Abstract
Introduction
The leukocyte adhesion cascade is important for the maintenance of homeostasis and the ability of immune cells to access sites of infection and inflammation. Despite much work identifying the molecular components of the cascade, and numerous simulations to predict the relationship between molecule density, identity, and adhesion, these relationships have not been measured experimentally.
Methods
Using surfaces functionalized with recombinant ICAM-1 and/or E-selectin along with immobilized SDF-1α, we used a flow chamber to measure rates of tethering, rolling and arrest of primary naïve human CD4+ T lymphocytes on different surface densities of ligand.
Results
Cells required a minimum level of ligand density to progress beyond tethering. E-selectin and ICAM-1 were found to have a synergistic relationship in promoting cell arrest. Surfaces with both ligands had the highest levels of arrest, while surfaces containing only E-selectin hindered the cell’s ability to progress beyond rolling. In contrast, surfaces of ICAM-1 allowed only tethering or arrest. Cells maintained constant rolling velocity and time to stop over large variations in surface density and composition. In addition, surface densities of only O(101) sites/µm2 allowed for rolling while surface densities of O(102) sites/µm2 promoted arrest, approximately equal to previously determined simulated values.
Conclusions
We have systematically and experimentally mapped out the state diagram of T cell adhesion under flow, directly demonstrating the quantitative requirements for each dynamic state of adhesion, and showing how multiple adhesion molecules can act in synergy to secure arrest.
Electronic supplementary material
The online version of this article (10.1007/s12195-018-0519-x) contains supplementary material, which is available to authorized users.
Keywords: E-selectin, ICAM-1, SDF-1α, CXCL12α, Flow chamber, Site density
Introduction
T cells must adhere in the vasculature to traffic into the lymphatics to perform immune surveillance and maintain homeostasis.12 In addition, T cells are often called upon to exit the bloodstream to enter the interstitial space in order to perform effector functions at sites of inflammation and injury.23 T cell adhesion is thought to follow the canonical leukocyte adhesion cascade where cells progress from tethering to rolling to firm adhesion on the endothelium as a prerequisite for transmigration.20,23 During tethering, cells within reactive distance of the endothelial layer begin to interact with P- and E-selectins, which begin to slow the cell from the free stream velocity and encourage closer contact between the cell and the endothelium.22 Specifically, E-selectins on the endothelial layer can interact with a number of receptors on the leukocyte, including PSGL-1, ESL-1, and CD44.15 During rolling, the cell forms and breaks multiple bonds with the P- and E-selectins and also partially activated integrins with their respective ligands.18,19 As the cell translates across the surface using these relatively weak adhesions, chemokine receptors on the cell scan the surface for the presence of activating chemokines.5 Upon chemokine receptor binding, the β2 integrins on the T cell, such as LFA-1, become fully activated and able to rapidly and strongly bind their cognate ligands, such as ICAM-1.4,14,17,26 The integrin-ligand interaction causes the firm arrest and activation of the T cell, which can then crawl.29 Finally, transmigration occurs after the cell has found an appropriate location between endothelial cells to enter the lymphatics or interstitial space.20,23
Despite extensive study on the individual molecular components of the adhesion cascade,1,27,32,33 there have been few experiments to measure how the combination of selectins, integrins, and chemokine affect the dynamics of tethering, rolling and firm arrest.31 Extensive computer simulations2,3,6,8 using Adhesive Dynamics and other techniques have been performed to predict the interrelationships between molecular type, density and the dynamics of adhesion. These results have been rendered into state diagrams, a consolidated representation in which one can map densities to dynamic states of adhesion.3,7 An interesting prediction of Adhesive Dynamics is that there would be a synergy between selectins and integrins in securing firm arrest.3 The idea is that by slowing down a cell, selectins enable slow reacting integrins to secure firm binding. This synergy was borne out in experiments using cell-free systems, combining selectin mediated-rolling and antibody-mediated firm arrest.9
Despite these predictions, there have been no reports an experimentally determined state diagram of adhesion, in which one has measured the effect of changing ligand density type or amount on the type of adhesion observed experimentally. Thus, this paper presents an experimentally determined state diagram of CD4+ T cells interacting with a surface containing recombinant E-selectin and ICAM-1, with the addition of the chemokine SDF-1α as shown in Fig. 1.
Figure 1.
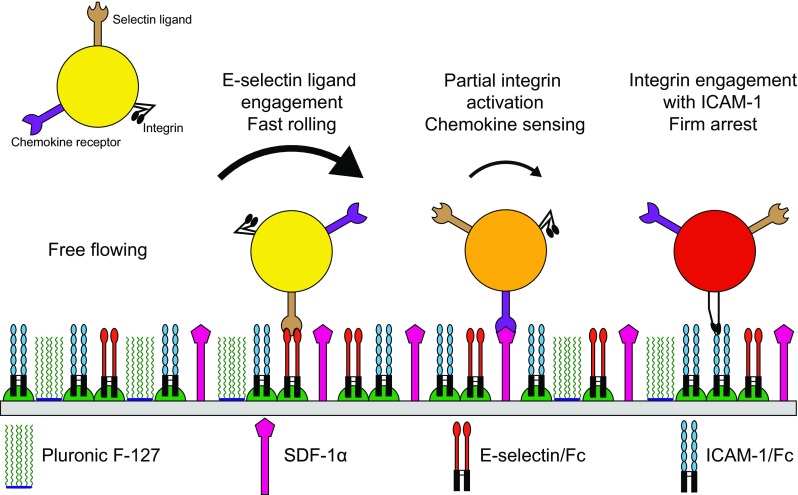
Schematic representation of T cell dynamic adhesion with a surface containing recombinant ICAM-1/Fc and E-selectin/Fc chimeras with SDF-1α.
Materials and Methods
Adsorption of Protein A/G and SDF-1α
Non-tissue culture treated polystyrene petri dishes (Corning, Corning, NY) were enclosed using a single well FlexiPerm gasket (Sigma-Aldrich, St. Loius, MO). A solution of 2 µg mL−1 of Protein A/G (Themo-Fisher Scientific, Waltham, MA) and 1 µg mL−1 of SDF-1α (R&D Systems, Minneapolis, MN) was applied and incubated overnight at 4 °C. The surfaces were then washed three times with PBS before a 30 min incubation of a 0.2% w/v solution of Pluronic F-127 (Sigma-Aldrich). The surfaces were washed again three times with PBS. Next, a solution of ICAM-1/Fc and/or E-selectin/Fc chimera (R&D Systems) was applied to the surface and incubated for 3 h at room temperature. The completed surfaces were then washed again three times with PBS before use. For experimentally tested points, molar ratios of 1:0, 10:1, 1:1, 1:10, and 0:1 E-selectin/Fc:ICAM-1/Fc were chosen to highlight differences between similar surfaces. In addition, total protein concentrations were chosen to yield total site densities of 4–5, 35–45, 130–160, 310–360, 550–610, and 1200–1300 sites/µm2.
CD4+ T Cells
Purified primary human CD4+ T cells from anonymized donors were obtained from the University of Pennsylvania Human Immunology Core. Cells were resuspended in RPMI-1640 media supplemented with 0.1% BSA and 2 mg mL−1 of glucose and used immediately.
Flow Chamber Assays
Flow chamber experiments were performed in a circular parallel plate flow chamber (GlycoTech, Gaithersburg, MD) with a gasket width of 0.25 cm, thickness of 127 µm, and length of 2 cm. Before flow chamber experiments, a functionalized dish was washed with prewarmed PBS and media to remove air bubbles in the flow path. The assembled flow chamber was mounted on an inverted Axiovert 200 (Carl Zeiss, Gottigen, Germany) enclosed by a XL-3 microscope incubator (PeCon, Ulm, Germany). All experiments were performed at 37 °C. T cells were suspended at a concentration of 5 × 105 mL−1 and were perfused into the flow chamber via syringe pump (Harvard Apparatus, Holliston, MA) at a flow rate corresponding to a calculated wall shear rate of 100 s−1. Rolling and adhesion of T cells on the immobilized ICAM-1 and E-selectin was observed via phase contrast microscopy under a 10× objective (NA = 0.2, Type A-Plan, Carl Zeiss). All points were tested twice on three different days with different anonymized donors and the results averaged.
Data Acquisition and Cell Tracking
A CCD camera (QImaging, Surrey, BC, Canada) was used to monitor T cell adhesion events with adhesive E-selectin/ICAM-1/SDF-1α substrates. Adhesion of T cells was recorded on DVD + RW discs for cell tracking analyses. Cell adhesion videos were redigitized to 640 × 480 pixels at 29.97 frames s−1 and deinterlaced with HandBrake software (http://handbrake.fr/) then converted to image stacks with MATLAB (MathWorks, Natick, MA). The stacked images were thresholded and converted to binary images. The coordinates of the centroid of interacting T lymphocytes with a surface every video frame were then acquired using the MTrack2 plugin (http://valelab.ucsf.edu/~nico/IJplugins/MTrack2.html) in the ImageJ program (http://imagej.nih.gov/ij; National Institutes of Health).
Analysis of Cellular Adhesion
MATLAB was used to analyze the tracked centroids obtained from the MTrack2 plugin. State diagrams were created in MATLAB using the Curve Fitting Toolbox. Surfaces were fit to experimental points with linear interpolation between points. Rolling was defined as the movement of an interacting cell over the substrate at an instantaneous velocity in any frame less than 20% of the calculated free stream velocity of a noninteracting cell13 while remaining in the field of view. Cell stopping was defined as reduction of its instantaneous velocity below 5% of the free stream velocity. Adherent T lymphocytes were further classified as tethering, rolling or firmly arrested cells based on their instantaneous velocities and the duration of their stopping. A tethering cell is defined as a cell that “rolls” less than 30 video frames (1 s) in a rolling period within the field of view. A firmly arrested cell is defined as a cell that “stops” stably more than 90 frames (3 s). Time and distance to stop were both calculated based on the initial time and location of the cell rolling. Values for figures were calculated based on cells that interacted with the surface. Cells which were tracked but did not interact with the surface were excluded from the analysis. A single flow chamber experiment resulted in 200–250 cells interacting with the surface on surfaces containing O(102) or more sites/µm2 to around 10 cells on surfaces containing O(100) sites/µm2. The number of cells interacting with the surface was also somewhat dependent on surface composition. To highlight differences in relative rates of adhesion, data are presented as fraction of cells undergoing a specific kind of motion.
Determination of Site Densities
A solution of 2 µg mL−1 of Protein A/G (Themo-Fisher Scientific) and 1 µg mL−1 of SDF-1α (R&D Systems) was added to the wells of a non-tissue culture treated 96 well polystyrene plate (Corning). This solution was allowed to coat the wells overnight at 4 °C. The wells were then washed three times with PBS before blocking with 0.2% Pluronic F127 (Sigma-Aldrich) for 30 min. The wells were again washed three times with PBS and then serial dilutions of human IgG1 (Invitrogen, Carlsbad, CA) were added and allowed to incubate for 3 h. Wells were washed three times with PBS and remaining binding sites on Protein A/G were blocked using 50 µg mL−1 murine IgG1 (Invitrogen) for 2 h at 37 °C. Next, an AlexaFluor 488-tagged anti-human IgG hinge antibody (Southern Biotech, Birmingham, AL) at 2.5 µg mL−1 was added to the wells and allowed to bind for 1 h at RT in the dark. After the incubation, wells were washed three times with PBS. Wells were then filled with PBS and fluorescence compared to dilutions of fresh AlexFluor-tagged antibody using a Tecan M200 Infinite plate reader. Fluorescence readings from the IgG1 treated wells were converted to an equivalent concentration and then into number of molecules per well, assuming two AlexaFluor 488 antibodies per IgG1 molecule, due to the two hinge regions available for binding and molar excess of antibody. The resulting number concentration was converted to a surface density by dividing by the wetted area of the well. This was performed on three different days and the results averaged. To determine site densities of the experimental conditions, the experimentally determined site densities were fitted to a four parameter logistic regression using R (R Core Team) and the drc package. Site densities are reported as monomer molecules per µm2 in order to facilitate comparison to previously reported studies.
Results
Determination of Site Densities
As described in the Materials and Methods section, we determined the site densities of surfaces using an isotype-matched IgG1 as a probe. The results of these experiments are shown in Supplementary Fig. 1. When using chimeric molecules, it was assumed that the chimeras had the same binding affinity with Protein A/G as the IgG1. In the case of mixtures of two different chimeras, it was assumed that they partitioned onto the surface at the same ratio as their molar ratio in the bulk. To confirm this assumption, diffusivities for each of the chimeras were calculated according to the procedure in Young, et al.,34 which showed a difference in diffusivities of only 5%. In addition, since both chimeras have the same Fc domain, we assumed that the resulting KD’s for binding protein A/G were the same. When plotting the state diagrams, the reported densities are derived from these calibration curves. This system allows us to study E-selectin concentrations above and below levels provided by stimulated HUVECs (100–150 sites/µm2).16 In addition, the density can reach levels roughly equivalent to activated HUVEC expression of ICAM-1 (1000–2000 sites/µm2).21,28,30
Flow Chamber Adhesion Experiments
We conducted a number of flow chamber experiments using surfaces functionalized with ICAM-1, E-selectin, and SDF-1α. These surfaces allowed for visualization of three states of T cell adhesion—tethering, rolling, and firm arrest. Because we could specifically vary the density and composition of the surfaces, we could probe the effects of different surface densities on the types of adhesion observed. For each combination of densities of ICAM-1 and E-selectin, along with a fixed concentration of SDF-1α, we mapped the fraction of cells displaying each kind of motion, as shown in Fig. 2. Surfaces containing ICAM-1 but lacking SDF-1α did not show significant levels of arrest, with fewer than 7% of cells arresting on these surfaces (data not shown). The presence or absence of SDF-1α did not change the cellular response to surfaces containing only E-selectin (data not shown). An additional set of experiments were carried out at a much higher ligand density, but these did not show any significant change from the points presented here (Supplementary Fig. 2).
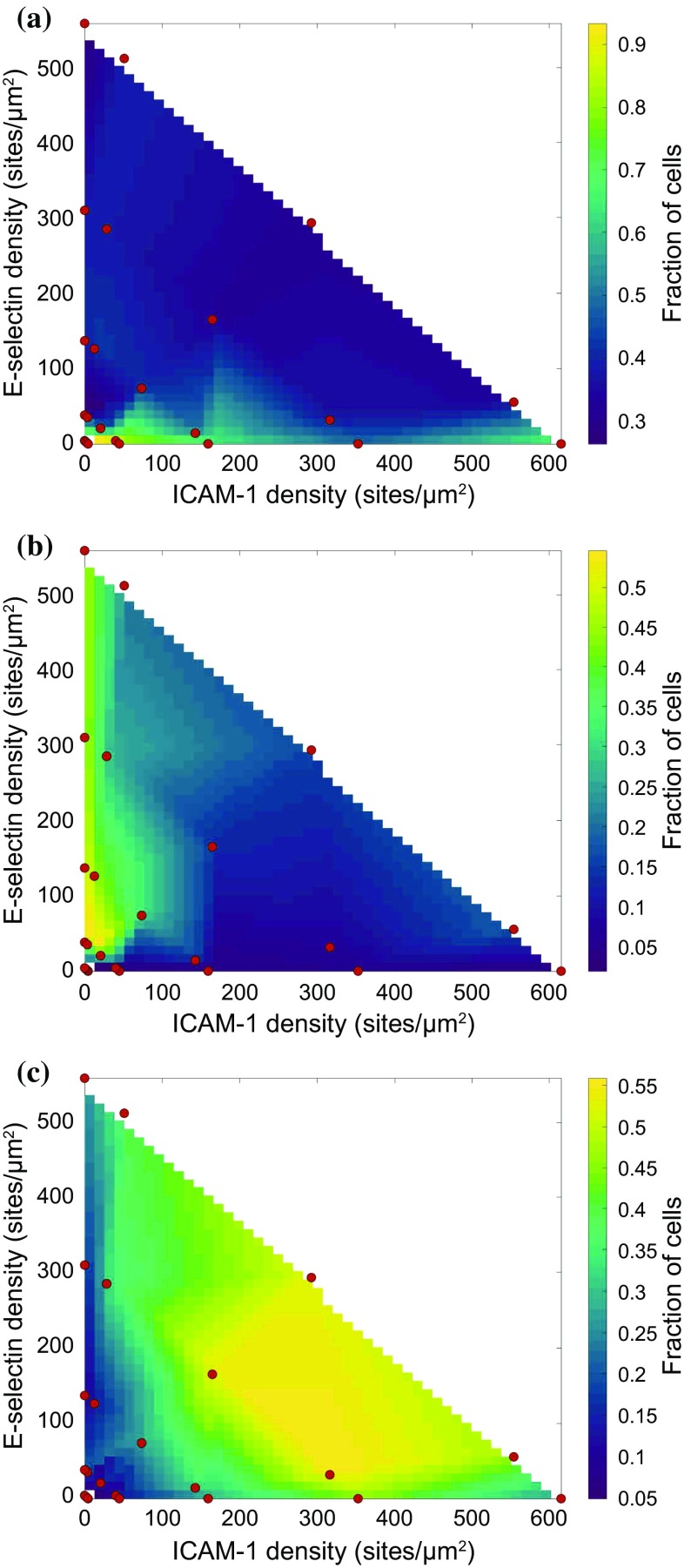
Figure 2.
State diagrams of the fraction of cells undergoing (a) tethering, (b) rolling, or (c) firm arrest. Red dots indicate experimentally tested points. All experiments were performed at a calculated wall shear rate of 100 s−1.
Tethering
Surfaces presenting low densities of molecules (O(100) sites/µm2) allowed only tethering. Overall, the rate of tethering decreased as surfaces contained increasing surface densities of ligand. In addition, surfaces containing only ICAM-1 showed higher rates of tethering at all densities compared to surfaces with similar levels of ICAM-1 paired with E-selectin.
Rolling
Rolling required a surface density of O(101) sites/µm2. Surfaces displaying only E-selectin exhibited the highest levels of rolling at all surface densities, due to a decrease in the level of firm arrest. T cells proved to be largely unable to roll on surfaces presenting only ICAM-1, with less than 5% of cells rolling during their interaction with the surface, compared to 40–50% of cells on surfaces with E-selectin.
Arrest
Surfaces containing both ICAM-1 and E-selectin at sufficient densities (O(102) sites/µm2) were found to be the most efficient at allowing cells to firmly adhere. Surfaces containing an equal density of ICAM-1 and E-selectin as well as surfaces with a tenfold higher density of ICAM-1 compared to E-selectin were the most efficient at causing the arrest of cells. Surfaces containing a tenfold higher density of E-selectin compared to ICAM-1 had a modestly lower level of arrest, with a concomitant increase in the level of rolling on these surfaces. Surfaces containing only E-selectin did not support robust levels of arrest. In addition, when cells did arrest, surfaces containing only E-selectin did not support significant levels of cell spreading in comparison to surfaces that also contained ICAM-1, as shown in Supplementary Fig. 3.
Synergy of Arrest
As seen in the state diagrams, reductions in the level of one of the ligands could be rescued by an increase in the level of the other ligand. This indicates the presence of synergy between the molecules. Surfaces with more E-selectin than ICAM-1 showed slight reductions in the level of firm arrest, but increases in ICAM-1 density fully rescued reductions in the level of E-selectin. However, fully removing one of the ligands reduced the level of firm adhesion on the surface, regardless of the remaining ligand density.
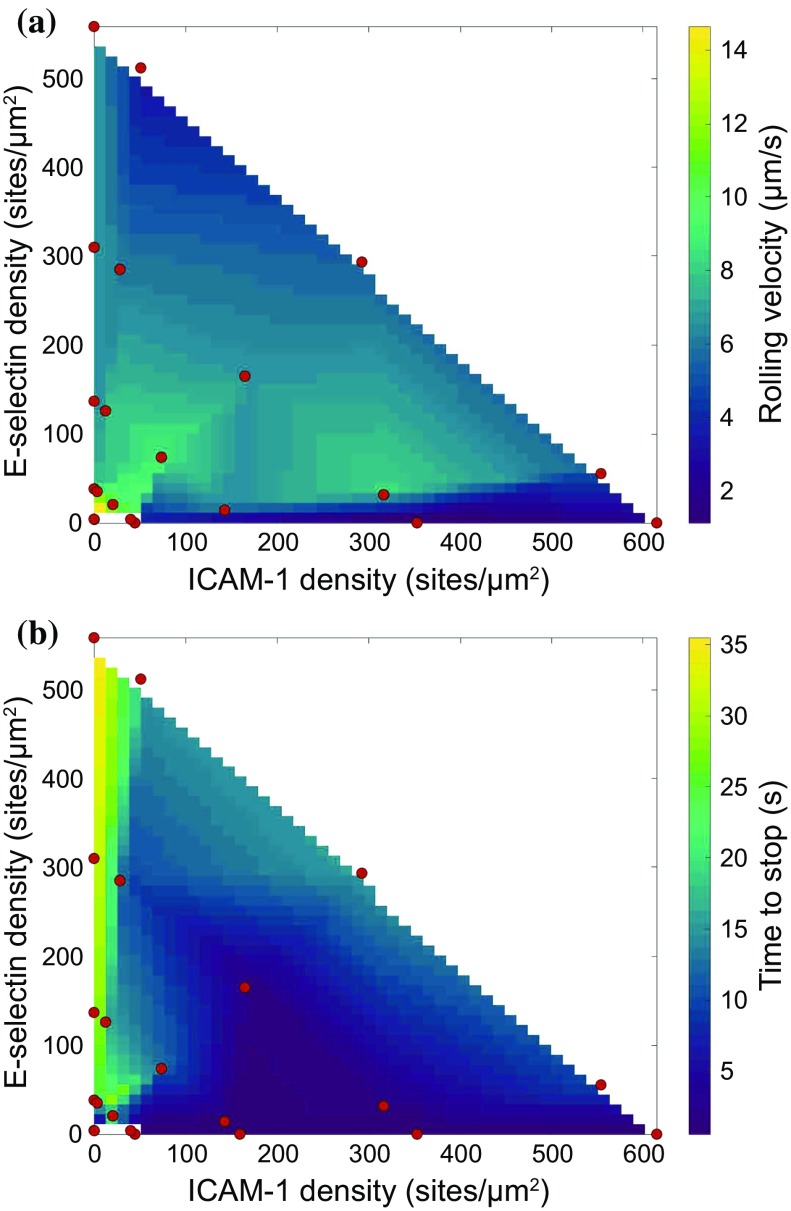
Rolling Velocity
In addition to types of adhesion, we measured the rolling velocity and time to stop, as shown in Fig. 3. Surprisingly, the rolling velocity of the naïve CD4+ T cells was nearly constant across large changes in surface density and surface composition. The two exceptions were on surfaces containing very low surface densities of molecules, where cells rolled much faster, and on surfaces consisting of only ICAM-1, where the very few cells rolled (typically fewer than five cells per flow chamber). These few cells had a lower rolling velocity compared to other cells, but not enough cells were in this category to make a definitive determination.
Figure 3.
Calculated surfaces showing the effect of ICAM-1 and E-selectin densities on (a) rolling velocity and (b) time to stop. Red dots indicate experimentally tested points.
Time to Stop
A metric of the strength of arrest is the time to stop for rolling cells to stop—that is, how long they roll until arrest. Cells which did not stop were excluded from this metric. Cells on surfaces containing only E-selectin rolled a very long time before they stopped, if at all. However, as the amount of ICAM-1 on the surface increased, the time for cells to stop showed a concomitant decrease. The shortest time to stop occurred on surfaces containing only ICAM-1, on which cells stopped extremely rapidly when compared to other surfaces. An additional metric, distance to stop (a convolution of rolling velocity and time to stop), is shown in Supplementary Fig. 4.
Discussion and Conclusion
In this work, we present the first experimentally determined state diagram for the adhesion of naïve CD4+ T cells on varying densities of ICAM-1 and E-selectin.
Synergy
Our results demonstrate the presence of a synergy between ICAM-1 and E-selectin, as predicted by simulation,3 as both ligands were required for the highest levels of arrest on the surfaces. In addition, reductions in the level of one ligand were compensated by increases in the density of the other, thus maintaining an overall constant density. In contrast, surfaces containing only E-selectin were less efficient at causing cells to arrest, perhaps due to the fast on–off rates of selectins and their ligands,1,7,10,11 coupled with the fact that the bonds are relatively weak.22 Consistent with numerous other reports, LFA-1/ICAM-1 interactions are needed for firm arrest.25,26 Surfaces displaying only ICAM-1 could support low levels of arrest, but the level of arrest was greatly enhanced by the presence of E-selectin interactions. Because the ICAM-1 only surfaces had a higher rate of tethering and a very low rate of rolling, we suggest that in this system, cells on an ICAM-1 only surface have a binary state space. In the brief time that these cells are initially interacting with the surface, either the requisite number of bonds for firm arrest is reached and the cell stops, or it is not achieved and the bonds that have formed are broken due to the shear force, thus resulting in a tethering event. With the addition of E-selectin, the cells interact with the surface for a longer period of time and are more able to form enough bonds to resist the shear forces applied.
Rolling Velocity
We measured the velocity of cells rolling on various surfaces. We found that rolling velocity of these cells was nearly constant across a large variety of surface densities and compositions, provided some amount of E-selectin was present. However, surfaces displaying solely ICAM-1 supported a much lower rolling velocity than surfaces containing ICAM-1 and E-selectin. ICAM-1-only surfaces, as mentioned previously, did not support robust levels of rolling, It has been shown previously that neutrophils, another type of leukocyte, can use LFA-1/ICAM-1 interactions to support rolling on surfaces with P-selectin.24,27 However, we did not see a significant difference in the rolling velocity between surfaces containing only E-selectin and surfaces with both ligands. We did not see these “slow rolling” interactions in our system, as the rolling velocity of cells on surfaces with ICAM-1 and E-selectin was comparable to surfaces containing E-selectin only. Our experiments do not allow us to determine whether E-selectin does not support the intracellular signaling required for slow rolling or if naïve human T cells do not support this functionality.
Time to Stop
We also tracked the time to stop of the cells, which was the difference in time between when cells initiated rolling and when they became arrested. Once again, the cells showed a surprising consistency in the time to stop across a wide range of surface densities and compositions. However, surfaces containing only ICAM-1 or E-selectin had divergent responses. For cells on surfaces containing only E-selectin, the time to stop was much higher than similar surfaces to which ICAM-1 was added. This once again highlights the importance of ICAM-1 in mediating the transition from rolling to firm arrest. In contrast, cells interacting with surfaces of only ICAM-1 stopped essentially immediately upon contact. This result highlights the binary state space that ICAM-1-only surfaces create.
In summary, we show that surfaces combining ICAM-1 and E-selectin allow for the most efficient arrest. This indicates the presence of synergy between these two molecules for the purposes of completing the leukocyte adhesion cascade. Surfaces containing only one of these molecules were not as efficient, but still managed a basal level of adhesion. These results point out that the bonds between E-selectin and its sialyated and fucosylated T-cell surface ligands and between LFA-1 and ICAM-1 are biomechanically different and tuned to support, by themselves, different dynamics of adhesion. Like many other adhesion systems,9,19,27 nature has evolved a system in which multiple molecules act in synergy to support robust and multi-level control of the dynamics of adhesion, and that one cannot compensate for mechanochemical deficiencies by increasing molecular density. In addition, our results can provide important information for the verification of computer models of cell rolling and adhesion, such as Adhesive Dynamics, especially regarding the effect of changing ligand identity and density.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (EPS 446 kb). Supplementary Fig. 1: Determination of site densities using an IgG1 probe. Protein A/G and SDF-1α were co-immobilized in polystyrene microwells and incubated with IgG1. Site density was determined by comparing to fresh dilutions of an AlexaFluor 488-tagged anti-human IgG1 hinge antibody. Results shown are mean ± SE of three independent experiments
Supplementary material 2 (EPS 4524 kb). Supplementary Fig. 2: Extended state diagrams of the fraction of cells undergoing (A) tethering, (B) rolling, or (C) firm arrest, including surfaces with high site densities. Red dots indicate experimentally tested points. All experiments were performed at a calculated wall shear rate of 100 s−1
Supplementary material 3 (EPS 2541 kb). Supplementary Fig. 3: Comparison between cell spreading after arrest on (A) E-selectin only surfaces and (B) surfaces with E-selectin and ICAM-1. Both images are the end of 10 min flow chamber experiments and are representative of repeated experiments. Orange arrowheads point to arrested cells that are not spread and green arrowheads identify arrested cells that have spread. Surfaces containing only E-selectin show fewer arrested cells than surfaces containing both E-selectin and ICAM-1. Cells arrested on E-selectin surfaces also did not spread efficiently, shown by the fewer cells marked with green. Cells not marked by arrowheads are rolling or tethering during the moment this frame was taken
Supplementary material 4 (EPS 1792 kb). Supplementary Fig. 4: Calculated surface showing the effect of ICAM-1 and E-selectin densities on the distance to stop. Red dots indicate experimentally tested points. All experiments were performed at a calculated wall shear rate of 100 s−1
Supplementary material 5 (EPS 999 kb). Supplementary Fig. 5: Comparison of adhesion on surfaces containing and lacking SDF-1α. Surfaces were functionalized with a 1:1 molar mixture of E-selectin/Fc:ICAM-1/Fc chimeras at an overall site density of 1250 sites/µm2 with and without SDF-1α. Surfaces were compared for their ability to support tethering, rolling, and arrest. Surfaces with SDF-1α showed a rate of arrest approximately 2.5 times higher than surfaces lacking SDF-1α, highlighting the importance of chemokine to cause arrest. Results are the average from three different experiments consisting of duplicate surfaces.
Acknowledgments
This work was supported by National Institutes of Health Grants to DAH AI082292 and GM123019.
Conflicts of interest
Nicholas R. Anderson, Dooyoung Lee, and Daniel A. Hammer declare that they have no conflicts of interest.
Ethical Standards
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
References
- 1.Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 2.Beste MT, Hammer DA. Selectin catch-slip kinetics encode shear threshold adhesive behavior of rolling leukocytes. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20716–20721. doi: 10.1073/pnas.0808213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia SK, King MR, Hammer DA. The state diagram for cell adhesion mediated by two receptors. Biophys. J. Elsevier. 2003;84:2671–2690. doi: 10.1016/S0006-3495(03)75073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolomini-Vittori M, et al. Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nat. Immunol. 2009;10:185–194. doi: 10.1038/ni.1691. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 6.Caputo KE, Lee D, King MR, Hammer DA. Adhesive dynamics simulations of the shear threshold effect for leukocytes. Biophys. J. 2007;92:787–797. doi: 10.1529/biophysj.106.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang K-C, Hammer DA. Adhesive dynamics simulations of sialyl-Lewis(x)/E-selectin-mediated rolling in a cell-free system. Biophys. J. 2000;79:1891–1902. doi: 10.1016/S0006-3495(00)76439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang K-C, Tees DFJ, Hammer DA. The state diagram for cell adhesion under flow: leukocyte rolling and firm adhesion. Proc. Natl. Acad. Sci. 2000;97:11262–11267. doi: 10.1073/pnas.200240897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eniola AO, Willcox PJ, Hammer DA. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys. J. 2003;85:2720–2731. doi: 10.1016/S0006-3495(03)74695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans E, Leung A, Hammer DA, Simon SI. Chemically distinct transition states govern rapid dissociation of single L-selectin bonds under force. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3784–3789. doi: 10.1073/pnas.061324998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans E, Leung A, Heinrich V, Zhu C. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11281–11286. doi: 10.1073/pnas.0401870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girard J-P, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 13.Goldman AJ, Cox RG, Brenner H. Slow viscous motion of a sphere parallel to a plane wall—II Couette flow. Chem. Eng. Sci. 1967;22:653–660. doi: 10.1016/0009-2509(67)80048-4. [DOI] [Google Scholar]
- 14.Herter J, Zarbock A. Integrin regulation during leukocyte recruitment. J. Immunol. 2013;190:4451–4457. doi: 10.4049/jimmunol.1203179. [DOI] [PubMed] [Google Scholar]
- 15.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang RB, Eniola-Adefeso O. Shear stress modulation of IL-1β-induced E-selectin expression in human endothelial cells. PLoS ONE. 2012;7:1–9. doi: 10.1371/annotation/e4e8ca0c-f6e8-4b32-aae1-b5f8e0c7ebc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin αLβ2. J. Cell Biol. 2004;167:1241–1253. doi: 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwano Y, Spelten O, Zhang H, Ley K, Zarbock A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood. 2010;116:617–624. doi: 10.1182/blood-2010-01-266122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-D. [DOI] [PubMed] [Google Scholar]
- 20.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 21.McCormick CJ, Craig A, Roberts D, Newbold CI, Berendt AR. Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J. Clin. Invest. 1997;100:2521–2529. doi: 10.1172/JCI119794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 2015;107:331–339. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41:694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Salas A, Shimaoka M, Kogan AN, Harwood C, Von Andrian UH, Springer TA. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity. 2004;20:393–406. doi: 10.1016/S1074-7613(04)00082-2. [DOI] [PubMed] [Google Scholar]
- 25.San Lek H, et al. The spontaneously adhesive leukocyte function-associated antigen-1 (LFA-1) integrin in effector T cells mediates rapid actin- and calmodulin-dependent adhesion strengthening to ligand under shear flow. J. Biol. Chem. 2013;288:14698–14708. doi: 10.1074/jbc.M112.430918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shamri R, et al. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat. Immunol. 2005;6:497–506. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 27.Shao B, et al. Signal-dependent slow leukocyte rolling does not require cytoskeletal anchorage of P-selectin glycoprotein ligand-1 (PSGL-1) or integrin αLβ2. J. Biol. Chem. 2012;287:19585–19598. doi: 10.1074/jbc.M112.361519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao W, et al. AFM investigation on Ox-LDL-induced changes in cell spreading and cell-surface adhesion property of endothelial cells. Scanning. 2013;35:119–126. doi: 10.1002/sca.21040. [DOI] [PubMed] [Google Scholar]
- 29.Shulman Z, et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon SI, Goldsmith HL. Leukocyte adhesion dynamics in shear flow. Ann. Biomed. Eng. 2002;30:315–332. doi: 10.1114/1.1467677. [DOI] [PubMed] [Google Scholar]
- 31.Sundd P, Pospieszalska MK, Cheung LSL, Konstantopoulos K, Ley K. Biomechanics of leukocyte rolling. Biorheology. 2011;48:1–35. doi: 10.3233/BIR-2011-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright RD, Cooper D. Glycobiology of leukocyte trafficking in inflammation. Glycobiology. 2014;24:1242–1251. doi: 10.1093/glycob/cwu101. [DOI] [PubMed] [Google Scholar]
- 33.Yago T, et al. Blocking neutrophil integrin activation prevents ischemia–reperfusion injury. J. Exp. Med. 2015;212:1267–1281. doi: 10.1084/jem.20142358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young ME, Carroad PA, Bell RL. Estimation of diffusion coefficients of proteins. Biothechnol. Bioeng. 1980;22(5):947–955. doi: 10.1002/bit.260220504. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (EPS 446 kb). Supplementary Fig. 1: Determination of site densities using an IgG1 probe. Protein A/G and SDF-1α were co-immobilized in polystyrene microwells and incubated with IgG1. Site density was determined by comparing to fresh dilutions of an AlexaFluor 488-tagged anti-human IgG1 hinge antibody. Results shown are mean ± SE of three independent experiments
Supplementary material 2 (EPS 4524 kb). Supplementary Fig. 2: Extended state diagrams of the fraction of cells undergoing (A) tethering, (B) rolling, or (C) firm arrest, including surfaces with high site densities. Red dots indicate experimentally tested points. All experiments were performed at a calculated wall shear rate of 100 s−1
Supplementary material 3 (EPS 2541 kb). Supplementary Fig. 3: Comparison between cell spreading after arrest on (A) E-selectin only surfaces and (B) surfaces with E-selectin and ICAM-1. Both images are the end of 10 min flow chamber experiments and are representative of repeated experiments. Orange arrowheads point to arrested cells that are not spread and green arrowheads identify arrested cells that have spread. Surfaces containing only E-selectin show fewer arrested cells than surfaces containing both E-selectin and ICAM-1. Cells arrested on E-selectin surfaces also did not spread efficiently, shown by the fewer cells marked with green. Cells not marked by arrowheads are rolling or tethering during the moment this frame was taken
Supplementary material 4 (EPS 1792 kb). Supplementary Fig. 4: Calculated surface showing the effect of ICAM-1 and E-selectin densities on the distance to stop. Red dots indicate experimentally tested points. All experiments were performed at a calculated wall shear rate of 100 s−1
Supplementary material 5 (EPS 999 kb). Supplementary Fig. 5: Comparison of adhesion on surfaces containing and lacking SDF-1α. Surfaces were functionalized with a 1:1 molar mixture of E-selectin/Fc:ICAM-1/Fc chimeras at an overall site density of 1250 sites/µm2 with and without SDF-1α. Surfaces were compared for their ability to support tethering, rolling, and arrest. Surfaces with SDF-1α showed a rate of arrest approximately 2.5 times higher than surfaces lacking SDF-1α, highlighting the importance of chemokine to cause arrest. Results are the average from three different experiments consisting of duplicate surfaces.