This article focuses on the challenge of maintaining cardiovascular health in long‐term childhood cancer survivors. A simple risk score is proposed, which can be used in clinical practice to identify patients at increased cardiovascular risk.
Keywords: Cancer, Childhood, Cardiovascular risk, Mortality, Survivorship
Abstract
Background.
Long‐term childhood cancer survivors (CCS) are at increased risk of adverse cardiovascular events; however, there is a paucity of risk‐stratification tools to identify those at higher‐than‐normal risk.
Subjects, Materials, and Methods.
This was a population‐based study using data from the Surveillance, Epidemiology, and End Results Program (1973–2013). Long‐term CCS (age at diagnosis ≤19 years, survival ≥5 years) were followed up over a median time period of 12.3 (5–40.9) years. Independent predictors of cardiovascular mortality (CVM) were combined into a risk score, which was developed in a derivation set (n = 22,374), and validated in separate patient registries (n = 6,437).
Results.
In the derivation registries, older age at diagnosis (≥10 years vs. reference group of 1–5 years), male sex, non‐white race, a history of lymphoma, and a history of radiation were independently associated with an increased risk of CVM among long‐term CCS (p < .05). A risk score derived from this model (Childhood and Adolescence Cancer Survivor CardioVascular score [CHACS‐CV], range: 0–8) showed good discrimination for CVM (Harrell's C‐index [95% confidence interval (CI)]: 0.73 [0.68–0.78], p < .001) and identified a high‐risk group (CHACS‐CV ≥6), with cumulative CVM incidence over 30 years of 6.0% (95% CI: 4.3%–8.1%) versus 2.6% (95% CI: 1.8%–3.7%), and 0.7% (95% CI: 0.5%–1.0%) in the mid‐ (CHACS‐CV = 4–5) and low‐risk groups (CHACS‐CV ≤3), respectively (plog‐rank < .001). In the validation set, the respective cumulative incidence rates were 4.7%, 3.1%, and 0.8% (plog‐rank < .001).
Conclusion.
We propose a simple risk score that can be applied in everyday clinical practice to identify long‐term CCS at increased cardiovascular risk, who may benefit from early cardiovascular screening, and risk‐reduction strategies.
Implications for Practice.
Childhood cancer survivors (CCS) are known to be at increased cardiovascular risk. Currently available prognostic tools focus on treatment‐related adverse events and late development of congestive heart failure, but there is no prognostic model to date to estimate the risk of cardiovascular mortality among long‐term CCS. A simple clinical tool is proposed for cardiovascular risk stratification of long‐term CCS based on easily obtainable information from their medical history. This scoring system may be used as a first‐line screening tool to assist health care providers in identifying those who may benefit from closer follow‐up and enable timely deployment of preventive strategies.
Introduction
It is estimated that 1 out of 285 children in the U.S. will be diagnosed with cancer before the age of 20, and 1 in 530 young adults between the ages of 20 and 39 years is a survivor of a childhood malignancy [1]. In recent decades, a growing and highly effective armamentarium in cancer therapy has led to significantly improved survival rates in children diagnosed with malignant neoplasms [2]. As a result, 5‐year survival rates among children diagnosed with cancer have increased from less than 50% in 1960 to more than 80% in the last decade [3]. As of 2013, it was estimated that there were more than 420,000 childhood cancer survivors in the U.S., and this number is expected to surpass 500,000 by 2020 [3].
However, improvements in observed survival rates have been accompanied by an increasing burden of treatment‐related late effects such as second cancers and adverse cardio‐pulmonary effects [4], [5], [6], [7]. An expanding body of evidence now suggests that long‐term childhood cancer survivors are at increased risk of developing multiple chronic health conditions [8], [9], as well as severe life‐threatening or fatal events years after their original cancer diagnosis and treatment [3], [5], [10], [11]. Cardiovascular complications in particular are of major concern given that several cancer treatments, such as mediastinal radiation, older anthracycline (e.g., doxorubicin), and newer non‐anthracycline chemotherapeutic agents have well‐known cardiotoxic effects [12]. Although these have shown to reduce cancer‐specific mortality, they have also been associated with a competing increase in cardiovascular morbidity and mortality [4], [5], [12]. Increasing awareness of these adverse effects has led to efforts to limit exposure at the time of treatment [12], [13], [14]. Nevertheless, because cardiotoxicity is progressive and often irreversible [15], identifying those at risk and instituting early detection and risk‐reduction strategies remain of utmost importance.
Given the importance of maintaining cardiovascular health in both patients undergoing treatment for cancer and long‐term cancer survivors, the field of cardio‐oncology has emerged with the key aim to reduce the effects of cancer treatment on the cardiovascular system, ensuring a good quality of life after treatment and, ultimately, improved long‐term survival rates [16], [17], [18]. Several scientific organizations, including the National Comprehensive Cancer Network and the Children's Oncology Group, as well as international, collaborative efforts such as the International Late Effects of Childhood Cancer Guideline Harmonization Group have issued detailed recommendations on the long‐term follow‐up of childhood cancer survivors including screening for adverse cardiovascular sequelae based on detailed exposure variables [14], [19], [20]. However, few adult childhood cancer survivors return to their cancer center for adult care, and, as a result, such detailed exposure variables may be hard to obtain in busy clinical practice [21]. Furthermore, although it is well established that cardiovascular morbidity and mortality is a significant issue of concern among childhood cancer survivors, these patients are usually young, which limits the potential value of traditional cardiovascular risk factors or adult risk scores for cardiovascular risk prediction. Moreover, their absolute risk, although elevated, remains relatively low when compared with that of older individuals with multiple risk factors and comorbidities. A cost‐effective clinical tool based on simple demographic and clinical variables that can be easily obtained in a routine medical encounter would be highly valuable as an entry point into existing guidelines helping health care providers identify individuals who are in need of closer follow‐up and more intensive risk‐reduction strategies.
Subjects, Materials, and Methods
Study Design
This was a population‐based study of prospectively collected data from the Surveillance, Epidemiology, and End Results (SEER) Program database of the National Cancer Institute. The SEER database is available for public research, and all patient data are de‐identified; hence, approval by the institutional review boards was not required. All authors with access to the individual de‐identified data have signed the SEER data use agreement form. The National Cancer Institute SEER*Stat software (seer.cancer.gov/seerstat) version 8.1.5 was used to extract information from the SEER Program SEER*Stat Database (Incidence—SEER 9 Regs Research Data, November 2015 Sub [1973–2013] Total U.S., released April 2016, based on the November 2015 submission). All registered cases of single, primary, malignant neoplasms diagnosed between 1973 and 2013 at an age of ≤19 years were identified, and pertinent clinical variables were extracted. Long‐term survivors were defined as individuals with at least 5 years of prospective follow‐up after their diagnosis. For the purpose of developing a prognostic model, analysis was restricted to those registries with continuous, prospective data collection since at least 1975.
Definitions
Primary, malignant neoplasms were defined according to the International Classification for Diseases in Oncology (3rd edition) and International Agency for Research on Cancer criteria [22]. In situ neoplasms and borderline malignancies were excluded from the analysis. To limit the effects of recurrent exposure to cancer treatment, individuals with second, primary malignancies were also excluded. The primary outcome of the study was early cardiovascular mortality (CVM), which was defined as any death due to proximate cardiac causes (including aortic aneurysm and dissection), cerebrovascular disease, peripheral vascular events, hypertension with or without heart disease, and other diseases of arteries, arterioles, and capillaries.
Statistical Analysis
Demographic variables are presented as number (percentage) or median (range). CVM incidence rates with corresponding 95% confidence intervals (CI) were calculated for each cancer type both before and after the first 5 years of diagnosis. Kaplan‐Meier curves were plotted to compare survival rates between different subgroups and were compared by the log‐rank test. Next, SEER registries with prospective data collection since at least 1975 were identified and split into a derivation (n = 7 registries) and validation set (n = 2 registries). All available demographic and treatment variables with <10% missing data were fitted in a multivariable Cox regression model with CVM as the dependent variable, and significant predictors were identified by bootstrapping with 1,000 replications for internal validation (confidence level set at 0.95). Next, a simplified score was created by assigning a weighted value to each one of the significant predictors. This was done by calculating their beta coefficients (ln[hazard ratio (HR)]) in multivariable Cox regression models, and dividing them by the smallest of these coefficients, before rounding to the nearest integer. Discrimination was assessed both by the C‐statistic and Harrell's concordance index (95% confidence intervals for Harrell's C‐index were calculated by a jackknife tool) [23]. Long‐term survivors were then assigned to three risk groups according to their Childhood and Adolescence Cancer Survivor CardioVascular (CHACS‐CV) score (high, mid, low) to compare their relative survival and cumulative incidence of CVM by the Kaplan‐Meier method as well as by Cox regression analysis. The proportional hazards assumption was tested by means of global and covariate‐specific Schoenfeld residuals (a p value >.05 indicated that the proportional hazards assumption was valid). The final risk score was validated in a separate (validation) group of two independent registries by measuring the same discrimination indices and plotting the respective Kaplan‐Meier curves for the different risk groups [24]. All statistical tests were performed using the Stata 14 software (StataCorp, College Station, TX). All tests were two‐sided and α was set at 0.05. Reporting of the methods and results was performed according to the Strengthening the Reporting of Observational Studies in Epidemiology Statement guidelines for reporting observational studies (supplemental online Table 1) [25].
Results
Cause‐Specific Mortality Rates per Malignancy Type
The study flowchart is summarized in Figure 1, and the baseline demographics and treatment data of the identified childhood cancer cases are presented in Table 1. Leukemia (n = 23,610, 26.7%), lymphoma (n = 13,372, 15.1%), and central nervous system tumor cases (n = 15,074, 17.1%) represented the majority of single, primary malignant neoplasms in childhood (Table 1; a detailed cancer classification of all malignancies included in our analysis is also available in supplemental online Table 2).
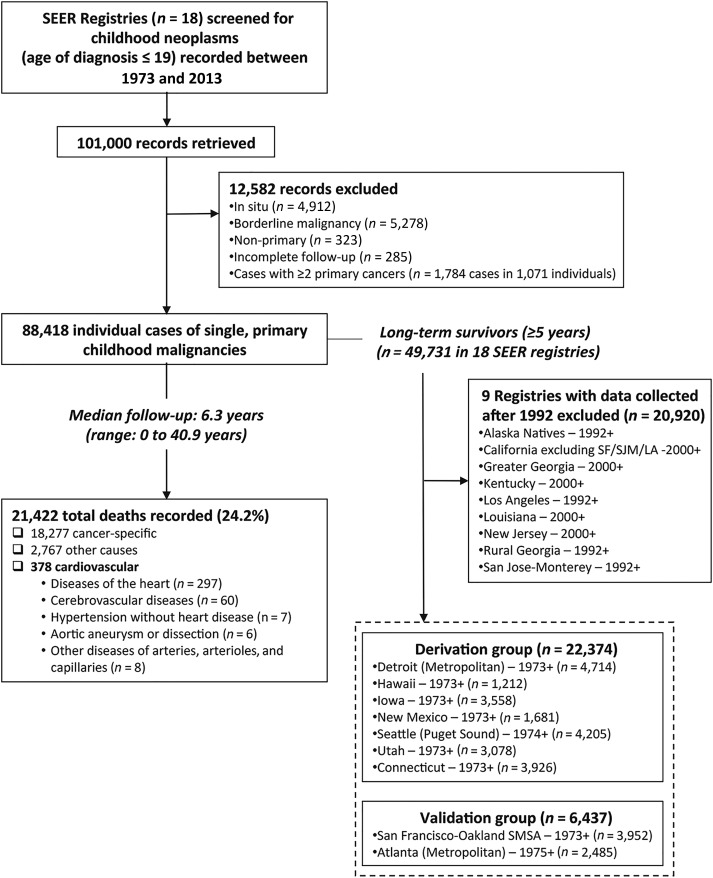
Figure 1.
Study flowchart. The SEER Program database of the National Cancer Institute was searched to identify cases of primary, malignant neoplasms diagnosed during childhood or adolescence. A total of 88,418 patients with a single, primary malignant tumor and recorded follow‐up were identified. Over a median follow‐up of 6.3 (0–40.9) years, 21,422 deaths were recorded, 378 of which were due to cardiovascular causes. Follow‐up at least 5 years after diagnosis was available in 49,731 long‐term survivors across 18 SEER registries. Nine registries with prospective data collection since at least 1975 were selected, reporting on a total of 28,811 long‐term survivors. These were split in a derivation (n = 22,374 in seven registries) and an external validation set (n = 6,437 in two independent registries) in order to develop and test a clinical prediction model of cardiovascular mortality risk among long‐term childhood cancer survivors.
Abbreviations: LA, Los Angeles; SEER, Surveillance, Epidemiology, and End Results; SF, San Francisco; SJM, San Jose Monterrey; SMSA, Standard Metropolitan Statistical Area.
Table 1. Basic demographics of childhood cancer patients with single, primary malignant tumor identified in the SEER database (1973–2013).
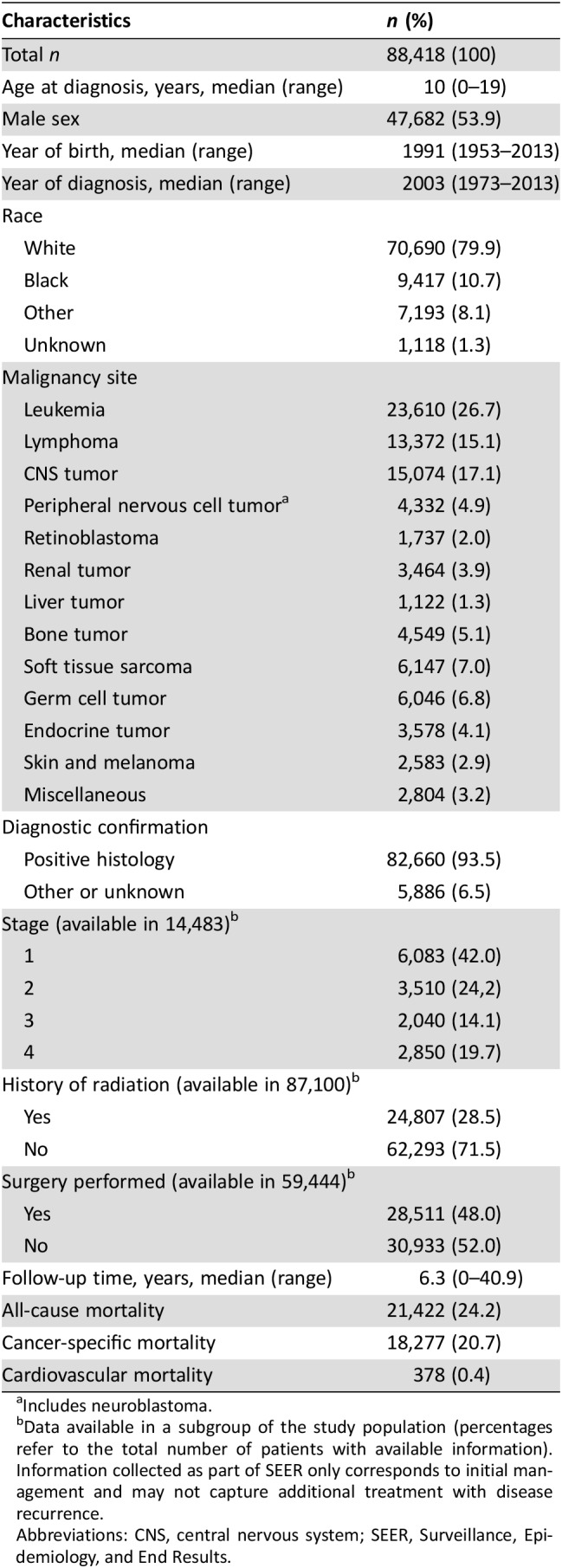
Includes neuroblastoma.
Data available in a subgroup of the study population (percentages refer to the total number of patients with available information). Information collected as part of SEER only corresponds to initial management and may not capture additional treatment with disease recurrence.
Abbreviations: CNS, central nervous system; SEER, Surveillance, Epidemiology, and End Results.
Cancer‐specific and CVM incidence rates varied significantly between different malignancy types (supplemental online Table 3). Overall cancer‐specific mortality rates dropped from an average of 50.3 (95% CI: 49.6–51.1) events per 1,000 person‐years in the first 5 years after diagnosis to just 4.1 (95% CI: 4.0–4.3) events per 1,000 person‐years after the 5‐year cutoff. On the other hand, average CVM incidence rates remained stable at relatively low levels, averaging at 0.44 (95% CI: 0.38–0.52) in the first 5 years and at 0.46 (95% CI: 0.41–0.53) events per 1,000 person‐years beyond the first 5 years of follow‐up.
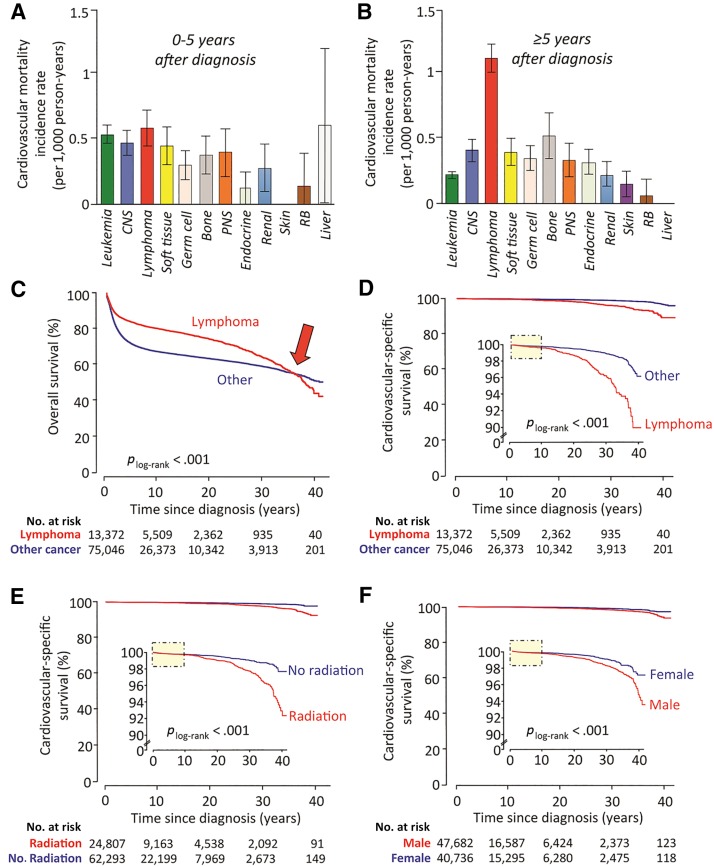
Nevertheless, analysis by type of malignancy revealed a significant rise in CVM incidence among individuals with a diagnosis of childhood lymphoma (compared with any other childhood malignancy) beyond the first 5 years after diagnosis (Fig. 2A, 2B). Indeed, when compared with other childhood malignancies, patients with a history of lymphoma had significantly better overall survival rates (Fig. 2C, plog‐rank <.001) but a significantly higher risk of late death due to cardiovascular causes (Fig. 2D, plog‐rank <.001). In addition, males as well as individuals who received radiation as part of their treatment regimen were also found to be at increased risk of CVM, compared with their female counterparts or those who did not undergo radiotherapy (Fig. 2E, 2F, plog‐rank <.001 for both). In all three cases, cardiovascular‐specific survival curves started splitting at least 10 years after diagnosis (Fig. 2D, 2E, 2F, yellow boxes).
Figure 2.
Temporal trends in cardiovascular mortality incidence across different malignancy types and patient subgroups. Cardiovascular mortality (CVM) incidence rates vary between different malignancy types as well as between early and long‐term survivors (A, B). Lymphoma patients in particular appear to carry the highest risk of CVM beyond the first 5 years of diagnosis, with an estimated annual incidence rate of 1.16 (95% confidence interval: 0.96–1.41) events per 1,000 long‐term survivors (B). Indeed, compared with a pooled group consisting of all other childhood malignancies, lymphoma patients have significantly better overall survival rates (C); however, this appears to be reversed at later stages (∼30–35 years after diagnosis, red arrow), possibly due to increased mortality rate attributed to cardiovascular causes (D). Of note, the cardiovascular‐specific survival Kaplan‐Meier curves do not split until 10 years after diagnosis (D, yellow shaded box), possibly suggesting delayed effects associated with cancer treatment. Indeed, survival analysis according to history of radiation use (E) or male gender (F) reveals similar trends of delayed, yet increased, risk of cardiovascular mortality among individuals with a history of childhood cancer. Taken together, these findings suggest that exposure and demographic variables are linked to a delayed but significant increase in the risk of CVM among long‐term childhood cancer survivors. Bar graphs represent average incidence rates, and error bars depict standard errors.
Abbreviations: CNS, central nervous system; PNS, peripheral nervous system (includes neuroblastoma); RB, retinoblastoma.
Predicting Adverse Cardiovascular Outcomes Among Childhood Cancer Survivors
Nine registries with prospective data collection since at least 1975 were included in the development and validation of a prognostic model for prediction of CVM among childhood cancer survivors. These were split in a derivation set (seven registries; 35,991 cases with 22,374 long‐term survivors) and an independent validation set (two registries; 19,937 cases with 6,437 long‐term survivors; Fig. 1; baseline demographics are summarized in supplemental online Table 4).
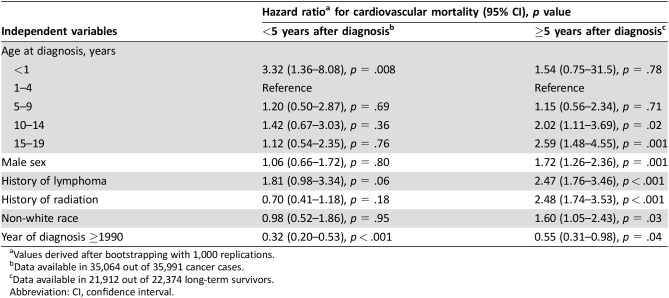
In the derivation set, male sex, older age at diagnosis, non‐white race, a history of lymphoma versus other childhood malignancies, a history of radiation, and later year of diagnosis (<1990 vs. ≥1990) were all independently linked to a higher risk of CVM among childhood cancer survivors who were alive 5 years after their diagnosis (Table 2). On the contrary, analysis of the first 5 years after diagnosis revealed that only year of diagnosis and younger (<1 year) rather than older age were associated with CVM (Table 2), whereas no significant association was found with either sex, type of malignancy or a history of radiation.
Table 2. Multivariable Cox regression model for prediction of cardiovascular mortality in long‐term survivors of childhood cancer (derivation cohort).
Values derived after bootstrapping with 1,000 replications.
Data available in 35,064 out of 35,991 cancer cases.
Data available in 21,912 out of 22,374 long‐term survivors.
Abbreviation: CI, confidence interval.
The CHACS‐CV Score
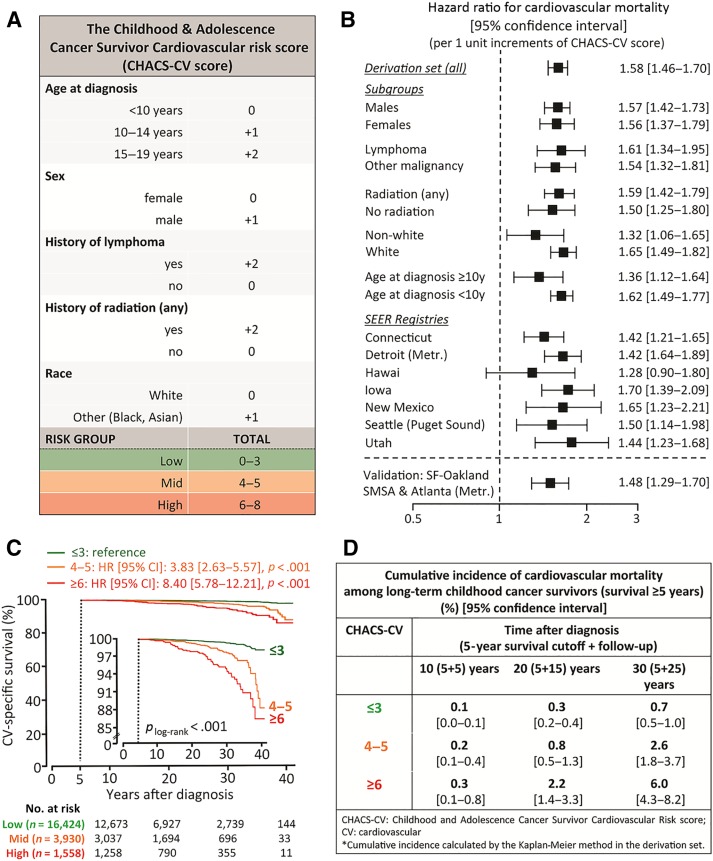
Based on the results of the multivariable Cox regression model for prediction of CVM among long‐term survivors, individual points were assigned to each one of the following predictors in order to create a simplified risk score. Briefly, the beta coefficients (ln[HR]) for all significant clinical predictors in the model were divided by the smallest of these coefficients (race: βrace = ln[HR] = 0.47) and then rounded to the nearest integer. As a result, two points were given if there was a history of radiation or lymphoma or if age at diagnosis was 15–19 years, and one point was given for male gender, non‐white race, and if age at diagnosis was 10–14 years (Fig. 3A). The sum of all points was defined as the CHACS‐CV risk score (range 0–8) and showed good discrimination for prediction of CVM both in the derivation (C‐statistic: 0.75 [95% CI: 0.71–0.79], p < .001 and Harrell's C‐index: 0.73 [95% CI: 0.68–0.78], p < .001) and the validation group (C‐statistic: 0.72 [95% CI: 0.65–0.80], p < .001 and Harrell's concordance index: 0.66 [95% CI: 0.57–0.74], p < .001). Of note, CHACS‐CV retained its predictive value in subgroup analyses per sex, age group, history of radiation, type of malignancy, and race, as well as in the separate, geographical SEER registries (Fig. 3B).
Figure 3.
The CHACS‐CV risk score. A clinical risk score of cardiovascular mortality was created by assigning one or two points to clinical variables that were independently associated with cardiovascular mortality (A). The sum of all points was then defined as the CHACS‐CV risk score (range 0–8). CHACS‐CV was predictive of cardiovascular mortality both in the overall derivation group and in the subgroup analysis per age, sex, race, history of radiation, and malignancy type (B). Long‐term survivors were then stratified in three distinct risk groups (high risk if CHACS‐CV ≥ 6; mid risk if CHACS‐CV = 4–5, and low risk if CHACS‐CV ≤ 3; C). Compared with the low‐risk group, those in the high‐risk and mid‐risk groups had an eightfold and an almost fourfold higher risk of cardiovascular mortality, respectively. Twenty‐five‐year cumulative incidence of CVM (calculated after the 5‐year survival cutoff) was found to be 6.0% in the high‐, 2.6% in the mid‐, and 0.7% in the low‐risk CHACS‐CV groups, respectively (plog‐rank < .001; D).
Abbreviations: CHACS‐CV, Childhood and Adolescence Cancer Survivor CardioVascular; CI, confidence interval; HR, hazard ratio; Metr, metropolitan; SEER, Surveillance, Epidemiology, and End Results; SF, San Francisco; SMSA, Standard Metropolitan Statistical Area; y, years.
CHACS‐CV was then used to stratify the derivation set in three distinct risk groups (high risk if CHACS‐CV ≥ 6; mid risk if CHACS‐CV = 4–5, and low risk if CHACS‐CV ≤ 3; Fig. 3A). Compared with the low‐risk group, those in the high‐risk and mid‐risk groups had an eightfold and a more than threefold higher risk of CVM, respectively (Fig. 3C). At 25 years after the 5‐year survival cutoff, cumulative incidence of CVM in the high‐, mid‐, and low‐risk groups was 6.0%, 2.6%, and 0.7%, respectively (plog‐rank <.001, Fig. 3C, 3D). These results were externally validated in an independent set of SEER registries (4.7%, 3.1%, and 0.8% in the high‐, mid‐, and low‐risk groups, respectively, plog‐rank <.001, supplemental online Fig. 1).
Discussion
In the present study, we have developed and validated a simple clinical risk prediction tool that can identify long‐term childhood cancer survivors at increased risk of CVM using easily accessible clinical information. Using prospectively collected data from a robust database, we were able to identify demographic and clinical factors that are independently associated with an increased risk of CVM. Our proposed risk score relies on simple risk factors that can be easily obtained during any routine patient encounter, identifying the need for a risk score that would be easily applicable to everyday clinical practice. We propose the use of this simple clinical tool in cardiovascular risk stratification of childhood cancer survivors at the time of their 5‐year follow‐up in order to identify individuals at high risk of early CVM and therefore in need of early and more intensive preventive and therapeutic management.
It is now established that several types of cancer treatment have well‐described adverse effects on the cardiovascular system, leading to a significant increase in cardiovascular morbidity [15]. For instance, mediastinal radiation has been linked to accelerated atherosclerosis and structural heart disease [26], [27], [28]. Adverse cardiovascular effects have also been described for several chemotherapeutic agents widely used in the management of childhood malignancies [29]. Anthracyclines (widely used in the treatment of lymphoma and sarcoma) increase the risk of congestive heart failure due to left ventricular systolic and, less commonly, diastolic dysfunction in a dose‐dependent manner [29]. Other drugs, such as alkylating agents, have also been associated with heart failure as well as arrhythmias, endomyocardial fibrosis, and myo/peri‐carditis [29], [30], [31]. As a result, it is now established that childhood cancer survivors are at increased risk of developing multiple cardiovascular risk factors in addition to coronary artery disease, heart failure, pericardial disease, conduction disturbances, and venous/arterial thrombosis, leading to early cardiovascular morbidity and mortality [16], [17].
Despite increasing awareness of the cardiotoxicity associated with several cancer therapies, no validated risk score exists to identify long‐term childhood cancer survivors at increased risk of CVM. Chow et al. recently described and validated the first‐of‐its‐kind risk calculator that predicts the risk of heart failure among long‐term survivors of childhood cancer using data from the Childhood Cancer Survivor Study (n = 13,060) with excellent discrimination and adequate external validation [17]. Nevertheless, the model predicts the sole clinical outcome of congestive heart failure and requires details on radiation and chemotherapy exposure, which are often not easily accessible.
In our study, we focused on cardiovascular mortality and used data from SEER, a large, publicly available database pooling population‐based cancer registries. We decided not to include complex variables that only apply to certain cancer types (e.g., histology type), as SEER database does not provide information on the specific chemotherapy or radiation regimens used. Nevertheless, reliable medical documentation about radiation dose and chemotherapy dose is quite often not available in everyday practice to guide decision‐making [21].
Therefore, in our final model, we only included five simple demographic and clinical factors based on clinical and statistical reasoning, which can be easily obtained during a patient encounter. Because CVM remains a relatively infrequent event in young populations, an easy‐to‐use, cost‐effective clinical tool is necessary to guide risk stratification of childhood cancer survivors. We found that male sex, non‐white race, a history of lymphoma versus other childhood malignancy, and a history of radiation therapy were all independent predictors of CVM. Male sex has been linked to an earlier development of atherosclerotic vascular disease in the general population [32], and non‐white race is associated with worse cancer‐specific survival rates as well as a higher risk of CVM among young people [33], [34], [35]. Older age might also contribute to increased cardiovascular risk due to the increasing presence of other cardiovascular risk factors as well as the effects of ageing per se on the cardiovascular system [36]. Moreover, a history of lymphoma is frequently linked to a history of cardiotoxic chemotherapeutic agents (e.g., anthracyclines) as well as mediastinal radiation [37], [38]. In fact, radiotherapy was also found to be an independent predictor of CVM most likely due to the well‐described adverse effects of mediastinal or chest radiotherapy on cardiac function as well as those of brain radiation on the development of obesity and metabolic syndrome‐associated cardiovascular disease [16], [26], [28]. More importantly, these risk factors were all shown to be independent predictors of CVM, allowing for the development of an integrated risk score.
By stratifying the study population based on the proposed CHACS‐CV score, we were able to identify distinct cardiovascular risk groups among long‐term survivors. For example, the annual incidence rate of CVM among long‐term survivors in the derivation set (calculated over 25 years of follow‐up after the 5‐year survival cutoff) was estimated at 174 per 100,000 (95% CI: 128–237) in the high‐risk compared with only 20 per 100,000 (95% CI: 14–26) in the low‐risk CHACS‐CV group. In comparison, the crude, annual rate of cardiovascular death (defined as death due to diseases of the circulatory system [I00–I99]) in the general U.S. population for individuals aged 20–45 years is approximately 19 events per 100,000 [11].
Our study is not without limitations. All cases included in the analysis were recorded in the U.S., raising some concerns about the external validity of this scoring system in the rest of the world. Therefore, further validation in geographically diverse cohorts is required. However, the proposed model performed well when applied separately to the included geographical SEER registries, despite the differences in the demographic composition of each group. In addition, information on the type or dosages of chemotherapeutic agents or radiation used was not included, because this information was not available as part of the SEER program.
Nevertheless, this scoring system was developed and is proposed to be used as a simple, cost‐effective tool that can flag childhood cancer survivors at increased cardiovascular risk at the time of their 5‐year follow‐up, based on easily obtainable clinical information. Based on our findings, patients in the highest CHACS‐CV group should be considered for rigorous cardiovascular screening as well as primary and secondary prevention. Although all patients should receive recommendations about risk factor modification, and nonpharmacologic lifestyle interventions especially in the early adult life, patients in the high, and possibly mid, CHACS‐CV groups could be candidates for earlier initiation of pharmacologic intervention with aspirin and/or statins. However, individualized decision‐making remains prudent. CHACS‐CV should be viewed as a screening tool, and decisions on diagnostic procedures and/or treatment should be individualized. In this regard, health care providers should always use their clinical reasoning when managing a childhood cancer survivor and should only use this score as an early screening tool to help them identify individuals who might qualify for more complex diagnostic or preventive management. A detailed history of the chemotherapeutic agents and radiation used, including dosages, should always be taken into account, whenever available, to guide more appropriate and personalized management strategies.
Conclusion
We propose a simple clinical tool for cardiovascular risk stratification of long‐term childhood cancer survivors based on easily obtainable information from their medical history. This clinical score (namely the CHACS‐CV score) was developed and validated using a large dataset from the SEER Program of the National Cancer Institute. It aims to assist health care providers caring for long‐term childhood cancer survivors in identifying those at increased cardiovascular risk and therefore in need of closer follow‐up and deployment of appropriate risk‐reduction strategies.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This work was supported by a Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748) to R.M.S. and D.G.
Author Contributions
Conception/design: Evangelos K. Oikonomou, Sofia G. Athanasopoulou, Dipti Gupta
Data analysis and interpretation: Evangelos K. Oikonomou, Damianos G. Kokkinidis
Manuscript writing: Evangelos K. Oikonomou, Sofia G. Athanasopoulou, Polydoros N. Kampaktsis, Christos Papanastasiou, Attila Feher, Richard M. Steingart, Kevin C. Oeffinger, Dipti Gupta
Final approval of manuscript: Evangelos K. Oikonomou, Sofia G. Athanasopoulou, Polydoros N. Kampaktsis, Damianos G. Kokkinidis, Christos Papanastasiou, Attila Feher, Richard M. Steingart, Kevin C. Oeffinger, Dipti Gupta
Disclosures
The authors indicated no financial relationships.
References
- 1. Ward E, DeSantis C, Robbins A et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- 2. Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev 2010;36:277–285. [DOI] [PubMed] [Google Scholar]
- 3. Armstrong GT, Chen Y, Yasui Y et al. Reduction in late mortality among five‐year survivors of childhood cancer. N Engl J Med 2016;374:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ng AK, Bernardo MP, Weller E et al. Long‐term survival and competing causes of death in patients with early‐stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol 2002;20:2101–2108. [DOI] [PubMed] [Google Scholar]
- 5. Castellino SM, Geiger AM, Mertens AC et al. Morbidity and mortality in long‐term survivors of Hodgkin lymphoma: A report from the childhood cancer survivor study. Blood 2011;117:1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong GT, Oeffinger KC, Chen Y et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013;31:3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carver JR, Shapiro CL, Ng A et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: Cardiac and pulmonary late effects. J Clin Oncol 2007;25:3991–4008. [DOI] [PubMed] [Google Scholar]
- 8. Phillips SM, Padgett LS, Leisenring WM et al. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev 2015;24:653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oeffinger KC, Mertens AC, Sklar CA et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355:1572–1582. [DOI] [PubMed] [Google Scholar]
- 10. Swerdlow AJ, Higgins CD, Smith P et al. Myocardial infarction mortality risk after treatment for hodgkin disease: A collaborative British cohort study. J Natl Cancer Inst 2007;99:206–214. [DOI] [PubMed] [Google Scholar]
- 11. Aleman BM, van den Belt‐Dusebout AW, Klokman WJ et al. Long‐term cause‐specific mortality of patients treated for Hodgkin's disease. J Clin Oncol 2003;21:3431–3439. [DOI] [PubMed] [Google Scholar]
- 12. Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments. Nat Rev Cardiol 2015;12:547–558. [DOI] [PubMed] [Google Scholar]
- 13. Lipshultz SE, Adams MJ, Colan SD et al. Long‐term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 2013;128:1927–1995. [DOI] [PubMed] [Google Scholar]
- 14. Armenian SH, Hudson MM, Mulder RL et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the international late effects of childhood cancer guideline harmonization group. Lancet Oncol 2015;16:e123–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albini A, Pennesi G, Donatelli F et al. Cardiotoxicity of anticancer drugs: The need for cardio‐oncology and cardio‐oncological prevention. J Natl Cancer Inst 2010;102:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meacham LR, Chow EJ, Ness KK et al. Cardiovascular risk factors in adult survivors of pediatric cancer–A report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev 2010;19:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow EJ, Chen Y, Kremer LC et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol 2015;33:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lenneman CG, Sawyer DB. Cardio‐oncology: An update on cardiotoxicity of cancer‐related treatment. Circ Res 2016;118:1008–1020. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network (NCCN): Clinical practice guidelines in oncology. Available at www.nccn.org. Accessed May 2, 2017. [DOI] [PMC free article] [PubMed]
- 20.Children's Oncology Group. Long‐term follow‐up guidelines for survivors of childhood, adolescent, and young adult cancers. Version 4.0. Children's Oncology Group, Arcadia, CA, USA (2013). Available at www.survivorshipguidelines.org. Accessed May 2, 2017.
- 21. Nathan PC, Greenberg ML, Ness KK et al. Medical care in long‐term survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol 2008;26:4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steliarova‐Foucher E, Stiller C, Lacour B et al. International Classification of Childhood Cancer, third edition. Cancer 2005;103:1457–1467. [DOI] [PubMed] [Google Scholar]
- 23. Newson RB. Comparing the predictive powers of survival models using Harrell's C or Somers' D. Stata J 2010;10:339–358. [Google Scholar]
- 24. Royston P, Altman DG. External validation of a Cox prognostic model: Principles and methods. BMC Med Res Methodol 2013;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Elm E, Altman DG, Egger M et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 26. Baker JE, Moulder JE, Hopewell JW. Radiation as a risk factor for cardiovascular disease. Antioxid Redox Signal 2011;15:1945–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sardar P, Chatterjee S, Nohria A et al. Long term cardiovascular mortality after radiotherapy for breast cancer: A meta‐analysis. Circulation; 2014;130:A11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta D, Pun SC, Verma S et al. Radiation‐induced coronary artery disease: A second survivorship challenge? Future Oncol 2015;11:2017–2020. [DOI] [PubMed] [Google Scholar]
- 29. Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev 2011;37:300–311. [DOI] [PubMed] [Google Scholar]
- 30. Mulrooney DA, Armstrong GT, Huang SJ et al. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy a cross‐sectional study. Ann Intern Med 2016;164:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bovelli D, Plataniotis G, Roila F et al. Cardiotoxicity of chemotherapeutic agents and radiotherapy‐related heart disease: ESMO clinical practice guidelines. Ann Oncol 2010;21(suppl 5):v277–v282. [DOI] [PubMed] [Google Scholar]
- 32. Maas AH, Appelman YE. Gender differences in coronary heart disease. Neth Heart J 2010;18:598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leigh JA, Alvarez M, Rodriguez CJ. Ethnic minorities and coronary heart disease: An update and future directions. Curr Atheroscler Rep 2016;18:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ward E, Jemal A, Cokkinides V et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004;54:78–93. [DOI] [PubMed] [Google Scholar]
- 35. Jolly S, Vittinghoff E, Chattopadhyay A et al. Higher cardiovascular disease prevalence and mortality among younger blacks compared to whites. Am J Med 2010;123:811–818. [DOI] [PubMed] [Google Scholar]
- 36. Pletcher MJ, Vittinghoff E, Thanataveerat A et al. Young adult exposure to cardiovascular risk factors and risk of events later in life: The Framingham offspring study. PLoS One 2016;11:e0154288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aleman BM, van den Belt‐Dusebout AW, De Bruin ML et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007;109:1878–1886. [DOI] [PubMed] [Google Scholar]
- 38. Limat S, Demesmay K, Voillat L et al. Early cardiotoxicity of the CHOP regimen in aggressive non‐Hodgkin's lymphoma. Ann Oncol 2003;14:277–281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.