Abstract Abstract
Brephallus Wang et al., gen. n. is established as a genus distinct from Pseudophoraspis Kirby, 1903 because of the lack of a well-developed apical outgrowth on sclerite L2D and substantial genetic differences. Two species are transferred to the new genus from Pseudophoraspis, i.e., Brephallusfruhstorferi (Shelford, 1910), comb. n. and Brephallustramlapensis (Anisyutkin, 1999), comb. n. We provide a detailed generic diagnosis of Brephallus Wang et al., gen. n. Based on COI data, males, females and nymphs of three Pseudophoraspis species (P.clavellata Wang et al., 2013, P.recurvata Wang et al., 2013 and P.kabakovi Anisyutkin, 1999) were successfully matched. The former two are sexually dimorphic with macropterous males and micropterous females. Photos of the species from China are presented.
Keywords: China, sexual dimorphism, species delimitation, taxonomy, cockroaches
Introduction
Pseudophoraspis Kirby, 1903 is a genus of Epilamprinae cockroaches from South-east Asia whose taxonomy and biogeography were recently discussed by Wang et al. (2013). They exhibit some parental care behaviors rare among cockroaches (Tallamy and Wood 1986; Clutton-Brock 1991). Pseudophoraspisnebulosa, the type species of the genus, has offspring that cling ventrally to the parent’s body after hatching and feed on their mother’s bodily secretions (Nalepa and Bell 1997). Yet, owing to the lack of research on Pseudophoraspis, this behavior in other members of the genus remains unknown.
Currently, the genus is composed of 18 species (Beccaloni 2014). According to original descriptions, 15 species are from South-east Asia (Cambodia, Thailand, Vietnam and Malaysia), and three from South China (Yunnan, Hainan). Among these, internal male genitalia are known for only 13 species (Anisyutkin 1999; Anisyutkin 2005; Wang et al. 2013). Meanwhile, only the external morphology of the remaining five species has been described, four of which are based on female specimens (Walker 1868; Hanitsch 1925, 1933).
In the past, some external morphological characters have been used to diagnose Pseudophoraspis (e.g. male and female with fully-developed tegmina and wings, and head entirely covered by the pronotum; Kirby 1903; Shelford 1910; Hanitsch 1915; Princis 1958; Wang et al. 2013). Additionally, the genus has been identified by the apical part of sclerite L2D having a well-developed apical outgrowth (Anisyutkin 1999; Wang et al. 2013). Yet, two species, P.fruhstorferiShelford 1910 and P.tramlapensisAnisyutkin 1999 are distinctively different from their congeners by the absence of this genital character (Anisyutkin 1999; Wang et al. 2013). Anisyutkin (1999) mentioned that P.fruhstorferi and P.tramlapensis were included conditionally in Pseudophoraspis. Wang et al. (2013) subdivided this genus into two species groups, the fruhstorferi group and the gorochovi group, but only according to pronotal characteristics and without information on the females of the gorochovi group. Males, females and nymphs in this genus from the same locality are difficult to match accurately (Wang ZZ, pers. obs.). Sexual dimorphism can exaggerate male-female differences to the extent that the sexes appear to be entirely different species. In the genera Escala Shelford, 1906 and Robshelfordia Princis, 1954, for example, most females have micropterous tegmina that are reduced to small lateral pads without wings, and in the genus Laxta Walker, 1868, the females are apterous. But the males of these three genera usually have fully-developed tegmina and wings (Roth 2003).
The commonly-adopted, standard COI sequence has proven to be highly informative and successful in resolving problems of polymorphism, sexual dimorphism and identification of nymphs in cockroaches (Evangelista et al. 2013; Yue et al. 2014; Che et al. 2017; Bai et al. 2018). These issues highlight the need for determining the taxonomic status of P.fruhstorferi and P.tramlapensis, and clarifying approaches toward solving sexual dimorphism in cockroach species.
In this study, Brephallus Wang et al., gen. n. is established for two species, Brephallusfruhstorferi (Shelford, 1910), comb. n. and Brephallustramlapensis (Anisyutkin, 1999), comb. n. A combination of newly generated and publicly available molecular data (COI) has been used to aid in associating adult sexual morphs and juveniles. Additionally, this study adds to the knowledge of cockroach diversity in China.
Material and methods
Specimen collection and morphological study
In this study, 32 specimens were collected at night with the help of headlight from dead leaves of grasses or shrubs in the litter layer. Other specimens were mostly collected with a net in daytime. Voucher specimens are deposited in the Institute of Entomology, College of Plant Protection, Southwest University (SWU), Chongqing, China.
Terminologies used for male genitalia mainly follow Klass (1997) and Anisyutkin (2014). The apical part of an abdomen was removed and macerated in 10% NaOH and observed in glycerin jelly using a Motic K400 stereomicroscope. The dissected genitalia were preserved in glycerin jelly. Specimens were photographed using a Canon50D with a Canon EF 100mm f/2.8L Macro IS USM Macro USM lens, and stacked with Helicon Focus software. All photos and images were edited with Adobe Photoshop CS5. Male adults were identified to species mainly based on morphological characters, including the apical part of sclerite L2D, the macula on the head, depressions and punctuation on the pronotal disk, and wing size.
Phylogenetic data collection and analysis
Tissue samples from adult females and nymphs were used directly for PCR analysis and DNA sequencing. The hind legs were used for DNA extraction. Other body parts were stored in 95% ethanol as voucher specimens. In total, 32 specimens were used for COI sequencing in this study and all sequences are deposited at the National Center for Biotechnology Information GenBank (Table 1).
Table 1.
Specimens for which COI DNA barcodes were sequenced.
| Species | Specimen voucher | Sequence ID | Location (China) | Accession Number |
|---|---|---|---|---|
| P. clavellata | I01.1M | RhicClav01 | Xishuangbanna, Yunnan | MH755944 |
| I01.2M | RhicClav03 | Pu’er, Yunnan | MH755945 | |
| I01.2F | RhicClav04 | Pu’er, Yunnan | MH755946 | |
| I01.3M | RhicClav02 | Xishuangbanna, Yunnan | MH755947 | |
| I01.4M | RhicClav05 | Pu’er, Yunnan | MH755948 | |
| I01.5N | RhicClav06 | Xishuangbanna, Yunnan | MH755949 | |
| P. recurvata | I02.1M | RhicRecu01 | Changjiang, Hainan | MH755950 |
| I02.2F | RhicRecu05 | Sanya, Hainan | MH755951 | |
| I02.3M | RhicRecu03 | Baoting, Hainan | MH755952 | |
| I02.3F | RhicRecu04 | Baoting, Hainan | MH755953 | |
| I02.4M | RhicRecu02 | Changjiang, Hainan | MH755954 | |
| I02.5F | RhicRecu06 | Sanya, Hainan | MH755955 | |
| P. kabakovi | E04.1F | RhicKaba02 | Menglun, Yunnan | MH755937 |
| E04.1M | RhicKaba01 | Menglun, Yunnan | MH755938 | |
| E04.2F | RhicKaba04 | Xishuangbanna, Yunnan | MH755939 | |
| E04.2N | RhicKaba05 | Menglun, Yunnan | MH755940 | |
| E04.2M | RhicKaba03 | Xishuangbanna, Yunnan | MH755941 | |
| E04.3M | RhicKaba06 | Menglun, Yunnan | MH755942 | |
| E04.4F | RhicKaba07 | Menglun, Yunnan | MH755943 | |
| B. fruhstorferi | E01.1M | PseuFruh01 | Jianfengling, Hainan | MH755924 |
| E01.2M | PseuFruh02 | Limushan, Hainan | MH755925 | |
| E01.4N | PseuFruh04 | Diaoluoshan, Hainan | MH755926 | |
| E01.5F | PseuFruh05 | Jianfengling, Hainan | MH755927 | |
| E01.5M | PseuFruh06 | Jianfengling, Hainan | MH755928 | |
| E01.7F | PseuFruh09 | Wuzhishan, Hainan | MH755929 | |
| E01.8F | PseuFruh11 | Yinggeling, Hainan | MH755930 | |
| E01.8M | PseuFruh10 | Yinggeling, Hainan | MH755931 | |
| E01.9F | PseuFruh12 | Yinggeling, Hainan | MH755932 | |
| E01.9M | PseuFruh13 | Yinggeling, Hainan | MH755933 | |
| B. tramlapensis | E02.1M | PseuTram01 | Damingshan, Guangxi | MH755934 |
| E02.2M | PseuTram02 | Dayaoshan, Guangxi | MH755935 | |
| E02.3M | PseuTram03 | Mangshan, Hunan | MH755936 |
DNA extraction, PCR amplification and sequencing follow Bai et al. (2018). COI specific primers were used: LCO1490 (GGTCAACAAATCATAAAGATATTGG); and HCO2198 (TAAACTTCAGGGTGACCAAAAAATCA).
PCR products were sent to BGI Technology Solutions Company Limited (BGI-Tech) (Beijing, China) for sequencing using the aforementioned primers.
A total of 48 COI sequences were analyzed (32 new sequences from this study, and 14 cockroach sequences and 2 mantis sequences downloaded from GenBank; Table 2). All COI sequences were aligned using MUSCLE 3.8 (Edgar 2004) and adjusted visually after translation into amino acid sequences. Intraspecific and interspecific genetic divergence values were quantified based on the Kimura 2-parameter (K2P) distance model (Kimura 1980), and variance was estimated by using bootstrap method with 1000 bootstrap replications in MEGA 6.0.6 (Tamura et al. 2013). Phylogenetic analysis was done using Maximum Likelihood (ML) in RAxML (Stamatakis et al. 2008) following the GTR GAMMA model with 1000 bootstrap replicates.
Table 2.
Ectobiidae and Mantodea (outgroup) used in this study.
| Species | Family | Accession number | Reference |
|---|---|---|---|
| Sorineuchora bivitta | Ectobiidae | KY349592, KY349593 | Che et al. 2017 |
| Sorineuchora nigra | Ectobiidae | KY349516-KY349522 | Che et al. 2017 |
| Allacta ornata | Ectobiidae | KY349665 | Che et al. 2017 |
| Balta jinlinorum | Ectobiidae | KY349666-KY349669 | Che et al. 2017 |
| Mantis religiosa | Mantidae | KR148854, KM529415 | Hebert et al. 2016, Dewaard et al. (Unpublished) |
Results
Phylogenetic analysis based on COI data
In this study, we acquired 32 COI sequences whose length, excluding primers, was 658bp each. All of the new sequences have been deposited in GenBank with accession numbers MH755924 to MH755955 (Table 1). The COI region we sequenced had a relatively high AT content (65.8%), with an average nucleotide composition of A = 30.3%, T = 35.5%, C = 18.3%, and G = 15.9%. Sequence analysis revealed that 156 (23.71%) sites were variable, of which 148 (22.49%) sites were parsimoniously informative.
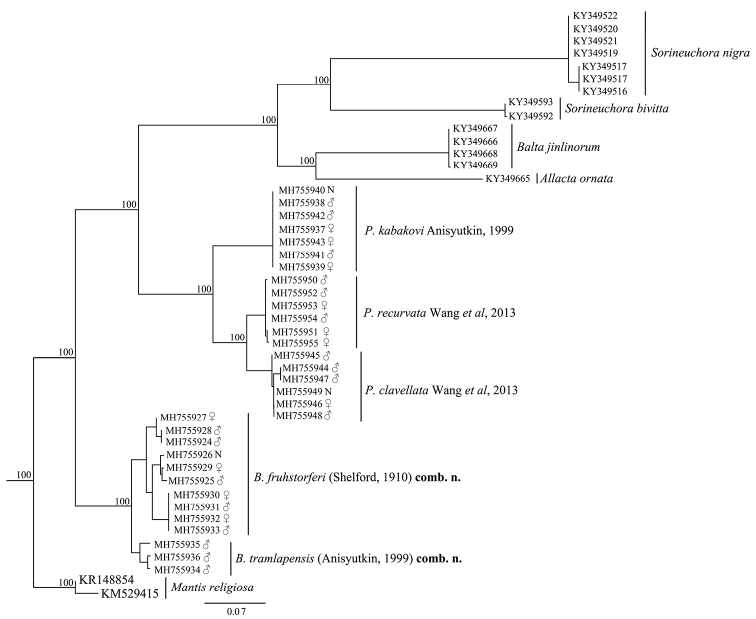
The ML phylogeny of the COI data revealed that clades from the same species, including females and nymphs, constitute monophyletic groups with very strong bootstrap values (all MLB = 100) (Figure 1). Three recognized major lineages of Pseudophoraspis (P.clavellata, P.recurvata and P.kabakovi) are recognized, and cluster with the ectobiids Sorineuchora, Allacta and Balta, with high support values, but are more distant from the other two Epilamprinae, P.fruhstorferi and P.tramlapensis.
Figure 1.
Maximum likelihood (ML) tree derived from COI gene analysis following GTR GAMMA model with 1000 bootstrap replicates. The bootstrap support are all 100%, in this phylogenetic tree.
Establishment of Brephallus Wang et al., gen. n.
Pseudophoraspisfruhstorferi and P.tramlapensis are easily distinguished from other congeners by the apical part of sclerite L2D lacking a well-developed apical outgrowth (Anisyutkin 1999; Wang et al. 2013). Other diagnostic morphological characters of these two species compared with other Pseudophoraspis members are shown in Table 3. Therefore, these two species are moved to Brephallus Wang et al., gen. n. (i.e., Brephallusfruhstorferi (Shelford, 1910), comb. n., and Brephallustramlapensis (Anisyutkin, 1999), comb. n.). In addition, these two species were recovered as sister groups and show a close relationship to each other, but were distant from the other three Pseudophoraspis species (Figure 1).
Table 3.
Diagnostic morphological characters among Brephallus Wang et al., gen. n., the nebulosa group and the gorochovi group of Pseudophoraspis.
| Species | Characters | ||||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | |
| Brephallus Wang et al., gen. n. | 0 | 1 | 1 | 2 | 0 | 1 | 0 |
| Pseudophoraspisnebulosa group | 0 | 1 | 0 | 0 | 1 | 0 | 1 |
| Pseudophoraspisgorochovi group | 1 | 0 | 0 | 1 | 1 | 0 | 1 |
I depressions and punctuation on pronotum present (1), absent (0) II females with tegmina and wings fully-developed (1), tegmina reduced to lateral scales without wings (0) III male tegmina about twice as long as broad (1), tegmina length more than twice as broad (0) IV facial part of head with large brown spot from ocellus to clypeus, basal margin of ocellus with brown spot (2), vertex to basal margin of ocellus with brown spot (1), inside and basal margin of ocellus with brown round spot (0) V female supra-anal plate with posterior margin distinctly exceeding posterior margin of subgenital plate (1), not beyond (0)VI R3 well-developed, widened caudally and fused with R5 of right phallomere (1), R3 not widened caudally and fused with R5 (0) VII the apical part of sclerite L2D with well-developed apical outgrowth (1), without (0)
Genus. Brephallus gen. n.
http://zoobank.org/6023C59C-4D25-4730-9FB1-90FEAA7CD51F
Figures 2A–F , 3F–G , 4D–E , 4G , 5G–H
Figure 2.
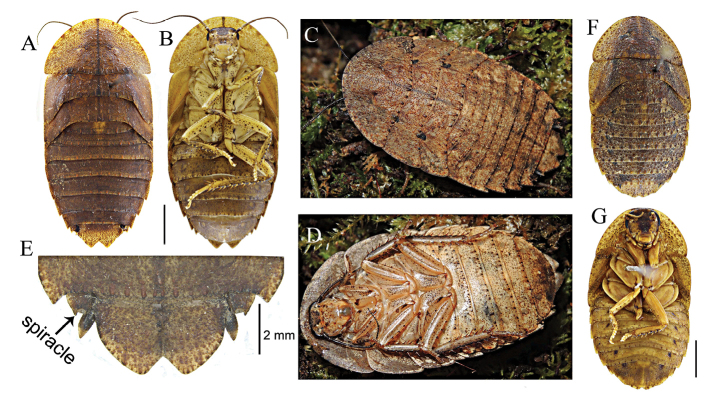
A–DBrephallusfruhstorferi (Shelford, 1910) comb. nov. (male A–B female C–D) E–FBrephallustramlapensis (Anisyutkin, 1999) comb. nov. (male) G–JP.recurvata (male G–H female I–J) K–NP.clavellata (male K–L female M–N) O–RP.kabakovi (male O–P female Q–R). Scale bars = 10 mm (A–F) Scale bars = 5 mm (G–R).
Figure 3.
A–BP.kabakovi (nymph) scale bars = 5 mm C–DP.clavellata (nymph) E abdomen of female of P.clavellataF–GBrephallusfruhstorferi (Shelford, 1910) comb. nov. (nymph).
Figure 4.
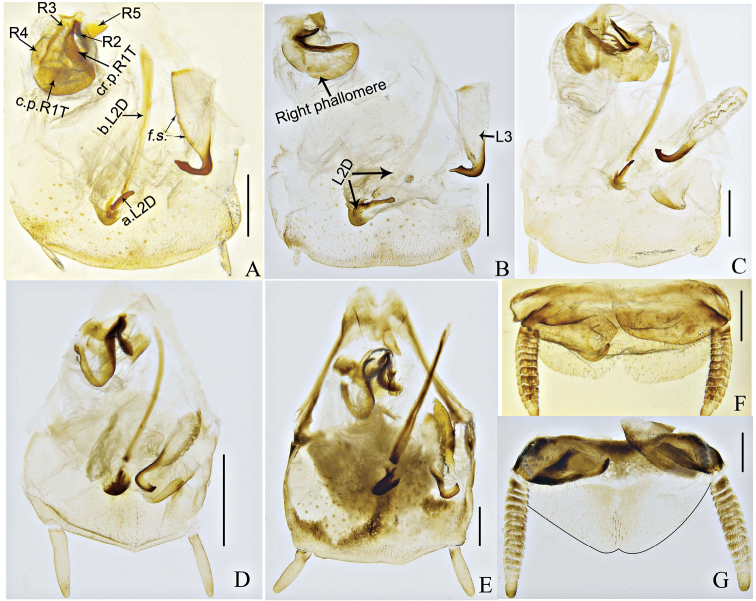
Male genitalia of Pseudophoraspis and Brephallus Wang et al., gen. nov. AP.recurvataBP.clavellataCP.kabakoviDBrephallusfruhstorferi (Shelford, 1910) comb. nov. EBrephallustramlapensis (Anisyutkin, 1999) comb. nov. F supra-anal plate of P.recurvataG supra-anal plate of Brephallustramlapensis (Anisyutkin, 1999) comb. nov. (Scale bars = 1 mm).
Figure 5.
A female of P.recurvata from Hainan Province. This specimen, collected as a nymph in 8 April 2015, was reared at Southwest University by Lu Qiu and adult emergence occurred in 21 May 2015 B male of P.recurvata from Hainan Province C male of P.clavellata from Yunnan Province D habitat of P.clavellataE male of P.kabakovi from Yunnan Province F female of P.kabakovi from Yunnan Province. A–F Photographed by Lu Qiu G–HBrephallusfruhstorferi (Shelford, 1910) comb. nov. from Hainan Province (Photographed by Xin-Ran Li).
Species included.
Brephallusfruhstorferi (Shelford, 1910), comb. n., Brephallustramlapensis (Anisyutkin, 1999), comb. n.
Type species.
Pseudophoraspisfruhstorferi Shelford, 1910, by present designation.
Generic diagnosis.
Coloration brownish yellow. Pronotum smooth, completely covering vertex, anterior margin curved and posterior margin obtusely produced. Tegmina and wings fully developed in both sexes, entirely covering abdomen, tegmina about twice as long as broad, apices rounded (Figure 2A–F). Hind metatarsus shorter than succeeding tarsal segments combined, with two equal rows of spines along most of its length, 2nd–4th segments with large euplantulae. Supra-anal plate and hypandrium nearly symmetrical, posterior margin emarginate near mid-line (Figure 4G).
Male genitalia (Figure 4D–E). Right phallomere similar to that in Morphna, Opisthoplatia, and Rhabdoblatta with well-developed caudal part of sclerite R1T subrectangular in shape, R2 rounded, R3 well developed, widened caudally and fused with R5. Sclerite L2D similar to Rhabdoblatta, divided into basal and apical parts, basal part rod-like, apical part more or less rounded, cap-shaped, but with more bristles. Sclerite L3 with terminal rectangular apex pointed and folded, scattered with bristles.
The new genus differs from other genera of Epilamprinae as follows: 1) male tegmina about twice as long as broad (Figure 2A, E); 2) facial part of head with large brown spot from ocellus to clypeus, basal margin of ocellus with brown spot (Figure 2B, D, F); 3) one third of radius vein of tegmen from base yellowish white (Figure 2A, C, E); 4) sclerite L3 with terminal rectangular, apex pointed (Figure 4D–E).
Etymology:
We propose the name Brephallus, based on the composition of two Latin words (“brevis” and “phallus”) meaning “short phallomere”, in reference to the short L2D sclerite of the male genitalia.
Remarks.
This genus differs from Pseudophoraspis in the apical part of sclerite L2D without a well-developed apical outgrowth. Meanwhile, the mean sequence divergence among species of Brephallus and Pseudophoraspis ranged from 15.2% to 18.8%, larger than that of congeners (Table 4). Although Brephallusfruhstorferi and B.tramlapensis only have the mean interspecific genetic distance of 4.1% (Table 4) between them, they show distinct morphological differences as follows: 1) mid-abdomen of B.tramlapensis (Anisyutkin, 1999) has two brown stripes while B.fruhstorferi (Shelford, 1910) lacks stripes (Figure 2A–F); and 2) the apical part of sclerite L2D of B.tramlapensis (Anisyutkin, 1999) is large and long, with a protrusion in the middle (Figure 4E) while in B.fruhstorferi (Shelford, 1910) it is short, without a protrusion in the middle (Figure 4D).
Table 4.
The variance of the underlying distribution of distances calculated by using K 2–P model and bootstrap method respectively in MEGA.
| Species | B. fruhstorferi | B. tramlapensis | P. kabakovi | P. clavellata | P. recurvata |
|---|---|---|---|---|---|
| Brephallus fruhstorferi | – | – | – | – | – |
| Brephallus tramlapensis | 0.041±0.007 | – | – | – | – |
| P. kabakovi | 0.188±0.018 | 0.172±0.017 | – | – | – |
| P. clavellata | 0.174±0.017 | 0.152±0.016 | 0.090±0.012 | – | – |
| P. recurvata | 0.171±0.017 | 0.157±0.016 | 0.087±0.011 | 0.041±0.008 | – |
Pseudophoraspis
Kirby, 1903
Figures 2G–R , 3A–E , 4A–C , 4F , 5A–F
Type species:
Epilampranebulosa Burmeister, 1838.
The species P.clavellata and P.recurvata exhibit sexual dimorphism (male with developed tegmina and wings, females with tegmina reduced to lateral scales and wings absent) (Figure 2G–N); therefore, we provide below supplementary information on the nymphs and females.
Generic description.
Body slender, general color yellowish brown, head entirely covered by pronotum. Pronotum with numerous brown spots, smooth, without or with scattered punctuation. Male with fully-developed tegmina and wings, female with tegmina reduced to lateral scales without wings or with fully-developed tegmina and wings (Figure 2G–R). Hind metatarsus shorter than other tarsal segments combined, with small apical euplantulae along its lower margin, with spinules, euplantulae occupying less than half of its length, with two equal rows of spines along most of its length. Tarsal claws symmetrical and unspecialized. Supra-anal plate semicircular, meso-posterior margin emarginate (Figure 4F).
Male genitalia (Figure 4A–C). Right phallomere with well-developed caudal sclerite, R1T subrectangular in shape (Figure 4A–C “c.p.R1T”), R2 rounded, R3 weakly sclerotized, without branch, narrowed caudally. Sclerite L2D divided into basal and apical parts, basal part rod-like, apical part with well-developed apical outgrowth (Figure 4A–C “a.L2D”), with bristles. Sclerite L3 with apex pointed and folded structure scattered with bristles (Figure 4A–C “f.s.”).
Remarks.
Wang et al. (2013) subdivided the Chinese Pseudophoraspis into two species groups: the fruhstorferi group and the gorochovi group, but the latter lacked information on females. The fruhstorferi group currently includes three species: P.fruhstorferi Shelford, 1910, P.tramlapensis Anisyutkin, 1999 and P.kabakovi Anisyutkin, 1999. Because we have transferred the former two species to the new genus, Brephallus Wang et al., gen. n., the fruhstorferi group is renamed as nebulosa group. Some diagnostic characters between the nebulosa group and the gorochovi group are shown in Table 3.
The mean interspecific sequence divergence among the three Pseudophoraspis members ranged from 4.1% to 9.0% (Table 4), but there are distinguishing differences among them, as described below.
Pseudophoraspisgorochovi group
Species included here.P.clavellata Wang et al., 2013; P.recurvata Wang et al., 2013; P.incurvata Wang et al., 2013; and P.gorochovi Anisyutkin, 1999.
Pseudophoraspis recurvata
Wang et al., 2013
Note.
Wang et al. (2013) described the male of P.recurvata including detailed information on male genital structures (Figures 2G–H, 4A, 4F, 5B). The description of the female is provided here.
Material examined.
China: Hainan: five males and one female, Baoting County, 2013.V.2, coll. Yan Shi and Shun-Hua Gui; three males, Changjiang County, Qicha Township, 2015.IV.28, coll. Lu Qiu and Qi-Kun Bai; one male and two females, Sanya City, Liupan Village, 2015.IV.8, coll. Lu Qiu and Qi-Kun Bai; two males (holotype and paratype), Baoting County, 1959.VII.10, coll. Yi-Chuan Hu. China: Guangxi: one male (paratype), Mt. Daqingshan, 1958.IX, coll. Yi-Xin Xu.
Female description.
(Figures 2I–J, 5A). Body brownish-yellow. Vertex, eyes and between the antennal sockets black-brown. Ocellar spots pale yellowish. Antennae, legs, thorax and abdomen brown. Maxillary palp with 1st–2nd segments pale yellowish and 3rd–5th segments brown. Cerci brown with apical segment yellow.
Head longer than wide. Interocular space slightly less wide than interocellar space, ocellar spots rather small, eyes elongate. Antennae short, not reaching to half length of body, first segment of flagellum twice length of next segment; interantennal portion of frons concave. Frons moderately punctuated; clypeus and labrum unmarked. Pronotum covering vertex of head, anterior margin arcuate, posterior margin truncate, with scattered punctuation and a pair of impressions on disc. Thoracic and abdominal tergites with small tubercles and longitudinal inflations along posterior margins. Tegmina reduced to lateral scales, with nearly indistinct venation, veins reduced, wings absent. Anterior margin of fore femur type B, with six large spines and one single apical spine. Tibial spines well developed. 3rd–7th abdominal tergites with paired rounded impressions. Hind metatarsus with spines along most of its length, equal to remaining joints, tarsal spines absent. Tarsal claws symmetrical, simple, arolia very small. Supra-anal plate transverse, beyond the subgenital plate, hind margin with a medial V-shaped excavation. Hypandrium widely rounded, caudal margin arcuate. Cerci abbreviated, apex blunt.
Variation.
Morphology of paratypes is same as female type described above, but with following variation: five to six large spines scattered along anterior margin of fore femora; color of clypeus, labrum and abdomen tergites brown or yellow. Overall length: 20.1 ± 0.2 mm; head length × width: 3.6 ± 0.1 mm × 2.9 ± 0.1 mm; pronotum length × width: 6.2 ± 0.1 mm × 10.7 ± 0.1 mm.
Known geographic range.
China (Hainan, Guangxi).
Pseudophoraspis clavellata
Wang et al., 2013
Figures 2K–N , 3C–E , 4B , 5C–D
Note.
Wang et al. (2013) described the male of P.clavellata including the male genitalic structures (Figures 2K–L, 4B and 5C). Description of the female and nymph is provided here.
Material examined.
China: Yunnan: Thirty males and one female, Pu’er City, Meizi Lake, 2016.V.20, coll. Lu Qiu and Zhi-Wei Qiu; two males, Jinhong City, Dadugang, 2014.VI.29, coll. Conlin McCat (= Xin-Ran Li) and Hong-Guang Liu; one nymph, Xishuangbanna, Menghai County, Bulong Natural Reserve, 2017.I.31, coll. Jian-Yue Qiu and Hao Xu; male (holotype), Xishuangbanna, 1981.V.27-30, coll. Zhi-Gang Zheng.
Female description
(Figures 2M–N, 3E). Identical to the female of P.recurvata but body larger; in addition, legs, venter of thorax and abdomen yellow.
Female measurements.
Overall length 28.1 mm; head length × width: 3.8 mm × 3.7 mm; pronotum length × width: 7.0 mm × 12.5 mm.
Nymph
(Figure 3C–D). Body flattened. Identical to adult female but lacking wings.
Known geographic range.
China (Yunnan).
Pseudophoraspisnebulosa group
According to the original descriptions of male genitalia of these species: P.kabakovi Anisyutkin, 1999, P.marginata Anisyutkin, 1999, P.grigorenkoi Anisyutkin, 1999, P.argillacea Anisyutkin, 1999, P.truncatulus Anisyutkin, 1999, P.buonluoiensis Anisyutkin, 1999 and P.doroshenkoi Anisyutkin, 2005, the apical part of sclerite L2D has a well-developed apical outgrowth, pronotum smooth without punctuation, and both male and female have fully developed tegmina and wings. We therefore assign these seven species to the Pseudophoraspisnebulosa group.
Pseudophoraspis kabakovi
Anisyutkin, 1999
Figures 2O–R , 3A–B , 4C , 5E–F
Note.
The male of P.kabakovi was described (Figures 2O–P, 4C, 5E) by Anisyutkin (1999) and Wang et al. (2013), but little was known about the female and nymph until now.
Materials examined.
China: Yunnan: One male, Xishuangbanna, 1974.IV.13, coll. Yao Zhou and Feng Yuan; twenty males, five females and one nymph, Xishuangbanna, Menglun Town, 2016.V.27, coll. Lu Qiu and Zhi-Wei Qiu; one male and two females, Xishuangbanna, Mengla County, Wangtianshu, 2016.V.23, coll. Lu Qiu and Zhi-Wei Qiu.
Female description
(Figures 2Q–R, 5F). Body yellowish brown. Eyes and antennae black, ocellar spots pale yellow. Pronotum with dense small brown spots. Tegmina with scattered large black spots. Abdominal sterna with small and fewer large black dots, large black dots along the hind margins of the segments. Cerci brown.
Similar to male in general appearance, but shorter and convex. Tegmina and wings shorter than in males. Fore femur with six spines along anterior margin and one single apical spine. Hind metatarsus with two rows of spines along most of its length. Claws symmetrical, simple; arolium well developed. Abdominal terga unspecialized. Supra-anal plate caudal margin with a medial V-shaped excavation. Hypandrium posterior margin emarginate near mid-line.
Female measurements.
Overall length 32 ± 0.2 mm; head length × width: 4.2 ± 0.1 mm × 3.6 ± 0.1 mm; pronotum length × width: 8.3 ± 0.2 mm × 12.1 ± 0.2 mm; tegmina length × width: 25.4 ± 0.1 mm × 10.3 ± 0.2 mm.
Nymph.
Identical to adult females of P.recurvata and P.clavellata except for undeveloped wing (Figure 3A–B).
Known geographic range.
China (Yunnan); Vietnam.
Discussion
Five Epilamprine species were identified mainly on the basis of morphological and male genitalia data. Due to the apical part of sclerite L2D lacking a well-developed apical outgrowth, two species of Pseudophoraspis are transferred to Brephallus Wang et al., gen. n.
Our molecular results show two members of the Pseudophoraspisgorochovi group, P.recurvata Wang et al., 2013 and P.clavellata Wang et al., 2013, collected in China were sexually dimorphic. However, the other species group within this genus, P.nebulosa group, is not sexually dimorphic. As we have applied it, and as others have shown (Che et al. 2017; Bai et al. 2018; Evangelista et al. 2013), the integration of morphological and DNA-based approaches is useful for cockroach species identification and to supplement morphological keys, which are typically limited to adult male morphological characters.
Supplementary Material
Acknowledgements
We thank Xin-Ran Li and Lu Qiu for photos of these species in the wild and thank other collectors who contributed important specimens discussed in this paper. We also thank Dr. John Richard Schrock (Department of Biological Sciences, Emporia State University) for revising the manuscript.
This study is supported by the National Natural Science Foundation of China (Nos. 31672329, 31772506).
Citation
Wang Zh, Zhao Q, Li W, Che Y, Wang Z (2018) Establishment of a new genus, Brephallus Wang et al., gen. nov. (Blattodea, Blaberidae, Epilamprinae) based on two species from Pseudophoraspis, with details of polymorphism in species of Pseudophoraspis. ZooKeys 785: 117–131. https://doi.org/10.3897/zookeys.785.26565
References
- Anisyutkin LN. (1999) Cockroaches of the subfamily Epilamprinae (Dictyoptera, Blaberidae) from the Indochina Peninsula. Entomological Review 79(4): 434–454. [Google Scholar]
- Anisyutkin LN. (2005) Two new species of Epilamprinae from Vietnam and Cambodia (Dictyoptera, Blattina: Blaberidae). Zoosystematica Rossica 14(1): 37–40. [Google Scholar]
- Anisyutkin LN. (2014) On cockroaches of the subfamily Epilamprinae (Dictyoptera: Blaberidae) from South India and Sri Lanka, with descriptions of new taxa. Zootaxa 3847(3): 301–332. 10.11646/zootaxa.3847.3.1 [DOI] [PubMed] [Google Scholar]
- Bai QK, Wang LL, Wang ZQ, Lo N, Che YL. (2018) Exploring the diversity of Asian Cryptocercus (Blattodea: Cryptocercidae): species delimitation based on chromosome numbers, morphology, and molecular analysis. Invertebrate Systematics 32(1): 69–91. 10.1071/IS17003 10.1071/IS17003 [DOI] [Google Scholar]
- Beccaloni GW. (2014) Cockroach Species File Online. Version 5.0/5.0. http://Cockroach.SpeciesFile.org[accessed 5 July 2016].
- Bey-Bienko GY. (1969) New genera and species of cockroaches (Blattoptera) from tropical and subtropical Asia. Entomologicheskoe Obozrenie 48(4): 831–862. [Google Scholar]
- Che YL, Gui SH, Lo N, Andrew R, Wang ZQ. (2017) Species delimitation and phylogenetic relationships in Ectobiid cockroaches (Dictyoptera, Blattodea) from China. PLoS ONE 12(1): e0169006. 10.1371/journal.pone.0169006 [DOI] [PMC free article] [PubMed]
- Clutton-Brock TH. (1991) The Evolution of Parental Care. Princeton University Press, Princeton, 352 pp.
- Edgar RC. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista DA, Bourne G, Ware JL. (2013) Species richness estimates of Blattodea s.s. (Insecta: Dictyoptera) from northern Guyana vary depending upon methods of species delimitation. Systematic Entomology 39: 150–158. 10.1111/syen.12043 [DOI] [Google Scholar]
- Hanitsch R. (1915) Malayan Blattidae. Part I. Journal Straits Branch Royal Asiatic Society, 69: 17–178. [Google Scholar]
- Hanitsch R. (1925) On a collection of Blattidae from Northern Sarawak, Chiefly Mt. Murud and Mt. Dulit. The Sarawak Museum Journal 8: 75–106. [Google Scholar]
- Hanitsch R. (1933) XXI. The Blattidae of Mount Kinabalu, British North Borneo. Journal of the Federated Malay States Museums 17: 297–337. [Google Scholar]
- Hebert PDN, Ratnasingham S, Zakharov EV, Telfer AC, Levesque-Beaudin V, Milton MA, Pedersen S, Jannetta P, Dewaard JR. (2016) Counting animal species with DNA barcodes: Canadian insects. Philosophical Transactions of the Royal Society B-Biological Sciences 371(1702): 1–10. 10.1098/rstb.2015.0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16, 111–120. 10.1007/BF01731581 [DOI] [PubMed]
- Kirby WF. (1903) Notes on Blattidae, with descriptions of new genera and species in the collection of the British Museum, South Kensington. No. II. The Annals and Magazine of Natural History 12(7): 273–280. 10.1080/00222930308678853 [DOI] [Google Scholar]
- Klass KD. (1997) The external male genitalia and the phylogeny of Blattaria and Mantodea. Bonner Zoologische Monographien 42: 1–341. [Google Scholar]
- Nalepa CA, Bell WJ. (1997) Postovulation parental investment and parental care in cockroaches. In Social Behavior in Insects and Arachnids. J.C. Choe and B.J. Crespi, editors. Cambridge University Press, Cambridge, 26–51. 10.1017/CBO9780511721953.004 [DOI]
- Princis K. (1958) Revision der Walkerschen und Kirbyschen Blattarientyen im British Museum of Natural History, London. II. Opuscula Entomologica 23(1–2): 59–75. [Google Scholar]
- Roth LM. (2003) Systematics and phylogeny of cockroaches (Dictyoptera: Blattaria). Oriental Insects 37: 1–186. 10.1080/00305316.2003.10417344 [DOI] [Google Scholar]
- Shelford R. (1910) Orthoptera. Fam. Blattidae. Subfam. Epilamprinae. In: Wytsman P. (Ed.), Genera Insectorum 101: 1–21.
- Stamatakis A, Hoover P, Rougemont J. (2008) A rapid bootstrap algorithm for the RAxML Web Servers. Systematic Biology 57: 758–771. 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- Tallamy DW, Wood TK. (1986) Convergence patterns in social insects. Annual Review of Entomology 31: 369–390. 10.1146/annurev.en.31.010186.002101 [DOI] [Google Scholar]
- Tamur K, Stecher G, Peterson D, Filipski A, Kumar S. (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FLS. (1868) Catalogue of the Specimens of Blattariae in the Collection of the British Museum. Printed for The Trustees of The British Museum, London, 239 pp. [Google Scholar]
- Wang ZQ, Wu KL, Che YL. (2013) New record of the cockroach genus Pseudophoraspis (Blaberidae, Epilamprinae) from China with descriptions of three new species. ZooKeys 273: 1–14. 10.3897/zookeys.273.4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue QY, Wu KL, Qiu D, Hu J, Liu D, Wei XY, Chen J, Cook CE. (2014) A formal re-description of the cockroach Hebardinaconcinna anchored on DNA barcodes confirms wing polymorphism and identifies morphological characters for field identification. PLoS ONE 9: e106789. 10.1371/journal.pone.0106789 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.