Abstract
Purpose
Transient Epileptic Amnesia (TEA) is a form of adult onset temporal lobe epilepsy characterised by ictal amnesia. The amnesic seizures are often accompanied by interical memory disturbance, involving autobiographical amnesia and accelerated long-term forgetting. Short-term follow-up studies suggest a relatively stable cognitive profile once treated, but recent case reports raise concerns regarding the risk of developing Alzheimer’s Disease (AD). The current study reports clinical and cognitive outcome in TEA patients over a 20-year period.
Methods
A cohort of ten TEA patients first reported in 1998 were followed up at two time intervals, each 10 years apart. Information regarding clinical outcomes and subjective reports of memory functioning was gained via GP records and clinical interview. Objective memory function was determined at each time point via a comprehensive neuropsychological assessment, where possible.
Results
Information was obtained for nine of the original 10 participants. Over the 20-year period, 4 participants died, with no indication of dementia prior to death. One participant was diagnosed with Vascular Dementia. Seizures were generally well controlled. Subjective reports of memory varied, including no concerns, stable memory difficulties, and worsening memory. Neuropsychological assessment at 10 years showed stable performances across most measures. At the 20-year follow up, there was no evidence of a general cognitive decline. Participants showed stability on some measures, with reductions on others. Performance was not consistent with AD.
Conclusions
No elevated risk of dementia was evident from this TEA series. Although memory difficulties persist over time, the prognosis of TEA appears generally benign.
Keywords: Transient epileptic amnesia, memory impairment, longitudinal study, prognosis
Introduction
Transient Epileptic Amnesia (TEA) is a form of adult onset temporal lobe epilepsy characterised by repeated transient episodes of memory impairment, which occur in the absence of other significant cognitive disruption. For the majority, this ictal amnesia is accompanied by some degree of persistent, interictal memory difficulty, usually autobiographical amnesia [1,2], accelerated long term-forgetting [3–6] or topographical amnesia [7]. i.e. memory for routes and places. Although cases of TEA have been described since 1889 [8], the recognition of TEA as a distinctive subtype of TLE is relatively recent, with specific diagnostic criteria outlined in 1998 [9]. As a result, while several studies characterise features at presentation, there is little information regarding long term prognosis. Evidence from shorter term follow up, however, suggests that while interictal memory problems may persist, the cognitive profile remains stable once the seizures are successfully treated with anticonvulsant medication [10–12]. Whether this is true over the longer term is currently unclear.
Longitudinal studies of other forms of TLE suggest that while memory performance of such patients may be below age-matched peers, declines over time do not generally exceed rates seen in normal ageing [13] and are therefore not usually suggestive of a neurodegenerative process. Prognosis in this group has been related to the type of seizure (with greater risk occurring if the patient’s epilepsy includes generalised seizures), the number of uncontrolled seizures, and the age of onset (earlier onset predicting a poorer outcome)[14]. This bodes well for people with TEA, as the majority do not experience generalised seizures, respond well to medication, and do not develop the condition until their midlife [7].
Nevertheless, TEA was recently described in a woman who developed Alzheimer’s Disease (AD) 16 years later[15]. While potentially coincidental, the authors suggest that TEA may have been the first sign of this disease. Similarly, cases diagnosed with Epileptic Amnesic Syndrome (EAS) have developed AD [16]. ‘EAS’ denotes a syndrome closely resembling TEA but also encompassing patients with interictal memory disturbance in the context of subtle, non-amnesic, temporal lobe seizures. Amnesic seizures have been also noted, occasionally as a presenting feature, among patients with Mild Cognitive Impairment and early AD[17,18]. While this is usually in the context of other seizure types, taken together, the emergence of such cases has served as a warning that TEA and EAS may not be as benign as originally thought.
The current study presents the clinical and cognitive outcome of patients with TEA first reported in 1998 who have been long-term participants in the TIME project (The Impairment of Memory in Epilepsy - http://projects.exeter.ac.uk/time/). From the original series of 10 participants[9], we present follow-up information on 9 individuals over a 20-year period, with 3 detailed case reports. These unique long-term data provide insights into the prognosis of TEA.
Method
Participants
All participants took part in the 1998 published study[9] of TEA (T1). The diagnosis of TEA was established via clinical assessment by an experienced behavioural neurologist (AZ). In all cases, the repeated amnesic episodes had been witnessed by the participant’s spouse. Evidence of epilepsy was confirmed through any combination of epileptiform abnormalities on EEG, reports of concurrent classically epileptic features (e.g. olfactory hallucinations, automatisms) and /or a positive treatment response to anticonvulsant therapy.
All available participants from the 1998 study were then recruited to the TIME study as part of the 2007 series (T2) [7], and invited to a third follow up 10 years later (T3).
The study was approved by the Multicentre Research Ethics Committee, United Kingdom (MREC 03/10/77). All participants gave written, informed consent.
Measures
Updated medical information regarding memory complaints, ongoing seizures and other significant medical events was collected either via clinical interview directly with the participant or through their General Practitioner. Where possible, participants also underwent neuropsychological re-assessment. This included administration of the following measures:
general intellectual functioning: National Adult Reading Test (NART) [19] was used at T1 to estimate IQ. At T2 and T3, the 4-subtest version of the Wechsler Abbreviated Scale of Intelligence (WASI)[20] was used.
Visuospatial/visuoconstructional skills: Rey Complex Figure Test (RCFT)[21] – copy trial.
Visual delayed recall: 30 minute free recall of the RCFT.
Verbal immediate and delayed recall: Story 1 (Anna Thompson) from the Logical Memory (LM) subtest of the Wechsler Memory Scale (WMS-R version at T1 and WMS-III version at T2)[22].
Recognition memory: Warrington Recognition Memory Test (RMT)[23] - Words and Faces subtests
Semantic memory: Graded Naming Test (GNT)[24,25] at T2 and T3
Executive function: verbal fluency [26,27]and Wisconsin Card Sorting Test – 64 Card Version[28] at T2 and T3.
Data include either age-corrected percentile ranks, or raw scores if these were not available, to interpret performance over time. Clinically significant impairment was defined as test performance that was less than or equal to the 10th percentile. Performance was considered stable if the percentile rank remained within a similar range (e.g. if scores remained within the normal range of 25-75th percentile, above 75th percentile, or below average range 10-25th percentile; or if scores were in overlapping percentile ranges, such as “75-95” and then 75th). Where test norms did not adjust for differences in age over time, stability was assumed if raw scores remained relatively unchanged (i.e. allowing for minor decrements that may be expected from measurement error).
Results
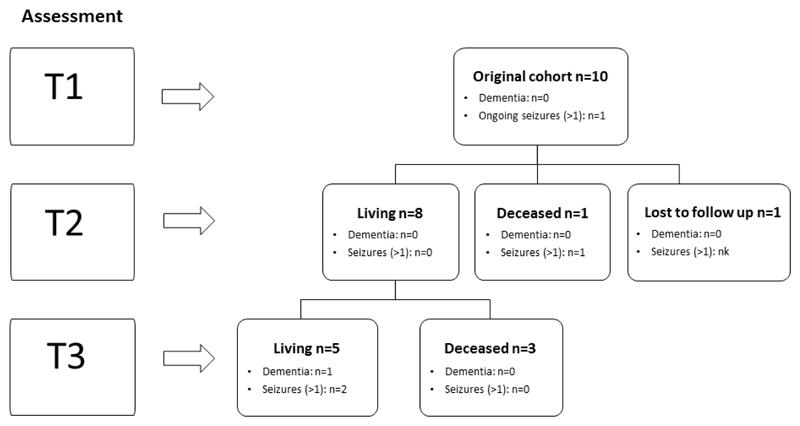
Table 1 summarises each participant’s clinical presentation, Table 2 the clinical outcomes over the 10 to 20-year period, and Table 3 the neuropsychological test performance. Figure 1 provides a summary of participant numbers at each time point.
Table 1. Clinical characteristics of TEA participants.
| ID | Sex | Age at onset | Duration of attacks | Approx Frequency of attacks | Seizure features | Other epilepsy | EEG | Initial treatment response |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | 30-60 mins | Monthly | All on waking, Olf hall | sps | Normal | Yes - LAM |
| 2 | M | 49 | 15-30 mins | Twice/month | All on waking, Pure amnesia | sps | epileptic | Yes - CBZ |
| 3 | M | 68 | Hrs-days | No pattern | On waking, Olf hall | sps, cps | epileptic | Yes -SVP |
| 4 | F | 52 | 2-24 hrs | Twice/ yr | On waking, Pure amnesia | none | epileptic | Yes - LAM |
| 5 | M | 78 | Hrs-days | Twice/ yr | On waking | none | epileptic | Equivocal reduction – CBZ |
| 6 | M | 69 | 15-30 mins | Monthly | On waking, automatisms, déjà vu | sps | Non-specific abnormalities | Not treated |
| 7 | M | 73 | 1-3 hrs | Monthly | On waking, Pure amnesia | none | Non-specific abnormalities | Yes - Phen |
| 8 | M | 60 | 2-24 hrs | Monthly | On waking, automatisms | cps, tcl (1) | Normal | Yes - SVP |
| 9 | M | 69 | 20-60 mins | Monthly | On waking, automatisms | cps | Some abnormalities | Yes - CBZ |
| 10 | M | 52 | 15-30 mins | Twice/wk | On waking, automatisms | cps, tcl (3) | Some abnormalities | Yes - CBZ |
CBZ = carbamazepine; LAM = lamotrigine; SVP = sodium valproate; Phen = phenytoin, sps = simple partial seizures; cps = complex partial seizures; tcl = tonic-clonic seizures; Olf hall = olfactory hallucination
Table 2. Clinical review – Seizure history, medication and subjective cognitive outcomes.
| ID | Current Status | Current Age | No. of amnesic seizures | Treatment history | Dementia Diagnosis | Subjective cognitive complaints | Additional clinical notes | ||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | |||||||
| 1 | Living | 83 | 50 | 1 | 0 | Initial change from CBZ to LAM. No change from 1996 | No | At T1, ALF and patchy RA extending 30 years. At T2, reported ongoing memory concerns since onset, with more pronounced forgetting of new information. At T3, reported similar ongoing concern of fast forgetting of new information. | No significant concerns regarding cognition by medical team |
| 2 | Living | 71 | 10 | 0 | 0 | Treated on CBZ for 10 years; phased out in 2005 due to associated fatigue | No | At T1, RA extending 30 years. At T2, reports of ongoing concerns, with RA now extending 40 years. At T3, stable memory complains | No significant concerns regarding cognition by medical team. Participant reports “life has returned to near normal apart from the loss of part of my memory” |
| 3 | Deceased | 74 at death | 5 | nk (>1) | - | Treated with SVP for 2 years; topirmate added in 1997 when seizures recurred | No | At T1, RA extending 30 years. Died in 1999, 5 years prior to T2 follow up. | Cause of death was ruptured aortic aneurysm. Neuropsychological assessment 9 months prior to death showed average performances across tasks, with no evidence of decline. |
| 4 | Living | 87 | 5 | 1 | ?2 | 2009 one increase to dosage of LAM | No | At T1, ALF. No concerns of RA, although remote memories reported to be “less vivid”. At T2, ongoing memory concerns were reported. At T3, concerns of worsening memory | No significant concerns regarding cognition by medical team |
| 5 | unknown | unknown | Treated with CBZ. Effectiveness unknown. Lost to follow up | No | At T1, patchy amnesia for recent events. | unknown | |||
| 6 | Deceased | 89 at death | 15 | 0 | 0 | Treated with CBZ for 7 weeks but discontinued due to a rash. No further treatment | No | At T1, difficulties with day to day memory. At T2, reported no persistent memory difficulties | Cognitive impairments (poor memory) but no indication of dementia. Assessed by medical team 3 months prior to death. Dementia screening concluded “no major concerns about cognition”. Cause of death was pneumonia. |
| 7 | Deceased | 84 at death | 6 | 0 | - | Stable on Phenytoin | No | At T1, minor forgetfulness. At T2, reported no persisting memory difficulties | No cognitive difficulties noted and independent in activities of daily living on medical review 4 months prior to death. Cause of death was mesothelomia |
| 8 | Living | 81 | 6 | 0 | nk | Stable on SVP | Yes - VD | At T1, patchy RA extending 30 years, and poor day-to-day memory. At T2, reports of ongoing memory concerns since onset. Between T2 and T3, increased cognitive difficulty associated with CVAs. | Impaired cognition associated with Vascular Dementia (diagnosed in 2013). Multiple CVAs, resulting in cognitive impairments. |
| 9 | Deceased | 83 at death | 5 | ?1 | - | Stable on CBZ | No | At T1 patchy RA extending 25 years. At T2 reported ongoing memory concerns since onset, with additional fading recall of recent events | Died awaiting a second aortic valve replacement. No record of significant concerns regarding cognition by medical team |
| 10 | Living | 77 | ≥20 | 0 | ≥ 10 | Stable on CBZ until 2008 then multiple reductions in dosage required; 2014 changed AED (LEV) | No | At T1, ALF and patchy RA extending 10 years. At T2, reports of ongoing memory concerns since onset. Additional cognitive difficulty from 2008 onwards | Patient and family report significant difficulties with fatigue, attention and concentration |
CBZ = carbamazepine; LAM = lamotrigine; SVP = sodium valproate; LEV = leveteracitam; ALF = accelerated long-term forgetting; RA = retrograde amnesia; VD = Vascular Dementia; nk = not known
Table 3a. Neuropsychological test performance for participants assessed over T1 and T2 only (Score or percentile).
| Case 2 | Case 6 | Case 7 | Case 8 | Case 9 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Assessment / age | T1 /49 | T2 /60 | T1 / 70 | T2 /80 | T1 / 73 | T2 /83 | T1 /60 | T2 /70 | T1 /69 | T2 /79 |
| IQ/ Estimated IQ | 110 | 113 | 114 | 132 | 105 | 100 | 116 | 120 | 120 | 129 |
| RCFT – copy (/36) | 32 | 32 | 33 | 35 | 34 | 34 | 33 | 34 | 35 | 35 |
| LM (Story 1) – Immediate (/25) | 9 | 6 | 16 | 16 | 7.5 | 12 | 14.5 | 14 | 15 | 18 |
| LM (Story 1) – Delay (/25) | 5* | 4* | 14 | 15 | 1.5* | 3* | 13 | 14 | 11 | 14 |
| RCFT – 30 minute delay (%ile) | 3* | 27 | 54 | 97 | 54 | 46 | 66 | 46 | 93 | 82 |
| RMT – Words (%ile) | 25 | 50 | 75-95 | 75 | 50-75 | 25 | 75 | >95 | 95 | 95 |
| RMT – Faces (%ile) | 25-50 | <5* | 50-75 | 50 | 5-10* | <5* | 25-50 | 5-10* | >95 | >95 |
| GNT (/30) | - | 11* | - | 22 | - | 20 | 21 | 23 | - | 23 |
| Letter fluency – FAS (%ile) | - | 2* | - | 11 | 3-5* | 29-40 | 60-71 | |||
| Category fluency (%ile) | - | <10* | - | 50-75 | - | 90 | 75-90 | 50-75 | - | >90 |
| WCST perseverative errors (%ile) | - | - | - | >99 | - | 39 | >99 | - | >99 | |
clinically impaired scores of ≤ 10% ile or -1.3 SDs below control mean; Norms used: RCFT [21]; LM [7]; RMT [23]; GNT [25]; Letter fluency [27], Category fluency [26]; WCST [28]. For RCFT – copy, all scores reflect performances ≥ 16th%ile unless marked otherwise. For RMT, age-adjusted percentiles only include 40-54 years and 55 years and above.
Figure 1.
Flow diagram of participant follow-up over the three time periods (T1, T2 and T3). Nk = not known. Only one participant is diagnosed with (vascular) dementia throughout the period of the study.
Participant characteristics at presentation (T1)
At T1, participants comprised 9 males and 1 female. Age of onset of TEA ranged from 49 to 78 years, with a mean age of 63 years. In half the cases, amnesic seizures lasted under an hour, but in two cases extended beyond a day (cases 3 and 5). Frequency of these seizures was commonly estimated at once a month (cases 1,6,7,8,9). All participants had experienced at least one amnesic episode on waking, with attacks exclusively on waking in two cases (cases 1 and 2). In three cases (cases 4, 5 and 7), attacks were purely amnesic, without additional epileptic features. The remaining 7 participants had other features at the time of at least some of their amnesic seizures, including olfactory hallucinations (cases 1 and 3), automatisms (cases 6,8,9,10), or déjà vu (case 6). Seven participants had additional simple partial, complex partial or tonic clonic seizures. EEG abnormalities were present in 8 cases: 4 which were epileptiform and 4 non-specific. Seizures ceased on initial treatment in 8 of the 10 cases.
All participants presented with memory complaints, most commonly retrograde amnesia (Cases 1,2,3,8,9,10), followed by accelerated forgetting of new information (Cases 1,4,10). Neuropsychological assessment at T1 indicated average to above average intellectual ability (Table 3a and 3b). Overall, as a group, performance on standard memory measures was normal, although some impairments on tests were observed at an individual level [9]. In particular, Case 2 showed poor verbal and visual delayed recall memory; Case 7 showed poor verbal recall and impoverished face recognition (all scoring below the 10th percentile).
Table 3b. Neuropsychological test performance for participants assessed over T1-T3 (Score or percentile).
| Case 1 | Case 4 | Case 10 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Assessment / age | T1 /63 | T2 /73 | T3 /83 | T1 / 66 | T2 /76 | T3/ 87 | T1 / 56 | T2 /66 | T3 /77 |
| IQ/ Estimated IQ | 110 | 117 | 112 | 123 | 138 | 129 | 120 | 116 | 112 |
| RCFT – copy (/36) | 34 | 31 | 21* | 36 | 36 | 36 | 36 | 36 | 33 |
| LM (Story 1) – Immediate (/25) | 16 | 17 | 11 | 16 | 18 | 16 | 16 | 14 | 7 |
| LM (story 1) – Delay (/25) | 12 | 15 | 11 | 10 | 15 | 8 | 9.5 | 12 | 6 |
| RCFT – 30 min delay (%ile) | 50 | 69 | 42 | 88 | 84 | 31 | 46 | 62 | 54 |
| RMT – Words (%ile) | >95 | 75-95 | 75-95 | 75-95 | 75-95 | 5* | 50-75 | 50-75 | 25-50 |
| RMT – Faces (%ile) | 50 | 50-75 | <5* | 75 | 75-95 | 75 | 75-95 | 25-50 | <5* |
| GNT (/30) | - | 22 | 22 | - | 25 | 21 | - | 19 | 15* |
| Letter fluency (%ile) | 82-89 | 60-71 | 82-89 | 29-40 | 19-28 | 2 | |||
| Category fluency (%ile) | >90 | >90 | >90 | 50-75 | 25 | <10 | |||
| WCST perseverative errors (%ile) | - | 90 | 16 | - | >99 | >99 | - | 21 | 63 |
clinically impaired scores of ≤ 10% ile or -1.3 SDs below control mean; Norms used: RCFT [21]; LM [7]; RMT [23]; GNT [25]; Letter fluency [27], Category fluency [26]; WCST [28]. For RCFT – copy, all scores reflect performances ≥ 16th%ile unless marked otherwise. For RMT, age-adjusted percentiles only include 40-54 years and 55 years and above.
Clinical and cognitive outcomes 10 years later (T2)
Information regarding 9 participants was available at the first 10-year follow-up. Of these, one participant (Case 3) had died, following a ruptured aortic aneurysm. Preceding his death there were no documented concerns regarding his cognition, beyond the original reports of marked autobiographical amnesia. A neuropsychological assessment completed 9 months prior to death showed no evidence of cognitive decline[29]. The remaining eight individuals underwent a full re-assessment [7]. Seven participants continued to have good seizure control on anti-convulsant medication (with ≤ 1 further episode per participant over the 10-year period). The remaining participant (Case 6), who had abandoned treatment after 7 weeks, also reported an absence of seizures during this period.
Self-reported cognitive outcomes varied from no persisting memory difficulty (Cases 6 & 7) to either stable memory deficits (Cases 4,8,10) or reports of worsening memory (Cases 1,2,9). On objective neuropsychological assessment, however, when accounting for age, performance typically remained steady or showed minor improvements (i.e. percentile rankings remained within a similar range or increased; see Table 3a and 3b). An exception to this was in face recognition, where clinically significant impairments were observed now in 3 cases (2,7 and 8). Some additional decline was also evident for immediate recall of a story for Case 2, however, on other tasks he remained stable or slightly improved (Table 3a).
Clinical and cognitive outcomes 20 years later (T3)
At the second 10-year follow-up, 3 additional participants had now died (cases 6,7,9, see Figure 1). Causes of death were pneumonia, mesothelomia, and cardiac failure. There was no indication of a dementia or significant cognitive concerns in these cases beyond the deficits initially recorded at the time of diagnosis. In each case, age at death exceeded current life expectancy estimates for males (79.35 years)[30]. Of the remaining 5 participants, 3 were now in their 80s. Four participants had continued their treatment on anticonvulsant medication, with no further reports of seizures in two participants. An additional participant was also reportedly seizure free, despite having ceased taking anticonvulsants in 2005. In the remaining 2 participants (cases 4 and 10), some re-emergence of episodes had occurred. One participant (case 8) suffered multiple strokes and was diagnosed with vascular dementia, with associated cognitive declines and did not participate further in the study.
Assessment of subjective memory complaints in the remaining 4 cases revealed either ongoing but stable impairments (cases 1 and 2) or reported worsening of memory (cases 4 and 10). All 4 participants were living at home independently. Three participants (cases 1, 4, 10) underwent a further neuropsychological re-assessment. We summarise the key features and findings in these cases in the following detailed descriptions.
Case 1 – 83 year old male, retired engineer
Case 1 presented in 1995 aged 63 with a 3-year history of monthly episodes of transient amnesia lasting for around one hour each, mostly on waking, involving a retrograde amnesia of up to 30 years. He sometimes complained of a strange smell or taste at attack onset. MRI and EEG were normal. The attacks ceased when he was treated with carbamazepine. He changed to lamotrigine because of drowsiness. Initial cognitive assessment estimated his intellectual functioning to be within the average range, with commensurate performance on standard memory tests.
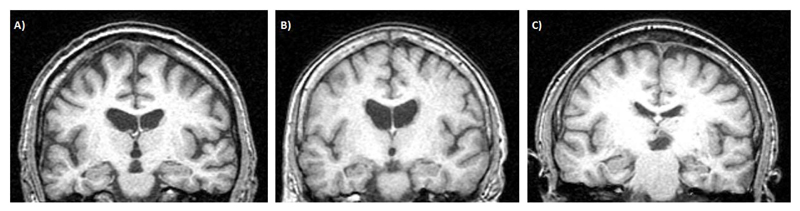
At follow-up in 2005, only one additional attack was reported, following dose reduction. On returning to his previous dose, he remained attack-free. He underwent a research brain MRI, with no abnormalities reported (Figure 2A). Despite this, at review he complained of worsening memory difficulties involving accelerated forgetting of new information and patchy loss of salient autobiographical memories. Neuropsychological assessment at T2, however, indicated a stable profile of skills – with small increases, rather than decreases, in scores of visual and verbal delayed recall. General cognitive ability was measured within the high average range and he performed well on measures of executive function.
Figure 2.
T1 coronal MR brain images for A) Case 1 (age 73), B) Case 4 (age 76), and C) Case 10 (age 66). All images were acquired at the first 10-year follow-up period (T2). Note the left hemisphere is shown on the left. Case 10 had moderate right hippocampal atrophy.
Medical review in 2015 indicated that he was well, with no further episodes of amnesia on continuing anticonvulsant treatment. He was living alone, independently. On self-report, he indicated ongoing difficulty with forgetting new information. Neuropsychological review at T3 revealed continued stability in general knowledge and problem solving, but some reduction in visual skills, as seen in both his drawing and recall of the complex figure. His recognition of faces, which are viewed briefly, was also reduced from T2, now falling within the clinically impaired range (below the 10th percentile). Although some decline in encoding verbal information (a story) was evident, his delayed recall remained very similar to that shown 20 years earlier. Semantic memory remained stable and he continued to perform well on speeded word generation tasks.
Case 4 – 87 year old female, previous almoner and housewife
Case 4 presented aged 67 with five episodes of transient amnesia over a 15-year period, with no other seizure features. Brain CT and wake EEG were normal, but a sleep study revealed unequivocal left temporal epileptiform abnormalities. She was treated with carbamazepine, with one further episode after which dosage was adjusted. Cognitive assessment suggested above average intellectual functioning, with performance on standard memory tests at least within the average range for her age.
At her clinical follow up in 2005, no further seizures had occurred. She underwent a research brain MRI, with no abnormalities reported (Figure 2B). Despite this, she complained of ongoing difficulty with retaining new information, which she felt faded over the course of a few hours. Her husband, however, had not observed any significant memory problem. Neuropsychological assessment at T2 showed no evidence of decline in any cognitive domain, with improved scores on verbal delayed recall (over a 30-minute period), despite her increasing age.
She had two possible minor amnesic seizures over the next 10 years. Following an increase in medication (CBZ), no further episodes were recorded. At reassessment in August 2015, she complained of worsening memory, but was living alone independently.
Neuropsychological performance remained generally stable over the 20-year period for visuospatial skills, initial verbal encoding and recognition of faces. Performance on a novel problem solving task remained at the 99th percentile. Declines, however, were evident in delayed recall of visual and verbal information when comparing performance from T2 to T3, and she now showed impaired recognition memory for words (below the 10th percentile). Performance on many tasks, however, remained within the normal range for her age.
Case 10 – 77 year old male, retired lecturer
Case 10 presented at age 57 with a 5-year history of numerous episodes of anterograde amnesia, commonly occurred on waking from naps, together with other seizures including three witnessed tonic-clonic convulsions. Prior to these episodes, his medical history included one fit at age 2 and another when aged 23. Brain MRI in 1995 showed focal atrophy of the right hippocampus, which was unchanged on serial imaging in 2000 and 2003. Treatment with carbamazepine abolished both the episodes of amnesia and overt seizures. His cognitive assessment suggested above average intellectual functioning, with performance on memory measures generally within the average range for his age. Although his initial verbal recall of a story was adequate, his retention of this information appeared somewhat reduced.
At his clinical follow up in 2005, no additional attacks were reported although he continued to complain of memory difficulties both in recalling events from his distant past, as well as learning new information. Results of his neuropsychological assessment at T2, however, indicated little change over the 10-year period, with slight improvements in his delayed recall of information. Recognition memory for words was stable, with some lowering in recognition of faces. His research brain MRI conducted at this time did not reveal any additional abnormalities (Figure 2C).
In 2008, he had an episode of encephalopathy associated with hyponatraemia. This improved with reduction in his dose of carbamazepine, but he was left with continuing fatigue, concentration difficulty and emotional lability. In July 2014, he reported experiencing 3-4 “vacant episodes” per year. Brain MRI was repeated, noting again the original hippocampal asymmetry, evidence of small vessel disease, and now moderate global atrophy. In December 2014, he had an episode of delirium and an exacerbation of his seizures following introduction of leveteracitam. At the time of his 2015 neuropsychological review, the last seizure had been 2 weeks prior. He reported difficulties concentrating and recognising faces from recent past. This included neighbours from the last 5 years and one of his grandchildren.
Neuropsychological assessment at T3 suggested little or no change in general intellectual ability or problem solving. Encoding of verbal information had dropped, although retention of information learned after a 30 minute delay was good. Visuoconstructional skills (drawing) were not impacted and recall of visual information, when adjusted for age, remained steady over the 20-year period (originally 46thpercentile, then 62nd, then 54th). Consistent with the participant’s self-reported difficulties, further decline in recognising faces was evident, with performance now within the clinically impaired range. Additional impairments arose in semantic memory and speeded thinking.
Telephone contact with the family 6 months later indicated seizures were now controlled. Although no objective assessment was conducted, reports from his wife suggested improvement in alertness and general cognition.
Discussion
The long-term outcome of a group of patients with TEA has not been reported previously, leaving the prognosis of this disorder uncertain. We describe longitudinal findings over 20 years in a group of patients first reported in 1998. Follow-up data were available for 9 out of these 10 participants. Results indicated that TEA had no major adverse impact on life expectancy. During the first 10-year period, the majority of patients remained seizure free, with little evidence of cognitive change, and in some cases, minor improvements on neuropsychological testing. Over the following 10 years, control of seizures remained generally good. One participant developed vascular dementia. Significant cognitive impairments were also observed in a second patient, associated with seizure recurrence and complications of therapy. In the remaining participants, while some declines occurred, considerable stability was observed for many tasks over the 20-year period. Where declines were found, the cognitive domain most often affected was visual recognition memory. Importantly, and in contrast to the previous case report by Cretin and colleagues[15], the results of this series do not suggest a substantially increased risk of AD among patients with TEA.
The initial stability observed for the majority of participants within this series is consistent with existing reports within the literature that have followed up patients at shorter time intervals (e.g. 9 months to 2 years). In these studies, anti-convulsants were successful in abolishing seizures, with cognitive performance either remaining stable [12] or showing some minor improvements on memory scores [10,31]. Likewise, in the current series, test performance tended to be unchanged or slightly improved at the first 10-year follow up, coinciding with good seizure control. Interestingly, neuropsychological test results did not always coincide with an individual’s subjective reports of memory, where worsening was reported at times. This discrepancy may in part reflect increasing awareness of memory gaps over time (e.g. through conversations with others, becoming aware of additional lost autobiographical memories), as well as the difficulty in capturing certain types of memory deficits with standardised neuropsychological tests. Regardless, the results at 10 years suggest that even in the context of increasing memory concerns for some patients, changes in broader memory skills were minimal. Where reductions were observed, this did not extend across multiple measures of memory (for example, Case 7 showed some lowering in scores for recognition of words and faces, but some improvements to verbal memory, with somewhat steady age-adjusted visual recall performance), and generally did not involve a striking change in score over a 10-year period.
Greater variability in cognitive performance was observed over the second 10-year period. One participant was now diagnosed with vascular dementia. This appears consistent with estimated base rates of dementia prevalence within this age bracket[32]. No participants, however, were diagnosed with AD, despite several participants living into their 80s. In the 4 deceased participants, there were no reports of significant cognitive decline prior to death. In the remaining participants who underwent neuropsychological assessment at T3, general cognitive ability remained above average, although some declines in memory were observed. The nature and degree of these declines, however, varied. The individual who showed the greatest cognitive impairment (Case 10), experienced changes within the context of re-emerging seizures and significant fatigue, with an overall neuropsychological profile that was not typical of AD[33] (given his visuoconstructional skills and problem solving remained stable and within the normal range and his ability to retrieve learned information after a delay was not indicative of rapid forgetting). In the other two cases assessed at 20 years, both continued to perform highly on certain neuropsychological tests for their age and were living alone independently, indicating a maintained functional ability in everyday life that would not support a dementia diagnosis.
Interestingly, the most consistent impairment on neuropsychological testing was on the Faces subtest of the Warrington Recognition Memory Test. Over the course of the study, 5 of the 9 participants performed within the clinically impaired range, suggesting that visual recognition impairments are a common neuropsychological feature of TEA. This has been noted in previous case reports[34,35] and is consistent with another recent study of TEA where significant impairments in picture recognition were found when using a challenging visual task [36]. Performance on the Warrington Faces has been found to correlate with right hippocampal volumes in TEA, with significant, although subtle, reductions in volume compared with matched healthy controls [37]. Under-functioning in adjacent regions of the brain has also been reported in a study of autobiographical memory, where TEA participants showed reduced activation in the right parahippocampal gyrus relative to controls [38]. Declines over time on the Warrington Faces task may therefore reflect increasing structural or functional changes within key right temporal brain regions, which should be explored in future studies.
Thus, while the case description by Cretin and colleagues raised the possibility that TEA was the first sign of dementia, this does not seem to be the case in our series. It is noteworthy that the woman described had a family history of Alzheimer’s Disease, given her mother’s diagnosis at age 70, which may have been an important predisposing factor in this case[15]. Although no participant in our series appeared to develop AD within the 20-year timeframe, variability in cognitive outcome was still observed and persistent memory complaints were common. We note that the average intelligence of our participants was high, which may have served as a protective factor against developing dementia [39]. Future longitudinal studies including individuals with lower IQ will be important in determining any potential differences in outcome.
Beyond the cognitive results, the current study also provides information regarding seizure outcomes in relation to the long term use of anticonvulsant medication in TEA. Consistent with short-term follow up reports in TEA, few if any seizures were reported over the 20-year period for those remaining on medication, with the exception of Case 10 and Case 4, where adjustments were required after 10 years. Two participants (Cases 2 and 6) discontinued treatment with anticonvulsants – either initially or after 10 years - and remained seizure free. These results suggest that, as in other adult-onset focal epilepsies, some patients with TEA benefit from continuing anticonvulsant treatment while others can achieve seizure freedom following treatment withdrawal.
Our study has some limitations. The evaluation of neuropsychological outcomes involved interpreting age-corrected values of standard measures where possible. However, the norms used were cross-sectional rather than longitudinal and thus only provided a crude measure to evaluate normal change over time. In addition, standard measures are limited in their ability to assess autobiographical memory deficits and forgetting that occurs beyond a half hour delay. We aim to address these limitations in a subsequent prognosis study.
The sample size is small. The demographic and clinical characteristics, however, mirror those reported in larger TEA groups, regarding male predominance, age of onset, seizure frequency, initial treatment response, and average to above intellectual functioning [7,31]. Thus while small, the group studied appears representative. Moreover we were able to follow up 9 of the 10 original cases, reducing selection bias in reporting of outcomes. While our data are reassuring with respect to the risk of dementia among patients presenting with classical features of TEA, we are unable to exclude Alzheimer pathology via either pathological confirmation at autopsy or biomarkers such as cerebrospinal fluid. Such studies are clearly needed. In the interim, this study provides the most comprehensive information currently available on long-term outcome in TEA.
Conclusion
We conclude that while TEA can be a prelude to AD this appears to be rare. Based on a detailed, very long-term follow-up of cases, treated TEA has a relatively benign outcome, with good long-term seizure control in the majority of patients, substantial stability of cognitive performance and no evidence, from this small group study, of an elevated risk of dementia.
Highlights.
Nine patients with TEA were followed up over a 20-year period
Subjective complaints of memory difficulty were common at all time points
Memory performance showed stability at 10 years, with some declines at 20 years
At 20 years, only one participant was diagnosed with (vascular) dementia
Cognitive decline in another case linked to recurrent seizures/drug side-effects
Acknowledgments
This research was supported by The Dunhill Medical Trust [grant number R322/1113]. Associate Professor Chris Butler was funded by an MRC Clinician Scientist award [MR/K010395/1]. Professor John Hodges was supported by funding to Forefront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neurone disease, from the National Health and Medical research Council of Australia program grant [grant number 1037746] and the Australian Research Council Centre of Excellence in Cognition and its Disorders Memory Program [grant number CE110001021].
Footnotes
Conflicts of interest: none.
References
- [1].Milton F, Muhlert N, Pindus DM, Butler CR, Kapur N, Graham KS, et al. Remote memory deficits in transient epileptic amnesia. Brain. 2010;133:1368–79. doi: 10.1093/brain/awq055. [DOI] [PubMed] [Google Scholar]
- [2].Ioannidis P, Balamoutsos G, Karabela O, Kosmidis MH, Karacostas D. Transient epileptic amnesia in a memory clinic setting: A report of three cases. Epilepsy Behav. 2011;20:414–7. doi: 10.1016/j.yebeh.2010.12.028. [DOI] [PubMed] [Google Scholar]
- [3].Muhlert N, Milton F, Butler CR, Kapur N, Zeman AZ. Accelerated forgetting of real-life events in Transient Epileptic Amnesia. Neuropsychologia. 2010;48:3235–44. doi: 10.1016/j.neuropsychologia.2010.07.001. [DOI] [PubMed] [Google Scholar]
- [4].Hoefeijzers S, Dewar M, Della Sala S, Zeman A, Butler C. Accelerated long-term forgetting in transient epileptic amnesia: An acquisition or consolidation deficit? Neuropsychologia. 2013;51:1549–55. doi: 10.1016/j.neuropsychologia.2013.04.017. [DOI] [PubMed] [Google Scholar]
- [5].Hoefeijzers S, Dewar M, Della Sala S, Butler C, Zeman A. Accelerated Long-Term Forgetting Can Become Apparent Within 3-8 Hours of Wakefulness in Patients With Transient Epileptic Amnesia. Neuropsychology. 2014 doi: 10.1037/neu0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Atherton KE, Nobre AC, Zeman AZ, Butler CR. Sleep-dependent memory consolidation and accelerated forgetting. Cortex. 2014;54:92–105. doi: 10.1016/j.cortex.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Butler CR, Graham KS, Hodges JR, Kapur N, Wardlaw JM, Zeman AZJ. The syndrome of transient epileptic amnesia. Ann Neurol. 2007;61:587–98. doi: 10.1002/ana.21111. [DOI] [PubMed] [Google Scholar]
- [8].Hughlings-Jackson J. On a Particular Variety of Epilepsy (“intellectual Aura”), One Case with Symptoms of Organic Brain Disease. Brain. 1888;11:179–207. doi: 10.1093/brain/11.2.179. [DOI] [Google Scholar]
- [9].Zeman AZJ, Boniface SJ, Hodges JR. Transient epileptic amnesia: a description of the clinical and neuropsychological features in 10 cases and a review of the literature. J Neurol Neurosurg Psychiatry. 1998;64:435–43. doi: 10.1136/jnnp.64.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Razavi M, Barrash J, Paradiso S. A longitudinal study of transient epileptic amnesia. Cogn Behav Neurol. 2010;23:142–5. doi: 10.1097/WNN.0b013e3181df3022. [DOI] [PubMed] [Google Scholar]
- [11].Midorikawa A, Kawamura M. Recovery of Long-Term Anterograde Amnesia, but Not Retrograde Amnesia, after Initiation of an Anti-Epileptic Drug in a Case of Transient Epileptic Amnesia. Neurocase. 2008;13:385–9. doi: 10.1080/13554790701851536. [DOI] [PubMed] [Google Scholar]
- [12].Del Felice A, Broggio E, Valbusa V, Gambina G, Arcaro C, Manganotti P. Transient epileptic amnesia mistaken for mild cognitive impairment? A high-density EEG study. Epilepsy Behav EB. 2014;36:41–6. doi: 10.1016/j.yebeh.2014.04.014. [DOI] [PubMed] [Google Scholar]
- [13].Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain J Neurol. 2009;132:2822–30. doi: 10.1093/brain/awp182. [DOI] [PubMed] [Google Scholar]
- [14].Helmstaedter C. Cognitive outcome of status epilepticus in adults. Epilepsia. 2007;48:85–90. doi: 10.1111/j.1528-1167.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- [15].Cretin B, Philippi N, Sellal F, Dibitonto L, Martin-Hunyadi C, Blanc F. Can the syndrome of transient epileptic amnesia be the first feature of Alzheimer’s disease? Seizure. 2014;23:918–20. doi: 10.1016/j.seizure.2014.07.008. [DOI] [PubMed] [Google Scholar]
- [16].Cretin B, Blanc F, Gaultier C, Sellal F. Epileptic Amnesic Syndrome revealing Alzheimer’s disease. Epilepsy Res. 2012;102:206–9. doi: 10.1016/j.eplepsyres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- [17].Cretin B, Sellal F, Philippi N, Bousiges O, Di Bitonto L, Martin-Hunyadi C, et al. Epileptic Prodromal Alzheimer’s Disease, a Retrospective Study of 13 New Cases: Expanding the Spectrum of Alzheimer’s Disease to an Epileptic Variant? J Alzheimers Dis JAD. 2016;52:1125–33. doi: 10.3233/JAD-150096. [DOI] [PubMed] [Google Scholar]
- [18].Vossel KA, Beagle AJ, Rabinovici GD, Shu H, Lee SE, Naasan G, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013;70:1158–66. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nelson H. The National Adult Reading Test (NART): test manual. NFER-NELSON; 1982. [Google Scholar]
- [20].Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- [21].Meyers JE, Meyers KR. Rey complex figure test and recognition trial: Professional manual. Odessa, FL: Psychological Assessment Resources, Inc; 1995. [Google Scholar]
- [22].Wechsler D. Wechsler Memory Scale. 3rd ed. Pearson Assessment; 1997. [Google Scholar]
- [23].Warrington EK. Recognition Memory Test. NFER-NELSON; 1984. [Google Scholar]
- [24].McKenna P, Warrington EK. Testing for nominal dysphasia. J Neurol Neurosurg Psychiatry. 1980;43:781–8. doi: 10.1136/jnnp.43.9.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Warrington EK. The Graded Naming Test: A Restandardisation. Neuropsychol Rehabil. 1997;7:143–6. doi: 10.1080/713755528. [DOI] [Google Scholar]
- [26].Tombaugh TN, Kozak J, Rees L. Normative Data Stratified by Age and Education for Two Measures of Verbal Fluency: FAS and Animal Naming. Arch Clin Neuropsychol. 1999;14:167–77. doi: 10.1016/S0887-6177(97)00095-4. [DOI] [PubMed] [Google Scholar]
- [27].Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ. Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Trail-Making Test, the Stroop Test, and MAE Controlled Oral Word Association Test. Clin Neuropsychol. 2005;19:329–77. doi: 10.1080/13854040590945210. [DOI] [PubMed] [Google Scholar]
- [28].Kongs S, Thompson L, Iverson G, Heaton R. Wisconsin Card Sorting Test - 64 Card Version (WCST-64) professional manual. Odessa, TX: Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- [29].Manes F, Hodges JR, Graham KS, Zeman A. Focal autobiographical amnesia in association with transient epileptic amnesia. Brain. 2001;124:499–509. doi: 10.1093/brain/124.3.499. [DOI] [PubMed] [Google Scholar]
- [30].National Life Tables, United Kingdom: 2012–2014: Trends for the UK and constituent countries in the average number of years people will live beyond their current age measured by “period life expectancy”, analysed by age and sex. Office for National Statistics; 2015. [Google Scholar]
- [31].Mosbah A, Tramoni E, Guedj E, Aubert S, Daquin G, Ceccaldi M, et al. Clinical, neuropsychological, and metabolic characteristics of transient epileptic amnesia syndrome. Epilepsia. 2014;55:699–706. doi: 10.1111/epi.12565. [DOI] [PubMed] [Google Scholar]
- [32].Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. The Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Weintraub S, Wicklund AH, Salmon DP. The Neuropsychological Profile of Alzheimer Disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kapur N. Transient epileptic amnesia--a clinical update and a reformulation. J Neurol Neurosurg Psychiatry. 1993;56:1184–90. doi: 10.1136/jnnp.56.11.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kapur N, Young A, Bateman D, Kennedy P. Focal Retrograde Amnesia: A Long Term Clinical and Neuropsychological Follow-Up. Cortex. 1989;25:387–402. doi: 10.1016/S0010-9452(89)80053-X. [DOI] [PubMed] [Google Scholar]
- [36].Dewar M, Hoefeijzers S, Zeman A, Butler C, Della Sala S. Impaired picture recognition in transient epileptic amnesia. Epilepsy Behav. 2015;42:107–16. doi: 10.1016/j.yebeh.2014.10.032. [DOI] [PubMed] [Google Scholar]
- [37].Butler CR, Bhaduri A, Acosta-Cabronero J, Nestor PJ, Kapur N, Graham KS, et al. Transient epileptic amnesia: regional brain atrophy and its relationship to memory deficits. Brain. 2009;132:357–68. doi: 10.1093/brain/awn336. [DOI] [PubMed] [Google Scholar]
- [38].Milton F, Butler CR, Benattayallah A, Zeman AZJ. The neural basis of autobiographical memory deficits in transient epileptic amnesia. Neuropsychologia. 2012;50:3528–41. doi: 10.1016/j.neuropsychologia.2012.09.027. [DOI] [PubMed] [Google Scholar]
- [39].Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]