Abstract
In recent years, the increased popularity of functional beverages such as herbal teas and decoctions has led to the search for new sources of raw materials that provide appropriate taste and functionality to consumers. The objective of this study was to investigate the nutritional, phytochemical profiles and bioactivities of possible functional beverages produced from F. ulmaria and its alternative substitutes (F. camtschatica, F. denudata, F. stepposa). The investigated decoctions were analyzed regarding their macronutrient, carbohydrate, organic acid, amino acid and mineral composition. Quantification of the main phenolic compounds in the decoctions of meadowsweet floral teas was performed by a microcolumn RP-HPLC-UV procedure; the highest content was revealed in F. stepposa tea. The investigation of the essential oil of four meadowsweet teas revealed the presence of 28 compounds, including simple phenols, monoterpenes, sesquiterpenes and aliphatic components. The dominance of methyl salicylate and salicylaldehyde was noted in all samples. Studies on the water soluble polysaccharides of Filipendula flowers allowed us to establish their general affiliation to galactans and/or arabinogalactans with an admixture of glucans of the starch type and galacturonans as minor components. The bioactivity data demonstrated a good ability of meadowsweet teas to inhibit amylase, α-glucosidase and AGE formation. Tea samples showed antioxidant properties by the DPPH•, ABTS•+ and Br• free radicals scavenging assays and the carotene bleaching assay, caused by the presence of highly active ellagitannins. The anti-complement activity of the water-soluble polysaccharide fraction of meadowsweet teas indicated their possible immune-modulating properties. Filipendula beverage formulations can be expected to deliver beneficial effects due to their unique nutritional and phytochemical profiles. Potential applications as health-promoting functional products may be suggested.
Keywords: Filipendula camschatica, Filipendula denudata, Filipendula stepposa, Filipendula ulmaria, tannins, flavonoids, polysaccharides, essential oils, anti-diabetic activity, antioxidant activity, anti-complement activity
1. Introduction
The food and beverage industry is growing solidly due to the increased demand of consumers. The business opportunity has attracted the herbal industry to penetrate the herbal-based food product market, and herbal-based products are becoming a widespread production trend among manufacturers [1]. From a global perspective and particularly with regards to products sold with a specific medical claim, Europe and Asia have been leading the way in terms of supplying herbal medicinal products [2]. Herbal teas are one type of herbal medicinal product. The term generally refers to any functional beverage made from an infusion or decoction of herbs, spices, or other plant materials in hot water. These beverages usually do not contain caffeine. The consumption of functional beverages has grown in popularity, primarily due to metabolism, immunity and oxidant status improving claims [3,4,5,6].
One of the most popular plant genera for herbal tea preparation is Filipendula [7]. This genus includes perennial herbaceous plants of the family Rosaceae that number about 30 species, commonly known as meadowsweets. These herbs are used due to the specific honey-like fragrance of the flowers and the pleasant taste of water decoctions [8]. Scientifically, the most studied species are F. ulmaria (L.) Maxim. and F. vulgaris Moench, which are officinal plant species in many countries. Researchers have found in these species derivatives of salicylic acid [9,10], 2-pyrone-4,6-dicarboxylic acid [11,12], flavonoids [13,14], tannins [15,16] and essential oils [17,18].
The growing demand for use of an officinal species, coupled with the simultaneous shortage of raw materials due to its low adaptability, agricultural peculiarities and unlimited gathering as a medicinal plant, necessitates scouting studies for the discovery of vicarious and chemically similar substitutes within the genus Filipendula [19,20]. Species F. denudata and F. stepposa relate to the same Ulmaria subgenus as F. ulmaria and species F. camtschatica belongs to the close subgenus Aceraria; accordingly, these plant species may be potential substitutes for the officinal species F. ulmaria. Until recently, the chemical composition of these species was not known. The presence of several compounds has been previously demonstrated for the genus Filipendula, such as dimeric and monomeric ellagitannins, in addition to flavonol-4′-O-glycosides [21]. Moreover, it is known that these plant species have a positive effect in diabetic diseases, illnesses of the immune system and may have possible antioxidant effects [22,23,24]. None of these aspects has been previously studied for the species F. camschatica, F. stepposa and F. denudata.
In this regard, the objective of this study was to comparatively investigate the nutritional composition (macronutrients, carbohydrates, organic acids, amino acids and minerals), and phytochemical profiles (phenolics, essential oils, polysaccharides) and biological effects of four meadowsweet teas (F. camschatica, F. denudata, F. stepposa and F. ulmaria).
2. Results and Discussion
2.1. Nutritional Profiles of Meadowsweet Teas
The results of the nutritional characterization of the meadowsweet floral teas, including organoleptic parameters (color, odor and taste) and the macronutrient composition are presented in Table 1. Teas extractives ranged from 310 mg/100 mL (F. camschatica) to 394 mg/100 mL (F. stepposa), demonstrating the good extractibility of Filipendula tea components in boiling water. The color of the four meadowsweet tea was typical for yellow floral teas, the odor was characterized as specific, methyl salicylate-like, and the taste was characterized as astringent, with bitterness of variable intensity. In general, these organoleptic characteristics allow us to describe these meadowsweet floral teas as specific products with atypical but pleasant tastes and odours. Concerning the macronutrients, carbohydrates were the most abundant compounds, reaching 132.05–173.81 mg/100 mL decoction, followed by proteins (8.57–12.49 mg/100 mL decoction). Moderate ash amount parameters (34.40–54.59 mg/100 mL decoction) were typical for Filipendula floral teas [25,26]. Low lipid (<1 mg/100 mL decoction) and energy (0.57–0.75 kcal/100 mL decoction) contents were detected in the meadowsweet floral teas.
Table 1.
Organoleptic, macronutrients characteristics, free sugars, organic acids, amino acids and minerals composition of four meadowsweet teas (mean ± SD) a.
| Parameter | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| Extractives b | 310 ± 12 | 390 ± 15 | 394 ± 15 | 374 ± 14 |
| Organoleptic characteristics | ||||
| Color | Yellow | Dark yellow | Yellow | Pale yellow |
| Odor | Specific, sharp, methyl salicylate like | Specific, sweet | Specific, sweet, honey like | Specific, sweet |
| Taste | Bitterish, astringent | Bitterish, astringent | Bitterish, astringent | Bitterish, astringent |
| Macronutrients | ||||
| Carbohydrates b | 173.81 ± 5.04 | 132.05 ± 3.82 | 163.25 ± 4.87 | 147.11 ± 3.97 |
| Protein b | 10.30 ± 0.35 | 8.57 ± 0.31 | 12.49 ± 0.40 | 9.73 ± 0.32 |
| Lipids b | <1.00 | <1.00 | <1.00 | <1.00 |
| Ash b | 54.59 ± 1.91 | 40.98 ± 1.59 | 34.40 ± 1.16 | 45.63 ± 1.59 |
| Energy c | 0.75 | 0.57 | 0.71 | 0.64 |
| Free sugars | ||||
| Fructose b | tr. | tr. | tr. | tr. |
| Galactose b | 11.22 ± 0.30 | 8.07 ± 0.21 | 9.02 ± 0.25 | 9.74 ± 0.30 |
| Glucose b | 66.31 ± 1.85 | 54.39 ± 1.63 | 67.31 ± 2.01 | 65.27 ± 2.02 |
| Sucrose b | 15.61 ± 0.43 | 11.86 ± 0.35 | 12.08 ± 0.34 | 10.18 ± 0.32 |
| Total free sugars b | 93.14 | 74.32 | 88.41 | 85.19 |
| Organic acids | ||||
| Citric acid b | 5.84 ± 0.15 | 10.84 ± 0.25 | 8.24 ± 0.19 | 7.11 ± 0.17 |
| Malic acid b | 6.52 ± 0.15 | 11.67 ± 0.29 | 10.30 ± 0.26 | 9.58 ± 0.24 |
| Oxalic acid b | 9.62 ± 0.24 | 6.04 ± 0.14 | 4.93 ± 0.12 | 5.74 ± 0.12 |
| Quinic acid b | 0.11 ± 0.00 | tr. | tr. | tr. |
| Succinic acid b | tr. | 0.10 ± 0.00 | tr. | tr. |
| Tartaric acid b | tr. | 0.35 ± 0.01 | 0.19 ± 0.00 | 0.27 ± 0.00 |
| Total organic acids b | 22.09 | 29.00 | 23.66 | 22.70 |
| Amino acids | ||||
| Alanine b | 0.89 ± 0.02 | 0.26 ± 0.00 | 0.62 ± 0.02 | 0.75 ± 0.02 |
| Arginine b | 1.28 ± 0.04 | 0.32 ± 0.01 | 0.98 ± 0.03 | 0.73 ± 0.02 |
| Asparagine b | 0.56 ± 0.01 | 0.39 ± 0.01 | 0.58 ± 0.02 | 0.26 ± 0.00 |
| Aspartic acid b | 3.35 ± 0.07 | 0.90 ± 0.02 | 3.71 ± 0.08 | 2.03 ± 0.05 |
| Cisteine b | 0.05 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 |
| Glutamine b | 0.02 ± 0.00 | 0.10 ± 0.00 | 0.14 ± 0.00 | 0.08 ± 0.00 |
| Glutamic acid b | 3.65 ± 0.09 | 0.68 ± 0.02 | 2.59 ± 0.06 | 1.48 ± 0.03 |
| Glycine b | 1.26 ± 0.04 | 0.29 ± 0.00 | 0.84 ± 0.02 | 0.67 ± 0.02 |
| Histidine b | 0.81 ± 0.02 | 0.08 ± 0.00 | 0.37 ± 0.01 | 0.35 ± 0.00 |
| Isoleicine b | 0.97 ± 0.03 | 0.17 ± 0.00 | 0.65 ± 0.01 | 0.59 ± 0.02 |
| Leucine b | 1.55 ± 0.04 | 0.72 ± 0.02 | 1.45 ± 0.03 | 1.04 ± 0.03 |
| Lysine b | 1.44 ± 0.04 | 0.28 ± 0.00 | 0.77 ± 0.02 | 0.86 ± 0.02 |
| Methionine b | 1.01 ± 0.02 | 0.12 ± 0.00 | 0.40 ± 0.01 | 0.14 ± 0.00 |
| Phenyalanine b | 0.89 ± 0.03 | 0.23 ± 0.00 | 0.57 ± 0.01 | 0.73 ± 0.01 |
| Proline b | 0.77 ± 0.02 | 0.18 ± 0.00 | 0.85 ± 0.02 | 0.72 ± 0.01 |
| Serine b | 1.43 ± 0.04 | 0.31 ± 0.01 | 0.78 ± 0.02 | 0.69 ± 0.01 |
| Treonine b | 0.75 ± 0.01 | 0.14 ± 0.00 | 0.38 ± 0.01 | 0.58 ± 0.01 |
| Tyrosine b | 0.65 ± 0.01 | 0.11 ± 0.00 | 0.02 ± 0.00 | 0.42 ± 0.01 |
| Valine b | 1.25 ± 0.03 | 0.32 ± 0.01 | 0.71 ± 0.01 | 0.76 ± 0.02 |
| Total amino acids b | 22.58 | 5.61 | 16.44 | 12.89 |
| Minerals | ||||
| Calcium d | 396.29 ± 28.93 | 295.84 ± 23.08 | 239.42 ± 22.51 | 273.72 ± 24.09 |
| Chromium d | 0.16 ± 0.01 | 0.23 ± 0.02 | 0.29 ± 0.02 | 0.20 ± 0.02 |
| Cobalt d | 0.11 ± 0.01 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 |
| Copper d | 4.83 ± 0.44 | 5.31 ± 0.45 | 4.38 ± 0.40 | 4.74 ± 0.42 |
| Iron d | 4.84 ± 0.42 | 4.70 ± 0.40 | 5.06 ± 0.41 | 11.12 ± 1.01 |
| Magnesium d | 4923.48 ± 428.34 | 3266.55 ± 264.59 | 2726.8 ± 245.41 | 2730.73 ± 251.23 |
| Manganese d | 2.78 ± 0.22 | 2.69 ± 0.22 | 1.21 ± 0.11 | 6.30 ± 0.60 |
| Molybdenum d | 0.03 ± 0.00 | 0.08 ± 0.01 | 0.01 ± 0.00 | 0.05 ± 0.00 |
| Nickel d | 0.33 ± 0.02 | 1.24 ± 0.10 | 1.43 ± 0.12 | 1.28 ± 0.11 |
| Selenium d | 0.34 ± 0.03 | 0.01 ± 0.00 | 0.16 ± 0.01 | 0.26 ± 0.02 |
| Zinc d | 14.51 ± 1.03 | 16.14 ± 1.48 | 11.88 ± 1.01 | 12.55 ± 0.99 |
a standard brewing—1 g plant material/100 mL water; b mg/100 mL decoction; c kcal/100 mL decoction; tr.—traces amounts (<limit of quantification); d μg/100 mL decoction.
The free sugar compositions of the four samples of meadowsweet teas are shown in Table 1. Glucose was found to be predominant in all samples, accounting for 71%–77% of the total free sugars. This compound was followed by sucrose and galactose, the contents of which were 10.18–15.61 and 8.07–11.22 mg/100 mL, respectively. Only trace amounts of fructose were detected in the meadowsweet teas. The total content of free sugars in the four Filipendula decoctions ranged from 74.32 to 93.14 mg/100 mL.
The contents of organic acids detected in the meadowsweet teas were also investigated. Citric and malic acids were present in the examined tea samples at the highest levels, i.e., 5.84–10.84 and 6.52–11.67 mg per 100 mL of decoction, respectively. Their proportions of the total organic acids content were 26%–37% and 30%–44%, respectively. The amount of oxalic acid, a known antinutrient, was minimal in F. stepposa tea (4.93 mg/100 mL) and maximal in F. camschatica tea (9.62 mg/100 mL). Some other acids like quinic, succinic and tartaric acid were found in trace or low levels.
Nineteen amino acids were identified and quantified in the investigated tea samples. Aspartic acid was predominant in F. denudata (0.90 mg/100 mL), F. stepposa (3.71 mg/100 mL) and F. ulmaria (2.03 mg/100 mL) teas, whereas glutamic acid was the main amino acid in F. camschatica tea (3.65 mg/100 mL). The percentages of the basic amino acids (aspartic acid, glutamic acid and leucine) were 25% (F. ulmaria tea), 38% (F. camschatica tea), 41% (F. denudata tea) and 47% (F. stepposa tea) of the total amino acid content. The lowest concentrations (<0.1 mg/100 mL) in teas were observed for glutamine in F. camschatica; cysteine, glutamine and histidine in F. denudata; cysteine and tyrosine in F. stepposa and cysteine and glutamine in F. ulmaria.
The content of nine microelements (Cr, Co, Cu, Fe, Mn, Mo, Ni, Se and Zn) and two macroelements (Ca and Mg) in the meadowsweet floral teas were determined. Among the major micronutrients measured in these floral teas, Mg predominated, but high levels of Ca were also found. High levels of Fe (11.12 μg/100 mL) and Mn (6.30 μg/100 mL) were observed in F. ulmaria floral tea compared with the other teas. F. camschatica floral tea was distinguished by high contents of Mg (4923.48 μg/100 mL), Ca (396.29 μg/100 mL), Se (0.34 μg/100 mL) and Co (0.11 μg/100 mL). The highest amounts of Cu (5.31 μg/100 mL), Mo (0.08 μg/100 mL) and Zn (16.14 μg/100 mL) were observed in F. denudata tea, whereas F. stepposa accumulated Cr (0.29 μg/100 mL) and Ni (1.43 μg/100 mL). Although the general trends in the concentration of major minerals were similar across the Filipendula species, substantial variation in the levels of individual elements occurred. In the absence of information on factors such as soil type and seasonal climate, a more detailed comparison is not justified. In order to estimate whether the floral teas prepared from Filipendula species represent an important source of microelements, these results were compared with the recommended daily dietary requirements. The concentrations of the micronutrients dissolved in floral teas are below the levels considered to be adequate to humans [27]. Our results provide evidence that the meadowsweet teas are the products with appropriate nutritional value and good sugars, organic acids, amino acids, macro- and micro-mineral content.
2.2. General Phytochemical Characteristics of Meadowsweet Teas
The contents of the major groups of phenolic compounds (flavonoids, tannins, catechins and proanthocyanidins) were determined for the phytochemical characterization of the meadowsweet floral teas. Water soluble (WS) polymers like polysaccharides are an obligate group of phytochemicals in all herbal teas. The potential bioactivity of WS-polysaccharides necessitated us to investigate their contents. The data presented in Table 2 demonstrate the general phytochemical characteristics of meadowsweet teas.
Table 2.
General phytochemical characteristics of four meadowsweet teas, mg/100 mL decoction (±SD).
| Parameter | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| Flavonoids | 18.34 ± 0.42 | 50.92 ± 1.22 | 52.25 ± 1.35 | 55.31 ± 1.43 |
| Tannins | 51.83 ± 1.50 | 114.81 ± 3.55 | 120.18 ± 3.72 | 100.88 ± 3.21 |
| Catechins | 9.57 ± 0.39 | 1.27 ± 0.05 | tr. | 5.69 ± 0.24 |
| Proanthocyanidins | 4.64 ± 0.15 | 2.15 ± 0.06 | 5.14 ± 0.16 | 3.83 ± 0.12 |
| WS-Polysaccharides | 21.39 ± 0.56 | 24.51 ± 0.61 | 30.47 ± 0.94 | 23.81 ± 0.59 |
tr.—traces amounts (<limit of quantification).
The amount of flavonoids varied from 18.34 mg/100 mL (F. camschatica) to 55.31 mg/100 mL (F. ulmaria). The highest concentration of tannins was observed in F. stepposa tea (120.18 mg/100 mL), whereas the lowest concentration was detected in F. camschatica tea (51.83 mg/100 mL). The content of catechins changed from 1.27 mg/100 mL (F. denudata) to 9.57 mg/100 mL (F. camschatica), while in F. stepposa, trace amounts of catechins were observed. The amount of proanthocyanidins varied from 2.15 mg/100 mL (F. denudata) to 5.14 mg/100 mL (F. stepposa). The difference between WS-polysaccharide contents was not significant, i.e., from 21.39 mg/100 mL (F. camschatica) to 30.47 mg/100 mL (F. stepposa). The general phytochemical profile results show that meadowsweet floral teas are a good source of phenolic compounds as well as WS-polysaccharides.
2.3. MC-RP-HPLC Quantification of the Main Phenolics of Meadowsweet Teas
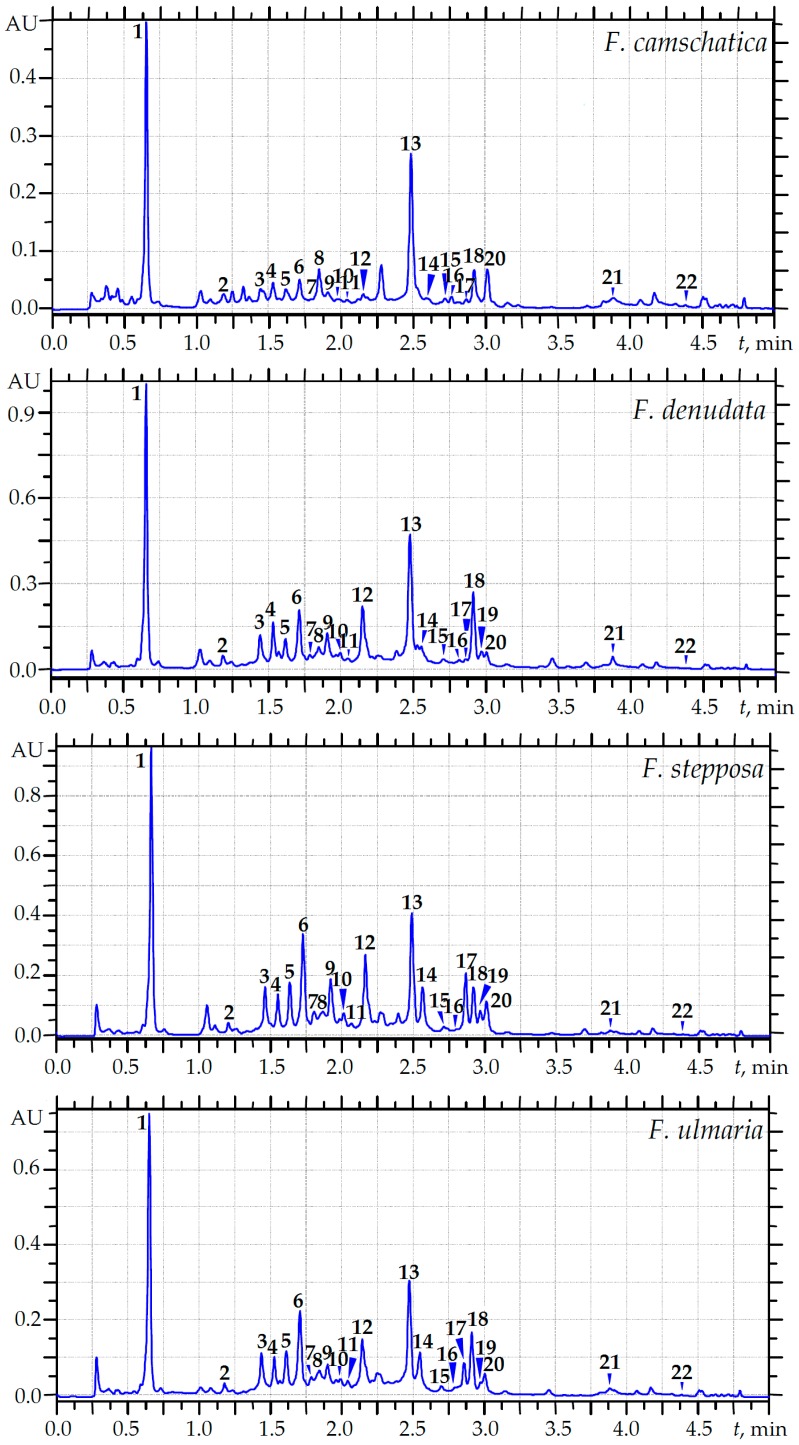
The presence of different classes of compounds, including simple phenolics, phenylpropanoids, catechins, flavonoids and ellagitannins, has been found in the flowers of F. camschatica, F. denudata, F. stepposa and F. ulmaria [21]. The present work aimed at the quantification of some principal compounds in meadowsweet floral teas by a microcolumn (MC)-RP-HPLC-UV procedure. Among the known phenolics described in Filipendula flowers, only 22 were found in quantifiable amounts in meadowsweet teas, including eight flavonols, eight ellagitannins, protocatechuic, gallic, ellagic, caffeic and 1,3-di-O-caffeoylquinic acids, and methyl salicylate (Figure 1). These contents are summarized in Table 3.
Figure 1.
MC-RP-HPLC-UV chromatograms of four meadowsweet floral tea decoctions at 270 nm. Compounds: 1—gallic acid; 2—protocatechuic acid; 3—tellimagrandin I1; 4—rugosin B1; 5—tellimagrandin I2; 6—rugosin B2; 7—caffeic acid; 8—1,3-di-O-caffeoylquinic acid; 9—rugosin E1; 10—rugosin E2; 11—tellimagrandin II; 12—rugosin D; 13—ellagic acid; 14—quercetin-3-O-β-d-glucoside (isoquercitrin); 15—quercetin-3-O-α-l-arabinoside (avicularin); 16—quercetin-3-O-β-d-glucuronide (miquelianin); 17—quercetin-3-O-α-l-rhamnoside (quercitin); 18—quercetin-4′-O-β-d-glucoside (spiraeoside); 19—kaempferol-3-O-α-l-rhamnoside (afzelin); 20—kaempferol-4′-O-β-d-glucoside; 21—quercetin; 22—methyl salicylate.
Table 3.
Content of the main phenolics in four meadowsweet tea decoctions, mg/100 mL decoction (±SD).
| Compound | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| Flavonoids | ||||
| Kaempferol-3-O-α-l-rhamnoside | tr. | 2.27 ± 0.07 | 2.78 ± 0.07 | 0.87 ± 0.02 |
| Kaempferol-4′-O-β-d-glucoside | 5.43 ± 0.14 | 4.70 ± 0.14 | 6.50 ± 0.19 | 5.71 ± 0.12 |
| Quercetin | 0.40 ± 0.01 | 1.71 ± 0.04 | 0.72 ± 0.02 | 1.20 ± 0.03 |
| Quercetin-3-O-β-d-glucoside | 0.74 ± 0.02 | 4.95 ± 0.14 | 10.18 ± 0.30 | 6.92 ± 0.16 |
| Quercetin-3-O-α-l-arabinoside | 0.76 ± 0.02 | 5.96 ± 0.17 | 4.21 ± 0.13 | 2.60 ± 0.05 |
| Quercetin-3-O-α-l-rhamnoside | 0.29 ± 0.01 | 1.18 ± 0.03 | 9.50 ± 0.27 | 3.92 ± 0.09 |
| Quercetin-4′-O-β-d-glucoside | 6.43 ± 0.17 | 28.50 ± 0.71 | 17.91 ± 0.45 | 17.53 ± 0.42 |
| Quercetin-3-O-β-d-glucuronide | 0.46 ± 0.01 | tr. | tr. | tr. |
| Subtotal | 14.51 | 49.27 | 51.80 | 38.75 |
| Ellagitannins | ||||
| Tellimagrandin I1 | 1.32 ± 0.03 | 7.61 ± 0.19 | 9.30 ± 0.25 | 4.56 ± 0.10 |
| Tellimagrandin I2 | 2.28 ± 0.06 | 6.58 ± 0.19 | 10.65 ± 0.29 | 6.71 ± 0.15 |
| Tellimagrandin II | 0.59 ± 0.02 | 2.27 ± 0.06 | 2.73 ± 0.07 | 2.02 ± 0.04 |
| Rugosin B1 | 2.42 ± 0.07 | 8.24 ± 0.25 | 7.69 ± 0.19 | 5.35 ± 0.12 |
| Rugosin B2 | 2.92 ± 0.09 | 12.41 ± 0.32 | 22.44 ± 0.67 | 12.36 ± 0.29 |
| Rugosin E1 | 1.75 ± 0.05 | 9.18 ± 0.23 | 13.42 ± 0.39 | 5.80 ± 0.12 |
| Rugosin E2 | 1.03 ± 0.03 | 3.28 ± 0.09 | 5.07 ± 0.14 | 2.52 ± 0.06 |
| Rugosin D | 0.78 ± 0.02 | 15.62 ± 0.42 | 19.92 ± 0.52 | 8.75 ± 0.20 |
| Subtotal | 13.09 | 65.19 | 91.22 | 48.07 |
| Other classes | ||||
| Protocatechuic acid | 0.78 ± 0.02 | 1.24 ± 0.03 | 1.32 ± 0.03 | 0.73 ± 0.01 |
| Gallic acid | 11.80 ± 0.34 | 25.76 ± 0.67 | 26.17 ± 0.68 | 19.25 ± 0.40 |
| Ellagic acid | 10.92 ± 0.32 | 26.72 ± 0.77 | 22.64 ± 0.61 | 15.27 ± 0.36 |
| Methyl salicylate | 0.71 ± 0.02 | 0.79 ± 0.02 | 0.97 ± 0.03 | 0.92 ± 0.02 |
| Caffeic acid | 1.08 ± 0.03 | 3.88 ± 0.12 | 7.66 ± 0.22 | 4.20 ± 0.09 |
| 1,3-Di-O-caffeoylquinic acid | 6.18 ± 0.17 | 11.24 ± 0.34 | 12.47 ± 0.31 | 10.43 ± 0.23 |
| Subtotal | 31.47 | 69.63 | 71.23 | 50.80 |
| Total | 59.07 | 184.09 | 214.25 | 137.62 |
tr.—traces amounts (<limit of quantification).
The amounts of the specific compounds varied among the different Filipendula species. The results show that the total content of quantifiable components in the meadowsweet tea samples displayed a 3.6-fold variation. F. stepposa tea had the highest total compound content (214.25 mg/100 mL), followed in order by F. denudata tea (184.09 mg/100 mL), F. ulmaria tea (137.62 mg/100 mL) and F. camschatica tea (59.07 mg/100 mL).
As we mentioned in our previous work, dimeric and monomeric ellagitannins are one of the chemotaxonomic markers of the Filipendula genus [21]. The compositions of ellagitannins in the investigated floral teas were typical, with a predominance of dimers over monomers. The principal constituents of this class were rugosins. A high content of rugosin D (15.62 mg/100 mL) was observed in the F. denudata tea sample and the dominance of rugosin B2 was typical for the F. stepposa, F. ulmaria and F. camschatica decoctions (22.44, 12.36 and 2.92 mg/100 mL, respectively).
Other marker compounds of the genus Filipendula are flavonoids, quercetin and kaempferol derivatives, found in the investigated decoctions primarily as the glycoside form in monoglycosides. The 4′-O-glycosides made up the principal part of the flavonoids of tea samples, and the predominance of quercetin-4′-O-β-D-glucoside (spiraeoside) was observed in all decoctions investigated. The highest content of spiraeoside was revealed in the F. denudata decoction (28.50 mg/100 mL) while the lowest was in F. camschatica tea (6.43 mg/100 mL). It should be noted that the percentage of kaempferol derivatives was high in F. camschatica tea (37.42%), whereas in other tea samples this value did not exceed 18% and the prevalence of quercetin derivatives was higher.
Speculating about the possible reasons for these results, it can be concluded that F. ulmaria, F. denudata and F. stepposa are relate to the same subgenus Ulmaria of the Filipendula genus, while F. camschatica belong to the subgenus Aceraria. Due to their high content of flavonoids and ellagitannins, F. denudata and F. stepposa may be considered as promising substitutes for the officinal species F. ulmaria.
The highest content of gallic and protocatechuic acids was noted in samples of F. stepposa tea (26.17 mg/100 mL and 1.32 mg/100 mL, respectively), while the lowest concentration of gallic acid was found in F. camschatica tea (11.80 mg/100 mL) and the lowest content of protocatechuic acid was revealed in F. ulmaria tea sample (0.73 mg/100 mL). The content of methyl salicylate varied slightly from 0.73 mg/100 mL (F. ulmaria tea) to 0.97 mg/100 mL (F. stepposa tea). Quantifiable amounts of phenylpropanoids are presented by caffeic and 1,3-di-O-caffeoylquinic acid; the highest content of both compounds was noted in samples of F. stepposa tea (7.66 and 12.47 mg/100 mL, respectively), while the lowest was noticed for F. camschatica tea (1.08 and 6.18 mg/100 mL, respectively). The F. denudata decoction was distinguished by the highest content of ellagic acid (26.72 mg/100 mL), whereas F. camschatica tea contained the least amount of this compound (10.92 mg/100 mL).
The data allowed us to calculate the total uptake of specific compounds after application of the standard dosage of meadowsweet teas (100 mL). This was 59.07 mg/100 mL for F. camschatica floral tea, 137.62 mg/100 mL for F. ulmaria floral tea, 184.09 mg/100 mL for F. denudata floral tea and 214.25 mg/100 mL for F. stepposa floral tea. The HPLC quantification results are clear evidence that Filipendula species accumulate phenolic compounds and their decoctions are a good source of flavonols, ellagitannins, simple phenols and phenylpropanoids.
2.4. Essential Oil Compositions of Meadowsweet Flowers
All samples of these meadowsweet teas were characterized by specific odours caused by the presence of essential oil components. The most studied essential oil of the Filipendula genus is F. ulmaria essential oil, which consists of salicylic acid derivatives like salicylaldehyde, methyl salicylate and other components responsible for the characteristic odors of the meadowsweet teas [28]. The other species investigated in the present work (F. camschatica, F. denudata, F. stepposa) have similar odours, but have not yet been assessed regarding their essential oil composition. The samples of the floral essential oils were isolated by hydrodistillation and the compositions were determined after GC/MS analysis. The yields of the essential oil were 0.02%–0.11%. All the samples were yellowish liquids with strong odors. Twenty-eight compounds were identified in the four essential oils, including simple phenols, monoterpenes, sesquiterpenes and aliphatic components (Table 4).
Table 4.
Composition of the essential oils from the flowers of four Filipendula species.
| Compound | RI | MI a | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|---|---|
| Simple phenols | ||||||
| Benzaldehyde | 956 | i, ii, iii | 0.9 | 3.2 | 2.9 | 2.3 |
| Benzyl alcohol | 1031 | i, ii, iii | 0.5 | 3.0 | 2.2 | 1.4 |
| Salicylaldehyde | 1043 | i, ii, iii | 9.0 | 45.9 | 25.7 | 35.7 |
| Ethyl benzoate | 1170 | i, ii, iii | 0.3 | 0.8 | 0.9 | 0.3 |
| Methyl salicylate | 1192 | i, ii, iii | 73.9 | 20.7 | 14.2 | 18.4 |
| Ethyl salicylate | 1386 | i, ii, iii | 5.2 | 2.4 | 0.3 | 1.1 |
| Vanillin | 1400 | i, ii, iii | tr. | 0.3 | 5.9 | 0.9 |
| Benzyl salicylate | 1872 | i, ii, iii | 4.4 | 1.2 | 0.3 | 6.3 |
| Subtotal | 94.2 | 77.5 | 52.4 | 66.4 | ||
| Monoterpenes | ||||||
| Linalool | 1098 | i, ii, iii | 0.2 | 2.2 | 5.7 | 2.3 |
| α-Terpineol | 1190 | i, ii, iii | 0.3 | 1.1 | 0.9 | 2.2 |
| Geraniol | 1252 | i, ii, iii | 0.1 | 0.5 | 1.3 | 0.3 |
| Subtotal | 0.6 | 3.8 | 7.9 | 4.8 | ||
| Sesquiterpenes | ||||||
| β-Caryophyllene | 1420 | i, ii, iii | 0.4 | 1.5 | 1.9 | 1.6 |
| Humulene | 1455 | i, ii, iii | 0.2 | 1.3 | 1.1 | 0.9 |
| Germacrene D | 1483 | i, ii, iii | 0.2 | 0.9 | 1.2 | 1.2 |
| β-(E)-Ionone | 1489 | i, ii, iii | 0.2 | 1.9 | 2.7 | 2.3 |
| δ-Amorphene | 1510 | i, ii | 0.3 | 0.4 | 0.3 | 0.4 |
| Caryophyllene oxide | 1587 | i, ii | tr. | 0.1 | tr. | tr. |
| (E)-Asarone | 1686 | i, ii | 0.1 | 0.2 | 0.3 | 0.6 |
| Subtotal | 1.4 | 6.3 | 7.5 | 7.0 | ||
| Aliphatic compounds | ||||||
| Decanal | 1205 | i, ii, iii | tr. | 0.9 | 2.9 | 1.6 |
| Dodecanal | 1408 | i, ii, iii | tr. | 0.2 | 1.3 | 0.3 |
| Tetradecanal | 1610 | i, ii | tr. | 0.3 | 0.9 | 0.4 |
| Pentadecanal | 1712 | i, ii | 0.1 | 1.0 | 2.2 | 1.9 |
| Hexadecanal | 1816 | i, ii | 2.9 | 1.3 | 6.3 | 5.2 |
| Heptadecanal | 1919 | i, ii | 0.2 | 5.7 | 9.4 | 6.9 |
| n-Docosane | 2200 | i, ii | tr. | 0.2 | 0.9 | 0.3 |
| n-Tricosane | 2300 | i, ii | tr. | 0.1 | 1.2 | 0.2 |
| n-Tetracosane | 2400 | i, ii | tr. | 0.1 | 0.3 | 0.7 |
| n-Pentacosane | 2500 | i, ii | tr. | 0.2 | 0.9 | 0.3 |
| Subtotal | 3.2 | 10.0 | 26.3 | 17.8 | ||
| Total | 99.4 | 97.6 | 94.1 | 96.0 | ||
| Yield, % b | 0.02 | 0.07 | 0.11 | 0.05 | ||
a Methods of identification: i—retention index, ii—mass spectrum, iii—co-injection with authentic sample; b yield, % of dry plant weight; tr.—traces amounts (<0.1%).
As expected, salicylaldehyde (35.7%) and methyl salicylate (18.4%) were the major components of F. ulmaria essential oil, as well as heptadecanal (6.9%) and hexadecanal (5.2%). The contents of salicylaldehyde and methyl salicylate in F. stepposa essential oil were 25.7% and 14.2%, respectively. This essential oil was characterized by the highest amount of aliphatic compounds like heptadecanal (9.4%), hexadecanal (6.3%) and decanal (2.9%) as well as the phenol vanillin (5.9%) and monoterpene linalool (5.7%). With respect to F. denudata essential oil, the composition was close to that of F. ulmaria essential oil, with a high content of salicylaldehyde (45.9%) and a moderate amount of methyl salicylate (20.7%). The flowers of F. camschatica produced an essential oil with a high content of methyl salicylate (73.9%), corroborated by the odor, which was described as very sharp. Salicylaldehyde (9.0%), ethyl salicylate (5.2%), benzyl salicylate (4.4%) and hexadecanal (2.9%) were also detected.
The data on the chemical composition of the essential oils of the Filipendula genus indicate the similarity of the volatile compounds. The main components of the essential oil from F. vulgaris (F. hexapetala) flowering shoots were n-tricosane (17.9%), salicylaldehyde (13.7%), benzyl salicylate (6.8%), methyl salicylate (6.7%) [18] and in the essential oil from F. vulgaris leaves salicylaldehyde (68.6%), 3-hexen-1-ol (6.0%), α-asarone (5.9%) and 2-hexenal (4.2%) [17]. Methyl salicylate (70.1%) together with eicosane (4.6%) and tricosane (2.3%) dominated in the essential oil from the flowers of F. palmata [29].
2.5. Water-Soluble Polysaccharide Characterization of Meadowsweet Teas
The yields of the WSP fraction from meadowsweet flowers ranged from 13.03 mg/g (F. denudata) to 17.24 mg/g (F. ulmaria) (Table 5). All WSP had a high carbohydrate content (973.64–989.47 mg/g) and low uronic acid (63.18–102.27 mg/g) and protein amounts (12.60–23.18 mg/g). The negative reaction with resorcinol declared the absence of fructose-containing polymers like inulin. However, positive reactions with iodine and the Yariv reagent indicated the presence of starch-related polysaccharides and arabinogalactan-protein complexes. The monosaccharide compositions of four WSP fractions from Filipendula flowers showed a dominance of neutral carbohydrates other than uronic acids (91.1–94.0 mol % vs. 5.9–8.8 mol %). The galactose content was maximal in F. camschatica (48.8 mol %) and minimal in F. ulmaria (40.5 mol %). Also, high amounts of glucose were observed in the range of 23.4–36.4 mol %. Arabinose and mannose were important components of the WSP fraction with contents of 4.2–10.5 mol % and 6.0–7.0 mol %, respectively. The minor monosaccharides were fucose (0.2–0.9 mol %), xylose (0.4–2.7 mol %) and rhamnose (0.8–3.1 mol %).
Table 5.
General parameters and monosaccharide compositions of water soluble polysaccharide fractions of four meadowsweet floral tea decoctions.
| Parameter | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| Yield, mg/g | 11.75 | 13.03 | 17.07 | 17.24 |
| Total carbohydrate content, mg/g | 973.64 ± 30.47 | 982.50 ± 28.63 | 989.47 ± 31.20 | 980.52 ± 30.39 |
| Uronic acid content, mg/g | 102.27 ± 3.40 | 63.18 ± 2.24 | 79.30 ± 3.01 | 90.45 ± 3.43 |
| Protein content, mg/g | 23.18 ± 0.61 | 14.37 ± 0.35 | 12.60 ± 0.30 | 17.22 ± 0.49 |
| Reaction with I2 | positive | positive | positive | positive |
| Reaction with resorcinol | negative | negative | negative | negative |
| Reaction with Yariv reagent | positive | positive | positive | positive |
| Monosaccharide composition, mol % | ||||
| Ara | 10.5 | 4.2 | 5.1 | 6.3 |
| Gal | 48.8 | 43.0 | 46.4 | 40.5 |
| Glc | 23.4 | 36.4 | 29.0 | 33.6 |
| Fuc | 0.2 | 0.5 | 0.4 | 0.9 |
| Man | 7.0 | 6.3 | 6.9 | 6.0 |
| Rha | 0.8 | 2.5 | 3.1 | 1.6 |
| Xyl | 0.4 | 1.1 | 1.2 | 2.7 |
| GalA | 8.8 | 5.9 | 7.8 | 8.3 |
tr.—traces amounts (<limit of quantification).
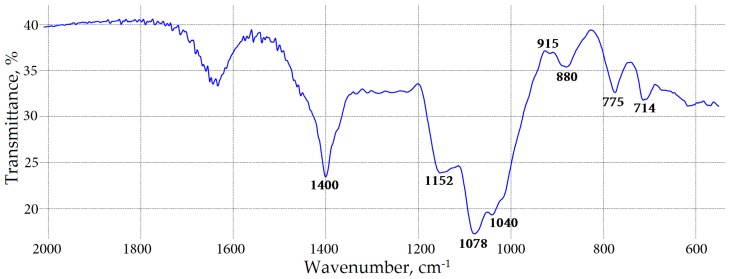
The FT-IR spectra of the WSP fractions of four meadowsweet floral teas were very close to each other and similar to those for (arabino)galactan polysaccharides [30]. The FT-IR spectrum of WSP of F. denudata is shown in Figure 2. It contains strong bands assigned to stretching vibrations of pyranose and furanose rings (1152, 1078, 1040 cm−1), bands of C1-Hβ deformation vibrations in the “anomeric region” (915, 880, 775, 714 cm−1) and less intensive bands of carboxylate groups (1400, 1650 cm−1).
Figure 2.
FT-IR spectra of water-soluble polysaccharide from F. denudata flowers.
The data allowed us to conclude that the WSP of Filipendula flowers are generally galactans and/or arabinogalactan with an admixture of glucans of the starch type and galacturonans as minor components. The known data on the biological activity of galactan-type polymers of plant origin suggest their effectiveness as immune modulators due to their influence on the complement system and phagocytosis [31]. For further structural investigations, Filipendula flower WSP should be subjected to bioactivity assessments such as immune modulatory testing to determine their role in the therapeutic effectiveness of meadowsweet teas.
2.6. Biological Activity of Meadowsweet Floral Teas Decoctions
2.6.1. Inhibitory Effect on Amylase, α-Glucosidase and AGEs Formation
The investigation of the influence of meadowsweet teas on amylase demonstrated their ability to inhibit enzymatic activity with the highest IC50 value for F. denudata tea (IC50 74.80 μg/mL) and the lowest value for F. camschatica (IC50 > 100 μg/mL) (Table 6).
Table 6.
Inhibitory effect on amylase (Amy), α-glucosidase (Glu) and AGEs formation (AGE) of four meadowsweet floral tea decoctions and reference compounds a.
| Method | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| Amy b | >100 | 74.80 ± 2.54 ii | 89.67 ± 2.78 | 85.52 ± 2.90 ii |
| Glu b | ~100 | 78.53 ± 2.28 | 71.35 ± 2.06 iv | 76.14 ± 2.74 iv |
| AGE c | 10.27 ± 0.41 v | 52.11 ± 1.87 vi | 61.18 ± 2.20 vi | 32.90 ± 1.22 v |
| Spiraeoside | Rugosin D | Methyl salicylate | Acarbose | |
| Amy b | >100 | 6.27 ± 0.21 i | >100 | 10.57 ± 0.34 i |
| Glu b | >100 | 4.82 ± 0.16 iii | >100 | 38.60 ± 1.34 iii |
| AGE d | <5 | 69.72 ± 2.37 | <5 | n.d. e |
a Average of three analyses (±SD); b IC50, μg/mL; c inhibitory activity at 1 mg/mL, %; d inhibitory activity at 0.5 mg/mL, %; e n.d.—not determined. All values correspond to mean values ± standard deviation of three replicates. Values with different letters (i–vi) indicate statistically significant differences among groups at p < 0.05 by one-way ANOVA.
A similar trend was seen in the α-glucosidase inhibition experiments. F. stepposa was the most active meadowsweet tea (IC50 71.35 μg/mL) and F. camschatica displayed no inhibition of α-glucosidase activity up to a concentration >100 μg/mL.
Separate compounds used as references in both experiments were either inactive, such as spiraeoside or methyl salicylate (IC50 > 100 μg/mL), or strongly active, such as a rugosin D. The latter compound was more active than acarbose, a known amylase and α-glucosidase inhibitor, used us as the positive control. The results regarding the inhibitory activity of these teas against AGE production displayed potent activity of F. denudata, F. stepposa and F. ulmaria teas with 52.11%, 61.18% and 32.90% percentage inhibition for solutions with a concentration of 1 mg/mL. The most active compound was rugosin D with an inhibition value of 69.72% at 0.5 mg/mL in contrast to inactive spiraeoside and methyl salicylate. Experiments with WSP of Filipendula flowers showed no inhibitory effects on the activity of enzymes and AGE formation.
Summarizing the results of the inhibitory potentials of the meadowsweet teas on the activity of amylase, α-glucosidase and AGE formation, we can confirm the potential of Filipendula plants as antidiabetic agents. The use of phenolic compounds as inhibitors of amylase and α-glucosidase in the treatment of diabetic disorders is related to their ability to reduce the postprandial blood glucose level [32]. A high level of the blood glucose leads to the accumulation of AGE, leading to the development of pathological conditions related to diabetes (nephropathy and neuropathy, cardiovascular disease and atherosclerosis) [33] Thus, the search for compounds that provide a combined positive effect on these negative processes is important.
Rosaceous plants are a known source of extracts with amylase inhibiting activity. Some species inhibit amylase, like Rosa gallica, R. suavissimus, Rubus idaeus and Sorbus aucuparia with IC50 values of 20–140 μg/mL [34]. The main chemical factors providing this activity are ellagitannins, which have demonstrated strong inhibition of amylase [35]. The potential of flavonoids as amylase inhibitors has been discussed previously [36], with a wide range of activity from inactive (or weakly active) to moderate. Close relationships have been observed between the ellagitannin content and the ability of crude plant extracts to inhibit α-glucosidase activity [37]. Despite this, only a few reports have discussed the inhibitory activity of rosaceous plants and ellagitannins on AGE formation in vitro [38]; the tannins of two Chamaerhodos species have been found to prevent AGE formation with IC50 values of 91–250 μM [39]. The anti-diabetic potential of Filipendula plants is discussed in this work and the results indicate no contradictions with known information about the bioactivity of Rosaceous plants and ellagitannins as a whole.
2.6.2. Antioxidant Activity
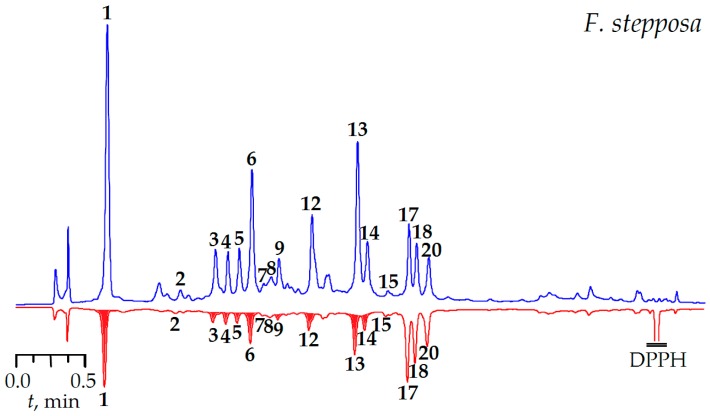
A preliminary characterization of antioxidant potential was carried out using the DPPH-MC-RP-HPLC-UV procedure, involving HPLC separation of samples pre-treated with an excess of DPPH•, followed by comparison with the HPLC chromatogram of an untreated sample. The reaction between an antioxidant and radical results in the oxidation of the antioxidant, which leads to a decrease in the corresponding peak areas in the chromatograms. The comparison of the HPLC chromatograms of untreated and radical-treated samples allows for the determination of the most active compounds. The chromatograms of the F. stepposa tea decoction spiked with DPPH• radicals are shown in Figure 3, and present obviously reduced peak areas for some compounds in comparison with untreated samples.
Figure 3.
DPPH-MC-RP-HPLC-UV chromatograms of F. stepposa floral tea at 270 nm before (blue) and after (red) reaction with DPPH•-radicals. Compounds: 1—gallic acid; 2—protocatechuic acid; 3—tellimagrandin I1; 4—rugosin B1; 5—tellimagrandin I2; 6—rugosin B2; 7—caffeic acid; 8—1,3-di-O-caffeoylquinic acid; 9—rugosin E1; 10—rugosin E2; 11—tellimagrandin II; 12—rugosin D; 13—ellagic acid; 14—quercetin-3-O-β-d-glucoside (isoquercitrin); 15—quercetin-3-O-α-l-arabinoside (avicularin); 16—quercetin-3-O-β-d-glucuronide (miquelianin); 17—quercetin-3-O-α-l-rhamnoside (quercitin); 18—quercetin-4′-O-β-d-glucoside (spiraeoside); 19—kaempferol-3-O-α-l-rhamnoside (afzelin); 20—kaempferol-4′-O-β-d-glucoside
Numerous compounds demonstrated a visible reduction of the peak area after spiking with DPPH• radicals (gallic acid, peak 1; tellimagrandin I1, peak 3; rugosin B1, peak 4; tellimagrandin I2, peak 5; rugosin B2, peak 6; rugosin E1, peak 9; rugosin D, peak 12; ellagic acid, peak 13; isoquercitrin, peak 14). The majority of ellagitannins possess antioxidant activity and they may be considered as the major active compounds, while only one flavonoid, isoquercetin, revealed a high antioxidant potential. The results show the leading role of ellagitannins in free radical scavenging of meadowsweet teas.
The antioxidant properties of four Filipendula decoctions were evaluated using various tests: the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) scavenging assay, the 2,2′-azino-bis(3-ethyl-benzthiazoline-6-sulphonic acid) radical (ABTS•+) scavenging assay, the Br•-radical scavenging activity and the carotene bleaching assay (CBA). All experiments included the determination and comparative estimation of the same antioxidant factors for spiraeoside, rugosin D and methyl salicylate, the representatives of the main classes of compounds dominating in the investigated tea samples; Trolox was used as a reference compound. As can be observed in Table 7, F. stepposa tea was the most active antioxidant in all assays, followed by F. denudata, F. ulmaria and F. camschatica teas.
Table 7.
Antioxidant activity of four meadowsweet floral tea decoctions and reference compounds a.
| Method b | F. camschatica | F. denudata | F. stepposa | F. ulmaria |
|---|---|---|---|---|
| DPPH• | 23.72 ± 0.59 ii | 8.13 ± 0.21 ii | 7.33 ± 0.19 ii | 10.43 ± 0.26 ii |
| ABTS•+ | 9.76 ± 0.23 iv | 4.97 ± 0.11 iii | 4.08 ± 0.08 iii | 5.74 ± 0.12 iii |
| Br•− | 111.89 ± 2.12 v | 241.95 ± 4.35 vi | 259.64 ± 4.67 vi | 228.11 ± 4.33 v, vi |
| CBA | 21.16 ± 0.76 | 4.11 ± 0.15 ix | 3.53 ± 0.12 viii, ix | 4.55 ± 0.16 ix |
| Spiraeoside | Rugosin D | Methyl salicylate | Trolox | |
| DPPH• | 82.40 ± 2.14 | 5.36 ± 0.11 i | >100 | 7.40 ± 0.14 i |
| ABTS•+ | 7.02 ± 0.14 iii, iv | 0.70 ± 0.01 | 59.18 ± 1.24 | 4.27 ± 0.08 iii |
| Br•− | 1046.82 ± 19.88 vii | 1293.63 ± 24.58 vii | 331.53 ± 5.96 vi | 663.25 ± 12.98 |
| CBA | >100 | 2.62 ± 0.09 | >100 | 2.70 ± 0.10 viii |
a Average of three analyses (±SD); b DPPH•—DPPH• radical scavenging activity (IC50, μg/mL); ABTS•+—ABTS•+ radical scavenging activity (IC50, μg/mL); Br•−—bromine anion radical scavenging activity (mg gallic acid equivalents per g extract); CBA—carotene bleaching assay (IC50, μg/mL). All values correspond to mean values ± standard deviation of three replicates. Values with different letters (i–ix) indicate statistically significant differences among groups at p < 0.05 by one-way ANOVA.
Early information about the quantitative content of the individual compounds in the meadowsweet teas allowed us to associate the significant antioxidant potential of F. denudata decoction with the highest ellagitannin content.
Decoctions of F. stepposa and F. denudata exhibited a similar ability to inactivate DPPH• and ABTS•+ free radicals and demonstrated the most pronounced scavenging activity of these radicals. Less pronounced activity was observed for F. ulmaria and F. camschatica teas in both tests. The scavenging value of the F. stepposa decoction against bromine radicals was the highest (259.64 mg/g), while the lowest value was observed for F. camschatica tea (111.89 mg/g). The examination of the influence of meadowsweet teas on the oxidative destruction of β-carotene in the oleic acid-DMSO-H2O2 system demonstrated a high degree of antioxidant activity for F. stepposa, F. denudata and F. ulmaria tea samples with IC50 values of 3.53, 4.11 and 4.55 μg/mL.
The efficiency of F. camschatica in this assay was the lowest (IC50 21.16 μg/mL). The investigation into the antioxidant activity of individual reference compounds demonstrated a wide range of activity. Rugosin D was the most active compound in all assays, while the least active compound was methyl salicylate.
Previously, the antioxidant properties of several Filipendula extractions were analyzed. The data on the antioxidant properties of the aqueous fraction from the aerial parts of F. ulmaria from Lithuania revealed lower efficiency in the scavenging of DPPH• and ABTS•+ free radicals (IC50 410 and 730 μg/mL, respectively) [40]. A possible reason for this phenomenon is the high contents of leaves and stems vs. flowers in a sample of the aerial parts. Also, a reduced content of ellagitannins in leaves reflects their lower effectiveness in radical scavenging [21]. The methanolic extract of F. ulmaria aerial parts collected in Serbia revealed effective scavenging of DPPH• and ABTS•+ radicals, i.e., 16.41 μg/mL and 36.75 μg/mL, respectively [41]. The methanolic extract from flowers of F. vulgaris (F. hexapetala; Serbian origin) has been shown to be effective in the DPPH• assay with an IC50 of 8.25 μg/mL [42]. In the ABTS•+ assay, the methanolic extract of the aerial parts of the same plant species demonstrated high efficiency in the scavenging of free radicals with an IC50 value of 34.52 μg/mL, whereas the scavenging ability of the aerial parts against DPPH• radicals was lower than in flowers, i.e., 13.47 μg/mL [43]. The scavenging value of the aqueous fraction from the leaves and stems of F. vulgaris against DPPH• radicals was less pronounced and amounted to an IC50 of 650 μg/mL [44].
The results obtained in the present study indicated a greater antioxidant effect of F. stepposa, F. denudata and F. ulmaria tea decoctions, indicating the effectiveness of meadowsweet tea for the regulation of antioxidant status in humans.
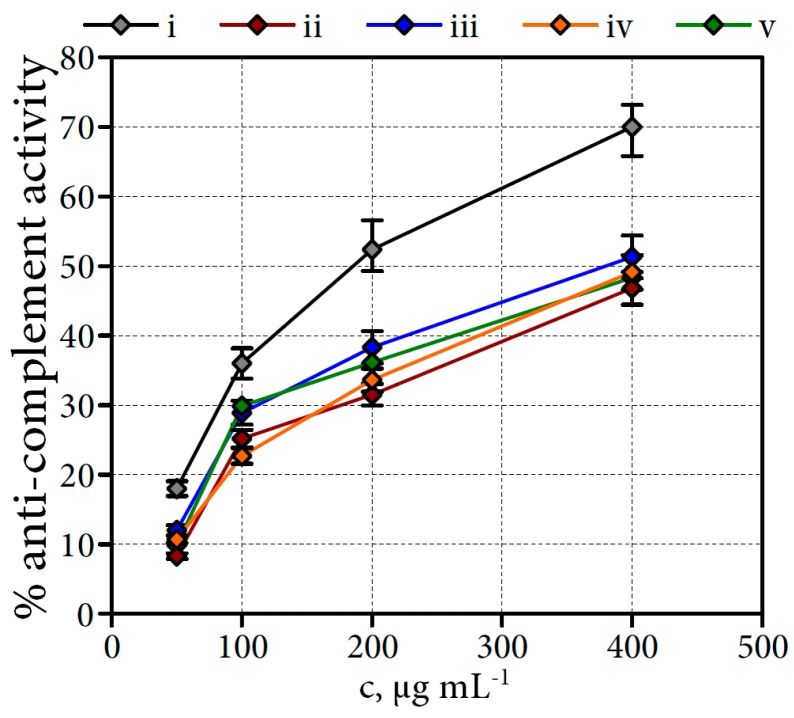
2.6.3. Anti-Complement Activity of Filipendula Flower Water-Soluble Polysaccharides
The test used for the biological activity investigation of WSP was the anti-complement activity test, which is an in vitro test to assess the ability of polysaccharides to interact with the complement cascade reaction [45]. Four crude WSP fractions from Filipendula flowers were tested for their anti-complement activity. As illustrated in Figure 4, all WSP fractions were less effective than the bioactive polysaccharide MPP′-2 from Mentha piperita used as a positive control. WSP from F. denudata flowers were found to have highest activity, i.e., 51.3% at a concentration 400 μg·mL−1 (vs. 70% for the polysaccharide MPP′-2).
Figure 4.
Anti-complement activity of crude water-soluble polysaccharide fractions (WSPS) from four meadowsweet floral teas. Samples: i—polysaccharide MPP′-2 from Mentha piperita (positive control); ii—WSPS from F. camschatica flowers; iii—WSPS from F. denudata flowers; iv—WSPS from F. stepposa flowers; v—WSPS from F. ulmaria flowers.
Polysaccharides from F. camschatica, F. stepposa and F. ulmaria flowers activated the complement system, to a similar extent as the WSP from F. denudata flowers, and are therefore considered to be biologically active in the same manner. The activity modes for all polysaccharide samples were dose-dependent.
Several anti-complement galactans have been isolated from plants. Examples of bioactive polysaccharides are (arabino)galactans from Angelica acutiloba [46], Plantago major [47] and Calendula officinalis [48]. This is the first report of the anti-complement activity of WSP from meadowsweet plants.
The complement system is a part of the immune system consisting of different serum proteins activated by means of a cascade mechanism. Activation of the complement system is important in initiating inflammatory processes and in inducing leukocyte activation and the degranulation of basophils and mast cells [45]. The present results demonstrate the potent anti-complement activity of the crude WSP fractions of Filipendula flowers, and indicate their potent anti-inflammatory and immune-modulating ability.
3. Materials and Methods
3.1. Plant Materials and Chemicals
The samples of meadowsweet flowers were collected in the appropriate flowering period: F. camschatica (Pall.) Maxim. (syn. F. camtschatica (Pall.) Maxim., F. kamtschatica auct.)—Zaozernyi (Kamchatka Krai; 14.VII.2012, 53°01′55″ N, 158°78′01″ E, voucher specimen No. FRo/ae-31/15-08/0712); F. denudata (J.Presl & C.Presl) Fritsch (syn. F. ulmaria subsp. denudata (J.Presl & C.Presl) Hayek)—Kremeno (Gatchina District, Leningrad Oblast; 11.VII.2012, 59°35′21′′ N, 30°29′21′′ E, voucher specimen No. FRo/ae-24/16-08/0712); F. stepposa Juz. (syn. F. ulmaria subsp. picbaueri (Podp.) Smejkal)—Suzun (Suzunsky District, Novosibirsk Oblast; 5.VII.2012, 53°77′08′′ N, 82°28′07′′ E, voucher specimen No. FRo/ae-22/14-08/0712); F. ulmaria—Yakutsk (Yakutskii region, Sakha (Yakutia) Republic; 14.VI.2015, 62°9′57″ N, 129°36′42″ E, voucher specimen No. FRo/ae-03/18-41/0615). The species was determined by Prof. T.A. Aseeva (IGEB SB RAS, Ulan-Ude). The flowers were dried in a convective drying oven UT-4610 (Ulab, Sankt-Petersburg, Russia) at 40 °C (20–24 h) up to the humidity level 9%–12%. The flowers were grounded in an analytical mill A11 basic (IKA®-WerkeGmbH&Co.KG, Staufen, Germany) and then sieved using sieving machine ERL-M1 (Zernotekhnika, Moscow, Russia) up to an average particle diameter of 0.5 mm. The chemicals were purchased in Biosupplies Australia Ply Ltd. (Victoria, Australia)—Yariv reagent kit; Extrasynthese (Lyon, France)—quercetin-3-O-β-d-glucoside (isoquercitrin), quercetin-3-O-β-d-glucuronide (miquelianin), quercetin-3-O-α-l-rhamnoside (quercitin), quercetin-4′-O-β-d-glucoside (spiraeoside); Sigma-Aldrich (St. Louis, MO, USA)—acarbose, α-amylase from Aspergillus oyzae (30 U/mg), 4-aminoantipyrin, anthrone, arabinose, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), bovine serum albumin (BSA), Bradford reagent, caffeic acid, β-carotene, citric acid, 1,3-di-O-caffeoylquinic acid, 3,5-dimethylphenol, 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), dimethylsulphoxide (DMSO), ellagic acid, fructose, galactose, galacturonic acid, gallic acid, α-glucosidase from Saccharomyces cerevisiae (type I, 10 U/mg), glucose, glucose oxidase from Aspergillus niger (type II, 15,000 U/g), glucuronic acid, hydrogen peroxide (ca. 30%), kaempferol-3-O-α-l-rhamnoside (afzelin), lithium perchlorate, malic acid, mannose, methyl salicylate, p-nitrophenyl α-d-glucopyranoside, oleic acid, oxalic acid, perchloric acid (≥70%, 99.999% trace metals basis), peroxidase from horseradish (150 U/mg), protocatechuic acid, starch soluble, quercetin, quercetin-3-O-α-l-arabinoside (avicularin), quinic acid, resorcinol, rhamnose, sodium persulphate, succinic acid, sucrose, tartaric acid, trichloroacetic acid (TCA), Tween® 80, xylose. Kaempferol-4′-O-β-d-glucoside, tellimagrandins I and II, rugosins B1, B2, E1, E2 and D were isolated previously from F. ulmaria [21]. Equipment used for UV-Vis spectrophotometry was SF-2000 UV-Vis-spectrophotometer (OKB Specter; St. Peterburg, Russia); analyt. MC-HPLC—microcolumn chromatograph MiLiChrom A-02 (Econova; Novosibirsk, Russia); mineral analysis—ELAN DRC II mass-spectrometer (PerkinElmer, Inc.; Shelton; CT; USA); GC/MS—6890 N gas chromatograph coupled to a 5973 N mass selective/quadrupole detector (Agilent Technologies; Santa Clara, CA, USA); fluorimetry—Fluorat 02-5M fluorimeter (Lumex; Saint Petersburg, Russia); coulometry—Expert-006 potentiostat (Econix-Expert; Moscow, Russia).
3.2. Organoleptic and Nutritional Analysis
3.2.1. Decoction Preparation
The sample of dried milled herb (1 g) in thermostable flat-bottomed flask (250 mL) was mixed with distilled water (100 mL), reflux condenser was attached with flask and then boiled on heater plate at 10 min. The mixture was left to stand at room temperature for 15 min, and then filtered throw the 0.45 μm PTFE filter under reduced pressure into the volumetric flask (100 mL) and volume was filled with distilled water up to 100 mL.
3.2.2. Crude Composition
Organoleptic parameters (color, odor, taste) of meadowsweet teas were determined accordingly AHPA guidance on Organoleptic Analysis [49]. Extractives and ash were determined accordingly WHO recommendations [50]. The protein content was estimated by Bradford method using BSA as a reference substance [51]. The lipid content was determined by extracting a known weight of dried meadowsweet tea with chloroform–methanol mixture (4:1) using Soxhlet apparatus. Carbohydrate content was determined with spectrophotometric phenol–sulphuric acid method [52]. Energy was calculated according to the following equation: Energy (kcal) = 4 × (g protein + g carbohydrate) + 9 × (g lipids). Free amino acids content was determined with ninhydrin method [50].
3.2.3. Free Sugars Composition
Free sugars were determined by a Milichrom A-02 microcolumn HPLC system, using Separon 5-NH2 column (1 mm × 60 mm, Ø 1 μm; Tessek Ltd.; Prague, Czech Republic), column temperature was 20 °C. Mobile phase was acetonitrile–water 75:25. The injection volume was 1 μL, and elution was at 100 μL/min. Detector wavelength was 190 nm. Decoctions of meadowsweet floral herb teas prepared accordingly a protocol described in Section 3.2.1 were filtered through a 0.22 μm PTFE syringe filter before injection into the HPLC system for analysis. Stock solutions of standards were made by accurately weighing 10 mg of fructose, galactose, glucose and saccharose and dissolving it in 10 mL of water in a volumetric flask. The appropriate amounts of stock solutions were diluted with water in order to obtain standard solutions containing 0.25–1.00 mg/mL. As all the compounds used for quantification were well-separated in experiment conditions mixtures of standards were analyzed. Prepared solutions were stored at 4 °C for no more than 10 h. The results are presented as mean values ± SD (standard deviations) of the three replicates.
3.2.4. Organic Acids Composition
Organic acids were determined by a Milichrom A-02 microcolumn HPLC system, using a ProntoSIL-120-5-C18 AQ column (1 mm × 70 mm, Ø 5 μm; Metrohm AG; Herisau, Switzerland), column temperature was 35 °C. Eluent A was 0.2 M LiClO4 in 0.01 M HClO4 and eluent B was acetonitrile. The injection volume was 1 μL, and elution was at 50 μL/min. Gradient programme: 0–20 min, 1% B; 20–25 min, 1%–10% B. Detector wavelength was 210 nm. Decoctions of meadowsweet floral herb teas prepared accordingly a protocol described in Section 3.2.1 were filtered through a 0.22 μm PTFE syringe filter before injection into the HPLC system for analysis. Stock solutions of standards were made by accurately weighing 2 mg of citric, malic, oxalic, quinic, succinic and tartaric acids and dissolving it in 10 mL of water in a volumetric flask. The appropriate amounts of stock solutions were diluted with water in order to obtain standard solutions containing 0.10–0.50 mg/mL. As all the compounds used for quantification were well-separated in experiment conditions mixtures of standards were analyzed. Prepared solutions were stored at 4 °C for no more than 10 h. The results are presented as mean values ± SD (standard deviations) of the three replicates.
3.2.5. Amino Acids Composition
Amino acids were determined as phenylthiocarbamyl derivatives using a Milichrom A-02 microcolumn HPLC system with a Nucleosil-100-C18 column (2 mm × 75 mm, Ø 5 μm; Macherey-Nagel GmbH & Co. KG; Düren, Germany), column temperature was 60 °C. Eluent A was mixture of 1 M CH3COONH4/H3PO4 (pH 5.25) and water (5:95) and eluent B was mixture of 1 M CH3COONH4/H3PO4 (pH 6.50), acetonitrile and water (5:35:60). The injection volume was 2 μL, and elution was at 150 μL/min. Gradient programme: 0–6.6 min, 0%–12% B; 6.6–21.3 min, 12%–65% B; 21.3–30 min, 100% B. Detector wavelength was 246 nm. Decoctions of meadowsweet floral teas prepared accordingly a protocol described in Section 3.2.1 were filtered through a 0.22 μm PTFE syringe filter before derivatization procedure using Scholtze method [53]. Sigma standard mixture of 22 amino acids (Cat. No. 094165, ≥99%) was used as a reference mixture. As all the compounds used for quantification were well-separated in experiment conditions mixtures of standards were analyzed. Prepared solutions were stored at 4 °C for no more than 72 h. The results are presented as mean values ± SD (standard deviations) of the three replicates.
3.2.6. Minerals Composition
The content of Ca, Cr, Co, Cu, Fe, Mg, Mn, Mo, Ni, Se, Zn were determined in meadowsweet floral tea decoctions by inductively coupled plasma mass spectrometry (ICP-MS) using ELAN DRC II mass-spectrometer (PerkinElmer, Inc., Shelton, CT, USA). The protocol used has been described elsewhere [54]. Decoctions of meadowsweet floral herb teas prepared accordingly a protocol described in Section 3.2.1 were filtered through a 0.22 μm PTFE syringe filter before analysis. The results are presented as mean values ± SD (standard deviations) of the three replicates.
3.3. MC-RP-HPLC Quantification of Phytochemicals in Meadowsweet Teas
MC-RP-HPLC experiments were performed on an MiLiChrom A-02 microcolumn chromatograph coupled with a UV-detector, using a ProntoSIL-120-5-C18 AQ column (1 × 50 mm, Ø 1 μm; Metrohm AG; Herisau, Switzerland); the column temperature was 35 °C. Eluent A was 0.2 M LiClO4 in 0.01 M HClO4 and eluent B was acetonitrile. The injection volume was 1 μL, and elution was at 600 μL/min. Gradient program: 0–1.9 min, 7%–22% B; 1.9–2.2 min, 22%–25% B; 2.2–3.0 min, 25%–27% B; 3.0–4.3 min, 27%–100% B; 4.3–5.0 min, 100% B. UV-detector wavelengths were 270 nm. Decoctions of meadowsweet floral herb teas prepared accordingly a protocol described in Section 3.2.1 were filtered through a 0.22 μm PTFE syringe filter before injection into the HPLC system for analysis. Stock solutions of standards were made by accurately weighing 1 mg of gallic acid, protocatechuic acid, caffeic acid, 1,3-di-O-caffeoylquinic acid, ellagic acid, methyl salicylate, tellimagrandins I and II, rugosins B1, B2, E1, E2, D, quercetin-3-O-β-d-glucoside (isoquercitrin), quercetin-3-O-α-l-arabinoside (avicularin), quercetin-3-O-β-d-glucuronide (miquelianin), quercetin-3-O-α-l-rhamnoside (quercitin), quercetin-4′-O-β-d-glucoside (spiraeoside), kaempferol-3-O-α-l-rhamnoside (afzelin), kaempferol-4′-O-β-d-glucoside and quercetin and dissolving it in 20 mL of methanol/DMSO in a volumetric flask. The appropriate amounts of stock solutions were diluted with methanol in order to obtain standard solutions containing 0.25–1.00 mg/mL. As all the compounds used for quantification were well-separated in experiment conditions mixtures of standards were analyzed. Prepared solutions were stored at 4 °C for no more than 72 h. The results are presented as mean values ± SD (standard deviations) of the three replicates.
3.4. Essential Oil Analysis
Dry flowers of Filipendula species (300 g) were subjected to hydrodistillation in Clevenger-type apparatus for 150 min, which gave the essential oil. GC/MS analysis was performed on an Agilent 6890 N gas chromatograph coupled to a Agilent Technologies 5973 N mass selective/quadrupole detector using a fused capillary column HP-5MS (30 m × 0.25 mm, film thickness 0.50 μm, 5% diphenyl- and 95% dimethylpolysiloxane stationary phase). Splitless injection of 0.2 μl sample solution in hexane (~1%) was performed using an injection port temperature of 250 °C. The carrier gas was helium at a flow of 1.0 mL/min. The column temperature was programmed from 150 to 250 °C at 2.0 °C/min. The ion source temperature was 230 °C. The EIMS spectra (70 eV) were obtained in the scan mode in m/z range 41–450 a.u.m. Identification of compounds was made by comparison of their retention times with those of analytical standards of available terpenoids, on comparison of mass spectra with those found in the literature, and the mass spectrometry data bank (NIST 05) and computer search of the Wiley library. For quantification purposes, relative area percentages by FID were used.
3.5. Polysaccharide Analysis
The sample of dried milled herb (100 g) was added to distilled water (10 L), heated on a boiled water bath (1 h) and af ter cooling to room temperature water extract was filtered under reduced pressure and concentrated down in vacuo to 200 mL. The concentrated residue was mixed with 95% ethanol (1:5) and after 2 h the precipitate was centrifuged at 3000 g. The crude polysaccharide fraction was redissolved in 200 mL of water. The Sevag method [55] was used for deproteinisation, and was followed by dialysis for 48 h against distilled water using dialysis tubes with an MW-cut off of 2 kDa (Sigma-Aldrich, St. Louis, MO, USA). The non-dialysed part was loaded on to a KU-2-8 cation-exchange resin column (H+-form, 200 g; Closed Joint-Stock Company Tokem, Kemerovo, Russia) which was eluted with 2 L of distilled water. Eluate was concentrated in vacuo up to 200 mL and then liophylized. WSPF obtained were off-white powders. Total carbohydrate content was determined with the spectrophotometric anthrone-sulphuric acid method [56]; content of uronic acids was estimated by the 3,5-dimethylphenol method calculated as galacturonic acid [57]; and the proteins were determined by the Bradford method using Coomassie G250 [51]. Reactions of WSPF solutions with iodine, resorcinol and Yariv reagent were performed accordingly [58,59,60]. IR spectra were registered in a spectral range of 4000–600 cm−1 using a FT-801 Fourier-transform infrared spectrometer (Simex, Novosibirsk, Russia) coupled with a single reflection ATR device. The hydrolysis procedure and HPLC analysis conditions of the released products were as described by us previously [61].
3.6. Biological Activity
3.6.1. Amylase Inhibitory Activity
Amylase inhibitory activity was assayed according to a previously published spectrophotometric protocol [62]. Sample solution in DMSO (10 μL), 30 μL of phosphate buffer (pH 5.0) and 10 μL of amylase from Aspergillus niger (3 U/mL) were incubated for 20 min at 45 °C. Then 10 μL of 2% starch solution, 40 μL of phosphate buffer (pH 5.0) and 100 μL of the reagent were added and incubated for 30 min at 50 °C. Absorbance was measured at 510 nm. The reagent was a solution of K2HPO4 (0.8 mM), KH2PO4 (0.4 mM), phenol (220 mM), 4-aminoantipyrine (1.5 μM), glucose oxidase from Aspergillus niger (3 U/mL), and peroxidase from horseradish (0.3 U/mL) in deionized water. A 2% solution of acarbose was used as a positive control (PC), and water was used as a negative control (NC). The ability to inhibit amylase was calculated using the following equation: Inhibitory ability (%) = [(A510 NC − A510 PC) – (A510 Sample − A510 PC) / (A510 NC − A510 PC)] × 100, where A510 NC is the absorbance of the negative control, A510 PC is the absorbance of the positive control and A510 Sample is the absorbance of the sample solution. The IC50 value is the effective concentration at which amylase activity was inhibited by 50%. Values are expressed as mean obtained from five independent experiments.
3.6.2. α-Glucosidase Inhibitory Activity
The α-glucosidase inhibition assay was performed using spectrophotometric method [63]. α-Glucosidase from Saccharomyces cerevisiae was dissolved in phosphate buffer (pH 6.8) containing BSA (0.2%) up to 0.5 U/mL concentration. Solution (10 μL) of sample in phosphate buffer (pH 6.8) at varying concentrations (10–1000 μg/mL) was premixed with 490 μL of phosphate buffer (pH 6.8) and 250 μL 5 mM p-nitrophenyl α-d-glucopyranoside. After preincubating at 37 °C for 5 min, 250 μL α-glucosidase (0.4 U/mL) was added and incubated at 37 °C for 15 min. The reaction was terminated by the addition of 2000 μL Na2CO3 (200 mM). Absorbance was measured at 400 nm. A 2% solution of acarbose was used as a positive control (PC), and water was used as a negative control (NC). The ability to inhibit amylase was calculated using the following equation: Inhibitory ability (%) = [(A400NC – A400 PC) – (A400Sample – A400 PC)/(A400 NC – A400 PC)] × 100, where A400 NC is the absorbance of the negative control, A400 PC is the absorbance of the positive control and A400 Sample is the absorbance of the sample solution. The IC50 value is the effective concentration at which α-glucosidase activity was inhibited by 50%. Values are expressed as mean obtained from five independent experiments.
3.6.3. AGEs Formation Inhibitory Activity
The inhibitory effect on AGEs formation reaction was carried out by fluorimetric method of Matsuura et al. [64]. The reaction mixture consisted of 4 mg BSA in 400 μL 50 mM sodium phosphate buffer (pH 7.4), 80 μL 1 M glucose, and 20 μL of sample (meadowsweet tea decoction or pure compound in water or DMSO). The reaction solution was incubated at 60 °C for 48 h. The blank sample which contained no meadowsweet tea decoction or pure compound was kept at 4 °C until measurement. After cooling aliquots of 250 μL were transferred to 1.5 mL plastic tubes, then, 25 μL TCA was added to each tube and stirred. The supernatant was removed after centrifugation (15,000 rpm) at 4 °C for 4 min and AGEs-BSA precipitate was dissolved with 1 mL of sodium phosphate buffer (pH 7.4). This solution was monitored fluorimetrically in fluorescence intensity (ex. 360 nm, em. 460 nm). The ability to inhibit AGEs formation was calculated using the following equation: Inhibitory ability (%) = [1 − (FS/FB)] × 100, where FS is the fluorescence of the sample, FB is the fluorescence of the blank. Values are expressed as mean obtained from three independent experiments.
3.6.4. DPPH-HPLC-UV Procedure
The DPPH-HPLC-UV procedure was realized as described previously [65]. Briefly, a sample of the meadowsweet tea decoction (100 μL) were added to DPPH• radical solution in methanol (250 μL, 20 mg/mL). The mixture was shaken for few seconds and left to stand in the dark for 30 min at room temperature. Then the sample was filtered through a 0.22 μm membrane filter. The untreated sample was prepared by adding a sample of the meadowsweet tea decoction (100 μL) to methanol (250 μL). HPLC analysis was performed on as described in a Section 3.4.
3.6.5. DPPH• Radical Scavenging Activity
The DPPH• radical scavenging activity (DPPH•) was assessed as described by Asker and Shawky [66]. 500 μL of a DPPH• methanol solution (freshly prepared, 100 μg/mL) was added to 500 μL of sample solution (meadowsweet tea decoction or pure compound in methanol). After 15 min absorbance was measured at 520 nm. A 0.01% solution of gallic acid was used as a positive control (PC), and water was used as a negative control (NC). The ability to scavenge DPPH• radicals was calculated using the following equation: Scavenging ability (%) = [(A520NC − A520PC) – (A520Sample − A520PC)/(A520 NC − A520 PC)] × 100, where A520NC is the absorbance of the negative control, A520PC is the absorbance of the positive control and A520Sample is the absorbance of the sample solution. The IC50 value is the effective concentration at which DPPH• radicals were scavenged by 50%. Values are expressed as mean obtained from five independent experiments.
3.6.6. ABTS•+ Radical Scavenging Activity
The ABTS•+ radical scavenging activity (ABTS•+) was measured using the method of Ding et al. [67]. ABTS was dissolved in water to a concentration of 7 mM. ABTS•+ radical cations were produced by reacting this ABTS•+ stock solution with 2.45 mM potassium persulphate (final concentration) and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. The ABTS•+ radical cation solution was diluted with methanol to an absorbance of 0.70 at 734 nm and equilibrated at 30 °C. An aliquot of sample solution (500 μL) was mixed with 500 μL of diluted ABTS•+ radical cation solution. After reaction at 30 °C for 20 min, the absorbance was measured at 734 nm. A 0.01% solution of gallic acid was used as a positive control (PC), and water was used as a negative control (NC). The ability to scavenge the ABTS•+ radical cation was calculated using the following equation: Scavenging ability (%) = [(A734NC – A734PC) – (A734Sample – A734PC) / (A734 NC – A734 PC)] × 100, where A734NC is the absorbance of the negative control, A734PC is the absorbance of the positive control and A734Sample is the absorbance of the sample solution. The IC50 value is the effective concentration at which ABTS•+ radicals were scavenged by 50%. Values are expressed as mean obtained from five independent experiments.
3.6.7. Br• Radical Scavenging Activity
The bromine radical scavenging activity (Br•-SA) was determined using culometric method [68] with electrogenerated bromine radicals. Coulometric measurements were carried out using Expert-006 potentiostat with four-electrode two-compartment electrochemical cell. A bare platinum foil with 1 cm2 surface area was used as the working electrode, and a platinum wire separated from the anodic compartment with a semipermeable diaphragm—as the auxiliary electrode. A pair of polarized platinum electrodes was used for detection of the titration end-point (ΔE = 200 mV). Surface of platinum electrodes was cleaned by HNO3 and then rinsed thoroughly with double distilled water. Electrochemical generation of titrant was carried out from 0.2 M KBr in 0.1 M H2SO4 at a current density 5 mA∙cm−2 providing 100% current yield. Coulometric titration was carried out in a 50 mL cell containing 20.0 mL of supporting electrolyte. The generating circuit was switched on and a certain value of the indicator current was attained. Then an aliquot portion (50 μL) of meadowsweet tea decoction (or gallic acid, 1 mg/mL, as a reference compound) was added to the cell and timer was simultaneously started. The titration end-point was detected by the attainment of the initial value of the indicator current. The timer was stopped, and the generating circuit was turned off. The time of titration were used for the Br•-SA calculation that were expressed in units of quantity of electricity (Coulombs (C)) spent for titration on 100 mL of meadowsweet tea decoction. Finally the value of Br•-SA was calculated as a mg gallic acid equivalents per g extract. Values are expressed as mean obtained from five independent experiments.
3.6.8. β-Carotene Bleaching Assay
β-Carotene bleaching assay (CBA) was performed in β-carotene–oleic acid–DMSO–H2O2 system [69]. Two milligrams of β-carotene, 16 mg of oleic acid, and 160 mg of Triton X-100 were dissolved in 50 mL of chloroform, and organic solvent was removed in a vacuum at 30 °С. The residue was solved in bidistilled water (50 mL); solution was removed to a volumetric flask (100 mL) and water was added until the total volume reached 100 mL. The reaction solutions was prepared in a tubes by mixing 50–250 μL of test solution, 500 μL DMSO, 500 μL β-carotene and 500 μL of 3% H2O2 solution. The total volume of reaction solution was reached by adding of bidistilled water up to 2 mL. The mixture was placed in a thermostat at 50 °С for 120 min. Absorbance was measured at 460 nm at 0 min and 120 min after incubation. The ability to protect β-carotene was calculated using the following equation: Inhibitory ability (%) = 100 − {[(A0 – A120)/A0] × 100}, where A0 is the absorbance after 0 min of incubation, A120 is the absorbance after 120 min of incubation. The IC50 value is the effective concentration allowed to protect 50% of β-carotene. Values are expressed as mean obtained from three independent experiments.
3.6.9. Complement Fixation Test
The complement fixation test based on inhibition of hemolysis of antibody sensitized sheep red blood cells by the complement from human sera was used [70]. Veronal buffer/bovine serum albumin, serum, and sensitized sheep erythrocytes were the control of the medium, and the rhamnogalacturonan MPP′-2 from Mentha piperita was used as positive control [71]. The indicator system in the assay is inhibition of haemolysis induced by human complement. Samples showing inhibition in the assay is thus having a direct effect on the human immune system. Inhibition of lysis induced by the test samples was calculated by the formula: Inhibition (%) = [(AC – AS)/AC] × 100, where AC is the absorbance the control, AS is the absorbance of the sample. Values are expressed as mean obtained from three independent experiments.
3.7. Statistical Analysis
Statistical analyses were performed using a one-way analysis of variance (ANOVA), and the significance of the mean difference was determined by Duncan’s multiple range test. Differences at p < 0.05 were considered statistically significant. The results are presented as mean values ± SD (standard deviations) of the three replicates.
4. Conclusions
The present study provides detailed data on the nutritional profile, phenolic, essential oil, water-soluble polysaccharide composition and bioactivity of four herbal products used in Siberia, namely Filipendula camschatica, F. denudata, F. stepposa and F. ulmaria floral teas, or meadowsweet teas in general. As far as we know, up to now, this is the first study to report data on these parameters for the studied products. Macronutrients were found in appropriate amounts in meadowsweet teas, with carbohydrates being the predominant component. Phenolic profiling of the meadowsweet teas revealed high contents in flavonols and ellagitannins. Interestingly, the meadowsweet teas were also found to be a source of bioactive volatiles such as salicyaldehyde and methyl salicylate, which are the components of the essential oils of Filipendula flowers. It should be noted that the water-soluble components were characterized by the presence of polymeric carbohydrates with a high content of galactose. The bioactivity data demonstrated the good ability of meadowsweet teas to inhibit amylase, α-glucosidase and AGE formation, and also expressed antioxidant properties. The anti-complement activity of the water-soluble polysaccharide fraction indicated their possible immune-modulating properties. The results highlight that meadowsweet can be considered as a new natural source of functional beverages due to the high content of health-promoting compounds, including anti-diabetic and antioxidant phenolics and immune-active polysaccharides.
Acknowledgments
The reported study was funded by RFBR and Government of Republic of Buryatia according to the research project No. 16-43-030857.
Author Contributions
D.N.O. and N.I.K. designed research; N.I.K. and N.K.C. performed research and analyzed the data; D.N.O. and N.I.K. wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of Filipendula camschatica, F. denudata, F. stepposa and F. ulmaria plants and extracts are available from the authors.
References
- 1.Zakaria N.Z.I., Masnan M.J., Zakaria A., Shakaff A.Y.M. A bio-inspired herbal tea flavor assessment technique. Sensors. 2014;14:12233–12255. doi: 10.3390/s140712233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukherjee P.K. Evidence-Based Validation of Herbal Medicine. 1st ed. Volume 1. Elsevier; Amsterdam, The Netherlands: 2015. Value chains of herbal medicines—Ethnopharmacological and analytical challenges in a globalizing world; pp. 29–42. [Google Scholar]
- 3.Junior E.L.C., Morand C. Interest of mate (Ilex paraguariensis A. St.-Hil.) as a new natural functional food to preserve human cardiovascular health—A review. J. Funct. Foods. 2016;21:440–454. doi: 10.1016/j.jff.2015.12.010. [DOI] [Google Scholar]
- 4.Zengin G., Uysal A., Gunes E., Aktumsek A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olennikov D.N., Kashchenko N.I., Chirikova N.K., Tankhaeva L.M. Iridoids and flavonoids of four Siberian gentians: Chemical profile and gastric stimulatory effect. Molecules. 2015;20:19172–19188. doi: 10.3390/molecules201019172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olennikov D.N., Kashchenko N.I., Chirikova N.K., Koryakina L.P., Vladimirov L.N. Bitter gentian teas: Nutritional and phytochemical profiles, polysaccharide characterisation and bioactivity. Molecules. 2015;20:20014–20030. doi: 10.3390/molecules201119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghedira K., Goetz P., Jeune R. Reine-des-prés (sommité fleurie de) Filipendula ulmariae (L.) Maxim. Phytothérapie. 2011;9:318–322. doi: 10.1007/s10298-011-0660-3. [DOI] [Google Scholar]
- 8.Lindeman A., Jounelaeriksson P., Lounasmaa M. The aroma composition of the flower of meadowsweet (Filipendula ulmaria (L.) Maxim) Lebensm. Wiss. Technol. 1982;15:286–289. [Google Scholar]
- 9.Toiu A., Vlase L., Oniga I., Benedec D., Tămaş M. HPLC analysis of salicylic derivatives from natural products. Farmacia. 2011;59:106–112. [Google Scholar]
- 10.Blazics B., Papp I., Kery A. LC-MS qualitative analysis and simultaneous determination of six Filipendula salicylates with two standards. Chromatographia. 2010;71:S61–S67. doi: 10.1365/s10337-010-1502-4. [DOI] [Google Scholar]
- 11.Wilkes S., Glasl H. Isolation, characterization, and systematic significance of 2-pyrone-4,6-dicarboxylic acid in Rosaceae. Phytochemistry. 2001;58:441–449. doi: 10.1016/S0031-9422(01)00256-4. [DOI] [PubMed] [Google Scholar]
- 12.Bijttebier S., Van der A.A., Voorspoels S., Noten B., Hermans N., Pieters L., Apers S. A first step in the quest for the active constituents in Filipendula ulmaria (meadowsweet): Comprehensive phytochemical identification by liquid chromatography coupled to quadrupole-orbitrap mass spectrometry. Planta Med. 2016;82:559–572. doi: 10.1055/s-0042-101943. [DOI] [PubMed] [Google Scholar]
- 13.Bączek K., Cygan M., Przybył J.L., Kosakowska O., Węglarz Z. Seasonal variation of phenolics content in above- and underground organs of dropwort (Filipendula vulgaris Moench) Herba Pol. 2012;58:24–32. [Google Scholar]
- 14.Pemp E., Reznicek G., Krenn L. Fast quantification of flavonoids in Filipendulae ulmariae flos by HPLC/ESI-MS using a nonporous stationary phase. J. Anal. Chem. 2007;62:669–673. doi: 10.1134/S1061934807070106. [DOI] [Google Scholar]
- 15.Abe I., Kashiwagi Y., Noguchi H., Tanaka T., Ikeshiro Y., Kashiwada Y. Ellagitannins and hexahydroxydiphenoyl esters as inhibitors of vertebrate squalene epoxidase. J. Nat. Prod. 2001;64:1010–1014. doi: 10.1021/np010100y. [DOI] [PubMed] [Google Scholar]
- 16.Piwowarski J.P., Granica S., Zwierzyńska M., Stefańska J., Schopohl P., Melzig M.F., Kiss A.K. Role of human gut microbiota metabolism in the anti-inflammatory effect of traditionally used ellagitannin-rich plant materials. J. Ethnopharmacol. 2014;155:801–809. doi: 10.1016/j.jep.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Radulović N., Mišić M., Aleksić J., Ðoković D., Palić R., Stojanović G. Antimicrobial synergism and antagonism of salicylaldehyde in Filipendula vulgaris essential oil. Fitoterapia. 2007;78:565–570. doi: 10.1016/j.fitote.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Pavlovic M., Petrovic S., Ristic M., Maksimovic Z., Kovacevic N. Essential oil of Filipendula hexapetala. Chem. Nat. Comp. 2007;43:228–229. doi: 10.1007/s10600-007-0088-z. [DOI] [Google Scholar]
- 19.Karimova O.A., Zhigunov O.Y. Introduction of some varieties of Filipendula Mill. genus in Ufa Botanic Garden. Biol. Sci. 2016;58:146–148. (In Russian) [Google Scholar]
- 20.Gudkova N.Y. Perspectives of introduction of meadowsweet (Filipendula Mill.) as a source of medicinal raw material. Agric. Biol. 2012;47:73–79. (In Russian) [Google Scholar]
- 21.Olennikov D.N., Kruglova M.Y. A new quercetin glycoside and other phenolic compounds from the genus Filipendula. Chem. Nat. Comp. 2013;49:610–616. doi: 10.1007/s10600-013-0691-0. [DOI] [Google Scholar]
- 22.Aseeva T.A. Tibetan Medicine of Buryats. Publishing House of Russian Academy of Science; Novosibirsk, Russia: 2008. [Google Scholar]
- 23.Makarov A.A. Plant remedies of the Traditional Yakutian Medicine. Yakutian Scientific Center; Yakutsk, Russia: 1974. [Google Scholar]
- 24.Anonymous. Buryats. Nauka; Moscow, Russia: 2004. [Google Scholar]
- 25.Zykova I.D., Efremov A.A., Gerasimov V.S., Leshok A.A. Features of the accumulation of macro- andmicronutrients in the aboveground parts of Filipendula ulmaria (L.) Maxim. in different phonological phases. Chem. Plant Raw Mater. 2013;16:189–193. [Google Scholar]
- 26.Enel M. Distribution of heavy metals in plants and their habitats in the outcrop area of Dictyonema shale. Oil Shale. 2003;20:459–476. [Google Scholar]
- 27.Szefer P., Nriagu J.O. Mineral Components in Foods. CRC Press, Taylor Francis Group; London, UK: New York, NY, USA: 2007. [Google Scholar]
- 28.Valle M.G., Nano G.M., Tira S. The essential oil of Filipendula ulmaria. Planta Med. 1988;54:181–182. doi: 10.1055/s-2006-962390. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y., Meng X., Liu X., Bao Y., Wang S. Analysis of essential oils from flower-buds, leaves and stems of Filipendula palmata (Pall.) Maxim. Chem. Res. Chin. Univ. 2005;21:658–662. [Google Scholar]
- 30.Kačuráková M., Capek P., Sasinková V., Wellner N., Ebringerová A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000;43:195–203. doi: 10.1016/S0144-8617(00)00151-X. [DOI] [Google Scholar]
- 31.Arifkhodzhaev A.O. Galactans and galactan-containing polysaccharides of higher plants. Chem. Nat. Comp. 2000;36:229–244. doi: 10.1007/BF02238327. [DOI] [Google Scholar]
- 32.De Sales P.M., de Souza P.M., Simeoni L.A., de Olivera Magalhães P., Silveira D. α-Amylase inhibitors: A review of raw material and isolated compounds from planr source. J. Pharm. Pharm. Sci. 2012;15:141–183. doi: 10.18433/J35S3K. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto K., Yasujima M., Yagihashi S. Role of advanced glycation end products in diabetic neuropathy. Curr. Pharm. Des. 2008;10:953–961. doi: 10.2174/138161208784139774. [DOI] [PubMed] [Google Scholar]
- 34.Etxeberria U., de la Garza A.L., Campión J., Martínez J.A., Milagro F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets. 2012;16:269–297. doi: 10.1517/14728222.2012.664134. [DOI] [PubMed] [Google Scholar]
- 35.Li H., Tanaka T., Zhang Y.J., Yang C., Kouno I. Rubusuaviins A-F, monomeric and oligomeric ellagitannins from Chinese sweet tea and their alpha-amylase inhibitory activity. Chem. Pharm. Bull. 2007;55:1325–1331. doi: 10.1248/cpb.55.1325. [DOI] [PubMed] [Google Scholar]
- 36.Xiao J., Ni X., Kai G., Chen X. A review on structure-activity relationships of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013;53:497–506. doi: 10.1080/10408398.2010.548108. [DOI] [PubMed] [Google Scholar]
- 37.Yin Z., Zhang W., Feng F., Zhang Y., Kang W. α-Glycosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness. 2014;3:136–174. doi: 10.1016/j.fshw.2014.11.003. [DOI] [Google Scholar]
- 38.Peng X., Ma J., Chen F., Wang M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct. 2011;2:289–301. doi: 10.1039/c1fo10034c. [DOI] [PubMed] [Google Scholar]
- 39.Selenge E., Odontuya G., Murata T., Sasaki K., Kobayashi K. Phytochemical constituents of Mongolian traditional medicinal plants, Chamaerhodos erecta and C. altaica, and its constituents prevents the extracellular matrix degradation factors. J. Nat. Med. 2013;67:867–875. doi: 10.1007/s11418-013-0748-1. [DOI] [PubMed] [Google Scholar]
- 40.Pukalskienė M., Venskutonis P.R., Pukalskas A. Phytochemical characterization of Filipendula ulmaria by UPLC/Q-TOF-MS and evaluation of antioxidant activity. Rec. Nat. Prod. 2015;9:451–455. [Google Scholar]
- 41.Katanić J., Boroja T., Stanković N., Mihailović V., Mladenović M. Bioactivity, stability and phenolic characterization of Filipendula ulmaria (L.) Maxim. Food Funct. 2015;6:1164–1175. doi: 10.1039/C4FO01208A. [DOI] [PubMed] [Google Scholar]
- 42.Maksimović Z., Petrović S., Pavlović M., Kovačević N., Kukić J. Antioxidant activity of Filipendula hexapetala flowers. Fitoterapia. 2007;78:265–267. doi: 10.1016/j.fitote.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Katanić J., Mihailović V., Stanković N., Boroja T., Mladenović M. Dropwort (Filipendula hexapetala Gilib.): Potential role as antioxidant and antimicrobial agent. EXCLI J. 2015;14 doi: 10.17179/excli2014-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pukalskiene M., Venskutonis P.R., Pukalaskas A. Phytochemical composition and antioxidant properties of Filipendula vulgaris as a source of healthy functional ingredients. J. Funct. Foods. 2015;15:233–242. doi: 10.1016/j.jff.2015.03.002. [DOI] [Google Scholar]
- 45.Samuelsen A.B., Lund I., Djahromi J.M., Paulsen B.S., Wold J.K. Structural features and anti-complementary activity of some heteroxylan polysaccharide fractions from the seeds of Plantago major L. Carbohydr. Polym. 1999;38:133–143. doi: 10.1016/S0144-8617(98)00115-5. [DOI] [Google Scholar]
- 46.Kiyohara H., Yamada H. Structure of an anti-complementary arabinogalactan from the root of Angelica acutiloba Kitagawa. Carbohydr. Res. 1989;193:173–192. doi: 10.1016/0008-6215(89)85117-1. [DOI] [PubMed] [Google Scholar]
- 47.Samuelsen A.B., Paulsen B.S., Wold J.K., Knutsen S.H., Yamada H. Characterization of a biologically active arabinogalactan from the leaves of Plantago major L. Carbohydr. Polym. 1998;35:145–153. doi: 10.1016/S0144-8617(97)00238-5. [DOI] [Google Scholar]
- 48.Varljen J., Lipták A., Wagner H. Structural analysis of a rhamnoarabinogalactan and arabinogalactans with immuno-stimulating activity from Calendula officinalis. Phytochemistry. 1989;28:2379–2383. doi: 10.1016/S0031-9422(00)97988-3. [DOI] [Google Scholar]
- 49.Anonymous. Organoleptic Analysis of Herbal Ingredients. American Herbal Products Association; Silver Spring, MD, USA: 2013. [Google Scholar]
- 50.Anonymous. Quality Control Methods for Herbal Materials. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 51.Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;76:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 52.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 53.Scholtze H. Determination of phenylthiocarbamyl amino acids by reversed-phase high-performance liquide chromatography. J. Chromatogr. 1985;350:453–460. doi: 10.1016/S0021-9673(01)93551-4. [DOI] [Google Scholar]
- 54.Wolf R.E., Adams M. Multi-Elemental Analysis of Aqueous Geochemical Samples by Quadrupole Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) 2015. [(accessed on 5 June 2015)]. U.S. Geological Survey Open-File Report 2015-1010. Available online: [DOI]
- 55.Sevag M.G., Lackman D.B., Smolens J. The isolation of the components of Streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938;124:425–436. [Google Scholar]
- 56.Olennikov D.N., Tankhaeva L.M., Samuelsen A.B. Quantitative analysis of polysaccharides from Plantago major using the Dreywood method. Chem. Nat. Comp. 2006;42:265–268. doi: 10.1007/s10600-006-0096-4. [DOI] [Google Scholar]
- 57.Usov A.T., Bilan M.I., Klochkova N.G. Polysaccharides of algae. 48. Polysaccharide composition of several calcareous red algae: Isolation of alginate from Corallina pilulitara P. et R. (Rhodophyta, Corallinaceae) Bot. Mar. 1995;35:43–51. doi: 10.1515/botm.1995.38.1-6.43. [DOI] [Google Scholar]
- 58.Olennikov D.N., Stolbikova A.V., Rokhin A.V., Khobrakova V.B., Tankhaeva L.M. Polysaccharides from Fabaceae. V. α-Glucan from Sophora flavescens roots. Chem. Nat. Comp. 2011;47:1–6. doi: 10.1007/s10600-011-9817-4. [DOI] [Google Scholar]
- 59.Olennikov D.N., Tankhaeva L.M. A quantitative assay for total fructans in burdock (Arctium spp.) roots. Russ. J. Bioorg. Chem. 2011;37:893–898. doi: 10.1134/S1068162011070181. [DOI] [Google Scholar]
- 60.Togola A., Inngjerdingen M., Diallo D., Barsett H., Rolstad B. Polysaccharides with complement fixing and macrophage stimulation activity from Opilia celtidifolia, isolation and partial characterization. J. Ethnopharmacol. 2007;115:423–431. doi: 10.1016/j.jep.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 61.Olennikov D.N., Rokhin A.V. Water-soluble glucans from true cardamom (Elettaria cardamomum White at Maton) seeds. Appl. Biochem. Microbiol. 2013;49:182–187. doi: 10.1134/S0003683813010134. [DOI] [PubMed] [Google Scholar]
- 62.Olennikov D.N., Kashchenko N.I. Componential profile and amylase inhibiting activity of phenolic compounds from Calendula officinalis L. leaves. Sci. World J. 2014;2014:654193. doi: 10.1155/2014/654193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elya B., Basah K., Mun’im A., Yuliastuti W., Bangun A., Septiana E.K. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. BioMed Res. Int. 2012;2012:281078. doi: 10.1155/2012/281078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsuura N., Aradate T., Sasaki C., Kojima H., Ohara M. Screening sysytem for the Maillard reaction inhibitor from natural product extract. J. Health Sci. 2002;48:520–526. doi: 10.1248/jhs.48.520. [DOI] [Google Scholar]
- 65.Olennikov D.N., Kashchenko N.I., Chirikova N.K. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules. 2014;19:18296–18316. doi: 10.3390/molecules191118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asker M.M.S., Shawky B.T. Structural characterization and antioxidant activity of an extracellular polysaccharide isolated from Brevibacterium otitidis BTS 44. Food Chem. 2010;123:315–320. doi: 10.1016/j.foodchem.2010.04.037. [DOI] [Google Scholar]
- 67.Ding H., Chou T., Liang C. Antioxidant and antimelanogenic properties of rosmarinic acid methyl ester from Origanum vulgare. Food Chem. 2010;123:254–262. doi: 10.1016/j.foodchem.2010.04.025. [DOI] [Google Scholar]
- 68.Ziyatdinova G., Salikhova I., Budnikov H. Coulometric titration with electrogenerated oxidants as a tool for evaluation of cognac and brandy antioxidant properties. Food Chem. 2014;150:80–86. doi: 10.1016/j.foodchem.2013.10.133. [DOI] [PubMed] [Google Scholar]
- 69.Olennikov D.N., Tankhaeva L.M., Agafonova S.V. Antioxidant components of Laetiporus sulphureus (Bull.: Fr.) Murr. fruit bodies. Appl. Biochem. Microbiol. 2011;47:419–425. doi: 10.1134/S0003683811040107. [DOI] [PubMed] [Google Scholar]
- 70.Michaelsen T.E., Gilje A., Samuelsen A.B., Hogasen K., Paulsen B.S. Interaction between human complement and a pectin type polysaccharide fraction, PMII, from the leaves of Plantago major L. Scand. J. Immunol. 2000;52:483–490. doi: 10.1046/j.1365-3083.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 71.Olennikov D.N., Tankhaeva L.M. Lamiaceae carbohydrates. 1. Pectinic substances and hemicelluloses from Mentha × piperita. Chem. Nat. Comp. 2007;43:501–507. doi: 10.1007/s10600-007-0177-z. [DOI] [Google Scholar]