Abstract
IL-33 has pleiotropic functions in immune responses and promotes the development of allergic diseases and asthma. IL-33 induces Th2 differentiation and enhances type 2 cytokine production by CD4+ T cells. However, the regulation of IL-33-driven type 2 cytokine responses is not fully defined. In this study, we investigated the effect of PGI2, a lipid mediator formed in the cyclooxygenase pathway of arachidonic acid metabolism, on naïve CD4+ T cell activation, proliferation, and differentiation by IL-33. Using wild-type and PGI2 receptor (IP) knockout (KO) mice, we found that the PGI2 analog cicaprost dose-dependently inhibited IL-33-driven IL-4, IL-5, and IL-13 production by CD4+ T cells in an IP-specific manner. In addition, cicaprost inhibited IL-33-driven IL-2 production and CD25 expression by CD4+ T cells. Furthermore, IP KO mice had increased IL-5 and IL-13 responses of CD4+ T cells to Alternaria sensitization and challenge in mouse lungs. Since IL-33 is critical for Alternaria-induced type 2 responses, these data suggest that PGI2 not only inhibits IL-33-stimulated CD4+ Th2 cell responses in vitro, but also suppresses IL-33-induced Th2 responses caused by protease-containing allergens in vivo.
INTRODUCTION
The differentiation of naïve CD4+ T cells toward Th2 subset is required for defense against parasite pathogens, while dysfunctional type 2 immune responses may cause immunopathological diseases such as allergic diseases and asthma. Classical Th2 differentiation of CD4+ T cells is induced by IL-4 which activates the STAT6 signaling pathway for the expression of type 2 cytokines including IL-4, IL-5, and IL-13. IL-33 is a pro-inflammatory cytokine constitutively expressed in the nucleus of epithelial and endothelial cells and promotes innate immunity and adaptive type 2 immune responses (1–4). IL-33 binds to IL-1RL1 (ST2) and increases IL-5 and IL-13 expression by mouse and human CD4+ T cells, indicating its ability to promote Th2 differentiation (5). The effect of IL-33 on naïve CD4+ T cell Th2 differentiation is mediated by the MyD88 signaling pathway, but is independent of IL-4, IL-4R, and STAT6 signaling (5). Thus, IL-33 activates an alternative Th2 differentiation pathway from the classical IL-4-driven STAT6-dependent Th2 cell polarization.
Lipid molecules such as prostaglandins formed in the cyclooxygenase pathway of arachidonic acid metabolism have regulatory functions in immune responses and inflammation (6–9). PGI2, also known as prostacyclin, suppressed Th2 cytokine (IL-5 and IL-13) expression, eosinophilia, and mucus production in the lung through PGI2 receptor (IP) signaling pathway in a mouse model of OVA-induced allergic inflammation (10–13). Recently, we reported that IP deficiency abrogated OVA-induced immune tolerance in mice, further supporting the modulatory functions of PGI2 not only on immune responses, but also on immune tolerance (14). In vitro, the PGI2 analogs cicaprost and iloprost inhibited the production of the effector cytokines IL-4 and IL-5 by Th2 cells that had been differentiated under IL-4 and anti-IFN-γ condition (15). However, the effect of PGI2 on IL-33-driven Th2 polarization has not been reported.
In this study, we tested the hypothesis that PGI2 inhibits IL-33-induced CD4+ T cell activation, proliferation, and type 2 cytokine expression. We found that the PGI2 analog cicaprost suppressed IL-33-induced production of IL-4, IL-5, and IL-13 by WT CD4+ T cells, but not by IP KO CD4+ T cells. In addition, cicaprost signaling through IP decreased IL-33-induced IL-2 production and CD25 surface expression by CD4+ T cells. The suppressive effect of the PGI2 analog cicaprost on IL-33-driven Th2 responses suggests that PGI2 limits the development of type 2 immune responses caused by IL-33-stimulating antigens such as protease-containing allergens. This notion is supported by our findings that IP KO mice had increased CD4+ cell IL-5 and IL-13 responses in the model of Alternaria-induced lung inflammation.
MATERIALS AND METHODS
Mice
Wild-type (WT) BALB/c mice were obtained from The Jackson Laboratory (Bar Harbor, ME). IP KO mice on a C57BL/6 background were generated by homologous recombination in embryonic stem cells and kindly provided by Dr. Garret FitzGerald at the University of Pennsylvania (16). The IP KO mice were backcrossed to a BALB/c background for 10 generations. Age-matched WT and IP KO mice were used at 8–12 weeks old. Animal experiments were reviewed, approved by the Institutional Animal Care and Use Committee at Vanderbilt University, and were conducted according to the guidelines for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, National Research Council.
CD4+ T cell culture
CD4+ T cells were purified from the splenic cells of WT or IP KO mice by a mouse naive CD4+ T cell isolation kit (Miltenyi Biotec, Auburn, CA, Cat. #130–106-643). An anti-CD11c antibody was included in this kit to deplete DCs. The isolated CD4+CD62L+ T cell population contains 97.5% CD3+CD4+ cells as determined by flow cytometry. The purified CD4+ T cells were resuspended at 1 ×106 cells/ml in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 10% FBS (HyClone, Logan, UT), 4 mM of L-glutamine, 1 mM of sodium pyruvate, 55 mM of 2-ME, 10 mM of HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were stimulated with plate-bound anti-CD3 (10 μg/ml) and anti-CD28 (2 μg/ml; BD Biosciences, San Diego, CA) in 96-well flat- bottom plates and treated with IL-33 (20 ng/ml, PeproTech, Rocky Hill, NJ ) plus cicaprost (a generous gift provided by Dr. Manuela Huebner, Bayer HealthCare, Berlin, Germany) at various concentrations or vehicle (water) control. The cells were cultured for 3 days.
Cell culture supernatant was collected at day 2 for IL-2 measurement because IL-2 production by activated CD4+ T cells peak at day 2 (our unpublished data). At day 3, the culture supernatant was harvested for IL-4, IL-5 and IL-13 measurement.
RT-PCR
Naïve CD4+ T cells and CD4+ T cells activated under the conditions with anti-CD3 and anti-CD28 in the absence or presence of IL-33 for 3 days were used for RT-PCR to determine IP, COX-2, PGI2 synthase (PGIS) and β-actin expression. TaqMan Real-Time PCR Assays (Thermo Fisher Scientific, Assay IDs: IP, Mm00801939_m1; COX-2, Mm00478374_m1; PGIS, Mm00447271_m1; and beta-actin, Mm02619580_g1) were used for the experiments. LPS-stimulated mouse lung tissue was used as positive control of these target genes.
ELISA
IL-2, IL-4, IL-5, and IL-13 in the culture supernatant were measured by DuoSet ELISA kits from R&D Systems according to the manufacturer’s instructions. Measurements below the limit of detection were assigned a value of half the lower limit of detection for purposes of statistical analyses.
Flow cytometry
At day 3 of cell culture, for intracellular IL-4, IL-5, and IL-13 flow cytometry, cells were stimulated with PMA (10 ng/ml), ionomycin (1 μM) and GolgiStop (2.25 μM Monensin, BD Biosciences) for 4–6 h. The cells were stained with Live/Dead Fixable Blue Dead Cell Stain Kit (Thermo Fisher Scientific), fixed, and permeabilized. The cells were then stained with fluorochrome-labeled antibodies against CD4+ (BioLegend, San Diego, CA), IL-4 (BD Biosciences, San Jose, CA), IL-5 (BD Biosciences), IL-13(Biolegend), Gata3 (Affymetrix, Santa Clara, CA), CD25 (BD Biosciences), and TSLP (Biolegend). 123count™ eBeads (Affymetrix) were added to the cell solution for cell counting purpose. An LSR II flow cytometer (BD Biosciences) was used for flow cytometry and the data were analyzed with FlowJo software (FlowJo, LLC, Ashland OR). For cell proliferation assay, naïve CD4+ T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE, 0.5 μM) before cell activation and treatment. At day 3, DAPI and 123count™ eBeads (Affymetrix) were added to the cell solution for dead cell staining and cell counting purpose by flow cytometry. CFSE intensity of the cultured cells was determined by flow cytometry. Cell division index was calculated with FlowJo software.
NFAT function assay
Cignal Lenti-NFAT luciferase reporter system (Qiagen, Hilden, Germany) was used for determining the effect of cicaprost on NFAT function according to manufacturer’s instructions. Briefly, purified naïve CD4+ T cells were transduced with Cignal Lenti-NFAT or Cignal Lenti-GFP reporter vectors in the presence of SureENTRY Transduction Reagent and cultured for 24 h to ensure lentivirus integration. The cells were washed and treated with vehicle or cicaprost in the absence or presence of IL-33 for 2 days. Lenti-NFAT-transduced cells were harvested and the cell pellets were used for luciferase assay with a Luciferase Assay System (Promega, Madison, WI) to determine the NFAT activity. Lenti-GFP-transduced cells were stained with dead cell dye and analyzed by flow cytometry to determine the transduction efficiency of live cells. We found that about 2% of transduced cells were GFP+.
Alternaria extract challenge in vivo
WT and IP KO mice were anesthetized with ketamine/xylazine and then challenged intranasally with either Alternaria extract (5 μg) or heat-inactivated (120 ᵒC for 2 h) Alternaria extract (5 μg) (Greer Laboratories, Inc., Lenoir, NC) in 100 μl PBS 4 times on days 0, 3, 6, and 9. Twenty-four hours after the last challenge, the mouse lungs were harvested and digested. Lung cells were stimulated with PMA (10 ng/ml), ionomycin (1 μM) and GolgiStop (2.25 μM Monensin, BD Biosciences) for 4–6 h. Cell surface marker and intracellular cytokine staining was performed to enumerate IL-5+CD4+ T cells and IL-13+CD4+ T cells. Lung homogenate was used for determining the levels of IL-5 and IL-13 by ELISA.
Statistical analysis
The results were presented as mean ± SEM. Statistical analyses were conducted by using Student t test or one-way ANOVA with a Bonferroni post hoc test for Fig. 8.
Results
The PGI2 analog cicaprost decreased IL-33-induced type 2 cytokine production by CD4+ T cells
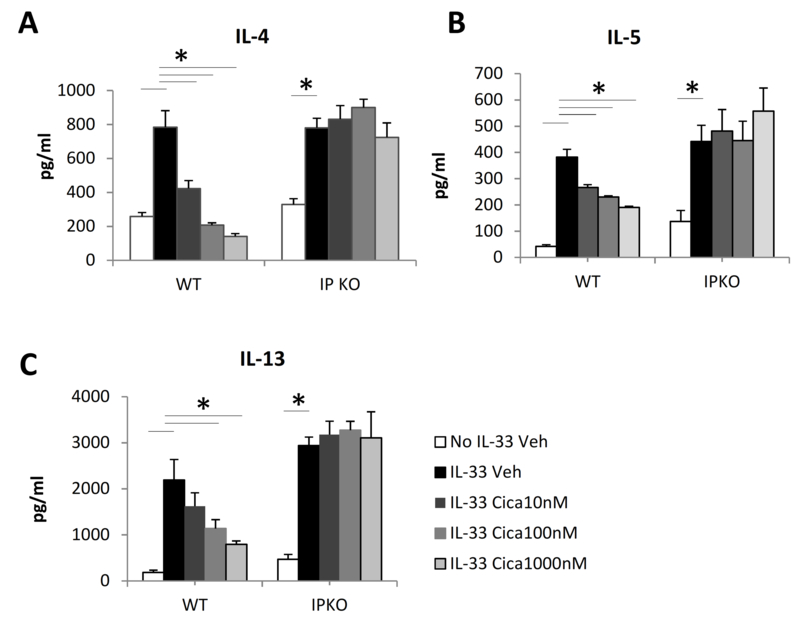
To determine the effect of PGI2 signaling on IL-33-induced Th2 differentiation, naïve CD4+ T cells of WT and IP KO mice, both on a BALB/c background, were activated with anti-CD3 and anti-CD28 either with or without IL-33 and treated with cicaprost or vehicle for 3 days. We found that naïve and activated CD4+ T cells expressed IP receptor as determined by RT-PCR (online supplementary Fig. S1), providing a molecular basis of T cell responsiveness to cicaprost treatment. Activated CD4+ cells, but not naïve CD4+ cells, expressed COX-2, whereas neither naïve nor activated CD4+ cells expressed PGIS, (Fig. S1). As shown in Fig. 1, treatment of the cells with IL-33 significantly increased the production of the type 2 cytokines IL-4, IL-5, and IL-13 compared to the cell culture without IL-33. In the presence of IL-33, cicaprost dose-dependently decreased IL-4, IL-5 and IL-13 production by WT CD4+ T cells, suggesting that PGI2 has an inhibitory effect on Th2 differentiation and type 2 cytokine production. Cicaprost did not change the production of IL-4, IL-5, or IL-13 by IP KO CD4+ T cells, indicating that cicaprost-mediated inhibition of type 2 cytokine production is dependent on IP signaling.
Fig. 1.
Cicaprost decreased IL-33-induced IL-4, IL-5, and IL-13 expression by CD4+ T cells. Naïve CD4+ T cells from WT and IP KO mice were stimulated with anti-CD3 and anti-CD28 Abs in the absence or presence of IL-33 and were treated with vehicle or cicaprost for 3 days. The levels of (A) IL-4, (B) IL-5, and (C) IL-13 in the culture supernatant were determined by ELISA. Data are combined of 3 experiments and presented as mean ± SEM. *, p < 0.05, n=9.
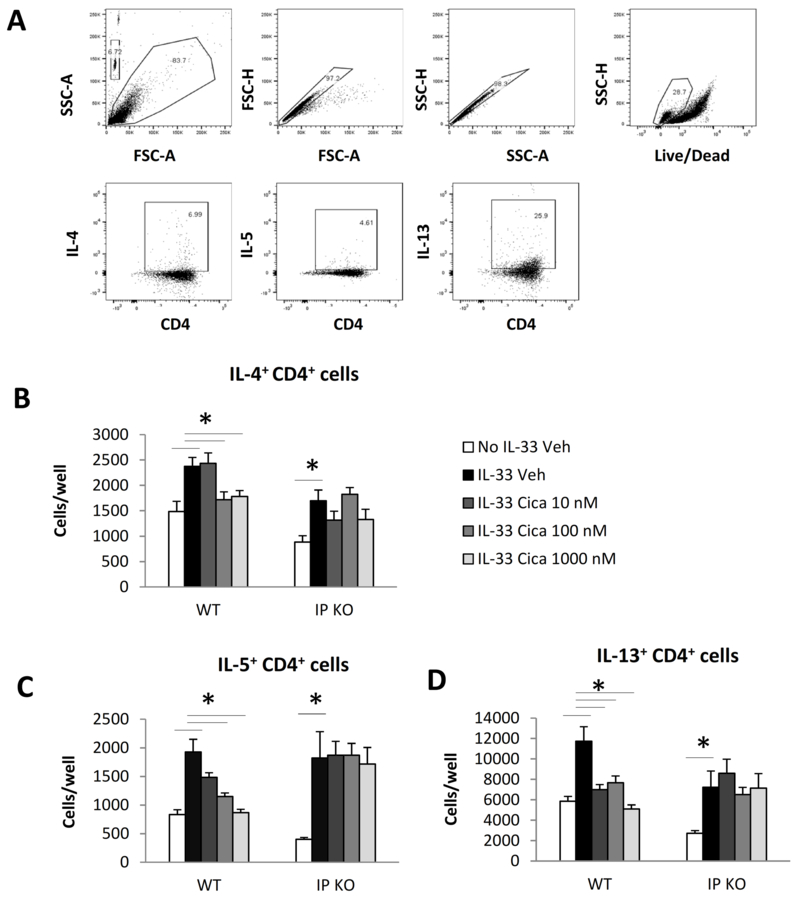
To determine the effect of cicaprost on type 2 cytokine expression at a single cell level, we activated and treated naïve CD4+ T cells with vehicle or cicaprost for 3 days and stimulated the cells with PMA and ionomycin in the presence of GolgiStop for intracellular cytokine measurement by flow cytometry. We found that IL-33 increased the numbers of IL-4+CD4+, IL-5+CD4+, and IL-13+CD4+ cells compared with the cell culture in the absence of IL-33 for both WT and IP KO T cells (Fig. 2). In the presence of IL-33, cicaprost dose-dependently decreased total numbers of IL-4+CD4+, IL-5+CD4+, and IL-13+CD4+ cells compared with vehicle control in WT T cell culture, but not in IP KO T cell culture (Fig. 2). These results indicate that the inhibitory effect of cicaprost on CD4+ T cell type 2 cytokine expression is dependent on the IP signaling pathway.
Fig. 2.
Cicaprost decreased the numbers of IL-4+CD4+, IL-5+CD4+ and IL-13+CD4+ T cells. Naïve CD4+ T cells from WT and IP KO mice were stimulated with anti-CD3 and anti-CD28 Abs in the absence or presence of IL-33 and treated with vehicle or cicaprost for 3 days. The cells were then stimulated with PMA and ionomycin in the presence of GolgiStop for 4h. The levels of intracellular cytokines were determined by flow cytometry. A. Flow gating strategy. The cells were gated for lymphocytes, single cells, and live cells and analyzed for IL-4, IL-5, and IL-13 expression in CD4+ cells. B-D. The number of IL-4+CD4+ (B), IL-5+CD4+ (C) and IL-13+CD4+ (D) T cells. Data are combined of 2 experiments and presented as mean ± SEM. *, p < 0.05, n=6.
Cicaprost did not affect the percentages of IL-4+CD4+, IL-5+CD4+, and IL-13+CD4+ cells. Neither did cicaprost affect MFI of IL-4, IL-5 and IL-13 of the cells in these experiments (Online supplementary Fig. S2). These data suggest that cicaprost inhibited IL-33-induced Th2 responses by limiting the total number of Th2 cells, rather than by affecting type 2 cytokine expression at a single cell level.
Cicaprost decreased Gata3 expression in CD4+ T cells
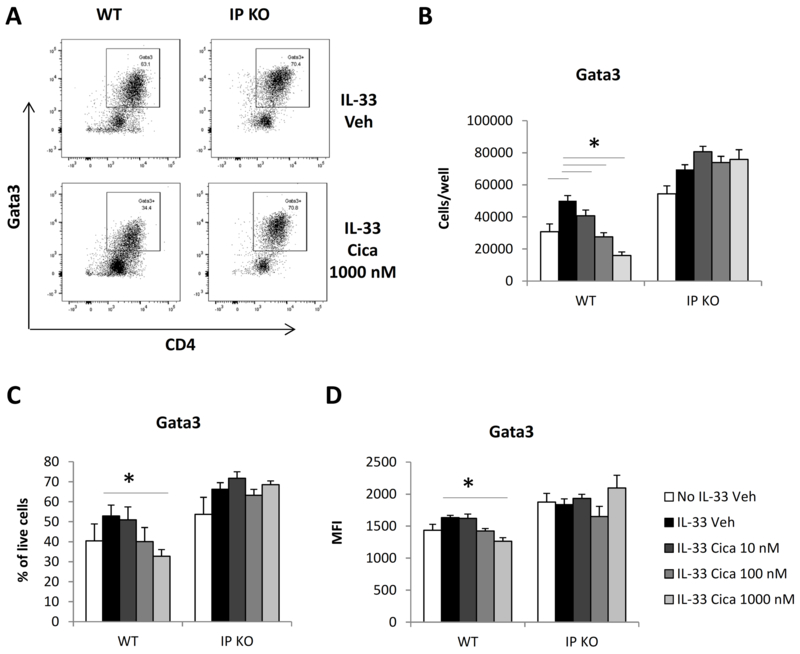
The differentiation of naïve CD4+ T cells into Th2 cells under IL-4 culture conditions requires the expression of the transcription factor Gata3 (17). In this study, IL-4 was endogenously produced in the presence of IL-33. To investigate whether Gata3 plays a role in the inhibitory effect of cicaprost on IL-33-induced type 2 cytokine production, we determined the protein expression of Gata3 in CD4+ T cells activated with IL-33 and treated with cicaprost at various concentrations or vehicle for 3 days. As shown in Fig. 3, we found that cicaprost at 1000 nM significantly decreased total numbers, the percentages, and Gata3 mean fluorescence intensity of Gata3-expressing CD4+ T cells from WT mice, but not IP KO mice. Therefore, the IP-dependent suppression of type 2 cytokine production by cicaprost was associated with decreased Gata3 protein expression, suggesting that PGI2/IP signaling inhibits Gata3 expression and suppresses Th2 differentiation.
Fig. 3.
Cicaprost decreased Gata3 expression in CD4+ T cells. Naïve CD4+ T cells from WT and IP KO mice were stimulated with anti-CD3 and anti-CD28 Abs in the absence or presence of IL-33 and were treated with vehicle or cicaprost for 3 days. The levels of Gata3 protein expression in the cells was determined by flow cytometry. A. The cells were analyzed for Gata3 expression after gating for lymphocytes and live cells. B. Total numbers of Gata3+CD4+ cells. C. The percentages of Gata3+ CD4+ cells. D. The mean fluorescent intensity (MFI) of Gata3. Data are combined of 3 experiments and presented as mean ± SEM. *, p < 0.05, n=9.
Cicaprost decreased IL-2 production, NFAT activation, and CD4+ T cell activation and proliferation
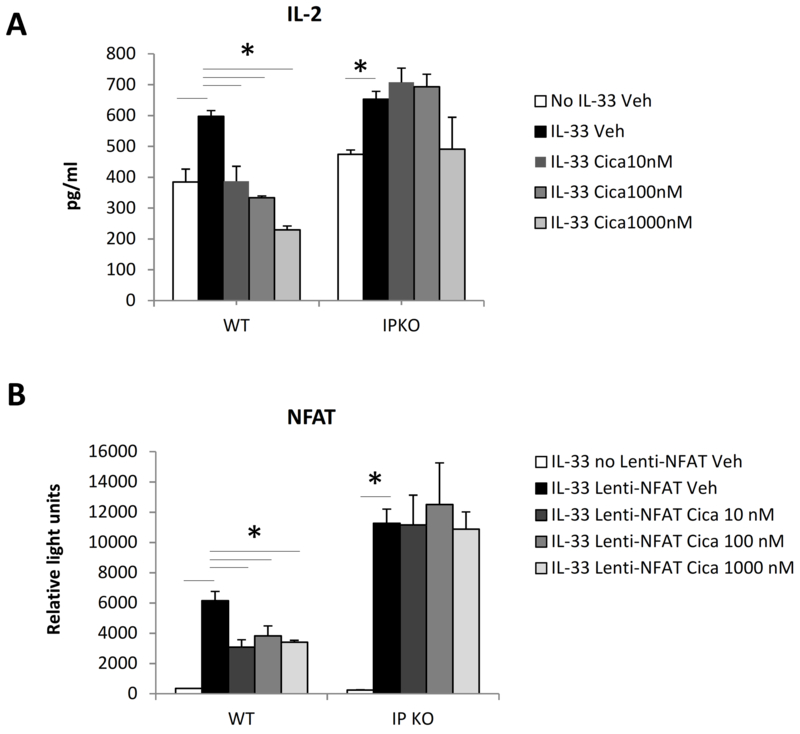
In vitro activation of mouse naïve CD4+ T cells results in the expression of IL-2, a growth factor critical for cell survival and proliferation (18). To investigate whether cicaprost inhibits IL-33-induced type 2 cytokine production by suppressing CD4+ T cell IL-2 production and cell activation, we measured IL-2 levels in the culture supernatant. We found that IL-33 significantly increased CD4+ T cell IL-2 expression (Fig. 4A). Cicaprost dose-dependently suppressed IL-2 production by WT CD4+ T cells, but not by IP KO CD4+ T cells, indicating that the suppressive effect of cicaprost is dependent on IP signaling (Fig. 4A).
Fig. 4.
Cicaprost decreased IL-2 production and NFAT activation in CD4+ T cells. Naïve CD4+ T cells from WT and IP KO mice were stimulated with anti-CD3 and anti-CD28 Abs in the absence or presence of IL-33 and were treated with vehicle or cicaprost. A. The levels of IL-2 in the culture supernatant at day 2 were determined by ELISA. B. Cells were transduced without or with NFAT-luciferase reporter lentiviral vector. Cells were harvested on day 3 for luciferase assay. Data are combined of 3 (A) and 2 (B) experiments and presented as mean ± SEM. *, p < 0.05, n=9 (A) and 6 (B).
To determine whether the suppressive effect of cicaprost on IL-2 production is associated with the inhibition of NFAT, a transcription factor that binds to the IL-2 gene promoter and activates IL-2 gene expression, we performed an NFAT functional assay with an NFAT-luciferase reporter lentiviral vector system. We found that cicaprost significantly suppressed NFAT function in WT cells, but not IP KO cells after 3 days of cell culture (Fig. 4B). These results suggest that cicaprost inhibits IL-2 production by attenuating NFAT activation.
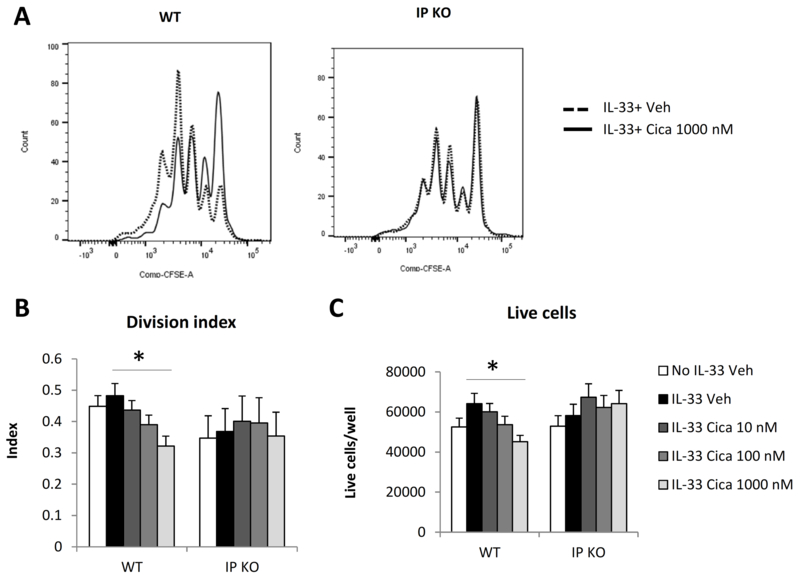
To further analyze the effect of cicaprost on cell proliferation, naïve CD4+ T cells were CFSE-labeled, activated with anti-CD3 and anti-CD28, and treated with IL-33 plus either vehicle or cicaprost. Cell proliferation was determined by CFSE dilution. As shown in Fig. 5, cicaprost at 1000 nM significantly decreased the cell division index and the total number of live cells of WT CD4+ T cells, but not IP KO CD4+ T cells (Fig. 5). These data indicate that cicaprost inhibited cell activation and proliferation in an IP-dependent manner.
Fig. 5.
Cicaprost inhibited CD4+ T cell proliferation. Naïve CD4+ T cells from WT or IP KO mice were CFSE-labeled and stimulated with anti-CD3 and anti-CD28 Abs in the absence or presence of IL-33, and were treated with vehicle or cicaprost for 3 days. A. Cell division was analyzed by flow cytometry. B. Cell division index. C. Total numbers of live cells. Data are combined of 5 (B and C) experiments and presented as mean ± SEM. *, p < 0.05, n=15.
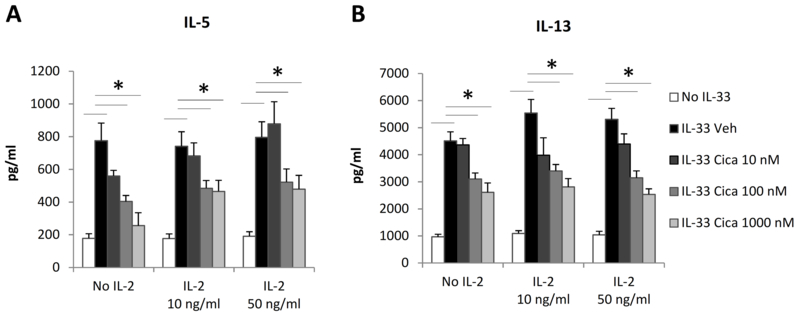
Cicaprost decreased IL-5 and IL-13 production by CD4+ T cells in the presence of exogenous IL-2
IL-2 not only stimulates CD4+ T cell survival and expansion, but also promotes Th2 differentiation in the presence of IL-4 and anti-IFN-γ (19). The correlation between IL-2 inhibition and type 2 cytokine suppression in cicaprost-treated cells suggests that decreased IL-2 expression may be a mechanism by which cicaprost subsequently inhibits IL-33-induced type 2 cytokine production. To test this hypothesis, we added IL-2 to the cell cultures, reasoning that if cicaprost inhibits type 2 cytokine production by affecting IL-2 production, addition of exogenous IL-2 should attenuate or abrogate the effect of cicaprost. We found that cicaprost still significantly inhibited IL-5 and IL-13 production in the presence of either 10 ng/ml or 50 ng/ml exogenous IL-2 (Fig. 6A and 6B), suggesting that other mechanisms downstream of IL-2 expression such as the IL-2 receptor signaling pathway are involved in the inhibitory effect of cicaprost on type 2 cytokine production.
Fig. 6.
Cicaprost decreased type 2 cytokine production by CD4+ T cells in the presence of exogenous IL-2. Naïve CD4+ T cells of WT mice were stimulated with anti-CD3 and anti-CD28 Abs in the absence or presence of IL-33 and IL-2 and were treated with vehicle or cicaprost for 3 days. The levels of (A) IL-5 and (B) IL-13 in the culture supernatant were determined by ELISA. Data are combined of 4 experiments and presented as mean ± SEM. *, p < 0.05, n=8–11.
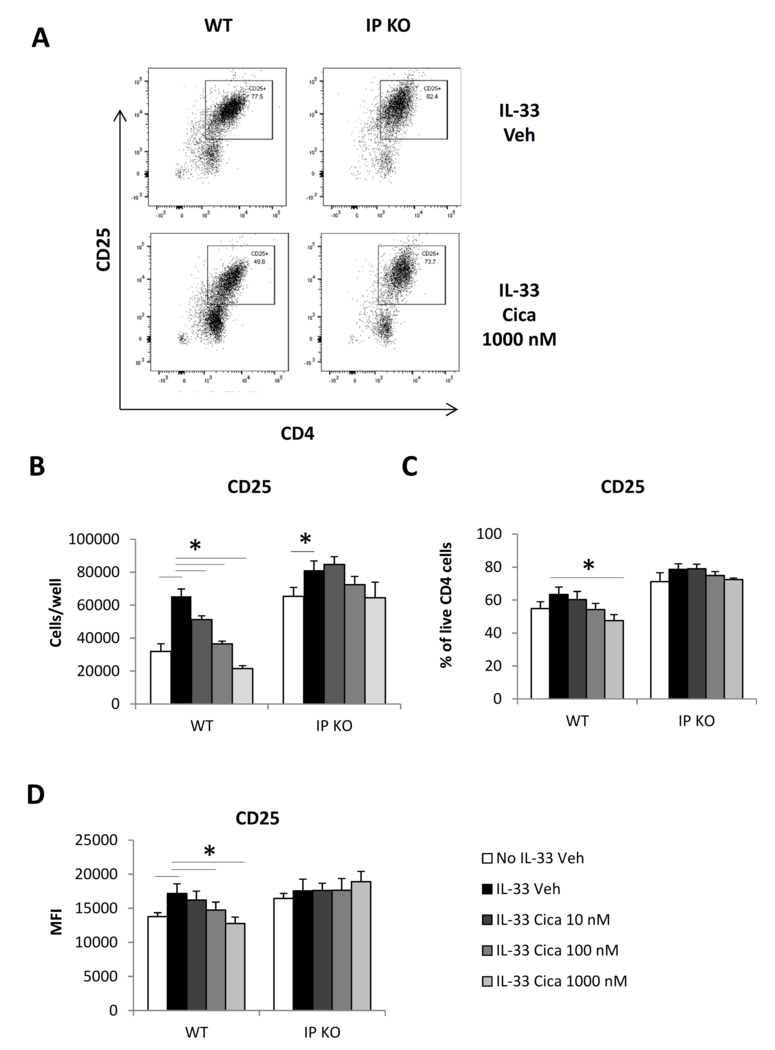
Cicaprost decreased CD25 expression on CD4+ T cells
To determine whether cicaprost attenuates the IL-2 signaling pathway, CD4+ T cells activated and treated with either cicaprost or vehicle for 3 days were stained with fluorochrome-labeled anti-CD25 antibody and analyzed for the expression of IL-2 receptor α chain (CD25) by flow cytometry. CD25 is a high-affinity IL-2 receptor subunit. The inducible expression of CD25 on naïve CD4+ T cells enables fully functional IL-2 receptors for the responses to IL-2 and is critical for naïve CD4+ T cell activation. We found that cicaprost dose-dependently decreased total numbers of CD25 expressing cells, and percentages, and CD25 mean fluorescence intensity compared with vehicle control (Fig. 7 A-D). As a comparison, cicaprost did not change percentages of TSLPR expressing cells and TSLPR MFI in WT or IP KO T cell culture in the presence of IL-33 (data not shown).
Fig. 7.
Cicaprost decreased CD25 expression in CD4+ T cells. Naïve CD4+ T cells from WT or IP KO mice were stimulated with anti-CD3 and anti-CD28 Abs in the absence or presence of IL-33 and were treated with vehicle or cicaprost for 3 days. The levels of CD25 protein surface expression were determined by flow cytometry. A. Flow plot of CD25 expression after the cells were gated for lymphocytes and live cells. B. Total numbers of CD25+CD4+ T cells. C. The percentages of CD25+CD4+ T cells. D. CD25 mean fluorescent intensity (MFI). Data are combined of 3 experiments and presented as mean ± SEM. *, p < 0.05, n=9.
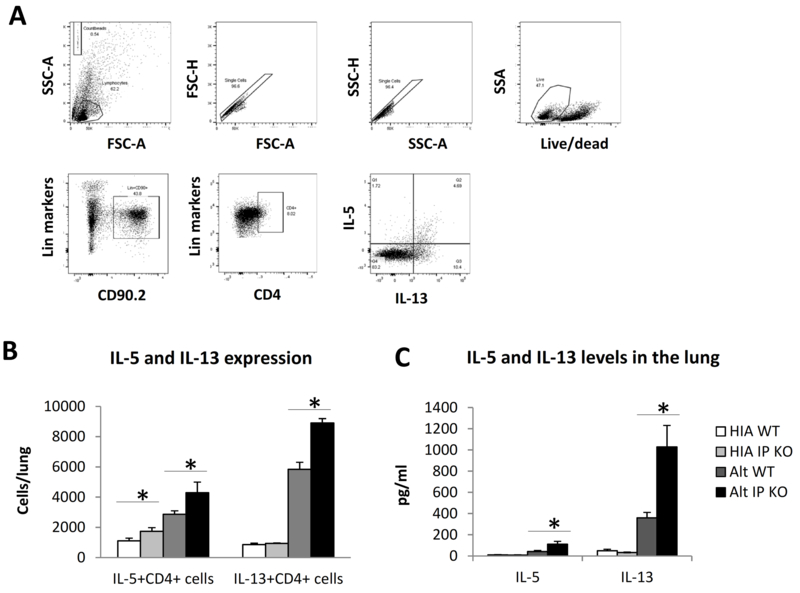
IP deficiency increased CD4+ T cell IL-5 and IL-13 responses in vivo
To investigate the in vivo relevance of IP-dependent inhibitory effect of cicaprost on IL-33-enhanced Th2 responses, we challenged WT and IP KO mice with Alternaria extract, a protease-containing substance that has been used as a model allergen to induce lung inflammation in mice (2). It has been shown that IL-33 receptor signaling is required for Alternaria-induced type 2 responses and exacerbation of allergic inflammation, eosinophilia, and IL-13 production in the lung (21, 22). We used a 10-day allergen sensitization and challenge model in which the adaptive immune system and CD4+ T cells are activated. We found that IP KO mice had significantly increased numbers of IL-5+CD4+ T cells and IL-13+CD4+ T cells in the lung and greater protein levels of IL-5 and IL-13 in the lung homogenate (Fig. 8). These results indicated that IP deficiency augmented type 2 CD4+ T cell responses and type 2 cytokine responses to Alternaria challenge, suggesting that endogenous PGI2 inhibits IL-33-enhanced Th2 responses.
Fig. 8.
IP deficiency increased Alternaria extract-induced Th2 responses in the lung. WT mice were challenged with Alternaria extract (Alt) (5 μg/mouse in 100 μl PBS) or heat-inactivated Alternaria extract (HIA) intranasally at days 0, 3, 6, and 9. The mouse lungs were harvested and lung cells were stimulated with PMA and ionomycin in the presence of GolgiStop for 4 h for flow cytometry analyses of intracellular IL-5 and IL-13 expression in lung CD4+ T cells. (A) Flow gating strategy. The cells were gated for lymphocytes, single cells, live cells, CD90.2+Lineage+ cells, and CD4+ cells for analyses of IL-5 and IL-13 expression. (B) The number of IL-5+CD4+ or IL-13+CD4+ cells cells in the lung. (C) The levels of IL-5 and IL-13 in the lung homogenate determined by ELISA. Data are combined of 3 experiments (B and C) and presented as mean ± SEM. *, p < 0.05, n=9–10.
DISCUSSION
The discovery of IL-33-induced Th2 polarization reveals an alternative Th2 differentiation pathway from the classical IL-4-driven Th2 differentiation pathway (5). We have previously published findings on the regulation of allergic responses and inflammation by lipid mediators formed in arachidonic acid metabolism (10, 14, 23). We have shown that PGI2 and IP signaling inhibit the effector cytokine production by Th2 cells that had been differentiated under classical Th2 conditions with IL-4 and anti-IFN-γ (15). In this study, we demonstrate that the PGI2 analog cicaprost and IP signaling negatively regulated IL-33-driven Th2 responses and reduced IL-4, IL-5, and IL-13 production and Gata3 protein expression in CD4+ T cells in a dose-dependent manner. The inhibitory effect of cicaprost is associated with decreased NFAT activation, IL-2 expression and CD4+ T cell proliferation, and correlated with lower surface expression of IL-2 receptor α chain (CD25) in CD4+ T cells. The data presented here suggest that IP signaling restrains IL-33-driven alternative Th2 responses in part by inhibiting IL-2 production and suppressing IL-2 receptor α chain expression. In addition, we demonstrated that IP KO mice had increased adaptive immunity, Th2 cell numbers, and type 2 cytokine responses in protease-containing allergen-induced lung inflammation. As IL-33 has been shown to be critical for Alternaria-induced lung type 2 responses (2, 21), these data suggest an in vivo inhibitory effect of PGI2 on IL-33-induced Th2 responses. To our knowledge, this is also the first report that PGI2 signaling inhibits Th2 immune responses in an allergen challenge model that does not use aluminum hydroxide as an adjuvant, increasing the potential clinical relevance of the inhibitory effect of PGI2 and its analogs in chronic allergic diseases such as asthma.
Naïve CD4+ T cells activated with anti-CD3 and stimulated by IL-33 produced IL-5 and IL-13, but not IL-4 (5). In our study, we found that IL-33 stimulated not only IL-5 and IL-13 expression, but also IL-4 production by either naïve WT BALB/c or IP KO CD4+ T cells after activation with anti-CD3 and anti-CD28. The difference in the findings may be explained by variations in the presence of costimulatory signals in our study and strength of TCR stimulation. In addition to studies focusing on the stimulatory functions of IL-33 on innate and adaptive type 2 responses (5, 24), more recent reports showed that IL-33 can act as an enhancer for effector cytokine production by Th1, Th17, and Treg cells (25–27). Our data here demonstrate that the PGI2 analog cicaprost and IP signaling restrain IL-33-induced Th2 cell expansion and type 2 cytokine production. Further research is needed to determine the effect of PGI2 on IL-33-mediated effects on other CD4+ T cell subsets.
IL-2 is a critical cytokine for CD4+ T cell activation and clonal expansion through autocrine and paracrine signaling. We demonstrate in this study that IL-33 significantly enhanced IL-2 production in WT and IP KO CD4+ T cells and cicaprost dose-dependently inhibited IL-33-induced IL-2 production in WT CD4+ T cells, but not in IP KO CD4+ T cells. Cicaprost also suppressed the IL-2 receptor α chain CD25 expression. The down-regulation of both IL-2 and IL-2R may contribute to cicaprost-mediated suppression of IL-2R signaling and Th2 differentiation. The suppressive effect of prostaglandins on CD25 expression and IL-2 receptor signaling has been reported in several studies. PGE2 inhibited IL-2 production and CD25 expression in human and bovine CD4+ T cells.(28–31) The cAMP-elevating agents forskolin and dbcAMP had similar inhibitory effect on IL-2 and CD25 expression by human CD4+ T cells, suggesting a role of cAMP pathway in PGE2-mediated inhibition of IL-2R expression, signaling and function.(28, 30) In a human cell culture study, the PGI2 analog iloprost inhibited CD25 expression by in vitro polarized Treg cells (32).
We found that cicaprost treatment decreased Gata3 expression in an IP-dependent manner, suggesting that cicaprost may inhibit Th2 responses mediated by Gata3 signaling pathway. Kurowska-Stolarska et al. reported that IL-33 by itself did not strongly induce Gata3 expression. IL-33 enhanced IL-5 and IL-13 expression in IL-4 KO and STAT6 KO CD4+ T cells, suggesting that the augmentation of IL-5 and IL-13 production by IL-33 is independent of IL-4 and STAT6 (5). However, in the presence of endogenous IL-4 as shown in this study, Gata3 protein was expressed at a high level and is likely play an enhancing role in Th2 differentiation. Although Kurowska-Stolarska et al. showed that IL-33 alone did not markedly increase GATA3 mRNA levels. Whether Gata3 is dispensable for IL-33-induced Th2 differentiation remains to be investigated. In this study, IL-33 did not further increase the levels of Gata3 expression; however, this does not exclude the possibility that cicaprost inhibits type 2 cytokine expression in the presence of endogenous IL-4 partially by suppressing Gata3 expression.
By using WT and IP KO mice, we were able to demonstrate that cicaprost-mediated inhibition of IL-33-induced CD4+ cell Th2 cytokine production, IL-2 production, and CD25 expression is IP-dependent. IL-33 plays an important role in the development of allergic diseases induced by protease-containing allergens such as Alternaria Alternata (2, 34). PGI2 analog-mediated inhibition of IL-33-induced Th2 responses in vitro suggests that PGI2 may limit Th2 differentiation and allergy development induced by protease-containing allergens in vivo. In a mouse model of Alternaria-induced lung inflammation (10 day protocol that induced adaptive immune responses), we found that IP KO mice had significantly augmented IL-5 and IL-13 responses and increased the numbers of IL-5+CD4+ cells and IL-13+CD4+ cells in the lung. These data suggest that PGI2 signaling through IP attenuates IL-33-induced Th2 cytokine responses in adaptive immune cells. We have recently reported that cicaprost inhibited IL-33-induced cytokine responses of type 2 innate lymphoid cells (ILC2) (33). Using a mouse model of Alternaria extract-induced lung inflammation (short protocol, 4 days of Alternaria challenge), we revealed that endogenous PGI2 has an inhibitory function on ILC2 cells (33). We demonstrated that IP-deficiency resulted in greater ILC2 responses compared to ILC2 responses in WT mice, and conversely cicaprost inhibited ILC2 response in WT mice (33). Based on our previously published findings and the results here, it appears that PGI2 and IP receptor signaling limits IL-33- and IL-4-induced Th2 cell responses, as well as ILC2 function, highlighting the broad regulatory properties of PGI2 in adaptive and innate immunity and inflammation.
Taken together, the data presented here indicate that the PGI2 analog cicaprost significantly down-regulates IL-33-induced Th2 differentiation and type 2 cytokine production, likely via suppressing IL-2 production, CD25 expression, and Gata3 expression. PGI2 and its analog iloprost are Food and Drug Administration (FDA)-approved drugs for the clinical use to treat pulmonary hypertension (35) and could be a potential therapeutic drug option for IL-33-related type 2 immune disorders such as allergic diseases and asthma.
Supplementary Material
Footnotes
Grant supports: This work was supported by the National Institute of Health (R01 HL 090664, U19 AI 95227, R01 AI 111820, T32 GM 007347, F30 AI118376–01, R56 AI076411), the Department of Veterans Affairs (2I01BX 000624)
REFERENCES
- 1.Moussion C, Ortega N, and Girard JP. 2008. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One 3: e3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iijima K, Kobayashi T, Hara K, Kephart GM, Ziegler SF, McKenzie AN, and Kita H. 2014. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J Immunol 193: 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, and Girard JP. 2007. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A 104: 282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, and Takei F. 2014. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, Niedbala W, McKenzie AN, Teixeira MM, Liew FY, and Xu D. 2008. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol 181: 4780–4790. [DOI] [PubMed] [Google Scholar]
- 6.Peebles RS Jr., Hashimoto K, Morrow JD, Dworski R, Collins RD, Hashimoto Y, Christman JW, Kang KH, Jarzecka K, Furlong J, Mitchell DB, Talati M, Graham BS, and Sheller JR. 2002. Selective cyclooxygenase-1 and −2 inhibitors each increase allergic inflammation and airway hyperresponsiveness in mice. Am.J.Respir.Crit Care Med 165: 1154–1160. [DOI] [PubMed] [Google Scholar]
- 7.Cheng J, Dackor RT, Bradbury JA, Li H, DeGraff LM, Hong LK, King D, Lih FB, Gruzdev A, Edin ML, Travlos GS, Flake GP, Tomer KB, and Zeldin DC. 2016. Contribution of alveolar type II cell-derived cyclooxygenase-2 to basal airway function, lung inflammation, and lung fibrosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 30: 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaffar Z, Ferrini ME, Buford MC, FitzGerald GA, and Roberts K. 2007. Prostaglandin I2-IP signaling blocks allergic pulmonary inflammation by preventing recruitment of CD4+ Th2 cells into the airways in a mouse model of asthma. J.Immunol 179: 6193–6203. [DOI] [PubMed] [Google Scholar]
- 9.Idzko M, Hammad H, van Nimwegen M, Kool M, Vos N, Hoogsteden HC, and Lambrecht BN. 2007. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. The Journal of clinical investigation 117: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W, Zhang J, Goleniewska K, Dulek DE, Toki S, Newcomb DC, Cephus JY, Collins RD, Wu P, Boothby MR, and Peebles RS Jr. 2016. Prostaglandin I2 Suppresses Proinflammatory Chemokine Expression, CD4 T Cell Activation, and STAT6-Independent Allergic Lung Inflammation. J Immunol 197: 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagao K, Tanaka H, Komai M, Masuda T, Narumiya S, and Nagai H. 2003. Role of prostaglandin I2 in airway remodeling induced by repeated allergen challenge in mice. Am.J.Respir.Cell Mol.Biol 29: 314–320. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi Y, Tokuoka S, Masuda T, Hirano Y, Nagao M, Tanaka H, Inagaki N, Narumiya S, and Nagai H. 2002. Augmentation of allergic inflammation in prostanoid IP receptor deficient mice. Br.J.Pharmacol 137: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffar Z, Wan KS, and Roberts K. 2002. A key role for prostaglandin I2 in limiting lung mucosal Th2, but not Th1, responses to inhaled allergen. J.Immunol 169: 5997–6004. [DOI] [PubMed] [Google Scholar]
- 14.Zhou W, Goleniewska K, Zhang J, Dulek DE, Toki S, Lotz MT, Newcomb DC, Boswell MG, Polosukhin VV, Milne GL, Wu P, Moore ML, FitzGerald GA, and Peebles RS. 2014. Cyclooxygenase inhibition abrogates aeroallergen-induced immune tolerance by suppressing prostaglandin I2 receptor signaling. The Journal of allergy and clinical immunology 134: 698–70500000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Blackwell TS, Goleniewska K, O’Neal JF, FitzGerald GA, Lucitt M, Breyer RM, and Peebles RS Jr. 2007. Prostaglandin I2 analogs inhibit Th1 and Th2 effector cytokine production by CD4 T cells. J.Leukoc.Biol 81: 809–817. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, and FitzGerald GA. 2002. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science 296: 539–541. [DOI] [PubMed] [Google Scholar]
- 17.Pai SY, Truitt ML, and Ho IC. 2004. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc.Natl.Acad.Sci.U.S.A 101: 1993–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sojka DK, Bruniquel D, Schwartz RH, and Singh NJ. 2004. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol 172: 6136–6143. [DOI] [PubMed] [Google Scholar]
- 19.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, and Paul WE. 2004. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A 101: 3880–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malek TR 2008. The biology of interleukin-2. Annu Rev Immunol 26: 453–479. [DOI] [PubMed] [Google Scholar]
- 21.Anderson EL, Kobayashi T, Iijima K, Bartemes KR, Chen CC, and Kita H. 2016. IL-33 mediates reactive eosinophilopoiesis in response to airborne allergen exposure. Allergy 71: 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snelgrove RJ, Gregory LG, Peiró T, Akthar S, Campbell GA, Walker SA, and Lloyd CM. 2014. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol 134: 583–592. e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou W, Newcomb DC, Moore ML, Goleniewska K, O’Neal JF, and Peebles RS Jr. 2008. Cyclooxygenase inhibition during allergic sensitization increases STAT6-independent primary and memory Th2 responses. J.Immunol 181: 5360–5367. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, and Kastelein RA. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23: 479–490. [DOI] [PubMed] [Google Scholar]
- 25.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, and Paul W. 2009. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A 106: 13463–13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumann C, Bonilla WV, Frohlich A, Helmstetter C, Peine M, Hegazy AN, Pinschewer DD, and Lohning M. 2015. T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc Natl Acad Sci U S A 112: 4056–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BM, Lohning M, Belkaid Y, Fallon PG, and Powrie F. 2014. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513: 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anastassiou ED, Paliogianni F, Balow JP, Yamada H, and Boumpas DT. 1992. Prostaglandin E2 and other cyclic AMP-elevating agents modulate IL-2 and IL-2R alpha gene expression at multiple levels. J.Immunol 148: 2845–2852. [PubMed] [Google Scholar]
- 29.Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc’h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, and Dubinett SM. 2005. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J.Immunol 175: 1483–1490. [DOI] [PubMed] [Google Scholar]
- 30.Rincón M, Tugores A, López-Rivas A, Silva A, Alonso M, De Landázuri MO, and López-Botet M. 1988. Prostaglandin E2 and the increase of intracellular cAMP inhibit the expression of interleukin 2 receptors in human T cells. Eur J Immunol 18: 1791–1796. [DOI] [PubMed] [Google Scholar]
- 31.Maślanka T, Spodniewska A, Barski D, Jasiecka A, Zuśka-Prot M, Ziółkowski H, Markiewicz W, and Jaroszewski JJ. 2014. Prostaglandin E₂ down-regulates the expression of CD25 on bovine T cells, and this effect is mediated through the EP4 receptor. Vet Immunol Immunopathol 160: 192–200. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Li H, Zhang X, Wen D, Yu F, Yang S, Jia X, Cong B, and Ma C. 2013. Prostaglandin I2-IP signalling regulates human Th17 and Treg cell differentiation. Prostaglandins Leukot Essent Fatty Acids 89: 335–344. [DOI] [PubMed] [Google Scholar]
- 33.Zhou W, Toki S, Zhang J, Goleniewksa K, Newcomb DC, Cephus JY, Dulek DE, Bloodworth MH, Stier MT, Polosuhkin V, Gangula RD, Mallal SA, Broide DH, and Peebles RS Jr. 2016. Prostaglandin I2 Signaling and Inhibition of Group 2 Innate Lymphoid Cell Responses. American journal of respiratory and critical care medicine 193: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kita H 2015. ILC2s and fungal allergy. Allergol Int 64: 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorris SL, and Peebles RS. 2011. PGI2 as a regulator of inflammatory diseases. Mediators of inflammation 2012: 926968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.