Abstract
The aim of this study was to evaluate the effect of a single dose of subcutaneous (s.c.) meloxicam administered at the time of knife and band castration on inflammatory response and wound healing over 56-d post-castration. Seventy-two Angus crossbred calves (47.3 ± 6.70 kg of body weight [BW] and 7 to 8 d of age) were randomly assigned according to a 3 × 2 factorial design assessing castration method: sham (CT), band (BA), and knife (KN) castration, and pain mitigation: non-medicated (NM) and medicated calves (M) injected s.c. with meloxicam (0.5 mg/kg of BW). Calf BW, rectal temperature, swelling (“0”: no swelling; “4”: swelling needing intervention) and healing score (“1” to “5” with “5” being completely healed), scrotal circumference, and maximum scrotal temperature were measured on d −1, immediately before castration (d 0), and weekly thereafter over a 56-d period. Blood samples for haptoglobin (Hp), serum amyloid-A (SAA), and complete blood cell count were collected according to the same schedule. Hair samples were collected on d −1, 28, and 56 to determine cortisol concentrations. Standing and lying behaviors were measured using accelerometers that were placed on the calves on d −1 until d 35, and visual observations of behaviors related to pain were recorded once a week for 35 d. Knife-castrated calves achieved swelling scores of “3” and “2” between d 7 and 14, which was sooner (Z< 0.05) than in BA calves (from d 14 to 35). In addition, greater (P = 0.03) concentrations of SAA were observed in BA calves (76.9 ± 0.12 g/liter) compared with CT (57.6 ± 0.12 g/liter) and KN (51.6 ± 0.12 g/liter) from d 7 to 35. Healing scores of “2” and “4” tended to be achieved sooner (Z < 0.10) in KN calves than in BA calves, although healing scores of “3” tended to be achieved sooner (Z < 0.10) in BA calves than KN calves. No differences (P > 0.10) were observed among treatments for hair cortisol on d −1 and 28, but on d 56, hair cortisol concentrations in BA–NM calves were greater (P > 0.05) than for CT-NM, BA-M, KN–NM, and KN–M, and tended to be greater (P = 0.08) than for CT–M calves. Lying duration tended (P = 0.10) to be greater and suckling behavior tended (P = 0.08) to be lower in NM than M calves. A single s.c. injection of meloxicam did not reduce long-term inflammatory responses or improve wound healing; however, it may be useful in reducing pain and stress in band castrated calves as evidenced by reduced hair cortisol concentrations up to 56 d post-castration.
Keywords: beef calves, castration, healing, pain mitigation, welfare
INTRODUCTION
In North America anesthetics and analgesics are not routinely administered to calves prior to castration (Dockweiler et al., 2013; Moggy et al., 2017), even though castration is a painful procedure (Molony et al., 1995; Stafford et al., 2002). Knife castration causes more acute pain at the time of the incision, but healing typically occurs more quickly (Molony et al., 1995; Meléndez et al., 2017), while band castration produces a longer-lasting pain and the lesion heals more slowly (Molony et al., 1995; Marti et al., 2017a).
The Canadian and American Veterinary Medical Associations (CVMA, 2012; AVMA, 2014) indicate that all ruminants benefit from the use of systemic analgesia regardless of the castration technique. Meléndez et al. (2018) observed that a single dose of subcutaneous (s.c.) meloxicam reduced concentrations of substance P and tail flicks after castration in 1-wk-old calves, and Creutzinger et al. (2017) reported a reduction in hair cortisol concentrations 14 d after knife castration in 1- to 3-mo-old calves. However, to the author’s knowledge, there are no studies evaluating the effect of s.c. meloxicam on the inflammatory response and wound healing after castration. Mintline et al. (2014) reported that flunixin meglumine did not reduce inflammation or improve the rate of wound healing in 1-mo knife castrated calves. However, meloxicam has an elimination half-life of 22 h (Stock and Coetzee, 2015) providing long-lasting analgesia and is a cyclo-oxygenase (COX) 2-preferential inhibitor, while flunixin meglumine has a half-life of 3 to 8 h and is a COX-1 preference inhibitor (Anderson et al., 1990). We hypothesized that s.c. meloxicam may reduce the inflammatory response and improve the healing process after knife or band castration as it has a longer half-life than other nonsteroidal anti-inflammatory drugs (NSAIDs). The aim of this study was to evaluate the effect of a single dose of s.c. meloxicam on inflammatory responses, wound healing, and long-term stress after knife or band castration in 1-wk-old beef calves.
MATERIALS AND METHODS
All procedures described within this study were approved by the Animal Care and Use Committee of the Lethbridge Research and Development Centre (ACC1410) and University of Calgary Animal Care Committee (AC14-0159) according to the guidelines established by the Canadian Council on Animal Care (2009).
This article evaluates the effect of s.c. meloxicam and methods of castration in 1-wk-old calves assessing the inflammatory response and wound healing, and was part of a larger study, which included the assessment of acute pain (Meléndez et al., 2018).
Animals, Housing, and Treatments
A total of 72 Angus crossbred cow–calf pairs were transported approximately 30 km on 2 separate days (group 1: 36 cow–calf pairs on March 28, 2015; group 2: 36 cow–calf pairs on April 3, 2015) from a local ranch to the LRC (Lethbridge Research and Development Centre, Agriculture and Agri-Food Canada, Lethbridge, Alberta, Canada) feedlot within the first few days after the calves were born. To ensure that calves were the same age at the time of the experiment, calves were divided into two groups (36 calves/group) that were castrated 10 d apart (36 calves on March 31, 2015 and, 36 calves on April 9, 2015) following the same experimental protocols. At the beginning of the experiment (d −1) calves weighed an average of 47.3 ± 6.70 kg and were 7 to 8 d of age. The experiment was conducted as a completely randomized block design where calves were blocked by body weight (BW) and distributed according to a 3 × 2 factorial design assessing castration method: 1) sham castration (CT) where calves were handled in a similar manner and for a similar amount of time as castrated calves, including testicle manipulation; 2) band castration (BA) using rubber rings (Elastrator Pliers and Rings, Kane Veterinary Suppliers Ltd., Edmonton); and 3) knife castration (KN) performed with a Newberry knife (Syrevet Inc., Waukee, IA) and pain mitigation; 1) medicated calves injected with meloxicam (M; 0.5 mg/kg of BW) (Metacam 20 mg/mL, Boheringer Ingelhein, Burlington, Ontario, Canada); 2) non-medicated calves injected with lactated ringer’s solution (NM; Lactated Ringer’s Irrigation, Baxter Canada, Mississauga, Ontario, Canada) with the same volume as medicated calves. During castration, calves were restrained using a tip table (Calf Roper, Ram-Bull Ltd., Barons, Alberta, Canada) and the castrations were performed by an experienced veterinarian. Medication was injected s.c. immediately prior to castration using a 1.6- × 25-mm needle in the neck of the calves.
During the first 35 d of the study, calves were allocated with their dams, into one of six pens (group 1: 3 pens of 36 × 22 m; 12 cow–calf pairs per pen; group 2: 3 pens of 40 × 27; 12 cow–calf pains per pen) containing wind break fencing, calf shelters (2.4 × 3.6 × 1.4 m) and deep straw bedding. Cows were fed alfalfa grass hay from bale feeders and were provided with fresh water ad libitum from a water trough (0.8 × 0.4 × 0.5 m) located at the side or middle of the pen depending on the group. After d 35 (May 4, 2015 for group 1, and May 14, 2015, for group 2), cow–calf pairs were moved to an adjacent pasture (206.3 ha) consisting of a mixture of bromegrass, orchardgrass and creeping red fescue, and annual cereal crops (fall triticale).
The day of castration was considered d 0 of the study. Samples were collected in the morning between 8:00 and 10:30.
Measurements and Sample Collection
Haptoglobin, serum amyloid-A, and complete blood cell count.
A 6-mL blood sample (non-additive tube; BD Vacutainer, BD Vacutainer, Becton Dickinson Co., Frankin Lakes, NJ) for the analysis of haptoglobin (Hp) and serum amyloid-A (SAA) and a 4-mL blood sample (ethylenediaminetetraacetate; BD Vacutainer, Becton Dickinson Co., Frankin Lakes, NJ) for complete blood cell count (CBC) were collected via jugular venipuncture from all calves on d −1 and d 0, and weekly thereafter until d 56. Samples for Hp and SAA were centrifuged at 1,600 × g at 4 °C for 15 min, and serum were decanted and stored at −20 °C until analysis. Haptoglobin assays were performed at the University of Guelph (Animal Health Laboratory, University of Guelph, Guelph, Ontario, Canada) using a Roche Cobas c501 biochemistry analyzer (Roche Diagnostics, Laval, QC, Canada). The interassay CV was 7.6%. The SAA samples were analyzed using an enzyme linked immunosorbent assay (Tridelta Phase Serum Amyloid A, Tridelta Development Ltd., Maynooth, Kildare, Ireland). The intra- and interassay CV were 13.7 and 7.5%, respectively. The CBC was measured using a HemaTrue Hematology Analyzer (Heska, Loveland, CO) to obtain white blood cell count (WBC), red blood cell count (RBC), and neutrophil-to-lymphocyte ratio (N:L).
Hair cortisol.
The hair on the forehead of each calf was shaved as close as possible to the skin with clippers (Lister, Wahl Clippers Corp., Sterling, IL) to determine hair cortisol concentrations on d −1, 28, and 56 d after castration. Hair was shaved on d −1 to remove hair growth prior to the castration event. Collection days were selected based on growth rate of the hair (0.6 to 1 cm/mo; Comin et al., 2011) such that sufficient hair had regrown to be analyzed. Hair samples were processed and quantification of cortisol was determined using an immunoassay technique (Salimetric Assay Kit, State College, PA) following Moya et al. (2013). The intra- and interassay CV were 6.3 and 11.2%, respectively.
Maximum scrotal temperature, scrotal circumference, and swelling and healing score.
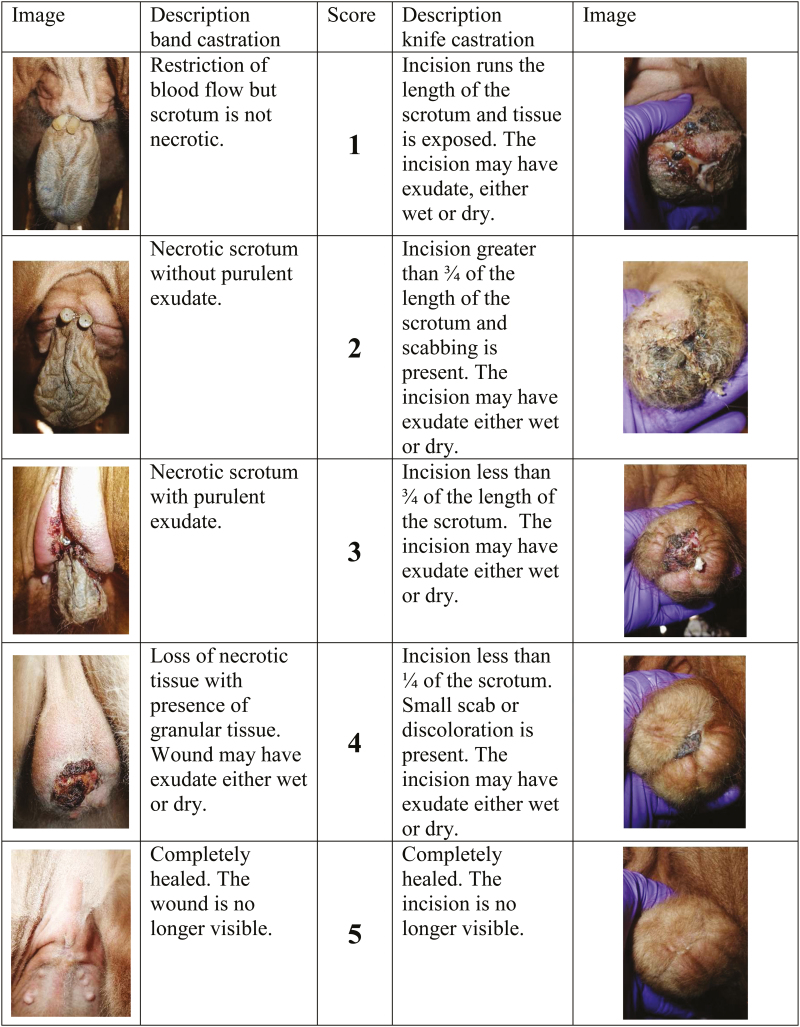
On d −1, immediately prior to castration (d 0) and weekly thereafter until d 56, thermographic images of the scrotal area were taken using a Flir E60 infrared camera and processed with a ThermCam QuickView 1.3 (Flir systems Inc. Burlington, ON, Canada) to measure maximum scrotal temperature (MST) to identify potential changes in blood flow and inflammation of the scrotal site. Scrotal circumference was measured using scrotal tape (Reliabull, Lane Manufacturing, Denver, CO) on the same sampling days as described above. The clinical state of the scrotum (swelling score) was scored using an 11-point scale (Molony et al., 1995), which was collapsed into the following five scores: 0) no swelling, inflammation or infection visible or palpable; 1) increasing degree of swelling without obvious erythema; 2) increasing degree of swelling with obvious erythema but without pus; 3) presence of pus with increasing inflammatory response; and 4) presence of pus with inflammatory response that needs an intervention. Wound healing or incision state for knife-castrated calves was assessed using a 5-point scale (Figure 1; previously described by Mintline et al. (2014). Wound healing of band castrated calves was also assessed using a 5-point scale (Figure 1; Marti et al., 2017a).
Figure 1.
Healing score (5-point score) used to evaluate wounds associated with knife (Mintline et al. 2014) and band castration in beef calves.
Stride length.
Calf stride length was measured 1 d before castration, and weekly until the end of the study (d 56). Calves were video recorded (Panasonic WVCP474; Panasonic Canada, Inc., Mississauga, ON, Canada) as they traversed a 3-m-long and 1-m-wide alley located immediately after the chute. Using video editing software (GOM Media Player, GOM lab, Seoul, South Korea), two pictures capturing when both hooves from the back limbs were in contact with the ground were retrieved from the video and step length was measured as the distance between the hind hooves using image editing software (ImageJ, Bethesda, MD).
Behavior.
Behavioral data were collected using accelerometers and visual observations for 35 d after castration when calves were housed within their experimental pens. On d −1 calves were fitted with an accelerometer data logger (Hobo Pedant G; Onset Computer Corp., Bourne, MA) that was attached between the hock and the fetlock of the hind legs with vet wrap (Professional Preference, Calgary, Canada) and covered with a foam pad to protect the animals from abrasions. The accelerometers were set to log data at 1 min intervals, and they were changed weekly and placed on the opposite leg to minimize lesions over the 35-d study period. Data from the data loggers were recovered using HOBOware software (Onset Computer Corporation, Bourne, MA) and exported to a text file, which was processed according to the method of Ledgerwood et al. (2010) to obtain standing and lying time (total time standing or lying during the day, %), standing and lying duration (mean standing and lying bouts duration, min) and standing and lying bouts (n) (UBC AWP, 2013). For visual observations of behaviors related to pain, calves were individually identified with numbered penning back tags (Wheelntree Enterprise, Sherwood Park, Canada) and two cameras per pen (2.0MP Bullet Camera, Avigilon, Vancouver, British Columbia, Canada) were mounted on steel poles 6 m above the pens to record video continuously for 8 h/d. The video recordings were analyzed with Observer XT software (Noldus Information Technology Inc., Wageningen, The Netherlands). Observation time was the same throughout the study, 2 min of continuous video every 10 min for a 3-h period, 2 to 5 h relative to when castration was done for each pen on d 0 (12 calves per pen; 6 calves per treatment), and after samples were collected once a week (d 7, 14, 21, 28, and 34 post-castration). An intraclass correlation coefficient with a 95% CI was used to determine inter-rater and intra-rater reliability for two different observers. The behaviors evaluated were: eating (time calves spent suckling or consuming straw/hay from the ground or feeder; min), tail flicking (number of times the tail moved to one side beyond the widest part of the rump), foot stamping (number of times the hind legs were lifted and forcefully placed on the ground or kicked outwards while standing), head turning (number of times the head was turned and touched the side of the calf’s body, including grooming), and lesion licking (number of times the head was turned to lick the scrotal area). All the behaviors related to pain were scored while animals were standing.
Performance and rectal temperature.
Body weight and rectal temperature (GLA M750 Livestock Thermometer, San Luis Obispo, CA) were collected on d −1 and d 0 prior to castration, and weekly until the scrotums of all band castrated calves sloughed off (d 56).
Calculations and Statistical Analyses
The schematic boxplot and proc univariate procedures of SAS (SAS 9.4, SAS Inst. Inc., Cary, NC) were used for outlier detection and normality testing. Hp, SAA, and N:L ratio were transformed to a log-scale (Napierian logarithm), and behavioral data were transformed to root-square + 1 to achieve a normal distribution prior to statistical analysis. Data from accelerometers were summarized by weeks.
Animal was the experimental unit and data were analyzed by two different periods, period 1, from d 7 to d 35 when calves were allocated in pens, and period 2, from d 42 to 56 when calves were allocated in pasture, if period did not differ, data were analyzed together. Performance data were analyzed using a mixed-effects model (SAS 9.4, SAS Inst. Inc., Cary, NC) including castration method, medication, and their interactions as a main effect and pen and animal nested within pen as random effects. Initial BW was used as a covariate for the analysis of performance. Hair cortisol, rectal temperature, MST, scrotal circumference, stride length, and all transformed data described above were analyzed using a mixed-effects model (SAS 9.4, SAS Inst. Inc., Cary, NC) with repeated measures. The model included castration method, medication, time, and their interactions as main effects and pen and animal within pen as random effects. Data collected on d −1 and d 0 was averaged and used as a covariate. For hair cortisol only data collected on d −1 was used as a covariate. There were no baseline data for behavior and for stride length as the data collected on d 0 contained animals that were already castrated. Time was considered a repeated factor, and for each analyzed variable, pen and animal within pen (the error term) was subjected to three variance–covariance structures: compound symmetry, autoregressive order 1, and unstructured. The covariance structure that minimized Schwarz’s Bayesian information criterion was considered the most desirable analysis. Significance was established at P ≤ 0.05 and trends at 0.05 > P ≤ 0.10.
The effect of castration method and medication (without including CT) on the time to reduce swelling from the maximum score (score 5) to no swelling (score 0), and to achieve each healing score were analyzed with a Wilcoxon–Mann–Whitney test (SAS 9.4, SAS Inst. Inc., Cary, NC). In addition, the univariate procedure (SAS 9.4, SAS Inst. Inc., Cary, NC) was used to calculate medians and the 95% distribution-free confidence limits. Significance was established at Z ≤ 0.05 and trends at 0.05 < Z ≤ 0.10.
RESULTS AND DISCUSSION
The lack of medication effect on APP concentrations in this study was similar to the findings of Brown et al. (2015), who reported no differences in Hp concentrations when new born calves were castrated with or without the administration of oral meloxicam. In contrast, in the same study conducted by Brown et al. (2015), as well as a study by Roberts et al. (2015), it was found that oral meloxicam reduced peak Hp concentrations in calves castrated at weaning. Roberts et al. (2018) observed that the reduction of Hp concentrations after oral meloxicam administration was only visible after surgical castration vs. band castration in weaned beef calves. The use of other NSAIDs (ketoprofen or carprofen) also mitigated inflammation after castration by decreasing Hp concentrations in 5.5-mo-old calves after knife and band methods (Early and Crowe, 2002; Pang et al., 2006). In addition, there were no effects of medication or medication × time on MST, scrotal circumference, or swelling score in this study.
There were considerable differences between methods of castration with regard to swelling score (Tables 1 and 2). Knife-castrated calves achieved swelling scores of “3” and “2” between d 7 and 14, which was sooner (Z = 0.01 and Z = 0.06, respectively) than in BA calves (from d 14 to 35; Table 1). Band castration causes ischemia, which results in necrosis and inflammation (Pang et al., 2006). Molony et al. (1995) explained that inflammation and sepsis appeared after contamination of the site when the skin breaks down approximately 6 d after band castration, and the inflammatory process might last until the testicles slough off, which in this study started on d 28 and lasted until d 56. In contrast, knife castration is conducted by creating an incision, and crushing and cutting the spermatic cords, which results in inflammation lasting up to 14 d (Marti et al., 2017b). Likewise, as observed in this study, scrotal circumference was greater (P < 0.05) in KN than BA and CT on d 7 (13.6 ± 0.43 cm vs. 11.5 ± 0.41 cm, 11.3 ± 0.42 cm, respectively) and 14 (12.7 ± 0.43 cm vs. 11.5 ± 0.41 cm, 11.3 ± 0.42 cm, respectively). The swelling score results were in agreement with the results obtained for the concentrations of acute phase proteins (APP; Table 3). The APPs such as SAA and Hp are extensively used as indicators of infection, inflammation, surgical trauma, and stress in cattle (Horadadoga et al., 1999; Murata et al., 2004). The initial response of the immune system to a noxious stimulus is local inflammation. When infection and tissue injuries overcome the normal reaction of the local defense system, the animal responds by activating APP. In this study, greater (P = 0.03) SAA concentrations were observed between d 7 and d 35 in BA (76.9 ± 0.12 μg/mL) compared with CT (57.6 ± 0.12 μg/mL) and KN (51.6 ± 0.12 μg/mL) calves. However, no differences (P > 0.10) in Hp concentrations were found over the duration of the experiment. The lack of differences in Hp observed between CT and KN calves may be related to when those samples were collected and not because knife castration did not cause inflammation. Meléndez et al. (2018) observed that KN calves had greater concentrations of SAA compared to CT and BA calves as samples were collected from d 1 to 7, which is when greater swelling scores are observed in knife-castrated calves. Furthermore, Horadadoga et al. (1999) found that SAA was elevated more by acute inflammatory cases rather than chronic inflammatory cases after clinical and postmortem examinations in 81 cattle. Similarly, Alsemgeest et al. (1994) compared serum concentrations of SAA and Hp in cattle, and found that the Hp/SAA ratio differed depending on the stage of the infection, with SAA concentrations being greater than Hp concentrations when acute inflammation was observed and the opposite when the inflammation was chronic. Thus, we hypothesize the lack of effect of castration on Hp may indicate that BA calves did not experience chronic inflammation although inflammation lasted for 35 d, or that greater stimulation associated with inflammation was needed to increase the concentrations of Hp (Kujala et al., 2010). In addition, it was not possible to determine which of the hypotheses was more likely as acute and chronic inflammatory reactions are mediated by different immune cells and they were not examined in this study. Another major factor to consider is the effect of age at the time of castration on pain mitigation, which may play an important role in the APP response and inflammation. Marti et al. (2017a) did not find differences in Hp concentrations between calves castrated on d 7, in 2- and 4-mo-old calves. However, Roberts et al. (2018) found Hp concentrations were greater in calves castrated on arrival to the feedlot, which may be associated with greater scrotal damage and inflammation experienced in older calves. Moreover, in this study, CBCs were found to be within the normal ranges for WBC, RBC, platelet count, and N:L ratio reported for cattle of similar age (Jones and Allison, 2007), although an effect of medication was observed for RBC (Table 3).
Table 1.
Swelling and healing score of band and knife castrated Angus crossbreed calves castrated at 1 wk of age from d 7 post-castration until the end of the study (d 56)
| Treatment1 | |||||||
|---|---|---|---|---|---|---|---|
| BA | KN | P-value2 | |||||
| N | Median | 95% FCL3 | N | Median | 95% FCL3 | ||
| Swelling score | |||||||
| 4 | — | — | — | — | — | — | — |
| 3 | 6 | 28 | 14–28 | 5 | 14 | 14 | 0.01 |
| 2 | 4 | 28 | 28–35 | 2 | 7 | 7 | 0.06 |
| 1 | 23 | 7 | 7–42 | 21 | 7 | 7–21 | 0.34 |
| 0 | 9 | 42 | 35–56 | 21 | 28 | 28–49 | <0.01 |
| Healing score | |||||||
| 1 | 23 | 7 | 7 | 8 | 7 | 7 | 0.99 |
| 2 | 21 | 14 | 14–21 | 22 | 7 | 7–14 | <0.001 |
| 3 | 20 | 28 | 14–42 | 15 | 21 | 21–35 | 0.07 |
| 4 | 21 | 35 | 21–56 | 20 | 28 | 28–49 | 0.01 |
| 5 | 9 | 42 | 42–56 | 20 | 49 | 35–56 | 0.97 |
1BA = calves castrated with rubber rings; KN = calves castrated with a Newberry knife.
2 P-value = castration effect.
3 n = number of animals observed at each score; median = median days to reach each healing score relative to day of castration; FCL = 95% confidence limits distribution free of the days to reach each healing score.
Table 2.
Swelling and healing scores of non-medicated and medicated with a single application of s.c. meloxicam of Angus crossbreed calves castrated at 1 wk, of age from d 7 post-castration until the end of the study (d 56)
| Treatment1 | |||||||
|---|---|---|---|---|---|---|---|
| NM | M | P-value2 | |||||
| N | Median | 95% FCL3 | N | Median | 95% FCL3 | ||
| Swelling score | |||||||
| 4 | — | — | — | — | — | — | — |
| 3 | 4 | 14 | 14 | 7 | 14 | 14–21 | 0.83 |
| 2 | 3 | 7 | 7 | 3 | 7 | 7 | 0.18 |
| 1 | 21 | 7 | 7–28 | 23 | 7 | 7–42 | 0.45 |
| 0 | 14 | 35 | 28–49 | 16 | 35 | 28–56 | 0.07 |
| Healing score | |||||||
| 1 | 14 | 7 | 7 | 17 | 7 | 7 | 0.99 |
| 2 | 20 | 7 | 7–21 | 23 | 7 | 7–21 | 0.45 |
| 3 | 14 | 21 | 21–42 | 21 | 28 | 14–42 | 0.67 |
| 4 | 19 | 28 | 28–49 | 22 | 35 | 21–56 | 0.12 |
| 5 | 14 | 49 | 35–56 | 15 | 49 | 35–56 | 0.24 |
1NM = single s.c. injection of lactated ringer’s at castration time; M = single injection of s.c. meloxicam (0.5 mg/kg) at castration time.
2 P-value = medication effect.
3 n = number of animals observed at each score; median: median days to reach each healing score relative to day of castration and castration; FCL = 95% confidence limits distribution free of the days to reach each healing score.
Table 3.
Mean serum amyloid-A, haptoglobin, and blood cell count of non-castrated, band and knife castrated with or without a single injection of s.c. meloxicam in Angus crossbreed calves castrated at 1 wk of age from d 7 post-castration until the end of the study (d 56)
| Treatment1 | |||||||||||
| CT | BA | KN | P-value2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item5 | NM | M | NM | M | NM | M | SEM | CAS | MED | CAS × MED | T |
| Serum amyloid-A3 d 7 to d 35, g/liter | 55.9 | 59.3 | 82.1 | 71.7 | 47.8 | 55.5 | 0.16 | 0.03 | 0.68 | 0.39 | <0.001 |
| Serum amyloid-A3 d 42 to d 56, g/liter | 66.0 | 55.5 | 62.6 | 51.8 | 44.8 | 45.3 | 0.24 | 0.41 | 0.75 | 0.85 | <0.001 |
| Haptoglobin3, g/liter | 0.19 | 0.21 | 0.19 | 0.19 | 0.19 | 0.18 | 0.038 | 0.52 | 0.45 | 0.52 | <0.001 |
| Blood cell count4 | |||||||||||
| WBC, 103/μL | 10.8 | 10.8 | 11.3 | 11.0 | 10.8 | 10.4 | 0.751 | 0.50 | 0.57 | 0.88 | <0.001 |
| RBC, 106/μL | 9.3 | 8.9 | 9.3 | 9.2 | 9.6 | 9.3 | 0.201 | 0.23 | 0.07 | 0.55 | <0.001 |
| Platelet, 103/μL | 573 | 507 | 474 | 517 | 490 | 510 | 39.8 | 0.32 | 0.95 | 0.19 | <0.001 |
| N:L ratio | 0.64 | 0.64 | 0.62 | 0.57 | 0.53 | 0.65 | 0.113 | 0.58 | 0.76 | 0.49 | <0.001 |
| Rectal temperature, °C | 39.2 | 39.3 | 39.2 | 39.3 | 39.3 | 39.3 | 0.09 | 0.67 | 0.06 | 0.21 | <0.001 |
| Maximum scrotal temperature, °C | 36.7ab | 36.7ab | 36.1c | 36.5ab | 36.8a | 36.4bc | 0.22 | 0.05 | 0.96 | 0.05 | <0.001 |
| Circumference to d 35, cm | 11.5 | 11.7 | 11.5 | 11.5 | 12.2 | 13.1 | 0.47 | 0.02 | 0.35 | 0.61 | 0.12 |
1CT = non-castrated but handled as castrated; BA = calves castrated with rubber rings; KN = calves castrated with a Newberry knife; NM = single s.c. injection of lactated ringer’s at castration time; M = single injection of s.c. meloxicam (0.5 mg/kg) at castration time.
2CAS = castration effect; MED = medication effect T = time effect.
3Data transformed to Napierian logarithm. Data presented herein are LSmeans without transformation, SEM and P-values form transformed values.
4Blood cell count: WBC = white blood cells; RBC = red blood cells; N:L = neutrophil-to-lymphocyte ratio.
5Within rows, values with different superscripts differ (P < 0.05).
The process of tissue repair or wound healing has four phases that overlap: hemostasis, inflammation, proliferation, and tissue remodeling (Boughton et al., 2006). Administration of s.c. meloxicam at the time of castration did not reduce the healing process in this study. Similarly, Mintline et al. (2014) did not observe differences in healing score after the administration of flunixin meglumine in 1-mo-old surgically castrated calves. In the present study, healing scores of “2” were achieved sooner (Z < 0.10) in KN calves than in BA calves (Table 1); although healing scores of “3” were achieved sooner in BA calves, median days to heal at score “3” tended to be shorter (Z < 0.10) in KN calves. Scores of “4” (Z = 0.01) were achieved between d 21 and 56 for BA calves and between d 28 and 49 in KN calves. Although no differences were observed in the number of days required to achieve a healing score of “5” between KN and BA calves, only 9 of 24 band-castrated calves had completely healed by d 56. As observed with swelling score, there were considerable differences between methods of castration with regard to healing. In knife-castrated calves, healing of the skin and spermatic cords should be accounted for when length of the healing process is evaluated, as scar tissue and inflammation persisted inside the scrotum due to the severing of the spermatic cords (Marti et al. 2017b). For example, Molony et al. (1995) indicated that wound healing after surgical castration in 1-wk-old calves lasted for 10 d, while Stafford et al. (2002) and Mintline et al. (2014) reported that wounds were completely healed by d 63 in 1- to 2-mo-old calves, and these differences might be associated with healing of the entire scrotum. Further research is needed to evaluate the internal tissue damage in knife-castrated calves. In band-castrated calves, wounds cannot be completely healed until the scrotum sloughs off. This is because the rubber-ring and/or band continues to break through the skin and spermatic cords until the scrotum has sloughed off, which is when the fourth phase of the healing process, tissue remodeling, occurs. Molony et al. (1995) observed that few calves had not healed after d 51 when measurements stopped, which was similar to the observations of this study (d 56).
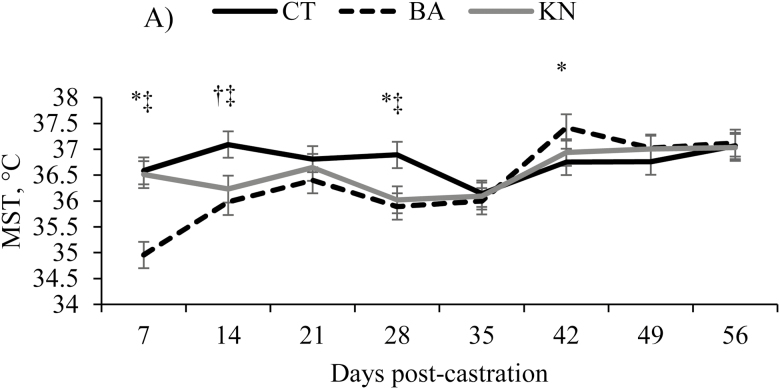
A castration effect (P = 0.05) and an interaction between castration and time (P < 0.05; Figure 2) were observed in MST; BA calves reduced (P < 0.05) their scrotal temperature by 1.6 °C on d 7 (P < 0.01) compared with CT and KN calves, and it might be due to the interruption of blood flow caused by the bands that reduced the temperature of the scrotum. In addition, when the testes are removed in knife-castrated calves, the temperature of the scrotum is reduced; however, an increase in temperature likely occurs due to the fact that the scrotum is inflamed as a result of tissue damage, which would increase the average temperature of the scrotum. After d 7, no differences (P > 0.11) in MST were observed between BA and KN, and their scrotal temperatures were lower (P < 0.05) than for CT calves between d 14 and 28. The testes that are within the scrotum produce heat but are typically between 2 and 5 °C lower than body temperature in cattle (Lunstra et al., 1997); therefore, it was expected that the scrotums of KN would have lower MST than CT calves. On d 42, MST for BA calves was greater (P < 0.05) than that of CT calves, which corresponded to the day that 43.5% of the scrotums had sloughed off, resulting in an open wound and consequently greater MST compared with previous days.
Figure 2.
Maximum scrotal temperature measured between d 7 and 56 of non-castrated (CT), band (BA), and knife (KN) castrated Angus crossbreed calves castrated at 1 wk, of age. * denotes differences between CT and BA; † denotes differences between CT and KN; and ‡ denotes differences between BA and KN (P < 0.10).
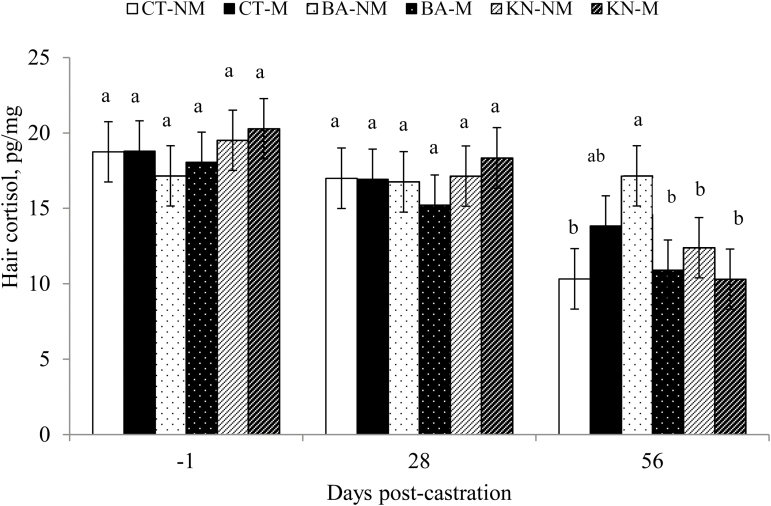
A castration × medication × time interaction (P = 0.03; Figure 3) was observed for hair cortisol concentration. Concentration of hair cortisol on d –1 and on d 28 did not differ (P > 0.10) for each treatment and were greater (P < 0.05) than that on d 56 for all treatments except for BA–NM where hair cortisol concentrations were similar (P > 0.10) between the 3 sampling days. Furthermore, on d 56, hair cortisol concentrations in BA–NM were greater (P > 0.05) than that for CT–NM, BA–M, KN–NM, and KN–M calves, and tended to be greater (P = 0.08) than in CT–M calves. Molony et al. (1995), using a combination of physiologic, behavioral, and clinical–pathological indices, suggested that rubber ring castration produced chronic pain in 1-wk-old calves. Thüer et al. (2007) used a similar approach and showed that chronic pain in 21- to 28-d-old rubber ring castrated calves was more pronounced than Burdizzo castration, while Marti et al. (2017a) reported that 4-mo-old calves suffered chronic pain for 21 d post-castration. To date, there is no consensus as to what constitutes chronic pain; Bonica (1953) defined chronic pain as pain persist after healing, Molony et al. (1995) used the term chronic pain after 24 h post-castration, and Meléndez et al. (2017) and Marti et al. (2017a) defined acute pain as occurring within the first 7 d after castration, while chronic pain was considered to occur following 7 d post-castration. Recently, hair cortisol has been analyzed as a potential indicator of long-term stress (Meyer and Novak, 2012) and some authors have been using hair cortisol to evaluate the long-term effect of castration on calves (Creutzinger et al. 2017; Marti et al. 2015; 2017a). Stafford et al. (2002) and Meléndez et al. (2017; 2018) observed a significant increase in serum and salivary cortisol between 1 and 3.5 h after knife and band castration, which returned to basal levels after that time. Serum and salivary cortisol have been used as a biomarker not only for stress, but also as a proxy for evaluating pain associated with castration (Molony et al. 1995; Stafford et al. 2002; Coetzee et al. 2007; González et al. 2010), which has led to the conclusion that knife and band castration cause acute pain. As a result of this acute response, it was expected that hair cortisol concentrations in KN and BA castrated calves would have been greater on d 28 compared with the other treatments, as was observed by Creutzinger et al. (2017) 14 d after surgical castration in 2- to 3-mo-old calves. However, as reported by Tallo-Parra et al. (2017) hair cortisol concentrations did not increase in 4.5-mo-old Holstein calves after they were subjected to two acute ACTH challenges, done 7 d apart, which increased serum cortisol concentrations for 3 h post-injection with maximum recorded concentrations of 13.8 mol/liter. However, pain in knife and band castration in 1-wk-old calves might not be severe enough to increase hair cortisol concentrations 28 d post-procedure. Meléndez et al. (2018) did not observe that meloxicam administered s.c. prior to knife or band castration in 1-wk-old calves reduced salivary cortisol concentrations up to 120 min post-procedure, which may explain why hair cortisol on d 28 was not reduced by the administration of s.c. meloxicam in this study. In contrast, Creuzinger et al. (2017) observed that hair cortisol concentrations in knife-castrated calves administered with s.c. meloxicam did not differ from the hair cortisol concentrations of non-castrated calves indicating that 1- to 3-mo-old calves benefit from the administration of s.c. meloxicam at the time of knife castration. Furthermore, the lack of differences in hair cortisol on d 28 in both knife and band castrated calves could be due to the elevated serum cortisol concentrations in neonate calves, which do not return to baseline levels until 28 d after birth (Griebel et al., 1987). Thus, hair cortisol deposition may have been elevated, which could have masked the effects of castration in the first sample obtained 28 d after the procedure. Research has found that band castration causes long-lasting pain. Molony et al. (1995) reported abnormal standing postures, abnormal walking, and increased licking frequencies at the site of the lesion in 1-wk-old calves, while Thüer et al. (2007) found that 21- to 28-d-old calves that were rubber ring castrated responded to palpation of the scrotal area for up to 4 wk post-castration and they also exhibited a greater number of abnormal behaviors. González et al. (2010) reported that weaned calves that were band-castrated reduced their feeding activity and had shorter stride lengths 3 wk after castration. It was expected that BA–NM calves in this study had greater levels of hair cortisol concentrations on d 56 as their hair shafts would contain the cortisol deposited during the entire inflammatory process and sloughing of the scrotum. Subcutaneous meloxicam has a half-life of 22.7 h (Stock and Coetzee, 2015), and therefore it was expected that, similar to BA–NM calves, BA–M calves would have greater concentrations of hair cortisol on d 56 post-castration. However, reasons for why BA–M calves had reduced hair cortisol concentrations are unknown. Long-lasting stress and pain are difficult to measure and before definitive conclusions can be made the study should be replicated with a greater number of calves.
Figure 3.
Hair cortisol concentrations of non-castrated (CT), band (BA), and knife (KN) castrated with (M) or without (NM) a single injection of s.c. meloxicam in Angus crossbreed calves castrated at 1 wk of age on d 28 and 56. Values with different superscripts differ (P < 0.10).
Millman (2013) explained that sometimes painful stimuli are not detectable by biochemical measures and therefore physical and behavioral measures should be considered. Tail flicks, in this study showed a castration × medication effect (P = 0.04; Table 4). BA–NM had an increased (P > 0.05) number of tail flicks compared with BA–M; in contrast, the number of tail flicks in KN–NM was reduced (P > 0.05) compared with KN–M. Molony et al. (1995) described abnormal immobile behaviors as an indicator of pain related to knife castration. Results from this study indicate that the administration of meloxicam may reduce abnormal behaviors such as excessive tail flicks expressed by the calves castrated using different methods. A tendency for a medication × time effect (P = 0.10) was observed for lying duration where NM calves tended to spend greater amounts of time lying between d 7 and 14 than M calves (58.4 ± 3.38 min and 51.6 ± 3.39 min, respectively). Another tendency toward a medication × time effect (P < 0.10) was seen for standing bouts, where NM calves (17.3 ± 0.76 bouts) tended to have fewer standing bouts than M calves (18.5 ± 0.76 bouts) between d 7 and 14 after castration. Moreover, a tendency (P = 0.08) for a medication effect was observed for suckling behavior where M calves tended (P = 0.08) to spend more time suckling than NM calves (7.4 ± 0.20 min and 6.0 ± 0.20 min, respectively). In this study, medication had minimal effects on the biochemical parameters measured; however, meloxicam did reduce the behavioral indicators of pain, although most of them were tendencies, which may be due to small sample size in this study. Few changes in behavioral indicators of pain have been reported in other studies following the administration of an NSAID in castrated calves. Brown et al. (2015) observed an increase in lateral lying duration in calves administered oral meloxicam; however, they could not identify if this change in behavior was related to pain or contentment. In contrast, Roberts et al. (2018) did not find changes in standing or lying time among castration methods for the first 7 d after administration of an NSAID in weaned calves. However, Roberts et al. (2018) found that a motion index indicative of the calf’s pain response was reduced in surgical castrated animals administered oral meloxicam. Similarly, Petherick et al. (2014) observed that calves castrated at weaning and administered ketoprofen spent more time feeding and had reduced tail flicks compared with NM calves on the day of castration. A castration (P = 0.04) effect was observed for suckling behavior; KN calves (8.2 ± 0.23 min) spent more time (P < 0.05) suckling than CT calves (5.2 ± 0.23 min), while no differences (P ≥ 0.11) were observed for suckling time among BA calves (6.8 ± 0.22 min), and CT and KN calves. Lesion licking differed among castration methods (P = 0.01); BA castrated calves licked their scrotal area more (P < 0.05) times compared with CT and KN calves (0.93 ± 0.013 licks, 0.17 ± 0.013 licks, and 0.22 ± 0.013 licks, respectively). No treatment effects (P ≥ 0.11) were observed for foot stamping or head turning. Molony et al. (1995), and Stafford and Mellor (2005) described that abnormal lying, lesion licking, and head turning were associated with rubber band or rubber ring castration. In this study, and according to Stafford and Mellor (2005), lesion licking was a behavior mostly observed in band-castrated calves.
Table 4.
Stride length and behavior of non-castrated, band and knife castrated with or without a single injection of s.c. meloxicam in Angus crossbreed calves castrated at 1 wk of age from d 7 post-castration until the end of the experiment (d 35)
| Treatment1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | BA | KN | P-value2 | ||||||||
| Item4 | NM | M | NM | M | NM | M | SEM | CAS | MED | CAS × MED | T |
| Stride length, cm | 38.7 | 38.3 | 38.3 | 39.0 | 39.6 | 38.7 | 0.68 | 0.57 | 0.66 | 0.48 | <0.001 |
| Standing, % | 28.4 | 26.4 | 28.6 | 28.3 | 26.7 | 27.1 | 0.97 | 0.27 | 0.41 | 0.44 | <0.001 |
| Standing duration, min | 25.0 | 24.8 | 27.3 | 24.8 | 27.6 | 25.5 | 2.03 | 0.54 | 0.20 | 0.73 | <0.001 |
| Lying duration, min | 48.5 | 50.8 | 49.2 | 49.3 | 50.9 | 49.1 | 3.36 | 0.96 | 0.93 | 0.73 | <0.001 |
| Standing bouts, n | 18.6 | 17.6 | 18.1 | 18.1 | 17.5 | 17.6 | 0.87 | 0.37 | 0.91 | 0.48 | 0.12 |
| Lying bouts, n | 26.1 | 25.8 | 26.5 | 25.3 | 27.4 | 25.6 | 1.31 | 0.85 | 0.27 | 0.81 | 0.03 |
| Suckling, min3 | 4.6 | 5.8 | 6.3 | 7.3 | 7.2 | 9.1 | 0.27 | 0.04 | 0.08 | 0.88 | 0.40 |
| Tail flick, n3 | 46.0ab | 58.3ab | 77.6a | 48.5ab | 30.4b | 98.2a | 0.14 | 0.88 | 0.35 | 0.04 | 0.61 |
| Foot stamping, n3 | 4.0 | 1.5 | 2.3 | 3.7 | 1.8 | 2.3 | 0.04 | 0.77 | 0.95 | 0.19 | 0.21 |
| Head turning, n3 | 9.6 | 6.9 | 6.2 | 7.3 | 9.8 | 8.3 | 0.05 | 0.49 | 0.71 | 0.61 | 0.45 |
| Lesion licking, n3 | 0.27 | 0.06 | 0.97 | 0.88 | 0.28 | 0.16 | 0.018 | 0.01 | 0.17 | 0.99 | 0.19 |
1CT = non-castrated but handled as castrated; BA = calves castrated with rubber rings; KN = calves castrated with a Newberry knife; NM = single s.c. injection of lactated ringer’s at castration time; M = single injection of s.c. meloxicam (0.5 mg/kg) at castration time.
2CAS = castration effect; T = time effect; CAS × T = interaction between castration and time effect.
3Data transformed to root square +1. Data presented herein are LSmeans without transformation, SEM and P-values form transformed values.
4Within rows, values with different superscripts differ (P < 0.05).
Our results indicated that the performance of calves castrated at 1 wk of age using either knife or band methods (regardless of s.c. meloxicam administration), was not impaired (P > 0.10) compared with non-castrated calves (Table 5). Similar results were reported in a study by Brown et al. (2015) in newborn knife-castrated calves either 66 d post-castration or at weaning (214 d post-castration).
Table 5.
Performance of non-castrated, band and knife castrated with or without a single application of s.c. meloxicam in Angus crossbred calves castrated at 1 wk of age from d 7 post-castration until the end of the study (d 56)
| Treatment1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT | BA | KN | P-value2 | |||||||
| Item | NM | M | NM | M | NM | M | SEM | CAS | MED | CAS × MED |
| Initial BW, kg | 48.4 | 46.7 | 47.7 | 47.9 | 49.2 | 48.3 | 2.18 | 0.80 | 0.60 | 0.90 |
| Final BW, kg | 112.9 | 109.6 | 109.5 | 113.5 | 111.7 | 111.9 | 3.44 | 0.98 | 0.86 | 0.53 |
| ADG d 56, kg/d | 1.16 | 1.10 | 1.09 | 1.17 | 1.14 | 1.14 | 0.061 | 0.98 | 0.87 | 0.53 |
1CT = non-castrated but handled as castrated; BA = calves castrated with rubber rings; KN = calves castrated with a Newberry knife; NM = single s.c. injection of lactated ringer’s at castration time; M = single injection of s.c. meloxicam (0.5 mg/kg) at castration time.
2CAS = castration effect; MED = medication effect.
CONCLUSION
The results of this study assessing long-term effects of band and knife castration after an administration of s.c. meloxicam such as inflammatory responses and wound healing rates in combination of the acute effects assessed by Melendez et al. (2018) (as part of a larger study) indicate that knife castration caused a greater number of pain related indicators during and up to 7 d post-procedure than band castration. In addition, meloxicam was effective in reducing substance P, and WBC concentrations as well as tail flick behavior during that period of time. Results of this study showed that s.c. administration of meloxicam during band or knife castration did not reduce the inflammatory response or improve the healing process in calves castrated at 1 wk of age from d 7 to 56 after the procedure. However, it might help to improve welfare after castration as lying duration was reduced and suckling behavior increased. Band castration resulted in a greater inflammatory response and extended period of wound healing as evidenced by greater values of SAA, MST, and a greater number of days to reduce swelling and improve lesion healing compared to knife castration. However, the administration of meloxicam in band castrated calves might reduce the stress caused by scrotal sloughing as evidenced by lower hair cortisol concentrations.
ACKNOWLEDGMENTS
We appreciate the invaluable help of Agriculture and Agri-Food Canada research feedlot staff and the beef welfare technicians Randy Wilde and Fiona Brown. In addition, we would like to thank all the students that helped to collect data and observe behavior including Jonathan Low, Louise Theron, Ashley Adams, Andrea Lippa, Nicole Desautels, Allecia Gheyssens, Chantel deBeurs, Nita Hynes, and Teryn Gilmet. We are very thankful for the funding provided by the Beef Cattle Research Council through the Canadian Beef Industry Science Cluster. Co-author Sonia Marti was supported by the CERCA program from Generalitat de Catalunya.
Footnotes
This is Lethbridge Research and Development Centre contribution 38717054.
LITERATURE CITED
- Alsemgeest S. P. M., Kalsbeek H. C., Wensing T. H., van der Kolk A. M., van Ederen D. H., Kim P., Westers P., and Gruys E.. 1994. The diagnostic value of acute phase proteins in bovine clinical chemistry. In: Blood concentrations of acute-phase proteins in cattle as markers for disease [thesis]. The Netherlands: University of Utrecht, The Netherlands: p. 39–53. [Google Scholar]
- Anderson K. L., Neff-Davis C. A., Davis L. E., and Bass V. D.. 1990. Pharmacokinetics of flunixin meglumine in lactating cattle after single and multiple intramuscular and intravenous administrations. Am. J. Vet. Res. 51:1464–1467. [PubMed] [Google Scholar]
- AVMA.. 2014. American Veterinary Medical Association Welfare implications of castration of cattle; https://www.avma.org/KB/Resources/LiteratureReviews/Pages/castration-cattle-bgnd.aspx (Accessed 10 September 2017). [Google Scholar]
- Bonica J. J. 1953. Management of pain. Lea & Febiger, Philadelphia. [Google Scholar]
- Boughton G., Jani J. E., and Attinger C. E.. 2006. The basic science of wound healing. Plast Reconstr Surg. 117(Suppl. 7):12–34. [DOI] [PubMed] [Google Scholar]
- Brown A. C., J. G. Powell, Kegley E. B., Gadberry M. S., Reynolds J. L., Hughes H. D., Carroll J. A., Burdick Sanchez N. C., Thaxton Y. V., Backes E. A., et al. 2015. Effect of castration timing and oral meloxicam administration on growth performance, inflammation, behavior, and carcass quality of beef calves. J. Anim. Sci. 93:2460–2470. doi: 10.2527/jas.2014-8695 [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care.. 2009. CCAC guidelines on: the care and use of farm animals in research, teaching, and testing. Canadian Council on Animal Care, Ottawa, Canada. [Google Scholar]
- Coetzee J. F., Gehring R., Bettenhausen A. C., Lubbers B. V., Toerber S. E., Thomson D. U., Kukanich B., and Apley M. D.. 2007. Attenuation of acute plasma cortisol response in calves following intravenous sodium salicylate administration prior to castration. J. Vet. Pharmacol. Ther. 30:305–313. doi: 10.1111/j.1365-2885.2007.00869.x [DOI] [PubMed] [Google Scholar]
- Comin A., Prandi A., Peric T., Corazzin M., Dovier S., S., and Bovolenta S.. 2011. Hair cortisol levels in dairy cows from winter housing to summer highland grazing. Livest. Sci. 138:69–73. doi: 10.1016/j.livsci.2010.12.009 [DOI] [Google Scholar]
- Creutzinger K. C., Stookey J. M., Marfleet T. W., Campbell J. R., Janz D. M., Marqués F. J., and Seddon Y. M.. 2017. An investigation of hair cortisol as a measure of long-term stress in beef cattle: results from a castration study. Can. J. Vet. Sci. 97:499–509. doi: 10.1139/CJAS-2016-0206 [DOI] [Google Scholar]
- CVMA.. 2012. Canadian Veterinary Medical Association. Castration of cattle, sheep, and goats- Position statement https://www.canadianveterinarians.net/documents/castration-cattle-sheep-goats (Accessed 11 May 2017).
- Dockweiler J. C., Coetzee J. F., Edwards-Callaway L. N., Bello N. M., Glynn H. D., Allen K. A., Theurer M. E., Jones M. L., Miller K. A., and Bergamasco L.. 2013. Effect of castration method on neurohormonal and electroencephalographic stress indicators in Holstein calves of different ages. J. Dairy Sci. 96:4340–4354. doi: 10.3168/jds.2012-6274 [DOI] [PubMed] [Google Scholar]
- Earley B. and Crowe M. A.. 2002. Effects of ketoprofen alone or in combination with local anesthesia during the castration of bull calves on plasma cortisol, immunological, and inflammatory responses. J. Anim. Sci. 80:1044–1052. [DOI] [PubMed] [Google Scholar]
- González L. A., Schwartzkopf-Genswein K. S., Caulkett N. A., Janzen E., McAllister T. A., Fierheller E., Schaefer A. L., Haley D. B., Stookey J. M., and Hendrick S.. 2010. Pain mitigation after band castration of beef calves and its effects on performance, behavior, Escherichia coli, and salivary cortisol. J. Anim. Sci. 88:802–810. doi: 10.2527/jas.2008-1752 [DOI] [PubMed] [Google Scholar]
- Griebel P. J., Schoonderwoerd M., and Babiuk L. A.. 1987. Ontogeny of the immune response: effect of protein energy malnutrition in neonatal calves. Can. J. Vet. Res. 51:428–435. [PMC free article] [PubMed] [Google Scholar]
- Horadagoda N. U., Knox K. M., Gibbs H. A., Reid S. W., Horadagoda A., Edwards S. E., and Eckersall P. D.. 1999. Acute phase proteins in cattle: discrimination between acute and chronic inflammation. Vet. Rec. 144:437–441. [DOI] [PubMed] [Google Scholar]
- Jones M. L. and Allison R. W.. 2007. Evaluation of the ruminant complete blood cell count. Vet. Clin. North Am. Food Anim. Pract. 23:377–402, doi: 10.1016/j.cvfa.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Kujala M., Orro T., and Soveri T.. 2010. Serum acute phase proteins as a marker of inflammation in dairy cattle with hoof diseases. Vet. Rec. 166:240–241. doi: 10.1136/vr.b4770 [DOI] [PubMed] [Google Scholar]
- Ledgerwood D. D. Winckler C., Tucker C. B.. 2010. Evaluation of data loggers, sampling intervals, and editing techniques for measuring the lying behavior of dairy cattle. J. Dairy Sci. 93:5129–5139. doi: 10.3168/jds.2009-2945 [DOI] [PubMed] [Google Scholar]
- Lunstra D. D. and Coulter G. H.. 1997. Relationship between scrotal infrared temperature patterns and natural-mating fertility in beef bulls. J. Anim. Sci. 75:767–774. [DOI] [PubMed] [Google Scholar]
- Marti S., Devant M., Amatayakul-Chantler S., Jackson J. A., Lopez E., Janzen E. D., and Schwartzkopf-Genswein K. S.. 2015. Effect of anti-gonadotropin-releasing factor vaccine and band castration on indicators of welfare in beef cattle. J. Anim. Sci. 93:1581–1591. doi: 10.2527/jas.2014-8346 [DOI] [PubMed] [Google Scholar]
- Marti S., Meléndez D. M., Pajor E. A., Moya D., Heuston C. E. M., Gellatly D., Janzen E. D., and Schwartzkopf-Genswein K. S.. 2017a. Effect of band and knife castration of beef calves on welfare indicators of pain at three relevant industry ages: II. Chronic pain. J. Anim. Sci. 95:4367–4380. doi: 10.2527/jas2017.1763 [DOI] [PubMed] [Google Scholar]
- Marti S., Schwartzkopf-Genswein K. S., Janzen E. D., Meléndez D. M., Gellatly D., and Pajor E. A.. 2017b. Use of topical healing agents on scrotal wounds after surgical castration in weaned beef calves. Can. Vet. J. 58:1081–1085. [PMC free article] [PubMed] [Google Scholar]
- Meléndez D. M., Marti S., Pajor E. A., Moya D., Gellatly D., Janzen E. D., and Schwartzkopf-Genswein K. S.. 2017. Effect of timing of subcutaneous meloxicam administration on indicators of pain after knife castration of weaned calves. J. Anim. Sci. 95:5218–5229. doi: 10.2527/jas2017.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meléndez D. M., Marti S., Pajor E. A., Moya D., Gellatly D., Janzen E. D., Schwartzkopf-Genswein K. S.. 2018. Effect of a single dose of meloxicam prior to band or knife castration in 1-wk-old beef calves: I. Acute pain. J. Anim. Sci. 96:1268–1280. doi: 10.1093/jas/sky034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. S. and Novak M. A.. 2012. Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology 153:4120–4127. doi: 10.1210/en.2012-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman S. T. 2013. Behavioral responses of cattle to pain and implications for diagnosis, management, and animal welfare. Vet. Clin. North Am. Food Anim. Pract. 29:47–58. doi: 10.1016/j.cvfa.2012.11.007 [DOI] [PubMed] [Google Scholar]
- Mintline E. M., Varga A., Banuelos J., Walker K. A., Hoar B., Drake D., Weary D. M., Coetzee J. F., Stock M. L., and Tucker C. B.. 2014. Healing of surgical castration wounds: a description and an evaluation of flunixin. J. Anim. Sci. 92:5659–5665. doi: 10.2527/jas.2014-7885 [DOI] [PubMed] [Google Scholar]
- Moggy M. A., Pajor E. A., Thurston W. E., Parker S., Greter A. M., Schwartzkopf-Genswein K. S., Campbell J. R., and Windeyer M. C.. 2017. Management practices associated with stress in cattle on western Canadian cow-calf operations: a mixed methods study. J. Anim. Sci. 95:1836–1844. doi: 10.2527/jas.2016.1310 [DOI] [PubMed] [Google Scholar]
- Molony V., Kent J. E., Robertson I. S.. 1995. Assessment of acute and chronic pain after different methods of castration of calves. Appl. Anim. Behav. Sci. 46:33–48. doi: 10.1016/0168-1591(95)00635-4 [DOI] [Google Scholar]
- Moya D., Schwatzkopf-Genswein K. S., and Veira D.M.. 2013. Standardization of a non-invasive methodology to measure cortisol in hair of beef cattle. Livest. Sci. 158:138–144. doi: 10.1016/j.livsci.2013.10.007 [DOI] [Google Scholar]
- Murata H., Shimada N., and Yoshioka M.. 2004. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet. J. 168:28–40. doi: 10.1016/S1090-0233(03)00119-9 [DOI] [PubMed] [Google Scholar]
- Pang W. Y., Earley B., Sweeney T., and Crowe M. A.. 2006. Effect of carprofen administration during banding or burdizzo castration of bulls on plasma cortisol, in vitro interferon-gamma production, acute-phase proteins, feed intake, and growth. J. Anim. Sci. 84:351–359. [DOI] [PubMed] [Google Scholar]
- Petherick J. C., Small A. H., Mayer D. G., Colditz I. G., Ferguson D. M., and Stafford K.. 2014. A comparison of welfare outcomes for weaner and mature Bos indicus bulls surgically or tension band castrated with or without analgesia: 2. Responses related to stress, health and productivity. Appl. Anim. Behav. Sci. 157:23–34. doi: 10.1016/j.applanim.2014.05.003 [DOI] [Google Scholar]
- Roberts S. L., Hughes H. D., Burdick Sanchez N. C., Carroll J. A., Powell J. G., Hubbell D. S., and Richeson J. T.. 2015. Effect of surgical castration with or without oral meloxicam on the acute inflammatory response in yearling beef bulls. J. Anim. Sci. 93:4123–4131. doi: 10.2527/jas.2015-9160 [DOI] [PubMed] [Google Scholar]
- Roberts S. L., Powell J. G., Hughes H. D., and Richeson J. T.. 2018. Effect of castration method and analgesia on inflammation, behavior, growth performance, and carcass traits in feedlot cattle. J. Anim. Sci. 96:66–75. doi: 10.1093/jas/skx022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford K. J. and Mellor D. J.. 2005. The welfare significance of the castration of cattle: a review. N. Z. Vet. J. 53:271–278. doi: 10.1080/00480169.2005.36560 [DOI] [PubMed] [Google Scholar]
- Stafford K. J., Mellor D. J., Todd S. E., Bruce R. A., and Ward R. N.. 2002. Effects of local anaesthesia or local anaesthesia plus a non-steroidal anti-inflammatory drug on the acute cortisol response of calves to five different methods of castration. Res. Vet. Sci. 73:61–70. [DOI] [PubMed] [Google Scholar]
- Stock M. L. and Coetzee J. F.. 2015. Clinical pharmacology of analgesic drugs in cattle. Vet. Clin. North Am. Food Anim. Pract. 31:113–38, vi. doi: 10.1016/j.cvfa.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Tallo-Parra O., Lopez-Bejar M., Carbajal A., Monclús L., Manteca X., and Devant M.. 2017. Acute ACTH-induced elevations of circulating cortisol do not affect hair cortisol concentrations in calves. Gen. Comp. Endocrinol. 240:138–142. doi: 10.1016/j.ygcen.2016.10.007 [DOI] [PubMed] [Google Scholar]
- Thüer S., Mellema S., Doherr M. G., Wechsler B., Nuss K., and Steiner A.. 2007. Effect of local anaesthesia on short- and long-term pain induced by two bloodless castration methods in calves. Vet. J. 173:333–342. doi: 10.1016/j.tvjl.2005.08.031 [DOI] [PubMed] [Google Scholar]
- University of British Columbia Animal Welfare Program.. 2013. UBC animal welfare program: SOP-HOBO data loggers. University of British Columbia, Vancouver, Canada: p. 1–23. [Google Scholar]