Abstract
Growing demonstrations of regenerative potential for some stem cells led recently to promising therapeutic proposals for neuromuscular diseases. We have shown that allogeneic MuStem cell transplantation into Golden Retriever muscular dystrophy (GRMD) dogs under continuous immunosuppression (IS) leads to persistent clinical stabilization and muscle repair. However, long-term IS in medical practice is associated with adverse effects raising safety concerns. Here, we investigate whether the IS removal or its restriction to the transplantation period could be considered. Dogs aged 4–5 months old received vascular infusions of allogeneic MuStem cells without IS (GRMDMU/no-IS) or under transient IS (GRMDMU/tr-IS). At 5 months post-infusion, persisting clinical status improvement of the GRMDMU/tr-IS dogs was observed while GRMDMU/no-IS dogs exhibited no benefit. Histologically, only 9-month-old GRMDMU/tr-IS dogs showed an increased muscle regenerative activity. A mixed cell reaction with the host peripheral blood mononucleated cells (PBMCs) and corresponding donor cells revealed undetectable to weak lymphocyte proliferation in GRMDMU/tr-IS dogs compared with a significant proliferation in GRMDMU/no-IS dogs. Importantly, any dog group showed neither cellular nor humoral anti-dystrophin responses. Our results show that transient IS is necessary and sufficient to sustain allogeneic MuStem cell transplantation benefits and prevent host immunity. These findings provide useful critical insight to designing therapeutic strategies.
Keywords: Stem cells, cell therapy, transplantation, immunosuppression, GRMD dog, Duchenne muscular dystrophy
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked recessive muscle disease, caused by mutations in the dystrophin gene that result in an absence of dystrophin protein at the fiber membrane1,2. It is the most common form of muscular dystrophy and is characterized by extensive and progressive degeneration of skeletal and cardiac muscles3,4. Clinically, it causes progressive muscle weakness leading to reduced motility, wheelchair dependency and severe limitation of life expectancy5,6. As yet, there is no effective treatment for DMD despite the recent development of several therapeutic strategies based on new drugs, viral vector-based dystrophin transfer, oligonucleotide-induced exon skipping, and progenitor/stem cell delivery7.
The first candidates tested in cell-based therapy were myoblasts, which are derived from satellite cells (SCs) and correspond to the natural precursors of muscle fibers8,9. Despite encouraging results showing that intramuscular (IM) injections of murine10,11 or human12,13 myoblasts restored dystrophin expression in the mdx mouse, a murine DMD model, subsequent clinical trials of the strategy were less successful, with few dystrophin+ fibers and no clinical benefit observed14,15. This outcome was attributed to the poor survival and limited migration of injected cells, a low number of donor-derived muscle fibers, and humoral and cellular immune responses of recipients against allogeneic donor cells16–19. The recent identification of tissue-specific progenitors/stem cell populations with in vivo myogenic potential and homing capacities following vascular delivery has provided new impetus to correct the dystrophic phenotype20–25. In scid/mdx mice, IM or intra-arterial (IA) injection of human blood- and muscle-derived AC133+ cells contributed to muscle regeneration, SC replenishment, dystrophin restoration, and recovery of muscle function26. Similar results have been obtained with genetically corrected AC133+ cells isolated from DMD patients27. Furthermore, IA delivery of wildtype mesoangioblasts (Mabs) corrected the dystrophic phenotype in α-sarcoglycan null mice28 and even improves mobility in Golden Retriever muscular dystrophy (GRMD) dogs treated with immunosuppressants29. By comparison, autologous canine Mabs genetically corrected to express dystrophin appear to be much less effective, suggesting that the allogeneic strategy holds the most promise29.
In addition to the successful demonstrations of myogenic potential, concomitant studies have reported that some of these tissue-specific stem cells show immune privileged behavior. After injection into mdx mice, murine muscle-derived stem cells (MDSCs) showed greater dystrophin-restoring ability than myoblasts. This is in part due to their low level of major histocompatibility complex (MHC) class 1 expression, which allows them to avoid rapid immune rejection30–32. Human adipose-derived stem cells (hADSCs), when injected intramuscularly into non-immunocompromised mdx mice, withstood rejection up to 6 months after injection and produced large numbers of dystrophin+ fibers. That these cells escape immune recognition may be due in part to their low levels of cell surface class I human leukocyte antigen (HLA) and their lack of class II HLA33. Non-immunosuppressed GRMD dogs have also been shown to engraft and express dystrophin several months after local or systemic delivery of hADSCs34. Overall, these results strongly suggest that these cells may have specific immunoregulatory properties, as previously demonstrated for mesenchymal stem cells (MSCs) and Mabs, which can modulate both innate and adaptive immunity35–38. Given the adverse effects associated with long-term immunosuppression (IS) in medical practice, these properties are of major interest for allogeneic stem cell-based strategies. In recent decades, the development of a large panel of new immunosuppressive molecules39,40 has significantly increased short-term graft survival rates following organ transplantation41,42. One of the main drugs used is cyclosporin A (CsA)43. However, long-term CsA use is associated with aggressive toxicity of the kidney44, liver45 and heart46,47 as well others adverse effects related to the immunosuppression itself including increased sensitivity to infections48 and lymphoma formation49,50. Myalgia, cramps, and weakness in skeletal muscle have also been reported51,52. Moreover, both in vitro and in vivo, CsA inhibits myogenic differentiation of the muscle precursor cells53,54, induces their apoptosis55 and delays muscle regeneration following injury56,57.
Over the past years, we have isolated delayed adherent MDSCs from healthy dogs, which we have named MuStem cells, and demonstrated that their vascular delivery into GRMD dogs submitted to IS that started 1 week before cell administration and then maintained throughout the experiment (mentioned then as continuous IS) produce striking clinical stabilization and long-term muscle repair58,59. In the present study, we sought to determine the optimal immunosuppressive regimen required to obtain beneficial effects using the aforementioned allogeneic MuStem cells infusion protocol. Specifically, we investigated whether IS could be reduced or limited to the injection time window, without impairing the phenotypic correction previously described with continuous IS.
To this end, we developed a protocol for IA delivery of MuStem cells in 4–5-month-old GRMD dogs using donor/recipient pairs of dogs with identical dog leukocyte antigen (DLA). Longitudinal clinical follow up was performed up to 9 months of age in non-immunosuppressed or transiently immunosuppressed recipient dogs and followed by a comprehensive tissue analysis. Overall, two additional mock groups of non-transplanted dogs receiving or not receiving the transient IS regimen were included in the analysis. Also, immune responses of recipient GRMD dogs to both transplanted MuStem cells and dystrophin protein were monitored.
Materials and Methods
Animals
A total of 27 male Golden Retrievers were included in this study. All dogs were obtained from the Centre d’Elevage du Domaine des Souches (CEDS, Mézilles, France) or the Boisbonne Center for gene and cell therapy (Oniris, Nantes, France). The dogs were housed at the Boisbonne Center in a controlled environment (temperature 21 ± 1°C, 12-hour light/dark cycle). Dogs with muscular dystrophy were identified at birth using polymerase chain reaction (PCR)-based genotyping, as previously described60. Diagnosis was confirmed by elevated serum creatine kinase levels. A total of seven 2.5-month-old healthy dogs were used for MuStem cell isolation, while control muscle samples were collected from four other dogs. A total of 16 GRMD dogs were subdivided into two main groups each composed of 8 dogs and used for clinical and/or pathophysiological investigations (see Table 1). The first group was composed of dogs that received MuStem cells without IS (GRMDMU/no-IS, n = 4) or with transient IS (GRMDMU/tr-IS, n = 4). The second (mock) group were not transplanted with MuStem cells and received either no IS (GRMDmo/no-IS, n = 3)58 or transient IS (GRMDmo/tr-IS, n = 5). The study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the French National Research Council. The protocol was approved by the Ethics Committee on Animal Experimentation of the Pays de la Loire Region, France (Permit Number: CEEA.2012.121). All surgeries were performed under anesthesia induced with ketamine (Imalgene 1000, Merial, Toulouse, France) / diazepam (Valium, Roche, Boulogne-Billancourt, France) and that was maintained using an inhalational mixture of isoflurane (Vetflurane, Virbac, Magny-en-Vexin, France) and oxygen. To minimize suffering, analgesia treatment was performed with tolfenamic acid (4 mg/kg, Tolfedine, Vetoquinol SA, Magny Vernois, France). Pain was evaluated daily as part of a complete clinical evaluation performed by a veterinarian and analgesia was provided if deemed necessary. Dogs were euthanized by intravenous administration of sodium pentobarbital (2000 mg; Dolethal, Vetoquinol SA, Magny Vernois).
Table 1.
Summary of dog experiments.a
| GRMD dogs | Immunosuppressive regimen | MuStem cell transplantation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | ID number | Name | Littermate | Age of death | Cause of death | Age at the setting up | Age at the arrest | Cell number (107/kg) | Age at first injection | Age at 2nd injection | Experiments | |
| MU/no-IS | 1 | ICÔNE | a | 8.75 | End of protocol | None | 6.3–10.2 | 4.25 | 4.75 | Complete evaluation | ||
| 2 | IDEM | a | 7.25 | End of protocol | None | 7.9–9.7 | 3.25 | 3.75 | Complete evaluation | |||
| 3 | INDOU | a | 7.75 | End of protocol | None | 8.2–10.0 | 3.5 | 4 | Complete evaluation | |||
| 4 | IRON | b | 8.75 | End of protocol | None | 9.7–11.7 | 4.5 | 5 | Complete evaluation | |||
| MU/tr-IS | 5 | GAROU | c | 9 | End of protocol | Transient | 4.25 | 5.75 | 10.0–11.1 | 4.5 | 5 | Complete evaluation |
| 6 | GAVROCHE | c | 9 | End of protocol | Transient | 4 | 5.75 | 11.0–11.3 | 4.25 | 4.75 | Complete evaluation | |
| 7 | GEPETTO | c | 9 | End of protocol | Transient | 4.25 | 5.75 | 9.7–11.2 | 4.5 | 5 | Complete evaluation | |
| 8 | HOLLIDAY | d | 9.5 | End of protocol | Transient | 4.75 | 6.25 | 9.8–10.0 | 5 | 5.5 | Complete evaluation | |
| mo/no-IS | 9 | DIEU | e | 12.75 | Aspiration pneumonia | None | Clinical follow up | |||||
| 10 | DOBBY | e | 27.25 | Digestive complication | None | Clinical follow up | ||||||
| 11 | GAMELLE | f | 13.5 | End of protocol | None | Clinical follow up | ||||||
| mo/tr-IS | 12 | GABIN | g | 9.75 | End of protocol | Transient | 5 | 6.5 | Complete evaluation | |||
| 13 | GRINGO | g | 9.75 | End of protocol | Transient | 5 | 6.5 | Complete evaluation | ||||
| 14 | HAMLET | h | 9.5 | End of protocol | Transient | 4.75 | 6.25 | Complete evaluation | ||||
| 15 | HITCHCOK | h | 9.5 | End of protocol | Transient | 4.75 | 6.25 | Complete evaluation | ||||
| 16 | HIZAC | i | 9.75 | End of protocol | Transient | 5 | 6.5 | Complete evaluation | ||||
GRMD: Golden Retriever muscular dystrophy; IS: immunosuppression.
aEach dog was assigned an identification number and a name. The age of dogs at the beginning of the study period is expressed in months. Cause of death, immunosuppressive treatment status (none or transient), and the number of cells injected are indicated.
Genotyping of DLA
Using blood samples collected from breeders and litters, DNA was extracted using the NucleoSpin® Blood L kit (Macherey-Nagel, Hoerdt, France), according to the manufacturer’s instructions. All samples were genotyped using the Illumina Infinium Canine 170 K SNP HD array by IntegraGen (Evry, France). To identify DLA-identical littermate donor/recipient pairs for dogs 1 to 8, haplotype phasing of MHC loci was performed manually based on single-nucleotide polymorphism (SNP) positions in CanFam3, CFA12: 0-3 496 085 containing MHC class I, II and III genes, and CFA35: 24 006 657-26 506 199 containing MHC class I genes, as previously described61.
Isolation of Canine MuStem Cells
Tissues were collected from a pool of hindlimb muscles from healthy 2.5-month-old dogs (n = 7) and immediately placed in cold phosphate-buffered saline (PBS; PAA; Les Rumeaux, France) supplemented with 2% 100 UI/mL penicillin/0.1mg/mL streptomycin/0.25 μg/mL amphotericin B (PSF; Sigma, St. Louis, MO, USA). Tissues were mechanically and enzymatically dissociated to produce a muscle-derived cell suspension. cMuStem cells were isolated using a modified pre-plating protocol, as previously described58. Cells were expanded in growth medium composed of 37% Dulbecco’s modified eagle’s medium (DMEM), 2.5 g/L glucose / 37% M199 (Invitrogen, Cergy-Pontoise, France) supplemented with 10% fetal calf serum (FCS; Eurobio, Les Ulis, France), 10% horse serum (Eurobio), 1% PSF, and human recombinant factors [10 ng/mL basic fibroblast growth factor, 50 ng/mL epidermal growth factor, and 25 ng/mL stem cell factor (PromoCell, Heidelberg, Germany)]. They were cultured under standard conditions (37°C in a humidified atmosphere containing 5% CO2) and passaged every 4 to 5 days when they exhibited approximately 75% confluence. The medium was replaced every 2 days. As described above58, MuStem cells exhibit a marked capacity for expansion and correspond to early myogenic progenitors and uncommitted cells.
Immunosuppressive Treatment
Immunosuppression of GRMD dogs was achieved by daily administration of 27 mg/kg of oral CsA (Neoral®; Novartis, Rueil-Malmaison, France) in combination with 6 mg/kg mycophenolate mofetil (CellCept®; Roche, Paris, France). Ketoconazole (10 mg/kg; Nizoral®; Janseen-Cilag, Issy-les-Moulineaux, France) was also added daily to decrease CsA catabolism. Blood levels of CsA were controlled twice per week and maintained between 250 and 350 ng/mL by individual dose adjustments. Transient IS treatment began 1 week before cell administration and was terminated 2 weeks after the last administration.
Systemic Delivery Procedure
MuStem cells (passage 2–4) were injected as previously described58. Briefly, cells were suspended at 1.5 × 107 cells/mL in 0.9% NaCl/2.5% homologous serum/10 U/mL heparin. A total of eight GRMD dogs (aged 3.25–5.50 months) received two intra-femoral injections of between 6.3 × 107 and 1.2 × 108 MuStem cells/kg at 2-week intervals, using laminar flow at a rate of 15 mL/min (Table 1).
Isolation of Canine Peripheral Blood Mononucleated Cells and Splenocytes
Blood samples were collected every 2 weeks from GRMD dogs until sacrifice, and peripheral blood mononuclear cells (PBMCs) were isolated by gradient-density centrifugation [2000 rpm for 20 min at room temperature (RT)] on Ficoll 1.077 (PAA). PBMC rings were collected in PBS (Lonza)-5% FCS and centrifuged (1800 rpm for 10 min at 4°C). The supernatant was removed and the pellet was washed twice with PBS-5% FCS before centrifugation (1400 rpm for 10 min at 4°C). Cells were counted in Türk and Trypan dye. At necropsy, the spleen was harvested and treated for 45 min with collagenase D (2 mg/mL; Roche, Basel, Switzerland). The reaction was stopped by adding 0.1 M ethylenediaminetetraacetic acid (EDTA Sigma-Aldrich, St-Louis, MO, USA), pH 7.2 for 5 min. Freshly isolated splenocytes were washed once with 50 mL PBS-5% FCS. Red blood cells were lysed for 10 min at RT in a lysis buffer (NH4Cl, KHCO3, Na2EDTA) and washed twice. PBMCs and splenocytes were frozen in FCS-10% dimethylsulfoxide (DMSO; Sigma-Aldrich, St-Louis, MO, USA) prior to testing.
Clinical Follow Up
GRMD dogs were clinically evaluated every week by the same doctor of veterinary medicine degree (DVM) in a blind manner regarding treatment using a modified version of the grid previously described58,62. A total of six musculoskeletal criteria and items related to general health status were semi-quantitatively scored from 0 to 2 (0 corresponding to a normal appearance, 1 to an intermediate phenotype and 2 to a severe alteration). These parameters, selected to be objective and weakly biased by animal willingness, corresponded to dysphagia, mouth opening, Triceps brachii muscle firmness, suppleness of the hind limbs, tibio-patellar reflex and ability to get over a fence (20 cm high for adult dogs). The clinical score was calculated by summing all parameters and expressed as a percentage of the healthy dog score, that is of 100%.
Muscle Sampling
Fragments of the Triceps brachii muscle were collected from 9-month-old GRMDMU/no-IS (n = 4), GRMDMU/tr-IS (n = 4), and GRMDmo/tr-IS (n = 5) dogs, as well as healthy dogs (n = 4), during a complete necropsy examination conducted at the end of the study protocol. Muscle samples were divided into two parts for immunohistochemistry and molecular/biochemical analyses.
Immunohistochemistry
Muscle samples were snap-frozen in isopentane (VWR international, Fontenay-sous-Bois, France) cooled in liquid nitrogen and stored at −80°C until processing. After incubation for 1 hour at RT in blocking buffer (PBS/5% goat serum), transversal cryosections (12 μm) were successively incubated with mouse monoclonal anti-dystrophin antibody (1:50, overnight at RT; Cat. # NCL-DYS2, Menarini, Rungis, France), Alexa Fluor 488-conjugated goat anti-mouse IgG (1:400, 1 hour at RT; Cat. # ab150113, Invitrogen, Carlsbad, CA, USA) and the fluorescent DNA-dye DRAQ5 (1:1000, 15 min, at RT; Cat. # DR05500, Biostatus, Leicestershire, UK). Immunofluorescence labeling was observed using a confocal microscope (Zeiss, Marly-le-Roi, France).
Histomorphometry
Muscle regenerative activity was evaluated using immunolabeling for the developmental isoform of the myosin heavy chain (MyHCd) (1:20; Cat. # NCL-MHCd, Menarini). Microscopic fields were randomly selected to evaluate at least 700 fibers (1010 ± 232 fibers). Endomysial fibrosis was analyzed after immunolabeling for collagen I (1:500; Cat. # 02150026, MP Biomedicals, Illkirch, France). Measurements were performed using Nikon Imaging Software (Nikon, Champigny sur Marne, France). Repeatability was tested by the same observer by analyzing the same sample five times. In all cases, intra-assay variation coefficients were <5%.
Evaluation of Peripheral Blood Mononucleated Cell Proliferation
cMuStem cells (n = 7 independent cell batches) were thawed and seeded at 1 × 104 cells/cm2 for 1 week. Next, host PBMCs harvested before and after cell delivery were thawed in cMuStem cell growth medium and cultured overnight at 37°C and 5% CO2. cMuStem cells were harvested and, together with splenocytes taken from another dog and used as a positive control, were irradiated at 35 Gy. PBMCs were then cultured in quadruplicate at 1 × 105 cells per well in 96-well plates with irradiated cMuStem cells. In parallel, PBMCs were also cultured with control splenocytes or stimulated with 10 μg/mL Concanavalin A (ConA; Sigma-Aldrich) to serve as a positive control of proliferation. cMuStem cells were diluted at 2:1, 1:1, 1:4, 1:16 and 1:64 (MuStem cells: PBMCs) to demonstrate a dose effect. After 5 days of co-culture, cells were incubated overnight with tritiated thymidine (1:40; NET027A001MC, Perkin Elmer, Waltham, MA, USA) at 37°C and 5% CO2. Cells were harvested on a filter using a Perkin Elmer Harvester and radioactivity was measured as counts per minute (cpm) using a MicroBeta plate counter (Perkin Elmer).
Interferon γ ELISpot
The enzyme-linked immunospot (ELISpot) assay was performed using a library of seven pools of 105 peptides each, covering the canine dystrophin sequence. These 15-mers peptides have an overlap of 10 amino acids (Pepscreen, Sigma). After incubation for 18 to 20 hours at 37°C and 5% CO2, thawed recipient splenocytes were seeded in polyvinylidene difluoride (PVDF) membrane-bottomed 96-well plates (Millipore, Billerica, MA, USA) coated overnight with mouse anti-canine interferon (IFN)γ capture antibody (Canine IFNγ ELISpot Development Module; Cat. # SEL781, R&D, Minneapolis, MN, USA). The splenocytes were then stimulated for 24 hours with the peptide pools. Cells and non-bound protein were washed away before the addition of biotinylated goat anti-canine IFNγ detection antibody (Canine IFNγ ELISpot Development Module, R&D) and subsequent overnight incubation at 4°C. Next, streptavidin-AP (ELISpot Blue Color Module) was added for 2 hours at RT. NBT/BCIP substrate (ELISpot Blue Color Module, R&D) was added in each well of the plate for 30 min, and the plates read using an iSpot Reader Spectrum (AID, Strassberg, Germany). The data obtained were analyzed using AID ELISpot software. As controls, splenocytes from transplanted GRMD dogs were either stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin (positive control, 10 ng/mL and 500 ng/mL, respectively) or cultured in medium alone (negative control). Responses were considered positive when the number of spot-forming colonies per million cells was >50 and at least three-fold higher than that observed for unstimulated cells.
Western blot Analysis
Presence of anti-dystrophin immunoglobulin (Ig)G antibodies in sera from transplanted dogs were determined by Western blot, as previously described63. Briefly, muscle protein extracts from healthy or GRMD dogs were subjected to polyacrylamide gel electrophoresis using 3-8% Tris-Acetate Precast gels (Invitrogen), and then transferred to a Hybond ECL nitrocellulose membrane (Invitrogen). After overnight saturation, membranes were incubated (2 hours, RT) with sera (dilution 1:500) obtained from transplanted dogs at different time points. Subsequently, protein detection was performed by hybridization with peroxidase-conjugated rabbit anti-dog IgG antibody (1:5000; Cat. # 304-001-003, Jackson ImmunoResearch, West Grove, PA, USA) followed by enhanced chemiluminescence detection (Pierce). Overall, two positive controls were used: (i) an anti-dystrophin IgG-positive canine serum (kindly provided by S. Blot, Veterinary School of Alfort, France) obtained from a GRMD dog immunized against the dystrophin protein and (ii) an anti-dystrophin antibody (1:100; Cat. # NCL-DYS1, Novocastra) revealed using a peroxidase-conjugated goat anti-mouse IgG antibody (1:2000; Cat. # P044701, Dako, Santa Clara, CA, USA).
Statistical Analysis
All data were represented as the mean ± standard deviation (SD). Clinical scores were compared between GRMD dog groups (from 3 to 8 months of age) using linear models followed by testing using R (version 3.3.2). The residuals of the models fitted were found to follow a normal distribution. Percentages of regenerative fibers and area fractions of fibrosis were compared between GRMDMU/no-IS, GRMDMU/tr-IS, and GRMDmo/tr-IS dogs using a two-tailed Mann–Whitney U test. A p-value <0.05 was considered statistically significant.
Results
Transiently Immunosuppressed GRMD Dogs Show Improved Clinical Status Following MuStem Cell Transplantation
The clinical course of the different dog groups was determined based on a weekly scored evaluation and analyzed by linear models that were shown as being significantly predictive of the dog groups (Table S1). The linear models revealed that the clinical scores were always significantly related to the age and to the dog group when GRMDmo/tr-IS versus GRMDmo/no-IS, GRMDMU/no-IS versus GRMDmo/no-IS, GRMDMU/tr-IS versus GRMDmo/tr-IS and GRMDMU/tr-IS versus GRMDMU/no-IS dog groups were compared (Fig. 1; Table 2). Also, the clinical scores were shown to significantly decrease with the age.
Fig. 1.
Clinical evaluation of GRMD dogs. Animals were clinically evaluated weekly from 2 months of age and a clinical score was expressed as a percentage of the score of a theoretical healthy dog. Scatterplots and best fit lines of clinical score versus age were sequentially represented by considering two experimental groups. (A). Mock GRMD dogs (GRMDmo/no-IS in purple, n=4) and MuStem cell-injected GRMD dogs without IS (GRMDMU/no-IS in orange, n=4): MuStem cell delivery (GRMDMU/no-IS dogs) did not mitigate the clinical impairment observed in GRMDmo/no-IS dogs; (B). MuStem cell-injected GRMD dogs with transient IS (GRMDMU/tr-IS in red, n=4) and mock GRMD dogs with transient IS (GRMDmo/tr-IS in green, n=4): Under transient IS, cell infusion allowed an increased clinical score (GRMDMU/tr-IS dogs); (C). MuStem cell-injected GRMD dogs without IS (GRMDMU/no-IS in orange, n=4) and MuStem cell-injected GRMD dogs with transient IS (GRMDMU/tr-IS in red, n=4): Benefits of MuStem cell delivery in the clinical score were observed when IS was applied.
GRMD: Golden Retriever muscular dystrophy; IS: immunosuppression.
Table 2.
Clinical evaluation of GRMD dogs.a
| Clinical score | GRMDmo/tr-IS versus GRMDmo/no-IS | GRMDMU/no-IS versus GRMDmo/no-IS | GRMDMU/tr-IS versus GRMDmo/tr-IS | GRMDMU/tr-IS versus GRMDMU/no-IS |
|---|---|---|---|---|
| Overall | adjusted R2 = 0.2341 p<3.4 × 10−9 |
adjusted R2 = 0.2983 p<2.7 × 10−9 |
adjusted R2 = 0.1748 p<1.1 × 10−6 |
adjusted R2 = 0.2520 p<1.9 × 10−7 |
| Age of dog | aage = −0.0554 p<9.5 × 10−10 |
aage = −0.0646 p<9.4 × 10−9 |
aage = −0.0372 p<2.3 × 10−6 |
aage = −0.0387 p<5.1 × 10−5 |
| Dog group | agroup = −0.0323 p = 0.229 |
agroup = −0.1007 p<2.0 × 10−3 |
agroup = 0.0483 p<4.5 × 10−2 |
agroup = 0.1125 p<7.2 × 10−5 |
GRMD: Golden Retriever muscular dystrophy; IS: immunosuppression.
aThe clinical status of the dogs included in the different groups was determined weekly based on a semi-quantitative evaluation. The resulting clinical scores were analyzed using linear models to determine the relation between the clinical score and the age as well as the dog group.
The linear model fitted with the GRMDmo/tr-IS and GRMDmo/no-IS dog groups showed that the transient IS was not a significant variable (agroup = −0.0323; p = 0.229), indicating that alone it did not affect the clinical scores of the GRMD dogs. A progressive clinical impairment in GRMDMU/no-IS dogs was observed clinically between 3 and 8 months of age, with a marked decline in locomotor ability, as also seen in GRMDmo/no-IS dogs. This was confirmed by the linear model that showed a significant decreased of the clinical score with the only MuStem cell infusion (agroup = −0.1007; p<2.0 × 10−3; Fig. 1A). This indicated that the sole MuStem cell transplantation has no positive clinical impact in absence of IS. In return, the linear model fitted with the GRMDMU/tr-IS and GRMDmo/tr-IS dog groups importantly pointed that the clinical scores were significantly increased with the MuStem cell transplantation (agroup = 0.0483; p<4.5 × 10−2; Fig. 1B), demonstrating that the cell infusion positively acts on the clinical status of the transplanted GRMD dogs when placed under transient IS. Finally, the linear model fitted with the GRMDMU/tr-IS and GRMDMU/no-IS dog groups indicated that the clinical score significantly increased with the transient IS (agroup = 0.1125; p<7.2 × 10−5; Fig. 1C), indicating that the last one is required to observe the beneficial clinical effect of the allogeneic MuStem cells.
Transiently Immunosuppressed GRMD Dogs Show Increased Muscle Regenerative Activity Following MuStem Cell Transplantation
Lesions typically described in GRMD dogs were observed in hematoxylin-eosin-saffron (HES)-stained sections from 9-month-old GRMDMU/tr-IS, GRMDmo/tr-IS, and GRMDMU/no-IS dogs. Briefly, muscle fibers displayed some degree of size heterogeneity with the presence of small fibers and large hyaline ones, some of which were centronucleated. Notably, sporadic calcium deposits eliciting focal mononuclear cell infiltration as well as mild endomysial and perimysial fibrosis were observed. Except around calcification foci, very few inflammatory cells were observed without any difference noted between dogs included in the different groups.
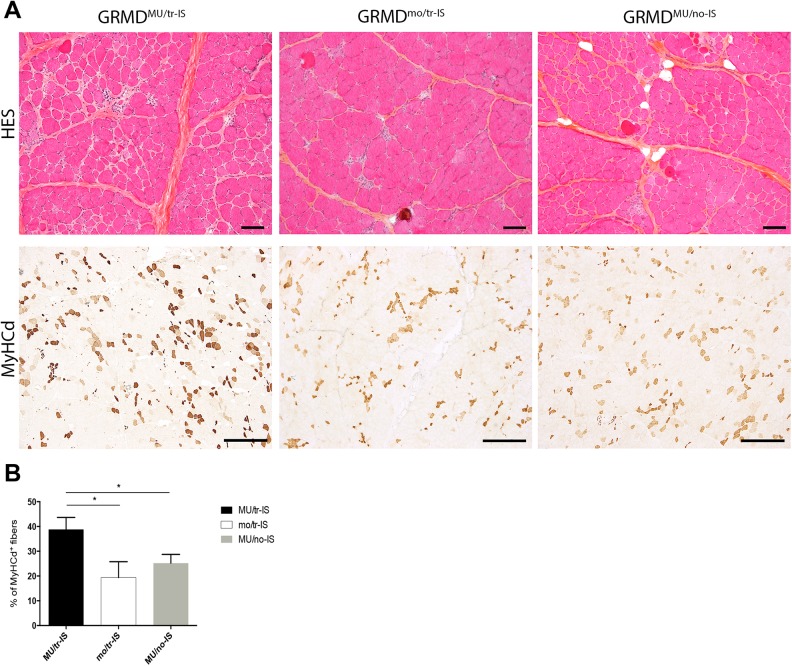
Muscle regenerative activity was assessed using specific immunolabeling for the MyHCd the expression of which is restricted to developmental and regeneration processes. In the transient IS dog group, the proportion of MyHCd+ fibers in the Triceps brachii muscle was 39.8 ± 4.8% and 19.3 ± 6.4% in GRMDMU/tr-IS and GRMDmo/tr-IS dogs respectively, indicating a significant effect of MuStem cell delivery (p = 0.005) (Fig. 2). Strikingly, the proportion of MyHCd+ fibers in GRMDMU/no-IS dogs was 25.2 ± 3.5% (p = 0.005), indicating that the formation of newly regenerated muscle fibers was compromised in the absence of IS. These results indicated that transient IS is required in order for MuStem cells to actively and persistently contribute to muscle fiber regeneration. Levels of subsarcolemmal dystrophin expression in muscle sections were low, and below the threshold of detection by Western blot.
Fig. 2.
Systemic delivery of MuStem cells into the Triceps brachii muscle: histological findings. (A). Figure shows HES topographic staining (upper panel) and MyHCd labeling for muscle regeneration (lower panel) in representative muscle sections. Representative images are shown for three groups of 9-month-old GRMD dogs: MuStem cell-injected dogs with transient IS (GRMDMU/tr-IS, left); non-MuStem cell-injected (mock) GRMD dogs with transient IS (GRMDmo/tr-IS, middle); and MuStem cell-injected dogs without IS (GRMDMU/no-IS, right). Note the heterogeneity of muscle fiber sizes and the presence of numerous small fibers, especially in GRMDMU/tr-IS dogs. MyHCd labeling revealed that most of these small fibers were newly regenerated, indicating a global increase in regenerative activity (brown staining) in GRMDMU/tr-IS versus GRMDMU/no-IS and GRMDmo/tr-IS muscle samples. Scale bar = 100 µm. (B). Histogram recapitulates percentage of MyHCd+ fibers in GRMDMU/tr-IS, GRMDMU/no-IS, and GRMDmo/tr-IS muscles, showing a positive effect of MuStem cell delivery that is abolished in absence of IS.
*indicates a significant difference between groups (Kruskal–Wallis, p<0.005).
GRMD: Golden Retriever muscular dystrophy; HES: hematoxylin-eosin-saffron; IS: immunosuppression; MyHCd: developmental isoform of the myosin heavy chain.
Transiently Immunosuppressed GRMD Dogs Show no Deleterious Immune Responses to Either MuStem Cells or Dystrophin Following Allogeneic Cell Transplantation
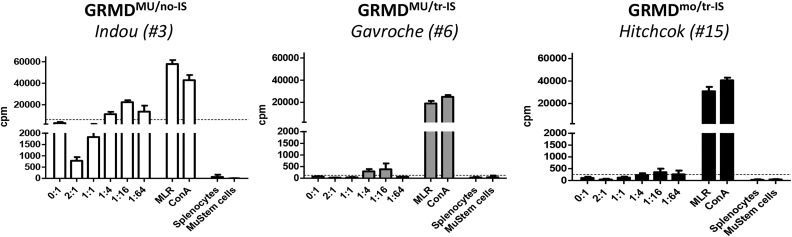
Host immunity against MuStem cells was examined in a mixed cell reaction (MCR)-based assay in which host PBMCs harvested at different time points (before transplantation, upon cessation of IS, and at euthanasia) were co-cultured with donor MuStem cells at ratios increasing from 1:64 to 2:1 (MuStem cells:PBMCs). In GRMDMU/no-IS dogs, significant PBMC proliferation was observed in the presence of donor MuStem cells (Fig. 3 and Table 3). By contrast, absent or weak PBMC proliferation was observed in GRMDMU/tr-IS dogs. Interestingly, similar results were obtained in the GRMDmo/tr-IS group, revealing that the presence of MuStem cells does not result in a host cellular response.
Fig. 3.
Cellular response to donor MuStem cells. Mixed cell reaction was done with recipient PBMCs collected at the moment of sacrifice. PBMCs were stimulated with donor MuStem cells at increasing MuStem cells: PBMC ratios were from 1:64 up to 2:1. Lymphocyte proliferation was measured using a thymidine uptake-based assay and expressed as counts per minute (cpm). The threshold of positivity was established as the basal proliferation rate of PBMCs (medium alone) + 3 × SD. The results of one representative recipient out of 4 to 5 animals from GRMDMU/no-IS (left panel), GRMDMU/tr-IS (middle panel), and GRMDmo/tr-IS (right panel) dog groups are presented.
GRMD: Golden Retriever muscular dystrophy; IS: immunosuppression; PBMC: peripheral blood mononucleated cell; SD: standard deviation.
Table 3.
Analysis of immune responses against both MuStem cells and dystrophin protein.a
| GRMD dogs | Anti-MuStem cell immunity | Anti-dystrophin immunity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cellular response (recipient PBMC) | Cellular response (splenocytes) | Humoral response | ||||||||
| Group | ID number | Name | Before injection | Arrest of IS | Sacrifice | Sacrifice | Before injection | Arrest of IS | Sacrifice | Heatmap |
| MU/no-IS | 1 | ICÔNE | 87 | 21,861 | 54,003 | Neg | NA | NA | NA | 0 |
| 2 | IDEM | 247 | ND | 8287 | Neg | Neg | Neg | Neg | 10,000 | |
| 3 | INDOU | 3119 | 3177 | 22,422 | Neg | Neg | Neg | Neg | 20,000 | |
| 4 | IRON | 214 | 3976 | 14,017 | Neg | Neg | Neg | Neg | 30,000 | |
| MU/tr-IS | 5 | GAROU | 127 | ND | ND | Neg | Neg | Neg | Neg | 40,000 |
| 6 | GAVROCHE | 379 | 247 | 384 | Neg | Neg | Neg | Neg | 50,000 | |
| 7 | GEPETTO | 248 | 59 | 827 | Neg | Neg | Neg | Neg | 60,000 | |
| 8 | HOLLIDAY | 53 | ND | ND | Neg | NA | NA | NA | ||
| Results were expressed in cpm | NA: Western blot was not performed | |||||||||
cpm, counts per minute; GRMD: Golden Retriever muscular dystrophy; IFN: interferon; Ig: immunoglobulin; IS: immunosuppression; NA: not applicable (Western blot not performed); ND: not detected; PBMC: peripheral blood mononucleated cell; SD: standard deviation.
aFor the anti-MuStem cell immunity, PBMCs of the recipient GRMD dogs were tested against the corresponding donor’s MuStem cells. The selected dilution was 1:16 with a detection threshold corresponding to the basal proliferation (PBMCs in medium alone + 3 × SD). For the cellular anti-dystrophin immunity, recipient splenocytes harvested at euthanasia were tested against overlapping dystrophin peptide library using an IFNγ ELISpot assay. For the humoral anti-dystrophin response (circulating specific IgG antibodies), transplanted dog’s sera were tested by a Western blot-based assay.
Importantly, cellular and humoral immune responses against dystrophin were also monitored in MuStem cell-transplanted dogs to determine whether the lack of dystrophin expression in these animals was associated with immune rejection. ELISpot assay revealed no IFNγ secretion after dystrophin peptide stimulation of splenocytes harvested at the end of the study protocol, regardless of IS regimen. This finding indicates that splenocytes do not become reactive following MuStem cell infusion (Fig. 4A). In agreement, Western blot revealed no anti-dystrophin IgG, regardless of time point or IS regimen (Fig. 4B).
Fig. 4.
Host immune response to dystrophin. (A). Cellular response investigated by IFNγ ELISpot. Negative (C-) and positive (C+) controls correspond to splenocytes in medium alone or after stimulation with PMA/ionomycin, respectively. P1 to P7 correspond to the seven pools of peptides. Responses were considered positive when the number of SFCs per million cells was >50 and at least three-fold higher than that of unstimulated cells. The results of one representative recipient out of 4 to 5 animals from GRMDMU/no-IS (left panel), GRMDMU/tr-IS (middle panel), and GRMDmo/tr-IS (right panel) dog groups are presented. (B). The humoral response was determined by Western blot analysis. Sera collected from GRMDMU/no-IS, GRMDMU/tr-IS and GRMDmo/tr-IS dogs before injection, upon cessation of IS treatment, and at sacrifice, were incubated with muscle extracts from healthy (H) or GRMD (G) dogs and analyzed by Western blot for the presence of dystrophin antibody. An IgG-positive canine serum and an anti-dystrophin antibody (DYS1) incubated with healthy muscle extract were used as positive controls (C+).
GRMD: Golden Retriever muscular dystrophy; IFN: interferon; Ig: immunoglobulin; IS: immunosuppression; PMA: phorbol 12-myristate 13-acetate; SFC: spot-forming cell.
Overall, these results summarized in Table 3 indicate that the MuStem cell infusion protocol performed in transiently immunosuppressed dogs generates no cellular or humoral responses against MuStem cells or dystrophin, whereas it triggers an anti-allogeneic antigen immune response when done in the absence of IS.
Discussion
In terms of clinical application, the use of allogeneic cells offers several advantages over ex-vivo genetically corrected autologous cells. These include a better in vitro proliferation capacity, which could facilitate whole-body treatment strategies and/or delivery protocols based on repeated cell infusions, and a higher muscle regenerative index64. Allogeneic cell-based therapy allows a better standardization of cell batches for injection by avoiding the use of patient muscle that is highly heterogeneous in terms of damage and cell yield. Finally, in the case of repeated cell delivery, cell banking allows for better clinical reactivity. The main concern regarding the use of allogeneic products for cell therapy is the possibility of immune rejection, which can be ameliorated with immunosuppressive treatment65–67. However, long-term clinical use of immunosuppressants is limited by their numerous and severe adverse effects46,47. In the last decade, certain immunomodulatory properties have been attributed to several somatic stem cells and in particular to MSCs37,68,69, suggesting that these cells may hold promise as a new strategy for IS70.
In 2011, we demonstrated persistent clinical stabilization and increased muscle regeneration following systemic delivery of allogeneic MuStem cells into continuously immunosuppressed GRMD dogs58. Here, we show that similar clinical benefits and tissue regeneration can be obtained following MuStem cell delivery into transiently immunosuppressed DLA-haplo- or geno-identical dogs. Cell transplantation performed in the absence of IS produced no beneficial effects and induced a cellular response against allogeneic cells, clearly demonstrating that transient IS is a requirement for MuStem cell transplantation. Longitudinal clinical evaluation revealed remarkable stabilization in transplanted recipients that received transient IS treatment. By contrast, the corresponding recipient group that received no IS regimen displayed progressive impairment. Furthermore, the non-transplanted mock group that received only the transient IS regimen displayed a similar clinical course as those described for the non-transplanted mock group that received any IS, revealing that the transient treatment alone has no positive impact. As a consequence of continuous degeneration, dystrophic muscle is characterized by the presence of pro-inflammatory cytokines and could display immune cell infiltrates, particularly lymphocytes and macrophages71,72. This inflammatory component makes dystrophic muscle tissue a particularly unfavorable environment for cell engraftment and partially explains the need for initial neutralization of the immune response, despite the use of DLA-identical donor-recipient pairs of dogs. Nevertheless, it cannot be disregarded the fact that a neutralization of the immune system subsequently to IS treatment could also interact negatively with the process of cell homing to injured muscles, as it has been demonstrated that immunosuppressants can limit secretion of chemoattractive molecules through their action on the calcineurin pathway73,74 and/or migration ability of immune cells such as dendritic cells, T lymphocytes or macrophages75–77. Our data demonstrating induction of the host immune response to allogeneic donor antigens are in agreement with previous results obtained after injection of MyoD-transduced canine CD271+ MSCs into the Tibialis anterior (TA) muscle of non-immunosuppressed CXMDJ dogs78. In that study, analysis of muscle tissue 8 weeks post-injection revealed the presence of numerous CD8+ and CD11+ cellular infiltrates around degenerating donor fibers, and a relatively low number of dystrophin+ cells. Another study in which non-immunosuppressed, double mutant Utrntm1Ked Dmdmdx/J mice received eight successive intra-peritoneal injections of human pericytes derived from four different tissues reported a lack of detection of both human cells, histological alterations, as well as motor ability79. By contrast, IV and IM injections of hADSCs into non-immunosuppressed SJL80 and mdx mice33, respectively, resulted in xenogeneic cell engraftment into host muscles. Indeed, both studies reported the detection of chimeric human/mouse muscle fibers and the re-expression of defective proteins, as well as improved muscle performance. Nonetheless, it is important to note that these data were obtained in two mouse models exhibiting a mild dystrophic phenotype, in contrast to previous results obtained in more clinically relevant DMD models (Dystrophin adn utrophin “double knockout” [DKO] mice and GRMD dogs).
In the present study, tissue regeneration and clinical benefits observed following MuStem cell transplantation in transiently immunosuppressed GRMD dogs were associated with very low subsarcolemmal dystrophin expression, as we previously reported in continuously immunosuppressed GRMD dogs58,59. It has been suggested that the global effect observed following MuStem cell transplantation is primarily due to stimulation of muscle regenerative activity, which promotes preservation of muscle architecture despite limited restoration of dystrophin expression. Similar results were described following intra-femoral injection of 5 × 108 MyoD-transduced CD271+ MSCs into immunosuppressed wildtype dogs subjected to prior cardiotoxin-induced TA muscle damage78. Few donor muscle fibers were detected in the damaged muscle 8 weeks post-transplantation, suggesting that the dispersion of infused cells throughout the body only resulted in scattered dystrophin+ fibers. We thus hypothesize that the number of donor MuStem cell nuclei per recipient muscle fiber was too low to enable sufficient dystrophin expression. Sampaolesi and coworkers demonstrated that the efficacy of IA delivery of allogeneic Mabs into immunosuppressed GRMD dogs could be significantly improved by increasing the number of successive injections of 5 × 107 cells from 3 to 5, resulting in a corresponding increase in the proportion of dystrophin+ fibers from 2% to 10%29. Another study described the presence of few human nuclei and a limited number of dystrophin+ fibers with weak immunolabeling 6 months after nine successive IV injections of 5 × 107 hADSCs/kg in GRMD dogs34. Importantly, the present findings demonstrate that this low level of dystrophin expression is not associated with host cellular or humoral responses against the protein, although we cannot exclude the possibility that serum antibody levels or cell responses were below the limits of detection of the tests used. Similarly, Nitahara-Kasahara and coworkers reported that anti-dystrophin antibodies were undetectable 8 weeks after IM injection of allogeneic MyoD-transduced CD271+ MSCs in non-immunosuppressed CXMDJ dogs, despite the presence of some dystrophin+ fibers78. We cannot rule out the possibility that the absence of a humoral anti-dystrophin response may be linked to the engraftment rate of MuStem cells. Interestingly however, GRMD dogs subjected to an adenovirus-associated virus-based gene therapy protocol also show no humoral anti-dystrophin immune response, despite marked restoration of dystrophin expression62. In the present study, the absence of an immune response to dystrophin may be explained by either low levels of dystrophin protein expression, and hence of potential antigens, or the presence in GRMD dogs of some dystrophin-expressing revertant fibers that exert a tolerogenic effect. Nevertheless, this revertant fiber hypothesis is not supported by findings in DMD patients, who can present preexisting circulating anti-dystrophin T-cells, the levels of which can increase in individuals not treated with corticosteroids81,82. Those observations in human patients suggest that anti-dystrophin immunity must be considered when designing cell therapy trials.
MSCs are implicated in a large number of immunomodulatory pathways owing to their interactions with a broad range of immune cell types83. For example, vascular cell adhesion molecule-1 and intracellular cell adhesion molecule-1 have been shown to mediate MSC immunomodulation via direct adhesion of MSCs to activated T-cells84. Furthermore, Mabs inhibit T-cell proliferation via a cell contact-independent mechanism involving indoleamine 2,3-dioxygenase and prostaglandin E237. We found that transient immunosuppressive treatment, limited to the infusion period, was sufficient to support the therapeutic effects of MuStem cell transplantation. This suggests that initial inhibition of the immune system is sufficient to allow donor MuStem cells to engraft into recipient muscle tissue, where they subsequently secrete soluble immunomodulatory factors and/or establish contact with immune cell subsets to enable continued engraftment. Further experiments to identify cell surface markers expressed by MuStem cells, as well as the soluble factors they secrete, could significantly contribute to our understanding of this therapeutic strategy.
In summary, the original data presented here show that long-term immunosuppressive treatment is not required to produce therapeutic effects in GRMD dogs undergoing allogeneic MuStem cell transplantation, and that transient IS limited to the transplantation period is sufficient to obtain a sustained beneficial response. While further experiments are required to determine how the therapeutic effects of MuStem cell delivery are maintained after cessation of IS regimen, our results provide critical insight that will enable the design of safer MuStem cell-based trials in the future.
Supplemental Material
Supplemental Material, Lorant_et_al_CT-1923_R1_Table_S1 for Vascular Delivery of Allogeneic MuStem Cells in Dystrophic Dogs Requires Only Short-Term Immunosuppression to Avoid Host Immunity and Generate Clinical/Tissue Benefits by Judith Lorant, Thibaut Larcher, Nicolas Jaulin, Benoît Hedan, Aurélie Lardenois, Isabelle Leroux, Laurence Dubreil, Mireille Ledevin, Hélicia Goubin, Sophie Moullec, Jack-Yves Deschamps, Chantal Thorin, Catherine André, Oumeya Adjali, and Karl Rouger in Cell Transplantation
Acknowledgements
We largely thank our colleague Yan Chérel (who died on 13 December 2013) for his helpful contribution to this work. He participated to the design of the study, the in vivo experiments (the systemic delivery procedure of the MuStem cells) and the muscle sampling. We thank Gillian Butler-Browne (Sorbonne Universités, UPMC Université Paris 06, INSERM UMRS974, CNRS FRE3617, Center for Research in Myology, Paris, France) for useful discussions that helped improve the manuscript. Also, we thank Emmanuelle Becker (INSERM U1085-IRSET, Université Rennes 1, Rennes, France) for her advice on statistical analysis of clinical scoring data. We are grateful to Maeva Dutilleul for technical assistance with animal care and operations, collection of biological samples, PBMC isolation and histology. Also, we are grateful to Celine Zuber who participated to in vitro experiments (technical isolation and expansion of muscle-derived cells). Finally, we thank the staff of the Boisbonne Center for gene and cell therapy (Oniris, Nantes, France) for their management and care of the GRMD dog colony.
Footnotes
Ethics Statements: The study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the French National Research Council.
Ethical Approval: This study was approved by the Ethics Committee on Animal Experimentation of the pays de la Loire region, France (Permit Number: CEEA.2012.121).
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects and so corresponding rights are not applicable.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the Association Française contre les Myopathies (AFM; N°15550 and N°15990) and the FEDER (Fond Européen de Développement Régional; N°37085 and N°38436). It was realized in the context of the IHU-Cesti project that received French Government financial support managed by the National Research Agency via the investment of the future program ANR-10-IBHU-005. The IHU-Cesti project was also supported by Nantes Metropole and the Pays de la Loire Region.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Bonilla E, Samitt CE, Miranda AF, Hays AP, Salviati G, DiMauro S, Kunkel LM, Hoffman EP, Rowland LP. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988;54(4):447–452. [DOI] [PubMed] [Google Scholar]
- 2. Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. [DOI] [PubMed] [Google Scholar]
- 3. Emery AEH. Population frequencies of inherited neuromuscular diseases – A word survey. Neuromuscul Disord. 1991;1(1):19–29. [DOI] [PubMed] [Google Scholar]
- 4. Moat SJ, Bradley DM, Salmon R, Clarke A, Hartley L. Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years’ experience in Wales. Eur J Hum Genet. 2013;21(10):1049–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubowitz V. Carrier detection and genetic counselling in Duchenne dystrophy. Dev Med Child Neurol. 1975;17(3):352–356. [DOI] [PubMed] [Google Scholar]
- 6. Manzur AY, Muntoni F. Diagnosis and new treatments in muscular dystrophies. J Neurol Neurosurg Psychiatry. 2009;80(7):706–714. [DOI] [PubMed] [Google Scholar]
- 7. Berardi E, Annibali D, Cassano M, Crippa S, Sampaolesi M. Molecular and cell-based therapies for muscle degenerations: a road under construction. Front Physiol. 2014;5:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35(8):1151–1156. [DOI] [PubMed] [Google Scholar]
- 10. Kinoshita I, Vilquin JT, Guérette B, Asselin I, Roy R, Tremblay JP. Very efficient myoblast allotransplantation in mice under FK506 immunosuppression. Muscle Nerve. 1994;17(12):1407–1415. [DOI] [PubMed] [Google Scholar]
- 11. Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. [DOI] [PubMed] [Google Scholar]
- 12. Huard J, Tremblay G, Verreault S, Labrecque C, Tremblay JP. Utilization of an antibody specific for human dystrophin to follow myoblast transplantation in nude mice. Cell Transplant. 1993;2(2):113–118. [DOI] [PubMed] [Google Scholar]
- 13. Huard J, Verreault S, Roy R, Tremblay M, Tremblay JP. High efficiency of muscle regeneration after human myoblast clone transplantation in SCID mice. J Clin Invest. 1994;93(2):586–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L, Blau HM. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992;356:435–438. [DOI] [PubMed] [Google Scholar]
- 15. Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW, Sahenk Z, Benson S, McAndrew PE, Rice R, Nagaraja H, Stephens R, Lantry L, Morris GE, Burghes AHM. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N Engl J Med. 1995;333(13):832–838. [DOI] [PubMed] [Google Scholar]
- 16. Guérette B, Asselin I, Vilquin JT, Roy R, Tremblay JP. Lymphocyte infiltration following allo- and xenomyoblast transplantation in mdx mice. Muscle Nerve. 1995;18(1):39–51. [DOI] [PubMed] [Google Scholar]
- 17. Huard J, Bouchard JP, Roy R, Malouin F, Dansereau G, Labrecque C, Albert N, Richards CL, Lemieux B, Tremblay JP. Human myoblast transplantation: preliminary results of 4 cases. Muscle Nerve. 1992;15(5):550–560. [DOI] [PubMed] [Google Scholar]
- 18. Tremblay JP, Malouin F, Roy R, Huard J, Bouchard JP, Satoh A, Richards CL. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993;2(2):99–112. [DOI] [PubMed] [Google Scholar]
- 19. Vilquin JT, Wagner E, Kinoshita I, Roy R, Tremblay JP. Successful histocompatible myoblast transplantation in dystrophin-deficient mdx mouse despite the production of antibodies against dystrophin. J Cell Biol. 1995;131(4):975–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chirieleison SM, Feduska JM, Schugar RC, Askew Y, Deasy BM. Human muscle-derived cell populations isolated by differential adhesion rates: phenotype and contribution to skeletal muscle regeneration in Mdx/SCID mice. Tissue Eng Part A. 2012;18(3-4):232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. [DOI] [PubMed] [Google Scholar]
- 22. Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–267. [DOI] [PubMed] [Google Scholar]
- 23. Meng J, Adkin CF, Xu SW, Muntoni F, Morgan JE. Contribution of human muscle- derived cells to skeletal muscle regeneration in dystrophic host mice. PLoS One. 2011:6(3):e17454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meng J, Chun S, Asfahani R, Lochmüller H, Muntoni F, Morgan J. Human skeletal muscle-derived CD133(+) cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol Ther. 2014;22(5):1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129(11):2773–2783. [DOI] [PubMed] [Google Scholar]
- 26. Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D’Antona G, Tonlorenzi R, Porretti L, Gavina M, Mamchaoui K, Pellegrino MA, Furling D, Mouly V, Butler-Browne GS, Bottinelli R, Cossu G, Bresolin N. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest. 2004;114(2):182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benchaouir R, Meregalli M, Farini A, D’Antona G, Belicchi M, Goyenvalle A, Battistelli M, Bresolin N, Bottinelli R, Garcia L, Torrente Y. Restoration of human dystrophin following transplantation of exonskipping- engineered DMD patient stem cells into dystrophic mice. Cell Stem Cell. 2007;24(1):646–657. [DOI] [PubMed] [Google Scholar]
- 28. Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, Bottinelli R, Cossu G. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301(5632):487–492. [DOI] [PubMed] [Google Scholar]
- 29. Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthélémy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444(7119):574–579. [DOI] [PubMed] [Google Scholar]
- 30. Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B, Tobita K, Keller BB, Cummins JH, Huard J. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12(6):1130–1141. [DOI] [PubMed] [Google Scholar]
- 31. Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142(5):1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157(5):851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C, Breittmayer JP, Groux H, Ailhaud G, Dani C. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201(9):1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vieira NM, Valadares M, Zucconi E, Secco M, Bueno CR, Jr, Brandalise V, Assoni A, Gomes J, Landini V, Andrade T, Caetano HV, Vainzof M, Zatz M. Human adipose-derived mesenchymal stromal cells injected systemically into GRMD dogs without immunosuppression are able to reach the host muscle and express human dystrophin. Cell Transplant. 2012;21(7):1407–1417. [DOI] [PubMed] [Google Scholar]
- 35. Yan Z, Zhuansun Y, Liu G., Chen R., Li J., Ran P. Mesenchymal stem cells suppress T-cells by inducing apoptosis and through PD-1/B7-H1 interactions. Immunol Lett. 2014;162(1 Pt A):248–255. [DOI] [PubMed] [Google Scholar]
- 36. François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–195. [DOI] [PubMed] [Google Scholar]
- 37. English K, Tonlorenzi R, Cossu G, Wood KJ. Mesoangioblasts suppress T-cell proliferation through IDO and PGE-2-dependent pathways. Stem Cells Dev. 2013;22(3):512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, Roelofs H. Multipotent stromal cells induce human regulatory T-cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31(9):1980–1991. [DOI] [PubMed] [Google Scholar]
- 39. Borel JF, Baumann G, Chapman I, Donatsch P, Fahr A, Mueller EA, Vigouret JM. In vivo pharmacological effects of ciclosporin and some analogues. Adv Pharmacol. 1996;35:115–246. [DOI] [PubMed] [Google Scholar]
- 40. Kahan BD. Immunosuppressive therapy. Curr Opin Immunol. 1992;4(5):553–560. [DOI] [PubMed] [Google Scholar]
- 41. Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605–612. [DOI] [PubMed] [Google Scholar]
- 42. Henry ML. Cyclosporine and tacrolimus (FK506): a comparison of efficacy and safety profiles. Clin Transplant. 1999;13(3):209–220. [DOI] [PubMed] [Google Scholar]
- 43. Burke JF, Jr, Pirsch JD, Ramos EL, Salomon DR, Stablein DM, Van Buren DM, West JC. Long-term efficacy and safety of cyclosporine in renal transplant recipients. N Engl J Med. 1994;331(6):358–363. [DOI] [PubMed] [Google Scholar]
- 44. Wongmekiat O, Thamprasert K. Investigating the protective effects of aged garlic extract on cyclosporin-induced nephrotoxicity in rats. Fundam Clin Pharmacol. 2005;19(5):555–62. [DOI] [PubMed] [Google Scholar]
- 45. Warren RB, Griffiths CE. Systemic therapies for psoriasis: methotrexate, retinoids, and cyclosporine. Clin Dermatol. 2008;26(5):438–447. [DOI] [PubMed] [Google Scholar]
- 46. Chi J, Zhu Y, Fu Y, Liu Y, Zhang X, Han L, Yin X, Zhao D. Cyclosporin A induces apoptosis in H9c2 cardiomyoblast cells through calcium-sensing receptor-mediated activation of the ERK MAPK and p38 MAPK pathways. Mol Cell Biochem. 2012;367(1–2):227–236. [DOI] [PubMed] [Google Scholar]
- 47. Zhu Y, Chi J, Liu Y, Sun Y, Fu Y, Zhang X, Ding X, Yin X, Zhao D. Knockdown of dishevelled-1 attenuates cyclosporine A-induced apoptosis in H9c2 cardiomyoblast cells. Mol Cell Biochem. 2013;374(1–2):113–123. [DOI] [PubMed] [Google Scholar]
- 48. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601–2614. [DOI] [PubMed] [Google Scholar]
- 49. Bottomley MJ, Harden PN. Update on the long-term complications of renal transplantation. Br Med Bull. 2013;106:117–134. [DOI] [PubMed] [Google Scholar]
- 50. Herman M, Weinstein T, Korzets A, Chagnac A, Ori Y, Zevin D, Malachi T, Gafter U. Effect of cyclosporin A on DNA repair and cancer incidence in kidney transplant recipients. J Lab Clin Med. 2001;137(1):14. [DOI] [PubMed] [Google Scholar]
- 51. Biring MS, Fournier M, Ross DJ, Lewis MI. Cellular adaptations of skeletal muscles to cyclosporine. J Appl Physiol. 1998;84(6):1967–1975. [DOI] [PubMed] [Google Scholar]
- 52. Fernández-Solá J, Campistol J, Casademont J, Grau JM, Urbano-Márquez A. Reversible cyclosporin myopathy. Lancet. 1990;335(8685):362–363. [DOI] [PubMed] [Google Scholar]
- 53. Friday BB, Horsley V, Pavlath GK. Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol. 2000;149(3):657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hardiman O, Sklar RM, Brown RH., Jr Direct effects of cyclosporin A and cyclophosphamide on differentiation of normal human myoblasts in culture. Neurology. 1993;43(7):1432–1434. [DOI] [PubMed] [Google Scholar]
- 55. Hong F, Lee J, Song JW, Lee SJ, Ahn H, Cho JJ, Ha J, Kim SS. Cyclosporin A blocks muscle differentiation by inducing oxidative stress and inhibiting the peptidyl-prolyl-cis-trans isomerase activity of cyclophilin A: cyclophilin A protects myoblasts from cyclosporin A- induced cytotoxicity. FASEB J. 2002;16(12):1633–1635. [DOI] [PubMed] [Google Scholar]
- 56. Sakuma K, Nishikawa J, Nakao R, Watanabe K, Totsuka T, Nakano H, Sano M, Yasuhara M. Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropathol. 2003;105(3):271–280. [DOI] [PubMed] [Google Scholar]
- 57. Sakuma K, Nakao R, Aoi W, Inashima S, Fujikawa T, Hirata M, Sano M, Yasuhara M. Cyclosporin A treatment upregulates Id1 and Smad3 expression and delays skeletal muscle regeneration. Acta Neuropathol. 2005;110(3):269–280. [DOI] [PubMed] [Google Scholar]
- 58. Rouger K, Larcher T, Dubreil L, Deschamps JY, Le Guiner C, Jouvion G, Delorme B, Lieubeau B, Carlus M, Fornasari B, Theret M, Orlando P, Ledevin M, Zuber C, Leroux I, Deleau S, Guigand L, Testault I, Le Rumeur E, Fiszman M, Chérel Y. Systemic delivery of allogenic muscle stem cells induces long-term muscle repair and clinical efficacy in duchenne muscular dystrophy dogs. Am J Pathol. 2011;179(5):2501–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Robriquet F, Lardenois A, Babarit C, Larcher T, Dubreil L, Leroux I, Zuber C, Ledevin M, Deschamps JY, Fromes Y, Chérel Y, Guevel L, Rouger K. Differential gene expression profiling of dystrophic dog muscle after mustem cell transplantation. PLoS One. 2015;10(5):e0123336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Honeyman K, Carville KS, Howell JM, Fletcher S, Wilton SD. Development of a snapback method of single-strand conformation polymorphism analysis for genotyping Golden Retrievers for the X-linked muscular dystrophy allele. Am J Vet Res. 1999;60(6):734–737. [PubMed] [Google Scholar]
- 61. Yuhki N, Beck T, Stephens R, Neelam B, O’Brien SJ. Comparative genomic structure of human, dog, and cat MHC: HLA, DLA, and FLA. J Hered. 2007;98(5):390–399. [DOI] [PubMed] [Google Scholar]
- 62. Thibaud JL, Monnet A, Bertoldi D, Barthélémy I, Blot S, Carlier PG. Characterization of dystrophic muscle in golden retriever muscular dystrophy dogs by nuclear magnetic resonance imaging. Neuromuscul Disord. 2007;17(7):575–584. [DOI] [PubMed] [Google Scholar]
- 63. Le Guiner C, Montus M, Servais L, Chérel Y, François V, Thibaud JL, Wary C, Matot B, Larcher T, Guigand L, Dutilleul M, Domenger C, Allais M, Beuvin M, Moraux A, Le Duff J, Devaux M, Jaulin N, Guilbaud M, Latournerie V, Veron P, Boutin S, et al. Forelimb treatment in a large cohort of dystrophic dogs supports delivery of a recombinant AAV for exon skipping in Duchenne patients. Mol Ther. 2014;22(11):1923–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Baroja-Mazo A, Revilla-Nuin B, Ramírez P, Pons JA. Immunosuppressive potency of mechanistic target of rapamycin inhibitors in solid-organ transplantation. World J Transplant. 2016;6(1):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cossu G, Previtali SC, Napolitano S, Cicalese MP, Tedesco FS, Nicastro F, Noviello M, Roostalu U, Natali Sora MG, Scarlato M, De Pellegrin M, Godi C, Giuliani S, Ciotti F, Tonlorenzi R, Lorenzetti I, Rivellini C, Benedetti S, Gatti R, Marktel S, Mazzi B, Tettamanti A, et al. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol Med. 2015;7(12):1513–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ventura-Aguiar P, Campistol JM, Diekmann F. Safety of mTOR inhibitors in adult solid organ transplantation. Expert Opin Drug Saf. 2016;15(3):303–319. [DOI] [PubMed] [Google Scholar]
- 68. Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18(2):128–134. [DOI] [PubMed] [Google Scholar]
- 69. Ichim TE, Alexandrescu DT, Solano F, Lara F, Campion Rde N, Paris E, Woods EJ, Murphy MP, Dasanu CA, Patel AN, Marleau AM, Leal A, Riordan NH. Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol. 2010;260(2):75–82. [DOI] [PubMed] [Google Scholar]
- 70. Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28(5):219–226. [DOI] [PubMed] [Google Scholar]
- 71. Guérette B, Skuk D, Célestin F, Huard C, Tardif F, Asselin I, Roy B, Goulet M, Roy R, Entman M, Tremblay JP. Prevention by anti- LFA-1 of acute myoblast death following transplantation. J Immunol. 1997;159(5):2522–2531. [PubMed] [Google Scholar]
- 72. Villalta SA, Rinaldi C, Deng B, Liu G, Fedor B, Tidball JG. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet. 2011;20(4):790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sales KJ, Maldonado-Pérez D, Grant V, Catalano RD, Wilson MR, Brown P, Williams AR, Anderson RA, Thompson EA, Jabbour HN. Prostaglandin F(2alpha)-F-prostanoid receptor regulates CXCL8 expression in endometrial adenocarcinoma cells via the calcium-calcineurin-NFAT pathway. Biochim Biophys Acta. 2009;1793(12):1917–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Boyd JH, Divangahi M, Yahiaoui L, Gvozdic D, Qureshi S, Petrof BJ. Toll-like receptors differentially regulate CC and CXC chemokines in skeletal muscle via NF-kappaB and calcineurin. Infect Immun. 2006;74(12):6829–6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Datta A, David R, Glennie S, Scott D, Cernuda-Morollon E, Lechler RI, Ridley AJ, Marelli-Berg FM. Differential effects of immunosuppressive drugs on T-cell motility. Am J Transplant. 2006;6(12):2871–2883. [DOI] [PubMed] [Google Scholar]
- 76. Chen T, Guo J, Yang M, Han C, Zhang M, Chen W, Liu Q, Wang J, Cao X. Cyclosporin A impairs dendritic cell migration by regulating chemokine receptor expression and inhibiting cyclooxygenase-2 expression. Blood. 2004;103(2):413–421. [DOI] [PubMed] [Google Scholar]
- 77. Drath DB, Kahan BD. Alterations in rat pulmonary macrophage function by the immunosuppressive agents cyclosporine, azathioprine, and prednisolone. Transplantation. 1983;35(6):588–592. [DOI] [PubMed] [Google Scholar]
- 78. Nitahara-Kasahara Y, Hayashita-Kinoh H, Ohshima-Hosoyama S, Okada H, Wada-Maeda M, Nakamura A, Okada T, Takeda S. Long-term engraftment of multipotent mesenchymal stromal cells that differentiate to form myogenic cells in dogs with Duchenne muscular dystrophy. Mol Ther. 2012;20(1):168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Valadares MC, Gomes JP, Castello G, Assoni A, Pellati M, Bueno C, Corselli M, Silva H, Bartolini P, Vainzof M, Margarido PF, Baracat E, Péault B, Zatz M. Human adipose tissue derived pericytes increase life span in Utrn (tm1Ked) DMD (mdx) /J mice. Stem Cell Rev. 2014;10(6):830–840. [DOI] [PubMed] [Google Scholar]
- 80. Vieira NM, Bueno CR, Jr, Brandalise V, Moraes LV, Zucconi E, Secco M, Suzuki MF, Camargo MM, Bartolini P, Brum PC, Vainzof M, Zatz M. SJL dystrophic mice express a significant amount of human muscle proteins following systemic delivery of human adipose-derived stromal cells without immunosuppression. Stem Cells. 2008;26(9):2391–2398. [DOI] [PubMed] [Google Scholar]
- 81. Flanigan KM, Campbell K, Viollet L, Wang W, Gomez AM, Walker CM, Mendell JR. Anti-dystrophin T-cell responses in Duchenne muscular dystrophy: prevalence and a glucocorticoid treatment effect. Hum Gene Ther. 2013;24(9):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, Bowles D, Gray S, Li C, Galloway G, Malik V, Coley B, Clark KR, Li J, Xiao X, Samulski J, McPhee SW, Samulski RJ, Walker CM. Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med. 2010;363(15):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. English K, French A, Wood KJ. Mesenchymal stromal cells: facilitators of successful transplantation? Cell Stem Cell. 2010;7(4):431–442. [DOI] [PubMed] [Google Scholar]
- 84. Ren G, Zhao X, Zhang L, Zhang J, L’Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine- induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184(5):2321–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Lorant_et_al_CT-1923_R1_Table_S1 for Vascular Delivery of Allogeneic MuStem Cells in Dystrophic Dogs Requires Only Short-Term Immunosuppression to Avoid Host Immunity and Generate Clinical/Tissue Benefits by Judith Lorant, Thibaut Larcher, Nicolas Jaulin, Benoît Hedan, Aurélie Lardenois, Isabelle Leroux, Laurence Dubreil, Mireille Ledevin, Hélicia Goubin, Sophie Moullec, Jack-Yves Deschamps, Chantal Thorin, Catherine André, Oumeya Adjali, and Karl Rouger in Cell Transplantation