Significance
The neocortex is a hallmark of mammalian evolution, and connections between both hemispheres integrate bilateral functions. In eutherians (e.g., rodents and humans), interhemispheric circuits course via the corpus callosum and share a similar connectome throughout species. Noneutherian mammals (i.e., monotremes and marsupials), however, did not evolve a corpus callosum; therefore, whether the eutherian connectome arose as consequence of callosal evolution or instead reflects ancient connectivity principles remains unknown. We studied monotreme and marsupial interhemispheric neocortical connectomes and compared these with eutherian datasets. This revealed interhemispheric connectivity features shared across mammals, with or without a corpus callosum, suggesting that an ancient connectome originated at least 80 million years before callosal evolution.
Keywords: diffusion tensor MRI, claustrum, cortical connectome, anterior commissure, corpus callosum
Abstract
The brain of mammals differs from that of all other vertebrates, in having a six-layered neocortex that is extensively interconnected within and between hemispheres. Interhemispheric connections are conveyed through the anterior commissure in egg-laying monotremes and marsupials, whereas eutherians evolved a separate commissural tract, the corpus callosum. Although the pattern of interhemispheric connectivity via the corpus callosum is broadly shared across eutherian species, it is not known whether this pattern arose as a consequence of callosal evolution or instead corresponds to a more ancient feature of mammalian brain organization. Here we show that, despite cortical axons using an ancestral commissural route, monotremes and marsupials share features of interhemispheric connectivity with eutherians that likely predate the origin of the corpus callosum. Based on ex vivo magnetic resonance imaging and tractography, we found that connections through the anterior commissure in both fat-tailed dunnarts (Marsupialia) and duck-billed platypus (Monotremata) are spatially segregated according to cortical area topography. Moreover, cell-resolution retrograde and anterograde interhemispheric circuit mapping in dunnarts revealed several features shared with callosal circuits of eutherians. These include the layered organization of commissural neurons and terminals, a broad map of connections between similar (homotopic) regions of each hemisphere, and regions connected to different areas (heterotopic), including hyperconnected hubs along the medial and lateral borders of the cortex, such as the cingulate/motor cortex and claustrum/insula. We therefore propose that an interhemispheric connectome originated in early mammalian ancestors, predating the evolution of the corpus callosum. Because these features have been conserved throughout mammalian evolution, they likely represent key aspects of neocortical organization.
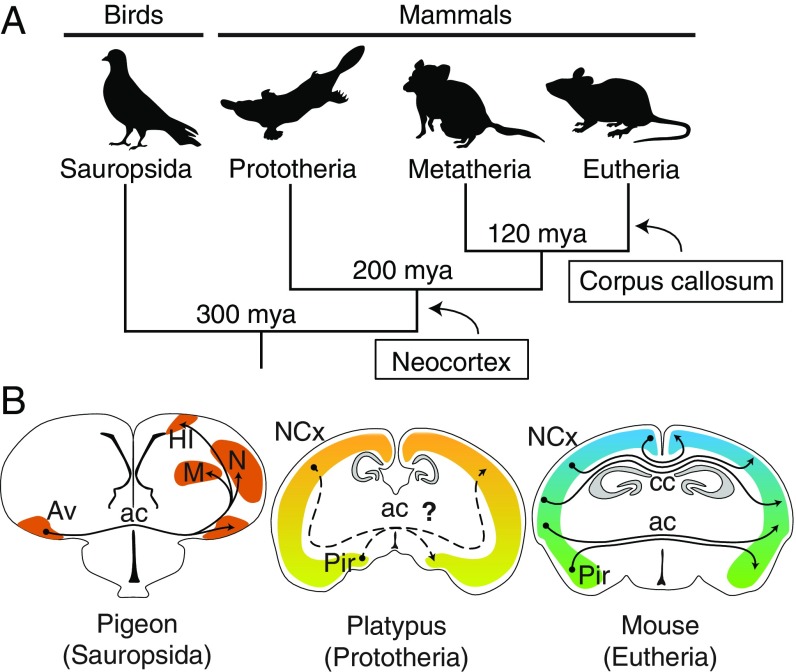
The vertebrate nervous system is organized into functional modules of spatially arranged neurons and fibers (1), where topographic maps shared between peripheral and central circuits mediate sensory-motor behaviors (2). Although such maps are abundant in the spinal cord, hindbrain, and midbrain, they are less evident in the telencephalic pallium of nonmammalian vertebrates, such as birds (3, 4). Mammals, however, evolved a highly topographic six-layered neocortex that recapitulates the peripheral sensory maps via point-to-point connections with subcortical regions (5–7). Moreover, the left and right cortical hemispheres of mammals are heavily interconnected compared with fewer such connections in birds, in which sensory-motor and associative regions of the telencephalic pallium receive limited input from the contralateral hemisphere (8, 9) (Fig. 1). A second key evolutionary innovation was the origin of the corpus callosum exclusively in eutherian (placental) mammals (10–12). Such an event likely involved rerouting neocortical axons medially to the dorsal region of the embryonic commissural plate, coupled with a process of midline tissue remodeling by embryonic astroglia (13), which is exclusively present in eutherians (14). The evolution of the corpus callosum as a distinct tract allowed a significant expansion of the number of interhemispheric neocortical connections in species with large brains (15). The corpus callosum carries fibers topographically arranged according to the position of their cell bodies (16–18) and connects mostly similar (homotopic) but also different (heterotopic) regions in each hemisphere (Fig. 1B). However, although the map of callosal fibers in eutherians is well-established, and includes connectivity features that are highly conserved across species, such as the presence of bilateral hubs (19–25), it remains unclear whether such features depend on the route taken by commissural axons or instead reflect more ancient organizational principles of neocortical connectivity.
Fig. 1.
Evolution of cortical commissures. (A) Cladogram of birds and mammals showing the origin of the neocortex (NCx) and the corpus callosum (cc). (B) Coronal brain schematics. The anterior commissure (ac) of pigeons contains mostly unidirectional projections from the ventral arcopallium (Av) to contralateral homotopic and heterotopic targets, including the mesopallium (M), nidopallium (N), and hyperpallium intercalatum (HI). In eutherians, callosal axons are topographically segregated and connect mostly homotopic regions, whereas the ac carries axons from the piriform cortex (Pir) and lateral NCx. Homologous circuits in noneutherians are carried exclusively through the ac.
To address these questions, here we studied the main connectivity features of interhemispheric cortical circuits in noneutherian mammals and compared these with known eutherian connectomes. We found that the spatial segregation of interhemispheric axons across the anterior commissure in marsupials and monotremes resembles the arrangement of fibers across the callosal tract in eutherians, including the presence of point-to-point homotopic circuits. Furthermore, single cell-level circuit mapping via in vivo retrograde and anterograde tracer injections in marsupials revealed a highly conserved layer distribution of contralaterally projecting neurons, as well as homotopic, heterotopic, and hyperconnected circuits through the anterior commissure that strongly suggest an ancient precallosal origin.
Results
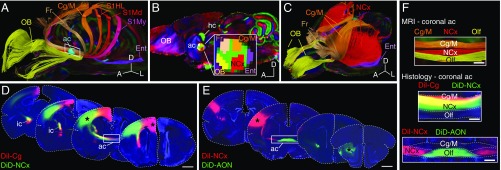
We first performed magnetic resonance imaging (MRI) and tractography on fixed brains of fat-tailed dunnarts (Sminthopsis crassicaudata: Marsupialia, Dasyuridae) by placing spherical regions of interest (ROIs) at several cortical regions and an inclusion ROI at the anterior commissure (Fig. 2A). This revealed that interhemispheric cortical fibers are spatially segregated within the anterior commissure according to the 3D arrangement of cortical areas, similar to the topographic segregation of callosal axons in humans and rodents (16–18) (Fig. 2A, SI Appendix, Fig. S1, and Movie S1). We then examined whether the relative position of fibers within the anterior commissure midline was sufficient to recapitulate cortical area topography by manually drawing 2D ROIs at the midsagittal plane (Fig. 2B). Color-coded tractographic reconstructions of fibers crossing through each territory of the midsagittal anterior commissure revealed a highly topographic interhemispheric map of connections between at least five well-defined homotopic bilateral domains (i.e., olfactory, frontal, cingulate/motor, neocortical, and entorhinal cortices; Fig. 2 B and C and SI Appendix, Fig. S2). To validate these MRI reconstructions with histology, we then performed double injections of carbocyanine fluorescent tracers (DiI and DiD) into separate regions of the same hemisphere of fixed adult dunnart brains [neocortex and cingulate/motor cortex (Fig. 2D) and neocortex and anterior olfactory nucleus (Fig. 2E)]. This resulted in segregated fibers along the anterior commissure, similar to previous reports in wallabies (26). Notably, experiments of neuronal tracer injections closely recapitulated the segregation of fibers found using MRI tractography and seeding ROIs in equivalent areas (Fig. 2F). Similarly, an equivalent cortical-area segregation was observed in axons within the internal capsule, which carries cortical-subcortical connections, using both histology (Fig. 2D) and MRI (SI Appendix, Fig. S1D), suggesting that similar developmental mechanisms of axon guidance may direct the areal topography of intra- and extracortical connections (12, 17).
Fig. 2.
Segregation of commissural fibers by cortical areas in marsupials. (A) HARDI tractography of the dunnart brain (marsupial) using a spherical ROI at the anterior commissure (ac, cyan) and inclusion ROIs at seven cortical regions (spheres), reveal segregated tracts into color-coded homotopic domains. (B) Midsagittal dunnart MRI scan showing five hand-drawn ROIs within the anterior commissure (Inset) that generate the color-coded tracts in C. (C) Tracts generated by ROIs in B reveal contralateral homotopy of the main cortical areas. (D and E) Coronal sections of dunnart brains after ex vivo injections of DiI (red) and DiD (green) in the regions indicated by asterisks. (F) Comparable coronal views of fiber segregation within the ac, as revealed by MRI and histology (from top down, high-magnification views of the areas shown in A, D, and E, respectively). AON, anterior olfactory nucleus; Cg/M, cingulate/motor cortex; Ent, entorhinal cortex; Fr, frontal cortex; ic, internal capsule; NCx, neocortex; OB, olfactory bulb; Olf, olfactory branch; Pir, piriform cortex; S1, primary somatosensory cortex; S1HL, S1 hindlimb; S1Md, S1 mandible; S1My, S1 mystacial whiskers. (Scale bars, 1 mm in D and E and 200 μm in F.)
Pan-Mammalian Commissural Axon Segregation.
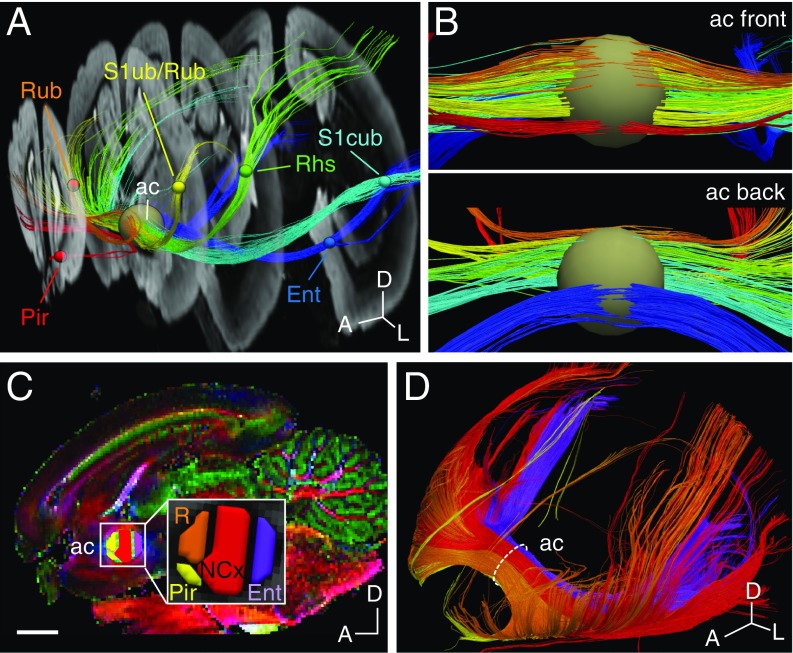
To elucidate whether the topographic segregation of interhemispheric axons based on cortical area position is a feature exclusive to the Therian clade (Metatheria + Eutheria), or instead they can be traced back to more evolutionarily distant mammals, we then performed brain tractography in an egg-laying monotreme mammal (Prototheria), the duck-billed platypus (Ornithorhynchus anatinus: Monotremata, Ornithorhynchidae). We examined MRI tractography of fixed platypus brains and found that well-defined, 3D-adjacent, and homotopic commissural tracts were evident by placing small adjacent regions of interest within the midsagittal anterior commissure, along anteroposterior, dorsoventral, and an oblique axis (anterodorsal to posteroventral; SI Appendix, Fig. S3 and Movie S2). Similar to in dunnarts and eutherians, placing ROIs in well-defined cortical areas of the platypus brain (5) resulted in fibers that crossed the midline in spatially segregated topographic domains (Fig. 3 A and B). Moreover, this arrangement could be reconstructed by manual parcellation of the midsagittal anterior commissure, which resulted in at least five broadly homotopic interhemispheric subsystems that include the olfactory/piriform, rostral cortical, neocortical, and entorhinal cortices (Fig. 3 C and D and Movie S3). The similar patterns of commissural fiber topography within the white matter tract, with shared spatial arrangement relative to each other and recapitulating the position of cortical areas, in species from all extant mammalian subclasses strongly suggest the ancient origin of neocortical axon guidance principles in the common ancestors of all modern mammals.
Fig. 3.
Topography of the anterior commissure of the platypus. (A) T1-weighted coronal series of a fixed platypus brain showing color-coded tracts between cortical ROIs (small spheres) and the anterior commissure (ac, large sphere). (B) Higher-magnification coronal views of the tracts generated in A as viewed from the front (Top) and the back (Bottom). (C) Midsagittal view of the platypus brain and the anterior commissure (Inset) showing the parcellation of ROIs that generate the tracts in D. (D) Interhemispheric tracts across ROIs of C, showing color-coded homotopic domains. A, anterior; D, dorsal; Ent, entorhinal cortex; L, lateral; Pir, piriform cortex; R, rostral somatosensory cortex; Rhs, R head-shield; Rub, R upper-bill; S1cub, primary somatosensory cortex central upper-bill; S1ub, primary somatosensory cortex upper-bill. (Scale bar, 4 mm.)
Single-Cell-Level Circuit Mapping in Dunnarts.
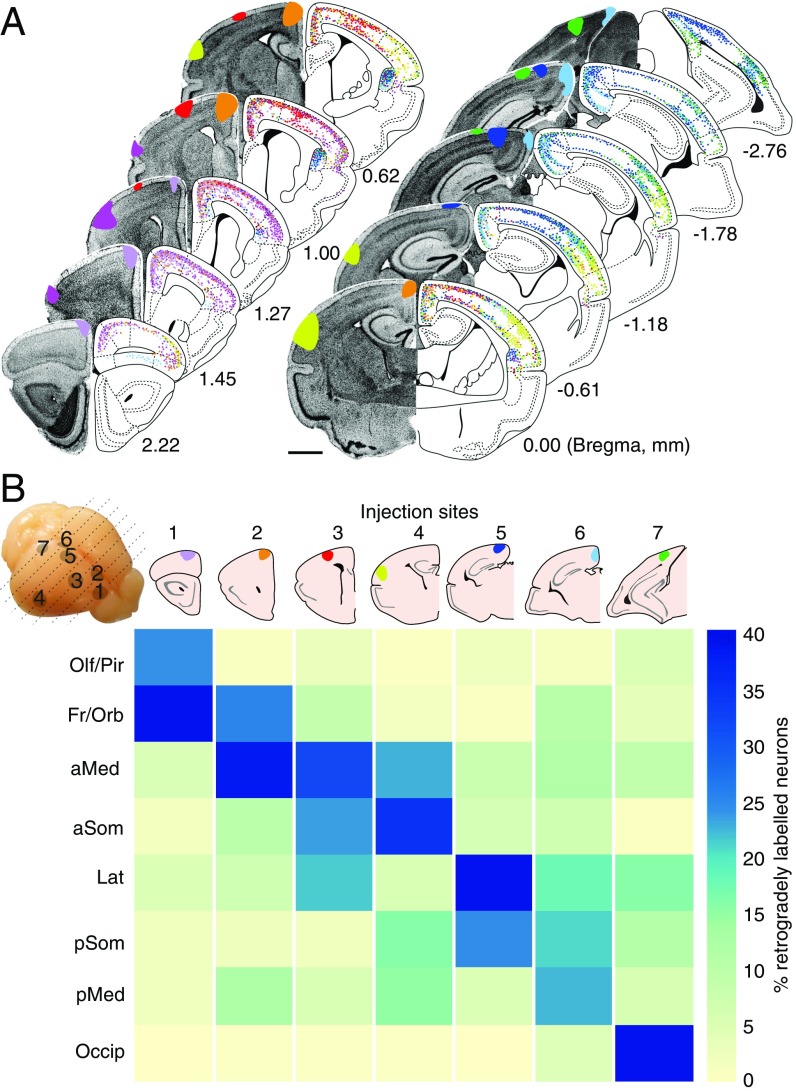
Although these results demonstrate conserved features that suggest a pan-mammalian topography of interhemispheric cortical connections at the macro- and mesoscale levels, features of microanatomy connectivity, such as neuronal projection diversity within areas, as well as branching, fasciculation, or crossing of axons, cannot be readily revealed via magnetic resonance imaging. To investigate whether and which features of the callosal connectome at the cellular level are present in marsupials, we performed retrograde interhemispheric circuit mapping in dunnarts. Stereotaxic injections of retrograde fluorescent tracers (carbocyanines and/or cholera toxin b subunit) into distinct cortical regions in 13 dunnarts in vivo revealed that, similar to callosal neurons in eutherians, contralaterally projecting neurons are located in regions that are broadly homotopic to the injection sites (Fig. 4 and SI Appendix, Table S1). Moreover, the distribution of commissural projecting neurons, particularly within primary sensory areas, differed across cortical layers (P < 0.0001, one-way ANOVA), whereby they are located primarily in layers 2/3 (65.3 ± 2.2%), followed by layer 5 (24.3 ± 1.6%), and to a lesser extent in layer 4 (7.6 ± 1.4%; SI Appendix, Fig. S4). These ratios closely resemble that of eutherian callosal neurons in primary sensory cortices (27–29). However, in both eutherian and noneutherian mammals, the layer distribution of commissural neurons is largely variable across cortical areas (Figs. 4 and 5). Notably, we found that the medial and caudal portions of the cerebral cortex of dunnarts (i.e., motor and cingulate/retrosplenial cortices) receive contralateral inputs not only from homotopic areas but also from several heterotopic regions of the contralateral hemisphere (e.g., site 6; Fig. 4B). Such heterotopic inputs included lateral regions of the cortex at similar rostrocaudal levels (SI Appendix, Fig. S5), as well as long-range contralateral projections from neurons located at more rostral regions, including the claustrum, a nonlayered derivative of the pallial subplate, and lateral portions of the frontal and orbital cortices (Fig. 5).
Fig. 4.
Homotopic and heterotopic commissural neurons in marsupials resemble the callosal connectome of eutherians. (A) Coronal sections of dunnart brains showing a summary of retrograde tracer injection sites (DAPI and color-coded in the Left) and the contralateral position of cell bodies (dots). (B) Connectivity matrix combining injection sites (columns; approximate brain positions on Top Left) and percentage of labeled neurons per brain area (rows). aMed, anterior medial cortex; aSom, anterior somatosensory cortex; Fr/Orb, frontal/orbital cortices; Lat, lateral cortex; pMed, posterior medial cortex; pSom, posterior somatosensory cortex; Olf/Pir, olfactory/piriform cortices; Occip, occipital cortex. (Scale bar, 1 mm.)
Fig. 5.
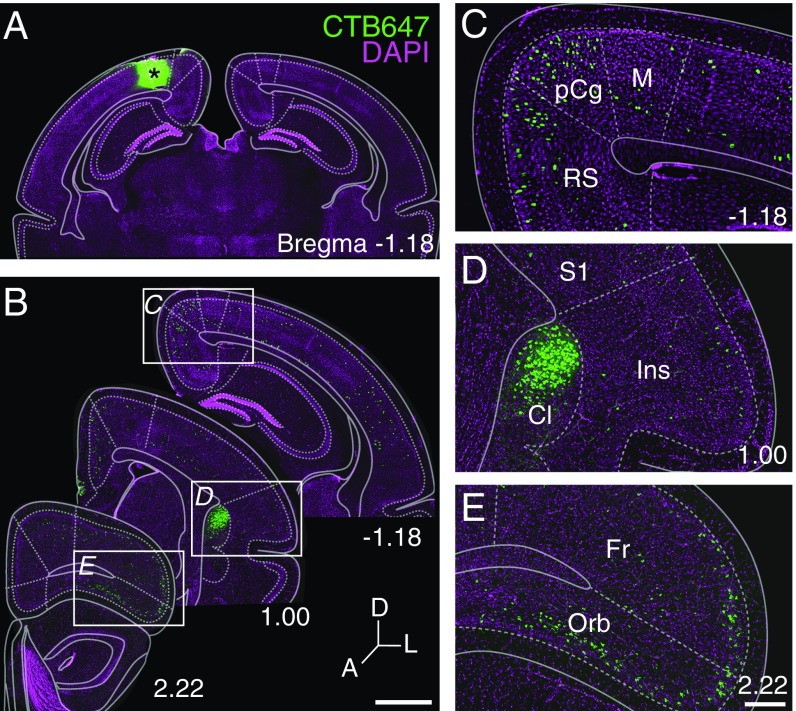
Heterotopic claustro-cortical commissural connections in dunnarts. (A) A posteromedial CTB injection and contralaterally projecting neurons across the rostrocaudal extent (B) in mm from Bregma. (C–E) Insets of the regions highlighted in B include neurons in the posterior cingulate (pCg), retrosplenial (RS), and motor (M) cortices in C, the claustrum (Cl) and insula (Ins) in D, and the frontal (Fr) and orbital cortices (Orb) in E. (Scale bars: 1 mm in B, and 250 μm in C and D.)
Similar long-range interhemispheric connections from the claustrum to heterotopic contralateral targets, including the dorsomedial portions of the cortex, have been previously described in rodents (19–21) and can be revealed after retrograde tracer injections in the posterior cingulate/retrosplenial or motor cortices (SI Appendix, Figs. S6 and S7, respectively). Moreover, the long-range contralateral projection neurons in the dunnart claustrum were topographically organized according to the mediolateral position of their termination sites (SI Appendix, Fig. S8), further resembling the ipsilateral and contralateral topography of the eutherian claustrum (19–23).
Anterograde Circuit Mapping via In-Pouch Electroporation in Dunnarts.
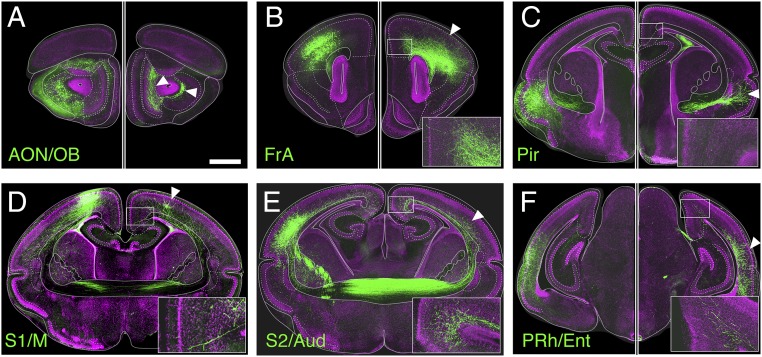
We next examined the patterns of contralateral axon innervation via in-pouch electroporation of fluorescent reporters in dunnarts during cortical development (30), followed by collection after completion of corticogenesis (31). We found that, whereas commissural neurons project largely to homotopic targets (Fig. 6, arrowheads), most neocortical regions, but not the olfactory or piriform areas, also send heterotopic axonal projections that terminate in the contralateral motor and cingulate areas (Fig. 6, Insets). This is consistent with the retrograde tract-tracing results and further demonstrates that the cingulate, retrosplenial, and motor cortices receive long-range interhemispheric connections from multiple heterotopic regions.
Fig. 6.
Anterograde mapping of contralateral projections in dunnarts reveals a conserved interhemispheric connectome. (A–F) In-pouch electroporated neurons are shown in the left hemisphere and their axon terminals in the right hemisphere in six different cases. Homotopic targets are shown with arrowheads, and Insets show heterotopic terminals in the contralateral motor/cingulate cortex. To reveal the cell bodies and their axons, the hemispheres have different brightness/contrast levels in A–C and F. AON/OB, anterior olfactory nucleus/olfactory bulb; FrA, anterior frontal cortex; PRh/Ent, perirhinal/entorhinal cortex; S1/M, primary somatosensory/motor cortex; S2/Aud, secondary somatosensory/auditory cortex. (Scale bar, 1 mm.)
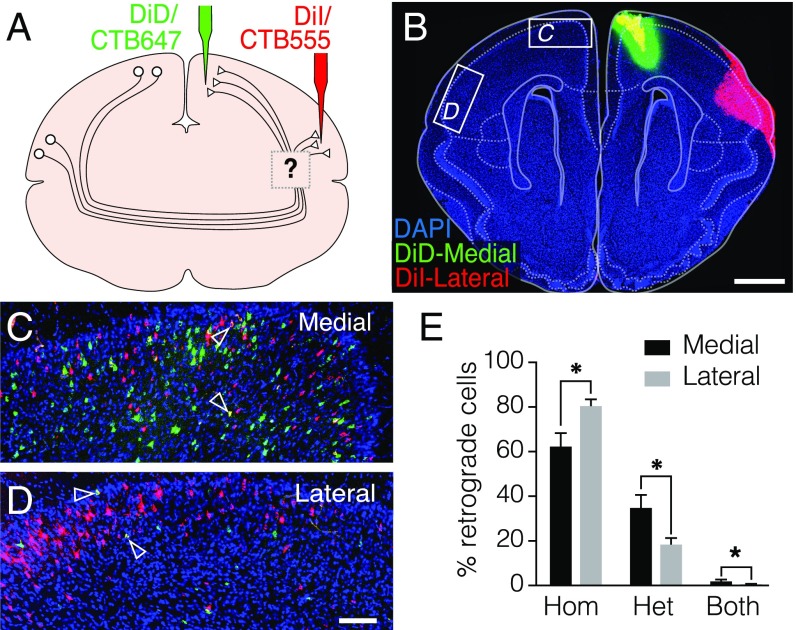
We next investigated whether projections from one cortical area to two or more different targets arise from the collateral branches of homogeneous neuronal populations, or instead from distinct neurons with independent projection profiles. To elucidate between these scenarios, we performed injections of retrograde tracers into the medial and lateral portions of the same hemisphere of the dunnart cortex in vivo and counted neurons that incorporated either one or both tracers at the medial and lateral cortices contralateral to the injection sites (Fig. 7 A and B). We found that the majority of neurons incorporated only one of the dies, with less than 3% of neurons incorporating both dies (Fig. 7 C and D, arrowheads). Moreover, both homotopic and heterotopic neurons were arranged in an intermingled, salt-and-pepper fashion, and their relative proportion differed across cortical areas (Fig. 7 C–E). The lateral regions of the cortex, including the insula, contained more homotopic neurons than the medial regions, including cingulate and motor cortices (80.8 ± 2.7% vs. 62.6 ± 5.7%, respectively). In contrast, these medial cortical regions had more heterotopic and branched (both tracers) contralaterally projecting neurons than the lateral cortex (heterotopic: 35.2 ± 5.5% medial vs. 18.8 ± 2.5% lateral; both: 2.2 ± 0.5% medial vs. 0.5 ± 0.3% lateral; P < 0.026; df = 8; n = 5; Student’s t test; Fig. 7E). Importantly, such an arrangement of intermingled commissural neurons with homotopic and heterotopic targets, with only few cells that demonstrate long-range contralateral branches, has also been reported in callosal neurons of rodents and primates (32–34). These findings, together with anterograde evidence of contralateral axons terminating in both homotopic and heterotopic targets in both marsupials and eutherians, suggest that the mechanisms of contralateral axon targeting include both positional and nonpositional cues (35).
Fig. 7.
Intermingled populations of homotopic and heterotopic commissural neurons in dunnarts. (A and B) Double retrograde injections with CTB647 and CTB555 and/or DiI and DiD in the medial and lateral cortices result in intermingled cell bodies in the contralateral homotopic cortices (C and D) with very few double-labeled cells (arrowheads). (E) The proportion of cells that project exclusively to homotopic (Hom), heterotopic (Het) and to both targets (double-labeled cells) over the total number of cells labeled in the section, differ between the medial and lateral cortices (mean + SEM, n = 5; *P < 0.026). (Scale bars, 1 mm in B, 100 μm in H.)
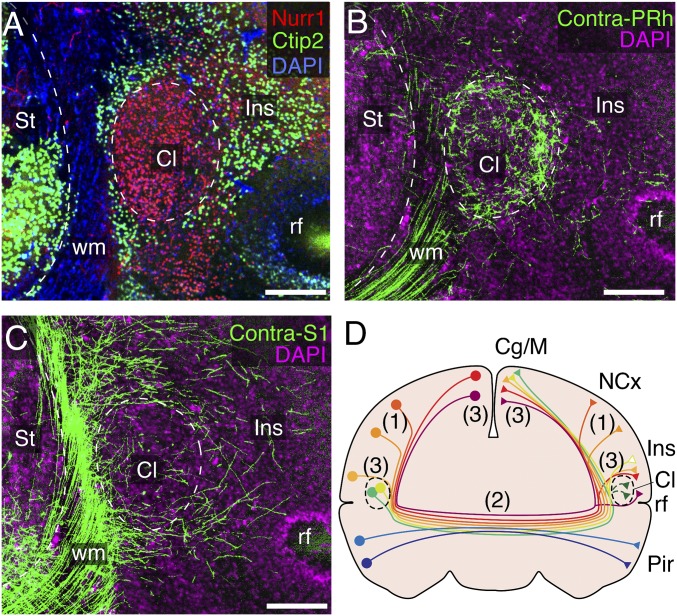
Finally, to get a better understanding of the homologies between the marsupial and eutherian cortical connectomes, we examined the general distribution and bilateral symmetry of heterotopic circuits in dunnarts, particularly within known interhemispheric hubs in eutherians such as the claustrum. Similar to eutherians, the claustrum of dunnarts was distinguished from the surrounding striatum and deep insular cortex by Nurr1+ and Ctip2− expression (Fig. 8A), and resembles the eutherian claustrum in that it is differentially interconnected with contralateral areas (19–23). For example, although the dunnart claustrum receives dense projections from heterotopic regions in the contralateral hemisphere, such as the perirhinal cortex (Fig. 8B), it is also largely avoided by axons from the contralateral primary somatosensory sensory cortex (Fig. 8C). Furthermore, the overall pattern of neocortical contralateral projections closely resembled the pattern of projections within the same hemisphere, in a mirrored fashion (SI Appendix, Fig. S9). A similar pattern of ipsilateral and contralateral symmetry of pyramidal neuron projections has been widely reported in the better-studied neocortex of rats (25), further suggesting the deep-time evolutionary conservation of cortical connectivity rules throughout mammals.
Fig. 8.
Heterotopic circuits and a pan-mammalian connectome. (A) Coronal dunnart brain section stained against Nurr1 (red), Ctip2 (green), and DAPI (blue) outline the claustrum (Cl) from the striatum (St), white matter (wm), and insula (Ins). (B and C) Axon terminals in the claustrum (Cl) were differentially found after electroporations in the perirhinal (PRh, B) or somatosensory (S1, C) cortices of the contralateral hemisphere. (D) Schematic of a coronal brain section representing the features of an interhemispheric connectome likely conserved by all mammals. Neocortical (NCx) neurons from layers 2/3 and 5 project to contralateral homotopic regions (1), following a topographic arrangement of axons across the midline (2), whereas hyperconnected and heterotopic circuits include the cingulate-motor (Cg/M) and insular (Ins) cortices, as well as the claustrum (3). rf, rhinal fissure. (Scale bars, 250 μm.)
Discussion
In all eutherians studied to date, including rodents, carnivores, monkeys, and humans, the axons that form the corpus callosum are spatially segregated within the tract into rostrocaudal and dorsoventral topographies that represent the broad position of neuronal cell bodies across the different cortical areas (16–18). Callosal circuits include connections between largely homotopic regions, as well as heterotopic circuits that importantly include the medial (i.e., cingulate, motor, and retrosplenial cortex) and lateral (i.e., perirhinal cortex, insula, and claustrum) borders of the neocortical sheet (25, 32–37). These medial and lateral regions also represent hyperconnected hubs within and between hemispheres and form part of the task-negative (also known as default mode) network (25, 38). Notably, despite its relatively small size, the nonlayered claustrum is a central contributor to large-scale neocortical networks in rodents (21) and humans (23). Our findings of the dunnart claustrum as a major component of long-range heterotopic and hyperconnected contralateral circuits suggest its early origin. Indeed, despite reports of the apparent absence of the claustrum in platypus (39), cytoarchitectonic and neuroanatomical studies in the claustrum of the short-beaked echidna (Tachyglossus aculeatus; Monotremata) suggest that Therian-like circuits including the claustrum/insula and the medial/frontal cortices may have originated early in mammalian evolution, possibly as a central hub of long-range heterotopic connectivity between and within neocortical hemispheres (40–42).
Previous studies in marsupials have described several features of cortical intra- and interhemispheric organization that are shared with eutherians. These include similar developmental sequence of neuronal generation (26, 30, 31, 43, 44), expression of cell type-specific and layer-specific genetic markers (44–46), and patterns of connections within and between cortical hemispheres, including homotopic and heterotopic circuits (26, 47–50), which laid the groundwork for the present study. Taken together, our findings in platypus and dunnarts further suggest the early origin and conservation of an interhemispheric cortical connectome that predates the evolution of the corpus callosum. The main features of such an ancient connectome are summarized in Fig. 8D and include coexistence of homotopic and heterotopic circuits, topographic segregation of commissural axons within the tract according to the position of cell bodies, and hyperconnected hubs at the medial (cingulate/motor) and lateral (claustrum/insula) borders of the cortex. In conclusion, our results suggest that the origin of the corpus callosum in early eutherian ancestors likely included the conservation of preexisting features of intra- and interhemispheric connectivity. Notably, humans with congenital absence of the corpus callosum, but with preserved interhemispheric integrative functions, often show compensatory wiring through the anterior commissure that resembles the noneutherian connectome (51). This suggests that, under certain unknown conditions, neocortical commissural neurons may exploit developmental plasticity of ancient mechanisms of axon guidance, resulting in functional interhemispheric circuits. Our findings provide a comparative framework to further elucidate the molecular underpinnings of interhemispheric wiring in individuals with and without a corpus callosum, as well as to investigate developmental hypotheses concerning the evolution of homologous circuits in the vertebrate brain.
Materials and Methods
Animal Ethics.
This study was approved by The University of Queensland Animal Ethics Committee and followed international guidelines on animal welfare.
Magnetic Resonance Imaging and Tractography.
Fixed brains of adult platypus (n = 2) and dunnarts (n = 3) were scanned at 16.4 or 9.4 T (Bruker Ultrashield Plus Avance I, 89 mm bore, or Bruker Biospec Avance III, 300 mm bore, respectively; Paravision 5.1). Diffusion MRI datasets were processed using HARDI/Q-ball reconstruction, and tractography was generated using Fiber Assignment by Continuous Tracing in TrackVis (www.trackvis.com).
In-Pouch Electroporation of Dunnart Joeys.
Electroporation of cortical neurons in dunnarts attached to the mother’s teat was performed as described (30). Briefly, 0.5–1 μL of a 1 mL/mg DNA solution of pCAG-eYFP plasmid was injected into the lateral ventricle, followed by delivery of five 100-ms square pulses of 30–35 V at 1 Hz (ECM 830, BTX; Harvard Bioscience).
Histology and Cell Counts.
Double injections of fine carbocyanine crystals (DiI and DiD) were performed in fixed dunnart brains, using a pulled-glass micropipette. Brains were kept from light in formalin at 4 °C for 7 d, and then at 38 °C for 7 mo for transport. In vivo stereotaxic injections (SI Appendix, Table S1) were performed under 2–5% isoflurane, using a reference atlas of the stripe-faced dunnart (S. macroura) (52). Brains were collected after 7 d, and histological images obtained with confocal microscopy. Cell counts were made blind to the injection sites. Statistically significant differences were considered as P < 0.05, using two-tailed Student’s t test (Fig. 7E) or one-way ANOVA (Prism 7).
Supplementary Information.
Supplementary material and full methods are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank the Queensland Nuclear Magnetic Resonance Network and the Australian National Imaging Facility for the operation of the scanners, the Queensland Brain Institute’s Advanced Microscopy Facility for histology imaging, and the University of Queensland Biological Resources and the Native Wildlife Teaching and Research Facility for all animal help. This work was supported by Australian Research Council Grants DP160103958 (to L.J.R. and R.S.) and DE160101394 (to R.S.), National Health and Medical Research Council Fellowships DP160103958 (to L.J.R. and R.S.) and DE160101394 (to R.S.), the Australian Postgraduate Award (to L.R.F. and L.R.M.), and the UQ-QBI Doctoral Scholarship award (to A.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808262115/-/DCSupplemental.
References
- 1.Ramón y Cajal S. Histología del Sistema Nervioso del Hombre y de los Vertebrados. Boletín Oficial del Estado; Madrid: 1904. [Google Scholar]
- 2.Sperry RW. Restoration of vision after crossing of optic nerves and after contralateral transplantation of eye. J Neurophysiol. 1945;8:15–28. [Google Scholar]
- 3.Fredes F, Tapia S, Letelier JC, Marín G, Mpodozis J. Topographic arrangement of the rotundo-entopallial projection in the pigeon (Columba livia) J Comp Neurol. 2010;518:4342–4361. doi: 10.1002/cne.22460. [DOI] [PubMed] [Google Scholar]
- 4.Elliott KC, Wu W, Bertram R, Hyson RL, Johnson F. Orthogonal topography in the parallel input architecture of songbird HVC. J Comp Neurol. 2017;525:2133–2151. doi: 10.1002/cne.24189. [DOI] [PubMed] [Google Scholar]
- 5.Krubitzer L, Manger P, Pettigrew J, Calford M. Organization of somatosensory cortex in monotremes: In search of the prototypical plan. J Comp Neurol. 1995;351:261–306. doi: 10.1002/cne.903510206. [DOI] [PubMed] [Google Scholar]
- 6.Oh SW, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaas JH. Topographic maps are fundamental to sensory processing. Brain Res Bull. 1997;44:107–112. doi: 10.1016/s0361-9230(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 8.Letzner S, Simon A, Güntürkün O. Connectivity and neurochemistry of the commissura anterior of the pigeon (Columba livia) J Comp Neurol. 2016;524:343–361. doi: 10.1002/cne.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paterson AK, Bottjer SW. Cortical inter-hemispheric circuits for multimodal vocal learning in songbirds. J Comp Neurol. 2017;525:3312–3340. doi: 10.1002/cne.24280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen R. On the structure of the brain in marsupial animals. Philos Trans R Soc Lond. 1837;127:87–96. [Google Scholar]
- 11.Suárez R. Evolution of telencephalic commissures: Conservation and change of developmental systems in the origin of brain wiring novelties. In: Kaas JH, editor. Evolution of Nervous Systems. 2nd Ed. Academic; Oxford: 2017. pp. 205–223. [Google Scholar]
- 12.Suárez R, Gobius I, Richards LJ. Evolution and development of interhemispheric connections in the vertebrate forebrain. Front Hum Neurosci. 2014;8:497. doi: 10.3389/fnhum.2014.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gobius I, et al. Astroglial-mediated remodeling of the interhemispheric midline is required for the formation of the corpus callosum. Cell Rep. 2016;17:735–747. doi: 10.1016/j.celrep.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gobius I, et al. Astroglial-mediated remodeling of the interhemispheric midline during telencephalic development is exclusive to eutherian mammals. Neural Dev. 2017;12:9. doi: 10.1186/s13064-017-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashwell KWS. Anterior commissure versus corpus callosum: A quantitative comparison across mammals. Zoology (Jena) 2016;119:126–136. doi: 10.1016/j.zool.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 16.de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. J Neuropathol Exp Neurol. 1985;44:578–591. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Zhou J, et al. Axon position within the corpus callosum determines contralateral cortical projection. Proc Natl Acad Sci USA. 2013;110:E2714–E2723. doi: 10.1073/pnas.1310233110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tovar-Moll F, et al. Neuroplasticity in human callosal dysgenesis: A diffusion tensor imaging study. Cereb Cortex. 2007;17:531–541. doi: 10.1093/cercor/bhj178. [DOI] [PubMed] [Google Scholar]
- 19.Sloniewski P, Usunoff KG, Pilgrim C. Retrograde transport of fluorescent tracers reveals extensive ipsi- and contralateral claustrocortical connections in the rat. J Comp Neurol. 1986;246:467–477. doi: 10.1002/cne.902460405. [DOI] [PubMed] [Google Scholar]
- 20.Smith JB, Alloway KD. Interhemispheric claustral circuits coordinate sensory and motor cortical areas that regulate exploratory behaviors. Front Syst Neurosci. 2014;8:93. doi: 10.3389/fnsys.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, et al. Organization of the connections between claustrum and cortex in the mouse. J Comp Neurol. 2017;525:1317–1346. doi: 10.1002/cne.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patzke N, Innocenti GM, Manger PR. The claustrum of the ferret: Afferent and efferent connections to lower and higher order visual cortical areas. Front Syst Neurosci. 2014;8:31. doi: 10.3389/fnsys.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torgerson CM, Irimia A, Goh SYM, Van Horn JD. The DTI connectivity of the human claustrum. Hum Brain Mapp. 2015;36:827–838. doi: 10.1002/hbm.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zingg B, et al. Neural networks of the mouse neocortex. Cell. 2014;156:1096–1111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson LW, Hahn JD, Sporns O. Organizing principles for the cerebral cortex network of commissural and association connections. Proc Natl Acad Sci USA. 2017;114:E9692–E9701. doi: 10.1073/pnas.1712928114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashwell KW, Marotte LR, Li L, Waite PM. Anterior commissure of the wallaby (Macropus eugenii): Adult morphology and development. J Comp Neurol. 1996;366:478–494. doi: 10.1002/(SICI)1096-9861(19960311)366:3<478::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson S, Trojanowski JQ. The cells of origin of the corpus callosum in rat, cat and rhesus monkey. Brain Res. 1974;74:149–155. doi: 10.1016/0006-8993(74)90118-8. [DOI] [PubMed] [Google Scholar]
- 28.Wise SP, Jones EG. The organization and postnatal development of the commissural projection of the rat somatic sensory cortex. J Comp Neurol. 1976;168:313–343. doi: 10.1002/cne.901680302. [DOI] [PubMed] [Google Scholar]
- 29.Granger EM, Masterton RB, Glendenning KK. Origin of interhemispheric fibers in acallosal opossum (with a comparison to callosal origins in rat) J Comp Neurol. 1985;241:82–98. doi: 10.1002/cne.902410107. [DOI] [PubMed] [Google Scholar]
- 30.Paolino A, Fenlon LR, Kozulin P, Richards LJ, Suárez R. Multiple events of gene manipulation via in pouch electroporation in a marsupial model of mammalian forebrain development. J Neurosci Methods. 2018;293:45–52. doi: 10.1016/j.jneumeth.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Suárez R, et al. Development of body, head and brain features in the Australian fat-tailed dunnart (Sminthopsis crassicaudata; Marsupialia: Dasyuridae); A postnatal model of forebrain formation. PLoS One. 2017;12:e0184450. doi: 10.1371/journal.pone.0184450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fenlon LR, Suárez R, Richards LJ. The anatomy, organisation and development of contralateral callosal projections of the mouse somatosensory cortex. Brain Neurosci Adv. 2017;1:1–9. doi: 10.1177/2398212817694888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanz F, et al. Distant heterotopic callosal connections to premotor cortex in non-human primates. Neuroscience. 2017;344:56–66. doi: 10.1016/j.neuroscience.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Olavarria JF, Safaeian P. Development of callosal topography in visual cortex of normal and enucleated rats. J Comp Neurol. 2006;496:495–512. doi: 10.1002/cne.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenlon LR, Richards LJ. Contralateral targeting of the corpus callosum in normal and pathological brain function. Trends Neurosci. 2015;38:264–272. doi: 10.1016/j.tins.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Suárez R, et al. Balanced interhemispheric cortical activity is required for correct targeting of the corpus callosum. Neuron. 2014;82:1289–1298. doi: 10.1016/j.neuron.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 37.De Benedictis A, et al. New insights in the homotopic and heterotopic connectivity of the frontal portion of the human corpus callosum revealed by microdissection and diffusion tractography. Hum Brain Mapp. 2016;37:4718–4735. doi: 10.1002/hbm.23339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stafford JM, et al. Large-scale topology and the default mode network in the mouse connectome. Proc Natl Acad Sci USA. 2014;111:18745–18750. doi: 10.1073/pnas.1404346111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler AB, Molnár Z, Manger PR. Apparent absence of claustrum in monotremes: Implications for forebrain evolution in amniotes. Brain Behav Evol. 2002;60:230–240. doi: 10.1159/000066698. [DOI] [PubMed] [Google Scholar]
- 40.Ashwell KW, Hardman C, Paxinos G. The claustrum is not missing from all monotreme brains. Brain Behav Evol. 2004;64:223–241. doi: 10.1159/000080243. [DOI] [PubMed] [Google Scholar]
- 41.Divac I, Holst MC, Nelson J, McKenzie JS. Afferents of the frontal cortex in the echidna (Tachyglossus aculeatus). Indication of an outstandingly large prefrontal area. Brain Behav Evol. 1987;30:303–320. doi: 10.1159/000118653. [DOI] [PubMed] [Google Scholar]
- 42.Divac I, Pettigrew JD, Holst MC, McKenzie JS. Efferent connections of the prefrontal cortex of echidna (Tachyglossus aculeatus) Brain Behav Evol. 1987;30:321–327. doi: 10.1159/000118654. [DOI] [PubMed] [Google Scholar]
- 43.Ashwell KWS, Waite PME, Marotte L. Ontogeny of the projection tracts and commissural fibres in the forebrain of the tammar wallaby (Macropus eugenii): Timing in comparison with other mammals. Brain Behav Evol. 1996;47:8–22. doi: 10.1159/000113225. [DOI] [PubMed] [Google Scholar]
- 44.Puzzolo E, Mallamaci A. Cortico-cerebral histogenesis in the opossum Monodelphis domestica: Generation of a hexalaminar neocortex in the absence of a basal proliferative compartment. Neural Dev. 2010;5:8. doi: 10.1186/1749-8104-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung AF, et al. The subventricular zone is the developmental milestone of a 6-layered neocortex: Comparisons in metatherian and eutherian mammals. Cereb Cortex. 2010;20:1071–1081. doi: 10.1093/cercor/bhp168. [DOI] [PubMed] [Google Scholar]
- 46.Wang WZ, et al. Comparative aspects of subplate zone studied with gene expression in sauropsids and mammals. Cereb Cortex. 2011;21:2187–2203. doi: 10.1093/cercor/bhq278. [DOI] [PubMed] [Google Scholar]
- 47.Bravo H, Olavarría J, Martinich S. Patterns of interhemispheric and striate-peristriate connections in visual cortex of the South American marsupial Marmosa elegans (mouse opossum) Anat Embryol (Berl) 1990;182:583–589. doi: 10.1007/BF00186465. [DOI] [PubMed] [Google Scholar]
- 48.Dooley JC, Franca JG, Seelke AM, Cooke DF, Krubitzer LA. A connection to the past: Monodelphis domestica provides insight into the organization and connectivity of the brains of early mammals. J Comp Neurol. 2013;521:3877–3897. doi: 10.1002/cne.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinich S, Pontes MN, Rocha-Miranda CE. Patterns of corticocortical, corticotectal, and commissural connections in the opossum visual cortex. J Comp Neurol. 2000;416:224–244. [PubMed] [Google Scholar]
- 50.Sheng XM, Marotte LR, Mark RF. Development of connections to and from the visual cortex in the wallaby (Macropus eugenii) J Comp Neurol. 1990;300:196–210. doi: 10.1002/cne.903000205. [DOI] [PubMed] [Google Scholar]
- 51.Tovar-Moll F, et al. Structural and functional brain rewiring clarifies preserved interhemispheric transfer in humans born without the corpus callosum. Proc Natl Acad Sci USA. 2014;111:7843–7848. doi: 10.1073/pnas.1400806111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ashwell KWS, McAllan BM, Mai JK. Atlas of the brain of the stripe-faced dunnart (Sminthopsis macroura) In: Ashwell KWS, editor. The Neurobiology of Marsupials. Cambridge Univ Press; New York: 2010. p. 241. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.