Abstract
Background
Aberrant glycosylation is a hallmark of cancer cells and plays an important role in oncogenesis and cancer progression including metastasis. This study aimed to assess alteration in cellular glycosylation, detected by lectin Helix pomatia agglutinin (HPA) binding, in adrenal cancers and to determine whether such altered glycosylation has prognostic significance.
Methods
HPA binding lectin histochemistry was performed on archival paraffin wax‐embedded specimens of adrenocortical cancers excised from patients attending two tertiary referral centres. Benign tumours were used as controls. Demographic, histological and survival data were collected and compared between patients with HPA‐positive and HPA‐negative tumours.
Results
Thirty‐two patients were treated for adrenal cancer between 2000 and 2016; their median age was 49 (range 23–79) years. Fifteen patients had functioning tumours (14 adrenal Cushing's tumours and 1 Conn's tumour). Mean(s.d.) tumour size was 127·71(49·70) mm. None of 10 control tumours expressed HPA‐binding glycoproteins. Invasion was associated with HPA‐binding glycoproteins (P = 0·018). Local recurrence or metastatic disease did not significantly differ between HPA‐positive and HPA‐negative adrenocortical cancers. Overall survival was significantly longer in patients with HPA‐negative tumours (median survival not reached versus 22 months in patients with HPA‐positive tumours; P = 0·002).
Conclusion
Altered cellular glycosylation detected by lectin HPA is associated with poor survival in patients with adrenocortical cancer.
Introduction
Adrenocortical cancers are rare endocrine tumours with an incidence of about 1–2 per million population. This disease appears to have a higher prevalence in women and is associated with extremely poor prognosis1, 2, 3, 4, 5. Factors that predict prognosis include stage of the disease and complete resection (R0) of the tumour6. Molecular markers of prognosis include Ki‐67 proliferation index above 10 per cent7, high steroidogenic factor 1 expression8, mutation of tumour suppressor gene TP53, and loss of retinoblastoma protein expression9.
Cell surface glycoproteins play an important role in cell recognition, signalling, proliferation and differentiation10. There are two main types of protein glycosylation: N‐linked (a N‐acetylglucosamine (GlcNAc) residue is added to the Asn residue within a consensus peptide sequence of Asn–X–Ser/Thr, where X can be any amino acid except proline) and O‐linked (a N‐acetylgalactosamine (GalNAc) residue is added to the hydroxyl group of Ser or Thr residue on the polypeptide) glycosylation.
Alteration in glycosylation is associated with invasive and metastatic potential in some cancer types11. Binding of the lectin Helix pomatia agglutinin (HPA) has been reported as a marker of altered O‐glycosylation in cancer. This has been shown in a wide range of human cancers including lung, breast, prostate, stomach, thyroid and colorectal cancer12, 13, 14, 15, 16, 17, 18. HPA recognizes a range of O‐linked glycans bearing the terminal monosaccharide GalNAc19, including blood group A substance, Forssman antigens20, Tn epitope21 and, to a lesser extent, terminal GlcNAc22. Studies on aberrant glycosylation in adrenocortical cancer have not been reported.
The aims of this study were to assess alteration in cellular glycosylation, detected by HPA binding in adrenal cancers, and to determine whether such altered glycosylation has prognostic significance.
Methods
Patient selection and follow‐up procedure
This was a retrospective study of patients who underwent surgery for adrenocortical cancer at two tertiary referral centres (Singapore and Oxford) between January 2000 and December 2016. Patients were eligible if archival paraffin wax‐embedded specimens were available and they had been followed up. Lectin histochemistry was performed to detect binding of HPA. All patients were followed until death or to the end of December 2016. Ethical approval was obtained from the institutional boards (IRB: 2014/00378; REC: 11/SC/0396).
Preoperative investigation
All patients underwent two 24‐h urine biochemical evaluations to exclude the diagnosis of phaeochromocytoma by measuring 24‐h urinary metadrenalines, excessive secretion of steroids and their precursors, aldosterone and cortisol, to differentiate non‐functional from functional tumours. CT of the chest and abdomen was performed with contrast injection to assess characteristics of the tumour, lymph node involvement, adjacent organ invasion (of kidney, distal pancreas, spleen, liver or diaphragm), presence of intravascular thrombus in the inferior vena cava (IVC) or renal vein, and metastasis. If intravascular thrombus was suspected, further evaluation was performed with MRI. [18F]fluorodeoxyglucose PET was performed annually as part of follow‐up surveillance to detect metastasis or if patients had progression of symptoms. Both institutions followed similar protocols in terms of biochemical assessments and radiological investigations. In later years, however, PET was used more frequently for staging and assessment for metastasis.
Demographic data
Demographic data, histological data, recurrence of disease, local invasion and mortality data were collected. Survival was estimated as overall survival (OS), defined as duration of survival from the date of surgery to the date of last follow‐up, and recurrence‐free survival (RFS), defined as the interval between surgery and diagnosis of recurrence for relapsed patients or from the date of surgery to date of last follow‐up for patients without recurrence.
Lectin histochemistry
Lectin immunohistochemistry was performed on 5‐μm thick paraffin wax‐embedded clinical adrenal tumour samples using an avidin–biotin labelling technique validated by Brooks and Wilkinson23 to reveal binding of lectin HPA. For positive controls, sections of rat kidney, which shows strong and characteristic HPA labelling, were included in each labelling experiment. For negative controls, the lectin was omitted and the specificity of binding was confirmed by incubating the sections with HPA in the presence of 0·1‐mol/l GalNAc. Ten benign adrenal tumours were also assessed for controls.
Paraffin wax sections were dewaxed in xylene and rehydrated through a series of graded alcohols (100, 90 and 70 per cent (v/v)) to water. Endogenous peroxidases were then quenched by soaking the sections in 3 per cent (v/v) hydrogen peroxide in methanol for 20 min at room temperature before washing gently in running tap water for 5 min. The sections were then trypsinized in 0·1 per cent (w/v) trypsin (crude type II from porcine pancreas) and 0·1 per cent (w/v) calcium chloride in lectin buffer (0·6 per cent (w/v) Tris, 0·085 per cent (w/v) sodium chloride, 0·02 per cent (w/v) magnesium chloride, 0·01 per cent calcium chloride, pH 7·6) at 37oC for 20 min, in order to reveal lectin‐binding sites. The slides were washed in gently running tap water for 5 min after the trypsinization procedure. Biotinylated HPA was diluted in blocking buffer (5 per cent (w/v) bovine serum albumin in lectin buffer) at a concentration of 10 μg/ml. The slides were incubated with this solution for 1 h, washed in several changes of lectin buffer, and then incubated with 5‐μg/ml horseradish peroxidase conjugated avidin for 30 min. The sections were washed again in several changes of lectin buffer, and then lectin binding was revealed by incubation with hydrogen peroxidase/diaminobenzidine (DAB) (DAB Perioxidase Substrate Kit; Vector Laboratories, Burlingame, California, USA), prepared according to manufacturer's instructions, for 10 min. The sections were washed for 5 min under running tap water and then lightly counterstained in Mayer's haematoxylin. After blueing under running tap water, the sections were dehydrated through a series of graded alcohols (70 per cent (v/v), 90 per cent (v/v), 100 per cent), cleared in xylene, and mounted in DPX resinous mountant.
Assessment of Helix pomatia agglutinin labelling
The guidelines described by Brooks and colleagues24 were used to classify the labelling of sections as positive or negative for HPA binding. Sections were scored as positive when 5 per cent or more of the cancer cells labelled positively for HPA binding and as negative when less than 5 per cent labelled positively. Assessments of HPA binding were performed by two observers who were blinded to the identity of the sample, and their results were compared. In cases of discordance, consensus was obtained after discussion. Interobserver variability was calculated by κ index using the formula: K = Po − Pe/1 − Pe, where Po is observed proportionate agreement and Pe is overall random agreement probability.
Statistical analysis
Statistical analyses were performed with SPSS® version 22.0 (IBM, Armonk, New York, USA). The association between positive HPA labelling and clinicopathological variables was analysed using the χ2 test and Wilcoxon signed rank test. Survival curves were determined by Kaplan–Meier analysis and compared with the log rank test. P < 0·050 was considered statistically significant.
Results
Patient and tumour characteristics
Forty‐seven patients were eligible for this study; 15 were excluded because they had no follow‐up (2) or tissue blocks were not available (13). Of the 32 included patients, 21 and 11 were operated on in Oxford and Singapore respectively; their demographic profile is shown in Table 1. Fifteen of the 32 tumours were functional and malignant (showed features of capsular and vascular invasion, extra‐adrenal extension, high mitotic figures, tumour necrosis and large size).
Table 1.
Demographics of the study population
| No. of patients (n = 32)* | |
|---|---|
| Age (years)† | 49 (23–79) |
| Sex ratio (M : F) | 15 : 17 |
| Diagnosis | |
| Non‐functioning tumour | 17 |
| Cushing's tumour | 14 |
| Conn's tumour | 1 |
| Tumour size (mm)‡ | 127·71(49·70) |
| Tumour stage | |
| I | 5 |
| II | 5 |
| III | 5 |
| IV | 17 |
| Treatment | |
| Biopsy only | 2 |
| Adrenalectomy | 13 |
| Adrenalectomy + en bloc resection | 17 |
| Adjuvant treatment | |
| Mitotane only | 22 |
| Mitotane + other chemotherapy | 4 |
| Radiotherapy | 2 |
| Local invasion | |
| Yes | 26 |
| No | 6 |
| Metastasis | |
| Yes | 14 |
| No | 18 |
| Died | 21 |
Unless indicated otherwise, values are
median (range) and
mean(s.d.).
Treatment modalities for the patients were: surgery in 30 and adjuvant mitotane in 22. Four patients who received mitotane also underwent salvage chemotherapy with doxorubicin, cisplatin and doxorubicin. In patients who had surgery, 13 underwent adrenalectomy only and 17 had en bloc resection with nephrectomy (10), splenectomy (9), pancreatectomy (5) and IVC thrombectomy (4). Two patients did not undergo surgery and diagnosis was confirmed by CT‐guided biopsy. In 13 patients who underwent adrenalectomy only, malignancy was suspected in five cases but there were no features of local invasion on imaging warranting contiguous organ resection.
Based on the AJCC criteria, 17 patients had stage IV disease, and local invasion was observed in 26 patients. Fourteen of the 32 patients developed metastases, predominantly pulmonary. The next most common site of metastasis was the liver, followed by bone.
Median OS was 30 (range 1–168) months, RFS was 30 (6–156) months. The mortality rate for the cohort was 66 per cent (21 of 32) over the study period. Five of the 13 patients who had adrenalectomy alone developed local recurrence, and six of 17 had local recurrence following a radical en bloc resection.
Helix pomatia agglutinin labelling of tissue samples
Positive labelling for HPA binding was observed in 19 of the 32 patients. The difference in labelling between positive and negative tumours is shown in Fig. 1. Labelling was not observed in negative controls where HPA was omitted, or where HPA incubation took place in the presence of inhibiting GalNAc. The κ index between observers was 0·75, indicating satisfactory inter‐rater reliability for the immunohistochemical assessment of tumour samples.
Figure 1.
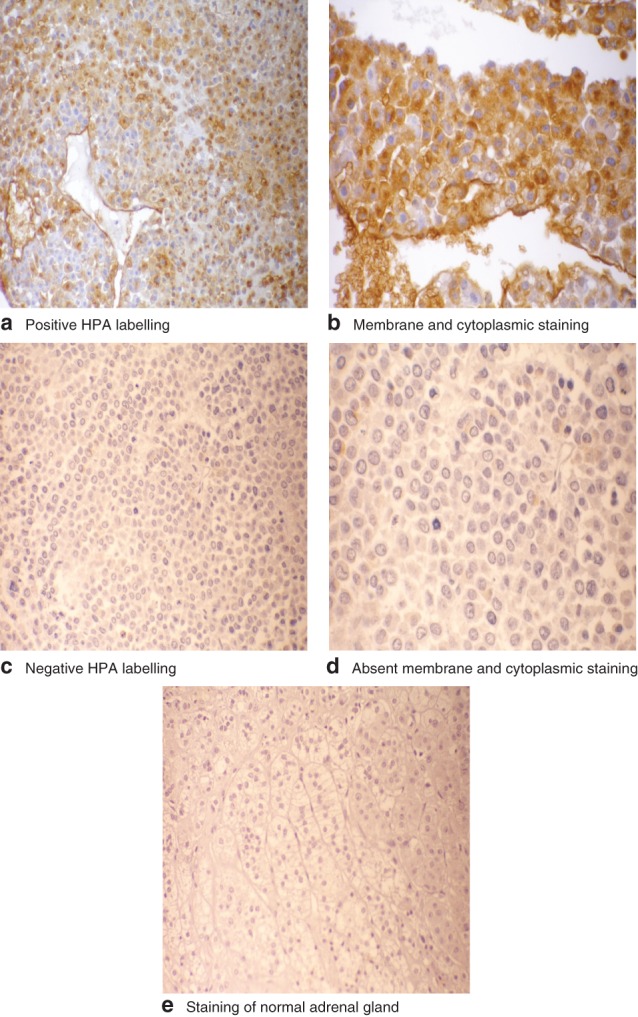
Results of Helix pomatia agglutinin (HPA) lectin immunohistochemistry in the tumours studied: a positive HPA labelling, as shown by intense deep brown staining, in adrenocortical cancer (ACC) (magnification ×10); b intense membrane and cytoplasmic staining of cancer cells (magnification ×20); c negative HPA binding in ACC with absence of any labelling (magnification ×10); d absent membrane and cytoplasmic staining of cancer cells (magnification ×20); e absent staining with HPA in a normal adrenal gland (magnification ×10)
Correlation between clinicopathological variables
The correlation between the various clinicopathological variables and HPA labelling is shown in Table 2. No significant correlation was observed between HPA labelling and sex, histological diagnosis, tumour recurrence, stage of the disease, presence or absence of metastasis and adjuvant therapy. Of the 26 patients with features of local invasion, 18 had positive HPA labelling and eight negative HPA labelling (P = 0·018). Similarly, positive HPA labelling was seen in six of the ten patients with tumour recurrence (P = 0·961).
Table 2.
Correlation between Helix pomatia agglutinin binding and clinicopathological variables
| Positive HPA binding (n = 19) | Negative HPA binding (n = 13) | P * | |
|---|---|---|---|
| Sex ratio (M : F) | 10 : 9 | 5 : 8 | 0·430 |
| Tumour type | 0·273 | ||
| Cushing's tumour | 10 | 4 | |
| Conn's tumour | 1 | – | |
| Non‐functioning tumour | 8 | 9 | |
| Type of surgery | 0·524 | ||
| Biopsy only | 1 | 1 | |
| Adrenalectomy | 8 | 5 | |
| En bloc resection | 10 | 7 | |
| Tumour stage | 0·451 | ||
| I | 4 | 1 | |
| II | 2 | 3 | |
| III | 2 | 3 | |
| IV | 11 | 6 | |
| Invasion | 0·018 | ||
| Yes | 18 | 8 | |
| No | 1 | 5 | |
| Recurrence | 0·961 | ||
| Yes | 6 | 4 | |
| No | 13 | 9 | |
| Metastasis | 0·618 | ||
| Yes | 9 | 5 | |
| No | 10 | 8 | |
| Mitotane therapy | 0·467 | ||
| Yes | 14 | 8 | |
| No | 5 | 5 | |
| Weiss score | 0·945 | ||
| ≥ 3 | 9 | 6 | |
| < 3 | 10 | 7 | |
| Died | 0·007 | ||
| Yes | 16 | 5 | |
| No | 3 | 8 |
HPA, Helix pomatia agglutinin.
Log rank test.
Significance of positive Helix pomatia agglutinin labelling
In univariable analysis, sex, type of surgery, stage, recurrence, metastasis, Weiss score and adjuvant therapy did not influence survival. Local invasion (P = 0·018) and positive HPA labelling (P = 0·007) were significantly correlated with survival (Table 2).
Differences in survival between patients with tumours that were positive or negative for HPA labelling are shown in Fig. 2. Patients with positive HPA labelling had a median survival of 22 (range 2–156) months; at the end of follow‐up, 16 of 19 had died. Of patients with negative HPA binding, all but three were alive at the end of the analysis period, with a mortality rate of 23 per cent (3 of 13); their survival was significantly better than that in patients with positive HPA labelling (P = 0·002).
Figure 2.
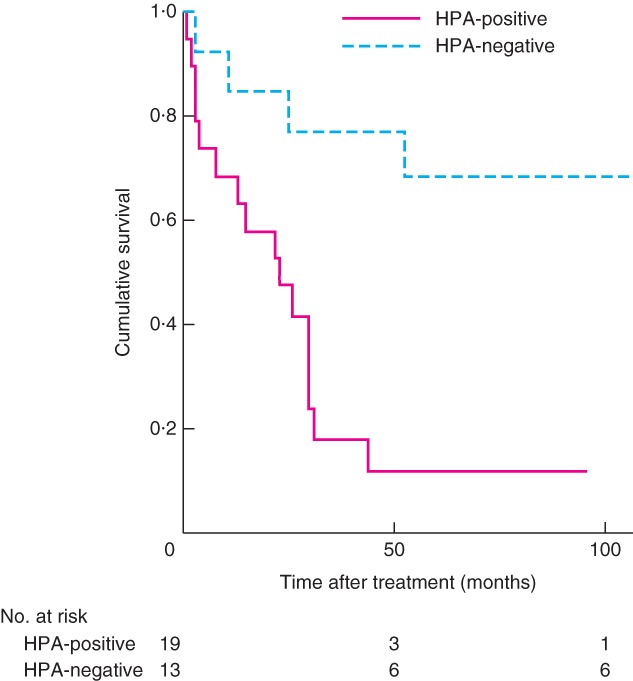
Kaplan–Meier survival curves for patients with positive and negative Helix pomatia agglutinin (HPA) immunolabelling. P = 0·002 (log rank test)
Discussion
This study has shown that aberrant glycosylation detectable by HPA lectin histochemistry is a predictor of poor survival in adrenal cancer. Glycosylation is the most common post‐translational modification of proteins and plays an important role in cell communication, interaction and adhesion25. Aberrant glycosylation is more likely to be seen in tumours with a more aggressive phenotype26.
In the present study, lectin cells showed significant cell membrane localization of HPA‐binding glycans where positive. The results were similar to those shown by Peiris and colleagues18 in metastatic colorectal cancer. The labelling pattern appeared to show an all‐or‐nothing effect: the cancers either labelled as clearly positive or completely negative. This is similar to the labelling pattern described by Brooks and Wilkinson in breast cancer23.
Adrenocortical carcinoma is an aggressive cancer with 5‐year survival rates of up to 60 per cent, with surgery the only effective curative treatment. Factors that affect survival include patient age, stage, completeness of resection, local invasion, functionality and metastasis27. In the present study there was no relationship between HPA lectin labelling and parameters including age, sex, histological type, surgery and chemotherapy. Positive HPA labelling was, however, associated with an increased risk of local invasion and significantly decreased median survival. Positive HPA labelling was not correlated with metastasis, unlike findings in studies of breast and colonic cancer18 28. In studies that showed a correlation with metastatic state, the two main binding partners of HPA were identified as integrin α5 and α6, and annexin 2 and 418 29. These two binding partners were not evaluated in the present study. The exact binding partners in adrenal cancer are yet to be identified; characterization using affinity chromatography and mass spectrometry is required.
Markers such as Ki‐67 labelling index have been used to determine prognosis in adrenal cancer7 30, 31 but continue to have significant variability in reporting amongst pathologists32. A recent publication32 on reporting variability described Ki‐67 as an unreliable marker. In the present cohort, any association between Ki‐67 and prognosis was not analysed as data pertaining to this variable were missing for several patients. HPA labelling was, in contrast, straightforward to interpret as positive or negative, making HPA lectin labelling a promising marker of prognosis in adrenal cancer.
Some studies33 34 have shown that cortisol‐secreting adrenocortical cancers are associated with a poor prognosis. There was no difference in HPA binding or survival between functioning and non‐functioning adrenocortical cancers in the present cohort. It has been reported35 36 that patients who received treatment with mitotane immediately after surgery had improved survival compared with those treated later after surgery, but another study37 did not show such a difference. The present study did not show a statistically significant difference in survival and HPA binding between patients who received mitotane and those who did not.
The limitations of this study include the small sample size with its inherent biases, the loss of 15 patients who could not be included in the study, and the retrospective design.
Positive HPA lectin labelling has the potential to be used as a marker of poor prognosis in patients with adrenocortical cancers, along with other currently used markers.
Acknowledgements
This project was funded by a British Association of Endocrine and Thyroid Surgeons grant, awarded in 2012.
Disclosure: The authors declare no conflict of interest.
Funding information
British Association of Endocrine and Thyroid Surgeons
References
- 1. Phan AT. Adrenal cortical carcinoma – review of current knowledge and treatment practices. Hematol Oncol Clin North Am 2007; 21: 489–507; viii–ix. [DOI] [PubMed] [Google Scholar]
- 2. Dackiw AP, Lee JE, Gagel RF, Evans DB. Adrenal cortical carcinoma. World J Surg 2001; 25: 914–926. [DOI] [PubMed] [Google Scholar]
- 3. Loncar Z, Djukic V, Zivaljevic V, Pekmezovic T, Diklic A, Tatic S et al Survival and prognostic factors for adrenocortical carcinoma: a single institution experience. BMC Urol 2015; 15: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chagpar R, Siperstein AE, Berber E. Adrenocortical cancer update. Surg Clin North Am 2014; 94: 669–687. [DOI] [PubMed] [Google Scholar]
- 5. Else T, Kim AC, Sabolch A, Raymond VM, Kandathil A, Caoili EM et al Adrenocortical carcinoma. Endocr Rev 2014; 35: 282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Przytulska J, Rogala N, Bednarek‐Tupikowska G. Current and emerging therapies for adrenocortical carcinoma – review. Adv Clin Exp Med 2015; 24: 185–193. [DOI] [PubMed] [Google Scholar]
- 7. Terzolo M, Boccuzzi A, Bovio S, Cappia S, De Giuli P, Alì A et al Immunohistochemical assessment of Ki‐67 in the differential diagnosis of adrenocortical tumors. Urology 2001; 57: 176–182. [DOI] [PubMed] [Google Scholar]
- 8. Duregon E, Volante M, Giorcelli J, Terzolo M, Lalli E, Papotti M. Diagnostic and prognostic role of steroidogenic factor 1 in adrenocortical carcinoma: a validation study focusing on clinical and pathologic correlates. Hum Pathol 2013; 44: 822–828. [DOI] [PubMed] [Google Scholar]
- 9. Ragazzon B, Libé R, Assié G, Tissier F, Barreau O, Houdayer C et al Mass‐array screening of frequent mutations in cancers reveals RB1 alterations in aggressive adrenocortical carcinomas. Eur J Endocrinol 2014; 170: 385–391. [DOI] [PubMed] [Google Scholar]
- 10. Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 1993; 3: 97–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Häuselmann I, Borsig L. Altered tumor‐cell glycosylation promotes metastasis. Front Oncol 2014; 4: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parameswaran R, Sadler G, Brooks S. Helix pomatia agglutinin binding glycoproteins in thyroid tumors. World J Surg 2011; 35: 2219–2227. [DOI] [PubMed] [Google Scholar]
- 13. Brooks SA. The involvement of Helix pomatia lectin (HPA) binding N‐acetylgalactosamine glycans in cancer progression. Histol Histopathol 2000; 15: 143–158. [DOI] [PubMed] [Google Scholar]
- 14. Brooks SA, Leathem AJ. Prediction of lymph node involvement in breast cancer by detection of altered glycosylation in the primary tumour. Lancet 1991; 338: 71–74. [DOI] [PubMed] [Google Scholar]
- 15. Schumacher DU, Randall CJ, Ramsay AD, Schumacher U. Is the binding of the lectin Helix pomatia agglutinin (HPA) of prognostic relevance in tumours of the upper aerodigestive tract? Eur J Surg Oncol 1996; 22: 618–620. [DOI] [PubMed] [Google Scholar]
- 16. Schumacher U, Brooks SA, Mester J. The lectin Helix pomatia agglutinin as a marker of metastases – clinical and experimental studies. Anticancer Res 2005; 25: 1829–1830. [PubMed] [Google Scholar]
- 17. Dwek MV, Ross HA, Streets AJ, Brooks SA, Adam E, Titcomb A et al Helix pomatia agglutinin lectin‐binding oligosaccharides of aggressive breast cancer. Int J Cancer 2001; 95: 79–85. [DOI] [PubMed] [Google Scholar]
- 18. Peiris D, Ossondo M, Fry S, Loizidou M, Smith‐Ravin J, Dwek MV. Identification of O‐linked glycoproteins binding to the lectin Helix pomatia agglutinin as markers of metastatic colorectal cancer. PLoS One 2015; 10: e0138345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brooks SA, Leathem AJ. Expression of alpha‐GalNAc glycoproteins by breast cancers. Br J Cancer 1995; 71: 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu AM, Song SC, Sugii S, Herp A. Differential binding properties of Gal/GalNAc specific lectins available for characterization of glycoreceptors. Indian J Biochem Biophys 1997; 34: 61–71. [PubMed] [Google Scholar]
- 21. Springer GF. Tn epitope (N‐acetyl‐d‐galactosamine alpha‐O‐serine/threonine) density in primary breast carcinoma: a functional predictor of aggressiveness. Mol Immunol 1989; 26: 1–5. [DOI] [PubMed] [Google Scholar]
- 22. Ito N, Imai S, Haga S, Nagaike C, Morimura Y, Hatake K. Localization of binding sites of Ulex europaeus I, Helix pomatia and Griffonia simplicifolia I‐B4 lectins and analysis of their backbone structures by several glycosidases and poly‐N‐acetyllactosamine‐specific lectins in human breast carcinomas. Histochem Cell Biol 1996; 106: 331–339. [PubMed] [Google Scholar]
- 23. Brooks SA, Wilkinson D. Validation of a simple avidin–biotin detection method for Helix pomatia lectin (HPA) binding as a prognostic marker in cancer. Acta Histochem 2003; 105: 205–212. [DOI] [PubMed] [Google Scholar]
- 24. Brooks SA, Leathem AJ, Camplejohn RS, Gregory W. Markers of prognosis in breast cancer – the relationship between binding of the lectin HPA and histological grade, SPF, and ploidy. Breast Cancer Res Treat 1993; 25: 247–256. [DOI] [PubMed] [Google Scholar]
- 25. Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell 2006; 126: 855–867. [DOI] [PubMed] [Google Scholar]
- 26. Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate‐mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci 2004; 95: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mihai R. Diagnosis, treatment and outcome of adrenocortical cancer. Br J Surg 2015; 102: 291–306. [DOI] [PubMed] [Google Scholar]
- 28. Fry SA, Sinclair J, Timms JF, Leathem AJ, Dwek MV. A targeted glycoproteomic approach identifies cadherin‐5 as a novel biomarker of metastatic breast cancer. Cancer Lett 2013; 328: 335–344. [DOI] [PubMed] [Google Scholar]
- 29. Saint‐Guirons J, Zeqiraj E, Schumacher U, Greenwell P, Dwek M. Proteome analysis of metastatic colorectal cancer cells recognized by the lectin Helix pomatia agglutinin (HPA). Proteomics 2007; 7: 4082–4089. [DOI] [PubMed] [Google Scholar]
- 30. Vassilopoulou‐Sellin R, Schultz PN. Adrenocortical carcinoma. Clinical outcome at the end of the 20th century. Cancer 2001; 92: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 31. Stojadinovic A, Brennan MF, Hoos A, Omeroglu A, Leung DH, Dudas ME et al Adrenocortical adenoma and carcinoma: histopathological and molecular comparative analysis. Mod Pathol 2003; 16: 742–751. [DOI] [PubMed] [Google Scholar]
- 32. Papathomas TG, Pucci E, Giordano TJ, Lu H, Duregon E, Volante M et al An international Ki67 reproducibility study in adrenal cortical carcinoma. Am J Surg Pathol 2016; 40: 569–576. [DOI] [PubMed] [Google Scholar]
- 33. Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P et al Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol‐secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab 2006; 91: 2650–2655. [DOI] [PubMed] [Google Scholar]
- 34. Berruti A, Fassnacht M, Haak H, Else T, Baudin E, Sperone P et al Prognostic role of overt hypercortisolism in completely operated patients with adrenocortical cancer. Eur Urol 2014; 65: 832–838. [DOI] [PubMed] [Google Scholar]
- 35. Baudin E, Pellegriti G, Bonnay M, Penfornis A, Laplanche A, Vassal G et al Impact of monitoring plasma 1,1‐dichlorodiphenildichloroethane (o,p′DDD) levels on the treatment of patients with adrenocortical carcinoma. Cancer 2001; 92: 1385–1392. [DOI] [PubMed] [Google Scholar]
- 36. Kasperlik‐Załuska AA, Migdalska BM, Zgliczyński S, Makowska AM. Adrenocortical carcinoma. A clinical study and treatment results of 52 patients. Cancer 1995; 75: 2587–2591. [DOI] [PubMed] [Google Scholar]
- 37. Venkatesh S, Hickey RC, Sellin RV, Fernandez JF, Samaan NA. Adrenal cortical carcinoma. Cancer 1989; 64: 765–769. [DOI] [PubMed] [Google Scholar]


