Abstract
Multiple system atrophy (MSA) is a rapidly-progressing fatal synucleinopathy of the aging population characterized by parkinsonism, dysautonomia, and in some cases ataxia. Unlike other synucleinopathies, in this disorder the synaptic protein, α-synuclein, (α-syn) predominantly accumulates in oligodendroglial cells (and to some extent in neurons), leading to maturation defects of oligodendrocytes, demyelination, and neurodegeneration. The mechanisms through which α-syn deposits occur in oligodendrocytes and neurons in MSA is not completely clear. While some studies suggest that α-syn might transfer from neurons to glial cells, others propose that α-syn might be aberrantly over-expressed by oligodendroglial cells. A number of in vivo models have been developed, including transgenic mice over-expressing α-syn under oligodendroglial promoters (eg: MBP, PLP, and CNP). Other models have been recently developed either by injecting synthetic α-syn fibrils or brain homogenates from patients with MSA into wild-type mice or by using viral vectors expressing α-syn under the MBP promoter in rats and non-human primates. Each of these models reproduces some of the neuropathological and functional aspects of MSA, however, none of them fully replicate the spectrum of MSA. Understanding better the mechanisms of how α-syn accumulates in oligodendrocytes and neurons will help in developing better models that recapitulate various pathogenic aspects of MSA in combination with translatable biomarkers of early stages of the disease that are necessary to devise disease modifying therapeutics for MSA.
1. What is MSA
Synucleinopathies [40] of the aging population are a group of neurodegenerative disorders that affect over 1.5 million people in the US alone [110] and include Parkinson’s disease (PD), PD dementia, dementia with Lewy bodies (DLB) and multiple system atrophy (MSA). While considerable effort has been devoted to understanding PD and DLB, less is known about MSA, which is a fatal, rapidly-progressing neurodegenerative disorder characterized by motor, autonomic, and non-motor deficits associated with oligodendroglial accumulation of α-synuclein (α-syn) [68, 151, 163] (Figure 1a). MSA is differentiated from other synucleinopathies both clinically, by the rapid progression of the disease and the lack of response to L-DOPA [156], and pathologically, by the extensive accumulation of α-syn within oligodendrocytes differentiates MSA from other synucleinopathies [22].
Figure 1.
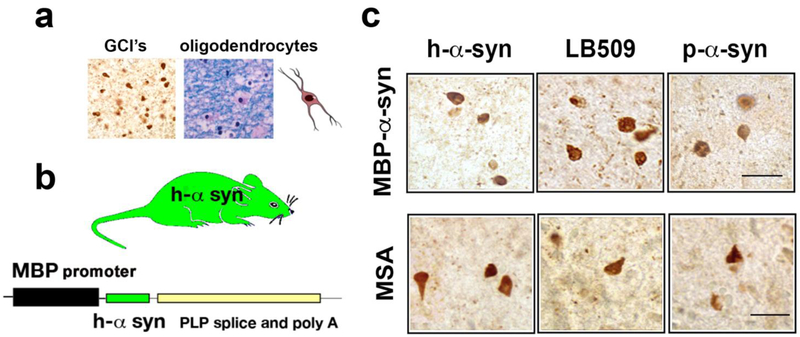
Comparison of α-synuclein accumulation in oligodendroglial cells in the MBP-α-syn and in MSA. (a) α-synuclein accumulation (left panel) in oligodendroglial cells forming glial cytoplasmic inclusions (GCI’s). Luxol fast blue staining of myelin and oligodendrocytes in MSA brain (right panel). (b) Comparison of α-synuclein inclusions in the MBP model and MSA, images are from the white matter tracts in the striatum immunostained with antibodies against h-α-synuclein, misfolded α-synuclein (LB509 clone) and p-Ser129-α-synuclein. Bar=25 μm. (c) Schematic representation of the MBP transgenic mouse model of MSA driving human α-synuclein.
The term MSA was first utilized in the late 1960’s to include three previously described neurodegenerative disorders that included, striatonigral degeneration, olivopontocerebellar ataxia, and “Shy-Drager syndrome” [45]. MSA is estimated to affect 3 out of every 100,000 in the 50 years and older population [42, 44, 121]. The mean age of disease onset is around 60 years and the mean survival ranges from 7–9 years following the appearance of clinical symptoms [115].
Disorders under the MSA spectrum can be divided into parkinsonian and cerebellar categories. Extra-pyramidal motor abnormalities such as bradykinesia, rigidity and postural instability are included in the Parkinsonian-type (MSA-P), while the cerebellar (MSA-C) form also manifests with ataxia [14, 43, 44, 62-64]. In addition, patients with MSA also develop behavioral alterations such as depression and executive dysfunction that suggest frontal lobe impairment [7, 26, 34, 113, 114]. Autonomic dysfunction, most commonly urogenital, gastrointestinal and cardiovascular dysfunction in the form of orthostatic hypotension, eventually develops in both MSA-P and MSA-C patients [98]. Population studies in the US, Europe [44], and Japan [160] indicate an ethnic variation with one study reporting that 60% of European patients had MSA-P and 13% exhibited MSA-C [44]. In contrast, the Japanese study reported a much higher percentage of patients (83.8%) exhibiting MSA-C features with only 16.2% of patients being categorized as MSA-P [160]. The basis for this variability remains unclear but might involve genetic and/or environmental factors.
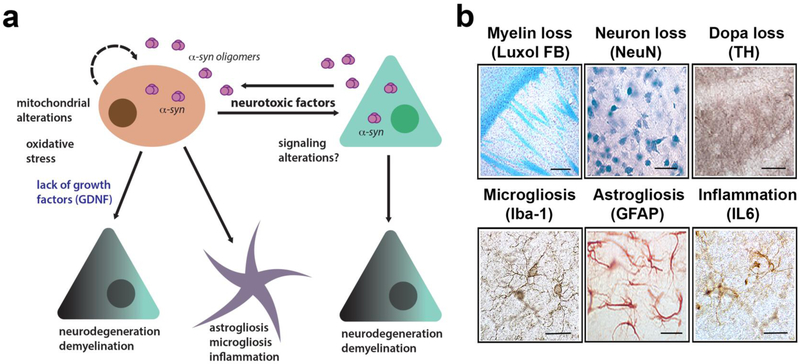
α-Synuclein is a 140 amino acid long synaptic protein [53, 143] that has a role in vesicular neurotransmitter release [89, 116]. The native structure of α-syn continues to be an area of scientific research. Under physiological conditions, α-syn is primarily produced by neuronal cells as a natively unfolded protein [28, 69, 155], which occasionally might arrange into a stable tetramer [5]. Recent studies continue to explore the membrane-bound structure of α-syn [37, 38, 149]; however, consensus on the native structure has yet to be achieved. Under pathological conditions such as those typical of PD, DLB, and MSA, α-syn accumulates in neuronal and non-neuronal cells forming low and high molecular weight oligomers, protofibrils and fibrils [17]. While in PD and DLB α-syn aggregates are more frequently observed in neuronal cell bodies, synapses, axons and astroglial [11, 120, 137] (and to a lesser extent in oligodendrocytes), in MSA α-syn aggregates primarily accumulate in oligodendroglial cells (Figure 1) and to a lesser extent in neurons [19, 47, 57]. In PD and DLB the intraneuronal α-syn inclusions are known as Lewy bodies (LBs) and Lewy neurites (LNs). In MSA, oligodendroglial inclusions containing α-syn are known as glial cytoplasmic inclusions, (GCIs) (Figure 1) and in neuronal cells as neuronal cytoplasmic inclusions (NCIs) [55, 68]. Most studies have focused on α-syn accumulation in glial cells and GCIs because of their abundance in MSA. However, a recent study centered on characterizing the neuronal accumulation of α-syn in MSA patients found a greater presence of NCIs than had been previously reported, most likely due to the sensitivity of the LB509 antibody [19], which detects pathological α-syn. α-synuclein was found in neurons not only in brain regions associated with MSA (striatum and substantia nigra) but also within the anterior cingulate cortex, amygdala, entorhinal cortex, basal forebrain and hypothalamus [19]. However, α-syn accumulated to a greater extent in oligodendrocytes than in neurons in those MSA cases. This accumulation was accompanied by neuroinflammation [127, 147], demyelination [80, 157] and neurodegeneration [54, 140] (Figure 2a) that in turn might lead to the classical clinical features and deficits of MSA-P and MSA-C.
Figure 2.
Pathogenic mechanisms in MSA triggered by α-synuclein leading to neurodegeneration. (a) Diagrammatic representation of the hypothetical mechanisms neurodegeneration in MSA. α-Synuclein transmits from neurons to oligodendroglial cells leading to mitochondrial dysfunction, oxidative stress and loss of trophic support of neurons (e.g.: GDNF), this, in turn, is associated with neuroinflammation, demyelination, and neuronal degeneration. (b) Characterization of neuropathology in the MBP-α-synuclein tg mice includes myelin loss (luxol fast blue, bar=100 μm), neuronal loss (NeuN, bar=50 μm), loss of DOPA fibers (tyr hydroxylase TH, bar=50 μm), microgliosis (Iba1, bar=25 μm), astrogliosis (GFAP, bar=25 μm) and IL6 expression (bar=25 μm). Images are from the striatum of the MBP Line1 age 12 months.
Taken together, these studies suggests that modeling of MSA might require a number of initial “hits” within neurons and oligodendroglial cells that trigger α-syn accumulation [19] followed by the typical neuropathological features of MSA such as neuroinflammation [127, 147], demyelination [80, 157] and neurodegeneration [54, 140] (Figure 2a) that results in the characteristic clinical manifestations of these disorders. Currently, available models mimic some of these aspects, but none fully reproduce the disease (Figure 2) (Table 1). The next sections will describe these models, as well as their advantages and pitfalls.
Table 1:
Summary of α-syn animal models
| Promoter | Gene | Neurological alterations |
α-Syn pathology |
Glial pathology | Neuronal pathology |
|---|---|---|---|---|---|
| PLP [59] | α-syn | Short stride [126] | Insoluble Pser129; GCI-like | Microglial activation | Loss of SN |
| CNP [161] | α-syn | Rotarod deficits | Insoluble aggregates GCI-like | Oligodendroglial loss | Spinal cord, axonal dystrophy |
| MBP [118] | α-syn | Tremor, Pole, RR beam walking test, grip strength and olfactory deficits and stride variability [118, 126, 142] |
α-syn inclusions | Oligodendroglial loss, astrogliosis, myelin loss | Loss of SN, axonal dystrophy, cortical neuron loss laterodorsal tegmental nucleus, pedunculopontine tegmental nucleus, Onuf’s nucleus, nucleus ambiguus, Barrington’s nucleus, raphe obscurus and pallidus [10, 35, 65, 66, 131] |
| AAV-mediated [6] | human wild-type alpha-synuclein | Stepping, adhesion removal | Insoluble α-syn aggregates; Insoluble Pser129 | Loss of striatal neurons, SN, |
2. What aspects of MSA do we want to model in vivo
Most patients with MSA do not respond to conventional PD treatments and no disease-modifying therapy is available [152]. Therefore, there is an urgent need to develop novel therapeutics for this devastating disorder. Modeling the disease in vitro and in animal models would help advance this goal by helping to better understand the pathogenesis and mechanisms of neurodegeneration in MSA and by allowing the testing of new treatment approaches [130]. The main problem is that although there are a few reports of familial MSA [31, 48, 119, 159] there are no unique gene mutations linked to familial forms of the disease that will help guide the development of animal models. MSA is a heterogeneous disorder with MSA-P being more predominant in the US and Europe while MSA-C is more common in Asia. Moreover, it is unclear if the process begins in neuronal cells and then spreads to non-neuronal cells (such as oligodendrocytes) and what is the relative contribution of genetic versus environmental factors in the disease [8] (Figure 2a). Furthermore, patients with MSA manifest a variety of extra-pyramidal, autonomic, and cerebellar features that are difficult to model in rodents [8]. Therefore, at best the current models might be able to reproduce some of the neuropathological and functional aspects of the disease that might translate into some predictive value toward therapeutic development. For example, studies in one of the α-syn transgenic overexpressing models [78, 118] (Figure 2 and Table 1), have led to a clinical trial to test the value of α-syn vaccination in MSA [77]. However, since the mechanisms through which α-syn accumulates within oligodendroglial cells in MSA are not completely understood [12] (Figure 3), as yet it is unclear what is the full translational value of the currently available models. Better paradigms will need to be developed in the near future [8].
Figure 3.
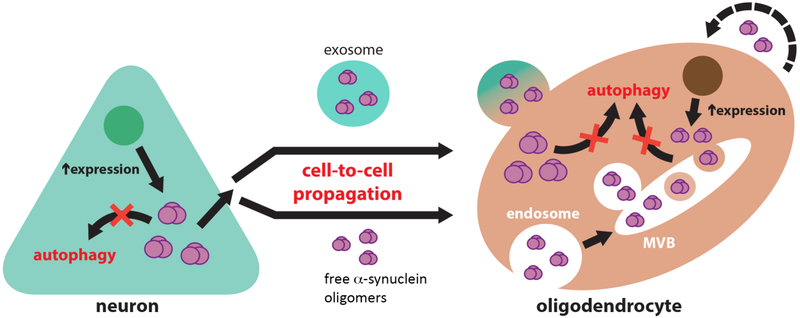
Diagrammatic representation of the potential role of defective clearance in the mechanisms of α-syn transmission from neurons to oligodendroglial cells. Inhibition of autophagic clearance in neurons overexpressing α-syn may lead to an increase in its cytosolic accumulation and to the release of toxic α-syn species to the extracellular space. Extracellular α-syn may propagate from neurons to oligodendrocytes either within extracellular vesicles (exosomes) or as free-floating protein species. Oligodendrocytes are able to uptake extracellular α-syn, and due to the inhibition of oligodendroglial autophagic clearance, accumulate in the form of glial cytoplasmic inclusions at an enhanced rate. It is also possible that α-syn expression is also concurrently elevated in oligodendroglial cells, which also may release α-syn to the extracellular space in a similar fashion to neurons, thus potentiating the cell-to-cell spreading of toxic conformations of this protein.
The key neuropathological feature of MSA is the presence of abundant α-synpositive GCIs in the white matter tracts, cortex, striatum, brainstem and cerebellum [56] and NCI’s in the cortex (Figure 1). For this reason, most efforts at modeling MSA have been focused at reproducing the accumulation of α-syn in oligodendroglial cells [8]. This fundamental finding presupposes that accumulation of α-syn in oligodendroglial cells triggers the demyelinating, neurodegenerative and neuroinflammatory cascades in MSA (Figure 2) that results in the characteristic clinical features of parkinsonism, dysautonomia, and ataxia [8].
The GCIs are found in proximity to or surrounding the nucleus of oligodendrocytes (Figure 1c). Ultrastructurally, the GCIs are composed of loosely packed filaments of α-syn [16, 94, 117]. However, GCIs also contain p25α, αβ-crystallin, MAP2, ubiquitin and tubulin among other proteins [39]. In MSA the α-syn in GCIs is phosphorylated at residue Ser-129 (Figure 1c) and ubiquitinated, as is the case in Lewy bodies of PD and DLB [36], however other post-transcriptional modifications such as oxidation, nitrosylation, and acetylation are possible. As mentioned before, in MSA α-syn also accumulates in neurons as so-called NCIs [19, 22, 68, 151]. Alterations in the solubility of α-syn are detected in extracts of MSA affected brains, with insoluble α-syn accumulation in SDS fractions specific to disease affected [15, 22, 25, 59, 138]. These observations suggest a shift in the solubility of α-syn out of the cytosolic compartment may be a key step in MSA pathogenesis.
A major unanswered question in the field that is critical toward developing valid models is why and how α-syn tends to accumulate to a greater extent in oligodendrocytes compared to neurons (Figure 3). One possibility is that since α-syn is typically expressed by neurons, levels of expression, clearance or release of α-syn in neuronal cells is altered resulting in a secondary accumulation in non-neuronal cells such as oligodendrocytes. Another option is that α-syn is produced by oligodendroglial cells which in turn over-express or fail to intrinsically clear out α-syn, or that α-syn that propagates from neurons cannot be cleared out by oligodendrocytes due to defective vesicle trafficking and/or clearance mechanisms (Figure 3). The source of α-syn in oligodendroglial cells in MSA is unclear. While previous studies initially reported an absence of α-syn mRNA in oligodendroglial cells [58, 82, 92] (Figure 3), more recent studies suggest that α-syn mRNA might be detected in oligodendroglial cells [4, 18, 67, 87, 103]. Given the expression levels [13, 83, 84] and widespread distribution of α-syn aggregates in MSA, it is possible that propagation from both neurons and oligodendroglial α-syn expression might be occurring simultaneously. Supporting the possibility of neuronal propagation, several studies have shown that α-syn aggregates can transmit from neuron to neuron [21], neuron to astroglial [72] and oligodendroglial cells [102], and oligodendroglial to astroglial cells [147], leading to neuronal dysfunction [60, 150], apoptosis [21] and neuroinflammation [72, 147] (Figure 2). Moreover, recent studies have shown that injection of homogenates from MSA brains propagate α-syn pathology in a prion-like fashion in the murine brain [153]. Neuronal cells (donors) release α-syn aggregates into the extracellular environment by exocytosis and in clear vesicles [70] and exosomes [20], and α-syn is taken up by other neurons, oligodendrocytes, and astrocytes (acceptors) via endocytosis [71] (Figure 3).
While neuronal loss is a well-known hallmark of PD, investigating to what extent large numbers of oligodendroglia are lost in MSA is ongoing. Using stereology, Nykjaer et al. did not observe any significant oligodendrocyte degeneration in MSA patients compared to controls [91], while another study reported a loss in the putamen and globus pallidus [108]. Other studies correlating immunohistochemical markers suggested evidence of oligodendrocyte degeneration in MSA patients [1, 106]. In light of these data indicating a limited loss of oligodendroglial cells despite myelin loss, an investigation into the status of oligodendrocyte precursor cells (OPCs) in MSA cases is of great interest since these cells are responsible for remyelination of the axons. Until recently, immunohistochemical visualization of OPCs was challenging due to the lability of OPC-specific antigens; however, a recent study showed that OPCs are increased in the cerebellar white matter with increasing severity of GCIs in MSA cases [1]. Moreover, other studies have shown that intracellular accumulation of α-syn in oligodendroglial cells retards the maturation of OPC’s and myelination in the striatum white matter tracts and corpus callosum in MSA patients and transgenic mice [29, 30, 81].
Therefore, the key problem is that it is difficult to determine how to model MSA in vivo when we do not know how or where the process starts. The next section describes models of MSA-like pathology that were developed assuming that key pathological events might occur in oligodendrocytes [8] (Table 1).
3. Transgenic models over-expressing α-syn in oligodendroglial cells
To date, the most widely used models of MSA-like pathology involve the over-expression of wild-type human α-syn from oligodendroglial promoters namely, the proteolipid protein promoter (PLP) [59]; the 2’,3’-cyclic nucleotide 3’- phosphodiesterase promoter (CNP) [162] and the myelin basic protein (MBP) promoter reported by Shults and colleagues [118] (Table 1). Each of these models displays extensive accumulation of α-syn in oligodendroglial cells (Figure 2) (Table 1) in the cortex, striatum, corpus callosum and brainstem with a distribution similar to MSA [8]. The accumulation of α-syn is accompanied by neuroinflammatory and neurodegenerative pathology (Figure 2b) and some functional deficits. However the behavioral alterations are mild to moderate and require high levels of expression of α-syn in oligodendroglial cells (Table 1) that might not be physiological or comparable to what it is observed in patients with MSA (Figure 2b), where there is controversial data from the increased expression of α-syn in oligodendrocytes [8]. Below we describe each of these models with their corresponding neuropathological and behavioral deficits. The last section of this review discusses the pros and cons of these animal models and what might be needed to develop better models of MSA. It is important to take into account that these models (PCP, CNP, and MBP) mostly reproduce the oligodendroglial component of MSA; however, they lack the neuronal pathology that recent studies have shown to be important [19]. In this context, in the following section, we describe a cross between the PDGFβ and MBP mice which displays both neuronal and oligodendroglial components.
The PLP- α-synuclein transgenic model
One of the most widely used models developed by Khale and colleagues involves expressing human α-syn under an oligodendrocyte-specific promoter, proteolipid protein promoter (PLP) [8, 59, 129] (Table 1). The resulting transgenic mice (PLP-α-syn) display α-syn-positive inclusions in oligodendrocytes similar to GCIs. These aggregates mimic some biochemical characteristics of GCIs including phosphorylated α-syn and SDS detergent-insolubility. The model is characterized by significant early microglial activation which may account for the progression of the MSA-like neurodegeneration [127]. However, no clear myelin or oligodendrocyte loss was detected, even in old tg mice. Only mild motor changes in mice after 12 months of age [33, 126] was found. These alterations correlated with mild neuronal loss in the substantia nigra and locus coeruleus in PLP-α-syn mice [126], age-related striatal dysfunction and cortical atrophy [33]. There was also a reduction in dopaminergic neurons in the SNc compared to non-transgenic mice. The PLP-α-syn mouse line also displays autonomic alterations [131] and neurodegeneration in the nucleus ambiguus [66]. Finally, a recent study reported urinary dysfunction in this line, corresponding to damage in micturition centers in the spinal cord and brainstem [10]. The PLP-α-syn model presents with REM sleep behavior disorder that typically occurs in MSA and other α-synucleinopathies [49] and is linked to degeneration of sleep centers in the brainstem including the pedunculopontine and the laterodorsal tegmental nucleus [131]. The PLP-α-syn mouse shows preserved olfaction despite the presence of human α-syn in the oligodendrocytes of the olfactory bulbs similar to the human disease [65]. In addition, this mouse model has been extensively utilized to test new therapies for MSA [130]. In summary, this model recapitulates several neuropathological and behavioral alterations of MSA including nigral degeneration, dysautonomia and other non-motor features (Table 1); however, the motor deficits are mild and demyelination is less prominent.
The CNP- α-synuclein transgenic model
The second MSA mouse model features expression of human α-syn under the 2’,3’-cyclic nucleotide 3’- phosphodiesterase promoter (CNP-α-syn) [8] (Table 1). These mice show age-related progressive motor deficits with the formation of GCI-like α-syn inclusions and neurodegeneration, most prominently in the cerebral cortex and spinal cord [162]. There was no neuronal loss in the cerebellum and pontine nuclei. The pattern of high molecular weight insoluble α-syn in CNP-α-syn mice was similar to what is observed in MSA tissue. Oligodendrocytes in these mice showed myelin damage, lysosomal alterations, and cytoplasmic myelin fragments, suggesting autophagocytosis of myelin. α-Syn positive filamentous inclusions were found in oligodendrocytes; only minimal α-syn was detected in axons. The authors concluded that the oligodendroglia accumulation of human α-syn resulted in the accumulation of mouse α-syn in neurons. The presence of NCIs is a consistent feature of MSA, though their pathogenic significance is unclear [90]. Moreover, recent studies have confirmed neuronal accumulation of α-syn in MSA [19], although the distribution and variety of such inclusions were not observed using previous silver stains [95]. Together, these studies suggested that the conformation of α-syn in NCI’s and GCIs might be different [46]. In summary, this model features several of the landmarks or MSA including GCIs, myelin neuropathology, severe motoric deficits and biochemical alterations of α-syn (Table 1). However, dysautonomia and other non-motor features are less obvious and the severity of the motor deficits is difficult to test. Moreover, some of these changes appear to be linked to α-syn accumulation in the spinal cord.
The MBP- α-synuclein transgenic model
Finally, the third MSA model overexpresses human α-syn from the oligodendrocyte myelin basic protein promoter [118] (MBP-α-syn mice) (Figure 2) (Table 1). Several lines were generated with varying levels from low to high α-syn expression. GCI-like perinuclear α-syn inclusions with a fibrillar structure under electron microscopy were observed in oligodendrocytes. The α-syn inclusions in oligodendrocytes similar to MSA were hyperphosphorylated and ubiquitinated (Figure 1b). The most extensive alterations followed regions affected in MSA such as white matter tracts, striatum, brainstem, and cerebellum. In addition, depending on the levels of α-syn expression these mice displayed neuro-inflammatory alterations with astrogliosis and loss of myelin in white matter tracts (Figure 1c). Ultrastructural examination of affected oligodendrocytes revealed prominent mitochondrial abnormalities including enlarged and irregularly shaped organelles. There were also extensive axonal alterations including decreased fiber density and irregular swelling of axons. Similar to MSA, these mice display defective maturation of oligodendroglial cells in connection with the myelin pathology [29, 30, 81]. In addition to the myelin alterations, oligodendrocyte alterations lead to a loss in expression of glial cell line-derived neurotrophic factor GDNF and other neurotrophic factors that in turn leads to neurodegeneration with decrease dopaminergic neurons in the substantia nigra [142] (Figure 2a). The severity of the GCI-like pathology and neurodegeneration was dependent on the levels of α-syn expression. Motor phenotype severity also varied dramatically from severe tremor, ataxia, seizures and premature death in the highest expressing line to mild tremor and variable motor impairment in intermediate and lower expressing lines. Some of the lines demonstrated olfactory deficits however autonomic alterations were mild or not observed. However, in contrast to PD, the significant olfactory disturbance is not a consistent feature of MSA [65, 135]. This model has been extensively used for testing on immunotherapy, anti-inflammatory and other therapies for MSA [144-146]. In summary, this model resembles several of the early stage alterations of MSA and display progressive α-syn positive GCI-like formation (Figure 1), myelin loss, neurodegeneration and neuroinflammation (Figure 2) (Table 1). The mice also show motoric deficits and dopaminergic loss. However, autonomic features are mild and pathology is dependent on levels of transgene expression.
Combined PDGFβ and MBP double transgenic mouse model
Although the single transgenic models under oligodendroglial promoters display several features consistent with MSA, the significance is unclear since the α-syn is overexpressed from oligodendrocytes. Moreover, in MSA more recent studies have shown extensive α-syn accumulation in neurons in addition to the GCI pathology [4]. The mechanisms underlying the oligodendroglial accumulation of α-syn in the brains of patients with MSA and its relationship to neurons have attracted a great deal of interest, given the primarily neuronal role reported for this protein. To investigate the interactions between neuronal and oligodendroglial α-syn, MBP1-α-syn tg mice were crossed with mice expressing α-syn under the neuronal platelet-derived growth factor promoter (PDGFβ-α-syn tg) [105]. Interestingly, the progeny from the cross displayed a re-localization of α-syn from neurons to oligodendroglial cells. The double transgenic mice displayed motor deficits and dopaminergic degeneration. These results suggest that the pathological CNS milieu in MSA might favor the re-distribution of α-syn to oligodendroglial cells [105]. This model is also of interest because it combines neuronal and oligodendroglial pathology that is now recognized as an important characteristic of MSA. Moreover, it might represent an example of “cell to cell” transmission of α-syn [73] similar to what has been shown utilizing viral vectors expressing α-syn in one cell population and transmission to other cells trans-synaptically or by other means [27]. Along these lines, in vivo neuron to oligodendrocyte α-syn transmission has been reported [3, 102]. In this model, embryonic rat tissue from the ventral mesencephalon was transplanted into the region of the transduction. The authors reported that α-syn transmitted both to neurons and oligodendrocytes in the grafted tissue. These models of oligodendrocyte α-syn accumulation are more consistent with the current evidence from human studies of MSA brain tissue [58, 82, 92]. However, an alternative hypothesis has been advanced that focuses on the “protein spreading” prion-like hypothesis [154, 158] for these models and is discussed in the next section.
4. α-Synuclein seeding mediated spreading and viral vector models
Recent studies have advanced the alternative hypothesis that in synucleinopathies α-syn might spread across the brain utilizing a seeding mediated prion-like mechanism [158]. Utilizing this approach, previous studies have shown that injection of selected species of (synthetic) α-syn fibrils (seeds) into wild-type mice [75] recapitulates several features of PD. In this regard, a recent study in rodents explored the effects of α-syn aggregates with a ribbon vs. fibrillar morphology to seed differential pathology mimicking MSA vs. PD in the presence and absence of α-syn expressed under viral vector control [97]. These studies led to the theory that strains could account for the different clinicopathological traits within synucleinopathies. In this study, although injection with ribbon α-syn induced more pronounced LB/LN-like inclusions, fibrils imposed the greatest neurotoxic burden on the striatonigral pathway in the presence of rAAV α-syn [97]. However, the actual potential of the ribbon morphology to seed oligodendroglial pathology needs to be determined. In a similar study, homogenates from MSA patients (rather than synthetic fibrils) were injected into the brains of wild-type mice or low-level α-syn expressing transgenic mice that did not develop neurological disease otherwise [153]. Inoculation with MSA brain homogenates (rather than PD brain homogenates) induced a progressive neurological disease characterized by neuronal α-synuclein deposition mostly in transgenic mice; however, uptake by oligodendrocytes, in particular, was not reported, although it is important to note that using transgenic models with increased neuronal α-syn expression are likely to have neuronal rather than glial pathology. In a subsequent study, this group confirmed the finding that brain extracts from MSA cases all propagate neurodegeneration to mice with the development of neuronal α-syn deposition [100]. Homogenates from PD did not promote aggregation of α-syn, supporting the notion, as reported by others [97]. that the strain of α-syn found in MSA might be different to that of PD brain [100], although earlier studies have demonstrated that Lewy body extracts from PD cases [101] or brain homogenates from DLB cases [79] can trigger robust α-syn pathology in wild-type mice. Recently MSA models in rats and non-human primates have been developed using viral vectors [6, 76]. For example, delivery of chimeric adeno-associated virus (AAV) vectors expressing human wild-type α-syn under the control of mouse myelin basic protein in the striatum resulted in abundant oligodendroglial expression in rats and non-human primates [6, 76]. Rats developed progressive motor alterations that were not affected by L-DOPA; loss of dopaminergic neurons was detected at 3 months with phosphorylated and proteinase-K-resistant α-syn accumulation in oligodendrocytes in the striatum and substantia nigra. The rat and non-human primates also showed demyelination in the white matter tracts of the corpus callosum and striatum. The advantage of these new in vivo models is that they reproduce several key aspects of the pathology including the degeneration of the striatal output neurons and clinical aspects of MSA and offers a new paradigm for testing therapeutics for MSA. The disadvantage, as with others, is the over-expression of α-syn in oligodendroglial cells without recapitulating how the MSA pathogenesis starts. In regards to the models utilizing injection of seeds, it is worth mentioning that it is unclear to what extent α-syn spreads in prion-like fashion in patients with MSA. A recent study investigated MSA transmission in mice under similar conditions known to result in another prion disease, PRP scrapie and found peripheral exposure to MSA resulted in neurological signs along with α-syn prions in the brain [158]. However, fibrillar forms of α-syn such as the one shown experimentally to spread have not been detected extracellularly in patients. In MSA and other synucleinopathies, the extracellular α-syn species appear to be small aggregates and oligomers and thus the need for alternative models.
5. In vitro models of MSA
While the in vivo models described above have proved useful in furthering understanding of MSA pathology, there are some noted disadvantages, and therefore the need for complementary models. In recent years the development of oligodendroglial cells lines and primary cultures have aided in this regard. For instance in vitro models of MSA using cell cultures using overexpressing of wild-type α-syn or mutant A35T α-syn in oligodendroglial cells (OLN-93) [104] suggested proteolytic and oxidative stress increased α-syn aggregation and insolubility. Other clonal cell lines include HOG [99] and KG1c [86], although the morphology and expression profiles were similar to immature oligodendroglia cells. Other in vitro models have employed glioblastoma/astrocytoma (U373) cells [128] or mixed rat glial cultures [125] transfected with full-length or C-terminally truncated (1-111) α-syn; however, they experience some drawbacks [96]. Primary cultures of oligodendrocytes and neurons from the brains of transgenic MSA animal models offer fully differentiated cells as an experimental system in which to evaluate α-syn accumulation and cytotoxicity, although they do not proliferate.
Primary human oligodendroglial cells could help; however, they are difficult to obtain and maintain. In recent years the development of inducible pluripotent stem cells (iPSCs) isolated from skin and bone marrow biopsies from patients with synucleinopathy that can be reprogrammed into a pluripotent state by overexpressing four transcription factors (Oct4, Sox2, Klf4, and c-Myc) have advanced the field considerably [136]. Recently, iPSC clones have been obtained from MSA patients [23] and differentiated into oligodendroglial cells [50]. A recent review paper provides a detailed list of such iPSC lines of MSA and PD [8, 9]. In brief, these lines have shown mild accumulation of α-syn and related deficits after long periods of incubation (+100 days). Cells display myelination deficits, increased the propensity to degenerate if exposed to challenges, and delayed differentiation [30].
Given that MSA is most likely a multifactorial disorder, MSA-derived iPSCs differentiated into oligodendrocytes might need additional epigenetic and genetic hits to display a more overt phenotype. Nonetheless, these human cell models in combination with the available α-syn transgenic models might be of great use in studies of pathogenesis and drug development.
6. Discussion- what do we need to do to develop better models
While most transgenic animal models of neurodegenerative disorders do not fully reproduce the pathogenesis of the disease, identification of genetic mutations in familial forms of Alzheimer’s disease, Parkinson’s disease, trinucleotide repeat disorders and frontotemporal dementia has allowed the development of better models. These studies have emphasized the need for developing novel models in species other than rodents, identifying translatable biomarkers in the models that mimic what is seen in patients, utilizing knock-in and CRISPR approaches that utilizes the endogenous promoter and physiological levels of expression of the mutant gene and developing models of sporadic disease that combine genetic susceptibility genes and environmental triggers.
Despite the currently available transgenic mice, prion-like seeding and iPSC models described above, developing valid in vivo paradigms of MSA has been difficult without clear genetic and environmental leads to guide the modeling of MSA in animals. As mentioned before, our models today depend on replication of the neuropathological hallmarks of the disease, namely accumulation of α-syn in oligodendrocytes. However, it is important to recognize that these models are useful in understanding how GCIs are formed, how α-syn accumulation in oligodendrocytes leads to neuroinflammation, demyelination, and selective neuronal degeneration and to develop potential new disease-modifying therapeutics [8].
Unlike other neurodegenerative disorders of the aging population such as AD, PD, and FTD, no unique gene mutations linked to familial forms of the disease have been identified. Only a few reports of familial MSA are available [31, 48, 119, 159]. Some studies have shown that genetic polymorphisms in the SNCA locus are associated with a risk of MSA [2, 107, 112]. However, this finding has been disputed by other studies [164]. Moreover, genes involved in lysosomal degradation activity, mitochondrial function, inflammation as well as ataxia-related genes have been proposed as susceptibility genes for MSA [132]. For example, COQ2 [88] involved in mitochondrial function and glucocerebrosidase (GBA) involved in lysosomal function [85], have been described as risk factors for MSA [8]. These studies await replication by others and validation in larger cohorts.
Since it is difficult in most of the cases to link specific genetic changes directly to the etiology of MSA, epigenetic and environmental factors have been investigated [134]. Environmental factors and oxidative stress were associated with the risk of developing MSA [93, 148, 165] (Figure 2a). Interestingly, exposure of transgenic mice overexpressing human α-syn in oligodendrocytes to oxidative or proteolytic stress was shown to trigger MSA-like phenotypes supporting a possible interaction between α-syn pathology and environmental toxins [124, 126, 139]. Dysfunctional histone acetylation was associated with oligodendroglial α-syn pathology in the PLP-α-syn mouse [133], supporting previous reports on the role of α-syn in the nucleus to inhibit histone acetylation [61]. However, studies on the histone acetylation profile in human MSA are still lacking. Other lines of investigation, such as RNA-Seq in MSA brains, have shown alterations in a number of genes including alpha and beta hemoglobin (HBA1, HBA2, and HBB) and transthyretin [83]. Interestingly, these findings have been replicated in the RNA-Seq analysis of substantia nigra and striatum of PLP-α-syn mice in the early pre-symptomatic stages of the disease, suggesting that the alterations in the alpha and beta hemoglobin genes may be related to the α-syn accumulation in oligodendrocytes [111]. Several changes in the miRNA-mRNA regulatory network have been linked to region-specific changes that preceded the neurodegenerative events in the PLP-α-syn mouse. Moreover, we investigated the profile of microRNAs in the brains of patients with MSA and in the MBP mice and found widespread dysregulation of microRNAs [141]. Changes to microRNA-96 resulted in downregulation of the solute carrier protein family SLC1A1 and SLC6A6 [141].
Demyelination is an important feature of advanced MSA [51] and recent studies have focused on the role of oligodendroglial maturation in MSA [1, 81], as well as myelin production [30]. Along these lines, a recent study showed that the myelin lipids (sphingomyelin, sulfatide, and galactosylceramide) were severely decreased in MSA white matter, specifically in disease-affected regions, suggesting that modeling of MSA might require targeting oligodendrocyte maturation and lipid synthesis pathways [9, 24].
Microglial activation is consistently found to accompany the neurodegenerative process in MSA both in post-mortem and in-vivo PET imaging studies [41, 52]. A large body of evidence has been collected over the last few years focusing on the pathogenic role of α-syn-induced microglial activation in PD, DLB, and MSA [32, 109]. The role of microglial activation in the context of GCI-like pathology has been extensively studied in the PLP-α-syn mouse model. It has been shown that early suppression of microglial activation by minocycline may rescue nigral neurons [127], whereas, in the same model, TLR4-linked clearance of α-syn by microglia was found to represent an important intrinsic mechanism that may modulate the progression of the degenerative process [122]. Therefore, the PLP-α-syn mouse is a suitable preclinical tool to study neuroinflammation related to microglial activation, while the MBP-α-syn mouse model presents with predominant astrogliosis [118] which may trigger a different neuroinflammatory profile and result in the phenotypic differences seen between the models.
Most of the tg mouse models described in this review (PLP, CNP, and MBP) are based on overexpression that does not necessarily occur in sporadic patients unless they harbor a polymorphism in the α-syn gene (SNCA) promoter region that increases expression. Given that transcripts of the SNCA have been found in white matter oligodendrocytes from MSA patients and IPSCs-derived oligodendrocytes, it is possible that at least in part the process of α-syn aggregation could start from oligodendroglial production. If knowing “where” the process starts is obviously a relevant issue, the key problem is rather “how” it starts. Thus the limited knowledge on the early events which trigger the disease is a major difficulty in recapitulating MSA in an in vivo model. It is assumed that α-syn dysfunction is the main source of the follow-up pathogenic events. The dysfunction has been related to the ectopic expression of SNCA mRNA in oligodendrocytes [4] and/or specific conformational changes of the α-syn protein which enable the pathogenic cell-to-cell spreading of MSA type [97]. However, the mechanisms of α-syn accumulation in oligodendrocytes remain unresolved. Therefore, the available murine models represent an excellent tool to study GCI-downstream mechanisms of MSA-like neurodegeneration as listed above. Their main limitation is linked to the fact that they are based on replicating the end pathology of the disease, and probably due to this issue, they lack in recapitulating the aggressive and quick progression of human MSA. The only mouse model of MSA with severe aggressive phenotype and shortened survival (MBP-α-syn line 29) confirmed the toxic role of high α-syn expression similar to cases with phenotypic MSA-like clinical presentation and multiplication of the SNCA [4]. Unfortunately, follow-up studies failed to confirm the role of SNCA multiplications in pathologically confirmed MSA cases [74]. It is generally accepted that rodent models replicate human pathology less effectively than non-human primates; however, the limited knowledge and understanding of the initial trigger(s) of MSA will currently set the same limitation in the development of evolutionary more appropriate models of the disease.
Animal models of α-syn cell-to-cell-spreading have not yet convincingly induced GCI-like pathology similar to the human MSA, although uptake of α-syn by oligodendrocytes has been shown in some instances [102] but not in others [100]. Furthermore, the understanding of the α-syn-species differences in MSA, as well as their generation during the pathogenic process is insufficient. Therefore, more extensive studies in this direction are warranted.
In spite of all the limitations listed above, the existing transgenic models of MSA provide a valuable tool not only for the study of disease mechanisms downstream of the GCI formation but also for targeted screening of candidate therapeutic approaches. Due to the differences between the various models, it is important to always select the appropriate one that represents the target of interest accordingly. Therefore, all MSA animal models may serve as a proof-of-concept test-bed when assessing α-syn lowering strategies, since all of them are based on inducing MSA-like pathology through α-syn oligodendrogliopathy or propagation. The MBP-α-syn mouse is especially suitable for strategies that target demyelination [29], neuroinflammatory responses linked to astrogliosis [147], and neurotrophic support [142], whereas the PLP-α-syn mouse is the MSA model of choice when testing strategies related to the progression of α-syn induced microglial activation and selective neurodegeneration [40, 123, 127]. Finally, MSA-derived iPSC-based in vitro models, when established and well-characterized, may provide a personalized therapeutic screening system in the future.
Acknowledgments
EV and ER were supported by NIH grants AG18440 and NS092803.
References
- 1.Ahmed Z, Asi YT, Lees AJ, Revesz T, Holton JL (2013) Identification and quantification of oligodendrocyte precursor cells in multiple system atrophy, progressive supranuclear palsy and Parkinson’s disease. Brain Pathol 23: 263–273 Doi 10.1111/j.1750-3639.2012.00637.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Chalabi A, Durr A, Wood NW, Parkinson MH, Camuzat A, Hulot JS, Morrison KE, Renton A, Sussmuth SD, Landwehrmeyer BG et al. (2009) Genetic variants of the alphα-synuclein gene SNCA are associated with multiple system atrophy. PLoS One 4: e7114 Doi 10.1371/journal.pone.0007114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angot E, Steiner JA, Lema Tome CM, Ekstrom P, Mattsson B, Bjorklund A, Brundin P (2012) Alphα-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in vivo. PLoS One 7: e39465 Doi 10.1371/journal.pone.0039465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asi YT, Simpson JE, Heath PR, Wharton SB, Lees AJ, Revesz T, Houlden H, Holton JL (2014) Alphα-synuclein mRNA expression in oligodendrocytes in MSA. Glia 62: 964–970 Doi 10.1002/glia.22653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartels T, Choi JG, Selkoe DJ (2011) alphα-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477: 107–110 Doi 10.1038/nature10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassil F, Guerin PA, Dutheil N, Li Q, Klugmann M, Meissner WG, Bezard E, Fernagut PO (2017) Viral-mediated oligodendroglial alphα-synuclein expression models multiple system atrophy. Mov Disord: Doi 10.1002/mds.27041 [DOI] [PubMed] [Google Scholar]
- 7.Benrud-Larson LM, Sandroni P, Schrag A, Low PA (2005) Depressive symptoms and life satisfaction in patients with multiple system atrophy. Mov Disord 20: 951–957 Doi 10.1002/mds.20450 [DOI] [PubMed] [Google Scholar]
- 8.Bleasel JM, Halliday GM, Kim WS (2016) Animal modeling an oligodendrogliopathy--multiple system atrophy. Acta neuropathologica communications 4: 12 Doi 10.1186/s40478-016-0279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleasel JM, Wong JH, Halliday GM, Kim WS (2014) Lipid dysfunction and pathogenesis of multiple system atrophy. Acta neuropathologica communications 2: 15 Doi 10.1186/2051-5960-2-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudes M, Uvin P, Pinto S, Voets T, Fowler CJ, Wenning GK, De Ridder D, Stefanova N (2013) Bladder dysfunction in a transgenic mouse model of multiple system atrophy. Mov Disord 28: 347–355 Doi 10.1002/mds.25336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braak H, Sastre M, Del Tredici K (2007) Development of alphα-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol 114: 231–241 Doi 10.1007/s00401-007-0244-3 [DOI] [PubMed] [Google Scholar]
- 12.Bruck D, Wenning GK, Stefanova N, Fellner L (2016) Glia and alphα-synuclein in neurodegeneration: A complex interaction. Neurobiol Dis 85: 262–274 Doi 10.1016/j.nbd.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brudek T, Winge K, Rasmussen NB, Bahl JM, Tanassi J, Agander TK, Hyde TM, Pakkenberg B (2016) Altered alphα-synuclein, parkin, and synphilin isoform levels in multiple system atrophy brains. J Neurochem 136: 172–185 Doi 10.1111/jnc.13392 [DOI] [PubMed] [Google Scholar]
- 14.Burn DJ, Jaros E (2001) Multiple system atrophy: cellular and molecular pathology. Mol Pathol 54: 419–426 [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell BC, McLean CA, Culvenor JG, Gai WP, Blumbergs PC, Jakala P, Beyreuther K, Masters CL, Li QX (2001) The solubility of alphα-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J Neurochem 76: 87–96 [DOI] [PubMed] [Google Scholar]
- 16.Conway KA, Harper JD, Lansbury PT Jr. (2000) Fibrils formed in vitro from alphα-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry 39: 2552–2563 [DOI] [PubMed] [Google Scholar]
- 17.Cremades N, Chen SW, Dobson CM (2017) Structural Characteristics of alphα-synuclein Oligomers. International review of cell and molecular biology 329: 79–143 Doi 10.1016/bs.ircmb.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 18.Culvenor JG, Rietze RL, Bartlett PF, Masters CL, Li QX (2002) Oligodendrocytes from neural stem cells express alphα-synuclein: increased numbers from presenilin 1 deficient mice. Neuroreport 13: 1305–1308 [DOI] [PubMed] [Google Scholar]
- 19.Cykowski MD, Coon EA, Powell SZ, Jenkins SM, Benarroch EE, Low PA, Schmeichel AM, Parisi JE (2015) Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138: 2293–2309 Doi 10.1093/brain/awv114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ (2012) Exosomal cell-to-cell transmission of alpha synuclein oligomers. Molecular neurodegeneration 7: 42 Doi 10.1186/1750-1326-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alphα-synuclein. Proc Natl Acad Sci USA 106: 13010–13015 Doi 10.1073/pnas.0903691106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickson DW, Liu W, Hardy J, Farrer M, Mehta N, Uitti R, Mark M, Zimmerman T, Golbe L, Sage J et al. (1999) Widespread alterations of alphα-synuclein in multiple system atrophy. Am J Pathol 155: 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djelloul M, Holmqvist S, Boza-Serrano A, Azevedo C, Yeung MS, Goldwurm S, Frisen J, Deierborg T, Roybon L (2015) Alphα-synuclein Expression in the Oligodendrocyte Lineage: an In Vitro and In Vivo Study Using Rodent and Human Models. Stem cell reports 5: 174–184 Doi 10.1016/j.stemcr.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Don AS, Hsiao JH, Bleasel JM, Couttas TA, Halliday GM, Kim WS (2014) Altered lipid levels provide evidence for myelin dysfunction in multiple system atrophy. Acta neuropathologica communications 2: 150 Doi 10.1186/s40478-014-0150-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duda JE, Giasson BI, Gur TL, Montine TJ, Robertson D, Biaggioni I, Hurtig HI, Stern MB, Gollomp SM, Grossman M et al. (2000) Immunohistochemical and biochemical studies demonstrate a distinct profile of alphα-synuclein permutations in multiple system atrophy. J Neuropathol Exp Neurol 59: 830–841 [DOI] [PubMed] [Google Scholar]
- 26.Dujardin K, Defebvre L, Krystkowiak P, Degreef JF, Destee A (2003) Executive function differences in multiple system atrophy and Parkinson’s disease. Parkinsonism Relat Disord 9: 205–211 Doi S1353802002000500 [pii] [DOI] [PubMed] [Google Scholar]
- 27.El-Agnaf O, Overk C, Rockenstein E, Mante M, Florio J, Adame A, Vaikath N, Majbour N, Lee SJ, Kim C et al. (2017) Differential effects of immunotherapy with antibodies targeting alphα-synuclein oligomers and fibrils in a transgenic model of synucleinopathy. Neurobiol Dis 104: 85–96 Doi 10.1016/j.nbd.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Eliezer D, Kutluay E, Bussell R Jr., Browne G (2001) Conformational properties of alphα-synuclein in its free and lipid-associated states. J Mol Biol 307: 1061–1073 [DOI] [PubMed] [Google Scholar]
- 29.Ettle B, Kerman BE, Valera E, Gillmann C, Schlachetzki JC, Reiprich S, Buttner C, Ekici AB, Reis A, Wegner M et al. (2016) alphα-Synuclein-induced myelination deficit defines a novel interventional target for multiple system atrophy. Acta Neuropathol 132: 59–75 Doi 10.1007/s00401-016-1572-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ettle B, Reiprich S, Deusser J, Schlachetzki JC, Xiang W, Prots I, Masliah E, Winner B, Wegner M, Winkler J (2014) Intracellular alphα-synuclein affects early maturation of primary oligodendrocyte progenitor cells. Mol Cell Neurosci 62: 68–78 Doi 10.1016/j.mcn.2014.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Federoff M, Schottlaender LV, Houlden H, Singleton A (2015) Multiple system atrophy: the application of genetics in understanding etiology. Clin Auton Res 25: 19–36 Doi 10.1007/s10286-014-0267-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fellner L, Jellinger KA, Wenning GK, Stefanova N (2011) Glial dysfunction in the pathogenesis of alphα-synucleinopathies: emerging concepts. Acta Neuropathol 121: 675–693 Doi 10.1007/s00401-011-0833-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernagut PO, Meissner WG, Biran M, Fantin M, Bassil F, Franconi JM, Tison F (2014) Age-related motor dysfunction and neuropathology in a transgenic mouse model of multiple system atrophy. Synapse 68: 98–106 Doi 10.1002/syn.21719 [DOI] [PubMed] [Google Scholar]
- 34.Fetoni V, Soliveri P, Monza D, Testa D, Girotti F (1999) Affective symptoms in multiple system atrophy and Parkinson’s disease: response to levodopa therapy. J Neurol Neurosurg Psychiatry 66: 541–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flabeau O, Meissner WG, Ozier A, Berger P, Tison F, Fernagut PO (2014) Breathing variability and brainstem serotonergic loss in a genetic model of multiple system atrophy. Mov Disord 29: 388–395 Doi 10.1002/mds.25804 [DOI] [PubMed] [Google Scholar]
- 36.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (2002) alphα-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4: 160–164 [DOI] [PubMed] [Google Scholar]
- 37.Fusco G, De Simone A, Arosio P, Vendruscolo M, Veglia G, Dobson CM (2016) Structural Ensembles of Membrane-bound alphα-Synuclein Reveal the Molecular Determinants of Synaptic Vesicle Affinity. Sci Rep 6: 27125 Doi 10.1038/srep27125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fusco G, De Simone A, Gopinath T, Vostrikov V, Vendruscolo M, Dobson CM, Veglia G (2014) Direct observation of the three regions in alphα-synuclein that determine its membrane-bound behaviour. Nat Commun 5: 3827 Doi 10.1038/ncomms4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gai WP, Power JH, Blumbergs PC, Culvenor JG, Jensen PH (1999) Alphα-synuclein immunoisolation of glial inclusions from multiple system atrophy brain tissue reveals multiprotein components. J Neurochem 73: 2093–2100 [PubMed] [Google Scholar]
- 40.Galvin JE, Lee VM, Trojanowski JQ (2001) Synucleinopathies: clinical and pathological implications. Arch Neurol 58: 186–190 [DOI] [PubMed] [Google Scholar]
- 41.Gerhard A, Banati RB, Goerres GB, Cagnin A, Myers R, Gunn RN, Turkheimer F, Good CD, Mathias CJ, Quinn N et al. (2003) [11C](R)-PK11195 PET imaging of microglial activation in multiple system atrophy. Neurology 61: 686–689 [DOI] [PubMed] [Google Scholar]
- 42.Geser F, Seppi K, Stampfer-Kountchev M, Kollensperger M, Diem A, Ndayisaba JP, Ostergaard K, Dupont E, Cardozo A, Tolosa E et al. (2005) The European Multiple System Atrophy-Study Group (EMSA-SG). J Neural Transm 112: 1677–1686 Doi 10.1007/s00702-005-0328-y[doi] [DOI] [PubMed] [Google Scholar]
- 43.Gilman S, Low P, Quinn N, Albanese A, Ben-Shlomo Y, Fowler C, Kaufmann H, Klockgether T, Lang A, Lantos P et al. (1998) Consensus statement on the diagnosis of multiple system atrophy. American Autonomic Society and American Academy of Neurology. Clin Auton Res 8: 359–362 [DOI] [PubMed] [Google Scholar]
- 44.Gilman S, May SJ, Shults CW, Tanner CM, Kukull W, Lee VM, Masliah E, Low P, Sandroni P, Trojanowski JQ et al. (2005) The North American Multiple System Atrophy Study Group. J Neural Transm 112: 1687–1694 [DOI] [PubMed] [Google Scholar]
- 45.Graham JG, Oppenheimer DR (1969) Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry 32: 28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halliday GM (2015) Re-evaluating the glio-centric view of multiple system atrophy by highlighting the neuronal involvement. Brain 138: 2116–2119 Doi 10.1093/brain/awv151 [DOI] [PubMed] [Google Scholar]
- 47.Halliday GM, Holton JL, Revesz T, Dickson DW (2011) Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol 122: 187–204 Doi 10.1007/s00401-011-0852-9 [DOI] [PubMed] [Google Scholar]
- 48.Hara K, Momose Y, Tokiguchi S, Shimohata M, Terajima K, Onodera O, Kakita A, Yamada M, Takahashi H, Hirasawa M et al. (2007) Multiplex families with multiple system atrophy. Arch Neurol 64: 545–551 Doi 10.1001/archneur.64.4.545 [DOI] [PubMed] [Google Scholar]
- 49.Hartner L, Keil TW, Kreuzer M, Fritz EM, Wenning GK, Stefanova N, Fenzl T (2016) Distinct Parameters in the EEG of the PLP alphα-syN Mouse Model for Multiple System Atrophy Reinforce Face Validity. Frontiers in behavioral neuroscience 10: 252 Doi 10.3389/fnbeh.2016.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmqvist S, Lehtonen Š, Chumarina M, Puttonen KA, Azevedo C, Lebedeva O, Ruponen M, Oksanen M, Djelloul M, Collin A et al. (2016) Creation of a library of induced pluripotent stem cells from Parkinsonian patients. Npj Parkinson’s Disease 2: 16009 Doi 10.1038/npjparkd.2016.9 https://www.nature.com/articles/npjparkd20169#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishizawa K, Komori T, Arai N, Mizutani T, Hirose T (2008) Glial cytoplasmic inclusions and tissue injury in multiple system atrophy: A quantitative study in white matter (olivopontocerebellar system) and gray matter (nigrostriatal system). Neuropathology 28: 249–257 Doi 10.1111/j.1440-1789.2007.00855.x [DOI] [PubMed] [Google Scholar]
- 52.Ishizawa K, Komori T, Sasaki S, Arai N, Mizutani T, Hirose T (2004) Microglial activation parallels system degeneration in multiple system atrophy. J Neuropathol Exp Neurol 63: 43–52 [DOI] [PubMed] [Google Scholar]
- 53.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T (1995) The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron 14: 467–475 [DOI] [PubMed] [Google Scholar]
- 54.Jellinger KA (2003) Neuropathological spectrum of synucleinopathies. Mov Disord 18 Suppl 6: S2–12 Doi 10.1002/mds.10557 [DOI] [PubMed] [Google Scholar]
- 55.Jellinger KA (2012) Neuropathology and pathophysiology of multiple system atrophy. Neuropathol Appl Neurobiol 38: 379–380; author reply 381 [DOI] [PubMed] [Google Scholar]
- 56.Jellinger KA (2014) Neuropathology of multiple system atrophy: new thoughts about pathogenesis. Mov Disord 29: 1720–1741 Doi 10.1002/mds.26052 [DOI] [PubMed] [Google Scholar]
- 57.Jellinger KA, Lantos PL (2010) Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol 119: 657–667 Doi 10.1007/s00401-010-0672-3[doi] [DOI] [PubMed] [Google Scholar]
- 58.Jin H, Ishikawa K, Tsunemi T, Ishiguro T, Amino T, Mizusawa H (2008) Analyses of copy number and mRNA expression level of the alphα-synuclein gene in multiple system atrophy. J Med Dent Sci 55: 145–153 [PubMed] [Google Scholar]
- 59.Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, Fuss B, Mallon B, Macklin WB, Fujiwara H et al. (2002) Hyperphosphorylation and insolubility of alphα-synuclein in transgenic mouse oligodendrocytes. EMBO Rep 3: 583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klucken J, Poehler AM, Ebrahimi-Fakhari D, Schneider J, Nuber S, Rockenstein E, Schlotzer-Schrehardt U, Hyman BT, McLean PJ, Masliah E et al. (2012) Alphα-synuclein aggregation involves a bafilomycin A 1-sensitive autophagy pathway. Autophagy 8: 754–766 Doi 10.4161/auto.19371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kontopoulos E, Parvin JD, Feany MB (2006) Alphα-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet 15: 3012–3023 [DOI] [PubMed] [Google Scholar]
- 62.Kosaka K (1990) Diffuse Lewy body disease in Japan. JNeurol 237: 197–204 [DOI] [PubMed] [Google Scholar]
- 63.Kosaka K (1978) Lewy bodies in cerebral cortex. Report of three cases. Acta Neuropathol(Berl) 42: 127–134 [DOI] [PubMed] [Google Scholar]
- 64.Kosaka K, Yoshimura M, Ikeda K, Budka H (1984) Diffuse type of Lewy body disease. Progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree - A new disease? ClinNeuropathol 3: 183–192 [PubMed] [Google Scholar]
- 65.Krismer F, Wenning GK, Li Y, Poewe W, Stefanova N (2013) Intact olfaction in a mouse model of multiple system atrophy. PLoS One 8: e64625 Doi 10.1371/journal.pone.0064625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuzdas D, Stemberger S, Gaburro S, Stefanova N, Singewald N, Wenning GK (2013) Oligodendroglial alphα-synucleinopathy and MSA-like cardiovascular autonomic failure: experimental evidence. Exp Neurol 247: 531–536 Doi 10.1016/j.expneurol.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langerveld AJ, Mihalko D, DeLong C, Walburn J, Ide CF (2007) Gene expression changes in postmortem tissue from the rostral pons of multiple system atrophy patients. Mov Disord 22: 766–777 [DOI] [PubMed] [Google Scholar]
- 68.Lantos PL, Papp MI (1994) Cellular pathology of multiple system atrophy: a review. J Neurol Neurosurg Psychiatry 57: 129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of alphα-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14: 38–48 Doi 10.1038/nrn3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee HJ, Patel S, Lee SJ (2005) Intravesicular localization and exocytosis of alphα-synuclein and its aggregates. J Neurosci 25: 6016–6024 Doi 10.1523/JNEUROSCI.0692-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee HJ, Suk JE, Bae EJ, Lee JH, Paik SR, Lee SJ (2008) Assembly-dependent endocytosis and clearance of extracellular alphα-synuclein. Int J Biochem Cell Biol 40: 1835–1849 Doi 10.1016/j.biocel.2008.01.017 [DOI] [PubMed] [Google Scholar]
- 72.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ (2010) Direct transfer of alphα-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem 285: 9262–9272 Doi 10.1074/jbc.M109.081125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E (2010) Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol 6: 702–706 Doi 10.1038/nrneurol.2010.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lincoln SJ, Ross OA, Milkovic NM, Dickson DW, Rajput A, Robinson CA, Papapetropoulos S, Mash DC, Farrer MJ (2007) Quantitative PCR-based screening of alphα-synuclein multiplication in multiple system atrophy. Parkinsonism Relat Disord 13: 340–342 Doi 10.1016/j.parkreldis.2006.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM (2012) Pathological alphα-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338: 949–953 Doi 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mandel RJ, Marmion DJ, Kirik D, Chu Y, Heindel C, McCown T, Gray SJ, Kordower JH (2017) Novel oligodendroglial alpha synuclein viral vector models of multiple system atrophy: studies in rodents and nonhuman primates. Acta neuropathologica communications 5: 47 Doi 10.1186/s40478-017-0451-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mandler M, Valera E, Rockenstein E, Mante M, Weninger H, Patrick C, Adame A, Schmidhuber S, Santic R, Schneeberger A et al. (2015) Active immunization against alphα-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Molecular neurodegeneration 10: 10 Doi 10.1186/s13024-015-0008-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mandler M, Valera E, Rockenstein E, Weninger H, Patrick C, Adame A, Santic R, Meindl S, Vigl B, Smrzka O et al. (2014) Next-generation active immunization approach for synucleinopathies: implications for Parkinson’s disease clinical trials. Acta Neuropathol 127: 861–879 Doi 10.1007/s00401-014-1256-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M (2013) Prion-like spreading of pathological alphα-synuclein in brain. Brain 136: 1128–1138 Doi 10.1093/brain/awt037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuo A, Akiguchi I, Lee GC, McGeer EG, McGeer PL, Kimura J (1998) Myelin degeneration in multiple system atrophy detected by unique antibodies. Am J Pathol 153: 735–744 Doi 10.1016/S0002-9440(10)65617-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.May VE, Ettle B, Poehler AM, Nuber S, Ubhi K, Rockenstein E, Winner B, Wegner M, Masliah E, Winkler J (2014) alphα-Synuclein impairs oligodendrocyte progenitor maturation in multiple system atrophy. Neurobiol Aging 35: 2357–2368 Doi 10.1016/j.neurobiolaging.2014.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miller DW, Johnson JM, Solano SM, Hollingsworth ZR, Standaert DG, Young AB (2005) Absence of alphα-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm 112: 1613–1624 Doi 10.1007/s00702-005-0378-1 [DOI] [PubMed] [Google Scholar]
- 83.Mills JD, Kim WS, Halliday GM, Janitz M (2015) Transcriptome analysis of grey and white matter cortical tissue in multiple system atrophy. Neurogenetics 16: 107–122 Doi 10.1007/s10048-014-0430-0 [DOI] [PubMed] [Google Scholar]
- 84.Mills JD, Ward M, Kim WS, Halliday GM, Janitz M (2016) Strand-specific RNA-sequencing analysis of multiple system atrophy brain transcriptome. Neuroscience 322: 234–250 Doi 10.1016/j.neuroscience.2016.02.042 [DOI] [PubMed] [Google Scholar]
- 85.Mitsui J, Matsukawa T, Sasaki H, Yabe I, Matsushima M, Durr A, Brice A, Takashima H, Kikuchi A, Aoki M et al. (2015) Variants associated with Gaucher disease in multiple system atrophy. Ann Clin Transl Neurol 2: 417–426 Doi 10.1002/acn3.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miyake E (1979) Establishment of a human oligodendroglial cell line. Acta Neuropathol 46: 51–55 [DOI] [PubMed] [Google Scholar]
- 87.Mori F, Tanji K, Yoshimoto M, Takahashi H, Wakabayashi K (2002) Demonstration of alphα-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp Neurol 176: 98–104 [DOI] [PubMed] [Google Scholar]
- 88.Multiple-System Atrophy Research C (2013) Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med 369: 233–244 Doi 10.1056/NEJMoa1212115 [DOI] [PubMed] [Google Scholar]
- 89.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH (2010) Increased expression of alphα-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65: 66–79 Doi 10.1016/j.neuron.2009.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishie M, Mori F, Yoshimoto M, Takahashi H, Wakabayashi K (2004) A quantitative investigation of neuronal cytoplasmic and intranuclear inclusions in the pontine and inferior olivary nuclei in multiple system atrophy. Neuropathol Appl Neurobiol 30: 546–554 Doi 10.1111/j.1365-2990.2004.00564.x [DOI] [PubMed] [Google Scholar]
- 91.Nykjaer CH, Brudek T, Salvesen L, Pakkenberg B (2017) Changes in the cell population in brain white matter in multiple system atrophy. Mov Disord 32: 1074–1082 Doi 10.1002/mds.26979 [DOI] [PubMed] [Google Scholar]
- 92.Ozawa T, Okuizumi K, Ikeuchi T, Wakabayashi K, Takahashi H, Tsuji S (2001) Analysis of the expression level of alphα-synuclein mRNA using postmortem brain samples from pathologically confirmed cases of multiple system atrophy. Acta Neuropathol 102: 188–190 [DOI] [PubMed] [Google Scholar]
- 93.Ozawa T, Revesz T, Paviour D, Lees AJ, Quinn N, Tada M, Kakita A, Onodera O, Wakabayashi K, Takahashi H et al. (2012) Difference in MSA phenotype distribution between populations: genetics or environment? Journal of Parkinson’s disease 2: 7–18 Doi 10.3233/JPD-2012-11056 [DOI] [PubMed] [Google Scholar]
- 94.Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci 94: 79–100 Doi 0022-510X(89)90219-0 [pii] [DOI] [PubMed] [Google Scholar]
- 95.Papp MI, Lantos PL (1994) The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain 117 (Pt 2): 235–243 [DOI] [PubMed] [Google Scholar]
- 96.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ (2008) Disease-specific induced pluripotent stem cells. Cell 134: 877–886 Doi 10.1016/j.cell.2008.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V (2015) alphα-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522: 340–344 Doi 10.1038/nature14547 [DOI] [PubMed] [Google Scholar]
- 98.Pfeiffer RF (2007) Multiple system atrophy. Handb Clin Neurol 84: 305–326 Doi S0072-9752(07)84046-2 [pii] 10.1016/S0072-9752(07)84046-2 [DOI] [PubMed] [Google Scholar]
- 99.Post GR, Dawson G (1992) Characterization of a cell line derived from a human oligodendroglioma. Mol Chem Neuropathol 16: 303–317 [DOI] [PubMed] [Google Scholar]
- 100.Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN et al. (2015) Evidence for alphα-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A 112: E5308–5317 Doi 10.1073/pnas.1514475112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C et al. (2014) Lewy body extracts from Parkinson disease brains trigger alphα-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75: 351–362 Doi 10.1002/ana.24066 [DOI] [PubMed] [Google Scholar]
- 102.Reyes JF, Rey NL, Bousset L, Melki R, Brundin P, Angot E (2014) Alphα-synuclein transfers from neurons to oligodendrocytes. Glia 62: 387–398 Doi 10.1002/glia.22611 [DOI] [PubMed] [Google Scholar]
- 103.Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM (2000) alphα-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res 62: 9–14 Doi [pii] [DOI] [PubMed] [Google Scholar]
- 104.Riedel M, Goldbaum O, Richter-Landsberg C (2009) alphα-Synuclein promotes the recruitment of tau to protein inclusions in oligodendroglial cells: effects of oxidative and proteolytic stress. J Mol Neurosci 39: 226–234 Doi 10.1007/s12031-009-9190-y [DOI] [PubMed] [Google Scholar]
- 105.Rockenstein E, Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Masliah E (2012) Neuronal to oligodendroglial alphα-synuclein redistribution in a double transgenic model of multiple system atrophy. Neuroreport 23: 259–264 Doi 10.1097/WNR.0b013e3283509842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rohan Z, Milenkovic I, Lutz MI, Matej R, Kovacs GG (2016) Shared and Distinct Patterns of Oligodendroglial Response in alphα-synucleinopathies and Tauopathies. J Neuropathol Exp Neurol 75: 1100–1109 Doi 10.1093/jnen/nlw087 [DOI] [PubMed] [Google Scholar]
- 107.Ross OA, Vilarino-Guell C, Wszolek ZK, Farrer MJ, Dickson DW (2010) Reply to: SNCA variants are associated with increased risk of multiple system atrophy. Ann Neurol 67: 414–415 Doi 10.1002/ana.21786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Salvesen L, Ullerup BH, Sunay FB, Brudek T, Lokkegaard A, Agander TK, Winge K, Pakkenberg B (2015) Changes in total cell numbers of the basal ganglia in patients with multiple system atrophy - A stereological study. Neurobiol Dis 74: 104–113 Doi 10.1016/j.nbd.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 109.Sanchez-Guajardo V, Tentillier N, Romero-Ramos M (2015) The relation between alphα-synuclein and microglia in Parkinson’s disease: Recent developments. Neuroscience 302: 47–58 Doi 10.1016/j.neuroscience.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 110.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA (2013) Incidence and Pathology of Synucleinopathies and Tauopathies Related to Parkinsonism. JAMA neurology: 1–7 Doi 10.1001/jamaneurol.2013.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schafferer S, Khurana R, Refolo V, Venezia S, Sturm E, Piatti P, Hechenberger C, Hackl H, Kessler R, Willi Met al (2016) Changes in the miRNA-mRNA Regulatory Network Precede Motor Symptoms in a Mouse Model of Multiple System Atrophy: Clinical Implications. PLoS One 11: e0150705 Doi 10.1371/journal.pone.0150705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scholz SW, Houlden H, Schulte C, Sharma M, Li A, Berg D, Melchers A, Paudel R, Gibbs JR, Simon-Sanchez J et al. (2009) SNCA variants are associated with increased risk for multiple system atrophy. Ann Neurol 65: 610–614 Doi 10.1002/ana.21685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schrag A, Selai C, Mathias C, Low P, Hobart J, Brady N, Quinn NP (2007) Measuring health-related quality of life in MSA: the MSA-QoL. Mov Disord 22: 2332–2338 Doi 10.1002/mds.21649[doi] [DOI] [PubMed] [Google Scholar]
- 114.Schrag A, Sheikh S, Quinn NP, Lees AJ, Selai C, Mathias C, Litvan I, Lang AE, Bower JH, Burn DJ et al. (2010) A comparison of depression, anxiety, and health status in patients with progressive supranuclear palsy and multiple system atrophy. Mov Disord 25: 1077–1081 Doi 10.1002/mds.22794[doi] [DOI] [PubMed] [Google Scholar]
- 115.Schrag A, Wenning GK, Quinn N, Ben-Shlomo Y (2008) Survival in multiple system atrophy. Mov Disord 23: 294–296 Doi 10.1002/mds.21839[doi] [DOI] [PubMed] [Google Scholar]
- 116.Scott D, Roy S (2012) alphα-synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci 32: 10129–10135 Doi 10.1523/JNEUROSCI.0535-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Serpell L, Berriman J, Jakes R, Goedert M, Crowther R (2000) Fiber diffraction of synthetic α–synuclein filaments shows amyloid-like cross-ß conformation. ProcNatlAcadSci 97: 4897–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shults CW, Rockenstein E, Crews L, Adame A, Mante M, Larrea G, Hashimoto M, Song D, Iwatsubo T, Tsuboi K et al. (2005) Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alphα-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J Neurosci 25: 10689–10699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soma H, Yabe I, Takei A, Fujiki N, Yanagihara T, Sasaki H (2006) Heredity in multiple system atrophy. J Neurol Sci 240: 107–110 Doi 10.1016/j.jns.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 120.Song YJ, Halliday GM, Holton JL, Lashley T, O’Sullivan SS, McCann H, Lees AJ, Ozawa T, Williams DR, Lockhart PJ et al. (2009) Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J Neuropathol Exp Neurol 68: 1073–1083 Doi 10.1097/NEN.0b013e3181b66f1b [DOI] [PubMed] [Google Scholar]
- 121.Stefanova N, Bucke P, Duerr S, Wenning GK (2009) Multiple system atrophy: an update. Lancet Neurol 8: 1172–1178 Doi S1474-4422(09)70288-1 [pii] 10.1016/S1474-4422(09)70288-1[doi] [DOI] [PubMed] [Google Scholar]
- 122.Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK (2011) Toll-like receptor 4 promotes alphα-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol 179: 954–963 Doi 10.1016/j.ajpath.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stefanova N, Georgievska B, Eriksson H, Poewe W, Wenning GK (2012) Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res 21: 393–404 Doi 10.1007/s12640-011-9294-3 [DOI] [PubMed] [Google Scholar]
- 124.Stefanova N, Kaufmann WA, Humpel C, Poewe W, Wenning GK (2012) Systemic proteasome inhibition triggers neurodegeneration in a transgenic mouse model expressing human alphα-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. Acta Neuropathol 124: 51–65 Doi 10.1007/s00401-012-0977-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stefanova N, Klimaschewski L, Poewe W, Wenning GK, Reindl M (2001) Glial cell death induced by overexpression of alphα-synuclein. J Neurosci Res 65: 432–438 [DOI] [PubMed] [Google Scholar]
- 126.Stefanova N, Reindl M, Neumann M, Haass C, Poewe W, Kahle PJ, Wenning GK (2005) Oxidative Stress in Transgenic Mice with Oligodendroglial {alpha}-Synuclein Overexpression Replicates the Characteristic Neuropathology of Multiple System Atrophy. Am J Pathol 166: 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK (2007) Microglial activation mediates neurodegeneration related to oligodendroglial alphα-synucleinopathy: implications for multiple system atrophy. Mov Disord 22: 2196–2203 Doi 10.1002/mds.21671[doi] [DOI] [PubMed] [Google Scholar]
- 128.Stefanova N, Reindl M, Poewe W, Wenning GK (2005) In vitro models of multiple system atrophy. Mov Disord 20 Suppl 12: S53–56 Doi 10.1002/mds.20540 [DOI] [PubMed] [Google Scholar]
- 129.Stefanova N, Wenning GK (2015) Animal models of multiple system atrophy. Clin Auton Res 25: 9–17 Doi 10.1007/s10286-014-0266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stefanova N, Wenning GK (2016) Review: Multiple system atrophy: emerging targets for interventional therapies. Neuropathol Appl Neurobiol 42: 20–32 Doi 10.1111/nan.12304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stemberger S, Poewe W, Wenning GK, Stefanova N (2010) Targeted overexpression of human alphα-synuclein in oligodendroglia induces lesions linked to MSA-like progressive autonomic failure. Exp Neurol 224: 459–464 Doi S0014-4886(10)00166-4 [pii] 10.1016/j.expneurol.2010.05.008[doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stemberger S, Scholz SW, Singleton AB, Wenning GK (2011) Genetic players in multiple system atrophy: unfolding the nature of the beast. Neurobiol Aging 32: 1924 e1925–1914 Doi 10.1016/j.neurobiolaging.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sturm E, Fellner L, Krismer F, Poewe W, Wenning GK, Stefanova N (2016) Neuroprotection by Epigenetic Modulation in a Transgenic Model of Multiple System Atrophy. Neurotherapeutics 13: 871–879 Doi 10.1007/s13311-016-0447-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sturm E, Stefanova N (2014) Multiple system atrophy: genetic or epigenetic? Exp Neurobiol 23: 277–291 Doi 10.5607/en.2014.23.4.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Suzuki M, Hashimoto M, Yoshioka M, Murakami M, Kawasaki K, Urashima M (2011) The odor stick identification test for Japanese differentiates Parkinson’s disease from multiple system atrophy and progressive supra nuclear palsy. BMC Neurol 11: 157 Doi 10.1186/1471-2377-11-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 Doi 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- 137.Tong J, Ang LC, Williams B, Furukawa Y, Fitzmaurice P, Guttman M, Boileau I, Hornykiewicz O, Kish SJ (2015) Low levels of astroglial markers in Parkinson’s disease: relationship to alphα-synuclein accumulation. Neurobiol Dis 82: 243–253 Doi 10.1016/j.nbd.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, Rajput AH, Muenter MD, Kish SJ, Hornykiewicz Oet al (2010) Brain alphα-synuclein accumulation in multiple system atrophy, Parkinson’s disease and progressive supranuclear palsy: a comparative investigation. Brain 133: 172–188 Doi awp282 [pii] 10.1093/brain/awp282 [DOI] [PubMed] [Google Scholar]
- 139.Ubhi K, Lee PH, Adame A, Inglis C, Mante M, Rockenstein E, Stefanova N, Wenning GK, Masliah E (2009) Mitochondrial inhibitor 3-nitroproprionic acid enhances oxidative modification of alphα-synuclein in a transgenic mouse model of multiple system atrophy. J Neurosci Res 87: 2728–2739 Doi 10.1002/jnr.22089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ubhi K, Low P, Masliah E (2011) Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci 34: 581–590 Doi 10.1016/j.tins.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ubhi K, Rockenstein E, Kragh C, Inglis C, Spencer B, Michael S, Mante M, Adame A, Galasko D, Masliah E (2014) Widespread microRNA dysregulation in multiple system atrophy - disease-related alteration in miR-96. Eur J Neurosci 39: 1026–1041 Doi 10.1111/ejn.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ubhi K, Rockenstein E, Mante M, Inglis C, Adame A, Patrick C, Whitney K, Masliah E (2010) Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci 30: 6236–6246 Doi 30/18/6236 [pii] 10.1523/JNEUROSCI.0567-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ueda K, Fukushima H, Masliah E, Otero D, Horsburgh K, Kondo J, Ihara Y (1991) Molecular cloning of a novel amyloid protein in Alzheimer’s disease. SocNeurosciAbstr 17: 1443 [Google Scholar]
- 144.Valera E, Masliah E (2016) Therapeutic approaches in Parkinson’s disease and related disorders. J Neurochem 139 Suppl 1: 346–352 Doi 10.1111/jnc.13529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Valera E, Monzio Compagnoni G, Masliah E (2016) Review: Novel treatment strategies targeting alphα-synuclein in multiple system atrophy as a model of synucleinopathy. Neuropathol Appl Neurobiol 42: 95–106 Doi 10.1111/nan.12312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Valera E, Spencer B, Fields JA, Trinh I, Adame A, Mante M, Rockenstein E, Desplats P, Masliah E (2017) Combination of alphα-synuclein immunotherapy with anti-inflammatory treatment in a transgenic mouse model of multiple system atrophy. Acta neuropathologica communications 5: 2 Doi 10.1186/s40478-016-0409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Valera E, Ubhi K, Mante M, Rockenstein E, Masliah E (2014) Antidepressants reduce neuroinflammatory responses and astroglial alphα-synuclein accumulation in a transgenic mouse model of multiple system atrophy. Glia 62: 317–337 Doi 10.1002/glia.22610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vanacore N (2005) Epidemiological evidence on multiple system atrophy. J Neural Transm (Vienna) 112: 1605–1612 Doi 10.1007/s00702-005-0380-7 [DOI] [PubMed] [Google Scholar]
- 149.Vermaas JV, Tajkhorshid E (2014) Conformational heterogeneity of alphα-synuclein in membrane. Biochim Biophys Acta 1838: 3107–3117 Doi 10.1016/j.bbamem.2014.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM (2011) Exogenous alphα-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72: 57–71 Doi 10.1016/j.neuron.2011.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wakabayashi K, Takahashi H (2006) Cellular pathology in multiple system atrophy. Neuropathology 26: 338–345 [DOI] [PubMed] [Google Scholar]
- 152.Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M et al. (2002) Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain : a journal of neurology 125: 1070–1083 [DOI] [PubMed] [Google Scholar]
- 153.Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB (2013) Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci U S A 110: 19555–19560 Doi 10.1073/pnas.1318268110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Watts JC, Prusiner SB (2014) Mouse models for studying the formation and propagation of prions. J Biol Chem 289: 19841–19849 Doi 10.1074/jbc.R114.550707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT Jr. (1996) NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry 35: 13709–13715 Doi 10.1021/bi961799nbi961799n[pii] [DOI] [PubMed] [Google Scholar]
- 156.Wenning GK, Ben Shlomo Y, Magalhaes M, Daniel SE, Quinn NP (1994) Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain : a journal of neurology 117 (Pt 4): 835–845 [DOI] [PubMed] [Google Scholar]
- 157.Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG (2008) Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol 64: 239–246 Doi 10.1002/ana.21465[doi] [DOI] [PubMed] [Google Scholar]
- 158.Woerman AL, Watts JC, Aoyagi A, Giles K, Middleton LT, Prusiner SB (2017) alphα-synuclein: Multiple System Atrophy Prions. Cold Spring Harb Perspect Med: Doi 10.1101/cshperspect.a024588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wullner U, Abele M, Schmitz-Huebsch T, Wilhelm K, Benecke R, Deuschl G, Klockgether T (2004) Probable multiple system atrophy in a German family. J Neurol Neurosurg Psychiatry 75: 924–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yabe I, Soma H, Takei A, Fujiki N, Yanagihara T, Sasaki H (2006) MSA-C is the predominant clinical phenotype of MSA in Japan: analysis of 142 patients with probable MSA. J Neurol Sci 249: 115–121 Doi S0022-510X(06)00282-6 [pii] 10.1016/j.jns.2006.05.064 [DOI] [PubMed] [Google Scholar]
- 161.Yazawa I, Giasson B, Sasaki R, Zhang B, Joyce S, Uryu K, Trojanowsk JQ, Lee V-M (2004) Mouse model of Multiple System Atrophy: alphα-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. submitted: [DOI] [PubMed] [Google Scholar]
- 162.Yazawa I, Giasson BI, Sasaki R, Zhang B, Joyce S, Uryu K, Trojanowski JQ, Lee VM (2005) Mouse model of multiple system atrophy alphα-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron 45: 847–859 [DOI] [PubMed] [Google Scholar]
- 163.Yoshida M (2007) Multiple system atrophy: alphα-synuclein and neuronal degeneration. Neuropathology 27: 484–493 [DOI] [PubMed] [Google Scholar]
- 164.Yun JY, Lee WW, Lee JY, Kim HJ, Park SS, Jeon BS (2010) SNCA variants and multiple system atrophy. Ann Neurol 67: 554–555 Doi 10.1002/ana.21889 [DOI] [PubMed] [Google Scholar]
- 165.Zhou L, Jiang Y, Zhu C, Ma L, Huang Q, Chen X (2016) Oxidative Stress and Environmental Exposures are Associated with Multiple System Atrophy in Chinese Patients. Can J Neurol Sci 43: 703–709 Doi 10.1017/cjn.2016.261 [DOI] [PubMed] [Google Scholar]