Abstract
We investigated the incidence of viral, fungal, bacterial, and parasitic infections observed in 57 patients with central nervous system lymphoma (CNSL) after TBC-ASCT and 79 patients with systemic NHL after traditional BEAM-ASCT. Twenty of 57 (35%) TBC-ASCT patients had detectable viremia with human herpesvirus 6 (HHV-6), cytomegalovirus (CMV), adenovirus (ADV), or BK virus (BKV), versus 9 of 79 (11%) BEAM-ASCT patients. Nine TBC-ASCT patients had clinically relevant viral infections (4 HHV-6, 2 CMV, 1 ADV, 2 BKV), versus 0 in the BEAM-ASCT group. Four TBC-ASCT patients suffered infections from either a fungal or parasitic pathogen versus 1 BEAM-ASCT patient. TBC was associated with greater risk of viral reactivation compared with BEAM, independent of other factors (HR 4.42, 95% CI 1.9–11.3, p<0.001). Prolonged lymphopenia and steroid use in the peri- and post-ASCT period did not explain these observed differences. The pathogenesis of these unusual infections in TBC-ASCT patients remains incompletely understood, and may involve more potent immune suppression with TBC conditioning. Clinicians should be aware of these differences in infection risk in TBC-ASCT patients, which more closely parallel those seen in allo-HCT recipients. New prophylactic approaches to help minimize these infections should be considered in this population.
Keywords: Central nervous system lymphoma, infectious complications, high-dose therapy and autologous stem cell transplantation, diffuse large B cell lymphoma
Introduction
Our group and others have shown that high-dose therapy and autologous stem cell transplantation (HDT-ASCT) results in highly favorable outcomes in patients with primary and secondary central nervous system lymphoma (CNSL) when utilizing an intensive conditioning regimen that combines thiotepa, busulfan, and cyclophosphamide (TBC).1–7 TBC-ASCT has become a standard consolidation approach in physiologically appropriate patients with CNSL in both the upfront and relapsed settings at our center and others. Despite impressive durable long-term remissions and often cures, TBC-ASCT is met with unfavorable regimen-related toxicity.4,5 In a recent publication, we noted many unforeseen infections in patients undergoing TBC-ASCT compared to more traditional ASCT conditioning regimens such as carmustine, etoposide, cytarabine, and melphalan (BEAM).5
Infections with viruses, fungi, and protozoa are frequently seen after allogeneic hematopoietic cell transplantation (allo-HCT) and contribute to morbidity and mortality.8,9 Because of the unique infectious toxicity profile in allo-HCT, systemic monitoring for these infections coupled with an empiric antimicrobial program is standard protocol. In contrast, systemic monitoring of infections is not as rigorous after ASCT as these are considered atypical events.8,9 In order to determine whether similar pre-emptive protocols should be considered in TBC-ASCT patients, we characterized and compared the incidence and severity of infectious complications between patients with CNSL undergoing TBC-ASCT and those with systemic lymphoma undergoing BEAM-ASCT. We hypothesized that patients who undergo TBC-ASCT carry an increased risk of severe infections, perhaps aligning their level of immunity more closely to recipients of allo-HCT.
Methods
The Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Board approved this observational study. We evaluated patients with chemosensitive CNSL who underwent consolidative TBC-ASCT between 2013 and 2016. We compared them to patients with systemic diffuse large B-cell lymphoma without CNS involvement who underwent standard BEAM-ASCT during the same period. This time period was chosen because our center began regularly employing molecular-based test methods for quantifying blood viral loads using real-time polymerase chain reaction (PCR) in 2013. As a workup for a clinical syndrome, we recorded all positive DNA viral reactivations by serum PCR, urine PCR for BK virus (BKV), blood stream infections (BSIs), diagnostic markers for fungal infection (serum and bronchial wash galactomannan and serum 1,3-β-D-glucan), and serologies for toxoplasmosis from the start of conditioning until 6 months post-ASCT among all patients. Patients were censored at the time of relapsed disease if that occurred before 6 months post-ASCT. We then assessed for evidence of clinically relevant infections that coincided with the above-mentioned laboratory tests during the same time period. Clinically relevant infections were defined as infections having a compatible syndrome whose treatment with an antimicrobial agent was initiated. DNA viral infections were defined as either a “possible” or “probable” infection as per previously published studies.10–12
The rates of viral infection, defined as any positive PCR value for human HHV-6, CMV, ADV, or BKV for TBC and BEAM groups were compared by the log rank test. Cox proportional hazard analysis was used to compare the risk of viral reactivation between both groups. The cumulative incidences of bacteremia, defined as any positive blood culture that was not coagulase-negative Staphylococcus aureus were also compared between TBC and BEAM. Patient and ASCT variables including age, gender, disease status, conditioning type (TBC v. BEAM), peri-ASCT absolute lymphocyte count (ALC), and use of post-ASCT corticosteroids were evaluated. Prolonged pre-ASCT lymphopenia was defined as having 2 measurements of an ALC less than 0.5 K/mcL in a 14-day period. Corticosteroid use was defined as having received equivalent doses of prednisone 20 mg with first dose greater than or equal to 20 mg treated as a time dependent variable. Time to neutrophil engraftment was treated as a time dependent variable. All variables with p-values of < 0.2 in the univariate analysis were included in the multivariate analysis. CD34+ stem cell doses were compared between both groups by Mann-Whitney test.
The TBC conditioning regimen was administered as previously described.5 The BEAM conditioning regimen was comprised of carmustine 300 mg/m2 I.V. on day –6; etoposide 100 mg/m2 and cytarabine 200 mg/m2 I.V. every 12 hours on days -5, -4, -3, -2, and melphalan 140 mg/m2 I.V. on day -1, with autologous stem cell infusion on day 0. For BEAM and TBC, standard anti-emetic prophylaxis includes dexamethasone 20 mg oral or I.V. daily from day -6 through day -1, and 20 mg oral or I.V. daily from days -9 through day -1, respectively. Corticosteroid use is otherwise not given peri-ASCT unless indicated by a specific clinical scenario such as engraftment syndrome. not In accordance with our institutional ASCT guidelines for both regimens, antiviral prophylaxis with oral acyclovir 400 mg twice daily was started on admission and continued until at least 1 year post-ASCT, antibacterial prophylaxis for febrile neutropenia with oral ciprofloxacin 500 mg twice daily was started on day -2 and continued until engraftment, and antifungal prophylaxis with fluconazole 400 mg/day was started on admission and continued until engraftment. In addition, pneumocystis jiroveci prophylaxis (PJP) with sulfamethoxazole-trimethoprim was started at day +30 and continued until 6 months post-ASCT.
Results
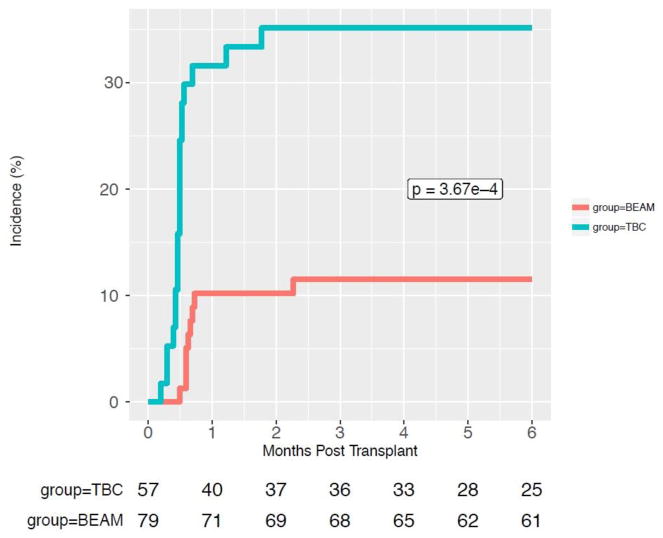
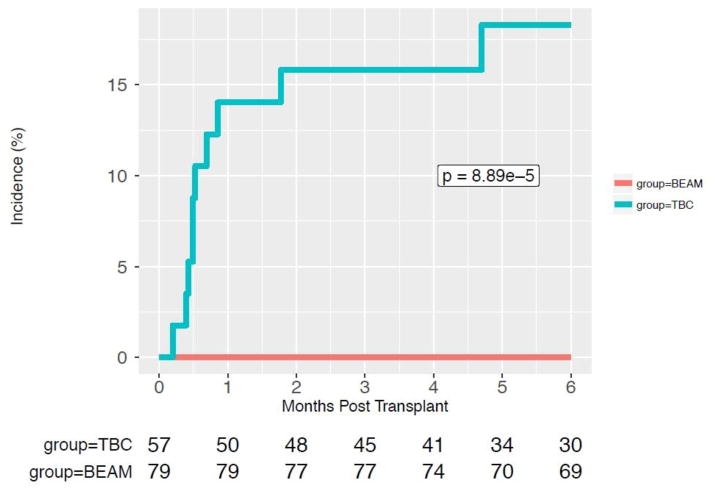
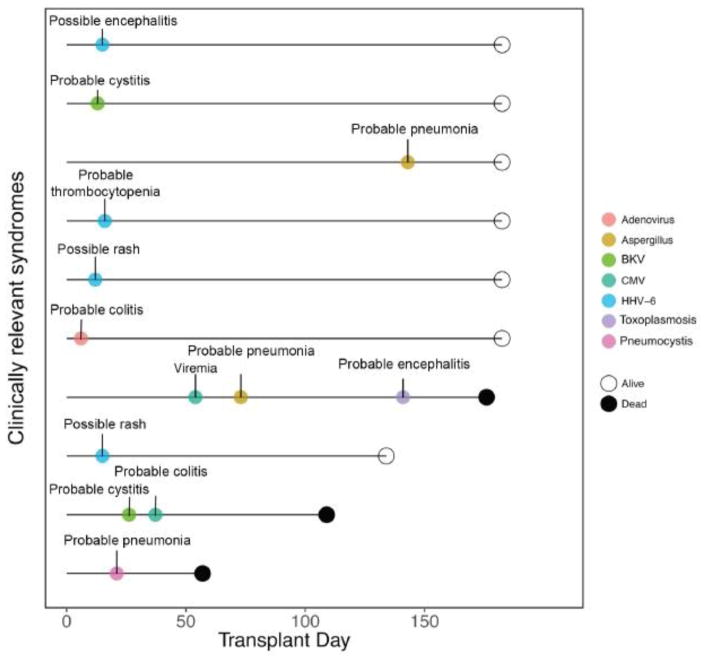
We compared 57 TBC-ASCT and 79 BEAM-ASCT patients. Baseline patient and ASCT characteristics including age, gender, disease status, pre-ASCT ALC (from day -100 to day 0) were similar between groups as shown in Table 1. There were 2 patients with HIV seropositivity in each of the ASCT groups. The CD34+ stem cell dose was not significantly different between BEAM- and TBC-ASCT patients, p=0.404. TBC-ASCT patients were more likely to receive prolonged steroid use in the post-ASCT period, 61% versus 26.6% (p<0.001). Twenty (35%) TBC-ASCT patients had detectable viremia with HHV-6, CMV, ADV, and/or BKV within 6 months of their transplantation versus 9 (11%) BEAM-ASCT patients, p=0.0003, as shown in Figure 1. In a univariate analysis, receiving TBC conditioning was associated with an increased risk of viral reactivation, hazard ratio (HR) 4.57 (2.06–11.29), p<0.001. In a multivariate analysis, receiving TBC conditioning was the only variable associated with an increased risk of viral reactivation, HR 4.42 (1.90–11.30), p<0.001 (Table 2). Prolonged lymphopenia and steroid use in the peri-ASCT period, were not significantly associated with an increased risk of infection, as illustrated in Supplemental Figures S1 and S2, respectively. Eight (14%) TBC-ASCT patients had a clinically relevant viral infection with HHV-6 (n=4), CMV (n=2), ADV (n=1), BKV (n=2) compared to 0 patients in the BEAM-ASCT group (p=0.0008) as shown in Figure 2. These infections led to various infectious syndromes including encephalitis, colitis, and cystitis as detailed in Figure 3. Four TBC-ASCT patients also had a infection from a fungal or parasitic pathogen, including 2 patients with Aspergillus pneumonia, 1 with PJP, and 1 with toxoplasmosis encephalitis. Two of these patients had more than 1 opportunistic infection leading to death, and another died of PJP alone. The 2 patients that developed Aspergillus infections had not previously been treated with ibrutinib prior to ASCT. In contrast, only 1 patient in the BEAM-ASCT had an opportunistic infection (PJP), and survived the event. Non-coagulase negative Staphylococcus aureus bacteremias were similar between the TBC and BEAM groups (p=0.73, Supplemental Figure S3). The non-coagulase negative Staphylococcus aureus bacteremias in both groups are detailed in Supplemental Table S1.
Table 1.
Patient and ASCT Characteristics
| BEAM | TBC | P-value | |
|---|---|---|---|
|
| |||
| N (Patients) | 79 | 57 | |
|
| |||
| Age (mean): | 55 | 55 | 0.89 |
|
| |||
| Gender (% male): | 48 | 33 | 0.87 |
|
| |||
| Disease Status (%): | |||
| PR | 23.0 (29.1) | 18.0 (31.6) | 0.67 |
| Refractory | 1.0 (1.3) | 0 (0.0) | |
| CR | 55.0 (69.6) | 39.0 (68.4) | |
|
| |||
| Steroid exposure post-ASCT: | 21 (26.6) | 35 (61.4) | < 0.001 |
|
| |||
| Pre-ASCT Lymphopenia: | 39 (49.4) | 30 (52.6) | 0.84 |
|
| |||
| Neutrophil Engraftment, days (mean): | 10.09 | 9.53 | 0.08 |
CR, complete response; PR, partial response, Pre-ALC, ALC < 0.5 K/mcL for 14 consecutive day period from 0 to day -100; Steroid exposure was defined as having received an equivalent dose of prednisone 20 mg from day 0–180.
Figure 1.
Cumulative Incidence of DNA Virus Reactivations Compared Between TBC and BEAM
Table 2.
Risk Factors for DNA Virus Reactivation
| Risk Factor | Univariate model HR (95% CI) |
P-value | Multivariate model HR (95% CI) |
P-value |
|---|---|---|---|---|
|
| ||||
| Group: | ||||
| TBC | 4.61 (2.08–11.4) | <0.001 | 4.58 (2.06 –11.3) | <0.001 |
| BEAM | 1.00 (referent) | 1.00 (referent) | ||
|
| ||||
| Age: | 0.98 (0.96 –1.01) | 0.21 | ||
|
| ||||
| Gender: | ||||
| Male | 1.96 (0.89–4.85) | 0.10 | 2.11 (0.95 –5.22) | 0.07 |
| Female | 1.00 (referent) | 1.00 (referent) | ||
|
| ||||
| Disease Status: | ||||
| CR | 1.00 (referent) | |||
| PR | 0.99 (0.42–2.15) | 0.96 | ||
| Refractory | 2.16 (0.02–15.7) | 0.63 | ||
|
| ||||
| Steroid exposure Post-ASCTa | 1.61 (0.74 –3.44) | 0.12 | 1.24 (0.53–2.82) | 0.62 |
|
| ||||
| Pre-ASCT Lymphopeniab: | 1.06 (0.50 –2.25) | 0.89 | ||
|
| ||||
| Neutrophil Engraftment, days, (mean): | 10.33 (0.71–127.6) | 0.08 | 8.52 (0.58–106) | 0.11 |
CI, confidence interval; HR, hazard ratio; PR, partial response; CR,complete response; ALC, absolute lymphocyte count TBC, thiotepa, busulfan, cyclophosphamide; BEAM, carmustine, etoposide, Ara-C and Melphalan
Analyzed as a time-varying predictor; defined as having received an equivalent dose of prednisone 20 mg or not in the post-transplant period (day 0–180)
Defined as having at least 1 period of an ALC of < 0.5 K/mcL 14 days pre-transplant (day -100 to day 0)
Figure 2.
Cumulative Incidence of Clinically Relevant DNA Virus Infections Compared Between TBC and BEAM
Figure 3.
Clinically Relevant Infections Among TBC-ASCT Patients
Timelines of all clinically relevant viral, fungal, and parasitic infections in 10 TBC-ASCT patients.
Discussion
Compared to allo-HCT, HDT-ASCT generally carries a much lower risk of infections because prophylactic or ex-vivo immunosuppression is not utilized, and because graft-versus-host disease (GVHD) is not a common complication of ASCT.8,9,13 Typical infections after HDT-ASCT consist mainly of BSI’s during pre-engraftment neutropenia and respiratory viruses.8,9,13 Herein, we call attention to a cohort of TBC-ASCT patients with notable infections from DNA viruses, fungi, and parasites that are generally considered rare events after ASCT. Recent analyses suggest that viral reactivations early after HDT-ASCT as measured by PCR may be more common than previously believed, but their clinical significance has not been fully elucidated.14–17 However, our results show TBC-ASCT patients are at an increased risk for early reactivation and subsequent clinically significant infections that contribute to morbidity and potential mortality when compared to patients receiving more traditional BEAM-ASCT. This suggests that the immune status of TBC-ASCT patients may align more closely with that of patients undergoing allo-HCT.18 The fungal and viral infections seen in TBC-ASCT patients occurred later in the course of transplant and were not observed during pre-engraftment neutropenia.
The reason for our observed infectious profile in TBC-ASCT patients remains unclear. We recently reported several cases of autologous GVHD after TBC-ASCT, which in part may have contributed to the marked immune alteration in some patients.5 Our findings could be explained by inherent differences in the immune function of patients with CNSL compared to those with systemic non-Hodgkin lymphoma (NHL), which could also be partly due to variations in the types of inductions and salvage regimens used to treat both entities prior to ASCT. 19,20 However, our multivariate model incorporated prior prolonged lymphopenia and time-dependent steroid use as covariates, and found the altered infectious profile of TBC-ASCT to be independent of these risk factors.21,22 This suggests that our findings may reflect the higher myeloablative and immunosuppressive intensity of TBC compared to BEAM.
Because bacterial, viral, fungal and parasitic infections are rare after ASCT, preventive and empiric treatment guidelines are less firm and vary between institutions.8,9 Our results suggest that patients undergoing TBC-ASCT are a specific population where consideration for early post-ASCT DNA viral PCR monitoring and pre-emptive therapy to prevent viral organ disease may be warranted. In contrast, the fungal and parasitic infections noted in our study were too few to advocate for an empiric program to monitor these types of infections.23
There are several limitations to our study that are inherent to a historical cohort analysis comparing two relatively small and unselected patient cohorts with similar disease histology, but different disease biology.20 There may be bias and variation in the frequency of which infectious studies, namely DNA viral PCRs, were evaluated between these patient groups, as there was no standard viral monitoring protocol. That is, these lab tests to detect infection were not obtained because of a preconceived impression regarding TBC having more potent immunosuppressive properties. However, given that serum and urine PCR monitoring for DNA viruses is not part of our institutional guidelines for any patient undergoing ASCT, these tests were sent in both groups when there was a specific clinical suspicion or when results of standard bacterial and fungal assays did not explain a clinical scenario where infection was strongly considered in the differential diagnosis.
As research efforts continue to examine the most efficacious consolidation approach for patients with primary and secondary CNSL, HDT-ASCT has emerged as one of the most promising platforms.4,20 HDT-ASCT with thiotepa-based conditioning regimens demonstrate high overall and durable response rates leading their use in several ongoing studies including multicenter Phase III studies (NCT02531841) comparing conventional chemotherapy versus HDT-ASCT consolidation.20,24 Our group and others have successfully utilized TBC-ASCT both in the up-front and relapsed settings even in patients > 70 years old.5 In our current analysis, receiving TBC was the only significant risk factor for the increased risk of infections, thereby identifying modification of the TBC conditioning platform as a focus for future studies. Our next objective is to extend TBC-ASCT to more patients by reducing its toxicity and safety profile. As the use of HDT-ASCT for CNSL continues to grow, clinicians should be aware of the inherent differences in infectious toxicities seen between patients undergoing TBC-ASCT and more commonly used conditioning regimens for systemic NHL. Patients undergoing TBC-ASCT with clinical syndromes unexplained by characteristic post-ASCT infections should be evaluated for infections including DNA viruses, with consideration given to preemptive treatment to avoid progression to clinically relevant infections.
Supplementary Material
Highlights.
TBC-ASCT patients have a higher risk of DNA virus infections compared to BEAM-ASCT.
Fungal and parasitic infections appear more common after TBC-ASCT.
Clinicians should be aware of the differences in infections between BEAM- and TBC-ASCT patients.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748, and supported by the Brownstein Fund.
Footnotes
Conflicts of Interest Statement: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol. 2008;26(15):2512–2518. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 2.Cote GM, Hochberg EP, Muzikansky A, et al. Autologous Stem Cell Transplantation with Thiotepa, Busulfan, and Cyclophosphamide (TBC) Conditioning in Patients with CNS Involvement by Non-Hodgkin Lymphoma. Biol Blood Marrow Transplant. 2012;18(1):76–83. doi: 10.1016/j.bbmt.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Welch MR, Sauter CS, Matasar MJ, et al. Autologous stem cell transplant in recurrent or refractory primary or secondary central nervous system lymphoma using thiotepa, busulfan and cyclophosphamide. Leuk Lymphoma. 2015;56(2):361–367. doi: 10.3109/10428194.2014.916800. [DOI] [PubMed] [Google Scholar]
- 4.Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403–1410. doi: 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scordo M, Bhatt V, Hsu M, et al. A Comprehensive Assessment of Toxicities in Patients with Central Nervous System Lymphoma Undergoing Autologous Stem Cell Transplantation Using Thiotepa, Busulfan, and Cyclophosphamide Conditioning. Biol Blood Marrow Transplant. 2017;23(1):38–43. doi: 10.1016/j.bbmt.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeFilipp Z, Li S, El-Jawahri A, et al. High-dose chemotherapy with thiotepa, busulfan, and cyclophosphamide and autologous stem cell transplantation for patients with primary central nervous system lymphoma in first complete remission. Cancer. 2017;123(16):3073–3079. doi: 10.1002/cncr.30695. [DOI] [PubMed] [Google Scholar]
- 7.Qualls D, Sullivan A, Li S, et al. High-dose Thiotepa, Busulfan, Cyclophosphamide, and Autologous Stem Cell Transplantation as Upfront Consolidation for Systemic Non-Hodgkin Lymphoma With Synchronous Central Nervous System Involvement. Clin Lymphoma Myeloma Leuk. 2017 Aug; doi: 10.1016/j.clml.2017.08.100. [DOI] [PubMed] [Google Scholar]
- 8.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for Preventing Infectious Complications among Hematopoietic Cell Transplantation Recipients: A Global Perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullmann AJ, Schmidt-Hieber M, Bertz H, et al. Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016. Ann Hematol. 2016;95(9):1435–1455. doi: 10.1007/s00277-016-2711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthes-Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: Summary of ECIL-4 (2011) Transpl Infect Dis. 2012;14(6):555–563. doi: 10.1111/tid.12022. [DOI] [PubMed] [Google Scholar]
- 11.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87–91. doi: 10.1093/cid/ciw668. [DOI] [PubMed] [Google Scholar]
- 12.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017 doi: 10.1056/NEJMoa1706640. NEJMoa1706640. [DOI] [PubMed] [Google Scholar]
- 13.Zaia J, Baden L, Boeckh MJ, et al. Viral disease prevention after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44(8):471–482. doi: 10.1038/bmt.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilgrami S, Aslanzadeh J, Feingold JM, et al. Cytomegalovirus viremia, viruria and disease after autologous peripheral blood stem cell transplantation: no need for surveillance. Bone Marrow Transpl. 1999;24(1):69–73. doi: 10.1038/sj.bmt.1701827. [DOI] [PubMed] [Google Scholar]
- 15.Fassas A, Bolaños-Meade J, Buddharaju LN, et al. Cytomegalovirus infection and non-neutropenic fever after autologous stem cell transplantation: High rates of reactivation in patients with multiple myeloma and lymphoma. Br J Haematol. 2001;112(1):237–241. doi: 10.1046/j.1365-2141.2001.02487.x. [DOI] [PubMed] [Google Scholar]
- 16.Marchesi F, Pimpinelli F, Gumenyuk S, et al. Cytomegalovirus reactivation after autologous stem cell transplantation in myeloma and lymphoma patients: A single-center study. World J Transplant. 2015;5(3):129–136. doi: 10.5500/wjt.v5.i3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inazawa N, Hori T, Nojima M, et al. Virus reactivations after autologous hematopoietic stem cell transplantation detected by multiplex PCR assay. J Med Virol. 2017;89(2):358–362. doi: 10.1002/jmv.24621. [DOI] [PubMed] [Google Scholar]
- 18.van den Brink MRM, Velardi E, Perales M-A. Immune reconstitution following stem cell transplantation. Hematol Am Soc Hematol Educ Progr. 2015;2015:215–219. doi: 10.1182/asheducation-2015.1.215. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y-B, Lane Aa, Logan BR, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(6):1046–1053. doi: 10.1016/j.bbmt.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grommes C, DeAngelis LM. Primary CNS Lymphoma. J Clin Oncol. 2017;35(21):2410–2418. doi: 10.1200/JCO.2017.72.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rostaing L, Malvezzi P. Steroid-Based Therapy and Risk of Infectious Complications. PLoS Med. 2016;13(5) doi: 10.1371/journal.pmed.1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Koning C, Nierkens S, Boelens JJ. Strategies before, during, and after hematopoietic cell transplantation to improve T-cell immune reconstitution. Blood. 2016;128(23):2607–2615. doi: 10.1182/blood-2016-06-724005. [DOI] [PubMed] [Google Scholar]
- 23.Pagano L, Fianchi L, Mele L, et al. Pneumocystis carinii pneumonia in patients with malignant haematological diseases: 10 Years’ experience of infection in GIMEMA centres. Br J Haematol. 2002;117(2):379–386. doi: 10.1046/j.1365-2141.2002.03419.x. [DOI] [PubMed] [Google Scholar]
- 24.High-dose Chemotherapy and ASCT or Consolidating Conventional Chemotherapy in Primary CNS Lymphoma (MATRix) https://clinicaltrials.gov/ct2/show/NCT02531841.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.