Abstract
Background
Survivors of sexual assault are vulnerable to long-term negative psychological and physical health outcomes, but few studies have investigated changes in cognition, emotional processing and brain function in the early stages after sexual assault. We used a multimodal approach to identify the cognitive and emotional correlates associated with sexual assault in women.
Methods
Twenty-seven female survivors of sexual assault were included within 4 weeks of the traumatic event, and they were compared with 20 age-matched controls. Participants underwent functional MRI while performing cognitive/emotional tasks (n-back, emotional go/no-go, mental imagery). We also measured diurnal salivary cortisol and conducted neuropsychological assessments of attention and memory abilities.
Results
Relative to the control group, the survivor group had lower levels of morning cortisol and showed attentional deficits. We observed no between-group differences in brain activation during the n-back or mental imagery tasks. During the emotional go/no-go task, however, the survivor group showed a lack of deactivation in the dorsal anterior cingulate cortex when processing emotional material, relative to neutral material. Exploratory analyses in the survivor group indicated that symptom severity was negatively associated with cerebellar activation when positive emotional (happy) content interfered with response inhibition, and positively associated with cerebellar activation when thinking of positive (happy) memories.
Limitations
The small sample size was the main limitation of this study.
Conclusion
Dysfunctions in the dorsal anterior cingulate cortex and the cerebellum may represent early functional brain modifications that alter higher cognitive processes when emotional material is involved.
Introduction
In France, sexual assault occurs at an alarming lifetime frequency of 16% in women and 5% in men.1 Similarly, data from the United States Department of Justice show that American women have about a 20% chance of exposure to sexual assault in their lifetime, although 54% of the estimated 300 000 to 1.3 million women raped every year in the United States do not report it.2 Sexual assault is associated with devastating psychological and physical health outcomes.3–10 Trauma survivors are vulnerable to emotional and behavioural symptoms such as hyper-arousal and intrusive trauma-related memories or thoughts, and they are at high risk of engaging in behaviours such as substance use.11,12 However, the early cognitive, emotional and biological modifications following trauma exposure are under-studied and remain unclear. This study aimed to identify the early neuropsychological, neuroendocrine and neural modifications that occur in women who experience sexual assault.
Acute responses to trauma (typically within a month after exposure) vary depending on the nature of the traumatic event. In healthy populations, including women who have been sexually assaulted but did not develop psychopathological traits,13 exposure to an acute experimental stressor (including psychosocial or physical stresses) has been associated with deficits in working memory14–17 and cognitive flexibility,18 and with aberrant dorsolateral prefrontal cortex (DLPFC) function.19–21 Similar deficits in higher cognitive function (such as working memory, attention or executive function, including cognitive flexibility or response inhibition)22,23 are also common features of acute24 and chronic posttraumatic stress disorder (PTSD).22,25
In addition to cognitive deficits, PTSD has been associated with deficits in emotional processing.26 Increased activation in frontal (ventromedial prefrontal cortex [vmPFC], DLPFC), posterior cingulate cortex/precuneus and sensory areas (occipital, temporal) are evident when patients with acute stress disorder or acute PTSD are exposed to personalized trauma-related recall or pictures,27–30 or when they appraise fearful faces.31 In contrast, when women with chronic sexual assault–related PTSD performed an n-back working memory task, researchers found no association between trauma-related material and changes in brain function. 32 A recent meta-analysis of functional neuroimaging studies indicated a critical role for the posterior cingulate cortex/precuneus, anterior cingulate cortex and angular gyrus when processing trauma-related stimulation.33 Deficits in retrieving specific autobiographical memories (a common feature of acutely traumatized populations24,34) were associated with increased activation in the amygdala and vmPFC and increased connectivity between these regions when patients with chronic PTSD retrieved negative memories.35 In contrast, retrieving positive memories was associated with decreased amygdala and vmPFC activation and decreased connectivity between these regions.35 The regions involved in cognition, autobiographical memory and emotional processing — including the DLPFC, vmPFC, anterior cingulate cortex, middle occipital cortex, left inferior parietal lobule, insula and regions from the medial temporal lobe (amygdala, hippocampus, parahippocampal gyrus, entorhinal gyrus) — are also activated during intrusive memories and are critical for mental imagery processes (see Clark and Mackay36 for a review). Although there is accumulating evidence for deficits in cognitive function, emotional processing, autobiographical memory and mental imagery processes in PTSD, along with brain-function alterations in overlapping regions across these dimensions, the neural correlates associated with these deficits in acutely traumatized individuals — particularly women who have been sexually assaulted — are unclear and under-studied.
Finally, exposure to acute stress activates the hypothalamic–pituitary–adrenal axis, resulting in the production of glucocorticoids (cortisol) in the adrenal gland, released into the blood. The hypothalamic–pituitary–adrenal axis is critical for adequate stress response and maintaining homeostasis.37 Patients with PTSD generally present with blunted levels of cortisol38,39; however, because cortisol concentrations are also affected by factors such as age, sex, type of trauma, time of collection and time since trauma, these findings are inconsistent.38,39 Notably, while trauma-exposed individuals (independent of sex) have shown lower daily cortisol output, 40 women with PTSD have shown elevated afternoon cortisol concentrations.38,39 However, despite this evidence, early variations in diurnal cortisol concentration in women exposed to sexual assault are not well characterized.
This study used a multimodal approach to identify the early cognitive, emotional and biological modifications associated with sexual assault in women. We recruited women exposed to sexual assault, along with nonexposed controls, and participants performed neuropsychological assessments (working memory and attention), provided diurnal salivary cortisol samples and underwent functional brain imaging. We expected that exposure to sexual assault would be associated with decreased cognitive performance as well as blunted morning and elevated afternoon cortisol concentrations. We also expected to observe decreased activation in the DLPFC and anterior cingulate cortex during working memory, response inhibition (emotional go/no-go) and mental imagery tasks, along with increased activation in the amygdala, the vmPFC and the posterior cingulate cortex/precuneus during the mental imagery task and emotional processing (emotional go/no-go).
Methods
Ethics
This study was approved by an independent research ethics committee (CPP Tours Ouest-1, France; 2010-R36). The project was registered on the ClinicalTrials.gov website (NCT01405495) and was supervised by a clinical investigation monitoring committee (Inserm CIC1415). All patients consented to participate after they were informed of the study’s purpose. Participants received €200 for completing the study.
Participants
In collaboration with several sexual assault referral centres and the University Hospital of Tours, France, 102 survivors of sexual assault were asked to participate in the study. Of those, 50 refused to participate and 25 reported 1 or more exclusion criteria: history of head injury, substance use, claustrophobia, current use of a psychotropic medication for more than 21 days, medical disorders affecting brain function (e.g., epilepsy, tumour), and MRI contraindications. Twenty-seven participants were included within 4 weeks of experiencing a sexual assault. We also recruited 20 age-matched healthy controls with no history of sexual assault from the general population. To ensure a sufficient number of participants with posttraumatic symptoms at a follow-up visit 6 months later (not reported in this article), we recruited more survivors than controls. A trained psychiatrist (W.E.) interviewed all participants. We used the Mini International Neuropsychiatric Interview41 to screen the control group for the presence of any neuropsychiatric disorder, and we used the Clinician-Administered PTSD Scale (CAPS)42 to assess symptom severity among the survivors. We assessed comorbid depressive symptoms using the 13-item version of the Beck Depression Inventory43 and anxiety levels using the State-Trait Anxiety Inventory. 44 Clinical interviews, neuropsychological assessments, cortisol collection and imaging acquisitions were all performed on the same day.
Neuropsychological assessment
We tested visual attention and task switching using a computerized version of the Trail-Making Test (TMT, http://pebl.sourceforge.net/wiki/index.php/PEBL_Trail-making_task).45,46 The task required the participant to connect consecutive targets. In part A, the targets are all numbers, and the participant had to connect them in sequential order (e.g., 1, 2, 3, …); in part B, the participant alternated between numbers and letters (e.g., 1, A, 2, B, …). In addition to accuracy and reaction to both the TMT-A and TMT-B, we derived an index of executive functioning from the differences between each part of the test (TMT-B minus TMT-A) for both accuracy and time to perform the task.47
We evaluated attentional abilities using 2 subtests of the Tests of Attentional Performance (TAP) battery.48 We tested the flexibility of focused attention by having participants alternate between 2 sets of targets, including verbal (letters and numbers) and nonverbal (angular and round shapes) stimuli. Two stimuli, 1 from each set, were presented simultaneously and randomly on the left or the right side of the fixation point. From one presentation to the next, the target changed from letters/numbers to angular/round shapes, and vice versa. The participants pressed a key corresponding to the side of the target (left or right) as quickly as possible. Reaction times and number of correct responses were measured.
Salivary cortisol levels
Trauma-exposed individuals show lower daily cortisol output40 and decreased morning cortisol,38,39 but women who have experienced sexual abuse are more likely to show increased cortisol levels in the afternoon.38,39 To better capture early diurnal changes in salivary cortisol following trauma exposure, we collected samples at 4 time points (8 am, 12 pm, 4 pm and 8 pm) using Salivettes (Sarstedt AG & Co). Participants were asked not to brush their teeth, drink, eat or smoke 90 minutes before saliva sampling, and they received an instruction sheet to increase adherence. They were asked to record when each sample was taken to ensure adherence to the timing protocol. After collection, samples were returned to the laboratory and stored at −20°C until the assay. Salivary cortisol concentration was determined using high-performance liquid chromatography coupled to tandem mass spectrometry and using a CHS MSMS steroid kit (PerkinElmer). Not all analyses could be completed because participants failed to collect a sample or because they collected an insufficient amount of saliva.
Functional MRI procedure
All stimuli were generated using E-PRIME (v2.0; Psychology Software Tools) and projected on a screen at the rear of the magnet using an LCD projector. Participants were given instructions and received a practice session on each task before each scan. Instructions were repeated before each task.
n-back working memory task
A series of letters was presented, and participants were asked to decide whether each item was the same as the item presented n-time back. We tested 4 levels of difficulty (0-back or identification condition, 1-back, 2-back and 3-back). This blocked-design experiment consisted of 3 blocks of each difficulty level presented in a pseudorandom order. In each block, 14 stimuli were presented (500 ms each, not jittered); the number of targets depended on the difficulty of the condition.
Emotional go/no-go task
Participants were instructed to target a category (sex: male or female), and asked to press a button only when they saw a face that matched the target category. The stimuli consisted of neutral, happy and sad facial expressions from 4 men and 4 women extracted from the Facial Expressions of Emotion Stimuli and Tests.49,50 Stimuli were presented in black and white on a grey background to better control the luminance effect. The experiment consisted of 4 blocks with 75% “go” stimuli. Each stimulus had a duration of 500 ms with a pseudorandomized interval between stimuli varied between 1000 ms and 7000 ms.
Mental imagery task
This task consisted of 3 conditions. In the rest condition, when a “0” appeared at the centre of the screen, participants were asked to rest, keeping mind-wandering to a minimum. In the positive condition, when a “+” appeared at the centre of the screen, participants were asked to remember their best (positive) memories. In the negative condition, when a “−” appeared at the centre of the screen, participants were asked to remember their worst (negative) memories. Each condition lasted for 30 seconds and was presented 3 times in a pseudorandom order. After the task, participants were asked to describe and rate their memories during the positive and negative conditions.
Data acquisition
Imaging data were acquired on a 3 T Siemens Magnetom Verio scanner (Siemens AG) using a 12-channel brain coil. We acquired high-resolution T1-weighted 3D anatomic scans for each participant (192 contiguous sagittal slices; 1 mm slice thickness; repetition time 1.9 s; echo time 2.48 ms; inversion time 0.9 ms; flip angle 9°; in-plane resolution 1 × 1 mm). We acquired functional images for each task (n-back: 136 volumes; emotional go/no-go: 182 volumes; mental imagery: 180 volumes) using a T2*-weighted gradient-echo echo-planar sequence (41 axial slices in ascending order; slice thickness 3 mm, no gap; repetition time 2.5 s, echo time 30 ms, flip angle 90°, field of view 240 mm, matrix 80 × 80, in-plane resolution 3 × 3 mm).
Image processing
We performed image preprocessing using statistical parametric mapping software (SPM12, Wellcome Department of Cognitive Neurology, University College London; www.fil.ion.ucl.ac.uk/spm). The first 4 acquisitions for each participant were discarded to allow for magnetization stability. Functional volumes were time-corrected, motion-corrected by spatial realignment to the first volume and then normalized to the Montreal Neurological Institute (MNI) reference brain. Finally, the normalized functional images were spatially smoothed with an 8 mm full-width at half-maximum Gaussian kernel. We included the 6 estimated movement parameters as covariates in the design matrix. We analyzed the 3 cognitive tasks separately. Individual contrasts of interest consisted of n-back (1-, 2-, 3-back) versus 0-back for the n-back task; no-go versus go, male versus female and emotional versus neutral for the emotional go/no-go task; and positive versus rest and negative versus rest for the mental imagery task.
Statistical analysis
In addition to the relatively small sample size, variables for sample characteristics, neuropsychological measures and cortisol measures did not meet the assumption of normal distribution. Therefore, we performed nonparametric Mann–Whitney tests to compare groups on continuous variables and reported the corresponding standardized statistics (z) and associated effect sizes (r = z/√N, where N is the sample size for the test). Statistical significance was set at p < 0.05. Within the survivor group, we also explored potential associations between symptom severity (CAPS total score), cortisol concentrations (8 am, 12 pm, 4 pm and 8 pm) and neuropsychological performance (accuracy and reaction time for the TMT-A, TMT-B, TMT-B minus TMT-A, TAP flexibility of focused attention with nonverbal and verbal stimuli) using Spearman correlations. Statistical significance for these exploratory analyses was set at p < 0.01.
Neuroimaging
Whole-brain analyses were carried out for the 3 functional MRI tasks using the statistical nonparametric mapping toolbox (SnPM13; http://warwick.ac.uk/snpm).51 Individual contrasts of interest for each task were entered into separate second-level 2-sample t-tests. Statistical significance was set at an initial uncorrected cluster-forming threshold of p < 0.001, to which we applied a family-wise error (FWE) correction resulting from permutation tests at the cluster level (pFWEc < 0.05). In addition, in the survivor group, we explored the associations between symptom severity (CAPS total score as regressor of interest) and brain activation for each contrast of interest using the regression model available from SnPM13 (pFWEc < 0.05). We identified MNI regions using the Automated Anatomic Labelling atlas.52
Results
Participant characteristics
Details of the participant characteristics are provided in Table 1. Briefly, groups were statistically matched for age, but the survivor group was slightly less educated and reported significantly higher levels of anxiety (State-Trait Anxiety Inventory) and depressive symptoms (Beck Depression Inventory) than the control group.
Table 1.
Participant characteristics
| Characteristic | Controls (n = 20); mean ± SD (range) | Survivors (n = 27); mean ± SD (range) | Statistics* |
|---|---|---|---|
| Age, yr | 28.30 ± 10.07 (18–52) | 27.22 ± 8.80 (18–53) | U = 261.00, z = −0.194, p = 0.849, r = −0.03 |
| Education, yr | 14.55 ± 1.99 (11–18) | 13.30 ± 2.09 (10–18) | U = 173.50, z = −2.146, p = 0.032, r = −0.31 |
| CAPS, mean total score | — | 58.78 ± 19.83 (19–97) | — |
| BDI, mean total score | 1.10 ± 1.62 (0–5) | 13.44 ± 7.33 (1–29) | U = 528.00, z = 5.603, p < 0.001, r = 0.82 |
| STAI, mean total score | 27.45 ± 6.93 (20–43) | 52.19 ± 13.85 (22–74) | U = 507.00, z = 5.107, p < 0.001, r = 0.74 |
BDI = Beck Depression Inventory; CAPS = Clinician-Administered PTSD Scale; SD = standard deviation; STAI = State-Trait Anxiety Inventory.
Significant differences are in bold.
Neuropsychological assessment
Statistical details of group differences on neuropsychological assessment are provided in Table 2. We found no significant group differences for accuracy or reaction time on the TMT-A. On the TMT-B, we found no difference in reaction time, but the survivor group performed significantly less accurately than the control group. The groups did not differ significantly in terms of performance or reaction time in the executive function measure (i.e., TMT-B minus TMT-A). Similarly, we found no significant between-group differences in accuracy of reaction time for the verbal or nonverbal versions of the TAP focused attention battery.
Table 2.
Group differences on neuropsychological assessment
| Assessment | Controls; mean ± SD | Survivors; mean ± SD | Statistics* |
|---|---|---|---|
| TMT-A accuracy, % | 95.04 ± 4.52 | 94.18 ± 4.79 | U = 192.50, z = −0.460, p = 0.653, r = −0.09 |
| TMT-A total time, s, | 19.67 ± 2.92 | 27.97 ± 16.19 | U = 221.00, z = 1.286, p = 0.214, r = 0.26 |
| TMT-B accuracy, % | 93.48 ± 3.70 | 89.46 ± 4.77 | U = 167.00, z = −2.021, p = 0.045, r = −0.41 |
| TMT-B total time, s | 26.20 ± 6.21 | 41.02 ± 21.71 | U = 231.00, z = 1.898, p = 0.061, r = 0.39 |
| TMT-B – TMT-A accuracy, % | −0.016 ± 0.044 | −0.047 ± 0.072 | U = 178.00, z = −1.348, p = 0.192, r = −0.28 |
| TMT-B – TMT-A total time, s | 6.53 ± 4.59 | 13.05 ± 12.24 | U = 222.00, z = 1.347, p = 0.192, r = 0.27 |
| TAP flexibility – verbal accuracy | 95.29 ± 4.11 | 92.00 ± 6.35 | U = 176.00, z = −1.079, p = 0.308, r = −0.22 |
| TAP flexibility – verbal reaction time, ms | 736.01 ± 176.45 | 962.74 ± 326.63 | U = 217.00, z = 1.670, p = 0.103, r = 0.35 |
| TAP flexibility – nonverbal accuracy | 96.57 ± 3.21 | 86.06 ± 23.81 | U = 163.00, z = −1.957, p = 0.055, r = −0.41 |
| TAP flexibility – nonverbal reaction time, ms | 737.51 ± 109.80 | 817.38 ± 308.98 | U = 205.00, z = 0.869, p = 0.413, r = 0.18 |
SD = standard deviation; TAP = Tests of Attentional Performance; TMT = Trail-Making Test.
Significant differences are in bold.
Salivary cortisol levels
Relative to the control group, the survivor group had significantly lower morning (8 am) cortisol concentration, but the groups did not differ significantly at the other time points (12 pm, 4 pm and 8 pm; Table 3). To determine whether there were differences in diurnal cortisol concentrations across the day, we performed an exploratory repeated-measures analysis of variance and found a significant effect for cortisol sample (Wilks’ Λ = 0.278, F3,41 = 35.428, p < 0.001, ηp2 = 0.722), but no significant effect for group (F1,43 = 1.274, p = 0.265, ηp2 = 0.029) or cortisol sample × group interaction (Wilks’ Λ = 0.955, F3,41 = 0.639, p = 0.594, ηp2 = 0.045).
Table 3.
Salivary cortisol levels for each collected sample, nmol/L
| Time point | Controls (n = 19); mean ± SD | Survivors (n = 26); mean ± SD | Statistics* |
|---|---|---|---|
| 8 am | 6.82 ± 4.60 | 5.36 ± 5.49 | U = 159.00, z = −2.022, p = 0.043, r = −0.30 |
| 12 pm | 2.68 ± 1.51 | 2.30 ± 1.79 | U = 204.00, z = −0.988, p = 0.323, r = −0.15 |
| 4 pm | 2.07 ± 1.04 | 1.78 ± 0.97 | U = 191.50, z = −1.276, p = 0.202, r = −0.19 |
| 8 pm | 1.00 ± 0.61 | 0.97 ± 0.52 | U = 228.50, z = −0.425, p = 0.671, r = −0.06 |
SD = standard deviation.
Significant differences are in bold.
Exploratory correlational analyses
In the survivor group, we found no significant associations between CAPS total and cortisol concentrations at any time point, or with neuropsychological performance (all p > 0.05). Exploratory associations between neuropsychological performance and cortisol measurements did indicate a significant negative association between cortisol at 8 am and executive functioning (TMT-B minus TMT-A; ρ = −0.646, p = 0.009) and a significant positive association with the time to perform the task (ρ = 0.704, p = 0.003).
Neuroimaging
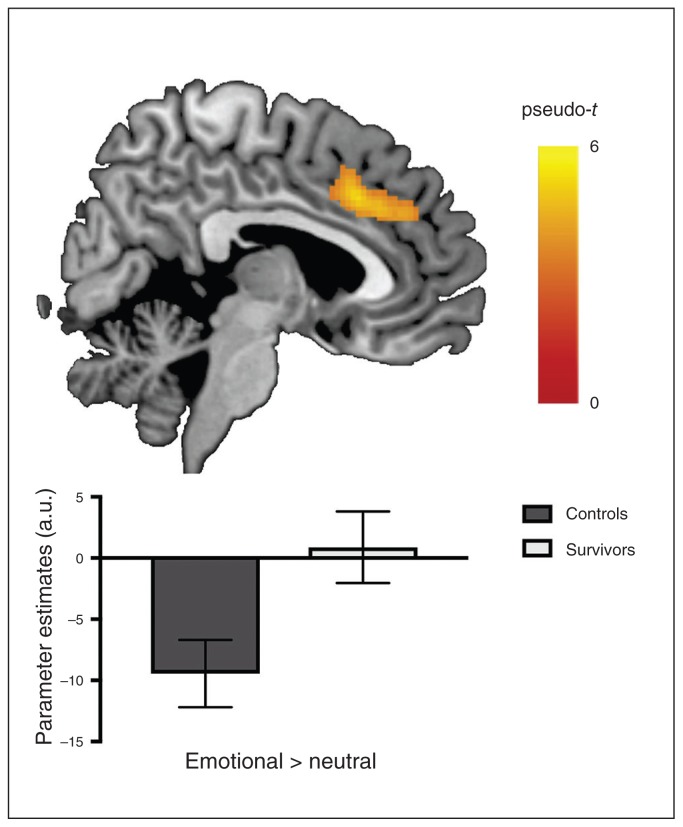
Whole-brain analyses indicated no significant differences between the control and survivor groups in terms of brain function during the n-back and mental imagery tasks. During the emotional go/no-go task, we found no significant group differences for the male versus female or no-go versus go contrasts. However, relative to the control group, the survivor group showed a significant lack of deactivation in a bilateral cluster that included the dorsal anterior cingulate cortex (dACC) and the medial superior frontal gyrus (peak MNI coordinates x, y, z = 4, 22, 36; k = 854; pseudo-t = 5.23; pFWEc = 0.013) during emotional processing (emotional > neutral; Fig. 1). In addition, using contrasts comparing emotional nogo (happy or sad, separately) versus neutral go trials, we explored potential group differences in emotional interference with response inhibition. These analyses yielded no significant between-group differences.
Fig. 1.
Group differences in brain activation when processing emotional material during an emotional go/no-go task. Compared to controls (black), the survivors (grey) showed a lack of deactivation in the dorsal anterior cingulate cortex/superior frontal gyrus. Colour bar represents pseudo-t values; error bars represent 95% confidence interval. Initial p < 0.001 uncorrected, with cluster-wise family-wise error (FWE) correction (pFWEc = 0.05). a.u. = arbitrary unit.
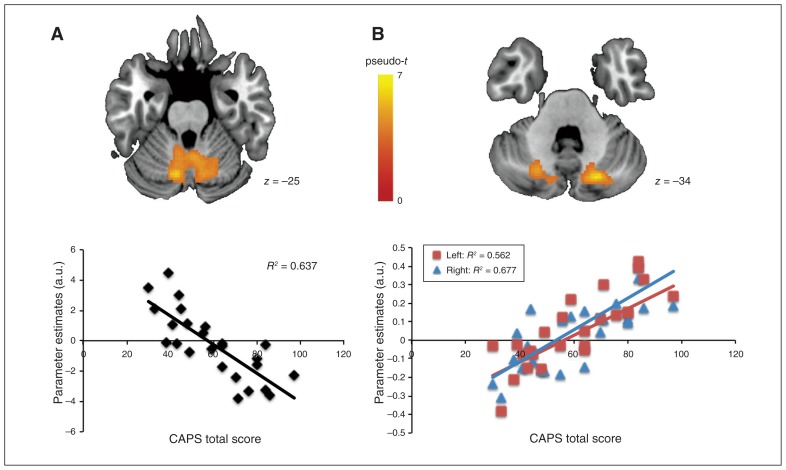
In the survivor group, we found no whole-brain associations between CAPS total scores and brain activation during the n-back task. However, bilateral decreased activation in the cerebellum, encompassing the cerebellar lobules VI, VIII, IX, crus I and vermis VIII–IX, for the happy no-go versus neutral go contrast (emotional interference with response inhibition; Fig. 2A) was significantly associated with higher CAPS total scores (R2 = 0.637; peak MNI coordinates x, y, z = −22, −48, −36; pseudo-t = 6.4; k = 2002; pFWEc = 0.002). Finally, for the positive versus rest contrast of the mental imagery task (Fig. 2B), the CAPS total score was significantly associated with increased activation in a cluster including the left cerebellar lobules VIII–IX and crus I–II (R2 = 0.562; peak MNI coordinates x, y, z = −26, −50, −46; pseudo-t = 5.53; k = 432; pFWEc = 0.034) and a cluster encompassing the right cerebellar lobule VIII, vermis VIII–IX and crus I (R2 = 0.677; peak MNI coordinates x, y, z = 20, −72, −36; pseudo-t = 6.94; k = 303; pFWEc = 0.049).
Fig. 2.
Associations between symptom severity and brain function when processing positive emotions. In the survivor group, symptom severity (CAPS total score) was (A) negatively associated with cerebellar function during the interference of positive emotion on response inhibition (happy no-go v. neutral go), and (B) positively associated with left (red squares) and right (blue triangles) cerebellar activation when retrieving positive memory during the mental imagery task. Colour bar represents pseudo-t values; slices are shown in axial view and marked with the z coordinate as distance in millimetres from the anterior-posterior commissure. Initial p < 0.001 uncorrected, with cluster-wise family-wise error (FWE) correction (pFWEc = 0.05). a.u. = arbitrary units; CAPS = Clinician-Administered PTSD scale. CAPS total score CAPS total score
Discussion
To the best of our knowledge, this multimodal study is the first to characterize the early neuropsychological and neurobiological changes occurring in female survivors of sexual assault compared with women who had never been sexually assaulted. Relative to the control group, the survivor group showed reduced accuracy in the TMT-B, lower morning (8 am) cortisol concentrations and increased levels of anxiety and depression. The survivor group also showed a lack of deactivation in the right dACC/superior frontal gyrus in response to emotional stimuli while performing an emotional go/no-go task. Finally, symptom severity in the survivor group was not associated with cortisol concentration or neuropsychological performance, but was associated with decreased left cerebellar activation in the potential emotional (happy) interference with response inhibition (emotional go/no-go task), as well as with increased bilateral cerebellar activation when thinking of positive memories (mental imagery task).
In this study, women who had experienced sexual assault showed impairments in cognitive flexibility (TMT-B). This was consistent with animal research showing similar deficits in a rat model (single prolonged stress) of PTSD,53 and importantly, with deficits in switching between different task demands as reported in people with acute24 and chronic PTSD.25 In addition, while the lack of deficits in executive function (TMT-B minus TMT-A) was consistent with previous findings in acute PTSD,24 the lack of working memory deficits was at odds with meta-analysis results in people with chronic PTSD.25 Interestingly, better performance on executive functioning was associated with lower morning (8 am) cortisol levels. It is therefore possible that survivors of sexual assault may not show the same cognitive deficit profile as survivors of other trauma (e.g., motor vehicular accident or other types of interpersonal violence). In addition, the cognitive deficits observed in acutely traumatized populations might be different from those observed in people with chronic PTSD. These differences have implications for personalized treatments or interventions following trauma exposure. Future studies should investigate these differences by comparing groups of survivors exposed to different types of traumatic events.
While processing emotional content in the go/no-go task, the survivors showed a lack of deactivation in the dACC extending to the superior frontal gyrus. This was consistent with similar meta-analysis findings, indicating a possible heightened threat evaluation during emotional processing in PTSD.23 Indeed, the dACC is involved in the modulation of fear54 and in the appraisal and expression of negative emotions. 55 The dACC is also a core region of the so-called salience network,56 implicated in the detection of task-relevant/salient stimuli to engage appropriate behaviour. This region is commonly reported to be hyperreactive in people with PTSD processing emotional material.23,57,58 Lack of deactivation in the dACC may represent inadequate modulation of emotional material during a cognitive task shortly after a sexual assault. Because of the limited sample size of the current study, larger studies are needed to replicate this finding and confirm this interpretation.
Unexpectedly, in the survivor group, symptom severity was associated with decreased left cerebellar activation when investigating the interference of positive emotion (happy) with response inhibition, but also with increased bilateral cerebellar activation with a positive memory. The participation of the cerebellum in higher cognitive and affective processes is well established as having a modulatory role in sending adaptive feedback to cortical areas during higher-order cognitive processes.59,60 The cerebellum is also involved in recognizing and discriminating emotional facial expressions, 61,62 processing self-related information through an affective regulatory loop including the frontal lobes and limbic system,63 and in retrieving autobiographical memories.64 It is possible that early alterations in cerebellar function when processing positive emotions may be a precursor of later alterations in the affective regulatory loop, especially between the vmPFC, the dACC and the amygdala, when PTSD symptoms become chronic. However, this interpretation remains speculative and will need to be formally tested using a longitudinal design.
Also, unexpectedly, the survivor group did not show alterations in brain function during working memory or response inhibition (no-go versus go) tasks, indicating that the function of the brain regions critical for these processes may be preserved in the early period following sexual assault. A complementary explanation would be that the participants who participated in this study were not those with more severe symptoms, presenting attenuated emotional and cognitive deficits and potentially less vivid memories of their traumatic event. Although these results are not consistent with previous neuropsychological studies using acute psychological stress16,20 or in chronic PTSD,65,66 to date no neuroimaging study has investigated these 2 cognitive abilities in acutely traumatized women. Further investigation in larger samples is therefore warranted to better characterize the early functional changes associated with trauma exposure.
Limitations
An obvious limitation of this study was the small sample size. Inclusion of survivors of sexual assault in this particular period of disorientation and distress was challenging. We also acknowledge that the acquisition method of the functional neuroimaging data (ascending, sequential with no gap) may have introduced blood-flow and slice-to-slice artifacts. However, we carefully inspected the data and found no gross artifact. In addition, our morning cortisol measure may have been confounded by the cortisol awakening response. To avoid this issue, future studies of morning cortisol should be performed in reference to wakening time. Finally, an important limitation is related to interpretation of the nonsignificant results. Because of our limited sample size, we could not rule out the influence of type-II errors. Larger studies with sufficient statistical power are needed to confirm the subtle changes in neuropsychological and neuroendocrine alterations following sexual assault that the current study was unable to detect.
Conclusion
The goal of this study was to provide a better and more global understanding of the early neurobiological changes that occur following exposure to a single traumatic event by combining neuropsychological, neuroendocrine and functional neuroimaging measures. Our results revealed that a lack of deactivation of the dACC/superior frontal gyrus was associated with atypical emotional processing in survivors of sexual assault. In addition, cerebellar activation was associated with symptom severity when processing positive stimuli. Future studies are needed to better understand whether or not these early functional modifications can predict the development of PTSD.
Acknowledgments
The authors are grateful for the invaluable contributions of the participants. This project was funded by a Hospital Clinical Research Program (Hôpital Promoteur, CHRU de Tours). The authors acknowledge the Fondation Pierre Deniker and the SFR FED4226 Neuroimagerie Fonctionnelle for their financial support. Y. Quidé was supported by a postgraduate scholarship from the French Ministry of Higher Education, Research and Innovation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. An abstract of the preliminary data from this study was presented as a poster at the 72nd Annual Scientific Convention and Meeting, Society of Biological Psychiatry Annual Meeting, May 19, 2017. The authors also thank the reviewers for their insightful comments on an earlier version of this manuscript.
Footnotes
Competing interests: None declared.
Contributors: Y. Quidé, F. Andersson and W. El-Hage designed the study. F. Andersson, C. Descriaud, P. Saint-Martin, L. Barantin, V. Gissot, M.-P. Carrey Le Bas, S. Osterreicher, B. Brizard, M. Ogielska and W. El-Hage acquired the data, which Y. Quidé, H. Cléry, F. Andersson, V. Gissot, D. Dufour-Rainfray and W. El-Hage analyzed. Y. Quidé, H. Cléry and W. El-Hage wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Bajos N, Bozon M. Contexte de la sexualité en France. 2007. [Google Scholar]
- 2.US Department of Justice. Extent, nature, and consequences of rape victimization: findings from the National Violence Against Women Survey. 2006. [Google Scholar]
- 3.Carper TL, Mills MA, Steenkamp MM, et al. Early PTSD symptom sub-clusters predicting chronic posttraumatic stress following sexual assault. Psychol Trauma. 2015;7:442–7. doi: 10.1037/tra0000060. [DOI] [PubMed] [Google Scholar]
- 4.Chivers-Wilson KA. Sexual assault and posttraumatic stress disorder: a review of the biological, psychological and sociological factors and treatments. McGill J Med. 2006;9:111–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Nickerson A, Steenkamp M, Aerka IM, et al. Prospective investigation of mental health following sexual assault. Depress Anxiety. 2013;30:444–50. doi: 10.1002/da.22023. [DOI] [PubMed] [Google Scholar]
- 6.Pegram SE, Abbey A. Associations between sexual assault severity and psychological and physical health outcomes: similarities and differences among African American and Caucasian survivors. J Interpers Violence. 2016 doi: 10.1177/0886260516673626. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peter-Hagene LC, Ullman SE. Social reactions to sexual assault disclosure and problem drinking: mediating effects of perceived control and PTSD. J Interpers Violence. 2014;29:1418–37. doi: 10.1177/0886260513507137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ullman SE. Sexual revictimization, PTSD, and problem drinking in sexual assault survivors. Addict Behav. 2016;53:7–10. doi: 10.1016/j.addbeh.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization and Pan American Health Organization. Understanding and addressing violence against women. Geneva: World Health Organization; 2012. [Google Scholar]
- 10.Ybarra ML, Espelage DL, Langhinrichsen-Rohling J, et al. Lifetime prevalence rates and overlap of physical, psychological, and sexual dating abuse perpetration and victimization in a national sample of youth. Arch Sex Behav. 2016;45:1083–99. doi: 10.1007/s10508-016-0748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard DE, Wang MQ. Psychosocial factors associated with adolescent boys’ reports of dating violence. Adolescence. 2003;38:519–33. [PubMed] [Google Scholar]
- 12.Wolitzky-Taylor KB, Ruggiero KJ, Danielson CK, et al. Prevalence and correlates of dating violence in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry. 2008;47:755–62. doi: 10.1097/CHI.0b013e318172ef5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchette I, Caparos S. Working memory function is linked to trauma exposure, independently of post-traumatic stress disorder symptoms. Cogn Neuropsychiatry. 2016;21:494–509. doi: 10.1080/13546805.2016.1236015. [DOI] [PubMed] [Google Scholar]
- 14.Oei NY, Everaerd WT, Elzinga BM, et al. Psychosocial stress impairs working memory at high loads: an association with cortisol levels and memory retrieval. Stress. 2006;9:133–41. doi: 10.1080/10253890600965773. [DOI] [PubMed] [Google Scholar]
- 15.Schoofs D, Pabst S, Brand M, et al. Working memory is differentially affected by stress in men and women. Behav Brain Res. 2013;241:144–53. doi: 10.1016/j.bbr.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Schoofs D, Preuss D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33:643–53. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Schoofs D, Wolf OT, Smeets T. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav Neurosci. 2009;123:1066–75. doi: 10.1037/a0016980. [DOI] [PubMed] [Google Scholar]
- 18.Shields GS, Sazma MA, Yonelinas AP. The effects of acute stress on core executive functions: a meta-analysis and comparison with cortisol. Neurosci Biobehav Rev. 2016;68:651–68. doi: 10.1016/j.neubiorev.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin S, Hermans EJ, van Marle HJ, et al. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Shansky RM, Lipps J. Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Front Hum Neurosci. 2013;7:123. doi: 10.3389/fnhum.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aupperle RL, Melrose AJ, Stein MB, et al. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62:686–94. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagarde G, Doyon J, Brunet A. Memory and executive dysfunctions associated with acute posttraumatic stress disorder. Psychiatry Res. 2010;177:144–9. doi: 10.1016/j.psychres.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Polak AR, Witteveen AB, Reitsma JB, et al. The role of executive function in posttraumatic stress disorder: a systematic review. J Affect Disord. 2012;141:11–21. doi: 10.1016/j.jad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cwik JC, Sartory G, Nuyken M, et al. Posterior and prefrontal contributions to the development posttraumatic stress disorder symptom severity: an fMRI study of symptom provocation in acute stress disorder. Eur Arch Psychiatry Clin Neurosci. 2016 doi: 10.1007/s00406-016-0713-6. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Cwik JC, Sartory G, Schurholt B, et al. Posterior midline activation during symptom provocation in acute stress disorder: an fMRI study. Front Psychiatry. 2014;5:49. doi: 10.3389/fpsyt.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels JK, Coupland NJ, Hegadoren KM, et al. Neural and behavioral correlates of peritraumatic dissociation in an acutely traumatized sample. J Clin Psychiatry. 2012;73:420–6. doi: 10.4088/JCP.10m06642. [DOI] [PubMed] [Google Scholar]
- 30.Nilsen AS, Blix I, Leknes S, et al. Brain activity in response to trauma-specific, negative, and neutral stimuli. A fMRI study of recent road traffic accident survivors. Front Psychol. 2016;7:1173. doi: 10.3389/fpsyg.2016.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Xie H, Cotton AS, et al. Preliminary study of acute changes in emotion processing in trauma survivors with PTSD symptoms. PLoS One. 2016;11:e0159065. doi: 10.1371/journal.pone.0159065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landré L, Destrieux C, Andersson F, et al. Working memory processing of traumatic material in women with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37:87–94. doi: 10.1503/jpn.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sartory G, Cwik J, Knuppertz H, et al. In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD) PLoS One. 2013;8:e58150. doi: 10.1371/journal.pone.0058150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey AG, Bryant RA, Dang ST. Autobiographical memory in acute stress disorder. J Consult Clin Psychol. 1998;66:500–6. doi: 10.1037//0022-006x.66.3.500. [DOI] [PubMed] [Google Scholar]
- 35.St Jacques PL, Botzung A, Miles A, et al. Functional neuroimaging of emotionally intense autobiographical memories in posttraumatic stress disorder. J Psychiatr Res. 2011;45:630–7. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark IA, Mackay CE. Mental imagery and post-traumatic stress disorder: a neuroimaging and experimental psychopathology approach to intrusive memories of trauma. Front Psychiatry. 2015;6:104. doi: 10.3389/fpsyt.2015.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 38.Meewisse ML, Reitsma JB, de Vries GJ, et al. Cortisol and posttraumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–92. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 39.Morris MC, Hellman N, Abelson JL, et al. Cortisol, heart rate, and blood pressure as early markers of PTSD risk: a systematic review and meta-analysis. Clin Psychol Rev. 2016;49:79–91. doi: 10.1016/j.cpr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 41.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (MINI): the development and validation of a structured diagnostic interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 42.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 43.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 44.Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press; 1983. [Google Scholar]
- 45.Piper BJ, Li V, Eiwaz MA, et al. Executive function on the Psychology Experiment Building Language tests. Behav Res Methods. 2012;44:110–23. doi: 10.3758/s13428-011-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mueller ST, Piper BJ. The Psychology Experiment Building Language (PEBL) and PEBL test battery. J Neurosci Methods. 2014;222:250–9. doi: 10.1016/j.jneumeth.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oosterman JM, Vogels RL, van Harten B, et al. Assessing mental flexibility: neuroanatomical and neuropsychological correlates of the Trail Making Test in elderly people. Clin Neuropsychol. 2010;24:203–19. doi: 10.1080/13854040903482848. [DOI] [PubMed] [Google Scholar]
- 48.Zimmermann P, Fimm B. Test for attentional performance (TAP) PsyTest, Herzogenrath. 1995:76–7. [Google Scholar]
- 49.Ekman P, Friesen W. The pictures of facial affect. Palo Alto (CA): Consulting Psychologists Press; 1976. [Google Scholar]
- 50.Young A, Perrett C, Calder A, et al. Facial Expressions of Emotion: Stimuli and Tests (FEEST) Bury St Edmunds, UK: Thames Valley Test Company; 2002. [Google Scholar]
- 51.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 53.George SA, Rodriguez-Santiago M, Riley J, et al. Alterations in cognitive flexibility in a rat model of post-traumatic stress disorder. Behav Brain Res. 2015;286:256–64. doi: 10.1016/j.bbr.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 54.Milad MR, Quirk GJ, Pitman RK, et al. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–4. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 55.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 57.Hughes KC, Shin LM. Functional neuroimaging studies of posttraumatic stress disorder. Expert Rev Neurother. 2011;11:275–85. doi: 10.1586/ern.10.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pannu Hayes J, Labar KS, Petty CM, et al. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keren-Happuch E, Chen SH, Ho MH, et al. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp. 2014;35:593–615. doi: 10.1002/hbm.22194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11:352–65. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- 61.Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- 62.Adamaszek M, Kirkby KC, D’Agata F, et al. Neural correlates of impaired emotional face recognition in cerebellar lesions. Brain Res. 2015;1613:1–12. doi: 10.1016/j.brainres.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 63.Ceylan ME, Dönmez A, Ülsalver BÖ. The contribution of the cerebellum in the hierarchial development of the self. Cerebellum. 2015;14:711–21. doi: 10.1007/s12311-015-0675-7. [DOI] [PubMed] [Google Scholar]
- 64.Addis DR, Moloney EEJ, Tippett LJ, et al. Characterizing cerebellar activity during autobiographical memory retrieval: ALE and functional connectivity investigations. Neuropsychologia. 2016;90(Suppl C):80–93. doi: 10.1016/j.neuropsychologia.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 65.Honzel N, Justus T, Swick D. Posttraumatic stress disorder is associated with limited executive resources in a working memory task. Cogn Affect Behav Neurosci. 2014;14:792–804. doi: 10.3758/s13415-013-0219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swick D, Honzel N, Larsen J, et al. Increased response variability as a marker of executive dysfunction in veterans with posttraumatic stress disorder. Neuropsychologia. 2013;51:3033–40. doi: 10.1016/j.neuropsychologia.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]