Abstract
Chronic obstructive pulmonary disease (COPD) was the fourth leading cause of death worldwide in 2015. Current treatments for patients ease discomfort and help decrease disease progression; however, none improve lung function or change mortality. COPD is heterogeneous in its molecular and clinical presentation, making it difficult to understand disease aetiology and define robust therapeutic strategies. Given the complexity of the disease we propose a precision medicine approach to understanding and better treating COPD. It is possible that multiOMICs can be used as a tool to integrate data from multiple fields. Moreover, analysis of electronic medical records could aid in the treatment of patients and in the predictions of outcomes. The Precision Medicine Initiative created in 2015 has made precision medicine approaches to treat disease a reality; one of these diseases being COPD.
Short abstract
MultiOMICs integration of clinical and molecular markers is needed to predict patient outcomes in COPD http://ow.ly/cnhj30kBxfd
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, complex, heterogeneous condition that is responsible for growing morbidity and mortality [1]. The complexity refers to components with nonlinear dynamic interactions, while heterogeneity suggests that not all components are present in all patients at the same time [2, 3]. Early versions of the Global Initiative for Obstructive Lung Disease (GOLD) therapeutic strategy recommended assessing disease severity and guiding therapeutic decisions as a function of the degree of airflow limitation. To address the complexity of COPD, some investigators suggested identifying clinical phenotypes as groups with similar clinical characteristics, prognosis and/or therapeutic needs [3]. Numerous groups have addressed innovative analytical methods that may guide future approaches to personalised medicine [4, 5]. In this review we focus on the practical clinical implications of current and future approaches to the evaluation and care of patients suffering from COPD. A framework for this approach has been presented previously (figure 1) [6].
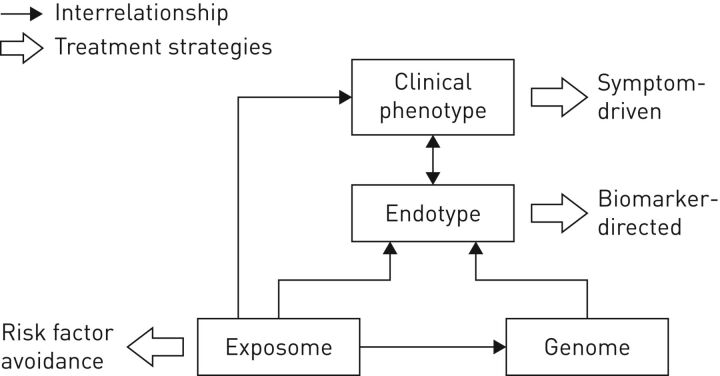
FIGURE 1.
Diagram of the interrelationships between the exposome (the totality of human environmental exposures, from conception onwards), genome (the genetic background of the individual), the endotype (biological networks that enable and restrict reactions) and the clinical phenotype (final clinical expression of the disease, e.g. symptoms, exacerbations, response to treatment, rate of disease progression or death). Reproduced from [6] with permission from the publisher.
Where are we now?
The terms “personalised”, “precision” and “individualised” medicine have been used interchangeably by many clinicians and investigators [7]. Precision medicine is an emerging strategy assessing genetic, biomarker, phenotypic and psychosocial characteristics to distinguish between patients with similar diagnoses [8]. Combined, this information may allow providers to anticipate disease course and patient responses to predict efficacious therapy and circumvent trial and error in finding effective therapies.
Over the past decade, the GOLD therapeutic strategy acknowledged the limitation of using spirometry alone to assess disease severity and guide therapy [9]. Treatment objectives were focused on relieving symptoms and reducing the risk of future exacerbations. A four-quadrant assessment system for initial pharmacotherapy was introduced to group patients into categories based on currently accepted phenotypes [10], including the following.
More symptomatic
Breathlessness and exertional limitation are cardinal manifestations in patients suffering from COPD [11]. Furthermore, dyspnoea level and impaired health status vary significantly between patients suffering from similar physiological abnormality [12]; these may be predictors of mortality [13].
Frequent exacerbator
Over a decade ago, it was found that patients with frequent exacerbations have worse survival [14]. This was further explored in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE) cohort, demonstrating that severity and frequency of exacerbations correlated with the severity of COPD and that across all GOLD stages, the single best predictor of exacerbations was a history of exacerbations [15]. Similarly, study of an unbiased prospective cohort COPD patients independently suggested that a history of two moderate or severe exacerbations was the best predictor of subsequent events [16]. However, a recent large observational cohort suggested that individuals meeting this threshold are rare and that variability in exacerbation rate over time is significant [3].
Chronic bronchitis
Chronic cough and sputum production are common clinical manifestations [17] associated with worse health status [17, 18] and a greater risk of clinical events [17] in population-based studies [19]. Current or former smokers with severe COPD in SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) had higher total mucin concentrations (MUC5B and MUC5AC) [20], as did participants with two or more respiratory exacerbations per year [20].
The symptom burden and risk of exacerbations (assessed using forced expiratory volume in 1 s (FEV1) and number of exacerbations in the previous year) assessment schema was an attempt to move COPD therapy into a personalised era [6]. For all GOLD groups, short-acting bronchodilators were recommended for symptom relief. For those with more symptoms, long-acting bronchodilators are effective in improving lung function and health status [21]. Patients at risk of exacerbations should use long-acting anticholinergics (LAMA) or combinations of inhaled long-acting β2-agonists (LABA) and corticosteroids (ICS) [10]. The LABA/LAMA combination was suggested for more symptomatic patients and those at greater exacerbation risk. Roflumilast, a phosphodiesterase-4 inhibitor, is an alternative approach to prevent exacerbations in those with chronic bronchitis and a history of prior exacerbations [22, 23]. This latter population serves as a unique example of phenotype-driven pharmacotherapy [24]. Although the overall approach was lauded for its personalised basis [25], many of the recommendations were not strictly evidence-based [26]. A key limitation of these recommendations reflected the unclear role of ICS in COPD with evidence suggesting that the widespread use of these agents persists [27].
The subsequent major GOLD therapeutic strategy revision further expanded the role of clinical phenotyping. Spriometry was removed for therapeutic decisions [4]. The impact of responses to a LABA/LAMA compared to single agents [28] was highlighted. Although there are few studies that assess the effect on risk of exacerbations, one study demonstrated a clear impact on the number needed to treat using ICS/LABA combination therapy [29]. Dual bronchodilator therapy was recommended for exacerbation reduction based on one comparative therapeutic trial [30] and inhaled LABA/LAMA/ICS as step-up therapy based on several comparative studies [31–33]. Further clinical phenotyping in chronic bronchitis was highlighted with response to roflumilast in patients with at least one respiratory hospitalisation in the prior year [34, 35]. All of these recommendations were placed within the context of adopting a benefit–risk approach for therapeutics (figure 2) [3]. This concept was particularly relevant, given the concerns that ICS increase the risk of pneumonia and systemic side-effects [36]. One investigative group described a greater increase in ICS-related pneumonia risk in current smokers, patients with prior pneumonia, those with a body mass index <25 kg·m−2 and severe airflow limitation [37]. Similarly, this benefit–risk approach was adopted by the GOLD therapeutic strategy in interventional lung volume reduction based on clinical phenotyping. Figure 3 illustrates the advocated approach that is dependent on the impact of emphysema severity and distribution coupled with severe airflow obstruction and persistent exertional limitation [38]. The Spanish guideline for COPD (GesEPOC) has been much more explicit in recommending COPD treatment according to four clinical phenotypes: non-exacerbator phenotype with either chronic bronchitis or emphysema; asthma–COPD overlap (ACO) syndrome; frequent exacerbator phenotype with emphysema; or frequent exacerbator phenotype with chronic bronchitis [39] (figure 4).
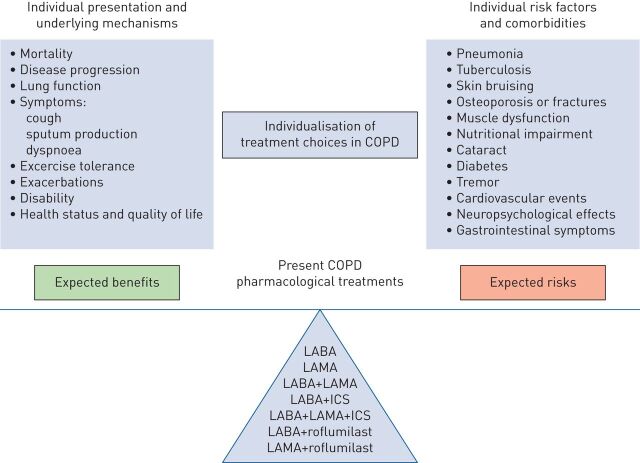
FIGURE 2.
Benefit–risk balance and its individual determinants with personalised chronic obstructive pulmonary disease (COPD) treatment choices. When a clinician is deciding which pharmacological treatment options to prescribe to a patient, they have to consider expected benefits (determined by individual presentation and underlying mechanisms of disease) and possible risks (which depend on individual risk factors and comorbidities). LABA: long-acting β2 agonists; LAMA: long-acting muscarinic antagonists; ICS: inhaled corticosteroids. Reproduced from [6] with permission from the publisher.
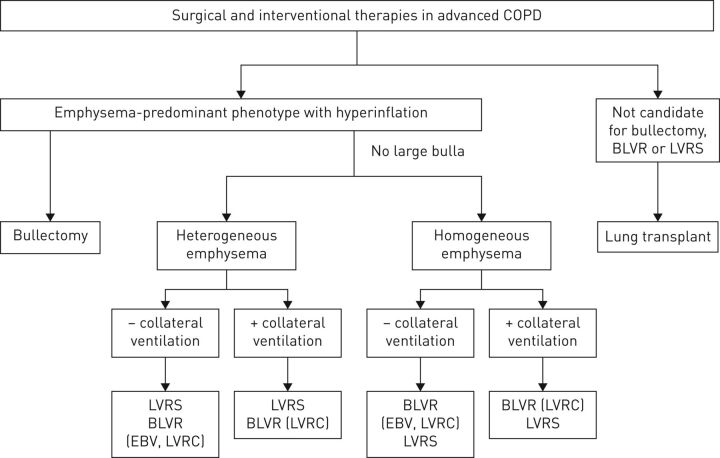
FIGURE 3.
Interventional bronchoscopic and surgical treatments for chronic obstructive pulmonary disease (COPD). Overview of therapeutic algorithm used to treat patients with COPD and emphysema. BLVR: bronchoscopic lung volume reduction; LVRS: lung volume reduction surgery; EBV: endobronchial valve; LVRC: lung volume reduction coil. Reproduced from [38] with permission from the publisher.
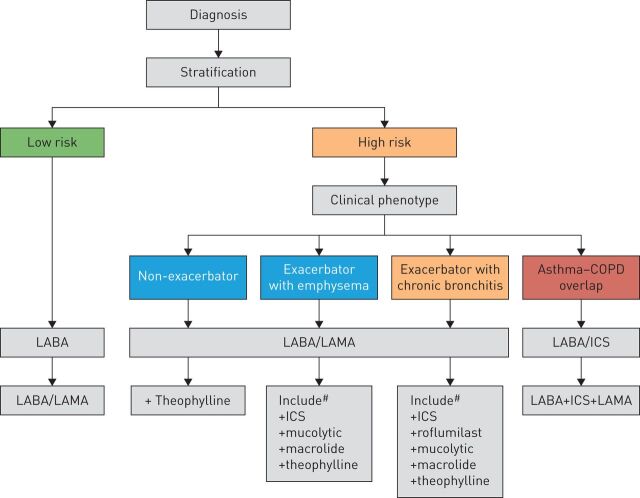
FIGURE 4.
Flow chart of chronic obstructive pulmonary disease (COPD) therapy as a function of risk and clinical phenotype. ICS: inhaled corticosteroids; LABA: long-acting β2 agonist; LAMA: long-acting muscarinic antagonist. #: treatments presented in order of suggested preference. Reproduced from [40] with permission from the publisher.
Where do we need to go?
It is clear that the investigative and clinical community needs to continue evolving phenotypic traits that influence COPD treatment and outcomes.
Targeting “early” COPD
Lung function trajectories in COPD differ significantly between patients [41, 42], and as we currently cannot reverse lung damage, defining early COPD is critical in mitigating disease progression. Although we have criteria to identify mild disease, there is no accepted definition of what constitutes early disease in COPD. Early COPD should be defined by the initial changes that ultimately lead to disease development, but this is not possible at this time. Definitions of early COPD have been proposed based on early symptoms or changes in lung structure on computed tomography (CT) imaging [43], which identifies subsets of individuals at risk of progression of disease. However, symptoms and lung structure may not always correlate. It has been proposed that “early COPD” should be studied in those aged <50 years with no other known chronic lung diseases, ≥10 pack-years smoking history and any of the following abnormalities: 1) early airflow limitation (post-bronchodilator FEV1/forced vital capacity (FVC) less than lower limit of normal); 2) compatible CT abnormalities; or 3) rapid decline in FEV1 (≥60 mL·year−1) that is accelerated relative to FVC [44]. The rationale behind these choices has been elaborated in detail [45], but is based on recognising the minimum exposure to cause lung function decline at point at which decline is detectable. However, these criteria identify disease that results from tobacco use. Strategies to objectively quantify other environmental exposures implicated in COPD development, such as biomass fuel inhalation are needed to better identify patients with “early COPD”.
Altering COPD disease progression
Although spirometry allows for diagnosing COPD, it cannot predict outcomes. Therefore, we need reliable clinical markers that predict disease outcomes such as lung function decline, exacerbation likelihood and mortality. Being able to predict patient outcomes is important for both basic and clinical research, because it can determine inclusion of patients in clinical trials and the study of molecular mechanisms in more defined subgroups. Recent data suggest that failure to achieve normal lung function in early adulthood followed by age-appropriate rates of decline causes up to half of COPD cases [46]. Smoke exposure in utero, in childhood or in adolescence is associated with increased adult COPD risk [41, 45]. Other causes of impaired lung growth during childhood can also reduce adult lung function [47], and even more importantly, may lead to more comorbidities and premature death [48]. While there are many hypotheses of mechanisms leading to this, such as stunted lung development and growth, epigenetic modifications and possible changes in the lung microbiome [45], more studies are needed before these criteria can be incorporated in a patient-specific strategy for medical care.
Asthma–COPD overlap
These studies along with others have substantiated the premise that subphenotyping may provide prognostic information. ACO is recognised as a distinct COPD clinical phenotype in international guidelines from GOLD and Global Initiative for Asthma (GINA) for COPD and asthma [49]. According to both GOLD and GINA, ACO is “characterised by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD” [38, 50]. This concept remains very controversial [51–53] as there is increasing appreciation of the involvement of the small airways and non-T-helper type 2 (Th2) type of inflammation in asthma as well as the involvement of large airways and eosinophils in COPD. Although the relevance of this phenotype remains unclear, patients with ACO appear to suffer a greater disease burden [54]. The therapeutic implications of this phenotype await a keener understanding of the underlying endotype [55], as currently the treatment is based on the most dominant clinical phenotype [38, 50].
Moving from personalised medicine to precision medicine
At this time, precision medicine is most commonly used in cancers in which tumours are heterogeneous and treatment can be tailored to the specific mutations in the tumour. Lung cancers frequently contain somatic mutations in the kinase domain of epidermal growth factor receptor (EGFR), and is most commonly found in women, nonsmokers and people of Japanese descent. A clinical trial studying the EGFR inhibitor gefinitib in lung cancer treatment did not show any significant difference in outcome. However, this trial did not specifically study patients with the EGFR mutation. Stratification based on genotype in addition to clinical presentation allowed for the identifications of patients likely to reach better outcomes with a given treatment [56].
Like cancer, COPD is complex with a wide array of molecular and cellular alterations that result in similar clinical presentations of cough, dyspnoea and wheeze. There have been many limitations to our understanding of patient characteristics and phenotypes of COPD and the development of new therapies, including molecular causes with several nonlinear interactions, which may or may not be present in any given patient at a given time point [57]. COPD is complex in both cause and presentation, which justifies the need for a precision medicine approach to improve assessment, treatment and outcomes. The hope is that by considering these biological factors, in combination with psychosocial ones, precision medicine will offer us the best chance to improve current COPD patient outcomes. This will require a robust approach to development of predictive biomarkers [58, 59].
α1-antitrypsin deficiency: the prototypical trait for precision medicine in COPD
One highly elucidated topic is α1-antitrypsin (α1-AT) deficiency in COPD. α1-AT is a glycoprotein protease inhibitor encoded by the SERPINA1 gene. SERPINA1 mutations lead to decreased α1-AT in lung tissue resulting in an increased risk of COPD emphysema [60]. α1-AT deficiency is a prototypical example for precision medicine in COPD as it has identifiably genetic underpinnings, with specific epidemiology and clinical characteristics. Most importantly, the recognition of this deficiency by identifiable biomarkers can be used to guide therapy [61] and with targeted therapy demonstrating improvement in lung density by CT scanning [62, 63]. However, this is a small subtype of patients that it is due to an identifiable mutation, unlike the majority of COPD patients.
COPD patients with an inflammatory state
Small airway fibrosis and obliteration probably contribute to physiological airway dysfunction and occur earlier than any development of emphysema. One potential mechanism contributing to small airway fibrosis/obliteration involves altered epithelial integrity [64, 65]. Triggers such as cigarette smoke and other environmental air pollutants can trigger these changes to cause inflammation implicated in COPD where cytokine expression increases, mucus production increases and permeability increases in airways [66]. Researchers developed a protein microarray to assess 14 cytokines in patient serum. Overall, cytokine concentration differences between groups were not statistically significant. However, the researchers uncovered that the total serum cytokine levels statistically correlated with GOLD-determined COPD severity [67]. Given the assay sensitivity and serum access, this may be a fruitful way to identify patient risks prior to phenotypic symptoms and exacerbations. For biomarker analysis to have global utility, considerations such as compartment sampling need to be standardised [68]. To determine if blood biomarkers could reliably predict exacerbation, data from participants from two cohorts were analysed (COPDgene and SPIROMICS). These data suggested that certain biomarkers within each cohort were associated with exacerbations, but there was minimal replication between the two cohorts [69]. Ultimately, the investigators found that clinical manifestations remain the strongest predictor of disease and improved understanding of mechanisms of exacerbation are needed before biomarkers of utility can be identified [69].
A study drew a connection between inflammatory-response cytokines and epigenetic changes in COPD patients undergoing an exercise regimen. Epigenetic modifications occur when external stimuli change gene expression without altering the inherent DNA code. The most common markers of epigenetic change are DNA methylation and histone H4 acetylation that silence and enhance transcription, respectively. It is well accepted that exercise is a critical part of effective treatment for COPD disease progression; however, the molecular mechanisms that modulate the effect have yet to be understood. The study collected blood from 10 patients at different times in a prolonged exercise training regimen. There was an initial decrease in DNA methylation and changes in histone H4 acetylation that were negatively correlated to interleukin (IL)-4 cytokine levels and positively correlated to IL-8 levels [70]. Correlations between epigenetic and cytokine changes in response to exercise regimens indicate a possible link between the two in modulating COPD progression. Fully elucidating the interplay between epigenetics and inflammatory response may reveal a tool for predicting patient outcomes.
“Eosinophilic” COPD
Eosinophilic airway inflammation occurs in ∼15–40% of COPD subjects [71] with increases in sputum eosinophils with exacerbations [72–74]. Eosinophilic levels may correlate with patient responses to medications and outcomes [75]. High eosinophilic levels may be associated with corticosteroid responsiveness [76–81], and the use of corticosteroids in subjects with low eosinophil counts (<2%) was associated with an increased risk of pneumonia [82]. High lung eosinophils may represent a distinct host endotype with predominance of a Th2 phenotype which is responsive to corticosteroids [83]. However, blood eosinophil levels do not necessarily correlate with levels present in the airways or lung parenchyma in smokers with and without COPD [84–86]. In addition, the stability of eosinophil counts is a concern in its use as a biomarker or to guide therapy [87]. Mepolizumab, an anti-IL-5 antibody that affects proliferation, differentiation and migration of eosinophils, showed no significant differences in the annual rate of moderate or severe exacerbations [88]. However, in subjects with higher blood eosinophil counts, a low dose of mepolizumab was associated with a lower annual rate of moderate or severe exacerbations. Whether eosinophil levels alone are sufficient as biomarkers to identify a treatable distinct trait of COPD requires further longitudinal investigation [89–91].
Treatable traits controversy
Perhaps, patients should be identified with a “label-free” strategy based on the identification of “treatable traits”, instead of by asthma or COPD [92]. For example, some traits, such as airflow limitation, airway mucosal oedema or loss of elastic recoil can be assessed by spirometry and/or CT imaging and these can be used to guide specific therapy. The strength of such an approach is that it allows for precise, individualistic treatment options based on patient phenotypes without making assumptions about the association of different treatable traits. Conversely, this approach may compartmentalise the disease to the extent of impairing research on more basic mechanisms of COPD and tobacco-related injury.
Biological markers and the evolution to endotypic therapy
Further identification of specific characteristics associated with response to treatment can be provided by several approaches: 1) post hoc exploratory analyses of therapeutic trials; 2) observational cohort studies (retrospective, e.g. using databases, or prospective), especially with a comparative effectiveness design; 3) pragmatic randomised controlled trials (RCT); and 4) large, long-term, “classical” RCTs. In addition to precise clinical characterisation (including physiology and imaging), biomarkers are likely to be of major interest to identify target patients and to assess treatment effects. Their identification is likely to come from systems biology and network medicine [93–95], and studies of gene signatures have already generated attractive hypotheses regarding mechanisms and predictors of response independent of the clinical phenotype [55, 96, 97]. Moreover, we know that exacerbations themselves serve as a marker for other disease. COPD patients with cardiovascular disease, or even those with risk factors for cardiovascular disease are at increased risk of cardiovascular events if they have COPD exacerbations. This is especially true in hospitalised patients and within the first 30 days post-exacerbation [98].
MultiOMICs: a tool for precision medicine
The lack of reliable biomarkers for COPD emphasises the inherent need for large data gathering and integration to help explicate the underlying molecular mechanisms involved in disease pathogenesis. MultiOMICs is a method of biological analysis in which data from multiple omics studies are integrated to enable a better understanding of complex data [99]. This is an emerging field that can potentially answer the deficiency in reliable biomarkers for COPD. One fundamental goal of multiOMICs use in COPD is the ability to stratify risk in patients quickly and cost effectively, based on specific markers. Large multiOMICs studies are becoming more prevalent as the methods become less expensive, allowing scientists to scan the genome, proteome, transcriptome and the microbiome. In addition, the ability to store all the data in a secure accessible database is increasing the feasibility of such large OMICs studies. Most importantly, the key is not only analysing the data quickly, but integrating it into a meaningful synthesis.
A large study integrating data from three different OMICs experiments showed differences in the transcriptome, proteome and metabolome in the lung tissue of rats. Rats were exposed to 8 weeks of cigarette smoke and infected with Klebsiella pneumoniae to induce rat airway changes representative of human COPD airway remodelling. Following this, rats were treated with control saline or an exacerbation treatment for 12 weeks and downstream omics experiments were performed on the lung tissue. The data was analysed by mapping the dysregulated genes and transcripts into common physiological processes, and indicated alterations in lipid metabolism. Furthermore, the researchers identified that arachidonic acid metabolism was inhibited by aminophylline treatment [100]. Further research is needed to elucidate the molecular mechanisms leading to the altered physiological processes, but large omics studies are able to identify quickly what processes may be of interest and what molecules are affected. Most importantly, it will be necessary to extend this type of analysis to human subjects in order to be relevant clinically. Understanding the mechanism can then allow for proper biomarker assessment for COPD patients.
Another compelling study involves next-generation sequencing focused on microRNAs (miRNAs) in peripheral leukocytes from patient blood. mRNAs are 17–24 nucleotide long noncoding RNA transcripts that bind to complementary base pair sequences on mRNA molecules to regulate gene expression post-transcriptionally. miRNAs are easily detectable due to their stability in serum as they are resistant to degradation and are highly detectable by quantitative PCR, microarrays and miRNA sequencing. Wang et al. [101] found that miR-106b-5p could be a biomarker of COPD severity. However, more about miR-106b-5p must be understood to elucidate any mechanism, as it has come up in several miRNA serum studies, including carcinogenesis. A study found that clear cell renal carcinoma cells had higher levels of miR-106b-5p and it was found to bind to and inhibit three Wnt signalling antagonists, suggesting a mechanism [102]. There are a multitude of other studies where biomarkers are shown to correlate to disease and outcomes, but many of these studies are small and have to yet to be validated numerous times prior to being implemented clinically. Understanding the specific role(s) that molecules such as miR-106b-5p and other correlative markers play in the lung in general, and more specifically in COPD pathogenesis, is critical to determining how to use them clinically to assess patient outcome and severity.
Role of the microbiome in COPD precision medicine
Many have suggested correlations between lung microbiota and disease manifestations [103]. There has been a debate as to whether the changes in the respiratory microbiome identified in patients with COPD are causal to disease exacerbations or indicative of disease severity and/or phenotype. Dickson et al. [104] modelled the idea that it is not specific lung microbiota that lead to COPD exacerbations, but rather dysbiosis in microbial populations resulting from differential growth conditions following inflammatory triggers that lead to exacerbations. The comparison between acute bacterial infections and exacerbations highlights why dysbiosis rather than the newly acquired bacteria contribute to frequent exacerbations. Bacterial density compared to baseline is high during infections, but remains normal during exacerbations. Moreover, while there is clear clinical benefit from antibiotic treatment during infections, there is only a mild benefit for patients during exacerbations, although antibiotics may help stave off exacerbations. These lung environment changes can lead to selective growth and killing of microbes, ultimately leading to microbial dysbiosis that feeds back mechanistically, perpetuating the dysbiosis.
This model may explain the frequent-exacerbator phenotype in which a subgroup of COPD patients experience frequent exacerbations. These patients may have either a specific alteration in lung microbiota or lung architectural modifications due to disease that is more susceptible to dysbiosis, preventing full recovery to a homeostatic state. Chronic azithromycin antibiotic treatments have decreased exacerbation frequency in COPD, but the mechanism is not understood. Potentially, azithromycin could be reducing dysbiosis occurrences by keeping a proper selective pressure on lung microbiota [104]. Other studies suggest azithromycin treatment increases anti-inflammatory bacterial metabolites that may contribute to its therapeutic effects [105]. The relationship between lung microbial environments and COPD exacerbations can be targeted in precision medicine treatments for frequent-exacerbator patients. Similarly, azithromycin was found to be more effective at reducing exacerbations in older patients with milder disease who have stopped smoking [106].
Electronic health records: a helpful tool for precision medicine
Electronic health records (EHR) are a helpful tool for precision medicine for all diseases and can be coupled with omics data and analytical programmes to identify at-risk patients, potential outcomes and personalised treatments. The ability to process large amounts of data is especially important in heterogeneous diseases like COPD that exhibit varying symptoms. Use of EHR in primary care practices had increased to 53% in Canada in 2014 [107]. Proper implementation involves monitoring usage by clinicians and nurses, record maintenance and security. Knowledge of second-hand smoke exposure, exercise frequency, environmental pollutants and tobacco usage can identify at-risk patients. Adding psychosocial factors that may influence the availability or use of medication, adherence to medicines and the frequency of other exposures would help clinicians identify the predicted outcomes and the best treatments for each patient.
One UK study targeted specific patients based on EHRs for health education, psychological counselling and smoking cessation. For participants, the 30-day readmission rates decreased from 13.4% to 1.9%, illustrating the potential for thorough EHRs to identify and aid COPD patients in managing the disease [108]. This highlights the need for EHRs to identify symptoms and clinical test results, as well as psychosocial factors like medication compliance and tobacco cessation for therapies that help slow disease pathogenesis. Moreover, there is a strong possibility that information that predicts outcomes is already buried in current EHRs. Organising this information using non-hypothesis-driven modelling may provide important prognostic information. Such a strategy has been demonstrated by the Intermountain Risk Score (IMRS) that predicts mortality and morbidity in medical and general populations [109]. In addition, IMRS predicts common morbidity end-points that lead to mortality, including COPD, leading some investigators to try to find a pulmonary-specific IMRS to prognosticate mortality [110].
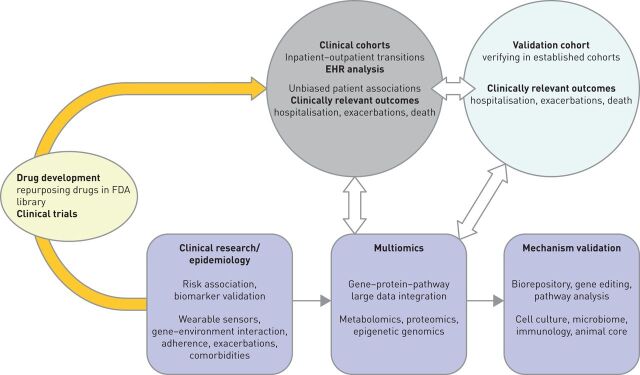
Studies have shown that most practices utilise EHRs, but EHRs have both positive and negative aspects, which must be balanced to improve the quality of care. Successful usage will need to be monitored closely as improper use can hurt the patient–physician relationship and decrease contextual knowledge of each patient or overall patient satisfaction. However, using computers to input patient data can streamline the process of recording patient history [111, 112]. EHRs can store much more information than paper records, and therefore proper care is required to keep the information concise to avoid hiding important data among the extraneous patient history. Security should be taken seriously, as electronic forms are more susceptible to security attacks [113]. Creating a partnership between investigators to integrate EHR analysis with clinical research and biomarker identification, multiOMICs analysis, to better understand basic mechanisms of disease could create a powerful approach to identify new treatment options for patients with COPD, as shown in figure 5.
FIGURE 5.
Integrated electronic health records (EHR), clinical, multiOMICs analysis to guide basic mechanisms and identify new treatment options for patients with chronic obstructive pulmonary disease. FDA: US Food and Drug Administration.
Precision medicine research initiative
US President Barack Obama announced the Precision Medicine Initiative (PMI) with the goal of developing personalised therapies for cancer and enhancing patient data collection, storage and research to better understand disease prevention, diagnosis and treatment. To fund the endeavour, a USD215 million budget was proposed for the 2016 fiscal year for PMI and allocated to the National Institutes of Health (NIH), NIH National Cancer Institute (NCI), Food and Drug Administration and the Office of the National Coordinator for Health Information Technology.
A critical factor in the success of PMI is patient involvement, which is problematic given the discrepancy between the US population and clinical trial patient populations. Chronic diseases such as COPD disproportionally affect lower socioeconomic and minority groups, while clinical trial participants are disproportionally Caucasian from higher socioeconomic groups. Minority group inclusion is important in clinical trials because it can elucidate unknown aspects. Heart disease is also associated with genetic predisposition combined with environmental and psychosocial factors. Antiplatelet therapy, including clopidogrel is shown to reduce cardiovascular mortality by 25% in smokers, but only by 8% in nonsmokers [114]. Clopidogrel acts on the adenosine diphosphate receptor (P2Y12) to irreversibly block activation of platelets. However, to act on the receptor, clopidogrel must be metabolised into its active form by cytochrome P450 2C19 (CYP2C19) [115]. CYP2C19*2 is a loss-of-function mutation that impedes bioactivation of clopidogrel, and at least one copy is present in 51% of Asians, 33% of African Americans, 18% of Mexican Americans and 24% in Caucasians. Knowing CYP2C19*2 genotypes among racial groups can allow clinicians to choose the best treatment for each patient [116].
A recent study among the five NCI-designated cancer centres highlighted the need for patient diversity in clinical trials and outlined specific hindrances to minority inclusion in cancer clinical trials, including 1) distrust and uncertainty in clinical research; 2) obstacles to enrolment; 3) lack of proper accommodations and dialogue with the community; and 4) insufficient referrals [117]. In addition, there is a lack of female data in animal studies and patient studies. In response to this disparity, in June 2015 the NIH announced that “sex” should be considered as a biological variable in research studies [118].
Acknowledging this lack of diversity and addressing the healthcare disparity, the PMI will enrol 1 million or more voluntary participants that represent the sex, racial, ethnic, socioeconomic and environmental diversity of the US. The study is specifically designed to include resources to alleviate the difficulties that minority groups often face. This includes multilingual investigators and recruiters, community involvement, self-reported data and more.
With the enrolment of appropriate patients into the PMI, it is important to have the proper data collection and data storage programmes and guidelines. Clinical information, genomic sequencing and microbiome assessments, as well as lifestyle, behaviour and environmental data will be collected. To aid in the collection of such lifestyle measures, smartphones and electronic sensors will be used. All data collected from patients will be de-identified and it is recommended that individual-level data be accessed in a secure computing area to prevent privacy breaches. Patients will be encouraged to access their data and have unrestricted rights to their own data. Moreover, assistance through the NIH and their precision medicine working group will be provided upon request for patients who need support in understanding their data.
The PMI is a powerful step toward elucidating the underlying aspects of many diseases and how we can improve disease prevention, diagnosis and treatment. It does so through active and diverse patient inclusion, encouraging patients to actively access and understand their data and how it is helping research studies. Although it will take time to understand specific parts of each disease, the PMI is promising for the future of precision medicine based disease management.
Conclusion
COPD is a complex and heterogeneous disease and treatments to reduce disease progression are lacking due to the deficiencies in our understanding of the disease. To improve assessment, treatment and outcomes we must explicate the relationship of phenotype and endotype and understand how features of the disease are modulated by cellular and molecular pathway(s) during disease pathogenesis. While there have been calls to have better subphenotyping of COPD patients to guide therapy [6, 55, 119–131], we are still in the early phases of approaching this goal.
FEV1/FVC is insufficient for predicting disease outcomes and we should utilise multiOMICs to understand what molecules are altered and how they affect physiological processes. Advances in omics data gathering and storage rationalise the procedure. The relationship described between lung microbiota dysbiosis and frequent exacerbations illustrate how omics data may aid in identifying patient outcomes. Patient records are being stored as EHRs that can be utilised in research studies and predict clinical care. The Precision Medicine Research Initiative is a step towards implementing precision medicine into all facets of disease care; one of which will be COPD. Our review of precision medicine for COPD highlights the research at both the basic and clinical level that needs to be addressed for COPD treatments.
Footnotes
Number 8 in the Series “Personalised medicine in respiratory diseases” Edited by Renaud Louis and Nicolas Roche
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Chung KF. Personalised medicine in asthma: time for action. Eur Respir Rev 2017; 26: 170064. No. 2: Bonsignore MR, Suarez Giron MC, Marrone O, et al. Personalised medicine in sleep respiratory disorders: focus on obstructive sleep apnoea diagnosis and treatment. Eur Respir Rev 2017; 26: 170069. No. 3: Mascaux C, Tomasini P, Greillier L, et al. Personalised medicine for nonsmall cell lung cancer. Eur Respir Rev 2017; 26: 170066. No. 4: Noell G, Faner R, Augusti A. From systems biology to P4 medicine: applications in respiratory medicine. Eur Respir Rev 2018; 27: 170110. No. 5: Wouters EFM, Wouters BBREF, Augustin IML, et al. Personalised pulmonary rehabilitation in COPD. Eur Respir Rev 2018; 27: 170125. No. 6: Kokosi MA, Margaritopoulos GA, Wells AU. Personalised medicine in interstitial lung diseases. Eur Respir Rev 2018; 27: 170117. No. 7: Savale L, Guignabert C, Weatherald J, et al. Precision medicine and personalising therapy in pulmonary hypertension: seeing the light from the dawn of a new era. Eur Respir Rev 2018; 27: 180004.
Author contributions: All authors contributed to the conception and design of the manuscript.
Conflict of interest: F.J. Martinez reports personal fees and non-financial support from the American College of Chest Physicians (personal fee honoraria and non-personal travel support for COPD CME programmes in India), personal fees and non-financial support from AstraZeneca (personal fees and non-personal travel support for COPD advisory boards, a study steering committee and an ALAT presentation), personal fees and non-financial support from Boehringer Ingelheim (personal fees and non-personal travel support for a COPD advisory board, and personal fees for an ATS presentation), non-financial support from ProterrixBio (support for an NIH study, but no direct financial compensation for a COPD scientific advisory board), personal fees and non-financial support from Continuing Education (personal fee honorarium and non-personal travel support for a cough CME programme), personal fees from Columbia University, Haymarket Communications, Integritas, Methodist Hospital Brooklyn, New York University, UpToDate, WebMD/MedScape and Western Connecticut Health Network (personal fee honoraria for COPD CME programmes), personal fees and non-financial support from ConCert, Pearl Pharmaceuticals, Roche, Sunovion and Theravance (personal fee honoraria and non-personal travel support for COPD advisory boards), personal fees and non-financial support from Genentech (personal fee and non-personal travel support for a COPD advisory board and non-financial support for an asthma data safety monitoring board), personal fees and non-financial support from GlaxoSmithKline (personal fee honoraria and non-personal travel support for COPD advisory boards, non-personal travel support for a study steering committee and an ERS presentation, and academic co-authorship for a data safety monitoring board), personal fees and non-financial support from Inova Fairfax Health System, Miller Communications, the National Association for Continuing Education, PeerView Communications, Prime Communications, the Puerto Rican Respiratory Society and Chiesi (personal fee honoraria and non-personal travel support for COPD CME programmes), personal fees from Inthought Research (personal fee honoraria for a COPD/asthma teleconference), personal fees from MD Magazine (personal fee honorarium and non-personal travel support for a COPD CME programme), personal fees and non-financial support from Novartis (personal fees honoraria and non-personal travel support for a COPD advisory board and international meeting COPD disease presentations), personal fees from Unity (personal fee honoraria for a COPD teleconference), personal fees from the American Thoracic Society (personal fee honoraria for being deputy editor of the AJRCCM), and a grant from the National Institutes of Health (COPD UO1/RO1).
Support statement: Supported by R01HL124099 (to V.K. Sidhaye) and SPIROMICS, which was supported by contracts from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune, Bayer, Bellerophon Therapeutics, Boehringer-Ingelheim Pharmaceuticals, Inc., Chiesi Farmaceutici SpA., Forest Research Institute, Inc., GlaxoSmithKline, Grifols Therapeutics, Inc., Ikaria, Inc., Nycomed GmbH, Takeda Pharmaceutical Company, Novartis Pharmaceuticals Corporation, ProterixBio, Regeneron Pharmaceuticals, Inc., Sanofi and Sunovion. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch Bronconeumol 2017; 53: 128–149. [DOI] [PubMed] [Google Scholar]
- 2.Faner R, Agustí A. Multilevel, dynamic chronic obstructive pulmonary disease heterogeneity. A challenge for personalized medicine. Ann Am Thorac Soc 2016; 13: Suppl. 5, S466–S470. [DOI] [PubMed] [Google Scholar]
- 3.Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med 2017; 5: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agustí A, Bafadhel M, Beasley R, et al. Precision medicine in airway diseases: moving to clinical practice. Eur Respir J 2017; 50: 1701655. [DOI] [PubMed] [Google Scholar]
- 5.Agustí A, Antó JM, Auffray C, et al. Personalized Respiratory Medicine: Exploring the Horizon, Addressing the Issues. Summary of a BRN-AJRCCM workshop held in Barcelona on June 12, 2014. Am J Respir Crit Care Med 2015; 191: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodruff PG, Agusti A, Roche N, et al. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised management. Lancet 2015; 385: 1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calverley PMA, Tetzlaff K, Vogelmeier C, et al. Eosinophilia, frequent exacerbations, and steroid response in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; 196: 1219–1221. [DOI] [PubMed] [Google Scholar]
- 8.Jameson JL, Longo DL. Precision medicine – personalized, problematic, and promising. N Engl J Med 2015; 372: 2229–2234. [DOI] [PubMed] [Google Scholar]
- 9.Ingebrigtsen TS, Marott JL, Lange P, et al. Medically treated exacerbations in COPD by GOLD 1–4: a valid, robust, and seemingly low-biased definition. Respir Med 2015; 109: 1562–1568. [DOI] [PubMed] [Google Scholar]
- 10.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 11.Marciniuk DD, Goodridge D, Hernandez P, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J 2011; 18: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonelli-Incalzi R, Imperiale C, Bellia V, et al. Do GOLD stages of COPD severity really correspond to differences in health status? Eur Respir J 2003; 22: 444–449. [DOI] [PubMed] [Google Scholar]
- 13.Casanova C, Marin JM, Martinez-Gonzalez C, et al. Differential effect of modified Medical Research Council dyspnea, COPD assessment test, and clinical COPD questionnaire for symptoms evaluation within the new GOLD staging and mortality in COPD. Chest 2015; 148: 159–168. [DOI] [PubMed] [Google Scholar]
- 14.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurst JR. The rhythm of chronic obstructive pulmonary disease exacerbations. J Clin Epidemiol 2010; 63: 1285–1286. [DOI] [PubMed] [Google Scholar]
- 16.Le Rouzic O, Roche N, Cortot AB, et al. Defining the ‘frequent exacerbator’ phenotype in COPD: a hypothesis-free approach. Chest 2018; 153: 1106–1115. [DOI] [PubMed] [Google Scholar]
- 17.Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heijdra YF, Pinto-Plata VM, Kenney LA, et al. Cough and phlegm are important predictors of health status in smokers without COPD. Chest 2002; 121: 1427–1433. [DOI] [PubMed] [Google Scholar]
- 19.Allinson JP, Hardy R, Donaldson GC, et al. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med 2016; 193: 662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kesimer M, Ford AA, Ceppe A, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med 2017; 377: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westwood M, Bourbeau J, Jones PW, et al. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res 2011; 12: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009; 374: 685–694. [DOI] [PubMed] [Google Scholar]
- 23.Rennard SI, Calverley PM, Goehring UM, et al. Reduction of exacerbations by the PDE4 inhibitor roflumilast – the importance of defining different subsets of patients with COPD. Respir Res 2011; 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010; 182: 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruffydd-Jones K, Pinnock H, Loveridge C, et al. Conflicting standards for diagnostic spirometry within-session repeatability are confusing. Prim Care Respir J 2012; 21: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin SH, Ji BC, Shih YM, et al. Comorbid pulmonary disease and risk of community-acquired pneumonia in COPD patients. Int J Tuberc Lung Dis 2013; 17: 1638–1644. [DOI] [PubMed] [Google Scholar]
- 27.Contoli M, Corsico AG, Santus P, et al. Use of ICS in COPD: from blockbuster medicine to precision medicine. COPD 2017; 14: 641–647. [DOI] [PubMed] [Google Scholar]
- 28.Martinez FJ, Rabe KF, Ferguson GT, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co-suspension delivery technology in patients with COPD. Chest 2017; 151: 340–357. [DOI] [PubMed] [Google Scholar]
- 29.Martinez FJ, Vestbo J, Anderson JA, et al. Effect of fluticasone furoate and vilanterol on exacerbations of chronic obstructive pulmonary disease in patients with moderate airflow obstruction. Am J Respir Crit Care Med 2017; 195: 881–888. [DOI] [PubMed] [Google Scholar]
- 30.Allinson JP, Wedzicha JA. Update in chronic obstructive pulmonary disease 2016. Am J Respir Crit Care Med 2017; 196: 414–424. [DOI] [PubMed] [Google Scholar]
- 31.Singh D, Miravitlles M, Vogelmeier C. Chronic obstructive pulmonary disease individualized therapy: tailored approach to symptom management. Adv Ther 2017; 34: 281–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 2017; 389: 1919–1929. [DOI] [PubMed] [Google Scholar]
- 33.Lipson DA, Barnacle H, Birk R, et al. Reply to Morice and Hart: Increased propensity for pneumonia with fluticasone in chronic obstructive pulmonary disease. Am J Respir Crit Care Medicine 2018; 197: 1230–1231. [DOI] [PubMed] [Google Scholar]
- 34.Martinez FJ, Rabe KF, Sethi S, et al. Effect of roflumilast and inhaled corticosteroid/long-acting β2-agonist on chronic obstructive pulmonary disease exacerbations (RE2SPOND). A randomized clinical trial. Am J Respir Crit Care Med 2016; 194: 559–567. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Roisin R, Rabe KF, Vestbo J, et al. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 20th anniversary: a brief history of time. Eur Respir J 2017; 50: 1700671. [DOI] [PubMed] [Google Scholar]
- 36.Kerkhof M, Sonnappa S, Postma DS, et al. Blood eosinophil count and exacerbation risk in patients with COPD. Eur Respir J 2017; 50: 1700761. [DOI] [PubMed] [Google Scholar]
- 37.Rutten EP, Spruit MA, McDonald ML, et al. Continuous fat-free mass decline in COPD: fact or fiction? Eur Respir J 2015; 46: 1496–1498. [DOI] [PubMed] [Google Scholar]
- 38.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017; 195: 557–582. [DOI] [PubMed] [Google Scholar]
- 39.Soriano JB, Lamprecht B, Ramírez AS, et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: a pooled analysis of individual patient data. Lancet Respir Med 2015; 3: 443–450. [DOI] [PubMed] [Google Scholar]
- 40.Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol 2017; 53: 324–335. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan JK, Martinez FJ. Lung function trajectories and chronic obstructive pulmonary disease: current understanding and knowledge gaps. Curr Opin Pulm Med 2018; 24: 124–129. [DOI] [PubMed] [Google Scholar]
- 42.Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med 2015; 373: 111–122. [DOI] [PubMed] [Google Scholar]
- 43.Bhatt SP, Han MK. Developing and implementing biomarkers and novel imaging in COPD. Chronic Obstr Pulm Dis 2016; 3: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez FJ, Han MK, Allinson JP, et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 197: 1540–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med 2016; 375: 871–878. [DOI] [PubMed] [Google Scholar]
- 46.Lange P, Celli B, Agustí A. Lung-function trajectories and chronic obstructive pulmonary disease. N Engl J Med 2015; 373: 1575. [DOI] [PubMed] [Google Scholar]
- 47.Allinson JP, Hardy R, Donaldson GC, et al. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am J Respir Crit Care Med 2017; 196: 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agustí A, Noell G, Brugada J, et al. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med 2017; 5: 935–945. [DOI] [PubMed] [Google Scholar]
- 49.Bateman ED, Reddel HK, van Zyl-Smit RN, et al. The asthma-COPD overlap syndrome: towards a revised taxonomy of chronic airways diseases? Lancet Respir Med 2015; 3: 719–728. [DOI] [PubMed] [Google Scholar]
- 50.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008; 31: 143–178. [DOI] [PubMed] [Google Scholar]
- 51.Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J 2016; 48: 664–673. [DOI] [PubMed] [Google Scholar]
- 52.Cazzola M, Rogliani P. Do we really need asthma-chronic obstructive pulmonary disease overlap syndrome? J Allergy Clin Immunol 2016; 138: 977–983. [DOI] [PubMed] [Google Scholar]
- 53.Woodruff PG, van den Berge M, Boucher RC, et al. American Thoracic Society/National Heart, Lung, and Blood Institute Asthma-Chronic Obstructive Pulmonary Disease Overlap Workshop Report. Am J Respir Crit Care Med 2017; 196: 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurashima K, Takaku Y, Ohta C, et al. COPD assessment test and severity of airflow limitation in patients with asthma, COPD, and asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis 2016; 11: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christenson SA, Steiling K, van den Berge M, et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191: 758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–2388. [DOI] [PubMed] [Google Scholar]
- 57.Faner R, Gutiérrez-Sacristán A, Castro-Acosta A, et al. Molecular and clinical diseasome of comorbidities in exacerbated COPD patients. Eur Respir J 2015; 46: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 58.Hollander Z, DeMarco ML, Sadatsafavi M, et al. Biomarker development in COPD: moving from p values to products to impact patient care. Chest 2017; 151: 455–467. [DOI] [PubMed] [Google Scholar]
- 59.Sin DD, Hollander Z, DeMarco ML, et al. Biomarker development for chronic obstructive pulmonary disease. From discovery to clinical implementation. Am J Respir Crit Care Med 2015; 192: 1162–1170. [DOI] [PubMed] [Google Scholar]
- 60.Russo R, Zillmer LR, Nascimento OA, et al. Prevalence of alpha-1 antitrypsin deficiency and allele frequency in patients with COPD in Brazil. J Bras Pneumol 2016; 42: 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strange C, Beiko T. Treatment of alpha-1 antitrypsin deficiency. Semin Respir Crit Care Med 2015; 36: 470–477. [DOI] [PubMed] [Google Scholar]
- 62.Chapman KR, Burdon JG, Piitulainen E, et al. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 386: 360–368. [DOI] [PubMed] [Google Scholar]
- 63.Miravitlles M. Alpha-1-antitrypsin and other proteinase inhibitors. Curr Opin Pharmacol 2012; 12: 309–314. [DOI] [PubMed] [Google Scholar]
- 64.Sohal SS, Ward C, Danial W, et al. Recent advances in understanding inflammation and remodeling in the airways in chronic obstructive pulmonary disease. Expert Rev Respir Med 2013; 7: 275–288. [DOI] [PubMed] [Google Scholar]
- 65.Nishida K, Brune KA, Putcha N, et al. Cigarette smoke disrupts monolayer integrity by altering epithelial cell-cell adhesion and cortical tension. Am J Physiol Lung Cell Mol Physiol 2017; 313: L581–L591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016; 138: 16–27. [DOI] [PubMed] [Google Scholar]
- 67.Selvarajah S, Todd I, Tighe PJ, et al. Multiple circulating cytokines are coelevated in chronic obstructive pulmonary disease. Mediators Inflamm 2016; 2016: 3604842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Neal WK, Anderson W, Basta PV, et al. Comparison of serum, EDTA plasma and P100 plasma for luminex-based biomarker multiplex assays in patients with chronic obstructive pulmonary disease in the SPIROMICS study. J Transl Med 2014; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keene JD, Jacobson S, Kechris K, et al. Biomarkers predictive of exacerbations in the SPIROMICS and COPDGene cohorts. Am J Respir Crit Care Med 2017; 195: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.da Silva IRV, de Araujo CLP, Dorneles GP, et al. Exercise-modulated epigenetic markers and inflammatory response in COPD individuals: a pilot study. Respir Physiol Neurobiol 2017; 242: 89–95. [DOI] [PubMed] [Google Scholar]
- 71.Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis 2006; 1: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bathoorn E, Kerstjens H, Postma D, et al. Airways inflammation and treatment during acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis 2008; 3: 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schleich F, Corhay JL, Louis R. Blood eosinophil count to predict bronchial eosinophilic inflammation in COPD. Eur Respir J 2016; 47: 1562–1564. [DOI] [PubMed] [Google Scholar]
- 74.Negewo NA, McDonald VM, Baines KJ, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J Chron Obstruct Pulmon Dis 2016; 11: 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Casanova C, Celli BR, de-Torres JP, et al. Prevalence of persistent blood eosinophilia: relation to outcomes in patients with COPD. Eur Respir J 2017; 50: 1701162. [DOI] [PubMed] [Google Scholar]
- 76.Bafadhel M, McKenna S, Terry S, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012; 186: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pascoe S, Locantore N, Dransfield MT, et al. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015; 3: 435–442. [DOI] [PubMed] [Google Scholar]
- 78.Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med 2016; 4: 390–398. [DOI] [PubMed] [Google Scholar]
- 80.Tashkin DP, Wechsler ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2018; 13: 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med 2018; 6: 117–126. [DOI] [PubMed] [Google Scholar]
- 82.Pavord ID, Lettis S, Anzueto A, et al. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir Med 2016; 4: 731–741. [DOI] [PubMed] [Google Scholar]
- 83.Barnes NC, Sharma R, Lettis S, et al. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J 2016; 47: 1374–1382. [DOI] [PubMed] [Google Scholar]
- 84.Turato G, Semenzato U, Bazzan E, et al. Blood eosinophilia neither reflects tissue eosinophils nor worsens clinical outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 197: 1216–1219. [DOI] [PubMed] [Google Scholar]
- 85.Hastie AT, Martinez FJ, Curtis JL, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med 2017; 5: 956–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roche N, Zysman M, Burgel PR. Blood eosinophil counts as a guide for COPD treatment strategies. Lancet Respir Med 2018; 6: 78–80. [DOI] [PubMed] [Google Scholar]
- 87.Oshagbemi OA, Burden AM, Braeken DCW, et al. Stability of blood eosinophils in patients with chronic obstructive pulmonary disease and in control subjects, and the impact of sex, age, smoking, and baseline counts. Am J Respir Crit Care Med 2017; 195: 1402–1404. [DOI] [PubMed] [Google Scholar]
- 88.Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613–1629. [DOI] [PubMed] [Google Scholar]
- 89.Bel EH, Ten Brinke A. New anti-eosinophil drugs for asthma and COPD: targeting the trait! Chest 2017; 152: 1276–1282. [DOI] [PubMed] [Google Scholar]
- 90.Hamad GA, Cheung W, Crooks MG, et al. Eosinophils in COPD: how many swallows make a summer? Eur Respir J 2018; 51: 1702177. [DOI] [PubMed] [Google Scholar]
- 91.Rabe KF, Beghé B, Fabbri LM. Peripheral eosinophil count as a biomarker for the management of COPD: not there yet. Eur Respir J 2017; 50: 1702165. [DOI] [PubMed] [Google Scholar]
- 92.Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47: 410–419. [DOI] [PubMed] [Google Scholar]
- 93.Wheelock CE, Goss VM, Balgoma D, et al. Application of 'omics technologies to biomarker discovery in inflammatory lung diseases. Eur Respir J 2013; 42: 802–825. [DOI] [PubMed] [Google Scholar]
- 94.Diez D, Agustí A, Wheelock CE. Network analysis in the investigation of chronic respiratory diseases. From basics to application. Am J Respir Crit Care Med 2014; 190: 981–988. [DOI] [PubMed] [Google Scholar]
- 95.Agustí A, Celli B, Faner R. What does endotyping mean for treatment in chronic obstructive pulmonary disease? Lancet 2017; 390: 980–987. [DOI] [PubMed] [Google Scholar]
- 96.van den Berge M, Steiling K, Timens W, et al. Airway gene expression in COPD is dynamic with inhaled corticosteroid treatment and reflects biological pathways associated with disease activity. Thorax 2014; 69: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noell G, Cosío BG, Faner R, et al. Multi-level differential network analysis of COPD exacerbations. Eur Respir J 2017; 50: 1700075. [DOI] [PubMed] [Google Scholar]
- 98.Kunisaki KM, Dransfield MT, Anderson JA, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med 2018; 198: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim S, Oesterreich S, Kim S, et al. Integrative clustering of multi-level omics data for disease subtype discovery using sequential double regularization. Biostatistics 2017; 18: 165–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li J, Zhao P, Yang L, et al. Integrating 3-omics data analyze rat lung tissue of COPD states and medical intervention by delineation of molecular and pathway alterations. Biosci Rep 2017; 37: BSR20170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang R, Xu J, Liu H, et al. Peripheral leukocyte microRNAs as novel biomarkers for COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu Q, Ji C, Zhao X, et al. Histopathologic analysis of tumor bed and peritumoral pseudocapsule after in vitro tumor enucleation on radical nephrectomy specimen for clinical T1b renal cell carcinoma. Urol Oncol 2017; 35: 603.e15–603.e620. [DOI] [PubMed] [Google Scholar]
- 103.Faner R, Sibila O, Agustí A, et al. The microbiome in respiratory medicine: current challenges and future perspectives. Eur Respir J 2017; 49: 1602086. [DOI] [PubMed] [Google Scholar]
- 104.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 2014; 384: 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Segal LN, Clemente JC, Wu BG, et al. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax 2017; 72: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med 2014; 189: 1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jones M, Koziel C, Larsen D, et al. Progress in the enhanced use of electronic medical records: data from the Ontario experience. JMIR Med Inform 2017; 5: e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu Y, Pleasants RA, Croft JB, et al. Smoking duration, respiratory symptoms, and COPD in adults aged ≥45 years with a smoking history. Int J Chron Obstruct Pulmon Dis 2015; 10: 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horne BD, May HT, Muhlestein JB, et al. Exceptional mortality prediction by risk scores from common laboratory tests. Am J Med 2009; 122: 550–558. [DOI] [PubMed] [Google Scholar]
- 110.Horne BD, Hegewald M, Muhlestein JB, et al. Pulmonary-specific intermountain risk score predicts all-cause mortality via spirometry, the red cell distribution width, and other laboratory parameters. Respir Care 2015; 60: 1314–1323. [DOI] [PubMed] [Google Scholar]
- 111.Manca DP. Rebuttal: Do electronic medical records improve quality of care? Yes. Can Fam Physician 2015; 61: e435, e437. [PMC free article] [PubMed] [Google Scholar]
- 112.Ramanan C, Gruber JM, Malý P, et al. The role of exciton delocalization in the major photosynthetic light-harvesting antenna of plants. Biophys J 2015; 108: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sulmasy LS, López AM, Horwitch CA, et al. Ethical implications of the electronic health record: in the service of the patient. J Gen Intern Med 2017; 32: 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gagne JJ, Bykov K, Choudhry NK, et al. Effect of smoking on comparative efficacy of antiplatelet agents: systematic review, meta-analysis, and indirect comparison. BMJ 2013; 347: f5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cattaneo M, Lecchi A, Ohno M, et al. Antiaggregatory activity in human platelets of potent antagonists of the P2Y 1 receptor. Biochem Pharmacol 2004; 68: 1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shuldiner AR, O'Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009; 302: 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Durant RW, Wenzel JA, Scarinci IC, et al. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT). Cancer 2014; 120: Suppl. 7, 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miller VM, Rocca WA, Faubion SS. Sex differences research, precision medicine, and the future of women's health. J Womens Health 2015; 24: 969–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bovornkitti S. Asthma-COPD overlap syndrome and precision medicine. Asian Pac J Allergy Immunol 2017; 35: 1–2. [PubMed] [Google Scholar]
- 120.Cazzola M, Calzetta L, Rogliani P, et al. The challenges of precision medicine in COPD. Mol Diagn Ther 2017; 21: 345–355. [DOI] [PubMed] [Google Scholar]
- 121.de Jong K, Vonk JM, Imboden M, et al. Genes and pathways underlying susceptibility to impaired lung function in the context of environmental tobacco smoke exposure. Respir Res 2017; 18: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Taube C. Asthma-COPD-Overlap-Syndrom (ACOS): Präzisionsmedizin oder diagnostische Sackgasse? [Asthma COPD overlap syndrome (ACOS): precision medicine or diagnostic dead end?]. Dtsch Med Wochenschr 2016; 141: 1161–1163. [DOI] [PubMed] [Google Scholar]
- 123.Sciurba FC, Chandra D, Bon J. Bronchoscopic lung volume reduction in COPD: lessons in implementing clinically based precision medicine. JAMA 2016; 315: 139–141. [DOI] [PubMed] [Google Scholar]
- 124.Laucho-Contreras M, de Oca MM, Owen CA. Asthma COPD overlap syndrome: an approach to a real-world endotype in obstructive lung disease? Curr Pharm Des 2016; 22: 6273–6282. [DOI] [PubMed] [Google Scholar]
- 125.Blasi F, Chalmers JD, Aliberti S. COPD and bronchiectasis: phenotype, endotype or co-morbidity? COPD 2014; 11: 603–604. [DOI] [PubMed] [Google Scholar]
- 126.Antoniu SA. Phenotype/endotype-driven therapy in COPD: potential economic implications. Expert Rev Pharmacoecon Outcomes Res 2013; 13: 421–423. [DOI] [PubMed] [Google Scholar]
- 127.Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the Diskus® dry powder inhaler. J Aerosol Med Pulm Drug Deliv 2013; 26: 174–179. [DOI] [PubMed] [Google Scholar]
- 128.Matkovic Z, Miravitlles M. Chronic bronchial infection in COPD. Is there an infective phenotype? Respir Med 2013; 107: 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McGarvey L. Keeping up appearances: the importance of maintaining health status in COPD. Thorax 2015; 70: 813–814. [DOI] [PubMed] [Google Scholar]
- 130.Roy K, Smith J, Kolsum U, et al. COPD phenotype description using principal components analysis. Respir Res 2009; 10: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang WH, Zhang Y, Cui YY, et al. Can β2-adrenoceptor agonists, anticholinergic drugs, and theophylline contribute to the control of pulmonary inflammation and emphysema in COPD? Fundam Clin Pharmacol 2012; 26: 118–134. [DOI] [PubMed] [Google Scholar]