Abstract
The formyl peptide receptors (FPRs) are G protein-coupled receptors that transduce chemotactic signals in phagocytes and mediate host-defense as well as inflammatory responses including cell adhesion, directed migration, granule release and superoxide production. In recent years, the cellular distribution and biological functions of FPRs have expanded to include additional roles in homeostasis of organ functions and modulation of inflammation. In a prototype, FPRs recognize peptides containing N-formylated methionine such as those produced in bacteria and mitochondria, thereby serving as pattern recognition receptors. The repertoire of FPR ligands, however, has expanded rapidly to include not only N-formyl peptides from microbes but also non-formyl peptides of microbial and host origins, synthetic small molecules and an eicosanoid. How these chemically diverse ligands are recognized by the three human FPRs (FPR1, FPR2 and FPR3) and their murine equivalents is largely unclear. In the absence of crystal structures for the FPRs, site-directed mutagenesis, computer-aided ligand docking and structural simulation have led to the identification of amino acids within FPR1 and FPR2 that interact with several formyl peptides. This review article summarizes the progress made in the understanding of FPR ligand diversity as well as ligand recognition mechanisms used by these receptors.
Keywords: G protein-coupled receptors, formyl peptides, inflammation, phagocytes
1. Introduction
The formyl peptide receptors (FPRs) are a group of G protein-coupled chemoattractant receptors that play important roles in host defense and inflammation [1,2]. They are so named because their cognate agonists are peptides bearing formylated methionine (fMet), such as those derived from bacterial and mitochondrial proteins [3,4]. The present review focuses on the FPR family members in humans and mice and their interaction with a wide variety of ligands. The reader is referred to recently published reviews on FPR signaling [5], synthetic non-peptide ligands for FPRs [6] and characterization of FPR knockout mice [7]. The reader is also referred to a general overview of FPRs and their nomenclature [2].
2. The Formyl Peptide Receptor Family
2.1. The Human Formyl Peptide Receptors
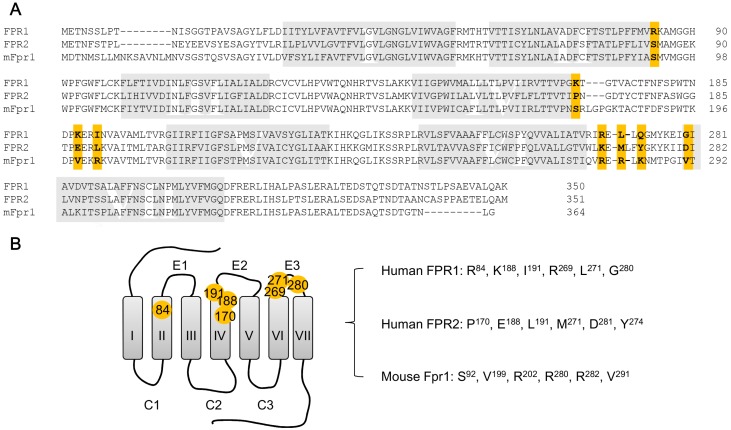
Three genes coding for human G protein-coupled formyl peptide receptors have been cloned, including FPR1, FPR2 and FPR3 [8,9,10,11,12,13]. Among them, FPR1 and FPR2 share an overall high sequence identity (Figure 1) and certain overlapping functions.
Figure 1.
Sequence alignment and location of positive selection sites in human formyl peptide receptors (FPRs): FPR1, FPR2 and mouse mFpr1. (A) Alignment of the protein sequence of human FPR1, FPR2 and mouse Fpr1. Positively selected amino acid sites [14] in three FPR isoforms are shown in color; (B) Schematic diagram for the location of the positive selection sites in the formyl peptide receptors, based on the sequence of human FPR1. The amino acids at these positions in each of the three receptors are marked on the right.
FPR1 and FPR2 are expressed in both monocytes and neutrophils, while FPR3 is found in monocytes but not neutrophils. Besides myeloid cells, FPR1 is expressed in astrocytes, microglial cells, hepatocytes and immature dendritic cells. FPR2 shows an even wider distribution pattern than FPR1 and is expressed in a variety of non-myeloid cells including astrocytoma cells, epithelial cells, hepatocytes, microvascular endothelial cells, neuroblastoma cells, in addition to phagocytic leukocytes. In recent studies, FPR1 has been associated with anti-bacterial inflammation and metastasis of malignant glioma cells, while FPR2 is implicated in the pathogenesis of chronic inflammatory diseases such as systemic amyloidosis, Alzheimer’s disease, atherosclerosis, systemic lupus erythematosus and ovarian cancer metastasis et al. [15,16,17,18,19]. More examples and details of FPR involvement in multiple diseases are provided in recent reviews [7,20].
2.2. The Mouse Formyl Peptide Receptors
Since the human FPR1 gene (FPR1) was first cloned, orthologs have been identified in other mammalian species, including rabbits, guinea pigs, horses, rats and mice (reviewed in [2]). The genes coding for FPR members comprise a large family that implicates a complex evolutionary path with evidence of positive selection [14]. Despite overall sequence homology, these genes vary considerably in their numbers and sequences between humans and other species. For example, the mouse FPR gene family has eight known members (mFpr1, mFpr2, mFpr-rs1, mFpr-rs3, mFpr-rs4, mFpr-rs6, mFpr-rs7 and mFpr-rs8) clustered on mouse chromosome 17A3.2. Among these genes, mFpr-rs5 (ψmFpr-rs3) is found as a pseudogene that does not code for a functional receptor.
Although several mouse Fpr genes are present in neutrophils, work on mFprs has been focused mostly on the gene products of mFpr1 and mFpr2, which are widely expressed in mouse phagocytic leukocytes and show high similarity to their human counterparts. Targeted deletion of mFpr1 or mFpr2 renders mice more susceptible to bacterial infection without changing their viability and fertility [21,22]. Under unstimulated conditions, mice lacking mFpr1 or mFpr2 behave normally. However, in models of human diseases, both mFpr1−/− and mFpr2−/− mice respond differently from their wildtype littermates, indicating regulatory roles of these receptors in host defense and inflammation. Targeted gene disruption of mFpr1 also suggested a homeostatic role for the mFpr1 in the production of glucocorticoids and anxiety-like behavior [23]. As the suggested ortholog of human FPR1, mFpr1 was identified as a low-affinity receptor for the classical formyl tri-peptide fMet-Leu-Phe (fMLF) [24], but this receptor responds well to several N-formyl peptides derived from other bacteria (e.g., fMIFL, fMIVIL, fMIVTLF) and mitochondria (e.g., fMMYALF) [25,26]. In general, mFpr2 is not a high-affinity receptor for either fMLF or other native formyl peptides tested so far [26]. However, it responds to endogenous peptide agonists for FPR2, including the amyloidogenic proteins SAA (serum amyloid A) [27] and Aβ42 [28]. Likewise, this receptor can be inhibited by FPR2-specific antagonists when expressed in neutrophil or by stably transfected cells. Besides this, mouse mFpr2 has also been reported as a receptor for F2L [29] that is a potent agonist for human FPR3 [30].
Published reports indicate that other members of the murine mFpr family are not coding for stereotypic formyl peptide receptors. In recent studies, mFpr-rs8 (ψmFpr-rs2) has been characterized as a constitutively expressed gene associated with mouse longevity [31]. The gene products of five other mFprs, including mFpr-rs1, mFpr-rs3, mFpr-rs4, mFpr-rs6, and mFpr-rs7, are recently described as mouse vomeronasal sensory receptors [32,33]. Phylogenetic analysis suggested that they belong to a chemosensory GPCR family, which is characterized with an olfactory function and has the ability to identify pathogenic states. Among them, the property of mFpr-rs1 is still uncertain as it is also expressed in neutrophils. It was believed that mFpr-rs1 might have some functional overlap with human FPR2 because a variant of mFpr-rs1 was reported to encode a mouse receptor for lipoxin A4 (LXA4) [34], an eicosanoid ligand for FPR2. However, functional and pharmacology assays conducted in stably transfected cells suggested that mFpr-rs1 responds poorly to most agonists that activate human and murine FPR receptors [35]. These observations add to studies using knockout animal models and together indicate that murine Fprs (especially mFpr1 and mFpr2) share numerous structural and pharmacological properties with human FPRs, but the evolutionary correlation between them is by no means linear.
3. Agonists for the Formyl Peptide Receptors
The FPR family is well known for the structural diversity of their ligands, including a variety of ligands with different chemical properties and origins ranging from natural peptides to synthetic non-peptide compounds [2,5] (Table 1). FPRs are therefore classified as a group of pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular pattern (DAMPs) [36,37,38].
Table 1.
Structures and sequences of representative FPR ligands. The table shows selected FPR ligands in different categories, based on their origins and chemical structures. Ac = acetyl; Me = methyl; Ph = phenyl; Pam = palmitoyl; N.D, binding affinity or potency not determined.
| Ligands Representatives | Sequence/Structure | Selectivity | Reference |
|---|---|---|---|
| Agonists | |||
| N-Formyl peptides | |||
|
|||
| fMLF | formyl-Met-Leu-Phe | FPR1 > FPR2 | [40] |
| PSMα peptide | formyl-MGIIAGIIKFI KGLIEKFTGK | FPR2 > FPR1 | [52] |
|
|||
| fMMYALF | formyl-Met-Met-Tyr-Ala-Leu-Phe | FPR1, FPR2 | [53] |
| Mitocryptide-2 | formyl-MTNIRKSHPLMKIIN | FPR2 | [54] |
| Non-formyl peptides | |||
|
|||
| Hp2-20 | AKKVFKRLEKLFSKIQNDK | FPR2 >> FPR3 | [55] |
|
|||
| SAA1.1 |  |
FPR2, others | [56,57] |
| Aβ42 | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA | FPR2 | [28,58] |
| Ac2–26 | Ac-AMVSEFLKQAWFIENEEQEYVQTVK | FPR1, FPR2 | [59,60] |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | FPR2 | [61] |
| uPAR88-92 | 88Ser-Arg-Ser-Arg-Tyr92 (SRSRY) | FPR1 | [62] |
| uPAR84-95 | AVTYSRSRYLEC | FPR2, FPR3 | [63] |
| PrP(106-126) | KTNMKHMAGAAAAGAVVGGLG | FPR2 | [64] |
| SHAAGtide | MLWRRKIGPQMTLSHAAG | FPR2 > CCR1 | [65] |
| VIP | HSDAVFTDNYTRLRKQMAVKKYLNSILN | FPR2, VPAC1 | [66] |
|
|||
| W peptides | WKYMVm(Trp-Lys-Tyr-Met-Val-D-Met-NH2) | FPR2 > FPR1 >> FPR3 | [67,68,69] |
| WKYMVM(Trp-Lys-Tyr-Met-Val-Met-NH2) | FPR2 >> FPR3 | [69] | |
| MMK1 | LESIFRSLLFRVM | FPR2 | [70] |
| L-37pA | DWLKAFYDKVAEKLKEAFPDWLKAFYDKVAEKLKEAF | FPR2 | [71] |
| CGEN-855A | TIPMFVPESTSKLQKFTSWFM | FPR2, FPR3 | [72] |
|
|||
| F2Pal16 | Pam-KIHKKGMIKSSRPLRV | FPR2 | [72,73] |
| Eicosanoids | |||
| Lipoxin A4 | 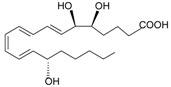 |
[74] | |
| Small molecules | |||
|
 |
FPR2 >> FPR1 | [75] |
|
 |
FPR1 | [76] |
|
 |
FPR2 | [77] |
|
 |
FPR2 > FPR1 | [78,79] |
|
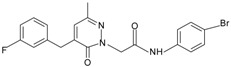 |
FPR1 | [80] |
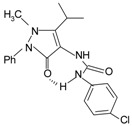
| Pyridazin-3(2H)-one derivative 2 | 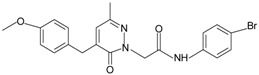 |
FPR2 | [80] |
|
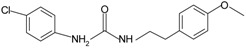 |
FPR2 | [76] |
|
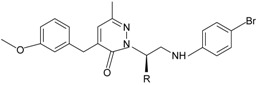 R = n-C3H7, i-C3H7, n-C4H9 |
FPR1, FPR2 | [81] |
|
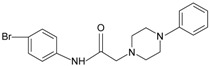 |
FPR2 | [76] |
|
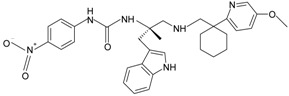 |
FPR2 | [82,83] |
|
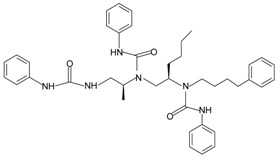 |
FPR1 | [84] |
| Antagonists | |||
| Peptides | |||
|
|||
| Boc-1 | N-tert-butoxycarbonyl-MLF | FPR1 >> FPR2 | [40] |
| Boc-2 | N-tert-butoxycarbonyl-FLFLF | FPR1 >> FPR2 | [40] |
| CHIP peptide Uteroglobin (UG) |
FTFEPF MKLAVTLTLVTLALCCSSASAEICPSFQRVIETLLMDTPSSYEAAMELFSPDQDMREAGAQLKKLVDTLPQKPRESIIKLMEKIAQSSLCN |
FPR1 FPR2 |
[85] [86] |
| Cyclosporin H (CsH) | 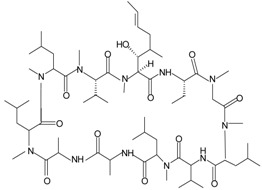 |
FPR1 | [87] |
| Cyclized uPAR88-92 | [88Ser-Arg-Ser-Arg-Tyr92]( cyclized SRSRY) | FPR1, N.D for FPR2 | [88] |
|
|||
| WRW4 | WRWWWW (Trp-Arg-Trp-Trp-Trp-Trp) | FPR2, FPR3 | [89] |
| PBP10 | RhoB-QRLFQVKGRR | FPR2, others | [90] |
| Lipopeptides | |||
| F1Pal16 | Pam-KIHKQGMIKSSRPLRV | FPR2 | [91] |
| Pam-(Lys-βNSpe)6-NH2 | 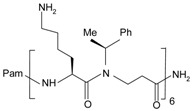 |
FPR2, mFpr2 | [92,93] |
| Non-peptide molecules | |||
| Isoflavone analog | 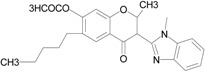 |
FPR1 | [94] |
| 1754-31 | 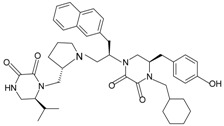 |
FPR2 | [84] |
| Quin-C7 | 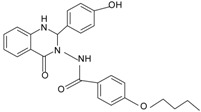 |
FPR2 | [95] |
3.1. Conventional Formyl Peptide Agonists for FPRs
Among all the ligands for the FPRs, N-formylated peptides, particularly fMLF (an E. coli-derived chemotactic peptide), are most often studied. fMLF is among the first characterized and also the shortest formyl peptides with full agonistic activities [39,40,41]. These peptides are initiated with N-formyl methionine and are generally cleavage products of bacterial and mitochondrial proteins. N-formylated peptides constitute the most commonly studied class of FPR agonists that trigger a variety of biological activities in myeloid cells, such as chemokinesis, chemotaxis, calcium flux, cytokine production and superoxide anion generation. Through binding with the high affinity receptor FPR1, fMLF and other N-formylated peptides serve as potent chemoattractants, which also include activated complements (C5a, C3a) and chemokines, in recruiting and guiding leukocytes to the site of bacterial infection and to damaged tissues. A wealth of literatures have described the proinflammatory properties of N-formyl peptides. As early as 30 years ago, right after the discovery of N-formyl peptides, studies have shown that fMLF was involved in the pathogenesis of multiple inflammatory diseases such as colitis [42,43,44], pouchitis, ulcerative colitis and Crohn’s disease [45,46] and juvenile peridotitis [47]. Studies also reported that inhalation or injection of fMLF can cause rapid neutropenia and bronchial inflammation in human and other mammals [48,49,50,51].
Human FPR2 is a low affinity receptor for fMLF and many potent formyl peptide agonists for FPR1. However, FPR2 displays relatively high affinity for N-formylated peptides of specific composition and longer length. For instance, FPR2 responds better to peptides carrying positive charges at the C-terminus (e.g., fMLFK, fMLFIK) than peptides with negative charges (e.g., fMLFE and fMLF) [96]. Studies also showed that FPR2 responds well to formyl peptides of microbial origin other than E. coli, such as fMIFL (S. aureus) and fMIVIL (L. monocytogenes) in calcium mobilization and cAMP release assays [96]. In general, most bacteria-derived formyl peptides are more potent at FPR1 than FPR2. A prominent exception is the recently described cytolytic peptides PSM (phenol-soluble modulins) that are α-helical peptides composed of 20–25 amino acids [97]. Although they are predominantly secreted from community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) in formylated form, PSMα peptides are more selective for FPR2 than for FPR1 [98,99,100]. Another exception is mitocryptide-2, a neutrophil-activating formyl peptide produced from mitochondrial cytochromes. Mitocryptide-2 can directly bind to FPR2 and activate it with an EC50 of 6.9 nM in calcium flux assay, whereas it manifests no interaction with FPR1 [54]. In addition, another group of formyl peptides derived from mitochondria (fMMYALF, fMYFINILTL, and fMLKLIV) were found equally potent on FPR1 and FPR2 with EC50 values ranging from 10 nM to 160 nM [53]. Despite the different potency and selectivity of these formyl peptides, the N-formyl group is important for optimal interaction with FPR1 and FPR2. It is suggested that FPR1 is a highly effective receptor recognizing formyl peptides and mediating phagocyte functions, while FPR2 has an overall lower affinity but it can discriminate between formyl peptides with different sizes and features. In contrast, no bacterial or mitochondrial formyl peptide has been described as agonist for FPR3 by far. Noting that the prototypic FPR1 agonist fMLF is not a potent activator for murine Fprs, including mFpr1 and mFpr2, when compared to human FPR1. In agreement with the research that murine neutrophils are more susceptible to bacteria other than E. coli [21], mFpr1 was found more responsive to formyl peptides derived from Listeria monocytogenes (fMIVTLF), Staphylococcus aureus (fMIFL), and mitochondria (fMMYALF) [25,26].
3.2. Other Peptide Agonists for FPRs
Except for formyl peptides, a number of different peptides/proteins, including microbial and host-derived non-formyl peptides/proteins and peptides from synthetic libraries, have been identified to be agonists for FPRs. It appears that the absence of N-formyl group and lack of overall sequence similarity with formyl peptides mentioned above do not impair their agonistic activity at FPRs. In general, the ligand diversity is more prominent for FPR2 than FPR1.
The non-formylated peptides/proteins derived from microbe are represented by virus envelope proteins (gp41 and gp120) of the human immunodeficiency virus type 1 (HIV-1), which contains at least five FPR-recognizing peptide sequences composed of 20–37 amino acids. Most of these sequences were found more potent at FPR2 than at FPR1 and FPR3 [101,102,103,104,105], except only one is selective for FPR1 and mFpr1 [101,102,106]. A cecropin-like peptide (Hp2-20) from Helicobacter pylori was also identified as an agonist for FPRs [55,107]. The studies demonstrated that in H. pylori infection, Hp2-20 was able to induce chemotaxis of monocytes and basophils, and evoke superoxide release via signaling through FPR2 and FPR3. Recently, two antimicrobial peptides (AMPs) isolated from Scolopendra subspinipes mutilans were reported to elicit neutrophil chemotactic migration through FPR1 [108].
The host-derived non-formyl peptide agonists for FPRs are generally peptides and proteins associated with human diseases and inflammation. This group of agonists activates FPRs independently of the N-formyl group and they also show a preference for FPR2. A prominent example of them is serum amyloid A (SAA), an acute-phase protein that is implicated in chronic inflammation and amyloidosis. As the first identified endogenous peptide agonist for FPR [56], documented studies have shown that it exerts pro-inflammatory activities through FPR2 in phagocytes, epithelial cells and T lymphocytes, including stimulating production of inflammatory mediators and enhancing the expression of cytokine receptors [109,110,111,112,113]. Recently, questions have been raised regarding the proinflammatory cytokine-like properties of native SAA, since most studies of SAA and FPR2 are performed using a commercially available recombinant protein that contains amino acid substitutions of SAA2 in SAA1 [5,114,115]. However, research conducted with SAA1 isoforms purified from an E. coli expression system suggested that major SAA1 isoforms have cytokine-inducing activity and minor substitutions of amino acids affected their potency at FPR2 [116]. In vivo studies have shown Th17-induction activity of SAA in gut epithelium, although whether these activities are attributed to FPR2 or other SAA receptors remain unclear [117,118].
Another amyloidogenic disease-associated FPR2 agonist is the prion protein fragment PrP (106–126), which was reported to interact with FPR2 in glial cells and induce calcium mobilization, chemotaxis as well as production of pro-inflammatory cytokines [64]. In addition to SAA and PrP (106–126), other two amyloidogenic disease-associated peptides were found to be agonists for FPR2: the 42-amino acid form of Aβ amyloid peptide (Aβ42) and humanin. Although both peptides use FPR2 to induce migration and activation of monocytic phagocytes in the brain, Aβ42 and humanin display divergent roles in the development of Alzheimer’s disease: Aβ42 is a major cause of fibrillary formation and deposition in brain of AD patients [119,120], whereas humanin is neuroprotective on the contrary [121]. Studies suggested that humanin reduces aggregation and fibrillary formation by inhibiting Aβ42/FPR2 interaction in mononuclear phagocytes [121]. FPR3 and mFpr2 are also functional receptors for humanin in humans and mice, respectively [121,122].
Annexin A1, a glucocorticoid-regulated protein, and its N-terminal peptides (Ac2-26 and Ac9-25) are FPR agonists that have dual roles in inflammation. At high concentrations, the annexin A1 peptides fully activate FPR1 like the conventional agonists that induce pro-inflammatory responses. In contrast, at low concentrations they only show partial activity at FPR1 [123], leading to neutrophil desensitization and inhibiting neutrophil migration induced by other chemoattractants. All three FPR members are implicated in the various functions of annexin A1 peptides. Some researchers thought that these peptides use FPR2 for anti-inflammatory actions [59], while others suggested the presence of receptors other than FPR2 and FPR3 as being responsible for the resolving effects [124]. The exact mechanism for the pro-/anti-inflammatory activity of annexin A1 peptides is not entirely clear.
The antimicrobial peptide LL-37 and its murine homolog CRAMP (Cathelicidin-Related Anti-Microbial Peptide) were identified as FPR2 agonists [61,125]. LL-37 is a cleavage product of the neutrophil granule protein cathelicidin found in leukocytes and epithelial cells. As a pro-inflammatory mediator, LL-37 activates FPR2 and evokes superoxide generation in human fibroblasts [126]. It also induces directional migration of human monocytes, neutrophils, and T lymphocytes [61]. However, LL-37 also inhibits SAA signaling in human neutrophils, including IL-8 production and chemotaxis through FPR2 [127]. LL-37 seems to be a pleiotropic peptide and its interaction with FPR2 is implicated in wound healing, cell proliferation [128], angiogenesis [129] and anti- or pro-tumorigenesis [130,131,132]. In a recent study, the proinflammatory circuits of LL-37 and leukotriene B4 was reported. In human neutrophils, LL-37/FPR2 promotes Leukotriene B4 production, which in turn elicits further LL-37 release through BLT1 (leukotriene B4 receptor 1) [133].
uPAR is the cell surface receptor for urokinase-type plasminogen activator (uPA) that is a serine protease involved in regulation of fibrinolysis, cell adhesion, migration and tissue repair. uPAR can be cleaved by different proteases, including uPA, generating soluble uPAR fragments that can be recognized by different FPR members. For instance, uPAR D2D388–274, a cleaved peptide corresponding to residues from 88 to 274 of uPAR, binds to and activates FPR2 in monocytes, inducing cell migration [134]. Moreover, uPAR88-95 (88SRSRY95) interacts with FPR1 [62], while uPAR84–95 activates both FPR2 and FPR3 in basophils [63].
In a more recent study, a new host-derived chemotaxis agonist FAM3D, for the FPR1 and FPR2 was identified [135]. FAM3D, a cytokine-like protein, is constitutively expressed in the gastrointestinal tract with significant induction in dextran sulfate sodium (DSS)-induced colitis [135]. FAM3D was found to strongly attract the chemotaxis of human peripheral blood neutrophils and monocytes. Through functional screen of a group of chemoattractant receptors, FPR1 and FPR2 were determined to be the receptors for FAM3D. In HEK293 cells transfected to express FPR1 or FPR2, FAM3D induced significant cell chemotaxis and calcium mobilization that was desensitized by other FPR agonists (fMLF and WKYMVm), and vice versa. The activation of ERK1/2 and p38 MAPK signaling caused by FAM3D in neutrophils was blocked by an antagonist of FPR2 (WRW4). This study suggested that FAM3D/FPR interaction might play a role in intestinal homeostasis and gastrointestinal inflammation.
The FPR receptors also have other endogenous peptide ligands, such as cathepsin G for FPR1, chemokine CCL23 (amino acids 22–137) and its N-terminal fragment SHAAGtide for FPR2, a vasoactive intestinal neuropeptide VIP (vasoactive intestinal polypeptide) for FPR2, and a heme-binding protein fragment F2L for FPR3 (Table 1). In addition, exogenous allergens including house dust mite and birch pollen extracts were recently shown as being agonistic for FPR1 and FPR2. The detailed properties of these FPR agonists are discussed in previous reviews [2,5,136].
Among all the synthetic peptide agonists for FPRs, a hexapeptide, Trp-Lys-Tyr-Met-Val-D-Met-NH2 (WKYMVm) isolated from a random peptide library [67], was identified to be a strong activator for FPR1, FPR2 and FPR3 [68,137]. WKYMVm conjugated with FITC in the second residue Lys was shown slightly more efficacious in binding to FPR2 than to FPR1 [96]. WKYMVm is by far the most potent peptide agonist for FPR2, being able to activate FPR2 at picomolar concentrations in chemotaxis assays. WKYMVM, a derivative of WKYMVm with the substitution of l-methionine at the carboxyl terminus, becomes highly selective for FPR2 and is weakly agonistic for FPR3 [69]. Another peptide identified from library screen, MMK-1 (LESIFRSLLFRVM), is a highly selective chemotactic agonist for FPR2 [70,138]. CGEN-855A, a 21 amino acids anti-inflammatory peptide obtained from a computational platform, was identified to be agonist for FPR2 and FPR3 [139]. In another study, a tethered library was screened and a peptide with the sequence MMWLL was identified as an FPR1 agonist [140]. This peptide becomes 1000-fold more potent when the first Met is N-formylated, consistent with the preferential recognition of fMet-containing peptides by FPR1. In a recent study [141], a series of AApeptides, a class of peptidomimetics based on N-acylated-N-aminoethyl amino acid residues, were designed and synthesized to mimic the structure and function of the prototypic tripeptide fMLF, the reference agonist of FPR1. Three of these fMLF-mimicking AApeptides were found effective in activating FPR-expressing cells in calcium mobilization assay, albeit at concentrations above 10 µM. Certain AApeptides were shown to be more efficacious than fMLF at a higher concentration (10 µM). Similar to fMLF, these AApeptides have receptor preference for FPR1 over FPR2.
3.3. Non-peptide Agonists Screened from Small Molecule Library
In comparison with peptide/protein FPR agonists, the small molecule agonists screened from chemical library are more stable and highly selective for certain FPR members. These properties make them valuable tools for detailed characterization of FPRs, which is important for a better understanding of the complex role of FPRs in vivo. FPRs are involved in a variety of signaling systems and their distribution and functions are not limited to the immune system. As a result, these small molecules screened by targeting FPRs as agonists or antagonists are expected to serve as potentially useful agents in therapeutic treatment.
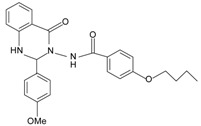
The first reported non-peptide agonist for FPR from library screening is a quinazolinone derivative named Quin-C1 [75]. Quin-C1 (4-butoxy-N-[2-(4-methoxy-phenyl)-4-oxo-1, 4-dihydro-2H-quinazolin-3-yl]-benzamide) was found highly selective for FPR2 as opposed to FPR1. It was identified as a biased agonist, as it stimulated calcium mobilization through FPR2 but did not induce substantial neutrophil superoxide generation, even at concentrations up to 100 µM. A later study revealed an anti-inflammatory role for Quin-C1 in bleomycin-induced lung injury in a mouse model [142]. The murine receptors for Quin-C1 were determined to be mFpr1 and mFpr2 [26]. Competitive binding analysis showed that Quin-C1 does not share the same recognition sites with formyl peptides in mFprs [26]. Given that Quin-C1 is non-competitive with formyl peptides, it is interesting to surmise that Quin-C1 may act at FPR2 as an ago-allosteric modulator. However, the precise mechanism for the agonistic activity of Quin-C1 requires further examination.
Since the first synthetic non-peptide FPR agonist was reported, an increasing number of small-molecule agonists have been discovered in recent years [6]. So far, a series of structurally divergent small-molecules with high-affinity have been identified as FPR ligands via cell-based assays for high throughput screening (HTS), as well as structure-activity relationship (SAR)-directed design and synthesis [82,83,84,143,144]. In general, the identification of new molecules relies on known structures, SAR analysis and computer-aided design. Using this strategy, around one hundred FPR agonists with high potency and clear selectivity have been identified and optimized in the reported research. Based on the structures with varying scaffolds and SAR-directed evaluation, these small compounds fall into at least 10 groups: benzimidazoles, pyrazolones (e.g., Compound 43), N-substituted benzimidazoles, pyridazin-3(2H)-ones, chiral pyridazines, N-phenylureas, chiral 3-(1H-indol-3-yl)-2-[3-(4-nitrophenyl)ureido] propanamides, 2-(N-Piperazinyl)acetamide derivatives, quinazolinones (exemplified by Quin-C1), and other derivatives (reviewed by [6]). Among these molecules, the most potent FPR1-selective agonist was identified to be a polyphenylure derivative with an EC50 of 131 nM in calcium flux assays and a Ki of 4 nM in binding assays. This compound, [3-phenyl-1-((R)-1-(3-phenyl-1-((S)-1-(3-phenylureido)propan-2-yl)ureido)hexan-2-yl)-1-(4-phenylbutyl)urea], coded as 1753-103 is also the most potent synthetic non-peptide agonist for FPR1 known to date [84]. In addition to 1753-103, other chemical agonists with high FPR1 specificity, such as the benzimidazole derivates [145] and the pyridazinone-based compounds [146], have been described, although they act with EC50 above micromolar (>1.5 μM). Compared with FPR1, FPR2 have more specific and potent agonists identified from screening of compound library and through rational design. Currently, more than 10 FPR2-slective agonists have been reported to have an EC50 in the nanomolar range in functional assays, including three pyrazolone derivatives [78], an N-substituted benzimidazole derivative [77], an N-phenylurea derivative [147], a chiral N-phenylurea derivative [148], two benzodioxole derivatives of N-phenylureas [148], six chiral ureidopropanamido derivatives [82,83], a phenylsubstituted urea compound [143], a compound with a unique 2-(4-phenyl-5-((phenylamino)methyl)-1,2,4-triazol-3-ylthio) acetamide scaffold [143], and a pyrrolidine bis-diketopiperazine derivative [84]. As expected, there are numerous agonists with dual specificity (FPR1/FPR2) (reviewed by [6]). Noting that a pyrazolone derivative, designated in most publications as “Compound 43”, was first identified as a FPR2-specific agonist [78], but it was redefined to be a mixed FPR1/FPR2 agonist in an independent screen recently [79]. It is not clear for most of the reported small molecule agonists whether they interact with murine formyl peptide receptors since they are designed and screened to target human FPRs and murine FPRs have distinct properties compared to their human counterparts.
3.4. Host-derived Lipid and Lipopeptide Agonists
Lipoxin A4 (LXA4), derived from arachidonic acid, has highly potent anti-inflammatory and pro-resolving activities based on in vivo studies [149,150,151,152]. This eicosanoid was reported to directly bind to FPR2 and a variant of mouse mFpr-rs1 [34,74,149,153]. LXA4 was shown to have a high affinity (Kd of ~ 1.7 nM) in binding to FPR2-expressing Chinese hamster ovary cells [74]. Despite the high-affinity binding, LXA4 is an atypical ligand for FPR2 as it cannot activate proinflammatory activities such as chemotaxis, enzyme release and ROS production. LXA4 also fails to activate FPR2-dependent signaling, such as calcium mobilization, in transfected cell lines [154,155,156]. These findings do not support the original report that LXA4 could stimulate GTPase activity in FPR2-expressing CHO cells [74]. It appears difficult to determine the role of LXA4 as an FPR2 agonist without establishing a standard in compound preparation. Moreover, FPR2 specific antagonists, stereoselective analogs, antibodies, and transgenic approaches are required in further studies, and the interaction of LXA4 with other receptors such as the cannabinoid receptor CB1 [157], aryl hydrocarbon receptor (AhR) [158] and estrogen receptor [159] will have to be considered.
Resolvins, protectins and maresins are novel pro-resolving mediators that are biosynthesized from omega-3 fatty acids, including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). These lipid mediators block neutrophil recruitment, promote monocyte activation, and enhance macrophage phagocytosis. Resolvin D1, a biosynthetic product from DHA, was reported to activate FPR2 and GPR32, an orphan GPCR. Similar with LXA4, Resolvin D1 cannot evoke calcium mobilization through FPR2 or GPR32. The interaction of Resolvin D1 with these GPCRs was examined by a GPCR-β-arrestin-coupled system [160]. Selective knock down or inhibition of FPR2 reduces the Resolvin D1 response, such as macrophage phagocytosis [160] and salivary epithelium migration [161]. Resolvin D1 and FPR2 are also implicated in pulmonary inflammation [162] and obesity [163].
Oxidized low-density lipoprotein (oxLDL), an atherogenesis associated molecule, was recently found to stimulate macrophages via FPR2 signaling [164]. The oxLDL-induced foam cell formation and TNF-α production could be inhibited by an FPR2 antagonist WRW4, but not affected by an FPR1 antagonist. In FPR2-expressing RBL-2H3 cells, oxLDL also stimulated significant calcium mobilization and chemotaxis [164]. In another study, the mimetic peptide L-37pA (Table 1) of apoA-I, a major component of circulating high-density lipoprotein (HDL), was reported to induce calcium flux and chemotaxis through FPR2. L-37pA is probably a biased agonist of FPR2 as it fails to induce superoxide generation in human neutrophils at a concentration up to 20 μM [71]. D-37pA, the D-stereoisomer of L-37pA, blocks L-37pA signaling and induces chemotaxis but not calcium flux. It is unclear whether D-37pA is an anti-inflammatory antagonist of FPR2. It has been noted that apoA-I itself and apoE, another important component of HDL, are described as being anti-inflammatory, as they suppress chemotaxis of leukocytes in the airway.
Currently, there has been an increasing interest in a group of newly emerging molecules that modulate GPCR activity by entirely novel mechanisms. These molecules, known as pepducins, comprise a lipid moiety conjugated with a peptide that is derived from a sequence of the cytoplasmic loops or the C-terminal tail of the target GPCR [165,166]. With the facilitation of the lipid moiety, pepducins are thought to pass across plasma membrane and anchor in the cytosolic interface to activate or inhibit signaling of receptors [166]. These lipidated peptides have been proposed as allosteric modulators, because the binding sites and action modes are different from those of conventional ligands that generally interact with extracellular domains of receptors [5,166]. Despite that the ‘allosteric modulation’ mechanism is not yet fully clarified, several receptor-specific pepducins have been identified, including an FPR2 agonistic pepducin F2Pal16. F2Pal16 is composed of palmitic acid linked to a 16-mer peptide with sequence identical to the third intracellular loop of FPR2. F2Pal16 was found to activate FPR2, like conventional FPR2 agonists, in phagocytes and stably transfected HL-60 cells [72,73]. A shorter variant F2Pal10, with higher potency compared to F2Pal16, was shown to act as a partial agonist for direct activation of FPR2 but as a full agonist for cross-talk triggered hPFR2 reactivation generated by PAF receptor and ATP receptor (P2Y2R) [73,167]. It is noteworthy that pepducins are not always specific for their designated target receptor. For example, the pepducins P2Y2PalIC2 and P2Y2PalIC3 that contain sequences of the second and third intracellular loops of P2Y2R, were identified to be FPR2 agonists and could activate neutrophils with responses inhibited by FPR2 antagonists but not P2Y2R antagonist [168]. In a recent study, a pepducin (ATI-2341) designed for CXCR4, a chemokine receptor, was found to activate neutrophil functions through FPR2-dependent signaling [169]. The selectivity of pepducins for the FPR2 appears not entirely relying on the segment of the cognate receptor. Specific sequence and amino acid are required for the action of FPR2 pepducins. It is also notable that pepducin derived from another intracellular loop has no effect [168] and pepducin with an amino acid substitution lost all activity [73]. More efforts are needed to delineate the mechanisms of action by which pepducins recognize and modulate FPR2. Of interest, although FPR1 and FPR2 share very similar sequence in the intracellular loops, there are no FPR1 specific pepducins so far. This suggests not only the divergence in the G protein signaling triggered from cytosolic domains of FPR1 and FPR2, but also the presence of different forms (such as homo- or heterodimers) involved in the activation of these two major human FPRs. However, this speculation needs verification. It also remains a question whether the pepducins mentioned above can interact with murine Fprs.
4. Antagonists for the Formyl Peptide Receptors
As FPRs are potentially attractive drug target, molecules that inhibit cell responses induced by FPR agonists are thought to have therapeutic value for FPR-related diseases. These molecules, generally originating from natural peptides and secondary metabolites, can interact with FPRs directly or interfere with their downstream signaling pathways. In addition to drug development, radio-labeled FPR antagonists can be used as tracking probes for in vivo imaging of infiltrating neutrophils [170,171].
4.1. Natural Peptides and Analogs
The first FPR antagonists were obtained by modifying the amino termini of the classical tripeptide fMLF. Early studies have shown that the N-formyl group is important for the formyl peptides to be recognized by FPR1. Freer et al. substituted this group with tert-butyloxycarbonyl group (t-Boc) and found the resulting peptide (t-Boc-MLF, Boc-1) exhibiting FPR1 antagonistic properties [40]. The switch from an agonist to an antagonist was also observed when other carbamates (such as iso-butyloxycarbonyl and benzyloxycarbonyl group) were used to replace the formyl group at the amino terminus [172]. Further C-terminal modification of iso-butyloxycarbonyl-MLF (i-Boc-MLF) did not alter the antagonistic potency, implying the different roles of amino and carboxyl terminus in conferring the activities to these peptides. The t-butyloxycarbonyl analog of formylated peptide f-FLFLF (t-Boc-FLFLF, Boc-2) is another antagonistic peptide that potently inhibits neutrophil NADPH-oxidase activity induced by fMLF [40,173,174]. There is evidence that Boc-1 and Boc-2 begin to lose their receptor preference to FPR1 at high concentrations (>5 µM) and display inhibitory effects on FPR2 and even the complement component 5a (C5a)-induced responses [174]. Thus, despite their fairly specific activity at low concentrations, these Boc peptides may be classified as pan-antagonists for FPRs. However, other disagree because receptor overlap was seen at high concentrations of all antagonists [5]. In a recent study, optimization at the Phe residues of Boc-2 following Ala-scanning led to a more potent FPR1-selective antagonist [175]. Nevertheless, among the available formyl peptide-derived antagonists, Boc-1 and Boc-2 are still most potent and commonly used in experiments.
Chemotaxis inhibitory protein of S. aureus (CHIPS) is another native protein (14.1 kDa) with FPR antagonistic activity. The full length CHIPS (121 amino acids) was first reported to be an antagonist for both FPR1 and the C5a receptor [176]. However, the N-terminal peptides FTFEPFPTNEEIESN and FTFEPF (the first six residues) display only anti-FPR1 properties [85], suggesting a mechanism of S. aureus invasion through inhibiting fMLF-induced neutrophil chemotaxis. Accordingly, neutralizing antibodies against CHIPS may be useful for treating S. aureus infection [177] given the fact that CHIPS can bind to FPR1 with high affinity (Kd ~ 35 nM) [176]. In addition, another S. aureus-derived 105-amino acid protein FLIPr (FPR-like 1 inhibitory protein), was reported to selectively inhibit the binding of and activation by FPR2 agonists [178].
Several peptides derived from viruses were also found inhibitory to FPR activation. These peptides include a retroviral p15E-derived hexapeptide (LDLLFL) that can inhibit fMLF-induced response in monocytes and granulocytes [179], and a coronavirus 229E-derived 12-mer peptide (ETYIKPWWVWL) that was identified as a potent antagonist of FPR1 with a Ki of 230 nM [180]. Additional peptides isolated from HIV-1, HIV-2, severe acute respiratory syndrome coronavirus and Ebola virus were reported as FPR1 antagonists with lower affinities [180]. It is interesting that N-formylation of ETYIKPWWVWL transforms this 12-mer peptide into an FPR1 agonist, which is consistent with the notion that modification of size and shape at the amino terminus can change the FPR agonistic/antagonistic property of some peptides.
Among the antagonistic cyclic peptides from natural sources, cyclosporin H (CsH) is one of the most potent and specific FPR1 antagonists. CsH and another cyclic undecapeptide CsA are derived from peptide metabolites of the fungus Tolypocladium inflatum [87,144,174]. Several cyclosporins were synthesized based on CsH and SAR analysis [181]. Competition assays conducted with radionuclide binding or N-acetyl-β-d-glucosaminidase release assay demonstrated that cyclosporin H and its derivatives are potent FPR1 antagonists with nanomolar IC50 values [181,182]. These compounds were shown to be specific to FPR1 only at low concentrations. The blocking effect on FPR2 signaling is also observed with CsH at high concentrations (>2.5 μM) [174]. It was reported that CsH could attenuate mouse acute inflammation evoked by cigarette smoking [183]. Another study revealed that administration of CsH reduced opioid peptide secretion and analgesia associated with neutrophil activation during Mycobacteria butyricum infection [184]. CsH and derivatives are anticipated to be useful in the treatment of airway, gastrointestinal tract and skin inflammatory disorders associated with neutrophil and eosinophil infiltration.
An endogenous allergen uteroglobin (UG) was previously identified as an FPR2 antagonist. UG, known as Clara cell 10 kDa protein (CC10) in humans, is an anti-inflammatory and anti-chemotactic protein that is constitutively expressed in mammalian airway epithelium. Before molecular cloning of FPR, UG was found to inhibit fMLF-induced chemotaxis of monocytes and neutrophils [185]. After molecular cloning of the FPR genes, the high affinity binding of UG to FPR2 was determined with a Kd of 33 nM in HEK293 cells stably expressing FPR2 [86]. The interaction of UG with FPR2 inhibits SAA expression and SAA-driven inflammation [86,186]. The activity of UG at FPR1 or other receptors has not been examined yet, thus the selectivity of this allergen is not fully understood.
An additional natural peptide with antagonistic activity is a urokinase receptor-derived cyclic peptide. Note that the linear form of this peptide 88Ser-Arg-Ser-Arg-Tyr92 is actually a dual agonist for FPR1 and FPR2 [62]. The cyclized SRSRY was reported to inhibit the binding of fluorescence labeled fMLF to FPR1-expressing RBL-2H3 cells and it inhibits fMLF-directed monocyte migration with a picomolar IC50 [88]. However, the activity of cyclized SRSRY on FPR2 was not verified in the study. An extract of secondary metabolites from a marine Bacillus sp. was also found to have FPR antagonistic properties [187]. The precise molecule responsible for the inhibitory activity has not yet been identified. Other endogenous non-cyclized peptides with antagonistic activity include spinorphin (LVVYPWT) [188] and sarpopeptate, an aurantiamide isolated from plants and fungi [189]. Spinorphin is an FPR1 antagonist [188,190]. Sarpopeptate and its dipeptide derivatives were shown to have potent inhibitory effects on fMLF-stimulated neutrophil responses [191,192] and exhibit a receptor preference for FPR1 over FPR2 [193]. However, the receptor specificity needs further identification because of the lack of data for binding analysis.
4.2. Other Peptide Antagonists
In a ligand screen of hexapeptide libraries, several peptides were found to inhibit the binding of FPR2 agonist WKYMVm. The peptide WRWWWW (WRW4) is the most potent of these peptides with antagonistic activity against FPR2 agonists [89]. In addition, WRW4 is the first FPR3 antagonist that can completely inhibit the activation of FPR3 by F2L in FPR3-transfected HEK293 cells [194]. It inhibits F2L-induced response in mature monocyte-derived dendritic cells, which express FPR3 but not other FPRs.
Recently, a cell permeable peptide of 10 amino acids conjugated with a rhodamine group (RhoB-QRLFQVKGRR, PBP10) was shown to have potent inhibitory activity on FPR2. The peptide sequence of PBP10 is derived from PIP2-binding domains of the cytoskeletal protein gelsolin, and the rhodamine group is essential for the peptide to pass through plasma membrane and exert antagonistic activity. This rhodamine-linked decapeptide can completely inhibit ROS production mediated by FPR2 but not by FPR1 [90]. In a shorter form, RhoB-QRLFQVG maintains potent antagonistic activity for FPR2 and displays the ability to partially inhibit FPR1. Since PBP peptides bind to the intracellular domain of receptors, the difference in length may reflect divergent structural features of the cytosolic side of FPR1 and FPR2. It is notable that PBP10 is not an FPR2-specific antagonist as it also affects non-FPR2 mediated signaling [90].
4.3. Lipopeptide Antagonists
The pepducin F1Pal16, derived from a segment of the third intracellular loop of FPR1, was found to inhibit FPR2-mediated cellular response, but had no effect on FPR1 signaling [91]. The FPR2 antagonistic property of F1Pal16 was confirmed by selective inhibition in the binding of a conventional FPR2 agonist (Cy5-WKYMVM). This is not the first pepducin designed to target another GPCR but instead was found to hijack FPR2; however it is the first FPR pepducin with antagonistic activity.
Pam-(Lys-βNSpe)6-NH2, a lapidated α-peptide/β-peptoid oligomers was recently found to be a cross-species antagonist with selectivity for FPR2 and mFpr2 [92,93]. Pam-(Lys-βNSpe)6-NH2 belongs to a group of immunomodulatory host defense peptides (HDPs), however, unlike most HDPs, this synthetic HDP mimic is resistant to proteolysis. It was revealed that both the lipid and peptidomimetic portions are important for its immunomodulatory function. Pam-(Lys-βNSpe)6-NH2 can directly bind to FPR2 and prevent the binding of fluorescently labeled FPR2-specific agonist (Cy5-WKYMWM) but not the FPR1-specific agonist (FITC-fNLFNYK) [92]. The inhibitory potency of Pam-(Lys-βNSpe)6-NH2 is close to that of PBP10. Both FPR2 antagonists are membrane permeable and display a reversible inhibition pattern [92]. Despite the similarity, their action mechanisms appear divergent, because the rhodamine group in PBP10 cannot be exchanged for hexadecanoic acid in the HDP mimic peptide [90]. It was suggested that Pam-(Lys-βNSpe)6-NH2 and its structural analogs are allosteric modulators of FPR2, because they can inhibit neutrophil responses induced by the FPR2 pepducin F2Pal10, which is assumed to act in a different way from conventional FPR2 agonists by anchoring to the intracellular loop of the receptor [73]. However, like other FPR2 antagonists such as WRW4, PBP10 and FLIPr were also found to inhibit F2Pal10 at FPR2, an assumption requiring further verification.
4.4. Non-Peptide Molecules and Derivatives
As FPRs are potential drug targets, the search for novel FPR antagonists/inhibitors with high affinity and selectivity remains one of the fundamental aims of biomedical research in this field. The first reported FPR1 competitive antagonist is a non-steroidal anti-inflammatory drug (NSAID), Sulfinpyrazone and its derivative 1,2-diphenyl-4-(3-(1-naphthyl)-propyl)-3,5-pyrazolidinedione (DNP) [195,196]. However, Sulfinpyrazone is a low affinity (Ki = 14 μM) and non-specific ligand at FPR1 [197,198]. In recent years, numerous small molecule compounds have been discovered to have antagonistic/inhibitory effects using HTS of either mixture-based combinatorial libraries or natural product-based drug designs combined with SAR analysis and computational modeling. Although there are hundreds of antagonistic/inhibitory small molecules in published reports, the fully characterized chemical antagonists with high potency (Ki < 10 µM) are no more than 10 compounds after excluding those without binding analysis and/or agonistic activity assays.
The 4H-chromone compound (6-hexyl-2-methyl-3-(1-methyl-1H-benzimidazol-2-yl)-4-oxo-4H-chromen-7-yl acetate) was identified as the most potent FPR1 antagonist (Ki ~ 100 nM) among the related synthetic and natural isoflavones. This isoflavone and analogs were found to suppress agonist-stimulated calcium flux and chemotaxis of neutrophils with IC50 values of 0.02~20 μM [94]. The competitive analysis showed that they are specific for FPR1 and did not inhibit FPR2-, FPR3-, CXCR1- or murine mFpr1-dependent responses. They have been also shown to be inactive as direct agonists of FPR1/FPR2 and mFpr1 [94]. A hederagenin SMG-1 [3-O-(3,4-Odi-acetyl-α-l-arabinopyranoside)-(1 → 3)-α-l-rhamnopyranosyl-(1 → 2)-α-l-arabinopyranoside], a saponin isolated from Sapindus mukorossi, was reported to block binding of fluorescently labeled FPR1 ligand fNLFNYK (Ki < 10 µM) and inhibit FPR1-mediated response in neutrophils and FPR1-expressing HL-60 cells [199,200]. It has been verified that SMG-1 has no direct agonist effects. In another study, a lignin PP-6 [(2R,3R)-2-(3′,4′-dihydroxybenzyl)-3-(3′′,4″-dimethoxybenzyl) butyrolactone], isolated from Piper philippinum, was found to inhibit fMLF-induced neutrophil response and block FITC-fMLF binding to neutrophil with a Ki of ~1.5 μM. In the studies of a pharmacophore model of FPR1 antagonists and molecular docking, PP-6 displays impressive properties of FPR1 antagonism [200]. However, it is difficult to consider PP-6 a competitive antagonist for FPR1 based on the experimental data because the antagonistic effect of PP-6 appears non-competitive and reversible [201]. Further studies are also necessary to address the specificity of this lignin.
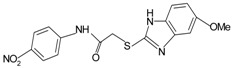
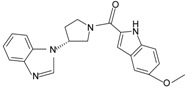
By screening combinatorial compound libraries, a pyrolidine bis-diketopiperazine-based compound 1754-31 was recently identified as the most potent non-peptide FPR2 antagonist with a Ki of 1 nM and an IC50 of 81 nM in calcium mobilization assays. The compound 1754-31 [(R)-4-(cyclohexylmethyl)-5-(4-hydroxybenzyl)-1-((R)-1-((S)-2-(((S)-6-isopropyl-2,3-dioxopiperazin-1-yl)methyl)pyrrolidin-1-yl)-3-(naphthalen-2-yl)propan-2-yl)piperazine-2,3-dione]) showed no agonistic activity at concentrations up to 12 μM [84]. A quinazolinone derivative was previously reported to suppress FPR agonist-stimulated inflammatory responses in vitro and in an in vivo model [96]. This Quin-C1 related quinazolinone, namely Quin-C7, was proven to have no agonist activity. It could displace [125I]WKYMVm binding to FPR2 with an estimated Ki of 6.7 μM. It did not inhibit the binding of [3H]fMLF to FPR1 at concentrations up to 100 µM, indicating a high preference for FPR2 over FPR1 [95]. Quin-C7 was described as an FPR2-specific antagonist, albeit its activity at other inflammation-related receptors (such as C5aR and CXCR) has not been fully examined. Additional FPR2-specific antagonists with an 2-phenylimidazo[1,2-a]pyrimidine scaffold were discovered through the use of a high-throughput HyperCyt flow cytometric platform [202,203,204,205]. They were reported to competitively bind to FPR2 with Ki values of 0.21, 1.8 and 3.2 μM. The subsequent functional analysis revealed no direct agonistic effects in one of the most potent compounds [200]. More information and analysis are provided in recent reviews [6,200].
In summary, future studies of binding properties and off-target effects are needed to determine the bona fide antagonists among compounds that have been identified to suppress FPR agonist-stimulated cell responses in functional activity assays. Moreover, most of the antagonists mentioned above have been characterized as human ligands, and their potency and specificity for the murine FPRs remain to be determined.
5. Structural Basis for Ligand Detection
Despite the lack of crystal structures, efforts in understanding FPR structure–function relationship has been propelled with approaches of molecular modification (including construction of receptor chimera and site-directed mutagenesis) and computational docking studies. Several clusters of key residues have been identified to be crucial for the interaction between FPRs and various modulators with highly divergent profiles and properties. Mutational studies have confirmed the individual functions of some of these residues in FPR–ligand interaction [96].
5.1. Binding Sites for Formyl Peptides
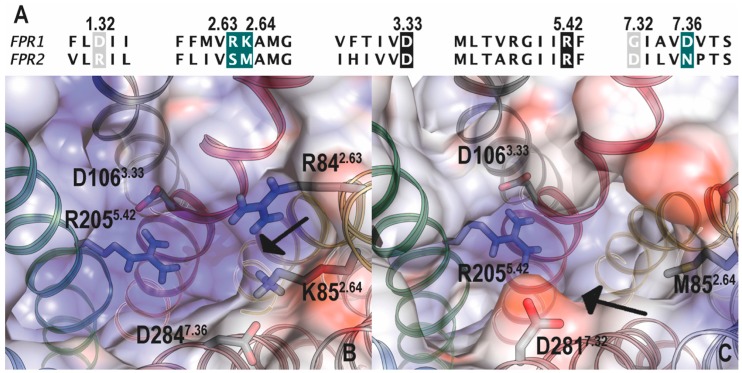
Using receptor chimeras constructed by domain swapping between FPR1/FPR2 [206] and FPR1/C5aR [207], early studies demonstrated that the first, second and third extracellular loops and adjacent membrane regions seem to be important for recognition of fMLF. This approach was also taken to study chemotaxis mediated by FPRs [208]. Following these studies, point mutations were introduced into both FPRs, resulting in the identification of multiple charged amino acids that are important for the interaction with fMLF [209,210,211,212]. These non-contiguous residues include Arg84, Lys85, Arg163, Arg205, and Asp284 in FPR1 (Figure 1). Among them, Lys85 in the second transmembrane segment (TM2) and Asp284 in TM7 were proven essential for the high-affinity binding of fMLF [212,213]. It is suggested that binding of fMLF to FPR1 disrupts the electrostatic interaction between Lys85 and Asp284, which might contribute to the receptor activation [212,214,215] (Figure 2). The lack of a ‘salt bridge’ formed by Lys/Asp in FPR2 (becoming Met and Asn in corresponding positions) is responsible for the low affinity binding of fMLF and most formyl peptide, although it cannot explain the relatively effective recognition by formyl peptides of other compositions and lengths.
Figure 2.
Comparison of FPR1 and FPR2 in selected regions. (A) Sequence alignment of FPR1 and FPR2. Residues referenced in the manuscript are boxed. Conserved side-chains between both receptors are shown in black, and major differences in amino acids are highlighted in green for N-formyl peptide binding to FPR1) or gray (for N-formyl peptide binding to FPR2). B and C, molecular electrostatic potential on the inner surface of the binding cavity of a computational model of FPR1 (B) and FPR2 (C), viewed from top of the receptors. FPR1 and FPR2 were modeled using the structure of the CXCR4 chemokine receptor as template. Arrows show key positive area in FPR1 due to Arg84 and Lys85, and negative area in FPR2 due to Asp281 sequence alignment and location of positive selection sites in FPR1, FPR2 and mFpr1 (see also Figure 1). Reproduced from [96] with permission.
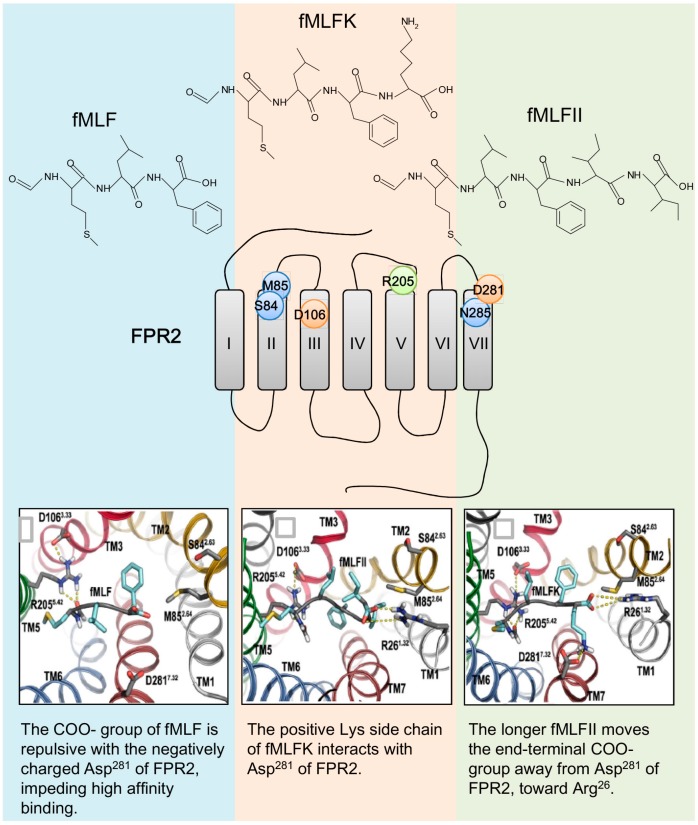
Using a similar approach of site-directed mutagenesis aided by computer modeling, another charged residue Asp281 in FPR2 (Gly280 in FPR1) was found to be crucial for the interaction of FPR2 with certain formyl peptides that contain a positively charged C-terminal residue (Figure 3), such as Lys in fMLFK [96]. In this case, the formation of ‘salt bridge’ seems still important because the D281G substitution of FPR2 alone failed to restore the potency of fMLF to the level in FPR1 [96]. Studies have also shown that longer formyl peptides with α-helical and amphipathic properties favor FPR2 over FPR1 [52,216,217]. A simple explanation is that the binding pocket of FPR2 is larger and deeper than the relatively shallow and narrow one in FPR1, thus providing an added advantage in accommodating longer peptides.
Figure 3.
CXCR4-based homology models of FPR2 (middle) in complex with three formyl peptides of different composition (top). The lower panel show the molecular interaction of FPR2 residues with the side chains of fMLF (left), fMLFK (middle) and fMLFII (right) in a computational model of FPR2. The lower panels were adapted from [96] with permission.
5.2. Binding Sites for Other FPR Ligands
To understand the structural basis for the selective activation of FPR2 by pro-resolving modulators, chimeric receptors were constructed between FPR2 and other receptors, including FPR1 and BLT1. The study using these chimeras demonstrated that TM7 and adjacent regions of FPR2 are essential for the recognition of LXA4. In contrast, the extracellular loops were required for high affinity binding for pro-inflammatory peptide agonists such as MMK1 and the MHC peptides. In addition, conserved N-glycosylation sites in FPR2 (Asn4 and Asn179) were also implicated in the interaction with these peptides but not with LXA4 [2,218]. In another study, a computational model of FPR1–annexin peptide interaction was built and it suggested that the transmembrane portions (TM2, TM5, TM6, TM7) of the binding pocket of FPR1 is important to optimal binding of the N-terminal residue in Ac-9QAWF12, the core structure of active annexin peptides [219]. Using the same set of chimeric FPR1/FPR2 receptor [206] stably expressed in HEK293 cells [208], the N-terminal region and the second extracellular loop of FPR2 were identified as required for annexin A1-mediated signaling [220].
Chimeric receptors were also constructed to dissect the mechanisms of action by which cell-penetrating lipopeptides (e.g., pepducins and PBP10) use unconventional access to modulate FPR2. It was suggested that the pepducins F2Pal16 and F2Pal10 act primarily through the third intracellular loop of FPR2. The third intracellular loops of FPR1 and FPR2 differ in only two amino acids. However, a substitution of these amino acids from FPR1 to FPR2 did not affect the selectivity of these pepducins [73]. Another study focused on PBP10, an antagonist against FPR2 agonists, suggested that neither the third intracellular loop nor the presumed signaling cytoplasmic tail of FPR2 alone is responsible for the specific inhibition of PBP10 [90]. Clearly, the current knowledge on the action mode and binding sites of these lipopeptides is still very limited. Perhaps new strategies should be exploited to understand the structure–function relationship of FPR in association with these allosteric modulators before crystal structures become available.
5.3. Docking Studies
Due to the lack of crystal structures for FPRs, homology models and molecular docking analysis based on the mutagenesis/chimera studies represent an alternative approach for explaining ligand binding and receptor activation. The current homology models of FPR receptors (including FPR1 and FPR2) are generally created by using the crystal structures of two classes of receptors: (1) the bovine rhodopsin receptor, that has a relatively low sequence identity to FPRs (~20%) and was selected as a template because of its higher resolution (2.2 Å) [221]; (2) the CXCR4-based templates, usually CXCR4 alone, which has a higher sequence identity with FPRs (28%) but has a lower resolution (3.2 Å) compared to that of rhodopsin [222]. A dual template strategy (CXCR4 and μOR opioid receptor) was developed in a recent docking study focused on FPR2 and non-peptide ligands [223].
Despite questions about the low similarity of rhodopsin receptor with FPRs, several interesting points have been made by using the homology models based on this receptor. The homology model derived from the rhodopsin template shows that the FPR1 ligand binding site comprises two channels, two cavities, and the bottom [145]. Several amino acids are found to be involved in the interaction of FPR1 with agonists, including Asn192, Thr199, Arg205, Tyr257, and Thr265 [80,145,146,212]. Docking studies with FPR1 non-peptide antagonists (including the bile acids DCA and CDCA) suggested that Thr177, Tyr257, and Thr265 are involved in the formation of three stable H-bond interactions with DCA and CDCA in the docked pose [224,225]. A pharmacophore model based on best docking poses of four FPR1 antagonists including CsH described three anchor points: two acceptors for H-bonding and one hydrophobic point [172,224,226]. In comparison with the agonists, FPR1 antagonists are characterized as containing more OH groups, which can serve as H-bond donors and/or acceptors upon binding to FPR1 [6]. However, the three-point model was further proved to be not only fitted by FPR1 antagonists but also by FPR1 agonists [147,227]. Thus, this model may be helpful in prediction for structures of FPR1 ligands without discriminating agonists and antagonists. In another study, a CXCR4-based homology model of FPR1 suggested an important role of water molecule in discrimination between FPR1 agonists and antagonists [228]. In a comparative model, the potent antagonist Boc-1 was found to interact with FPR1 by forming H-bonds with Arg84 and Lys85, similarly to the interaction between fMLF and FPR1. However, Boc-1 directly forms an H-bond with Asp284 while water molecule mediates H-bonding between the fMLF carbonyl group and Asp284 [228].
The rhodopsin-based model with five FPR2 agonists described the binding pocket of FPR2 as dumb-bell shaped, with a large cavity and a small cavity linked by a narrow channel. The proposed model comprises three sub-pockets, the pharmacophore sub-pocket I (within the small cavity) surrounded by positive residues, and the hydrophobic sub-pockets II and III (within the large cavity) surrounded by polar residues [82]. The FPR2-specific peptide agonist WKYMVM fits well with this model by occupying all three sub-pockets, with the N-terminal indole moiety located in sub-pocket I in the best docked pose [6]. In another docking simulation, residues His102, Val105, Asp106, Leu109, and Trp254 were identified as being critical for agonist binding [229]. In a more recent study, comparative docking analysis was performed on a homology model of FPR2 that used CXCR4 and μOR as a dual template [223]. The model proposed that three hydrophobic clusters are important in binding non-peptide ligands, including agonists and antagonists. The first cluster is formed by TM2 and TM3 while His102 is crucial in the interaction with docked ligands. The second cluster is located between TM6 and TM7 with Phe257 as the key residue in binding to aromatic groups of docked compounds. The third cluster is buried in the protein consisting of residues Phe74, Val105, Phe257 and Phe292 [223]. The docking model suggested that the proper orientation is required for penetration into the binding site: the peptide ligands are placed upright while non-peptide ligands prefer a more horizontal position. These findings have yet to be substantiated by mutational studies. Still, the differences in binding modes between antagonists and agonists to FPR2 are not fully understood. To date, there are no reports describing modeling of FPR3 and mouse mFprs.
6. Conclusions
The FPRs have evolved to be a class of receptors that not only recognize bacteria-derived formyl peptides but also ligands with drastically different structures, including non-formyl peptides of microbial origins, endogenous peptides and synthetic small molecules. Because of these features, the FPRs are highly ‘promiscuous’ in terms of ligand recognition. How FPRs recognize these different ligands remains unclear at present without available crystal structures. Molecular docking and computer simulation approaches combined with site-directed mutagenesis, however, have provided clues to our understanding of how formyl peptides interact with FPR1 and to a lesser extent, FPR2. Over a very long period of time, adaptive evolution has occurred in FPRs, especially FPR1, with positive selection for receptor interaction with the bona fide or orthosteric ligands. Indeed, several residues involved in H-bonding with amino acids in formyl peptides are located within the predicted binding sites undergoing positive selection, suggesting that human FPR1 is under selection pressure for its binding of formyl peptides. As for other FPR1 agonists as well as for FPR3, the picture remains unclear. There is no doubt that some agonists for the FPRs have been in existence only recently and they are likely candidates for allosteric or ago-allosteric modulators of the FPRs. With respect to FPR2, it apparently has diverged from the evolutionary path taken by FPR1, resulting in a different recognition profile including binding of longer formyl peptides and a wide range of endogenous and microbial peptides. A better understanding of the mechanisms by which FPRs interact with their ligands will help to sort out agonists and antagonists that are crucial for the biological functions of these receptors, and to speed up identification of therapeutically important molecules.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (Grant 31470856 to R.D.Y. and Grant 81401302 to H.Q.H.), the Science and Technology Development Fund of Macau (FDCT 072/2015/A2), and the University of Macau (CPG20015-00018-ICMS and SRG2015-00047-ICMS-QRCM).
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| AhR | aryl hydrocarbon receptor |
| Aβ | β-amyloid |
| BLT1 | Leukotriene B4 receptor 1 |
| CB1 | cannabinoid receptor type 1 |
| C5aR | complement component 5a receptor |
| CDCA | chenodeoxycholic acid |
| CHIPS | Chemotaxis inhibitory protein of S. aureus |
| CHO | Chinese hamster ovary |
| CsA | Cyclosporin A |
| CsH | Cyclosporin H |
| CXCR1 | C-X-C motif chemokine receptor 1 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DAMP | damage-associated molecular pattern |
| DCA | deoxycholic acid |
| EC50 | half maximal effective concentration |
| E. coli | Escherichia coli |
| FAM3D | family with sequence similarity 3 member D |
| FPR | Formyl peptide receptor |
| GPCR | G protein-coupled receptor |
| H. pylori | Helicobacter pylori |
| HTS | high throughput screening |
| i-BOC | iso-butyloxycarbonyl |
| IC50 | half maximal inhibitory concentration |
| Kd | dissociation constant |
| Ki | inhibitor constant |
| L. monocytogenes | Listeria monocytogenes |
| LXA4 | lipoxin A4 |
| μOR | μ-opioid receptor |
| oxLDL | oxidized low-density lipoprotein |
| PAMP | pathogen-associated molecular pattern |
| P2Y2R | P2Y purinoceptor 2 |
| ROS | reactive oxygen species |
| SAA | serum amyloid A |
| SAR | structure-activity relationships |
| S. aureus | Staphylococcus aureus |
| t-Boc | tert-butyloxycarbonyl |
| uPA | Urokinase plasminogen activator |
| UG | Uteroglobin |
Author Contributions
H.Q.H. and R.D.Y. initiated and designed the study, collected the literature and wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Migeotte I., Communi D., Parmentier M. Formyl peptide receptors: A promiscuous subfamily of g protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Ye R.D., Boulay F., Wang J.M., Dahlgren C., Gerard C., Parmentier M., Serhan C.N., Murphy P.M. International union of basic and clinical pharmacology. Lxxiii. Nomenclature for the formyl peptide receptor (fpr) family. Pharmacol. Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffmann E., Corcoran B.A., Wahl S.M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc. Natl. Acad. Sci. USA. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J. Exp. Med. 1982;155:264–275. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlgren C., Gabl M., Holdfeldt A., Winther M., Forsman H. Basic characteristics of the neutrophil receptors that recognize formylated peptides, a danger-associated molecular pattern generated by bacteria and mitochondria. Biochem. Pharmacol. 2016;114:22–39. doi: 10.1016/j.bcp.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Schepetkin I.A., Khlebnikov A.I., Giovannoni M.P., Kirpotina L.N., Cilibrizzi A., Quinn M.T. Development of small molecule non-peptide formyl peptide receptor (fpr) ligands and molecular modeling of their recognition. Curr. Med. Chem. 2014;21:1478–1504. doi: 10.2174/0929867321666131218095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L., Chen K., Xiang Y., Yoshimura T., Su S., Zhu J., Bian X.W., Wang J.M. New development in studies of formyl-peptide receptors: Critical roles in host defense. J. Leukoc. Biol. 2016;99:425–435. doi: 10.1189/jlb.2RI0815-354RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulay F., Tardif M., Brouchon L., Vignais P. Synthesis and use of a novel n-formyl peptide derivative to isolate a human n-formyl peptide receptor cdna. Biochem. Biophys. Res. Commun. 1990;168:1103–1109. doi: 10.1016/0006-291X(90)91143-G. [DOI] [PubMed] [Google Scholar]
- 9.Boulay F., Tardif M., Brouchon L., Vignais P. The human N-formylpeptide receptor. Characterization of two cdna isolates and evidence for a new subfamily of g-protein-coupled receptors. Biochemistry. 1990;29:11123–11133. doi: 10.1021/bi00502a016. [DOI] [PubMed] [Google Scholar]
- 10.Ye R.D., Cavanagh S.L., Quehenberger O., Prossnitz E.R., Cochrane C.G. Isolation of a cdna that encodes a novel granulocyte N-formyl peptide receptor. Biochem. Biophys. Res. Commun. 1992;184:582–589. doi: 10.1016/0006-291X(92)90629-Y. [DOI] [PubMed] [Google Scholar]
- 11.Murphy P.M., Ozcelik T., Kenney R.T., Tiffany H.L., McDermott D., Francke U. A structural homologue of the n-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. J. Biol. Chem. 1992;267:7637–7643. [PubMed] [Google Scholar]
- 12.Bao L., Gerard N.P., Eddy R.L., Jr., Shows T.B., Gerard C. Mapping of genes for the human c5a receptor (c5ar), human fmlp receptor (fpr), and two fmlp receptor homologue orphan receptors (fprh1, fprh2) to chromosome 19. Genomics. 1992;13:437–440. doi: 10.1016/0888-7543(92)90265-T. [DOI] [PubMed] [Google Scholar]
- 13.Perez H.D., Holmes R., Kelly E., McClary J., Andrews W.H. Cloning of a cdna encoding a receptor related to the formyl peptide receptor of human neutrophils. Gene. 1992;118:303–304. doi: 10.1016/0378-1119(92)90208-7. [DOI] [PubMed] [Google Scholar]
- 14.Muto Y., Guindon S., Umemura T., Kohidai L., Ueda H. Adaptive evolution of formyl peptide receptors in mammals. J. Mol. Evol. 2015;80:130–141. doi: 10.1007/s00239-015-9666-z. [DOI] [PubMed] [Google Scholar]
- 15.Cui Y., Le Y., Yazawa H., Gong W., Wang J.M. Potential role of the formyl peptide receptor-like 1 (fprl1) in inflammatory aspects of Alzheimer’s disease. J. Leukoc. Biol. 2002;72:628–635. [PubMed] [Google Scholar]
- 16.Cui Y.H., Le Y., Gong W., Proost P., Van Damme J., Murphy W.J., Wang J.M. Bacterial lipopolysaccharide selectively up-regulates the function of the chemotactic peptide receptor formyl peptide receptor 2 in murine microglial cells. J. Immunol. 2002;168:434–442. doi: 10.4049/jimmunol.168.1.434. [DOI] [PubMed] [Google Scholar]
- 17.Bennett L., Palucka A.K., Arce E., Cantrell V., Borvak J., Banchereau J., Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iribarren P., Zhou Y., Hu J., Le Y., Wang J.M. Role of formyl peptide receptor-like 1 (fprl1/fpr2) in mononuclear phagocyte responses in Alzheimer disease. Immunol. Res. 2005;31:165–176. doi: 10.1385/IR:31:3:165. [DOI] [PubMed] [Google Scholar]
- 19.Wan W., Gao J.L. Leukocyte chemoattractant receptor fpr2 may accelerate atherogenesis. Med. Hypotheses. 2012;79:101–103. doi: 10.1016/j.mehy.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prevete N., Liotti F., Marone G., Melillo R.M., de Paulis A. Formyl peptide receptors at the interface of inflammation, angiogenesis and tumor growth. Pharmacol. Res. 2015;102:184–191. doi: 10.1016/j.phrs.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Gao J.L., Lee E.J., Murphy P.M. Impaired antibacterial host defense in mice lacking the n-formylpeptide receptor. J. Exp. Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M., Chen K., Yoshimura T., Liu Y., Gong W., Wang A., Gao J.L., Murphy P.M., Wang J.M. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci. Rep. 2012;2:786. doi: 10.1038/srep00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J.L., Schneider E.H., Dimitrov E.L., Haun F., Pham T.M., Mohammed A.H., Usdin T.B., Murphy P.M. Reduced fear memory and anxiety-like behavior in mice lacking formylpeptide receptor 1. Behav. Genet. 2011;41:724–733. doi: 10.1007/s10519-011-9467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartt J.K., Barish G., Murphy P.M., Gao J.L. N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two n-formylpeptide receptor (fpr) subtypes. Molecular characterization of fpr2, a second mouse neutrophil fpr. J. Exp. Med. 1999;190:741–747. doi: 10.1084/jem.190.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Southgate E.L., He R.L., Gao J.L., Murphy P.M., Nanamori M., Ye R.D. Identification of formyl peptides from Listeria monocytogenes and Staphylococcus aureus as potent chemoattractants for mouse neutrophils. J. Immunol. 2008;181:1429–1437. doi: 10.4049/jimmunol.181.2.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He H.Q., Liao D., Wang Z.G., Wang Z.L., Zhou H.C., Wang M.W., Ye R.D. Functional characterization of three mouse formyl peptide receptors. Mol. Pharmacol. 2013;83:389–398. doi: 10.1124/mol.112.081315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang T.S., Wang J.M., Murphy P.M., Gao J.L. Serum amyloid a is a chemotactic agonist at fpr2, a low-affinity n-formylpeptide receptor on mouse neutrophils. Biochem. Biophys. Res. Commun. 2000;270:331–335. doi: 10.1006/bbrc.2000.2416. [DOI] [PubMed] [Google Scholar]
- 28.Tiffany H.L., Lavigne M.C., Cui Y.H., Wang J.M., Leto T.L., Gao J.L., Murphy P.M. Amyloid-beta induces chemotaxis and oxidant stress by acting at formylpeptide receptor 2, a g protein-coupled receptor expressed in phagocytes and brain. J. Biol. Chem. 2001;276:23645–23652. doi: 10.1074/jbc.M101031200. [DOI] [PubMed] [Google Scholar]
- 29.Gao J.L., Guillabert A., Hu J., Le Y., Urizar E., Seligman E., Fang K.J., Yuan X., Imbault V., Communi D., et al. F2l, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating fpr2, the low-affinity n-formylpeptide receptor. J. Immunol. 2007;178:1450–1456. doi: 10.4049/jimmunol.178.3.1450. [DOI] [PubMed] [Google Scholar]
- 30.Migeotte I., Riboldi E., Franssen J.D., Gregoire F., Loison C., Wittamer V., Detheux M., Robberecht P., Costagliola S., Vassart G., et al. Identification and characterization of an endogenous chemotactic ligand specific for fprl2. J. Exp. Med. 2005;201:83–93. doi: 10.1084/jem.20041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiffany H.L., Gao J.L., Roffe E., Sechler J.M., Murphy P.M. Characterization of fpr-rs8, an atypical member of the mouse formyl peptide receptor gene family. J. Innate Immun. 2011;3:519–529. doi: 10.1159/000327718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riviere S., Challet L., Fluegge D., Spehr M., Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- 33.Liberles S.D., Horowitz L.F., Kuang D., Contos J.J., Wilson K.L., Siltberg-Liberles J., Liberles D.A., Buck L.B. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc. Natl. Acad. Sci. USA. 2009;106:9842–9847. doi: 10.1073/pnas.0904464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takano T., Fiore S., Maddox J.F., Brady H.R., Petasis N.A., Serhan C.N. Aspirin-triggered 15-epi-lipoxin a4 (lxa4) and lxa4 stable analogues are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J. Exp. Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z.G., Ye R.D. Characterization of two new members of the formyl peptide receptor gene family from 129s6 mice. Gene. 2002;299:57–63. doi: 10.1016/S0378-1119(02)01012-0. [DOI] [PubMed] [Google Scholar]
- 36.McDonald B., Pittman K., Menezes G.B., Hirota S.A., Slaba I., Waterhouse C.C., Beck P.L., Muruve D.A., Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q., Raoof M., Chen Y., Sumi Y., Sursal T., Junger W., Brohi K., Itagaki K., Hauser C.J. Circulating mitochondrial damps cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honda M., Takeichi T., Hashimoto S., Yoshii D., Isono K., Hayashida S., Ohya Y., Yamamoto H., Sugawara Y., Inomata Y. Intravital imaging of neutrophil recruitment reveals the efficacy of fpr1 blockade in hepatic ischemia-reperfusion injury. J. Immunol. 2017;198:1718–1728. doi: 10.4049/jimmunol.1601773. [DOI] [PubMed] [Google Scholar]
- 39.Schiffmann E., Showell H.V., Corcoran B.A., Ward P.A., Smith E., Becker E.L. The isolation and partial characterization of neutrophil chemotactic factors from escherichia coli. J. Immunol. 1975;114:1831–1837. [PubMed] [Google Scholar]
- 40.Freer R.J., Day A.R., Radding J.A., Schiffmann E., Aswanikumar S., Showell H.J., Becker E.L. Further studies on the structural requirements for synthetic peptide chemoattractants. Biochemistry. 1980;19:2404–2410. doi: 10.1021/bi00552a019. [DOI] [PubMed] [Google Scholar]
- 41.Marasco W.A., Phan S.H., Krutzsch H., Showell H.J., Feltner D.E., Nairn R., Becker E.L., Ward P.A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J. Biol. Chem. 1984;259:5430–5439. [PubMed] [Google Scholar]
- 42.Chester J.F., Ross J.S., Malt R.A., Weitzman S.A. Acute colitis produced by chemotactic peptides in rats and mice. Am. J. Pathol. 1985;121:284–290. [PMC free article] [PubMed] [Google Scholar]
- 43.LeDuc L.E., Nast C.C. Chemotactic peptide-induced acute colitis in rabbits. Gastroenterology. 1990;98:929–935. doi: 10.1016/0016-5085(90)90017-U. [DOI] [PubMed] [Google Scholar]
- 44.Colucci M., Mastriota M., Maione F., Di Giannuario A., Mascolo N., Palmery M., Severini C., Perretti M., Pieretti S. Guinea pig ileum motility stimulation elicited by n-formyl-met-leu-phe (fmlf) involves neurotransmitters and prostanoids. Peptides. 2011;32:266–271. doi: 10.1016/j.peptides.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Nast C.C., LeDuc L.E. Chemotactic peptides. Mechanisms, functions, and possible role in inflammatory bowel disease. Dig. Dis. Sci. 1988;33:50S–57S. doi: 10.1007/BF01538131. [DOI] [PubMed] [Google Scholar]
- 46.Anton P.A., Targan S.R., Shanahan F. Increased neutrophil receptors for and response to the proinflammatory bacterial peptide formyl-methionyl-leucyl-phenylalanine in crohn’s disease. Gastroenterology. 1989;97:20–28. doi: 10.1016/0016-5085(89)91410-8. [DOI] [PubMed] [Google Scholar]
- 47.Perez H.D., Kelly E., Elfman F., Armitage G., Winkler J. Defective polymorphonuclear leukocyte formyl peptide receptor(s) in juvenile periodontitis. J. Clin. Investig. 1991;87:971–976. doi: 10.1172/JCI115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berend N., Armour C.L., Black J.L. Formyl-methionyl-leucyl-phenylalanine causes bronchoconstriction in rabbits. Agents Actions. 1986;17:466–471. doi: 10.1007/BF01965515. [DOI] [PubMed] [Google Scholar]
- 49.Peters M.J., Breslin A.B., Kemp A.S., Chu J., Berend N. Haematological effects of inhalation of n-formyl-methionyl-leucyl-phenylalanine in man. Thorax. 1992;47:284–287. doi: 10.1136/thx.47.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jonsson M., Tzanela M., Kolbeck R.C., McCormick J.R. Hemodynamic and metabolic effects of intravenous formyl-methionyl-leucyl-phenylalanine (fmlp) in rabbits. In Vivo. 1997;11:133–139. [PubMed] [Google Scholar]
- 51.Tzanela M., Orfanos S., McCormick J.R. Endotoxin augments hemodynamic and metabolic effects of formyl-methionyl-leucyl-phenylalanine (fmlp) in rabbits. In Vivo. 2007;21:81–87. [PubMed] [Google Scholar]
- 52.Forsman H., Winther M., Gabl M., Skovbakke S.L., Boulay F., Rabiet M.J., Dahlgren C. Structural changes of the ligand and of the receptor alters the receptor preference for neutrophil activating peptides starting with a formylmethionyl group. Biochim. Biophys. Acta. 2015;1853:192–200. doi: 10.1016/j.bbamcr.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Rabiet M.J., Huet E., Boulay F. Human mitochondria-derived n-formylated peptides are novel agonists equally active on fpr and fprl1, while Listeria monocytogenes-derived peptides preferentially activate fpr. Eur. J. Immunol. 2005;35:2486–2495. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 54.Seki T., Fukamizu A., Kiso Y., Mukai H. Mitocryptide-2, a neutrophil-activating cryptide, is a specific endogenous agonist for formyl-peptide receptor-like 1. Biochem. Biophys. Res. Commun. 2011;404:482–487. doi: 10.1016/j.bbrc.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Betten A., Bylund J., Christophe T., Boulay F., Romero A., Hellstrand K., Dahlgren C. A proinflammatory peptide from Helicobacter pylori activates monocytes to induce lymphocyte dysfunction and apoptosis. J. Clin. Investig. 2001;108:1221–1228. doi: 10.1172/JCI13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su S.B., Gong W., Gao J.L., Shen W., Murphy P.M., Oppenheim J.J., Wang J.M. A seven-transmembrane, g protein-coupled receptor, fprl1, mediates the chemotactic activity of serum amyloid a for human phagocytic cells. J. Exp. Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu J., Yu Y., Zhu I., Cheng Y., Sun P.D. Structural mechanism of serum amyloid a-mediated inflammatory amyloidosis. Proc. Natl. Acad. Sci. USA. 2014;111:5189–5194. doi: 10.1073/pnas.1322357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Le Y., Gong W., Tiffany H.L., Tumanov A., Nedospasov S., Shen W., Dunlop N.M., Gao J.L., Murphy P.M., Oppenheim J.J., et al. Amyloid (beta)42 activates a g-protein-coupled chemoattractant receptor, fpr-like-1. J. Neurosci. 2001;21:RC123. doi: 10.1523/JNEUROSCI.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perretti M., Chiang N., La M., Fierro I.M., Marullo S., Getting S.J., Solito E., Serhan C.N. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin a4 receptor. Nat. Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayhoe R.P., Kamal A.M., Solito E., Flower R.J., Cooper D., Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: Indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- 61.Yang D., Chen Q., Schmidt A.P., Anderson G.M., Wang J.M., Wooters J., Oppenheim J.J., Chertov O. Ll-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (fprl1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and t cells. J. Exp. Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gargiulo L., Longanesi-Cattani I., Bifulco K., Franco P., Raiola R., Campiglia P., Grieco P., Peluso G., Stoppelli M.P., Carriero M.V. Cross-talk between fmlp and vitronectin receptors triggered by urokinase receptor-derived srsry peptide. J. Biol. Chem. 2005;280:25225–25232. doi: 10.1074/jbc.M412605200. [DOI] [PubMed] [Google Scholar]
- 63.De Paulis A., Montuori N., Prevete N., Fiorentino I., Rossi F.W., Visconte V., Rossi G., Marone G., Ragno P. Urokinase induces basophil chemotaxis through a urokinase receptor epitope that is an endogenous ligand for formyl peptide receptor-like 1 and -like 2. J. Immunol. 2004;173:5739–5748. doi: 10.4049/jimmunol.173.9.5739. [DOI] [PubMed] [Google Scholar]
- 64.Le Y., Yazawa H., Gong W., Yu Z., Ferrans V.J., Murphy P.M., Wang J.M. The neurotoxic prion peptide fragment prp(106–126) is a chemotactic agonist for the g protein-coupled receptor formyl peptide receptor-like 1. J. Immunol. 2001;166:1448–1451. doi: 10.4049/jimmunol.166.3.1448. [DOI] [PubMed] [Google Scholar]
- 65.Miao Z., Premack B.A., Wei Z., Wang Y., Gerard C., Showell H., Howard M., Schall T.J., Berahovich R. Proinflammatory proteases liberate a discrete high-affinity functional fprl1 (ccr12) ligand from ccl23. J. Immunol. 2007;178:7395–7404. doi: 10.4049/jimmunol.178.11.7395. [DOI] [PubMed] [Google Scholar]
- 66.El Zein N., Badran B., Sariban E. Vip differentially activates beta2 integrins, cr1, and matrix metalloproteinase-9 in human monocytes through camp/pka, epac, and pi-3k signaling pathways via vip receptor type 1 and fprl1. J. Leukoc. Biol. 2008;83:972–981. doi: 10.1189/jlb.0507327. [DOI] [PubMed] [Google Scholar]
- 67.Seo J.K., Choi S.Y., Kim Y., Baek S.H., Kim K.T., Chae C.B., Lambeth J.D., Suh P.G., Ryu S.H. A peptide with unique receptor specificity: Stimulation of phosphoinositide hydrolysis and induction of superoxide generation in human neutrophils. J. Immunol. 1997;158:1895–1901. [PubMed] [Google Scholar]
- 68.Le Y., Gong W., Li B., Dunlop N.M., Shen W., Su S.B., Ye R.D., Wang J.M. Utilization of two seven-transmembrane, g protein-coupled receptors, formyl peptide receptor-like 1 and formyl peptide receptor, by the synthetic hexapeptide wkymvm for human phagocyte activation. J. Immunol. 1999;163:6777–6784. [PubMed] [Google Scholar]
- 69.Christophe T., Karlsson A., Dugave C., Rabiet M.J., Boulay F., Dahlgren C. The synthetic peptide trp-lys-tyr-met-val-met-nh2 specifically activates neutrophils through fprl1/lipoxin a4 receptors and is an agonist for the orphan monocyte-expressed chemoattractant receptor fprl2. J. Biol. Chem. 2001;276:21585–21593. doi: 10.1074/jbc.M007769200. [DOI] [PubMed] [Google Scholar]
- 70.Klein C., Paul J.I., Sauve K., Schmidt M.M., Arcangeli L., Ransom J., Trueheart J., Manfredi J.P., Broach J.R., Murphy A.J. Identification of surrogate agonists for the human fprl-1 receptor by autocrine selection in yeast. Nat. Biotechnol. 1998;16:1334–1337. doi: 10.1038/4310. [DOI] [PubMed] [Google Scholar]
- 71.Madenspacher J.H., Azzam K.M., Gong W., Gowdy K.M., Vitek M.P., Laskowitz D.T., Remaley A.T., Wang J.M., Fessler M.B. Apolipoproteins and apolipoprotein mimetic peptides modulate phagocyte trafficking through chemotactic activity. J. Biol. Chem. 2012;287:43730–43740. doi: 10.1074/jbc.M112.377192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee H.Y., Kim S.D., Shim J.W., Kim H.J., Kwon J.Y., Kim J.M., Baek S.H., Park J.S., Bae Y.S. Activation of human monocytes by a formyl peptide receptor 2-derived pepducin. FEBS Lett. 2010;584:4102–4108. doi: 10.1016/j.febslet.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 73.Forsman H., Bylund J., Oprea T.I., Karlsson A., Boulay F., Rabiet M.J., Dahlgren C. The leukocyte chemotactic receptor fpr2, but not the closely related fpr1, is sensitive to cell-penetrating pepducins with amino acid sequences descending from the third intracellular receptor loop. Biochim. Biophys. Acta. 2013;1833:1914–1923. doi: 10.1016/j.bbamcr.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 74.Fiore S., Maddox J.F., Perez H.D., Serhan C.N. Identification of a human cdna encoding a functional high affinity lipoxin a4 receptor. J. Exp. Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nanamori M., Cheng X., Mei J., Sang H., Xuan Y., Zhou C., Wang M.W., Ye R.D. A novel nonpeptide ligand for formyl peptide receptor-like 1. Mol. Pharmacol. 2004;66:1213–1222. doi: 10.1124/mol.104.004309. [DOI] [PubMed] [Google Scholar]
- 76.Kirpotina L.N., Khlebnikov A.I., Schepetkin I.A., Ye R.D., Rabiet M.J., Jutila M.A., Quinn M.T. Identification of novel small-molecule agonists for human formyl peptide receptors and pharmacophore models of their recognition. Mol. Pharmacol. 2010;77:159–170. doi: 10.1124/mol.109.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frohn M., Xu H., Zou X., Chang C., McElvaine M., Plant M.H., Wong M., Tagari P., Hungate R., Burli R.W. New ‘chemical probes’ to examine the role of the hfprl1 (or alxr) receptor in inflammation. Bioorg. Med. Chem. Lett. 2007;17:6633–6637. doi: 10.1016/j.bmcl.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 78.Burli R.W., Xu H., Zou X., Muller K., Golden J., Frohn M., Adlam M., Plant M.H., Wong M., McElvain M., et al. Potent hfprl1 (alxr) agonists as potential anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2006;16:3713–3718. doi: 10.1016/j.bmcl.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 79.Sogawa Y., Ohyama T., Maeda H., Hirahara K. Formyl peptide receptor 1 and 2 dual agonist inhibits human neutrophil chemotaxis by the induction of chemoattractant receptor cross-desensitization. J. Pharmacol. Sci. 2011;115:63–68. doi: 10.1254/jphs.10194FP. [DOI] [PubMed] [Google Scholar]
- 80.Crocetti L., Claudia V., Cilibrizzi A., Graziano A., Khlebnikov A.I., Kirpotina L.N., Schepetkin I.A., Quinn M.T., Giovannoni M.P. Synthesis and pharmacological evaluation of new pyridazin-based thioderivatives as formyl peptide receptor (fpr) agonists. Drug Dev. Res. 2013;74:12. doi: 10.1002/ddr.21076. [DOI] [Google Scholar]
- 81.Cilibrizzi A., Schepetkin I.A., Bartolucci G., Crocetti L., Dal Piaz V., Giovannoni M.P., Graziano A., Kirpotina L.N., Quinn M.T., Vergelli C. Synthesis, enantioresolution, and activity profile of chiral 6-methyl-2,4-disubstituted pyridazin-3(2h)-ones as potent n-formyl peptide receptor agonists. Bioorg. Med. Chem. 2012;20:3781–3792. doi: 10.1016/j.bmc.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schepetkin I.A., Kirpotina L.N., Khlebnikov A.I., Leopoldo M., Lucente E., Lacivita E., De Giorgio P., Quinn M.T. 3-(1h-indol-3-yl)-2-[3-(4-nitrophenyl)ureido]propanamide enantiomers with human formyl-peptide receptor agonist activity: Molecular modeling of chiral recognition by fpr2. Biochem. Pharmacol. 2013;85:404–416. doi: 10.1016/j.bcp.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lacivita E., Schepetkin I.A., Stama M.L., Kirpotina L.N., Colabufo N.A., Perrone R., Khlebnikov A.I., Quinn M.T., Leopoldo M. Novel 3-(1h-indol-3-yl)-2-[3-(4-methoxyphenyl)ureido]propanamides as selective agonists of human formyl-peptide receptor 2. Bioorg. Med. Chem. 2015;23:3913–3924. doi: 10.1016/j.bmc.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pinilla C., Edwards B.S., Appel J.R., Yates-Gibbins T., Giulianotti M.A., Medina-Franco J.L., Young S.M., Santos R.G., Sklar L.A., Houghten R.A. Selective agonists and antagonists of formylpeptide receptors: Duplex flow cytometry and mixture-based positional scanning libraries. Mol. Pharmacol. 2013;84:314–324. doi: 10.1124/mol.113.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haas P.J., de Haas C.J., Kleibeuker W., Poppelier M.J., van Kessel K.P., Kruijtzer J.A., Liskamp R.M., van Strijp J.A. N-terminal residues of the chemotaxis inhibitory protein of staphylococcus aureus are essential for blocking formylated peptide receptor but not c5a receptor. J. Immunol. 2004;173:5704–5711. doi: 10.4049/jimmunol.173.9.5704. [DOI] [PubMed] [Google Scholar]
- 86.Ray R., Zhang Z., Lee Y.C., Gao J.L., Mukherjee A.B. Uteroglobin suppresses allergen-induced th2 differentiation by down-regulating the expression of serum amyloid a and socs-3 genes. FEBS Lett. 2006;580:6022–6026. doi: 10.1016/j.febslet.2006.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wenzel-Seifert K., Seifert R. Cyclosporin h is a potent and selective formyl peptide receptor antagonist. Comparison with n-t-butoxycarbonyl-l-phenylalanyl-l-leucyl-l-phenylalanyl-l- leucyl-l-phenylalanine and cyclosporins a, b, c, d, and e. J. Immunol. 1993;150:4591–4599. [PubMed] [Google Scholar]
- 88.Yousif A.M., Minopoli M., Bifulco K., Ingangi V., Di Carluccio G., Merlino F., Motti M.L., Grieco P., Carriero M.V. Cyclization of the urokinase receptor-derived ser-arg-ser-arg-tyr peptide generates a potent inhibitor of trans-endothelial migration of monocytes. PLoS ONE. 2015;10:e0126172. doi: 10.1371/journal.pone.0126172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bae Y.S., Lee H.Y., Jo E.J., Kim J.I., Kang H.K., Ye R.D., Kwak J.Y., Ryu S.H. Identification of peptides that antagonize formyl peptide receptor-like 1-mediated signaling. J. Immunol. 2004;173:607–614. doi: 10.4049/jimmunol.173.1.607. [DOI] [PubMed] [Google Scholar]
- 90.Forsman H., Andreasson E., Karlsson J., Boulay F., Rabiet M.J., Dahlgren C. Structural characterization and inhibitory profile of formyl peptide receptor 2 selective peptides descending from a pip2-binding domain of gelsolin. J. Immunol. 2012;189:629–637. doi: 10.4049/jimmunol.1101616. [DOI] [PubMed] [Google Scholar]
- 91.Winther M., Gabl M., Welin A., Dahlgren C., Forsman H. A neutrophil inhibitory pepducin derived from fpr1 expected to target fpr1 signaling hijacks the closely related fpr2 instead. FEBS Lett. 2015;589:1832–1839. doi: 10.1016/j.febslet.2015.05.050. [DOI] [PubMed] [Google Scholar]
- 92.Skovbakke S.L., Heegaard P.M., Larsen C.J., Franzyk H., Forsman H., Dahlgren C. The proteolytically stable peptidomimetic pam-(lys-betanspe)6-nh2 selectively inhibits human neutrophil activation via formyl peptide receptor 2. Biochem. Pharmacol. 2015;93:182–195. doi: 10.1016/j.bcp.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Skovbakke S.L., Winther M., Gabl M., Holdfeldt A., Linden S., Wang J.M., Dahlgren C., Franzyk H., Forsman H. The peptidomimetic lau-(lys-betanspe)6-nh2 antagonizes formyl peptide receptor 2 expressed in mouse neutrophils. Biochem. Pharmacol. 2016;119:56–65. doi: 10.1016/j.bcp.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 94.Schepetkin I.A., Kirpotina L.N., Khlebnikov A.I., Cheng N., Ye R.D., Quinn M.T. Antagonism of human formyl peptide receptor 1 (fpr1) by chromones and related isoflavones. Biochem. Pharmacol. 2014;92:627–641. doi: 10.1016/j.bcp.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou C., Zhang S., Nanamori M., Zhang Y., Liu Q., Li N., Sun M., Tian J., Ye P.P., Cheng N., et al. Pharmacological characterization of a novel nonpeptide antagonist for formyl peptide receptor-like 1. Mol. Pharmacol. 2007;72:976–983. doi: 10.1124/mol.107.037564. [DOI] [PubMed] [Google Scholar]
- 96.He H.Q., Troksa E.L., Caltabiano G., Pardo L., Ye R.D. Structural determinants for the interaction of formyl peptide receptor 2 with peptide ligands. J. Biol. Chem. 2014;289:2295–2306. doi: 10.1074/jbc.M113.509216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Otto M. Community-associated mrsa: What makes them special? Int. J. Med. Microbiol. 2013;303:324–330. doi: 10.1016/j.ijmm.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kretschmer D., Gleske A.K., Rautenberg M., Wang R., Koberle M., Bohn E., Schoneberg T., Rabiet M.J., Boulay F., Klebanoff S.J., et al. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mader D., Rabiet M.J., Boulay F., Peschel A. Formyl peptide receptor-mediated proinflammatory consequences of peptide deformylase inhibition in Staphylococcus aureus. Microbes Infect. 2010;12:415–419. doi: 10.1016/j.micinf.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 100.Forsman H., Christenson K., Bylund J., Dahlgren C. Receptor-dependent and -independent immunomodulatory effects of phenol-soluble modulin peptides from Staphylococcus aureus on human neutrophils are abrogated through peptide inactivation by reactive oxygen species. Infect. Immun. 2012;80:1987–1995. doi: 10.1128/IAI.05906-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su S.B., Gao J., Gong W., Dunlop N.M., Murphy P.M., Oppenheim J.J., Wang J.M. T21/dp107, a synthetic leucine zipper-like domain of the hiv-1 envelope gp41, attracts and activates human phagocytes by using g-protein-coupled formyl peptide receptors. J. Immunol. 1999;162:5924–5930. [PubMed] [Google Scholar]
- 102.Su S.B., Gong W.H., Gao J.L., Shen W.P., Grimm M.C., Deng X., Murphy P.M., Oppenheim J.J., Wang J.M. T20/dp178, an ectodomain peptide of human immunodeficiency virus type 1 gp41, is an activator of human phagocyte n-formyl peptide receptor. Blood. 1999;93:3885–3892. [PubMed] [Google Scholar]
- 103.Deng X., Ueda H., Su S.B., Gong W., Dunlop N.M., Gao J.L., Murphy P.M., Wang J.M. A synthetic peptide derived from human immunodeficiency virus type 1 gp120 downregulates the expression and function of chemokine receptors ccr5 and cxcr4 in monocytes by activating the 7-transmembrane g-protein-coupled receptor fprl1/lxa4r. Blood. 1999;94:1165–1173. [PubMed] [Google Scholar]
- 104.Le Y., Jiang S., Hu J., Gong W., Su S., Dunlop N.M., Shen W., Li B., Wang J.M. N36, a synthetic n-terminal heptad repeat domain of the hiv-1 envelope protein gp41, is an activator of human phagocytes. Clin. Immunol. 2000;96:236–242. doi: 10.1006/clim.2000.4896. [DOI] [PubMed] [Google Scholar]
- 105.Shen W., Proost P., Li B., Gong W., Le Y., Sargeant R., Murphy P.M., Van Damme J., Wang J.M. Activation of the chemotactic peptide receptor fprl1 in monocytes phosphorylates the chemokine receptor ccr5 and attenuates cell responses to selected chemokines. Biochem. Biophys. Res. Commun. 2000;272:276–283. doi: 10.1006/bbrc.2000.2770. [DOI] [PubMed] [Google Scholar]
- 106.Hartt J.K., Liang T., Sahagun-Ruiz A., Wang J.M., Gao J.L., Murphy P.M. The hiv-1 cell entry inhibitor t-20 potently chemoattracts neutrophils by specifically activating the n-formylpeptide receptor. Biochem. Biophys. Res. Commun. 2000;272:699–704. doi: 10.1006/bbrc.2000.2846. [DOI] [PubMed] [Google Scholar]
- 107.De Paulis A., Prevete N., Fiorentino I., Walls A.F., Curto M., Petraroli A., Castaldo V., Ceppa P., Fiocca R., Marone G. Basophils infiltrate human gastric mucosa at sites of Helicobacter pylori infection, and exhibit chemotaxis in response to h. Pylori-derived peptide hp(2–20) J. Immunol. 2004;172:7734–7743. doi: 10.4049/jimmunol.172.12.7734. [DOI] [PubMed] [Google Scholar]
- 108.Park Y.J., Lee S.K., Jung Y.S., Lee M., Lee H.Y., Kim S.D., Park J.S., Koo J., Hwang J.S., Bae Y.S. Promotion of formyl peptide receptor 1-mediated neutrophil chemotactic migration by antimicrobial peptides isolated from the centipede scolopendra subspinipes mutilans. BMB Rep. 2016;49:520–525. doi: 10.5483/BMBRep.2016.49.9.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He R., Sang H., Ye R.D. Serum amyloid a induces il-8 secretion through a g protein-coupled receptor, fprl1/lxa4r. Blood. 2003;101:1572–1581. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- 110.O’Hara R., Murphy E.P., Whitehead A.S., FitzGerald O., Bresnihan B. Local expression of the serum amyloid a and formyl peptide receptor-like 1 genes in synovial tissue is associated with matrix metalloproteinase production in patients with inflammatory arthritis. Arthritis Rheum. 2004;50:1788–1799. doi: 10.1002/art.20301. [DOI] [PubMed] [Google Scholar]
- 111.Sodin-Semrl S., Spagnolo A., Mikus R., Barbaro B., Varga J., Fiore S. Opposing regulation of interleukin-8 and nf-kappab responses by lipoxin a4 and serum amyloid a via the common lipoxin a receptor. Int. J. Immunopathol. Pharmacol. 2004;17:145–156. doi: 10.1177/039463200401700206. [DOI] [PubMed] [Google Scholar]
- 112.Lee H.Y., Jo S.H., Lee C., Baek S.H., Bae Y.S. Differential production of leukotriene b4 or prostaglandin e2 by wkymvm or serum amyloid a via formyl peptide receptor-like 1. Biochem. Pharmacol. 2006;72:860–868. doi: 10.1016/j.bcp.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 113.Bozinovski S., Uddin M., Vlahos R., Thompson M., McQualter J.L., Merritt A.S., Wark P.A., Hutchinson A., Irving L.B., Levy B.D., et al. Serum amyloid a opposes lipoxin a(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bjorkman L., Karlsson J., Karlsson A., Rabiet M.J., Boulay F., Fu H., Bylund J., Dahlgren C. Serum amyloid a mediates human neutrophil production of reactive oxygen species through a receptor independent of formyl peptide receptor like-1. J. Leukoc. Biol. 2008;83:245–253. doi: 10.1189/jlb.0607-408. [DOI] [PubMed] [Google Scholar]
- 115.Christenson K., Bjorkman L., Ahlin S., Olsson M., Sjoholm K., Karlsson A., Bylund J. Endogenous acute phase serum amyloid a lacks pro-inflammatory activity, contrasting the two recombinant variants that activate human neutrophils through different receptors. Front. Immunol. 2013;4:92. doi: 10.3389/fimmu.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen M., Zhou H., Cheng N., Qian F., Ye R.D. Serum amyloid a1 isoforms display different efficacy at toll-like receptor 2 and formyl peptide receptor 2. Immunobiology. 2014;219:916–923. doi: 10.1016/j.imbio.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sano T., Huang W., Hall J.A., Yang Y., Chen A., Gavzy S.J., Lee J.Y., Ziel J.W., Miraldi E.R., Domingos A.I., et al. An il-23r/il-22 circuit regulates epithelial serum amyloid a to promote local effector th17 responses. Cell. 2015;163:381–393. doi: 10.1016/j.cell.2015.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Atarashi K., Tanoue T., Ando M., Kamada N., Nagano Y., Narushima S., Suda W., Imaoka A., Setoyama H., Nagamori T., et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell. 2015;163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Le Y., Oppenheim J.J., Wang J.M. Pleiotropic roles of formyl peptide receptors. Cytokine Growth Factor Rev. 2001;12:91–105. doi: 10.1016/S1359-6101(01)00003-X. [DOI] [PubMed] [Google Scholar]
- 120.Yazawa H., Yu Z.X., Takeda, Le Y., Gong W., Ferrans V.J., Oppenheim J.J., Li C.C., Wang J.M. Beta amyloid peptide (abeta42) is internalized via the g-protein-coupled receptor fprl1 and forms fibrillar aggregates in macrophages. FASEB J. 2001;15:2454–2462. doi: 10.1096/fj.01-0251com. [DOI] [PubMed] [Google Scholar]
- 121.Ying G., Iribarren P., Zhou Y., Gong W., Zhang N., Yu Z.X., Le Y., Cui Y., Wang J.M. Humanin, a newly identified neuroprotective factor, uses the g protein-coupled formylpeptide receptor-like-1 as a functional receptor. J. Immunol. 2004;172:7078–7085. doi: 10.4049/jimmunol.172.11.7078. [DOI] [PubMed] [Google Scholar]
- 122.Harada M., Habata Y., Hosoya M., Nishi K., Fujii R., Kobayashi M., Hinuma S. N-formylated humanin activates both formyl peptide receptor-like 1 and 2. Biochem. Biophys. Res. Commun. 2004;324:255–261. doi: 10.1016/j.bbrc.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 123.Ernst S., Lange C., Wilbers A., Goebeler V., Gerke V., Rescher U. An annexin 1 n-terminal peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J. Immunol. 2004;172:7669–7676. doi: 10.4049/jimmunol.172.12.7669. [DOI] [PubMed] [Google Scholar]
- 124.Karlsson J., Fu H., Boulay F., Dahlgren C., Hellstrand K., Movitz C. Neutrophil nadph-oxidase activation by an annexin ai peptide is transduced by the formyl peptide receptor (fpr), whereas an inhibitory signal is generated independently of the fpr family receptors. J. Leukoc. Biol. 2005;78:762–771. doi: 10.1189/jlb.0305153. [DOI] [PubMed] [Google Scholar]
- 125.Kurosaka K., Chen Q., Yarovinsky F., Oppenheim J.J., Yang D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J. Immunol. 2005;174:6257–6265. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 126.Iaccio A., Cattaneo F., Mauro M., Ammendola R. Fprl1-mediated induction of superoxide in ll-37-stimulated imr90 human fibroblast. Arch. Biochem. Biophys. 2009;481:94–100. doi: 10.1016/j.abb.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 127.Lee H.Y., Kim S.D., Shim J.W., Lee S.Y., Yun J., Bae Y.S. Ll-37 inhibits serum amyloid a-induced il-8 production in human neutrophils. Exp. Mol. Med. 2009;41:325–333. doi: 10.3858/emm.2009.41.5.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shaykhiev R., Beisswenger C., Kandler K., Senske J., Puchner A., Damm T., Behr J., Bals R. Human endogenous antibiotic ll-37 stimulates airway epithelial cell proliferation and wound closure. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L842–L848. doi: 10.1152/ajplung.00286.2004. [DOI] [PubMed] [Google Scholar]
- 129.Koczulla R., von Degenfeld G., Kupatt C., Krotz F., Zahler S., Gloe T., Issbrucker K., Unterberger P., Zaiou M., Lebherz C., et al. An angiogenic role for the human peptide antibiotic ll-37/hcap-18. J. Clin. Investig. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coffelt S.B., Marini F.C., Watson K., Zwezdaryk K.J., Dembinski J.L., LaMarca H.L., Tomchuck S.L., Honer zu Bentrup K., Danka E.S., Henkle S.L., et al. The pro-inflammatory peptide ll-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc. Natl. Acad. Sci. USA. 2009;106:3806–3811. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coffelt S.B., Tomchuck S.L., Zwezdaryk K.J., Danka E.S., Scandurro A.B. Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells. Mol. Cancer Res. 2009;7:907–915. doi: 10.1158/1541-7786.MCR-08-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li M., Chen Q., Tang R., Shen Y., Liu W.D. The expression of beta-defensin-2, 3 and ll-37 induced by candida albicans phospholipomannan in human keratinocytes. J. Dermatol. Sci. 2011;61:72–75. doi: 10.1016/j.jdermsci.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 133.Wan M., Godson C., Guiry P.J., Agerberth B., Haeggstrom J.Z. Leukotriene b4/antimicrobial peptide ll-37 proinflammatory circuits are mediated by blt1 and fpr2/alx and are counterregulated by lipoxin a4 and resolvin e1. FASEB J. 2011;25:1697–1705. doi: 10.1096/fj.10-175687. [DOI] [PubMed] [Google Scholar]
- 134.Resnati M., Pallavicini I., Wang J.M., Oppenheim J., Serhan C.N., Romano M., Blasi F. The fibrinolytic receptor for urokinase activates the g protein-coupled chemotactic receptor fprl1/lxa4r. Proc. Natl. Acad. Sci. USA. 2002;99:1359–1364. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peng X., Xu E., Liang W., Pei X., Chen D., Zheng D., Zhang Y., Zheng C., Wang P., She S., et al. Identification of fam3d as a new endogenous chemotaxis agonist for the formyl peptide receptors. J. Cell Sci. 2016;129:1831–1842. doi: 10.1242/jcs.183053. [DOI] [PubMed] [Google Scholar]
- 136.Cattaneo F., Parisi M., Ammendola R. Distinct signaling cascades elicited by different formyl peptide receptor 2 (fpr2) agonists. Int. J. Mol. Sci. 2013;14:7193–7230. doi: 10.3390/ijms14047193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kang H.K., Lee H.Y., Kim M.K., Park K.S., Park Y.M., Kwak J.Y., Bae Y.S. The synthetic peptide trp-lys-tyr-met-val-d-met inhibits human monocyte-derived dendritic cell maturation via formyl peptide receptor and formyl peptide receptor-like 2. J. Immunol. 2005;175:685–692. doi: 10.4049/jimmunol.175.2.685. [DOI] [PubMed] [Google Scholar]
- 138.Hu J.Y., Le Y., Gong W., Dunlop N.M., Gao J.L., Murphy P.M., Wang J.M. Synthetic peptide mmk-1 is a highly specific chemotactic agonist for leukocyte fprl1. J. Leukoc. Biol. 2001;70:155–161. [PubMed] [Google Scholar]
- 139.Hecht I., Rong J., Sampaio A.L., Hermesh C., Rutledge C., Shemesh R., Toporik A., Beiman M., Dassa L., Niv H., et al. A novel peptide agonist of formyl-peptide receptor-like 1 (alx) displays anti-inflammatory and cardioprotective effects. J. Pharmacol. Exp. Ther. 2009;328:426–434. doi: 10.1124/jpet.108.145821. [DOI] [PubMed] [Google Scholar]
- 140.Chen J., Bernstein H.S., Chen M., Wang L., Ishii M., Turck C.W., Coughlin S.R. Tethered ligand library for discovery of peptide agonists. J. Biol. Chem. 1995;270:23398–23401. doi: 10.1074/jbc.270.40.23398. [DOI] [PubMed] [Google Scholar]
- 141.Hu Y., Cheng N., Wu H., Kang S., Ye R.D., Cai J. Design, synthesis and characterization of fmlf-mimicking aapeptides. ChemBioChem. 2014;15:2420–2426. doi: 10.1002/cbic.201402396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.He M., Cheng N., Gao W.W., Zhang M., Zhang Y.Y., Ye R.D., Wang M.W. Characterization of quin-c1 for its anti-inflammatory property in a mouse model of bleomycin-induced lung injury. Acta Pharmacol. Sin. 2011;32:601–610. doi: 10.1038/aps.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Forsman H., Kalderen C., Nordin A., Nordling E., Jensen A.J., Dahlgren C. Stable formyl peptide receptor agonists that activate the neutrophil nadph-oxidase identified through screening of a compound library. Biochem. Pharmacol. 2011;81:402–411. doi: 10.1016/j.bcp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 144.Cevik-Aras H., Kalderen C., Jenmalm Jensen A., Oprea T., Dahlgren C., Forsman H. A non-peptide receptor inhibitor with selectivity for one of the neutrophil formyl peptide receptors, fpr 1. Biochem. Pharmacol. 2012;83:1655–1662. doi: 10.1016/j.bcp.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 145.Khlebnikov A.I., Schepetkin I.A., Kirpotina L.N., Brive L., Dahlgren C., Jutila M.A., Quinn M.T. Molecular docking of 2-(benzimidazol-2-ylthio)-n-phenylacetamide-derived small-molecule agonists of human formyl peptide receptor 1. J. Mol. Model. 2012;18:2831–2843. doi: 10.1007/s00894-011-1307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Giovannoni M.P., Schepetkin I.A., Cilibrizzi A., Crocetti L., Khlebnikov A.I., Dahlgren C., Graziano A., Dal Piaz V., Kirpotina L.N., Zerbinati S., et al. Further studies on 2-arylacetamide pyridazin-3(2H)-ones: Design, synthesis and evaluation of 4,6-disubstituted analogs as formyl peptide receptors (fprs) agonists. Eur. J. Med. Chem. 2013;64:512–528. doi: 10.1016/j.ejmech.2013.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schepetkin I.A., Kirpotina L.N., Khlebnikov A.I., Quinn M.T. High-throughput screening for small-molecule activators of neutrophils: Identification of novel n-formyl peptide receptor agonists. Mol. Pharmacol. 2007;71:1061–1074. doi: 10.1124/mol.106.033100. [DOI] [PubMed] [Google Scholar]
- 148.Schepetkin I.A., Kirpotina L.N., Khlebnikov A.I., Jutila M.A., Quinn M.T. Gastrin-releasing peptide/neuromedin b receptor antagonists pd176252, pd168368, and related analogs are potent agonists of human formyl-peptide receptors. Mol. Pharmacol. 2011;79:77–90. doi: 10.1124/mol.110.068288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Serhan C.N. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot. Essent. Fat. Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 150.Chiang N., Serhan C.N., Dahlen S.E., Drazen J.M., Hay D.W., Rovati G.E., Shimizu T., Yokomizo T., Brink C. The lipoxin receptor alx: Potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 151.O’Meara S.J., Rodgers K., Godson C. Lipoxins: Update and impact of endogenous pro-resolution lipid mediators. Rev. Physiol. Biochem. Pharmacol. 2008;160:47–70. doi: 10.1007/112_2006_0606. [DOI] [PubMed] [Google Scholar]
- 152.Serhan C.N., Brain S.D., Buckley C.D., Gilroy D.W., Haslett C., O’Neill L.A., Perretti M., Rossi A.G., Wallace J.L. Resolution of inflammation: State of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vaughn M.W., Proske R.J., Haviland D.L. Identification, cloning, and functional characterization of a murine lipoxin a4 receptor homologue gene. J. Immunol. 2002;169:3363–3369. doi: 10.4049/jimmunol.169.6.3363. [DOI] [PubMed] [Google Scholar]
- 154.Forsman H., Dahlgren C. Lipoxin a(4) metabolites/analogues from two commercial sources have no effects on tnf-alpha-mediated priming or activation through the neutrophil formyl peptide receptors. Scand. J. Immunol. 2009;70:396–402. doi: 10.1111/j.1365-3083.2009.02311.x. [DOI] [PubMed] [Google Scholar]
- 155.Forsman H., Onnheim K., Andreasson E., Dahlgren C. What formyl peptide receptors, if any, are triggered by compound 43 and lipoxin a4? Scand. J. Immunol. 2011;74:227–234. doi: 10.1111/j.1365-3083.2011.02570.x. [DOI] [PubMed] [Google Scholar]
- 156.Hanson J., Ferreiros N., Pirotte B., Geisslinger G., Offermanns S. Heterologously expressed formyl peptide receptor 2 (fpr2/alx) does not respond to lipoxin a(4) Biochem. Pharmacol. 2013;85:1795–1802. doi: 10.1016/j.bcp.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 157.Pamplona F.A., Ferreira J., Menezes de Lima O., Jr., Duarte F.S., Bento A.F., Forner S., Villarinho J.G., Bellocchio L., Wotjak C.T., Lerner R., et al. Anti-inflammatory lipoxin a4 is an endogenous allosteric enhancer of cb1 cannabinoid receptor. Proc. Natl. Acad. Sci. USA. 2012;109:21134–21139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schaldach C.M., Riby J., Bjeldanes L.F. Lipoxin a4: A new class of ligand for the ah receptor. Biochemistry. 1999;38:7594–7600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- 159.Russell R., Gori I., Pellegrini C., Kumar R., Achtari C., Canny G.O. Lipoxin a4 is a novel estrogen receptor modulator. FASEB J. 2011;25:4326–4337. doi: 10.1096/fj.11-187658. [DOI] [PubMed] [Google Scholar]
- 160.Krishnamoorthy S., Recchiuti A., Chiang N., Yacoubian S., Lee C.H., Yang R., Petasis N.A., Serhan C.N. Resolvin d1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Odusanwo O., Chinthamani S., McCall A., Duffey M.E., Baker O.J. Resolvin d1 prevents tnf-alpha-mediated disruption of salivary epithelial formation. Am. J. Physiol. Cell Physiol. 2012;302:C1331–C1345. doi: 10.1152/ajpcell.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Eickmeier O., Seki H., Haworth O., Hilberath J.N., Gao F., Uddin M., Croze R.H., Carlo T., Pfeffer M.A., Levy B.D. Aspirin-triggered resolvin d1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013;6:256–266. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Claria J., Dalli J., Yacoubian S., Gao F., Serhan C.N. Resolvin d1 and resolvin d2 govern local inflammatory tone in obese fat. J. Immunol. 2012;189:2597–2605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee H.Y., Oh E., Kim S.D., Seo J.K., Bae Y.S. Oxidized low-density lipoprotein-induced foam cell formation is mediated by formyl peptide receptor 2. Biochem. Biophys. Res. Commun. 2014;443:1003–1007. doi: 10.1016/j.bbrc.2013.12.082. [DOI] [PubMed] [Google Scholar]
- 165.Miller J., Agarwal A., Devi L.A., Fontanini K., Hamilton J.A., Pin J.P., Shields D.C., Spek C.A., Sakmar T.P., Kuliopulos A., et al. Insider access: Pepducin symposium explores a new approach to gpcr modulation. Ann. N. Y. Acad. Sci. 2009;1180(Suppl. S1):E1–E12. doi: 10.1111/j.1749-6632.2009.05326.x. [DOI] [PubMed] [Google Scholar]
- 166.O’Callaghan K., Kuliopulos A., Covic L. Turning receptors on and off with intracellular pepducins: New insights into g-protein-coupled receptor drug development. J. Biol. Chem. 2012;287:12787–12796. doi: 10.1074/jbc.R112.355461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gabl M., Winther M., Skovbakke S.L., Bylund J., Dahlgren C., Forsman H. A pepducin derived from the third intracellular loop of fpr2 is a partial agonist for direct activation of this receptor in neutrophils but a full agonist for cross-talk triggered reactivation of fpr2. PLoS ONE. 2014;9:e109516. doi: 10.1371/journal.pone.0109516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gabl M., Holdfeldt A., Winther M., Oprea T., Bylund J., Dahlgren C., Forsman H. A pepducin designed to modulate p2y2r function interacts with fpr2 in human neutrophils and transfers atp to an nadph-oxidase-activating ligand through a receptor cross-talk mechanism. Biochim. Biophys. Acta. 2016;1863:1228–1237. doi: 10.1016/j.bbamcr.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 169.Holdfeldt A., Winther M., Gabl M., Dahlgren C., Forsman H. Data on human neutrophil activation induced by pepducins with amino acid sequences derived from β2ar and cxcr4. Data Brief. 2016;8:411–414. doi: 10.1016/j.dib.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Babich J.W., Tompkins R.G., Graham W., Barrow S.A., Fischman A.J. Localization of radiolabeled chemotactic peptide at focal sites of escherichia coli infection in rabbits: Evidence for a receptor-specific mechanism. J. Nucl. Med. 1997;38:1316–1322. [PubMed] [Google Scholar]
- 171.Locke L.W., Chordia M.D., Zhang Y., Kundu B., Kennedy D., Landseadel J., Xiao L., Fairchild K.D., Berr S.S., Linden J., et al. A novel neutrophil-specific pet imaging agent: Cflflfk-peg-64cu. J. Nucl. Med. 2009;50:790–797. doi: 10.2967/jnumed.108.056127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Derian C.K., Solomon H.F., Higgins J.D., 3rd, Beblavy M.J., Santulli R.J., Bridger G.J., Pike M.C., Kroon D.J., Fischman A.J. Selective inhibition of n-formylpeptide-induced neutrophil activation by carbamate-modified peptide analogues. Biochemistry. 1996;35:1265–1269. doi: 10.1021/bi952087k. [DOI] [PubMed] [Google Scholar]
- 173.Aswanikumar S., Schiffmann E., Corcoran B.A., Pert C.B., Morell J.L., Gross E. Antibiotics and peptides with agonist and antagonist chemotactic activity. Biochem. Biophys. Res. Commun. 1978;80:464–471. doi: 10.1016/0006-291X(78)90700-3. [DOI] [PubMed] [Google Scholar]
- 174.Stenfeldt A.L., Karlsson J., Wenneras C., Bylund J., Fu H., Dahlgren C. Cyclosporin h, boc-mlf and boc-flflf are antagonists that preferentially inhibit activity triggered through the formyl peptide receptor. Inflammation. 2007;30:224–229. doi: 10.1007/s10753-007-9040-4. [DOI] [PubMed] [Google Scholar]
- 175.Hayashi R., Kitajima T., Mizuguchi H., Fujimoto M., Yamaguchi A., Koga S., Koga Y., Osada S., Kodama H. Development of potent antagonists for formyl peptide receptor 1 based on boc-phe-d-leu-phe-d-leu-phe-oh. Bioorg. Med. Chem. 2014;22:3824–3828. doi: 10.1016/j.bmc.2014.06.048. [DOI] [PubMed] [Google Scholar]
- 176.Postma B., Poppelier M.J., van Galen J.C., Prossnitz E.R., van Strijp J.A., de Haas C.J., van Kessel K.P. Chemotaxis inhibitory protein of Staphylococcus aureus binds specifically to the c5a and formylated peptide receptor. J. Immunol. 2004;172:6994–7001. doi: 10.4049/jimmunol.172.11.6994. [DOI] [PubMed] [Google Scholar]
- 177.Wright A.J., Higginbottom A., Philippe D., Upadhyay A., Bagby S., Read R.C., Monk P.N., Partridge L.J. Characterisation of receptor binding by the chemotaxis inhibitory protein of Staphylococcus aureus and the effects of the host immune response. Mol. Immunol. 2007;44:2507–2517. doi: 10.1016/j.molimm.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Prat C., Bestebroer J., de Haas C.J., van Strijp J.A., van Kessel K.P. A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J. Immunol. 2006;177:8017–8026. doi: 10.4049/jimmunol.177.11.8017. [DOI] [PubMed] [Google Scholar]
- 179.Oostendorp R.A., Knol E.F., Verhoeven A.J., Scheper R.J. An immunosuppressive retrovirus-derived hexapeptide interferes with intracellular signaling in monocytes and granulocytes through n-formylpeptide receptors. J. Immunol. 1992;149:1010–1015. [PubMed] [Google Scholar]
- 180.Mills J.S. Peptides derived from HIV-1, HIV-2, Ebola virus, SARS coronavirus and coronavirus 229E exhibit high affinity binding to the formyl peptide receptor. Biochim Biophys Acta. 2006;1762:693–703. doi: 10.1016/j.bbadis.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Loor F., Tiberghien F., Wenandy T., Didier A., Traber R. Cyclosporins: Structure-activity relationships for the inhibition of the human fpr1 formylpeptide receptor. J. Med. Chem. 2002;45:4613–4628. doi: 10.1021/jm010987v. [DOI] [PubMed] [Google Scholar]
- 182.Yan P., Nanamori M., Sun M., Zhou C., Cheng N., Li N., Zheng W., Xiao L., Xie X., Ye R.D., et al. The immunosuppressant cyclosporin a antagonizes human formyl peptide receptor through inhibition of cognate ligand binding. J. Immunol. 2006;177:7050–7058. doi: 10.4049/jimmunol.177.10.7050. [DOI] [PubMed] [Google Scholar]
- 183.Cardini S., Dalli J., Fineschi S., Perretti M., Lungarella G., Lucattelli M. Genetic ablation of the fpr1 gene confers protection from smoking-induced lung emphysema in mice. Am. J. Respir. Cell Mol. Biol. 2012;47:332–339. doi: 10.1165/rcmb.2012-0036OC. [DOI] [PubMed] [Google Scholar]
- 184.Rittner H.L., Hackel D., Voigt P., Mousa S., Stolz A., Labuz D., Schafer M., Schaefer M., Stein C., Brack A. Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils. PLoS Pathog. 2009;5:e1000362. doi: 10.1371/journal.ppat.1000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vasanthakumar G., Manjunath R., Mukherjee A.B., Warabi H., Schiffmann E. Inhibition of phagocyte chemotaxis by uteroglobin, an inhibitor of blastocyst rejection. Biochem. Pharmacol. 1988;37:389–394. doi: 10.1016/0006-2952(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 186.Antico G., Aloman M., Lakota K., Miele L., Fiore S., Sodin-Semrl S. Uteroglobin, a possible ligand of the lipoxin receptor inhibits serum amyloid a-driven inflammation. Mediators Inflamm. 2014;2014:876395. doi: 10.1155/2014/876395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang S.C., Lin C.F., Chang W.Y., Kuo J., Huang Y.T., Chung P.J., Hwang T.L. Bioactive secondary metabolites of a marine bacillus sp. Inhibit superoxide generation and elastase release in human neutrophils by blocking formyl peptide receptor 1. Molecules. 2013;18:6455–6468. doi: 10.3390/molecules18066455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liang T.S., Gao J.L., Fatemi O., Lavigne M., Leto T.L., Murphy P.M. The endogenous opioid spinorphin blocks fmet-leu-phe-induced neutrophil chemotaxis by acting as a specific antagonist at the n-formylpeptide receptor subtype fpr. J. Immunol. 2001;167:6609–6614. doi: 10.4049/jimmunol.167.11.6609. [DOI] [PubMed] [Google Scholar]
- 189.Nwodo N.J., Okoye F.B., Lai D., Debbab A., Brun R., Proksch P. Two trypanocidal dipeptides from the roots of zapoteca portoricensis (fabaceae) Molecules. 2014;19:5470–5477. doi: 10.3390/molecules19055470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yamamoto Y., Kanazawa T., Shimamura M., Ueki M., Hazato T. Inhibitory effects of spinorphin, a novel endogenous regulator, on chemotaxis, o2- generation, and exocytosis by n-formylmethionyl-leucyl-phenylalanine (fmlp)-stimulated neutrophils. Biochem. Pharmacol. 1997;54:695–701. doi: 10.1016/S0006-2952(97)00221-9. [DOI] [PubMed] [Google Scholar]
- 191.Tsai P.L., Wang J.P., Chang C.W., Kuo S.C., Chao P.D. Constituents and bioactive principles of polygonum chinensis. Phytochemistry. 1998;49:1663–1666. doi: 10.1016/S0031-9422(98)00322-7. [DOI] [PubMed] [Google Scholar]
- 192.Yen C.T., Hwang T.L., Wu Y.C., Hsieh P.W. Design and synthesis of new n-(fluorenyl-9-methoxycarbonyl) (fmoc)-dipeptides as anti-inflammatory agents. Eur. J. Med. Chem. 2009;44:1933–1940. doi: 10.1016/j.ejmech.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 193.Hwang T.L., Hung C.H., Hsu C.Y., Huang Y.T., Tsai Y.C., Hsieh P.W. Design and synthesis of tryptophan containing dipeptide derivatives as formyl peptide receptor 1 antagonist. Org. Biomol. Chem. 2013;11:3742–3755. doi: 10.1039/c3ob40215k. [DOI] [PubMed] [Google Scholar]
- 194.Shin E.H., Lee H.Y., Kim S.D., Jo S.H., Kim M.K., Park K.S., Lee H., Bae Y.S. Trp-arg-trp-trp-trp-trp antagonizes formyl peptide receptor like 2-mediated signaling. Biochem. Biophys. Res. Commun. 2006;341:1317–1322. doi: 10.1016/j.bbrc.2006.01.098. [DOI] [PubMed] [Google Scholar]
- 195.Dahinden C., Fehr J. Receptor-directed inhibition of chemotactic factor-induced neutrophil hyperactivity by pyrazolon derivatives. Definition of a chemotactic peptide antagonist. J. Clin. Investig. 1980;66:884–891. doi: 10.1172/JCI109955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Levesque L., Gaudreault R.C., Marceau F. The interaction of 3,5-pyrazolidinedione drugs with receptors for f-met-leu-phe on human neutrophil leukocytes: A study of the structure-activity relationship. Can. J. Physiol. Pharmacol. 1991;69:419–425. doi: 10.1139/y91-064. [DOI] [PubMed] [Google Scholar]
- 197.Del Maschio A., Livio M., Cerletti C., De Gaetano G. Inhibition of human platelet cyclo-oxygenase activity by sulfinpyrazone and three of its metabolites. Eur. J. Pharmacol. 1984;101:209–214. doi: 10.1016/0014-2999(84)90158-4. [DOI] [PubMed] [Google Scholar]
- 198.Young S.M., Bologa C., Prossnitz E.R., Oprea T.I., Sklar L.A., Edwards B.S. High-throughput screening with hypercyt flow cytometry to detect small molecule formylpeptide receptor ligands. J. Biomol. Screen. 2005;10:374–382. doi: 10.1177/1087057105274532. [DOI] [PubMed] [Google Scholar]
- 199.Hwang T.L., Wang C.C., Kuo Y.H., Huang H.C., Wu Y.C., Kuo L.M., Wu Y.H. The hederagenin saponin smg-1 is a natural fmlp receptor inhibitor that suppresses human neutrophil activation. Biochem. Pharmacol. 2010;80:1190–1200. doi: 10.1016/j.bcp.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 200.Schepetkin I.A., Khlebnikov A.I., Kirpotina L.N., Quinn M.T. Antagonism of human formyl peptide receptor 1 with natural compounds and their synthetic derivatives. Int. Immunopharmacol. 2016;37:43–58. doi: 10.1016/j.intimp.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang Y.J., Chen I.S., Tseng C.P., Day Y.J., Lin Y.C., Liao C.H. (2r,3r)-2-(3′,4′-dihydroxybenzyl)-3-(3′′,4′′-dimethoxybenzyl)butyrolactone suppresses fmlp-induced superoxide production by inhibiting fmlp-receptor binding in human neutrophils. Biochem. Pharmacol. 2008;75:688–697. doi: 10.1016/j.bcp.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 202.Edwards B.S., Young S.M., Ivnitsky-Steele I., Ye R.D., Prossnitz E.R., Sklar L.A. High-content screening: Flow cytometry analysis. Methods Mol. Biol. 2009;486:151–165. doi: 10.1007/978-1-60327-545-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Edwards B.S., Young S.M., Oprea T.I., Bologa C.G., Prossnitz E.R., Sklar L.A. Biomolecular screening of formylpeptide receptor ligands with a sensitive, quantitative, high-throughput flow cytometry platform. Nat. Protoc. 2006;1:59–66. doi: 10.1038/nprot.2006.9. [DOI] [PubMed] [Google Scholar]
- 204.Strouse J.J., Young S.M., Mitchell H.D., Ye R.D., Prossnitz E.R., Sklar L.A., Edwards B.S. A novel fluorescent cross-reactive formylpeptide receptor/formylpeptide receptor-like 1 hexapeptide ligand. Cytom. A. 2009;75:264–270. doi: 10.1002/cyto.a.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Young S.M., Bologa C.M., Fara D., Bryant B.K., Strouse J.J., Arterburn J.B., Ye R.D., Oprea T.I., Prossnitz E.R., Sklar L.A., et al. Duplex high-throughput flow cytometry screen identifies two novel formylpeptide receptor family probes. Cytom. A. 2009;75:253–263. doi: 10.1002/cyto.a.20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Quehenberger O., Prossnitz E.R., Cavanagh S.L., Cochrane C.G., Ye R.D. Multiple domains of the n-formyl peptide receptor are required for high-affinity ligand binding. Construction and analysis of chimeric n-formyl peptide receptors. J. Biol. Chem. 1993;268:18167–18175. [PubMed] [Google Scholar]
- 207.Gao J.L., Murphy P.M. Species and subtype variants of the n-formyl peptide chemotactic receptor reveal multiple important functional domains. J. Biol. Chem. 1993;268:25395–25401. [PubMed] [Google Scholar]
- 208.Le Y., Ye R.D., Gong W., Li J., Iribarren P., Wang J.M. Identification of functional domains in the formyl peptide receptor-like 1 for agonist-induced cell chemotaxis. FEBS J. 2005;272:769–778. doi: 10.1111/j.1742-4658.2004.04514.x. [DOI] [PubMed] [Google Scholar]
- 209.Miettinen H.M., Mills J.S., Gripentrog J.M., Dratz E.A., Granger B.L., Jesaitis A.J. The ligand binding site of the formyl peptide receptor maps in the transmembrane region. J. Immunol. 1997;159:4045–4054. [PubMed] [Google Scholar]
- 210.Quehenberger O., Pan Z.K., Prossnitz E.R., Cavanagh S.L., Cochrane C.G., Ye R.D. Identification of an n-formyl peptide receptor ligand binding domain by a gain-of-function approach. Biochem. Biophys. Res. Commun. 1997;238:377–381. doi: 10.1006/bbrc.1997.7298. [DOI] [PubMed] [Google Scholar]
- 211.Lala A., Gwinn M., De Nardin E. Human formyl peptide receptor function role of conserved and nonconserved charged residues. Eur. J. Biochem. 1999;264:495–499. doi: 10.1046/j.1432-1327.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- 212.Mills J.S., Miettinen H.M., Cummings D., Jesaitis A.J. Characterization of the binding site on the formyl peptide receptor using three receptor mutants and analogs of met-leu-phe and met-met-trp-leu-leu. J. Biol. Chem. 2000;275:39012–39017. doi: 10.1074/jbc.M003081200. [DOI] [PubMed] [Google Scholar]
- 213.Mills J.S., Miettinen H.M., Barnidge D., Vlases M.J., Wimer-Mackin S., Dratz E.A., Sunner J., Jesaitis A.J. Identification of a ligand binding site in the human neutrophil formyl peptide receptor using a site-specific fluorescent photoaffinity label and mass spectrometry. J. Biol. Chem. 1998;273:10428–10435. doi: 10.1074/jbc.273.17.10428. [DOI] [PubMed] [Google Scholar]
- 214.Bokoch M.P., Zou Y., Rasmussen S.G., Liu C.W., Nygaard R., Rosenbaum D.M., Fung J.J., Choi H.J., Thian F.S., Kobilka T.S., et al. Ligand-specific regulation of the extracellular surface of a g-protein-coupled receptor. Nature. 2010;463:108–112. doi: 10.1038/nature08650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jongejan A., Bruysters M., Ballesteros J.A., Haaksma E., Bakker R.A., Pardo L., Leurs R. Linking agonist binding to histamine h1 receptor activation. Nat. Chem. Biol. 2005;1:98–103. doi: 10.1038/nchembio714. [DOI] [PubMed] [Google Scholar]
- 216.Cheung G.Y., Kretschmer D., Queck S.Y., Joo H.S., Wang R., Duong A.C., Nguyen T.H., Bach T.H., Porter A.R., DeLeo F.R., et al. Insight into structure-function relationship in phenol-soluble modulins using an alanine screen of the phenol-soluble modulin (psm) alpha3 peptide. FASEB J. 2014;28:153–161. doi: 10.1096/fj.13-232041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kretschmer D., Rautenberg M., Linke D., Peschel A. Peptide length and folding state govern the capacity of staphylococcal beta-type phenol-soluble modulins to activate human formyl-peptide receptors 1 or 2. J. Leukoc. Biol. 2015;97:689–697. doi: 10.1189/jlb.2A0514-275R. [DOI] [PubMed] [Google Scholar]
- 218.Chiang N., Fierro I.M., Gronert K., Serhan C.N. Activation of lipoxin a(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J. Exp. Med. 2000;191:1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Movitz C., Brive L., Hellstrand K., Rabiet M.J., Dahlgren C. The annexin i sequence gln(9)-ala(10)-trp(11)-phe(12) is a core structure for interaction with the formyl peptide receptor 1. J. Biol. Chem. 2010;285:14338–14345. doi: 10.1074/jbc.M109.080465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bena S., Brancaleone V., Wang J.M., Perretti M., Flower R.J. Annexin a1 interaction with the fpr2/alx receptor: Identification of distinct domains and downstream associated signaling. J. Biol. Chem. 2012;287:24690–24697. doi: 10.1074/jbc.M112.377101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Palczewski K., Kumasaka T., Hori T., Behnke C.A., Motoshima H., Fox B.A., Le Trong I., Teller D.C., Okada T., Stenkamp R.E., et al. Crystal structure of rhodopsin: A g protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 222.Wu B., Chien E.Y., Mol C.D., Fenalti G., Liu W., Katritch V., Abagyan R., Brooun A., Wells P., Bi F.C., et al. Structures of the cxcr4 chemokine gpcr with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Stepniewski T.M., Filipek S. Non-peptide ligand binding to the formyl peptide receptor fpr2—A comparison to peptide ligand binding modes. Bioorg. Med. Chem. 2015;23:4072–4081. doi: 10.1016/j.bmc.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 224.Edwards B.S., Bologa C., Young S.M., Balakin K.V., Prossnitz E.R., Savchuck N.P., Sklar L.A., Oprea T.I. Integration of virtual screening with high-throughput flow cytometry to identify novel small molecule formylpeptide receptor antagonists. Mol. Pharmacol. 2005;68:1301–1310. doi: 10.1124/mol.105.014068. [DOI] [PubMed] [Google Scholar]
- 225.Ferrari C., Macchiarulo A., Costantino G., Pellicciari R. Pharmacophore model for bile acids recognition by the fpr receptor. J. Comput. Aided Mol. Des. 2006;20:295–303. doi: 10.1007/s10822-006-9055-1. [DOI] [PubMed] [Google Scholar]
- 226.Dalpiaz A., Ferretti M.E., Vertuani G., Traniello S., Scatturin A., Spisani S. C- and n-terminal residue effect on peptide derivatives’ antagonism toward the formyl-peptide receptor. Eur. J. Pharmacol. 2002;436:187–196. doi: 10.1016/S0014-2999(01)01627-2. [DOI] [PubMed] [Google Scholar]
- 227.Schepetkin I.A., Kirpotina L.N., Tian J., Khlebnikov A.I., Ye R.D., Quinn M.T. Identification of novel formyl peptide receptor-like 1 agonists that induce macrophage tumor necrosis factor alpha production. Mol. Pharmacol. 2008;74:392–402. doi: 10.1124/mol.108.046946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yuan S., Ghoshdastider U., Trzaskowski B., Latek D., Debinski A., Pulawski W., Wu R., Gerke V., Filipek S. The role of water in activation mechanism of human n-formyl peptide receptor 1 (fpr1) based on molecular dynamics simulations. PLoS ONE. 2012;7:e47114. doi: 10.1371/journal.pone.0047114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fujita H., Kato T., Watanabe N., Takahashi T., Kitagawa S. Stimulation of human formyl peptide receptors by calpain inhibitors: Homology modeling of receptors and ligand docking simulation. Arch. Biochem. Biophys. 2011;516:121–127. doi: 10.1016/j.abb.2011.09.017. [DOI] [PubMed] [Google Scholar]