Abstract
Mating behavior in males and females is dependent on olfactory cues processed through both the main olfactory epithelium (MOE) and the vomeronasal organ (VNO). Signaling through the MOE is critical for the initiation of male mating behavior, and the loss of MOE signaling severely compromises this comportment. Here, we demonstrate that dosage of the homeodomain gene Six3 affects the degree of development of MOE but not the VNO. Anomalous MOE development in Six3 heterozygote mice leads to hyposmia, specifically disrupting male mounting behavior by impairing detection of volatile female estrus pheromones. Six3 is highly expressed in the MOE, main olfactory bulb (MOB), and hypothalamus; all regions essential in the proper migration of the gonadotropin-releasing hormone (GnRH) neurons, a key reproductive neuronal population that migrates along olfactory axons from the developing nose into the brain. Interestingly, we find that the reduction in Six3 expression in Six3 heterozygote mice compromises development of the MOE and MOB, resulting in mis-migration of GnRH neurons due to improper olfactory axon targeting. This reduction in the hypothalamic GnRH neuron population, by 45% in adulthood, leads to female subfertility, but does not impact male hormone levels, suggesting that male infertility is not related to GnRH neuron numbers, but exclusively linked to abnormal olfaction. We here determine that Six3 is haploinsufficient for MOE development, GnRH neuron migration, and fertility, and represents a novel candidate gene for Kallmann syndrome, a form of inherited infertility.
Keywords: Kallmann Syndrome, GnRH Neuron Migration, Olfactory Development, Anosmia, Reproductive Behavior
Introduction
Aggression, mating behavior, and fear responses depend on an intact olfactory circuit in mammals [1]. Olfaction is required to distinguish sex, social, and reproductive status, as well as to express sexual behavior and induce neuroendocrine responses necessary for reproduction [2–5]. In rodents, these odors are processed via two anatomically and functionally separate sets of neurons localized in the main olfactory epithelium (MOE) and the vomeronasal organ (VNO) [6]. In the MOE, complex chemosignals are detected and differentiated by sensory neurons that project to glomeruli in the main olfactory bulb (MOB) [6]. It is believed that MOE neurons respond mainly to volatile odorants in the environment, whereas VNO neurons respond mainly to non-volatile pheromones, with some overlap between the functions of these anatomically distinct organs [7, 6]. Interestingly, volatile odorants that cue mating behavior in mice are processed by the MOE [8–12], and exclusive impairment of the MOE results in disrupted male reproductive behavior due to the inability to recognize estrus females [13, 10, 14]. Although humans do not need their sense of smell for reproduction, olfaction is an essential part of our everyday lives, and it is clear that proper development of the olfactory system is required for normal sexual maturation [15].
The development of olfactory neurons is closely linked to the development of the key reproductive neurons, gonadotropin-releasing hormone (GnRH) neurons [16–18, 14, 19, 20]. GnRH neurons originate in the primordial MOE (the olfactory placode), and migrate along olfactory nerves into the forebrain [18, 21, 22]. Due to the topographical and migratory link between the development of the olfactory system and GnRH neurons, it is not surprising that their fates are tied together and that both are compromised in the developmental disorder termed Kallmann syndrome [23, 24]. Kallmann syndrome is a rare genetic disorder of complex and heterogeneous genetic etiology, leading to various degrees of subfertility, including complete infertility (hypogonadal hypogonadism), along with anosmia/hyposmia in humans [24, 25]. Genetic analysis in familial cases of Kallmann syndrome has demonstrated that there are multiple genes involved in the process of regulating GnRH and olfactory neuron migration, including compound heterozygosity in patients [26, 27]. Despite recent advances, ~68% of the cases of Kallmann syndrome are of an unknown genetic origin, thus identification of new genes of interest in this condition is critical [28, 27].
Several newly discovered genes [29, 30] and haploinsufficiencies [31, 32] that alter GnRH neurons in development in mouse models are candidates for hypogonadal hypogonadism without anosmia. One potential candidate for Kallmann syndrome is the homeodomain gene Six3, which is highly expressed in the MOE, MOB, and hypothalamus, areas essential for maintaining reproduction and olfaction (www.brain-map.org, consulted Feb 2017). Due to early postnatal lethality of Six3 knock-out (KO) mice [33], they have only been studied during early embryogenesis, with a focus on craniofacial and eye development [34, 35]. Haploinsufficiency of Six3 has been recently studied and shown to produce a phenotype in holoprosencephaly; demonstrating that dosage of SIX3 can be directly proportional to the severity of a phenotype [36–38]. Here, we present the first study of the fertility of adult Six3 heterozygote (HET) mice, finding that Six3 haploinsufficiency alters male fertility, with various degrees of subfertility to infertility attributed to disrupted development of the MOE producing an incapacity to express normal sexual behavior.
Materials and Methods
Mouse lines and animal housing
All animal procedures were performed in accordance with the University of California, San Diego, Institutional Animal Care and Use Committee regulations.
Mice were group-housed with ~4 to a cage on a 12-hour light, 12-hour dark cycle (on 6:00 A.M., off 6:00 P.M.), with ad libitum chow and water. All mice were kept on a C57BL/6J mouse background. Six3-flox mice were provided by Dr. Guillermo Oliver [33]. Six3 KO mice were created by crossing Six3-flox with a Zp3-Cre mouse [39] (https://www.jax.org/strain/006888), allowing germ-line recombination. Six3-flox mice were crossed with GnRH-Cre [40] mice to create Six3flox/flox:GnRHcre mice homozygous for the Six3 deletion within GnRH neurons. The mice were killed by a CO2 or isoflurane (Vet One) overdose. Unless specified, all animals used were ~3 months of age.
Behavioral experiments
All behavioral tests were performed during the first three hours of the dark phase using red light illumination. The experimenter was blind to the genotype of the subjects. Before each assay, the mice were habituated for 1 hour in a new cage with fresh bedding and no food or water. All females presented to males in behavioral tests were virgin ovariectomized and primed with 1 μg estradiol benzoate diluted in sesame oil at 9 A.M. the day before testing and 500 μg progesterone diluted in sesame oil at 2 P.M. on the day of testing.
Collection of tissue and histology
Diestrus ovaries, brains, olfactory bulbs, embryos, noses, ovaries, and testes were fixed overnight (~16 h) at 4°C in freshly made mixture of 6:3:1 absolute alcohol: 37% formaldehyde (Fisher F79-4): glacial acetic acid. Tissues were paraffin embedded and serially sectioned at 10 μm. Ovaries, testes, brains, and noses were stained with hematoxylin and eosin (H&E; Sigma-Aldrich). In ovaries, histology was examined and the number of corpora lutea in a single ovary per mouse was recorded blindly.
Immunohistochemistry
Adult brains, olfactory bulbs, and embryos were sectioned at 10 μm. Immunohistochemistry (IHC) was performed as described previously [41]. Sections were immunostained with rabbit anti-GnRH (1:1000; Novus Biologicals, catalog number: NB300-506, RRID:AB_2110266), anti-peripherin (1:200; Abcam, catalog number: ab4573, RRID:AB_2171346), or rabbit anti-OMP (1:100; Santa Cruz Biotechnology, catalog number: sc-67219, RRID:AB_2158005). To increase the visibility of immunostained neurons, adjustments of brightness, contrast, and color balance were done with Image J (National Institutes of Health, Health, Bethesda) and applied to the entire image. Peripherin quantification was conducted by counting all fibers within the MOE/MOB region in the HET and WT mice. Such analysis could not be performed on the KO mice, as all the fibers were contained in one large mass and could not be individually quantified. Counterstaining was performed using vector Hematoxylin counterstain (H-3401) for 15 seconds, followed by running tap water for two minutes. Slides were then incubated in bluing solution for one minute and then rinsed in distilled H2O.
GnRH neuron counting and OMP staining were conducted throughout the entire embryonic head, and throughout the adult brain beginning with the front of the olfactory bulb (bregma 4.46) to bregma −2.80 [42]. Each slide was collected and evaluated for GnRH and Olfactory Marker Protein (OMP) staining in a blinded manner using a Nikon Eclipse E400 microscope. Although all collected sections were assessed for staining, neuroanatomical landmarks were used to identify the regions of interest (ROI) as depicted in the corresponding figures. Slides were coded to blind the researcher to treatment group during analysis. In KO mice where recognizable structures were absent, images were taken in locations corresponding to where structures of interest are normally seen. Adult brains analyzed for GnRH include equal numbers of both male and female subjects, although a sex difference in GnRH neurons has not been recorded to our knowledge. Embryos were not sexed.
Lineage tracing
For lineage tracing, RosaRFP reporter mice (https://www.jax.org/strain/007908) were used [43] and mated to Six3 HET and GnRHcre mice to create the Six3HET:RosaRFP:GnRHcre line. IHC was performed with the anti-RFP antibody (1:1000, Abcam, catalog number: ab62341, RRID: AB_945213).
GnRH-pituitary stimulation tests
For two weeks prior to the hormonal challenge, mice were adapted to handling stress such that they would be unaffected by stress during serial sampling. Baseline tail blood was collected from male and female metestrus/diestrus WT and Six3 HET littermates. Ten minutes after receiving an IP injection of 1 μg/kg GnRH (Sigma #L7134) diluted in physiological serum, tail blood was collected again. For kisspeptin challenge, 20 minutes after ip injection of 30 nmoles of kisspeptin (Tocris #4243) diluted in physiological serum was injected, tail blood was collected again. The total volume of blood collected did not exceed 100 μL. Blood was collected between 11:00 AM and 12:00 PM and was allowed to clot for 1 hour at room temperature. Blood was then centrifuged for 15 min at 2600 X g. Serum was collected and stored at −20°C before Luminex analysis was used to measure LH. The LH assay detection limit was 0.24 ng/mL, inter-assay CV was 15.2 and intra-assay CV was 11.5. This experiment was conducted as previously described [30].
Sperm motility and total sperm count
Epididymides were collected in M2 media at room temperature (Sigma-Aldrich). One epididymis was cut in half and sperm were gently expelled by manual pressure. The numbers of motile and immotile sperm were counted in a hemocytometer 15 minutes after sperm were expelled. To immobilize motile sperm for a total sperm count, the hemocytometer was placed for 5 minutes on a 55°C heat block. The second epididymis was chopped into small pieces and left 30 minutes at room temperature in M2 media. The solution was filtered through a 70 μm filter (Falcon), and the sperm were diluted in PBS before counting the total number of sperm heads in one epididymis. This experiment was performed as described previously [31].
Vaginal plug formation, mating assay, generation of timed embryos, and estrous cycling
To monitor plug formation, a WT or Six3 HET female mouse was housed with either a WT or Six3 HET male mouse at 12 weeks of age and plug formation was monitored for ten consecutive days. Embryos were obtained using timed matings in which one male and one female mouse were housed together, and vaginal plug formation was monitored. If a plug was present, the day was noted as day 0.5 of pregnancy and used to collect embryos for timed mating at embryonic day e13.5 or e17.5. A second cohort of virgin Six3 WT and HET mice were housed in pairs, and the number of litters born and the number of pups per litter were recorded over a period of 14 weeks. Mice were assessed for estrous cyclicity as previously described [31]. To assess estrous cyclicity, vaginal smears were performed daily between 9:00 and 10:00 A.M. on 120-day-old mice by vaginal lavage as described previously [31]. A cycle was determined by counting the days from one estrus period to the next estrus period.
Determination of day of vaginal opening and preputial separation
Starting at 21 days-of-age, Six3 WT and HET mice were inspected daily and pubertal onset was determined by the age at vaginal opening in females or by preputial separation in males as previously described [31].
Mounting assay
Male mice were habituated to new cages for 1 hour. Ovariectomized and estrogen primed female mice were then introduced to WT and HET male mice of 16 weeks of age. This test was conducted three times with only the third trial quantified and reported. Trials were conducted one week apart from each other, and the same estrogen primed females were used in all assays. Pairs were videotaped and behavior quantitated in a blind manner with incidences of mounting being recorded over a period of 15 minutes. Some of the same males were used in the mounting assay, the buried food test, and the habituation-dishabituation experiments.
Buried food test
A buried food test was conducted to check for gross malfunction of the main olfactory system as described previously [44]. All mice were food-deprived overnight (18 hrs). A small piece of mouse chow was buried (~3 cm deep) at a random location in a clean mouse cage containing fresh bedding. One mouse was placed in the cage and timed for the latency to find the mouse chow during a period of up to 15 minutes. Mice were videotaped and behavior quantitated in a blind manner.
Sugar water test
Six3 HET and Six3 WT mice were housed under normal conditions, except a second water bottle was placed on the cage that contained 4% dissolved sugar. Prior to beginning testing, mice were habituated to the presence of two drinking bottles (one containing 4% sucrose and the other water) for 3 days in their home cage. Following this acclimation, mice had the free choice of either drinking the 4% sucrose solution or plain water for a period of 4 days. The location of the bottles was switched each day to avoid a confounding factor of cage-side preference. The water bottles were weighed at the beginning of the study and at the end, the change in weight was reported.
Wheel-running behavior
To determine if Six3 HET mice had normal activity on running wheels, Six3 HET and WT male mice were housed individually in cages with running wheels. Food and water was available ad libitum during the entire experiment. After 1 week acclimation to the polypropylene cages (17.8 × 25.4 × 15.2 cm) containing a metal running wheel (11.4 cm diameter), locomotor activity rhythms were monitored with a Vitalview data collection system (Version 4.2, Minimitter, Bend OR) that compiled in 6 min bins the number of electrical closures triggered by half wheel rotations. Running wheel activity was monitored for 2 weeks on a 12h Light:12h Dark cycle. Cage changes were scheduled at 3-week intervals. Wheel-running activity was analyzed using ClockLab Analysis (ActiMetrics Software).
Territorial marking assay
Six3 HET and Six3 WT male mice were placed in a clean empty cage to habituate for 1 hour. After habituation, the bottom of the cage was covered with Whatman paper for 15 minutes to record a baseline for normal urination. This paper was then removed, and the bottom of the cage was covered with a new piece of Whatman paper with 60 μL of estrus female mouse urine placed in the center of the paper. Male mice were allowed to mark the paper for 15 minutes. The Whatman papers were then dried overnight and sprayed with 0.2% ninhydrin diluted in 100% EtOH (Sigma) and air dried until urine spots developed a purple color. Papers were photographed and images were converted to binary and the number of urine marks was determined using Image J software. The number of marks elicited by sensing estrus female urine was determined by subtracting the number of urine marks in baseline markings. This experiment was conducted as previously described [45].
Male countermarking behavior against a strange male’s urine
Six3 HET and WT males, who had been singly housed and who were sexually inexperienced, were placed in a cage lined with Whatman paper for 30 min. Fifty μL of male urine pooled from 5 adult male mice was placed in the center of the Whatman paper. The Whatman papers were then dried overnight and sprayed with 0.2% ninhydrin diluted in 100% EtOH (Sigma) and air dried until urine spots developed a purple color. Papers were photographed and images were converted to binary and the number of urine marks was determined using Image J software. The number of marks elicited by sensing male urine was determined by subtracting the number of urine marks in baseline markings. Urine was collected from male mice by holding mice by the scruff of the neck over a piece of clean parafilm.
Urine collection
Urine was collected from stimulator estrus female mice by holding mice by the scruff of the neck over a piece of clean parafilm. Female stimulator mice were prepared by ovariectomizing female mice and inserting a low-dose estrogen pellet of 0.05 cm diameter subcutaneously. On the day of urine collection, stimulator females were injected with 1 μg of estradiol benzoate diluted in sesame oil 24 hours before collection, followed by 500 μg of progesterone diluted in sesame oil at 2 P.M. Urine was collected at 6 P.M. Urine from 5 different mice of each sex was pooled into separate male and female urine collections on the day of testing. This experiment was performed as described previously [46, 7].
Urine preference test
Preference for investigating the urine of one mouse sex over the other was assessed in a 5 min test during which male and estrus female urine were presented simultaneously on pieces of filter paper. The odor presentations were attached to weigh boats that were placed at opposite ends inside a cage with either a Six3 HET or WT male mouse. Tests were videotaped and seconds spent investigating the urine stimulus were quantitated in a blind manner. This experiment was conducted as described previously [47].
c-Fos immunohistochemistry
Prior to experimentation, males were isolated from female scent in a room separate from other mice for at least 2 days. On the day of the experiment, male mice were singly housed in a clean cage in a quiet room for three hours prior to female exposure. The male mice were then exposed (at 6:00 PM under dim red light) to urine (60 μL) collected from hormonally primed estrus stimulator female mice. The urine was placed just out of reach of the mice to prevent direct contact. After 90 minutes, males were killed, and brains were collected in fixative. Sections were stained with c-Fos antibody (Santa Cruz Biotechnology; catalog number sc-52 RRID:AB_10160513; 1:1000). Neuroanatomical landmarks were used to identify the counting region as depicted in the corresponding figure. Quantification was performed on biological replicates consisting of c-Fos nuclei within the defined region from a minimum of three unilateral sections. C-Fos-positive cells were quantified by an experimenter blinded to the treatment group. While the c-fos IHC produced a range of staining intensity, only those cells that were darkly stained were included in the quantification. The numbers from each biological replicate were then averaged across all the animals in that group. Slides were coded to blind the researcher to treatment group during analysis.
Resident–intruder aggression assay
Male mutant and control mice were isolated at 9–11 weeks of age for a period of 2 weeks before testing. Mice were singly housed in cages with bedding. Testing lasted 15 min, and began when a group-housed, sexually inexperienced, adult WT “intruder” male was placed in the home cage of the test mouse, whose bedding had not been changed for 4 days. Aggressive behavior was defined as biting, chasing, or wrestling. Cumulative attack duration, and number of attacks were video recorded, then quantitated by a blinded assessment. This experiment was performed as described previously [48, 6].
Testosterone replacement assay
Male mice were gonadectomized, given a testosterone pellet, and left to recover for 1 week before any behavioral tests were conducted. Pellets made of tubing with an inner diameter of 1.02 mm and an outer diameter of 2.16 mm filled for 1.2 cm with testosterone (Sigma T1500-1G) and inserted into the gonadectomy incision site of Six3 HET and WT mice. These implants have been shown previously to produce elevated physiological levels of T (11.1 ± 0.8 ng/mL) [49].
Volatile and non-volatile odor detection
To assess the ability of Six3 HET mice to detect volatile and non-volatile odors, and to discern these odors from each other, we used a home-cage habituation-dishabituation test, as described previously [47, 44]. In these tests, the mice become habituated to a urinary scent after three trials, and are then dishabituated when a new odorant is presented.
Six3 HET and WT males were tested in three different paradigms. First males were presented with (1) volatile/non-contact stimuli, (2) non-volatile and volatile/contact stimuli, and (3) non-volatile (MUPs)/contact stimuli. Six3 HET and WT males were presented with 20 μL of an odorant sample (either water or urine) pipetted onto a piece of Whatman paper taped to a plastic weigh boat. In tests (1) and (2), mice were presented with deionized water for two minutes three times in a row with one-minute intervals in between, followed by three 2-minute presentations of male urine and finally by three 2-minute presentations of a female estrus urine. In test (3), mice were presented with deionized water for two minutes three times in a row with one-minute intervals in between, followed by three 2-minute presentations of MUPs. Tests were videotaped and quantitated by an experimenter blinded to treatment group. The number of seconds that the mice stretched upwards to smell the filter paper containing the stimulus and the number of seconds mice touched their nose to the odorant stimulus were recorded. If the mouse does not increase their interest in the new urinary stimulus, they cannot differentiate between the two stimuli. If the interest in the odorant is similar to baseline water, then the mouse cannot detect the stimulus.
For each day of testing, one-half of the subjects in the Six3 HET and WT groups were presented with the male urinary stimulus followed by the estrus female urinary stimulus, and the other half were presented with the same urinary odors but in the reverse order. Subsequently, on a second day of testing, all of the subjects were re-exposed to the same odors but in the opposite order from their first day of exposure. The order of presentation of urine had no effect on the results of these trials; thus, we analyze and present here data from the trials where male urine was presented first followed by female urine. Tests were videotaped and behavior quantitated in a blind manner. Within groups, statistical analysis compared (1) the difference between the number of seconds that subjects spent investigating the third water stimulus versus the male urinary odorant presented, and (2) the difference between the number of seconds spent investigating the third male urinary stimulus versus the first presentation of the estrus female urinary stimulus.
In experiment (1), male Six3 HET mice were tested for their ability to detect and distinguish exclusively volatile odors. Volatile odors are airborne and do not need to be directly contacted to be sensed, as opposed to non-volatile odor components. Thus, to separate the volatile odors from the non-volatile, a cage lid with holes to let volatile odors pass through was placed on top of the cage, and the weigh boat was placed on this cage top such that only volatile odorants were available at body level, with subjects having no physical access to the stimulus. As the mice could not contact the stimulus, they could not detect non-volatile odorant components. The number of seconds spent investigating the stimulus by stretching up to reach the weigh boat on top of the cage was quantitated. In experiment (3), mice were tested for their ability to detect non-volatile odorants exclusively by presenting mice with MUPs.
Female odor detection was assessed with four separate odor presentations given consecutively for two minutes separated by one-minute intervals. First, water was given on Whatman paper taped to a weigh boat placed inside of the cage, to record a baseline for interest in an odorless presentation. Then male urine was presented inside of the cage where mice could contact the stimulus. The third presentation was water once more presented outside of the cage where the mice could not physically touch the stimulus but could stretch up and smell it, to record a baseline for investigation of odors presented in this new location. The final stimulus given was male urine on top of the cage, to test for volatile odor detection.
Collection of MUPs
Experiment was conducted as previously described [48]. In brief, urine was collected fresh from group-housed adult C57BL/6J male mice, from multiple cages and combined. To fractionate the MUPs, urine was applied to Amicon Ultra Centrifugal Filters (10,000 MWCO regenerated cellulose, Millipore) and centrifuged at 6000 × g, in a table-top centrifuge at room temperature for 15 min. The first effluent collected was considered the LMW fraction. The HMW fraction was washed by adding sterile 1X PBS (equal to starting urine volume), and centrifuged five times at 6000 × g at room temperature for 15 min. The washed protein fraction was then diluted to starting urine volume with 1X PBS.
Statistical analysis
All the statistical analysis was performed using R data analysis software. A P-value of <0.05 was considered statistically significant. For all experiments, data are expressed as the mean ± SEM. Unpaired two-tailed t-tests are used in all cases, except in cases where data was abnormal, or the variances were unequal, where the Wilcoxon rank sum tests were used. Additionally, in several cases, Two-way ANOVA with Bonferroni post-hoc was used to analyze data. Power analyses were performed before experiments to determine n values. Experimental groups were defined by genotype and thus no sorting mechanism was used. n values stated for each experiment report the number of mice in each group.
Results
Loss of one allele of Six3 is sufficient to cause subfertility
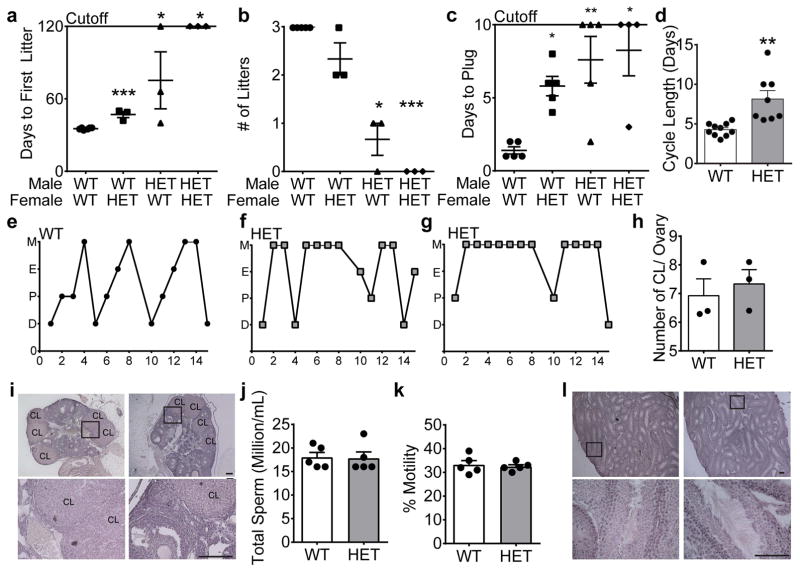
To test the hypothesis that Six3 heterozygosity affects reproductive competence, a 120-day fertility assay was performed. We found that both Six3 HET males and females took longer to generate their first litter when paired with wild-type (WT) mates (Fig. 1a). This subfertility was amplified when Six3 HET males and females were paired together, in which no litters were sired over the 120-day test period (Fig. 1a, b). Furthermore, Six3 HET males fathered significantly fewer litters than controls (Fig. 1b). Litter size was not significantly different between genotypes in either sex (WT: males 8.3 ± 2.4, HET: males 7.0 ± 1.0 pups/litter; WT: females 8.3 ± 2.4, HET: females 6.9 ± 2.3 pups/litter; Students t-test, p>0.05). As reproductive competence relies on successful completion of puberty, we determined whether the subfertility was due to delayed pubertal onset. Both Six3 HET males and females had normal pubertal onset as determined by preputial separation in the male and vaginal opening in the female, two external markers of pubertal onset in males and females, respectively (preputial separation WT: 26.7 ± 0.7 days, HET: 26.8 ± 0.8; vaginal opening WT: 27 ± 0.7, HET: 26.7 ± 1.0 days; Students t-test, p>0.05). Thus, the subfertility was not due to a delay in pubertal onset. Instead, the female delay to first litter was associated with significantly increased time to vaginal plug by a WT male (Fig. 1c), although all of these females plugged before the end of the 10-day assay, suggesting prolonged estrous cycles. Indeed, Six3 HET females had prolonged estrous cycles (Fig. 1d–g). Interestingly, we noted a heterogeneous impact of Six3 haploinsufficiency on estrous cyclicity wherein five Six3 HET females had prolonged estrous cycles (Fig. 1f), one was in persistent estrus (data not shown), and one spent 80% of the time in persistent metestrus (Fig. 1g). As expected, based on the normal litter size, Six3 HET females had a similar number of ovarian corpora lutea, a marker of successful ovulation, compared to controls (Fig. 1h, i).
Fig. 1.
Six3 HET mice are subfertile. a, Days until first litter (Mann Whitney test as compared to WTxWT, n=3–5) (WTxHET, p=0.0003), (HETxWT, p= 0.024), (HETxHET, p= 0.024). b, Average number of litters in a 120-day mating assay (Mann-Whitney test as compared to WTxWT, n=3–5) (WTxHET, p= 1.07), (HETxWT, p=0.018), (HETxHET, p= 0.018). c, Days until WT or HET males create a vaginal plug in WT or HET females during a 10-day assay (Mann-Whitney test as compared to WTxWT, n=4–5), (WTxHET, p=0.008), (HETxWT, p=0.024), (HETxHET, p=0.008). d, Length of estrous cycles monitored daily in Six3 HET and WT females over 15 days (Student’s t-test, p=0.001, t(16)=3.93, n=8–10). M, metestrus; E, estrus; P, proestrus; D, diestrus. Representative cycles are shown for e, WT female, f, and g, Six3 HET females. h, Number of corpora lutea (Student’s t-test, p= 0.574, t(4)= 0.529, n=3) and i, pictures of corpora lutea (CL) in Six3 HET and WT ovaries, n=3. j, Total number (Student’s t-test, p=0.910, t(8)= 0.117, n=5), and k, percent motile sperm (Student’s t-test, p=0.688, t(8)= 0.416, n=5) in Six3 HET and WT mice. l, Representative images of testes from Six3 HET and WT mice, n=3. Boxes on images indicate where the higher magnification images were taken. *p<0.05, **p<0.005, ***p<0.001. Scale bars, 2 mm, 20 μm.
To discern whether the deficiency in Six3 HET mating efficiency was due to a defect in spermatogenesis, sperm analysis was conducted. Examination in the Six3 HET mice showed that total sperm count (Fig. 1j) and motility (Fig. 1k) were not significantly different between Six3 HET and WT males, and that testis morphology was normal (Fig. 1l). To further ascertain the cause for the significantly reduced fertility of the Six3 HET males, we evaluated their ability to produce a vaginal plug in female mice. Six3 HET male mice plugged significantly fewer females compared to WT mice, with three of five pairs of mice never plugging over the 10-day assay (Fig. 1c). This effect was exacerbated in Six3 HET/HET matings in which only one out of four pairs of mice provided a plug during the assay.
Defective GnRH neuron migration in Six3 HET mice is associated with absence of olfactory fibers
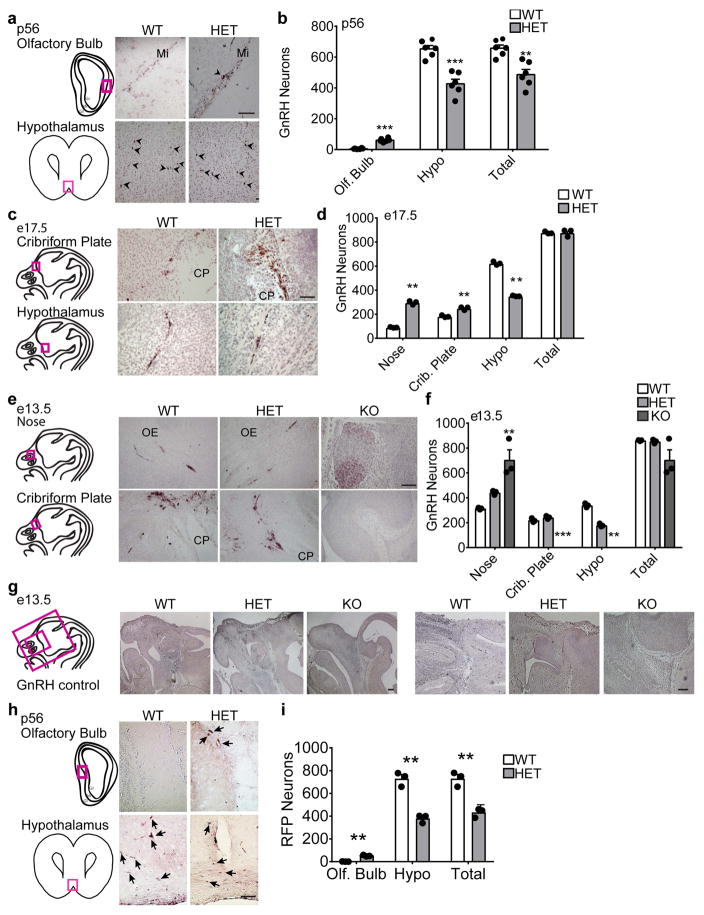
Fertility depends on correct maturation, localization and function of GnRH neurons. Even a modest decrease in the number of GnRH neurons is sufficient to engender irregular or lengthened estrous cycles. To determine if the subfertility of Six3 HET mice originated at the level of GnRH neurons, we performed GnRH immunohistochemistry and counted GnRH neurons. Adult male and female Six3 HET mice had ~45% fewer GnRH-immunoreactive neurons in the hypothalamus with ~10% of the total GnRH neuronal population halted along their migratory route through the olfactory bulb, indicating a defect in migration (Fig. 2a, b). The total number of GnRH neurons in Six3 HET brains was equivalent between sexes. To determine if the abnormal GnRH neuron migration arose during development, we counted GnRH neurons at e13.5 and e17.5. Despite the total number of GnRH neurons in the entire head being normal at both ages (Fig. 2c–f), the number of GnRH neurons was significantly higher in the nose and lower in the hypothalamus in Six3 HET than WT (Fig. 2c–f). At e13.5, a time point when we were also able to collect Six3 KO embryos, we found that KO heads were misshapen and most brain structures (including the MOB) unrecognizable, as has been shown previously [33]. In these embryos, all the GnRH neurons were found in one large tangled group in the region normally occupied by the MOE (Fig. 2e, f). This remarkable mass of GnRH neurons was observed in each of the KO mouse heads. The immunostaining was very specific, with no off-target staining observed (Fig. 2g), as has been previously reported [30]. Thus, in Six3 HET and KO embryos, GnRH neurons are inhibited in their migration from the nasal region. In addition, the migration of GnRH neurons is more severely disrupted in Six3 KO than in Six3 HET mice indicating a dose-dependence of Six3.
Fig. 2.
Six3 HET and KO mice showed delayed migration of GnRH neurons. Six3 HET and WT mice were processed for IHC staining for GnRH. a, c, and e, Representative images of staining in Six3 WT and HET mice are shown with boxed areas on the drawings of the sagittal mouse head sections to the left of the panels indicating where the (a, c, and e) images were taken. b, GnRH neurons at p56 (Student’s t-test, n=6 (3 females, 3 males)). Counting was conducted throughout the adult brain beginning with the front of the olfactory bulb to bregma −2.80. (olf. bulb, p= 0.00004, t(6)=11.1), (crib. plate, p=0.0001, t(9)=−6.17), (total, p=0.002, t(8)=−4.34). d, GnRH neurons at e17.5 (Student’s t-test, n=3). Counting was conducted throughout the entire embryonic head (nose, p=0.001, t(2)=−22.4; crib. plate, p=0.003, t(4)=−7.40; hypo, p=0.0004, t(2)=31.1; total, p=0.973, t(2)=−0.03). f, GnRH neurons at e13.5 (Student’s t-test, n=3; nose, p=0.002, t(3)=−12.2; crib. plate, p=0.116 t(4)=−2.06; hypo, p=0.0002, t(4)=13.6; total, p=0.488, t(2)=0.821). g, Controls for GnRH staining specificity, images are from e13.5 mice processes for anti-GnRH IHC. h and i, lineage tracing, IHC for RFP in Six3HET:RosaRFP:GnRHcre adult mice. RFP marks all GnRH neurons for the duration of the life of the neuron regardless of GnRH expression, to determine presence of GnRH neurons lacking GnRH expression. i, Number of RFP-labelled GnRH neurons in Six3HET:RosaRFP:GnRHcre adult mice. (Student’s t-test, n=3; olf. bulb, p=0.004, t(2)=−14.0; hypo, p=0.003, t(3)=8.41; total, p=0.004, t(3)=6.87). Arrows indicate RFP neurons. Mi, mitral layer; CP, cribriform plate; OE, olfactory epithelium. **p<0.005, ***p<0.001. Scale bars, 100 μm (a, c, e, h), 2 mm (g left), 200 μm (g right).
In embryos late in development, at e17.5, GnRH neurons were found delayed in anterior portions of the migratory pathway. Additionally, in the adult Six3 HET mouse, GnRH neurons were found delayed in their migratory pathway, and more GnRH neurons were seen in the olfactory bulb. However, it is possible that there is a population of GnRH neurons in the brain that stop expressing GnRH in late development, and therefore is undetectable using anti-GnRH antibody IHC. To exclude this possibility, we created a Six3HET:RosaRFP:GnRHcre mouse in which GnRH neurons are labeled with RFP for the life of the neuron regardless of the expression of GnRH. Therefore, if there are GnRH neurons in the brain, that are no longer expressing GnRH, they will be detectable using IHC for RFP. Six3 HET brains were assessed for RFP-positive neurons. In counting the anti-rfp IHC, a ~40% reduction in the number of GnRH neurons was observed in the HET mice as compared to WT mice (the same result that was found using the GnRH antibody) (Fig. 2h, i). Therefore, there was no detection of GnRH neurons that were no longer expressing GnRH. Thus, the source for the loss of hypothalamic GnRH neurons seen previously is solely due to mis-migration.
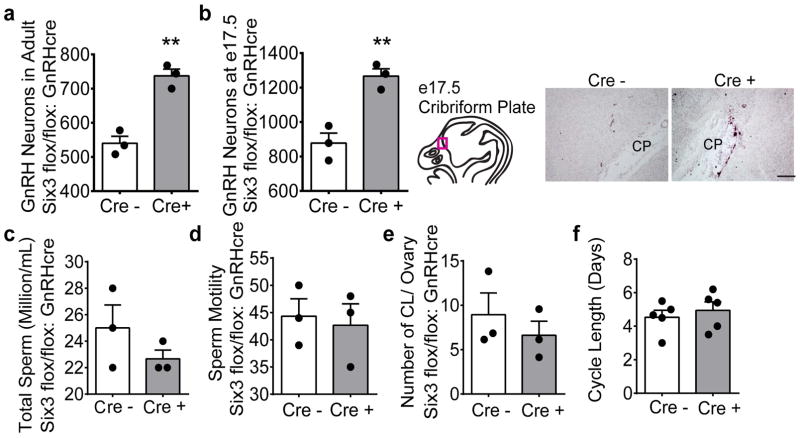
To investigate whether the abnormal GnRH neuron migration was due to effects within the GnRH neuron, a separate mouse model was used, termed Six3flox/flox:GnRHcre, where Six3 is deleted within GnRH neurons. GnRH neuron numbers were assessed in this model and surprisingly GnRH neurons migrated normally, but the total numbers were increased by ~30% in both the adult mouse (Fig. 3a) and in e17.5 embryos (Fig. 3b). This indicates that Six3 does not act within the GnRH neuron to inhibit the migration of this neuronal population. As expected, the increased number of GnRH neurons did not affect male or female gonadal function as evidenced by normal total amount of sperm and sperm motility in the male (Fig. 3c, d), and comparable number of corpora lutea and estrous cycle length of females (Fig. 3e, f). Thus, the source of impaired GnRH neuron migration stems from the role of Six3 outside of the GnRH neuron along the migratory pathway.
Fig. 3.
Six3flox/flox:GnRHcre mice have increased numbers of GnRH neurons and normal gonadal function. a, and b and In the Six3flox/flox: GnRHcre mice, the number of GnRH neurons at (a) p56 (Students t-test, p=0.0023, t(4)=6.91, n=3) and (b) at e17.5 (Students t-test, p= 0.006, t(4)=5.42, n=3) with representative images from e17.5 of anti-GnRH IHC antibody staining. d, Total number (Student’s t-test, p=0.277, t(4) =1.26, n=3) and e, percent motility (Student’s t-test, p=0.758, t(4)=0.330, n=3) of sperm. f, Number of corpora lutea in control and Six3flox/flox:GnRHcre mouse ovaries (Student’s t-test, p=0.471, t(4)=0.796, n=3). g, Length of estrous cycles monitored daily in control and Six3flox/flox:GnRHcre mice (Student’s t-test, p=0.471, t(8)=0.616, n=5). CP, cribriform plate. **p<0.005. Scale bars, 100 μm.
While gonadal function was unaffected by the loss of Six3, we next sought to determine whether pituitary function was altered by the loss of Six3. Since GnRH numbers were strongly reduced in Six3 HET animals, we sought to determine whether pituitary function was intact, as the secretion of GnRH can impact pituitary development and function. Fertility depends on sufficient GnRH stimulation of pituitary gonadotropes to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which are required for normal gonadal function. Given the decrease in hypothalamic GnRH neurons in Six3 HET mice, we measured the extent to which the reduction of hypothalamic GnRH neurons affects the reproductive physiology of the severely subfertile male mice. First, we analyzed the ability of the pituitary to respond to endogenous GnRH by measuring the basal level of LH in Six3 HET males. There was no significant difference in basal LH levels (WT: 2015 ± 793, HET: 1568 ± 612 pg/ml; n= 5, Students t-test, p>0.05), or in the capacity of the pituitary to respond to a GnRH challenge, as evaluated by the fold change in LH (Fold change WT: 1.78 ± 0.3, HET: 2.10 ± 0.25; n= 5, Students t-test, p>0.05) between Six3 WT and HET mice. Male fertility, particularly the secretion of LH, is very sensitive to appropriate GnRH levels. To test whether the number of GnRH neurons in Six3 HET mice is sufficient to generate a LH response, Six3 HET and WT males were injected with Kisspeptin, a stimulator of GnRH neurons, and the fold change of LH from baseline was determined. Again, no significant difference between genotypes was observed (Fold change WT: 1.16 ± 0.24, HET: 1.80 ± 0.37, n=5, Students t-test, p>0.05).
Six3 HET males lack normal mating behavior due to anosmia
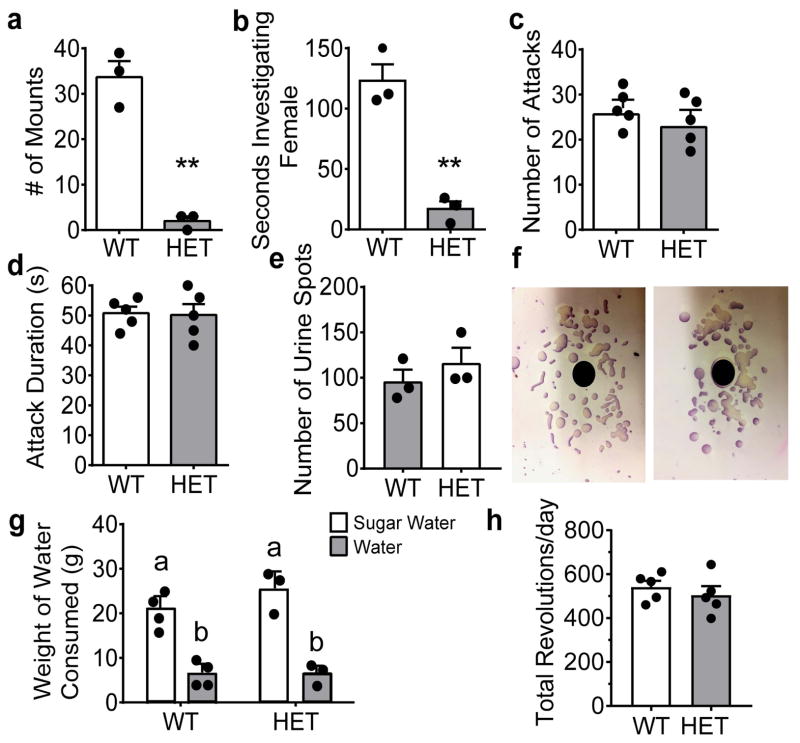
Given intact spermatogenesis, the presence of corpora lutea, and normal hormone levels in Six3 HET mice, it does not appear that the loss of GnRH neurons is sufficient to produce the subfertility observed in these male mice. Therefore, we sought to investigate mating behavior. Male mouse sexual behavior relies on a functional olfactory system and the capacity to detect pheromones [9, 50, 51, 47, 52, 53]. To determine whether the deficit in plugging was due to altered sexual behavior, we determined whether a WT female could elicit sexual behavior in Six3 HET males. When male mice were presented with an estrogen primed female (ovariectomized and primed with progesterone and estrogen injections), WT males mounted 9 times more frequently than Six3 HETs (Fig. 4a). Mounting behavior is preceded by chemoinvestigation, wherein males detect female pheromones. Interestingly, Six3 HET males spent significantly less time chemoinvestigating females than WT males, suggesting a defect in the odor processing in the Six3 HET males (Fig. 4b). In consideration of the clear alteration of reproductive behavior, pheromone driven aggressive behavior was assessed. Interestingly, Six3 HET males responded normally to the pheromones of WT male intruders as evidenced by their number of attacks toward the intruder male (Fig. 4c) and the total duration of the attacks (Fig. 4d). Mating behavior follows cues detected primarily by the MOE, while male-male aggressive behavior is driven by detection of pheromones in the VNO [53]. To further assess the functionality of the VNO, territorial countermarking behavior against a strange male’s urine was tested. This VNO-mediated behavior was tested by counting urine marking in response to the presentation of male urine. The number of urine marks made was not significantly different between Six3 HET and WT mice (Fig. 4e, f). These data indicate that the VNO is functional in the Six3 HET male, while the MOE is impaired. In addition to pheromones, mating behavior is also driven by neural circuits that regulate sexual motivation [53]. To determine if the lack of mounting behavior was due to a defect in motivation-regulated behavior, mice were tested for their preference of sugar versus water, a test that is an indicator of anhedonia. Anhedonia is the inability to find pleasure in in an activity that would carry hedonic value in a normal situation [54]. Both WT and Six3 HET mice displayed a preference for the sugar water over unsweetened water, showing that the neuronal motivation circuitry is intact (Fig. 4g), as is the sense of taste. This test also demonstrates that the sweet taste receptors of mice are intact, as identified by the preference towards the sweetened drink. In line with this, we found that Six3 HET males had normal levels of running wheel activity (Fig. 4h), a rewarding behavior, which also requires normal locomotor function [55]. Additionally, as expected, activity onset of wheel running in normal light:dark conditions (12h light: 12h dark) was normal for Six3 HET male mice, indicating normal eye development and sensitivity to light (WT: 23.9 ± 0.11, HET: 24.03 ± 0.014; n=5, Student’s t-test, t(8)=1.16, p= 0.279)
Fig. 4.
Six3 HET males do not respond normally to estrus female cues, but maintain normal aggression patterns towards male intruders. a, Six3 HET males mounting of estrus females, assay was 15 min (Student’s t-test, p=0.001, t(4)=8.64, n=3). b, Male chemoinvestigation of estrus females, assay was five min (Student’s t-test, p=0.002, t(4)=7.09, n=3). c, and d, Male aggression towards WT intruder males over 15 min as measured in (c) number of attacks (Student’s t-test, p=0.387, t(8)=0.918, n=5) and (d) duration of attacks (Student’s t-test, p=0.890, t(8)=0.143, n=5). e, Quantification of Territorial marking on Whatman paper f, in response to 60 μL pooled male urine (Student’s t-test, p=0.392, t(4)=0.959, n=3). g, Anhedonia of Six3 HET and WT mice measured by their intake of sugar water versus unsweetened water (different letters indicate statistical difference by Two-way ANOVA followed by Tukey post hoc, n= 3–4). h, Running wheel activity in 12h light:12h dark conditions for 1 week of Six3 WT and HET male mice. **p<0.01.
To further determine whether the deficiency in normal reproductive behavior of Six3 HET males was due to altered testosterone, we gonadectomized Six3 HET male mice and inserted a testosterone pellet. Sexual behavior was then reassessed with no change in the discrepancy in mounting behavior (WT: 130 ± 25, HET: 30 ± 18 mounts; n=3, Students t-test, t(4)=8.56, p=0.0001) and chemoinvestigation (WT: 30 ± 8, HET: 4 ± 7 seconds; n=3, Students t-test, t(4)=7.84, p=0.0007) seen between Six3 HET and WT male mice. The impaired response of Six3 HET male mice to estrus females was not altered when testosterone levels were equalized between the WT and HET mice; thus, the abnormal mating behavior of Six3 HET is not due to a paucity of testosterone.
Abnormal MOE development leads to an inability of Six3 HET males to detect and respond to volatile odors
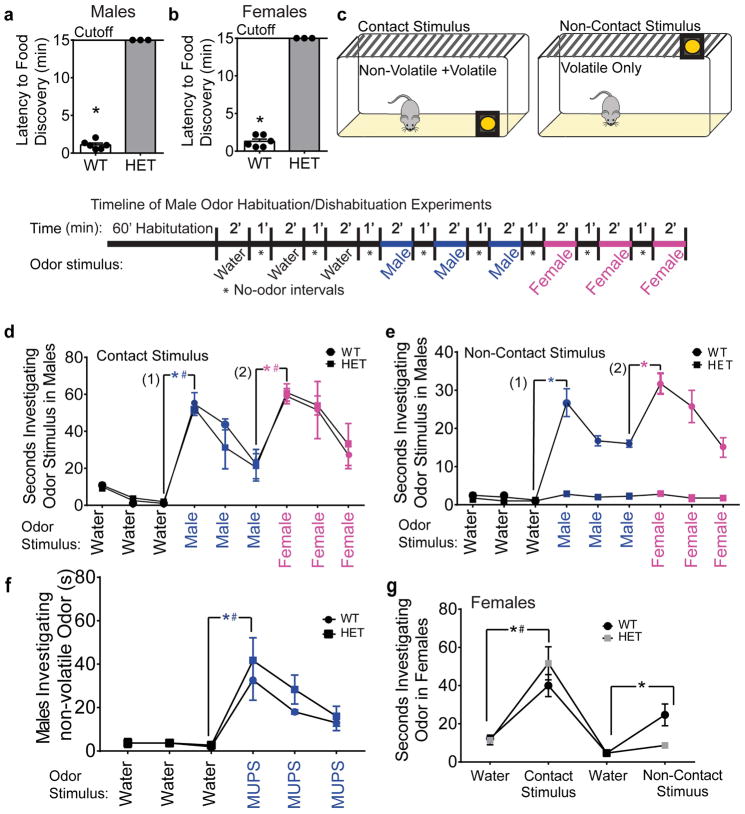
The normal motivational behavior displayed in the sucrose preference test and running-wheel activity indicate that the absence of mounting behavior in Six3 HET males is caused by a defect outside of the motivational circuit. To discern whether the altered mating behavior could be engendered by an olfactory deficit, we evaluated general olfaction using the buried food test [44]. Indeed, the Six3 HET males and females were unable to locate a buried food stimulus during the 15-minute assay (Fig. 5a, b). Odor processing occurs via two morphologically distinct circuits: the MOE and the VNO. The MOE neurons respond mainly to volatile odorants (including those that cue mating behavior) in the environment and VNO neurons respond mainly to non-volatile pheromones detected through direct contact with the odor; however, the VNO can also bind volatile odorants [48, 6]. To determine whether Six3 HET mice can detect and discriminate volatile and non-volatile odors, habituation/dishabituation tests were performed using intact male and estrus female urinary odorants (Fig. 5c). To test the ability of Six3 HET mice to detect and differentiate smells, we measured significant increases in investigation times (dishabituation) between (1) the third presentation of water and the initial presentation of male urinary stimulus and (2) the third presentation of male urine and the first presentation of estrus female urine in Six3 WT and HET males. The first test conducted provided mice with both volatile and nonvolatile odorants with presentation of odors inside the cage. Both WT and Six3 HET males could detect and differentiate between non-volatile/volatile urine components of male and estrus female urine (Fig. 5d). However, when the odor stimuli were moved outside of the cage (therefore only volatile odorants would be available to mice), Six3 HET males were unable to detect the male urine or estrus female urine (Fig. 5e). This locates the olfactory deficit to the MOE. However, to provide further evidence that the VNO is functional, non-volatile odorants were exclusively presented to Six3 HET and WT mice by using MUPs. MUPs are processed by the VNO and relay information about the donor animal [12]. To test VNO function, three presentations of MUPs were given to HET and WT mice following three presentations of water. Both Six3 HET and WT mice detected these components, indicating proper VNO function (Fig. 5f).
Fig. 5.
Six3 HET mice are unable to detect volatile odors. a and b, Identification of anosmia using a buried food test (a) male (Mann-Whitney, p=0.024, n=3–6) and (b) female (Mann Whitney, p=0.025, n=3–6). c, Schematic and time line for the habituation/dishabituation tests conducted to discern the ability to detect and discriminate odors. d, e, and f, Habituation/dishabituation tests were conducted to discern the ability to detect and discriminate odors. Results from males (e and f) and females (g). Odor placement was used to separate volatile and non-volatile odor components. Mice must be able to physically contact the odor stimulus to detect non-volatile odor components. Thus, when the odor was placed outside of the cage (f and g), only volatile odors were detectable. We compared the difference between (1) the number of seconds that subjects spent investigating the third water stimulus versus the first urinary odorant presented and (2) the difference between the number of seconds spent investigating the third presentation of the first urinary stimulus versus the first presentation of the second urinary stimulus. d, Contact stimulus detection in male mice (Mann-Whitney, n=4; water/male urine: WT, p=0.006; HET, p=0.009; male urine/female urine: WT, p=0.013; HET, p=0.024). e, Non-contact stimulus detection in male mice (Mann-Whitney, n=4; water/male urine: WT, p=0.005; HET, p=0.523; male urine/female urine: WT, p=0.015; HET, p=0.264). f, VNO function was tested more directly using three odor presentations of MUPs after three presentations of water (Student’s t-test, n=3; WT: p=0.028, t(2)=−5.61; HET: p=0.023, t(2)=−6.41). g, Odor investigation in female mice. Comparison of water to non-volatile/volatile odor (contact odor stimulus) (Two-way ANOVA followed by Tukey post hoc, n=3). In graphs with overlapping lines, * indicates WT mice and # indicates HET. *,#p<0.05.
The ability of Six3 HET females to detect volatile odorants processed by the MOE was assessed in a similar experiment. The same result was obtained in this experiment as was seen in the Six3 HET males, with Six3 HET females being able to detect non-volatile male urine components, but not volatile male urine (Fig. 5g). Female Six3 HET mice were not able to detect either male or female urine presentation (Fig. 5g). This test was similar in principle to the test conducted on male Six3 HET mice (Fig. 5c–e), but the methodology was simplified as the mating defect observed in Six3 HET females was not as severe as that observed in the Six3 HET males.
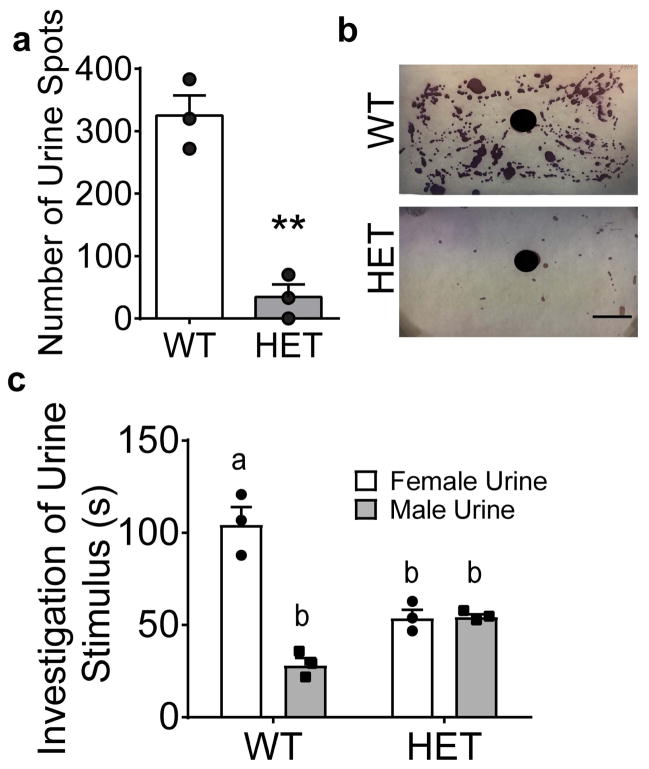
Given the inability of Six3 HET males to detect volatile female urine components, the response of Six3 HET male mice to estrus female urine was assessed. When presented with estrus female urine, male Six3 HET mice did not exhibit normal territorial marking, making significantly fewer urine spots around the estrus female urine than the WT mice (Fig. 6a, b). The abnormal response to female estrus cues in Six3 HET males was confirmed in a separate experiment, where Six3 HET males were presented with both estrus female and male urine simultaneously within the cage. Six3 HET mice, unlike the WT mice, did not preferentially sniff estrus female urine over male urine (Fig. 6c).
Fig. 6.
Six3 HET mice respond abnormally to estrus female cues. a, Territorial marking in response to female estrus urine assessed by placing 60 μL of estrus female urine in the center of Whatman paper (black circle) and quantifying the number of urine spots made by Six3 WT and HET mice after imaging these pieces of paper b, and subtracting a baseline sample with no odor exposure (Student’s t-test, p=0.002, t(4)= 7.66, n=3). c, Urine preference test with Six3 HET and WT mice presented with male urine and female estrus urine simultaneously. The amount of time mice spent investigating each odorant was measured over 5 minutes (different letters indicate statistical difference by Two-way ANOVA followed by Tukey post hoc, n=3). **p<0.01. Scale bars, 2.54 cm.
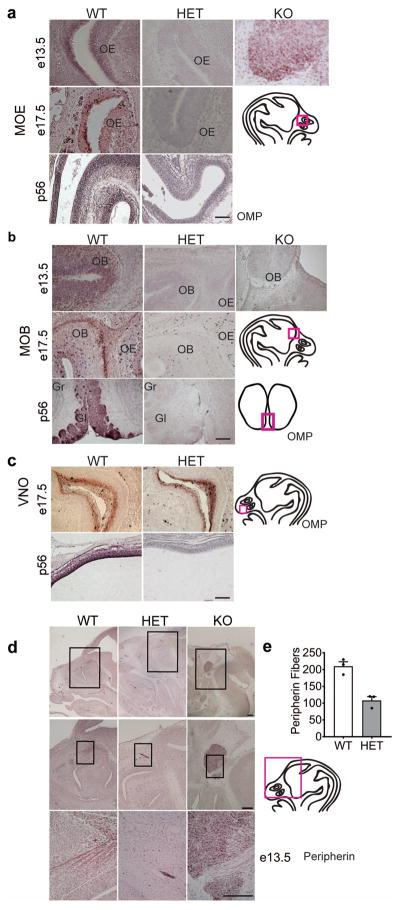
To determine how Six3 haploinsufficiency disrupted MOE-processed olfaction, we used immunohistochemistry for olfactory marker protein (OMP), which specifically localizes in the primary neurons of the olfactory system of vertebrates. A total loss of OMP staining of the MOE (Fig. 7a) and MOB (Fig. 7b) was observed in the Six3 HET mice at e13.5, e17.5, and p56. In the e13.5 Six3 KO embryos, morphologically recognizable olfactory structures were not detectable; however, a bundle of neurons and axonal fibers with OMP immunoreactive neurons was detected in the region where the MOE normally exists (Fig. 7a). In contrast, OMP staining was preserved in the VNO of Six3 HET and WT mice (Fig. 7c), supporting the normal non-volatile olfaction in these mice.
Fig. 7.
Six3 HET mice lack olfactory neurons. a, b, and c, Olfactory Marker Protein (OMP) IHC to mark all mature olfactory sensory neurons (OSNs) of Six3 WT, HET and KO mice at e13.5, 17.5, and p56 in (a) the MOE, (b) the MOB, and (c) the VNO, n=3. Boxes on drawings of the brain to the right of representative IHC images indicate where the pictures were taken. d, IHC for Peripherin-positive axons in the olfactory system at e13.5 in Six3 WT, HET, and KO mice, n=3. e, quantification of peripherin fibers in Six3 WT and HET embryos (Student’s t-test, p=0.004, t(4)=5.82, n=3). Boxes on drawings of adult brain (a) or embryo head (b, c) indicate where the representative images were taken. OB, olfactory bulb; OE, olfactory epithelium; Gr, granular layer; Gl, glomerular layer. Scale bars, 100 μm (a, b, c), panel d, 2 mm, 10 μm, 100 μm.
Olfaction requires olfactory fibers from the olfactory bulb projecting to the MOE. These olfactory fibers are also responsible for guiding GnRH neuron migration from the VNO into the forebrain during development. Peripherin, an intermediate filament protein can be used as a marker for these fibers [56]. Alteration of these fibers, including mistargeting or absence of olfactory axons along the migratory path of GnRH neurons, could be causing the defects in GnRH neuron migration observed in the Six3 KO and Six3 HET mice (Fig. 2). To determine the destiny and localization of peripherin-expressing olfactory fibers, we stained for peripherin in Six3 WT, HET and KO mice at e13.5. Whereas peripherin fibers appeared to be normal in the Six3 WT, Six3 KO mice displayed a complete absence of peripherin fibers in the upper olfactory system, and Six3 HET mice displayed a decrease in the number of projections reaching the MOB (Fig. 7d, e). The only peripherin fibers of the Six3 KO lead into the mass of neurons where GnRH- and OMP-immunopositive neurons were previously observed (Fig. 2b and 7d).
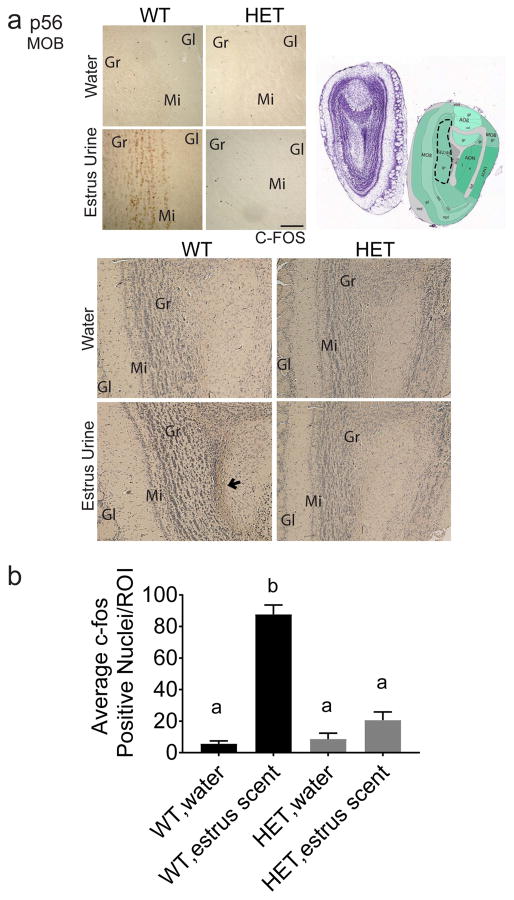
To confirm that odor processing fails to occur in Six3 HET mice, immunostaining for c-Fos, a marker of neuronal activation, was conducted after exposure to female mouse urine or water. c-Fos activation was absent from the target region of MOE neurons, the mitral layer of the dorsal MOB (Fig. 8a, b). Thus, the neuronal circuits that process olfactory cues from female estrus urine are not activated in Six3 HET males.
Fig. 8.
Six3 HET mice lack MOB neuron activation in response to estrus scent. a, Dotted outline indicates quantified portion. Images obtained from the Allen Institute web site, Allen Mouse Brain Atlas, p56 coronal image 20, (http://mouse.brain-map.org). IHC for c-Fos performed to identify regions in the MOB activated by the female urinary volatile odors presented to Six3 WT and HET male mice. Water was used as a negative control. Images are taken from the glomerular layer of the MOB, n=3. c, Quantification of ROI depicted in (b). Quantification was performed on biological replicates consisting of c-Fos positive nuclei within the defined region from a minimum of three unilateral sections. c-Fos-positive cells were quantified by an experimenter blinded to the treatment group. The numbers from each biological replicate were then averaged across all the animals in that group (different letters indicate statistical difference by two-way ANOVA followed by Tukey post hoc). Boxes on images of brains indicate where the higher magnification images below were taken. Gr, granular layer; Gl, glomerular layer; Mi, Mitral layer. Image reproduced with permission from the Allen Institute, Image credit: Allen Institute. Scale bar, 200 μm.
Discussion
Disrupted volatile olfaction leads to infertility of Six3 HET males
Loss of olfactory processing in the MOE has been shown to disrupt male sexual behavior in various studies [10, 7, 57]. Here, we describe for the first time a role for the homeodomain protein Six3 in MOE development. The impact of Six3 loss on MOE development is dosage sensitive (haploinsufficient), in that the effect of Six3 deficiency was significantly more dramatic in Six3 KO than HET mice. In Six3 KO embryos olfactory structures failed to develop into a morphologically recognizable form, and OMP-immunoreactive neurons were found in a cluster halted off their normal migratory pathway. This is markedly different from the Six3 HET mice where all olfactory structures were morphologically recognizable, although developmentally impaired with loss of OSNs and loss of neuronal activation within the MOB. IHC for cFos was conducted, revealing that some error in MOS development leads to the loss of neuronal activity, which would normally produce male sexual behavior in response to estrus cues. Loss of this neuronal activation results in the reduction in fertility seen in the Six3 Het males. To specifically determine which set of neurons in the olfactory system is resulting in this impairment, neuron activation would need to be tested.
The loss of olfactory processing due to developmental errors has been described in several mouse lines including Pax-6 SeyNeu/SeyNeu and Dlx5 KO [17], showing that when cells from the olfactory epithelium do not migrate properly or innervate the brain, the olfactory bulb can still develop. To elicit appropriate sexual behavior in rodents, correct processing of olfactory cues, via two key olfactory circuits, the MOE/MOB and VNO/Accessory Olfactory Bulb (AOB), are required. The MOE is known to process volatile odors, while the VNO is responsible for transmitting signals about water-soluble non-volatile compounds mediating innate behaviors [58]. Volatile odorants processed in the MOE and the efferent signals that they cue have been strongly implicated in the initiation of male sexual behavior [59]. The Six3 HET males displayed a severe impairment in their ability to detect volatile odors, explaining their reproductive incompetence. The reduced mounting in response to estrus female cues offers an explanation for the finding that Six3 HET males were unable to father litters during the fertility assay. Other mouse models with deficits in the MOE have shown similar sexual impairments, such as the Cnga2 mice [57] and Ac3 null male mice [9]. Similar to the Six3 HET mice, both the Cnga2 and Ac3 null males had an intact functional VNO but failed to mount female mice. The impaired preference for the scents of opposite-sex urine observed in Six3 HET mice was also observed in the Cnga1 and Ac3 nulls, and MOE-ablated mice, supporting our conclusion that Six3 HET males do not mount due to an incapacity to process volatile odors in the MOE [52]. The presence of aggressive behavior, and countermarking behavior is further evidence of the proper functioning of the VNO. While aggressive behavior is thought to be regulated by both MOE and VNO circuits [57], there is evidence that under loss of MOE function, the VNO compensates to produce normal aggressive behavior. Thus, even under loss of MOE function, as is observed in the Six3 HET mice, aggressive behavior can be mediated through the VNO to produce a normal response [59]. Indeed, the VNO developed normally and was functional in Six3 HET males, allowing the VNO to support normal aggressive response of Six3 HET males towards WT intruders, and allowing the detection of MUPs. We speculate that deletion of Six3 in adulthood might also impact function of the olfactory system, as Six3 is a transcription factor and is expressed in the nose into adulthood. However, at this time, we have only identified the role of Six3 in olfaction when heterozygous during development and in adulthood, not in adulthood alone. In conclusion, reduction in Six3 expression in Six3 heterozygote mice compromises development of the MOE and MOB, resulting in mismigration of GnRH neurons due to improper olfactory axon targeting. In addition, the impairment in development leads to an incapacity of the males to smell estrous females, and thus impairs their normal male sexual behaviors.
Six3 haploinsufficiency impairs GnRH neuron migration
Fertility depends on correct generation and migration of GnRH neurons. Numerous genes have been jointly implicated in the migration of GnRH neurons and olfactory neurons, both of which migrate along axons of the terminal olfactory pathways [17]. Mis-migration of GnRH neurons, in association with anosmia, gives rise to Kallmann syndrome, an infertility syndrome commonly due to the interruption of axonal guidance molecules that control development of the olfactory system [52]. While there is a clear GnRH neuron migratory defect observed in the Six3 HET mice, the reduction of hypothalamic GnRH neurons was not sufficient to produce either hypogonadism or hypogonadotropism. This is consistent with past findings stating that only 12% of GnRH neurons are required for pulsatile gonadotropin secretion and proper stimulation of the gonads [60]. Therefore, the Six3 HET males are subfertile due to defects in olfactory development producing altered mating behavior, rather than being due to insufficient GnRH secretion.
Our data support that the inability of a proportion of GnRH neurons to reach their appropriate destinations in the Six3 HET mice is associated with the loss or mistargeting of olfactory peripherin fibers. Six3 HET mice show a reduction in the peripherin fibers targeting the MOB, associated with a reduction in the number of GnRH neurons, while Six3 KO mice show a complete absence of olfactory fibers extending from the MOE to the MOB, giving rise to a bundle of GnRH neurons mislocalized in the MOE. A similar tangled mass of olfactory and GnRH neurons was observed in Sox10 KO mice [61], a gene identified as causing Kallmann syndrome [17]. Indeed, defects in MOB formation, such as those seen in the Six3 KO mouse, can directly affect the ability of the olfactory fibers to connect to the brain, which explains the loss of olfactovomeronasal axons in this mouse, accompanied by a loss of GnRH neuron migration [17]. Data from the Six3flox/flox:GnRHcre mouse indicate that the reduction in the number of GnRH neurons found in the adult hypothalamus is mediated through SIX3 actions external to the GnRH neurons themselves, and localized in cells along their migratory route. This is apparent because deletion of Six3 exclusively within the GnRH neuron in the Six3flox/flox:GnRHcre mouse does not reduce the number of GnRH neurons found in the adult hypothalamus. Instead, the increase in the number of GnRH neurons observed in this mouse model is likely due either to improved survival of migrating neurons, increased GnRH neuron proliferation, maintained expression of GnRH in neurons which lose GnRH expression in late development, or reduced death of GnRH neurons. Interestingly, Six3 is not the only protein that has been found to be a repressor of GnRH. MSX1 has been shown to be a repressor of GnRH promoter expression through binding to homeodomain elements within the GnRH regulatory region [62]. Therefore, MSX1 KO mice, like the Six3flox/flox:GnRHcre mice, show an increase in the number of GnRH expressing neurons within the hypothalamus [62].
Importantly, the Six3 HET mouse is, to our knowledge, the first example of a gene that in the heterozygous state gives rise to a fully penetrant phenotype in reproduction. Thus, this may be a candidate gene for Kallmann’s syndrome. While Six3 haploinsufficiency alone is sufficient to cause subfertility, Kallmann syndrome can arise from haploinsufficiency of several genes cumulatively producing complete infertility. Other genetic mutations affecting central components of the olfactory system, such as Prokr2, Pax6, Chd7, Fgf8/Fgfr1, Prok2, Sox10, and Sema3A, have been identified as causes of Kallmann Syndrome or idiopathic hypogonadal hypogonadism in various mouse models [17, 63–65]. Cases of haploinsufficiencies resulting in compromised fertility and hyposmia/anosmia have thus far been identified in both rodents and humans [47, 66, 32, 67, 31].
Sex differences in subfertility/infertility of Six3 HET mice
Despite comparable neuroanatomy in Six3 HET males and females, males were more severely subfertile than females. Extended fertility assays revealed that the Six3 HET males could plug a few WT females, albeit at a very reduced rate compared to WT mice. A potential explanation for the rare ability of the Six3 HET males to mount and produce litters despite their loss of MOE input, is that it is possible that some of the Six3 HET males were able to use tactile, visual, or auditory cues to trigger mating behavior; however, it does not necessary follow that Six3 HET males can discern ovulating females [9]. Our data support that the more severe reproductive impairment of the Six3 HET males is caused by impaired MOE development and function, a structure demonstrated to be key in male, but not female sexual behavior [59]. In contrast, female sexual behavior relies on the accessory olfactory system, regulating female sexual receptivity in the form of lordosis behavior [46, 7]. Although there are some cases in which MOE lesions produced alterations in female mating behavior [46, 68], in the majority of cases, female mating behavior is markedly less disrupted than male mating behavior.
A similar differential impact on male and female fertility by gene deletions affecting anosmia and the number of GnRH neurons was observed in studies of the B3gnt1 KO mice, a gene involved in the formation of axonal connections. B3gnt1 KO females were fertile, whereas the B3gnt1 KO males were unable to sire litters at a normal rate despite having normal reproductive organs [9]. Additionally, they displayed impaired sexual response to females, and olfactory neuron loss [9]. While fertility in Six3 HET females was not as severely disrupted as it was in the males, they remained subfertile as evidenced by a delay in being plugged, a reduced number of litters and a delay to the first litter during the fertility assay. The decrease in litters mothered correlated with increased estrous cycle length. This agrees with other studies, in which it has been shown that a decreased number of GnRH neurons in the hypothalamus can impact female fertility more severely than male fertility and often is associated with longer and irregular estrous cycles [60, 69, 31]. We believe the prolonged and irregular estrous cycles to be the cause of reduced fertility, as opposed to a behavioral deficit in mating behavior [31]. Mating behavior in females has repeatedly shown to be more strongly regulated by the VNO (a structure that was unaltered in the Six3 HET mice) than the MOE [59]. While the reason behind the subfertility/infertility of Six3 HET males and females differs, both sexes lose their ability to smell, and have a reduction in the number of GnRH neurons.
The findings presented here reveal that SIX3 dosage is essential in the proper development of the MOE and MOB olfactory structures. Furthermore, both alleles of Six3 are necessary for the proper migration of GnRH neurons and the detection of volatile odors. It is possible that SIX3 functions within the nose, in similar capacity to its role in the eye, by regulating the balance in proliferation and differentiation of olfactory structures [70]. These findings have broader implications for human health, as expanding the knowledge basis of the mechanism through which Six3 regulates neuronal development will provide insight into the diseases engendered by mutations in Six3, such as schizencephaly and holoprosencephaly [70]. In conclusion, our study is the first to address the impact of Six3 haploinsufficiency in adulthood and demonstrates Six3 to be a key transcription factor in the development of the MOE, olfaction, GnRH neuron migration, and normal fertility, remarkably producing anosmia and subfertility/infertility in the heterozygous state.
Acknowledgments
The authors thank Lauren D. Sitts and Jason D. Meadows for technical assistance on this project. This work was supported by National Institutes of Health grants R01 HD082567 and R01 HD072754 (to P.L.M.) and by National Institute of Child Health and Human Development/National Institutes of Health P50 HD012303 as part of the National Centers for Translational Research in Reproduction and Infertility (P.L.M.). P.L.M. was partially supported by P30 DK063491, P42 ES101337, and P30 CA023100. E.C.P. was partially supported by National Institutes of Health R25 GM083275 and National Institutes of Health F31 HD098652. H.M.H. was partially supported by K99 HD084759. E.L.S. was partially supported by T32 HD007203, The Lalor Foundation, P42 ES101337, and T32 DK007044.
Funding: This work was supported by NIH
Footnotes
AUTHOR CONTRIBUTIONS
E.C.P., H.M.H., E.L.S., M.R.G. and P.L.M. designed the experiments; E.C.P., H.M.H., and E.L.S performed the experiments; E.C.P., H.M.H., E.L.S., and P.L.M. analyzed the data; E.C.P., H.M.H., E.L.S., M.R.G., and P.L.M. discussed the results; and E.C.P., H.M.H., E.L.S., M.R.G., and P.L.M. wrote the manuscript.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest: The authors declare that they have no conflict of interest.
All animal procedures were performed in accordance with the UCSD Institutional Animal Care and Use Committee regulations.
References
- 1.Stowers L, Logan DW. Olfactory mechanisms of stereotyped behavior: on the scent of specialized circuits. Curr Opin Neurobiol. 2010;20(3):274–280. doi: 10.1016/j.conb.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown RE. Mammalian Social Odors: A Critical Review. In: Rosenblat JS, Hinde RA, Beer C, Busnell M-C, editors. Advances in the Study of Behavior. Vol. 10. Academic Press; New York, New York: 1979. pp. 107–161. [Google Scholar]
- 3.Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444(7117):308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- 4.Thompson ML, Edwards DA. Olfactory bulb ablation and hormonally induced mating in spayed female mice. Physiol Behav. 1972;8(6):1141–1146. doi: 10.1016/0031-9384(72)90210-7. [DOI] [PubMed] [Google Scholar]
- 5.Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51(4):457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- 6.Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nature reviews Neuroscience. 2003;4(7):551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- 7.Pierman S, Douhard Q, Balthazart J, Baum MJ, Bakker J. Attraction thresholds and sex discrimination of urinary odorants in male and female aromatase knockout (ArKO) mice. Horm Behav. 2006;49(1):96–104. doi: 10.1016/j.yhbeh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Ma D, Allen ND, Van Bergen YC, Jones CM, Baum MJ, Keverne EB, Brennan PA. Selective ablation of olfactory receptor neurons without functional impairment of vomeronasal receptor neurons in OMP-ntr transgenic mice. Eur J Neurosci. 2002;16(12):2317–2323. doi: 10.1046/j.1460-9568.2002.02303.x. [DOI] [PubMed] [Google Scholar]
- 9.Biellmann F, Henion TR, Burki K, Hennet T. Impaired sexual behavior in male mice deficient for the beta1-3 N-acetylglucosaminyltransferase-I gene. Molecular reproduction and development. 2008;75(5):699–706. doi: 10.1002/mrd.20828. [DOI] [PubMed] [Google Scholar]
- 10.Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123(4):669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 2006;103(11):4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stowers L, Marton TF. What is a pheromone? Mammalian pheromones reconsidered. Neuron. 2005;46(5):699–702. doi: 10.1016/j.neuron.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd-Thomas A, Keverne EB. Role of the brain and accessory olfactory system in the block to pregnancy in mice. Neuroscience. 1982;7(4):907–913. doi: 10.1016/0306-4522(82)90051-3. [DOI] [PubMed] [Google Scholar]
- 14.Rattazzi L, Cariboni A, Poojara R, Shoenfeld Y, D’Acquisto F. Impaired sense of smell and altered olfactory system in RAG-1(--) immunodeficient mice. Front Neurosci. 2015;9:318. doi: 10.3389/fnins.2015.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keverne EB. Importance of olfactory and vomeronasal systems for male sexual function. Physiol Behav. 2004;83(2):177–187. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Wray S. From nose to brain: development of gonadotrophin-releasing hormone-1 neurones. J Neuroendocrinol. 2010;22(7):743–753. doi: 10.1111/j.1365-2826.2010.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forni PE, Wray S. GnRH, anosmia and hypogonadotropic hypogonadism--where are we? Front Neuroendocrinol. 2015;36:165–177. doi: 10.1016/j.yfrne.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wray S. Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol. 2002;23(3):292–316. doi: 10.1016/s0091-3022(02)00001-8. [DOI] [PubMed] [Google Scholar]
- 19.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- 20.Schwanzel-Fukuda M. Origin and migration of luteinizing hormone-releasing hormone neurons in mammals. Microsc Res Tech. 1999;44(1):2–10. doi: 10.1002/(SICI)1097-0029(19990101)44:1<2::AID-JEMT2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Wray S. Development of luteinizing hormone releasing hormone neurones. J Neuroendocrinol. 2001;13(1):3–11. doi: 10.1046/j.1365-2826.2001.00609.x. [DOI] [PubMed] [Google Scholar]
- 22.Tobet SA, Schwarting GA. Minireview: recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology. 2006;147(3):1159–1165. doi: 10.1210/en.2005-1275. [DOI] [PubMed] [Google Scholar]
- 23.Bless E, Raitcheva D, Henion TR, Tobet S, Schwarting GA. Lactosamine modulates the rate of migration of GnRH neurons during mouse development. Eur J Neurosci. 2006;24(3):654–660. doi: 10.1111/j.1460-9568.2006.04955.x. EJN4955 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Cadman SM, Kim SH, Hu Y, Gonzalez-Martinez D, Bouloux PM. Molecular pathogenesis of Kallmann’s syndrome. Hormone research. 2007;67(5):231–242. doi: 10.1159/000098156. [DOI] [PubMed] [Google Scholar]
- 25.Balasubramanian R, Dwyer A, Seminara SB, Pitteloud N, Kaiser UB, Crowley WF., Jr Human GnRH deficiency: a unique disease model to unravel the ontogeny of GnRH neurons. Neuroendocrinology. 2010;92(2):81–99. doi: 10.1159/000314193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117(2):457–463. doi: 10.1172/JCI29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamou MI, Cox KH, Crowley WF. Discovering Genes Essential to the Hypothalamic Regulation of Human Reproduction Using a Human Disease Model: Adjusting to Life in the “-Omics” Era. Endocr Rev. 2015:er20151045. doi: 10.1210/er.2015-1045. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Chan YM, de Guillebon A, Lang-Muritano M, Plummer L, Cerrato F, Tsiaras S, Gaspert A, Lavoie HB, Wu CH, Crowley WF, Jr, Amory JK, Pitteloud N, Seminara SB. GNRH1 mutations in patients with idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2009;106(28):11703–11708. doi: 10.1073/pnas.0903449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaczok D, DiVall S, Matsuo I, Wondisford FE, Wolfe AM, Radovick S. Deletion of Otx2 in GnRH neurons results in a mouse model of hypogonadotropic hypogonadism. Mol Endocrinol. 2011;25(5):833–846. doi: 10.1210/me.2010-0271. me.2010-0271 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann HM, Trang C, Gong P, Kimura I, Pandolfi EC, Mellon PL. Deletion of Vax1 from GnRH neurons abolishes GnRH expression and leads to hypogonadism and infertility. J Neurosci. 2016;36(12):3506–3518. doi: 10.1523/JNEUROSCI.2723-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann HM, Tamrazian A, Xie H, Perez-Millan MI, Kauffman AS, Mellon PL. Heterozygous deletion of ventral anterior homeobox (Vax1) causes subfertility in mice. Endocrinology. 2014;155(6):4043–4053. doi: 10.1210/en.2014-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larder R, Kimura I, Meadows J, Clark DD, Mayo S, Mellon PL. Gene dosage of Otx2 is important for fertility in male mice. Mol Cell Endocrinol. 2013;377(1–2):16–22. doi: 10.1016/j.mce.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HR, McKinnon PJ, Solnica-Krezel L, Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17(3):368–379. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121(12):4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- 35.Anderson AM, Weasner BM, Weasner BP, Kumar JP. Dual transcriptional activities of SIX proteins define their roles in normal and ectopic eye development. Development. 2012;139(5):991–1000. doi: 10.1242/dev.077255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubourg C, Lazaro L, Pasquier L, Bendavid C, Blayau M, Le Duff F, Durou MR, Odent S, David V. Molecular screening of SHH, ZIC2, SIX3, and TGIF genes in patients with features of holoprosencephaly spectrum: Mutation review and genotype-phenotype correlations. Human mutation. 2004;24(1):43–51. doi: 10.1002/humu.20056. [DOI] [PubMed] [Google Scholar]
- 37.Pasquier L, Dubourg C, Blayau M, Lazaro L, Le Marec B, David V, Odent S. A new mutation in the six-domain of SIX3 gene causes holoprosencephaly. Eur J Hum Genet. 2000;8(10):797–800. doi: 10.1038/sj.ejhg.5200540. [DOI] [PubMed] [Google Scholar]
- 38.Pasquier L, Dubourg C, Gonzales M, Lazaro L, David V, Odent S, Encha-Razavi F. First occurrence of aprosencephaly/atelencephaly and holoprosencephaly in a family with a SIX3 gene mutation and phenotype/genotype correlation in our series of SIX3 mutations. J Med Genet. 2005;42(1):e4. doi: 10.1136/jmg.2004.023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7(2):148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe A, Divall S, Singh SP, Nikrodhanond AA, Baria AT, Le WW, Hoffman GE, Radovick S. Temporal and spatial regulation of CRE recombinase expression in gonadotrophin-releasing hormone neurones in the mouse. J Neuroendocrinol. 2008;20(7):909–916. doi: 10.1111/j.1365-2826.2008.01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark DD, Gorman MR, Hatori M, Meadows JD, Panda S, Mellon PL. Aberrant development of the suprachiasmatic nucleus and circadian rhythms in mice lacking the homeodomain protein six6. J Biol Rhythms. 2013;28(1):15–25. doi: 10.1177/0748730412468084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paxinos G, Franklin KBJ. The mouse brain in stereotxic coordinates. Academic Press; 2004. [Google Scholar]
- 43.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Current protocols in neuroscience/editorial board, Jacqueline N Crawley [et al] 2009;Chapter 8(Unit 8):24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neuroscience and biobehavioral reviews. 2008;32(7):1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006;23(2):521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24(42):9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295(5559):1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 49.Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology. 2012;153(2):782–793. doi: 10.1210/en.2011-1838. en.2011-1838 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448(7157):1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 51.Matsuo T, Hattori T, Asaba A, Inoue N, Kanomata N, Kikusui T, Kobayakawa R, Kobayakawa K. Genetic dissection of pheromone processing reveals main olfactory system-mediated social behaviors in mice. Proc Natl Acad Sci U S A. 2015;112(3):E311–320. doi: 10.1073/pnas.1416723112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slotnick B, Restrepo D, Schellinck H, Archbold G, Price S, Lin W. Accessory olfactory bulb function is modulated by input from the main olfactory epithelium. Eur J Neurosci. 2010;31(6):1108–1116. doi: 10.1111/j.1460-9568.2010.07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suarez R, Garcia-Gonzalez D, de Castro F. Mutual influences between the main olfactory and vomeronasal systems in development and evolution. Frontiers in neuroanatomy. 2012;6:50. doi: 10.3389/fnana.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction. 2006;132(3):379–392. doi: 10.1530/rep.1.00614. [DOI] [PubMed] [Google Scholar]
- 56.Fueshko S, Wray S. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol. 1994;166(1):331–348. doi: 10.1006/dbio.1994.1319. [DOI] [PubMed] [Google Scholar]
- 57.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8(12):1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 58.Baum MJ. Contribution of pheromones processed by the main olfactory system to mate recognition in female mammals. Frontiers in neuroanatomy. 2012;6:20. doi: 10.3389/fnana.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller M, Baum MJ, Bakker J. Olfactory control of sex-recognition and sexual behavior in mice. In: Hurst J, Beynon RJ, Roberts SC, Wyatt T, editors. Chemical Signals in Vertebrates. Vol. 11. Springer; New York: 2008. pp. 241–250. [Google Scholar]
- 60.Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone (GnRH) neuron requirements for puberty, ovulation and fertility. Endocrinology. 2008;149(2):597–604. doi: 10.1210/en.2007-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pingault V, Bodereau V, Baral V, Marcos S, Watanabe Y, Chaoui A, Fouveaut C, Leroy C, Verier-Mine O, Francannet C, Dupin-Deguine D, Archambeaud F, Kurtz FJ, Young J, Bertherat J, Marlin S, Goossens M, Hardelin JP, Dode C, Bondurand N. Loss-of-function mutations in SOX10 cause Kallmann syndrome with deafness. Am J Hum Genet. 2013;92(5):707–724. doi: 10.1016/j.ajhg.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Givens ML, Rave-Harel N, Goonewardena VD, Kurotani R, Berdy SE, Swan CH, Rubenstein JL, Robert B, Mellon PL. Developmental regulation of gonadotropin-releasing hormone gene expression by the MSX and DLX homeodomain protein families. The Journal of biological chemistry. 2005;280:19156–19165. doi: 10.1074/jbc.M502004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308(5730):1923–1927. doi: 10.1126/science.1112103. 308/5730/1923 [pii] [DOI] [PubMed] [Google Scholar]
- 64.Dode C, Rondard P. PROK2/PROKR2 Signaling and Kallmann Syndrome. Frontiers in endocrinology. 2013;4:19. doi: 10.3389/fendo.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Layman WS, McEwen DP, Beyer LA, Lalani SR, Fernbach SD, Oh E, Swaroop A, Hegg CC, Raphael Y, Martens JR, Martin DM. Defects in neural stem cell proliferation and olfaction in Chd7 deficient mice indicate a mechanism for hyposmia in human CHARGE syndrome. Hum Mol Genet. 2009;18(11):1909–1923. doi: 10.1093/hmg/ddp112. ddp112 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD, Illingworth P, Handelsman DJ. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology. 2007;148(8):3674–3684. doi: 10.1210/en.2007-0248. [DOI] [PubMed] [Google Scholar]
- 67.Kim HG, Herrick SR, Lemyre E, Kishikawa S, Salisz JA, Seminara S, MacDonald ME, Bruns GA, Morton CC, Quade BJ, Gusella JF. Hypogonadotropic hypogonadism and cleft lip and palate caused by a balanced translocation producing haploinsufficiency for FGFR1. J Med Genet. 2005;42(8):666–672. doi: 10.1136/jmg.2004.026989. 42/8/666 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edwards DA, Burge KG. Olfactory control of the sexual behavior of male and female mice. Physiol Behav. 1973;11(6):867–872. doi: 10.1016/0031-9384(73)90282-5. [DOI] [PubMed] [Google Scholar]
- 69.Larder R, Clark DD, Miller NL, Mellon PL. Hypothalamic dysregulation and infertility in mice lacking the homeodomain protein Six6. J Neurosci. 2011;31(2):426–438. doi: 10.1523/JNEUROSCI.1688-10.2011. 31/2/426 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu CC, Dyer MA, Uchikawa M, Kondoh H, Lagutin OV, Oliver G. Six3-mediated auto repression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development. 2002;129(12):2835–2849. doi: 10.1242/dev.129.12.2835. [DOI] [PubMed] [Google Scholar]