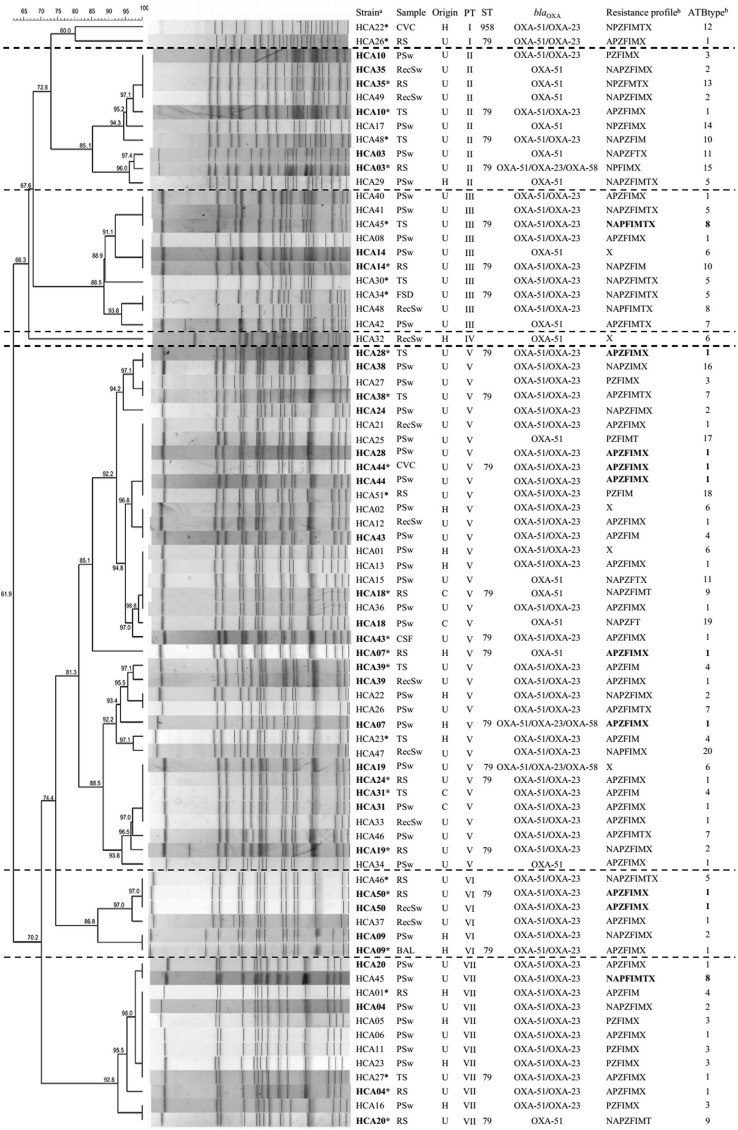
FIG. 2.
PFGE profile of the 78 Acinetobacter baumannii isolates obtained from DRC and clinical samples during the study period. Different pulsetypes are separated by horizontal dotted lines. *Clinical sample; aIsolate numbers in boldface represent those in which both the clinical sample and the DRC sample displayed identical pulsetypes. bResistance profiles and antibiotpyes in boldface indicate matching resistance profiles between the clinical isolate and the DRC sample. BAL, bronchoalveolar lavage; C, community; CSF, cerebrospinal fluid; CVC, central venous catheter; FSD, facial sinus drainage; H, hospital; PFGE, pulsed-field gel electrophoresis; PSw, pharyngeal swab; PTA, pulse type; RecSw, rectal swab; RS, respiratory secretion; ST, sequence type; TS, tracheal secretion; U, intensive care unit. A, amikacin; F, cefepime; I, imipenem; M, meropenem; N, gentamicin; P, ciprofloxacin; T, tobramycin; X; trimethoprim-sulfamethoxazole; Z, ceftazidime.
The 78 A. baumanii isolates (29 clinical, 49 colonizing) yielded 20 different resistance profiles, 12 of which included the 29 clinical isolates. Figure 2 depicts the different antibiotypes (ATBtype) detected. The largest profile, ATBtype 1, featured resistance to amikacin, ciprofloxacin, ceftazidime, cefepime, imipenem, meropenem, and trimethoprim-sulfamethoxazole; this profile included 37.9% and 34.7% of the clinical and colonizing isolates, respectively. Although ATBtype 1 was found in 6/7 pulsetypes, PTA- V and VI comprised 71.4% of isolates displaying ATBtype 1.
Colistin was the only antibiotic active against the 78 studied isolates, constituting the only therapeutic option available, among the tested antibiotics, for 5 isolates (the 3 clinical isolates already mentioned and 2 colonizing isolates). These isolates belonged to ATBtype 5 (Fig. 2).
Interestingly, matching resistance profiles between colonizing and clinical isolates were detected in only five patients, four belonged to ATBtype 1 and the remaining to ATBtype 8 (Fig. 2).
Clonal relationship between colonizing/infecting isolates
We compared by PFGE 78 A. baumannii isolates, 49 from DRC and 29 from clinical samples. The 78 isolates were clustered in 7 pulsetypes designated PTA-I to PTA-VII. PTA-II, III, V, and VII comprised 91.8% of the colonizing isolates and 82.8% of the clinical isolates (Fig. 2). PTA-V was the largest, comprising 47.4% of isolates recovered from 27 patients (41.4% of clinical isolates and 51.0% of colonizing isolates); PTA-VII was the second largest, including 12 isolates (4 clinical and 8 colonizing) (Fig. 2).
Sixty-three percent of patients (n = 18) with A. baumannii clinical isolates were previously colonized by isolates featuring the same pulsetype (p < 0.001, RR: 4.33, 95% CI: 2.64–7.12). In 15 of these 18 patients, isolates belonged to PTA-V, II, and VI (Fig. 2).
MLST analysis was performed on 22 clinical isolates belonging to the different pulsetypes. In this sense, 21 corresponded to the ST79, whereas the remaining isolate belonged to a new sequencetype, namely ST958. The latter constitutes a single-locus variant of ST79, since it features allele 6 of gene cpn60 instead of allele 26.
Detection of resistance genes
Carbapenemases were sought in all the studied isolates (n = 78). All of them harbored the A. baumannii cognate blaOXA-51. In addition, 62 isolates carried blaOXA-23; furthermore, 3 of such isolates also carried blaOXA-58. Neither blaOXA-143 nor KPC-like or NDM-like class A or B carbapenemases were detected in this study.
Regarding 16S-RMTase genes, rmtC was identified in two of the six clinical isolates displaying high-level aminoglycoside resistance (namely, isolates HCA30 and HCA45).
Discussion
CRAB was the main bacterium responsible for infections within the ICU under study, accounting for 35.4% of the microorganisms identified at the laboratory. Other species included P. aeruginosa, K. pneumoniae, and MSSA, constituting 33.2% of infectious agents. In 2014, Luna et al. reviewed the available information regarding the distribution of gram-negative bacteria circulating in ICUs in South America and the Caribbean, showing a relatively heterogeneous situation between the isolated pathogens.30 Most of the reviewed publications indicated that K. pneumoniae, P. aeruginosa, and A. baumannii were among the most frequently detected microorganisms, with A. baumannii fluctuating between 3% and 50% of the recovered isolates.
Although the proportion of A. baumannii isolates detected in our study is within such range, the impact of carbapenem resistance among such isolates is alarming; in this sense, 100% isolates were resistant to meropenem, whereas 96.6% isolates showed resistance to imipenem. Recently, Labarca et al. addressed the issue of carbapenem resistance among clinical isolates of P. aeruginosa and A. baumannii in Latin America; their findings showed resistance levels ranging between 7% and 74% for A. baumannii.31
Conversely, the SENTRY program for Latin America (which includes results from Mexico, Brasil, Argentina, and Chile) indicates that among A. baumannii isolates, resistance levels to imipenem and meropenem are 67.8% and 66.1%, respectively.32
The studies mentioned above analyzed isolates obtained from various hospital wards, besides ICUs; taking into consideration only those isolates obtained from patients in the ICU, resistance levels rise to 76.8%33; nevertheless, this resistance level remains below that detected in our center.
MLST analysis of clinical isolates indicated an overwhelming majority of ST79, represented by 21 of the studied isolates. Interestingly, Rodriguez et al. recently reported (in a multicentric study that included isolates from Argentina, Ecuador, Bolivia, Chile, Paraguay, and Uruguay) that the main sequencetype detected in Latin America was ST25, followed distantly by ST79 and ST15.15 A. baumannii ST25 strains were detected in 4/6 analyzed countries, including 10 isolates from Uruguay. However, in our study, none of the studied isolates belonged to ST25, thus highlighting the relevance of regional epidemiological differences, even within the same country.
On the other hand, Adams et al. demonstrated recently that the most epidemiologically relevant A. baumannii clones, including ST79, harbor on average 33 copies of insertion sequences.34 These insertion sequences and transposons confer on A. baumannii a great plasticity, reflected by insertions/deletions as well as changes in the expression of resistance and virulence genes, and metabolic routes.34,35 This constant remodeling could account for the great variability in resistance profiles and even in pulsetypes among DRC and clinical isolates. In this regard, in our study, strains displaying PFGE homology levels as low as 62% were shown to belong to the same sequence type. This genomic plasticity further complicates the analysis of outbreaks caused by such microorganisms.
In our work, blaOXA-23 was the most frequently detected carbapenemase gene (present in 79.5% of isolates). Similar data have been reported previously by other authors in the region.15,36 On the other hand, the three isolates harboring blaOXA-58 also carried blaOXA-51 and blaOXA-23. Finally, in 16 isolates, the only detected carbapenemase gene was blaOXA-51, 11 of which were CRAB and the remaining 5 were susceptible to antibiotics; more studies are required to determine if this gene is under control of a strong promoter, such as the one provided by the insertion of ISAba1.33
Rodriguez et al. have recently suggested the occurrence of a progressive substitution of OXA-58 for OXA-23 in A. baumannii isolates.15 In this regard, different studies have suggested that the expression of certain β-lactamases, including OXA-24, could impose a biological cost on their hosts.37–39 Accordingly, the simultaneous expression of several class D carbapenemases could favor the loss, even in presence of carbapenems, of that enzyme which imposes the biggest biological cost on its host.
Further studies are required to determine whether there are fitness variations in those A. baumannii isolates harboring different OXA carbapenemases.
Regarding 16S-RMTases, rmtC was identified in two A. baumannii clinical isolates. To the best of our knowledge, this is the first identification of rmtC associated to this species. These RmtC-producing A. baumannii isolates belonged to PTA-III and sequencetype ST79; both isolates harbored blaOXA-23 and blaOXA-51 carbapenemase genes. This perilous combination of resistance mechanisms, associated with a successful A. baumannii clone, is alarming for the public health.40 It would be interesting to determine the potential for mobilization of rmtC to other high-risk clones of A. baumannii as well as any fitness cost associated to the expression of rmtC; in this regard, these studies could help predict the stability and spreading potential of this gene in this microorganism.41
We have already reported the epidemiological aspects of A. baumannii isolates, obtained between 2005 and 2006, from respiratory samples in our ICU.18 In this regard, DRC and infections by A. baumannii increased from 35.4% to 71% (p < 0.001, odds ratio [OR]: 4.47, 95% CI: 2.44–8.18) and 12% to 24.6% (p = 0.016, OR: 2.53, 95% CI: 1.24–5.20), respectively. We also witnessed a significant increase, although to a lesser degree, in the number of patients already colonized with A. baumannii upon admission to the ICU, from 19.4% to 24.6% (p = 0.009, OR: 3.15; CI: 1.36–7.31).
This increase in the incidence of A. baumannii along with high levels of resistance to critically important antibiotics calls for continuous clinical and microbiological surveillance, and the implementation of measures aimed at reducing this tendency.
Acknowledgments
This work was partially supported by grants from Comisión Sectorial de Investigación Científica (CSIC, Montevideo, Uruguay) to R.V. and I.B., as well as grants from Agencia Nacional de Investigación e Innovación (ANII) to I.B.
Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic Resistance Threats in the United States, 2013. CDC Atlanta, Atlanta: [Google Scholar]
- 2.World Health Organization. 2017. Global Priority List of Antibiotic-Resistant Bacteria to Guide Reserch, Discovery and Development of New Antibiotics. Available at http://apps.who.int/medicinedocs/documents/s23171en/s23171en.pdf
- 3.World Health Organization. 2017. Critically Important Antimicrobials for Human Medicine–5th rev. WHO, Geneva. Licence: CC BY-NC-SA 3.0 IGO; [Google Scholar]
- 4.Doi Y., Wachino J.-i., and Arakawa Y. 2016. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect. Dis. Clin. North Am. 30:523–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tada T., Miyoshi-Akiyama T., Kato Y., Ohmagari N., Takeshita N., Hung N.V., Phuong D.M., Thu T.A., Binh N.G., Anh N.Q., Nga T.T.T., Truong P.H., Xuan P.T., Thu L.T.A., Son N.T., and Kirikae T. 2013. Emergence of 16S rRNA methylase-producing Acinetobacter baumannii and Pseudomonas aeruginosa isolates in hospitals in Vietnam. BMC Infect. Dis. 13:251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montravers P., Harpan A., and Guivarch E. 2016. Current and future considerations for the treatment of hospital-acquired pneumonia. Adv. Ther. 33:151–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan X., He L., Hu B., Hu J., Huang X., Lai G., Li Y., Liu Y., Ni Y., Qiu H., Shao Z., Shi Y., Wang M., Wang R., Wu D., Xie C., Xu Y., Yang F., Yu K., Yu Y., Zhang J., and Zhuo C. 2016. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin. Microbiol. Infect. 22(Suppl 1):S15–S25 [DOI] [PubMed] [Google Scholar]
- 8.Hidalgo L., Gutierrez B., Ovejero C.M., Carrilero L., Matrat S., Saba C.K.S., Santos-Lopez A., Thomas-Lopez D., Hoefer A., Suarez M., Santurde G., Martin-Espada C., and Gonzalez-Zorn B. 2013. Klebsiella pneumoniae sequence type 11 from companion animals bearing ArmAm, DHA-1 β-lactamase, and QnrB4. Antimicrob. Agents Chemother. 57:4532–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gniadek T.J., Carroll K.C., and Simner P.J. 2016. Carbapenem-resistant non-glucose-fermenting gram-negative bacilli: the missing piece to the puzzle. J. Clin. Microbiol. 54:1700–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neves F.C., Clemente W.T., Lincopan N., Paião I.D., Neves P.R., Romanelli R.M., Lima S.S., Paiva L.F., Mourão P.H., and Nobre-Junior V.A. 2016. Clinical and microbiological characteristics of OXA-23- and OXA-143-producing Acinetobacter baumannii in ICU patients at a teaching hospital, Brazil. Braz. J. Infect. Dis. 20:556–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poirel L., Naas T., and Nordmann P. 2010. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob. Agents Chemother. 54:24–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antunes N.T., and Fisher J.F. 2014. Acquired class D beta-lactamases. Antibiotics (Basel) 3:398–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagano M., Martins A.F., and Barth A.L. 2016. Mobile genetic elements related to carbapenem resistance in Acinetobacter baumannii. Braz. J. Microbiol. 47:785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodford N., Turton J.F., and Livermore D.M. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 35:736–755 [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez C.H., Balderrama Yarhui N., Nastro M., Nunez Quezada T., Castro Canarte G., Magne Ventura R., Ugarte Cuba T., Valenzuela N., Roach F., Mota M.I., Burger N., Velazquez Aguayo G., Ortellado-Canese J., Bruni G., Pandolfo C., Bastyas N., and Famiglietti A. 2016. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in South America. J. Med. Microbiol. 65:1088–1091 [DOI] [PubMed] [Google Scholar]
- 16.Camargo C.H., Tiba M.R., Saes M.R., Vasconcellos F.M., Santos L.F., Romero E.C., and Garcia Dde O. 2016. Population structure analysis of carbapenem-resistant Acinetobacter baumannii clinical isolates from brazil reveals predominance of clonal complexes 1, 15, and 79. Antimicrob. Agents Chemother. 60:2545–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sennati S., Villagran A.L., Bartoloni A., Rossolini G.M., and Pallecchi L. 2016. OXA-23-producing ST25 Acinetobacter baumannii: First report in Bolivia. J. Glob. Antimicrob. Resist. 4:70–71 [DOI] [PubMed] [Google Scholar]
- 18.Medina-Presentado J.C., Seija V., Vignoli R., Pontet J., Robino L., Cordeiro N.F., Bado I., Garcia-Fulgueiras V., Berro M., Bazet C., Savio E., and Rieppi G. 2013. Polyclonal endemicity of Acinetobacter baumannii in ventilated patients in an intensive care unit in Uruguay. Int. J. Infect. Dis. 17:e422–e427 [DOI] [PubMed] [Google Scholar]
- 19.The European Committee on Antimicrobial Susceptibility Testing. Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. Version 2.0, www.eucast.org. 2017
- 20.Hidalgo L., Hopkins K.L., Gutierrez B., Ovejero C.M., Shukla S., Douthwaite S., Prasad K.N., Woodford N., and Gonzalez-Zorn B. 2013. Association of the novel aminoglycoside resistance determinant RmtF with NDM carbapenemase in Enterobacteriaceae isolated in India and the UK. J. Antimicrob. Chemother. 68:1543–1550 [DOI] [PubMed] [Google Scholar]
- 21.Woodford N., Ellington M.J., Coelho J.M., Turton J.F., Ward M.E., Brown S., Amyes S.G., and Livermore D.M. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents. 27:351–353 [DOI] [PubMed] [Google Scholar]
- 22.Seija V., Medina-Presentado J.C., Bado I., Ezdra R.P., Batista N., Gutierrez C., Guirado M., Vidal M., Nin M., and Vignoli R. 2015. Sepsis caused by New Delhi metallo-β-lactamase (blaNDM-1) and qnrD-producing Morganella morganii, treated successfully with fosfomycin and meropenem: case report and literature review. Int. J. Infect. Dis. 30:20–26 [DOI] [PubMed] [Google Scholar]
- 23.Higgins P.G., Lehmann M., and Seifert H. 2010. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents. 35:305 [DOI] [PubMed] [Google Scholar]
- 24.Doi Y., and Arakawa Y. 2007. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 45:88–94 [DOI] [PubMed] [Google Scholar]
- 25.Davis M.A., Baker K.N.K., Orfe L.H., Shah D.H., Besser T.E., and Call D.R. 2010. Discovery of a gene conferring multiple-aminoglycoside resistance in Escherichia coli. Antimicrob. Agents Chemother. 54:2666–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bueno M.F.C., Francisco G.R., O'Hara J.A., de Oliveira Garcia D., and Doi Y. 2012. Coproduction of 16S rRNA Methyltransferase RmtD or RmtG with KPC-2 and CTX-M Group Extended-Spectrum β-Lactamases in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 57:2397–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fritsche T.R., Castanheira M., Miller G.H., Jones R.N., and Armstrong E.S. 2008. Detection of methyltransferases conferring high-level resistance to aminoglycosides in Enterobacteriaceae from Europe, North America, and Latin America. Antimicrob. Agents Chemother. 52:1843–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rafei R., Dabboussi F., Hamze M., Eveillard M., Lemarié C., Gaultier M.-P., Mallat H., Moghnieh R., Husni-Samaha R., Joly-Guillou M.-L., and Kempf M. 2014. Molecular analysis of Acinetobacter baumannii strains isolated in Lebanon using four different typing methods. PLoS One 9:e115969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diancourt L., Passet V., Nemec A., Dijkshoorn L., and Brisse S. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5:e10034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luna C.M., Rodriguez-Noriega E., Bavestrello L., and Guzman-Blanco M. 2014. Gram-negative infections in adult intensive care units of latin america and the Caribbean. Crit. Care Res. Pract. 2014:480463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labarca J.A., Salles M.J., Seas C., and Guzman-Blanco M. 2014. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Crit. Rev. Microbiol. 42:276–292 [DOI] [PubMed] [Google Scholar]
- 32.Gales A.C., Castanheira M., Jones R.N., and Sader H.S. 2012. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010). Diagn. Microbiol. Infect. Dis. 73:354–360 [DOI] [PubMed] [Google Scholar]
- 33.Castilho S.R.A., Godoy C.S.M., Guilarde A.O., Cardoso J.L., Andre M.C.P., Junqueira-Kipnis A.P., and Kipnis A. 2017. Acinetobacter baumannii strains isolated from patients in intensive care units in Goiania, Brazil: molecular and drug susceptibility profiles. PLoS One 12:e0176790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams M.D., Bishop B., and Wright M.S. 2016. Quantitative assessment of insertion sequence impact on bacterial genome architecture. Microb. Genom. 2:e000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H., Liu F., Zhang Y., Wang X., Zhao C., Chen H., Zhang F., Zhu B., Hu Y., and Wang H. 2015. Evolution of carbapenem-resistant Acinetobacter baumannii revealed through whole-genome sequencing and comparative genomic analysis. Antimicrob. Agents Chemother. 59:1168–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dias V.C., Diniz C.G., Peter A.C., Bastos A.N., Bastos V.Q., Bastos L.Q., and Da Silva V.L. 2016. Epidemiological characteristics and antimicrobial susceptibility among carbapenem-resistant non-fermenting bacteria in Brazil. J. Infect. Dev. Ctries. 10:544–553 [DOI] [PubMed] [Google Scholar]
- 37.Cordeiro N.F., Chabalgoity J.A., Yim L., and Vignoli R. 2014. Synthesis of metallo-beta-lactamase VIM-2 is associated with a fitness reduction in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 58:6528–6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordeiro N.F., Yim L., Betancor L., Cejas D., García-Fulgueiras V., Mota, Varela M.I., Anzalone G.L., Algorta G., Gutkind G., Ayala J.A., Chabalgoity J.A., and Vignoli R. 2013. Identification of the first blaCMY-2 gene in Salmonella enterica serovar Typhimurium isolates obtained from cases of paediatric diarrhoea illness detected in South America. J. Glob. Antimicrob. Resist. 1:143–148 [DOI] [PubMed] [Google Scholar]
- 39.Fernandez A., Perez A., Ayala J.A., Mallo S., Rumbo-Feal S., Tomas M., Poza M., and Bou G. 2012. Expression of OXA-type and SFO-1 beta-lactamases induces changes in peptidoglycan composition and affects bacterial fitness. Antimicrob. Agents Chemother. 56:1877–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hopkins K.L., Escudero J.A., Hidalgo L., and Gonzalez-Zorn B. 2010. 16S rRNA methyltransferase RmtC in Salmonella enterica serovar Virchow. Emerg. Infect. Dis. 16:712–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutierrez B., Escudero J.A., San Millan A., Hidalgo L., Carrilero L., Ovejero C.M., Santos-Lopez A., Thomas-Lopez D., and Gonzalez-Zorn B. 2012. Fitness cost and interference of Arm/Rmt aminoglycoside resistance with the RsmF housekeeping methyltransferases. Antimicrob. Agents Chemother. 56:2335–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]