Abstract
Background
The cerebellum supports many cognitive functions disrupted in ADHD. Prior neuroanatomic studies have been often limited by small sample sizes, inconsistent findings and a reliance on cross-sectional data, limiting inferences about cerebellar development. Here, we conduct a multi-cohort study using longitudinal data, to characterize cerebellar development.
Methods
Growth trajectories of the cerebellar vermis, hemispheres and white matter were estimated using piecewise linear regression from 1656 youth; of whom 63% had longitudinal data, totaling 2914 scans. Four cohorts participated, all contained childhood data (age 4 to 12 years); two had adolescent data (12 to 25 years). Growth parameters were combined using random effects meta-analysis.
Results
Diagnostic differences in growth were confined to the corpus medullare (cerebellar white matter). Here, the ADHD group showed slower growth in early childhood compared to the typically developing group (left corpus medullare z=2.49, p=0.01; right z=2.03, p=0.04). This reversed in late childhood, with faster growth in ADHD in the left corpus medullare (z=2.06, p=0.04). Findings held when gender, intelligence, comorbidity and psychostimulant medication were considered.
Discussion
Across four independent cohorts, containing predominately longitudinal data, we found diagnostic differences in the growth of cerebellar white matter. In ADHD, slower white matter growth in early childhood was followed by faster growth in late childhood. The findings are consistent with the concept of ADHD as a disorder of the brain’s structural connections, formed partly by developing cortico-cerebellar white matter tracts.
Keywords: Cerebellum, meta-analysis, neuroanatomy, attention deficit hyperactivity disorder, growth, white matter
Introduction
The cerebellum is richly interconnection with the cerebral cortex, forming a series of closed-loops that support not just motor skills but also affective and cognitive processes (Schmahmann, 2004). Several research strands point to the structure as pivotal in attention deficit hyperactivity disorder (ADHD). Firstly, the cerebellum is a core component of the neural circuitry underlying many cognitive functions pertinent to (ADHD), including working memory, response inhibition, attention shifting and the processing of rewards and temporal information (Durston, van Belle, & de Zeeuw, 2011; Noreika, Falter, & Rubia, 2013; Strick, Dum, & Fiez, 2009; Toplak, Dockstader, & Tannock, 2006). Secondly, functional imaging studies provide direct evidence of cerebellar compromise in ADHD. Meta-analysis of functional imaging studies show that ADHD is associated with hypoactivation of the left midline cerebellum during tasks that involve prediction of temporal events, along with the interconnected inferior parietal lobules (pertinent for attention to temporal information) and lateral inferior prefrontal cortex regions (temporal foresight and planning) (Hart, Radua, Mataix-Cols, & Rubia, 2012). Others highlight deficient response inhibition and impaired working memory as core deficits in ADHD, and both in turn have been linked to cerebellar hypoactivation (Rubia, Smith, Taylor, & Brammer, 2007; Szekely, Sudre, Sharp, Leibenluft, & Shaw, 2017; Wolf, 2009). Psychostimulant medication, the mainstay of treatment for ADHD, exerts a particularly potent effect on cerebellar activation, pointing to the structure as important in symptom mediation (Czerniak et al., 2013). There is also evidence of disrupted structural and functional connections between the cerebellum and cortex. Microstructural anomalies in white matter tracts are found in ADHD, with meta-analysis pointing to compromise in the white matter of the left cerebellum (van Ewijk, Heslenfeld, Zwiers, Buitelaar, & Oosterlaan, 2012). Studies of the brain’s intrinsic functional connections (using resting state functional imaging data) also delineate anomalies connectivity between cerebellum and multiple cortical regions (Tomasi & Volkow, 2011). Such demonstrations of anomalies in structural and functional connectivity in the disorder have led to suggestions that it is one example of a developmental dysconnectome (Di Martino et al., 2014). Finally, neuroanatomic imaging studies generally report that the cerebellum is smaller in those with ADHD compared to typically developing children, and the degree of volume loss correlates with symptom severity. Others have further pinpointed these anatomic changes to the posterior and superior vermis with volume loss again correlating with ADHD symptom severity (Bledsoe, Semrud-Clikeman, & Pliszka, 2009; Ivanov, Murrough, Bansal, Hao, & Peterson, 2014; Krain & Castellanos, 2006; Mackie et al., 2007). Meta-analysis of voxel based morphometric studies, which map volume change at a voxel level, found focal compromise in regions of the cerebellar hemispheres that form part of the ventral and dorsal attention networks (Stoodley, 2014). While neuroanatomic studies thus implicate the cerebellum as a key structure in the disorder, there are some important limitations. First, findings are inconsistent with no consensus on the cerebellar regions most affected in the disorder (Ivanov et al., 2014; Mackie et al., 2007; Stoodley, 2014; Valera, Faraone, Murray, & Seidman, 2007). In part, this reflects small sample sizes and differences in factors such as rates of comorbid disorders, gender composition, proportion of children with ADHD receiving psychostimulant treatment and varied approaches to cerebellar measurement. Additionally, with few exceptions, prior studies have used cross-sectional data, limiting the inferences that can be made about cerebellar development in ADHD (Kraemer, Yesavage, Taylor, & Kupfer, 2000).
To overcome these limitations, we use longitudinal data to determine if there are regional differences in cerebellar growth that can be detected consistently across four independent cohorts. The large sample size of this collaborative study allowed us to examine the anatomic correlates of comorbidity, medication and sex. To our knowledge, this is the first multi-cohort longitudinal study of cerebellar development in the disorder.
Methods
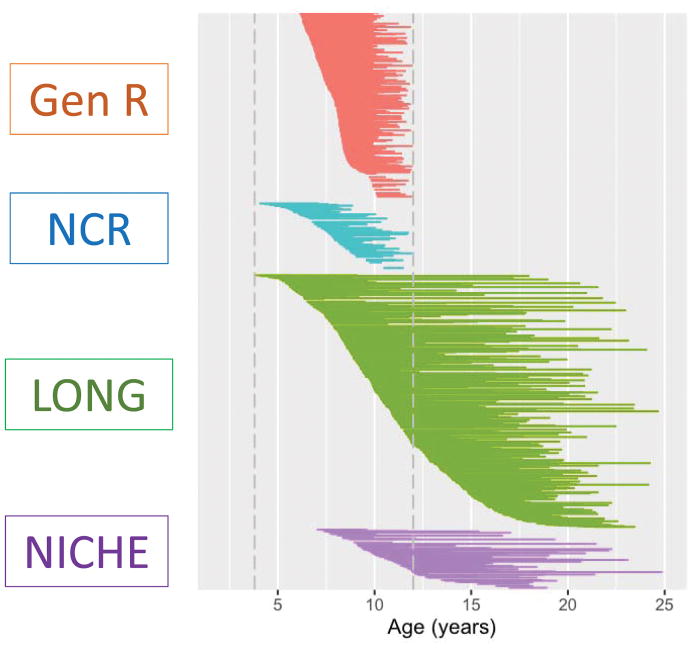
Four independent cohorts participated. In the final analyses, a total of 1656 individuals contributed 2914 neuroanatomic scans. Overall, 1031 (63%) participants had two or more scans, providing 2289 longitudinal observations (79% of all data). All cohorts had neuroimaging data that covered childhood (defined here as ages 4 through 12 years). Two of the cohorts also had substantial data covering adolescence into early adulthood (from 12 to 25 years). Overall 494 (30%) of participants had ADHD; 1026 (62%) were male. Age distributions are shown in Figure 1; inclusion/exclusion criteria in Table S1; psychostimulant treatment status at each scan in Table S2; race/ethnicity and intelligence in Tables S3, S4; and comorbidities in Table S5. Further details given below pertain to those who entered the final analyses, passing quality control steps. Details of imaging protocols are given in Table S6. The data call went out through the Enhancing Neuroimaging Genetics through Meta-Analysis (ENIGMA)-ADHD network in July 2015 and data collection was frozen in October 2016.
Figure 1.
Each subject is represented by a line that joins the ages at which that individual had a neuroanatomic scan. All four cohorts covered childhood (defined as between 4 to 12 years) and the LONG and NICHE cohorts also had data covering adolescence.
LONG cohort
The LONG (Longitudinal Observations, NeuroImaging) cohort was based at intramural NIH and had 727 participants (453 (62%) male) from 528 different families. The participants provided 1339 scans that passed quality control, of which 1062 (79%) were longitudinal (i.e. from a participant who had had two or more scans). The upper age range was set at 25 years as no other cohort had data beyond this age, and the lower age was 3.8years, (mean 12.0 [SD 4.32] years). There were 233 (32%) participants with ADHD. T1-weighted images with contiguous 1.5-mm slices in the axial plane were obtained on all participants using a 3-dimensional spoiled gradient-recalled echo in the steady state acquired on a 1.5-T General Electric (Milwaukee, WI) Signa scanner.
Generation R cohort
This cohort was a subsample of a population-based Dutch birth cohort, consisting of 547 participants (283 males)-described in detail elsewhere (White et al., 2013). The mean age was 9.01 (SD 1.51) years, with a range of 6.11 to 11.98 years. Out of its 985 scans, 876 (89%) were longitudinal. Fifty-nine children (11%) were categorized as having ADHD (predominately based on the Diagnostic Interview Schedule for Children and Adolescents) reflecting its community-based nature. Most children had two scans: baseline data were acquired on a 3-T GE Discovery MR-750 scanner (Milwaukee, WI) and the second scan on a 3-T GE Discovery MR-750w scanner. At baseline, a sagittal high-resolution T1-weighted inversion recovery fast spoiled gradient recalled sequence was collected. For the second scan, images were acquired using a coronal high-resolution T1-weighted inversion recovery fast spoiled gradient recalled BRAVO sequence.
Neuroimaging in Childhood (NICHE) cohort
This Dutch cohort, from the University Medical Centre, Utrecht contained 178 subjects, with 152 (85%) males.; 91 had ADHD (51%) (details elsewhere (van Hulst, de Zeeuw, & Durston, 2015)). Of its total of 282 scans, 175 (62%) were longitudinal; acquired between 6.3 to 24.8 years of age, with an overall mean of 12.8 (SD 3.89) years. A T1-weighted three-dimensional fast field echo scan was acquired on all participants on a 1.5-T MRI-scanner (Philips, Best, The Netherlands).
The Neurobehavioral Clinical Research (NCR) cohort
This cohort was based at the NIH like the LONG cohort, but was independent from the LONG cohort with no overlapping individuals (Shaw et al., 2016). It comprised 204 individuals (138 [67%] males) from 176 different families; 111 [55%] participants had ADHD. The upper age limit was set at 12 years as data beyond this age were sparse. The lower age bound was 4 years, with a mean of 8.36 (SD 1.75) years. Of its 308 scans, 176[57%] were longitudinal. A high-resolution T1 weighted volumetric structural image was obtained using a magnetization prepared rapid gradient echo sequence with ASSET preparation on a 3 T General Electric Signa scanner (USA).
The institutional review board of each institute approved study protocols (IRBs of the National Institutes of Health for the LONG and NCR cohorts, and the Medical Ethics Committee of the Erasmus Medical Center for the Generation R Study and the University Medical Center Utrecht). Written informed consent to participate in the study was obtained from parents and assent from children.
Cerebellar measurement
Cerebellar volumes were estimated using the publically available Multiple Automatically Generated Templates Brain Segmentation Algorithm (MAGeT) technique (Park et al., 2014) (http://cobralab.ca/software/; http://cobralab.ca/atlases/), and the MINC processing suite (http://www.bic.mni.mcgill.ca/ServicesSoftware/MINC). To summarize, an atlas for the cerebellum was defined through expert manual labeling of five cerebella. This atlas was then customized to 21 randomly selected scans of good quality within each cohort using non-linear transformations (M. M. Chakravarty, Sadikot, Germann, Bertrand, & Collins, 2008; M. Mallar Chakravarty et al., 2013), using Advanced Open-Source Tools for Normalization and Neuroanatomy. This set of subjects acted as a set of templates and all other subjects were warped to these templates. This provided 21 candidate segmentations for each subject’s cerebellum. The final segmentation was decided using a voxel-wise majority vote (Collins & Pruessner, 2010). Cerebellar MAGeT performs well against the gold standard of manual labels, with acceptable kappa values (>0.7) which compare well against other regional segmentation tools (e.g. Spatially Unbiased infratentorial Template (M. Mallar Chakravarty et al., 2013; Diedrichsen, Balsters, Flavell, Cussans, & Ramnani, 2009; Park et al., 2014). Quality control of the cerebellar segmentations was by visual inspection of serial sagittal images by two trained raters. Segmentations were rated as ‘1’ if no errors were detected; ‘2’ if minor errors were noted (e.g. poor segmentation of the inferior regions on one or two of the serial slices); ‘3’ if moderate errors were found (e.g. segmentation errors noted on 3 or more consecutive slices); 4 if there were gross errors. If ratings differed by more than one point, the segmentations were re-inspected and a consensus rating reached. Segmentations rated as 1 or 2 were retained. As a result of these measures, 1407 scans were excluded from an original total of 4321 (32%), leaving 2914 scans in the final analyses. Data were more likely to be excluded in younger and male participants, but there was no significant over-representation of those with ADHD and no significant differences in IQ.
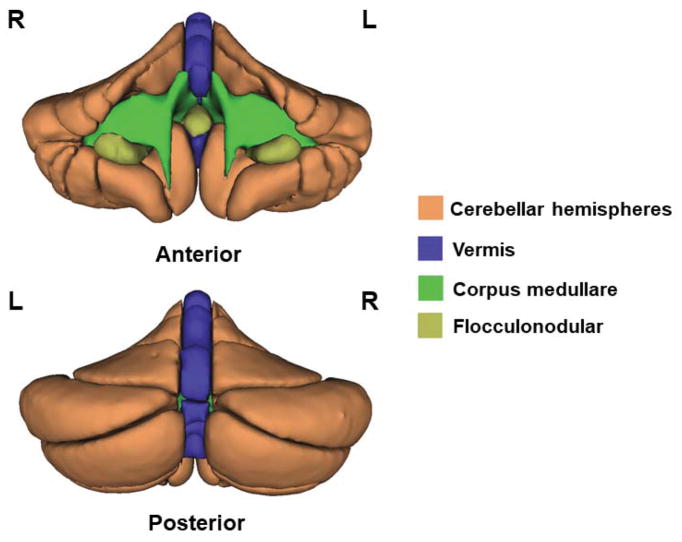
In the principal analyses, we considered the five major cerebellar divisions: the vermis, right and left cerebellar hemispheres and the right and left corpus medullare (the central cerebellar white matter)-shown in Figure 2. Given the functional specificity of different hemispheric and vermal subregions (Ivanov et al., 2014; Stoodley, 2014; Valera et al., 2007) we also conducted exploratory analyses, which further divided the hemispheres and vermis into subregions (the right and left hemispheric anterior, superior posterior and inferior posterior regions, and the corresponding vermal regions and flocculus-see Figure S1.
Figure 2.
Regions of the cerebellum measured.
Modelling childhood growth
All four cohorts provided data in childhood age range (up to 12 years); only two had data in adolescence. There was thus a natural division in the data at age 12 and we modeled growth before and after 12 years of age separately.
To model childhood growth, we used piecewise (segmented) linear regression in which the relationship between the volumes and age was represented by two straight lines connected at a breakpoint (Vieth, 1989). We chose this approach for three reasons. First, prior studies and visual inspection of the data suggested that volumes do not simply increase between 4 to 12 years, but there are non-linear trends (Tiemeier et al., 2010). Piecewise linear regression provides a good approximation of the shape of this underlying relationship. We fit two linear pieces rather than three or more pieces given the relatively narrow age range covered. Second, the approach provides linear growth parameters for each age piece or segment that are readily interpretable and can be combined in meta-analysis. Third, our approach allows us to include random terms thus accounting for the non-independence of observations within individuals, and the non-independence of individuals within families.
We estimated the breakpoints in childhood growth through an iterative, bootstrapped procedure, implemented in the R package ‘Segmented’ (Muggeo, 2003, 2008). At each iteration, a standard linear model is fitted using an initial breakpoint and this value is updated until the algorithm converges on an optimal breakpoint. We estimated breakpoints for all five cerebellar regions, and adopted the average breakpoint at age 8 in further modelling-Tables S7, S8. The proportions of participants with and without ADHD did not differ significantly between the early and late childhood stages for any cohort (all p>0.2). In the final piecewise linear model, age was replaced with two dummy age variables-one representing ages up to age 8, the second representing ages between 8 and 12 (throughout age refers to chronological age). Within each cohort, the jth volume in the ith individual in the kth diagnostic group was modelled as: -
where d is a random effect modeling within-person dependence, which was nested within a random term for family for the LONG and NCR cohorts. The intercept and β terms are fixed effects and eijk represents the residual error. Group difference in trajectories are given by the β3 and β5 term. To aid interpretability, the growth parameters were also expressed as percentage change in volume each year. For the early childhood phase (4 to 8 years), we estimated percentage change over volumes at age 4 and so on.
Growth parameters for each cohort were estimated separately and then combined using a random effect meta-analyses, implemented in the R package mvmeta (Gasparrini, Armstrong, & Kenward, 2012). We used random-effects meta-analysis as there was heterogeneity between cohorts in cerebellar trajectories.
Modeling adolescent growth
Only two cohorts had substantial data covering adolescence. We used linear mixed models to model age related change but did not introduce any additional breakpoints given the relative sparsity of data.
We also repeated analyses adjusting for: IQ, gender, total cerebellar volumes, psychostimulant treatment status and comorbidity.
Results
Cerebellar growth in youth with and without ADHD
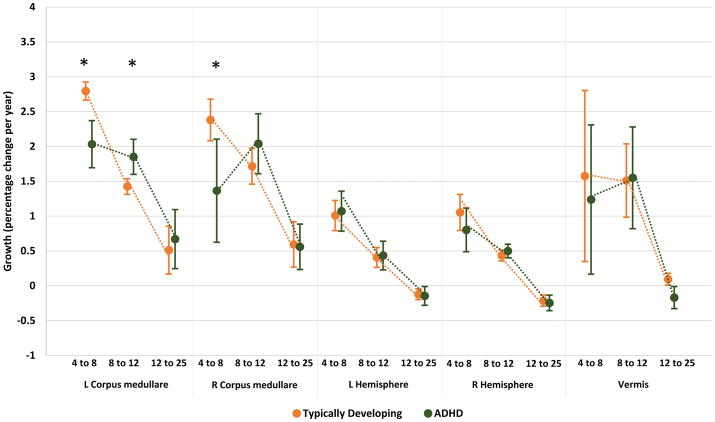
Diagnostic differences in growth emerged for cerebellar white matter only (corpus medullare). Here, the ADHD group showed significantly slower growth in early childhood more pronounced on the left than right (z=2.49, p=0.01; right z=2.03, p=0.04)-Figures 3,4. For the left corpus medullare the growth rate of the ADHD group in early childhood was 2.03[0.33]% per year, compared to the typical rate of 2.79[0.13]% per year. For the right corpus medullare, the ADHD growth rates was 1.36[0.74]% per year, compared to the typical rate of 2.38[0.3]% per year.
Figure 3.
Growth rates (expressed as percentage volume change per year) are shown for each cerebellar region for typically developing and ADHD groups. Rates differed significantly by diagnosis for the left corpus medullare in early and late childhood and for the right corpus medullare in early childhood. * diagnostic difference at p<0.05.
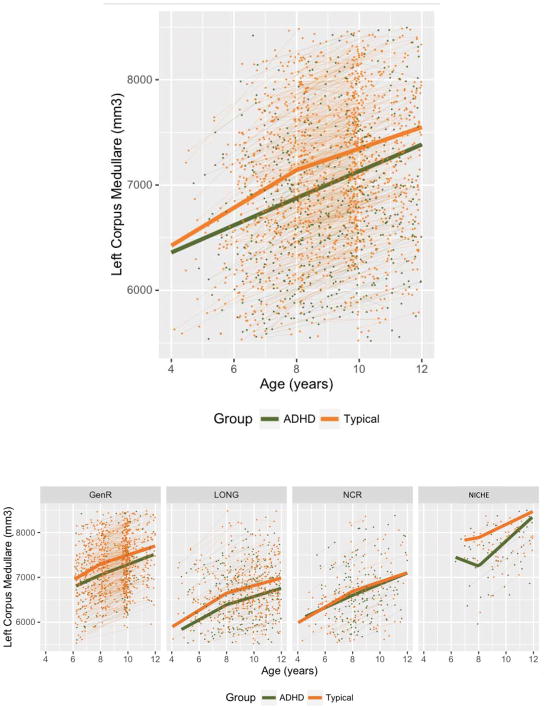
Figure 4.
Top: The thick lines show the results of the meta-analysis for growth of the left corpus medullare in early (4–8 years) and late childhood (8–12years). Individual data points and growth are also shows. Lower: left corpus medullare growth within each cohort.
This pattern reversed in late childhood (from 8 to 12 years). At this stage, the ADHD group showed significantly faster growth of the left corpus medullare (1.85 [0.25]% per year) compared to the typical group (1.43 [0.11]% per year, z=2.06, p=0.04). These diagnostic differences in corpus medullare growth were present in all cohorts but only attained significance in the meta-analysis. Table S9 gives the estimated values and slopes (rates) for each structure.
There were no other diagnostic differences in growth for the rest of the cerebellum-Figures 3. For example, for the right hemisphere in early childhood the typical group increased by 1.05 [0.26]% per year and the ADHD group by 0.8 [0.31]% per year, a non-significant difference in rates (z=0.87, p=0.38). This slowed in both groups between the ages of 8 and 12 (typically developing 0.43[0.08]%; ADHD 0.5[0.1]% per year, z=−0.44, p=0.66). Growth rates for vermis showed a similar early childhood growth in both groups (typical 1.58[1.22]% per year.; ADHD 1.24[1.07]% p.a.; z=0.64, p=0.53) that continued into late childhood (typical 1.51[0.53]% per year.; ADHD 1.55[0.73]% per year.; z=0.001, p=0.99).
During adolescence, there were no diagnostic differences in growth (all p>0.1). The corpus medullare showed volume increase in both groups, whereas the vermis and both hemispheres showed minimal change-Figure 3.
In exploratory analyses, we tested for subregional diagnostic differences in trajectories (examining the anterior, inferior posterior, superior posterior hemispheric and vermal regions). No diagnostic trajectories differences were found except for the right anterior hemisphere in late childhood, where the typical group showed faster growth (z=2.48, p=0.01)-see Table S10. Thus, in general, the lack of diagnostic differences in growth for the hemispheres and vermis held when their subdivisions were considered.
Heterogeneity between cohorts
We tested for cohort differences in the growth rates. Rates did not differ significantly between cohorts for the left corpus medullare in early childhood F[3,708]=1.8, p=0.15; the right corpus medullare in early childhood (F=2.43, p=0.06) and the right hemisphere in late childhood (F=0.64, p=0.59). However, significant cohort differences in growth rates emerged for the other structures - see Fig S2 and Table S11. Such cohort heterogeneity in growth rates supports our use of random effects meta-analytic approach to integrate the growth parameters.
Impact of other variables and robustness analyses
We considered the possibility of gender dimorphism in cerebellar growth in the typical and ADHD groups, by allowing gender to interact with age and diagnosis. No significant high order interactions emerged and thus gender was treated as a covariate in further analyses. We also adjusted for IQ and for total cerebellar volumes. Entering these covariates did not substantially alter the pattern of findings. Thus, growth of the left corpus medullare remained slower in ADHD early childhood at a trend level (z=1.91, p=0.06), faster in late childhood (z=2.22, p=0.03) and in adolescence compared to the typically developing group (z=2.94, p=0.003). The diagnostic difference for the right corpus medullare in the adjusted analysis was significant only in early childhood (z=2.7, p=0.007).
Analyses that evaluated the impact of removing each cohort one by one further tested the robustness of the diagnostic trajectory differences for the corpus medullare: the same overall pattern of findings held in all combinations of three cohorts – Table S12. Moving the breakpoint between the ages of 7 and 9 in half yearly increments also did not substantially alter the early childhood pattern for the corpus medullare– Table S13.
Psychostimulant treatment
Entering medication status at the time of each scan as a covariate did not alter the cerebellar trajectories; the corpus medullare still showed slower growth during early childhood in ADHD (left z=−2.97, p=0.003; right z=−1.96, p=0.05) with a trend to faster growth in late childhood (left; z=1.82, p=0.07; right z=1.63, p=0.1). As in the main analyses, no other diagnostic differences in trajectories were found in analyses adjusting for medication.
Comorbid diagnoses
Findings were largely unchanged when we excluded participants with comorbid diagnoses. Thus, the ADHD group with no comorbidities showed slower early childhood growth of the corpus medullare (left z=−2.52, p=0.01; right z=−2.1, p=0.04) and a trend to faster growth in late childhood for the left corpus medullare (z=1.87, p=0.06). Entering the presence of comorbid disorders as a covariate also did not alter trajectories.
Discussion
In both children with and without ADHD the cerebellar vermis and hemispheres show rapid early childhood growth that subsides to slower growth in late childhood ending with a phase of minimal growth or decrease in adolescence. Diagnostic differences emerged only in the growth of the corpus medullare-the white matter regions that contains the major white matter tracts connecting the cerebellum and cortex. Compared to typically developing children, those with ADHD show significantly slower growth in early childhood, that switches to significantly faster growth in late childhood. The diagnostic trajectory differences were most pronounced in early childhood, and remained when controlling for gender, intelligence, cerebellar volume, comorbid disorders and treatment with psychostimulant medication. This study complements ongoing efforts by ENIGMA that have already reported on subcortical structures with work underway on the cerebral cortex (Hoogman et al., 2017).
The diagnostic trajectory differences in white matter growth rates are consistent with the concept of ADHD as a disorder of the brain’s structural and functional connections-the ‘connectome’ (Di Martino et al., 2014; Konrad & Eickhoff, 2010; Shenton, Kubicki, & Makris, 2014). This model posits that ADHD partly arises from disruption to developing cerebello-cortical networks that are interconnected through white matter bundles carried in the corpus medullare. This is compatible with the imaging literature demonstrating disrupted structural and functional cerebellar connectivity, along with anomalies in cognitive facets supported by cerebellar processing. It is noteworthy that the ventral attention network, sometimes implicated in ADHD, is lateralized to the right cerebral cortex and thus we would expect and indeed find that cerebellar developmental anomalies are left lateralized, given its contralateral connections with the cerebrum (F. X. Castellanos & Proal, 2012; Cortese et al., 2012). The trajectory anomaly found in ADHD– ‘slower then faster’-could be viewed as an instance of delayed development. Equally, it might represent an early childhood partial arrest of development that adjusts itself; or indeed a complete deviation away from typical developmental templates, which nonetheless reaches the same endpoint (in terms of cerebellar volumes).
We can only speculate on mechanisms driving these findings. The cerebellum starts as one of the least heritable brain structures in infancy, but its heritability increases through childhood into adulthood (Gilmore et al., 2010; Wallace et al., 2006). Genes that show increasing developmental expression in the cerebellum are perhaps implicated by this rising heritability. Studies of the transcriptome in the post-mortem cerebellum of adolescent donors free from any psychiatric disorders, find increased expression in multiple ribosome-related genes moving from early to late childhood, such as RN5-8S6, which shows a 55-fold increase in expression (Hawrylycz et al., 2012). This raises the possibility that atypical regulation of these ribosomal genes in children with ADHD could contribute to the dynamic cerebellar anomalies we detect. The cerebellum also shows somewhat distinct patterns of gene expression from cerebral cortex in late childhood, with, for example, increased expression of genes such as SST (somatostatin), CCK (cholecystokinin), and DDN (dendrin, a component of cytoskeletal modifications at the synapse). Of particular interest, myocyte enhancer factor 2C (MEFC2), a gene involved in neuronal maturation, showed 26-fold greater expression in the cerebellum compared to the cerebral cortex, and single nucleotide polymorphisms implicating this gene area associated with ADHD at genome wide levels of significance (Demontis et al., 2017). Less is known about possible environmental exposures. Twin modelling studies can help guide our search, as they find that environmental factors unique to the child (e.g. peer friendships) rather than shared environments (e.g. the local neighborhood) are important in the onset and course of ADHD (Pingault et al., 2015).
While we examined white matter volumes, other imaging modalities such as diffusion tensor imaging (DTI) are needed to parse the microstructure of the inferior, middle and superior peduncles that constitute the corpus medullare. Meta-analysis of cross-sectional DTI studies point to focal decreases in a measure of tract organization in the left corpus medullare in ADHD (van Ewijk et al., 2012). The next step is to determine if developmental changes in white matter microstructure in ADHD parallel those of white matter volume.
Trajectories for the entire cerebellum did not differ by diagnosis, consistent with an earlier findings from one of the few studies to use longitudinal data (originating from the LONG cohort) (F. Castellanos et al., 2002). Another small study of 36 subjects with ADHD found that different adolescent outcomes of childhood ADHD were underpinned by different cerebellar trajectories (Mackie et al., 2007). However, the current data sets largely lack the requisite clinical data and length of follow-up to explore further the links between symptom trajectories and cerebellar trajectories, although this is a central goal for future work.
An advantage of this study is that meta-analyses were conducted on ‘raw’ data rather than a retrospective meta-analysis, based on aggregated results from each site. Nonetheless, in common with all previous multi-site imaging genetic studies, the imaging data were acquired on different scanners. The sites used different field strengths (1.5T and 3T) which could result in differences in contrast to noise ratios and introduce different partial volume effects. Additionally, the Generation R used a 3 Tesla GE system, 750w and this has a wide bore (the other scanners had standard bore sizes). This could introduce variances as keeping a constant field of 3T in the ‘sweet spot’ of a large bore can be challenging. While integrating such data is a major challenge for the field, we note several mitigating factors in this study. First, it was reassuring that similar patterns of regional cerebellar growth were found in all cohorts. Growth rates did not differ significantly by cohort in the left corpus medullare in early childhood, which showed the most prominent diagnostic differences. Second, we attenuated some effects of cohort heterogeneity by processing data on the same servers and software and by having the same raters conduct quality assurance (Gronenschild et al., 2012). Additionally, Generation R is a population cohort and thus differed in its inclusion and exclusion criteria (e.g. in IQ and handling of comorbid disorders) from the three clinical cohorts. To handle these sources of heterogeneity, we conducted analyses separately for each cohort, and only then combined results using a random-effects meta-analytic approach, which assumes the study-specific outcomes are randomly sampled from a multivariate normal distribution of studies.
We note several further limitations. While ODD, the most common comorbidity, did not have a major impact on trajectories we could not consider other important comorbidities such as autistic spectrum disorder, as this was exclusionary in three of the cohorts. We also had relatively few subjects in the pre-school age range and thus could have missed even earlier diagnostic differences in trajectories. Additionally, too few subjects were medication naïve throughout the study to consider them as a separate group in trajectory analyses. However, analyses considering psychostimulant treatment at the time of the scan found it had no significant impact on trajectories or baseline volumes. Previous studies have mostly found psychostimulant medication is associated with an attenuation rather than accentuation of cerebellar anatomic differences between those with and without ADHD (Bledsoe et al., 2009; Ivanov et al., 2014). Combined, these factors suggest that psychostimulant medication is unlikely to have had a major impact on the central findings. While we considered both sex and IQ as covariates, we did not include socio-economic status as we did not have these data on all cohorts and given the international nature of the study, it would be complicated to harmonize the measures used.
We found that the pattern of diagnostic differences in the corpus medullare was similar when we confined analyses to any 3 of the 4 cohorts. This makes it unlikely that the findings are driven by a disproportionate effect of any one cohort. This also suggests that the overall finding is robust to cohort differences in rates of diagnosis, comorbidity and psychostimulant medication. We chose a meta-analytic over mega-analytic approach given the heterogeneity between cohorts in cerebellar trajectories. While other approaches such as spline fitting can model nonlinear relationships, we used segmented regression models given the ready interpretability of the parameters and the ability to model the non-independence of repeated observations.
Integrating neuroanatomic data collected across different sites and across development is a complex endeavor. Notwithstanding the challenges, we find a diagnostic difference in the growth of cerebellar white matter that is consistently present across four independent cohorts. The central findings are consonant with models of ADHD as a disorder of the developing connectome.
Supplementary Material
Subregions of the cerebellar hemispheres and vermis.
Growth rates for each cohort did not differ for the right and left corpus medullare in early childhood and the right hemisphere in late childhood.
Table S1. Major inclusion and exclusion criteria for each cohort.
Table S2. Proportion of ADHD participants on psychostimulant medication at each scan.
Table S3. Parent reported race/ethnicity.
Table S4. Intelligence by cohort and diagnostic group.
Table S5. Comorbid disorders.
Table S6. Imaging parameters for each cohort.
Table S7. Breakpoints for the childhood data under 12 years, estimated by the Segmented R package.
Table S8. Number of observations in each cohort for each age period.
Table S9. The estimated volumes (from the fitted regression) at ages 4 and 8 year for the major divisions of the cerebellum.
Table S10. Diagnostic differences in growth rates for hemispheric and vermal subregions.
Table S11. The estimated volumes at ages 4 and 8 in mm3 for the major divisions of the cerebellum for each cohort.
Table S12. Differences in corpus medullare growth rates between ADHD and typical groups.
Table S13. Moving the breakpoint from age 7 through 9 in half yearly increments.
Key points.
The cerebellum is a key component of several cognitive systems that are disrupted in ADHD, such as working memory and response inhibition.
Prior studies mostly find a smaller cerebellum in association with ADHD, although there is limited data on the development of the structure and some inconsistency in findings.
Here, we use predominately longitudinal data from four independent cohorts to chart cerebellar anatomic development.
We find diagnostic differences in growth were confined to cerebellar white matter. Compared to unaffected children, those with ADHD showed slower growth in early childhood (ages 4 to 8) and faster growth in later childhood (8 to age 12). No diagnostic difference in cerebellar growth emerged in adolescence.
Defining the cerebellar anatomic growth in ADHD can inform interpretation of cognitive anomalies in the disorder.
In this observational study, we did not find any association with psychostimulant medication and volumes of the cerebellum or its growth.
Acknowledgments
The LONG and NCR cohorts are funded by the Intramural Programs of the NHGRI and NIMH. The Generation R Study is supported by the Sophia Children’s Hospital Research Foundation (SSWO) Project #639 and the Netherlands Organization for Health Research and Development (ZonMw) TOP project number 91211021. Supercomputing computations were supported by the NWO Physical Sciences Division (Exacte Wetenschappen) and SURFsara (Lisa compute cluster, www.surfsara.nl). The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. The authors gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Bledsoe J, Semrud-Clikeman M, Pliszka SR. A Magnetic Resonance Imaging Study of the Cerebellar Vermis in Chronically Treated and Treatment-Naïve Children with Attention-Deficit/Hyperactivity Disorder Combined Type. Biological Psychiatry. 2009;65(7):620–624. doi: 10.1016/j.biopsych.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F, Lee P, Sharp W, Jeffries N, Greenstein D, Clasen L, … Rapoport J. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: Beyond the prefrontal-striatal model. Trends in Cognitive Sciences. 2012;16(1):17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty MM, Sadikot AF, Germann J, Bertrand G, Collins DL. Towards a validation of atlas warping techniques. Med Image Anal. 2008;12(6):713–726. doi: 10.1016/j.media.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Steadman P, van Eede MC, Calcott RD, Gu V, Shaw P, … Lerch JP. Performing label-fusion-based segmentation using multiple automatically generated templates. Human Brain Mapping. 2013;34(10):2635–2654. doi: 10.1002/hbm.22092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Pruessner JC. Towards accurate, automatic segmentation of the hippocampus and amygdala from MRI by augmenting ANIMAL with a template library and label fusion. NeuroImage. 2010;52(4):1355–1366. doi: 10.1016/j.neuroimage.2010.04.193. [DOI] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. American Journal of Psychiatry. 2012;169(10):1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniak SM, Sikoglu EM, King JA, Kennedy DN, Mick E, Frazier J, Moore CM. Areas of the brain modulated by single-dose methylphenidate treatment in youth with ADHD during task-based fMRI: a systematic review. Harvard review of psychiatry. 2013;21(3):151. doi: 10.1097/HRP.0b013e318293749e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, … Neale BM. Discovery Of The First Genome-Wide Significant Risk Loci For ADHD. bioRxiv. 2017 doi: 10.1101/145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, … Zuo X-N. Unraveling the miswired connectome: a developmental perspective. Neuron. 2014;83(6):1335–1353. doi: 10.1016/j.neuron.2014.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Durston S, van Belle J, de Zeeuw P. Differentiating Frontostriatal and Fronto-Cerebellar Circuits in Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2011;69(12):1178–1184. doi: 10.1016/j.biopsych.2010.07.037. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward M. Multivariate meta-analysis for non-linear and other multi-parameter associations. Statistics in medicine. 2012;31(29):3821–3839. doi: 10.1002/sim.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Schmitt JE, Knickmeyer RC, Smith JK, Lin W, Styner M, … Neale MC. Genetic and environmental contributions to neonatal brain structure: a twin study. Human Brain Mapping. 2010;31(8):1174–1182. doi: 10.1002/hbm.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenschild EH, Habets P, Jacobs HI, Mengelers R, Rozendaal N, Van Os J, Marcelis M. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PloS one. 2012;7(6):e38234. doi: 10.1371/journal.pone.0038234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Radua J, Mataix-Cols D, Rubia K. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD) Neuroscience & Biobehavioral Reviews. 2012;36(10):2248–2256. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, … Jones AR. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391. doi: 10.1038/nature11405. https://www.nature.com/articles/nature11405 - supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, … Franke B. Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. The Lancet Psychiatry. 2017;4(4):310–319. doi: 10.1016/S2215-0366(17)30049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I, Murrough JW, Bansal R, Hao X, Peterson BS. Cerebellar morphology and the effects of stimulant medications in youths with attention deficit-hyperactivity disorder. Neuropsychopharmacology. 2014;39(3):718–726. doi: 10.1038/npp.2013.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping. 2010;31(6):904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How Can We Learn About Developmental Processes From Cross-Sectional Studies, or Can We? Am J Psychiatry. 2000;157(2):163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clinical Psychology Review. 2006;26(4):433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, 3rd, … Rapoport JL. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder.[see comment] American Journal of Psychiatry. 2007;164(4):647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- Muggeo VM. Estimating regression models with unknown break-points. Statistics in medicine. 2003;22(19):3055–3071. doi: 10.1002/sim.1545. [DOI] [PubMed] [Google Scholar]
- Muggeo VM. Segmented: an R package to fit regression models with broken-line relationships. R news. 2008;8(1):20–25. [Google Scholar]
- Noreika V, Falter CM, Rubia K. Timing deficits in attention-deficit/hyperactivity disorder (ADHD): evidence from neurocognitive and neuroimaging studies. Neuropsychologia. 2013;51(2):235–266. doi: 10.1016/j.neuropsychologia.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Park MTM, Pipitone J, Baer LH, Winterburn JL, Shah Y, Chavez S, … Voineskos AN. Derivation of high-resolution MRI atlases of the human cerebellum at 3T and segmentation using multiple automatically generated templates. NeuroImage. 2014;95:217–231. doi: 10.1016/j.neuroimage.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Pingault JB, Viding E, Galera C, Greven C, Zheng YRP, Rijsdijk F. Genetic and environmental influences on the developmental course of attention-deficit/hyperactivity disorder symptoms from childhood to adolescence. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0469T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Shaw P, Weingart D, Bonner T, Watson B, Park M, Sharp W, … Chakravarty M. Defining the neuroanatomic basis of motor coordination in children and its relationship with symptoms of attention-deficit/hyperactivity disorder. Psychological Medicine. 2016;46(11):2363–2373. doi: 10.1017/S0033291716000660. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kubicki M, Makris N. Understanding alterations in brain connectivity in attention-deficit/hyperactivity disorder using imaging connectomics. Biol Psychiatry. 2014;76(8):601–602. doi: 10.1016/j.biopsych.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Frontiers in Systems Neuroscience. 2014:8. doi: 10.3389/fnsys.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Szekely E, Sudre GP, Sharp W, Leibenluft E, Shaw P. Defining the Neural Substrate of the Adult Outcome of Childhood ADHD: A Multimodal Neuroimaging Study of Response Inhibition. American Journal of Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.16111313. appi. ajp. 2017.16111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. NeuroImage. 2010;49(1):63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2011;71(1):443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak ME, Dockstader C, Tannock R. Temporal information processing in ADHD: findings to date and new methods. Journal of Neuroscience Methods. 2006;151(1):15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61(12):1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews. 2012;36(4):1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- van Hulst BM, de Zeeuw P, Durston S. Distinct neuropsychological profiles within ADHD: a latent class analysis of cognitive control, reward sensitivity and timing. Psychological Medicine. 2015;45(04):735–745. doi: 10.1017/S0033291714001792. [DOI] [PubMed] [Google Scholar]
- Vieth E. Fitting piecewise linear regression functions to biological responses. Journal of applied physiology. 1989;67(1):390–396. doi: 10.1152/jappl.1989.67.1.390. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Eric Schmitt J, Lenroot R, Viding E, Ordaz S, Rosenthal MA, … Neale MC. A pediatric twin study of brain morphometry. Journal of Child Psychology and Psychiatry. 2006;47(10):987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- White T, El Marroun H, Nijs I, Schmidt M, van der Lugt A, Wielopolki PA, … Verhulst FC. Pediatric population-based neuroimaging and the Generation R Study: the intersection of developmental neuroscience and epidemiology. Eur J Epidemiol. 2013;28(1):99–111. doi: 10.1007/s10654-013-9768-0. [DOI] [PubMed] [Google Scholar]
- Wolf RC. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit/hyperactivity disorder. Human Brain Mapping. 2009;30(7):2252. doi: 10.1002/hbm.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subregions of the cerebellar hemispheres and vermis.
Growth rates for each cohort did not differ for the right and left corpus medullare in early childhood and the right hemisphere in late childhood.
Table S1. Major inclusion and exclusion criteria for each cohort.
Table S2. Proportion of ADHD participants on psychostimulant medication at each scan.
Table S3. Parent reported race/ethnicity.
Table S4. Intelligence by cohort and diagnostic group.
Table S5. Comorbid disorders.
Table S6. Imaging parameters for each cohort.
Table S7. Breakpoints for the childhood data under 12 years, estimated by the Segmented R package.
Table S8. Number of observations in each cohort for each age period.
Table S9. The estimated volumes (from the fitted regression) at ages 4 and 8 year for the major divisions of the cerebellum.
Table S10. Diagnostic differences in growth rates for hemispheric and vermal subregions.
Table S11. The estimated volumes at ages 4 and 8 in mm3 for the major divisions of the cerebellum for each cohort.
Table S12. Differences in corpus medullare growth rates between ADHD and typical groups.
Table S13. Moving the breakpoint from age 7 through 9 in half yearly increments.