Abstract
Marrow adipose tissue (MAT) in humans is distributed differentially across age and skeletal site. We have shown impaired microarchitecture and reduced bone strength at appendicular sites in conditions associated with high MAT of the axial skeleton in adults (including conditions of over- and undernutrition). Data are lacking regarding differences in MAT content of the appendicular versus the axial skeleton, and its relationship with bone microarchitecture and strength. Furthermore, data are conspicuously lacking in adolescents, a time when hematopoietic marrow is progressively converted to fatty marrow. The purpose of our study was to examine differential associations between appendicular (distal tibia) and axial (lumbar spine) MAT and bone microarchitecture and strength estimates of the distal tibia in adolescents with obesity. We hypothesized that compared to MAT of the axial skeleton (lumbar spine), MAT of the appendicular skeleton (distal tibia) would show stronger associations with bone microarchitecture and strength estimates of the appendicular skeleton (distal tibia). We evaluated 32 adolescents and young adults (27 females) with obesity; with a mean age of 17.8±2.1 years and median body mass index (BMI) of 41.34 kg/m2, who underwent dual energy x-ray absorptiometry (DXA) for total fat mass, proton MR spectroscopy (1H-MRS) of the distal tibia and 4th lumbar vertebra for MAT, high resolution peripheral quantitative computed tomography (HR-pQCT) of the distal tibia for volumetric bone mineral density (vBMD) and microarchitecture, and micro finite element analysis (FEA) for distal tibial strength estimates. Linear correlations between bone parameters and MAT were determined using the Spearman or Pearson methods, depending on data distribution. Lumbar spine MAT was inversely associated with age (r= −0.36; p=0.037). Total and trabecular vBMD and trabecular number at the distal tibia were inversely associated with MAT at the distal tibia (r= −0.39, p=0.025; r= −0.51, p=0.003; r= −0.42, p=0.015 respectively) but not with lumbar spine MAT (r= −0.19, p=0.27; r= −18, p=0.3; r= 0.005, p=0.97 respectively). In adolescents and young adults with obesity, the associations between MAT and appendicular bone parameters differ depending on the site of MAT assessment i.e. axial vs. appendicular. Studies evaluating these endpoints in adolescents and young adults with obesity should take the site of MAT assessment into consideration.
Keywords: Marrow adipose tissue, proton MR spectroscopy, Bone, Distal tibia, lumbar spine, Microarchitecture, Adolescents, Obesity
1. INTRODUCTION
Bone strength is determined not only by bone mineral density (BMD) and bone microarchitecture but also by its microenvironment, such as marrow adipose tissue (MAT) [1]. The distribution of MAT varies by age and skeletal site [2]. At birth, most of the marrow cavity is filled with red marrow which is a site of hematopoiesis. During childhood and adolescence, red marrow is progressively replaced by yellow or fatty marrow, and in the long bones this process begins in the epiphyses, followed by the diaphyses, the distal metaphyses and the proximal metaphyses. In contrast, red marrow may persist in the axial skeleton (pelvis and spine) well into adulthood [2]. MAT maturation reaches equilibrium in adulthood and may constitute up to 70% of the composition of bone marrow [3]. However, bone marrow is a dynamic organ and MAT content and composition can change in response to hormonal stimuli, and over- and undernutrition [4, 5].
In translational studies, we have identified two different types of MAT; constitutive MAT (cMAT), which remains relatively stable to different hormonal and nutritional changes and is more prominent in the distal tibia, and regulated MAT (rMAT), which is more dynamic and responds to hormonal and nutritional changes and is found in the proximal tibia, femur and lumbar vertebra [6]. In humans, the different properties of distal and proximal MAT are highlighted by the varied degree of unsaturation of marrow adipocytes between the distal tibia and proximal femur suggesting MAT composition varies by site [6]. Hence, it is crucial to consider the age, and the site (appendicular vs. axial) when assessing the effects of MAT on bone health.
Most studies assessing MAT content in humans have quantified MAT in the axial skeleton (lumbar spine and proximal femurs) and assessed its relationship with bone microarchitecture at appendicular sites (distal radius and distal tibia) [1, 4, 7, 8]. To our knowledge, there are no data in humans evaluating the effect of MAT on bone parameters at the same site.
The purpose of our study was to examine differential associations between appendicular (distal tibia) and axial (lumbar spine) MAT and bone microarchitecture and strength estimates of the distal tibia in adolescents with obesity. We hypothesized that bone microarchitecture and strength estimates of the appendicular skeleton (distal tibia) would have stronger associations with MAT of the appendicular skeleton (distal tibia) as compared to MAT of the axial skeleton (lumbar spine).
2. METHODS
We evaluated cross-sectional data from 32 adolescents and young adults between the ages of 14 – 21 years with obesity from an ongoing trial at Massachusetts General Hospital. Subjects had a BMI> 35 kg/m2 and did not have any known condition that would affect bone health. Our study was IRB approved and HIPAA compliant. Written informed consent/assent was obtained. A previously published subset of 14 healthy adolescents of normal weight, who had undergone proton MR Spectroscopy (1H-MRS) of the L4 vertebral body for MAT quantification using identical methods and equipment as in the present study, were used as a control group to compare the amount of L4 MAT between adolescents of normal weight and with obesity [9].
2.1. Experimental Protocol:
A detailed history was obtained, with attention to the use of medications and/or conditions that affect bone health. Participants were weighed on a calibrated electronic scale wearing a hospital gown, and height was measured in triplicate on a stadiometer. BMI was calculated using the formula: weight (kg) / height2 (m2).
2.2. Proton MR Spectroscopy (1 H-MRS):
Single voxel 1 H-MRS was used for assessment of MAT of the L4 vertebral body and the distal tibia using a 3.0T MR imaging system (Siemens Trio, Siemens Medical Systems, Erlangen, Germany) (Figure 1). Single-voxel 1H MRS data was acquired using point-resolved spatially localized spectroscopy (PRESS) pulse sequence without water suppression with the following parameters: TE of 30 ms, TR of 3,000 ms, 8 acquisitions, 1024 data points, and receiver bandwidth of 2000 Hz. Automated procedures for optimization of gradient shimming and transmit and receive gain were used. MAT is reported as the quotient of the lipid peak to the water peak.
Figure 1:
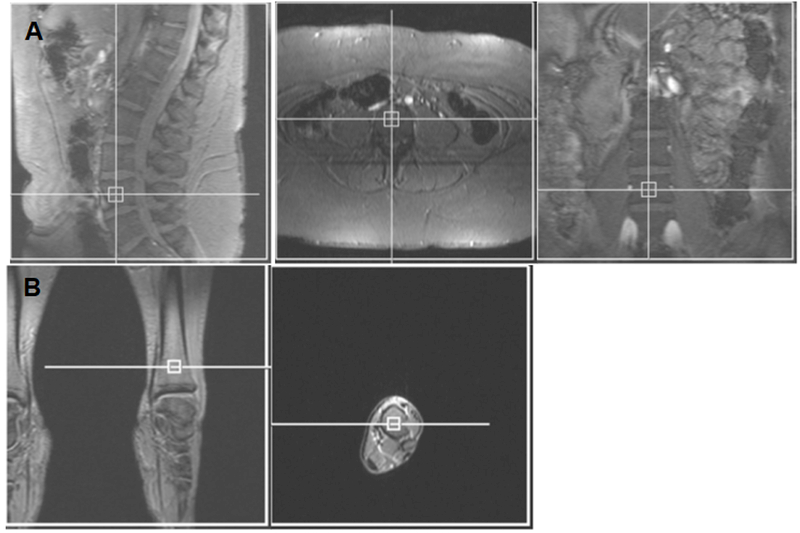
Voxel placement for lumbar spine (A) and tibia (B) marrow adipose tissue quantification.
2.4. Dual-energy x-ray absorptiometry (DXA):
DXA was used to assess total fat mass. The same scanner (Hologic 4500 A, Waltham, MA) was used for all subjects.
2.5. High resolution quantitative computed tomography (HR-pQCT):
HR-pQCT was used to assess bone size parameters, volumetric BMD (vBMD), and trabecular bone microarchitecture at the distal tibia (Xtreme CT; Scanco Medical AG, Bruttisellen, Switzerland) with an isotropic voxel size of 82μm3. Measurements were performed at the non-dominant leg unless there was a prior fracture at that site. 2D scout views were obtained to locate distal CT slice site at 22.5 mm from the tibial endplate.
2.6. Micro-finite element analysis (FEA):
FEA was performed to estimate the biomechanical properties of bone in the setting of simulated axial compression. Failure load and stiffness (kN) were estimated by scaling the resultant load from a 1% apparent compressive strain until 2% of all elements reached an effective strain > 7000 μstrain, per previously published methods [10].
2.7. Statistical analysis:
Statistical analysis was performed using JMP software (SAS Institute, Carey, NC). Normally distributed data are reported as means ± standard deviation (mean ± SD). In the case of non-normally distributed data, the median and interquartile range are reported. Pearson or Spearman correlations (depending on the distribution of the data) were used to determine univariate associations of MAT with clinical and bone parameters. Analyses were controlled for sex using standard least squares regression modeling. To adjust for multiple comparisons we used false discovery rate correction of <0.05 for estimating q-values for assessing statistical significance [11].
3. RESULTS
3.1. Subject Characteristics:
Table 1 shows the clinical characteristics of study participants. Participants (27 female and 5 male) had a mean age of 17.8 ± 2.1 years and a median BMI of 41.34 (38.31–45.60) kg/m2. None of the participants exercised for more than 2 hours per week in the preceding year and none had a history of diabetes or hypothyroidism. We present their body composition including the MAT content at the distal tibia and lumbar spine in Table 1.
Table 1.
Participant Characteristics and Body Composition
| N=32 | ||
|---|---|---|
| mean±SD, median (IQR) | min-max value | |
| Age (years) | 17.8 ± 2.1 | 14.5 – 21.4 |
| Gender F/M | 27/5 | |
| Height (cm) | 166.5 ± 5.6 | 157.6 – 180.0 |
| Weight (kg) | 118.3 ± 16.9 | 90.5 – 152.1 |
| BMI (kg/m2) | 41.3 (38.3- 45.6) | 35.2 – 55.5 |
| 25-hydroxy-vitamin D (ng/ml) | 24.3 ± 9.6 | 10.7 – 47.6 |
| Calcium (mg/dl) | 9.24 ± 0.26 | 8.7 – 9.8 |
| TSH (uIU/ml) | 1.9 (1.5 - 2.5) | 1.0 – 6.4 |
| HbA1C | 5.6 (5.4 – 5.7) | 5.0 – 6.5 |
| Total Fat Mass (kg) | 57.8 ± 11.4 | 39.0– 83.0 |
| Tibia MAT (Lipid/Water) | 10.1 ± 2.9 | 2.6 – 15.3 |
| L4 MAT (Lipid/Water) | 0.37 ± 0.17 | 0.15 – 0.76 |
Data are reported as means ± standard deviation (SD) for normally distributed data and as median and interquartile range (IQR) for non-normally distributed data as well as minimum (min) and maximum (max) values.
MAT content of L4 was lower compared to normal-weight controls of similar age: mean age 18.6 ± 1.6 (normal weight) vs 17.8 ± 2.1 years (obese), p=0.2; L4 MAT 0.53 ± 0.27 (normal weight) vs 0.37 ± 0.17 (obese), p=0.02.
3.2. Correlations of MAT at the Distal Tibia and L4 with Age and Body Composition
MAT at the distal tibia was not associated with age, but we found a negative correlation of lumbar spine MAT with age (r= −0.36; p=0.037), which remained significant after controlling for sex, but lost significance after false discovery rate correction. We found no correlation of axial and appendicular MAT with BMI or total fat mass (Table 2).
Table 2.
Correlation of Body Composition and Bone Parameters at the Distal Tibia with MAT at the Distal Tibia and L4 Vertebra
| Distal Tibial MAT | L4 MAT | |||||
|---|---|---|---|---|---|---|
| R | P | q-value | R | P | q-value | |
| With Age and Body Composition | ||||||
| Age at Baseline (yrs) | 0.23 | 0.18 | 0.33 | −0.36 | 0.037* | 0.11 |
| BMI (kg/m2) | −0.21 | 0.22 | 0.33 | −0.07 | 0.68 | 0.70 |
| Total Fat (kg) | −0.06 | 0.71 | 0.71 | 0.06 | 0.70 | 0.70 |
| With Distal Tibia vBMD and Microarchitecture | ||||||
| Total vBMD (mgHA/cm3) | −0.39 | 0.025* | 0.03 | −0.19 | 0.27 | 0.52 |
| Trabecular vBMD (mgHA/cm3) | −0.51 | 0.003* | 0.01 | −0.18 | 0.30 | 0.52 |
| Trabecular number (1/mm) | −0.42 | 0.015* | 0.025 | 0.005 | 0.97 | 0.97 |
| Trabecular Thickness (mm) | −0.01 | 0.94 | 0.94 | −0.18 | 0.31 | 0.52 |
| Trabecular Separation (mm) | 0.50 | 0.004* | 0.01 | 0.007 | 0.96 | 0.97 |
| With Distal Tibial Strength Estimates | ||||||
| Stiffness (N/mm) | −0.31 | 0.07 | 0.07 | 0.007 | 0.96 | 0.96 |
| Failure Load (N) | −0.33 | 0.05 | 0.07 | 0.04 | 0.81 | 0.96 |
Bold indicates p<0.05
significant after controlling for sex
3.3. Correlations of MAT at Tibia and L4 Spine with Bone vBMD, Microarchitecture and Strength Estimates at the Distal Tibia
Distal tibial MAT correlated negatively with distal tibial total and trabecular vBMD (r=−0.39, p=0.025, and r=−0.51, p=0.003 respectively) and trabecular number (r=−0.42, p=0.015), and positively with trabecular separation (r=0.50, p=0.0035), independent of sex and false discovery rate correction (Table 2). There was a trend for a negative association between distal tibial MAT and distal tibial strength estimates, namely stiffness and failure load (r=−0.31, p=0.07; r=−0.33, p=0.05). These parameters were not associated with spine MAT.
4. DISCUSSION
This is the first study to simultaneously compare the associations of bone parameters at the distal tibia with MAT at the same appendicular site, as well as MAT at a distant axial (L4 vertebra) site.
We found that spine MAT was inversely associated with age but did not find any association of distal tibial MAT with age. The differential association between spinal and tibial MAT with age suggests that lumbar MAT, which is an axial site, is dynamic and responsive to the state of overnutrition (regulated MAT), unlike MAT at distal tibia which is constitutive marrow and does not change under systemic challenges [6]. Contrary to data in normal weight adolescents and adults with obesity that indicate that spine MAT content increases with age [2, 12], we found that spine MAT was inversely associated with age in adolescents and young adults with obesity. In addition, MAT of L4 was lower compared to normal weight subjects of similar age. An explanation for these findings might be marrow reconversion from fatty to hematopoietic marrow, due to reduced oxygen carrying capacity and hypoxia which has been described in adults with obesity [13, 14]. This is the first report evaluating these associations in adolescents and young adults with severe obesity, who may develop MAT differently compared to other populations. Although, adults with obesity have higher MAT [15], data are lacking in adolescents with obesity. Further studies are needed to establish the evolution of MAT with increasing age in adolescents with obesity.
The lack of correlation of MAT (both at axial and appendicular skeletal site) with total fat mass suggests that MAT regulation differs from white fat regulation in adolescents with obesity. The independent regulation of MAT compared to white fat depots is also supported by the respective changes in these depots over a human lifetime – white adipose tissue peaks in middle age and then declines, whereas MAT continues to increase with aging [16]. Moreover, data from mice suggest that overfeeding, which increases white adipose tissue may increase regulated MAT but not constitutive MAT [17]. Data in premenopausal women with abdominal obesity have shown a positive correlation between lumbar MAT and visceral adipose tissue but not BMI or subcutaneous or total abdominal adipose tissue [5]. Thus, MAT regulation is likely different from that of other fat depots, and this relationship needs further clarification in adolescents with obesity.
Our results demonstrating negative associations of MAT with total and trabecular vBMD and microarchitectural parameters at the distal tibia (as well as weaker associations with strength estimates) are consistent with previous reports [9, 18]. However, the presence of these associations with appendicular MAT (distal tibia) and not with the axial MAT (lumbar spine) with bone endpoints is reported for the first time. We have previously reported that in adult men with obesity, MAT at the lumbar spine was negatively associated with distal radius trabecular microarchitecture. This is an association of axial MAT with bone endpoints at an appendicular site [18]. These associations between axial MAT and appendicular bone microarchitecture have also been reported in other osteoporotic states such as anorexia nervosa, hypogonadal female athletes, and adults with Type 2 diabetes mellitus [1, 8, 12, 19].
The differential associations of appendicular and axial with appendicular bone microarchitecture in adolescents with obesity may result from differing MAT composition at these sites – e.g. constitutive vs. regulated which are controlled differently. From our knowledge of mouse models, MAT at the distal tibia is constitutive and less dynamic compared to lumbar MAT, which is more regulated and influenced by changes in the hormonal and nutritional milieu [6].
The differential association of tibial vs. spine MAT with distal tibia bone parameters may also arise from the local paracrine and physical impact of MAT at the same site. There are multiple local interactions between marrow fat cells and the nearby microenvironment [20]. Moreover, adipocytes in MAT make adipokines that may have different paracrine (local) and endocrine (distant) effects [21]. For example, adiponectin, which is made by marrow adipocytes, has bone anabolic effects locally but may have an opposing effect systemically [22]. Moreover, mesenchymal stem cells in the bone marrow are more likely to respond to subtle local (vs. distant) changes in osteogenic vs. adipogenic stimuli. Hence, it is not surprising that we found associations between appendicular bone microarchitecture and same site appendicular MAT, but none with the distant axial MAT.
Our data combined with other preclinical data highlight the need to take site specificity of MAT assessments into account when studying bone outcomes, particularly in adolescents and young adults with obesity. MAT regulation and its unique response to excess calories, compared to other fat depots in adolescents and young adults with obesity, should be a focus of further research. Moreover, these data emphasize the need to assess bone outcomes with MAT content locally and to be cautious while deriving conclusions about such associations when MAT at the same site cannot be assessed.
Our study has the following limitations. First, the cross-sectional design limits our ability to determine causality. Second, our cohort is limited to adolescents and young adults with obesity and hence results cannot be generalized. Third, our sample size is relatively small which increases the likelihood of a Type 2 error. However, with 32 subjects, we were able to detect significant differences between associations of bone parameters with MAT at two different skeletal sites.
In conclusion, in adolescents and young adults with obesity, associations between MAT and appendicular bone parameters differ depending on the site of MAT assessment. Studies evaluating these endpoints should take the site of MAT assessment into consideration.
Highlights.
1. Marrow Adipose Tissue (MAT) content varies by age and skeletal site.
2. Associations differ between axial vs appendicular MAT and appendicular bone parameters.
3. BMD and trabecular microarchitecture at distal tibia have stronger associations with MAT content at distal tibia than lumbar spine MAT.
Acknowledgments
Sources of Funding: K24 DK109940, R01 DK103946, R24 DK092759, UL1 RR025758, P30DK040561, K23DK110419, K24 HD071843-04
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors have no relevant conflicts of interest to disclose
REFERENCES:
- 1.Patsch JM, et al. , Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res, 2013. 28(8): p. 1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kricun ME, Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol, 1985. 14(1): p. 10–9. [DOI] [PubMed] [Google Scholar]
- 3.Fazeli PK, et al. , Marrow fat and bone--new perspectives. J Clin Endocrinol Metab, 2013. 98(3): p. 935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredella MA, et al. , Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab, 2009. 94(6): p. 2129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bredella MA, et al. , Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring), 201119(1): p. 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheller EL, et al. , Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun, 2015. 6: p. 7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredella MA, et al. , Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone, 2017. 95: p. 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singhal V, et al. , Regional fat depots and their relationship to bone density and microarchitecture in young oligo-amenorrheic athletes. Bone, 2015. 77: p. 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singhal V, et al. , Impaired bone strength estimates at the distal tibia and its determinants in adolescents with anorexia nervosa. Bone, 2018. 106: p. 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pistoia W, et al. , High-resolution three-dimensional-pQCT images can be an adequate basis for in-vivo microFE analysis of bone. J Biomech Eng, 2001. 123(2): p. 176–83. [DOI] [PubMed] [Google Scholar]
- 11.Hsueh HM, Chen JJ, and Kodell RL, Comparison of methods for estimating the number of true null hypotheses in multiplicity testing. J Biopharm Stat, 2003. 13(4): p. 675–89. [DOI] [PubMed] [Google Scholar]
- 12.Yu EW, et al. , Marrow adipose tissue composition in adults with morbid obesity. Bone, 2017. 97: p. 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosentini T, et al. , Magnetic resonance imaging evaluation of bone marrow changes in obstructive sleep apnoea syndrome in adults. Radiol Med, 2006. 111(4): p. 572–84. [DOI] [PubMed] [Google Scholar]
- 14.Poulton TB, et al. , Bone marrow reconversion in adults who are smokers: MR Imaging findings. AJR Am J Roentgenol, 1993. 161(6): p. 1217–21. [DOI] [PubMed] [Google Scholar]
- 15.Bredella MA, et al. , Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology, 2013. 269(2): p. 534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visser M, et al. , One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol (1985), 2003. 94(6): p. 2368–74. [DOI] [PubMed] [Google Scholar]
- 17.Devlin MJ, et al. , Early-onset type 2 diabetes impairs skeletal acquisition in the male TALLYHO/JngJ mouse. Endocrinology, 2014. 155(10): p. 3806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bredella MA, et al. , Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab, 2012. 97(11): p. 4115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen A, et al. , Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab, 2012. 97(8): p. 2782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshita S, et al. , Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem, 2014. 289(24): p. 16699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cawthorn WP, et al. , Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab, 2014. 20(2): p. 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinoda Y, et al. , Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem, 2006. 99(1): p. 196–208. [DOI] [PubMed] [Google Scholar]


