Supplemental Digital Content is available in the text
Keywords: meta-analysis, nonalcoholic fatty liver disease, omega-3 polyunsaturated fatty acids
Abstract
Background:
Clinical trials examining the therapeutic benefit of omega-3 polyunsaturated fatty acids (ω-3 PUFAs) on nonalcoholic fatty liver disease (NAFLD) have reported inconsistent results. We performed a meta-analysis of randomized controlled trials (RCTs) examining the effect of ω-3 PUFA supplementation on NAFLD, and provide substantial evidence on whether ω-3 PUFA supplementation has a favorable effect for treating NAFLD.
Methods:
We searched the PubMed, Cochrane Library, Springer Link, China National Knowledge Infrastructure (CNKI), Wanfang, and Chinese Scientific and Technological Journal (VIP) databases for RCTs on oral ω-3 PUFA supplementation in patients with NAFLD. The data were pooled; meta-analyses were conducted using random-effect or fixed-effect models.
Results:
Eighteen studies involving 1424 patients were included. We found a significant benefit for ω-3 PUFAs vs control for liver fat, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transferase, triglycerides, insulin resistance, and glucose. However, there was significant interstudy heterogeneity. Subgroup and regression analyses showed no significantly clear methodologic discrepancy. Publication bias and serious adverse events were not detected.
Conclusions:
Our meta-analysis suggests that ω-3 PUFA supplementation may decrease liver fat and hepatic enzyme parameters. However, more large-scale, well-designed RCTs are needed to confirm the effect of ω-3 PUFA supplementation on these parameters.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses a histological spectrum that ranges from simple steatosis to nonalcoholic steatohepatitis (NASH), which can lead to cirrhosis and liver cancer and is projected to be the principal factor for liver transplantation by 2020.[1,2] The prevalence of NAFLD is steadily increasing and is currently 20% to 30% in Western countries and 5% to 18% in Asia.[1–3] NAFLD is more prevalent in patients with metabolic diseases such as obesity and type 2 diabetes mellitus (T2DM),[4] and is recognized as the hepatic manifestation of the metabolic syndrome (MS).[5,6] Moreover, its prevalence is expected to increase in the near future as an aftermath of the increasing adoption of a sedentary lifestyle and unhealthy diet.[3,4] Therefore, new therapeutic approaches for managing NAFLD are warranted.
Currently, the primary treatment for NAFLD is lifestyle therapy, which involves diet alteration and exercise, which are imperative for achieving weight loss and reducing insulin resistance (IR) and hepatic steatosis/inflammation in patients with NAFLD.[7,8] However, the effectiveness of lifestyle modification is low and wanes with time, underlining the need for pharmacologic therapy.[7] Small cohorts have trialed pharmacotherapies including vitamin E,[9] pioglitazone,[10] and obeticholic acid[11]; their effectiveness is limited by poor compliance. Therefore, there is a pressing need to develop more effective and safe agents for this common disease.
Studies investigating the dietary patterns of patients with NAFLD vs controls have reported that individuals with NAFLD have lower omega-3 polyunsaturated fatty acid (ω-3 PUFA) intake and higher ω-6/ω-3 PUFA intake ratio[12–14]; animal studies have confirmed these findings.[15,16] ω-3 PUFAs may reduce hepatic lipogenesis and inflammation, representing a simple and practical alternative therapy. Therefore, dietary guidelines on nutrient intake for improving NAFLD generally recommend increasing the intake of ω-3PUFA-rich foods and reducing ω-6 PUFA intake. However, clinical trials examining the therapeutic benefit of ω-3 PUFAs in NAFLD have reported inconsistent results. Here, we conducted a meta-analysis of randomized controlled trials (RCTs) on oral ω-3 PUFA treatment of NAFLD to assess the efficacy of ω-3 PUFA supplementation in modifying liver fat content, serum aminotransferases, and other clinical parameters, such as serum lipids, glucose metabolism, and anthropometric parameters.
2. Methods
2.1. Data sources and search strategy
We searched English and non-English language publications on the PubMed, Cochrane Library, Springer Link, China National Knowledge Infrastructure (CNKI), Wanfang, and Chinese Scientific and Technological Journal (VIP) databases from inception to May 2018. The search terms used were: omega-3, ω-3, n-3, PUFA, fish oil, EPA, DHA, PUFAs, eicosapentaenoic acid, docosahexaenoic acid, NAFLD, NASH, NAFLD, nonalcoholic steatohepatitis, fatty liver, and hepatic steatosis. The reference lists of reviewed articles were manually searched for additional relevant studies.
2.2. Study selection
We determined the inclusion and exclusion criteria a priori. Studies were included if they met the following inclusion criteria: population of any age or sex or ethnic origin with NAFLD diagnosed based on histologic or imaging evidence; intervention involving oral administration of ω-3 PUFA supplementation of any dose or duration; comparison with placebo or no intervention; outcomes concerning improvement in liver fat or serum aminotransferases.
The exclusion criteria were: nonhuman studies; fatty liver that was due to excessive alcohol intake, drug-induced, total parenteral nutrition-induced, viral, or genetic; uncontrolled, crossover, cross-sectional, or other non-RCT studies; and not reporting outcomes of interest or primary data.
2.3. Data extraction
Two authors extracted the data independently using a predefined data extraction sheet; discrepancies were resolved by mutual discussion. The outcome measures assessed were number of patients with improvement in the degree of steatosis quantified by biopsy or imaging, mean (and standard deviation) of biochemical levels and anthropometric parameters, and adverse events. If the articles contained insufficient information, we contacted the authors to obtain the missing details. Where this was unsuccessful, the missing data were calculated from the raw numbers and P-values reported.[17]
2.4. Quality assessment
Two authors assessed study quality using the Cochrane risk of bias tool,[17,18] which includes the following items: sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting and other bias with possible risk of low, unclear, or high bias; and intention-to-treat (ITT) analysis. If the authors omitted the methodologic details in their published reports, we subsequently contacted them to obtain information on the methodologies used in their RCTs. Disagreement was resolved by consensus after discussion.
2.5. Statistical analysis
Meta-analysis was performed in Review Manager 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark) and Stata 12.0 (Stata Corporation, College Station, TX). Dichotomous variables are presented as relative risk (RR) and 95% confidence interval (CI). Continuous variables are presented as weighted mean difference (WMD) (statistics were unit-consistent) or standardized mean differences (SMDs) (statistics were unit-inconsistent) with 95% CI. The I2 index was used to quantify the extent of heterogeneity, and statistical significance was defined as P < .05. A random-effect model was used to pool the study data if I2 ≥ 50% or P < .05, which indicated significant heterogeneity; otherwise, a fixed-effect model was used. Heterogeneity was detected using sensitivity analysis, subgroup analysis, and meta-regression analysis. Publication bias was evaluated using the Begg and Egger regression test.
2.6. Ethics
This study was approved by the local ethics committees of Fujian Medical University and all methods were performed in accordance with the relevant guidelines and regulations.
3. Results
3.1. Literature search and study characteristics
Figure 1 shows the flow diagram of the study selection process. A total 3207 studies were identified after duplicates had been removed. Full texts were obtained for articles where the title and abstract alone were insufficient for determining the eligibility for inclusion. An eventual 18 studies involving 1424 patients were deemed eligible for inclusion in the study: 13 were published in English and 5 were published in languages other than English. Table 1 summarizes the characteristics of the included studies. All included studies were RCTs published in 2008 to 2016: 55.83%, 25.56%, and 18.61% had been conducted in Asian, Caucasian, and European populations, respectively. Fifteen studies involved adult participants; 3 studies involved child participants. All studies used ω-3 PUFA supplementation only. Seven studies advised lifestyle modification for overweight or obese participants. The median ω-3 PUFA supplementation dose was 2.7 g/d (range, 0.25–5.00 g/d) and the median treatment duration was 6 months (range, 3–18 months). Only slight abdominal discomfort was reported in the reviewed studies, and there were no reports of serious adverse effects.
Figure 1.
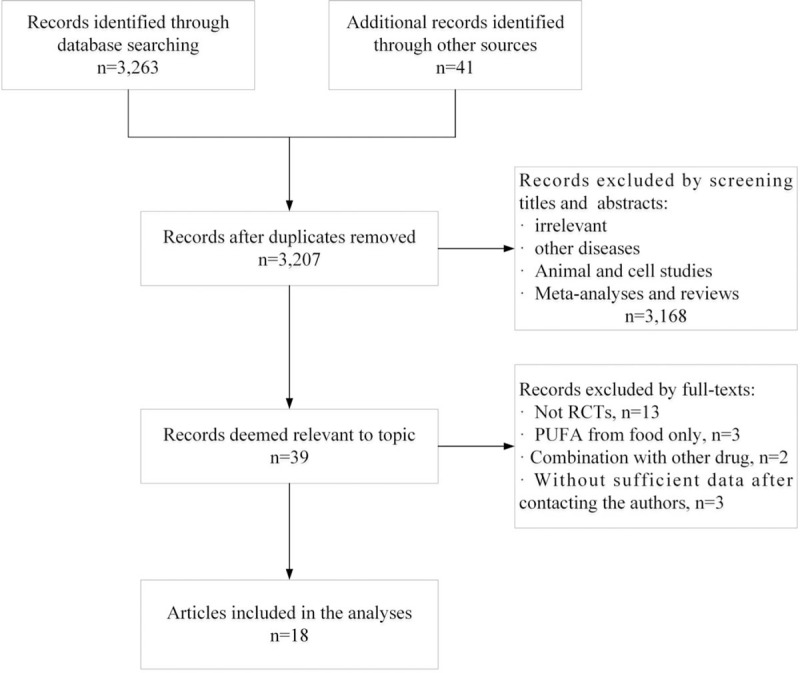
Flowchart of the study inclusion process.
Table 1.
Characteristics of the included studies.
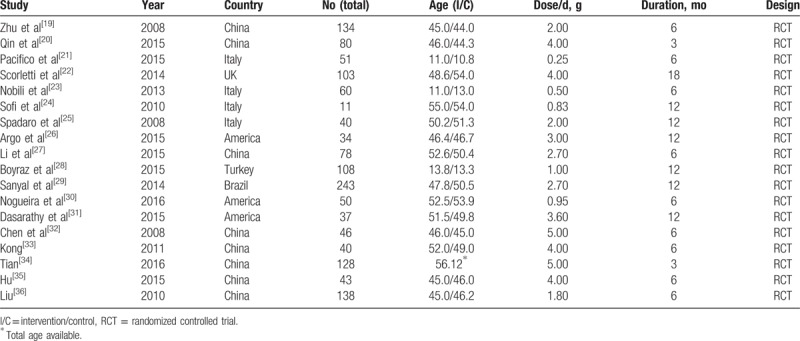
3.2. Methodologic quality
Table 2 and Figure 2 present the results of the study quality assessment. Most included studies had low risk of bias in sequence generation, allocation concealment, blinding, and analysis, and were of high methodologic quality.
Table 2.
Methodologic quality of the included studies.
Figure 2.
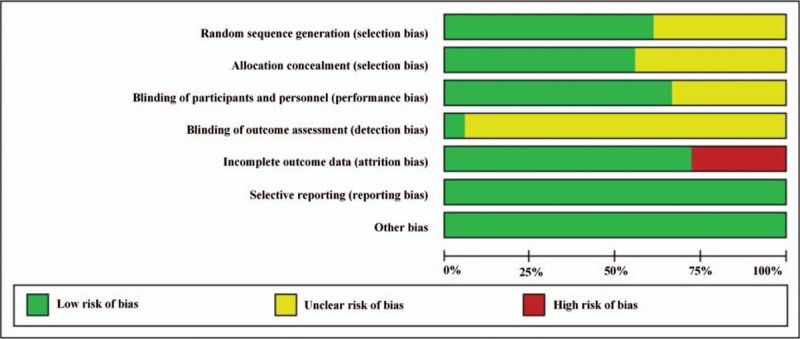
Methodologic quality and risk of bias of trials included in systematic review.
3.3. Effect of ω-3 PUFA supplementation on liver fat
Seven studies reported data on liver fat improvement. There was significant heterogeneity among these studies (I2 = 60.7%, P = .018). Therefore, a random-effect model was used. The meta-analysis showed that participants treated with ω-3 PUFAs were more likely to have improvement in liver fat compared with placebo-treated participants (RR = 1.56, 95% CI: 1.23–1.97) (Fig. 3).
Figure 3.
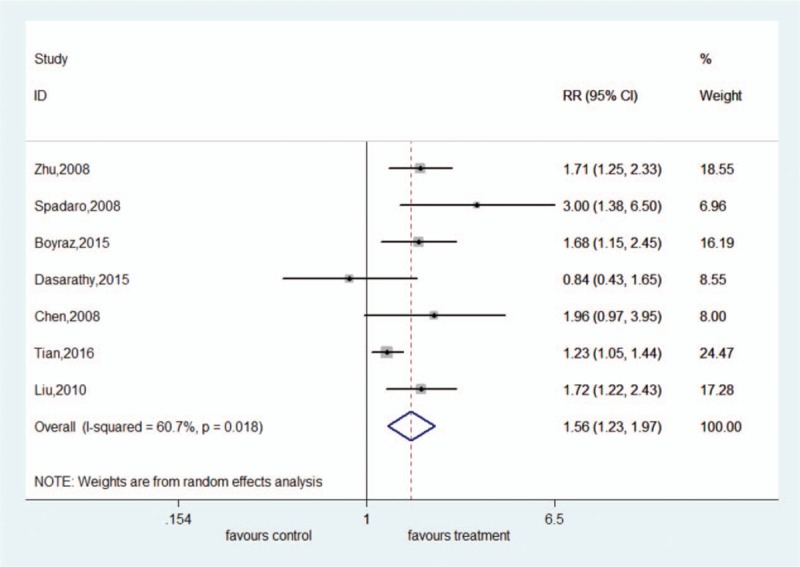
Forest plot of analysis of effect of omega-3 polyunsaturated fatty acids on liver fat.
3.4. Effect of ω-3 PUFAs on hepatic enzyme parameters
Fourteen, 12, and 8 studies had sufficient data for inclusion in analyses of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl transferase (GGT), respectively.
There was significant heterogeneity among these studies (ALT: I2 = 86.4%, P < .001; AST: I2 = 91.2%, P < .001). The random-effect model showed that ω-3 PUFA therapy had a statistically significant beneficial effect on ALT and AST; the pooled SMDs and their 95% CIs were −0.50 (95% CI: −0.88 to −0.11) and −0.54 (95% CI: −1.04 to −0.05), respectively. There was no significant heterogeneity among these studies for GGT (I2 = 41.6%, P = .101). The fixed-effect model showed significant pooled SMD for the efficacy of ω-3 PUFA therapy on GGT (SMD = −0.48, 95% CI: −0.64 to −0.31) (Figs. 4–6).
Figure 4.
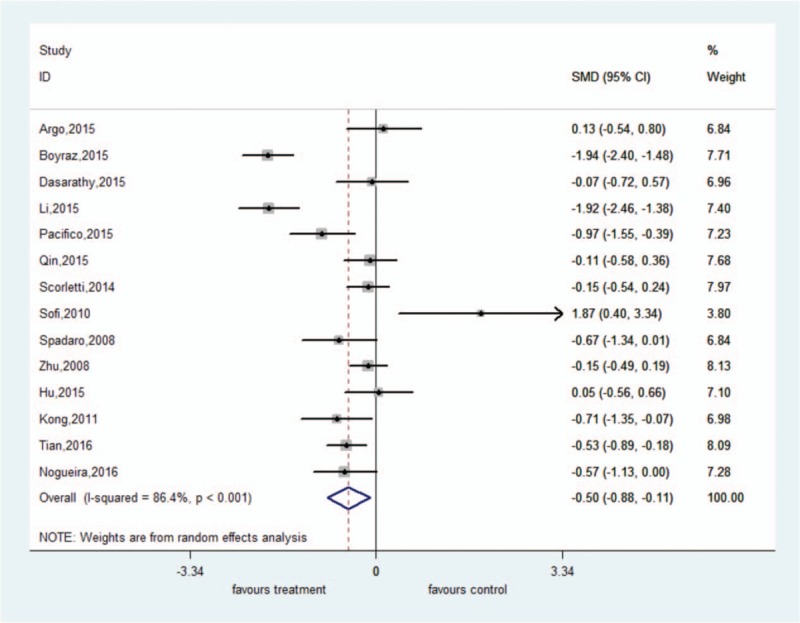
Forest plot of analysis of effect of omega-3 polyunsaturated fatty acids on alanine aminotransferase.
Figure 6.
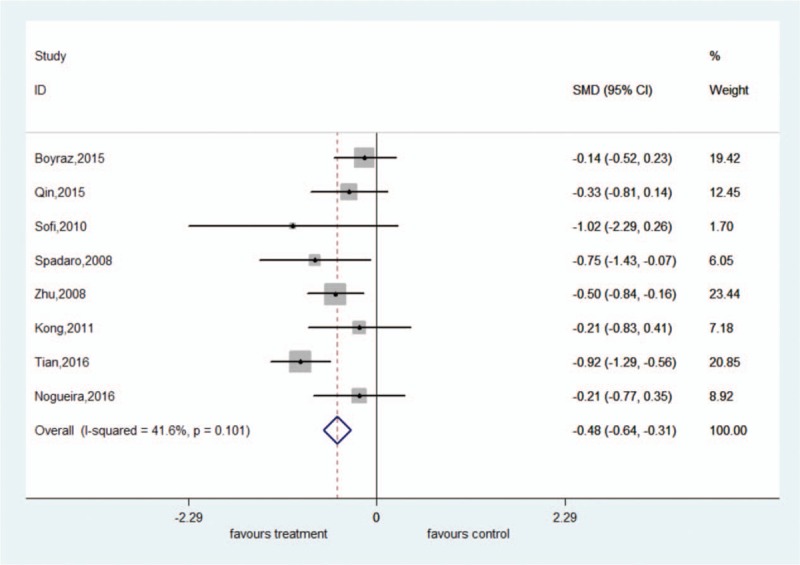
Forest plot of analysis of effect of omega-3 polyunsaturated fatty acids on γ-glutamyl transferase.
Figure 5.
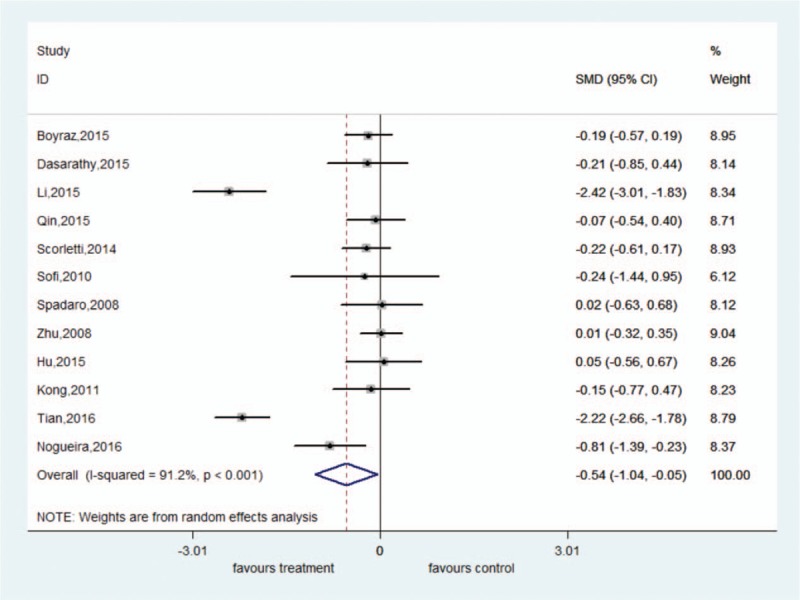
Forest plot of analysis of effect of omega-3 polyunsaturated fatty acids on aspartate aminotransferase.
3.5. Effect of ω-3 PUFAs on serum lipids
Sixteen, 15, 11, and 9 studies had sufficient data for inclusion in analyses of triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), respectively.
There was significant heterogeneity for TG (I2 = 79.6%, P < .001), TC (I2 = 90.3%, P < .001), and HDL-C (I2 = 74.8%, P < .001). The random-effect model showed significant pooled SMD favoring ω-3 PUFA therapy vs control for TG (SMD = −0.47, 95% CI: −0.76 to −0.19). However, there were no significant pooled SMDs for the efficacy of ω-3 PUFA therapy on TC (SMD = −0.09, 95% CI: −0.50 to 0.33) and HDL-C (SMD = 0.24, 95% CI: −0.08 to 0.55). There was no significant heterogeneity among these studies for LDL-C (I2 = 21.9%, P = .249). The fixed-effect model showed no significant pooled SMD for the efficacy of ω-3 PUFA therapy on LDL-C (SMD = −0.10, 95% CI: −0.25 to 0.06) (Figure S1).
3.6. Effect of ω-3 PUFAs on glucose metabolism
Eight, 8, and 7 studies had sufficient data for inclusion in analyses of homeostasis model assessment of IR (HOMA-IR), glucose, and insulin, respectively.
We found no significant heterogeneity among these studies (HOMA-IR: I2 = 16.6%, P = .299; glucose: I2 = 43.0%, P = .092; insulin: I2 = 20.7%, P = .272). The fixed-effect model showed a significant pooled MD favoring ω-3 PUFA therapy vs control for HOMA-IR (WMD = −0.40, 95% CI: −0.58 to −0.22) and glucose (SMD = −0.25, 95% CI: −0.43 to −0.06). However, there was no significant pooled SMD for the efficacy of ω-3 PUFA therapy on insulin (SMD = −0.08, 95% CI: −0.29 to 0.13) (Figure S2).
3.7. Effect of ω-3 PUFAs on anthropometric parameters
Nine, 5, 2, and 3 studies had sufficient data for inclusion in analyses of body mass index (BMI), waist circumference (WC), systolic blood pressure (SBP), and diastolic blood pressure (DBP), respectively.
We found no significant heterogeneity among the studies for BMI (I2 = 42.5%, P = .084), WC (I2 = 5.1%, P = .378), SBP (I2 = 0.0%, P = 1.000), and DBP (I2 = 0.0%, P = .958). The fixed-effect models showed no significant pooled WMD for the efficacy of ω-3 PUFA therapy on BMI (WMD = −0.30, 95% CI: −0.71 to 0.11), WC (WMD = 2.24, 95% CI: −0.17 to 4.65), SBP (WMD = 1.00, 95% CI: −4.40 to 6.40), and DBP (WMD = −0.14, 95% CI: −2.49 to 2.21) (Figure S3).
3.8. Publication bias and sensitivity analysis
Both Begg and Egger tests did not reveal statistically significant publication bias for any of the main outcomes (Table S1). This was confirmed based on visual inspection of the corresponding funnel plot (Figure S4). In the influence analyses, 2 study appeared to influence the pooled data.[28,36] So we excluded the study by Liu[36] from the analyses of ALT, AST, and GGT and excluded the study by Boyraz et al[28] from the analysis of SBP. The final results of sensitivity analysis were shown in Figure S5.
3.9. Subgroup and meta-regression analysis
Hypothesizing that differences in the effects of ω-3 PUFA supplementation on liver fat, ALT, AST, and GGT were not at random, we explored prespecified potential sources of heterogeneity. These included exposure characteristics, that is, dose per day (<3 g/d or ≥3 g/d) and treatment duration (≤6 months or >6 months), and participant characteristics, that is, ethnicity (European, Asian, or Caucasian) and age (adult or child). Consequently, more than half of the subgroup's heterogeneity was decreased inordinately (Figure S6–S9).
A meta-regression analysis was performed to investigate the effect of exposure and participant characteristics in determining the heterogeneity of estimates. The results indicated that the attributions of these covariates ranged from 1.32% to 52.19%; however, no statistical significant associations were observed between them (Table S1).
3.10. Adverse events
Overall, ω-3 PUFA supplementation was well-tolerated, and there were only minor gastrointestinal symptoms. However, the short duration of the trials, which did not exceed 2 years, prevents any conclusion from being drawn on the long-term safety of the proposed treatments.
4. Discussion
The present investigation is an updated systematic review of RCTs exploring the efficacy of ω-3 PUFA supplementation for treating NAFLD. Despite the significant heterogeneity in study design, there was high methodologic quality and absence of evidence of publication bias. The 8 RCTs included in the study suggest that ω-3 PUFA supplementation is associated with a beneficial effect on liver fat in humans. In addition, we found that ω-3 PUFA therapy had a significant beneficial effect on ALT, AST, GGT, TG, IR, and glucose. However, ω-3 PUFA supplementation was not significantly associated with TC, HDL-C, LDL-C, insulin, SBP, DBP, BMI, and WC. These results suggest that ω-3 PUFAs have therapeutic potential against NAFLD.
The effects of ω-3 PUFA supplementation on NAFLD have remained largely unchanged since a similar review by Parker et al in 2012.[37] The authors, who first reported ω-3 PUFA supplementation in humans with NAFLD, found that ω-3 PUFA therapy had a beneficial effect on liver fat, whether they included only RCTs or all trials. A recent meta-analysis by Lu et al[13] demonstrated that ω-3 PUFA supplementation improves liver fat. Our meta-analysis also confirms this conclusion. ω-3 PUFAs may influence NAFLD through specific mechanisms. They are known to downregulate sterol-regulatory-element-binding protein 1c (SREBP-1c) and upregulate peroxisome–proliferator-activated receptor α (PPAR-α), which would favor fatty acid oxidation and reduce steatosis.[38] Moreover, ω-3 PUFAs can give rise to resolvins, which are anti-inflammatory.[39] However, ω-3 PUFAs cannot be synthesized de novo, but oral administration can alter their levels. Based on these observations, it has been suggested that ω-3 PUFA supplementation is efficacious for managing NAFLD.[40]
Elevated liver enzymes in patients with NAFLD are associated with a clinically significant risk of developing end-stage liver disease.[41] It is unclear whether ω-3 PUFA supplementation affects the levels of hepatic enzymes such as ALT, AST, and GGT. Parker et al[37] reported that ω-3 PUFA therapy had a significant beneficial effect on AST and a tendency toward a beneficial effect on ALT, but that these effects were not significant after analyzing data from only RCTs. However, a meta-analysis of another 7 RCTs involving 442 patients reported a significant effect of ω-3 PUFAs on ALT and a nonsignificant effect on AST and GGT,[42] while a recent meta-analysis showed a significant effect of ω-3 PUFAs on GGT and a nonsignificant effect on ALT and AST.[13] In the present study, ω-3 PUFA therapy had significant effects on ALT, AST, and GGT. The possible reasons for the inconsistent findings are that the previous meta-analyses excluded trials involving children (<18 years old)[37,42]; by contrast, we did not restrict participant age in our study. The 5 studies[43–47] excluded from our analysis for being non-RCTs have been included in other meta-analysis; meanwhile, we included the 7 most recent RCTs published in the past 3 years.[20,21,28,30,34,35] The previous reviews involved 9, 7, and 10 trials, respectively, which were all published in English; however, we included in our study eighteen RCTs published in both English and languages other than English; 2 previous studies[13,42] assessed the risk of bias using the Jadad score; here, we used the Cochrane risk of bias tool.
The NAFLD is recognized as the hepatic manifestation of MS, which is associated with increased risk for T2DM and cardiovascular disease (CVD).[48,49] Therefore, the potential hepatic benefits of ω-3 PUFA therapy should be considered in combination with its effects on MS and cardiometabolic risk factors. Based on the cohorts included in the present meta-analyses, in which pooled MDs were measured, ω-3 PUFA supplementation benefited TG, IR, and glucose statistically significantly; however, the changes for TC, HDL-C, LDL-C, insulin, SBP, DBP, BMI, and WC were nonsignificant. Earlier systematic reviews have reported improvements in TG following ω-3 PUFA therapy,[13,42] and the improvements in IR and glucose are novel findings. Although the mechanism of ω-3 PUFAs for preventing MS and CVD adverse outcomes is unclear, the effect of ω-3 PUFAs may be due to their ability to decrease TG levels, BP, and glucose.[20,50] There is also clear evidence from meta-analysis that ω-3 PUFAs are linked to glucose, IR, and insulin secretion capacity.[51] However, our data suggest that the reductions in SBP and DBP are not significant.
There were several limitations in our study, especially heterogeneity. Although we detected statistical significant heterogeneity among some of the RCTs, exploration of the potential source of heterogeneity revealed that it may have been due to differences in treatment dose, treatment duration, ethnicity and age. However, the meta-regression analysis indicated no significant associations between them, and the random-effect model was applied. There may also have been other sources of heterogeneity, such as clinician skill, different measurement techniques, and inconsistent definition of disease criteria. In addition, the amount of ω-3 PUFAs would be easily obtained through diet, and the dietary intakes were not uniform across the studies. These heterogeneities may have restricted the interpretation of the pooled effects.
The strengths of this meta-analysis include: the large sample size enabled assessment of the association of ω-3 PUFAs and NAFLD and rendered it more powerful. Second, most included studies had low risk of bias in key domains, and were of high methodologic quality. Third, the prospective nature of the included studies avoided the influence of recall bias. Fourth, the compliance rates were high in most of the studies, which were determined by tablet-counting. These data provide a comprehensive view of the association between ω-3 PUFAs and NAFLD based on the current evidence.
In conclusion, the present analysis provides an updated systematic review and meta-analysis involving only RCTs on ω-3 PUFAs and NAFLD. The results suggest that ω-3 PUFA supplementation can improve liver fat, ALT, AST, GGT, TG, IR, and glucose in patients with NAFLD. So ω-3 PUFA supplementation may improve metabolic and cardiovascular risk factors and surrogate makers for liver disease progression. However, further studies are warranted to confirm whether ω-3 PUFA supplementation improves hard outcomes including mortality, progression to cirrhosis, or histologic inflammations. In addition, it is too early to validate these findings on liver fat, ALT, AST, GGT, and TG, given the heterogeneity among the studies. More large-scale, well-designed RCTs are needed to confirm the effect of ω-3 PUFA supplementation on these parameters. And future studies also need to confirm the dose-dependent effects and assess the long-term durability and safety of ω-3 PUFA supplementation.
Author contributions
Xian-E Peng, Jian-Hui Yan and Bing-Jie Guan conceived the study and wrote the manuscript. Jian-Hui Yan and Hai-Yan Gao contributed to collect data and evaluate literature. Jian-Hui Yan and Bing-Jie Guan analyzed and interpreted the data. All authors reviewed the manuscript.
Conceptualization: Jianhui Yan, Bing-Jie Guan, Xian-E Peng.
Data curation: Jianhui Yan, Bing-Jie Guan, Hai-Yan Gao.
Formal analysis: Jianhui Yan, Bing-Jie Guan.
Methodology: Jianhui Yan, Bing-Jie Guan, Hai-Yan Gao.
Software: Jianhui Yan, Bing-Jie Guan, Hai-Yan Gao.
Writing – original draft: Jianhui Yan, Bing-Jie Guan, Xian-E Peng.
Writing – review & editing: Jianhui Yan, Bing-Jie Guan, Xian-E Peng.
Supplementary Material
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CNKI = China National Knowledge Infrastructure, CVD = cardiovascular disease, DBP = diastolic blood pressure, GGT = γ-glutamyl transferase, HDL-C = high-density lipoprotein cholesterol, HOMA-IR = homeostasis model assessment of IR, IR = insulin resistance, ITT = intention-to-treat, LDL-C = low-density lipoprotein cholesterol, MS = metabolic syndrome, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, PPAR-α = peroxisome-proliferator-activated receptor α, PUFA = polyunsaturated fatty acid, RCT = randomized controlled trial, RR = relative risk, SBP= systolic blood pressure, SREBP-1c = sterol-regulatory-element-binding protein 1c, T2DM = type 2 diabetes mellitus, TC = total cholesterol, TGs = triglycerides, VIP = Chinese Scientific and Technological Journal, WC = waist circumference, WMD = weighted mean difference.
JHY and BJG contributed equally to this work.
This work was supported by grants from the National Natural Science Foundation of China (No: 81473047), the joint innovation project of science and technology from Fujian province (No: 2017Y9104), and the Training Program Foundation for Middle-aged and Young Talents from Sanitation System of Fujian province (No: 2014-ZQN-ZD-23).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723–30. [DOI] [PubMed] [Google Scholar]
- [2].Bayard M, Holt J, Boroughs E. Nonalcoholic fatty liver disease. Am Fam Physician 2006;73:1961–8. [PubMed] [Google Scholar]
- [3].Chitturi S, Wong VW, Farrell G. Nonalcoholic fatty liver in Asia: firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol 2011;26:163–72. [DOI] [PubMed] [Google Scholar]
- [4].Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274–85. [DOI] [PubMed] [Google Scholar]
- [5].Musso G, Gambino R, Tabibian JH, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014;11:e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pirola CJ, Sookoian S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a meta-analysis. Hepatology 2015;62:1742–56. [DOI] [PubMed] [Google Scholar]
- [7].Bellentani S, Dalle GR, Suppini A, et al. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology 2008;47:746–54. [DOI] [PubMed] [Google Scholar]
- [8].Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–23. [DOI] [PubMed] [Google Scholar]
- [9].Shu X, Zhang L, Ji G. Vitamin E therapy in non-alcoholic fatty liver disease. Int J Clin Med 2014;5:87–92. [Google Scholar]
- [10].Akbar HA, Amir Z, Sonia O, et al. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic fatty liver disease: a randomized controlled pilot study. Hepat Mon 2012;12:e6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Neuschwandertetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Musso G, Gambino R, De MF, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003;37:909–16. [DOI] [PubMed] [Google Scholar]
- [13].Lu W, Li S, Li J, et al. Effects of omega-3 fatty acid in nonalcoholic fatty liver disease: a meta-analysis. Gastroenterol Res Pract 2016;2016: 1459790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci 2004;106:635–43. [DOI] [PubMed] [Google Scholar]
- [15].Putti R, Migliaccio V, Sica R, et al. Skeletal muscle mitochondrial bioenergetics and morphology in high fat diet induced obesity and insulin resistance: focus on dietary fat source. Front Physiol 2016;6:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koska J, Ozias MK, Deer J, et al. A human model of dietary saturated fatty acid induced insulin resistance. Metab Clin Exp 2016;65:1621–8. [DOI] [PubMed] [Google Scholar]
- [17].Wiley-Blackwell, Green SR, Higgins J. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [Internet].2011. [Google Scholar]
- [18].Higgins JP, Altman DG. Assessing Risk of Bias in Included Studies. Chichester, UK: John Wiley & Sons, Ltd; 2011. [Google Scholar]
- [19].Zhu FS, Liu S, Chen XM, et al. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol 2008;14:6395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qin Y, Zhou Y, Chen SH, et al. Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin E2 in nonalcoholic fatty liver disease associated with hyperlipidemia: a randomized clinical trial. PLoS One 2015;10:e0133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pacifico L, Bonci E, Martino MD, et al. A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease. Nutr Metab Cardiovasc Dis 2015;25:734–41. [DOI] [PubMed] [Google Scholar]
- [22].Scorletti E, Bhatia L, Mccormick KG, et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: results from the Welcome∗ study. Hepatology 2014;60:1211–21. [DOI] [PubMed] [Google Scholar]
- [23].Nobili V, Bedogni G, Alisi A, et al. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child 2011;96:350–3. [DOI] [PubMed] [Google Scholar]
- [24].Sofi F, Giangrandi I, Cesari F, et al. Effects of a 1-year dietary intervention with n-3 polyunsaturated fatty acid-enriched olive oil on non-alcoholic fatty liver disease patients: a preliminary study. Int J Food Sci Nutr 2010;61:792–802. [DOI] [PubMed] [Google Scholar]
- [25].Spadaro L, Magliocco O, Spampinato D, et al. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis 2008;40:194–9. [DOI] [PubMed] [Google Scholar]
- [26].Argo CK, Patrie JT, Lackner C, et al. Effects of N-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol 2015;62:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li YH, Yang LH, Sha KH, et al. Efficacy of poly-unsaturated fatty acid therapy on patients with nonalcoholic steatohepatitis. World J Gastroenterol 2015;21:7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boyraz M, Pirgon Ö, Dündar B, et al. Long-term treatment with n-3 polyunsaturated fatty acids as a monotherapy in children with nonalcoholic fatty liver disease. J Clin Res Pediatr Endocrinol 2015;7:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology 2014;147:377–84. [DOI] [PubMed] [Google Scholar]
- [30].Nogueira MA, Oliveira CP, Ferreira Alves VA, et al. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2016;35:578–86. [DOI] [PubMed] [Google Scholar]
- [31].Dasarathy S, Dasarathy J, Khiyami A, et al. Double blind randomized placebo controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol 2015;49:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen R, Guo Q, Zhu WJ. Therapeutic efficacy of omega-3 polyunsaturated fatty acid capsule in treatment of patients with non-alcoholic fatty liver disease. World Chinese J Digestol 2008;16:2002–6. [Google Scholar]
- [33].Kong X. A basal and clinical research of ω-3 polyunsaturated fatty acids to seal non-alcoholic fatty liver disease. Dalian Medical University 2011. [Google Scholar]
- [34].Tian Z. A analyses for 128 cases of ω-3 polyunsaturated fatty acids to seal non-alcoholic fatty liver disease. Psychol Doctor 2016;22:91–2. [Google Scholar]
- [35].Hu ZW. Therapeutic effect of seal oil on non-alcoholic fatty liver disease. Acad J Guangzhou Med Univ 2015. 31–3. [Google Scholar]
- [36].Liu XL. Observation of clinical curative effect of pupa oil α-linoleic acid ethyl ester soft gelatin capsule to treat non-alcoholic fatty liver disease. Drugs Clin 2010;07:6–38. [Google Scholar]
- [37].Parker HM, Johnson NA, Burdon CA, et al. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;56:944–51. [DOI] [PubMed] [Google Scholar]
- [38].Pettinelli P, Del PT, Araya J, et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta 2009;1792:1080–6. [DOI] [PubMed] [Google Scholar]
- [39].Calder P. Marine omega-3 fatty acids and inflammation. J Lipid Nutr 2010;19:233–44. [Google Scholar]
- [40].Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr 2007;86:285–300. [DOI] [PubMed] [Google Scholar]
- [41].Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865. [DOI] [PubMed] [Google Scholar]
- [42].He XX, Wu XL, Chen RP, et al. Effectiveness of omega-3 polyunsaturated fatty acids in non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. PLoS One 2016;11:e0162368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Capanni M, Calella F, Biagini MR, et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther 2006;23:1143–51. [DOI] [PubMed] [Google Scholar]
- [44].Cussons AJ, Watts GF, Mori TA, et al. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab 2009;94:3842–8. [DOI] [PubMed] [Google Scholar]
- [45].Hatzitolios A, Savopoulos C, Lazaraki G, et al. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol 2004;23:131–4. [PubMed] [Google Scholar]
- [46].Tanaka N, Sano K, Horiuchi A, et al. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J Clin Gastroenterol 2008;42:413–8. [DOI] [PubMed] [Google Scholar]
- [47].Vega GL, Chandalia M, Szczepaniak LS, et al. Effects of N-3 fatty acids on hepatic triglyceride content in humans. J Investig Med 2008;56:780–5. [DOI] [PubMed] [Google Scholar]
- [48].Brea A, Puzo J. Non-alcoholic fatty liver disease and cardiovascular risk. World J Gastrointest Pathophysiol 2017;149:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wilson PW, D’Agostino RB, Parise H, et al. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–72. [DOI] [PubMed] [Google Scholar]
- [50].Rizos EC, Ntzani EE, Bika E, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012;308:1024–33. [DOI] [PubMed] [Google Scholar]
- [51].Fumiaki I, Renata M, Wu JHY, et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med 2016;13: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


