Abstract
Triterpenic acids (TTAs) are well known for their relevant biological properties and have been facing an increasing interest for nutraceutical and pharmaceutical applications. To overcome the concerns associated to the commonly used volatile organic solvents for their extraction from biomass, here we investigate the potential of aqueous solutions of ionic liquids (ILs) as alternative solvents. The solubility of ursolic acid (UA) was firstly determined in several aqueous solutions of ILs (hydrotropes or surface-active) at 30°C to appraise the dissolution phenomenon. Conventional surfactants were also investigated for comparison purposes. The collected data reveal a remarkable enhancement in the solubility of UA (8 orders of magnitude) in surface-active ILs aqueous solutions when compared to pure water. Afterwards, the potential of these ILs aqueous solutions was confirmed by their use in the extraction of TTAs from apple peels. Total extractions yield of TTAs of 2.62 wt.% were obtained using aqueous solutions of surface-active ILs at moderate conditions, overwhelming the extraction yields of 2.48 wt.% obtained with chloroform and 1.37 wt.% with acetone using similar conditions.
Keywords: Biorefinery, Triterpenic Acids, Solubility, Extraction, Surface-Active Ionic Liquids, Apple Peel
Introduction
It is well known that there is a strong link between the consumption of fruits and vegetables and improved human health.1,2 Some compounds present in fruits and vegetables have high potential to modulate many processes involved in the development of some diseases and degenerative disorders, including cancer,3 cardiovascular disorders,4 and diabetes.5 Amongst the vast plethora of bioactive natural compounds with potential to improve human health, are flavonoids, phenolic acids, carotenoids, tocopherols, alkaloids, lignans, tannins, triterpenoids, among others.6,7
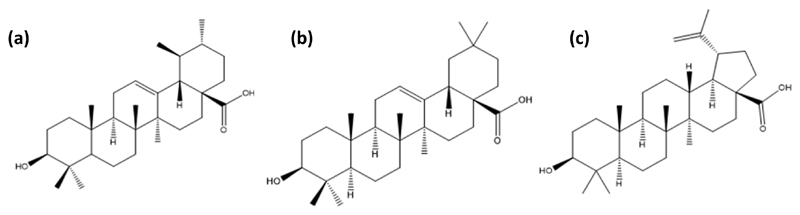
Triterpenoids are a vast class of C-30 terpenic compounds, which can be classified into different groups depending on their carbon backbone, including, for instance, lupane, oleanane and ursane-based compounds.8 Triterpenoids are widely distributed in medicinal and edible plants,9 and are part of the regular human diet due to their relevant health benefits.10 In the past few years, there has been a growing trend on the incorporation of triterpenoids-rich extracts in new functional foods, cosmetics, healthcare products and drugs.9–11 To turn possible the application of these novel products, it is however required to have abundant natural sources of triterpenoids, as well as safe and cost-effective extraction techniques. Agro-food industry by-products are an obvious resource to tackle this challenge, e.g. fruit peels which are rich in triterpenoids.12 Apples are rich in triterpenoids, and particularly in triterpenic acids (TTAs), such as ursolic, oleanolic and betulinic acids (Figure 1).13 In general, the occurrence of ursolic acid in apple peels is well documented;14 yet, there is a growing list of other triterpenoids that are also present and still need to be fully characterized.10,15 According to the United Nations Food and Agriculture Organization (FAO) database (2013), the global fruit production in 2013 was of 610 million tons, among which apples comprised 81 million tons, i.e., 13% of the total fruit production.16 Moreover, during the industrial processing of apples for the preparation of juices, jams, etc., large amounts of residues are generated, such as apple peels, being these a promising raw-material to obtain triterpenoids-rich extracts.
Figure 1.
Chemical structures of some triterpenoid acids present in apple peels: (a) ursolic, (b) oleanolic and (c) betulinic acids.
To obtain extracts rich in TTAs from apple peels, several extraction methods and solvents have been used, including extractions with ethanol (yields ranging between 0.01 and 1.3 wt.%),17 with chloroform (yield of 0.7 wt.%),18 and accelerated solvent extraction (ASE) with ethyl acetate (yields ranging between 0.2 and 2.1 wt.%).9 All these studies have been carried out with volatile, and some hazardous, organic solvents. Taking into account the envisaged application of TTA-rich extracts in nutraceutical and pharmaceutical products, there is a crucial demand to use safer and more biocompatible solvents, and to develop cost-effective extraction processes. Amongst the possible solvents, water appears as the greener and safer solvent overall; however, TTAs display negligible solubility in water.19 Therefore, aqueous solutions of ionic liquids (ILs) can be envisioned as promising solvents if an increase in the solubility of TTAs and further extraction ability from biomass are verified.
ILs are organic salts with melting temperatures below 100°C, typically composed of a large organic cation and an inorganic or organic anion.20 Their chemical nature is responsible for a number of unique properties, such as negligible volatility and nonflammability at ambient conditions, and high thermal and chemical stabilities.21,22 Furthermore, physicochemical properties of ILs can be modulated by an adequate selection and combination of their ions, allowing the design of ILs with target properties.20,23 Due to these features, ILs display a good solvation capacity for a wide range of solutes and are well known as potential substitutes of conventional organic solvents for the extraction of bioactive compounds from biomass.24,25 Despite the potential of pure ILs in the extraction of bioactive components from biomass it was recently demonstrated that ILs aqueous solutions display a tremendous potential in this domain, in which they can act either as hydrotropes or as surface-active agents, by promoting an increase in the solubility of bioactive compounds in aqueous media and by favouring their extraction from raw materials.26–28 In addition, the use of ILs aqueous solutions leads to foremost advantages since the IL consumption is reduced, and the viscosity of the extraction solvent is decreased, leading to enhancements in mass transfer phenomena and to a decrease in the energy consumption.25 Furthermore, ILs aqueous solutions also shown to be advantageous since they are more selective to target compounds while avoiding the dissolution of the biomass lignocellulosic part.25
TTAs (Figure 1) are aliphatic polycyclic structures with a low solubility in water.19 Aiming at finding alternative water-rich solvents, in this work, we investigated the potential of ILs aqueous solutions to increase the solubility of TTAs, using ursolic acid (UA) as a representative compound of this class, and further applied the most promising IL aqueous solutions in the extraction of TTAs (ursolic, oleanolic and betulinic acids) from green apple peels. For comparative purposes, the solubility and extraction of TTAs using common surfactants aqueous solutions and organic solvents, respectively, were also addressed. To the best of our knowledge, no attempts have been previously reported in the literature on the use of ILs aqueous solutions for improving the solubility and extraction of TTAs from biomass.
Experimental Section
Materials
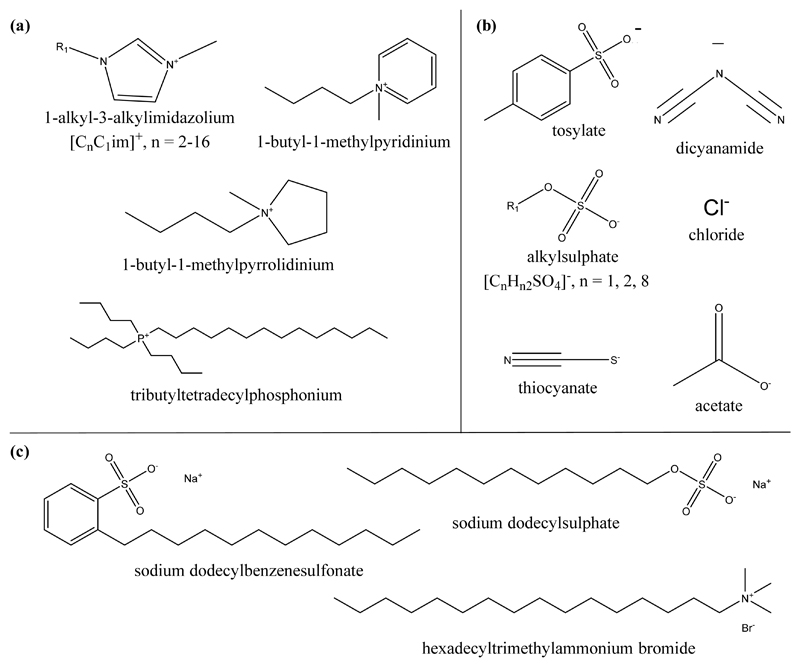
To infer the molecular structure characteristics which enhance the solubility of TTAs in aqueous media, a large array of ILs was investigated (chemical structures shown in Figure 2): 1-ethyl-3-methylimidazolium acetate, [C2C1im][CH3CO2]; 1-butyl-3-methylimidazolium ethylsulphate, [C4C1im][C2H5SO4]; 1-butyl-3-methylimidazolium octylsulphate, [C4C1im][C8H17SO4]; 1-butyl-3-methylimidazolium chloride, [C4C1im]Cl; 1-butyl-3-methylimidazolium dicyanamide, [C4C1im][N(CN)2]; 1-butyl-3-methylimidazolium thiocyanate, [C4C1im][SCN]; 1-butyl-3-methylimidazolium methylsulfate, [C4C1im][CH3SO4]; 1-butyl-3-methylimidazolium tosylate, [C4C1im][TOS]; 1-butyl-1-methylpyrrolidinium chloride, [C4C1pyr]Cl; 1-butyl-1-methylpyridinium dicyanamide [C4C1py][N(CN)2], 1-methyl-3-octylimidazolium chloride, [C8C1im]Cl; 1-decyl-3-methylimidazolium chloride, [C10C1im]Cl; 1-dodecyl-3-methylimidazolium chloride, [C12C1im]Cl; 1-tetradecyl-3-methylimidazolium chloride, [C14C1im]Cl; 1-hexadecyl-3-methylimidazolium chloride, [C16C1im]Cl; 1-octadecyl-3-methylimidazolium chloride, [C18C1im]Cl; and tributyltetradecylphosphonium chloride, [P444,14]Cl. The imidazolium-, pyridinium-, and pyrrolidinium-based ILs were purchased from Iolitec. The phosphonium-based IL was kindly offered by Cytec Industries Inc. All ILs used have a purity higher than 98 wt.%, according to the information provided by suppliers.
Figure 2.
Chemical structures of (a) cations and (b) anions composing the ILs and (c) conventional surfactants used in this work.
In addition to ILs, conventional surfactants were also studied for comparison purposes (chemical structures depicted in Figure 2), namely sodium dodecylsulphate (SDS) from Alfa Aesar, sodium dodecylbenzenesulfonate (SDBS) from Sigma Aldrich, and hexadecyltrimethylammonium bromide (CTAB) from Fluka. All conventional surfactants have a purity higher than 99 wt.%.
Ursolic acid (UA), oleanolic acid (OA) and betulinic acid (BA) standards, with a purity higher than 98 wt.%, were acquired from Sigma. The solvents used for the extraction of TTAs in addition to aqueous solutions of ILs and surfactants, included distilled water, acetone and chloroform (purity ≥ 99.99 wt.%) from VWR chemicals. The mobile phase used in the HPLC analysis was composed of methanol (purity ≥ 99.99 wt.%) from VWR Chemicals, and ultra-pure water (purity ≥ 99.99 wt.%) from Merck, both HPLC grade.
Solubility of ursolic acid in aqueous solutions of ILs
While there is a large diversity of apple cultivars available for consumption and, consequently, a broad variation in their bioactive compounds composition,12 ursolic acid (UA) is the most well documented triterpenic acid present in apple peels.14 Thus, we have chosen UA as a major representative of the TTAs class for carrying out screening studies of solubility in aqueous solutions of ILs, as well as in aqueous solutions of conventional surfactants for comparison purposes.
Pure UA (solid) was added in excess amounts to aqueous solutions of ILs and conventional surfactants of different concentrations (50, 250, 500, 750 and 1000 mM). Samples were kept under constant agitation using an Eppendorf Thermomixer Comfort equipment at (30±0.5) ºC. Previously optimized equilibration conditions were established as follows: stirring velocity of 750 rpm, and equilibration time of at least 72 h. At least two independent samples were prepared to determine the average solubility value and respective standard deviation. After saturation of the aqueous solutions, and always assuring the presence of a solid phase and thus of UA in excess, a 200 μL aliquot was taken, diluted with 800 μL of methanol, carefully filtered using a 0.20 μm syringe filter, and subsequently quantified using a GILSON HPLC unit coupled to an oven and with manual injector, using a previously established calibration curve (R2 > 0.9990). Data acquisition and evaluation were performed using the Jasco-Borwin 1.21 software. An analytical C18 reversed-phase column (250 × 4.60 mm), kinetex 5 μm C18 100 A, from Phenomenex, was used. The mobile phase consisted of 87 (v/v) % of methanol, 13 (v/v) % of water + 0.1 (v/v) % of trifluoroacetic acid (TFA). Separations were conducted in isocratic mode, at a flow rate of 1 mL.min−1 and using an injection volume of 10 μL. The wavelength was set at 210 nm. Each sample was analysed at least two times. The column oven and the autosampler were operated at 30°C. Illustrative HPLC chromatograms of standards TTAs and of an apple peel extract obtained using aqueous solution of [C14C1im]Cl (500 mM) is provided in supporting information (Figure S.I.1)
The pH of ILs and surfactants aqueous solutions was determined using a pH meter (Digimed, model DM21), previously calibrated with buffer solutions (pH 7.0 and pH 4.0, Reagent QM).
Due to the amphiphilic character of the ILs studied, and aiming at better understanding the role played by surface-active ILs in the solubility enhancement, the critical micellar concentration (CMC) of the studied ILs was determined by electric conductivity.29 The conductivity of several aqueous solutions of different concentrations of IL was determined using a Russel RL105 Conductivity Meter at 25°C, by continuous dilution of an IL concentrated solution in water. Each conductivity value was recorded when its fluctuation was less than 1% within 2 min.
Extraction of TTAs from apple peels using aqueous solutions of ILs
Apple peels from Portuguese origin Golden apples were manually removed. The apples peels were dried at 25°C for 2 days, and then their grinding with a commercial coffee grinder was carried out. The samples of ground apple peels were further divided and classified according to the particle size by means of stainless steel sieves. Samples with a diameter smaller than 1 mm were used.
Weighted amounts (with an uncertainty of 10-4 g) of ground apple peels were added to aqueous solutions of ILs with a concentration selected from the UA solubility studies, at a fixed solid-liquid ratio (RS/L, weight of dried biomass per weight of the IL aqueous solution) of 0.1. The extractions were carried out at different temperatures (25, 50, 80 and 90°C, within ± 0.5°C), a fixed extraction time (60 minutes) and constant stirring (1000 rpm), in order to appraise the effect of temperature on the extraction yield. A similar procedure was applied in the extractions with conventional organic solvents, in which the IL aqueous solution was replaced by organic solvents, however under reflux due to the lower boiling temperature of organic solvents. At least three independent extractions were carried out for each condition and solvent.
After the extraction step, the overall solution and extract were centrifuged and the supernatant filtered using a 0.20 μm syringe filter. A 200 μL aliquot was taken, mixed with 800 μL of methanol, filtered over a 0.2 μm syringe filter, and the TTAs content determined by HPLC-DAD at 210 nm. Three major TTAs have been identified in the extracts, namely oleanolic acid (OA), betulinic acid (BA), and ursolic acid (UA), according to the respective standards and retention time values. Previous calibration curves have been established for each TTA (R2 > 0.9992, 0.9995, 0.9990, for oleanolic, betulinic and ursolic acids, respectively). HPLC-DAD (Shimadzu, model PROMINENCE) analyses were performed using the same column and conditions described previously in the solubility tests. The TTAs extraction yield is expressed as the percentage ratio between the weight of TTAs and the total weight of the dried biomass.
Results and Discussion
Solubility of ursolic acid in aqueous solutions of ILs
TTAs (Figure 1) are almost insoluble in water,19 and their extraction from biomass is usually carried out with volatile organic solvents.9,17,18 Aiming at improving the TTAs solubility in aqueous-rich media and to favor their extraction from biomass, we first investigated the potential of ILs aqueous solutions to increase the solubility of TTAs, using ursolic acid (UA) as a representative compound of this class since it is the most well documented triterpenic acid present in apple peels.14 The solubility of ursolic acid was determined in several aqueous solutions of ILs, as well as aqueous solutions of conventional surfactants for comparison purposes, at concentrations of 50, 150, 250, 500, 750 and 1000 mM.
Although pure ILs have been described as potential solvents for the extraction of value-added compounds from biomass, e.g. betulin from birch bark30, aqueous solutions of ILs also display a high potential and additional advantages since they imply lower amounts of IL as solvent25, with additional benefits in terms of solvent toxicity and cost, and reduce the overall viscosity of the extraction media, thus enhancing the mass transfer phenomena and reducing energy consumptions. In summary, whenever possible, ILs aqueous solutions should be the preferred choice.25 When dealing with compounds with a low solubility in water, such as TTAs, two classes of ILs can be selected to improve their solubility and extraction in aqueous media: ILs that act as hydrotropes26 or as surfactants.28 Based on this possibility, both classes of ILs were studied to infer the main IL structural characteristics which rule the solubility and extraction of TTAs. It should be remarked that it was not possible to determine the solubility of ursolic acid in pure water, as found to be below the detection limit of the analytical method used. Still, the water solubility of ursolic acid reported in the literature19 is 1.02 × 10-7 g.L-1, used as a reference in this work.
In a first approach, ILs that behave as hydrotropes26,31 were selected, namely [C4C1im][N(CN)2], [C4C1im][TOS], [C4C1im][SCN], [C4C1im][C2H5SO4], [C4C1py][N(CN)2] and [C4C1pyrr]Cl, and tested in aqueous solutions in concentrations ranging from 50-1000 mM at 30°C to dissolve ursolic acid. These ILs comprise a butyl chain as the longest alkyl chain at the cation, and do not present a critical micellar concentration (CMC) nor are surface-active. With these ILs, and at different concentrations, the target TTA was not detected by HPLC analysis in any of the aqueous solutions, meaning that the solubility of UA is below the detection limit of the analytical equipment and method used (0.002 g.L-1, as determined by us). Therefore, hydrotropy does not play a significant role in enhancing the solubility of TTAs in aqueous media. Aqueous solutions of hydrotrope ILs seem thus more valuable for enhancing the solubility of moderately hydrophobic compounds, such as phenolic acids, and as previously reported.26
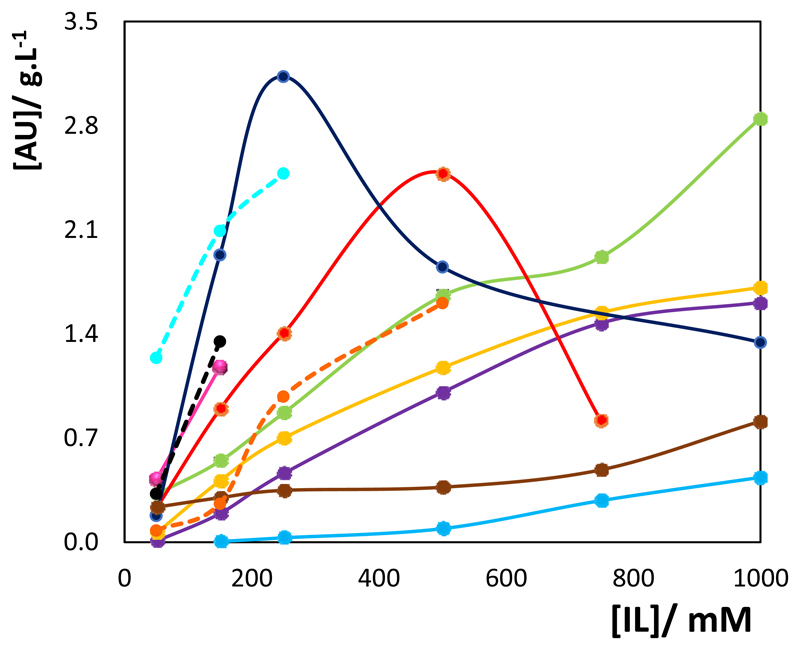
Subsequently, surface-active ILs, both cationic and anionic, as well as composed of different cations and anions, were investigated. In particular, different ILs constituted by long alkyl side chains with known surface-active characteristics28,32 have been studied ([CnC1im]X with n = 8, 10, 12, 14, 16 and 18 and X = Cl and [C8H17SO4], and [P444,14]Cl). Figure 3 shows the solubility data of UA at 30°C in the different surface-active ILs aqueous solutions in a 50-1000 mM concentration range; detailed data are provided in the Supporting Information (Tables S.I.1 to S.I.2). It should be remarked that some ILs were exploited up to lower concentrations due to the high viscosity obtained with more concentrated solutions and difficulties encountered in their handling for subsequent quantification. Based on the amphiphilic character of the studied ILs, we also determined their CMC values by conductivity, aiming a better understanding of the role played by the IL in what regards the dissolution mechanism which enhances the solubility of ursolic acid in aqueous media. These results are reported in the Supporting Information (Table S.I.3).
Figure 3.
Solubility of UA in aqueous solutions of surface-active ionic liquids and conventional surfactants at different concentrations at 30°C: (●) [C8C1im]Cl, (●) [P444,14]Cl, (●) [C10C1im]Cl, (●) [C12C1im]Cl, (●) [C14C1im]Cl, (●) [C16C1im]Cl, (●) [C18C1im]Cl, (●) [C4C1im][C8H17SO4], (– -) SDBS, (– -) SDS and (– -) CTAB.
In general, the addition of all the investigated surface-active ILs leads to an increase in the solubility of ursolic acid in aqueous solutions. The pH of almost all ILs aqueous solutions is below the pKa of UA (pKa = 4.90),33 meaning that UA is being solubilized in its protonated or neutral form. Thus, the gathered solubility results are a main result of the IL chemical structure and respective CMC and not a direct result of the solution pH. The pH detailed data combined with the solubility results are presented in the Supporting Information (Figure S.I.2).
For most ILs it was found a monotonous increase in the solubility of the TTA along the IL concentration, whereas for [C16C1im]Cl and [C4C1im][C8H17SO4], a maximum in the solubility was observed, occurring at 500 and 250 mM, respectively. This behaviour is analogous to that observed with hydrotrope-based ILs, although for these the maximum in solubility is observed at higher IL concentrations.26 As also shown in Figure 3, an increase in the IL cation alkyl side chain leads to an increased capacity to solubilise UA in aqueous media. It is well known that an increase in the IL alkyl side chain decreases the CMC and promotes the IL aggregation32 - as shown in the Supporting Information (Table S.I.3) with the CMC values determined in this work and respective comparison with literature values – supporting therefore the higher capacity of ILs comprising longer alkyl side chains to solubilize UA. For instance, at 500 mM of IL, the solubility of UA increases in the following order: [C16C1im]Cl (2.48 g.L-1) > [C4C1im][C8H17SO4] (1.85 g.L-1) > [C14C1im]Cl (1.66 g.L-1) > [C12C1im]Cl (1.17 g.L-1) > [C10C1im]Cl (1.01 g.L-1) > [P444,14]Cl (0.37 g.L-1) > [C8C1im]Cl (0.09 g.L-1). This trend follows the CMC values of all ILs investigated. However, an exception occurs with [C4C1im][C8H17SO4], since it presents a CMC (43.3 mM) between those displayed by [C10C1im]Cl (58.7 mM) and [C12C1im]Cl (15.2 mM), meaning that anionic surfactants may be promising options for enhancing the solubility of UA in aqueous solutions. Although a maximum solubility of ursolic acid would be expected with [C18C1im]Cl, as shown in Figure 3 for lower IL concentrations and according to its lower CMC, only a maximum concentration of 150 mM was used due to the high viscosity of [C18C1im]Cl aqueous solutions and difficulties in handling such solutions for further quantification. Similar viscosity problems have been described by Ressmann et al.28 with long alkyl chain ILs for the extraction of piperine from biomass.
When comparing the solubility of UA achieved using an imidazolium-based ([C14C1im]Cl (1.66 g.L-1)) with a phosphonium-based ([P444,14]Cl (0.37 g.L-1)) IL at 500 mM, it is clear that the presence of an aromatic ring in the structure of the IL cation is a relevant factor to increase the solubility of ursolic acid. According to these results, the role of the aromatic ring may be highly relevant not only to increase the solubility of UA in aqueous solutions, as demonstrated in Figure 3, but possibly also will influence the extraction process of TTAs.
Since a significant increase in the solubility of UA in surface-active ILs aqueous solutions was observed, in order to truly confirm the potential of ILs we further compared the results obtained with those gathered with aqueous solutions of conventional surfactants, such as sodium dodecylsulphate (SDS), sodium dodecylbenzenesulfonate (SDBS) and hexadecyltrimethylammonium bromide (CTAB). The results obtained are depicted in Figure 3. Although conventional surfactants aqueous solutions may lead to some competitive solubility data, these are limited by the lower solubility of conventional surfactants in water (if compared with ILs), and thus are more restricted in their ability to enhance the solubility of UA in aqueous media. A similar trend is expected with non-ionic surfactants, and for this reason this type of surfactants was not investigated in this work. Taking into account the surfactants molecular structures (Figure 1) it is evident the relevant role of anionic surfactants, which is in agreement with the high performance discussed above with the IL [C4C1im][C8H17SO4]. Moreover, the presence of aromatic rings also appears as a relevant factor toward the enhancement of the solubility of UA in aqueous solutions. Overall, the obtained results emphasize the potential of ILs aqueous solutions to solubilize poorly-water soluble compounds, such as TTAs.
Maximum solubility values of UA of 2.48 and 3.13 g.L-1, respectively, have been obtained with the two best ILs identified, namely [C16C1im]Cl and [C4C1im][C8H17SO4], that if compared with the solubility of the target compound in pure water (1.02×10-7 g.L-1),19 represents an increase in the solubility of UA of 8 orders of magnitude. An increase in the solubility of UA up to 5 orders of magnitude has been obtained with volatile organic solvents, such as tetrahydrofuran, cyclohexane and ethyl acetate34 and methanol, ethanol and 2-propanol35. This remarkable enhancement in solubility discloses the high potential of ILs aqueous solutions as alternative solvents for the extraction of TTAs from biomass, as shown below.
This remarkable increment in the solubility of UA along the IL concentration (up to 8 orders of magnitude) can be also tackled as a way of recovering the target solutes from the IL-water solvent, by a simple addition of water as an anti-solvent. To test this hypothesis we prepared and aqueous solutions of [C4C1im][C8H17SO4] at 250 mM containing 2.5 mg/mL of UA. At room temperature (ca. 25 ºC) we added water under constant agitation to reach a concentration of IL down to 50 mM. During the addition of water, it was macroscopically visible the precipitation of UA (cf. the Supporting Information – Figure S.I.3). This precipitate was recovered by filtration and washed several times with water at room temperature, aiming at removing any traces of IL present. The precipitate was then dried up to constant weight at 50ºC, allowing the recovery of 89% of the initial UA added to the system. The IL can be further recovered by an evaporation step to remove the excess of water and thus recycled and reused. Despite the good performance of ILs to solubilize and extract value-added compounds from biomass, the isolation/purification of the target compounds from the IL-rich medium remains a challenge, mainly because of the inability to apply a simple solvent evaporation step due to the non-volatile nature of the most studied aprotic ILs. Aiming at overcoming this drawback, some strategies have been proposed, including back-extractions with organic solvents, precipitation with anti-solvents, and use of macroporous and ion-exchange resins.24,25 Herein, taking advantage of the remarkable solubility dependence of TTAs along the IL concentration, water can be used as an appropriate anti-solvent, therefore making use of the greenest anti-solvent overall.
Extraction of triterpenic acids from green apple peels
After the previous screening on the ILs chemical features to enhance the solubility of TTAs in aqueous solutions, we selected [C14C1im]Cl, at 500 mM, to be used as an extraction solvent of TTAs from green apple peels. This IL and concentration were chosen since good solubility data for TTAs have been obtained (well above of what could be extracted from biomass taking into account the apple peels composition)13 and to work with ILs aqueous solutions of lower viscosity – as previously discussed an increase in the cation alkyl side chain length leads to aqueous solutions of high viscosity. Moreover, it is generally accepted that the decrease on the extraction yields from biomass observed at higher IL concentrations mainly result from the increased solution viscosity which hinders an efficient solvent penetration into the plant tissues.25,28
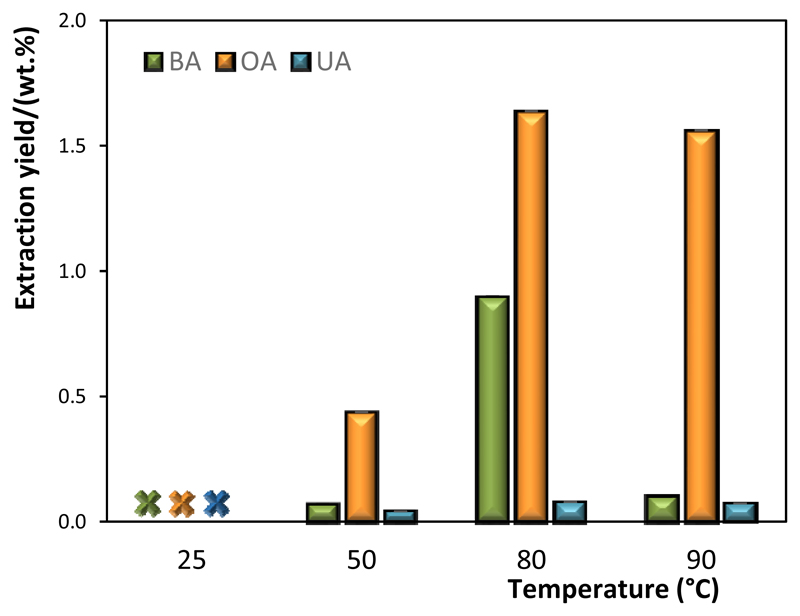
The extraction of TTAs from apple peels was carried out at 25, 50, 80 and 90°C, while keeping the other operational conditions constant, namely a biomass-solvent ratio of 1:10, an IL concentration of 500 mM and 60 min of extraction time. Three TTAs have been identified by HPLC-DAD, namely ursolic, oleanolic and betulinic acids, according to a wide range of standards tested. The extraction yields of the three identified TTAs at the studied temperatures are shown in Figure 4.
Figure 4.
Extraction yields of TTAs (BA (green), OA (yellow) and UA (blue)) from green apple peels with [C14C1im]Cl at different temperatures and other fixed conditions ([IL] = 500 mM, t = 60 min, RS:L = 1:10).
Negligible values have been obtained at 25°C (below the analytical equipment detection limit), increasing from 50°C to 80°C, followed by a decrease at 90°C. Therefore, temperature strongly influences the extraction yield of TTAs from biomass. Maximum extraction yields for the three identified TTAs have been obtained at 80°C, namely 0.079 wt.% for UA, 0.90 wt.% for BA and 1.64 wt.% for OA.
The increase in the extraction yield observed from 25 to 80°C may result both from a solubility increment and from the solvent viscosity decrease at higher temperatures, thereby increasing both the solution and the target compounds diffusion. The decrease in the extraction yields observed at higher temperatures may result from the co-dissolution of plant polysaccharides that turns the aqueous media into a gel, turning the recovery of TTAs highly difficult, as discussed in other works.36,37 Based on the results obtained, and amongst the temperatures range investigated, 80°C is the best temperature for the extraction of TTAs from apple peels using [C14C1im]Cl aqueous solutions at 500 mM.
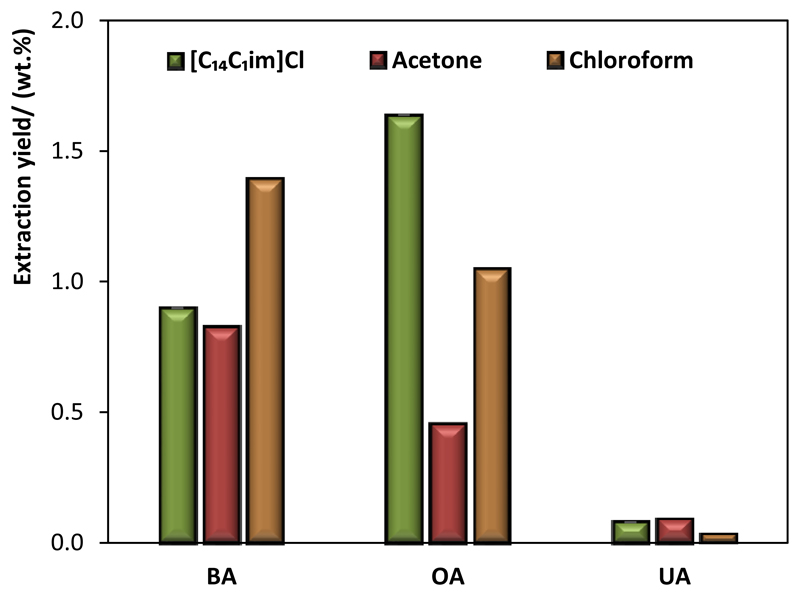
The TTAs extraction yield using [C14C1im]Cl aqueous solutions was then compared with common organic solvents well described in the literature for the extraction of TTAs from biomass,18,38 such as acetone and chloroform, under reflux at 80°C. The results obtained are shown in Figure 5.
Figure 5.
Extraction yields of TTAs (BA, OA and UA) from green apple peel with several solvents at fixed conditions (T = 80°C, [IL] = 500 mM, t = 60 min, RS:L = 1:10; organic solvents were used as pure and not as aqueous solutions).
[C14C1im]Cl aqueous solutions are particularly more relevant than the studied volatile and hazardous organic solvents for the extraction of OA. When comparing the total amount of extracted TTAs while envisaging the preparation of TTAs-rich extracts for incorporation in new functional foods, cosmetics, healthcare products and drugs, [C14C1im]Cl aqueous solutions appear as the most promising solvents, with a total extraction yield of TTAs of 2.62 wt.%, when compared with 2.48 wt.% obtained with chloroform and 1.37 wt.% with acetone under similar conditions.
The interest on TTAs has increased over the past few years, as well as the number of extraction studies of these target compounds from a broad range of biomass sources (apple,15 grape,36 tomato,37 olive38), while using several extraction methods (microwave,39 maceration,40 solid-liquid36 and supersonication41) and different solvents (ethyl acetate,36 n-hexane,15 and ethanol17). The results obtained in this work have been obtained with aqueous solutions of ILs, instead of the commonly used volatile organic solvents, under moderate temperatures and without using energy-intensive methods. Extraction processes based on aqueous solutions of ILs are thus potential platforms for enhancing the solubility and extraction of triterpenic acids and other value-added compounds present in biomass, performing even better than the pure ILs commonly investigated in the literature,25 while avoiding the dissolution of the lignocellulosic fraction and allowing the recovery of richer value-added extracts. Moreover, based on the solubility trends of TTAs along the IL concentration and the previous demonstrated induced-precipitation approach by using water as anti-solvent, it is envisioned the possibility of applying the same procedure to recover TTAs-rich extracts from the IL-water solvent, and for which the IL can be recovered and reused after an evaporation step to remove the excess of water.
Conclusions
In the past years, there has been a growing trend on the incorporation of triterpenoids-rich extracts in new functional foods, cosmetics, healthcare products and drugs. To turn possible the application of these products, it is yet required to have abundant natural sources of triterpenoids, preferentially agro-food industry by-products, as well as to use safer and more cost-effective extraction techniques. Based on these requirements, in this work, we investigated the potential of aqueous solutions of ILs as alternative solvents over the commonly used volatile organic solvents for the extraction of TTAs from biomass. Aiming at identifying the most promising IL aqueous solutions for the extraction of TTAs, we first addressed a comprehensive study based on the solubility of ursolic acid in aqueous solutions of ILs, allowing us to better understand the dissolution phenomenon and the IL chemical structure features which enhance the TTAs solubility and further extraction yield. The collected data reveal that hydrotropy does not play a significant role in the improvement of solubility of TTAs in aqueous media. However, surface-active ILs allow a significant increase in the solubility of ursolic acid in aqueous solutions – with an observed enhancement of 8 orders of magnitude when compared with its solubility in pure water. Based on the remarkable increase in the solubility of ursolic acid, a major representative of the TTAs class, aqueous solutions of surface-active ILs were then tested in the extraction of TTAs from apple peels, allowing the simultaneous extraction of betulinic, oleanolic and ursolic acids. A total extraction yield of TTAs of 2.62 wt.% was obtained using the best identified conditions, overwhelming the total extraction yields of 2.48 wt.% obtained with chloroform and 1.37 wt.% with acetone (under the same conditions and determined in this work for comparison purposes). The results obtained clearly confirm that aqueous solutions of ILs are an improved alternative for the extraction of TTAs from biomass, representing a promising alternative over the commonly used volatile organic solvents.
Supplementary Material
Solubility of ursolic acid in aqueous solutions of ILs and conventional surfactants, CMC values of the studied surface-active ILs, pH values of the ILs aqueous solutions, extraction yields of TTAs from green apple peels, and chromatograms used for the TTAs identification and quantification.
Acknowledgment
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, POCI-01-0145-FEDER-007679 (FCT Ref. UID /CTM /50011/2013), and projects Multibiorefinery (POCI-01-0145-FEDER-016403) and ReStoragePear (POCI-01-0247-FEDER-017777), financed by national funds through the FCT/MEC and when appropriate co-financed by FEDER under the PT2020 Partnership Agreement. FCT/MEC is also acknowledged for the contract under Investigator FCT to C.S.R. Freire (IF/01407/2012). E.L.P. Faria acknowledges CNPq for the PhD grant (200908/2014-6). M.G. Freire acknowledges the European Research Council (ERC) for the starting grant ERC-2013-StG-337753.
References
- 1.Yahia EM, de la Rosa LA, Alvarez-Parrilla E, González-Aguilar GA. The Contribution of Fruit and Vegetable Consumption to Human Health. In: Alvarez-Parrilla E, González-Aguilar GA, editors. Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value, and Stability. L. A. de la Rosa; Oxford: 2009. pp. 3–51. [Google Scholar]
- 2.Buttriss J, Salter A, Wiseman H, Tucker G. Plant Foods and Health. In: Salter A, Wiseman H, Tucker G, editors. Phytonutrients. Wiley-Blackwell; Oxford: 2012. pp. 1–51. [Google Scholar]
- 3.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113:71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB. Plant-based foods and prevention of cardiovascular disease: an overview. Am J Clin Nutr. 2003;78:544S–551S. doi: 10.1093/ajcn/78.3.544S. [DOI] [PubMed] [Google Scholar]
- 5.Nestle M. Animal v. plant foods in human diets and health: is the historical record unequivocal? Proc Nutr Soc. 1999;58:211–218. doi: 10.1017/s0029665199000300. [DOI] [PubMed] [Google Scholar]
- 6.Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, da Silva Pinto M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br J Clin Pharmacol. 2013;75:588–602. doi: 10.1111/j.1365-2125.2012.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrman TM, Barlow DJ, Hylands PJ. Phytochemical databases of Chinese herbal constituents and bioactive plant compounds with known target specificities. J Chem Inf Model. 2007;47:254–263. doi: 10.1021/ci600288m. [DOI] [PubMed] [Google Scholar]
- 8.Hill RA, Connolly JD. Triterpenoids. Nat Prod Rep. 2013;30:1028–1065. doi: 10.1039/c3np70032a. [DOI] [PubMed] [Google Scholar]
- 9.Jäger S, Trojan H, Kopp T, Laszczyk MN, Scheffler A. Pentacyclic triterpene distribution in various plants - rich sources for a new group of multi-potent plant extracts. Molecules. 2009;14:2016–2031. doi: 10.3390/molecules14062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szakiel A, Pączkowski C, Pensec F, Bertsch C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem Rev. 2012;11:263–284. doi: 10.1007/s11101-012-9241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domingues R, Guerra AR, Duarte MF, Freire CSR, Neto CS, Silvestre AJD. Bioactive Triterpenic Acids: From Agroforestry Biomass Residues to Promising Therapeutic Tools. Mini Rev Org Chem. 2014;11:382–399. [Google Scholar]
- 12.Wolfe KL, Liu RH. Apple peels as a value-added food ingredient. J Agric Food Chem. 2003;51:1676–1683. doi: 10.1021/jf025916z. [DOI] [PubMed] [Google Scholar]
- 13.Belding RD, Blankenship SM, Young E, Leidy RB. Composition and variability of epicuticular waxes in apple cultivars. J Am Soc Hortic Sci. 1998;123:348–356. [Google Scholar]
- 14.Frighetto RTS, Welendorf RM, Nigro EN, Frighetto N, Siani AC. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008;106:767–771. [Google Scholar]
- 15.Ellgardt K. Triterpenes in apple cuticle of organically and IP cultivated apples. Bachelor project in the Dannish-Swedish Horticulture Programme. Sveriges Lantbruksuniversitet. 2006 Nov;:1652–1579. [Google Scholar]
- 16.FAO. Food and Agriculture Organization. [Accessed on March 2017]; at http://www.fao.org/statistics/databases/en/
- 17.Siani AC, Nakamura MJ, Dos Santos DS, Mazzei JL, do Nascimento AC, Valente LM. Efficiency and selectivity of triterpene acid extraction from decoctions and tinctures prepared from apple peels. Pharmacogn Mag. 2014;10:225–231. doi: 10.4103/0973-1296.133236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi H, Noshita T, Kidachi Y, Umetsu H, Hayashi M, Komiyama K, Funayama S, Ryoyama K. Isolation of Ursolic Acid from Apple Peels and Its Specific Efficacy as a Potent Antitumor Agent. J Health Sci. 2008;54:654–660. [Google Scholar]
- 19.O’Neil MJ. The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Stn. 2006:1699. [Google Scholar]
- 20.Welton T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem Rev. 1999;99:2071–2083. doi: 10.1021/cr980032t. [DOI] [PubMed] [Google Scholar]
- 21.Ventura SPM, Gonçalves AMM, Sintra T, Pereira JL, Gonçalves F, Coutinho JAP. Designing ionic liquids: the chemical structure role in the toxicity. Ecotoxicology. 2013;22:1–12. doi: 10.1007/s10646-012-0997-x. [DOI] [PubMed] [Google Scholar]
- 22.Holbrey JD, Rogers RD, Mantz RA, Trulove PC, Cocalia VA, Visser AE, Anderson JL, Anthony JL, Brennecke JF, Maginn EJ, Welton T, et al. Physicochemical Properties. In: Wasserscheid P, Welton T, editors. Ionic Liquids in Synthesis. Wiley-VCH Verlag GmbH & Co. KGaA; Germany: 2008. pp. 57–174. [Google Scholar]
- 23.Earle MJ, Seddon KR. Ionic liquids: Green solvents for the future. Pure Appl Chem. 2000;72:1391–1398. [Google Scholar]
- 24.Bogdanov MG. Ionic Liquids as Alternative Solvents for Extraction of Natural Products. In: Chemat F, Vian MA, editors. Green Chemistry and Sustainable Technology. Springer; Heidelberg: 2014. pp. 127–166. [Google Scholar]
- 25.Passos H, Freire MG, Coutinho JAP. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014;16:4786–4815. doi: 10.1039/C4GC00236A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cláudio AFM, Neves MN, Shimizi K, Lopes JNC, Freire MG, Coutinho JAP. The magic of aqueous solutions of ionic liquids: ionic liquids as a powerful class of catanionic hydrotropes. Green Chem. 2015;17:3948–3963. doi: 10.1039/C5GC00712G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cláudio AFM, Ferreira AM, Freire MG, Coutinho JAP. Enhanced extraction of caffeine from guarana seeds using aqueous solutions of ionic liquids. Green Chem. 2013;15:2002–2010. [Google Scholar]
- 28.Ressmann AK, Zirbs R, Pressler M, Gaertner P, Bica K. Surface-active ionic liquids for micellar extraction of piperine from black pepper. Z Naturforsch A. 2013;68:1129–1137. [Google Scholar]
- 29.Inoue T, Ebina H, Dong B, Zheng L. Electrical conductivity study on micelle formation of long-chain imidazolium ionic liquids in aqueous solution. J Colloid Interface Sci. 2007;314:236–241. doi: 10.1016/j.jcis.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 30.Ressmann AK, Strassl K, Gaertner P, Zhao B, Greiner L, Bica K. New aspects for biomass processing with ionic liquids: towards the isolation of pharmaceutically active betulin. Green Chem. 2012;14:940–944. [Google Scholar]
- 31.Lin H, Zhang Y, Han M, Yang L. Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason Sonochem. 2013;20:680–684. doi: 10.1016/j.ultsonch.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Łuczak J, Hupka J, Thöming J, Jungnickel C. Self-organization of imidazolium ionic liquids in aqueous solution. Colloid Surfaces A. 2008;329:125–133. [Google Scholar]
- 33.Royal Society of Chemistry. ChemSpider – The free chemical database. [Accessed on March 2017]; at http://www.chemspider.com/
- 34.Schneider P, Hosseiny SS, Szczotka M, Jordan V, Schlitter K. Rapid solubility determination of the triterpenes oleanolic acid and ursolic acid by UV-spectroscopy in different solvents. Phytochem Lett. 2009;2:85–87. [Google Scholar]
- 35.Fan JP, Kong T, Zhang L, Tong S, Tian ZY, Duan YH, Zhang XH. Solubilities of ursolic acid and oleanolic acid in four solvents from (283.2 to 329.7 K) J Chem Eng Data. 2011;56:2723–2725. [Google Scholar]
- 36.Zhang Y, Jayaprakasam B, Seeram NP, Olson LK, DeWitt D, Nair MG. Insulin Secretion and Cyclooxygenase Enzyme Inhibition by Cabernet Sauvignon Grape Skin Compounds. J Agric Food Chem. 2004;52:228–233. doi: 10.1021/jf034616u. [DOI] [PubMed] [Google Scholar]
- 37.Leide J, Hildebrandt U, Vogg G, Riederer M. The positional sterile (ps) mutation affects cuticular transpiration and wax biosynthesis of tomato fruits. J Plant Physiol. 2011;168:871–877. doi: 10.1016/j.jplph.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 38.Bianchi G, Murelli C, Vlahov G. Surface waxes from olive fruits. Phytochemistry. 1992;31:3503–3506. [Google Scholar]
- 39.Liu T, Sui X, Zhang R, Yang L, Zu Y, Zhang L, Zhang Y, Zhang Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J Chromatogr A. 2011;1218:8480–8489. doi: 10.1016/j.chroma.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 40.Guinda Á, Rada M, Delgado T, Gutiérrez-Adánez P, Castellano JM. Pentacyclic triterpenoids from olive fruit and leaf. J Agric Food Chem. 2010;58:9685–9691. doi: 10.1021/jf102039t. [DOI] [PubMed] [Google Scholar]
- 41.Ma C-M, Cai S-Q, Cui J-R, Wang R-Q, Tu P-F, Hattori M, Daneshtalab M. The cytotoxic activity of ursolic acid derivatives. Eur J Med Chem. 2005;40:582–589. doi: 10.1016/j.ejmech.2005.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.