Abstract
Background:
The purpose of this meta-analysis from randomized controlled trials (RCTs) was to determine the efficacy and safety of the preoperative use of gabapentin for the treatment of acute and chronic postoperative pain following breast cancer surgery.
Methods:
In November 2017, a systematic computer-based search was conducted in PubMed, Embase, Web of Science, Cochrane Library, and Google databases. RCTs comparing gabapentin with placebo in patients undergoing breast cancer surgery were retrieved. The primary endpoint was the visual analog scale (VAS) after surgery and 24 hours after surgery and total morphine consumption. The secondary outcomes were incidence of chronic pain and complications (the incidence of nausea). Software Stata 12.0 was used for meta-analysis.
Results:
Finally, 9 RCTs were included in the meta-analysis. Results indicated that gabapentin was associated with reduced pain scores after surgery and 24 hours after surgery. Meanwhile, oral gabapentin was associated with a reduction of the total morphine consumption after breast cancer surgery. Similarly, gabapentin was associated with a reduction in the incidence of chronic pain and the incidence of nausea.
Conclusions:
Preoperative use of gabapentin was able to reduce acute and chronic postoperative pain, total morphine consumption and the occurrence of nausea following breast cancer surgery. Further studies should determine the optimal dose of gabapentin for pain control after breast cancer surgery.
Keywords: acute pain, breast cancer surgery, chronic pain, gabepentin, meta-analysis
1. Introduction
Breast cancer is the most commonly diagnosed cancer worldwide.[1] Modified radical mastectomy combined variably with chemotherapy or radiotherapy is currently the treatment of choice for breast cancer. Acute and chronic pain is a common complication after breast cancer surgery, with accounts between 20% to 50%, and higher.[2,3] Chronic neuropathic pain was particularly occurs after dissection of axillary lymph nodes and injury of the brachial plexus.[4] Adequate pain control after breast cancer is of vital for patient satisfaction and early mobilization.[5,6] Additionally, optimal management of the acute pain may influence the development of chronic pain[7]. Several modalities (oral opioids and patient controlled anesthesia) have been applied to reduce acute pain after breast cancer surgery.[8,9]
In recent years, gabapentin has been introduced as an adjunct in the multimodal management of postoperative pain after breast cancer surgery. Gabapentin is an anticonvulsant agent that can bind to the alpha2delta subunit of presynaptic voltage-gated calcium channels, thus reducing the calcium influx into presynaptic terminals.[10,11] However, whether perioperative oral gabapentin was effective in reducing acute pain and chronic pain among patients undergoing breast cancer surgery has not been determined.
The purpose of this meta-analysis was to systematically review randomized controlled trials (RCTs) and identify whether preoperative oral gabapentin was associated with less acute and chronic pain among women undergoing breast cancer surgery.
2. Materials and methods
This meta-analysis was conducted in compliance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions[12] and was written following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) checklist.[13]
2.1. Search strategies
The following databases were searched in March 2017 without language restriction: PubMed (1950–November 2017), Embase (1974–November 2017), Web of Science (1950–November 2017), Cochrane Library (November 2017 Issue 3), and Chinese Wanfang databases (1950–November 2017). The MeSH terms and their combinations used in the search were as follows: “analgesia” OR “pain management” OR “anesthetic agents” OR “breast cancer surgery” OR “mastectomy” OR “breast surgery” AND “gabapentin” OR “gabapentin” (MeSH terms).
The reference lists of related reviews and original articles were searched for any relevant studies, including RCTs involving adult humans. When multiple reports describing the same sample were published, the most recent or complete report was used. Because this is a meta-analysis, no ethics committee or institutional review board approval was necessary for the study.
2.2. Inclusion criteria and study selection
Patients (P): women (age >18 years) undergoing breast cancer surgery; Intervention (I): perioperative gabapentin as an intervention group; Comparison (C): placebo; Outcomes (O): visual analog scale (VAS) after surgery and 24 hours after surgery, total morphine consumption, incidence of chronic pain, and the occurrence of nausea; Study design (S): RCTs.
Two independent reviewers screened the titles and abstracts of the identified studies after removing duplicates in the search results. Any disagreements about the inclusion or exclusion of a study were resolved by discussion or consultation with an expert. The reliability of the study selection was determined by Cohen's kappa test; the acceptable threshold value was set at 0.61.[14,15]
2.3. Data abstraction
A specific extraction was performed to collect the following data from the included trials: patients’ general characteristics, country, sample size of the control group and intervention group, preoperative and postoperative doses, and the timing and frequency of gabapentin use. Outcomes such as VAS after surgery and 24 hours after surgery, total morphine consumption, incidence of chronic pain and the occurrence of nausea were abstracted and recorded on a form.
Postoperative pain intensity was measured by a 110-point VAS. When the numerical rating scale (NRS) was reported, it was converted to a VAS. Additionally, a 11-point VAS was converted to a 110-point VAS.[16] Data in other forms (i.e., median, interquartile range, and mean ± 95% confidence interval (CI)) were converted to the mean ± standard deviation (SD) according to the Cochrane Handbook.[17] And opioid drugs were converted to equivalent morphine consumption according to previously published literature (iv morphine 10 mg = oral morphine 30 mg = iv hydromorphone 1.5 mg = oral hydromorphone 7.5 mg = iv pethidine 75 mg = oral oxycodone 20 mg = iv tramadol 100 mg = iv piritramide 7.5 mg)).[18] If the data were not reported numerically, we extracted these data using the GetData Graph Digitizer software from the published figures. All data were extracted by 2 independent reviewers, and disagreements were resolved by discussion.
2.4. Quality assessment and quality evidence
The methodological quality of all included trials was independently assessed by 2 reviewers using the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (http://handbook.cochrane.org/). A total of 7 items (random sequence generation, allocation concealment, blinding to the participant and personnel, blinding to the outcome assessment, incomplete outcome, selective reporting, and other bias) were measured. Each of the items was measured as “low risk of bias,” “unclear risk of bias,” and “high risk of bias”. The risk of bias summary and risk of bias graph were obtained using Review Manager 5.3.0 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Two reviewers (XF and KJG) independently evaluated the quality of evidence assessment in accordance with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.[19] Risk of bias, inconsistency, indirectness, imprecision, and publication bias were the assessment items.[19,20] Each result was classified as high, moderate, low, or very low. GRADE Pro software was used to construct summary tables for the included studies.
2.5. Outcome measures and statistical analysis
Continuous outcomes (VAS after surgery and 24 hours after surgery, total morphine consumption) were expressed as the weighted mean differences (WMD) and respective 95% CI. Dichotomous outcomes (incidence of chronic pain and the occurrence of nausea) were expressed as the risk ratio (RR) with 95% CI. Statistical significance was set at P < .05 to summarize the findings across the trials. The meta-analysis was calculated by Stata software, version 13.0 (Stata Corp., College Station, TX). Statistical heterogeneity was tested using the chi-squared test and I2 statistic. When there was no statistical evidence of heterogeneity (I2 < 50%, P > .1), a fixed-effects model was adopted; otherwise, a random-effect model was chosen. Publication bias was tested using funnel plots. Publication bias was assessed by funnel plot and quantitatively assessed by Begg's test. We considered there to be no publication bias if the funnel plot was symmetrical and the P value was >.05. Subgroup analysis was conducted according to the dose of gabapentin (<900 or ≥900 mg/d).
3. Results
3.1. Search results
The search result is summarized in the PRISMA flowchart (Fig. 1). In the initial research, a total of 185 papers were identified from the electronic databases (PubMed = 77, Embase = 56, Web of Science = 23, Cochrane Library = 19, and Chinese Wanfang database = 10); 0 additional records were identified through other sources. Thus, a total of 185 papers were obtained in the initial search. These bibliographical references were introduced into Endnote Software (Version X7, Thompson Reuters, CA). Duplicates were then removed and 151 papers were reviewed. After screening the titles and abstracts of these 151 studies, 142 papers were excluded because they were irrelevant or did not meet the criteria. Ultimately, 9 clinical studies with 576 patients (gabapentin group = 287, placebo = 289) were finally included in the meta-analysis.[8–28] The general characteristic of the included studies can be seen in Table 1. The sample of included studies ranged from 20 to 50. And the gabapentin doses ranged from 300 mg to 1200 mg. The duration of follow-up ranged from 48 hours to 6 months.
Figure 1.
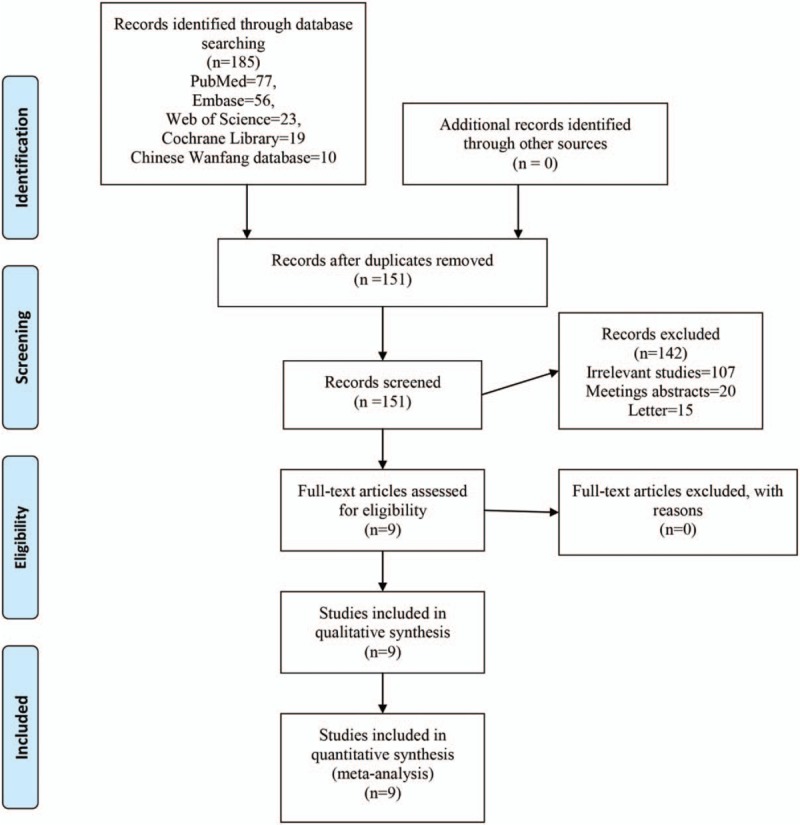
Flowchart of study search and inclusion criteria.
Table 1.
The general characteristic of the included studies; 1, VAS after surgery; 2, VAS at 24 hours after surgery; 3, total morphine consumption; 4, the occurrence of nausea; 5, chronic pain incidence.

3.2. Quality assessment
Quality assessment of the included studies can be seen in Figures 2 and 3. The risk of bias for random sequence generation, allocation concealment, blinding to the participant and outcome assessment, selection bias are all with low risk of bias. Two studies are with unclear risk of bias in other bias. Reasons for unclear were mainly due to the sample size was not calculated and we thus did not know whether the sample was enough to reach the statistically significance.
Figure 2.

Risk of bias of included randomized controlled trials. +, no bias; −, bias;?, bias unknown.
Figure 3.

the risk of bias graph.
3.3. Results of meta-analysis
3.3.1. VAS after surgery
VAS scores after surgery was reported in six studies, there was a middle heterogeneity between the included studies (I2 = 46.3%, P = .097). And the pooled results indicated that administration of gabapentin can decrease VAS score after surgery by 16.14 points (WMD = −16.14, 95% CI −21.85, −10.24, P = .000, low evidence, Fig. 4).
Figure 4.
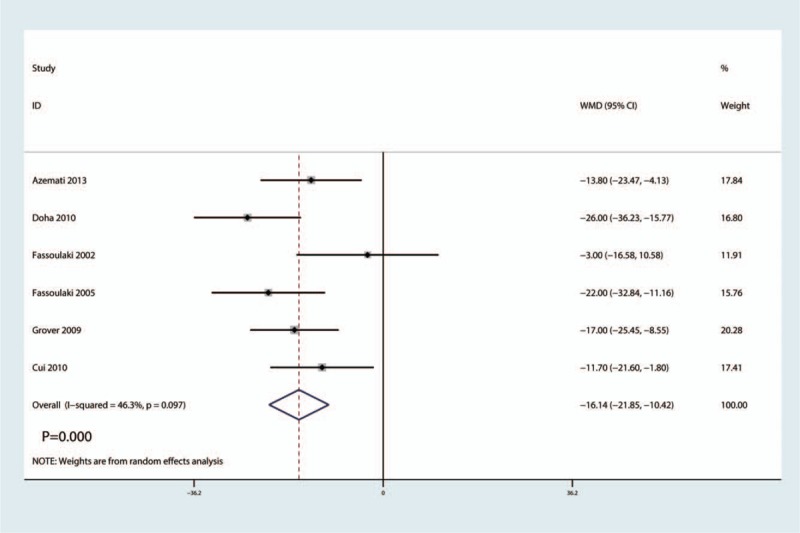
Forest plots of the included studies comparing the VAS after surgery. VAS = visual analog scale.
3.3.2. VAS at 24 hours after surgery
VAS scores after surgery was reported in four studies, there was a high heterogeneity between the included studies (I2 = 95.7%, P = .000). And the pooled results indicated that administration of gabapentin can decrease VAS score at 24 hours after surgery by 27.33 points (WMD = −27.33, 95% CI −51.03, −3.63, P = .024, low evidence, Fig. 5). We then performed sensitivity by omitting one study in turn and found that overall effects was in 95% CI limit (Fig. 6).
Figure 5.

Forest plots of the included studies comparing the VAS at 24 hours after surgery. VAS = visual analog scale.
Figure 6.

Sensitivity analysis of the VAS at 24 hours after surgery. VAS = visual analog scale.
3.4. Total morphine consumption
Total morphine consumption after surgery was reported in 8 studies, there was a high heterogeneity between the included studies (I2 = 97.4%, P = .000). And the pooled results indicated that administration of gabapentin can decrease total morphine consumption after surgery by 4.59 mg (WMD = −4.59, 95% CI −7.07, −2.11, P = .000, middle evidence, Fig. 7).
Figure 7.
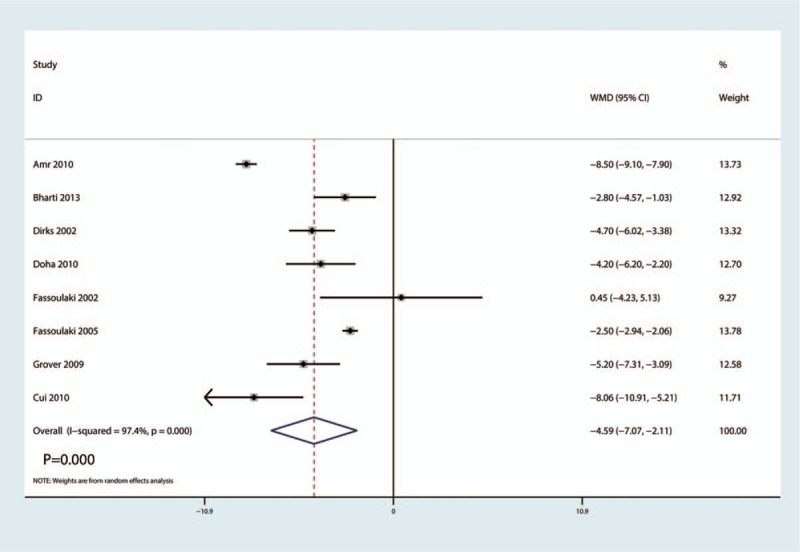
Forest plots of the included studies comparing the total morphine consumption.
3.5. The occurrence of nausea
The occurrence of nausea after surgery was reported in 4 studies, there was no heterogeneity between the included studies (I2 = 0.0%, P = .711). In addition, the pooled results indicated that administration of gabapentin can decrease the occurrence of nausea after surgery (RR = 0.54, 95% CI 0.38, 0.78, P = .001, middle evidence, Fig. 8).
Figure 8.

Forest plots of the included studies comparing the occurrence of nausea.
3.6. Chronic pain incidence
The chronic pain incidence after surgery was reported in 5 studies, there was no heterogeneity between the included studies (I2 = 0.0%, P = .463). Also the pooled results indicated that administration of gabapentin can decrease the chronic pain incidence (RR = 0.57, 95% CI 0.47, 0.68, P = .000, low evidence, Fig. 9).
Figure 9.
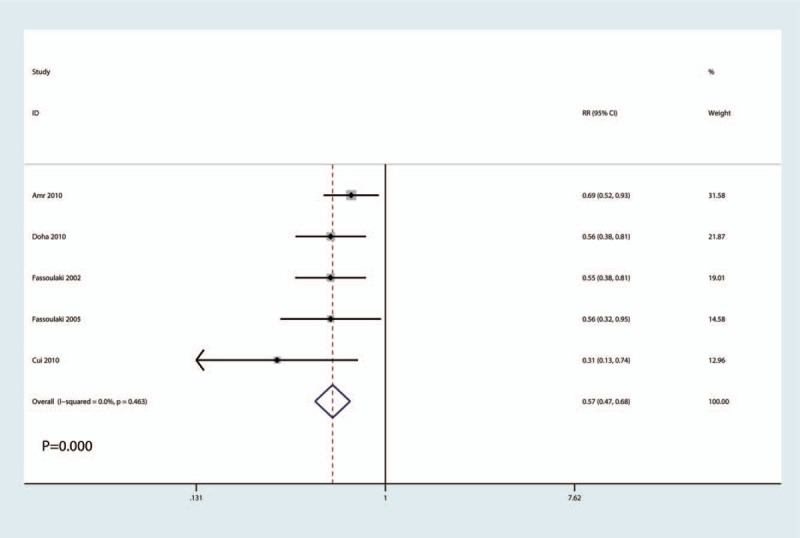
Forest plots of the included studies comparing the chronic pain incidence.
3.7. Subgroup analysis, publication bias, and sensitivity analysis
Subgroup analyses were conducted according to a low dose (<900 mg/d) and a high dose of gabapentin (≥900 mg/d). The detailed results can be seen in Table 2. The pooled results indicated that a high dose of gabapentin was superior than low dose of gabapentin in terms of (P < .05). Publication bias was detected by the funnel plot (Fig. 10) and Begg's test (P = .216, Fig. 11) and results revealed that there was no publication bias between the included studies for the VAS after surgery. Sensitivity analysis was then performed and results shown that the result was stable and excluded one of the study will not change the final result (Fig. 12).
Table 2.
Subgroup analysis for the VAS after surgery, at 24 hours after surgery, total morphine consumption, the occurrence of nausea, and chronic pain incidence.

Figure 10.

Funnel plot of the VAS after surgery. VAS = visual analog scale.
Figure 11.

Begg's test of the VAS after surgery. VAS = visual analog scale.
Figure 12.

Sensitivity analysis of the VAS after surgery. VAS = visual analog scale.
4. Discussion
4.1. Main findings
Our meta-analysis indicated that gabapentin has a positive role in reducing acute pain intensity, total morphine consumption, the occurrence of nausea and the chronic pain incidence. Moreover, high dose of gabapentin was superior to low dose of gabapentin in terms of the outcomes. Our analysis found low- to middle-quality evidence that the preoperative use of gabapentin for pain control and morphine-saving.
4.2. Strength of this meta-analysis
A major strength of current meta-analysis was that we limited the inclusion criteria restricted to a surgical breast cancer population and administration with gabapentin alone. Another new knowledge of this meta-analysis was that we performed a subgroup analysis and revealed that high dose of gabapentin was effective than low dose of gabapentin for alleviating acute and chronic pain after breast cancer surgery.
4.3. Implications for clinical practice
Our meta-analysis showed that the benefit existed in gabapentin compared with control group. Therefore, preoperative oral gabapentin might be the best guide for patients prepared for breast cancer surgery. And when choosing the dosage of gabapentin, high dose of gabapentin was preferable.
The anticonvulsant gabapentin has shown promising results in relieving acute and chronic neuropathic pain.[29,30] And preoperative use of gabapentin was associated with a pain relieving in total knee and hip arthroplasty, spinal surgery and laparoscopic cholecystectomy.[31–35] Results from current meta-analysis indicated that gabapentin can decrease acute and chronic pain after breast cancer surgery. Results showed that gabapentin can decrease the pain scores by 16.14 and 27.33 points on 110-points after surgery and 24 hours, respectively. There was a high heterogeneity for pain scores at 24 hours after surgery. We performed subgroup analysis according to the dosage of gabapentin (<900 or ≥900 mg/d). The dosage of gabapentin only contributed to heterogeneity of pain scores.
Rai et al[36] reported that gabapentin has an efficacy to reduce acute pain intensity and does not reported the chronic pain. What is more, they included gabapentin and pregabalin and thus a mixed results will weak the final conclusion. Axillary node dissection was associated with a significant risk of chronic pain. Thus, the axillary node dissection may be a source of heterogeneity.[36]
We assessed the total morphine consumption and the occurrence of nausea. Final results indicated that gabapentin was associated with a significantly reduction of the total morphine consumption and the occurrence of nausea. Nausea was a significant source of morbidity for patients after surgery and may contribute to delay in discharge from hospital. Khan et al[37] reported that gabapentin combined with other agents will enhance experience benefit.
The optimal dose of gabapentin for breast cancer surgery was not determined. For other surgery (total knee and hip arthroplasty), gabapentin (600 mg) given before the operation or 2 hour before operation can decrease postoperative opioid consumption and improve knee range of motion.[38] For breast cancer surgery, the dosage of gabapentin ranged from 300 to 1200 mg. And we could not determine the optimal dose of gabapentin. Future studies should be focused on the dose-response efficacy of gabapentin and the potential adverse complications.
4.4. Limitations
There were a total of 5 limitations in this meta-analysis: only 9 RCTs with small sample (20–50) were included, which might have affected the precision of the effect size estimations; follow-up was relatively short and the long-term benefit of gabapentin was unknown; dosage and timing of gabapentin administration differed between the studies and thus may cause the heterogeneity; we could not determine whether there was a publication bias for the final outcomes; different surgery with or without axillary dissection were included in this meta-analysis, which would cause selection bias.
5. Conclusion
In conclusion, immediate and chronic analgesic efficacy and opioid-sparing effects were obtained with the administration of gabapentin in breast cancer surgery. Because the sample size and the number of included studies were limited, a multicenter RCT is needed to identify the optimal dose and intervals of gabapentin.
Author contributions
Conceptualization: Huasheng Lin, Qing Zhang.
Data curation: Yunfeng Jiang, Huasheng Lin, Shijie Zhang.
Formal analysis: Qiaotong Huang, Xiong Jun.
Investigation: Tongbiao Wang, Shijie Zhang.
Project administration: Tongbiao Wang.
Resources: Qing Zhang.
Software: Qiaotong Huang, Shijie Zhang, Qing Zhang.
Supervision: Qiaotong Huang.
Validation: Shijie Zhang.
Visualization: Xiong Jun.
Writing – original draft: Junhong Li, Zheng Rong, Xiong Jun.
Writing – review & editing: Zheng Rong, Xiong Jun.
Footnotes
Abbreviations: CI = confidence interval, PRISMA = preferred reporting items for systematic reviews and meta-analyses, RCTs = randomized controlled trials, RR = risk ratio, SD = standard deviation, VAS = visual analog scale, WMD = weighted mean differences.
This study was funded by National Natural Science Fund regional fund (81760850 and 81660774), Guangxi medical and health appropriate technology research and development project (S201308-03) and Youth Science Foundation of the First Affiliated Hospital of Guangxi University of traditional Chinese (GZYQJ08).
The authors have no conflicts of interest to disclose.
References
- [1].Khosravi-Shahi P, Cabezon-Gutierrez L, Custodio-Cabello S. Metastatic triple negative breast cancer: optimizing treatment options, new and emerging targeted therapies. Asia Pac J Clin Oncol 2017;14:32–9. [DOI] [PubMed] [Google Scholar]
- [2].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [3].Bruce J, Thornton AJ, Powell R, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain 2014;155:232–43. [DOI] [PubMed] [Google Scholar]
- [4].Tasmuth T, Estlanderb AM, Kalso E. Effect of present pain and mood on the memory of past postoperative pain in women treated surgically for breast cancer. Pain 1996;68:343–7. [DOI] [PubMed] [Google Scholar]
- [5].Brodner G, Mertes N, Buerkle H, et al. Acute pain management: analysis, implications and consequences after prospective experience with 6349 surgical patients. Eur J Anaesthesiol 2000;17:566–75. [DOI] [PubMed] [Google Scholar]
- [6].Cheng GS, Ilfeld BM. An evidence-based review of the efficacy of perioperative analgesic techniques for breast cancer-related surgery. Pain Med 2017;18:1344–65. [DOI] [PubMed] [Google Scholar]
- [7].Katz J, Jackson M, Kavanagh BP, et al. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain 1996;12:50–5. [DOI] [PubMed] [Google Scholar]
- [8].Bharti N, Bala I, Narayan V, et al. Effect of gabapentin pretreatment on propofol consumption, hemodynamic variables, and postoperative pain relief in breast cancer surgery. Acta Anaesthesiol Taiwan 2013;51:10–3. [DOI] [PubMed] [Google Scholar]
- [9].Gartner R, Kroman N, Callesen T, et al. Multimodal prevention of pain, nausea and vomiting after breast cancer surgery. Minerva Anestesiol 2010;76:805–13. [PubMed] [Google Scholar]
- [10].Wang YM, Xia M, Shan N, et al. Pregabalin can decrease acute pain and postoperative nausea and vomiting in hysterectomy: A meta-analysis. Medicine (Baltimore) 2017;96:e7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang Y, Wang Y, Zhang X. Effect of pre-emptive pregabalin on pain management in patients undergoing laparoscopic cholecystectomy: a systematic review and meta-analysis. Int J Surg 2017;44:122–7. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JPT GS. Cochrane handbook for systematic reviews of interventions version 5.1.0, 2011. [Google Scholar]
- [13].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 1977;33:363–74. [PubMed] [Google Scholar]
- [15].Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- [16].Wang C, Cai X-Z, Yan S-G. Comparison of periarticular multimodal drug injection and femoral nerve block for postoperative pain management in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2015;30:1281–6. [DOI] [PubMed] [Google Scholar]
- [17].GS HJ. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. In, Vol. 2011. Available at: http://www.cochrane-handbook.org. [Google Scholar]
- [18].Berdine HJ, Nesbit SA. Equianalgesic dosing of opioids. J Pain Palliat Care Pharmacother 2006;20:79–84. [PubMed] [Google Scholar]
- [19].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ 2008;336:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of Venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain 2010;26:381–5. [DOI] [PubMed] [Google Scholar]
- [22].Azemati S, Dokouhaki AG, Talei A, et al. Evaluation of the effect of a preoperative single dose of gabapentin on emergence agitation in patients undergoing breast cancer surgery. Middle East J Cancer 2013;4:1–4. [Google Scholar]
- [23].Dirks J, Fredensborg BB, Christensen D, et al. A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology 2002;97:560–4. [DOI] [PubMed] [Google Scholar]
- [24].Doha NM, Rady A, Azab SRE. Preoperative use of gabapentin decreases the anesthetic and analgesic requirements in patients undergoing radical mastectomy. Egyptian J Anaesth 2010;26:287–91. [Google Scholar]
- [25].Fassoulaki A, Patris K, Sarantopoulos C, et al. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer. Anesth Analg 2002;95:985–91. table of contents. [DOI] [PubMed] [Google Scholar]
- [26].Fassoulaki A, Triga A, Melemeni A, et al. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg 2005;101:1427–32. [DOI] [PubMed] [Google Scholar]
- [27].Grover VK, Mathew PJ, Yaddanapudi S, et al. A single dose of preoperative gabapentin for pain reduction and requirement of morphine after total mastectomy and axillary dissection: randomized placebo-controlled double-blind trial. J Postgrad Med 2009;55:257–60. [DOI] [PubMed] [Google Scholar]
- [28].Xiude C, Feng L, Peng L, et al. Effect of gabapentin on patient controlled intravenous analgesia after modified radical mastectomy. Chin J Postgrad Med 2010;33:13–6. [Google Scholar]
- [29].Iftikhar IH, Alghothani L, Trotti LM. Gabapentin enacarbil, pregabalin and rotigotine are equally effective in restless legs syndrome: a comparative meta-analysis. Eur J Neurol 2017;24:1446–56. [DOI] [PubMed] [Google Scholar]
- [30].Wang J, Zhu Y. Different doses of gabapentin formulations for postherpetic neuralgia: a systematical review and meta-analysis of randomized controlled trials. J Dermatolog Treat 2017;28:65–77. [DOI] [PubMed] [Google Scholar]
- [31].Wang L, Dong Y, Zhang J, et al. The efficacy of gabapentin in reducing pain intensity and postoperative nausea and vomiting following laparoscopic cholecystectomy: a meta-analysis. Medicine (Baltimore) 2017;96:e8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peng C, Li C, Qu J, et al. Gabapentin can decrease acute pain and morphine consumption in spinal surgery patients: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hamilton TW, Strickland LH, Pandit HG. A Meta-analysis on the use of gabapentinoids for the treatment of acute postoperative pain following total knee arthroplasty. J Bone Joint Surg Am 2016;98:1340–50. [DOI] [PubMed] [Google Scholar]
- [34].Zhai L, Song Z, Liu K. The effect of gabapentin on acute postoperative pain in patients undergoing total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2016;95:e3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mao Y, Wu L, Ding W. The efficacy of preoperative administration of gabapentin/pregabalin in improving pain after total hip arthroplasty: a meta-analysis. BMC Musculoskelet Disord 2016;17:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rai AS, Khan JS, Dhaliwal J, et al. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: a systematic review and meta-analysis of randomized controlled trials. J Plast Reconstr Aesthet Surg 2017;70:1317–28. [DOI] [PubMed] [Google Scholar]
- [37].Khan JS, Margarido C, Devereaux PJ, et al. Preoperative celecoxib in noncardiac surgery: a systematic review and meta-analysis of randomised controlled trials. Eur J Anaesthesiol 2016;33:204–14. [DOI] [PubMed] [Google Scholar]
- [38].Clarke H, Pereira S, Kennedy D, et al. Gabapentin decreases morphine consumption and improves functional recovery following total knee arthroplasty. Pain Res Manag 2009;14:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


