Abstract
Background:
Plantar fasciitis is a common cause of heel pain, which often results in significant morbidity. There have been several treatment options that are used for plantar fasciitis, including nonsteroidal anti-inflammatory drugs, orthoses, physical therapy, and steroid injections.
Objectives:
The aim of this meta-analysis was to compare the effects of platelet-rich plasma (PRP) and other treatments in patients with plantar fasciitis.
Search methods:
Medline, Web of Science, and Embase were systematically searched to identify relevant trials.
Selection criteria:
Randomized controlled trials (RCTs) that compared the effects of PRP and other treatments on plantar fasciitis were included.
Data collection and analysis:
The main outcomes included changes from baseline in visual analog scale (VAS) score, American Orthopaedic Foot and Ankle Society Score (AOFAS), and Roles–Maudsley score (RMS). Results were expressed as weight mean difference (WMD) with 95% confidence interval (95% CI). The meta-analysis was performed using a fixed-effects or random-effects model according to heterogeneity.
Main results:
Ten RCTs involving a total of 445 patients with plantar fasciitis were included. Among these studies, 9 compared PRP with steroid, and 1 compared PRP with whole blood.
Four studies were categorized as being at low risk of bias, and the remaining 6 as being at unclear risk of bias.
Pooled estimates suggested that PRP had greater changes in VAS and AOFAS scores than other treatments. However, it had no benefit effect in the RMS.
Subgroup analysis for VAS and AOFAS showed that PRP had superior effect than other treatments at 12 months, but not at the 1, 3, 6 months.
Subgroup analysis based on treatment regimens demonstrated that PRP was more effective than steroid in the change from baseline in AOFAS, but not in VAS and RMS scores.
Authors’ conclusion:
PRP was as effective as other treatments in reducing pain and improving function in patients with plantar fasciitis. Subgroup analysis indicated that PRP had better effect than steroid in AOFAS Score and its effect was durable in a long term. However, considering the potential limitations in this study, more large-scale RCTs are needed to confirm the current findings.
Keywords: meta-analysis, plantar fasciitis, platelet-rich plasma
1. Introduction
Plantar fasciitis is the most common cause of heel pain.[1] The pathophysiology is not fully understood and seems to be multifactorial. Several established risk factors include prolonged weight bearing, obesity, and reduced plantar flexion.[2] Although plantar fasciitis is a self-limiting condition, the rehabilitation may require several months. Moreover, pain may become chronic, and disabling would carry a heavy healthcare burden for patents[3] and influence their quality of life.[4]
A variety of conservative treatment options include rest, heel cups, eccentric stretching exercises, nigh splints, orthotics, and nonsteroidal anti-inflammatory medication.[5] These treatment measures could resolve about 80% of cases.[5] However, in cases who are not responsive to these treatments, invasive procedures are required. Infiltration with intralesional steroids is commonly used in the treatment of chronic plantar fasciitis.[6] This procedure is effective, but only produces short-term pain relief.[7] Moreover, it is also accompanied by complications, such as application site infections, heel fat pad atrophy, and plantar fascia rupture.[7]
Local injection of platelet-rich plasma (PRP) is an emerging therapeutic alternative for plantar fasciitis. PRP, derived by centrifuging whole blood, has a platelet concentration higher than that of whole blood.[8] The platelet releases a variety of growth factors and cytokines, which can stimulate and accelerate the nature physiological tissue healing process.[8] Current evidence has shown the promising results of PRP in the treatment of plantar fasciitis.[9–11] However, whether it is more effective in reducing pain and improving function than other treatments (such as steroid injection, or whole blood) remains controversial. Therefore, we conducted this meta-analysis to compare the effects of PRP with other treatments for patients with plantar fasciitis.
2. Material and methods
2.1. Ethical review
Ethics approval was waived because this study does not involve any human participants or animals.
2.2. Literature search
We conducted this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[12] Medline, Embase, and Web of Science were systematically reviewed for studies that assessed the effects of PRP in the treatment of patients with plantar fasciitis. These electronic databases were searched from their inception through February 15, 2017. The structured search strategies used were as followings: (“fasciitis, plantar” [MeSH Terms] OR (“fasciitis” [All Fields] AND “plantar” [All Fields]) OR “plantar fasciitis” [All Fields] OR (“plantar” [All Fields] AND “fasciitis” [All Fields])) AND (“platelet-rich plasma” [MeSH Terms] OR (“platelet-rich”[All Fields] AND “plasma” [All Fields]) OR “platelet-rich plasma” [All Fields] OR (“platelet” [All Fields] AND “rich” [All Fields] AND “plasma” [All Fields]) OR “platelet rich plasma”[All Fields]). Details of the search strategy are shown in Appendix 1. The search was limited to human subjects and RCTs, and language was restricted into English. In addition, we also manually searched the reference lists of the included studies and previous reviews until no potentially eligible studies were identified.
2.3. Study selection and exclusion criteria
The following inclusion criteria were applied: study design: RCT; population: adult patients with plantar fasciitis who had no history of surgical interventions in the ankle and the heel; intervention: PRP; comparison intervention: steroid, whole blood or, placebo; outcome measures: pain and function assessed by visual analog score (VAS), Roles–Maudsley score (RMS), and American Orthopaedic Foot and Ankle Society Score (AOFAS). The exclusion criteria were used as followings: reviews, comments, editors, letters, case reports, and conference abstracts; studies unrelated with our topics.
2.4. Data extraction
We used a standardized data collection form to extract the following data from each RCT: first author, year of publication, country, participant characteristics, number of patients in each arm, and the outcomes (including changes from baseline in VOA, AOFAS, and RMS scores). The data extraction was performed by 2 independent investigators. Any disagreements between them were resolved by discussion and consensus.
2.5. Risk of bias and evidence of grade assessment
The assessment for risk of bias was performed using the method recommended by Cochrane Collaboration.[13] This method comprises of the following 7 domains: random sequence generation; allocation concealment; blinding of outcome participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting and other bias.[13] Two independent investigators objectively reviewed all the included studies, and assigned a value of “high,” “low,” or “unclear.”
The evidence quality of outcome measures was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.[14] Each outcome measure was identified by the GRADE system for the 4 grades: high, moderate, low, very low. The GRADE profiler software (version 3.6) was used to rate the level of evidence.
2.6. Statistical analysis
We estimated the weighted mean difference (WMD) with 95% confidence interval (CI) for continuous outcomes. Before the data were synthesized, we used the Cochrane Q chi-square test and I2 statistic to test the heterogeneity across studies, in which P value <.1 or I2 > 50% were considered to be significant.[15] When significant heterogeneity was identified, a random-effects model (DerSimonian–Laird method)[16] was used to pool the data; otherwise, a fixed-effects model (Mantel–Haenszel method)[17] was used. We also conducted sensitivity analysis and meta-regression to explore the potential sources of heterogeneity and to further identify the influence of various exclusion criteria on the overall estimates. A subgroup analysis was conducted based on the duration of follow-up (1, 3, 6, 12-month of follow-up), control regimens (steroid vs whole blood vs placebo), and the quality of study (low vs unclear risk of bias). Publication bias was assessed by Begg[18] and Egger et al[19] test. A P value <.05 was judged as statistically significant, except where otherwise specified. All analyses were performed by using STATA version 12.0 (Stata Corporation, College Station, TX).
3. Results
3.1. Study identification and selection
The initial search yielded 1252 relevant publications, of which 685 were excluded because of duplicate records. Then 567 were left for title/abstract review, of which 550 were excluded based on various reasons (reviews, letters, non-RCTs, or not relevant with our topics). Seventeen potentially relevant studies were identified for full-text analysis, however, 4 were excluded because of sing-arm study design,[9–11,20] and 2 RCTs were excluded because they did not provide outcomes of our interest, and one RCT was excluded because of designing type (a protocol article). Finally, 10 RCTs[21–30] that met the inclusion criteria were included in this meta-analysis (Fig. 1).
Figure 1.
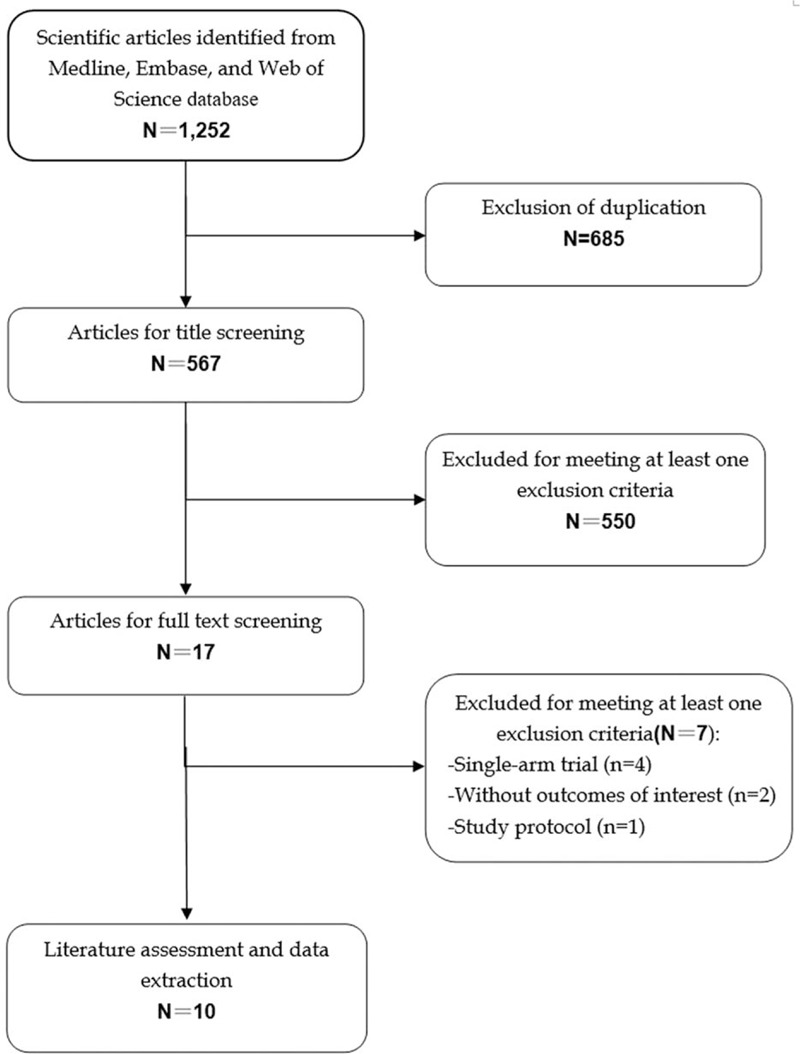
Eligibility of studies for inclusion in meta-analysis.
3.2. Study characteristics
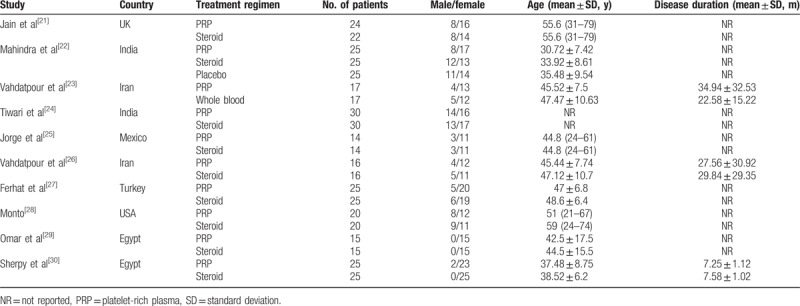
The main characteristics of the 8 RCTs were presented in Table 1. These studies were published between 2012 and 2017. The sample size of these studies ranged from 28 to 60 (total 445, 128 males and 317 females). Among the included studies, 2 were conducted in India,[22,24] 2 in Iran[23,26] and Egypt,[29,30] each one in UK,[21] Mexio,[25] Turkey,[27] and USA.[28] Nine trials compared the effects of PRP with steroid,[21,22,24,25,27–30] and one compared PRP with whole blood.[23] In the study conducted by Mahindra et al,[26] patients with plantar fasciitis were randomly assigned to the local PRP, corticosteroid, or placebo groups. Since the data for changes in VAS and AOFAS scores were presented in all the 3 arms, we included these data in this meta-analysis.
Table 1.
Baseline characteristics of patients in the trials included in the meta-analysis.
3.3. Quality assessment and risk of bias
The assessment of risk of bias is summarized in Figure 2. Overall, 4 trials were categorized as being at low risk of bias,[22,25,26,30] and the remaining 6 as being at unclear risk of bias.[21,23,24,27–29] The main reasons for the trials being at unclear risk of bias were that, they did not adequately describe the methods for randomized sequence, allocation sequence concealment, blinding of participants/ persons, and outcome assessment.
Figure 2.
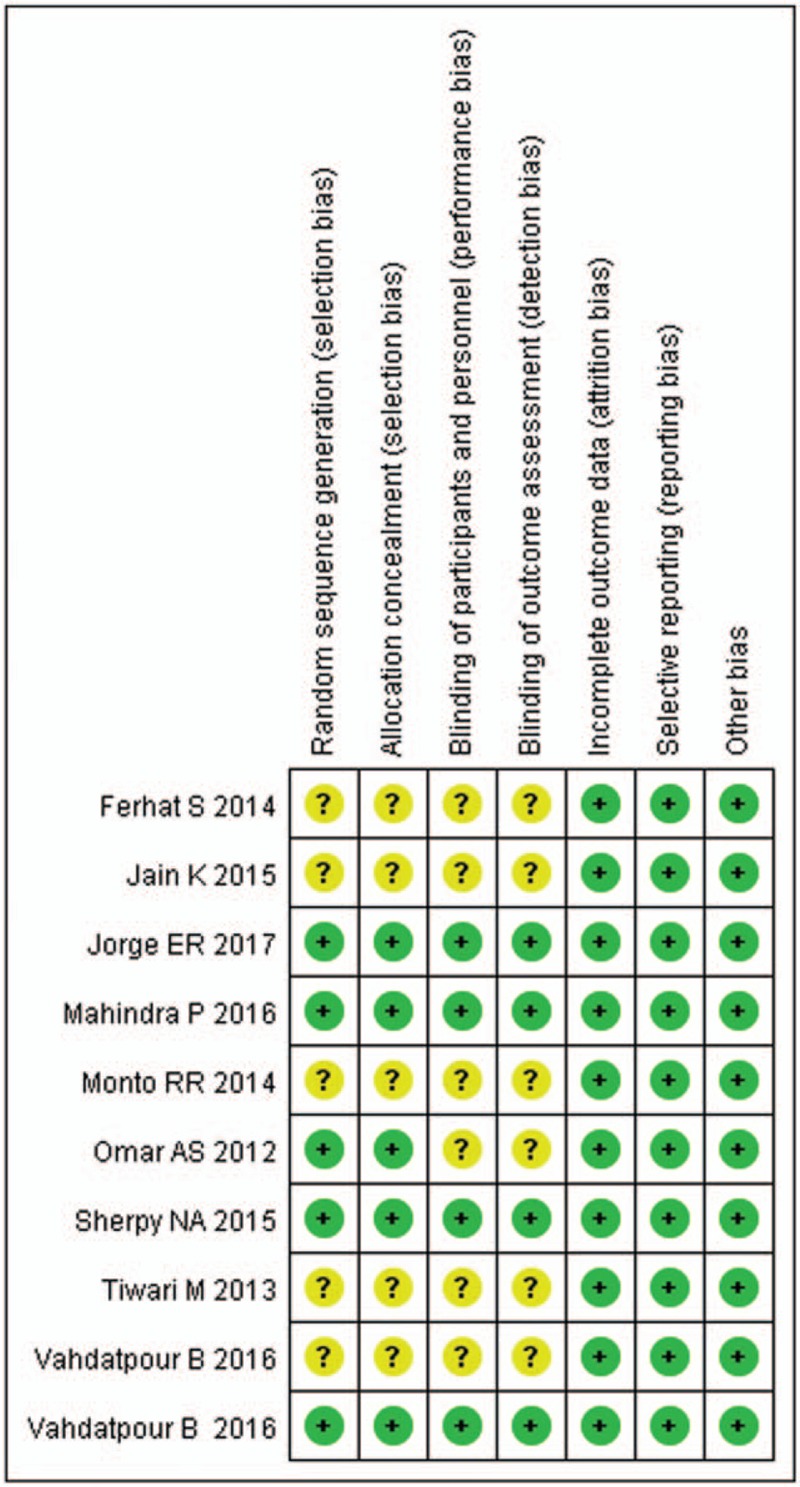
Risk of bias summary.
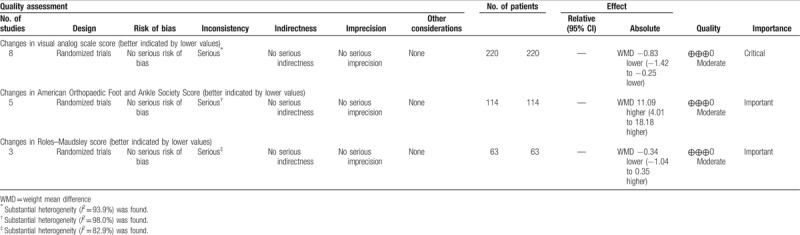
The GRADE evidence profiles for the outcomes are shown in Table 2. The GRADE level of evidence was moderate for changes from baseline in VAS score, AOFAS score, and RMS.
Table 2.
GRADE evidence profile.
3.4. Changes in visual analog scale score
Eight studies reported the data of VAS score.[21,22,24–27,29,30] The aggregated results of these studies suggested that the change from baseline in VAS score was greater in the PRP group than that in the control group (WMD = −0.83, 95% CI: −1.42, −0.25; P = .005). The test for heterogeneity was significant (I2 = 92.7%, P < .001). Subsequently, we performed sensitivity analysis to explore the potential sources of heterogeneity. Exclusion of the study with the small sample size[25] did not change the overall estimation substantially (WMD = −1.21, 95% CI: −1.89, −0.63; P = .001), however, significant heterogeneity was still present (I2 = 92.7%, P < .001). Exclusion of the study conducted by Mahindra et al[22] resulted in similar overall estimate (WMD = −0.44, 95% CI: −0.96, −0.27; P = .001), but the heterogeneity did not disappear (I2 = 86.7.6%, P < .001). We also further excluded any single study, however, the heterogeneity was still present, and the overall estimate of the remaining studies changed slightly.
Subgroup analysis based on the duration of follow-up showed that, PRP was associated with a significant reduction in VAS score at 12 months (WMD = −2.06, 95% CI: −3.87, −0.25; P = .026) (Fig. 3).Whereas, at 1, 3, 6 months, this benefic effect of PRP was not observed (at 1 month: WMD = −0.65, 95% CI: −1.70, 0.40, P = .227; at 3 months, WMD = −0.76, 95% CI: −2.41, 0.88, P = .982, at 6 months: WMD = −0.92, 95% CI: −1.87, 0.03, P = .058).
Figure 3.
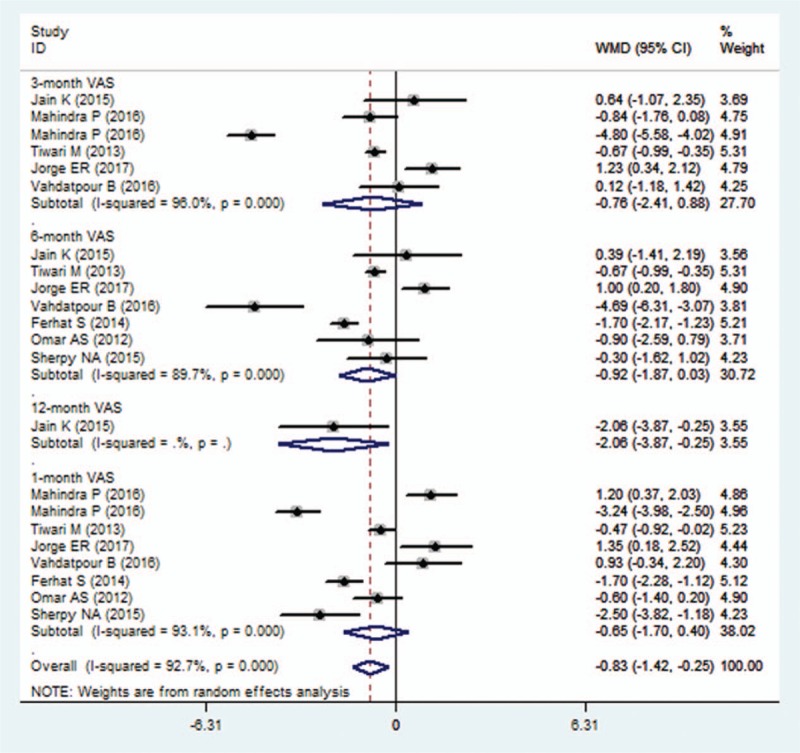
Forest plot showing the comparison between PRP and other treatments in change from baseline in VAS score. PRP = platelet-rich plasma, VAS = visual analog scale.
Subgroup analysis based on the methodology showed that, among the studies with unclear risk of bias, PRP resulted in a more reduction in VAS score than control treatment (WMD = −0.87, 95% CI: −1.35, −0.29; P = .001); whereas in studies with low risk of bias, the superior effect of PRP was not observed (WMD = −0.59, 95% CI: −2.33, 1.27; P = .059).
Subgroup analysis based on the control regimens demonstrated that, PRP had a greater decrease in VAS score than placebo (WMD = −4.01, 95% CI: −5.54, −2.49; P < .001), but a comparable change with steroid (WMD = −0.47, 95% CI: −0.94, 0.01; PP = .247).
Meta-regression was performed to investigate the potential effects of follow-up and comparators on the change from baseline in VAS score. The results showed that there was a significant relationship between VAS score and comparators (P = .010), but no significant association between VAS score and follow-up (P = .790).
3.5. Changes in American Orthopaedic Foot and Ankle Society score
Five studies reported data of AOFAS score.[21,22,25,27,28] Pooled estimates suggested that, the change from baseline in AOFAS score was not significant difference between the PRP and control group (WMD = 11.09, 95% CI: 4.01, 18.18; P = .002) (Fig. 4). The test for heterogeneity was significant (I2 = 98.0%, P < .001).
Figure 4.
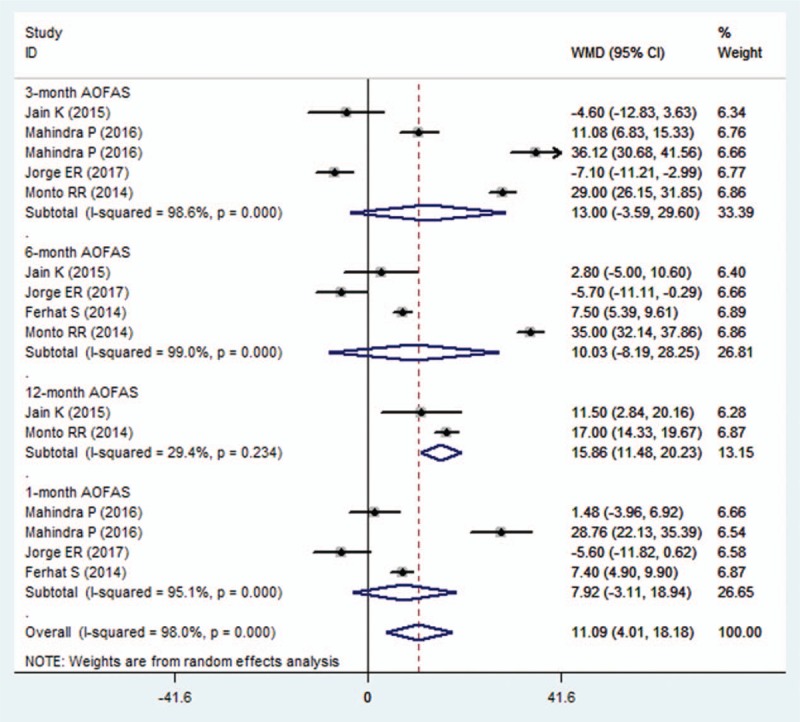
Forest plot showing the comparison between PRP and other treatments in change from baseline in AOFAS score. AOFAS = American Orthopaedic Foot and Ankle Society Score.
Subgroup analysis based on the duration of follow-up showed that, compared with control treatment, PRP had a similar increase in AOFAS score at the 1, 3, 6 months (1month: WMD = 7.92, 95% CI: −3.11, 18.94; P = .159; 3 months: WMD = 13, 95% CI: −3.59, 29.60; P = .125; 6 months: WMD = 10.03, 95% CI: −8.19, 28.25; P = .281) (Fig. 4). However, at the 12 months, the increase in AOFAS score was greater in the PRP group than that in the control group (WMD = 15.86, 95% CI: 11.48, 20.23; P < .001) (Fig. 4).
Subgroup analysis based on the methodology showed that, among the studies with unclear risk of bias, PRP was associated with a greater increase in AOFAS score than control treatment (WMD = 13.54, 95% CI: 4.77, 22.30; P = .002). Whereas, in the studies with low risk of bias, this effect was not found (WMD = 8.39, 95% CI: −4.06, 20.84; P = .187).
Subgroup analysis based on control regimen demonstrated that, PRP had a significant increase in AOFAS score when compared with steroid (WMD = 7.85, 95% CI: 0.48, 15.23; P = .037) or placebo (WMD = 32.7, 95% CI: 25.5, 39.89; P < .001).
Meta-regression was performed to investigate the potential effects of follow-up and comparators on the change from baseline in AOFAS score. The results showed that there was a significant relationship between AOFAS score and comparators (P = .023), but no significant association between AOFAS score and follow-up (P = .470).
3.6. Changes in Roles–Maudsley score
Three studies reported the data of RMS.[21,23,26] Pooled estimate suggested that, PRP had a comparable effect with other treatments in the change from baseline in RMS (WMD = −0.34, 95% CI: −1.04, 0.35; P = .334) (Fig. 5). There was significant heterogeneity among the included studies (I2 = 82.9%, P < .001).
Figure 5.
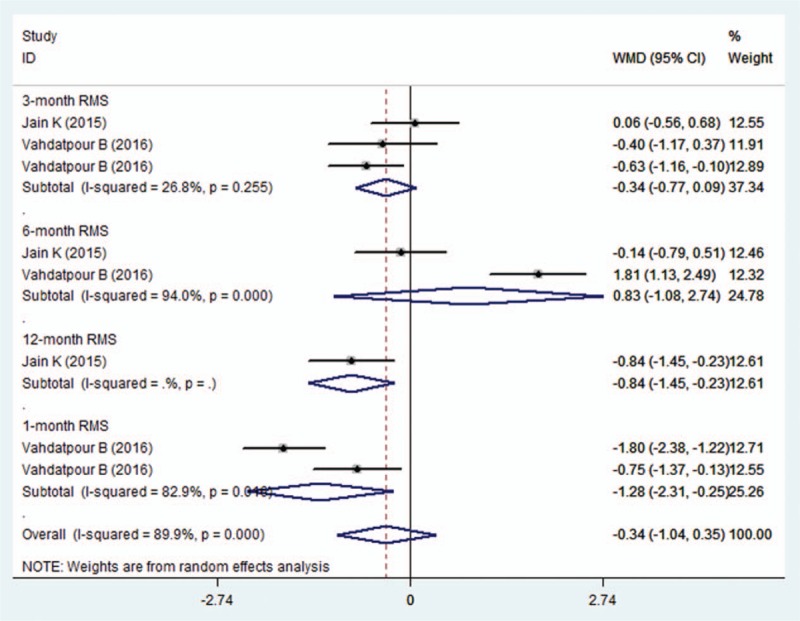
Forest plot showing the comparison between PRP and other treatments in change from baseline in RMS. PRP = platelet-rich plasma, RMS = Roles–Maudsley score.
Subgroup analysis based on the duration of follow-up showed that, PRP was associated with a significant reduction in RMS at 1 month (WMD = −1.28, 95% CI: −2.31, −0.25; P = .015) and 12 months (WMD = −0.84, 95% CI: −1.45, −0.23; P = .007). However, this benefic effect of PRP was not observed at the 3, 6 months (3 months, WMD = −0.34, 95% CI: −0.77, 0.09, P = .117;6 months: WMD = −0.83, 95% CI: −1.08, 2.74; P = .393).
Subgroup analysis based on the methodology showed that, PRP had similar effect in the reduction of RMS with control treatment irrespective of the study quality (studies with low risk of bias: WMD = 0.13, 95% CI: −1.4, 1.67, P = .864; studies with unclear risk of bias: WMD = −0.63, 95% CI: −1.32, 0.05, P = .071).
Subgroup analysis based on control regimen demonstrated that, PRP did not have an advantage effect in the decrease of RMS when compared with steroid (WMD = −0.09, 95% CI: −0.84, 0.65; P = .809) and whole blood (WMD = −1.12, 95% CI: −2.5, 0.25; P = .108).
3.7. Publication bias
Assessment of publication bias using Begg's and Egger's tests showed that there was no evidence of potential publication bias among the included studies (Begg's test: P = . 633; Egger's test: P = .687).
4. Discussion
The major purpose of this meta-analysis was to assess the effect of PRP in the treatment of patients with plantar fasciitis. Our results suggested that, PRP was associated with greater changes in VAS and AOFAS scores than other treatments. However, for RMS, there was not significant different between the 2 groups. Subgroup analysis demonstrated that, the advantaged effect of PRP over other treatments was only observed at the 12 month, but not at the 1, 3, 6 months. Moreover, PRP was more effective than steroid and placebo in the change of AOFAS score. Our results indicate that PRP has a long-term benefit in the management of plantar fasciitis and should be used as an alternative approach for patients with plantar fasciitis.
There have been 2 published systematic review and meta-analysis of autologous whole blood or autologous blood-derived products (ABPs) for plantar fasciitis.[31,32] However, their results were different from the findings of the present meta-analysis. In the study conducted by Hsiao et al,[31] the authors compared the efficacy of ABPs, corticosteroids or shock-wave therapy in the treatment of plantar fasciitis. Based on 3 of 10 included studies, PRP showed a significant more reduction in VAS score with corticosteroids at 3 months (standardized mean differences (SMD) = 1.81, 95% CI: 0.02, 3.60), but a similar reduction at 6 months (SMD = 0.66, 95% CI: −0.60, 1.92).[31] Whereas, in another meta-analysis, the authors compared the efficacy of autologous whole blood with that of corticosteroid injection on plantar fasciitis.[32] In that study, trials assessing the efficacy of PRP were excluded. The authors found that, corticosteroid was more effective than autologous whole blood for pain relief in the short term (SMD = 0.52, 95% CI: 0.18, 0.86; P < .001).[32] Our meta-analysis expands on the previous studies to provide a better characterization of evidence base for PRP in plantar fasciitis. First, our study had a more enlarged sample size than the previous studies, which increased the statistical power to evaluate the effects. In this study, the number of eligible studies for data analysis was 8. Whereas in the study of Hsiaoet al,[31] there were 7 RCTs and 3 quasi-experimental studies were included, and the number of contributing data for PRP was only 2 or 3. Second, in this study, we assessed the effects of PRP not only in a short term, but also in a long term. Whereas, in the previous study, the authors only evaluated the effects in the short term (at 3, 6 months).[32] Thus, whether PRP was superior to corticosteroids in a long term remains uncertain. Third, in this study, all the included studies were of well-conducted randomized controlled design; whereas, in the previous study, 3 of the 10 included studies were quasi-experimental design.[32] These studies had a lower quality than RCTs, which would reduce the reliability of the results. The enlarged sample size in this study has enhanced the statistical power of our results, and the high quality of included studies has ensured the reliability and credibility of the effect estimates.
In this study, our results showed that, PRP was effective at reducing pain and improving physical function in patients with plantar fasciitis. Subgroup analysis demonstrated that 12 months of long-term PRP could improve pain and physical function, but 1 to 6 months of short-term of PRP could not. Our results were in consistent with previous findings. Aksahin et al[33] compared the effects of local injection of PRP with corticosteroids in the treatment of plantar fasciitis. In that study, sixty patients who had no response to conservative treatment were enrolled (30 in each group).[33] At 3 weeks and 6 months after the treatment, the VAS score and RMS were significantly improved in both groups, however, the differences between them were not significant.[33] Similar effects between PRP and steroid were also found in another study.[21] In that study, the authors compared the efficacy of PRP with steroid at 3, 6, and 12 months after injection.[21] And they found that, at 3 months, the VAS, AOFAS and RM scores were marginally better in steroid group than that in the PRP group; at 6 months, these outcome scores were better in PRP group than that in the steroid group; however, these outcome scores were not statistically significant different between the 2 groups at 3 and 6 months.[21] Thus, the authors suggested that, PRP was as effective as steroid at achieving symptom relief at 3 and 6 months after injection.[21]
Contrary to the negative findings of the above-mentioned 2 studies, some other studies observed controversial results. In the study of Shetty et al,[34] the authors compared the efficacy of traditional corticosteroid injection (30 patients) with PRP injection (30 patients). At the 3-month of follow-up, the postoperative measure outcomes (VAS, American Foot and Ankle Score (AFAS), and Food & Ankle Disability Index (FADI)) were significantly improved in both groups.[34] And these results were much better in the PRP group than that in the steroid group.[34] Similarly, Say et al[27] compared the effects of PRP and steroid in patients with plantar fasciitis. In that study, 50 patients were included, and each group had a sample size of 25 patients.[27] At the 6 weeks and 6 months of follow-up, mean AFAS in PRP group was 85.5 ± 4.2 and 90.6 ± 2.6, compared with 75.3 ± 4.8 and 80.3 ± 4.7 in the steroid group, respectively.[27] The difference between the 2 groups was statistically significant (P < .001) at 6 weeks and 6 months.[27] PRP had a larger change in AFAS and VAS scores than that of the steroid.[27]
With regarding of the long-term effect of PRP, our results suggested that PRP was associated with greater changes in VAS, AOFAS, and RMS scores compared with other treatments. Our findings were in line with the results of the previous studies. In the trial conducted by Jain et al,[21] the mean VAS, AOFAS, and RMS scores in the PRP group (3.3, 88.5, and 1.9) were significantly better than the steroid group (5.3, 75, and 2.6) at the 12 months after treatment.[21] Likewise, in the study of Monto et al,[28] the AOFAS score in PRP group was 94 at 12 months and 92 at 24 months, compared with 58 and 56 in the steroid group, respectively.[28] The difference in AOFAS score between the PRP and steroid groups was clinically significant at the 12- and 24-month follow-up evaluations (P = .001).[28] Thus, it could be concluded that PRP was more effective than steroid in a long term in the management of plantar fasciitis.
PRP was administered at the point of maximum tenderness of the heel. Previous studies have advocated the use of ultrasound guidance for the injection in plantar fasciitis,[35,36] since this could allow for more accurate placement of the injection. However, results from the trials conducted by Tsai[37] and Kane[38] suggested that ultrasound-guided injection did not appear to more effective than palpation-guided injection in the treatment of idiopathic plantar fasciitis. Due to the limited data of the included studies, we did not conduct a subgroup analysis to explore whether ultrasound-guided injection was more effective than palpation-guided injection.
There were several limitations in this meta-analysis. First, our study was conducted based on 10 RCTs, all of which had a relatively small sample size (N < 100). Compared with the larger trials, smaller trials were more likely to result in an overestimation of the treatment effects. Second, there was substantial heterogeneity among the included studies. However, it should not be surprising given the variation in injection regimens and needle-guidance techniques. These factors may result in the heterogeneity and have potential impact on our results. Third, our data analysis for RMS was based on 3 RCTs; thus, the conclusion about the effects of PRP in RMS should be interpreted with caution.
In conclusion, the present study suggested that PRP was as effective as other treatments in terms of pain and functional results in the treatment of patients with plantar fasciitis. Our results demonstrated the long-term benefit of PRP for plantar fasciitis. Thus, it should be used as an alternative approach for patients with plantar fasciitis. However, considering the small sample size and heterogeneity among the studies, additional larger scale RCTs are needed to verify these findings.
Author contributions
Conceptualization: Yan Ling.
Data curation: Shu Wang.
Investigation: Yan Ling.
Methodology: Yan Ling.
Supervision: Yan Ling.
Writing – original draft: Shu Wang.
Footnotes
Abbreviations: AOFAS = American Orthopaedic Foot and Ankle Society Score, GRADE = Grading of Recommendations Assessment, Development and Evaluation, PRP = platelet-rich plasma, RCTs = randomized controlled trials, RMS = Roles–Maudsley score, VAS = visual analog scale, WMD = weight mean difference.
The authors have no conflicts of interest to disclose.
References
- [1].Riddle DL, Schappert SM. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int 2004;25:303–10. [DOI] [PubMed] [Google Scholar]
- [2].Irving DB, Cook JL, Menz HB. Factors associated with chronic plantar heel pain: a systematic review. J Sci Med Sport 2006;9:11–22. discussion 23–14. [DOI] [PubMed] [Google Scholar]
- [3].Tong KB, Furia J. Economic burden of plantar fasciitis treatment in the United States. Am J Orthop (Belle Mead NJ) 2010;39:227–31. [PubMed] [Google Scholar]
- [4].Irving DB, Cook JL, Young MA, et al. Impact of chronic plantar heel pain on health-related quality of life. J Am Podiatr Med Assoc 2008;98:283–9. [DOI] [PubMed] [Google Scholar]
- [5].Toomey EP. Plantar heel pain. Foot Ankle Clin 2009;14:229–45. [DOI] [PubMed] [Google Scholar]
- [6].Cheung JT, An KN, Zhang M. Consequences of partial and total plantar fascia release: a finite element study. Foot Ankle Int 2006;27:125–32. [DOI] [PubMed] [Google Scholar]
- [7].Tatli YZ, Kapasi S. The real risks of steroid injection for plantar fasciitis, with a review of conservative therapies. Curr Rev Musculoskelet Med 2009;2:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sanchez M, Anitua E, Orive G, et al. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med 2009;39:345–54. [DOI] [PubMed] [Google Scholar]
- [9].Martinelli N, Marinozzi A, Carni S, et al. Platelet-rich plasma injections for chronic plantar fasciitis. Int Orthop 2013;37:839–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].O’Malley MJ, Vosseller JT, Gu Y. Successful use of platelet-rich plasma for chronic plantar fasciitis. HSSJ 2013;9:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kumar V, Millar T, Murphy PN, et al. The treatment of intractable plantar fasciitis with platelet-rich plasma injection. Foot (Edinb) 2013;23:74–7. [DOI] [PubMed] [Google Scholar]
- [12].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [17].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [18].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [19].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Egmond JC, Breugem SJ, Driessen M, et al. Platelet-rich-plasma injection seems to be effective in treatment of plantar fasciitis: a case series. Acta Orthop Belg 2015;81:315–20. [PubMed] [Google Scholar]
- [21].Jain K, Murphy PN, Clough TM. Platelet rich plasma versus corticosteroid injection for plantar fasciitis: a comparative study. Foot (Edinb) 2015;25:235–7. [DOI] [PubMed] [Google Scholar]
- [22].Mahindra P, Yamin M, Selhi HS, et al. Chronic plantar fasciitis: effect of platelet-rich plasma, corticosteroid, and placebo. Orthopedics 2016;39:e285–9. [DOI] [PubMed] [Google Scholar]
- [23].Vahdatpour B, Kianimehr L, Ahrar MH. Autologous platelet-rich plasma compared with whole blood for the treatment of chronic plantar fasciitis; a comparative clinical trial. Adv Biomed Res 2016;5:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tiwari M, Bhargava R. Platelet rich plasma therapy: a comparative effective therapy with promising results in plantar fasciitis. J Clin Orthop Trauma 2013;4:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Acosta-Olivo C, Elizondo-Rodriguez J, Lopez-Cavazos R, et al. Plantar fasciitis. A comparison of treatment with intralesional steroids versus platelet-rich plasma (PRP). A randomized, blinded study. J Am Podiatr Med Assoc 2016;107:490–6. [DOI] [PubMed] [Google Scholar]
- [26].Vahdatpour B, Kianimehr L, Moradi A, et al. Beneficial effects of platelet-rich plasma on improvement of pain severity and physical disability in patients with plantar fasciitis: a randomized trial. Adv Biomed Res 2016;5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Say F, Gurler D, Inkaya E, et al. Comparison of platelet-rich plasma and steroid injection in the treatment of plantar fasciitis. Acta Orthop Traumatol Turc 2014;48:667–72. [DOI] [PubMed] [Google Scholar]
- [28].Monto RR. Platelet-rich plasma efficacy versus corticosteroid injection treatment for chronic severe plantar fasciitis. Foot Ankle Int 2014;35:313–8. [DOI] [PubMed] [Google Scholar]
- [29].Omar AS, Ibrahim ME, Ahmed AS, et al. Local injection of autologous platelet rich plasma and corticosteroid in treatment of lateral epicondylitis and plantar fasciitis: randomized clinical trial. Egypt Rheumatol 2012;34:43–9. [Google Scholar]
- [30].Sherpy NA, Hammad MA, Hagrass HA, et al. Local injection of autologous platelet rich plasma compared to corticosteroid treatment of chronic plantar fasciitis patients: a clinical and ultrasonographic follow-up study. Egypt Rheumatol 2016;38:247–52. [Google Scholar]
- [31].Hsiao MY, Hung CY, Chang KV, et al. Comparative effectiveness of autologous blood-derived products, shock-wave therapy and corticosteroids for treatment of plantar fasciitis: a network meta-analysis. Rheumatology (Oxford) 2015;54:1735–43. [DOI] [PubMed] [Google Scholar]
- [32].Tsikopoulos K, Tsikopoulos A, Natsis K. Autologous whole blood or corticosteroid injections for the treatment of epicondylopathy and plantar fasciopathy? A systematic review and meta-analysis of randomized controlled trials. Phys Ther Sport 2016;22:114–22. [DOI] [PubMed] [Google Scholar]
- [33].Aksahin E, Dogruyol D, Yuksel HY, et al. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg 2012;132:781–5. [DOI] [PubMed] [Google Scholar]
- [34].Shetty VD, Dhillon M, Hegde C, et al. A study to compare the efficacy of corticosteroid therapy with platelet-rich plasma therapy in recalcitrant plantar fasciitis: a preliminary report. Foot Ankle Surg 2014;20:10–3. [DOI] [PubMed] [Google Scholar]
- [35].Tsai WC, Wang CL, Tang FT, et al. Treatment of proximal plantar fasciitis with ultrasound-guided steroid injection. Arch Phys Med Rehabil 2000;81:1416–21. [DOI] [PubMed] [Google Scholar]
- [36].Cunnane G, Brophy DP, Gibney RG, et al. Diagnosis and treatment of heel pain in chronic inflammatory arthritis using ultrasound. Semin Arthritis Rheum 1996;25:383–9. [DOI] [PubMed] [Google Scholar]
- [37].Tsai WC, Hsu CC, Chen CP, et al. Plantar fasciitis treated with local steroid injection: comparison between sonographic and palpation guidance. J Clin Ultrasound 2006;34:12–6. [DOI] [PubMed] [Google Scholar]
- [38].Kane D, Greaney T, Shanahan M, et al. The role of ultrasonography in the diagnosis and management of idiopathic plantar fasciitis. Rheumatology (Oxford) 2001;40:1002–8. [DOI] [PubMed] [Google Scholar]


