Abstract
The microenvironment within a solid tumor is heterogeneous with regions being both acidic and hypoxic. As a result of this, cancer cells upregulate genes that allow survival in such environments. Some of these genes are pH regulatory factors, including carbonic anhydrase IX (CA IX) and in some cases XII (CA XII). CA IX helps to maintain normal cytoplasmic pH (pHi) while simultaneously contributing to the extracellular pH (pHe). CA XII is also thought to be responsible for stabilizing pHe at physiological conditions. Extracellular acidification of the tumor microenvironment promotes local invasion and metastasis while decreasing the effectiveness of adjuvant therapies, thus contributing to poor cancer clinical outcomes. In this review, we describe the properties of CA IX and CA XII that substantiate their potential use as anticancer targets. We also discuss the current status of CA isoform-selective inhibitor development and patents of CA IX/XII targeted inhibitors that show potential for treating aggressive tumors. Some of the recently published patents discussed include sulfonamide-based small molecule inhibitors including derivatives of boron cluster compounds; metal complexes of poly(carboxyl)amine-containing ligands; nitroi-midazole-, ureidosulfonamide-, and coumarin-based compounds; as well as G250 and A610 monoclonal antibodies for cancer treatment.
Keywords: Bicarbonate transport, cancer therapeutics, carbonic anhydrase, carbonic anhydrase IX, carbonic anhydrase XII, CO2 transport, coumarins, drug development, ERα, estrogen response element, HIF-1α, hypoxia response element, immunotherapy, pH regulation, prognostic marker, RNAi technology, sulfonamides, therapeutic target, tumor microenvironment, zinc metalloenzymes
1. INTRODUCTION
Carbonic anhydrase (CA, EC 4.2.1.1) is a family of metalloenzymes (mainly zinc) that catalyze the reversible hydration/dehydration of CO2 to bicarbonate: CO2 + H2O ⇌ HCO−3 + H+ via a two-step ping-pong mechanism. Present in both prokaryotes and eukaryotes, these enzymes have evolved into six distinct classes: α, β, γ, δ, ζ, and η [1–21]. Of these classes, only α-CAs are present in humans, of which fifteen isoforms are encoded [1, 9, 22]. Among these isoforms, only twelve coordinate a zinc in the active site and are catalytically active (CAs I–IV, CAs VA–VB, CAs VI–VII, CA IX, and CAs XII–XIV). Isoforms VIII, X, XI are termed CA-related proteins (CARPS) as they lack at least one amino acid in the active site to coordinate the zinc and therefore are catalytically inactive. The active human CA isoforms differ in kinetic properties along with cell and tissue distribution. CAs play crucial roles in many physiological processes including CO2 and bicarbonate transport between metabolizing tissues and lungs, pH and CO2 homeostasis, bone resorption, and electrolyte secretion [1, 9, 11 – 13, 22]. Of the human isoforms, only the membrane-associated isoforms IX and XII have been implicated in tumorigenicity, cancer metastasis, and as clinical prognosticators [19 – 25]. In this chapter, the characteristics, expression patterns, and functions both in normal and neoplastic tissue of the cancer associated CA IX and CA XII will be discussed. We will also discuss the anti-cancer targeting strategies for both isoforms.
1.1. Active Site Structure and Catalysis of CA IX and CA XII
The amino acids that form the active site of α-CAs are highly conserved, and at the core of the active site is a zinc (Zn2+) ion that is essential for catalysis (Fig. (1)) [25 – 27]. The zinc is located at the base of a 15Å deep active site cleft and is tetrahedrally coordinated by three histidines (His94, His96, His119; CA II numbering used throughout this manuscript unless otherwise specified) and a water/hydroxyl [28 – 30]. The first step of catalysis in the hydration direction is the nucleophilic attack by the Zn-bound hydroxyl group on CO2 to produce HCO−3. The HCO−3 is then displaced by an active site water and released. In the second step of catalysis, the Zn-bound water is regenerated to a hydroxyl through the transfer of a proton mediated by a highly ordered solvent network and conserved histidine residue (His64) found in most isoforms, termed the proton shuttle residue [1, 25, 31].
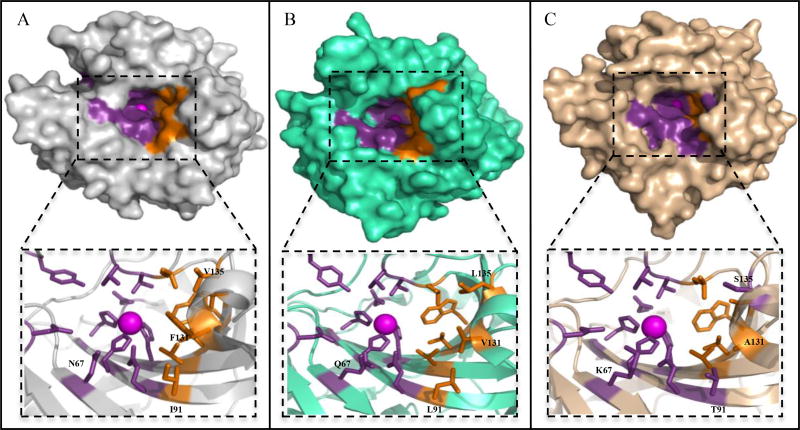
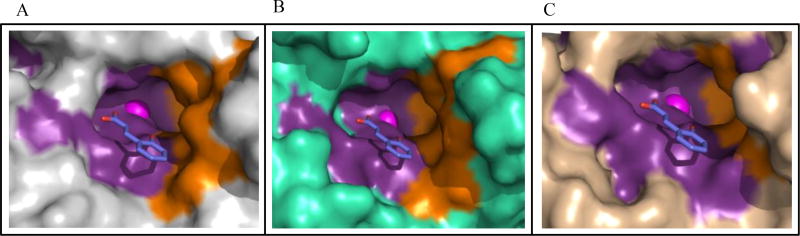
Fig. (1).
Structure of human α-CAs. Surface depiction of (A) CA II, PDB ID 3KS3 (grey), (B) CA IX, PDB ID 3IAI (green), C) CA XII, PDB ID IJCZ (wheat). Active site zinc (magenta sphere), hydrophilic (purple) and hydrophobic (orange) residues are shown. Residues within the active site that are different between these CAs are labeled (CA II numbering). CA II (N67, I91, F131, V135), CA IX (Q67, L91, V131, L135) and CA XII (K67, T91, A131, S135).
The proton transfer step is the rate-limiting step in this catalysis reaction. Isoforms CA II, CA IX and CA XII exhibit extremely fast catalytic activity (Table 1) [1, 22]. It is thought that this catalytic efficiency is in part due to the ability of His64 to shuttle protons with a low energy barrier between an inward (pointing towards the active site) and outward (pointing away from the active site) conformations [1, 25, 31]. The active sites of CAs also exhibit a divided active site cleft with a hydrophobic and a hydrophilic side (Fig. (1)). The hydrophobic side supports the pathway for substrate and product entry and exit, while the hydrophilic side provides the amino acids that define an ordered solvent network for proton transfer [32].
Table 1.
Catalytic Efficiency, Distribution and Associated Diseases of CA II, IX and XII [1].
| Isozyme | Kcat (s−1) |
KM (mM) |
Kcat /KM (M−1 s−1) |
Oligomeric State |
Tissue/Organ Localization |
Subcellular Localization/Associated disease |
|---|---|---|---|---|---|---|
| CA II | 1.4 × 106 | 9.3 | 1.5 × 108 | Monomer | RBC, kidney, brain, lung, testis, osteoclasts, eye, GI tract | Cytosol/ Glaucoma, epilepsy, edema, altitude sickness |
| CA IX | 3.8 × 105 | 6.9 | 5.5 × 107 | Dimer | GI mucosa, tumors | Transmembrane/ Cancer |
| CA XII | 4.2 × 105 | 12.0 | 3.5 × 107 | Dimer | Eye, tumors, reproductive epithelia, intestines, and kidney | Transmembrane/ Cancer, glaucoma |
Although there is high conservation of amino acid type and position lining the active sites of the CAs, X-ray crystallography studies have identified at least four amino acids within the active site (positions 67, 91, 131 and 135) that differ between CA II, CA IX and CA XII (Fig. (1)) [32]. These differences could be used to design CA isoform specific inhibitors to CA IX and XII and reduce other off target inhibition of CAs, particularly the ubiquitous CA II.
1.2. Biochemical and Biophysical Properties of CA IX and CA XII
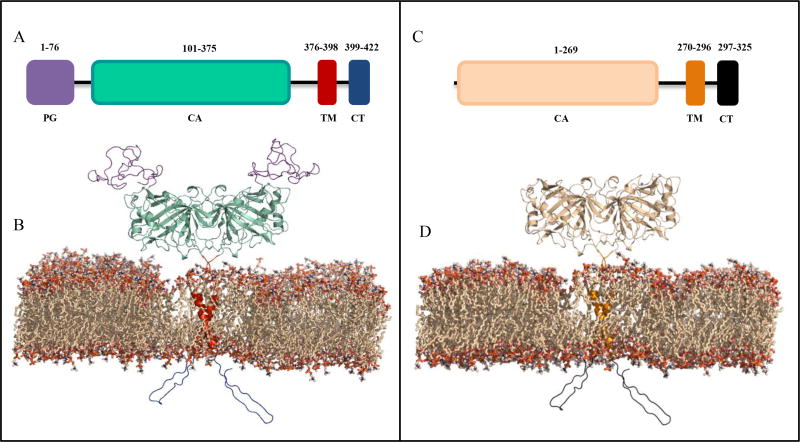
CA IX and CA XII are transmembrane glycoproteins with their N-termini facing the extracellular milieu (Table 1) (Fig. (2)) [33 – 36]. The CA9 and CA12 genes are mapped to chromosome 9p12–13 and 15q22, respectively [33 – 36]. CA IX is 414 amino acids (aa) in length, consisting of a signal peptide (37aa), a proteoglycan-like (PG) domain (59aa), a catalytic domain (257aa), a transmembrane domain (20aa), and a C-terminal intracellular domain (25aa) [35–38]. CA XII is shorter in length (354aa) and divided into only four distinct domains, as the PG domain is absent [39]. These include a 269aa catalytic domain, a 26aa transmembrane domain, and a 28aa C-terminal intracellular domain. For both isoforms, the signal peptide domain is cleaved in the mature protein [39]. Mass spectroscopic analysis indicates that CA IX has distinct N-linked and O-linked glycosylation sites at Asn309 and Thr78, respectively [40]. Similarly, CA XII is also a glycoprotein but containing two N-linked glycosylation sites at Asn52 and Asn136. Intermolecular disulfide bonds stabilize both isoforms: between Cys 119-Cys299 in CA IX and between Cys23-Cys203 in CA XII [39, 40]. If the sequences of the catalytic domains of CA IX and CA XII were aligned, these two Cys residue pairs would be at the same position. Three phosphorylation sites have also been confirmed on the intracellular domain of CA IX corresponding to Thr443, Ser448, and Tyr449 [2, 40, 41]. Although present in CA XII, studies have yet to show phosphorylation of the analogous Tyr residue. These post-translational modifications in CA IX are thought to play important roles in modulating catalytic activity and signaling [2, 40, 41]. The molecular weight of CA IX is predicted to be 49.5kDa, but it migrates as a doublet with molecular weights of 54 and 58kDa, both of which are glycosylated [34, 42]. The observed differences in molecular weight may result from differences or deletions in primary sequence. This is supported by data showing that two molecular weight species exist after removal of the N-linked glycans [42]. Under non-reducing conditions, both CA IX and CA XII are obs-erved in their homodimeric forms [33, 42]. Interestingly, soluble forms of both CA IX (consisting of only the PG and CA domains) and CA XII have been observed in culture medium of highly invasive tumor cell lines and the sera of cancer patients [43 – 45]. Although the purpose of the secreted form is not fully understood, recent studies suggest an involvement in cell signaling. Moreover, circulating forms of CA IX have shown great potential as biomarkers for therapeutic outcomes in cancer patients [46 – 49].
Fig. (2).
Schematic and structural models of CA IX* and XII. (A) CA IX domains include the proteoglycan-like domain (PG, purple), catalytic domain (CA, cyan), a transmembrane anchor (TM, red), and intracellular C-terminal domain (CT, blue). (C) CA XII domains include the catalytic domain (CA, wheat), the transmembrane domain (TM, gold), and intracellular C-terminal domain (CT, black). (B) Homodimeric membrane bound form of CA IX includes the extracellular PG and CA domains (purple and green), the TM anchor (red), and the CT domain (blue). (D) Homodimeric membrane bound form of CA XII includes the CA domain (wheat), the TM anchor (gold), and the CT domain (black). The TM domains are predicted to form helix-helix interactions within the lipid bilayer. The CT domains are predicted to exist as unstructured loops. * Structural model of CA IX provided by Brian Mahon [21].
1.3. Regulation of CA IX and CA XII Expression in Cancer
Expression of CA IX is modulated by hypoxia-inducible factors (HIFs) in response to decreased oxygen levels and increased cell density [50, 51]. HIF-1, typically associated with aggressive tumors, consists of a heterodimeric complex of α- and β-subunits (HIF-1α and HIF-1β) [1, 50 – 52]. Formation of the HIF-1 complex mediates a transcriptional response to hypoxic stress by interacting with target genes such as the CA9 gene that contain hypoxia response elements (HRE) in the promoter region [50]. HIF-1α expression is tightly regulated and activated by both hypoxia-dependent and hypoxia-independent oncogenic signaling. It can be down-regulated by hydroxylation-induced proteosomal degradation via von Hippel Lindau tumor suppressor protein (VHL), which mediates protein ubiquitination. HIF-1α may also be inactivated by the Akt-mammalian target of rapamycin (mTOR) signaling pathway [53 – 55].
Under normoxic conditions, although constitutively expressed, HIF-1α is rapidly degraded or inactivated by preventing interaction with its transcriptional co-activators [56]. Under hypoxic conditions however, hydroxylation and degradation is prevented, which in turn inhibits VHL binding to HIF-1α. Non-hydroxylated HIF-1α binds with transcriptional co-activators and is translocated to the nucleus for heterodimerization with HIF-1β, producing HIF-1. HIF-1 then binds to target genes that contain a HRE site, inducing transcription of glucose transporter 1 (GLUT1) and glucose transporter 3 (GLUT3), vascular epidermal growth factor (VEGF), insulin-like growth factor-2 (IGF2), ATP-binding cassette transporter B1 (ABCB1), sodium-hydrogen exchanger (NHE-1) and CA IX, among others [55 – 60]. Expression of these hypoxia-induced genes are involved in regulating iron, glucose and cell matrix metabolism, cell proliferation and viability, vascular remodeling and plasticity, cell adhesion, angiogenesis, pH regulation and other cellular processes [55, 57 – 60].
In contrast to CA IX, CA XII expression, though observed under hypoxic conditions, is not controlled by HIF-1α. Studies have shown that CA XII is robustly regulated in breast cancer cells by estrogen via estrogen receptor alpha (ERα) [61]. This regulation involves a distal estrogen-responsive enhancer region that communicates with the transcriptional start site of the CA12 gene via intrachromosomal looping [61]. The interaction between estrogen and ERα occurs through the estrogen response element (ERE) and results in the recruitment of RNA polymerase II and receptor co-activators. These cause changes in histone acetylation and enhanced transcription of the CA12 gene [61].
Estrogens stimulate normal cell growth and proliferation thus increasing the frequency of mutations and chromosomal aberrations. The effects of estrogenic steroid hormones are largely mediated through interaction and activation of ERα and estrogen receptor beta (ERβ). Estrogens are potent modulators of the pathophysiology, homeostasis, and development of many tissues such as skeletal, cardiovascular, adipose breast, male and female reproductive tracts. Cumulative exposure of estrogens (specifically ERα) causes an increased risk of cancer [61–64].
1.4. Functions and Expression of CA IX and CA XII in Normal Tissue
CA IX expression in normal tissues is restricted to stomach and epithelial tissues of the gut particularly the basolateral surfaces of the crypt enterocytes of the duodenum, jejunum and ileum of the small intestine [65, 66]. In the large intestine, CA IX expression is restricted to the base of the glands in the cecum and colon [65, 66]. However, abundant levels of CA IX are observed in developing embryonic tissues of the gut epithelium, skeleton muscle and lung, most of which are decreased in adult tissue [67]. The biological and molecular functions of CA IX include carbon dioxide and bicarbonate transport, acid base balance, and signal transduction.
In contrast, expression of CA XII has been observed in many organs and at different developmental stages with high expression reported for the pancreas, appendix, colon, rectum, kidney, prostate, intestine, and activated lymphocytes, moderate expression in oral mucosa, esophagus, urinary bladder, breast, vagina, cervix, uterine, endometrium and skin [68, 69]. Its biological functions range from facilitating the directed movement of bicarbonate in cells to maintenance of an internal steady state concentration of chloride ions within an organism or cell. Similar to CA IX, CA XII is also involved in chemical reactions and pathways involving small molecule metabolism.
1.5. Functions and Expression of CA IX and CA XII in Neoplastic Tissue
Although CA IX expression in normal tissues is limited, it has been observed in a many aggressive tumors including the brain, breast, bladder, cervix, colon, colorectal, head and neck, pancreas, kidney, lung, ovaries, stomach oral cavity, B and T-cell lymphomas [67, 69 – 72]. In advanced renal cell carcinomas (RCC) high CA IX expression seems to correlate to better response to IL-2 therapy [73]. However, no consensus has been reached thus far about its potential to predict clinical outcome in RCC [73 – 76]. Nevertheless, CA IX overexpression in most cancers is linked to poor prognosis, chemotherapeutic resistance, and poor clinical outcome [23]. This expression is attributable to the existence of hypoxic regions within solid tumors linked to defective tumor vasculature, which results in insufficient blood supply to certain areas. In response to this, tumor cells make oncogenic alternations in metabolism and express genes that are essential for survival [23, 24, 55 – 60]. These clonogenic transformations result in expansion of cells with more aggressive phenotypes and increased metastatic potential.
CA IX is upregulated in these tumors in response to hypoxia and, because its enzymatic activity is to facilitate the conversation of CO2 to HCO−3 and H+ in the presence of H2O, it functions to maintain physiological pH inside the cell (pHi) while simultaneously regulating the extracellular pH (pHe). Moreover, membrane ghost studies show that the hydration activity (conversion of CO2 to bicarbonate and a proton) increases as pH increases. However, at pH values lower than ~6.8, the dehydration (conversion of bicarbonate to CO2 and water) reaction is favored [52]. This supports the hypothesis that CA IX balances the pHe of the bicarbonate system within the range (6.5 to 6.8) that favors cancer cell survival and aggression while causing apoptosis of surrounding normal cells. While it is well known that extracellular acidification promotes tumor invasion and metastasis [23, 24], the reasons for this are not completely understood.
CA XII is optimally active at higher pH values (pKa of ~7.1) than CA IX (pKa of ~6.3), so it is therefore considered to be responsible for stabilizing pHe under more physiological conditions, which in turn contributes to more favorable outcomes at least in breast cancer [77]. At pH values lower than ~6.8, CA XII is shown to be inactive (unpublished data). Because its expression is tightly regulated by estrogen, CA XII is strongly associated with luminal cancers of the breast (Estrogen receptor (ER)/Progesterone receptor (PR)-positive) and better clinical outcomes than patients with Human epidermal growth factor receptor II (Her2) and the triple negative phenotype (ER/PR)-negative [61, 78, 79]. The latter represents a more aggressive form of breast cancer that is strongly associated with CA IX expression. Cancers other than breast cancer show variability in CA XII as a prognostic marker: good prognosis in lung cancer, equivocal prognosis in brain, and poor prognosis in colorectal cancer [80 – 82]. High expression of CA XII has been observed in glioblastomas, astrocytomas, lung carcinomas, urinary bladder transitional cell carcinomas ductal breast carcinoma cells and T-cell lymphomas, with moderate to low expression observed in cells obtained from ovarian cyst adenocarcinoma and metastatic breast adenocarcinoma [69, 72, 80, 82]. Recent studies have shown an upregulation of CA XII on the surface of chemoresistant cells, suggesting its potential as a therapeutic target to overcome chemoresistance in cancer cells [83].
2. CA IX AND CA XII AS ANTI-CANCER TARGETS FOR THERAPY
CA IX and CA XII both play important roles in tumor progression, acidification and metastasis, suggesting that both make important therapeutic cancer targets [23, 24, 50]. There are several factors that make them attractive therapeutic targets. Their catalytic domains face the extracellular milieu (Fig. (2)) thus eliminating many drug delivery complications often exhibited by cytosolic targets.
In addition, constitutive expression of CA IX is limited which reduces the potential for off target effects on normal tissue [65, 66]. Hence, targeting CA IX/CA XII alone, or in combination and with other therapies, may prove beneficial in fighting specific cancers with high CA IX/CA XII expression. Importantly, knockdown of CA IX in xenograft models resulted in decreased primary tumor growth and metastatic potential as well as an increase in tumor susceptibility to treatment [84]. As a result, interest in developing inhibitors against CA IX and CA XII are being explored by many research and pharmaceutical groups.
2.1. CA IX and CA XII Targeted Inhibitor Drug Design
The α-CAs are similar in both amino acid sequence and structure. These similarities lead to difficulties in the design of CA inhibitors (CAIs) that are isoform selective, as the majority of the active site residues are both identical in amino acid type and spatial location [32]. Hence, the lack of differences leads to inhibitors targeting multiple isoforms. In designing selective inhibitors against CA IX or CA XII, binding to CAII may also occur leading to decreased inhibitor effectiveness and unwanted side effects. Therefore, the design of highly selective CAIs for CA IX and CA XII requires the exploitation of active site differences, interaction with residues near the active site, and/or reduced cell permeability [32].
Since both isoforms expose their catalytic sides to the extracellular environment, designing targeted membrane impermeable inhibitors may also prove useful. Both the catalytic and inhibitory mechanisms of the α-CAs have been studied extensively and have aided in designing potent CA inhibitors that have a wide range of clinical applications. A typical CAI has three components; a zinc-binding group (ZBG), a linker region (heterocyclic or benzene ring), and a variable “tail” region [27, 32]. The zinc-binding CAIs can be divided into two groups based on their coordination to the metal: those that form tetrahedral adducts and interact directly with the zinc (e.g., sulfonamides and bisulfites), and those that form trigonal-bipyramidal adducts through binding to the zinc-bound hydroxyl/water (e.g., cyanates and formates) [19, 20, 27, 31, 85 – 88]. Both types of zinc binding CAIs effectively prevent catalysis [19, 20, 27, 89].
2.1.1. Classical CAIs
There are two types of “classical” CA inhibitors. These differ in their coordination of the catalytic zinc. The sulfonamides and their isosters (sulfamides/sulfamates), which are the most studied of the CAIs, interact directly with the zinc by forming tetrahedral adducts [19, 27, 85, 88]. The second type of classical CAIs include the metal chelating anions that bind to the zinc in three different geometries: a trigonal-bipyramidal, a distorted tetrahedral, or a tetrahedral geometry [19, 27, 88].
2.1.1.1. Sulfonamide Based Inhibitors
The “classical” inhibitors include drugs such as: acetazolamide, brinzolamide, dorzolamide, metazolamide, and zonisamide [1, 85, 87]. Sulfonamides and their isosters bind in deprotonated forms to the zinc, displacing the zinc-bound hydroxyl/water while maintaining a tetrahedral coordination about the active site [85, 86]. These inhibitors have been used to treat a range of diseases from glaucoma to epilepsy. More recently, the focus has been on applying these inhibitors in cancer treatment [20, 90 – 95]. However, few show selective inhibition for a single isoform including CA IX/CA XII (Fig. (3)) [1, 9, 27].
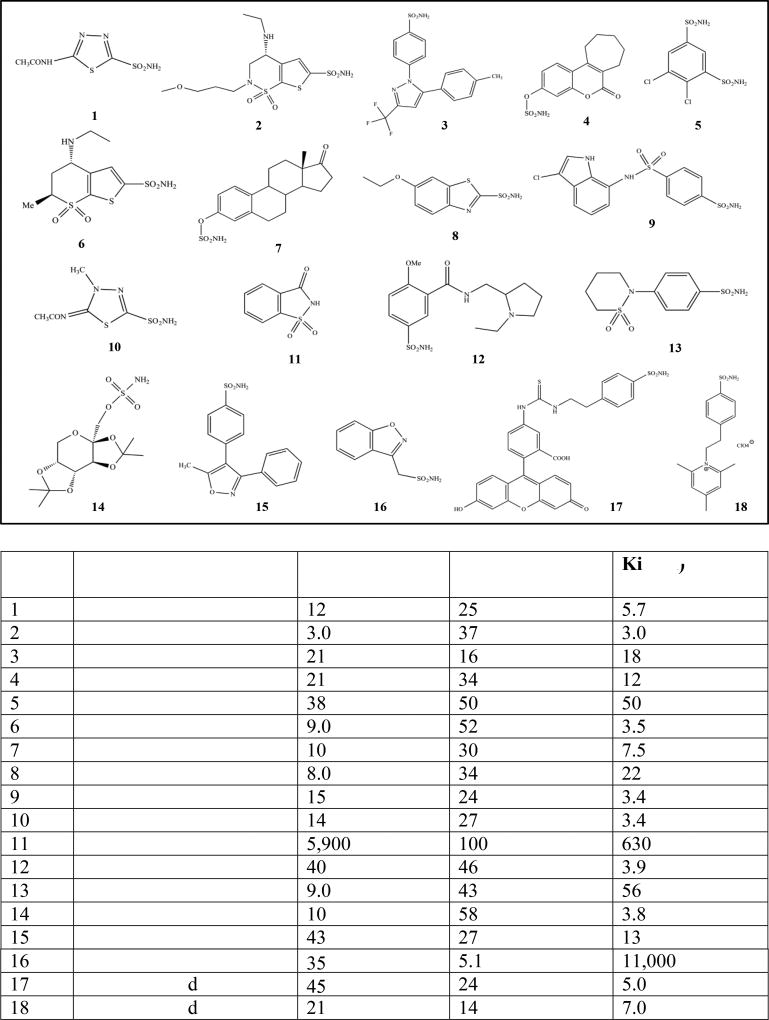
Fig. (3).
Structures of carbonic anhydrase inhibitors 1–18 and inhibition data. Structure of inhibitors 1–18 (top). Inhibition data with selected sulfonamide and sulfamate-based inhibitors against isozymes IX and XII (bottom). Values given to 2 significant digits [1].
These inhibitors are now considered as first/second generation CAIs, which form compact structures to interact deep within the CA active site cavity (Fig. (4)). But even within these compounds some, such as celecoxib, saccharin, and valdecoxib, show slightly higher affinity for CA IX/ CA XII over off target CA II (Fig. (3)) [1, 9, 27]. Hence, to design more CA isoform specific inhibitors that target CA IX and CA XII, the minor differences in the active site residues have to be taken into consideration. For example, compound 18, which belongs to a class of positively charged membrane impermeant compounds, is a potent inhibitor of both CA IX and CA XII and is under investigation as a target for CA IX (Fig. (3)) [94, 96].
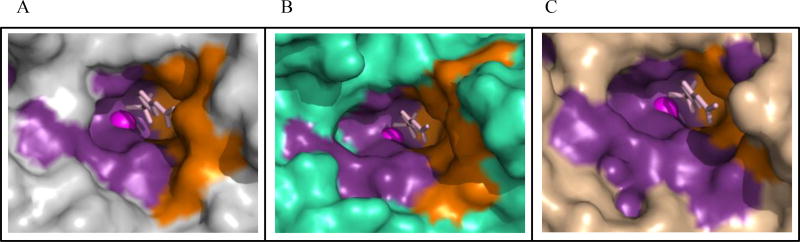
Fig. (4).
Structure of human α-CAs bound to acetazolamide, a “classical” sulfonamide inhibitor. Surface depiction of acetazolamide bound to (A) CA II, PDB ID 3HS4 (grey), (B) CA IX, PDB ID 3IAI (green), and (C) CA XII, PDB ID IJCZ (wheat). Active site zinc (magenta sphere), hydrophilic (purple) and hydrophobic (orange) residues are shown.
2.1.1.2. Metal-chelating Anions
The metal-chelating anions, unlike the sulfonamides and their isosters, may bind to the zinc in one of three possible geometries: a trigonal-bipyramidal geometry, a tetrahedral geometry, or in a distorted tetrahedral geometry [87]. The ability to bind in multi-faceted geometries is due primarily to the structural features of the ligand. These inorganic anions are weaker CA inhibitors than sulfonamides [30, 86, 87, 97].
2.1.2. Non-Classical CAIs
The “non-classical” CAIs include both small molecule inhibitors and several biologics such as monoclonal antibodies and RNAi based molecules [21, 72, 98 – 107]. Some “non-classical” small molecule CA inhibitors do not bind directly to the zinc and they include thiocarbonates, phenols, coumarins, and polyamines (Fig. (5)) [1, 27]. The carbohydrate based sulfonamide derivates and steroid sulfatases [98 – 104] bind the zinc but have additional moieties (longer variable tail regions) that allow for greater isoform specificity [105, 106].
Fig. (5).
Structure of human a-CAs bound to a “non-classical” sulfonamide inhibitor. Surface depiction of a substituted coumarin bound to the active site of (A) CA II, PDB ID 5BNL (grey), (B) CA IX, PDB ID 3IAI (green), (C) CA XII, PDB ID IJCZ (wheat). Active site zinc (magenta sphere), hydrophilic (purple) and hydrophobic (orange) residues are shown.
2.1.2.1. Carbohydrate-Based Sulfonamides
The carbohydrate-based sulfonamide derivatives and steroid sulfatases are derived from classic sulfonamide CAIs and may exhibit antitumor & antimitotic activities [105 – 107]. These compounds have primary sulfonamides attached to mono- or disaccharide moieties instead of an aromatic ring [102, 108, 109]. The inhibitors exhibit high affinity for CA IX/CA XII and their sugar moieties cause reduced membrane permeability and thus greater selectivity for targeting the extracellular facing of both tumor associated isoforms [100, 102, 103, 106, 107]. The observed lack of membrane permeability exerted by these CAIs may be attributed to their high molecular weights, and bulky sugar tails that are not easily transported. Furthermore, the presence of sugar moieties maintains water solubility and good bioavailability [102, 108, 109].
2.1.2.2. Immunotherapy
In addition to small molecule inhibitor development, there are several biologics used for CA inhibition. Mouse monoclonal antibodies such as VII/20, M75, G250 (that recognize the PG domain of CA IX) and more recently 6A10 (that recognizes CA XII) show great potential for anti-cancer therapy [110 – 113]. These antibodies are highly selective for binding to, and internalization by, CA IX/CA XII-expressing cancer cells. The use of antibody-based targeting systems to deliver cytotoxic payloads or radiolabeled antibodies to tumor cells provides an alternative approach to targeted therapy [21, 114 – 116]. Ligand conjugates consisting of either bivalent small molecules or a CA IX-specific, ligand-dye conjugate linked to a cytotoxic drug (maytansinoid DM1) were shown to specifically target tumor tissue and deliver the cytotoxic agent [114].
Recently, a Phase II clinical trial was initiated for use of Lutetium 177- labeled anti CA IX monoclonal antibody girentuximab (cG250) in patients with advanced clear cell renal cell carcinoma (ccRCC) [115, 116]. For the aforementioned tumor type, the radiolabeled chimeric monoclonal antibody cG250 was extensively investigated both for radioimmunodetection and radioimmuno therapy (RIT). Results from these studies show significant antitumor effects and less toxicity to normal cells [115, 116]. These effects were observed because of the specific targeting of cG250 to CA IX expressing lesions, which is a potent antibody-based carrier for tumor-targeted delivery of β-emitting radionuclides [115, 116].
Phage display technology has also been utilized for the development of novel peptide based CA IX-specific antibodies with the ability to potently inhibit CA activity and induce endocytosis [117]. These antibodies have the advantage of specifically disrupting the catalytic activity of the enzyme and induce surface CA IX internalization into endosomes. These novel peptide based antibodies thus target CA IXs tumorigenic functions, including pH regulation [112, 118]. New monoclonal antibodies against human recombinant CA XII for immunodetection and immunotherapy are also currently being developed and characterized [119, 120].
Recently, clinical trial data have been reported for use of chimeric antibody (CAR) engineered T cells against CA IX for treatment of metastatic ccRCC [121]. Results from these studies show that CA IX targeting CAR T cells exert antigen-specific effects in vivo and induce liver toxicities at low doses of T-cells [121]. This illustrates the potency of receptor modified T cells. The observed antigen-directed “on target” toxicity was successfully prevented by pretreatment with G250, consequently blocking antigen sites in off target organs and allowing higher T cell doses [121]. However, the effects of this therapeutic strategy on long-term patient survival are yet to be reported.
2.1.2.3. RNAi Technology
Several studies have utilized RNAi technology to modulate CA IX expression in several cancers [84, 122, 123]. The mechanism of RNAi uses the endogenous miRNA pathway that regulates protein expression by gene silencing through post-transcriptional mechanisms [123 – 125]. Ultimately, this activates translational repression and induces mRNA cleavage or degradation [123 – 125]. Typically, efficient siRNAs are designed with exact base complementation to the target mRNA and induce complete degradation. In contrast, shRNAs are typically exported to the cytosol and processed to form viable siRNA [124]. Successful knockdown of CA IX by siRNA or shRNA mediation has been shown to reduce growth, proliferation, and enhance the effects of commonly used inhibitors in several types of cancers [21, 40, 70, 84, 126 – 128].
Specifically, knockdown of CA IX using shRNA strategies in human breast cancer cells, when implanted into mouse mammary tissue, significantly inhibited primary tumor growth and metastasis formation [84]. A similar effect was noted in the 4T1 breast tumor model. The 4T1 mammary carcinoma is a “transplantable” mouse tumor cell line that is both tumorigenic and metastatic. When compared to the 4T1 shRNA control (non-silencing RNAi) group, the 4T1 shCA IX (CA IX-silencing RNAi) group showed 100% survival post tumor inoculation [84]. Furthermore, RNAi delivery systems designed to successfully deliver siRNA or shRNA to target cells, such as viral vectors or liposomes, promote siRNA longevity and evade inciting an immune response [21, 124]. As such, RNAi technology shows promise as a novel cancer therapeutic option or can possibly be used as an alternative to developing isoform specific CAIs in certain selected cancers. However, more research is needed to validate the use of RNAi technology as a viable option for therapy.
3. RECENT PATENTS FOR USE IN CANCER THERAPY
This section discusses recent patents granted during 2012–2016 on CA IX and CA XII for the use of small molecule inhibitors and immunotherapy for cancer treatment. It also includes some of the CA IX/CA XII inhibitors that are currently in clinical trials for treatment of the several cancers.
3.1. Small Molecule Inhibitors
3.1.1. Derivatives of Boron Cluster Compounds (Patent: WO2013060307)
The invention described in this patent, published by Prague et al., describes derivatives of boron cluster compounds and their pharmacologically acceptable salts and solvents and their specific inhibitory effects on CA IX [129]. The patent includes methods of synthesis, use, diagnosis and/ or therapy for CA IX expressing cancers [129 – 132]. The selective inhibition achieved by these novel compounds is because of a parallel presence of two substituted structural factors [131, 132]; the boron containing clusters (as a hydrophobic pharmacophore) and a group capable of binding to the active site of the enzyme. In the case of CA IX, the substituted group is a sulfonamide [129].
X-ray crystallography studies of one of the boron cluster derivatives, in complex with CA II (resolution: 1.60 Å), showed that the compounds bind tightly to the active site of CA II [129]. However, the inhibition efficacy of the compounds in vitro, assayed using conventional colorimetric methods, showed greater specificity for CA IX than CA II. The cytotoxicity of the derivatives was also tested on cancer cells versus normal cells. The results showed consistently less cytotoxicity to normal cells in comparison to tumor cells [129]. In order to demonstrate pharmaceutical exploitation of the compounds in this invention, the maximum tolerated dose and limiting toxicities of two promising CA IX inhibitors were tested in mice. The dose limiting toxicities observed included drowsiness, apathy, and local irritation [129]. However, the maximal tolerated dose observed and achieved supports further pharmacological use of the CA IX inhibitors for cancer therapy.
3.1.2. Metal Complexes of Poly(Carboxyl) Amine Containing Ligands (Patent: WO2013103813)
As described in this patent filed by Molecular Insight Pharmaceuticals, the invention is directed to the synthesis and use of metal complexes of poly(carboxyl)-amine containing compounds [133, 134]. The metal complexes include radionuclide and non-radionuclide metal complexes such as Pt, Zn, 64Cu, 186Re, 188Re and 99mTc [135]. These compounds are specific inhibitors of CA IX and may find use as candidate agents for imaging tumors and chemotherapy [133]. Biological studies for the compounds described in this invention were performed in cell culture to determine binding affinity and in human xenograft bearing mice for analysis of tissue biodistribution [133].
The in vitro binding affinity studies showed that radionuclide complexes of these compounds are potent inhibitors of CA IX with IC50 values in the nanomolar range. In contrast, the free (uncomplexed) compounds exhibit 2-250-fold higher IC50 values than the corresponding complex [133]. Tissue distribution studies of the CA IX inhibitors in vivo showed that the compounds were detected in blood, heart, liver, lungs, spleen, small and large intestine, stomach, kidneys and skeletal muscles [133]. Especially high concentrations of the inhibitor were detected in the kidney and liver of mice. Results from these studies also showed rapid clearance of the CA IX inhibitors from non-tumor tissue [133]. According to the inventors and results discussed, these compounds are suitable candidate agents for imaging and targeting tumor tissues with high expression of CA IX.
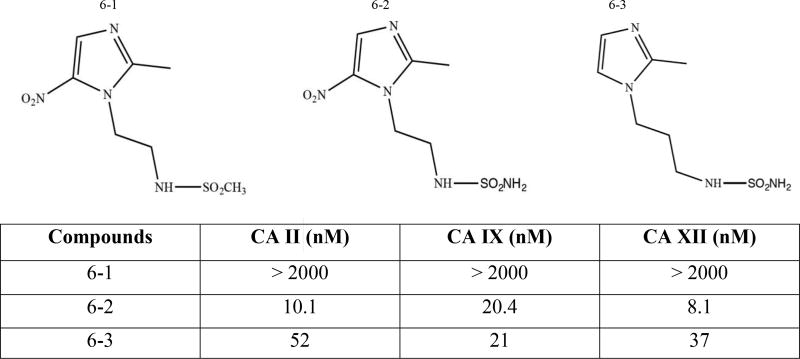
3.1.3. Nitroimidazole Based Compounds (Patent: WO2014030142)
Lambin et al. recently patented nitroimidazole-containing compounds for use in radiotherapy (Fig. (6)) [96, 136, 137]. The basic principle of irradiation is to damage the cancer cells to such an extent that they will die. The irradiated cells form free radicals and immediately damage the DNA or react with oxygen, creating reactive oxygen species that damage the cell. Under hypoxic conditions when there is little to no oxygen present, less reactive oxygen species are formed and the irradiation is not as effective. Studies have shown that extracellular acidosis also makes tumors less sensitive to irradiation treatment [138]. Nitroimidazole scaffolds are well known radiosensitizers and this patent describes the development of dual drugs that can selectively inhibit CA IX and enhance hypoxic tumor sensitization toward cancer therapy [137, 139]. These compounds include a panel of sulfonamide/sulfamide/sulfamate derivates containing 2- or 5-nitroimidazoles moieties [140]. Note that compound 6-1 (Fig. (6)) has no inhibitory effect on CA activity because it is not a sulfonamide derivative (NH2 is substituted for CH3). Crystallographic studies show binding of these novel nitroimidazole compounds in the active site cavity of CAs. These structural studies were validated in vitro in HT29 and HeLa cell lines, where selective CA IX inhibition reduced hypoxia induced tumor acidosis [140].
Fig. (6).
General structural formulae of patented nitroimidazole compounds.
In vivo studies using nitroimidazole and sulfonamide duel targeting drugs show reduced extracellular acidification, reduced tumor growth at non-toxic doses, and increased tumor sensitization to irradiation in a CA IX dependent manner [139]. These compounds, as described in the patent, are potent inhibitors targeted against CA IX that increase the sensitivity of tumor cells to radiation suggesting a novel approach to drug design [137]. Thus, specifically targeting CA IX in hypoxic tumors using nitroimidazole derivatives can be developed as a new strategy for therapeutic intervention in cancer.
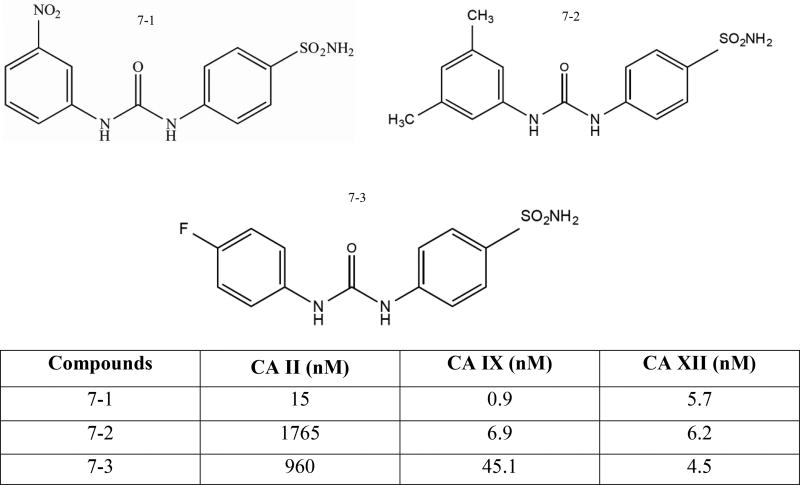
3.1.4. Ureido-Sulfonamide Based Compounds (Patent: WO2012021963)
Metasignal Therapeutics Inc. patented therapeutic ureido-sulfonamide compounds with the formula R-Q-Ar--SO2NH2 (Fig. (3): Compound 18) wherein R is an aryl, hetaryl, alkyl or cycloalkyl group, Q is the group -L(CH2)n-, wherein n = 0, 1 or 2 and L is the group - NHCXNH-, -NHC(S)SNH-, -NHCONHCSNH- or -SO2NH-, X is O or S and Ar is a C6-C10 aromatic or a heteroaromatic group that contains at least one heteroatom of oxygen, nitrogen or sulfur [141, 142]. Two compounds of this series: 4-{[(3nitrophenyl) carbamoyl] amino} benzene sulfonamide (Fig. (7-1)) and 4-{[(4-fluorophenyl) carbamoyl] amino} benzene sulfonamide (Fig. (7-2)) selectively inhibit CA IX and CA XII, and have been shown to effectively inhibit hypoxic tumor growth, suppress metastases, and impair and deplete cancer stem cells in mammals [84, 126]. In vitro studies showed a significant decrease in invasion and/or induced cell death when treated with an ureido-sulfonamide derivative. Furthermore, inhibition of CA IX activity impaired maintenance and depleted the cancer stem cell population in human breast cancer [126]. A reduction in tumor growth and size was also observed with studies performed in vivo [84, 143]. One of the ureidosulfonamide derivatives (Fig. (7-3)) under the name SLC-0111 is currently in Phase I clinical trials initiated by SignalChem
Fig. (7).
Structure of ureido-sulfonamide compounds.
Lifesciences Corp. (SLC) in Vancouver, British Columbia (2014- NCT02 215850). The first arm of this study evaluates the safety of SLC-0111 in dose escalation studies in patients with advanced solid tumors [139]. The second arm of this clinical trial will focus on treating only patients whose tumors express CA IX. SLC is also developing a backup small molecule inhibitor of CA IX (SLC-021) and in collaboration with the National Research Council of Canada, a monoclonal antibody (SLC-0131) targeting CA IX. Both programs are in the preclinical development stage.
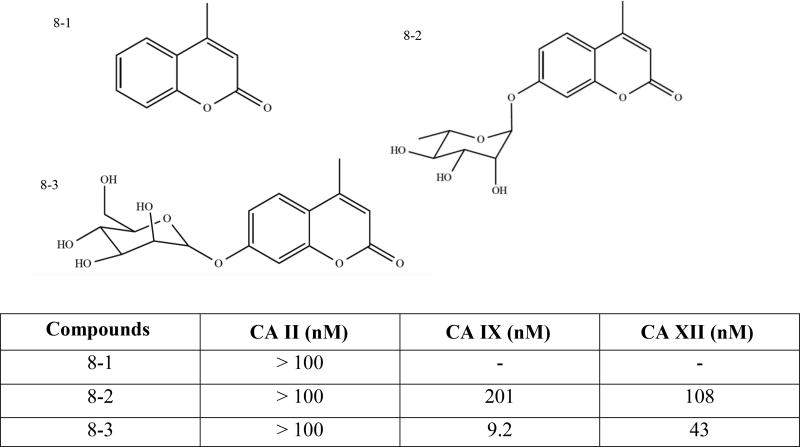
3.1.5. Coumarin Based Compounds (Patent: WO2012070024)
Metasignal Therapeutics Inc. also recently patented novel coumarins, thiocoumarins, and glycosylated coumarins, and their use as inhibitors of carbonic anhydrase IX and XII for treatment of hypoxic and metastatic cancer (Fig. (8)) [144]. Unsubstituted coumarins/thiocoumarins are nonselective to most CA isoforms and bind in hydrolyzed form at the entrance of CA active site with no interaction with the zinc ion (Fig. (8-1)) [98]. However, substituted coumarins are selective for membrane-bound CA isoforms due to the addition of bulky tail moieties. Coumarins are considered mechanism-based inhibitors, which undergo enzymatic hydrolysis of the lactone moiety to give the corresponding 2-hydroxycinnamic acid. The latter is responsible for CA IX inhibition in the high nanomolar range by means of the occlusion of the entrance to the enzyme site [27, 108, 145].
Fig. (8).
Structure of coumarin based compounds. (A) General structural formula of coumarin/thiocoumarins. (B) and (C) Glycosyl-substituted derivatives.
In this patent, the inventors claimed 40 novel (thio) coumarin derivatives substituted at positions C4 (hydrogen, cyano, methyl, O-allyl), C6 (hydrogen, hydroxy, O-allyl, O- glycosyl, etc.) and C7 (hydrogen, hydroxy, O-allyl, O-glycosyl, linker + heterocycles, such as 1,2,3 trizole, coumarin, pyridinium, aryl, imidazole or branched chains) of the main ring [144]. Among the different strategies developed in order to design selective hCA IX inhibitors, the ‘sugar approach’ represents one of the most promising tools and was used in this patent to decorate some of the coumarin-based inhibitors. These substituted derivatives contain bulky glycoside moieties, which decrease membrane permeability and exert multiple inhibitory effects. Addition of a sugar moiety also improves the biophysical & biochemical properties such as water solubility and bioavailability of these compounds [107, 144, 146].
The glycosylated coumarins strongly attenuate primary tumor growth and the formation of metastasis in 4T1 mouse model expressing high levels of CA IX [147]. Conversely, no such effects were observed in a breast cancer cell with limited CA IX expression. These selective inhibitors were also effective in limiting colonization to the lungs. One of the glycosyl coumarin derivatives was also shown to inhibit the action of cancer stem cells within breast tumors [84, 126]. The use of carbohydrate scaffolds in the design of CA inhibitors has desirable physicochemical properties for the treatment of metastatic cancer [145].
3.2. Antibody
3.2.1. Monoclonal Anti CA IX G250 Antibody (Patent: WO2007065027A2)
Patents were filed and clinical trials conducted for the use of antibodies that recognize and target CA IX [148]. These antibodies (mAbG250 & derivatives) alone or in combination with IL-2 or IFN-alpha, have been studied extensively in clinical settings for use in cancer therapy [149 – 151]. The G-250 antibody was patented for treatment of G250/CA IX antigen-expressing tumors, in particular renal cell carcinoma, using G250-antigen-specific antibodies as an adjuvant treatment modality to high-risk patients diagnosed with non-metastatic disease [148].
Since then, G250 antibodies and a chimeric version of G250 (cG250) have been used in combination with cytokines, cytotoxins and radionuclides to elicit antibody dependent cytotoxicity, as well as receptor-mediated internalization allowing for targeted delivery of various therapeutic payloads [115, 116]. This approach thus increases therapeutic efficacy by mediating tumor cell destruction and decreased cytotoxicity of surrounding normal tissue [115, 116]. Phase I and II clinical trials demonstrate that the cG250 antibody (RENCAREX) is safe, well tolerated, and able to positively impact disease burden alone and together with cytokines [152]. These studies recently completed Phase III clinical trials as adjuvant therapy aimed at reducing recurrence in surgically treated renal cell carcinoma (RCC) patients who have a high risk of relapse [139].
However, results from the Phase III trials showed that the antibody did not meet its primary end point. The analysis showed no improvement in median disease free-survival following RENCAREX treatment compared with placebo. However, a biomarker analysis showed that response to treatment was directly correlated to CA IX expression. The patient population with high CA IX levels treated with cG250 showed a clinically and statistically significant improvement when compared to placebo and patients with low CA IX score. Therefore, an immunotherapy for anti-CA IX ccRCC in the adjuvant setting may still be an option.
A Phase I trial was recently completed and a Phase II trial initiated for the treatment of metastatic ccRCC with Leutetium-177 (177Lu)-cG250/Girentuximab [115, 116]. The Phase I trials were designed to access the maximum tolerated dose, dositometry, pharmacokinetic and incidence of human anti-chimeric antibody formation [116]. Results from these dose escalation studies were very promising as (177Lu)-cG250 radioimmuno- therapy was generally well tolerated and resulted in disease stabilization in the majority of patients [116]. Because of these encouraging results, a Phase II trial was initiated in patients with advanced ccRCC [115]. Interim results of this ongoing radioimmunotherapy trial are also promising in terms of clinical response in patients with progressive metastatic ccRCC. The toxicity profile of (177Lu)-cG250 seems to be generally mild, apart from transient myelotoxicity [115]. Final analysis of the Phase II trials will shed more light on the therapeutic efficacy and safety of this treatment modality in patients with metastatic ccRCC.
3.2.2. GM-CSF (Patent: US20130230483)
More recently, a chimeric molecule comprised of a granulation macrophage colony-stimulating factor (GM-CSF) was patented for the treatment of renal cell carcinomas, cervical and bladder cancers [153]. The GM-CSF is a monomeric glycoprotein that functions as a cytokine and is secreted by fibroblasts, endothelial cells, T cells, mast cells, natural killer cells, and macrophages. It facilitates development of the immune system and promotes defense against infections [153]. The GM-CSF molecule attached to a CA IX variant (CA IXv) serves as a kidney cancer specific antigen and, as indicted in the patent application, provides a highly effective “vaccine” that raises an immune response directed against renal cell cancers [153 – 155]. Results from preclinical studies showed that this fusion protein induced specific cytotoxic T lymphocytes in vitro and inhibited tumor growth in severe compromised immunodeficiency disease (SCID) mice injected with human carcinoma cell line stably transfected with the fusion protein [155]. The GM-CSF and CA IX chimeric molecule can be used as a traditional vaccine or in adoptive immunotherapeutic applications.
3.2.3. Monoclonal Anti CA XII 6A10 Antibody (Patent: WO2011138279)
Although there are currently no CA XII-specific monoclonal antibodies in clinical trials, a recent patent was filed for use of 6A10 monoclonal antibody to specifically target CA XII [156]. This antibody was generated from stable monoclonal hybridoma producing IgG antibodies that bound to the surface of lung cancer cells [157]. The 6A10 monoclonal antibody showed > 20 times higher specificity for human CA XII than CA IX [157]. Results from these studies also showed that 6A10 inhibited exofacial CA activity in CA XII expressing cancer cells in nanomolar concentrations, without interacting with normal peripheral blood mononuclear cells [113, 157].
The mechanism of action for this antibody appears to be through inhibition of the catalytic activity of CA XII rather than on antigen binding per se. In vitro, 6A10 exerts growth limiting effects on tumor spheroids under hypoxic culture conditions where CA XII was active and where its catalytic activity was likely rate limiting [113, 157]. Results from studies in vivo corroborate the in vitro findings suggesting that membrane bound CAs are critical for cancer cell physiology. Specifically, the tumor xenograft model of breast cancer used in these studies displayed a significant delay in tumor outgrowth when 6A10 was administered [113, 157]. This clearly establishes the importance of targeting CA XII as a therapeutic approach in cancer treatment.
4. CURRENT & FUTURE DEVELOPMENTS
CA IX and CA XII are promising targets for systemic anticancer therapy [23, 24, 27]. Due to the limited presence of CA IX in normal tissue and its upregulation in many tumor types, it has gained significant attention since its discovery [67, 70, 82]. However, the physiological properties of CA XII and its overexpression in tumors have also incited research interest [69, 80, 82, 83]. CA IX expression and in some cases CA XII have been associated with poor prognosis and tumor progression to metastasis as a consequence of their roles in pH regulation [23, 24, 50, 82]. CA IX functions to balance pHi while simultaneously contributing to acidification of the tumor microenvironment. In contrast, CA XII is believed to operate more efficiently at physiologic pHe [23, 24, 77]. The actions of both enzymes are important for metabolic regulation, cell motility, chemoresistance and metastatic behavior in solid tumors [23 – 25].
Furthermore, inhibition of CA IX/CA XII activity by either small molecules or specific antibodies in several cancers has proven to slow overall tumor growth and metastatic properties [84]. Many classes of CA IX/CA XII inhibitors have been extensively studied in the preclinical setting and demonstrated promising results [1, 9, 27, 108]. Most specifically, patents have recently been published for use of sulfonamide based small molecule inhibitors to target CA IX/ CA XII for the treatment of cancer. Some of these include derivatives of boron cluster compounds, metal complexes of poly(carboxyl)amine containing ligands, nitroimidazole, ureidosulfonamide and coumarin based compounds [129, 133, 137, 141, 144]. These inhibitors have increased specificity and selectivity for tumor associated CA IX and CA XII over other possible off target CAs [129, 133, 137, 141, 144]. One possible reason for the increased isoform selectivity observed, as in the case of coumarin derivatives, is the addition of bulky tail moieties such as sugars that decrease membrane permeability and increase bioavailability [89, 144]. These patented compounds have shown great promise as anticancer agents by inhibiting tumor growth, metastasis, and enhancing tumor sensitization towards chemotherapy both in vitro and in vivo [129, 133, 137, 141, 144]. To this extent, one the ureidosulfonamide derivatives (SLC-0111) is currently in Phase I clinical trials for the treatment of solid tumors expressing CA IX [91, 139, 141].
Antibody-based targeting of CA IX and CA XII is another strategy that is currently being used for isoform specific and selective inhibition. These monoclonal antibodies include G250 (the first CA IX targeted inhibitor to reach clinical trials for the treatment of cancer), the anti CA XII 6A10 antibody, and GM-CSF a chimeric glycoprotein attached to a CA IX variant [148, 153, 155, 156]. This chimeric molecule serves as a kidney cancer-specific antigen and provides a highly effective “vaccine” that raises an immune response directed against renal cell cancers [153]. Although, the G250 antibody did not meet its primary end point goals in a Phase III clinical trial, it however showed a direct correlation between CA IX expression and response to treatment. Since then, the G250 antibody and a chimeric version cG250 have been used in combination with cytokines, cytotoxins and radionuclides to elicit antibody dependent cytotoxicity and receptor induced internalization allowing for targeted delivery of various therapeutic payloads [115, 116]. This approach increases therapeutic efficacy by mediating tumor cell destruction and decreased cytotoxicity of surrounding normal tissue as observed with (177Lu)cG250/Girentuximab which, is currently in Phase II clinical trials [115].
Although concerns still remain about off-target toxicities of CA inhibitors, mainly because of possible interactions with intracellular CAs and lack of isoform specificity [32], much progress has been made towards designing and developing CA IX/CA XII selective inhibitors. Most of these show great promise as anti-cancer therapeutics [32]. Despite such progress and promise, no CA IX or CA XII inhibitors have yet been approved for use in a clinical setting for the treatment of cancer. Moving forward, more attention should be focused on knocking down CA IX and CA XII expression using RNAi technology as a possible therapeutic approach for treating cancer [21]. The development of RNAi technology and RNAi delivery systems to completely eradicate CA IX/CA XII expressing in tumor cells may be more therapeutically beneficial than targeting activity alone. This will also prevent off target toxicities from inhibition of other CA isoforms. Alternatively, CAIs (alone or conjugated to cytotoxic agents) or siRNA can be used in combination with chemotherapy or radiation to combat chemoresistance, decrease tumor metastasis and improve patient outcome and survival. While preclinical evidence suggests that these concepts have great potential for success, more attention to clinical research is required to bring benefit to cancer patients.
Acknowledgments
The authors acknowledge support from the National Institutes of Health, Project CA165284 (SCF) and Minority Supplement CA165284-03S1 (MYM).
Footnotes
CONFLICT OF INTEREST
The authors confirm that they have no conflict of interest to declare for this publication.
References
- 1.Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7(2):168–81. doi: 10.1038/nrd2467. [ ] [DOI] [PubMed] [Google Scholar]
- 2.Ditte P, Dequiedt F, Svastova E, et al. Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res. 2011;71(24):7558–67. doi: 10.1158/0008-5472.CAN-11-2520. [ ] [DOI] [PubMed] [Google Scholar]
- 3.Brown BF, Quon A, Dyck JR, Casey JR. Carbonic anhydrase II promotes cardiomyocyte hypertrophy. Can J Physiol Pharmacol. 2012;90(12):1599–610. doi: 10.1139/y2012-142. [ ] [DOI] [PubMed] [Google Scholar]
- 4.Sly WS. The membrane carbonic anhydrases: From CO2 transport to tumor markers. EXS. 2000;90(90):95–104. doi: 10.1007/978-3-0348-8446-4_5. [DOI] [PubMed] [Google Scholar]
- 5.Aspatwar A, Tolvanen ME, Parkkila S. Phylogeny and expression of carbonic anhydrase-related proteins. BMC Mol Biol. 2010;11:25. doi: 10.1186/1471-2199-11-25. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chegwidden WR, Dodgson SJ, Spencer IM. The roles of carbonic anhydrase in metabolism, cell growth and cancer in animals. EXS. 2000;90(90):343–63. doi: 10.1007/978-3-0348-8446-4_16. [DOI] [PubMed] [Google Scholar]
- 7.Hilvo M, Innocenti A, Monti SM, De Simone G, Supuran CT, Parkkila S. Recent advances in research on the most novel carbonic anhydrases, CA XIII and XV. Curr Pharm Des. 2008;14(7):672–8. doi: 10.2174/138161208783877811. [ ] [DOI] [PubMed] [Google Scholar]
- 8.Henry RP, Swenson ER. The distribution and physiological significance of carbonic anhydrase in vertebrate gas exchange organs. Respir Physiol. 2000;121(1):1–12. doi: 10.1016/S0034-5687(00)00110-9. [ ] [DOI] [PubMed] [Google Scholar]
- 9.Supuran CT. Carbonic anhydrases-an overview. Curr Pharm Des. 2008;14(7):603–14. doi: 10.2174/138161208783877884. [ ] [DOI] [PubMed] [Google Scholar]
- 10.Biswas UK, Kumar A. Study on the changes of carbonic anhydrase activity in insulin resistance and the effect of methylglyoxal. J Pak Med Assoc. 2012;62(5):417–21. [PubMed] [Google Scholar]
- 11.Sterling D, Reithmeier RA, Casey JR. Carbonic anhydrase: In the driver’s seat for bicarbonate transport. JOP. 2001;2(4 (Suppl.)):165–70. [PubMed] [Google Scholar]
- 12.Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int. 2007;71(2):103–15. doi: 10.1038/sj.ki.5002020. [ ] [DOI] [PubMed] [Google Scholar]
- 13.Henry RP. Multiple roles of carbonic anhydrase in cellular transport and metabolism. Annu Rev Physiol. 1996;58:523–38. doi: 10.1146/annurev.ph.58.030196.002515. [ ] [DOI] [PubMed] [Google Scholar]
- 14.Chaput CD, Dangott LJ, Rahm MD, Hitt KD, Stewart DS, Wayne Sampson H. A proteomic study of protein variation between osteopenic and age-matched control bone tissue. Exp Biol Med (Maywood) 2012;237(5):491–8. doi: 10.1258/ebm.2012.011374. [ ] [DOI] [PubMed] [Google Scholar]
- 15.Frasseto F, Parisotto TM, Peres RC, Marques MR, Line SR, Nobre Dos Santos M. Relationship among salivary carbonic anhydrase VI activity and flow rate, biofilm pH and caries in primary dentition. Caries Res. 2012;46(3):194–200. doi: 10.1159/000337275. [ ] [DOI] [PubMed] [Google Scholar]
- 16.Gilmour KM. Perspectives on carbonic anhydrase. Comp Biochem Physiol A Mol Integr Physiol. 2010;157(3):193–7. doi: 10.1016/j.cbpa.2010.06.161. [ ] [DOI] [PubMed] [Google Scholar]
- 17.Kuo WH, Yang SF, Hsieh YS, Tsai CS, Hwang WL, Chu SC. Differential expression of carbonic anhydrase isoenzymes in various types of anemia. Clin Chim Acta. 2005;351(1–2):79–86. doi: 10.1016/j.cccn.2004.07.009. [ ] [DOI] [PubMed] [Google Scholar]
- 18.Del Prete S, Vullo D, Fisher GM, et al. Discovery of a new family of carbonic anhydrases in the malaria pathogen Plasmodium falciparum--the η-carbonic anhydrases. Bioorg Med Chem Lett. 2014;24(18):4389–96. doi: 10.1016/j.bmcl.2014.08.015. [ ] [DOI] [PubMed] [Google Scholar]
- 19.Supuran CT, Winum JY. Designing carbonic anhydrase inhibitors for the treatment of breast cancer. Expert Opin Drug Discov. 2015;10(6):591–7. doi: 10.1517/17460441.2015.1038235. [ ] [DOI] [PubMed] [Google Scholar]
- 20.Supuran CT, Winum JY. Carbonic anhydrase IX inhibitors in cancer therapy: an update. Future Med Chem. 2015;7(11):1407–14. doi: 10.4155/fmc.15.71. [ ] [DOI] [PubMed] [Google Scholar]
- 21.Mahon BP, Pinard MA, McKenna R. Targeting carbonic anhydrase IX activity and expression. Molecules. 2015;20(2):2323–48. doi: 10.3390/molecules20022323. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverman DN, Lindskog S. The catalytic mechanism of carbonic anhydrase - implication of a rate limiting protolysis of water. Acc Chem Res. 1988;21:30–6. doi: 10.1021/ar00145a005. [ ] [DOI] [Google Scholar]
- 23.McDonald PC, Winum JY, Supuran CT, Dedhar S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget. 2012;3(1):84–97. doi: 10.18632/oncotarget.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10(10):767–77. doi: 10.1038/nrd3554. [ ] [DOI] [PubMed] [Google Scholar]
- 25.Supuran CT. Structure and function of carbonic anhydrases. Biochem J. 2016;473(14):2023–32. doi: 10.1042/BCJ20160115. [ ] [DOI] [PubMed] [Google Scholar]
- 26.Fisher SZ, Maupin CM, Budayova-Spano M, et al. Atomic crystal and molecular dynamics simulation structures of human carbonic anhydrase II: Insights into the proton transfer mechanism. Biochemistry. 2007;46(11):2930–7. doi: 10.1021/bi062066y. [ ] [DOI] [PubMed] [Google Scholar]
- 27.Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem. 2016;31(3):345–60. doi: 10.3109/14756366.2015.1122001. [ ] [DOI] [PubMed] [Google Scholar]
- 28.Pocker Y, Sarkanen S. Carbonic anhydrase: structure catalytic versatility, and inhibition. Adv Enzymol Relat Areas Mol Biol. 1978;47:149–274. doi: 10.1002/9780470122921.ch3. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamurthy VM, Kaufman GK, Urbach AR, et al. Carbonic anhydrase as a model for biophysical and physical-organic studies of proteins and protein-ligand binding. Chem Rev. 2008;108(3):946–1051. doi: 10.1021/cr050262p. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eriksson AE, Jones TA, Liljas A. Refined structure of human carbonic anhydrase II at 2.0 A resolution. Proteins. 1988;4(4):274–82. doi: 10.1002/prot.340040406. [ ] [DOI] [PubMed] [Google Scholar]
- 31.Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev. 2003;23(2):146–89. doi: 10.1002/med.10025. [ ] [DOI] [PubMed] [Google Scholar]
- 32.Pinard MA, Mahon B, McKenna R. Probing the surface of human carbonic anhydrase for clues towards the design of isoform specific inhibitors. Biomed Res Int. 2015;2015:453543. doi: 10.1155/2015/453543. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whittington DA, Waheed A, Ulmasov B, et al. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proc Natl Acad Sci USA. 2001;98(17):9545–50. doi: 10.1073/pnas.161301298. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pastorek J, Pastoreková S, Callebaut I, et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9(10):2877–88. [PubMed] [Google Scholar]
- 35.Opavský R, Pastoreková S, Zelník V, et al. Human MN/CA9 gene, a novel member of the carbonic anhydrase family: Structure and exon to protein domain relationships. Genomics. 1996;33(3):480–7. doi: 10.1006/geno.1996.0223. [ ] [DOI] [PubMed] [Google Scholar]
- 36.Grabmaier K, Vissers JL, De Weijert MC, et al. Molecular cloning and immunogenicity of renal cell carcinoma-associated antigen G250. Int J Cancer. 2000;85(6):865–70. doi: 10.1002/(SICI)1097-0215(20000315)85:6<865::AID-IJC21>3.0.CO;2-Q. [ ] [DOI] [PubMed] [Google Scholar]
- 37.De Simone G, Supuran CT. Carbonic anhydrase IX: biochemical and crystallographic characterization of a novel antitumor target. Biochim Biophys Acta. 2010;1804:494–09. doi: 10.1016/j.bbapap.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 38.Alterio V, Hilvo M, Di Fiore A, et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc Natl Acad Sci USA. 2009;106(38):16233–8. doi: 10.1073/pnas.0908301106. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Türeci O, Sahin U, Vollmar E, et al. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA. 1998;95(13):7608–13. doi: 10.1073/pnas.95.13.7608. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilvo M, Baranauskiene L, Salzano AM, et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem. 2008;283(41):27799–809. doi: 10.1074/jbc.M800938200. [ ] [DOI] [PubMed] [Google Scholar]
- 41.Dorai T, Sawczuk IS, Pastorek J, Wiernik PH, Dutcher JP. The role of carbonic anhydrase IX overexpression in kidney cancer. Eur J Cancer. 2005;41(18):2935–47. doi: 10.1016/j.ejca.2005.09.011. [ ] [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Wang H, Tu C, Shiverick KT, Silverman DN, Frost SC. Role of hypoxia and EGF on expression, activity, localization and phosphorylation of carbonic anhydrase IX in MDA-MB-231 breast cancer cells. Biochim Biophys Acta. 2011;1813(1):159–67. doi: 10.1016/j.bbamcr.2010.09.018. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi M, Matsumoto T, Ryuge S, et al. CAXII is a sero-diagnostic marker for lung cancer. PLoS One. 2012;7(3):e33952. doi: 10.1371/journal.pone.0033952. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zatovicova M, Sedlakova O, Svastova E, et al. Ectodomain shedding of the hypoxia-induced carbonic anhydrase IX is a metalloprotease-dependent process regulated by TACE/ADAM17. Br J Cancer. 2005;93(11):1267–76. doi: 10.1038/sj.bjc.6602861. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Závada J, Závadová Z, Zat’ovicová M, Hyrsl L, Kawaciuk I. Soluble form of carbonic anhydrase IX (CA IX) in the serum and urine of renal carcinoma patients. Br J Cancer. 2003;89(6):1067–71. doi: 10.1038/sj.bjc.6601264. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown-Glaberman U, Marron M, Chalasani P, et al. Circulating carbonic anhydrase IX and antiangiogenic therapy in breast cancer. Dis Markers. 2016;2016:9810383. doi: 10.1155/2016/9810383. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carney WP. Circulating oncoproteins HER2/neu, EGFR and CAIX (MN) as novel cancer biomarkers. Expert Rev Mol Diagn. 2007;7(3):309–19. doi: 10.1586/14737159.7.3.309. [ ] [DOI] [PubMed] [Google Scholar]
- 48.Woelber L, Kress K, Kersten JF, et al. Carbonic anhydrase IX in tumor tissue and sera of patients with primary cervical cancer. BMC Cancer. 2011;11:12. doi: 10.1186/1471-2407-11-12. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg V, Pastorekova S, Zatovicova M, Vidlickova I, Jelenska L, Slezak P. High serum carbonic anhydrase IX predicts shorter survival in head and neck cancer. Bratisl Lek Listy (Tlacene Vyd) 2016;117(4):201–4. doi: 10.4149/bll_2016_038. [DOI] [PubMed] [Google Scholar]
- 50.Sadri N, Zhang PJ. Hypoxia-inducible factors: Mediators of cancer progression; prognostic and therapeutic targets in soft tissue sarcomas. Cancers (Basel) 2013;5(2):320–33. doi: 10.3390/cancers5020320. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60(24):7075–83. [PubMed] [Google Scholar]
- 52.Li Y, Tu C, Wang H, Silverman DN, Frost SC. Catalysis and pH control by membrane-associated carbonic anhydrase IX in MDA-MB-231 breast cancer cells. J Biol Chem. 2011;286(18):15789–96. doi: 10.1074/jbc.M110.188524. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yasinska IM, Gibbs BF, Lall GS, Sumbayev VV. The HIF-1 transcription complex is essential for translational control of myeloid hematopoietic cell function by maintaining mTOR phosphorylation. Cell Mol Life Sci. 2014;71(4):699–710. doi: 10.1007/s00018-013-1421-2. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–42. doi: 10.1038/nature11986. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5. doi: 10.1038/20459. [ ] [DOI] [PubMed] [Google Scholar]
- 56.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272(36):22642–7. doi: 10.1074/jbc.272.36.22642. [ ] [DOI] [PubMed] [Google Scholar]
- 57.Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60(24):7075–83. [PubMed] [Google Scholar]
- 58.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–78. doi: 10.1146/annurev.cellbio.15.1.551. [ ] [DOI] [PubMed] [Google Scholar]
- 59.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70(5):1469–80. doi: 10.1124/mol.106.027029. [ ] [DOI] [PubMed] [Google Scholar]
- 60.Kallio PJ, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia-inducible factor 1alpha: Posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci USA. 1997;94(11):5667–72. doi: 10.1073/pnas.94.11.5667. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnett DH, Sheng S, Charn TH, et al. Estrogen receptor regulation of carbonic anhydrase XII through a distal enhancer in breast cancer. Cancer Res. 2008;68(9):3505–15. doi: 10.1158/0008-5472.CAN-07-6151. [ ] [DOI] [PubMed] [Google Scholar]
- 62.Nilsson S, Gustafsson JA. Estrogen receptor action. Crit Rev Eukaryot Gene Expr. 2002;12(4):237–57. doi: 10.1615/CritRevEukaryotGeneExpr.v12.i4.10. [ ] [DOI] [PubMed] [Google Scholar]
- 63.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346(5):340–52. doi: 10.1056/NEJMra000471. [ ] [DOI] [PubMed] [Google Scholar]
- 64.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–70. doi: 10.1172/JCI27987. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saarnio J, Parkkila S, Parkkila AK, et al. Immunohistochemistry of carbonic anhydrase isozyme IX (MN/CA IX) in human gut reveals polarized expression in the epithelial cells with the highest proliferative capacity. J Histochem Cytochem. 1998;46(4):497–504. doi: 10.1177/002215549804600409. [ ] [DOI] [PubMed] [Google Scholar]
- 66.Saarnio J, Parkkila S, Parkkila AK, et al. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol. 1998;153(1):279–85. doi: 10.1016/S0002-9440(10)65569-1. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao SY, Lerman MI, Stanbridge EJ. Expression of transmembrane carbonic anhydrases, CAIX and CAXII, in human development. BMC Dev Biol. 2009;9:22. doi: 10.1186/1471-213X-9-22. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kummola L, Hämäläinen JM, Kivelä J, et al. Expression of a novel carbonic anhydrase, CA XIII, in normal and neoplastic colorectal mucosa. BMC Cancer. 2005;5:41. doi: 10.1186/1471-2407-5-41. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kivelä AJ, Parkkila S, Saarnio J, et al. Expression of transmembrane carbonic anhydrase isoenzymes IX and XII in normal human pancreas and pancreatic tumours. Histochem Cell Biol. 2000;114(3):197–204. doi: 10.1007/s004180000181. [DOI] [PubMed] [Google Scholar]
- 70.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158(3):905–19. doi: 10.1016/S0002-9440(10)64038-2. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen LQ, Howison CM, Spier C, et al. Assessment of carbonic anhydrase IX expression and extracellular pH in β-cell lymphoma cell line models. Leuk Lymphoma. 2015;56(5):1432–9. doi: 10.3109/10428194.2014.933218. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lounnas N, Rosilio C, Nebout M, et al. Pharmacological inhibition of carbonic anhydrase XII interferes with cell proliferation and induces cell apoptosis in T-cell lymphomas. Cancer Lett. 2013;333(1):76–88. doi: 10.1016/j.canlet.2013.01.020. [ ] [DOI] [PubMed] [Google Scholar]
- 73.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11(10):3714–21. doi: 10.1158/1078-0432.CCR-04-2019. [ ] [DOI] [PubMed] [Google Scholar]
- 74.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: Implications for prognosis and therapy. Clin Cancer Res. 2003;9(2):802–11. [PubMed] [Google Scholar]
- 75.Leibovich BC, Sheinin Y, Lohse CM, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol. 2007;25(30):4757–64. doi: 10.1200/JCO.2007.12.1087. [ ] [DOI] [PubMed] [Google Scholar]
- 76.Yoo CW, Nam BH, Kim JY, et al. Carbonic anhydrase XII expression is associated with histologic grade of cervical cancer and superior radiotherapy outcome. Radiat Oncol. 2010;5:101. doi: 10.1186/1748-717X-5-101. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Griffiths JR. Are cancer cells acidic? Br J Cancer. 1991;64(3):425–7. doi: 10.1038/bjc.1991.326. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Creighton CJ, Cordero KE, Larios JM, et al. Genes regulated by estrogen in breast tumor cells in vitro are similarly regulated in vivo in tumor xenografts and human breast tumors. Genome Biol. 2006;7(4):R28. doi: 10.1186/gb-2006-7-4-r28. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wykoff CC, Beasley N, Watson PH, et al. Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am J Pathol. 2001;158(3):1011–9. doi: 10.1016/S0002-9440(10)64048-5. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ilie MI, Hofman V, Ortholan C, et al. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. Int J Cancer. 2011;128(7):1614–23. doi: 10.1002/ijc.25491. [ ] [DOI] [PubMed] [Google Scholar]
- 81.Kivelä A, Parkkila S, Saarnio J, et al. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol. 2000;156(2):577–84. doi: 10.1016/S0002-9440(10)64762-1. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nordfors K, Haapasalo J, Korja M, et al. The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: An association of CA IX with poor prognosis. BMC Cancer. 2010;10:148. doi: 10.1186/1471-2407-10-148. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kopecka J, Campia I, Jacobs A, et al. Carbonic anhydrase XII is a new therapeutic target to overcome chemoresistance in cancer cells. Oncotarget. 2015;6(9):6776–93. doi: 10.18632/oncotarget.2882. [ http://dx.doi.org/10.18632/oncotarget.2882] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: Suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71(9):3364–76. doi: 10.1158/0008-5472.CAN-10-4261. [ ] [DOI] [PubMed] [Google Scholar]
- 85.McKenna R, Supuran CT. Carbonic anhydrase inhibitors drug design. Subcell Biochem. 2014;75:291–323. doi: 10.1007/978-94-007-7359-2_15. [ ] [DOI] [PubMed] [Google Scholar]
- 86.Aggarwal M, Kondeti B, McKenna R. Insights towards sulfonamide drug specificity in α-carbonic anhydrases. Bioorg Med Chem. 2013;21(6):1526–33. doi: 10.1016/j.bmc.2012.08.019. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem Rev. 2012;112(8):4421–68. doi: 10.1021/cr200176r. [ ] [DOI] [PubMed] [Google Scholar]
- 88.Winum JY, Supuran CT. Recent advances in the discovery of zinc-binding motifs for the development of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem. 2015;30(2):321–4. doi: 10.3109/14756366.2014.913587. [ ] [DOI] [PubMed] [Google Scholar]
- 89.Wilkinson BL, Bornaghi LF, Houston TA, et al. Inhibition of membrane-associated carbonic anhydrase isozymes IX, XII and XIV with a library of glycoconjugate benzenesulfonamides. Bioorg Med Chem Lett. 2007;17(4):987–92. doi: 10.1016/j.bmcl.2006.11.046. [ ] [DOI] [PubMed] [Google Scholar]
- 90.Thiry A, Dogné JM, Supuran CT, Masereel B. Carbonic anhydrase inhibitors as anticonvulsant agents. Curr Top Med Chem. 2007;7(9):855–64. doi: 10.2174/156802607780636726. [ ] [DOI] [PubMed] [Google Scholar]
- 91.Pacchiano F, Carta F, McDonald PC, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J Med Chem. 2011;54(6):1896–902. doi: 10.1021/jm101541x. [ ] [DOI] [PubMed] [Google Scholar]
- 92.Ward C, Meehan J, Mullen P, et al. Evaluation of carbonic anhydrase IX as a therapeutic target for inhibition of breast cancer invasion and metastasis using a series of in vitro breast cancer models. Oncotarget. 2015;6(28):24856–70. doi: 10.18632/oncotarget.4498. [ http://dx.doi.org/10.18632/oncotarget.4498] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carradori S, Secci D, De Monte C, et al. A novel library of saccharin and acesulfame derivatives as potent and selective inhibitors of carbonic anhydrase IX and XII isoforms. Bioorg Med Chem. 2016;24(5):1095–105. doi: 10.1016/j.bmc.2016.01.038. [ ] [DOI] [PubMed] [Google Scholar]
- 94.Scozzafava A, Briganti F, Ilies MA, Supuran CT. Carbonic anhydrase inhibitors: Synthesis of membrane-impermeant low molecular weight sulfonamides possessing in vivo selectivity for the membrane-bound versus cytosolic isozymes. J Med Chem. 2000;43(2):292–300. doi: 10.1021/jm990479+. [ ] [DOI] [PubMed] [Google Scholar]
- 95.Mincione F, Menabuoni L, Briganti F, Mincione G, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of isozymes I, II and IV with N-hydroxysulfonamides-a novel class of intraocular pressure lowering agents. J Enzyme Inhib. 1998;13(4):267–84. doi: 10.3109/14756369809021475. [ ] [DOI] [PubMed] [Google Scholar]
- 96.Dubois L, Douma K, Supuran CT, et al. Imaging the hypoxia surrogate marker CA IX requires expression and catalytic activity for binding fluorescent sulfonamide inhibitors. Radiother Oncol. 2007;83(3):367–73. doi: 10.1016/j.radonc.2007.04.018. [ ] [DOI] [PubMed] [Google Scholar]
- 97.Håkansson K, Carlsson M, Svensson LA, Liljas A. Structure of native and apo carbonic anhydrase II and structure of some of its anion-ligand complexes. J Mol Biol. 1992;227(4):1192–204. doi: 10.1016/0022-2836(92)90531-N. [ ] [DOI] [PubMed] [Google Scholar]
- 98.Maresca A, Temperini C, Vu H, et al. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J Am Chem Soc. 2009;131(8):3057–62. doi: 10.1021/ja809683v. [ ] [DOI] [PubMed] [Google Scholar]
- 99.Maresca A, Temperini C, Pochet L, Masereel B, Scozzafava A, Supuran CT. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem. 2010;53(1):335–44. doi: 10.1021/jm901287j. [ ] [DOI] [PubMed] [Google Scholar]
- 100.Lopez M, Trajkovic J, Bornaghi LF, et al. Design, synthesis, and biological evaluation of novel carbohydrate-based sulfamates as carbonic anhydrase inhibitors. J Med Chem. 2011;54(5):1481–9. doi: 10.1021/jm101525j. [ ] [DOI] [PubMed] [Google Scholar]
- 101.Carta F, Temperini C, Innocenti A, Scozzafava A, Kaila K, Supuran CT. Polyamines inhibit carbonic anhydrases by anchoring to the zinc-coordinated water molecule. J Med Chem. 2010;53(15):5511–22. doi: 10.1021/jm1003667. [ ] [DOI] [PubMed] [Google Scholar]
- 102.Rodríguez OM, Maresca A, Témpera CA, Bravo RD, Colinas PA, Supuran CT. N-β-Glycosyl sulfamides are selective inhibitors of the cancer associated carbonic anhydrase isoforms IX and XII. Bioorg Med Chem Lett. 2011;21(15):4447–50. doi: 10.1016/j.bmcl.2011.06.031. [ ] [DOI] [PubMed] [Google Scholar]
- 103.Lopez M, Paul B, Hofmann A, et al. S-Glycosyl primary sulfonamides-a new structural class for selective inhibition of cancer-associated carbonic anhydrases. J Med Chem. 2009;52(20):6421–32. doi: 10.1021/jm900914e. [ ] [DOI] [PubMed] [Google Scholar]
- 104.Carta F, Aggarwal M, Maresca A, Scozzafava A, McKenna R, Supuran CT. Dithiocarbamates: A new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chem Commun (Camb) 2012;48(13):1868–70. doi: 10.1039/c2cc16395k. [ ] [DOI] [PubMed] [Google Scholar]
- 105.Morris JC, Chiche J, Grellier C, et al. Targeting hypoxic tumor cell viability with carbohydrate-based carbonic anhydrase IX and XII inhibitors. J Med Chem. 2011;54(19):6905–18. doi: 10.1021/jm200892s. [ ] [DOI] [PubMed] [Google Scholar]
- 106.Winum JY, Colinas PA, Supuran CT. Glycosidic carbonic anhydrase IX inhibitors: A sweet approach against cancer. Bioorg Med Chem. 2013;21(6):1419–26. doi: 10.1016/j.bmc.2012.10.043. [ ] [DOI] [PubMed] [Google Scholar]
- 107.Stander BA, Joubert F, Tu C, Sippel KH, McKenna R, Joubert AM. In vitro evaluation of ESE-15-ol, an estradiol analogue with nanomolar antimitotic and carbonic anhydrase inhibitory activity. PLoS One. 2012;7(12):e52205. doi: 10.1371/journal.pone.0052205. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lomelino CL, Supuran CT, McKenna R. Non-classical inhibition of carbonic anhydrase. Int J Med Mol Sci. 2016;17(7):1150. doi: 10.3390/ijms17071150. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lomelino C, McKenna R. Carbonic anhydrase inhibitors: A review on the progress of patent literature (2011–2016) Expert Opin Ther Pat. 2016;26(8):947–56. doi: 10.1080/13543776.2016.1203904. [ ] [DOI] [PubMed] [Google Scholar]
- 110.Oosterwijk E, Ruiter DJ, Hoedemaeker PJ, et al. Monoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer. 1986;38(4):489–94. doi: 10.1002/ijc.2910380406. [ ] [DOI] [PubMed] [Google Scholar]
- 111.Pryma DA, O’Donoghue JA, Humm JL, et al. Correlation of in vivo and in vitro measures of carbonic anhydrase IX antigen expression in renal masses using antibody 124I-cG250. J Nucl Med. 2011;52(4):535–40. doi: 10.2967/jnumed.110.083295. [ ] [DOI] [PubMed] [Google Scholar]
- 112.Ahlskog JK, Schliemann C, Mårlind J, et al. Human monoclonal antibodies targeting carbonic anhydrase IX for the molecular imaging of hypoxic regions in solid tumours. Br J Cancer. 2009;101(4):645–57. doi: 10.1038/sj.bjc.6605200. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gondi G, Mysliwietz J, Hulikova A, et al. Antitumor efficacy of a monoclonal antibody that inhibits the activity of cancer-associated carbonic anhydrase XII. Cancer Res. 2013;73(21):6494–503. doi: 10.1158/0008-5472.CAN-13-1110. [ ] [DOI] [PubMed] [Google Scholar]
- 114.Krall N, Pretto F, Neri D. A bivalent small molecule-drug conjugate directed against carbonic anhydrase IX can elicit complete tumour regression in mice. Chem Sci (Camb) 2014;5(9):3640–4. doi: 10.1039/C4SC00685B. [ ] [DOI] [Google Scholar]
- 115.Muselaers CH, Boers-Sonderen MJ, van Oostenbrugge TJ, et al. Phase 2 study of lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur Urol. 2016;69(5):767–70. doi: 10.1016/j.eururo.2015.11.033. [ ] [DOI] [PubMed] [Google Scholar]
- 116.Stillebroer AB, Boerman OC, Desar IM, et al. Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur Urol. 2013;64(3):478–85. doi: 10.1016/j.eururo.2012.08.024. [ ] [DOI] [PubMed] [Google Scholar]
- 117.Ahlskog JK, Schliemann C, Mårlind J, et al. Human monoclonal antibodies targeting carbonic anhydrase IX for the molecular imaging of hypoxic regions in solid tumours. Br J Cancer. 2009;101(4):645–57. doi: 10.1038/sj.bjc.6605200. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Murri-Plesko MT, Hulikova A, Oosterwijk E, et al. Antibody inhibiting enzymatic activity of tumour-associated carbonic anhydrase isoform IX. Eur J Pharmacol. 2011;657(1–3):173–83. doi: 10.1016/j.ejphar.2011.01.063. [ ] [DOI] [PubMed] [Google Scholar]
- 119.Dekaminaviciute D, Kairys V, Zilnyte M, et al. Monoclonal antibodies raised against 167–180 aa sequence of human carbonic anhydrase XII inhibit its enzymatic activity. J Enzyme Inhib Med Chem. 2014;29(6):804–10. doi: 10.3109/14756366.2013.856424. [ ] [DOI] [PubMed] [Google Scholar]
- 120.Dekaminaviciute D, Lasickiene R, Parkkila S, et al. Development and characterization of new monoclonal antibodies against human recombinant CA XII. Biomed Res Int. 2014;2014:309307. doi: 10.1155/2014/309307. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21(4):904–12. doi: 10.1038/mt.2013.17. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Banerjee D, Slack F. Control of developmental timing by small temporal RNAs: A paradigm for RNA-mediated regulation of gene expression. BioEssays. 2002;24(2):119–29. doi: 10.1002/bies.10046. [ ] [DOI] [PubMed] [Google Scholar]
- 123.Tiram G, Scomparin A, Ofek P, Satchi-Fainaro R. Interfering cancer with polymeric siRNA nanomedicines. J Biomed Nanotechnol. 2014;10(1):50–66. doi: 10.1166/jbn.2014.1715. [ ] [DOI] [PubMed] [Google Scholar]
- 124.Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013;14(8):475–88. doi: 10.1038/nrm3611. [ ] [DOI] [PubMed] [Google Scholar]
- 125.Sakurai Y, Hatakeyama H, Sato Y, Hyodo M, Akita H, Harashima H. Gene silencing via RNAi and siRNA quantification in tumor tissue using MEND, a liposomal siRNA delivery system. Mol Ther. 2013;21(6):1195–203. doi: 10.1038/mt.2013.57. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lock FE, McDonald PC, Lou Y, et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32(44):5210–9. doi: 10.1038/onc.2012.550. [ ] [DOI] [PubMed] [Google Scholar]
- 127.Radvak P, Repic M, Svastova E, et al. Suppression of carbonic anhydrase IX leads to aberrant focal adhesion and decreased invasion of tumor cells. Oncol Rep. 2013;29(3):1147–53. doi: 10.3892/or.2013.2226. [DOI] [PubMed] [Google Scholar]
- 128.Yu SJ, Yoon JH, Lee JH, et al. Inhibition of hypoxia-inducible carbonic anhydrase-IX enhances hexokinase II inhibitor-induced hepatocellular carcinoma cell apoptosis. Acta Pharmacol Sin. 2011;32(7):912–20. doi: 10.1038/aps.2011.24. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bryna J, Cigler P, Gruner B, et al. Carbonic anhydrase inhibitors and method of their production. World Patent WO2013060307. 2013 [Google Scholar]
- 130.Cianchi F, Vinci MC, Supuran CT, et al. Selective inhibition of carbonic anhydrase IX decreases cell proliferation and induces ceramide-mediated apoptosis in human cancer cells. J Pharmacol Exp Ther. 2010;334(3):710–9. doi: 10.1124/jpet.110.167270. [ ] [DOI] [PubMed] [Google Scholar]
- 131.Lee CH, Jin GF, Kim HS, Nakamura H, Chung Y, Lee JD. Preparation of boronated heterocyclic compounds using intramolecular cyclization reaction. Bull Korean Chem Soc. 2008;29(2):357–62. doi: 10.5012/bkcs.2008.29.2.357. [ ] [DOI] [Google Scholar]
- 132.Lee JD, Lee CH, Nakamura H, Ko JJ, Kang SO. A convenient synthesis of the novel carboranyl-substituted tetrahydroisoquinoline-s: Application to the biologically active agent for BNCT. Tetrahedron Lett. 2002;43(31):5483–6. doi: 10.1016/S0040-4039(02)01034-1. [ ] [DOI] [Google Scholar]
- 133.Babich JW, Zimmerman C, Joyal J, et al. Metal complexes of poly(carboxyl)amine-containing ligands having an affinity for carbonic anhydrase IX. World Patent WO2013103813. 2013 [Google Scholar]
- 134.Zimmerman C, Babich JW, Joyal JL, Lu G, Maresca KP, Barone C. Inhibitors of carbonic anhydrase IX. European patent EP2823826A3. 2015 [Google Scholar]
- 135.Liu G, Dou S, He J, Vanderheyden JL, Rusckowski M, Hnatowich DJ. Preparation and properties of 99mTc(CO)3+-labeled N,N-bis(2-pyridylmethyl)-4-aminobutyric acid. Bioconjug Chem. 2004;15(6):1441–6. doi: 10.1021/bc049866a. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dubois L, Peeters SG, van Kuijk SJ, et al. Targeting carbonic anhydrase IX by nitroimidazole based sulfamides enhances the therapeutic effect of tumor irradiation: A new concept of dual targeting drugs. Radiother Oncol. 2013;108(3):523–8. doi: 10.1016/j.radonc.2013.06.018. [ ] [DOI] [PubMed] [Google Scholar]
- 137.Hay M, Wang J, Pruijn FB, Bonnet M, Hicks K. Nitroimidazole compounds and their use in cancer therapy. World Patent WO2014030142. 2014 [Google Scholar]
- 138.Doyen J, Parks SK, Marcié S, Pouysségur J, Chiche J. Knock-down of hypoxia-induced carbonic anhydrases IX and XII radiosensitizes tumor cells by increasing intracellular acidosis. Front Oncol. 2012;2:199. doi: 10.3389/fonc.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dubois LJ, Niemans R, van Kuijk SJ, et al. New ways to image and target tumour hypoxia and its molecular responses. Radiother Oncol. 2015;116(3):352–7. doi: 10.1016/j.radonc.2015.08.022. [ ] [DOI] [PubMed] [Google Scholar]
- 140.Rami M, Dubois L, Parvathaneni NK, et al. Hypoxia-targeting carbonic anhydrase IX inhibitors by a new series of nitroimidazole-sulfonamides/sulfamides/sulfamates. J Med Chem. 2013;56(21):8512–20. doi: 10.1021/jm4009532. [ ] [DOI] [PubMed] [Google Scholar]
- 141.Supuran C, Dedhar S, McDonald PC, Carta F. Novel sulfonamide compounds for inhibition of metastatic tumor growth. World Patent WO2012021963. 2012 [Google Scholar]
- 142.Pastorekova S, Vullo D, Casini A, et al. Carbonic anhydrase inhibitors: Inhibition of the tumor-associated isozymes IX and XII with polyfluorinated aromatic/heterocyclic sulfonamides. J Enzyme Inhib Med Chem. 2005;20(3):211–7. doi: 10.1080/14756360400028101. [ ] [DOI] [PubMed] [Google Scholar]
- 143.Gieling RG, Babur M, Mamnani L, et al. Antimetastatic effect of sulfamate carbonic anhydrase IX inhibitors in breast carcinoma xenografts. J Med Chem. 2012;55(11):5591–600. doi: 10.1021/jm300529u. [ ] [DOI] [PubMed] [Google Scholar]
- 144.Supuran C, Dedhar S, Carta F, Winum JY, McDonald PC. Carbonic anhydrase inhibitors with antimetastatic activity. World Patent WO2012070024. 2012 [Google Scholar]
- 145.Carradori S. Selective carbonic anhydrase IX inhibitors based on coumarin scaffold as promising antimetastatic agents: WO2012070024. Expert Opin Ther Pat. 2013;23(6):751–6. doi: 10.1517/13543776.2013.769523. [ ] [DOI] [PubMed] [Google Scholar]
- 146.Sippel KH, Stander A, Tu C, Venkatakrishnan B, Robbins AH, Agbandje-McKenna M, et al. Characterization of carbonic anhydrase isozyme specific inhibition by sulfamated 2-ethylestra compounds. Lett Drug Des Discov. 2011;8(8):678–84. doi: 10.2174/157018011796576105. [ ] [DOI] [Google Scholar]
- 147.Touisni N, Maresca A, McDonald PC, et al. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J Med Chem. 2011;54(24):8271–7. doi: 10.1021/jm200983e. [ ] [DOI] [PubMed] [Google Scholar]
- 148.Marasco WA, Lo A, Xu C. Carbonic anhydrase IX (G250) antibodies and methods of use thereof. World Patent WO2012070024. 2007 [Google Scholar]
- 149.Davis ID, Wiseman GA, Lee FT, et al. A phase I multiple dose, dose escalation study of cG250 monoclonal antibody in patients with advanced renal cell carcinoma. Cancer Immun. 2007;7:13–20. [PMC free article] [PubMed] [Google Scholar]
- 150.Mulders P, Bleumer I, Debruyne F, Oosterwijk E. Specific monoclonal antibody-based immunotherapy by targeting the RCC-associated antigen carbonic anhydrase-IX(G250/MN) Urologe A. 2004;43(3 (Suppl. 3)):S146–7. doi: 10.1007/s00120-004-0609-3. [ ] [DOI] [PubMed] [Google Scholar]
- 151.Bleumer I, Oosterwijk E, Oosterwijk-Wakka JC, et al. A clinical trial with chimeric monoclonal antibody WX-G250 and low dose interleukin-2 pulsing scheme for advanced renal cell carcinoma. J Urol. 2006;175(1):57–62. doi: 10.1016/S0022-5347(05)00040-6. [ ] [DOI] [PubMed] [Google Scholar]
- 152.Siebels M, Rohrmann K, Oberneder R, et al. A clinical Phase I/II trial with the monoclonal antibody cG250 (RENCAREX®) and interferon-alpha-2a in metastatic renal cell carcinoma patients. World J Urol. 2011;29(1):121–6. doi: 10.1007/s00345-010-0570-2. [ ] [DOI] [PubMed] [Google Scholar]
- 153.Li Z, Belldegrun A. Fusion molecule based on novel taa variant. US Patent US20130230483. 2013 [Google Scholar]
- 154.Kim BR, Yang EK, Kim DY, et al. Generation of anti-tumour immune response using dendritic cells pulsed with carbonic anhydrase IX-Acinetobacter baumannii outer membrane protein A fusion proteins against renal cell carcinoma. Clin Exp Immunol. 2012;167(1):73–83. doi: 10.1111/j.1365-2249.2011.04489.x. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hernández JM, Bui MH, Han KR, et al. Novel kidney cancer immunotherapy based on the granulocyte-macrophage colony-stimulating factor and carbonic anhydrase IX fusion gene. Clin Cancer Res. 2003;9(5):1906–16. [PubMed] [Google Scholar]
- 156.Zeidler R, Battke C, Kremmer E, Flatley A, Supuran C. Novel antibody to a carbonic anhydrase. World Patent WO2011138279. 2011 [Google Scholar]
- 157.Battke C, Kremmer E, Mysliwietz J, et al. Generation and characterization of the first inhibitory antibody targeting tumour-associated carbonic anhydrase XII. Cancer Immunol Immunother. 2011;60(5):649–58. doi: 10.1007/s00262-011-0980-z. [ ] [DOI] [PMC free article] [PubMed] [Google Scholar]