Abstract
Background
Fish oil supplementation has been shown to delay spontaneous delivery, but the levels and clinical significance remain uncertain. We examined the association between plasma fatty acids quantified in pregnancy and subsequent risk of early preterm birth.
Methods
In a case-control design nested in the Danish National Birth Cohort, we identified 376 early preterm cases (<34 gestational weeks, excluding preeclampsia cases) and 348 random controls. Plasma eicosapentaenoic acid plus docosahexaenoic acid (EPA+DHA% of total fatty acids), were measured twice in pregnancy, at gestation weeks 9 and 25 (medians). Odds ratios and 95% confidence intervals (CI's) for associations between EPA+DHA and early preterm risk were estimated by logistic regression, adjusted for the woman's age, height, pre-pregnancy BMI, parity, smoking, and socioeconomic factors. Hypotheses and analytical plan were defined and archived a priori.
Findings
Analysis using restricted cubic splines of the mean of 1st and 2nd sample measurements showed a strong and significant non-linear association (p < 0.0001) in which the risk of early preterm birth steeply increased when EPA+DHA concentrations were lower than 2% and flattened out at higher levels. Women in the lowest quintile (EPA+DHA < 1.6%) had 10.27 times (95% confidence interval 6.80–15.79, p < 0.0001) increased risk, and women in the second lowest quintile had 2.86 (95% CI 1.79–4.59, p < 0.0001) times increased risk, when compared to women in the three aggregated highest quintiles (EPA+DHA ≥ 1.8%).
Interpretation
Low plasma concentration of EPA and DHA during pregnancy is a strong risk factor for subsequent early preterm birth in Danish women.
Keywords: Early preterm birth, Long chained n-3 fatty acids, Biomarkers, Prospective study, Danish National Birth Cohort
Abbreviations: EPB, early preterm birth.; LC-n3FA, long chain n-3 fatty acids.; EPA, eicosapentaenoic acid.; DHA, docosahexaenoic acid.; ALA, alpha-linolenic acid.; FADS, fatty acid desaturase.; SD, standard deviation.; SEM, standard error of mean.; OR, odds ratio
Research in Context
Evidence Before This Study
Preterm birth, i.e. birth prior to 37 weeks of gestation, has been estimated to affect one in nine infants worldwide and is a leading cause of neonatal and childhood mortality. Although the majority of preterm births occur in the late preterm period, those born earlier experience disproportionately higher rates of prematurity-related complications. Despite vast research efforts, the aetiology of early delivery remains a conundrum. Several meta-analyses of published RCTs have concluded that intake of long chain n-3 fatty acids in pregnancy are associated with longer mean gestational length, a hypothesis which was first published in The Lancet.
Added Value of This Study
This is the first study to examine if a low plasma concentration of long chain n-3 fatty acids in early and mid-pregnancy is associated with increased risk of subsequent early preterm birth, i.e. birth prior to 34 weeks of gestation. This was possible because the large Danish National Birth Cohort has stored plasma samples from around 100,000 pregnancies drawn in gestation weeks 9 and 25, and because the diagnosis of early preterm cases occurring in the cohort has been validated and confirmed against hospital records. In a case-control design nested in the cohort, we identified 376 early spontaneous preterm cases (<34 gestational weeks, excluding preeclampsia cases) and 348 random controls. Concentrations of eicosapentaenoic acid plus docosahexaenoic acid (EPA+DHA), expressed as % of total fatty acids, were measured in both plasma samples for all case and control women. When taking the average of 1st and 2nd sample measurements, women in the lowest quintile of the EPA+DHA distribution had a 10 times (95% confidence interval 6.8 to 16, p < 0.0001) increased risk, and women in the second lowest quintile had a 2.9 (95% CI 1.8 to 4.6, p < 0.0001) times increased risk of early preterm birth, when compared to women in the three aggregated highest quintiles (EPA+DHA > 1.8%). Spline curves showed a highly significant bending, with a threshold effect at EPA+DHA concentrations somewhere between 2.0% and 2.5 % and with increases in risk at low levels, which then flattens out at higher levels. Hypotheses and analytical plan of our study were defined and archived a priori.
Implications of All Available Evidence
Our findings of a relatively strong association between plasma concentrations of total EPA and DHA in pregnancy and risk of early spontaneous preterm birth may suggest that that body levels of these fatty acids are causally implicated in the physiologic processes leading up to premature labour and delivery. These findings also suggest that plasma measurements of EPA and DHA in pregnancy may be used to identify women at risk of early preterm birth. Because our study was undertaken in Denmark, where rates of preterm birth are low, population-based studies should be conducted elsewhere to determine if the associations observed can be replicated in other populations. A further note of caution is that variation in genes might explain some of the observed association between EPA+DHA and early preterm risk and research is needed to understand the relative contributions of dietary versus genetic factors.
Alt-text: Unlabelled Box
1. Introduction
Preterm birth, defined as birth prior to 37 weeks of gestation, has been estimated to affect one in nine infants worldwide [1]. It is a leading cause of neonatal and childhood mortality and surviving children may suffer cognitive deficiencies and be at increased risk of cardio-metabolic and pulmonary diseases in adult life. Although the majority of preterm births occur in the late preterm period (34 0/7 to 36 6/7 gestation weeks), those born before 34 weeks of gestation experience disproportionately higher rates of prematurity-related complications [2].
Despite vast research efforts, the aetiology of early delivery remains a conundrum. Observations of high birth weights and long gestations [3, 4] in the fish eating community of the Faroe Islands led to the hypothesis, published three decades ago in The Lancet [5], that a high intake of the long chain n-3 fatty acids in pregnancy can delay timing of spontaneous delivery. Although not all intervention [6, 7]or observational [8, 9] studies have supported the hypothesis, at least one observational [10, 11] and five intervention [[12], [13], [14], [15], [16]] studies have; and several [[17], [18], [19]] meta-analyses of published RCTs have consistently concluded that intake of long chain n-3 fatty acids in pregnancy is associated with a few days longer mean gestational length.
The clinical relevance of this modest effect size has been questioned. While supplementation trials remain the principal gold standard for examining such associations, large prospective pregnancy cohorts can play an important role in answering questions about the relationship between magnitude of deficiency at different time points in pregnancy and risk of adverse outcomes, particularly if the outcomes studied are rare and if bio-samples are available for exposure assessment [20]. One such cohort is the Danish National Birth Cohort (DNBC) [21], where 101,042 women recruited in their early pregnancies gave blood samples in the 1st and 2nd trimester, and for whom pregnancy complications – including the early preterm diagnosis – have been verified by review of hospital records [22].
Several biomarker studies have been conducted in this field. Two early studies found gestation length to be associated with the concentration of long chain n-3 fatty acids assessed in erythrocytes sampled in gestation week 37 [23] or at delivery [24], respectively, and we know of one study that has related fatty acid concentrations in erythrocytes sampled in mid-pregnancy to later risk of preterm birth [25]. However, it seems that no-one has been able to examine the association between n-3 fatty acids quantified in blood samples obtained in early and mid-pregnancy and the woman's subsequent risk of early preterm birth, i.e. birth prior to 34 weeks of gestation.
The objective of the present study was to examine, in the Danish National Birth Cohort, if a low plasma concentration of long chain n-3 fatty acids in early and mid-pregnancy is associated with increased risk of subsequent early preterm birth. Understanding threshold effects of potential non-linear associations was a particularly important research priority given that some prior intake studies had suggested the existence of a ‘saturation’ threshold above which no further effect of intake seemed achievable [10, 12, 26].
2. Materials and Methods
2.1. Study Design
We conducted a case-control study nested in the Danish National Birth Cohort (DNBC), a nationwide prospective population-based study in Denmark [21]. For the majority of enrolled women, recruitment took place in 1st trimester at the woman's first antenatal visit to her general practitioner (GP) at which time a blood sample (‘1st sample’ in the present study) was taken for the project; a second blood sample, ‘2nd sample’ was taken for the cohort at a routine GP visit in 2nd trimester. Approximately one third of all pregnancies during the recruitment period (1996–2002) were enrolled and women gave consent for long term follow up for themselves and their children, resulting in a total of 101,042 pregnancies in 91,661 women. Data for the core DNBC database were derived from national registry extractions, questionnaires, and telephone interviews with the women [21]. The present study was approved by the Regional Scientific Ethical Committee of Capital Region of Denmark (H-2-2013-108).
2.2. Study Sample and Identification of Early Preterm Birth
Only singleton pregnancies resulting in a live born child were included, and if a woman had multiple pregnancies in the DNBC, only the first pregnancy was included.
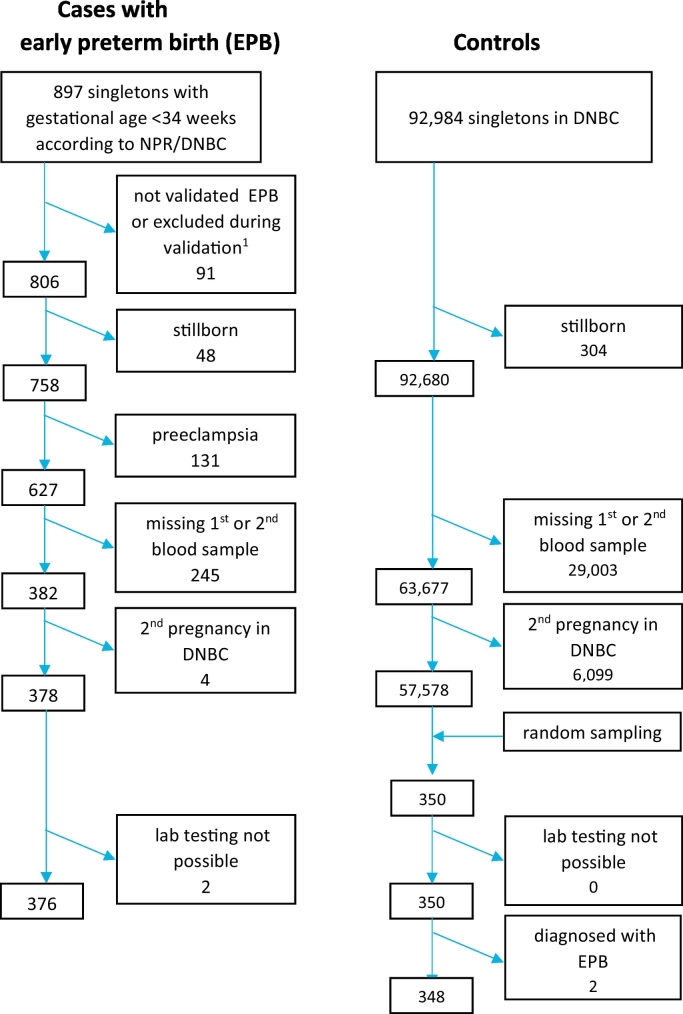
Cohort participants with suspected early preterm birth (before 34 0/7 weeks) were identified, and their diagnoses verified against hospital records [22]. Gestational age was based on ultrasound evaluation or last menstruation period if no ultrasound evaluation was performed (<2%). We excluded women with uterine malformations, preexisting medical conditions including hypertension and diabetes, cervical conization, placenta accreta or previa, preeclampsia, as well as fetal malformation, as our interest was to study aetiology of preterm birth without a known cause (see Fig. 1 with flowchart).
Fig. 1.
Flow chart for identifying early preterm cases and controls.
Random controls were drawn from the entire DNBC using a computed random process in SAS (SAS Institute Inc., Cary, NC, USA), excluding those with early preterm birth.
2.3. Exposure Assessment
Blood was collected in EDTA vials that were sent by mail to the national biobank at Statens Serum Institut, separated into plasma and buffy coat, and frozen and stored at −30 °C for later analysis. Fatty acid samples were analyzed in the Nutritional Biomarker Lab at the Harvard TH Chan School of Public Health from January through November 2015 and vitamin D samples were analysed at the Statens Serum Institut (a laboratory certified in the Vitamin D External Quality Assessment Scheme) from July through October 2015. Fatty acids were determined as previously described by Baylin et al. [27]. At both labs, samples were processed in a random order and technicians were blinded to the case status of the samples.
To represent exposure to long chain n-3 fatty acids, we used the aggregated fatty acids, EPA and DHA, expressed as % of total plasma fatty acids (later referred to as ‘EPA+DHA’). Fatty acids were determined as previously described by Baylin et al. [27]. Briefly, they were extracted and trans-methylated with methanol and sulfuric acid as described by Zock P et al.; Zock P et al., [28, 29]. After esterification the fatty acid methyl esters were re-dissolved in isooctane and quantitated by gas-liquid chromatography as follows: fused silica capillary cis/trans column SP2560, 100 m × 250 micrometers internal diameters × 0.20 micrometers film (Supelco, Belefonte, PA); splitless injection port at 240 °C; hydrogen carrier gas at 1.3 mL/min, constant flow; Hewlett-Packard Model (now Agilent) GC 6890 FID gas chromatograph with 7673 Autosampler injector (Palo Alto, CA); 1 microliter of sample injected; temperature program of 90 to 170 °C at 10 °C/min, 170 °C for 5 min, 170 to 175 °C at 5 °C/min, 175 to 185 °C at 2 °C/min, 185 to 190 °C at 1 °C/min, 190 to 210 at 5 °C/min, 210 °C for 5 min, 210 to 250 °C at 5 °C/min, 250 °C for 10 min. Peak retention times were identified by injecting known standards of purity above 99% (NuCheck Prep, Elysium, MN), using Agilent Technologies ChemStation A.08.03 software for analysis. Sample processing and freezing did not affect the fatty acid measurements, as determined by comparison of 2 pools of frozen and fresh samples and short vs. long term freezing. CVs for all the fatty acids studied were monitored continuously by analysis of a pooled control sample (indistinguishable from other study samples) run with each extraction and analysis batch. The mean CVs for EPA and DHA in the 36 pairs of controls were 1.8% and 1.9% respectively. In general, peaks that are near the sensitivity limit (1 to 10% of the total area) had larger CVs. Quality control was maintained by external validation through participation in programs offered by both the American Oil Chemists Society and the National Institute of Standards and Technology.
25-OH-vitamin D3 and 25-OH-vitamin D2 in plasma were determined as previously described [30], using liquid chromatography–tandem mass spectrometry (LC-MS) using Perkin Elmer's MSMS vitamin D kit (Perkin Elmer, Waltham MA), and were aggregated (25-OH-vitamin D) for the purpose of the present analyses.
2.4. Statistical Methods
A detailed statistical analysis plan (SAP, please see Supplementary Materials) was prepared prior to conducting the analysis and filed by Dr. Nils Axelsen, MD, DMSci, Office of Research Integrity, Statens Serum Institut (email address NA@ssi.dk). The analysis protocol was strictly adhered to. Logistic regression was used to model early preterm birth risk, which in a case-control study estimates odds ratios as an approximation for the relative risks in the whole cohort. Likelihood Ratio confidence intervals and tests were used throughout. When not stated otherwise, we used a significance level of 0.05.
For the main analyses we applied a sequential hierarchical testing procedure specified in the SAP as follows. In brief, we first fitted the mean of the 1st and 2nd measurements of EPA+DHA with a restricted cubic spline with 5 predefined knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles (Test A1 in SAP). If significant, we proceeded to two Bonferroni corrected tests (significance level 0.025) for alternative descriptions of the data: a test for linearity (Test A2a), and a test comparing the 1st quintile of EPA+DHA with a reference group consisting of the 3rd to 5th quintiles (Test A2b). If the 1st quintile was significantly different from the 3rd to 5th, we further compared the 2nd quintile to the reference group (Test A3 in SAP). The same procedure was used including only the women with concordant quintiles of 1st and 2nd EPA+DHA measurements (Tests B1-B3). The overall effect tests in the spline models with all women and only those with concordant quintiles, respectively, was Bonferroni corrected for each other giving a significance level of 0.025. In extra analyses (E2 in SAP), we made the same analyses for 1st and 2nd sample, respectively.
Potential confounders were chosen a priori using a theory based approach. Maternal age at birth, maternal height, and maternal pre-pregnancy BMI were in all analyses modelled as restricted cubic splines with 3 knots at the 10th, 50th, and 90th percentiles, whereas parity, socioeconomic status (SES), cohabitation, residence, and smoking were categorized as in Table 1. As a sensitivity analysis, we also adjusted for 25-OH-vitamin plasma concentrations (S2); this was because fish, in addition to containing EPA and DHA, is also an important dietary source of vitamin D, and vitamin D could therefore in principle act as a confounder for the association between EPA+DHA plasma concentrations and early preterm risk.
Table 1.
Participant characteristics in relation to early preterm risk and EPA+DHA plasma concentrations.
| Covariates | Controls |
Preterm Birth Cases |
Adjusted Odds |
EPA+DHA% (Controls) |
|||
|---|---|---|---|---|---|---|---|
| (n = 348) |
(n = 376) |
Ratioa (95% CI) | Mean of 1st and 2nd sample |
||||
| No. | Pct. | No. | Pct. | Mean (SEM) | Median [IQR] | ||
| Maternal age at birth (y) | |||||||
| Mothers age 16–20 | 3 | 0.9 | 8 | 2.1 | 1.79 (0.44; 9.40) | 2.20 (0.61) | 2.13 [1.18; 3.29] |
| Mothers age 21–30 | 210 | 60.3 | 200 | 53.2 | 1 | 1.98 (0.04) | 1.89 [1.63; 2.28] |
| Mothers age 31–45 | 133 | 38.2 | 168 | 44.7 | 1.87 (1.32; 2.68) | 2.08 (0.05) | 2.0 [1.61; 2.44] |
| Missing | 2 | 0.6 | 0 | 0.0 | .. | 1.68 (0.14) | 1.68 [1.54; 1.82] |
| Parity | |||||||
| Parity 0 | 165 | 47.4 | 245 | 65.2 | 1 | 2.07 (0.05) | 1.99 [1.69; 2.38] |
| Parity 1 | 123 | 35.3 | 86 | 22.9 | 0.42 (0.29; 0.61) | 1.98 (0.05) | 1.92 [1.63; 2.32] |
| Parity 2+ | 43 | 12.4 | 44 | 11.7 | 0.44 (0.25; 0.75) | 1.93 (0.10) | 1.82 [1.47; 2.17] |
| Missing | 17 | 4.9 | 1 | 0.3 | .. | 2.02 (0.18) | 1.83 [1.50; 2.23] |
| Maternal height (cm) | |||||||
| Height 150–165 | 86 | 24.7 | 117 | 31.1 | 1.33 (0.92; 1.93) | 2.01 (0.06) | 1.89 [1.67; 2.29] |
| Height 165–172 | 165 | 47.4 | 181 | 48.1 | 1 | 2.02 (0.05) | 1.95 [1.63; 2.38] |
| Height 172–188 | 80 | 23.0 | 78 | 20.7 | 0.87 (0.59; 1.28) | 2.04 (0.07) | 1.97 [1.60; 2.32] |
| Missing | 17 | 4.9 | 0 | 0.0 | .. | 2.02 (0.18) | 1.83 [1.50; 2.23] |
| Maternal pre-pregnancy BMI | |||||||
| BMI 14.2–18.5 | 21 | 6.0 | 22 | 5.9 | 0.93 (0.48; 1.83) | 1.79 (0.11) | 1.82 [1.54; 1.97] |
| BMI 18.5–24.9 | 217 | 62.4 | 248 | 66.0 | 1 | 2.06 (0.04) | 1.97 [1.66; 2.39] |
| BMI 25.0–29.9 | 68 | 19.5 | 60 | 16.0 | 0.87 (0.57; 1.31) | 1.98 (0.07) | 1.99 [1.64; 2.18] |
| BMI 30–47.8 | 19 | 5.5 | 39 | 10.4 | 1.92 (1.06; 3.56) | 1.84 (0.11) | 1.76 [1.57; 2.06] |
| Missing | 21 | 6.0 | 6 | 1.6 | .. | 2.0 (0.14) | 1.83 [1.55; 2.23] |
| SES | |||||||
| SES High | 33 | 9.5 | 28 | 7.4 | 0.73 (0.39; 1.37) | 2.23 (0.14) | 2.12 [1.54; 2.51] |
| SES Medium | 85 | 24.4 | 84 | 22.3 | 1 | 2.04 (0.06) | 1.98 [1.69; 2.43] |
| SES Skilled | 49 | 14.1 | 67 | 17.8 | 1.34 (0.82; 2.20) | 2.10 (0.08) | 2.04 [1.71; 2.42] |
| SES Student | 38 | 10.9 | 38 | 10.1 | 0.88 (0.49; 1.59) | 2.10 (0.10) | 2.03 [1.69; 2.32] |
| SES Unskilled | 86 | 24.7 | 87 | 23.1 | 0.87 (0.56; 1.35) | 1.97 (0.06) | 1.95 [1.58; 2.27] |
| SES Unemployed | 29 | 8.3 | 58 | 15.4 | 1.74 (0.97; 3.18) | 1.80 (0.08) | 1.77 [1.52; 1.99] |
| Missing | 28 | 8.0 | 14 | 3.7 | .. | 1.90 (0.12) | 1.67 [1.52; 2.17] |
| Cohabitation | |||||||
| Single | 7 | 2.0 | 6 | 1.6 | 0.45 (0.13; 1.47) | 1.72 (0.10) | 1.68 [1.47; 2.03] |
| Couple | 323 | 92.8 | 370 | 98.4 | 1.. | 2.03 (0.03) | 1.95 [1.64; 2.35] |
| Missing | 18 | 5.2 | 0 | 0.0 | 1.99 (0.17) | 1.79 [1.52; 2.18] | |
| Residence | |||||||
| Rented | 82 | 23.6 | 135 | 35.9 | 1.58 (1.10; 2.27) | 1.98 (0.07) | 1.84 [1.62; 2.21] |
| Owned | 244 | 70.1 | 239 | 63.6 | 1 | 2.04 (0.04) | 1.99 [1.64; 2.37] |
| Without | 5 | 1.4 | 2 | 0.5 | 0.31 (0.04; 1.54) | 1.91 (0.34) | 1.51 [1.39; 2.57] |
| Missing | 17 | 4.9 | 0 | 0.0 | .. | 2.02 (0.18) | 1.83 [1.50; 2.23] |
| Smoking | |||||||
| Non-smoker | 242 | 69.5 | 236 | 62.8 | 1 | 2.07 (0.04) | 2.01 [1.66; 2.40] |
| Occasional smoker | 39 | 11.2 | 49 | 13.0 | 1.14 (0.70; 1.86) | 2.0 (0.09) | 1.88 [1.69; 2.25] |
| Daily smoker, < 15 | 53 | 15.2 | 75 | 19.9 | 1.56 (1.02; 2.40) | 1.90 (0.08) | 1.87 [1.53; 2.17] |
| Daily smoker, 15+ | 11 | 3.2 | 16 | 4.3 | 1.64 (0.68; 4.03) | 1.72 (0.08) | 1.63 [1.54; 1.90] |
| Missing | 3 | 0.9 | 0 | 0.0 | .. | 1.49 (0.16) | 1.41 [1.27; 1.79] |
| Gestational Age (days), 1st sample | |||||||
| Gest. Age 25–49 | 82 | 23.6 | 98 | 26.1 | .. | 2.01 (0.05) | 1.94 [1.66; 2.22] |
| Gest. Age 49–70 | 167 | 48.0 | 183 | 48.7 | .. | 2.00 (0.05) | 1.88 [1.62; 2.30] |
| Gest. Age 70–135 | 84 | 24.1 | 85 | 22.6 | .. | 2.07 (0.06) | 2.05 [1.59; 2.45] |
| Missing | 15 | 4.3 | 10 | 2.7 | .. | .. | .. |
| Gestational Age (days), 2nd sample | |||||||
| Gest. Age 139–168 | 71 | 20.4 | 89 | 23.7 | .. | 2.01 (0.07) | 1.94 [1.56; 2.32] |
| Gest. Age 168–182 | 179 | 51.4 | 187 | 49.7 | .. | 2.03 (0.04) | 1.93 [1.65; 2.32] |
| Gest. Age 182–256 | 92 | 26.4 | 90 | 23.9 | .. | 2.01 (0.06) | 1.97 [1.61; 2.39] |
| Missing | 6 | 1.7 | 10 | 2.7 | .. | .. | .. |
Table also presents the relationship between timing of blood sample and EPA+DHA plasma concentrations.
Mutual adjustment for covariates: Maternal age at birth, maternal height, parity, maternal pre-pregnancy BMI, SES, cohabitation, residence and smoking.
Missing data for confounders was imputed with mode/mean imputation. The full SAP can be shared on request.
2.5. Data Sharing
DNBC is an open database and the data underlying the presented results in this paper can be shared by sending a request via the regular mechanism (for this, please contact SFO).
3. Results
Gestational age at delivery ranged between 171 and 237 (mean 219.8) days in early preterm cases (n = 376) and between 238 and 307 (mean 280.8) days in controls (n = 348); mean gestational age at 1st blood sampling was 59.7 (SD 17.4) days in cases and 60.1 (SD 16.0) days in controls, and for 2nd sample it was 175.7 (SD 11.8) days in cases and 177.3 (SD 13.0) days in controls (Supplementary Table 1). Early preterm risk varied with age, parity, pre-pregnancy BMI, and daily smoking, and these factors also tended to be associated with EPA+DHA concentrations in both 1st and 2nd sample (Table 1). In cases, mean plasma concentration of EPA+DHA was 1.57% (SEM 0.03) in the 1st and 1.41% (SEM 0.03) in the 2nd sample, which was about 25% lower compared to what was observed among controls where the corresponding numbers were 2.06% (SEM 0.04) in the 1st and 1.99% (SEM 0.04) in the 2nd sample (Supplementary Table 1).
3.1. Tests of Primary Hypotheses Relating Plasma EPA+DHA to Early Preterm Risk (SAP pages 2 and 5 (please see Supplementary Materials))
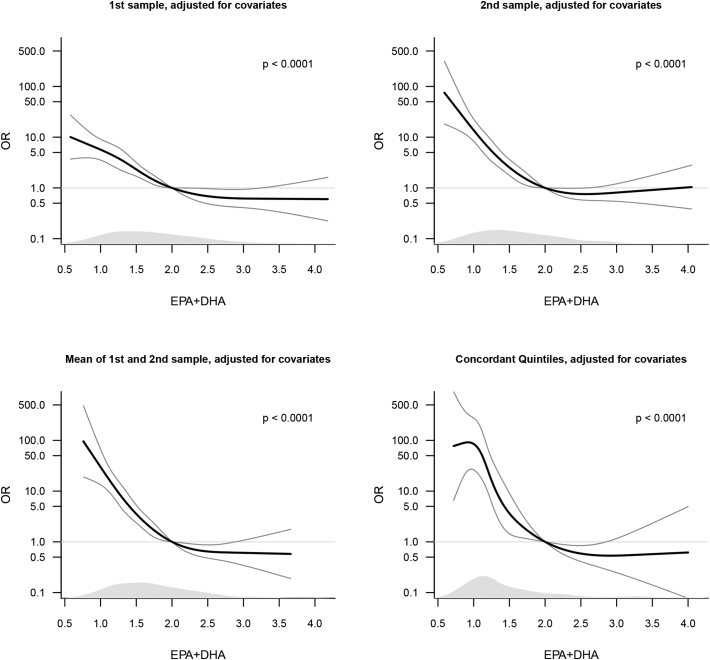
The primary hypothesis specified using the mean values from 1st and 2nd sample EPA+DHA measurements in the spline model. There was a strongly significant association (p < 0.0001), which was non-linear (p < 0.0001) (Table 2C and Fig. 2). Inspection of the spline curves indicated an inflection point located between EPA+DHA concentrations of 2.0% and 2.5%, below which early preterm risk increased steeply and above which curves became flat. Women in the lowest quintile (Q1) had 10.27 times (95% confidence interval 6.80 to 15.79, p < 0.0001) increased odds of early preterm birth, whereas women in the second to the lowest quintile (Q2) had 2.86 (95% CI 1.79 to 4.59, p < 0.0001) times increased odds, compared to women in the aggregated highest three quintiles (Q3 + Q4 + Q5). When limiting the study population to concordant quintiles, being in the lowest quintile at both occasions was associated with 48.3 (95% CI 20.2 to 129.0) times increased risk of early preterm birth, whereas women in the second to the lowest quintile (Q2) had a 6.4 (95% CI 2.4; 18.4) times increase risk, compared to being in one of the highest three quintiles (Table 2D and Fig. 2).
Table 2.
Association between EPA+DHA concentrations and risk of early preterm birth. Shown are results for EPA+DHA measurements solely based on sample 1 (Panel A), solely based on sample 2 (Panel B), mean of sample 1 and 2 (Panel C), and mean of sample 1 and 2 after restricting the analysis to only comprise women in concordant quintiles across sample 1 and 2 (Panel D).
| Controls | Cases | Crude |
Adjusteda |
Controls | Cases | Further adjusted for 2nd//1st sample |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | OR (95% CI) | P-Value | ||||||
| A: 1st trimester sample | |||||||||||
| Test for association in spline regression | – | <0.0001 | – | <0.0001 | – | <0.0001 | |||||
| Test for linear v. spline association | – | 0.0002 | – | 0.001 | – | 0.007 | |||||
| EPA+DHA categorized into quintiles | – | <0.0001 | – | <0.0001 | – | <0.0001 | |||||
| Q1: 0.37–1.48 | 67 | 192 | 5.54 (3.86; 8.02) | <0.0001 | 5.47 (3.72; 8.14) | <0.0001 | 66 | 186 | 3.62 (2.37; 5.58) | <0.0001 | |
| Q2: 1.48–1.81 | 67 | 71 | 2.05 (1.36; 3.09) | 0.0006 | 1.90 (1.23; 2.95) | 0.004 | 65 | 68 | 1.54 (0.95; 2.49) | 0.08 | |
| Q3-Q5: 1.81–4.74 | 199 | 103 | 1 | – | 1 | – | 196 | 102 | 1 | – | |
| B: 2nd trimester sample | |||||||||||
| Test for association in spline regression | – | <0.0001 | – | <0.0001 | – | <0.0001 | |||||
| Test for linear v. spline association | – | <0.0001 | – | <0.0001 | – | <0.0001 | |||||
| EPA+DHA categorized into quintiles | – | <0.0001 | – | <0.0001 | – | <0.0001 | |||||
| Q1: 0.47–1.42 | 68 | 226 | 9.33 (6.42; 13.74) | <0.0001 | 9.61 (6.46; 14.50) | <0.0001 | 66 | 220 | 7.41 (4.87; 11.42) | <0.0001 | |
| Q2: 1.43–1.74 | 69 | 67 | 2.73 (1.78; 4.20) | <0.0001 | 2.71 (1.72; 4.29) | <0.0001 | 66 | 65 | 2.51 (1.56; 4.07) | <0.0001 | |
| Q3-Q5: 1.74–4.95 | 205 | 73 | 1 | <0.0001 – |
1 | <0.0001 – |
195 | 71 | 1 | 0.0002 – |
|
| C: Mean of 1st and 2nd trimester samples | |||||||||||
| Test for association in spline regression | – | <0.0001 | – | <0.0001 | |||||||
| Test for linear v. spline association | – | <0.0001 | – | <0.0001 | |||||||
| EPA+DHA categorized into quintiles | – | <0.0001 | – | <0.0001 | |||||||
| Q1: 0.69–1.56 | 66 | 224 | 9.64 (6.58,14.31) | <0.0001 | 10.27 (6.80,15.79) | <0.0001 | |||||
| Q2: 1.56–1.82 | 65 | 63 | 2.75 (1.77,4.29) | <0.0001 | 2.86 (1.79,4.59) | <0.0001 | |||||
| Q3-Q5: 1.82–4.11 | 196 | 69 | 1 | – | 1 | – | |||||
| D: Mean of 1st and 2nd trimester samples: concordant quintiles only | |||||||||||
| Test for association in spline regression | – | <0.0001 | – | <0.0001 | |||||||
| Test for linear v. spline association | – | <0.0001 | – | <0.0001 | |||||||
| EPA+DHA categorized into concordant quintiles | – | <0.0001 | – | <0.0001 | |||||||
| Q1: | 1st: 0.37–1.48 2nd: 0.47–1.42 |
21 | 140 | 32.12 (15.03,74.33) | <0.0001 | 48.29 (20.18,128.99) | <0.0001 | ||||
| Q2: | 1st: 1.48–1.81 2nd: 1.43–1.74 |
18 | 18 | 4.82 (1.95,12.46) | <0.0001 | 6.42 (2.36,18.61) | <0.0001 | ||||
| Q3-Q5: | 1st: 1.81–4.74 2nd: 1.74–4.95 |
53 | 11 | 1 | – | 1 | – | ||||
| Q4: | 1st: 2.16–2.54 2nd: 2.05–2.45 |
||||||||||
| Q5: | 1st: 2.55–4.74 2nd: 2.47–4.95 |
||||||||||
In the covariate-adjusted analyses, maternal age at birth, maternal height, and maternal pre-pregnancy BMI were in all analyses modelled as restricted cubic splines with 3 knots at the 10th, 50th, and 90th percentiles, whereas parity, socioeconomic status (SES), cohabitation, residence, and smoking were categorized as in Table 1.
Adjusted for covariates: Maternal age at birth, maternal height, parity, maternal pre-pregnancy BMI, SES, cohabitation, residence and smoking.
Fig. 2.
Odds Ratios with 95% CI for early preterm birth given measurements of EPA+DHA modelled as restricted cubic splines with 5 knots. Above the x-axes is shown the density of EPA+DHA measurements estimated in controls. The graphs (but not the spline fitting) was restricted to EPA+DHA measurements between the 1 to 99 percentiles. P-values are for tests of no association in the spline model.
3.2. Other Results (SAP pages 6–7)
When 1st and 2nd sample were examined separately, similar patterns were seen, with highly significant effects and non-linearity (all p < 0.0001) (Table 2A and B and Fig. 2). EPA+DHA concentrations measured in the 2nd sample tended to be more strongly associated with early preterm risk than those in the 1st sample. Thus, for the 1st sample, Q1 women had 5.47 (95% CI 3.72 to 8.14) increased odds of early preterm birth compared to Q3 + Q4 + Q5 women, whereas Q2 women had 1.90 (95% CI 1.23 to 2.95) times increased odds of early preterm birth compared to Q3 + Q4 + Q5 women (Table 2A). For 2nd sample, Q1 women had 9.61 times (95% CI 6.46 to 14.50) and Q2 women had 2.71 (95% CI 1.72 to 4.29) increased odds of early preterm birth compared to Q3 + Q4 + Q5 women (Table 2B). When 1st and 2nd sample EPA+DHA values were adjusted for one another by including them in the same model, the associations of each of the measurements with early preterm birth were slightly attenuated, but each remained highly significant (Table 2A and B, right column) Fig. 2.
Adjustments of the a priori elected covariates had in general no impact on the estimated measures of association between EPA+DHA concentrations and early preterm risk; this was true for the factors listed in Table 1 and also (Supplementary Fig. 1) for 25-OH-vitamin D plasma concentrations measured in the same plasma samples as EPA+DHA.
3.3. Post-hoc Analyses
During the review process we discovered that samples had been stored at different temperatures during the last 2 years before they were taken out of the freezers for analysis. However, adjusting for storage temperature did not affect the association markedly. Thus, for the first sample, after adjustment for freezer temperature, women in the lowest quintile (Q1) had 5.54 times (95% confidence interval 3.86 to 8.02, p < 0.0001) increased odds of early preterm birth, whereas women in the second to the lowest quintile (Q2) had 2.05 (95% CI 1.36 to 3.09, p < 0.0006) times increased odds, compared to women in the aggregated highest three quintiles (Q3 + Q4 + Q5) (the ORs before these adjustments were made can be seen in Table 2). For the second sample, the corresponding ORs after temperature adjustment were also near-identical to the unadjusted (shown in Table 2), i.e. 9.61 (6.42 to 13.7, p < 0.0001) times increased odds in Q1 v. Q3 + Q4 + Q5 and 2.71 (1.78 to 4.20, p < 0.0001) times increased odds in Q2 v. Q3 + Q4 + Q5. In additional analyses we investigated mean levels according to storage temperature, see Supplementary Table 2.
During the process we also made adjustments for certain other fatty acids measured in the plasma samples, several of which were closely correlated with EPA+DHA (correlation matrix for 2nd samples of controls are shown in Supplementary Table 3 (similar patterns were seen for 1st samples, and for cases)). For the first sample, after simultaneous adjustment for linoleic acid (LA), alpha-linolenic acid (ALA), arachidonic acid (AA), the aggregated monounsaturated fatty acids (MUFAs), and the aggregated saturated fatty acids (SFAs), (each of these five variables were modelled with splines) women in the lowest EPA+DHA quintile (Q1) had 1.52 times (95% confidence interval 0.82 to 2.83) increased odds of early preterm birth, whereas women in the second to the lowest EPA+DHA quintile (Q2) had 1.09 (95% CI 0.64 to 1.86) times increased odds, compared to women in the aggregated highest three EPA+DHA quintiles (Q3 + Q4 + Q5) (the associations for 1st sample, before this adjustment, were 5.47 (3.72; 8.14) and 1.90 (1.23; 2.95), respectively; see Table 2). For the second sample, the corresponding ORs after simultaneous adjustment for LA, ALA, AA, MUFAs and SFAs, were 4.08 times (95% confidence interval 2.13 to 7.87) increased odds in EPA+DHA Q1 v. EPA+DHA Q3 + Q4 + Q5 and 2.18 times (95% confidence interval 1.23 to 3.91) increased odds in EPA+DHA Q2 v. EPA+DHA Q3 + Q4 + Q5 (the corresponding associations for 2nd sample, before this adjustment, were 9.61 (6.46; 14.50) and 2.71 (1.72; 4.29), respectively (Table 2)).
4. Discussion
This is the first study to examine if a low plasma concentration of long chain n-3 fatty acids in early and mid-pregnancy is associated with increased risk of subsequent early preterm birth. When taking the average of 1st and 2nd trimester measurements, women in the lowest quintile of the EPA+DHA distribution had a 10 times greater risk of early preterm birth compared to women in the three highest quintiles. When limiting the study population to only those women who were consistently classified into the same quintile in both 1st and 2nd trimester, being in the lowest EPA+DHA quintile at both occasions was associated with 48 times increased risk of early preterm birth, compared to being in one of the highest three quintiles. Spline curves showed a threshold effect at EPA+DHA concentrations somewhere between 2.0% and 2.5%, with sharp increase in risk at low levels which then flattens rapidly out at higher levels.
Earlier studies exploring the relationship between intake of long chain n-3 fatty acids and preterm birth have shown inconsistent results. Several intervention [6, 7] and observational [8, 9] studies have not been able to detect an association between intake of long chain n-3 fatty acids on the one hand and timing of delivery or preterm risk on the other, possibly because these studies were unable to define a group for reference with zero or very low intake of long chain n-3 fatty acids throughout. However, the present study is in agreement with our own early trials [12, 13] and some more recent trials by others [[14], [15], [16]], that showed beneficial effects on preterm risk or related parameters.
A smaller observational prospective study in Danish women [10, 11], which used an analytic strategy that was similar to that used here but with exposure based on dietary instead of biomarker information, is noteworthy because of its findings' similarity to those of the present study. Compared to women who in both early and mid-pregnancy consistently reported eating fish at least once a week, women who on both occasions reported never to consume fish had 19.6 (95% CI 2.3 to 165) times greater odds of preterm birth (Table 3 in [11]), an effect size comparable to those observed in the present study. We know of one other biomarker study, where erythrocyte fatty acid levels assessed in mid-pregnancy was related to subsequent risk of preterm birth. This study, by Klebanoff and colleagues [25], was undertaken within the framework of a clinical trial in high risk women with a history of previous preterm birth, who were treated with weekly injections of 17 alpha-hydroxyprogesterone Caproate, and half of whom were randomized to receive a supplement with EPA and DHA. The trial could detect no effect of the supplement on preterm risk. However, after adjustment for randomization group, women who at baseline belonged to the lowest quartile of erythrocyte EPA+DHA in mid-pregnancy tended to have lower risk of subsequent preterm birth, as compared to women in the upper three quartiles.
Our study had many strengths. It was based on a large national birth cohort [21] enabling adjustment for many potential confounders including smoking, parity, vitamin D status, and BMI. All early preterm cases were verified by review of the actual clinical records [22]. Our comprehensive statistical analyses of the relationship between the EPA+DHA and preterm risk strictly followed a detailed Statistical Analysis Plan that had been defined a priori. Because many statistical tests were foreseen, we were careful to prioritize and define a priori a small core set of primary hypotheses, which all tested highly significant. A potential weakness of the study was its observational design and the potential for unmeasured confounding. However, given the strength of the observed associations and the prospective design–with exposure assessment many weeks prior to the studied event–confounding or reverse causation would seem unlikely to fully explain away the results.
Our findings may help increase our understanding of the underlying biology of preterm birth, and how to prevent it and its related complications. Several mechanisms have been suggested to explain an effect of dietary long chain fatty acids on preterm birth. They may impact the production of eicosanoids involved in the parturition process [5], the electrical activity of the myometrium [31, 32], the regulation of oxytocin signalling [33] and inflammatory pathways by an increase in resolvin R3 production [34]. The strong inverse associations observed between plasma concentrations of EPA+DHA and subsequent early preterm birth suggest that body levels of essential fatty acids (or their close correlates) are causally implicated in the physiologic processes leading up to premature labour and delivery.
These observations may support the utility of an intervention focused on changing diet or supplementation based on circulating levels. Extending pregnancy even by a few days could be of significant clinical value in foetuses in danger of being delivered prior to 34 weeks [1, 2].
A cautious interpretation of the observations reported here is warranted, however. Firstly, our study was undertaken in Denmark, wherein the rates of preterm birth are historically very low. How supplementation would work in populations with high rates of co-morbidities with other conditions like hypertension, diabetes, or infection, is a question that needs to be explored. Population-based observational studies should also be conducted elsewhere to determine if the observed associations here can be replicated.
Secondly, it is possible that non-dietary factors could, partially or fully, explain the associations observed; in other words, that the patterns we see might mechanistically be non-dietary in nature. Plasma concentrations of EPA+DHA at any point during pregnancy is influenced not only by a woman's intake of fatty acids [20] before and during pregnancy but also by placental transfer of essential fatty acids from the mother to the growing foetus, as well as by her own genetically determined capacity to metabolize polyunsaturated fatty acids [35]. A recent study demonstrated that gestation duration is associated with fatty acid desaturase (FADS) gene variants known to affect polyunsaturated fatty acid metabolism [36]. In principle, it is therefore possible that variation in FADS genes could underlie the observed association between EPA+DHA and early preterm risk, and even underlie the observed weakening of this association after adjustment for other fatty acids if these are also controlled by FADS genes. Research is needed to understand the relative contributions of dietary versus genetic factors in influencing the associations between n-3 fatty acid status and risk of early preterm delivery. Irrespective of which mechanisms may be underlying the associations we observed, plasma measurements of EPA and DHA in pregnancy may be used to identify women at risk in clinical practice. This, together with the suggestion that the observed inverse relationship between the plasma concentration of EPA+DHA and early preterm risk is dose-dependent – strongest at low and absent at high concentrations – may turn out to be the main implications of the present study.
In conclusion, we found that low plasma concentrations of total EPA and DHA in pregnancy is a strong risk factor for subsequent early preterm birth in Danish women.
Funding Sources
Financial support was received from March of Dimes Birth Defects Foundation (6-FY-96-0240, 6-FY97-0553, 6-FY97-0521, 6-FY00-407), Innovation Fund Denmark (grant no. 09-067124, ‘Centre for Fetal Programming’), Danish Council for Independent Research (grant no. 1331-00229A, 9601842 and 22-03-0536), the Health Foundation (11/263-96), and the Heart Foundation (96-2-4-83-22450), Danish National Research Foundation. This research has been conducted using the Danish National Biobank resource, supported by the Novo Nordisk Foundation, grant number 2010-11-12 and 2009-07-28. The funding agencies did not have any role in design and conduct of the study; collection, management, and interpretation of the data; or preparation, review, or approval of the manuscript.
Declaration of Interests
The authors declare no potential, perceived, or real conflict of interest regarding the content of this manuscript.
Author Contributions
SFO: conception and design of the work; acquisition and interpretation of data for the work; drafting the work and revising it critically for important intellectual content. TIH: Design of the work; interpretation of data for the work, revising the work critically for important intellectual content. ALT-L: design of the work; interpretation of data for the work; revising the work critically for important intellectual content. MS: design of the work; interpretation of data for the work; revising the work critically for important intellectual content. SG: analysis and interpretation of data for the work; revising the work critically for important intellectual content. CG: analysis and interpretation of data for the work; revising the work critically for important intellectual content. PHN: analysis of data for the work; revising the work critically for important intellectual content. JW: interpretation of data for the work; revising the work critically for important intellectual content. JAL: acquisition of data for the work; drafting the work and revising it critically for important intellectual content. JL-R: acquisition of data for the work; drafting the work and revising it critically for important intellectual content. ASC: acquisition of data for the work; revising the work critically for important intellectual content. JDF: acquisition of data for the work; revising the work critically for important intellectual content. ELG: interpretation of data for the work; revising the work critically for important intellectual content. WZ: interpretation of data for the work; revising the work critically for important intellectual content. All authors: final approval of the version to be published; agree to be accountable for all aspects for the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The following are the supplementary data related to this article.
Odds Ratios with 95% CI for early preterm birth given measurements of EPA+DHA modelled as restricted cubic splines with 5 knots, with covariates as in Fig. 1 and with additional adjustment for 25-OH-vitamin D. The splines for 1st and 2nd sample EPA+DHA is adjusted for 25-OH-vitamin D in the 1st and 2nd sample, respectively, whereas the splines for the mean of 1st and 2nd sample EPA+DHA and for concordant quintiles is adjusted for both 1st and 2nd sample 25-OH-vitamin D measurements. Above the x-axes is shown the density of EPA+DHA measurements. The graphs (but not the spline fitting) was restricted to EPA+DHA measurements between the 1 to 99 percentiles. P-values is for tests of no association in the spline model.
Supplementary Tables
Statistical Analysis Plan (SAP) for the study “EPA+DHA plasma concentrations at two points in pregnancy and risk of early preterm birth: A case control study within the Danish National Birth Cohort”, SAP Version 2.0, 12 July 2017.
Acknowledgments
Acknowledgements
Medical Student, Bjarki Enni, provided excellent assistance in relation to literature searches and text editing.
References
- 1.Blencowe H., Cousens S., Oestergaard M.Z. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (Lond Engl) 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Frey H.A., Klebanoff M.A. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. 2016;21(2):68–73. doi: 10.1016/j.siny.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Olsen S.F., Joensen H.D. High liveborn birth weights in the Faroes: a comparison between birth weights in the Faroes and in Denmark. J Epidemiol Community Health. 1985;39(1):27–32. doi: 10.1136/jech.39.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen S.F., Hansen H.S. Marine fat, birthweight, and gestational age: a case report. Agents Actions. 1987;22(3–4):373–374. [Google Scholar]
- 5.Olsen S.F., Hansen H.S., Sorensen T.I. Intake of marine fat, rich in (n-3)-polyunsaturated fatty acids, may increase birthweight by prolonging gestation. Lancet (Lond Engl) 1986;2(8503):367–369. doi: 10.1016/s0140-6736(86)90055-3. [DOI] [PubMed] [Google Scholar]
- 6.Harper M., Thom E., Klebanoff M.A. Omega-3 fatty acid supplementation to prevent recurrent preterm birth: a randomized controlled trial. Obstet Gynecol. 2010;115(2 Pt 1):234–242. doi: 10.1097/AOG.0b013e3181cbd60e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramakrishnan U., Stein A.D., Parra-Cabrera S. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr Bull. 2010;31(2 Suppl):S108–S116. doi: 10.1177/15648265100312S203. [DOI] [PubMed] [Google Scholar]
- 8.Oken E., Kleinman K.P., Olsen S.F., Rich-Edwards J.W., Gillman M.W. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol. 2004;160(8):774–783. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers I., Emmett P., Ness A., Golding J. Maternal fish intake in late pregnancy and the frequency of low birth weight and intrauterine growth retardation in a cohort of British infants. J Epidemiol Community Health. 2004;58(6):486–492. doi: 10.1136/jech.2003.013565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen S.F., Secher N.J. Low consumption of seafood in early pregnancy as a risk factor for preterm delivery: prospective cohort study. BMJ (Clin Res Ed) 2002;324(7335):447. doi: 10.1136/bmj.324.7335.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen S.F., Osterdal M.L., Salvig J.D. Duration of pregnancy in relation to seafood intake during early and mid pregnancy: prospective cohort. Eur J Epidemiol. 2006;21(10):749–758. doi: 10.1007/s10654-006-9053-6. [DOI] [PubMed] [Google Scholar]
- 12.Olsen S.F., Sorensen J.D., Secher N.J. Randomised controlled trial of effect of fish-oil supplementation on pregnancy duration. Lancet (Lond Engl) 1992;339(8800):1003–1007. doi: 10.1016/0140-6736(92)90533-9. [DOI] [PubMed] [Google Scholar]
- 13.Olsen S.F., Secher N.J., Tabor A., Weber T., Walker J.J., Gluud C. Randomised clinical trials of fish oil supplementation in high risk pregnancies. Fish Oil Trials In Pregnancy (FOTIP) team. BJOG. 2000;107(3):382–395. doi: 10.1111/j.1471-0528.2000.tb13235.x. [DOI] [PubMed] [Google Scholar]
- 14.Makrides M., Gibson R.A., McPhee A.J., Yelland L., Quinlivan J., Ryan P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010;304(15):1675–1683. doi: 10.1001/jama.2010.1507. [DOI] [PubMed] [Google Scholar]
- 15.Carlson S.E., Colombo J., Gajewski B.J. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97(4):808–815. doi: 10.3945/ajcn.112.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauner H., Much D., Vollhardt C. Effect of reducing the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: an open-label randomized controlled trial. Am J Clin Nutr. 2012;95(2):383–394. doi: 10.3945/ajcn.111.022590. [DOI] [PubMed] [Google Scholar]
- 17.Chen B., Ji X., Zhang L., Hou Z., Li C., Tong Y. Fish oil supplementation improves pregnancy outcomes and size of the newborn: a meta-analysis of 21 randomized controlled trials. J Matern Fetal Neonatal Med. 2016;29(12):2017–2027. doi: 10.3109/14767058.2015.1072163. [DOI] [PubMed] [Google Scholar]
- 18.Saccone G., Berghella V. Omega-3 supplementation to prevent recurrent preterm birth: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2015;213(2):135–140. doi: 10.1016/j.ajog.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Makrides M., Duley L., Olsen S.F. Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD003402.pub2. (Cd003402) [DOI] [PubMed] [Google Scholar]
- 20.Hodson L., Skeaff C.M., Fielding B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47(5):348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Olsen J., Melbye M., Olsen S.F. The Danish National Birth Cohort--its background, structure and aim. Scand J Public Health. 2001;29(4):300–307. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 22.Lykke J.A., Bare L.A., Olsen J. Thrombophilias and adverse pregnancy outcomes: results from the Danish national birth cohort. J Thromb Haemost. 2012;10(7):1320–1325. doi: 10.1111/j.1538-7836.2012.04773.x. [DOI] [PubMed] [Google Scholar]
- 23.Olsen S.F., Hansen H.S., Jensen B., Sorensen T.I. Pregnancy duration and the ratio of long-chain n-3 fatty acids to arachidonic acid in erythrocytes from Faroese women. J Intern Med Suppl. 1989;731:185–189. doi: 10.1111/j.1365-2796.1989.tb01454.x. [DOI] [PubMed] [Google Scholar]
- 24.Olsen S.F., Hansen H.S., Sommer S. Gestational age in relation to marine n-3 fatty acids in maternal erythrocytes: a study of women in the Faroe Islands and Denmark. Am J Obstet Gynecol. 1991;164(5):1203–1209. doi: 10.1016/0002-9378(91)90683-i. Pt 1. [DOI] [PubMed] [Google Scholar]
- 25.Klebanoff M.A., Harper M., Lai Y. Fish consumption, erythrocyte fatty acids, and preterm birth. Obstet Gynecol. 2011;117(5):1071–1077. doi: 10.1097/AOG.0b013e31821645dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen S.F., Osterdal M.L., Salvig J.D., Weber T., Tabor A., Secher N.J. Duration of pregnancy in relation to fish oil supplementation and habitual fish intake: a randomised clinical trial with fish oil. Eur J Clin Nutr. 2007;61(8):976–985. doi: 10.1038/sj.ejcn.1602609. [DOI] [PubMed] [Google Scholar]
- 27.Baylin A., Kabagambe E.K., Siles X., Campos H. Adipose tissue biomarkers of fatty acid intake. Am J Clin Nutr. 2002;76(4):750–757. doi: 10.1093/ajcn/76.4.750. [DOI] [PubMed] [Google Scholar]
- 28.Zock P.L., Gerritsen J., Katan M.B. Partial conservation of the sn-2 position of dietary triglycerides in fasting plasma lipids in humans. Eur J Clin Invest. 1996;26(2):141–150. doi: 10.1046/j.1365-2362.1996.t01-1-105263.x. [DOI] [PubMed] [Google Scholar]
- 29.Zock P.L., Mensink R.P., Harryvan J., de Vries J.H., Katan M.B. Fatty acids in serum cholesteryl esters as quantitative biomarkers of dietary intake in humans. Am J Epidemiol. 1997;145(12):1114–1122. doi: 10.1093/oxfordjournals.aje.a009074. [DOI] [PubMed] [Google Scholar]
- 30.Thorsen S.U., Marild K., Olsen S.F. Lack of association between maternal or neonatal vitamin D status and risk of childhood type 1 diabetes: a Scandinavian case-cohort study. Am J Epidemiol. 2018;187(6):1174–1181. doi: 10.1093/aje/kwx361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baguma-Nibasheka M., Brenna J.T., Nathanielsz P.W. Delay of preterm delivery in sheep by omega-3 long-chain polyunsaturates. Biol Reprod. 1999;60(3):698–701. doi: 10.1095/biolreprod60.3.698. [DOI] [PubMed] [Google Scholar]
- 32.Olsen S.F., Secher N.J., Bjornsson S., Weber T., Atke A. The potential benefits of using fish oil in relation to preterm labor: the case for a randomized controlled trial? Acta Obstet Gynecol Scand. 2003;82(11):978–982. doi: 10.1034/j.1600-0412.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim P.Y., Zhong M., Kim Y.S., Sanborn B.M., Allen K.G. Long chain polyunsaturated fatty acids alter oxytocin signaling and receptor density in cultured pregnant human myometrial smooth muscle cells. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0041708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamashita A., Kawana K., Tomio K. Increased tissue levels of omega-3 polyunsaturated fatty acids prevents pathological preterm birth. Sci Rep. 2013;3:3113. doi: 10.1038/srep03113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Neill C.M., Minihane A.M. The impact of fatty acid desaturase genotype on fatty acid status and cardiovascular health in adults. Proc Nutr Soc. 2017;76(1):64–75. doi: 10.1017/S0029665116000732. [DOI] [PubMed] [Google Scholar]
- 36.Bernard J.Y., Pan H., Aris I.M. Long-chain polyunsaturated fatty acids, gestation duration, and birth size: a Mendelian randomization study using fatty acid desaturase variants. Am J Clin Nutr. 2018 Jul 1;108(1):92–100. doi: 10.1093/ajcn/nqy079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Odds Ratios with 95% CI for early preterm birth given measurements of EPA+DHA modelled as restricted cubic splines with 5 knots, with covariates as in Fig. 1 and with additional adjustment for 25-OH-vitamin D. The splines for 1st and 2nd sample EPA+DHA is adjusted for 25-OH-vitamin D in the 1st and 2nd sample, respectively, whereas the splines for the mean of 1st and 2nd sample EPA+DHA and for concordant quintiles is adjusted for both 1st and 2nd sample 25-OH-vitamin D measurements. Above the x-axes is shown the density of EPA+DHA measurements. The graphs (but not the spline fitting) was restricted to EPA+DHA measurements between the 1 to 99 percentiles. P-values is for tests of no association in the spline model.
Supplementary Tables
Statistical Analysis Plan (SAP) for the study “EPA+DHA plasma concentrations at two points in pregnancy and risk of early preterm birth: A case control study within the Danish National Birth Cohort”, SAP Version 2.0, 12 July 2017.