Abstract
Calmodulin (CaM) serves as a pervasive regulatory subunit of CaV1, CaV2, and NaV1 channels, exploiting a functionally conserved carboxy-tail element to afford dynamic Ca2+-feedback of cellular excitability in neurons and cardiomyocytes. Yet this modularity counters functional adaptability, as global changes in ambient CaM indiscriminately alter its targets. Here, we demonstrate that two structurally unrelated proteins, SH3 and cysteine-rich domain (stac) and fibroblast growth factor homologous factors (fhf) selectively diminish Ca2+/CaM-regulation of CaV1 and NaV1 families, respectively. The two proteins operate on allosteric sites within upstream portions of respective channel carboxy-tails, distinct from the CaM-binding interface. Generalizing this mechanism, insertion of a short RxxK binding motif into CaV1.3 carboxy-tail confers synthetic switching of CaM regulation by Mona SH3 domain. Overall, our findings identify a general class of auxiliary proteins that modify Ca2+/CaM signaling to individual targets allowing spatial and temporal orchestration of feedback, and outline strategies for engineering Ca2+/CaM signaling to individual targets.
Research organism: Other
Introduction
Supporting vital biological functions, voltage-gated calcium (CaV1 and CaV2) and sodium (NaV1) channels are tuned by the Ca2+-binding protein, calmodulin (CaM) (Ben-Johny et al., 2015; Catterall et al., 2017; Saimi and Kung, 2002). NaV1 supports action potential initiation and propagation (Hille, 2001), while CaV1/2 initiate muscle contraction, neurotransmission, and gene transcription (Berridge et al., 2000; Clapham, 2007; Maier and Bers, 2002). Despite divergent functions, these channel families share a conserved carboxy-tail element, termed Ca2+-inactivating (CI) module, that harbors CaM. Functionally, the CI module confers dynamic Ca2+-dependent regulation to CaV1, CaV2, and NaV1 that manifests as either inactivation (CDI) or facilitation (CDF), negative and positive feedback, respectively (Ben-Johny et al., 2015; Minor and Findeisen, 2010). Yet, this modularity poses a challenge – mechanisms that tune Ca2+/CaM-feedback must distinguish between structurally similar targets. Global inhibition of CaM indiscriminately alters many processes (Persechini and Stemmer, 2002). Given the abundance of CaM-regulated proteins, mechanisms that adjust CaM signaling to individual targets are crucial (Marshall et al., 2015; Saimi and Kung, 2002). Physiologically, Ca2+-regulation of CaV1 is critical for cardiac electrical stability (Limpitikul et al., 2014; Mahajan et al., 2008), rhythmicity of oscillatory neurons (Chan et al., 2007; Huang et al., 2012), and vesicle release at ribbon synapses (Joiner and Lee, 2015). CaV2 modulation contributes to short-term synaptic plasticity and spatial learning (Adams et al., 2010; Jackman and Regehr, 2017; Nanou et al., 2016), while NaV1 modulation tunes excitability of skeletal and cardiac muscle (Pitt and Lee, 2016; Van Petegem et al., 2012). Consequently, aberrant channel regulation underlies numerous maladies including cardiac arrhythmias (Venetucci et al., 2012; Zimmer and Surber, 2008), neurological and neuropsychiatric disorders (Adams and Snutch, 2007; Striessnig et al., 2010; Zamponi, 2016), and skeletal myotonia (Cannon, 2015).
Src homology 3 (SH3) and cysteine-rich domain (C1) proteins (stac) have emerged as attractive candidates that modulate CaV gating and trafficking (Polster et al., 2015; Suzuki et al., 1996). Initially identified in association with congenital skeletal myopathies as a structural protein that abets CaV1.1 plasmalemmal trafficking (Horstick et al., 2013; Linsley et al., 2017c; Polster et al., 2015), stac also suppresses CaV1.2 CDI (Campiglio et al., 2018; Wong King Yuen et al., 2017). Even so, the specificity of stac in tuning Ca2+-regulation of the broader CaV/NaV family, the underlying elementary mechanisms, and molecular determinants remain to be fully elucidated (Wong King Yuen et al., 2017). Stac isoforms (stac1-3) share a common architecture containing a C1 and two SH3 domains fused via a linker, and exhibit tissue-specific expression (Suzuki et al., 1996). Stac1/2 are expressed throughout the brain (Nelson et al., 2013; Suzuki et al., 1996), the peripheral nervous system (Legha et al., 2010), the retina (Wilhelm et al., 2014), and the inner ear (Cai et al., 2015), while stac3 is limited to the skeletal muscle (Nelson et al., 2013). Resolving mechanisms by which stac modulates CaV may furnish long-sought physiological insights (Suzuki et al., 1996).
Evolutionarily distinct from stac, fibroblast growth factor (fgf) homologous factors (fhf1-4, fgf11-14) are unconventional fgf that lack a secretory signal and serve as intracellular regulators of NaV gating and trafficking (Goldfarb, 2005; Pablo and Pitt, 2016). Curiously, fhf interacts with the NaV CI module in close proximity to the CaM binding interface, suggesting interplay between these modulators (Wang et al., 2012; Wang et al., 2011b). Yet, functionally, fhf is thought to modulate only voltage-dependent fast inactivation (Goldfarb et al., 2007; Lou et al., 2005; Wang et al., 2011a), with changes in Ca2+-regulation unknown. Fhf isoforms are differentially expressed in the brain (Smallwood et al., 1996; Yan et al., 2014), peripheral nervous system (Ornitz and Itoh, 2001), and cardiac (Wei et al., 2011) and skeletal muscle (Kraner et al., 2012). Genetic variation in fhf4 is linked to spinocerebellar ataxia 27 (Coebergh et al., 2014) and fhf1 to cardiac arrhythmias (Wei et al., 2011), hinting at their relevance for regulating neuronal and cardiac excitability.
By leveraging synergistic insights from CaV and NaV channels, we demonstrate that stac selectively diminishes Ca2+-regulation of CaV1. In-depth analysis shows that stac binds to a distinct channel interface from CaM and uses an allosteric mechanism to lock CaV1 into a high open probability (PO) gating mode. We further localize a minimal motif that recapitulates stac modulation of CaV1 gating. Paralleling stac-effect on CaV1, fhf reduces CDI of NaV1 with no effect on CaV1. In all, our findings point to a general class of auxiliary proteins that intercept CaM signaling to individual targets, allowing spatial and temporal orchestration of Ca2+-feedback.
Results
Stac selectively suppresses Ca2+-feedback of CaV1 channels
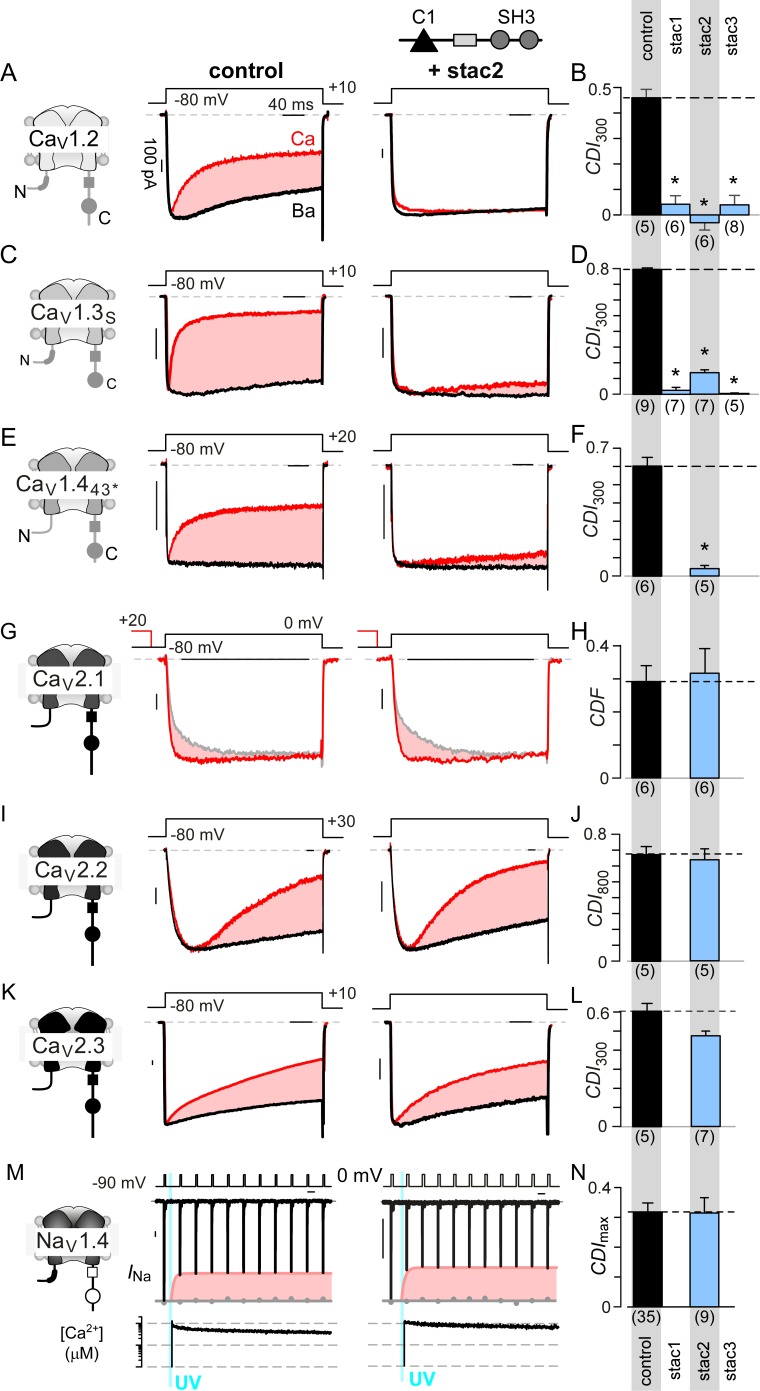
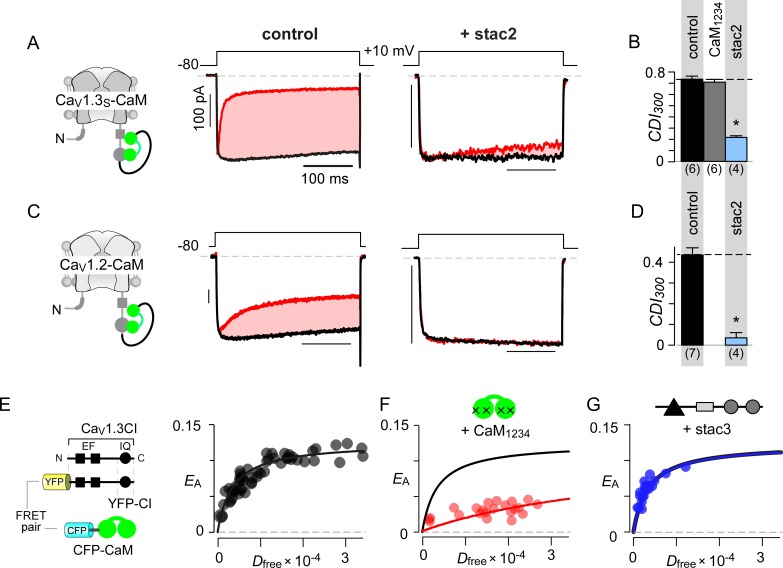
We sought to determine stac effect on CaV1, CaV2, and NaV1 channels in heterologous systems. Figure 1A shows baseline effects of stac on CaV1.2 (Campiglio et al., 2018; Polster et al., 2015; Wong King Yuen et al., 2017). Devoid of stac, CaV1.2 exhibits CaM-mediated CDI manifesting as enhanced decay of Ca2+ (red) versus Ba2+ current (black) when elicited using a step depolarization (Figure 1A, middle subpanel). As Ba2+ binds CaM poorly (Linse and Forsén, 1995), Ba2+-currents furnish a baseline measure of voltage-dependent inactivation (VDI) without CDI. Upon stac2 co-expression, CDI is diminished (Figure 1A, right subpanel). To quantify steady-state extent of inactivation, we measured the fraction of peak Ca2+ and Ba2+ current remaining after 300 ms depolarization, rCa and rBa (Figure 1—figure supplement 1A). The strength of CDI is quantified as CDI300 = 1 – rCa/rBa, the fractional Ca2+-dependent component of inactivation. Thus quantified, the population data confirm a reduction in CDI of CaV1.2 with stac2 (p=3.6 × 10−5; Figure 1B). Further analysis shows that both stac1 and stac3 isoforms also diminish CDI (p=2.0 × 10−5 and 7.1 × 10−5, respectively, Figure 1B and Figure 1—figure supplement 1A). Similarly, CaV1.3 short variant (CaV1.3S), a close homolog of CaV1.2, exhibits strong baseline CDI that is reduced on co-expression of stac1, stac2, and stac3 (p<1 × 10−5; Figure 1C–D and Figure 1—figure supplement 1B). Generalizing this phenomenon, stac2 also reduces CDI of CaV1.443* (p=3.2 × 10−5; Figure 1E–F; Figure 1—figure supplement 1C) (Tan et al., 2012).
Figure 1. Stac specifically abolishes Ca2+/CaM-regulation in CaV1 channels.
(A) Stac2 diminishes CDI of CaV1.2. Left, cartoon schematic shows CaV1.2. Middle, exemplar current traces evoked in response to +10 mV voltage-step shows robust CDI (rose shaded area) evident as enhanced current decay with Ca2+ (red) versus Ba2+ (black) as the charge carrier. Right, stac2 abolishes CDI. Steady-state levels of inactivation are assessed as the fraction of peak current remaining following 300 ms depolarization (rCa and rBa) and CDI = 1 – rCa/rBa. (B) Bar graph displays population data of CDI300 for CaV1.2 in the absence and in the presence of stac1, stac2, or stac3. Dashed line shows baseline CDI in the absence of stac for comparison. Each bar, mean ±S.E.M. obtained from specified number of cells (n). (C–D) Stac isoforms suppress CDI of CaV1.3S, the canonical short variant, as confirmed by both exemplar traces (C) and population data (D). Format as in (A) and (B). (E–F) Stac2 abolishes CDI of CaV1.443* assessed in response to +20 mV test pulse. Format as in (A) and (B). (G–H) Stac2 spares CDF of CaV2.1, as evaluated using a prepulse protocol. An isolated test pulse to 0 mV elicits Ca2+ currents with biphasic activation (gray, G). With a + 20 mV prepulse, channels are partially facilitated and the slow component of activation is reduced (red, G). The area between the two current traces (ΔQ), divided by τslow, yields facilitation (g). Bar graph plots, CDF = gCa – gBaH). Each bar, mean ±S.E.M from specified number of cells (n). (I–J) Stac2 spares CDI of CaV2.2 assessed in response to +30 mV test pulse. Here, CDI is evaluated following 800 ms of depolarization to accommodate slow inactivation kinetics, yielding CDI800. Format as in (A) and (B). (K–L) Stac2 spares CDI of CaV2.3. Format as in (A) and (B). (M–N) Stac2 spares CDI of NaV1.4. Both in the absence and presence of stac, NaV1.4 peak currents decline following a Ca2+ step (rose fit) (M). Gray dots, peak currents before uncaging. CDI = 1 – average peak INa of last three to four responses after Ca2+ uncaging / peak INa before uncaging. Bar graph plots maximal CDI observed with Ca2+ steps > 5 μM (N). Each bar, mean ±S.E.M.
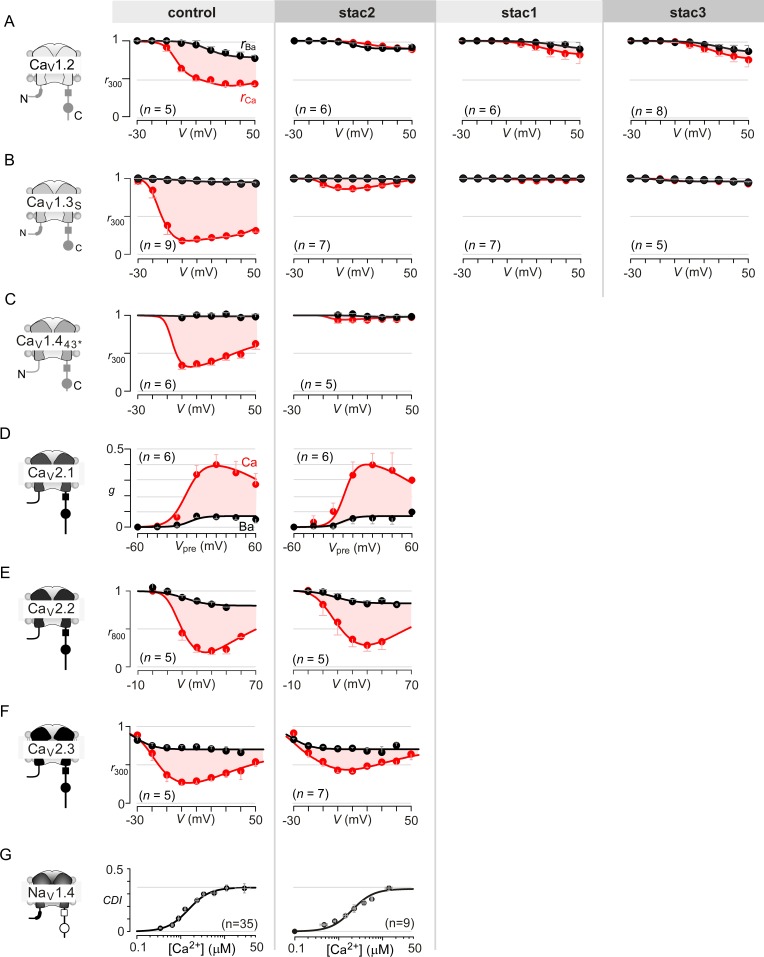
Figure 1—figure supplement 1. Extended data highlight selectivity of stac in modulating CaV1 versus CaV2 and NaV1 channels.
Encouraged by its pervasiveness, we considered whether stac alters Ca2+-dependent modulation of CaV2 isoforms that are abundant in the central nervous system. For CaV2.1, CaM elaborates both CDF and CDI (DeMaria et al., 2001; Lee et al., 2000). However, the Ca2+-sensitivity of CDI process is over 50-fold weaker than that of CDF, casting this negative feedback beyond physiological bounds (Lee et al., 2015). As such, we probed whether stac tunes CDF of CaV2.1 using a well-established prepulse protocol (DeMaria et al., 2001; Thomas and Lee, 2016). Figure 1G displays wildtype CaV2.1 currents in the absence of stac2. On presentation of an isolated test pulse to 0 mV, the activation of Ca2+ current follows a biphasic response (gray trace). Following a brief voltage prepulse, however, the ensuing test pulse yields enhanced Ca2+-currents with monophasic activation reflecting CDF (red trace). Further quantification revealed no change in CDF of CaV2.1 following the addition of stac2 in both exemplar current recordings (Figure 1G) and population data (Figure 1H; Figure 1—figure supplement 1D). For CaV2.2, CaM-regulation manifests as a kinetically slow CDI (Figure 1I) (Liang et al., 2003), that persists despite stac co-expression (Figure 1I–J; Figure 1—figure supplement 1E). Here CDI is quantified by metric CDI800 = 1 – rCa/rBa, measured following 800 ms of depolarization. Likewise, neuronal CaV2.3 also possesses robust CDI, which is spared with stac2 co-expression (Figure 1Figure 1K–L; Figure 1—figure supplement 1F).
Lastly, we tested whether stac suppresses Ca2+-regulation of NaV1, related to CaV1. Although all NaV1 possess a CI module homologous to both CaV1 and CaV2 (Babitch, 1990), CDI that bears mechanistic similarity to CaV has been identified only in NaV1.4 (Ben-Johny et al., 2014). Unlike CaV, NaV channels do not convey Ca2+ influx that triggers Ca2+-feedback. We used rapid photo-uncaging of Ca2+ to produce a step-like increase in intracellular [Ca2+]i, whose magnitude is simultaneously monitored via fluorescent indicators. Figure 1M displays baseline Ca2+-regulation of NaV1.4. As CDI is kinetically slow in comparison to fast inactivation, we applied a train of step depolarizations evoked at 10 Hz to probe Ca2+-dependent effects (Ben-Johny et al., 2014). Without Ca2+-uncaging, peak NaV1.4 currents remained steady (gray dots). In response to an ~10 μM Ca2+ step, the peak Na current declined rapidly revealing CDI (red envelope). Stac overexpression, however, does not disrupt NaV1.4 CDI (Figure 1M–N; Figure 1—figure supplement 1G). Overall, these results show the specificity of stac in tuning Ca2+-regulation of CaV1 channels.
Stac interacts with CaV1 CI module to elicit CDI suppression
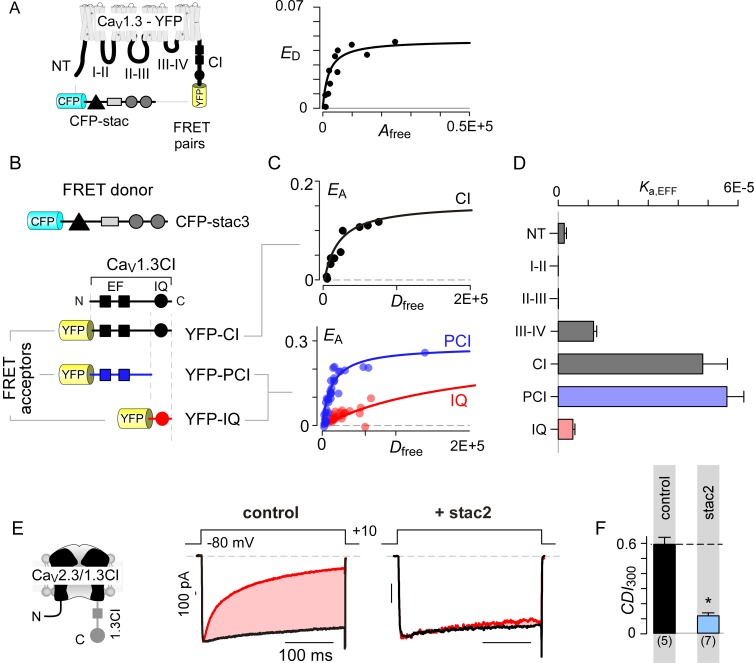
We sought to identify molecular mechanisms that underlie selective CaV1 modulation by stac. As the stac effect here is inferred based on overexpression analysis, we determined relative concentration requirements for stac binding to CaV1 holo-channel complexes within the milieu of living cells. We used live cell FRET 2-hybrid assay (Erickson et al., 2001) to probe the interaction of CFP-tagged stac3 with YFP-linked CaV1.3S. As all three stac variants suppress the CDI of all CaV1 isoforms, we chose CaV1.3 as YFP-tethered channels and a repertoire of YFP-tagged intracellular loop peptides are readily available for in-depth binding analysis (Yang et al., 2014). Stac3 was selected for its high potency in suppressing CaV1.3 CDI (Figure 1D). Accordingly, we quantified FRET efficiency (ED) between FRET pairs co-expressed in individual cells. By leveraging stochastic expression of the FRET pairs in cells, we obtained a saturating Langmuir relationship between ED and the free acceptor concentration (Afree) permitting estimation of relative binding affinities (Kd,EFF). Thus probed, we obtained a CaV1.3 holo-channel affinity for stac3 of Kd,EFF = 1458 ± 251 Dfree units proportional to ~47 nM (Figure 2A). By comparison, similar analysis of CaM binding to CaV1.3 showed Kd,EFF = 700 Dfreeunits ~ 22 nM (Yang et al., 2014). Consequently, stac’s relatively high binding-affinity for CaV1.3 suggests that it may be a potent modulator even at low concentrations.
Figure 2. Stac interacts with the channel carboxy-terminus.
(A) Live-cell FRET 2-hybrid assay shows high-affinity interaction between CFP-tagged stac3 with YFP-tethered holo-CaV1.3 channels in the presence of auxiliary β2A and α2δ subunits. (B) Cartoon shows FRET pairs, CFP-stac3 with YFP-CI, YFP-PCI, and YFP-IQ of CaV1.3. (C) FRET-binding curves show robust binding of stac3 to both the CI and PCI segment while binding to IQ is weaker. (D) Bar graph summarizes the relative association constant, Ka,EFF, of stac2 binding to major channel intracellular domains. (E–F) Transferring CaV1.3S CI to CaV2.3 (CaV2.3/1.3 CI) unveils latent stac2-mediated suppression of CDI. Format as in Figure 1A – B.
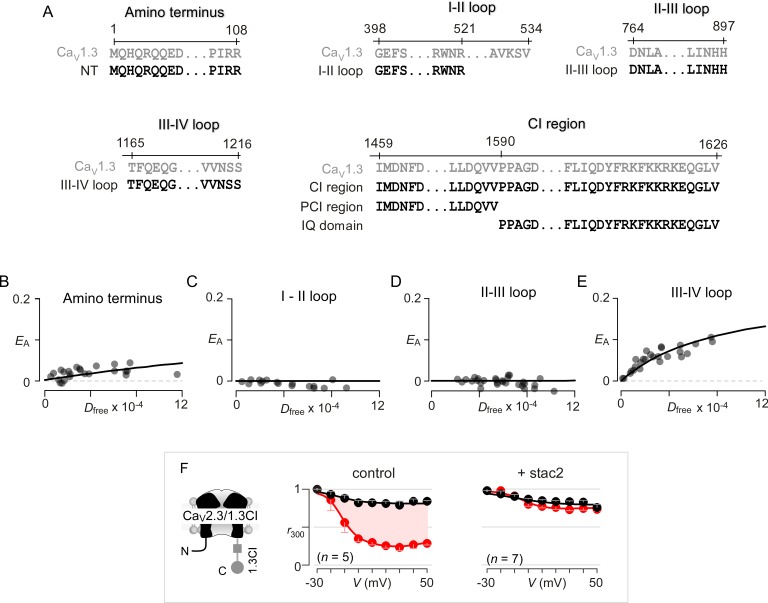
Figure 2—figure supplement 1. Systematic FRET 2-hybrid scan of major intracellular loops of CaV1.3 with stac.
With holo-channel binding assured, we systematically scanned YFP-tagged CaV1.3 intracellular domains (Yang et al., 2014) for stac binding sites (Figure 2B; Figure 2—figure supplement 1A). We found that stac3 binds well to the CI region (Kd,EFF = 20697 ± 3023 Dfree~0.67 ± 0.1 μM, Figure 2C). By contrast, analysis of the amino-terminus, intracellular loops between domains I and II (I-II loop), domains II and III (II-III loop), and domains III and IV (III-IV loop) revealed far weaker binding (Figure 2D; Figure 2—figure supplement 1B–E). To further localize the putative binding loci, we subdivided the CI module into two: (1) a proximal CI segment (PCI) composed of dual vestigial EF hand and preIQ segments and (2) the IQ domain (IQ). The YFP-tagged PCI segment bound stac3 with approximately tenfold higher affinity (Kd,EFF = 17725 ± 3990 Dfree~0.58 ± 0.1 μM) than the downstream IQ domain (Kd,EFF = 204739 ± 25465 Dfree~6.67 ± 0.8 μM) (Figure 2C–D). In all, systematic FRET analysis reveals that stac binds to CaV1 CI relying on upstream elements including the dual vestigial EF hand and preIQ domains, an interface distinct from that for CaM (Bazzazi et al., 2013; Minor and Findeisen, 2010).
To test for functional relevance of stac binding to the CaV1 CI module, we sought to confer stac-sensitivity to a stac-insensitive channel via a chimeric approach. We turned to CaV2.3 that lacks strong stac-mediated CDI suppression, yet forms functional chimeras with CaV1 (Mori et al., 2008; Yang et al., 2014). We replaced the CI region of CaV2.3 with the corresponding segment from CaV1.3 (CaV2.3/1.3 CI). Devoid of stac, CaV2.3–1.3 CI channels exhibit CDI isolated by high intracellular buffering (Figure 2E–F; Figure 2—figure supplement 1F). In contrast to wildtype CaV2.3, stac2 co-expression attenuated CDI (p=4.7 × 10−4, Figure 2E–F; Figure 2—figure supplement 1F), suggesting that CaV1 CI module is necessary for stac-mediated CDI suppression.
Stac uses an allosteric mechanism to suppresses CaM signaling
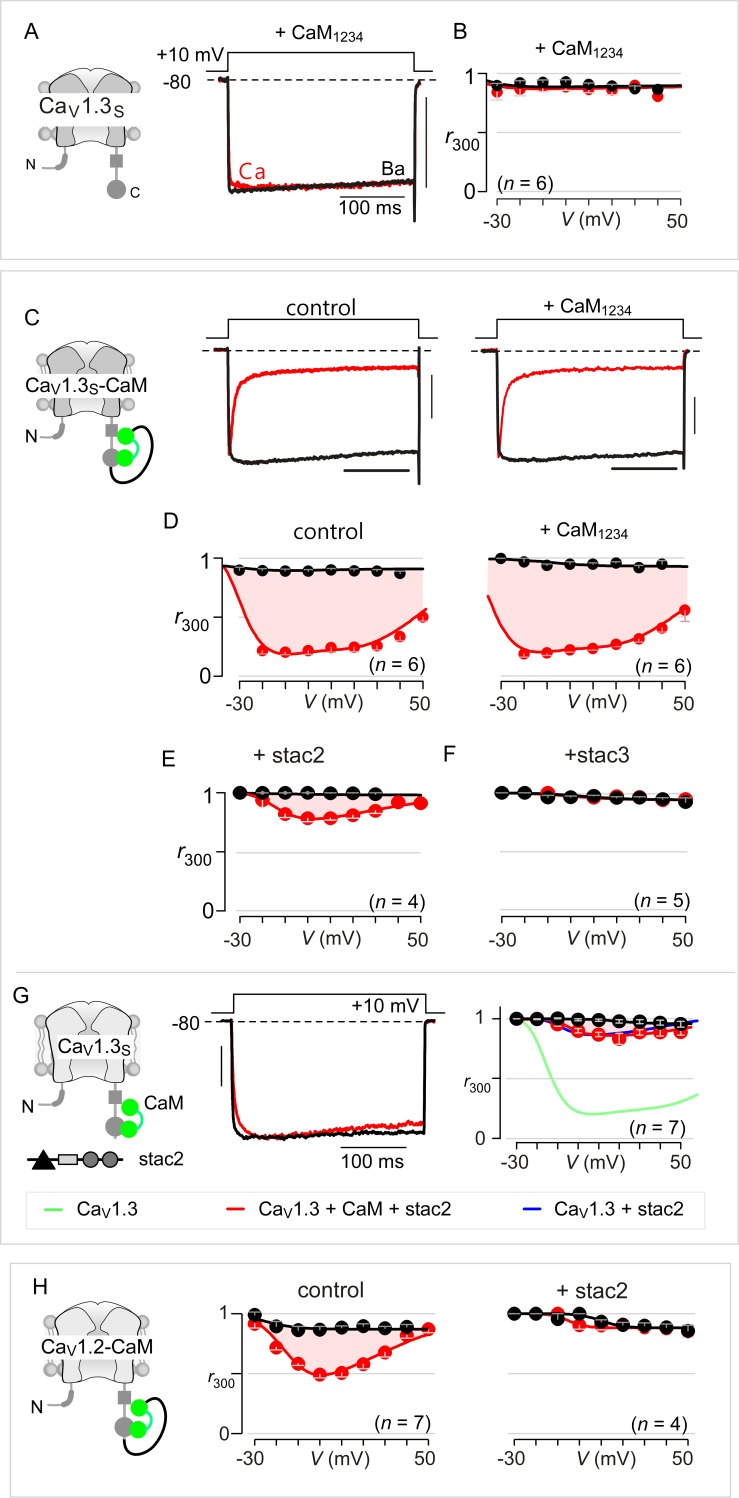
Given that both CaM and stac share the CI module as an effector site, two disparate mechanistic possibilities may allow suppression of Ca2+-regulation. First, stac may competitively displace Ca2+-free CaM (apoCaM) from its preassociation site. Second, stac may supersede CaM signaling to the channel pore via an allosteric mechanism. Systematic FRET analysis suggests that stac preferentially binds upstream CI elements (Figure 2D) while high-affinity CaM preassociation is supported via the IQ domain (Bazzazi et al., 2013; Minor and Findeisen, 2010), hinting that the two modulatory proteins may bind concurrently. To rule out competitive displacement of CaM preassociation, we covalently tethered CaM onto the CaV1.3 carboxy-tail using a poly-glycine linker (CaV1.3S-CaM) (Mori et al., 2004; Yang et al., 2014). This maneuver preserves CDI (Figure 3A left) and ensures a high local CaM concentration near CaV1 extending into the millimolar range, sufficient to protect the channel from a competitive inhibitor (Mori et al., 2004). Dominant-negative CaM1234 with its Ca2+-binding sites disabled, typically displaces intact apoCaM from the CI module thereby resulting in a loss of CDI for wildtype channels (Figure 3—figure supplement 1A–B) (Yang et al., 2014). CDI of CaV1.3S-CaM persists despite CaM1234 co-expression (gray bar, Figure 3B; Figure 3—figure supplement 1C–D). By contrast, CDI of CaV1.3S-CaM is diminished by co-expression of stac2 (p=3.8 × 10−6, Figure 3A–B; Figure 3—figure supplement 1E) and stac3 (p=4.5 × 10−4, Figure 3—figure supplement 1F). As a further test, co-expression of untethered CaV1.3S with both CaM and stac2 also showed low CDI (Figure 3—figure supplement 1G). We observed a similar fate for CaV1.2-CaM with stac2 (p=1.5 × 10−5, Figure 3C–D; Figure 3—figure supplement 1H). These findings suggest that stac does not need to dislodge CaM from its CaV1.3 carboxy-tail binding interface to exert functional modulation.
Figure 3. Allosteric regulation of stac by interaction with the channel carboxy-terminus.
(A–B) Stac2 suppresses CDI of CaV1.3S tethered to CaM. In contrast, fusion of CaM protects CaV1.3S from competitive inhibitors such as CaM1234. Format as in Figure 1A–B. (C–D) Stac2 suppresses CDI of CaV1.2 tethered to CaM. Format as in Figure 1A–B. (E) FRET 2-hybrid assay shows the high-affinity interaction of YFP-tagged CaV1.3 CI to CFP-tagged CaM with relative dissociation constant Kd,EFF ~4000 ± 291 Dfree units. (F) Co-expression of untagged CaM1234 with FRET pairs YFP-tagged CaV1.3 CI and CFP-tagged CaM results in a marked reduction in FRET efficiency. (G) Co-expression of untagged stac3 spares the binding of YFP-tagged CaV1.3 CI with CFP-tagged CaM, yielding an identical EA-Dfree relationship to that in the absence of stac3 (E).
Figure 3—figure supplement 1. Extended data show that stac acts concurrently with CaM.
To test this possibility, we undertook FRET 2-hybrid assay comparing binding of CFP-tagged CaM to YFP-tagged CaV1.3 CI in the presence and absence of unlabeled stac3. If stac3 were to competitively dislodge CaM, then this binding is predicted to be weakened. At baseline, CaM binds to CaV1.3 CI with a relative dissociation constant, Kd,EFF ~4000 ± 291 Dfree units (Figure 3E) (Ben Johny et al., 2013). Upon co-expression of CaM1234, this baseline binding is weakened ~11 fold, yielding a relative affinity of 47153 ± 4815 Dfree units (Figure 3F). By contrast, co-expression of stac3 did not appreciably perturb CaM binding to the CI module with Kd,EFF = 4182 ± 330 Dfree units (Figure 3G). These results suggest that both stac and CaM act concurrently via distinct sites on the channel carboxy-tail, in contradiction with a competitive mechanism.
Elementary mechanisms underlying stac-regulation of CaV1
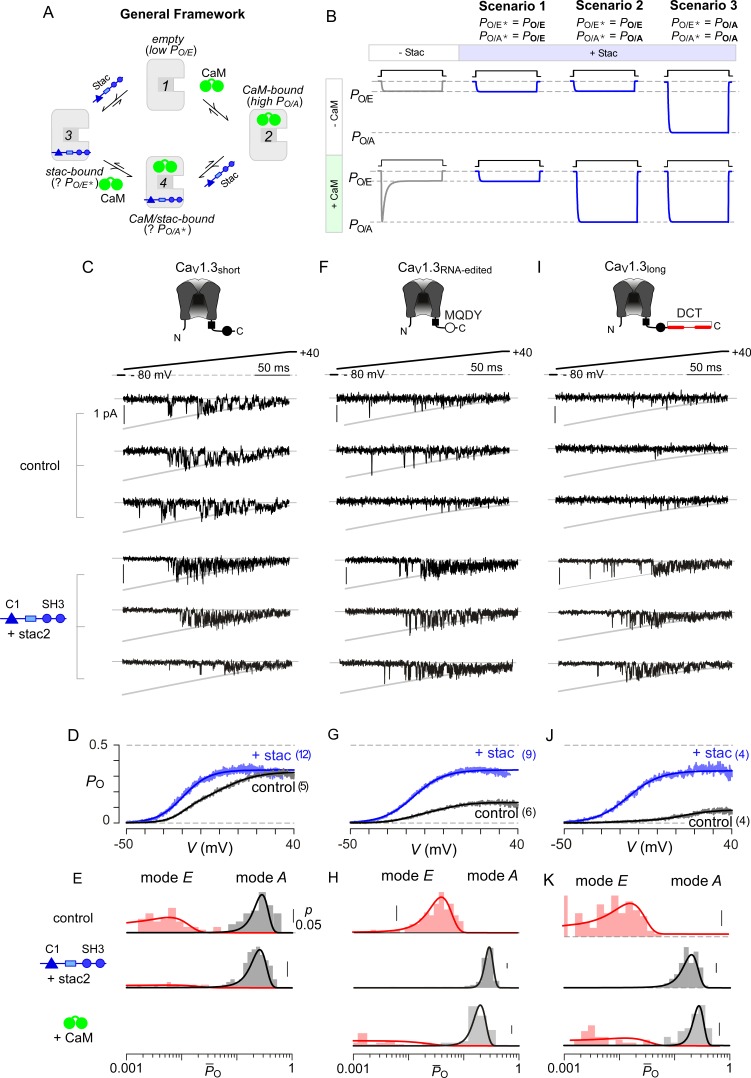
Beyond Ca2+-dependent regulation, apoCaM binding tunes the baseline activity of CaV channels (Adams et al., 2014). Absent stac, CaV1 lacking prebound CaM adopts a low PO configuration (empty configuration, PO/E) while apoCaM binding switches channels into a high PO mode (CaM-bound configuration, PO/A) (Adams et al., 2014). Ca2+/CaM divests this initial enhancement in PO and returns channels into a low PO gating mode (PO/E) manifesting as CDI. The addition of stac as a regulatory agent enriches this modulatory scheme (Figure 4A). Three distinct scenarios may underlie suppression of Ca2+-regulation by stac (Figure 4B): (1) stac binding may pre-inhibit channels into the low PO configuration (PO/E) akin to Ca2+-inactivated channels and prevent further Ca2+-modulation, (2) stac may obstruct Ca2+/CaM regulation while allowing apoCaM to change baseline PO, (3) stac binding may allosterically lock channels into a high PO mode irrespective of CaM-binding status. For Scenario 3, it is possible that the baseline PO of CaV1.3 with stac may be distinct from that observed with CaM-overexpression. These three scenarios may be distinguished at the single-molecule level by assessing CaV1 PO under various CaM-bound conditions using low-noise electrophysiology. We focused on CaV1.3 given the rich assortment of post-transcriptionally modified variants with distinct CaM binding affinities (Bazzazi et al., 2013; Liu et al., 2010; Singh et al., 2008). We focused on three variants, CaV1.3S with high apoCaM affinity, and CaV1.3MQDY and CaV1.3L with low affinities. These variants possess distinct baseline PO and CDI and constitute a convenient platform to identify stac-dependent effects (Adams et al., 2014; Tan et al., 2011).
Figure 4. Stac enhances the PO of CaV1.3.
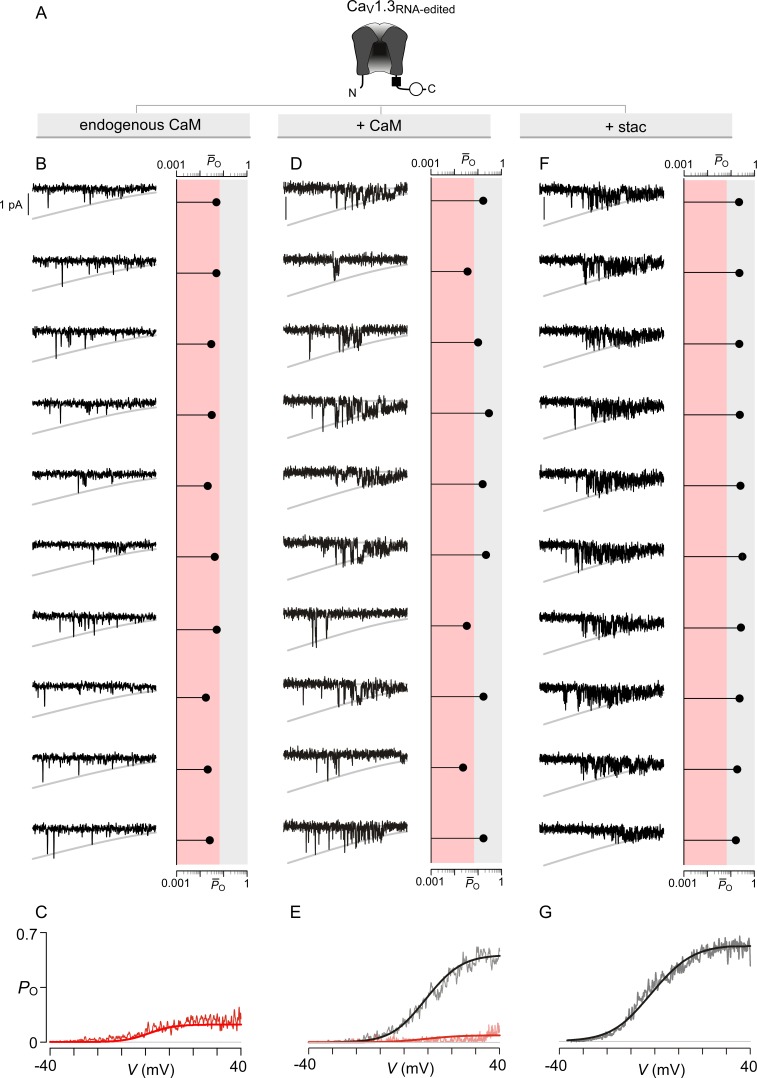
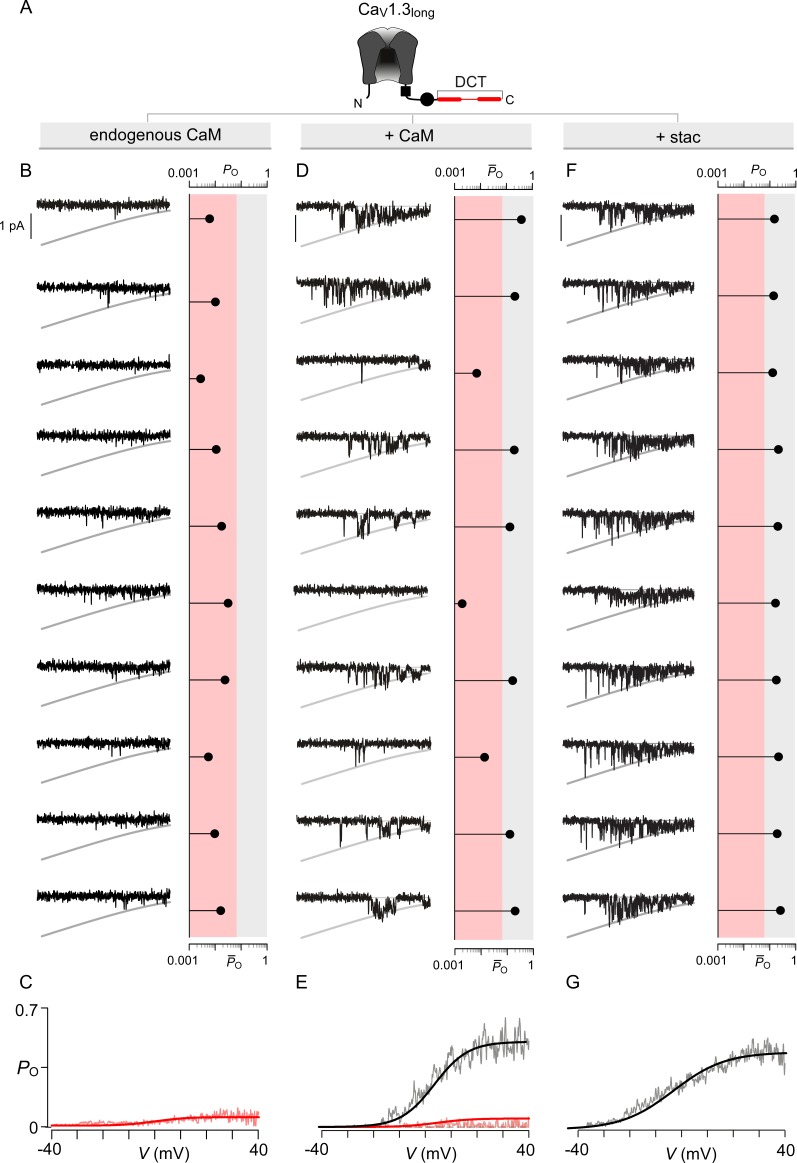
(A) A general four-state scheme for stac and CaM modulation. (1) CaV1.3S devoid of CaM and stac possess a low baseline PO (PO/E). (2) Without stac, apoCaM binding to CaV1.3S upregulates baseline PO (PO/A). Baseline PO of CaV1.3S bound to stac in the absence (configuration 3, PO/E*) and the presence of apoCaM (configuration 4, PO/A*) are unknown. (B) Schematic outlines three mechanistic possibilities for stac binding to CaV1 and their functional outcomes. Scenario 1, stac uniformly suppresses PO of CaV1 (PO/E) and abolishes CDI. Scenario 2, apoCaM tunes baseline PO of CaV1 despite concurrent stac binding. Stac, nonetheless, abrogates CDI. Scenario 3, stac uniformly upregulates the baseline PO of CaV1 and abolishes CDI (PO/A). (C) Top, cartoon shows the canonical CaV1.3S variant with a high apoCaM binding affinity. Single-channel analysis of recombinant CaV1.3S in the absence (middle) and presence of stac (bottom). In both panels, the unitary Ba2+ currents during voltage-ramp are shown between −50 mV and +40 mV (slanted gray lines, GHK fit). Robust CaV1.3 openings are detected in the absence and presence of stac. (D) Average single-channel PO-voltage relationship for CaV1.3S obtained from multiple patches in the absence (gray) and presence of stac2 (blue). Error bars indicate ±S.E.M. for specified number of patches and 80–150 stochastic records per patch. (E) Histogram shows distribution of single-trial average PO (O) for the voltage range -30 mV ≤ V ≤ +25 mV under control (top), stac-bound (middle), and CaM-bound (bottom) conditions. O-distribution is bimodal in the absence of stac corresponding to high PO (gray) and low PO (rose) gating modes. With stac, O-distribution is largely restricted to the high PO mode. (F–H) Single-channel analysis of a recombinant CaV1.3RNA-edited variant reveals a marked upregulation in the baseline PO in the presence of stac compared with control conditions in which apoCaM preassociation is weak. Absent stac or CaM, single-trial O-distribution is restricted to the low PO limits, With either stac or CaM, the high PO gating mode re-emerges. Format as in (C–E). (I–K) Stac also upregulates the baseline PO of CaV1.3L, an alternatively spliced variant, by stabilizing the high PO gating configuration. Format as in (C–E).
Figure 4—figure supplement 1. Extended data show that stac2 preferentially biases a high PO gating mode for CaV1.3.
Figure 4—figure supplement 2. Extended data show that stac2 and CaM enhance the PO of CaV1.3RNA-edited variant via discreet transitions into a high PO gating mode.
Figure 4—figure supplement 3. Extended data show that both stac2 and CaM enhance the PO of CaV1.3L variant.
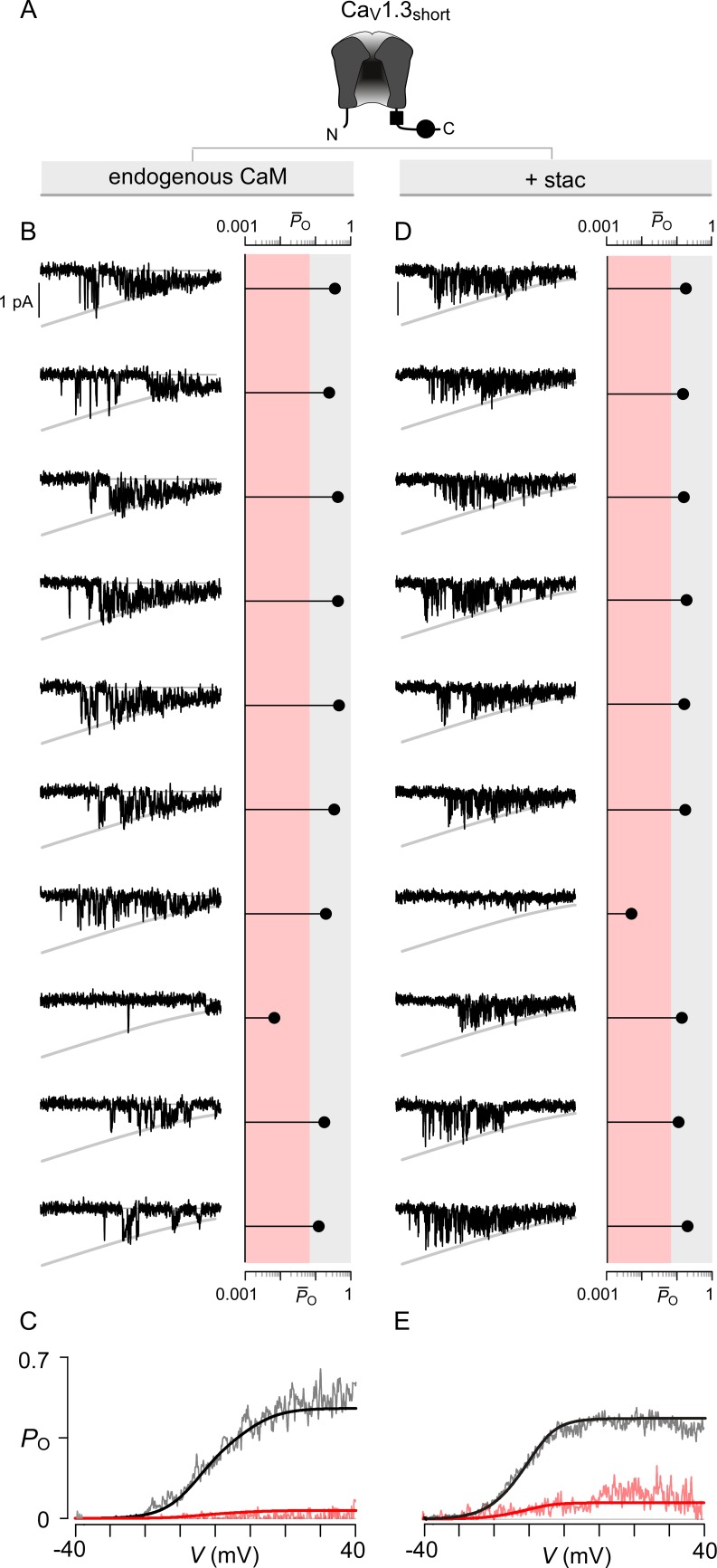
First, we analyzed CaV1.3S in the presence and absence of stac (Figure 4C–E) to determine whether stac may promote channel entry into a low PO gating configuration. CaV1.3S is typically prebound to CaM at endogenous CaM concentrations given its high affinity (Adams et al., 2014). Ba2+ is used as a charge carrier to estimate baseline behavior of channels without confounding effects of CDI. A slow voltage-ramp (shown between −50 and +40 mV) evokes stochastic channel openings that approximate near steady-state PO at each voltage. Stochastic records displayed in Figure 4C show channel openings as downward deflections to the open level (gray curves) and closures correspond to the zero-current portions of the trace. Robust openings are detected both in the presence and absence of stac (Figure 4C). To estimate the steady-state PO – voltage relationship, we averaged many stochastic records to obtain a mean current that is divided into the open level and averaged over multiple patches. CaV1.3S variant exhibits high PO in the absence of stac (Figure 4D) (Adams et al., 2014). Upon stac2 co-expression, the open probability remains high with ~10 mV hyperpolarizing shift in the voltage-dependence of activation (Figure 4D). We scrutinized single-channel trials to assess changes in gating modes. Figure 4—figure supplement 1 displays 10 sequential trials of CaV1.3 single-channel activity evoked by voltage-ramps introduced at 12 s intervals both in the presence and absence of stac. In the absence of stac, CaV1.3S activity switches between epochs of high and low activity, as evident from the diary plot of average PO within individual trials () (Figure 4—figure supplement 1B). Analysis of single-trial distribution reveals a bimodal distribution denoting discrete high and low PO gating modes (Figure 4E). Upon stac overexpression, channel activity is high as evident from -diary plots (Figure 4—figure supplement 1D) and single-trial distribution (Figure 4E). In contradiction with Scenario 1, stac-bound channels are not pre-inhibited; rather, channels preferentially adopt a high PO mode.
To distinguish between the second and third mechanistic possibilities, we considered CaV1.3 variants with weakened apoCaM binding affinity that largely reside in the low PO configuration (Adams et al., 2014). Accordingly, we tested the baseline PO of CaV1.3MQDY, an RNA-edited variant whose central isoleucine within the IQ domain is substituted to a methionine, (Bazzazi et al., 2013; Huang et al., 2012) and an alternative splice variant CaV1.3L containing an autoinhibitory domain that competitively displaces CaM (Liu et al., 2010; Singh et al., 2008). In the absence of stac and under endogenous CaM levels, both CaV1.3MQDY (Figure 4F–G) and CaV1.3L (Figure 4I–J) open sparsely, exhibiting a diminished maximal PO consistent with channels lacking CaM (Adams et al., 2014; Bock et al., 2011). Indeed, single-channel trials of CaV1.3MQDY (Figure 4—figure supplement 2A–C) and CaV1.3L (Figure 4—figure supplement 3A–C) under endogenous levels of CaM reveal uniformly low activity, with single-trial P̄O distribution restricted to low limits (Figure 4H for CaV1.3MQDY; Figure 4K for CaV1.3L). CaM overexpression with both channel variants reveals the resurgence of epochs of high activity (Figure 4—figure supplement 2D–E; Figure 4—figure supplement 3D–E) and a bimodal P̄O distribution with a substantial fraction of trials corresponding to a high PO configuration (Figure 4H and K for CaV1.3MQDY and CaV1.3L respectively). Upon stac co-expression, robust channel openings re-emerge for both CaV1.3MQDY (Figure 4F–G) and CaV1.3L (Figure 4I–J) yielding an enhanced baseline PO akin to CaV1.3S variant (Adams et al., 2014). Scrutiny of single-channel trials for both channel variants reveal uniformly high channel activity (Figure 4—figure supplement 2F–G for CaV1.3MQDY; Figure 4—figure supplement 3F–G for CaV1.3L) and single-trial P̄O distributions are now within the high activity limits (Figure 4H and K) reminiscent of the CaM overexpression. The steady-state PO-V relations for CaV1.3S, CaV1.3MQDY, and CaV1.3L in the presence of stac closely approximate each other (Figures 4D, G and J). These findings demonstrate that consistent with Scenario 3, stac-binding locks CaV1.3 channels in the high PO configuration and effectively decouples the channel pore from CaM-dependent conformational changes. Moreover, these results highlight the dominance of stac over CaM in CaV1 modulation.
U-domain constitutes a minimal motif for CaV1 CDI suppression
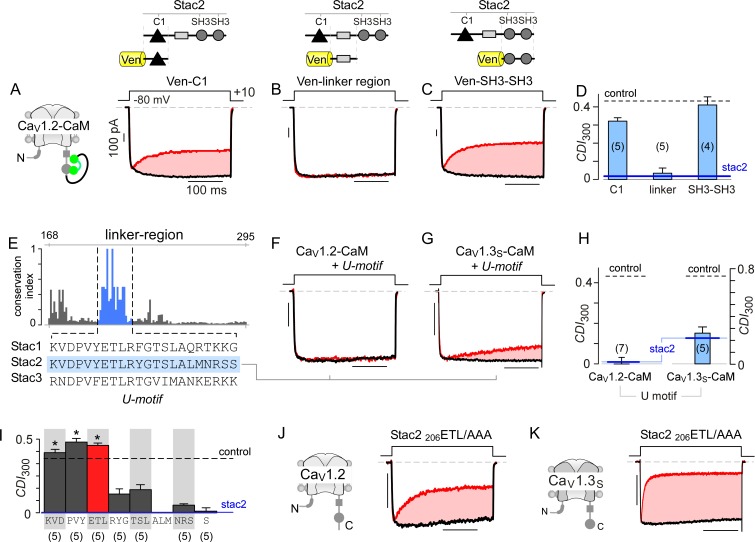
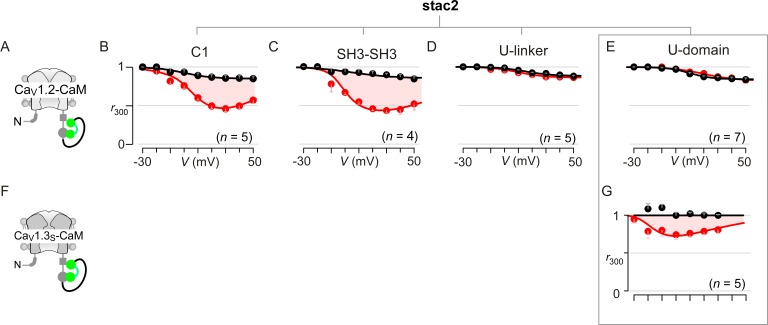
With elementary mechanisms discerned, we turned to identify stac motifs functionally critical for allosteric suppression of CaM-regulation. Structurally, stac isoforms share a modular architecture composed of a C1 domain linked to two SH3 domains via a largely unstructured linker segment (U-linker region) (Suzuki et al., 1996). As these modular subcomponents typically recognize distinct ligands, we reasoned that their molecular functions may be separable (Cohen et al., 1995; Colon-Gonzalez and Kazanietz, 2006). We trisected stac2 to assess whether individual subcomponents can recapitulate functional regulation. We focused initially on C1 and tandem SH3 domains as these segments were recently shown to be critical for CaV1.1 binding and triadic localization in skeletal muscle (Campiglio and Flucher, 2017; Wong King Yuen et al., 2017). Co-expression of either segment, however, only minimally perturbed CDI of CaV1.2-CaM (Figures 5A, C and D; Figure 5—figure supplement 1A–1C). By contrast, the linker region by itself fully abolished CDI of these channels (p=8.9 × 10−6, Figure 5B and D; Figure 5—figure supplement 1D), recapitulating the effect of stac2 on CaV1.2.
Figure 5. Stac U-domain is a minimal effect domain for suppression of CaV1 CDI.
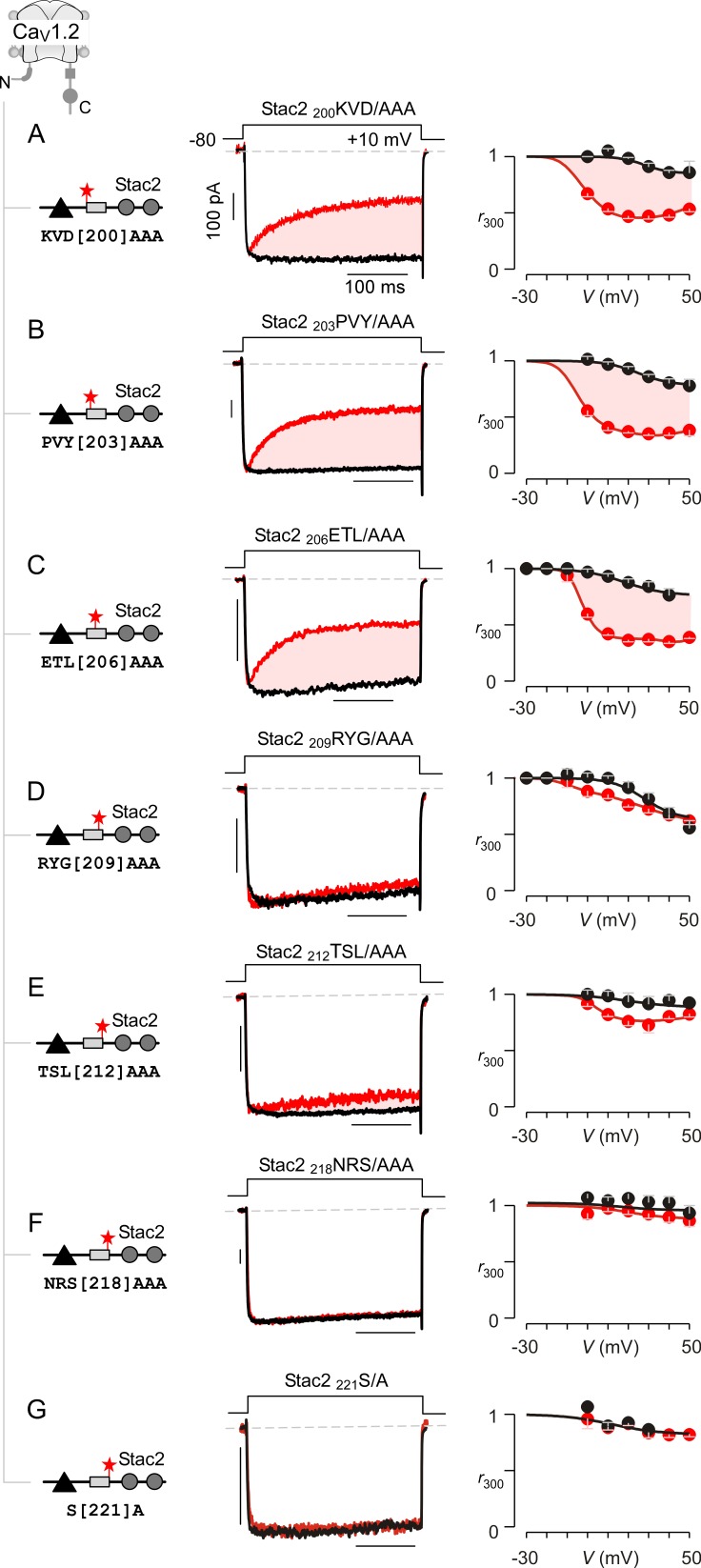
(A–C) To localize an effector motif for stac2, CDI of CaV1.2-CaM was quantified in the presence of three stac subdomains: (1) C1, (2) linker region, and (3) SH3-SH3. Exemplar traces in response to a +10 mV voltage-step depolarization show robust CDI of CaV1.2-CaM in the presence of C1 (A), and SH3-SH3 (C) domains. Co-expression of the linker-region is sufficient to suppress CDI of CaV1.2-CaM (B). Format as in Figure 1A. (D) Bar graph summarizes population data for CaV1.2-CaM CDI in the presence of the three stac subdomains. Each bar, mean ±S.E.M of CDI300 at +10 mV from specified number of cells. CDI levels in the presence (solid blue line) and absence (dashed gray line) of full-length stac2 is reproduced for comparison. (E) Bar graph shows degree of conservation for the linker region across 770 orthologs of stac2. A well conserved subsegment termed U-domain is shaded blue. (F–G) Co-expression of U-domain is sufficient to abolish CDI of CaV1.2-CaM (F) and CaV1.3-CaM (G). Format as in Figure 1A. (H) Bar graph displays population data for CDI of CaV1.2-CaM and CaV1.3-CaM in the presence of U-domain. Each bar, mean ±S.E.M of CDI300 at +10 mV from specified number of cells. Dashed line, baseline CDI for both channels in the absence of stac2. Blue line, CDI of both channels in the presence of full-length stac2. (I) Systematic alanine scanning mutagenesis of the U-domain reveals critical determinants for stac-mediated suppression of CaV1.2 CDI. For comparison, CaV1.2 CDI in the presence (blue line) and absence (black dashed line) of stac2 are shown. Stac2 mutants 200KVD/AAA, 203PVY/AAA, 206ETL/AAA fully abolish stac2-mediated CDI suppression. (J) Exemplar currents show that stac2 mutant 206ETL/AAA eliminates stac’s ability to suppress CaV1.2 CDI. Format as in Figure 1A. (K) Stac2 206ETL/AAA also fails to inhibit CDI of CaV1.3S. Format as in Figure 1A.
Figure 5—figure supplement 1. Extended data demonstrate that the U-motif is a minimal domain for suppressing CDI of CaV1.2 and CaV1.3.
Figure 5—figure supplement 2. Systematic alanine scanning mutagenesis of minimal U-motif reveals structural determinants for stac modulation of CaV1.
To localize functional segments within the U-linker, we undertook bioinformatic analysis to identify highly conserved regions. We performed multiple sequence alignment of complete sequences of 770 stac orthologs using the MUSCLE algorithm (Edgar, 2004) and subsequently computed an empirical measure for the degree of protein sequence conservation at each position. The degree of conservation is defined as the likelihood of observing the consensus residue at each sequence position divided by the number of distinct residues observed at this position. By this algorithm, perfectly conserved residues will yield a unitary value, whereas poorly conserved residues will have a lower score. We identified a 22-amino acid stretch, termed the U-domain (‘unknown’ domain), exhibiting high conservation (Figure 5E, blue shaded region). Co-expression of U-domain diminishes CDI of both CaV1.2-CaM and CaV1.3-CaM (Figure 5F–H, Figure 5—figure supplement 1E–G). Thus informed, we undertook systematic alanine scanning mutagenesis of the stac2 U-domain to identify key residues (Figure 5I; Figure 5—figure supplement 2). Co-expression of mutant stac2 with triple alanine substitution of residues ETL[206-208] resulted in minimal disruption of CaV1.2 and CaV1.3 CDI (Figure 5J–K), suggesting that these residues are necessary. Further analysis revealed residues PVY[203-205] and KVD[200-202] to be critical for stac function (Figure 5I; Figure 5—figure supplement 2A–2C). Residues outside these loci minimally affected stac modulation of CaV1 (Figure 5I; Figure 5—figure supplement 2D–G). These findings confirm the necessity and sufficiency of U-domain as a minimal motif for preventing CaM-regulation of CaV1.
U-domain modulates native CaV1 and reshapes cardiac action potentials
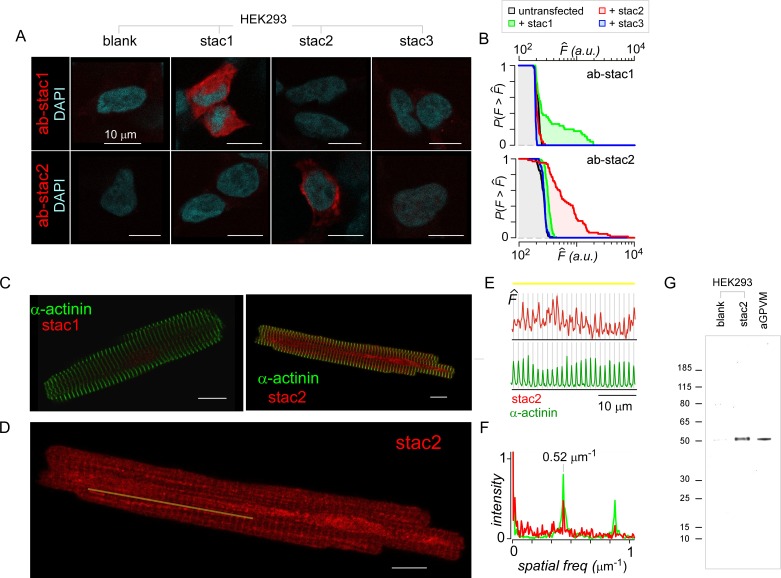
Having identified a minimal U-domain for CDI suppression, we sought to assess potential physiological consequences of stac regulation in cardiac myocytes. As stac expression is yet to be identified in myocytes, we first probed its presence using immunohistochemistry with stac1- and stac2-specific antibodies. To ensure that the two antibodies reliably probe the two isoforms, we first evaluated the ability to detect stac isoforms exogenously expressed in HEK293 cells (Figure 6—figure supplement 1). Untransfected cells show minimal stac1 and stac2 immunostaining (Figure 6—figure supplement 1A–B), as confirmed by confocal imaging and population data. By contrast, immunostaining with stac1 antibody shows labeling with cells expressing stac1 but not stac2 or stac3. Similarly, labeling with stac2 antibody reveals substantial fluorescence (F > 300) in cells transfected with stac2 but not stac1 or stac3. Thus informed, we assessed expression and localization of stac isoforms in cardiac myocytes (Figure 6—figure supplement 1C–F). Analysis of acutely dissociated adult guinea pig ventricular myocytes (aGPVMs) showed stac2 labeling but not stac1 (Figure 6—figure supplement 1C). Consistent with these findings, immunoblotting with stac2 antibody showed ~50 kDa signal in stac2-transfected HEK293 cells but absent from untransfected cells, confirming the ability of the antibody to detect stac2 (Figure 6—figure supplement 1G). Analysis of aGPVM lysate revealed likely endogenous stac2 with a similar molecular weight to that of recombinant stac2 in HEK293 cells (Figure 6—figure supplement 1G).
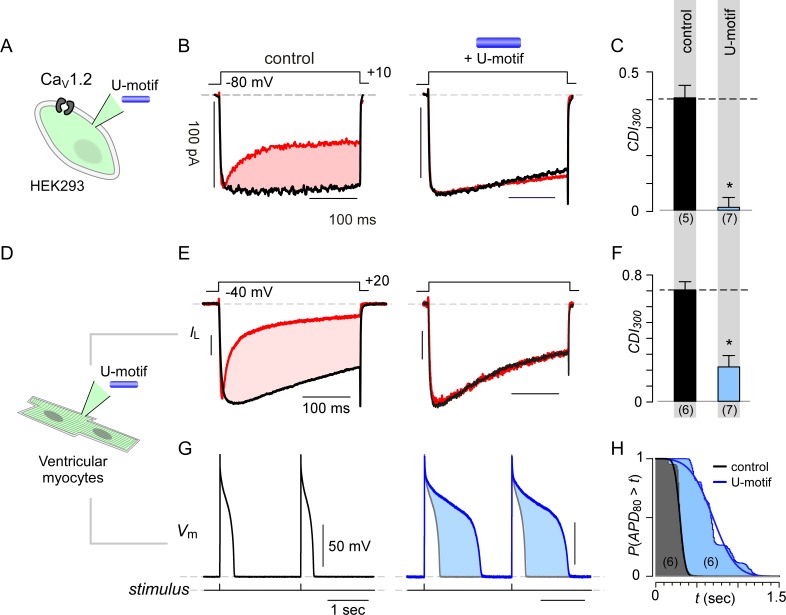
Given this baseline expression, we next considered potential effects of fluctuations in ambient stac levels. We synthesized the U-domain of stac2 as a peptide and delivered it via pipette dialysis to acutely elevate the myocyte’s cytosolic concentration. We validated the synthesized peptide by testing its effect on recombinant CaV1.2 expressed in HEK293 cells (Figure 6A). Following pipette dialysis of the U-peptide, CDI of CaV1.2 was reduced as evident from exemplar currents and population data (Figure 6B–C; Figure 6—figure supplement 2A–B). Thus affirmed, we isolated ventricular myocytes from adult guinea pigs (aGPVMs) to probe changes in CDI of native CaV channels and action potential duration in response to changes in stac levels (Figure 6D). Devoid of U-domain peptide, endogenous Ca2+ currents in ventricular myocytes displayed CDI, establishing baseline levels of CaM-regulation (Figure 6E, Figure 6—figure supplement 2D). Pipette dialysis of U-peptide reduced CDI in myocytes (Figure 6E–F, Figure 6—figure supplement 2E). The reduction in overall inactivation of Ca2+ currents suggest that fluctuations in stac levels may markedly alter action potential waveforms. To test this possibility, we obtained current-clamp recordings of aGPVMs and compared action potential waveforms in the presence and absence of U-peptide. Figure 6G shows typical voltage profiles of action potentials in aGPVMs paced at 0.5 Hz. Waveforms are stable between traces and the mean action potential duration (APD80), the duration of time when the action potential is at least 80% of its peak voltage, is 277.9 ± 31.37 ms (mean ±S.E.M., n = 6). Figure 6H displays the complement of the cumulative distribution of APD80. When the peptide is added to the internal solution, APD80 is enhanced to 740.1 ± 105.49 ms (n = 6) (Figure 6G–H). Thus, the U-peptide both alters the CDI of endogenous cardiac CaV1, prolongs APD, and may ultimately destabilize rhythmicity of the heart.
Figure 6. Synthetic U-domain peptide is sufficient for physiological perturbations.
(A) Schematic illustrates pipette dialysis of custom synthesized U-domain peptide in CaV1.2 heterologously expressed in HEK293 cells, a strategy that emulates acute elevation of cytosolic stac2 levels. (B–C) Exemplar traces and population data confirm that pipette dialysis of U-domain suppresses CDI of recombinant CaV1.2 in HEK293 cells. Format as in Figure 1A–B. Control relation in (C) is duplicated from Figure 1B. (D–F) Pipette dialysis of U-domain abolishes CDI of endogenous L-type current in freshly dissociated ventricular myocytes from adult guinea pigs as evident from exemplar traces and bar graph summary of population data. To eliminate T-type current, the cells were depolarized to −40 mV for a period of 100 ms. Format as in (A-–C). (G) Exemplar action potential traces of aGPVMs paced at 0.5 Hz with (blue) and without (black) 0.5 μM U-domain in the internal solution. In the presence of U-domain, the action potentials are markedly prolonged (blue shaded area) consistent with a loss of CDI of native L-type current. (H) Complement of cumulative distribution (P(APD80 >t) of action potential durations (APD80) obtained in the presence (blue) and absence (black) of U-domain in the internal solution.
Figure 6—figure supplement 1. Baseline expression of stac in cardiac myocytes.
Figure 6—figure supplement 2. Pipette dialysis of U-motif as a peptide abolishes CDI of both recombinant and native L-type currents in ventricular myocytes.
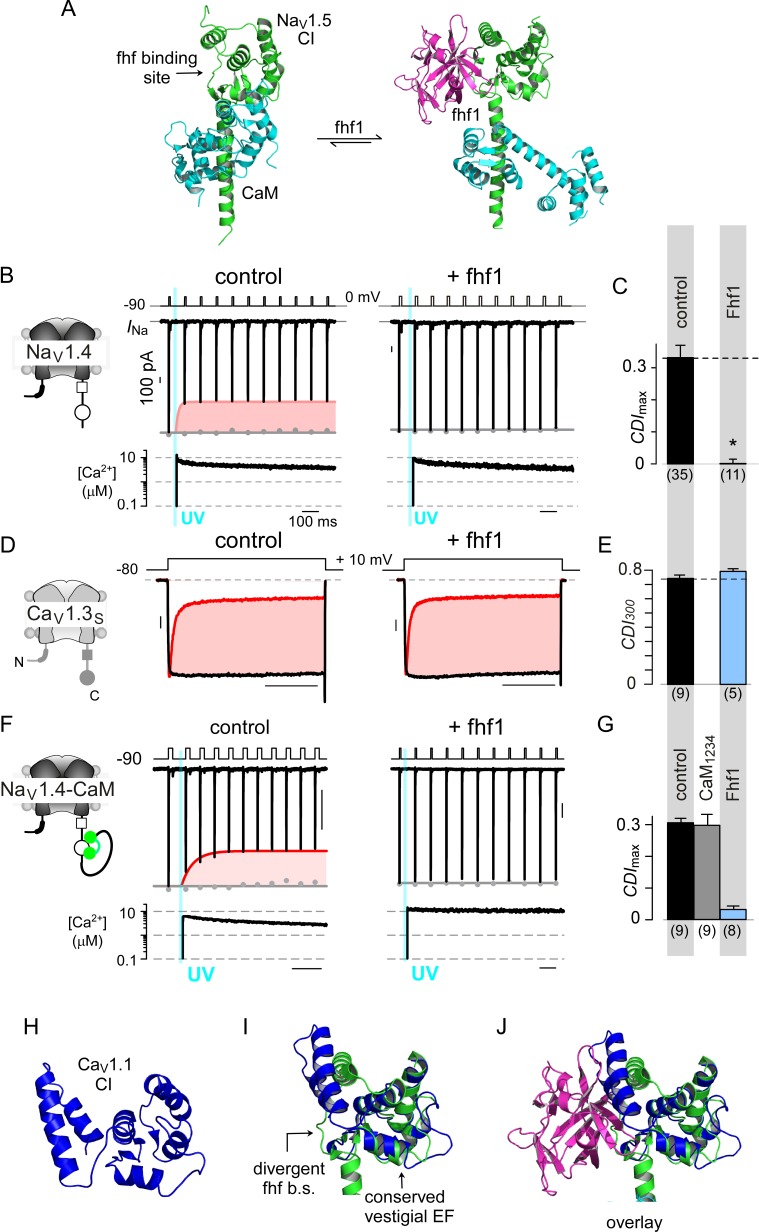
Fhf selectively abrogate CaM signaling to NaV1
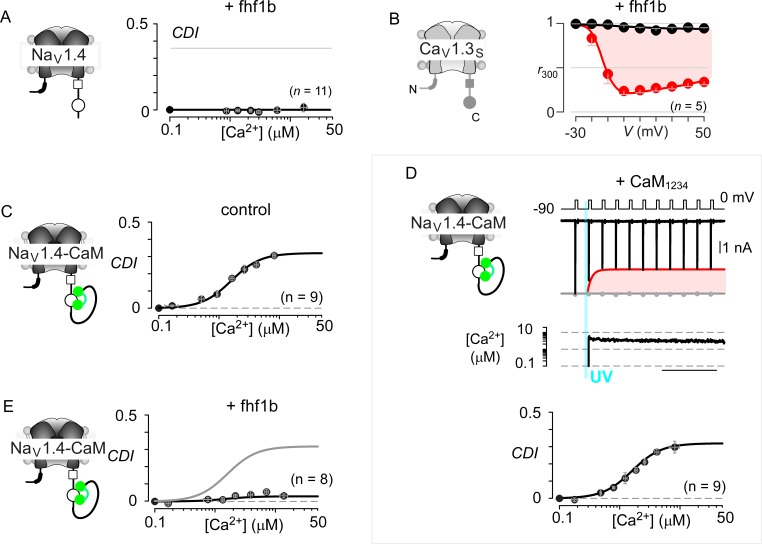
Encouraged by the selectivity of stac for CaV1, we sought to identify other regulatory proteins that may tune CaM-signaling to related channel families. However, recognizing such modulators amidst ion channel signalosomes is challenging. Given that stac interacts with CaV1 CI module via the PCI element, we reasoned that other CaV and NaV interacting proteins that engage a similar interface may suppress CaM-feedback. Intriguingly, recent atomic structures show that fhf interacts with NaV1 CI module via the PCI interface (Figure 7A) (Wang et al., 2012). Yet, functionally, fhf isoforms are thought to modulate only voltage-dependent gating properties, with effects on Ca2+/CaM-regulation unknown (Goldfarb et al., 2007; Lou et al., 2005; Wang et al., 2012). To test whether fhf alters NaV CDI, we undertook quantitative Ca2+ photo-uncaging of the skeletal muscle NaV1.4 isoform. We focused here on fhf1b given its modest baseline expression in skeletal muscle and pathological enrichment in critical illness myopathies (Kraner et al., 2012). Figure 7B reproduces baseline levels of CDI for NaV1.4 under control conditions. Co-expression of fhf1b abolished CDI (Figure 7B–C; Figure 7—figure supplement 1A), unveiling a novel role of fhf in tuning Ca2+-feedback of NaV channels. To assess selectivity, we probed whether fhf alters CDI of CaV1.3. In comparison to control conditions, fhf co-expression spared CaV1.3 CDI (Figure 7D–E; Figure 7—figure supplement 1B) suggesting that fhf may be a selective modulator of NaV1.
Figure 7. Fhf uses an allosteric mechanism to abrogate Ca2+-feedback of NaV1.4.
(A) Structural comparison of NaV1.5 CI (green) in the presence of CaM alone (cyan, left) or both CaM (cyan) and fhf1b (purple). Fhf binding changes baseline conformation of CaM on NaV1 CI. (B–C) Co-expression of fhf1b abolishes CDI in NaV1.4 evoked via Ca2+ photo-uncaging. Format as in Figure 1M–N. Control data are reproduced from Figure 1M–N for comparison. (D–E) In sharp contrast, strong overexpression of fhf1b does not alter CDI of CaV1.3S. Format as in Figure 1A–B. Control data reproduced from Figure 1D for comparison. (F–G) Fhf1 suppresses CDI of NaV1.4 tethered to CaM. Fusion of CaM protects NaV1.4 from competitive inhibitors such as CaM1234 (G). Format as in Figure 1M–N. (H) Structure of CaV1.1 upstream CI elements (blue) composed of dual vestigial EF hands and preIQ segments isolated from cryo-EM structure of CaV1.1 (PDBID, 5GJV). This domain is the primary interface for stac interaction in the CaV1 CI. (I) Structural overlay of upstream CI elements of CaV1.1 (PDBID, 5GJV) and NaV1.5 (PDBID, 4DCK) shows highly conserved dual vestigial EF hand segments while the fhf binding site is structurally divergent. (J) The divergence in the fhf binding interface in CaV1.1 in comparison to NaV1.5 would introduce a steric clash that prohibits fhf binding to CaV channels.
Figure 7—figure supplement 1. Extended data confirm the selectivity of fhf in modulating NaV versus CaV channels.
Mechanistically, functional results along with atomic structures of NaV1 CI bound to CaM and fhf yield insights on mechanisms for CDI suppression (Gabelli et al., 2014; Wang et al., 2012; Wang et al., 2014). Both fhf and CaM bind concurrently to NaV1 CI (Wang et al., 2012; Wang et al., 2014), with fhf binding triggering a conformational rearrangement of CaM (Figure 7A) (Gabelli et al., 2014; Wang et al., 2012). To experimentally validate allostery, we followed our strategy with CaV1.3 and tethered CaM to NaV1.4 carboxy-tail. Reassuringly NaV1.4-CaM exhibits robust baseline CDI (Figure 7F; Figure 7—figure supplement 1C). Whereas dominant negative CaM1234 typically abolishes CDI of NaV1.4 (Ben-Johny et al., 2014), NaV1.4-CaM exhibits robust CDI despite CaM1234, confirming the protective nature of tethered CaM against competitive inhibitors (Figure 7G; Figure 7—figure supplement 1D). Co-expression of fhf1b, however, reduces CDI of NaV1.4-CaM (Figure 7F–G; Figure 7—figure supplement 1E). Thus, like stac modulation of CaV1, fhf overrides CaM signaling to NaV1.4 despite a tethered CaM, suggesting that fhf acts in allostery.
To garner a structural perspective, we turn to NaV1.5 CI/fhf complex (Figure 7A) as the atomistic basis of the stac/CaV1 CI interaction is unknown (Wang et al., 2012; Wang et al., 2014; Wong King Yuen et al., 2017). Whereas the dual-vestigial EF hand segments of NaV1.5 and CaV1.1 are similar (Figure 7H–I), the fhf binding interface of NaV1.5, including the preIQ loop diverges from corresponding segments of CaV1.1 and introduces a steric clash (Figure 7I–J) (Wang et al., 2012; Wu et al., 2016). Thus, by leveraging structurally distinct loci on the CI module, fhf selectively diminish CaM signaling to NaV channels. These findings point to a class of auxiliary proteins that selectively adjust Ca2+-dependent feedback to individual ion channel targets.
Engineering synthetic modulation of CaV channels
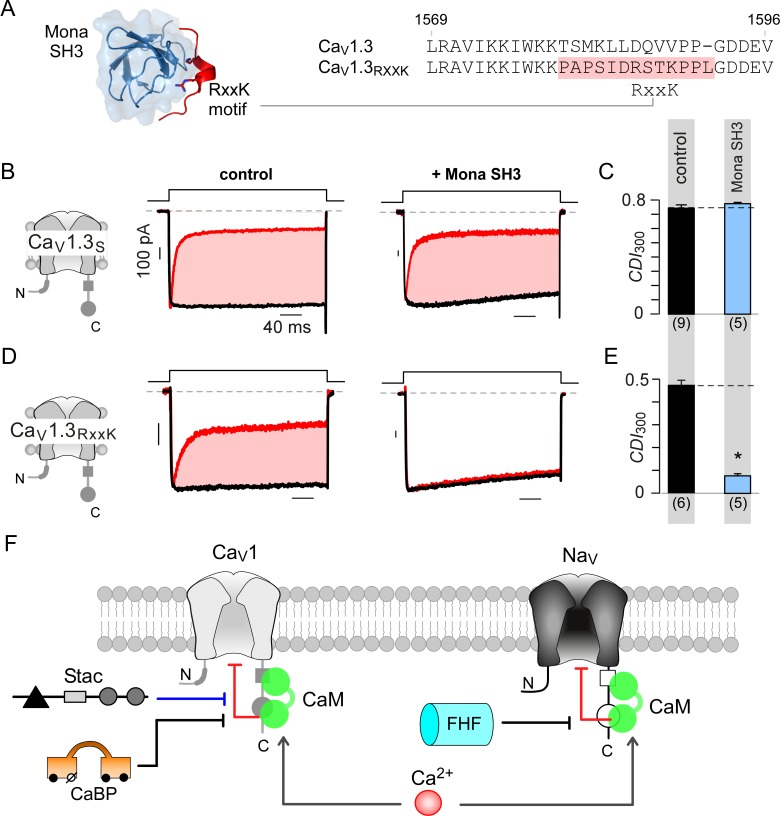
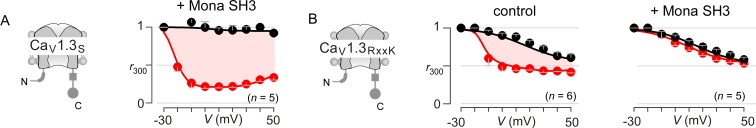
As both stac and fhf tune Ca2+-feedback to individual CaV and NaV targets by interacting with respective PCI segments, this mechanism furnishes a strategy to engineer synthetic channel modulators. We reasoned that introducing a short interaction motif into the PCI locus may permit inhibition of CaV1 Ca2+-feedback by a novel protein. We chose the well-characterized RxxK motif from SLP-76 for its small size and high-affinity interaction with SH3 domain of Mona (Harkiolaki et al., 2003) (Figure 8A). Co-expression of Mona SH3 with wildtype CaV1.3S demonstrated the persistence of CDI, confirming the suitability of these channels as a ‘blank slate’ to confer synthetic modulation (Figure 8B–C; Figure 8—figure supplement 1A). We replaced a 12-residue segment in the preIQ domain with the RxxK motif, as highlighted in Figure 8A, yielding CaV1.3RxxK engineered channels. As this locus is situated upstream of the IQ domain, this maneuver spares apoCaM prebinding. Under endogenous levels of CaM, CaV1.3RxxK exhibit robust baseline CDI (Figure 8D–E; Figure 8—figure supplement 1B). Co-expression of Mona SH3 with CaV1.3RxxK markedly diminished CDI (Figure 8D–E; Figure 8—figure supplement 1B) , thus revealing engineered CDI suppression. These findings illustrate the versatility of the CI module as a regulatory hub and highlight the feasibility of developing synthetic modulators to tune Ca2+-feedback of ion channels.
Figure 8. Engineering synthetic modulation of CaV1 channels.
(A) Left, schematic shows the atomic structure of Mona SH3 domain in complex with RxxK motif. Right, sequence alignment outlines strategy for insertion of RxxK motif into CaV1.3, yielding CaV1.3RxxK to confer synthetic suppression of CaV1.3 CDI by Mona SH3. (B–C) CaV1.3S expressed with and without Mona SH3 shows full CDI, confirming that wildtype CaV1.3 CDI is insensitive to Mona SH3. Format as in Figure 1A–B. Control data are reproduced from Figure 1D for comparison. (D–E) Mona SH3 strongly diminishes CDI of CaV1.3RxxK. Format as in Figure 1A–B. (F) Cartoon summarizes selective modulation of Ca2+/CaM signaling to CaV1, and NaV1 channels with CaM, stac, and fhf.
Figure 8—figure supplement 1. Extended data demonstrate feasibility of engineering synthetic modulators of CaM signaling to CaV1.3.
Discussion
CaM is a dynamic regulator of CaV1, CaV2, and NaV1, affording millisecond-precision Ca2+-feedback of channel activity. Our findings suggest that distinct auxiliary regulatory proteins tune CaM signaling to individual targets selectively. Stac prevents CaM signaling to CaV1, while fhf reduces signaling to NaV1 (Figure 8F). Parallel analysis of the two proteins delineates mechanisms and sets the stage for in-depth physiological analysis.
Relationship to prior studies of stac-CaV modulation
Stac regulation of CaV1 modifies multiple aspects of CaV1 function. For CaV1.1, stac3 enhances plasmalemmal trafficking (Linsley et al., 2017b; Niu et al., 2018; Polster et al., 2015; Wong King Yuen et al., 2017; Wu et al., 2018), and promotes conformational coupling to RyR (Linsley et al., 2017a; Polster et al., 2016). For CaV1.2, however, stac1-3 isoforms slow inactivation (Campiglio et al., 2018; Polster et al., 2015; Wong King Yuen et al., 2017). Our work generalizes the latter effect to the CaV1 family and further identifies a change in baseline channel openings (PO).
A few mechanistic nuances merit attention. First, stac binds to multiple CaV1 segments including (1) the II-III linker (Polster et al., 2018; Wong King Yuen et al., 2017), (2) the III-IV linker (Figure 2D), and (3) the carboxy-tail (Figure 2D) (Campiglio et al., 2018; Niu et al., 2018). Previous studies have shown that stac interaction with the II-III linker is important for CaV1 trafficking in skeletal muscle (Polster et al., 2018; Wong King Yuen et al., 2017). Chimeric analysis here suggests that stac interaction with the carboxy-tail is critical for tuning CDI. Prior analysis of CaV1.2 triadic localization in myotubes suggested that the channel IQ domain may be important for stac binding (Campiglio et al., 2018). However, FRET 2-hybrid assay indicates that stac interaction with the IQ is around tenfold weaker than with the PCI segment. Second, prior work also suggested that stac-mediated reduction in CDI results from competitive displacement of CaM by stac (Campiglio et al., 2018). Functional experiments using CaV1 tethered to CaM, however, suggest that stac does not compete with CaM. Consistent with this scheme, FRET 2-hybrid analysis shows that CaM binding with the CI module is intact even in the presence of stac. Third, key domains within stac relevant for CaV modulation remain controversial. Previous studies have identified the dual SH3 and C1 domains to be critical for stac effect on trafficking and coupling to RyR (Campiglio and Flucher, 2017; Linsley et al., 2017a; Linsley et al., 2017b; Polster et al., 2016), while the C1 has been proposed to be critical for modifying CaV1 CDI (Campiglio et al., 2018; Wong King Yuen et al., 2017). Our findings instead suggest that the U-domain in the stac2 linker region is sufficient to fully recapitulate reduction in CaV1 CDI. Notably, prior analysis of the C1 domain also included this linker (Wong King Yuen et al., 2017). Given these experimental findings, a simple possibility is that distinct subdomains within stac interact with disparate channel segments to support multifunctionality of stac. While the U-domain modifies channel inactivation, other subdomains may support plasmalemmal trafficking and conformational coupling.
Defining a general class of auxiliary modulators of CaM signaling
Although functionally divergent, CaV1, CaV2, and NaV1 feature a modular CI element with a common CaM interaction fingerprint and subsequently, shared mechanistic basis for Ca2+-regulation. For all three families, apoCaM prebinds the CI module while Ca2+/CaM interaction switches channels between discrete high and low PO gating modes (Ben-Johny et al., 2015). How do allosteric regulators override CaM-signaling? First, stac and fhf use unique interfaces on the channel CI to selectively tune Ca2+-feedback. Second, stac locks CaV1 into a high PO gating mode irrespective of whether apoCaM or Ca2+/CaM is bound, effectively disengaging the pore from CaM-conformational changes. For NaV1, despite fhf binding, CaM undergoes a profound Ca2+-dependent rearrangement (Wang et al., 2012; Wang et al., 2014) suggesting that fhf does not prevent Ca2+ binding to CaM or Ca2+/CaM interaction with effector interfaces. Instead, like stac and CaV1, fhf may override CaM-dependent changes to NaV, akin to a clutch disengaging power transmission in mechanical systems. As fhf elicits a change in apoCaM conformation (Figure 7A) (Gabelli et al., 2014; Wang et al., 2012), baseline gating of NaV may also be altered (Goldfarb et al., 2007; Lou et al., 2005). This parallelism between stac and fhf hints at a shared mechanism.
Ca2+-binding proteins (CaBPs) (Haeseleer et al., 2000) also suppress CaM signaling to CaV1 (Lee et al., 2002; Yang et al., 2006). Mechanistically, CaBPs exploit a mixed allosteric scheme – at low concentrations, they engage distinct interfaces from CaM but at higher concentrations displace CaM (Findeisen and Minor, 2010; Oz et al., 2013; Yang et al., 2014). The existence of other regulatory proteins that curtail Ca2+-feedback points to a general class of auxiliary regulators of CaM-signaling to targets beyond NaV1 and CaV1. Identifying such molecular players is critical to understand how CaM signaling is orchestrated.
Biological implications of stac modulation of CaV1
Stac1/2 isoforms are widely expressed in multiple brain regions, including both the hippocampus and the midbrain (Nelson et al., 2013; Suzuki et al., 1996). Our experiments hint at low basal stac2 expression in guinea pig ventricular cardiac myocytes, although previous studies have failed to detect stac2 in murine heart (Nelson et al., 2013). Further quantitative analysis will help establish ambient stac levels including species-specific differences and potential modulatory effects on cardiac function. Interestingly, endogenous CaV1 in both hippocampal and midbrain neurons (Bazzazi et al., 2013; Oliveria et al., 2012) as well as ventricular cardiac myocytes exhibit CDI. As all stac variants shunted CDI of CaV1 in HEK293, it is possible that stac function may be tightly regulated in native settings. One possibility is that stac abundance may be tuned developmentally (Suzuki et al., 1996), pathologically, or via interacting proteins (Satoh et al., 2006). For instance, the transcription factor, NFAT binds to an upstream promoter region of stac2 gene to upregulate stac2 expression in osteoclasts as well as during hypoxic conditions in neural stem cells (Jeong et al., 2018; Moreno et al., 2015). Physiologically, as CaV1 CDI is a potent homeostatic mechanism that prevents pathological Ca2+-overload (Dunlap, 2007), a low concentration regime of stac may be advantageous. By modulating a subpopulation of CaV1, stac may circumvent homeostatic requirements to amplify local Ca2+-signals via sustained Ca2+ influx. The C1 and SH3 domains may serve as scaffolds to localize stac to specific signaling complexes (Campiglio and Flucher, 2017; Cohen et al., 1995; Colon-Gonzalez and Kazanietz, 2006). It is also possible that phosphorylation of stac may dynamically tune its function (Huttlin et al., 2010). Resolving these complexities may unveil mechanisms that tune CaV function spatially and temporally.
In cardiac myocytes, CDI of CaV1 is a key factor for action potential duration (Limpitikul et al., 2014; Mahajan et al., 2008). Experimentally, this importance is inferred from prolongation of action potentials upon expression of mutant CaM1234 (Alseikhan et al., 2002). Yet, constitutive CaM expression may yield nonspecific effects (Hall et al., 2013; Wang et al., 2007) that obscure the net contribution of CaV1 CDI (Zhang et al., 2015). Acute elevation of the U-domain bypasses these ambiguities and confirms a key role for CaV1 CDI for cardiac action potentials. Pathophysiologically, differential expression of stac2 has been reported in right ventricular heart failure, hinting at a potential role in calcium remodeling during heart failure (di Salvo et al., 2015).
Post-transcriptional modification of CaV1.3 generates an assortment of variants with modified carboxy-termini (Bock et al., 2011; Huang et al., 2012). The apoCaM affinities of these variants are such that CaM fluctuations may redistribute channels between populations lacking or endowed with apoCaM (Bazzazi et al., 2013), evoking concomitant changes in maximal PO and CDI of CaV1.3 (Adams et al., 2014). Stac uniformly locks these variants into a high PO configuration incapable of CDI, thereby supporting reliable and persistent Ca2+-influx in spite of CaM. Notably, functional effects of CaV1.3 alternative splicing have been shown to be cell-type specific suggesting that auxiliary regulators may tune channel properties (Scharinger et al., 2015). Fitting with these regulatory possibilities, disruption of stac modulation of CaV1.3 in Drosophila alters circadian rhythm (Hsu et al., 2018).
Biological implications of fhf modulation of NaV1
Unlike canonical fibroblast growth factors, fhf lack a secretory signal sequence (Smallwood et al., 1996) and serve as intracellular proteins (Schoorlemmer and Goldfarb, 2001). Four distinct fhf isoforms have been identified with tissue-specific expression in neurons, cardiomyocytes, and skeletal muscle (Goldfarb, 2005; Kraner et al., 2012; Smallwood et al., 1996). Functionally, fhf isoforms promote NaV1 trafficking and fast inactivation (Pablo and Pitt, 2016). More specifically, fhf adjust steady-state voltage-dependence of inactivation (Lou et al., 2005), elicit a kinetically distinct long-term inactivation (Dover et al., 2010), and modify resurgent current (Yan et al., 2014). Our present findings suggest that fhf1 also tunes CDI of NaV1. Physiologically, NaV CDI may be prominent during repetitive activity, as excess Ca2+ accumulation may inhibit Na currents. Thus, suppression of NaV1 CDI by fhf may enhance repetitive firing. Interestingly, loss of fhf1 and/or fhf4 result in diminished firing properties of cerebellar Purkinje neurons (Bosch et al., 2015; Goldfarb et al., 2007), while loss of fhf2 reduces cardiac conduction (Park et al., 2016; Wang et al., 2011a). It is possible that loss of fhf may enhance net CDI thus contributing to diminished excitability in these cells. As mutations in fhf1 are associated with epileptic encephalopathy (For CENetDDD Study group‡* et al., 2016) and cardiac conduction disorders (Hennessey et al., 2013) while mutations in fhf4 are linked to spinocerebellar ataxia (Brusse et al., 2006), resolving the dynamic interplay between CaM and fhf in tuning NaV1 may be critical for understanding pathogenic mechanisms.
New strategy for synthetic ion channel modulation
Finally, our results highlight the possibility of engineering synthetic regulation to tune CaM signaling. While CaV1.3 is insensitive to Mona SH3, insertion of an RxxK motif (Harkiolaki et al., 2003) into the carboxy-tail preIQ segment allows latent modulation by Mona SH3. Given the structural similarity of the CI modules of CaV1, CaV2, and NaV1, and sequence variability within the preIQ domain, emerging protein engineering methods may be used to screen for synthetic modulators of related ion channel families. As the ligand specificity of SH3 domains can be custom-engineered (Nguyen et al., 2000) and subcellular localization tuned via targeting motifs (Komatsu et al., 2010), a custom library of synthetic regulators may be developed to combinatorially modify kinetic properties of CaV1, CaV2, or NaV1 channels with spatiotemporal specificity. Generalizing this approach may lead to the development of new tools to manipulate Ca2+ signaling.
In all, our findings unravel the elegant interplay between a novel class of allosteric regulators and CaM in orchestrating the activity of CaV and NaV channels.
Materials and methods
Key resources table.
| Reagent type (species) or resource |
Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (rat) | β2A | PMID: 1370480 | GenBank: M80545 | |
| Gene (rat) | α2δ | PMID: 8107966 | NCBI: NM_012919 | |
| Gene (Oryctolagus cuniculus) |
CaV1.2 | PMID: 1718988 | NCBI: NM_001136522 | |
| Gene (rat) | CaV1.3S | PMID: 20139964 | GenBank: AF370009.1 | |
| Gene (rat) | CaV1.3MQDY | PMID: 24120865, 22284185 |
||
| Gene (human) | CaV1.443* | PMID: 22069316 | Laboratory of Dr. Soong Tuck Wah (National University of Singapore) |
|
| Gene (human) | CaV2.1 splice variant 37a(EFa) with 43+/44-/47- |
PMID: 12451115 | ||
| Gene (human) | CaV2.2 | PMID: 1321501, 10233069 |
GenBank: M94172.1 | |
| Gene (rat) | CaV2.3 | PMID: 8388125, 18400181 |
NCBI: NM_019294.2 | |
| Gene (rat) | NaV1.4 | PMID: 2175278 | ||
| Gene (human) | stac1 | Origene | NCBI: NP_003140.1 | |
| Gene (mouse) | stac2 | Origene | NCBI: NP_666140.1 | |
| Gene (human) | stac3 | Origene | NCBI: NP_659501.1 | |
| Gene (human) | fhf | PMID: 8790420 | Laboratory of Dr. Jeremy Nathans (Johns Hopkins University). |
|
| Gene (human) | Mona SH3 | PMID: 12773374 | Synthesized by Genscript based on sequence in publication |
|
| Peptide (mouse) |
U-peptide | this paper | Peptide sequence KVDPVYETLRYGTSLALM NRSS synthesized by Genscript |
|
| Competent cells (E. coli) |
DH5α | Invitrogen: 18265017 |
||
| Cell line (human) | HEK293 | other | RRID: CVCL_0045 | |
| Biological sample (guinea pig) |
aGPVM | PMID: 24076394 | Generated from Hartley strain guinea pigs |
|
| Antibody | anti-stac1 | Abcam: ab181157 |
1:100 | |
| Antibody | anti-stac2 | Abcam: ab156080 |
IHC – 1:100 WB – 1:250 |
|
| Antibody | anti-α-actinin | Sigma Aldrich: A7811 |
RRID: AB_476766 | 1:300 |
| Antibody | goat anti- rabbit IgG Alexa Fluor 594 |
Abcam: ab150080 |
RRID: AB_2650602 | 1:1000 |
| Antibody | goat anti-mouse IgG1 Alexa Fluor 488 |
Thermo Fischer: A21121 |
RRID: AB_2535764 | 1:1000 |
| Antibody | Goat Anti-Rabbit IgG (H + L) |
Jackson ImmunoResearch: 111-035-144 |
RRID: AB_2307391 | 1:10,000 |
| Recombinant DNA reagent | CaV1.3L | PMID: 20139964 | Engineered from CaV1.3S and human long distal carboxyl tail (NCBI: NM_000718) |
|
| Recombinant DNA reagent | CaV2.3/1.3 CI | PMID: 24441587 | ||
| Recombinant DNA reagent | CaV1.3- CaMWT | PMID: 24441587 | ||
| Recombinant DNA reagent | CaV1.2- CaMWT | PMID: 15087548 | ||
| Recombinant DNA reagent | NaV1.4-CaM | this paper | Engineered by fusing CaMWT carboxy-tail of NaV1.4 |
|
| Recombinant DNA reagent | CaV1.3RxxK | this paper | Engineered from CaV1.3S | |
| Recombinant DNA reagent | CFP-stac3 | this paper | stac3 was cloned into CFP vector with NotI and XbaI |
|
| Recombinant DNA reagent | YFP-CaV1.3 CI | PMID: 23591884 | ||
| Recombinant DNA reagent | YFP-CaV1.3 PCI | PMID: 23591884 | ||
| Recombinant DNA reagent | YFP-CaV1.3 IQ | PMID: 23591884 | ||
| Recombinant DNA reagent | Ven-C1 | this paper | stac2 C1 was cloned into Venus vector (PMID: 26997269) with NotI and XbaI |
|
| Recombinant DNA reagent | Ven-linker region | this paper | stac2 linker region was cloned into Venus vector (PMID: 26997269) with NotI and XbaI |
|
| Recombinant DNA reagent | Ven-SH3-SH3 | this paper | stac2 SH3-SH3 was cloned into Venus vector (PMID: 26997269) with NotI and XbaI |
|
| Recombinant DNA reagent | Ven-U-motif | this paper | stac2 U-motif was cloned into Venus vector (PMID: 26997269) with NotI and XbaI |
|
| Recombinant DNA reagent | stac2 (KVD/AAA) | this paper | Quickchange PCR with stac2 |
|
| Recombinant DNA reagent | stac2 (PVY/AAA) | this paper | Quickchange PCR with stac2 |
|
| Recombinant DNA reagent | stac2 (ETL/AAA) | this paper | Quickchange PCR with stac2 |
|
| Recombinant DNA reagent | stac2 (RYG/AAA) | this paper | Quickchange PCR with stac2 |
|
| Recombinant DNA reagent | stac2 (TSL/AAA) | this paper | Quickchange PCR with stac2 |
|
| Recombinant DNA reagent | stac2 (NRS/AAA) | this paper | Quickchange PCR with stac2 |
|
| Recombinant DNA reagent | stac2 (S/A) | this paper | Quickchange PCR with stac2 |
|
| Sequence-based reagent | Ven-C1 forward primer |
this paper | cttctcgcggccgc tatgaccgaa atga gcgagaa |
|
| Sequence-based reagent | Ven-C1 reverse primer |
this paper | tcagaattctagattat tgctggt gggagatctc |
|
| Sequence-based reagent | Ven-linker region forward primer |
this paper | cttctcgcggccgcta catctttt cgacgcaact |
|
| Sequence-based reagent | Ven-linker region reverse primer |
this paper | tcagaattctagatta gtacatg ggccccacg |
|
| Sequence-based reagent | Ven-SH3-SH3 forward primer |
this paper | cttctcgcggccgc ttcctacgt cgccctct |
|
| Sequence-based reagent | Ven-SH3-SH3 reverse primer |
this paper | tcagaattctagattat cagatctct gccaaggag |
|
| Sequence-based reagent | Ven-U-motif forward primer |
this paper | cttctcgcggccgctaagg tggac ccagtttatga |
|
| Sequence-based reagent | Ven-U-motif reverse primer |
this paper | tcagaattctagattag ctggaa cggttcatcag |
|
| Sequence-based reagent | stac2 (KVD/AAA) sense | this paper | ctactgggaccagcgg ggcggcgg ccccagt ttatgagacgc |
|
| Sequence-based reagent | stac2 (KVD/AAA) antisense | this paper | gcgtctcataaactggg gccgccgc cccgctgg tcccagtag |
|
| Sequence-based reagent | stac2 (PVY/AAA) sense | this paper | ccagcgggaaggtggac gcagc tgctgagacgct gcgctatg |
|
| Sequence-based reagent | stac2 (PVY/AAA) antisense | this paper | catagcgcagcgtctc agcagct gcgtccacc ttcccgctgg |
|
| Sequence-based reagent | stac2 (ETL/AAA) sense | this paper | ggtggacccagttt atgcggcgg cgcgct atggcacctcc |
|
| Sequence-based reagent | stac2 (ETL/AAA) antisense | this paper | ggaggtgccatagcgc gccgcc gcataaact gggtccacc |
|
| Sequence-based reagent | stac2 (RYG/AAA) sense | this paper | cccagtttatgagacgc tggccgc tgccacctcc ctggcactgatg |
|
| Sequence-based reagent | stac2 (RYG/AAA) antisense | this paper | catcagtgccaggg aggtggca gcggccagc gtctcataaactggg |
|
| Sequence-based reagent | stac2 (TSL/AAA) sense | this paper | acgctgcgctatgg cgccgccgc ggcactga tgaaccg |
|
| Sequence-based reagent | stac2 (TSL/AAA) antisense | this paper | cggttcatcagtgc cgcggcggc gccatag cgcagcgt |
|
| Sequence-based reagent | stac2 (NRS/AAA) sense | this paper | gatgtgctgctga agctggcagcg gccatc agtgccagggaggtg |
|
| Sequence-based reagent | stac2 (NRS/AAA) antisense | this paper | cacctccctggc actgatggccgc tgcc agcttcagcagcacatc |
|
| Sequence-based reagent | stac2 (S/A) sense | this paper | cactgatgaacc gttccgccttc agcag cacatctg |
|
| Sequence-based reagent | stac2 (S/A) antisense | this paper | cagatgtgctgctga aggcgg aacggttca tcagtg |
|
| Software, algorithm | PyMOL | http://www.pymol.org/ | RRID: SCR_000305 |
Molecular biology and peptide synthesis
CaV1.2, CaV1.3, CaV1.443*, CaV2.1, CaV2.2, CaV2.3, and NaV1.4 variants were unmodified from previously published constructs: CaV1.2 (NM001136522) (Wei et al., 1991), CaV1.2-CaMWT (Mori et al., 2004), CaV1.3S (AF370009.1), CaV1.3L engineered from CaV1.3S and human long distal carboxyl tail (NM000718) (Liu et al., 2010), RNA-edited variant CaV1.3MQDY (Bazzazi et al., 2013; Huang et al., 2012), CaV1.443* was gifted from Dr. Soong Tuck Wah (National University of Singapore), CaV2.1 splice variant 37a(EFa) with 43+/44-/47− (Soong et al., 2002) was gifted from Dr. Terry Snutch (University of British Columbia), CaV2.2 (Jones et al., 1999), CaV2.3 (Mori et al., 2008), NaV1.4 (Trimmer et al., 1990). Stac variants were purchased from Origene: human stac1 mRNA transcript 1 (NP003140.1), mouse stac2 (NP666140.1), and human stac3 isoform 2 (NP659501.1). U-peptide was synthesized by Genscript (KVDPVYETLRYGTSLALMNRSS). Fhf variants were gifted from Dr. Gordon Tomaselli and Dr. Jeremy Nathans (Johns Hopkins University).
Cell culture and transfection of HEK293 cells
For whole-cell electrophysiology, single-channel electrophysiology, and immunohistochemistry, HEK293 cells (ATCC; mycoplasma tested negative) were cultured on glass coverslips in 10 cm dishes and transfected by a calcium phosphate method (Peterson et al., 1999) with the following amounts of DNA: 3 µg of SV40 T antigen to enhance expression, 2–8 µg of α1-subunit of Ca2+ or Na+ channel depending on expression, 8 µg from rat β2A (Perez-Reyes et al., 1992) (M80545), 8 µg from rat α2δ (Tomlinson et al., 1993) (NM012919.2), and 8 µg of the stac1, stac2, or stac3 variants indicated.
For FRET two-hybrid experiments, cells were cultured on glass-bottom dishes and transfected with a standard polyethylenimine protocol (Lambert et al., 1996). Epifluorescence measurements were recorded 1–2 days after transfection.
Adult guinea pig ventricular myocyte isolation
Adult guinea pig ventricular myocytes (aGPVMs) were isolated from whole hearts of Hartley strain guinea pigs 3–4 weeks old (250–350 g). Guinea pigs were anesthetized via intraperitoneal injection with pentobarbital (35 mg/kg). Hearts were then excised, and single ventricular myocytes were isolated following a previously published protocol (Joshi-Mukherjee et al., 2013). Cells were plated on glass coverslips that were laminin (20 µg/mL) coated overnight at 4°C.
Immunohistochemistry aGPVMs plated on glass coverslips were first washed three times with cold PBS and then fixed in 3.7% paraformaldehyde (15710, Electron Microscopy Sciences) in PBS for 15 min. After washing three times with PBS, cells were permeabilized in cold 0.5% Triton X-100 in tris buffered saline (TBS) for 20 min and then blocked with 10% goat serum in PBS for 1 hr at room temperature. Cells were incubated overnight at 4°C in primary antibodies diluted in antibody diluent solution (IW-1000, IHC World): monoclonal anti-α-actinin (sarcomeric) antibody produced in mouse (1:300, A7811), anti-STAC (stac1) antibody [EPR12805]-N-terminal (1:100, ab181157) or anti-STAC2 (stac2) antibody-N-terminal (1:100, ab156080) produced in rabbit. Next day, cells were rinsed three times with 0.05% TWEEN20 (Sigma P9416) in TBS (TBS-T) for 5 min each. In the dark, cells were incubated with secondary antibodies (1:1000): goat anti-mouse IgG1 Alexa Fluor 488 (1:1000, A21121), goat anti-rabbit IgG Alexa Fluor 594 (1:1000), and DAPI (1:10000) diluted in antibody solution for 45 min at room temperature and then washed three times with TBS-T for 5 min each. Stained cells were mounted with prolong gold mounting media (Invitrogen) on a microscope slide (Fischer Scientific).
Transfected HEK293 were immunostained following a similar protocol to that of aGPVM, but were not labelled with sarcomeric primary antibody and its respective secondary antibody.
Western blot aGPVMs and HEK293 cells were washed twice with PBS buffer. Cells were harvested with 1 mL 1x RIPA buffer (20–188, Sigma Aldrich) containing half a tablet of complete mini-EDTA-free protease inhibitor (11836170001, Sigma Aldrich) and incubated at 4°C for 30 min. Samples were centrifuged at 15,000 RPM for 15 min, and the pellet was discarded. Then, 2–5 µg of proteins in the supernatant were heated at 37°C for 30 min with 2x Laemmli sample buffer (S3401, Sigma Aldrich). Samples were loaded into 4–12% gradient gel (NP0335BOX, Invitrogen) with PageRuler plus prestained protein ladder (26619, Invitrogen) and run at 100 V for 2 hr at room temperature in running buffer: 1x NuPAGE MOPS SDS running buffer: 50 mM MOPS, 50 mM Tris base, 0.1% SDS, 1 mM EDTA, pH to 7.7. Proteins were transferred on ice from the gel to nitrocellulose membrane (10600003, GE Healthcare Life science) for 75 min at 10 V in transfer buffer: 24 mM Tris base, 192 mM glycine, 20% v/v methanol. Membrane was blocked with 5% (w/v) Blotting-Grade-Blocker (1706404, Bio-Rad) in 1x TRIS-buffered saline for 1 hr at 4°C. Primary antibody for stac2 (1:250) was added to the blocking buffer with 0.1% (v/v) Tween 20 (1706404, Bio-Rad) and incubated overnight at 4°C. Next day, the membrane was washed three times for 5 min each with TBS with 0.1% (v/v) Tween 20 (TBS-T). The secondary antibody (111-035-144, Jackson ImmunoResearch; 1:10,000) was added to the blocking buffer with 0.1% (v/v) Tween 20 and incubated for 1 hr. The membrane was washed again three times for 5 min each with TBS-T. Finally, western blots were developed with SuperSignal West Pico Chemiluminescent Substrate (34580, ThermoFischer) and images were collected on an Alpha InnoTech FluorChem HD2 imaging system.
Confocal optical imaging
Images of immunostained tissue slices and cells were captured with either an Olympus Fluorview FV300 confocal laser scanning microscope or an LSM780 (Carl Zeiss, Oberkochen, Germany) confocal microscope. For the FV300, we used Fluoview software (Olympus) with a PlanApo 403 or 603 oil objective (NA 1.40, PLAPO60XO3; Olympus). Argon laser (488 nm) was used to excite Alexa Fluor 488 (green), and Helium Neon (HeNe) Green Laser was used to excite Alexa Fluor 594 (red). Olympus optical filters used were 442/515 nm excitation splitter (FV-FCV), 570 nm emission splitter (FV-570CH), BA510 IF and BA530RIF for green emission channel, and 605 BP filter for red channel. Images were processed in ImageJ. Similar settings were used for the LSM780 setup.
Whole-cell electrophysiology
Whole-cell voltage-clamp recordings for HEK293 were collected at room temperature 1–2 days after transfection with Axopatch 200A (Axon Instruments). Glass pipettes (BF150-86-10, Sutter Instruments) were pulled with a horizontal puller (P-97; Sutter Instruments Company) and fire polished (Microforge, Narishige, Tokyo, Japan) to have 1–3 MΩ resistance. Recordings were low-pass filtered at 2 kHz and sampled at 10 kHz with P/8 leak subtraction and 70% series resistance and capacitance compensation. For recordings of CaV1.2 (Figure 1A–B, Figure 5I–K, Figure 6B), CaV1.3S (Figure 1C–D, Figure 3—figure supplement 1A–B and and G, Figure 7D–E, and Figure 8B–C), CaV1.443* (Figure 1E–F), CaV2.2 (Figure 1I–J), CaV2.3/1.3 CI chimera (Figure 2E–F), CaV1.3-CaM (Figure 3A–B, Figure 3—figure supplement 1C–F, and Figure 5G–H), CaV1.2-CaM (Figure 2C–D, Figure 5A–F) and CaV1.3RxxK (Figure 8D–E) exogenously expressed in HEK293 cells, the internal solution contained (in mM): CsMeSO3, 114; CsCl2, 5; MgCl2, 1; MgATP, 4; HEPES, 10; BAPTA, 10; adjusted to 295 mOsm with CsMeSO3 and pH 7.4 with CsOH. The external solution contained (in mM): TEA-MeSO3, 140; HEPES, 10; CaCl2, or BaCl2 40; adjusted to 300 mOsm with TEA-MeSO3 and pH 7.4 with TEA-OH. For recordings of CaV2.1 (Figure 1G–H) and CaV2.3 (Figure 1K–L), the internal solution contained (in mM): CsMeSO3, 135; CsCl2, 5; MgCl2, 1; MgATP, 4; HEPES, 10; EGTA, 1; adjusted to 295 mOsm with CsMeSO3 and pH 7.4 with CsOH. The external solution contained (in mM): TEA-MeSO3, 140; HEPES, 10; CaCl2, or BaCl2 5; adjusted to 300 mOsm with TEA-MeSO3 and pH 7.4 with TEA-OH. At a holding potential of −80 mV, we used a family of test pulses from −30 mV to +50 mV with repetition intervals of 20 s. Custom MATLAB (Mathworks) software (https://github.com/manubenjohny/WCDTY; copy archived at https://github.com/elifesciences-publications/WCDTY) was used to determine peak current and fraction of peak current remaining after either 300 ms (r300) or 800 ms (r800) of depolarization.
We incubated aGPVMs for 20–48 hr after isolation in 5 µM ryanodine for 5–10 min before we collected whole-cell recordings. The internal recording solution contained (in mM) CsMeSO3, 114; CsCl2, 5; MgCl2, 1; MgATP, 4; HEPES, 10; BAPTA, 10; ryanodine, 0.005 adjusted to 295 mOsm with CsMeSO3 and pH 7.4 with CsOH. Cells were sealed in Tyrodes solution, which contained (in mM): NaCl, 135; KCl, 5.4; CaCl2, 1.8; MgCl2, 0.33; NaH2PO4, 0.33; HEPES, 5; glucose, 5 (pH 7.4). For CDI measurements, external solutions containing (in mM): TEA-MeSO3, 140; HEPES, 10; CaCl2, or BaCl2 40; adjusted to 300 mOsm with TEA-MeSO3 and pH 7.4 with TEA-OH were perfused. Welch’s T-test was used to verify statistical significance among the population data.
For CDI recordings, we determined required sample size based on power analysis. Based on historical estimates of normal variation in CDI/CDF measurements, we computed the sample size required such that type I and type II errors are 5% to be 3.5. Thus, we obtained at least four independent measurements for all electrophysiological experiments.
Current-clamp recordings of aGPVMs were performed on the same setup and were filtered at 5 kHz and sampled at 25 kHz. The internal solution contained (in mM): K glutamate, 130; KCl, 9; NaCl, 10; MgCl2, 0.5; EGTA, 0.5, MgATP, 4; HEPES, 10; adjusted to pH 7.3 with KOH. The external solution contained (in mM): NaCl, 135; KCl, 5.4; CaCl2, 1.8; MgCl2, 0.33; NaH2PO4, 0.33; HEPES, 5; glucose, 5 (pH 7.4). The time from upstroke to 80% repolarization (APD80) was measured with MATLAB (Mathworks) and used as a metric for comparing physiological output between peptide treated and untreated. For experiments with U-peptide, peptide was dissolved in ddH2O to 2 mg/mL and then diluted to 500 μM in the appropriate internal solution.
Single-channel electrophysiology
Single-channel recordings were performed at room temperature using an on-cell configuration previously established in the laboratory (Tay et al., 2012) with the same setup as used for whole-cell electrophysiology. Glass pipettes were pulled and polished from ultra-thick-walled borosilicate glass (BF200-116-10, Sutter Instruments) and coated with sylgard to have 5–10 MΩ resistance. Recordings were filtered at 2–5 kHz. The pipette solution contained (in mM): TEA-MeSO3, 140; HEPES, 10; BaCl2 40; adjusted to 300 mOsm with TEA-MeSO3 and pH 7.4 with TEA-OH. The external solution contained (in mM): K glutamate, 132; KCl, 5; NaCl, 5; MgCl2, 3; EGTA, 2; HEPES, 10; adjusted to 300 mOsm with glucose and pH 7.4 with KOH. Cell-attached single-channel currents were measured during 200 ms voltage ramps between −80 and +70 mV (portions between −50 and 40 mV displayed and analyzed) as previously described. For each patch, we recorded 80–150 sweeps with a repetition interval of 12 s. Patches were analyzed as follows: (1) The leak for each sweep was fit and subtracted from each trace. (2) The unitary current relation, i(V), was fit to the open-channel current level using the following equation:
where g is the single-channel conductance (~0.2 pA/mV), z is the apparent valence of permeation (~2.1), F is Faraday’s constant, R is the gas constant, and T is the temperature in degrees Kelvin (assumed room temperature). These parameters were held constant for all patches, except for slight variations in the voltage-shift parameter Vs ~ 35 mV, as detailed below. (3) All leak-subtracted traces for each patch were averaged (and divided by the number of channels in the patch) to yield an I–V relation for that patch. As slight variability in VS was observed among patches, we calculated an average VS for each construct, VS,AVE. The data from each patch were then shifted slightly in voltage by an amount ΔV = VS,AVE – VS, with ΔV typically about ±5 mV. This maneuver allowed all patches for a given construct to share a common open-channel GHK relation. Thus shifted, the I–V relations obtained from different patches for each condition/construct were then averaged together. (4) PO at each voltage was determined by dividing the average I (determined in step three above) into the open-channel GHK relation. Channel number was determined by the maximal number of overlapping opening events upon application of the channel agonist Bay K8644 (5 μM) at the end of each recording. For modal analysis, a dashed line discriminator was chosen to be the average single-trial PO = 0.075 such that traces with average single-trial PO >0.075 were categorized as high PO while the remaining traces were considered to be low PO.
Quantitative calcium photo-uncaging
All Ca2+-uncaging experiments were conducted on a Nikon TE2000 inverted microscope with a Plan Fluor Apo 40 × oil objective as previously described (Ben-Johny et al., 2014). Briefly, a classic Cairn UV flash photolysis system was used for Ca2+-uncaging with brief UV pulses of ~1.5 ms in duration powered by a capacitor bank of up to 4000 μF charged to 200–290V. For concurrent Ca2+ imaging, Fluo4FF and Alexa568 dyes were dialyzed via patch pipette and imaged using Argon laser excitation (514 nm). Background fluorescence for each cell was measured prior to pipette dialysis of dyes and subtracted subsequently. A field-stop aperture was used to isolate fluorescence from individual cells. Dual-color fluorescence emission was attained using a 545DCLP dichroic mirror, paired with a 545/40 BP filter for detecting Fluo4FF, and a 580LP filter for detecting Alexa568. Typically, uncaging experiments were conducted after ~2 min of dialysis of internal solution. Welch’s T-test was used to verify statistical significance between the population data.
For all Ca2+-uncaging experiments, the internal solution contained (in mM): CsMeSO3, 120; CsCl, 5; HEPES (pH 7.4 with CsOH), 10; Fluo-4FF pentapotassium salt (Invitrogen), 0.01; Alexa 568 succinimidyl ester (Invitrogen), 0.0025; Citrate, 1; DM-Nitrophen EDTA (DMN) and CaCl2 were adjusted to obtain the desired Ca2+ flash. Typically, for flashes in the range 0.5–2 μM, DMN, 1 mM; and CaCl2, 0.7 mM. For the 2–8 μM range, DMN, 2 mM; and CaCl2, 1.4 mM. For larger Ca2+ steps, DMN, 4 mM; and CaCl2, 3.2 mM. As DMN can bind Mg2+, all experiments were conducted with 0 mM Mg2+ internally. For all Na channel experiments, the bath solution contained (in mM): TEA-MeSO3, 45; HEPES (pH 7.4), 10; NaCl, 100; at 300 mOsm, adjusted with TEA-MeSO3.
FRET-two-hybrid assay
To collect a range of donor molecule (Dfree) concentrations, HEK293 cells were transfected with combinations of DNA ratios. Cells were immersed in 2 mM Ca2+ Tyrodes solution, which contained (in mM): NaCl, 138; KCl, 4; CaCl2, 2; MgCl2, 1; HEPES, 10; glucose, 10. Three-cube FRET fluorescence measurements were performed under resting Ca2+ concentrations on an inverted fluorescence microscope. FRET efficiency (EA and ED) was calculated for each cell (Erickson et al., 2001) and a binding curve, either EA = [Dfree]/(Kd,EFF + [Dfree]) · EA,max or ED = [Afree]/(Kd,EFF + [Afree]), was fit to compute the effective dissociation constant (Kd,EFF).
Acknowledgements
We thank Deborah DeSilvestre, Rebeca Joca, and Travis Babola for assistance with immunohistochemistry, Hikeki Nakamura for confocal microscope training, Trudeau lab (Ashley Johnson and Sara Coddin) and Colecraft lab (Travis Morgenstern and Scott Kanner) for western blotting expertise. We are grateful for insightful discussions with the Calcium Signals Laboratory and Inoue Synthetic Biology Lab. Finally, we are indebted to the inspiration of Dr. David T Yue, who taught us to pursue science with a passion for the truth. This work was supported by grants from NINDS (DTY, IED, TI), NIMH (DTY, MBJ), NHLBI (GFT) and NSF (JN).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Takanari Inoue, Email: jctinoue@jhmi.edu.
Manu Ben-Johny, Email: mbj2124@cumc.columbia.edu.
Indira M Raman, Northwestern University, United States.
Richard Aldrich, The University of Texas at Austin, United States.
Funding Information
This paper was supported by the following grants:
National Science Foundation to Jacqueline Niu.
National Institute of Neurological Disorders and Stroke to Ivy E Dick, David T Yue, Takanari Inoue.
National Institute of Mental Health to David T Yue, Manu Ben-Johny.
National Heart, Lung, and Blood Institute to Gordon Tomaselli, Manu Ben-Johny.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Methodology, Writing—original draft, Writing—review and editing.
Resources, Data curation, Funding acquisition, Writing—review and editing.
Resources.
Data curation.
Funding acquisition.
Resources, Funding acquisition, Writing—review and editing.
Conceptualization, Supervision, Writing—review and editing.
Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing—original draft, Writing—review and editing.
Ethics
Animal experimentation: This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All of the animals were handled according to approved institutional animal care and use committee (IACUC) protocols of the Johns Hopkins University (GP15M172). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Johns Hopkins University. All surgery was performed under sodium pentobarbital anesthesia, and every effort was made to minimize suffering.
Additional files
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
References
- Adams PJ, Snutch TP. Calcium channelopathies: voltage-gated calcium channels. Sub-Cellular Biochemistry. 2007;45:215–251. doi: 10.1007/978-1-4020-6191-2_8. [DOI] [PubMed] [Google Scholar]
- Adams PJ, Rungta RL, Garcia E, van den Maagdenberg AM, MacVicar BA, Snutch TP. Contribution of calcium-dependent facilitation to synaptic plasticity revealed by migraine mutations in the P/Q-type calcium channel. PNAS. 2010;107:18694–18699. doi: 10.1073/pnas.1009500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PJ, Ben-Johny M, Dick IE, Inoue T, Yue DT. Apocalmodulin itself promotes ion channel opening and ca(2+) regulation. Cell. 2014;159:608–622. doi: 10.1016/j.cell.2014.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. PNAS. 2002;99:17185–17190. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babitch J. Channel hands. Nature. 1990;346:321–322. doi: 10.1038/346321b0. [DOI] [PubMed] [Google Scholar]
- Bazzazi H, Ben Johny M, Adams PJ, Soong TW, Yue DT. Continuously tunable ca(2+) regulation of RNA-edited CaV1.3 channels. Cell Reports. 2013;5:367–377. doi: 10.1016/j.celrep.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Johny M, Yang PS, Bazzazi H, Yue DT. Dynamic switching of calmodulin interactions underlies Ca2+ regulation of CaV1.3 channels. Nature Communications. 2013;4:1717. doi: 10.1038/ncomms2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Johny M, Yang PS, Niu J, Yang W, Joshi-Mukherjee R, Yue DT. Conservation of Ca2+/calmodulin regulation across na and Ca2+ channels. Cell. 2014;157:1657–1670. doi: 10.1016/j.cell.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Johny M, Dick IE, Sang L, Limpitikul WB, Kang PW, Niu J, Banerjee R, Yang W, Babich JS, Issa JB, Lee SR, Namkung H, Li J, Zhang M, Yang PS, Bazzazi H, Adams PJ, Joshi-Mukherjee R, Yue DN, Yue DT. Towards a unified theory of calmodulin regulation (Calmodulation) of Voltage-Gated calcium and sodium channels. Current Molecular Pharmacology. 2015;8:188–205. doi: 10.2174/1874467208666150507110359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Reviews Molecular Cell Biology. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bock G, Gebhart M, Scharinger A, Jangsangthong W, Busquet P, Poggiani C, Sartori S, Mangoni ME, Sinnegger-Brauns MJ, Herzig S, Striessnig J, Koschak A. Functional properties of a newly identified C-terminal splice variant of Cav1.3 L-type Ca2+ channels. The Journal of Biological Chemistry. 2011;286:42736–42748. doi: 10.1074/jbc.M111.269951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MK, Carrasquillo Y, Ransdell JL, Kanakamedala A, Ornitz DM, Nerbonne JM. Intracellular FGF14 (iFGF14) Is required for spontaneous and evoked firing in cerebellar purkinje neurons and for motor coordination and balance. Journal of Neuroscience. 2015;35:6752–6769. doi: 10.1523/JNEUROSCI.2663-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusse E, de Koning I, Maat-Kievit A, Oostra BA, Heutink P, van Swieten JC. Spinocerebellar ataxia associated with a mutation in the fibroblast growth factor 14 gene (SCA27): A new phenotype. Movement Disorders. 2006;21:396–401. doi: 10.1002/mds.20708. [DOI] [PubMed] [Google Scholar]
- Cai T, Jen HI, Kang H, Klisch TJ, Zoghbi HY, Groves AK. Characterization of the transcriptome of nascent hair cells and identification of direct targets of the Atoh1 transcription factor. Journal of Neuroscience. 2015;35:5870–5883. doi: 10.1523/JNEUROSCI.5083-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campiglio M, Costé de Bagneaux P, Ortner NJ, Tuluc P, Van Petegem F, Flucher BE. STAC proteins associate to the IQ domain of CaV1.2 and inhibit calcium-dependent inactivation. PNAS. 2018;115:1376–1381. doi: 10.1073/pnas.1715997115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campiglio M, Flucher BE. STAC3 stably interacts through its C1 domain with CaV1.1 in skeletal muscle triads. Scientific Reports. 2017;7:41003. doi: 10.1038/srep41003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon SC. Channelopathies of skeletal muscle excitability. Comprehensive Physiology. 2015;5:761–790. doi: 10.1002/cphy.c140062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Wisedchaisri G, Zheng N. The chemical basis for electrical signaling. Nature Chemical Biology. 2017;13:455–463. doi: 10.1038/nchembio.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. 'Rejuvenation' protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Coebergh JA, Fransen van de Putte DE, Snoeck IN, Ruivenkamp C, van Haeringen A, Smit LM. A new variable phenotype in Spinocerebellar ataxia 27 (SCA 27) caused by a deletion in the FGF14 gene. European Journal of Paediatric Neurology. 2014;18:413–415. doi: 10.1016/j.ejpn.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Ren R, Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Colon-Gonzalez F, Kazanietz M. C1 domains exposed: from diacylglycerol binding to protein–protein interactions. Biochimica Et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2006;1761:827–837. doi: 10.1016/j.bbalip.2006.05.001. [DOI] [PubMed] [Google Scholar]
- DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- di Salvo TG, Yang K-C, Brittain E, Absi T, Maltais S, Hemnes A. Right Ventricular Myocardial Biomarkers in Human Heart Failure. Journal of Cardiac Failure. 2015;21:398–411. doi: 10.1016/j.cardfail.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover K, Solinas S, D'Angelo E, Goldfarb M. Long-term inactivation particle for voltage-gated sodium channels. The Journal of Physiology. 2010;588:3695–3711. doi: 10.1113/jphysiol.2010.192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Calcium channels are models of self-control. The Journal of General Physiology. 2007;129:379–383. doi: 10.1085/jgp.200709786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated ca(2+) channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/S0896-6273(01)00438-X. [DOI] [PubMed] [Google Scholar]
- Findeisen F, Minor DL. Structural basis for the differential effects of CaBP1 and calmodulin on ca(V)1.2 calcium-dependent inactivation. Structure. 2010;18:1617–1631. doi: 10.1016/j.str.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For DDD Study group*. For CENet Study group‡. Al-Mehmadi S, Splitt M, Ramesh V, DeBrosse S, Dessoffy K, Xia F, Yang Y, Rosenfeld JA, Cossette P, Michaud JL, Hamdan FF, Campeau PM, Minassian BA. FHF1 (FGF12) epileptic encephalopathy. Neurology Genetics. 2016;2:e115. doi: 10.1212/NXG.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelli SB, Boto A, Kuhns VH, Bianchet MA, Farinelli F, Aripirala S, Yoder J, Jakoncic J, Tomaselli GF, Amzel LM. Regulation of the NaV1.5 cytoplasmic domain by calmodulin. Nature Communications. 2014;5:5126. doi: 10.1038/ncomms6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. Fibroblast growth factor homologous factors: evolution, structure, and function. Cytokine & Growth Factor Reviews. 2005;16:215–220. doi: 10.1016/j.cytogfr.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M, Schoorlemmer J, Williams A, Diwakar S, Wang Q, Huang X, Giza J, Tchetchik D, Kelley K, Vega A, Matthews G, Rossi P, Ornitz DM, D'Angelo E. Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron. 2007;55:449–463. doi: 10.1016/j.neuron.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Sokal I, Verlinde CL, Erdjument-Bromage H, Tempst P, Pronin AN, Benovic JL, Fariss RN, Palczewski K. Five members of a novel ca(2+)-binding protein (CABP) subfamily with similarity to calmodulin. Journal of Biological Chemistry. 2000;275:1247–1260. doi: 10.1074/jbc.275.2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DD, Dai S, Tseng PY, Malik Z, Nguyen M, Matt L, Schnizler K, Shephard A, Mohapatra DP, Tsuruta F, Dolmetsch RE, Christel CJ, Lee A, Burette A, Weinberg RJ, Hell JW. Competition between α-actinin and Ca²⁺-calmodulin controls surface retention of the L-type Ca²⁺ channel ca(V)1.2. Neuron. 2013;78:483–497. doi: 10.1016/j.neuron.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkiolaki M, Lewitzky M, Gilbert RJ, Jones EY, Bourette RP, Mouchiroud G, Sondermann H, Moarefi I, Feller SM. Structural basis for SH3 domain-mediated high-affinity binding between mona/Gads and SLP-76. The EMBO Journal. 2003;22:2571–2582. doi: 10.1093/emboj/cdg258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey JA, Marcou CA, Wang C, Wei EQ, Wang C, Tester DJ, Torchio M, Dagradi F, Crotti L, Schwartz PJ, Ackerman MJ, Pitt GS. FGF12 is a candidate brugada syndrome locus. Heart Rhythm. 2013;10:1886–1894. doi: 10.1016/j.hrthm.2013.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 3rd edn. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- Horstick EJ, Linsley JW, Dowling JJ, Hauser MA, McDonald KK, Ashley-Koch A, Saint-Amant L, Satish A, Cui WW, Zhou W, Sprague SM, Stamm DS, Powell CM, Speer MC, Franzini-Armstrong C, Hirata H, Kuwada JY. Stac3 is a component of the excitation-contraction coupling machinery and mutated in native american myopathy. Nature Communications. 2013;4:1952. doi: 10.1038/ncomms2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu IU, Linsley JW, Varineau JE, Shafer OT, Kuwada JY. Dstac is required for normal circadian activity rhythms in Drosophila. Chronobiology International. 2018;35 doi: 10.1080/07420528.2018.1454937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tan BZ, Shen Y, Tao J, Jiang F, Sung YY, Ng CK, Raida M, Köhr G, Higuchi M, Fatemi-Shariatpanahi H, Harden B, Yue DT, Soong TW. RNA editing of the IQ domain in Cav1.3 channels modulates their Ca²⁺-dependent inactivation. Neuron. 2012;73:304–316. doi: 10.1016/j.neuron.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villén J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Regehr WG. The mechanisms and functions of synaptic facilitation. Neuron. 2017;94:447–464. doi: 10.1016/j.neuron.2017.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong E, Choi HK, Park JH, Lee SY. STAC2 negatively regulates osteoclast formation by targeting the RANK signaling complex. Cell Death & Differentiation. 2018;25:1364–1374. doi: 10.1038/s41418-017-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner ML, Lee A. Voltage-Gated Cav1 channels in disorders of vision and hearing. Current Molecular Pharmacology. 2015;8:143–148. doi: 10.2174/1874467208666150507104937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LP, DeMaria CD, Yue DT. N-type calcium channel inactivation probed by gating-current analysis. Biophysical Journal. 1999;76:2530–2552. doi: 10.1016/S0006-3495(99)77407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi-Mukherjee R, Dick IE, Liu T, O'Rourke B, Yue DT, Tung L. Structural and functional plasticity in long-term cultures of adult ventricular myocytes. Journal of Molecular and Cellular Cardiology. 2013;65:76–87. doi: 10.1016/j.yjmcc.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Kukelyansky I, McCaffery JM, Ueno T, Varela LC, Inoue T. Organelle-specific, rapid induction of molecular activities and membrane tethering. Nature Methods. 2010;7:206–208. doi: 10.1038/nmeth.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraner SD, Novak KR, Wang Q, Peng J, Rich MM. Altered sodium channel-protein associations in critical illness myopathy. Skeletal Muscle. 2012;2:17. doi: 10.1186/2044-5040-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert RC, Maulet Y, Dupont JL, Mykita S, Craig P, Volsen S, Feltz A. Polyethylenimine-mediated DNA transfection of peripheral and central neurons in primary culture: probing Ca2+ channel structure and function with antisense oligonucleotides. Molecular and Cellular Neuroscience. 1996;7:239–246. doi: 10.1006/mcne.1996.0018. [DOI] [PubMed] [Google Scholar]
- Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2000;20:6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Westenbroek RE, Haeseleer F, Palczewski K, Scheuer T, Catterall WA. Differential modulation of ca(v)2.1 channels by calmodulin and Ca2+-binding protein 1. Nature Neuroscience. 2002;5:210–217. doi: 10.1038/nn805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Adams PJ, Yue DT. Large Ca²⁺-dependent facilitation of ca(V)2.1 channels revealed by Ca²⁺ photo-uncaging. The Journal of Physiology. 2015;593:2753–2778. doi: 10.1113/JP270091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legha W, Gaillard S, Gascon E, Malapert P, Hocine M, Alonso S, Moqrich A. stac1 and stac2 genes define discrete and distinct subsets of dorsal root ganglia neurons. Gene Expression Patterns. 2010;10:368–375. doi: 10.1016/j.gep.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+channel family. Neuron. 2003;39:951–960. doi: 10.1016/S0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- Limpitikul WB, Dick IE, Joshi-Mukherjee R, Overgaard MT, George AL, Yue DT. Calmodulin mutations associated with long QT syndrome prevent inactivation of cardiac L-type ca(2+) currents and promote proarrhythmic behavior in ventricular myocytes. Journal of Molecular and Cellular Cardiology. 2014;74:115–124. doi: 10.1016/j.yjmcc.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linse S, Forsén S. Determinants that govern high-affinity calcium binding. Advances in Second Messenger and Phosphoprotein Research. 1995;30:89–151. doi: 10.1016/S1040-7952(05)80005-9. [DOI] [PubMed] [Google Scholar]
- Linsley JW, Hsu IU, Groom L, Yarotskyy V, Lavorato M, Horstick EJ, Linsley D, Wang W, Franzini-Armstrong C, Dirksen RT, Kuwada JY. Congenital myopathy results from misregulation of a muscle Ca2+ channel by mutant Stac3. PNAS. 2017a;114:E228–E236. doi: 10.1073/pnas.1619238114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley JW, Hsu IU, Wang W, Kuwada JY. Transport of the alpha subunit of the voltage gated L-type calcium channel through the sarcoplasmic reticulum occurs prior to localization to triads and requires the beta subunit but not Stac3 in skeletal muscles. Traffic. 2017b;18:622–632. doi: 10.1111/tra.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley JW, Hsu IU, Wang W, Kuwada JY. Transport of the alpha subunit of the voltage gated L-type calcium channel through the sarcoplasmic reticulum occurs prior to localization to triads and requires the beta subunit but not Stac3 in skeletal muscles. Traffic. 2017c;18:622–632. doi: 10.1111/tra.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yang PS, Yang W, Yue DT. Enzyme-inhibitor-like tuning of ca(2+) channel connectivity with calmodulin. Nature. 2010;463:968–972. doi: 10.1038/nature08766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou JY, Laezza F, Gerber BR, Xiao M, Yamada KA, Hartmann H, Craig AM, Nerbonne JM, Ornitz DM. Fibroblast growth factor 14 is an intracellular modulator of voltage-gated sodium channels. The Journal of Physiology. 2005;569:179–193. doi: 10.1113/jphysiol.2005.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan A, Sato D, Shiferaw Y, Baher A, Xie LH, Peralta R, Olcese R, Garfinkel A, Qu Z, Weiss JN. Modifying L-type calcium current kinetics: consequences for cardiac excitation and arrhythmia dynamics. Biophysical Journal. 2008;94:411–423. doi: 10.1529/biophysj.106.98590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier LS, Bers DM. Calcium, Calmodulin, and calcium-calmodulin kinase II: heartbeat to heartbeat and beyond. Journal of Molecular and Cellular Cardiology. 2002;34:919–939. doi: 10.1006/jmcc.2002.2038. [DOI] [PubMed] [Google Scholar]
- Marshall CB, Nishikawa T, Osawa M, Stathopulos PB, Ikura M. Calmodulin and STIM proteins: two Major calcium sensors in the cytoplasm and endoplasmic reticulum. Biochemical and Biophysical Research Communications. 2015;460:5–21. doi: 10.1016/j.bbrc.2015.01.106. [DOI] [PubMed] [Google Scholar]
- Minor DL, Findeisen F. Progress in the structural understanding of voltage-gated calcium channel (CaV) function and modulation. Channels. 2010;4:459–474. doi: 10.4161/chan.4.6.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Fernández V, Monllau JM, Borrell V, Lerin C, de la Iglesia N. Transcriptional profiling of hypoxic neural stem cells identifies Calcineurin-NFATc4 signaling as a Major regulator of neural stem cell biology. Stem Cell Reports. 2015;5:157–165. doi: 10.1016/j.stemcr.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori MX, Erickson MG, Yue DT. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 2004;304:432–435. doi: 10.1126/science.1093490. [DOI] [PubMed] [Google Scholar]
- Mori MX, Vander Kooi CW, Leahy DJ, Yue DT. Crystal structure of the CaV2 IQ domain in complex with Ca2+/calmodulin: high-resolution mechanistic implications for channel regulation by Ca2+ Structure. 2008;16:607–620. doi: 10.1016/j.str.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanou E, Sullivan JM, Scheuer T, Catterall WA. Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to short-term synaptic plasticity in hippocampal neurons. PNAS. 2016;113:1062–1067. doi: 10.1073/pnas.1524636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BR, Wu F, Liu Y, Anderson DM, McAnally J, Lin W, Cannon SC, Bassel-Duby R, Olson EN. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. PNAS. 2013;110:11881–11886. doi: 10.1073/pnas.1310571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JT, Porter M, Amoui M, Miller WT, Zuckermann RN, Lim WA. Improving SH3 domain ligand selectivity using a non-natural scaffold. Chemistry & Biology. 2000;7:463–473. doi: 10.1016/S1074-5521(00)00130-7. [DOI] [PubMed] [Google Scholar]
- Niu J, Yang W, Yue DT, Inoue T, Ben-Johny M. Duplex signaling by CaM and Stac3 enhances CaV1.1 function and provides insights into congenital myopathy. The Journal of General Physiology. 2018;150:jgp.201812005. doi: 10.1085/jgp.201812005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Dittmer PJ, Youn DH, Dell'Acqua ML, Sather WA. Localized calcineurin confers Ca2+-dependent inactivation on neuronal L-type Ca2+ channels. Journal of Neuroscience. 2012;32:15328–15337. doi: 10.1523/JNEUROSCI.2302-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biology. 2001;2:reviews3005.1. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz S, Benmocha A, Sasson Y, Sachyani D, Almagor L, Lee A, Hirsch JA, Dascal N. Competitive and non-competitive regulation of calcium-dependent inactivation in CaV1.2 L-type Ca2+ channels by calmodulin and Ca2+-binding protein 1. The Journal of Biological Chemistry. 2013;288:12680–12691. doi: 10.1074/jbc.M113.460949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pablo JL, Pitt GS. Fibroblast growth factor homologous factors: new roles in neuronal health and disease. The Neuroscientist : A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2016;22:19–25. doi: 10.1177/1073858414562217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DS, Shekhar A, Marra C, Lin X, Vasquez C, Solinas S, Kelley K, Morley G, Goldfarb M, Fishman GI. Fhf2 gene deletion causes temperature-sensitive cardiac conduction failure. Nature Communications. 2016;7:12966. doi: 10.1038/ncomms12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain beta subunit of the L- type calcium channel. The Journal of Biological Chemistry. 1992;267:1792–1797. [PubMed] [Google Scholar]
- Persechini A, Stemmer PM. Calmodulin is a limiting factor in the cell. Trends in Cardiovascular Medicine. 2002;12:32–37. doi: 10.1016/S1050-1738(01)00144-X. [DOI] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/S0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Pitt GS, Lee SY. Current view on regulation of voltage-gated sodium channels by calcium and auxiliary proteins. Protein Science. 2016;25:1573–1584. doi: 10.1002/pro.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster A, Perni S, Bichraoui H, Beam KG. Stac adaptor proteins regulate trafficking and function of muscle and neuronal L-type Ca2+ channels. PNAS. 2015;112:602–606. doi: 10.1073/pnas.1423113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster A, Nelson BR, Olson EN, Beam KG. Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling that is disrupted by a myopathy-causing mutation. PNAS. 2016;113:10986–10991. doi: 10.1073/pnas.1612441113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster A, Nelson BR, Papadopoulos S, Olson EN, Beam KG. Stac proteins associate with the critical domain for excitation-contraction coupling in the II-III loop of CaV1.1. The Journal of General Physiology. 2018;150:jgp.201711917. doi: 10.1085/jgp.201711917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saimi Y, Kung C. Calmodulin as an ion channel subunit. Annual Review of Physiology. 2002;64:289–311. doi: 10.1146/annurev.physiol.64.100301.111649. [DOI] [PubMed] [Google Scholar]
- Satoh J, Nanri Y, Yamamura T. Rapid identification of 14-3-3-binding proteins by protein microarray analysis. Journal of Neuroscience Methods. 2006;152:278–288. doi: 10.1016/j.jneumeth.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Scharinger A, Eckrich S, Vandael DH, Schönig K, Koschak A, Hecker D, Kaur G, Lee A, Sah A, Bartsch D, Benedetti B, Lieb A, Schick B, Singewald N, Sinnegger-Brauns MJ, Carbone E, Engel J, Striessnig J. Cell-type-specific tuning of Cav1.3 Ca(2+)-channels by a C-terminal automodulatory domain. Frontiers in Cellular Neuroscience. 2015;9:309. doi: 10.3389/fncel.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoorlemmer J, Goldfarb M. Fibroblast growth factor homologous factors are intracellular signaling proteins. Current Biology. 2001;11:793–797. doi: 10.1016/S0960-9822(01)00232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Gebhart M, Fritsch R, Sinnegger-Brauns MJ, Poggiani C, Hoda JC, Engel J, Romanin C, Striessnig J, Koschak A. Modulation of voltage- and Ca2+-dependent gating of CaV1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. The Journal of Biological Chemistry. 2008;283:20733–20744. doi: 10.1074/jbc.M802254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood PM, Munoz-Sanjuan I, Tong P, Macke JP, Hendry SH, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. Fibroblast growth factor (FGF) homologous factors: new members of the FGF family implicated in nervous system development. PNAS. 1996;93:9850–9857. doi: 10.1073/pnas.93.18.9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong TW, DeMaria CD, Alvania RS, Zweifel LS, Liang MC, Mittman S, Agnew WS, Yue DT. Systematic identification of splice variants in human P/Q-type channel alpha1(2.1) subunits: implications for current density and Ca2+-dependent inactivation. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2002;22:10142–10152. doi: 10.1523/JNEUROSCI.22-23-10142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J, Bolz HJ, Koschak A. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflügers Archiv - European Journal of Physiology. 2010;460:361–374. doi: 10.1007/s00424-010-0800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kawai J, Taga C, Yaoi T, Hara A, Hirose K, Hayashizaki Y, Watanabe S. Stac, a novel neuron-specific protein with cysteine-rich and SH3 domains. Biochemical and Biophysical Research Communications. 1996;229:902–909. doi: 10.1006/bbrc.1996.1900. [DOI] [PubMed] [Google Scholar]
- Tan BZ, Jiang F, Tan MY, Yu D, Huang H, Shen Y, Soong TW. Functional characterization of alternative splicing in the C terminus of L-type CaV1.3 channels. The Journal of Biological Chemistry. 2011;286:42725–42735. doi: 10.1074/jbc.M111.265207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GM, Yu D, Wang J, Soong TW. Alternative splicing at C terminus of ca V 1.4 Calcium Channel Modulates Calcium-dependent Inactivation, Activation Potential, and Current Density. The Journal of Biological Chemistry. 2012;287:832–847. doi: 10.1074/jbc.M111.268722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay LH, Dick IE, Yang W, Mank M, Griesbeck O, Yue DT. Nanodomain Ca²⁺ of Ca²⁺ channels detected by a tethered genetically encoded Ca²⁺ sensor. Nature Communications. 2012;3:778. doi: 10.1038/ncomms1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JR, Lee A. Measuring Ca2+-Dependent modulation of Voltage-Gated Ca2+ channels in HEK-293T cells. Cold Spring Harbor Protocols. 2016;2016 doi: 10.1101/pdb.prot087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson WJ, Stea A, Bourinet E, Charnet P, Nargeot J, Snutch TP. Functional properties of a neuronal class C L-type calcium channel. Neuropharmacology. 1993;32:1117–1126. doi: 10.1016/0028-3908(93)90006-O. [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Cooperman SS, Agnew WS, Mandel G. Regulation of muscle sodium channel transcripts during development and in response to denervation. Developmental Biology. 1990;142:360–367. doi: 10.1016/0012-1606(90)90356-N. [DOI] [PubMed] [Google Scholar]
- Van Petegem F, Lobo PA, Ahern CA. Seeing the forest through the trees: towards a unified view on physiological calcium regulation of voltage-gated sodium channels. Biophysical Journal. 2012;103:2243–2251. doi: 10.1016/j.bpj.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venetucci L, Denegri M, Napolitano C, Priori SG. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nature Reviews Cardiology. 2012;9:561–575. doi: 10.1038/nrcardio.2012.93. [DOI] [PubMed] [Google Scholar]
- Wang HG, George MS, Kim J, Wang C, Pitt GS. Ca2+/calmodulin regulates trafficking of Ca(V)1.2 Ca2+ channels in cultured hippocampal neurons. Journal of Neuroscience. 2007;27:9086–9093. doi: 10.1523/JNEUROSCI.1720-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Hennessey JA, Kirkton RD, Wang C, Graham V, Puranam RS, Rosenberg PB, Bursac N, Pitt GS. Fibroblast growth factor homologous factor 13 regulates Na+ channels and conduction velocity in murine hearts. Circulation Research. 2011a;109:775–782. doi: 10.1161/CIRCRESAHA.111.247957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang C, Hoch EG, Pitt GS. Identification of novel interaction sites that determine specificity between fibroblast growth factor homologous factors and voltage-gated sodium channels. Journal of Biological Chemistry. 2011b;286:24253–24263. doi: 10.1074/jbc.M111.245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chung BC, Yan H, Lee SY, Pitt GS. Crystal structure of the ternary complex of a NaV C-terminal domain, a fibroblast growth factor homologous factor, and calmodulin. Structure. 2012;20:1167–1176. doi: 10.1016/j.str.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Chung BC, Yan H, Wang HG, Lee SY, Pitt GS. Structural analyses of Ca²⁺/CaM interaction with NaV channel C-termini reveal mechanisms of calcium-dependent regulation. Nature Communications. 2014;5:4896. doi: 10.1038/ncomms5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XY, Perez-Reyes E, Lacerda AE, Schuster G, Brown AM, Birnbaumer L. Heterologous regulation of the cardiac Ca2+ channel alpha 1 subunit by skeletal muscle beta and gamma subunits. implications for the structure of cardiac L-type Ca2+ channels. The Journal of Biological Chemistry. 1991;266:21943–21947. [PubMed] [Google Scholar]
- Wei EQ, Barnett AS, Pitt GS, Hennessey JA. Fibroblast growth factor homologous factors in the heart: a potential locus for cardiac arrhythmias. Trends in Cardiovascular Medicine. 2011;21:199–203. doi: 10.1016/j.tcm.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Schlegl J, Hahne H, Gholami AM, Lieberenz M, Savitski MM, Ziegler E, Butzmann L, Gessulat S, Marx H, Mathieson T, Lemeer S, Schnatbaum K, Reimer U, Wenschuh H, Mollenhauer M, Slotta-Huspenina J, Boese JH, Bantscheff M, Gerstmair A, Faerber F, Kuster B. Mass-spectrometry-based draft of the human proteome. Nature. 2014;509:582–587. doi: 10.1038/nature13319. [DOI] [PubMed] [Google Scholar]
- Wong King Yuen SM, Campiglio M, Tung CC, Flucher BE, Van Petegem F. Structural insights into binding of STAC proteins to voltage-gated calcium channels. PNAS. 2017;114:E9520–E9528. doi: 10.1073/pnas.1708852114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Yan Z, Li Z, Qian X, Lu S, Dong M, Zhou Q, Yan N. Structure of the voltage-gated calcium channel Ca(v)1.1 at 3.6 Å resolution. Nature. 2016;537:191–196. doi: 10.1038/nature19321. [DOI] [PubMed] [Google Scholar]
- Wu F, Quinonez M, DiFranco M, Cannon SC. Stac3 enhances expression of human CaV1.1 in Xenopus oocytes and reveals gating pore currents in HypoPP mutant channels. The Journal of General Physiology. 2018:475–489. doi: 10.1085/jgp.201711962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Pablo JL, Wang C, Pitt GS. FGF14 modulates resurgent sodium current in mouse cerebellar Purkinje neurons. eLife. 2014;3:e04193. doi: 10.7554/eLife.04193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PS, Alseikhan BA, Hiel H, Grant L, Mori MX, Yang W, Fuchs PA, Yue DT. Switching of Ca2+-dependent inactivation of Ca(v)1.3 channels by calcium binding proteins of auditory hair cells. Journal of Neuroscience. 2006;26:10677–10689. doi: 10.1523/JNEUROSCI.3236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PS, Johny MB, Yue DT. Allostery in Ca2+ channel modulation by calcium-binding proteins. Nature Chemical Biology. 2014;10:231–238. doi: 10.1038/nchembio.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nature Reviews Drug Discovery. 2016;15:19–34. doi: 10.1038/nrd.2015.5. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Lieu DK, Chiamvimonvat N. Small-conductance Ca2+ -activated K+ channels and cardiac arrhythmias. Heart Rhythm. 2015;12:1845–1851. doi: 10.1016/j.hrthm.2015.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer T, Surber R. SCN5A channelopathies--an update on mutations and mechanisms. Progress in Biophysics and Molecular Biology. 2008;98:120–136. doi: 10.1016/j.pbiomolbio.2008.10.005. [DOI] [PubMed] [Google Scholar]