Abstract
Recent years have witnessed the growing interest in the remote functionalization of alkenes for it offers a strategy to activate the challenging C–H bonds distant from the initiation point via alkene isomerization/functionalization. However, the catalytic enantioselective isomerization/functionalization with one single transition metal catalyst remains rare. Here we report a highly regio- and enantioselective cobalt-catalyzed remote C–H bond borylation of internal alkenes via sequential alkene isomerization/hydroboration. A chiral ligand featured twisted pincer, anionic, and non-rigid characters is designed and used for this transformation. This methodology, which is operationally simple using low catalyst loading without additional activator, shows excellent enantioselectivity and can be used to convert various internal alkenes with regio- and stereoisomers to valuable chiral secondary organoboronates with good functional group tolerance.
Sequential alkene isomerization/functionalization enables enantioselective transformations of remote C–H bonds. Here, the authors report a chiral cobalt catalytic system for the highly enantioselective, remote C–H borylation of internal alkenes via an isomerization/hydroboration sequence.
Introduction
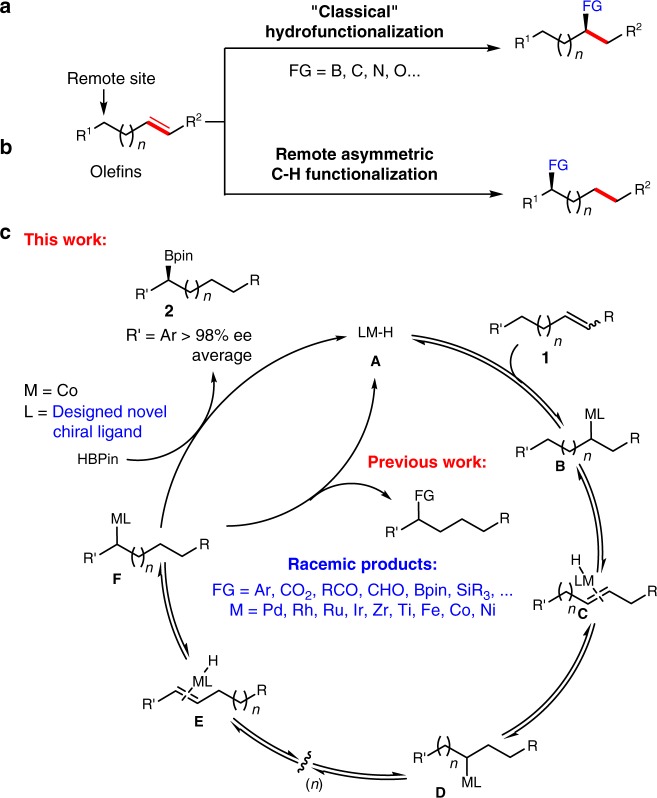
Alkenes containing multiple unactivated C(sp3)-H bond are readily available and abundant feedstock starting materials. Catalytic asymmetric strategies based on alkenes for construction of chiral organic molecules are commonly used. Asymmetric hydrofunctionalization of unactivated alkenes via metal-hydride species has been well established for efficient construction of chiral carbon centers (Fig. 1a)1–3. Among these transformations, alkene isomerization is considered to be a side-reaction to produce regio- and stereoisomers. However, this sequential alkene isomerization/functionalization offers an opportunity for the direct and enantioselective transformation of remote unactivated C(sp3)-H bonds to carbon–carbon or carbon–heteroatom bonds, which is fundamentally important and challenging for highly efficient organic synthesis (Fig. 1b)4–15. A general pathway for the remote functionalization of alkenes via isomerization is illustrated in Fig. 1c. Alkene 1 undergoes coordination and insertion into metal hydrogen bond to form alkyl metal species B that initiates alkene isomerization. Species B goes through β-H elimination to generate species C. After a serial of chain-walking process, a more stable alkyl metal species F, such as terminal alkyl or benzylic metal species, is formed as a terminal intermediate. Finally, species F could be trapped with a variety of reagents to afford products and regenerate catalyst, which offered a favorable thermodynamic driving force.
Fig. 1.
Remote functionalization of alkenes via sequential alkene isomerization/functionalization. a Asymmetric hydrofunctionalization of alkenes. b The concept of asymmetric remote functionalization of alkenes via isomerization. c A general pathway for the remote functionalization of alkenes via isomerization
Recent years have witnessed the important progress in the field of catalytic alkene isomerization/functionalization with various coupling reagents5,16, such as ArX17–19, CO220,21, CO/H222, HBpin23–26, R3SiH27–29, and so on11,30,31, to afford the corresponding coupling products. Additionally, the catalytic asymmetric sequential functionalization/isomerization of alkenes terminated by oxygen-motif has been demonstrated by Sigman32,33. However, the catalytic enantioselective isomerization/functionalization with one single transition metal catalyst is restricted to only few examples5,16. Nishimura and coworkers34 used Iridium catalyst to achieve alkene isomerization terminated by ether group and the following asymmetric hydroarylation. The development of asymmetric alkene isomerization/functionalization processes using single catalyst system is highly desirable.
Chiral organoboronates are of significant utility in asymmetric synthesis for constructing a wide range of other functional groups through C-B bond transformation in a stereospecific fashion35,36. To date, several strategies37, such as stereospecific organoboronate homologation38,39, borylation of benzylic electrophiles40,41, asymmetric hydrogenation of alkenylboronic esters42,43, and asymmetric hydroboration of alkenes44–53, have been developed for construction of chiral secondary organoboronates. However, asymmetric hydroboration of a mixture of alkenes isomers to deliver chiral products has not been previously reported. Our group is continuously investigating asymmetric iron- or cobalt-catalyzed hydrofunctionalization of alkenes based on the ligand design54–59. Recently, we have developed a cobalt-catalyzed asymmetric sequential hydroboration/hydrogenation of internal alkynes, affording a series of chiral secondary organoboronates56. The control experiment demonstrated that cobalt-catalyzed asymmetric hydroboration of internal alkenes afforded secondary organoboronates with poor enantioselectivity. It would be ideal to develop a highly enantioselective cobalt-catalyzed hydroboration of internal alkenes.
Here, we report a cobalt-catalyzed asymmetric remote C–H borylation of internal alkenes via isomerization/hydroboration using a imidazoline phenyl picoliamide (ImPPA) ligand with high enantioselectivity (>97% ee in most cases) (Fig. 1c).
Results
Reaction optimization
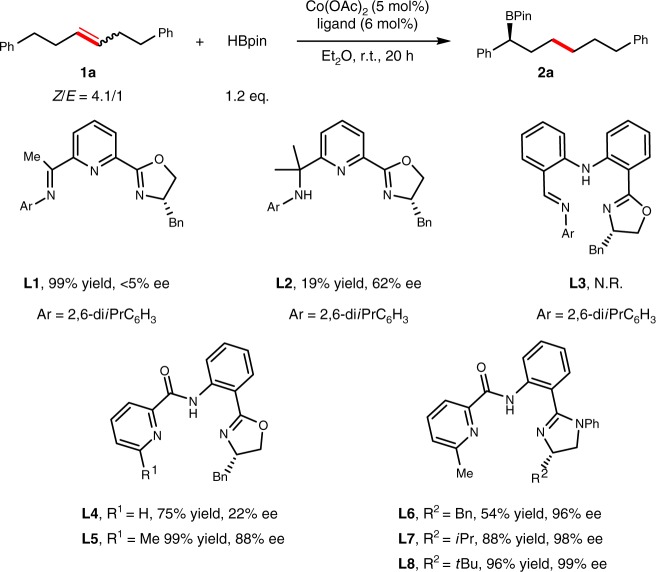
The simple internal alkene 1a was chosen as a model substrate (see Fig. 2 and Supplementary Tables 1, 2). When chiral OIP ligand L1 was used54, the cobalt-catalyzed isomerization/hydroboration reaction of 1a with HBpin was carried out to deliver 2a in 99% yield, however, with less than 5% ee. The use of amino-derivated ligand L260 or iminoaniline-derivated ligand L361 led to a significant drop-off in yield, whereas the use of L2 improved the enantioselectivity to 62% ee. Using a well-defined ligand L462, the remote borylative reaction could afford 2a in 75% yield with 22% ee. Encouragingly, when ligand L5 containing a methyl group at 6-position on pyridine was used, the enantioselectivity was dramatically promoted to 88% ee. Replacement of substituents on pyridine or oxazoline improved the enantioselectivity to 93% ee (see Supplementary Table 1). To our delight, the use of a more electron-rich phenyl-protected imidazoline (L6) instead of oxazoline led to a significant improvement in enantioselectivity (96% ee). Assessment of various imidazolines showed that L8 with a more bulky tert-butyl group was the most effective ligand to afford 2a in 96% yield with 99% ee. Catalyst loading could be further decreased to 2.5 mol% to afford 2a in 90% yield with 98% ee. The standard conditions are identified as 1 mmol of alkene, 1.2 mmol of HBpin, 2.5 mol% of Co(OAc)2, 3 mol% of L8 in 1.0 mL of Et2O for 20 h.
Fig. 2.
Ligands screen for asymmetric isomerization/hydroboration. Reaction conditions: 1a (1 mmol), HBpin (1.2 mmol), Co(OAc)2 (5.0 mol%), ligand (6.0 mol%), Et2O (1 M), r.t., 20 h
Substrate scope
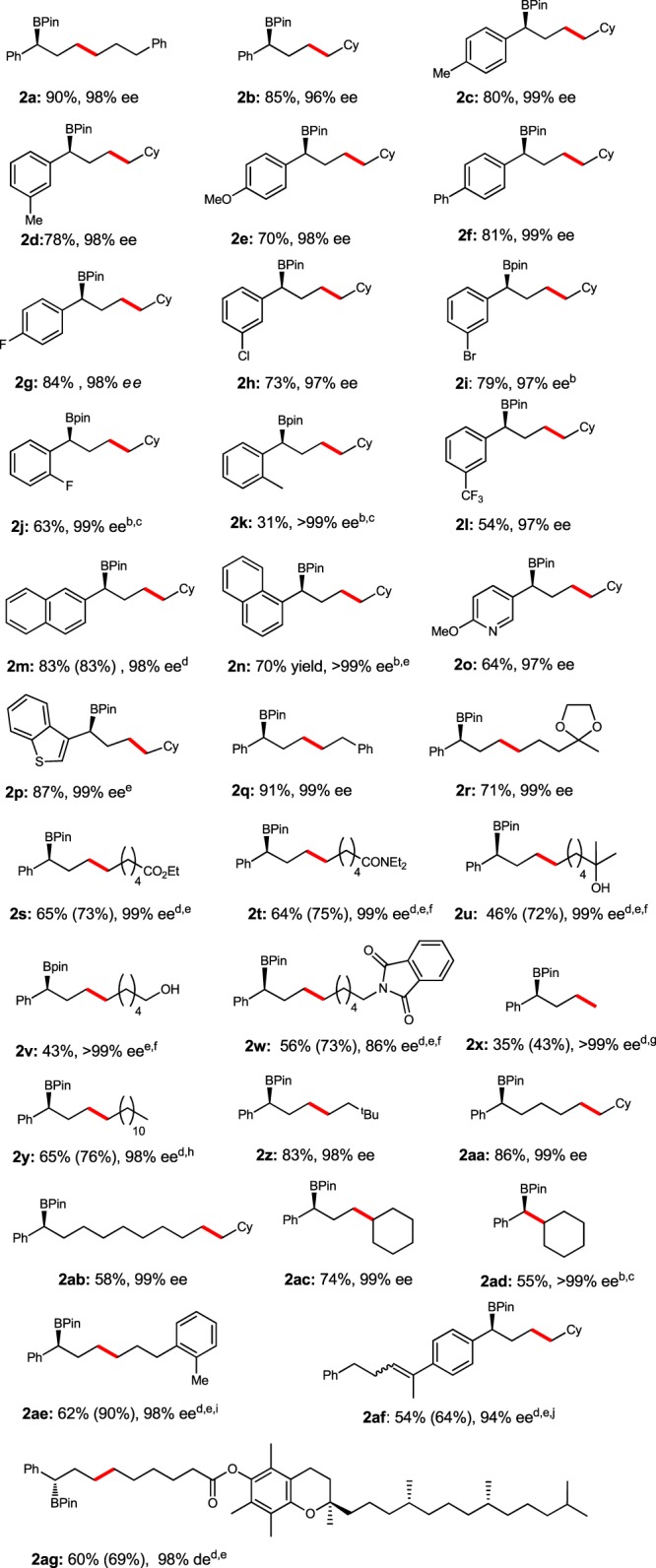
With the optimized conditions in hand, we explored the scope of the olefins (Table 1). The cyclohexyl alkene could participate to deliver the isomerization/hydroboration product 2b in 85% yield with 96% ee. The electron-donating and electron-withdrawing groups on phenyl ring were tolerated to afford 2c–2l in 31–84% yields with 97–>99% ee. Particularly, ortho-substituted alkene 1k could also participate in the reaction to afford 2k in 31% yield with excellent enantioselectivity (>99% ee). The alkenes containing polycyclic ring and heterocycle, such as 2-naphthyl (1 m), 1-naphthyl (1n), 3-pyridyl (1o) and 3-benzo[b]thiophenyl (1p), could be converted to the corresponding products 2m–2p in 64–87% yields with 97–>99% ee. Alkenes containing various functional groups, such as acetal (1r), ester (1s), amide (1t), tertiary alcohol (1u), and protected amine (1w) could be tolerated to afford corresponding boronic esters in 46–71% yields with 86–99% ee. Particularly, alkene 1v with primary alcohol could also participate in the reaction and afford the product in 43% yield with 99% ee. The reaction of terminal alkene 1× with HBpin afforded a mixture of the desired product 2× with 99% ee and terminal borylated product with a b/l ratio of 1/1. The alkene with a linear undecyl group could be reacted to afford 2y in 65% yield and 98% ee with a b/l ratio of 4/1. The reactions of alkenes containing terminal tert-butyl (1z) and cyclohexyl group (1aa) gave the benzylic borylated products with high regio- and enantioselectivities, even walking over eight carbon–carbon bonds (2ab, 58% yield, 99% ee). Remarkably, the trisubstituted alkene 1ac and 1ad could also participate in the transformation to afford the corresponding products in 74% yield with 99% ee and 55% yield with >99% ee, respectively. Alkene 1ae could also be transformed to 2ae in 67% yield with 11/1 rr and 98% ee. Alkene 1af containing a disubstituted and a trisubstituted olefin has also been tested in the reaction which afforded a mixture of (E)-2af and (Z)-2af both in 94% ee. Vitamin E-derivated olefin 1ag was smoothly converted to the corresponding product 2ag in 60% yield with 98% de. Due to the unstability, some products (2m, 2s–2w, 2ae–2ag) were obtained after being directly oxidized to the corresponding alcohols. The absolute configuration was verified by comparison of the optical rotation of 2q with previously reported data and the other products were then assigned by analogy63.
Table 1.
Substrate scope of enantioselective isomerization/hydroboration of alkenes
aStandard conditions: 1 (1 mmol), HBpin (1.2 mmol), Co(OAc)2 (2.5 mol%), L8 (3 mol%), Et2O (1 M), r.t., 20 h
b48 h
cCo(OAc)2 (10 mol%), L8 (12 mol%)
dNMR yield for boronic ester in the parentheses; isolated yield for corresponding alcohol outside the parentheses
eCo(OAc)2 (5 mol%), L8 (6 mol%)
fHBpin (2.0 eq.)
g1/1 rr
h4/1 rr
i11/1 rr
jZ/E = 1.2/1
Gram-scale reaction and synthesis of bioactive molecule
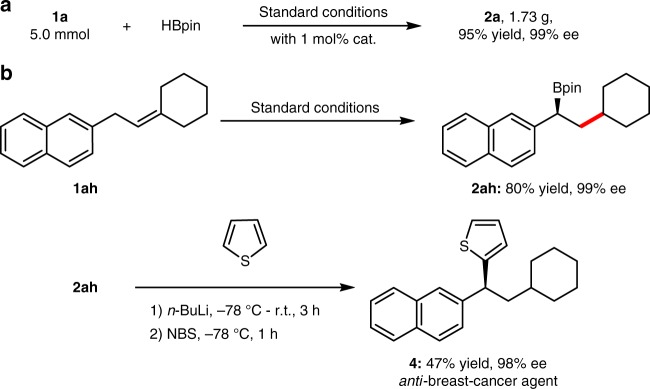
Notably, the preparation of 2a could be scaled up in 95% yield with 99% ee using 1 mol% of Co(OAc)2 and 1.2 mol% of ligand (Fig. 3a). Alkene (1ah) could be transformed smoothly to afford 2ah in 80% yield with 99% ee which could easily undergo C–C bond cross-coupling in a stereospecific manner64 to synthesize anti-breast-cancer agent 4 (Fig. 3b).
Fig. 3.
Applications. a Gram-scale reaction. b Synthesis of anti-breast-cancer agent 4
Mechanistic study and other applications
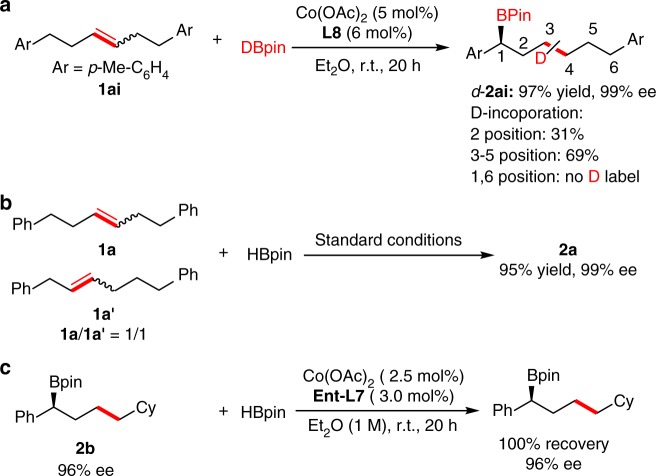
Cobalt-catalyzed deuterium labeling experiment was also conducted with DBpin (Fig. 4a). Stirring a mixture of 1ai and DBpin in the presence of 5 mol% of Co(OAc)2 and 6 mol% of L8 furnished d-2ai with 31% d-incorporation in 2-position. Detectable amounts of deuterium were also located in the interior (3–5) positions with 69% d-incorporation in total. No deuterium was detected at benzylic positions showed that species E underwent olefin reinsertion step to prefer to form more stable benzylic cobalt species F rather than non-benzylic alkyl cobalt species. It should be note that a mixture of 1a and 1a′ (1/1) could be transformed smoothly to a single product 2a in 95% yield with 99% ee under the standard conditions (Fig. 4b), which demonstrated the power of this catalytic system to utilize a mixture of geometrical and positional alkene isomers. Alkenes (E)-1aj and (Z)-1aj were subjected to the reaction system and the result shows that the stereochemistry of the starting olefin has no impact on the kinetics of the reaction (see Supplementary Fig. 246). The reaction of product 2b under the standard conditions using ligand Ent-L7 (the enantiomer of L7) was conducted and no reaction occurred. The ee value of boronate 2b did not change, which indicated that the formation of the carbon boron bond was irreversible (Fig. 4c).
Fig. 4.
Isotope labeling and control experiments. a Deuterium labeling experiment. b Utilization of a mixture of geometrical and positional alkene isomers. c The reaction of product 2b with HBpin using enantiomer of L7
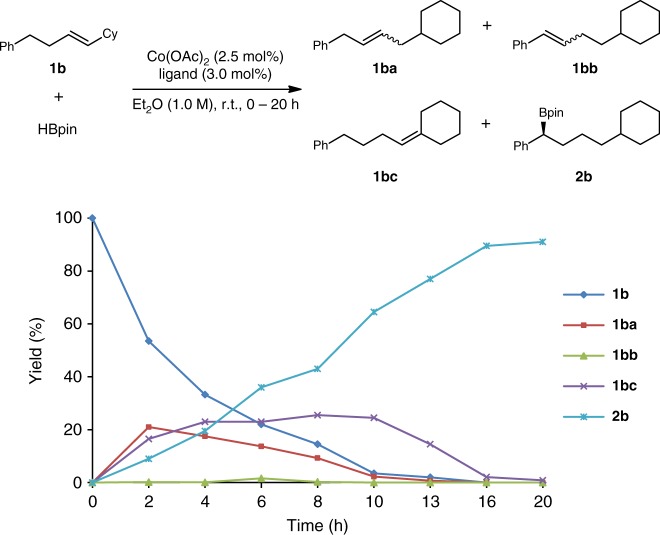
Time course study
The time course experiment (detail see Supplementary Table 5) of 1b was conducted (Fig. 5). The observation of alkenes 1ba, 1bb, and 1bc in the process showed that the internal alkene 1b underwent a double bond walking process to both the benzylic position and cyclohexyl position. Only a small amount of the benzylic alkene 1bb (<5%) was observed during the whole process, which demonstrated that the benzylic alkyl cobalt species F might undergo a rapid σ-bond metathesis with HBpin to afford the chiral organoboronic ester 2b.
Fig. 5.
The time course study of 1b. Reaction conditions: 1b (0.5 mmol), HBpin (0.6 mmol), Co(OAc)2 (2.5 mol%), L8 (3.0 mol%), Et2O (1 M), r.t., 0–20 h
Discussion
In summary, a highly regio- and enantioselective cobalt- catalyzed remote C–H bond borylation of internal alkenes via sequential alkene isomerization/hydroboration is developed. A chiral ImPPA ligand featured twisted pincer, anionic, and non-rigid characters is designed and used. This protocol is operationally simple and additional activator-free. The commonly useless mixture of internal alkenes is used for highly efficient and selective construction of valuable chiral secondary organoboronates with good functional group tolerance. The development of asymmetric transformations based on ligand design will be continuously carried out at our laboratory.
Methods
Materials
For NMR spectra of compounds in this manuscript, see Supplementary Figs. 1–204. For HPLC spectra of compounds in this manuscript, see Supplementary Figs. 205–245. For the optimization of reaction conditions and control experiments of alkene 1a, see Supplementary Tables 1, 2. For the experimental procedures and analytic data of compounds synthesized, see Supplementary Methods.
General procedure for remote C–H borylation of internal alkenes
To a 25 mL flame-dried Schlenk flask cooled under nitrogen, Co(OAc)2 (0.025 mmol), L8 (0.03 mmol), Et2O (1 mL) were added. The mixture was stirred at room temperature for 5 min. Then, alkene (1.0 mmol), HBpin (180 μL, 1.2 mmol) were added in sequence and stirred at room temperature for 20 h. The resulting solution was filtered by a short pad of silica gel and washed by ether (10 mL × 2). The combined filtrate was concentrated and purified by flash column chromatography using PE/EtOAc = 20/1 as the eluent to afford the corresponding product.
Electronic supplementary material
Acknowledgements
Financial support for this study was provided by the National Basic Research Program of China (2015CB856600), NSFC (21772171, 21472162), “Thousand Youth Talents Plan”, the Fundamental Research Funds for the Central Universities of China (2018QNA3009), and Zhejiang University. We also thank Professor BingFeng Shi in Zhejiang University for helpful suggestion and discussion.
Author contributions
X.C. and Z.C. performed the experiments. X.C., Z.C., and J.G. prepared the supporting information. Z.L. and X.C. designed the experiments. Z.L., X.C., and J.G. prepared the manuscript.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information file. The X-ray crystallographic coordinates for structures of (L8-H)·PdOAc has been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition nos. CCDC 1588226. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. The experimental procedures and characterization of all new compounds are provided in the Supplementary Information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-06240-y.
References
- 1.Mlynarski SN, Schuster CH, Morken JP. Asymmetric synthesis from terminal alkenes by cascades of diboration and cross-coupling. Nature. 2014;505:386–390. doi: 10.1038/nature12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Shi SL, Niu DW, Liu P, Buchwald SL. Catalytic asymmetric hydroamination of unactivated internal olefins to aliphatic amines. Science. 2015;349:62–66. doi: 10.1126/science.aab3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan AJ, Lalic G, Sadighi JP. Coinage metal hydrides: synthesis, characterization, and reactivity. Chem. Rev. 2016;116:8318–8372. doi: 10.1021/acs.chemrev.6b00366. [DOI] [PubMed] [Google Scholar]
- 4.Larionov E, Li HH, Mazet C. Well-defined transition metal hydrides in catalytic isomerizations. Chem. Commun. 2014;50:9816–9826. doi: 10.1039/C4CC02399D. [DOI] [PubMed] [Google Scholar]
- 5.Sommer H, Juliá-Hernández F, Martin R, Marek I. Walking metals for remote functionalization. ACS Cent. Sci. 2018;4:153–165. doi: 10.1021/acscentsci.8b00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, et al. Enantioselective cyanation of benzylic C-H bonds via copper-catalyzed radical relay. Science. 2016;353:1014–1018. doi: 10.1126/science.aaf7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G, et al. Ligand-accelerated enantioselective methylene C(sp3)-H bond activation. Science. 2016;353:1023–1027. doi: 10.1126/science.aaf4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi GJ, Zhu QL, Miller DC, Gu CJ, Knowles RR. Catalytic alkylation of remote C-H bonds enabled by proton-coupled electron transfer. Nature. 2016;539:268–271. doi: 10.1038/nature19811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao KB, Negretti S, Musaev DG, Bacsa J, Davies HML. Site-selective and stereoselective functionalization of unactivated C-H bonds. Nature. 2016;533:230–234. doi: 10.1038/nature17651. [DOI] [PubMed] [Google Scholar]
- 10.Li HH, Mazet C. Iridium-catalyzed selective isomerization of primary allylic alcohols. Acc. Chem. Res. 2016;49:1232–1241. doi: 10.1021/acs.accounts.6b00144. [DOI] [PubMed] [Google Scholar]
- 11.Kochi T, Hamasaki T, Aoyama Y, Kawasaki J, Kakiuchi F. Chain-walking strategy for organic synthesis: catalytic cycloisomerization of 1,n-dienes. J. Am. Chem. Soc. 2012;134:16544–16547. doi: 10.1021/ja308377u. [DOI] [PubMed] [Google Scholar]
- 12.Masarwa A, et al. Merging allylic carbon-hydrogen and selective carbon-carbon bond activation. Nature. 2014;505:199–203. doi: 10.1038/nature12761. [DOI] [PubMed] [Google Scholar]
- 13.Lin LQ, Romano C, Mazet C. Palladium-catalyzed long-range deconjugative isomerization of highly substituted α, β-unsaturated carbonyl compounds. J. Am. Chem. Soc. 2016;138:10344–10350. doi: 10.1021/jacs.6b06390. [DOI] [PubMed] [Google Scholar]
- 14.Romano C, Mazet C. Multicatalytic stereoselective synthesis of highly substituted alkenes by sequential isomerization/cross-coupling reactions. J. Am. Chem. Soc. 2018;140:4743–4750. doi: 10.1021/jacs.8b02134. [DOI] [PubMed] [Google Scholar]
- 15.Bruffaerts J, Vasseur A, Marek I. Alkene-zipper catalyzed selective and remote retro-ene reaction of alkenyl cyclopropylcarbinol. Adv. Synth. Catal. 2018;360:1389–1396. doi: 10.1002/adsc.201701481. [DOI] [Google Scholar]
- 16.Vasseur A, Bruffaerts J, Marek I. Remote functionalization through alkene isomerization. Nat. Chem. 2016;8:209–219. doi: 10.1038/nchem.2445. [DOI] [PubMed] [Google Scholar]
- 17.He YL, Cai YL, Zhu SL. Mild and regioselective benzylic C-H functionalization: Ni-catalyzed reductive arylation of remote and proximal olefins. J. Am. Chem. Soc. 2017;139:1061–1064. doi: 10.1021/jacs.6b11962. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Bruffaerts J, Vasseur A, Marek I. A unique Pd-catalysed Heck arylation as a remote trigger for cyclopropane selective ring-opening. Nat. Commun. 2017;8:14200. doi: 10.1038/ncomms14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dupuy S, Zhang KF, Goutierre AS, Baudoin O. Terminal-selective functionalization of alkyl chains by regioconvergent cross-coupling. Angew. Chem. Int. Ed. 2016;55:14793–14797. doi: 10.1002/anie.201608535. [DOI] [PubMed] [Google Scholar]
- 20.Juliá-Hernández F, Moragas T, Cornella J, Martin R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature. 2017;545:84–88. doi: 10.1038/nature22316. [DOI] [PubMed] [Google Scholar]
- 21.Gaydou M, Moragas T, Juliá-Hernández F, Martin R. Site-selective catalytic carboxylation of unsaturated hydrocarbons with CO2 and water. J. Am. Chem. Soc. 2017;139:12161–12164. doi: 10.1021/jacs.7b07637. [DOI] [PubMed] [Google Scholar]
- 22.Seayad A, et al. Internal olefins to linear amines. Science. 2002;297:1676–1678. doi: 10.1126/science.1074801. [DOI] [PubMed] [Google Scholar]
- 23.Scheuermann ML, Johnson EJ, Chirik PJ. Alkene isomerization-hydroboration promoted by phosphine-ligated cobalt catalysts. Org. Lett. 2015;17:2716–2719. doi: 10.1021/acs.orglett.5b01135. [DOI] [PubMed] [Google Scholar]
- 24.Obligacion JV, Chirik PJ. Bis(imino)pyridine cobalt-catalyzed alkene isomerization-hydroboration: a strategy for remote hydrofunctionalization with terminal selectivity. J. Am. Chem. Soc. 2013;135:19107–19110. doi: 10.1021/ja4108148. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa T, Ruddy AJ, Sydora OL, Stradiotto M, Turculet L. Cobalt- and iron-catalyzed isomerization-hydroboration of branched alkenes: terminal hydroboration with pinacolborane and 1,3,2-diazaborolanes. Organometallics. 2017;36:417–423. doi: 10.1021/acs.organomet.6b00823. [DOI] [Google Scholar]
- 26.Ruddy AJ, Sydora OL, Small BL, Stradiotto M, Turculet L. (N-Phosphinoamidinate)cobalt-catalyzed hydroboration: alkene isomerization affords terminal selectivity. Chem. Eur. J. 2014;20:13918–13922. doi: 10.1002/chem.201403945. [DOI] [PubMed] [Google Scholar]
- 27.Atienza CCH, et al. Bis(imino)pyridine cobalt-catalyzed dehydrogenative silylation of alkenes: scope, mechanism, and origins of selective allylsilane formation. J. Am. Chem. Soc. 2014;136:12108–12118. doi: 10.1021/ja5060884. [DOI] [PubMed] [Google Scholar]
- 28.Buslov I, Becouse J, Mazza S, Montandon-Clerc M, Hu XL. Chemoselective alkene hydrosilylation catalyzed by nickel pincer complexes. Angew. Chem. Int. Ed. 2015;54:14523–14526. doi: 10.1002/anie.201507829. [DOI] [PubMed] [Google Scholar]
- 29.Jia XQ, Huang Z. Conversion of alkanes to linear alkylsilanes using an iridium-iron-catalysed tandem dehydrogenation-isomerization-hydrosilylation. Nat. Chem. 2016;8:157–161. doi: 10.1038/nchem.2417. [DOI] [PubMed] [Google Scholar]
- 30.Fukuyama T, Doi T, Minamino S, Omura S, Ryu R. Ruthenium hydride catalyzed regioselective addition of aldehydes to enones to give 1,3-diketones. Angew. Chem. Int. Ed. 2007;46:5559–5561. doi: 10.1002/anie.200701005. [DOI] [PubMed] [Google Scholar]
- 31.Zhou F, Zhu J, Zhang Y, Zhu SL. NiH-catalyzed reductive relay hydroalkylation: a strategy for the remote C(sp3)-H alkylation of alkenes. Angew. Chem. Int. Ed. 2018;57:4058–4062. doi: 10.1002/anie.201712731. [DOI] [PubMed] [Google Scholar]
- 32.Werner EW, Mei TS, Burckle AJ, Sigman MS. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science. 2012;338:1455–1458. doi: 10.1126/science.1229208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mei TS, Patel HH, Sigman MS. Enantioselective construction of remote quaternary stereocentres. Nature. 2014;508:340–344. doi: 10.1038/nature13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebe Y, Onoda M, Nishimura T, Yorimitsu H. Iridium-catalyzed regio- and enantioselective hydroarylation of alkenyl ethers by olefin isomerization. Angew. Chem. Int. Ed. 2017;56:5607–5611. doi: 10.1002/anie.201702286. [DOI] [PubMed] [Google Scholar]
- 35.Brown HC, Singaram B. The development of a simple general procedure for synthesis of pure enantiomers via chiral organoboranes. Acc. Chem. Res. 1988;21:287–293. doi: 10.1021/ar00152a001. [DOI] [Google Scholar]
- 36.Sandford C, Aggarwal VK. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 2017;53:5481–5494. doi: 10.1039/C7CC01254C. [DOI] [PubMed] [Google Scholar]
- 37.Collins BSL, Wilson CM, Myers EL, Aggarwal VK. Asymmetric synthesis of secondary and tertiary boronic esters. Angew. Chem. Int. Ed. 2017;56:11700–11733. doi: 10.1002/anie.201701963. [DOI] [PubMed] [Google Scholar]
- 38.Stymiest JL, Dutheuil G, Mahmood A, Aggarwal VK. Lithiated carbamates: chiral carbenoids for iterative homologation of boranes and boronic esters. Angew. Chem. Int. Ed. 2007;46:7491–7494. doi: 10.1002/anie.200702146. [DOI] [PubMed] [Google Scholar]
- 39.Burns M, et al. Assembly-line synthesis of organic molecules with tailored shapes. Nature. 2014;513:183–188. doi: 10.1038/nature13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Q, Srinivas HD, Zhang SN, Watson MP. Accessing both retention and inversion pathways in stereospecific, nickel-catalyzed Miyaura borylations of allylic pivalates. J. Am. Chem. Soc. 2016;138:11989–11995. doi: 10.1021/jacs.6b07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tobisu M, et al. Nickel-catalyzed borylation of aryl and benzyl 2-pyridyl ethers: a method for converting a robust ortho-directing group. Adv. Synth. Catal. 2016;358:2417–2421. doi: 10.1002/adsc.201600336. [DOI] [Google Scholar]
- 42.Ueda M, Saitoh A, Miyaura N. Asymmetric hydrogenation of 1-phenylethenylboronic acid and esters for the synthesis of chiral organoboron compounds. J. Organomet. Chem. 2002;642:145–147. doi: 10.1016/S0022-328X(01)01239-6. [DOI] [Google Scholar]
- 43.Morgan JB, Morken JP. Catalytic enantioselective hydrogenation of vinyl bis(boronates) J. Am. Chem. Soc. 2004;126:15338–15339. doi: 10.1021/ja044396g. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi T, Matsumoto Y, Ito Y. Catalytic asymmetric hydroboration of styrenes. J. Am. Chem. Soc. 1989;111:3426–3428. doi: 10.1021/ja00191a049. [DOI] [Google Scholar]
- 45.Crudden CM, Hleba YB, Chen AC. Regio- and enantiocontrol in the room-temperature hydroboration of vinyl arenes with pinacol borane. J. Am. Chem. Soc. 2004;126:9200–9201. doi: 10.1021/ja049761i. [DOI] [PubMed] [Google Scholar]
- 46.Lee YM, Hoveyda AH. Efficient boron-copper additions to aryl-substituted alkenes promoted by NHC-based catalysts. enantioselective Cu-catalyzed hydroboration reactions. J. Am. Chem. Soc. 2009;131:3160–3161. doi: 10.1021/ja809382c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noh D, Chea H, Ju J, Yun J. Highly regio- and enantioselective copper-catalyzed hydroboration of styrenes. Angew. Chem. Int. Ed. 2009;48:6062–6064. doi: 10.1002/anie.200902015. [DOI] [PubMed] [Google Scholar]
- 48.Noh D, Yoon SK, Won J, Lee JY, Yun J. An efficient copper(I)-catalyst system for the asymmetric hydroboration of β-substituted vinylarenes with pinacolborane. Chem. Asian J. 2011;6:1967–1969. doi: 10.1002/asia.201100146. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Zuo ZQ, Wan XL, Huang Z. Cobalt-catalyzed enantioselective hydroboration of 1,1-disubstituted aryl alkenes. J. Am. Chem. Soc. 2014;136:15501–15504. doi: 10.1021/ja5093908. [DOI] [PubMed] [Google Scholar]
- 50.Xi YM, Hartwig JF. Diverse asymmetric hydrofunctionalization of aliphatic internal alkenes through catalytic regioselective hydroboration. J. Am. Chem. Soc. 2016;138:6703–6706. doi: 10.1021/jacs.6b02478. [DOI] [PubMed] [Google Scholar]
- 51.Jang WJ, Song SM, Moon JH, Lee JY, Yun J. Copper-catalyzed enantioselective hydroboration of unactivated 1,1-disubstituted alkenes. J. Am. Chem. Soc. 2017;139:13660–13663. doi: 10.1021/jacs.7b08379. [DOI] [PubMed] [Google Scholar]
- 52.Smith JR, et al. Enantioselective rhodium(III)-catalyzed Markovnikov hydroboration of unactivated terminal alkenes. J. Am. Chem. Soc. 2017;139:9148–9151. doi: 10.1021/jacs.7b05149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai Y, et al. Copper-catalyzed enantioselective Markovnikov protoboration of α-olefins enabled by a buttressed N-heterocyclic carbene ligand. Angew. Chem. Int. Ed. 2018;57:1376–1380. doi: 10.1002/anie.201711229. [DOI] [PubMed] [Google Scholar]
- 54.Chen JH, Cheng B, Cao MY, Lu Z. Iron-catalyzed asymmetric hydrosilylation of 1,1-disubstituted alkenes. Angew. Chem. Int. Ed. 2015;54:4661–4664. doi: 10.1002/anie.201411884. [DOI] [PubMed] [Google Scholar]
- 55.Cheng B, Lu P, Zhang HY, Cheng XP, Lu Z. Highly enantioselective cobalt-catalyzed hydrosilylation of alkenes. J. Am. Chem. Soc. 2017;139:9439–9442. doi: 10.1021/jacs.7b04137. [DOI] [PubMed] [Google Scholar]
- 56.Guo J, Cheng BA, Shen XZ, Lu Z. Cobalt-catalyzed asymmetric sequential hydroboration/hydrogenation of internal alkynes. J. Am. Chem. Soc. 2017;139:15316–15319. doi: 10.1021/jacs.7b09832. [DOI] [PubMed] [Google Scholar]
- 57.Guo J, Lu Z. Highly chemo-, regio-, and stereoselective cobalt-catalyzed Markovnikov hydrosilylation of alkynes. Angew. Chem. Int. Ed. 2016;55:10835–10838. doi: 10.1002/anie.201605501. [DOI] [PubMed] [Google Scholar]
- 58.Guo J, Shen XZ, Lu Z. Regio- and enantioselective cobalt-catalyzed sequential hydrosilylation/hydrogenation of terminal alkynes. Angew. Chem. Int. Ed. 2017;56:615–618. doi: 10.1002/anie.201610121. [DOI] [PubMed] [Google Scholar]
- 59.Cheng B, Liu WB, Lu Z. Iron-catalyzed highly enantioselective hydrosilylation of unactivated terminal alkenes. J. Am. Chem. Soc. 2018;140:5014–5017. doi: 10.1021/jacs.8b01638. [DOI] [PubMed] [Google Scholar]
- 60.Zhang HY, Lu Z. Dual-stereocontrol asymmetric cobalt-catalyzed hydroboration of sterically hindered styrenes. ACS Catal. 2016;6:6596–6600. doi: 10.1021/acscatal.6b02278. [DOI] [Google Scholar]
- 61.Chen X, Lu Z. Iminophenyl oxazolinylphenylamine for enantioselective cobalt-catalyzed hydrosilylation of aryl ketones. Org. Lett. 2016;18:4658–4661. doi: 10.1021/acs.orglett.6b02260. [DOI] [PubMed] [Google Scholar]
- 62.Decken A, Gossage RA, Yadav PN. Oxazoline chemistry. Part VIII. Synthesis and characterization of a new class of pincer ligands derived from the 2-(o-anilinyl)-2-oxazoline skeleton—applications to the synthesis of group X transition metal catalysts. Can. J. Chem. 2005;83:1185–1189. doi: 10.1139/v05-163. [DOI] [Google Scholar]
- 63.Lovinger GJ, Morken JP. Ni-catalyzed enantioselective conjunctive coupling with C(sp3) electrophiles: a radical-ionic mechanistic dichotomy. J. Am. Chem. Soc. 2017;139:17293–17296. doi: 10.1021/jacs.7b10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonet A, Odachowski M, Leonori D, Essafi S, Aggarwal VK. Enantiospecific sp2-sp3 coupling of secondary and tertiary boronic esters. Nat. Chem. 2014;6:584–589. doi: 10.1038/nchem.1971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information file. The X-ray crystallographic coordinates for structures of (L8-H)·PdOAc has been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition nos. CCDC 1588226. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. The experimental procedures and characterization of all new compounds are provided in the Supplementary Information.