Abstract
Analysis of non‐Gal antibody induced after pig‐to‐baboon cardiac xenotransplantation identified the glycan produced by porcine beta‐1,4‐N‐acetyl‐galactosaminyltransferase 2 (B4GALNT2) as an immunogenic xenotransplantation antigen. The porcine B4GALNT2 enzyme is homologous to the human enzyme, which synthesizes the human SDa blood group antigen. Most humans produce low levels of anti‐SDa IgM which polyagglutinates red blood cells from rare individuals with high levels of SDa expression. The SDa glycan is also present on GM2 gangliosides. Clinical GM2 vaccination studies for melanoma patients suggest that a human antibody response to SDa can be induced. Expression of porcine B4GALNT2 in human HEK293 cells results in increased binding of anti‐SDa antibody and increased binding of Dolichos biflorus agglutinin (DBA), a lectin commonly used to detect SDa. In pigs, B4GALNT2 is expressed by vascular endothelial cells and endothelial cells from a wide variety of pig backgrounds stain with DBA, suggesting that porcine vascular expression of B4GALNT2 is not polymorphic. Mutations in B4GALNT2 have been engineered in mice and pigs. In both species, the B4GALNT2‐KO animals are apparently normal and no longer show evidence of SDa antigen expression. Pig tissues with a mutation in B4GALNT2, added to a background of alpha‐1,3‐galactosyltransferase deficient (GGTA1‐KO) and cytidine monophosphate‐N‐acetylneuraminic acid hydroxylase deficient (CMAH‐KO), show reduced antibody binding, confirming the presence of B4GALNT2‐dependent antibodies in both humans and non‐human primates. Preclinical xenotransplantation using B4GALNT2‐deficient donors has recently been reported. Elimination of this source of immunogenic pig antigen should minimize acute injury by preformed anti‐pig antibody and eliminate an induced clinical immune response to this newly appreciated xenotransplantation antigen.
Keywords: antibody‐mediated rejection; beta‐1,4‐N‐acetyl‐galactosaminyltransferase 2; cardiac xenotransplantation; genetic modification; xenotransplantation
Abbreviations
- ANXA2
Annexin A2
- B4GALNT2
beta‐1,4‐N‐acetyl‐galactosaminyltransferase 2
- CMAH
cytidine monophosphate‐N‐acetylneuraminic acid hydroxylase
- DBA
Dolichos biflorus agglutinin
- EC
endothelial cell
- Gal
galactose alpha 1,3, galactose
- GalNAc
N‐acetylgalactosamine
- GGTA‐1
alpha‐1,3‐galactosyltransferase
- GI
gastrointestinal
- GTKO
GGTA1 mutant
- Neu5Gc
N‐acetylneuraminic acid
- NHP
non‐human primates
- PBMC
peripheral blood mononuclear cell
- PROC
endothelial cell protein C receptor
- RBC
red blood cell
- TACA
tumor‐associated carbohydrate antigen
- THGP
Tamm‐Horsfall glycoprotein
- XTx
xenotransplantation
1. INTRODUCTION
Xenotransplantation (XTx) is limited by recalcitrant antibody‐mediated rejection occurring either hyperacutely immediately after transplant (HAR) or at later times, referred to as delayed xenograft rejection.1, 2, 3 These rejection mechanisms result from the abundance of human and non‐human primate (NHP) antibodies directed to the classic xenogeneic antigen galactose alpha 1,3 galactose (Gal) which leads to chronic or induced antibody‐mediated vascular endothelial cell (EC) injury or activation.4, 5 Gal is not expressed in humans or Old World NHPs but is expressed at high levels in porcine tissues. Genetically modified pigs, with a mutation in the GGTA‐1 locus (GTKO), do not express the Gal antigen.6 The introduction of GTKO donor organs eliminated anti‐Gal‐mediated xenograft rejection, but did not eliminate antibody‐mediated rejection and instead highlighted the importance of antibody directed to non‐Gal pig antigens.7, 8
Non‐Gal antibody in human and NHP serum is reactive to both protein and carbohydrate antigens.9, 10, 11, 12, 13 The currently identified immunogenic EC carbohydrate antigens include Gal, glycans modified with N‐glycolylneuraminic acid (Neu5Gc), and a carbohydrate antigen (SDa) produced by the porcine β1,4 N‐acetylgalactosaminyltransferase‐2 (B4GALNT2). The human blood group A antigen is also potentially immunogenic14; however, high rates of A‐type blood group polymorphism in the pig permit exclusion of this antigen by selective breeding.15, 16 Collectively, antibody reactivity to the 3 major xenogeneic glycans, Gal, Neu5Gc‐modified glycans, and SDa, accounts for the majority of preformed human anti‐pig antibody reactivity.17, 18
Humans, but not NHPs, make an array of antibodies to Neu5Gc‐modified glycans.19, 20 This antibody reactivity is expected to contribute to clinical xenograft rejection, but determining its impact remains difficult due to the absence of anti‐Neu5Gc antibody in experimental NHP models. There have been excellent recent reviews on Neu5Gc and the potential immunogenicity of Neu5Gc‐modified glycans in XTx.21, 22, 23 Less is known about the expression and immunogenicity of the glycan produced by porcine B4GALNT2. The clinical contribution of B4GALNT2 and SDa, the glycan it synthesizes, has been recently reviewed.24 The purpose of this review is to summarize the current experimental and clinical information on B4GALNT2 gene expression and the SDa antigen, with an emphasis of its potential impact on future clinical use of porcine organs and XTx.
2. THE SDA HUMAN BLOOD GROUP
The SDa blood group, synthesized by the human B4GALNT2 glycosyltransferase, was independently identified 50 years ago by Renton et al25 and by Macvie et al.26 The initial identification was based on a small set of unrelated serum samples which agglutinated the majority of Caucasian red blood cell (RBC) samples. The degree of RBC agglutination varied widely, presenting a mixed field reaction with variably sized agglutinates against a large background of free RBCs. Further analysis, using agglutination inhibition, identified SDa+ expression in human saliva, milk, feces, and urine with more than 50% of people with SDa− RBCs being SDa+ in saliva or urine.26, 27 Based on urinary secretions, persons of European ancestry are 96% SDa+ and the frequency of SDa− individuals, lacking SDa expression on both RBCs and secretions, is about 4%.27
The initial descriptions of anti‐SDa antibody, based on RBC agglutination studies, indicated only 1%‐2% of individuals produced anti‐SDa IgM which weakly agglutinated SDa+ RBCs, preferentially at low temperature.25, 26 The apparent frequency of anti‐SDa antibody increased, however, when cells with stronger SDa expression are used. Clear evidence supporting the classification of SDa as a high‐frequency polyagglutinable antigen28 was evident with the discovery of a Mauritan family in which B‐ or O‐type RBCs were unexpectedly agglutinated by Dolichos biflorus agglutinin (DBA). DBA normally recognizes the N‐acetylgalactosamine (GalNAc) component of blood group A1. The target of this unusual B‐ or O‐type RBC DBA agglutination, called CAD, was subsequently shown to react strongly with anti‐SDa and some non‐CAD, O‐type SDa+ RBCs with high SDa antigen levels were shown to bind DBA.29, 30 This suggested that CAD, also termed Sd(a++), shared a common antigen and that CAD RBCs represent an extreme high level of SDa expression. Serum from most individual agglutinates CAD RBCs,29, 31 indicating that there is commonly a low level of anti‐SDa IgM in human serum. Notably, non‐A1‐type human RBCs are not agglutinated by DBA except, as noted above, in cases of high SDa expression. Due to the generally low expression of SDa on RBC in most individuals, the rarity of CAD individuals and the low level and low‐temperature reactivity of anti‐SDa IgM, the SDa antigen, and antibody do not create a significant transfusion risk. Rare hemolytic reactions have been reported, however, in association with RBCs with high levels of SDa expression.32, 33 This suggests that porcine ECs and organs expressing SDa (discussed below) may be at risk of early antibody‐mediated immune injury.
The B4GALNT2 enzyme, isolated from various human34, 35, 36 and animal tissues,37, 38, 39, 40 catalyzes the synthesis of the SDa blood group antigen by the addition of beta 1,4‐linked GalNAc to an alpha 2,3 sialic acid‐modified N‐acetyl lactosamine acceptor oligosaccharide. The B4GALNT2 enzyme preferentially utilizes N‐acetylneuraminic acid (Neu5Ac) α2,3 galactose β1,4 glucose as an acceptor molecule and does not react with Neu5Ac α2,6‐modified lactose. Additional alpha 2,3 sialylated acceptor molecules that can be modified by B4GALNT2 include both type 1 and 2 lactosamine, Core 1 and 3 O‐linked glycans, and paraglobosides.24 For each of these, the SDa trisaccharide (Table 1) present on human red blood cells (RBCs), rare CAD RBC protein, and glycolipids,41, 42 and the major urinary mucin protein Tamm‐Horsfall glycoprotein (THGP),43 is the terminal B4GALNT2‐dependent structure. The SDa epitope is commonly detected by reactivity to DBA30, 44 or by anti‐SDa monoclonal antibodies.40, 45, 46
Table 1.
SDa containing glycans
| Carbohydrate | Source | Reference |
|---|---|---|
| GalNAc β1,4 (Neu5Ac α2,3) Gal β1,4 GlcNAc β1,3 Gal | RBC glycophorin, THGP | 43 |
|
GalNAc β1,4 (Neu5Ac α2,3) Gal β1,3 (Neu5Ac α2,6) GalNAc‐Ser/Thr
GalNAc β1,4 (Neu5Ac α2,3) Gal β1,4 GlcNAc β1,3 Gal β1,4 Glc‐ceramide |
CAD RBCs | 41, 93 |
| GalNAc β1,4 (Neu5Ac α2,3) Gal β1,4 Glc‐ceramide | GM2 ganglioside | 94 |
| GalNAc β1,4 (Neu5Ac α2,3) Gal β‐R | SDa trisaccharide | a |
This terminal trisaccharide structure is shared between each of the major sources of SDa antigen.
The SDa trisaccharide present on the GM2 ganglioside (Table 1) is synthesized by the distinct B4GALNT1 transferase which shows only limited homology with B4GALNT2.47 This is analogous to the relationship between rat GGTA‐1, which adds Gal antigen to glycoproteins, and iGB3 synthase, which adds the Gal antigen to iGb3, but not glycoproteins.48 Excessive accumulation of GM2 ganglioside, due to a deficiency in β‐N‐acetylhexosaminidase, contributes to ongoing cellular damage associated with Tay‐Sachs disease. Mutations in B4GALNT1 have been identified, which lead to deficiency in GM2 synthesis and are associated with a hereditary spastic paraplegia.49 This suggests that targeted mutation of the porcine B4GALNT1 gene, to eliminate SDa antigen on GM2 gangliosides, may have deleterious effects on donor animals. Mutation of B4GALNT1 would not affect SDa modification of glycoproteins or of other glycolipids that are present in CAD RBCs. For xenotransplantation, the utility of mutating B4GALNT1 remains uncertain as the primary immune stimulation, at least in NHPs, appears to be from B4GALNT2‐dependent antigens.
3. INDUCTION OF ANTI‐SDA ANTIBODY
The GM2 ganglioside is a tumor‐associated carbohydrate antigen (TACA) detected at higher levels in malignant melanoma and other forms of cancer.50 Significant efforts have been made to develop tumor vaccines based on TACA targets including GM2.51 Clinical vaccination studies with purified GM252 or GM2 conjugated to keyhole limpet hemocyanin (GM2‐KLH)53, 54 have shown variable induction of cytolytic anti‐GM2 IgM and IgG. Most patients show an induced antibody response, but only about 10% show sustained induction of cytolytic IgM and IgG which react in both GM2 ELISA and whole‐cell binding (flow cytometry) assays.53 Other patients develop lower levels of cytolytic antibody or antibodies to epitopes not present on the cell surface. It is unclear from these studies what proportion of induced anti‐GM2 antibodies reacts specifically with the SDa trisaccharide or with the glycan ceramide or other GM2 epitopes. In an early clinical study of stage III melanoma patients, those patients receiving GM2 vaccines demonstrated an induced IgM and IgG responses and some evidence of prolonged relapse‐free survival.55 Subsequent phase 3 clinical studies consistently report induction of anti‐GM2 antibody but failed to show a therapeutic benefit for the primary endpoints of relapse‐free survival or overall survival.56, 57 Subset analysis, however, showed a trend for improvement in patients with high titers of anti‐GM2, suggesting that the effects of GM2‐KLH vaccination might be limited by a heterogeneous immune response. More recently, GM2 vaccine development has focused on using neoconjugates made with synthetic SDa tetrasaccharides.58, 59 These conjugates induce strong cytolytic anti‐GM2 antibody in Balb/c mice, which clearly bind the SDa glycan. Clinical GM2 vaccination studies consistently induce an antibody response, which is at least in part directed to the SDa glycan. This suggests that humans, challenged with SDa‐positive xenogeneic tissue, may induce an anti‐SDa response.
4. EXPRESSION OF B4GALNT2 AND SDA
Human B4GALNT2 is predominantly expressed in gastrointestinal (GI) epithelium of the colon with lower levels of expression, evident by Northern blot, in the kidney, ileum, stomach, and rectum.47, 60, 61 These sites also show reactivity to DBA or anti‐SDa antibody.62 Consistent with this analysis, B4GALNT2 enzyme has been purified from human and guinea pig kidney36, 38 and human and porcine large intestine.35, 39 There is also low expression of B4GALNT2, detected by reverse transcription PCR, in most other human tissues.47 This very low expression does not always correlate with detectable SDa staining; however, this does not mean that the antigen is not present as human RBCs exhibit widely variant levels of SDa, but only the highest levels of expression react with DBA.29, 30 Human mast cells, present in connective tissues, may also express B4GALNT2 as they show strong, blood group‐independent reactivity with DBA.63
There is a significant loss of B4GALNT2 expression in colonic tumors compared to normal tissue.64 The reduction of B2GALNT2 expression often correlates with a change in promoter methylation 65, 66 and with an increase in the synthesis of sialyl Lewis antigens.60 The increase in sialyl Lewis antigens appears to result from the reduction in B4GALNT2 expression, which alters the balance of competition between B4GALNT2 and fucosyltransferases in colonic tumors for the same acceptor substrate.24
Expression of B4GALNT2 and SDa in other species is chiefly characterized in mice and as a whole has not been systematically studied in other mammals. Common murine laboratory strains (BALB/c, C57BL/6) exhibit a similar GI epithelial cell‐dominated pattern of B4GALNT2 and SDa antigen expression, with a notable lack of EC expression, as seen in humans. This expression profile is broadly thought to occur in other mammals where, except for the pig, there is a consistent absence of DBA reactivity to vascular ECs.67 The RIII/SJ68 and other laboratory mouse strains which carry the modifier of von Willebrand factor‐1 (Mvwf1) mutation are exceptions, however, as they carry an altered regulatory domain of B4GALNT2 which causes a switch from GI epithelial to vascular EC expression.69, 70 Endothelial cell expression of B4GALNT2 in mice results in altered glycosylation of vWF, which leads to its rapid clearance from circulation and low plasma vWF levels characteristic of Mvwf1 mice. This polymorphic expression is not unique to laboratory mice and is detected at high frequency in wild murine populations.71 Similar cisregulatory variations affecting B4GALNT2 expression are also present Mus spretus and Mus musculus subspecies,72 suggesting that a balanced GI epithelial and vascular endothelial polymorphism of B4GALNT2 expression in mice has been maintained for over 2.8 million years. The selective pressure affecting B4GALNT2 expression may derive from host pathogen interactions as the absence of B4GALNT2 expression and altered glycosylation of the intestinal epithelia is associated with significant shifts in the composition of the microbiota,73 which can effect host sensitivity and pathologic responses to infection.74 Other variations in B4GALNT2 expression are reported in ovarian follicles of FecLL carrier sheep, where it is linked to increased fecundity75 and in respiratory tract expression in the ferret where high SDa expression reduces influenza infectivity.76
5. THE B4GALNT2 GENE IN HUMANS AND NHPS
The murine B4GALNT2 gene was isolated from a cDNA expression library made from a cytotoxic T lymphocyte cell line.40 This expression library was screened for reactivity to hybridoma IgM antibodies (CT1 and CT2) which bound activated cytolytic T lymphocytes, blocked target cell lysis, and reacted with the human SDa blood group antigen.77 The human B4GALNT2 gene was identified based on homology with the mouse gene.47 Both genes are composed of 11 coding exons. The murine B4GALNT2 gene produces a single transcript, whereas the human B4GALNT2 gene uniquely produces alternative splice variants resulting in a short and long form of the human protein.47 The long form of human B4GALNT2 includes an unusual 67 amino acid cytoplasmic domain which may affect cellular localization of this enzyme. The significance of this long isoform and its prevalence in non‐human primates remains under investigation. In both humans and mice, the encoded B4GALNT2 protein is a type II transmembrane protein containing an acidic DXD amino acid motif conserved in glycosyltransferase using UDP sugar as a donor substrate. The human protein shows 74% amino acid identity to mouse polypeptide.
Although human B4GALNT2 expression is essential for synthesis of the SDa antigen, the molecular mechanism, which affects polymorphic SDa expression in human RBCs, has not been identified. Genome‐wide analysis of copy number variation in humans, based on array comparative genomic hybridization (aCGH) or direct deep sequencing, has identified some limited structural variations and deletions in the intron between exons 3 and 4.78, 79, 80 These structural variations occur rarely, are not reported to impact B4GALNT2 expression and thereby appear unlikely to account for polymorphic expression of SDa. Microdeletions in chromosome 17q21.31, which occur near or include portions of the B4GALNT2 locus,81 cause pleiotropic developmental and neurologic effects. These microdeletions are also rare, and their effects have not been attributed to the loss of B4GALNT2 expression. Changes in DNA methylation within the B4GALNT2 promoter affect gene expression 66 and have been noted in Gl cancer cells.65 It is unclear whether this epigenetic mechanism contributes to normal B4GALNT2 expression and polymorphic SDa synthesis in RBCs.
Expression of B4GALNT2 in NHPs is poorly characterized. There has been a report of frequent B4GALNT2 copy number variation in the chimpanzee genome 82 which likely effects B4GALNT2 expression. In an aCGH study of human and chimpanzee DNA, Perry et al82 detected an approximately 68‐kb deletion of the regulatory region, including the first exon of B4GALNT2, in 22 of 26 chimpanzees studied (84%). The deletion was homozygous in 38% of the samples. The effect of this deletion on B4GALNT2 expression and SDa distribution in chimpanzees was not reported; however, the location and magnitude of the deletion would suggest a potential for altered expression or loss of B4GALNT2 function. Several additional aCGH analyses have been performed comparing humans and NHPs. An extensive comparison of human, bonobo, chimpanzee, gorilla, orangutan, gibbon, macaque, baboon, marmoset and lemur was reported by Dumas et al.83 This study was designed to detect duplications or loss of coding sequences and did not observe any such variation in B4GALNT2 in these species. Comparative genomic studies of human, bonobo, chimpanzee, gorilla and orangutan 84, 85, 86 have not reported additional instances of regulatory deletions in B4GALNT2 other than chimpanzee. Comparable genome screening has not been reported for baboons. The predicted Papio anubis B4GALNT2 gene (XM_021928974.1) encodes a protein with 94.6% amino acid identity to human. The current baboon genome assembly (assembly Panu_3.0), unlike the chimpanzee, does not show evidence of a regulatory deletion preceding B4GALNT2 gene. The Papio anubis B4GALNT2 5′ regulatory sequences adjacent to exon 1 of B4GALNT2 (Chr16:30698372‐30778728, assembly Panu_3.0) contain over 70 kb of sequence with a high level of homology to human chromosome 17 (Chr17:49056269‐49137578, GRCh38.p7 Primary Assembly) which overlaps the beginning of the human B4GALNT2 gene. This suggests that B4GALNT2 expression in baboon may be similar to human and that the induced immune response to B4GALNT2‐positive porcine organs seen in baboons 9 may model the immune response in future clinical XTx. Further study of B4GALNT2 expression and SDa antigen distribution in the baboon would be useful.
6. PORCINE B4GALNT2
The porcine B4GALNT2 gene was originally identified by screening a porcine EC cDNA library for non‐Gal antigens expressed in human HEK293 cells.10 Purified IgG enriched in non‐Gal reactivity, derived from pig‐to‐baboon cardiac XTx recipients, was used to identify human HEK293 cell‐expressing library‐encoded non‐Gal porcine antigens on the cell surface. Six porcine gene products were identified. Most of these non‐Gal porcine target genes corresponded to well‐known EC membrane proteins (PROC, CD9, CD46, CD59 and ANXA2). HEK293 expression of these porcine gene products is subject to HEK‐derived glycosylation, suggesting that non‐Gal baboon IgG reactivity to these cell lines is directed to porcine peptide epitopes and not carbohydrates, which would be present on all HEK293 cells. In contrast, the porcine B4GALNT2 gene is a Golgi expressed glycosyltransferase not typically expressed on the cell surface. This indicates that B4GALNT2 expression in HEK293 cells altered the glycosylation of HEK293 cell membrane proteins to create a non‐Gal carbohydrate antigen.
The porcine B4GALNT2 cDNA is derived from an 11‐exon gene and encodes a protein with 76% amino acid identity to the human protein and less than 50% amino acid identity to human B4GALNT1. Expression of porcine B4GALNT2 in HEK293 cells (HEK‐B4T) results in increased reactivity to anti‐B4GALNT2 antibody, anti‐SDa (KM694), and DBA reactivity.9 There is also a 20‐fold enhancement of HEK‐B4T cell, compared to HEK293 cell, sensitivity to antibody‐dependent complement‐mediated lysis using serum from cardiac XTx recipients.
In agriculture‐based strains of pigs, mRNA for B4GALNT2 is expressed in ECs, peripheral blood mononuclear cells (PBMCs), in the GI system and major vascularized organs.9 Porcine EC expression of B4GALNT2 is independent of AO blood group or GGTA‐1 (GTKO) status. Pig ECs are strongly agglutinated by DBA lectin, and cultured ECs from agricultural strains, Yucatan hairless minipigs,87 and Panepinto micropigs88 have been reported to bind DBA, independent of AO blood group status. In vivo vascular B4GALNT2 EC expression, measured by DBA stain, may not be uniform in all vascular beds but is prominent in cardiac capillaries, large renal blood vessels, and glomerular ECs, in reticuloendothelial cells of the liver9 and ECs of the femoral artery.89 Cardiac capillary staining with DBA and EC expression of B4GALNT2 is also evident in Gottingen minipigs (Figure 1A,B). This is consistent with previous studies of ECs from variant porcine backgrounds and suggests that porcine EC expression of B4GALNT2 is not polymorphic and will be commonly present in most pig strains.
Figure 1.
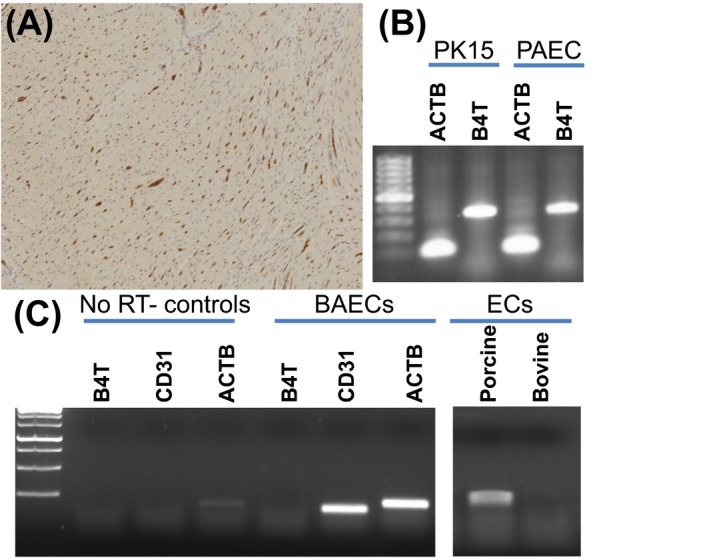
Expression of B4GALNT2 in porcine and bovine cells. A, Dolichos biflorus agglutinin (DBA) lectin staining of Gottingen minipig heart tissue showing capillary endothelial cell staining. B, Reverse transcriptase polymerase chain reaction analysis (RT‐PCR) of B4GALNT2 (B4T) expression in porcine PK15 and porcine aortic ECs (PAEC). RT‐PCR was performed as previously reported 9 using beta‐actin primers (ACTB, forward: CAAGATCATCGCGCCTCCA and reverse: ACTCCTGCTTGCTGATCCACATCT) and B4GALNT2 primers (B4T, forward: TACAGCCCTAGATGTCTGTC and reverse: CTCTCCTCTGAAAGTGTTCGAG). C, RT‐PCR analysis of B4GALNT2 (B4T) total RNA (400 ng/reaction) expression in bovine (BAEC) and porcine endothelial cells (EC). Primers specific for bovine B4GALNT2 (B4T, forward: CTCCAGAGCATTCGTGAGTATT and reverse: TTTGGTGGTGACCTGAGATATG), bovine beta‐actin (ACTB, forward: GTGACATCAAGGAGAAGCTCTG and reverse: AGGAAGGAAGGCTGGAAGA), and CD31 (CD31, forward: GGTCAACGTCACAGAGCTATT and reverse: CACAGTCATGCTTCCCTTCT) were used. Reactions run without a reverse transcriptase step (NO RT controls) show no gene expression. Cultured BAECs show prominent expression of CD31 but do not express B4GALNT2 (1C, left). Additional analysis (1C, right) using RT‐PCR primers conserved in B4GALNT2 in both the porcine and bovine mRNA (forward: ACAAGCTCATGACCATGCTC and reverse: TTTGGTGGTGACCTGAGATATG) detects porcine but not bovine B4GALNT2 expression. RT‐PCR for 1C was performed using one‐step RT‐PCR reaction (USB‐Affymetrix, Santa Clara, CA). Reverse transcription was performed at 42°C for 30 min, followed by 30 cycles of 95°C for 30 s, 58ºC for 30 s, and 72ºC for 50 s, and a final extension for 10 min at 72ºC. Amplification products in 1B and 1C were run in a 1.5% agarose gel
Porcine B4GALNT2 expression is not confined to ECs and is present in PBMCs, spleen,9 and epithelial cells such as PK15 (Figure 1B). Porcine primordial germ cells are also reported to express SDa and stain with anti‐SDa antibody90. In contrast, cultured bovine ECs do not express B4GALNT2 (Figure 1C), which is comparable to human and most murine strains, suggesting that clinical products produced from bovine tissues, such as heart valves, may be less likely to express B4GALNT2‐dependent glycans.
Porcine B4GALNT2 expression in HEK‐B4T cells results in increased anti‐SDa (KM694) and DBA reactivity; however, porcine ECs, which are strongly agglutinated by DBA, do not bind this anti‐SDa monoclonal antibody.9 A similar disparity between lectin and antibody binding was reported for ovine glycoproteins secreted by granulosa cells.75 The strict specificity of KM694, compared to DBA, suggests that on porcine cells, variation in the structure or presentation of the glycan(s) produced by porcine B4GALNT2 may occur. What is clear is that NHP non‐Gal antibody reactivity to HEK‐B4T cells can be eliminated by pre‐absorption with porcine ECs but not by human ECs, which do not express B4GALNT2 or the SDa antigen.9 This indicates that the porcine EC and HEK‐B4T cells share a common, B4GALNT2‐dependent, non‐Gal antigen. In NHPs after cardiac XTx with minimal immune suppression, non‐Gal antibody with preferential binding to HEK‐B4T cells, compared to control HEK cells, is consistently induced.10 This immune response is also evident in GTKO pig‐to‐baboon cardiac XTx recipients with full immune suppression, when a non‐Gal antibody response, determined by antibody reactivity to GTKO pig ECs, is also present.
7. MUTATIONS IN B4GALNT2
Mutations in B4GALNT2 have been produced in mice (John Lowe, Consortium of Functional Glycomics, 69) and pigs.18 The mutant mice are generally healthy and show no histological changes to the major organs but do show variations in the level of neutrophils, plasma cells, and some T‐cell subsets.24 Mutant mice lack DBA staining in GI epithelia and exhibit changes in microfloral composition.73 The loss of B4GALNT2 expression in the GI is associated with greater resistance to Salmonella infection.74 Mutations in B4GALNT2 in the pig have been reported, but only in conjunction with mutations in GGTA‐1 and CMP‐Neu5Ac hydroxylase (CMAH).17, 18 In these triple knockout pigs (GGTA‐1‐KO, CMAH‐KO, and B4GALNT2‐KO), loss of porcine B4GALNT2 gene eliminates DBA lectin binding to pig PBMCs, RBCs, and renal microvascular ECs91, 92 demonstrating the dependence of DBA binding on B4GALNT2 activity. The addition of the B4GALNT2 mutation to the GTKO, CMAH‐KO background, also significantly reduces the level of human non‐Gal IgM and IgG binding to pig PBMCs, confirming the presence of human antibody which binds to the porcine glycan(s) produced by the B4GALNT2 gene. As noted above, the presentation of antigen may differ when porcine B4GALNT2 is expressed in human and porcine cells, but the dependence of human antibody reactivity to B4GALNT2 expression clearly indicates that the SDa trisaccharide, present in both SDa and CAD RBCs, is likely the primary B4GALNT2‐dependent antigenic determinant.
This combination of porcine mutations, however, may have unexpected effects on the glycome which could affect human antibody reactivity in unexpected ways. This may contribute to the rather large apparent reduction in human anti‐SDa IgG binding to these cells, as IgG is not a frequent isotype for human anti‐SDa antibody. The triple knockout pigs are apparently healthy but, to our knowledge, have only been produced through nuclear transfer technology.
8. CONCLUSIONS
The SDa blood group, produced by the B4GALNT2 enzyme, is a recently appreciated xenogeneic antigen. The SDa glycan is commonly expressed in human GI epithelial cells and at widely variant levels in human RBCs and other tissues and fluids. Despite this antigen expression, most humans make low levels of cold reactive anti‐SDa IgM making SDa a polyagglutinable red cell antigen. Recent genetic engineering of the porcine B4GALNT2 locus confirms the presence of preformed NHP and human antibody to B4GALNT2‐dependent antigens. Although the SDa blood group is not a significant transfusion risk, the expression of B4GALNT2 in porcine ECs, the induced antibody response seen in NHPs and the results of cancer vaccination studies suggest that B4GALNT2‐dependent pig glycans may be immunogenic in humans. The major known xenogeneic glycans (Gal, Neu5Gc‐modified glycans, and SDa) account for the majority of preformed human anti‐pig antibody reactivity, with residual antibody reactivity apparently binding to a restricted set of SLA antigens.17 There remains, however, the possibility of additional immunogenic glycans and proteins. Identifying the B4GALNT2‐dependent non‐Gal antigens using serum from XTx recipients with minimal immune suppression underscores the utility of such transplants and suggests that future similar studies using “triple knockout” glycan‐depleted donor organs will be useful for further identification of residual preformed and induced non‐Gal antibody specificities.
AUTHOR CONTRIBUTIONS
Dr. Byrne was responsible for drafting and revision of the manuscript, research design, and data acquisition. Dr Ahmad‐Villiers and Dr Du contributed to data acquisition, analysis, and interpretation. Dr. McGregor contributed to the concept and design of the research. All authors provided the final approval for the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We acknowledge the technical help contributed by Karen Schumacher for writing this manuscript.
Byrne G, Ahmad‐Villiers S, Du Z, McGregor C. B4GALNT2 and xenotransplantation: A newly appreciated xenogeneic antigen. Xenotransplantation. 2018;25:e12394 10.1111/xen.12394
Funding information
NIH Grant AI66310, MRC Grant MR/R006393, and the NIHR UCL Biomedical Research Centre.
REFERENCES
- 1. Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC. Delayed xenograft rejection. Immunol Today. 1996;17:379‐384. [DOI] [PubMed] [Google Scholar]
- 2. Shimizu A, Meehan SM, Kozlowski T, et al. Acute humoral xenograft rejection: destruction of the microvascular capillary endothelium in pig‐to‐nonhuman primate renal grafts. Lab Invest. 2000;80:815‐830. [DOI] [PubMed] [Google Scholar]
- 3. Byrne GW, Azimzadeh AM, Ezzelarab M, et al. Histopathologic insights into the mechanism of anti‐non‐Gal antibody‐mediated pig cardiac xenograft rejection. Xenotransplantation. 2013;20:292‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3‐galactosyltransferase gene‐knockout pigs in baboons. Am J Pathol. 2008;172:1471‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGregor CG, Ricci D, Miyagi N, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93:686‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Byrne GW, McGregor CG, Breimer ME. Recent investigations into pig antigen and anti‐pig antibody expression. Int J Surg. 2015;23:223‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tazelaar HD, Byrne GW, McGregor CG. Comparison of Gal and non‐Gal‐mediated cardiac xenograft rejection. Transplantation. 2011;91:968‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3‐galactosyltransferase gene‐knockout pigs as donors: initial experience. Nat Med. 2005;11:29‐31. [DOI] [PubMed] [Google Scholar]
- 9. Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG. Cloning and expression of porcine beta1,4 N‐acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21:543‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Byrne GW, Stalboerger PG, Du Z, Davis TR, McGregor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011;91:287‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non‐Gal antibody targets after pig‐to‐primate cardiac xenotransplantation. Xenotransplantation. 2008;15:268‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burlak C, Wang ZY, Chihara RK, et al. Identification of human preformed antibody targets in GTKO pigs. Xenotransplantation. 2012;19:92‐101. [DOI] [PubMed] [Google Scholar]
- 13. Chihara RK, Lutz AJ, Paris LL, et al. Fibronectin from alpha 1,3‐galactosyltransferase knockout pigs is a xenoantigen. J Surg Res. 2013;184:1123‐1133. [DOI] [PubMed] [Google Scholar]
- 14. Bravery CA, Hunt BJ, Thorpe SJ, Yacoub M. Expression of ABH antigens in porcine heart tissue. Transplant Proc. 1992;24:445‐446. [PubMed] [Google Scholar]
- 15. Yamamoto F, Yamamoto M. Molecular genetic basis of porcine histo‐blood group AO system. Blood. 2001;97:3308‐3310. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen DT, Choi H, Jo H, et al. Molecular characterization of the human ABO blood group orthologus system in pigs. Anim Genet. 2011;42:325‐328. [DOI] [PubMed] [Google Scholar]
- 17. Martens GR, Reyes LM, Butler JR, et al. Humoral reactivity of renal transplant‐waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class i knockout pigs. Transplantation. 2017;101:e86‐e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Estrada JL, Martens G, Li P, et al. Evaluation of human and non‐human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22:194‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muchmore EA, Diaz S, Varki A. A structural difference between the cell surfaces of humans and the great apes. Am J Phys Anthropol. 1998;107:187‐198. [DOI] [PubMed] [Google Scholar]
- 20. Chou HH, Takematsu H, Diaz S, et al. A mutation in human CMP‐sialic acid hydroxylase occurred after the Homo‐Pan divergence. Proc Natl Acad Sci U S A. 1998;95:11751‐11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Padler‐Karavani V, Varki A. Potential impact of the non‐human sialic acid N‐glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amon R, Reuven EM, Leviatan Ben‐Arye S, Padler‐Karavani V. Glycans in immune recognition and response. Carbohydr Res. 2014;389:115‐122. [DOI] [PubMed] [Google Scholar]
- 23. Salama A, Evanno G, Harb J, Soulillou JP. Potential deleterious role of anti‐Neu5Gc antibodies in xenotransplantation. Xenotransplantation. 2015;22:85‐94. [DOI] [PubMed] [Google Scholar]
- 24. Dall'Olio F, Malagolini N, Chiricolo M, Trinchera M, Harduin‐Lepers A. The expanding roles of the Sd(a)/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT2. Biochim Biophys Acta. 2014;1840:443‐453. [DOI] [PubMed] [Google Scholar]
- 25. Renton PH, Howell P, Ikin EW, Giles CM, Goldsmith KLG. Anti‐Sda, a new blood group antibody. Vox Sang. 1967;13:493‐501. [Google Scholar]
- 26. Macvie SI, Morton JA, Pickles MM. The reaction and inheritance of a new blood group antigen, Sda. Vox Sang. 1967;13:485‐492. [Google Scholar]
- 27. Morton JA, Pickles MM, Terry AM. The Sda blood group antigen in tissues and body fluids. Vox Sang. 1970;19:472‐482. [DOI] [PubMed] [Google Scholar]
- 28. Danials G. Human Blood Groups, 3rd edn Chinchester: Wiley‐Blackwell; 2013. [Google Scholar]
- 29. Cazal P, Monis M, Caubel J, Brives J. Hereditary dominant polyagglutinability: private antigen (Cad) corresponding to a public antibody and a lectin of Dolichos biflorus . Rev Fr Transfus. 1968;11:209‐221. [DOI] [PubMed] [Google Scholar]
- 30. Sanger R, Gavin J, Tippett P, Teesdale P, Eldon K. Plant agglutinin for another human blood‐group. Lancet. 1971;1:1130. [DOI] [PubMed] [Google Scholar]
- 31. Lopez M, Gerbal A, Bony V, Salmon C. Cad antigen: comparative study of 50 samples. Vox Sang. 1975;28:305‐313. [DOI] [PubMed] [Google Scholar]
- 32. Peetermans ME, Cole‐Dergent J. Haemolytic transfusion reaction due to anti‐Sda. Vox Sang. 1970;18:67‐70. [DOI] [PubMed] [Google Scholar]
- 33. Reznicek MJ, Cordle DG, Strauss RG. A hemolytic reaction implicating Sda antibody missed by immediate spin crossmatch. Vox Sang. 1992;62:173‐175. [DOI] [PubMed] [Google Scholar]
- 34. Takeya A, Hosomi O, Kogure T. Identification and characterization of UDP‐GalNAc: NeuAc alpha 2‐3Gal beta 1‐4Glc(NAc) beta 1‐4(GalNAc to Gal)N‐acetylgalactosaminyltransferase in human blood plasma. J Biochem. 1987;101:251‐259. [DOI] [PubMed] [Google Scholar]
- 35. Malagolini N, Dall'Olio F, Di Stefano G, Minni F, Marrano D, Serafini‐Cessi F. Expression of UDP‐GalNAc:NeuAc alpha 2,3Gal beta‐R beta 1,4(GalNAc to Gal) N‐acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989;49:6466‐6470. [PubMed] [Google Scholar]
- 36. Piller F, Blanchard D, Huet M, Cartron JP. Identification of a alpha‐NeuAc‐(2‐3)‐beta‐D‐galactopyranosyl N‐acetyl‐beta‐D‐galactosaminyltransferase in human kidney. Carbohydr Res. 1986;149:171‐184. [DOI] [PubMed] [Google Scholar]
- 37. Serafini‐Cessi F, Dall'Olio F, Malagolini N. Characterization of N‐acetyl‐beta‐D‐galactosaminyltransferase from guinea‐pig kidney involved in the biosynthesis of Sda antigen associated with Tamm‐Horsfall glycoprotein. Carbohydr Res. 1986;151:65‐76. [DOI] [PubMed] [Google Scholar]
- 38. Serafini‐Cessi F, Dall'Olio F. Guinea‐pig kidney beta‐N‐acetylgalactosaminyltransferase towards Tamm‐Horsfall glycoprotein. Requirement of sialic acid in the acceptor for transferase activity. Biochem J. 1983;215:483‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Malagolini N, Dall'Olio F, Guerrini S, Serafini‐Cessi F. Identification and characterization of the Sda beta 1,4,N‐acetylgalactosaminyltransferase from pig large intestine. Glycoconj J. 1994;11:89‐95. [DOI] [PubMed] [Google Scholar]
- 40. Smith PL, Lowe JB. Molecular cloning of a murine N‐acetylgalactosamine transferase cDNA that determines expression of the T lymphocyte‐specific CT oligosaccharide differentiation antigen. J Biol Chem. 1994;269:15162‐15171. [PubMed] [Google Scholar]
- 41. Cartron JP, Blanchard D. Association of human erythrocyte membrane glycoproteins with blood‐group Cad specificity. Biochem J. 1982;207:497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herkt F, Parente JP, Leroy Y, et al. Structure determination of oligosaccharides isolated from Cad erythrocyte membranes by permethylation analysis and 500‐MHz 1H‐NMR spectroscopy. Eur J Biochem. 1985;146:125‐129. [DOI] [PubMed] [Google Scholar]
- 43. Donald AS, Yates AD, Soh CP, Morgan WT, Watkins WM. A blood group Sda‐active pentasaccharide isolated from Tamm‐Horsfall urinary glycoprotein. Biochem Biophys Res Commun. 1983;115:625‐631. [DOI] [PubMed] [Google Scholar]
- 44. Bird GW, Wingham J. Cad(super Sda) in a British family with eastern connections: a note on the specificity of the Dolichos biflorus lectin. J Immunogenet. 1976;3:297‐302. [DOI] [PubMed] [Google Scholar]
- 45. Dohi T, Ohta S, Hanai N, Yamaguchi K, Oshima M. Sialylpentaosylceramide detected with anti‐GM2 monoclonal antibody. Structural characterization and complementary expression with GM2 in gastric cancer and normal gastric mucosa. J Biol Chem. 1990;265:7880‐7885. [PubMed] [Google Scholar]
- 46. Kawamura YI, Kawashima R, Fukunaga R, et al. Introduction of Sd(a) carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res. 2005;65:6220‐6227. [DOI] [PubMed] [Google Scholar]
- 47. Montiel MD, Krzewinski‐Recchi MA, Delannoy P, Harduin‐Lepers A. Molecular cloning, gene organization and expression of the human UDP‐GalNAc:Neu5Acalpha2‐3Galbeta‐R beta1,4‐N‐acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: evidence for an unusual extended cytoplasmic domain. Biochem J. 2003;373:369‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taylor SG, McKenzie IF, Sandrin MS. Characterization of the rat alpha(1,3)galactosyltransferase: evidence for two independent genes encoding glycosyltransferases that synthesize Galalpha(1,3)Gal by two separate glycosylation pathways. Glycobiology. 2003;13:327‐337. [DOI] [PubMed] [Google Scholar]
- 49. Harlalka GV, Lehman A, Chioza B, et al. Mutations in B4GALNT1 (GM2 synthase) underlie a new disorder of ganglioside biosynthesis. Brain. 2013;136:3618‐3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Daniotti JL, Lardone RD, Vilcaes AA. Dysregulated expression of glycolipids in tumor cells: from negative modulator of anti‐tumor immunity to promising targets for developing therapeutic agents. Front Oncol. 2015;5:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ozao‐Choy J, Lee DJ, Faries MB. Melanoma vaccines: mixed past, promising future. Surg Clin North Am. 2014;94:1017‐1030, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Livingston PO, Natoli EJ, Calves MJ, Stockert E, Oettgen HF, Old LJ. Vaccines containing purified GM2 ganglioside elicit GM2 antibodies in melanoma patients. Proc Natl Acad Sci U S A. 1987;84:2911‐2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kitamura K, Livingston PO, Fortunato SR, et al. Serological response patterns of melanoma patients immunized with a GM2 ganglioside conjugate vaccine. Proc Natl Acad Sci U S A. 1995;92:2805‐2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chapman PB, Morrissey DM, Panageas KS, et al. Induction of antibodies against GM2 ganglioside by immunizing melanoma patients using GM2‐keyhole limpet hemocyanin + QS21 vaccine: a dose‐response study. Clin Cancer Res. 2000;6:874‐879. [PubMed] [Google Scholar]
- 55. Livingston PO, Wong GY, Adluri S, et al. Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol. 1994;12:1036‐1044. [DOI] [PubMed] [Google Scholar]
- 56. Kirkwood JM, Ibrahim JG, Sosman JA, et al. High‐dose interferon alfa‐2b significantly prolongs relapse‐free and overall survival compared with the GM2‐KLH/QS‐21 vaccine in patients with resected stage IIB‐III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370‐2380. [DOI] [PubMed] [Google Scholar]
- 57. Eggermont AM, Suciu S, Rutkowski P, et al. Adjuvant ganglioside GM2‐KLH/QS‐21 vaccination versus observation after resection of primary tumor > 1.5 mm in patients with stage II melanoma: results of the EORTC 18961 randomized phase III trial. J Clin Oncol. 2013;31:3831‐3837. [DOI] [PubMed] [Google Scholar]
- 58. Yin Z, Dulaney S, McKay CS, et al. Chemical synthesis of GM2 glycans, bioconjugation with bacteriophage Qbeta, and the induction of anticancer antibodies. ChemBioChem. 2016;17:174‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bay S, Fort S, Birikaki L, et al. Induction of a melanoma‐specific antibody response by a monovalent, but not a divalent, synthetic GM2 neoglycopeptide. ChemMedChem. 2009;4:582‐587. [DOI] [PubMed] [Google Scholar]
- 60. Groux‐Degroote S, Wavelet C, Krzewinski‐Recchi MA, et al. B4GALNT2 gene expression controls the biosynthesis of Sda and sialyl Lewis X antigens in healthy and cancer human gastrointestinal tract. Int J Biochem Cell Biol. 2014;53:442‐449. [DOI] [PubMed] [Google Scholar]
- 61. Dohi T, Nishikawa A, Ishizuka I, et al. Substrate specificity and distribution of UDP‐GalNAc:sialylparagloboside N‐acetylgalactosaminyltransferase in the human stomach. Biochem J. 1992;288(Pt 1):161‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsubokawa D, Goso Y, Kawashima R, et al. The monoclonal antibody HCM31 specifically recognises the Sd(a) tetrasaccharide in goblet cell mucin. FEBS Open Bio. 2012;2:223‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hormia M, Kariniemi AL, Laitinen L, Virtanen I. Dolichos biflorus agglutinin (DBA) reacts selectively with mast cells in human connective tissues. J Histochem Cytochem. 1988;36:1231‐1237. [DOI] [PubMed] [Google Scholar]
- 64. Dohi T, Yuyama Y, Natori Y, Smith PL, Lowe JB, Oshima M. Detection of N‐acetylgalactosaminyltransferase mRNA which determines expression of Sda blood group carbohydrate structure in human gastrointestinal mucosa and cancer. Int J Cancer. 1996;67:626‐631. [DOI] [PubMed] [Google Scholar]
- 65. Kawamura YI, Toyota M, Kawashima R, et al. DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology. 2008;135:142‐151. e143. [DOI] [PubMed] [Google Scholar]
- 66. Wang HR, Hsieh CY, Twu YC, Yu LC. Expression of the human Sd(a) beta‐1,4‐N‐acetylgalactosaminyltransferase II gene is dependent on the promoter methylation status. Glycobiology. 2008;18:104‐113. [DOI] [PubMed] [Google Scholar]
- 67. Roussel F, Dalion J. Lectins as markers of endothelial cells: comparative study between human and animal cells. Lab Anim. 1988;22:135‐140. [DOI] [PubMed] [Google Scholar]
- 68. Ponder BA, Wilkinson MM. Organ‐related differences in binding of Dolichos biflorus agglutinin to vascular endothelium. Dev Biol. 1983;96:535‐541. [DOI] [PubMed] [Google Scholar]
- 69. Mohlke KL, Purkayastha AA, Westrick RJ, et al. Mvwf, a dominate modifier of murine von Willebrand factor, results from altered lineage‐specific expression of a glycosyltransferase. Cell. 1999;96:111‐120. [DOI] [PubMed] [Google Scholar]
- 70. Johnsen JM, Levy GG, Westrick RJ, Tucker PK, Ginsburg D. The endothelial‐specific regulatory mutation, Mvwf1, is a common mouse founder allele. Mamm Genome. 2008;19:32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Johnsen JM, Teschke M, Pavlidis P, et al. Selection on cis‐regulatory variation at B4galnt2 and its influence on von Willebrand factor in house mice. Mol Biol Evol. 2009;26:567‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Linnenbrink M, Johnsen JM, Montero I, Brzezinski CR, Harr B, Baines JF. Long‐term balancing selection at the blood group‐related gene B4galnt2 in the genus Mus (Rodentia; Muridae). Mol Biol Evol. 2011;28:2999‐3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Staubach F, Kunzel S, Baines AC, et al. Expression of the blood‐group‐related glycosyltransferase B4galnt2 influences the intestinal microbiota in mice. ISME J. 2012;6:1345‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rausch P, Steck N, Suwandi A, et al. Expression of the blood‐group‐related gene B4galnt2 alters susceptibility to Salmonella infection. PLoS Pathog. 2015;11:e1005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Drouilhet L, Mansanet C, Sarry J, et al. The highly prolific phenotype of Lacaune sheep is associated with an ectopic expression of the B4GALNT2 gene within the ovary. PLoS Genet. 2013;9:e1003809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jia N, Barclay WS, Roberts K, et al. Glycomic characterization of respiratory tract tissues of ferrets: implications for its use in influenza virus infection studies. J Biol Chem. 2014;289:28489‐28504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Conzelmann A, Lefrancois L. Monoclonal antibodies specific for T cell‐associated carbohydrate determinants react with human blood group antigens CAD and SDA. J Exp Med. 1988;167:119‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zarrei M, MacDonald JR, Merico D, Scherer SW. A copy number variation map of the human genome. Nat Rev Genet. 2015;16:172‐183. [DOI] [PubMed] [Google Scholar]
- 79. Mills RE, Walter K, Stewart C, et al. Mapping copy number variation by population‐scale genome sequencing. Nature. 2011;470:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Coe BP, Witherspoon K, Rosenfeld JA, et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet. 2014;46:1063‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rooryck C, Burgelin I, Stef M, et al. A 580 kb microdeletion in 17q21.32 associated with mental retardation, microcephaly, cleft palate, and cardiac malformation. Eur J Med Genet. 2008;51:74‐80. [DOI] [PubMed] [Google Scholar]
- 82. Perry GH, Yang F, Marques‐Bonet T, et al. Copy number variation and evolution in humans and chimpanzees. Genome Res. 2008;18:1698‐1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dumas L, Kim YH, Karimpour‐Fard A, et al. Gene copy number variation spanning 60 million years of human and primate evolution. Genome Res. 2007;17:1266‐1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sudmant PH, Huddleston J, Catacchio CR, et al. Evolution and diversity of copy number variation in the great ape lineage. Genome Res. 2013;23:1373‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gokcumen O, Babb PL, Iskow RC, et al. Refinement of primate copy number variation hotspots identifies candidate genomic regions evolving under positive selection. Genome Biol. 2011;12:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gazave E, Darre F, Morcillo‐Suarez C, et al. Copy number variation analysis in the great apes reveals species‐specific patterns of structural variation. Genome Res. 2011;21:1626‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Darr D, McCormack KM, Manning T, et al. Comparison of Dolichos biflorus lectin and other lectin‐horseradish peroxidase conjugates in staining of cutaneous blood vessels in the hairless mini‐pig. J Cutan Pathol. 1990;17:9‐15. [DOI] [PubMed] [Google Scholar]
- 88. Johnson EK, Schelling ME, Quitadamo IJ, Andrew S, Johnson EC. Cultivation and characterization of coronary microvascular endothelial cells: a novel porcine model using micropigs. Microvasc Res. 2002;64:278‐288. [DOI] [PubMed] [Google Scholar]
- 89. Solanes N, Rigol M, Ramirez J, et al. Histological basis of the porcine femoral artery for vascular research. Anat Histol Embryol. 2005;34:105‐111. [DOI] [PubMed] [Google Scholar]
- 90. Klisch K, Contreras DA, Sun X, Brehm R, Bergmann M, Alberio R. The Sda/GM2‐glycan is a carbohydrate marker of porcine primordial germ cells and of a subpopulation of spermatogonia in cattle, pigs, horses and llama. Reproduction. 2011;142:667‐674. [DOI] [PubMed] [Google Scholar]
- 91. Wang ZY, Li P, Butler JR, et al. Immunogenicity of renal microvascular endothelial cells from genetically modified pigs. Transplantation. 2016;100:533‐537. [DOI] [PubMed] [Google Scholar]
- 92. Wang ZY, Martens GR, Blankenship RL, et al. Eliminating xenoantigen expression on swine RBC. Transplantation. 2016;101:517‐523. [DOI] [PubMed] [Google Scholar]
- 93. Blanchard D, Cartron JP, Fournet B, Montreuil J, van Halbeek H, Vliegenthart JF. Primary structure of the oligosaccharide determinant of blood group Cad specificity. J Biol Chem. 1983;258:7691‐7695. [PubMed] [Google Scholar]
- 94. Varki A, Cummings RD, Esko JD, et al. Essentials of Glycobiology, 3rd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2017. [PubMed] [Google Scholar]


