Abstract Abstract
Classiculasinensis, isolated from decaying leaves from Mozigou, Chongqing Municipality, China, is described as a new species. The new species is a member of basidiomycetous aquatic hyphomycetes which represent a small proportion of all aquatic hyphomycetes. This species falls within the genus Classicula (Classiculaceae, Pucciniomycotina) and is closely related to C.fluitans, based on multiple gene sequence analyses. Morphologically, it is characterised by the apical, hyaline, obclavate or navicular conidia with several hair-like lateral appendages and by its holoblastic and monoblastic conidiogenesis, with a flat un-thickened conidiogenous locus. Clamp connections and haustorial branches were often observed in culture.
Keywords: fresh water fungi, mycoparasites, Pucciniomycotina , taxonomy
Introduction
Aquatic hyphomycetes constitute a dominant mycoflora on submerged decaying plant debris, both in lotic and lentic systems (Khan 1987). Phylogenetically, most aquatic hyphomycetes belong to Ascomycota, with only a small percentage belonging to Basidiomycota (Shearer 2007). Most known basidiomycetous aquatic hyphomycetes have been reported from North America (Marvanová 1977, Marvanová and Barlocher 1988, 1998, 2000, Marvanová and Suberkropp 1990, Raj 1981), Australia (Shaw 1972), Asia (Hudson and Ingold 1960, Nawawi 1985, Marvanová and Bandoni 1987, Kirschner 2013) and Europe (Scheuer 2008).
There are more than 8000 known species in the Pucciniomycotina (previously Urediniomycetes) and these comprise about one-third of all described basidiomycetes (Aime et al. 2006). Classification of the Pucciniomycotina has been reviewed and revised multiple times. Based on sequences at large and small subunits of the nuclear rDNA, Aime et al. (2006) grouped them into 8 classes. More recently, two new classes, Tritirachiomycetes (Schell et al. 2011) and Spiculogloeomycetes (Wang et al. 2015), were added to Pucciniomycotina. Amongst these 10 classes in Pucciniomycotina, one class, the Classiculomycetes, contains a single order, the Classiculales, with only two monotypic fungi, Jaculispora H. J. Huds. & Ingold and Classicula R. Bauer, Begerow, Oberw. & Marvanová. As early as 1960, Jaculispora was erected to accommodate a single anamorphic fungal species, J.submerse H. J. Huds. & Ingold. The genus is characterised by having narrow and delicate conidiophores and obclavate conidia with 0–3 hair-like lateral appendages (Hudson and Ingold 1960). Later, Naiadella Marvanova & Bandoni was established with N.fluitans Marvanova & Bandoni (synomy of C.fluitans) as the type species (Marvanová and Bandoni 1987). Bauer et al. (2003) observed the basidial stage of N.fluitans and connected it to the teleomorphic state Classicula. Classicula was recommended over Naiadella because Classicula is the base of the higher level taxonomy (Aime et al. 2018). The conidia of Classicula are similar to those of Jaculispora in shape. Classicula is characterised by the production of clamped hyphae with tremelloid haustorial cells and binucleate fusoid conidia with 3–4 bristle-like lateral branches (Marvanová and Bandoni 1987). Bauer et al. (2003) defined the phylogenetic positions of genera Jaculispora and Classicula based on the small subunit of ribosomal DNA (18S rDNA). Subsequent analyses of both the 18S and the large subunit ribosomal DNA (28S rDNA) data also supported the conclusion that the two genera are closely related and both belong to class of Classiculomycetes (Schell et al. 2011).
During a study of aquatic hyphomycetes on submerged decaying leaves collected from a stream in south-western China, we encountered two fungi which resembled species in the genus Classicula. Combining the morphological and phylogenetic analyses, we identified that the fungi belonged to Classicula. In this paper, we describe these specimens as a new species and discuss its phylogenetic placement based on the combined sequences of the 18S and 28S rDNA, the internal transcribed spacer regions of rDNA (ITS 1 and 2, including the 5.8S rDNA gene) and the translation elongation factor 1-a (TEF1).
Materials and methods
Collection of samples, isolation and characterisation
Samples of submerged dicotyledonous plant leaves collected from a stream in Chongqing Municipality were transported to the laboratory in zip-locked plastic bags. The rotten leaves were cut to several 0.5–1.5 × 1–1.5 cm sized fragments in the laboratory and spread on to the CMA medium (20 g cornmeal, 18 g agar, 40 mg streptomycin, 30 mg ampicillin, 1000 ml distilled water). After incubation at 27 °C for about 10 days, a single conidium was isolated and cultivated on CMA in Petri plates using a sterilised toothpick under a BX51 microscope. Morphological observations were made from cultures on CMA after incubation at 27 °C for one week. Pure cultures and a permanent slide were deposited in the Herbarium of the Laboratory for Conservation and Utilization of Bio-resources, Yunnan University, Kunming, Yunnan, P.R. China (YMF; formerly Key Laboratory of Industrial Microbiology and Fermentation Technology of Yunnan). Ex-holotype living cultures were deposited in the China General Microbiological Culture Collection Center (CGMCC).
DNA extraction, PCR and sequencing
The cultures were grown on potato dextrose agar (PDA) and incubated at 27 °C for about 10 days. Fungal mycelia were harvested and transferred to a 2.0 ml Eppendorf tube. Total DNA was extracted using a CTAB method as described by Pratibha et al. (2014).
Three regions of the nuclear ribosomal DNA gene cluster and one nuclear protein-coding genes, translation elongation factor 1a (TEF1) were amplified: Primer pairs ITS4 and ITS5 (White et al. 1990) were used to amplify the complete ITS regions (including 5.8 S); NS1 and NS8 for the 18S rDNA; and LR5 and LROR for the 28S rDNA (Vilgalys and Hester 1990). Primer pairs EF1-983F and EF1-2218R were used for amplifying the TEF1 gene (Rehner and Buckley 2005). PCR amplifications were performed using the methods described previously (Wang et al. 2014). The PCR products were then sent to the Beijing Tsingke Biotechnology Co. of China, Ltd and sequenced on both strands with the same primers that were used for amplification.
Sequence alignment and phylogenetic analysis
Preliminary BLAST searches with 18S and 28S rDNA gene sequences of the new isolates indicated that they had a close phylogenetic relationship with sequences from the genera Jaculiapora and Naiadella (Classicula). Based on the phylogenetic positions of the two genera, we downloaded 18S, 28S, ITS and TEF1 sequences of representative species of 8 class within Pucciniomycotina, but Cryptomycocolacomycetes and Spiculogloeomycetes were not included as Cryptomycocolacomycetes only includes two known species, Cryptomycocolaxabnormis and Colacosiphonfiliformis and only 18S rDNA of two species are available. Spiculogloeomycetes only comprises yeast and yeast-like species, which has an affinity to Mixiomycetes within Pucciniomycotina. Based on our main aim of identifying new hyphomycetes species within Classiculomycetes, another 8 classes were chosen to carry out phylogenetic analysis. Four sequences of each representative strain of 8 classes were combined with those from our own cultures. (see Table 1 for all GenBank accession numbers).
Table 1.
The species used in the phylogenetic analyses. Also included in the Table are the representative isolate name of each species and the GenBank accession numbers for each of the four analysed gene fragments of each isolate.
| Class | Species | Isolate No. | GenBank accession No. | Reference | |||
|---|---|---|---|---|---|---|---|
| ITS | 28S | 18S | TEF | ||||
| Agaricostilbomycetes | Bensingtonia changbaiensis | AS 2.2310 | AY233339 | AY233339 | AY233339 | KJ707751 | Wang et al. 2003; 2015 |
| Agaricostilbum hyphaenes | CBS 7811 | AF444553 | AF177406 | AY665775 | KJ707749 | Scorzetti et al. 2002; Wang et al. 2015 | |
| Chionosphaera apobasidialis | CBS 7430 | AF444599 | AF177407 | U77662 | KJ707883 | Scorzetti et al. 2002; Wang et al. 2015 | |
| Bensingtonia ciliata | AS 2.1945 | AF444563 | AF189887 | D38234 | KF706486 | Scorzetti et al. 2002; Wang et al. 2015 | |
| Kurtzmanomyces insolitus | JCM 10409 | AF444594 | AF177408 | KJ708424 | KJ707893 | Scorzetti et al. 2002; Wang et al. 2015 | |
| Sporobolomyces sasicola | AS 2.1933 | AF444548 | AF177412 | AB021688 | KJ707900 | Scorzetti et al. 2002; Wang et al. 2015 | |
| Mycogloea nipponica | CBS 11308 | KJ778629 | KJ708456 | KJ708370 | KJ707882 | Wang et al. 2015 | |
| Sterigmatomyces elviae | JCM 1602 | AB038053 | KP216512 | KP216516 | KJ707852 | Wang et al. 2015 | |
| Kondoa aeria | CBS 8352 | AF444562 | AF189901 | KJ708417 | KJ707905 | Scorzetti et al. 2002 | |
| Cystobasidiomycetes | Bannoa sp. | MP 3490 | DQ631900 | DQ631898 | DQ631899 | DQ631902 | Matheny et al. 2006 |
| Naohidea sebacea | CBS 8477 | DQ911616 | DQ831020 | KP216515 | KF706487 | Wang et al. 2015 | |
| Sporobolomyces coprosmae | JCM 8772 | AF444578 | AF189980 | D66880 | KJ707798 | Scorzetti et al. 2002 | |
| Sakaguchia dacryoidea | JCM 3795 | AF444597 | AF189972 | D13459 | KP216514 | Scorzetti et al. 2002 | |
| Sporobolomyces bischofiae | JCM 10338 | AB035721 | AB082572 | AB035721 | KJ707777 | Hamamoto et al. 2002 | |
| Rhodotorula armeniaca | JCM 8977 | AF444523 | AF189920 | AB126644 | KJ707762 | Scorzetti et al. 2002; Wang et al. 2015 | |
| Occultifur externus | JCM 10725 | AF444567 | AF189910 | AB055193 | KJ707829 | Scorzetti et al. 2002; Wang et al. 2015 | |
| Cyrenella elegans | CBS 274.82 | KJ778626 | KJ708454 | KJ708360 | KJ707830 | Wang et al. 2015 | |
| Erythrobasidium hasegawianum | AS 2.1923 | AF444522 | AF189899 | D12803 | KJ707776 | Scorzetti et al. 2002; Wang et al. 2015 | |
| Pucciniomycetes | Chrysomyxa arctostaphyli | CFB22246 | DQ200930 | AY700192 | AY657009 | DQ435789 | Matheny et al. 2007 |
| Endocronartium harknessii | CFB22250 | DQ206982 | AY700193 | AY665785 | DQ234567 | Matthias et al. 2004 | |
| Helicobasidium mompa | CBS 278.51 | AY292429 | AY254179 | U77064 | EF100614 | Matthias et al. 2004 | |
| Platygloea disciformis | IFO32431 | DQ234556 | AY629314 | DQ234563 | DQ056288 | Matheny et al. 2007 | |
| Puccinia graminis tritici | CRL75-36-700-3/ECS | AF468044 | AF522177 | AY125409 | XM_003333024 | Weber et al. 2003 | |
| Insolibasidium deformans | TDB183-1 | – | AF522169 | AY123292 | – | Wang et al. 2015 | |
| Septobasidium canescens | DUKE:DAH(323) | DQ241446 | DQ241479 | DQ241410 | – | Henket al. 2007 | |
| Tritirachiomycetes | Tritirachium oryzae | CBS 164.67 | GQ329853 | KF258732 | JF779647 | JF779645 | Schell et al. 2011 |
| Tritirachium sp. | CBS 473.93 | JF779664 | JF779649 | JF779650 | JF779651 | Schell et al. 2011 | |
| Tritirachium sp. | CBS 265.96 | JF779668 | JF779652 | JF779653 | - | Schell et al. 2011 | |
| Mixiomycetes | Mixia osmundae | CBS 9802 | DQ831010 | DQ831009 | D14163 | KJ707837 | Matheny et al. 2006 |
| Microbotryomycetes | Leucosporidium scottii | JCM 9052 | AF444495 | AF070419 | X53499 | KJ707788 | Scorzetti et al. 2002; Wang et al. 2015 |
| Sphacelotheca hydropiperis | CBS 179.24 | KJ708463 | KJ708463 | KJ708394 | KJ707807 | Wang et al. 2015 | |
| Microbotryum violaceum | CBS 143.21 | KJ708462 | KJ708462 | KJ708388 | KJ707811 | Wang et al. 2015 | |
| Sporobolomyces bannaensis | AS 2.2285 | AY274824 | AY274823 | KJ708405 | KJ707934 | Zhao et al. 2003 | |
| Rhodosporidium babjevae | JCM 9279 | AF444542 | AF070420 | AB073270 | KJ707874 | Scorzetti et al. 2002; Wang et al. 2015 | |
| Rhodotorula rosulata | CBS 10977 | EU872492 | EU872490 | KJ708384 | KJ707854 | Wang et al. 2015 | |
| Atractiellomycetes | Helicogloea lagerheimii | FO 36341 | – | AY512849 | AY124476 | – | Bauer et al. 2003 |
| Helicogloea variabilis | KW 1540 | – | L20282 | U78043 | – | Berres et al. 1995 | |
| Platygloea vestita | DB 1280 | – | AY512872 | AY124480 | – | Bauer et al. 2003 | |
| Classiculomycetes | Classicula fluitans | ATCC 64713 | – | AY512838 | AY124478 | – | Schell et al. 2011 |
| Classicula sinense | YMF 1.04613 | KY548838 | KY548836 | KY468515 | MG787169 | This study | |
| Classicula sinense | YMF 1.04389 | KY548837 | KY548835 | KY468514 | MG787170 | This study | |
| Jaculispora submersa | CCM 8127 | – | AY512853 | AY124477 | – | Schell et al. 2014 | |
| Agaricomycotina | Auricularia sp. | AFTOL-ID 676 | DQ200918 | AY634277 | DQ234542 | DQ408144 | Schell et al. 2014 |
| Coprinus comatus | AFTOL-ID 626 | AY854066 | AY635772 | AY665772 | AY881026 | Schell et al. 2014 | |
Raw sequences were aligned using CLUSTAL W 1.6 (Thompson et al. 1994); then manually adjusted to minimise the number of uninformative gaps and to improve alignments using MEGA 6.06 (Kumar et al. 2012). Ambiguously aligned regions were excluded from downstream analyses. Missing data at the 5'- and 3'-end of partial sequences were coded by a ‘?’. To select the most appropriate model of sequence evolution, JMODEL TEST 2.1.1 was run for each gene (ITS, TEF1, 18S, 28S) and the GTR þ I þ G model was chosen according to the Akaike information criterion (AIC). Before phylogenetic analysis, the ITS, TEF1, 18S and 28S matrices were concatenated with BIOEDIT 7.5.0.3. The tree construction procedure was performed in MrBAYES 3.2 (Ronquist et al. 2012). Maximum likelihood was performed with MEGA 6.06. Auricularia sp. and Coprinuscomatus of Agaricomycotina were used as outgroups. Phylogenetic trees were imported into FIGURETREE 1.4.2 and exported as SVG vector graphics for Figure assembly in ADOBE ILLUSTRATOR CS6. The phylogenetic analyses of different datasets were performed using Bayesian and maximum likelihood algorithms.
Results
Phylogenetic analysis
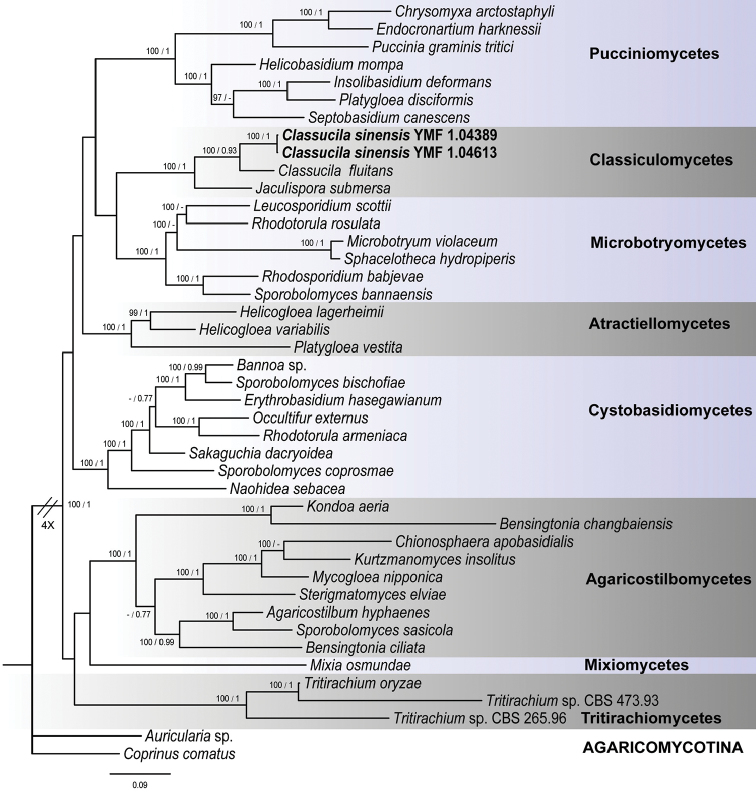
In our Bayesian and maximum likelihood analyses (Figure 1), our isolates representing the new species named C.sinensis was a sister group to C.fluitans and consistently had J.submerse as the next closest relative with a strong statistical support. The close relationship between C.sinensis and C.fluitans was supported with a posterior probability of 1.00 in the Bayesian analysis and with a bootstrap value of 0.93 in the maximum likelihood analysis. Phylogenetic relationships amongst the taxa inferred from the combined four gene sequences are in general agreement with those based on SSU rDNA and LSU rDNA D1/D2 domains by Wang et al. (2015). Although there are some minor variations in the relationships amongst the classes between the two studies, taxa within each class still formed a single clade.
Figure 1.
Phylogenetic tree based on Bayesian analysis of the combined ITS, TEF1, 18S and 28S rDNA sequences. Auricularia sp. and Coprinuscomatus of Agaricomycotina are used as outgroups. Clades and taxa are labelled according to Schell et al. (2011). Bayesian posterior probabilities, greater than 0.95, are given above the nodes (out of 100). Maximum likelihood bootstrap values, greater than 75%, are given below the nodes (out of 100). The scale bar shows the expected changes per site.
BLAST searches using the complete ITS regions of our C.sinensis strains (YMF 1.04613 and YMF 1.04389) aligned them only to the 5.8S rDNA of a variety of uncultured fungus. There are a few ITS1 matches at about 87% sequence identity to specimens in Pucciniomycotina. Since the study of Classiculomycetes by Bauer et al. (2003) did not employ ITS sequences, we were unable to use ITS sequences for species confirmation with those in Classicula. Sequences of accession numbers AY512838 and AY512853 were those of 18S rDNA of C.fluitans R. Bauer, Begerow, Oberw. & Marvanová and J.submerse, respectively, but were mistaken for ITS by Wang et al. (2015).
Taxonomy
Classicula sinensis
Y. Huang & Z.F. Yu sp. nov.
819813
Figure 2.
Microscopic features of Classiculasinensis (holotype YMF 1.04613). a, b Conidia c empty conidia d clamp connection on conidia e Haustorial branches with basal clamps on hyphae f Conidiogenous cells (black arrow) and clamp connection on hyphae (white arrow). Scale bar: 10 µm (a–f).
Etymology.
Sinensis refers to the country in which this species was found.
Diagnosis.
Classiculasinensis differs from C.fluitans by having fusiform conidiogenous cells growing from the hyphae directly.
Type.
CHINA. From leaves of an unidentified dicotyledonous plant submerged in a stream, Chongqing Municipality, Mozigou, 29°25'38"N, 107°24'19"E, ca. 750 m elev. Oct 2014, ZeFen Yu, YMF 1.04613–holotype[live culture], YMFT1.04613 [dried specimen], CGMCC–3.18938–ex-type culture. Other strain: YMF 1.04389, CGMCC–3.18937, Chongqing Municipality, Mozigou, 29°28'N, 107°25'E, ca. 750 m elev.
Description.
Colonies on CMA reach about 10 mm diameter after incubating for 7 days at 27 °C. Colony effuse, mycelium partly superficial, partly immersed in substratum, composed of hyaline, branched, thin-walled, septate, smooth, binucleated hyphae, 1.5–4.8 µm wide, often 1.8–2.7 µm wide. Clamp connection and haustorial branches on hyphae present. Haustorial branches with basal clamps, tapering distally or obclavate, 9–14.2 (–16.5) µm long, 1.2–2.6 µm wide, one or two terminal filaments of 3–8.5 × 1.3 µm located on the top of it. Conidiophores absent. Conidiogenous cells fusiform, monoblastic, 7.5–11×2–2.8 µm, attaching directly on the hyphae, solitary or in aggregates of two. Conidia solitary, acrogenous, navicular or obclavate, attenuating upwards, 25–38 (–42) µm long, 3.8–6.2 µm wide, 1.3–3.4 µm wide at the truncate base, (0–) 2–5 (–7) septa appear in those conidia without cytoplasm, with 1–5 (mainly 3–4) lateral appendages, attaching to the upper part of conidia, opposite or verticillate, filiform, smooth, divergent, pendulous or straight (–7) 13–21 (–25) µm long, 0.8–1.2 µm wide, 0–2(–3) septate. Occasionally, 1(–2) appendages also arise from apex of the main axis. Sometimes clamp connections appear at the top of conidia.
Discussion
Classicula is phylogenetically related to Jaculispora and morphologically similar to the latter. When Jaculispora was established, Hudson and Ingold (1960) did not mention clamp connections or haustorial cells. Later, Matsushima (1987) observed clamps on hyphae from J.submersa (isolate MFC 12864), but did not see haustorial cells. Bauer et al. (2003) reported that J.submersa also presented tremelloid haustorial cells similar to C.flutitans and both species have septal-spore architecture surrounded by microbodies. Further phylogenetic analysis inferred from the 18S rDNA gene revealed that the two species belonged to the family Classiculaceae of Urediniomycetes. In fact, both Classicula and Jaculispora are very similar in having navicular conidia with 3–4 distal setose branches. However, conidiogenous cells of Classicula are discrete fusiform, differentiated obviously and those of Jaculispora are integrated. Conidiogenous cells of C.sinensis are also integrated, but in the analysis of concatenated dataset of four sequences, C.sinensis and C.flutitans formed a well-supported clade separated from Jaculispora, so we treated our strains as a member of the genus Classicula.
C.fluitans is similar to C.sinensis in having haustorial branches and obclavate or navicular conidia with hair-like lateral and apex appendages. However, their conidiophores and conidiogenous cells were totally different. First, C.sinensis has no conidiophore and its conidiogenous cells grow from hyphae directly, while conidiophores of C.fluitans are determinate, micronematous to semi-macronematous. Second, typical conidiogenous cells of C.fluitans are discrete fusiform formed successively, clamped basally, but C.sinensis has no clamps at the base of conidiogenous cells and conidiogenous cells of C.sinensis are integrated, which resemble that of Jaculispora. Besides the main differences described above, conidia of C.fluitans are shorter and wider [(18–)25– 32(–45) × (4–)5–6.5(–9)] than those of C.sinensis, lateral branches of C.fluitans are 2–3, while 4 lateral branches often appear in C.sinensis. Furthermore, coralloid structures were interpreted as appressoria in C.fluitans but were not observed in C.sinensis (Bauer et al. 2003).
C.sinensis is similar to J.submersa in conidia form, but conidia of the latter grow on the tip of long micronematous conidiophores, while that of C.sinensis grow from conidiogenous cells directly produced on hyphae. Besides, conidia of J.submersa are longer than those of C.sinensis (type strain: 35–55 × 5–7, MFC-12864:35–56 × 4–6 µm). Septa of conidia without cytoplasm were not mentioned in the type strain of J.submerse. In strain MFC-12864, there is a septum obscurely presented at the attenuated part, while conidia of C.sinensis have 3–4 septa after cytoplasm drained out of the conidia.
A combination of morphological and molecular characters was used to establish C.sinensis. Conidiogenous cells of C.sinensis and C.flutitans were sufficiently different to support the molecular data and to suggest the new species. This situation has not been observed often in other fungi of the same genus, thus more isolates belonging to Classiculomycetes are needed to circumscribe genus characteristics of Classicula better and in more detail.
Supplementary Material
Acknowledgements
This work was financed by the National Natural Science Foundation Program of PR China (31770026, 31570023). We are grateful to two reviewers for critically reviewing the manuscript and providing helpful suggestions to improve this paper.
Citation
Qiao M, Li W, Huang Y, Xu J, Zhang L, Yu Z (2018) Classicula sinensis, a new species of basidiomycetous aquatic hyphomycetes from southwest China. MycoKeys 40: 1–12. https://doi.org/10.3897/mycokeys.40.23828
References
- Aime MC, Matheny PB, Henk DA, Frieders EM, Nilsson RH, Piepenbring M, McLaughlin DJ, Szabo LJ, Begerow D, Sampaio JP, Bauer R. (2006) An overview of the higher level classification of Pucciniomycotina based on combined analyses of nuclear large and small subunit rDNA sequences. Mycologia 98: 896–905. 10.1080/15572536.2006.11832619 [DOI] [PubMed] [Google Scholar]
- Aime MC, Castlebury LA, Abbasi M, Begerow D, Berndt R, Kirschner R, Marvanová L, Ono Y, Padamsee M, Scholler M, Thines M, Rossman AY. (2018) Competing sexual and asexual generic names in Pucciniomycotina and Ustilaginomycotina (Basidiomycota) and recommendations for use. IMA FUNGUS 9: 75–90. 10.5598/imafungus.2018.09.01.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Begerow D, Oberwinkler F, Marvanova L. (2003) Classicula: the teleomorph of Naiadellafluitans. Mycologia 95: 756–764. 10.1080/155725362004.11833077 [DOI] [PubMed] [Google Scholar]
- Hudson HJ, Ingold CT. (1960) Aquatic hyphomycetes from Jamaica. Transactions of the British Mycological Society 43: 469–478. 10.1016/S0007-1536(60)80029-0 [DOI] [Google Scholar]
- Khan MA. (1987) Interspecies interactions in aquatic hyphomycetes. The botanical magazine Tokyo 100: 295–303. 10.1007/BF02492836 [DOI] [Google Scholar]
- Kumar S, Stecher G, Peterson D, Tamura K. (2012) MEGA-CC: computing core of molecular evolutionary genetics analysis program for automated and iterative data analysis. Bioinformatics 28: 2685–2686. 10.1093/bioinformatics/bts507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner R, Okuda T. (2013) A new species of Pseudocercospora and new record of Bartheletiaparadoxa on leaves of Ginkgo biloba. Mycological Progress 12: 421–426. 10.1007/s11557-012-0849-3 [DOI] [Google Scholar]
- Marvanová L. (1977) Taeniosporagracilis gen. et sp. nov. Transactions of the British Mycological Society 69: 146–148. [DOI] [Google Scholar]
- Marvanová L, Bandoni RJ. (1987) Naiadellafluitans gen. et sp. nov.: a conidial basidiomycete. Mycologia 79: 578–586. 10.2307/3807598 [DOI] [Google Scholar]
- Marvanová L, Barlocher F. (1988) Hyphomycetes from Canadian streams. I. Basidiomycetous anamorphs. Mycotaxon 32: 339–351. [Google Scholar]
- Marvanová L, Suberkropp K. (1990) Camptobasidiumhydrophilum and its anamorph, Crucellasubtilis: a new heterobasidiomycete from streams. Mycologia 82: 208–217. 10.2307/3759849 [DOI] [Google Scholar]
- Marvanová L, Bärlocher F. (1998) Hyphomycetes from Canadian streams. III. Arcisporabisagittaria anam. gen. and sp. nov. Mycologia 90: 531–536. 10.2307/3761413 [DOI] [Google Scholar]
- Marvanova L, Barlocher F. (2000) Hyphomycetes from Canadian streams. V. Two new conidial basidiomycetes. Mycotaxon 75: 409–423. [Google Scholar]
- Matsushima T. (1987) Matsushima mycological memoirs No. 5. Matsushima Fungus Collection, Kobe, Japan, 100 pp. [Google Scholar]
- Nawawi A. (1985) Basidiomycetes with branched, waterborne conidia. Botanical Journal of the Linnean Society 91: 51–60. 10.1111/j.1095-8339.1985.tb01134.x [DOI] [Google Scholar]
- Pratibha J, Nguyen HDT, Mel’nik VA, Bhat DJ, White GP, Seifert KA. (2014) Lectotypification, epitypification, and molecular phylogeny of the synnematous hyphomycete Pseudogliophragmaindicum, the second genus in the Wiesneriomycetaceae. Mycoscience 56: 387–395. 10.1016/j.myc.2014.12.002 [DOI] [Google Scholar]
- Raj TRN, Kendrick B. (1981) Infundibura, a new hyphomycete with unique appendages. Canadian Journal of Botany 59: 542–546. 10.1139/b81-076 [DOI] [Google Scholar]
- Rehner SA, Buckley EA. (2005) Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorp-hs. Mycologia 97(1): 84–98. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference andmodel choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell WA, Lee AG, Aime MC. (2011) A new lineage in Pucciniomycotina: class Tritirachiomycetes, order Tritirachiales, family Tritirachiaceae. Mycologia 103: 1331–1340. 10.3852/10-333 [DOI] [PubMed] [Google Scholar]
- Shaw DE. (1972) Ingoldiellahamata gen. et sp. nov., a fungus with clamp connexions from a stream in north Queensland. Transactions of the British Mycological Society 59: 255–259. 10.1016/S0007-1536(72)80010-X [DOI] [Google Scholar]
- Shearer CA, Descals E, Kohlmeyer B, Kohlmeyer J, Marvanová L, Padgett D, Porter D, Raja HA, Schmit JP, Thorton HA, Voglymayr H. (2007) Fungal biodiversity in aquatic habitats. Biodiversity and Conservation 16: 49–67. 10.1007/s10531-006-9120-z [DOI] [Google Scholar]
- Scheuer C, Bauer R, Lutz M, Stabentheiner E, Mel’nik VA, Grube M. (2008) Bartheletiaparadoxa is a living fossil on Ginkgo leaf litter with a unique septal structure in the Basidiomycota. Mycological Research 112: 1265–1279. 10.1016/j.mycres.2008.06.008 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Groenewald M, Takashima M, Theelen B, Liu XZ. (2015) Phylogeny of yeasts and related filamentous fungi within Pucciniomycotina determined from multigene sequence analyses. Studies in Mycology 81: 27–53. 10.1016/j.simyco.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Theelen B, Groenewald M, Bai FY, Boekhout T. (2014) Moniliellomycetes and Malasseziomycetes, two new classes in Ustilaginomycotina. Persoonia: Molecular Phylogeny and Evolution of Fungi 33: 41–47. 10.3767/003158514X682313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innins MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR Protocols: a guide to methods and applications.Academic Press, San Diego, California, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.