Abstract
Non-alcoholic fatty liver disease (NAFLD) is a clinical condition characterized by lipid infiltration of the liver, highly prevalent in the general population affecting 25% of adults, with a doubled prevalence in diabetic and obese patients. Almost 1/3 of NAFLD evolves in Non-Alcoholic SteatoHepatitis (NASH), and this can lead to fibrosis and cirrhosis of the liver. However, the main causes of mortality of patients with NAFLD are cardiovascular diseases. At present, there are no specific drugs approved on the market for the treatment of NAFLD, and the treatment is essentially based on optimization of lifestyle. However, some nutraceuticals could contribute to the improvement of lipid infiltration of the liver and of the related anthropometric, haemodynamic, and/or biochemical parameters. The aim of this paper is to review the available clinical data on the effect of nutraceuticals on NAFLD and NAFLD-related parameters. Relatively few nutraceutical molecules have been adequately studied for their effects on NAFLD. Among these, we have analysed in detail the effects of silymarin, vitamin E, vitamin D, polyunsaturated fatty acids of the omega-3 series, astaxanthin, coenzyme Q10, berberine, curcumin, resveratrol, extracts of Salvia milthiorriza, and probiotics. In conclusion, Silymarin, vitamin E and vitamin D, polyunsaturated fatty acids of the omega-3 series, coenzyme Q10, berberine and curcumin, if well dosed and administered for medium–long periods, and associated to lifestyle changes, could exert positive effects on NAFLD and NAFLD-related parameters.
Keywords: NAFLD, dietary supplements, nutraceuticals, clinical trials
1. Introduction
The liver is the largest gland in the human body. It has an antrophrenic action (both endocrine and exocrine) and more than 150 other functions. In particular, the liver interacts with the glyco-lipid metabolism, being responsible for gluconeogenesis, glycogenolysis, glycogenosynthesis, apolipoprotein synthesis, cholesterol, and triglycerides, and Low-Density Lipoprotein (LDL) cholesterol elimination through biliary route. The bile produced by the liver is also essential for emulsifying lipids in the intestine allowing absorption. The liver is also responsible for maintaining plasma osmolarity, through the production of albumin and globulins, for the production of coagulation factors, such as factor I (fibrinogen), II (thrombin), V, VII, IX, X, XI, and other proteins involved in coagulation processes, such as protein C, protein S, hepcidin, and antithrombin. In addition to these, it also produces other proteins and enzymes essential for survival (e.g., alpha-1 antitrypsin). The liver is also responsible for the catabolism of endogenous toxicants, for the storage of glycogen, vitamin B12, iron and copper, and it mainly contributes to the function of the immune system. For all these reasons, it is evident that liver health is essential for maintenance of the health of the entire organism and must be preserved as much as possible [1].
Intracellular infiltration of fat in the liver is called liver steatosis and could be due to both excessive alcohol intake (Alcoholic Fatty Liver Disease—AFLD) and other metabolic factors (Non-Alcoholic Fatty Liver Disease—NAFLD). The international threshold level chosen by the Scientific Community to distinguish AFLD from NAFLD is 2 drinks, the equivalent of 20 g, per day. NAFLD is an extremely common condition affecting 25–30% of the general adult population, 15% of children, and more than 50% of overweight, obese, and type 2 diabetics. NAFLD could not be considered a real disease even if absolutely reversible [2].
The clinically aggressive variant of NAFLD, non-alcoholic steatohepatitis (NASH), characterized by inflammation and progressive tissue degeneration, affects about 5% of the general adult population, and 20% of obese people [3]. The gold standard for NAFLD and NASH diagnosis is liver biopsy. However, the diagnosis of NAFLD is usually made by ultrasounds (“bright liver”), after excluding other causes of chronic liver disease, an alcohol intake lower of 20 g/day, and by using validated scores, such as the Fatty Liver Index (FLI), or fibrosis score or others [4].
The main risk factors for primary NAFL and NAFLD (Table 1) are overweight/obesity, insulin resistance/type 2 diabetes, hypertriglyceridaemia and the related dietary-behavioural triggers, primarily the intake of beverages sweetened with fructose. By itself, in different observational studies [5], only consumption of sugared soft drinks (mainly with fructose) increases the risk of developing NAFLD of around 55% [6]. Among the emerging risk factors there are also the smoking habit and the Obstructive Sleep Apnoea Syndrome (OSAS), but also insomnia and excessive daytime sleepiness unrelated to nocturnal sleep apnoea [7,8]. However, the first risk factor is often related to poor lifestyle habits. Finally, the strong association between hypothyroidism and NAFLD has recently been confirmed by a meta-analysis of 13 prospective studies that showed how hypothyroidism could increase the risk of NAFLD to more than 50%. The risk increases up to 70% if subclinical hypothyroidism is excluded [9].
Table 1.
Main risk factors for the development of Non-Alcoholic Fatty Liver Disease (NAFLD).
| Defined | Emerging |
|---|---|
| Diet rich in refined foods, carbohydrates with a high glycemic index, drinks sweetened with fructose Sedentary Overweight/Obesity Insulin resistance/Type II diabetes Intake of cortisones, methotrexate, some antiretrovirals |
Sarcopenia Hypothyroidism Hyperuricemia Cigarette smoke Chronic obstructive pulmonary disease (COPD) Polycystic ovary syndrome Helicobacter pylori infection |
NAFLD is the first step for the development of irreversible alterations of the liver parenchyma leading to cirrhosis (about 1/3 of the cases of NAFLD tend to become NASH, and 15% of these can degenerate into cirrhosis), while on the other hand, NAFLD is itself a risk factor for the development of cardiovascular disease [10] and type 2 diabetes, and preliminary data suggest that it may also be associated with a greater incidence of hepatic and extra-hepatic oncology pathologies [11]. A recent meta-analysis of 9 observational studies that included data from 96,595 adult subjects (34.1% of whom were affected by NAFLD) with 4654 cases of moderate-to-severe renal failure over a median observation period of 5.2 years showed a risk of also developing chronic renal failure 37% higher in patients with NAFLD. This risk was the greater the degree of lipid infiltration of the liver. Considering that both renal failure and NAFLD in themselves are then risk factors for cardiovascular disease, it is easy to understand how this epidemiological association is of particular relevance [12].
Another meta-analysis of six studies that included 25,837 patients (of whom 5953 were affected by NAFLD) showed that patients with NAFLD had a relative risk of total cardiovascular events of 1.77 (95% CI 1.26–2.48, p < 0.001). Specifically, the relative risk increased to 2.26 (95% CI: 1.04–4.92, p < 0.001) with regard to coronary artery disease and to 2.09 (95% CI: 1.46–2.98, p < 0.001) relative to ischemic stroke. Furthermore, the presence of NAFLD seems to significantly increase the relative risk of mortality due to cardiovascular causes (RR 1.46, 95% CI 1.31–1.64, p < 0.001) [13].
Pending specific drug therapies, actually in phase 3 clinical trials, the main treatment of NAFLD is currently the improvement of lifestyle aimed at weight loss and increased physical activities, in order to reduce insulin resistance [14]. Since the risk factors for NAFLD are similar to the ones for cardiovascular diseases, the suggestions for the management of NAFLD are the same suggested by the guidelines for the prevention of cardiovascular diseases [15]. Therefore, the general indication is to prescribe relatively low-calorie diets (with a caloric quantity proportional to energy consumption), with carbohydrates predominantly with a low glycemic index and minimizing the consumption of fructose, alcohol, saturated and trans-unsaturated fats [16]. In particular, the adherence to Mediterranean Diet is a significant predictor of changes in the fat content of the liver in patients with NAFLD [17]. The consumption of coffee will not be prescribed but if consumed by the patient it will not be suggested to quit it, since there are many evidences, summarised in a recent metanalysis, showing a lower risk of fibrosis in coffee drinkers with NAFLD [18].
The role of physical activity is fundamental, to be implemented as much as possible according to the degree of training and any co-morbidities. In fact, a recent meta-analysis of 6 cohort studies involving 142,781 participants with 32,657 incident cases of NAFLD, as well as 4 case-control studies involving 382 affected patients and 302 controls, shows that the difference in risk of developing NAFLD among sedentary and physically active is 21% in observational studies and 57% in case-control studies [19]. On the other hand, regardless of diet, the greater the frequency and intensity of physical activity, the greater the reduction of transaminase levels and the degree of hepatosteatosis, especially in overweight subjects [20].
To this dutiful approach, increasing evidence suggests that the prescription of specific supplements or nutraceuticals with demonstrated hepatoprotective action can be suggested to accelerate the improvement of the alteration of liver enzymes and maybe of liver steatosis or at least to slow down its evolution [9]. This rationale becomes stronger when using nutraceuticals simultaneously active on the modulation of cardiovascular risk.
2. Methods
For the purpose of this review, a systematic search strategy was developed to identify trials in both MEDLINE (National Library of Medicine, Bethesda, Maryland, MD, USA; January 1980 to May 2018) and the Cochrane Register of Controlled Trials (The Cochrane Collaboration, Oxford, UK). The terms ‘nutraceuticals’, ‘dietary supplements’, ‘food bioactives’, ‘herbal drug’, ‘liver steatosis’, ‘non-alcoholic fatty liver disease’, ‘NAFLD’, ‘clinical trial’, and ‘human’ were incorporated into an electronic search strategy. The references of all identified studies and review articles were reviewed to look for additional studies of interest. The authors reviewed all of the citations retrieved from the electronic search to identify potentially relevant articles for this review. We excluded in vitro data and animal studies and focused on human data, in order to limit our report to food components and nutraceuticals for which safety and tolerability in humans are already known. Therefore, we preferably selected papers reporting recent comprehensive reviews or meta-analyses, or original clinical trials on substances with a lipid-lowering effect and those improving action on vascular health.
3. Results
From a “nutraceutical point of view”, there are relatively few molecules adequately clinically studied for their effects on NAFLD: among these, we will analyse in detail the role of silymarin, vitamin E, vitamin D, polyunsaturated fatty acids of the omega-3 series, astaxanthin, coenzyme Q10, berberine, curcumin, resveratrol, extracts of Salvia milthiorriza, and probiotics.
3.1. Silymarin
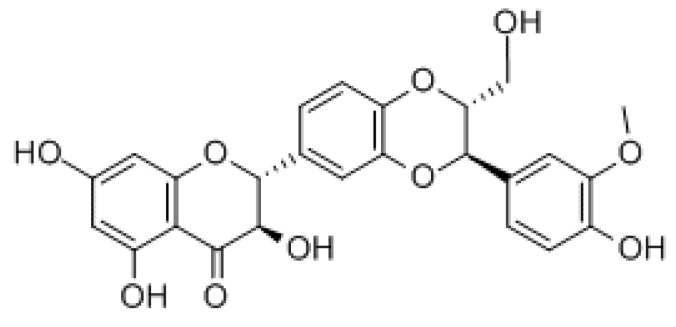
Silymarin is a powerful antioxidant agent extracted from milk thistle (Silybum marianum) with a specific liver tropism. Silymarin is actually a set of antioxidant substances of which the most concentrated are six flavolignans (silibine A and B—usually in isoconcentration—, isosilibine, silidianin, silicristine, isosilicristine) and a flavonoid (taxifolin) [21]. Of these substances, the one with the highest concentration and the most evident biological effects is silybin (Figure 1), (ID IUPAC: (2R, 3R) -3,5,7-trihydroxy-2-(2R, 3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl) -2,3-dihydrobenzo [b] [1,4] dioxin-6-yl] chroman-4-one, molecular mass 482,44 g/mol), constituting up to 70% of silymarin in its diastereoisomeric silybin A and silybin B forms [22].
Figure 1.
Chemical structure of silybin.
Silymarin has poor oral bioavailability, both due to poor intestinal absorption and high hepatic first-pass metabolism. However, this limitation can be bypassed with particular pharmaceutical techniques, for example by complexing silymarin in a phytosome with phosphatidylcholine, which increases its solubility while maintaining its antioxidant properties [23]. Silymarin is undoubtedly one of the most studied nutraceuticals of plant origin in hepatopathic patients, even with severe disease [24]. The pharmacological mechanisms by which silymarin exerts its hepatoprotective action in patients with NAFLD are numerous and summarized in Table 2 [25], which also highlights those that could have a positive impact on vascular health.
Table 2.
Biological effects of silymarin involved in its hepatoprotective action in patients with NAFLD.
| Effect | Proposed Mechanism of Action |
|---|---|
| Antioxidant |
|
| Anti-inflammatory |
|
| Anti-apoptotic |
|
| Antifibrotic |
|
| Endocrine-metabolic |
|
| Choleretic |
|
GLUT4 = glucose transporter type 4, IL = Interleukin, NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells, PPAR = peroxisome proliferator-activated receptor, TGF-β = transforming growth factor beta, TNF = tumor necrosis factor, * potentially positive effects on vascular health.
The available data show the ability of silymarin (administered alone, or usually in combination with low dosages of vitamin E) to improve insulin resistance and indirect markers of hepatosteatosis (Hepatic Steatosis Index, Lipid Accumulation Product) already after 3 months of treatment [26].
In a recent multicenter randomized double-blind phase 3 study conducted on 180 patients with histological diagnosis of NAFLD/NASH, the administration of silybin and vitamin E (silibine 188 mg, phosphatidylcholine 388 mg, vitamin E 180 mg) for 12 months determined the normalisation of transaminase, a significant reduction of gamma-glutamyl transferase levels and the significant decrease of liver steatosis measured with both ultrasound scan and, in one-fifth of patients, with a second liver biopsy. As expected, there was also a proportional improvement of fasting glucose, basal insulinemia and insulin resistance index [27]. These data were confirmed by a meta-analysis of 8 controlled clinical trials involving 587 patients [28]. There is also a preliminary report showing that supplementation with 420 mg/day of silymarin reduced the 4-years risk of mortality in patients with cirrhosis [29]. Overall tolerability is usually good, even for high doses and long-term administration [30]. For these reasons, the guidelines of the Mayo Clinic on food supplements classifies the use of silymarin for hepatoprotection as Grade B (“Good scientific evidence for this use”) [31].
Of particular interest from a cardiometabolic point of view is the clinical effect of silymarin on the metabolic control of the diabetic patient. A recent meta-analysis of five controlled clinical trials that enrolled 270 patients showed how the administration of silymarin significantly improved fasting glycemic control (−26.86 mg/dL; 95% CI −35.42–18.30) and glycated hemoglobin values (−1.07; 95% CI −1.73–0.40), plausibly for the insulin-sensitizing action of this nutraceutical [32].
The greatest limitation in the use of silymarin is usually the cost of effective dose treatment, which needs to be administered continuously and protracted over time at least until optimisation of the lifestyle, and normalisation of ultrasound liver scan.
3.2. Vitamin E
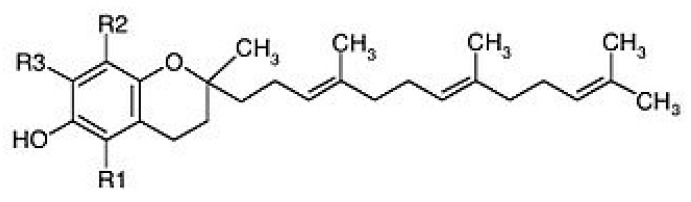
A nutraceutical tested extensively in patients with NAFLD, although almost always in association with silymarin, is vitamin E (alpha-tocopherol or (2R)-2,5,7,8-Tetramethyl-2-[(4R,8R)-(4,8,12-trimethyltridecyl)]chroman-6-ol) (Figure 2).
Figure 2.
Chemical structure of tocotrienols.
The most effective dosage of the active form of Vitamin E in order to reduce inflammation and liver fibrosis is 40 times higher than the Recommended Daily Allowance (RDA) (800 IU/day). However, doses 20 times higher (400 IU/day) are associated with increased risk of mortality from all causes. Therefore, caution is necessary to set up long-term therapies with vitamin E at the effective dosage. The majority of clinicians use Vitamin E either at a lower, less-effective, but safer dosages or by associating it with other active drugs that are probably effective in the management of NAFLD [33]. A recent meta-analysis of 16 controlled clinical trials has shown that the long-term administration of low-dose Vitamin E and alone (not in association with other antioxidant vitamins) is able to reduce the risk of myocardial infarction (RR 0.82; 95%CI, 0.70–0.96; p = 0.01) [34]. Furthermore, another meta-analysis involving 303 subjects enrolled in seven studies showed that vitamin E supplementation is associated with a 2.5% increase in flow-mediated vasodilatation [35]. This result is important since it has been estimated that a 1% improvement in flow-mediated vasodilatation would be associated with a 12% reduction in cardiovascular risk [36].
3.3. Vitamin D
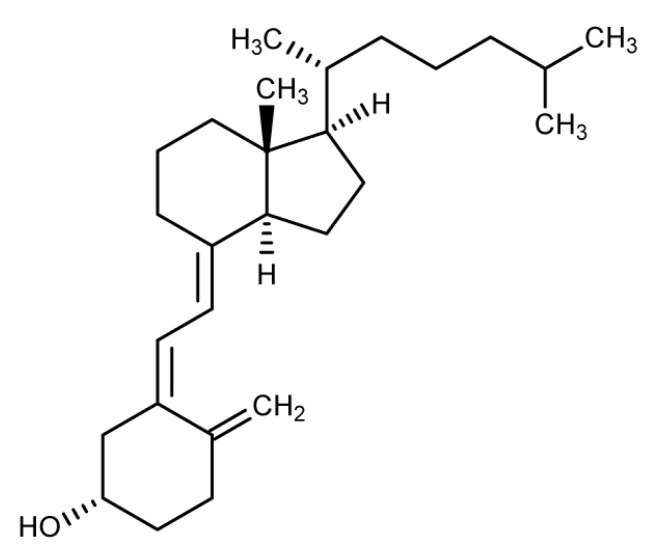
Vitamin D3 (1,25 OH cholecalciferol; C27H44O; ID IUPAC: (3β, 5Z, 7E)-9,10-secocholesta-5,7,10 (19)-trien-3-ol) (Figure 3) is a secosteroid hormone with a molar mass of 384.64 g/mol which plays a fundamental role in mineral metabolism, but also in the regulation of immune response, cell differentiation and inflammation, with important repercussions on both liver and cardiovascular health. In the human body, vitamin D is derived for approximately 10% from the diet, while from 90% from the cutaneous conversion of 7-dehydrocholesterol to colecalciferol for exposure to ultraviolet B (UVB). Then the cholecalciferol is hydroxylated from the liver by a 25-hydroxylase and then from the kidney from an alpha-1 hydroxylase, resulting in activation of 1,25-hydroxy-cholecalciferol or calcitriol vitamin D. The decline in exposure to the sun linked to the modern lifestyle, associated with the reduction in age of the capacity of hepatic and renal hydroxylation, make a large part of the population relatively and absolutely deficient in vitamin D [37].
Figure 3.
Chemical structure of Vitamin D3.
Vitamin D deficiency and non-alcoholic hepatosteatosis could be associated only for the high prevalence of both conditions in the general population. However, recent epidemiological evidence shows that in patients with NAFLD they are more frequently deficient in vitamin D than in the general population, and the circulating vitamin D levels are proportional to the degree of fibrotic evolution of NAFLD [38]. Nevertheless, not all the studies are in agreement: a recent metanalysis of observational studies involving 974 NAFLD patients, showed no difference in 25-hydroxyvitamin D levels among NAFLD patients with high NAFLD activity score (NAS) versus low NAS (MD = −0.93, 95% CI −2.45 to 0.58) and also high fibrosis score versus low fibrosis score (MD = 0.88, 95% CI −2.65 to 4.42). Despite evidence implicating vitamin D in NAFLD pathogenesis, serum 25-hydroxyvitamin D may not be associated with NAFLD histologic severity [39]. In addition, in the study of Barchetta et al. oral vitamin D supplementation (2000 IU/day) over 24 weeks did not improve hepatic steatosis or metabolic/cardiovascular parameters in diabetic patients with NAFLD [40]. In contrast, the study of Lim et al. suggests that serum 25-hydroxyvitamin D levels may be a risk factor for metabolic syndrome in patients with NAFLD [41].
Table 3 summarizes the available evidence that binds vitamin D and NAFLD from a pathophysiological point of view, justifying vitamin D supplementation in patients affected by this condition [42,43]. Moreover, integration is justified by the virtual absence of side effects for non-pharmacological dosages of supplementation, because vitamin D deficiency is almost pandemic, and for the positive actions that vitamin performs not only at the level of the bone and liver, but also on the immune and cardiovascular system [44,45].
Table 3.
Pathophysiological Mechanisms That Bind Vitamin D and NAFLD.
| Proposed Mechanism | Support Tests | Ref. |
|---|---|---|
| Insulin-sensitivity improvement |
|
[46,47] |
| Reduction of inflammation of adipose tissue |
|
[48,49,50] |
| Reduction of hepatic inflammation |
|
[51,52] |
| Slowdown of liver fibrosis |
|
[53] |
GLUT4 = glucose transporter type 4, IL = Interleukin, NF-kB = nuclear factor kappa-light-chain-enhancer of activated B cells, TIMP-1 = tissue inhibitors of metalloproteinases-1.
Some clinical studies show that daily vitamin D supplementation improves insulin resistance and related parameters in patients with NAFLD [54,55]. Again, vitamin D supplementation can also have positive implications on the cardiovascular system. In fact, while its deficiency has been associated with a risk of hypertension and vascular ageing. Its supplementation, on the contrary, would significantly reduce the levels of high sensitivity C reactive protein, known as an independent risk factor for cardiovascular diseases, as demonstrated by the meta-analysis of 10 studies involving 924 participants [56].
3.4. Polyunsaturated Fatty Acids of the Omega-3 Series
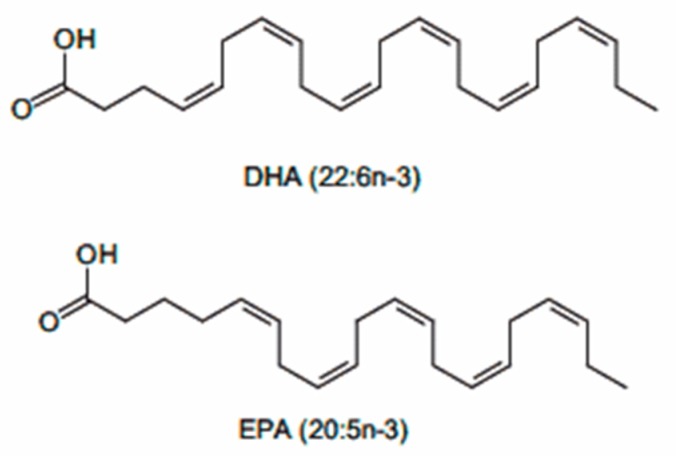
The polyunsaturated fatty acids of the series omega-3 (Figure 4) are essential fatty acids that the human body is unable to synthesize and must take them with the diet.
Figure 4.
Chemical structure of Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA).
In the human organism, omega-3 fatty acids are involved in a very high number of biological activities that make them fundamental for adequate development and for the maintenance of the health status of different organs and tissues. Although a balanced diet can theoretically provide an adequate amount of omega-3 fatty acids, the qualitative and biodiversity impoverishment of the components of the diet, the methods of processing and cooking, and the increase in functional demands of the organism makes supplementation with omega-3 fatty acids increasingly necessary [57]. A recent meta-analysis of controlled clinical trials has shown that supplementation with polyunsaturated fatty acids of the omega-3 series, mainly docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA), contributes significantly to the reduction of circulating levels of AST and gamma-glutamyl transferase [58]. This effect, associated with the known hypotriglyceridemic and anti-inflammatory actions of omega-3 fatty acids, places them among the potentially active and effective nutraceuticals in the management of NAFLD and NASH [59]. A meta-analysis of 4 randomized clinical trials conducted in 263 children showed that long-term supplementation with EPA and DHA is associated with a 25% reduction of both circulating levels of AST and ALT, and the degree of steatosis assessed by liver ultrasound scan, with no side effects [60].
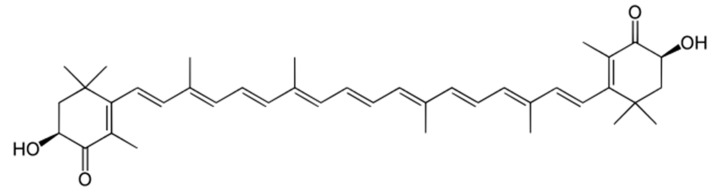
3.5. Astaxanthin
A possible nutraceutical alternative to vitamin E (to be considered to avoid the problem of balancing between hepatoprotective dose and cardio-injurious dose) is the use of astaxanthin (C40H52O4: ID IUPAC: (6S)-6-Hydroxy-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxo-1-cyclohexenyl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaenyl]-2,4,4-trimethyl-1-cyclohex-2-enone), a carotenoid of marine origin of the colour purplish-red, very stable, with an antioxidant activity in vitro extremely more powerful than the most common natural antioxidants (e.g., vitamins A, E, and C, lycopene, resveratrol), with a molar mass of 596,841 g/mol (Figure 5) [61]. In preclinical experimental models, astaxanthin is more effective than vitamin E in reducing lipogenesis, insulin resistance, hepatic inflammation, and fibrogenesis, and therefore appears to be the ideal natural antioxidant for the prevention of liver injury induced by NAFLD [62]. However, there is still no direct evidence on humans of these promising data.
Figure 5.
Chemical structure of Astaxanthin.
3.6. Coenzyme Q10
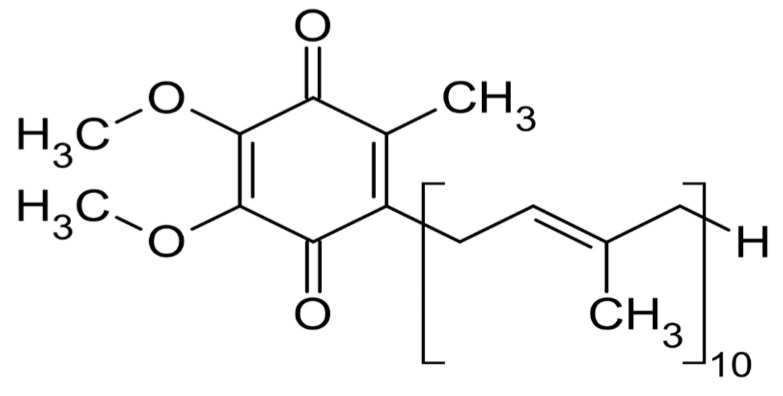
Another antioxidant of recent interest for the management of NAFLD is Coenzyme Q10 (Ubidecarenone; C59H90O4; ID IUPAC: 2-[(2E, 6E, 10E, 14E, 18E, 22E, 26E, 30E, 34E)-3.7, 11,15,19,23,27,31,35,39-Decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaenyl]-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione), molecule of molar mass 863.34 g/mol, particularly concentrated in heart, striated muscle and liver, but present in all cells of the organism (Figure 6) [63].
Figure 6.
Chemical structure of coenzyme Q10.
In a recent double-blind randomized placebo clinical study, 100 mg/day of Coenzyme Q10 for 3 weeks resulted in a significant reduction of transaminases, gamma-GT, hsCRP and degrees of NAFLD, as well as improvement of the adiponectin/leptin ratio [64]. In addition, coenzyme Q10 could help to improve the lipid pattern typically associated with NAFLD [65], as well as reducing oxidized LDL levels and arterial pressure. The main limitation of the use of coenzyme Q10 is the need for high dosages (>100 mg/day) of pharmaceutically modified formulations to have an increased bioavailability, since coenzyme Q10 by itself has poor oral bioavailability. On the other hand, it has a very high safety profile without any risk of drug interactions [66].
3.7. Berberine
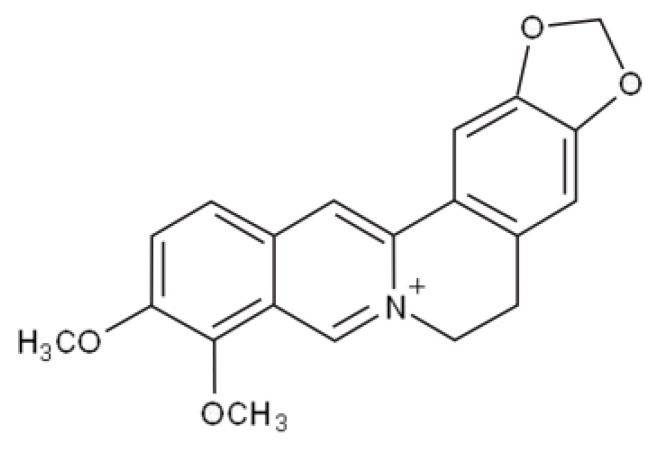
Berberine hydrochloride is a quaternary ammonium salt belonging to the group of alkaloids benzylisoquinoline (C20H18NO4 +) of a molar mass 336.3612 g/mol (Figure 7), extracted from numerous medicinal plants (in particular those of the Berberis genus) and endowed with lipid-lowering and insulin-sensitizing actions clearly demonstrated in humans [67].
Figure 7.
Chemical structure of berberine.
Some preliminary clinical reports confirmed that these actions of berberine are also related to the improvement of levels of indirect markers of hepatosteatosis (Hepatic Steatosis Index, Lipid Accumulation Product) for short-term supplements (2–4 months) at doses of 500 mg/day [68]. These data were recently collected in a meta-analysis of six randomized clinical trials that evaluated 501 patients, confirming the positive effect of berberine on lipid parameters, insulin resistance, hepatic markers and degree of hepatic steatosis in patients with NAFLD. However, these studies have used relatively high doses (1000–1500 mg/day), which may be associated with intestinal disorders [69]. Even berberine has a poor oral bioavailability that can, however, be improved by ad hoc pharmaceutical techniques.
3.8. Curcumin
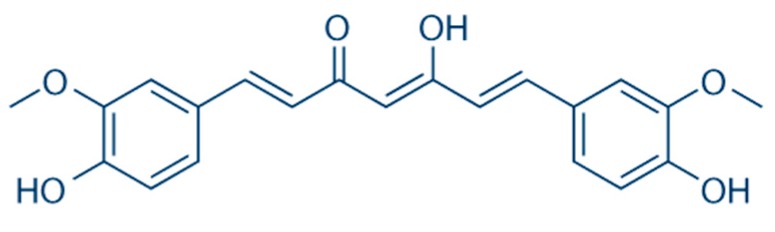
Curcumin (C21H20O6, ID IUPAC: (1E, 6E)-1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, molar mass: 368.38 g/mol) (Figure 8), another known insulin-sensitizing agent extracted from Curcuma longa, has been associated in numerous preclinical studies and in recent preliminary clinical trials with a significant improvement in NAFLD-related parameters [70]. In particular, a clinical study of 100 Asian patients with metabolic syndrome has shown a statistically significant improvement in the degree of hepatic steatosis assessed by liver ultrasound with a daily administration of 400 mg of curcumin [71]. In another controlled clinical study conducted on 102 Iranian patients, supplementation with phytosomal curcumin, 500 mg b.i.d. for 8 weeks led to a significant reduction in transaminase levels, waist circumference, body mass index, but especially the degree of hepatic steatosis in 75% of the treated subjects. Although these data are particularly promising, it should be noted that the positive effects are often observed for high supplementations (usually >1500 mg/day) of pure curcumin, with consequent problems of treatment compliance and costs (partially mitigated with the use of new, more bioavailable pharmaceutical formulations) [72]. This is because curcumin also has a poor oral bioavailability, that can, however, be improved by “ad hoc” pharmaceutical techniques.
Figure 8.
Chemical structure of curcumin.
3.9. Resveratrol
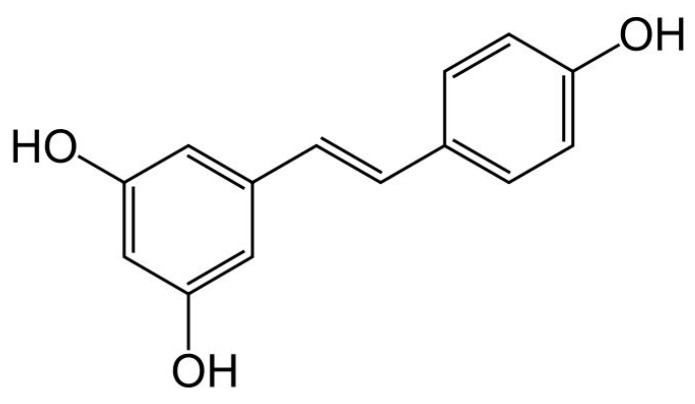
Resveratrol (3,5,4′-trihydroxy-trans-stilbene, C14H12O3, molar mass: 228.25 g/mol) is a non-flavonoid phenol (Figure 9) particularly concentrated in the grape skin peel, but also in the extracts of Polygonum cuspidatum, poorly orally bioavailable (if not chemically modified), characterized by an important antioxidant, vasoprotective (both cerebral and peripheral) and insulin-sensitizing activity [73,74]. Preliminary clinical data appear to contradict preclinical literature, suggesting a potential efficacy of resveratrol in improving NAFLD-related parameters [75]. However, these studies are usually short (too short to have an impact on the liver structure) and are conducted with resveratrol doses incompatible with the insulin-sensitizing action that could improve NAFLD. In fact, when used at the appropriate dose and for long periods, resveratrol has already shown to exert an important antihypertensive effect in those affected by NAFLD [76].
Figure 9.
Chemical structure of resveratrol.
3.10. Salvia Miltiorrhiza
A meta-analysis of 8 controlled clinical trials that included 800 Asian patients showed how Salvia miltiorrhiza dry extract supplementation (red or Chinese sage, Danshen) leads to a significant reduction in plasma levels of transaminases associated with an increase in computerized tomographic contrast between liver and spleen, indicative of a reduction in the degree of hepatosteatosis [77]. Despite the interest in these evidences, clinical data on Caucasian subjects are currently lacking.
3.11. Probiotics
Growing literature shows that even chronic supplementation with probiotics and/or symbiotics could improve several parameters related to NAFLD (insulin resistance, plasma levels of transaminases, degree of lipid infiltration of the liver), but the available studies have all been conducted with different probiotic strains or associations and it is therefore difficult to provide a specific indication for supplementation [78]. Undoubtedly, if probiotics are to be taken for other indications, the effect on the possibly co-hosted NAFLD could only be positive [79].
Promising results may come from certain probiotic strains. Preliminary RCTs have shown that treatment with L. bulgaris or S. thermophilus reduces the levels of alanine aminotransferase, aspartate aminotransferase, and gamma glutamyltransferase [80]. In obese children with NAFLD, supplementation with L. rhamnosus GG resulted in a significant improvement in liver function [81]. Alisi et al. found a significant improvement in the severity of fatty liver and a significant decrease in Body Mass Index (BMI) of children with NAFLD treated for 4 months with bifidobacteria, lactobacilli and S. thermophilus strains. These data suggest that probiotics could reduce liver fat and thus prevent the progression of NAFLD [82]. Similar results were obtained with the association L. acidophilus and B. lactis for 8 weeks in adult patients with NAFLD: at the end of treatment, there was a significant improvement of transaminases and cholesterolaemia compared to the control group [83].
4. Discussion
At the state of the art, in the absence of specific pharmacological therapies available, some nutraceuticals could play an important role in improving the NAFLD frameworks in association with diet and lifestyle based on weight loss and the reduction of insulin resistance. In particular, data concerning silymarin, vitamin E polyunsaturated fatty acids of the omega-3 series, coenzyme Q10, berberine and curcumin (Table 4), all nutraceuticals with both hepatoprotective activity and positive actions on the cardiovascular system, seem particularly convincing. Results of vitamin D still remain conflicting. As regards herbal extract, the clinical literature often does not report details about the extracts standardization.
Table 4.
Nutraceuticals with clinical effects on NAFLD: Main mechanisms of action, clinical effects, tested dosages, side effects, and level of clinical Evidence.
| Nutraceutical | Tested Dosages | Proposed Mechanism of Actions | Clinical Effects | Side Effects | Level of Scientific Evidence [Ref.] |
|---|---|---|---|---|---|
| Berberine | 500–500 mg/day | Activation of AMPK and the expression of LDL receptors, inhibition of PCSK9 | Improvement of levels of indirect markers of hepatosteatosis (Hepatic Steatosis Index, Lipid Accumulation Product), lipid parameters and insulin resistance | Mild gastrointestinal side effects | Meta-analysis of RCTs [69,84] |
| Coenzyme Q10 | 100–300 mg/day | Antioxidant activity, sensitizing of Ca++ channels, inductor of the synthesis of ATP, reduction of oxidative stress and lipid peroxidation | Reduction of transaminases, gamma-GT, hsCRP and degrees of NAFLD and hepatic steatosis, improvement of the adiponectin/leptin ratio | Not reported | RCTs [64,65,68] |
| Curcumin | 400–2000 mg/day | Inhibition of the expression of NPC1L1 transporter, increases the efflux of cholesterol, downregulation of the expression of PCSK9, reduction of TNF-α levels, inhibition of NF-κB activation, lipid peroxidation and lysosomal enzyme activities, induction of PPAR-γ and Nrf2 activation | Improvement in the degree of hepatic steatosis, reduction in transaminase levels, waist circumference and body mass index | Mild nausea, stomach cramps and/or upset, diarrhea, dizziness | Meta-analysis of RCTs [71,72] |
| Polyunsaturated Omega-3 fatty acids | 1–4 g/day of eicosapentanoic and/or docosahexaenoic acid | Reduction of the release and synthesis of inflammatory cytokines, activation of eNOS, prostaglandins synthesis balance toward vasodilating ones, insulin-sensitivity, vascular tone regulation by parasympathetic nervous system stimulation, and suppression of the renin–angiotensin–aldosterone system | Reduction of transaminases, serum triglycerides, blood pressure (SBP 1–5 mmHg) | Mild aftertaste, nausea, gastroesophageal reflux, bloating and dyspepsia | Meta-analysis of RCTs [58,59] |
| Probiotics | >3,5 CFU/day (extremely variable depending on strains, associations, and vehicle of administration used) | Reduction of lucky gut syndrome, intestinal permeability, modulation of bile salt hydrolases | Improvement of insulin resistance, plasma levels of transaminases, degree of lipid infiltration of the liver | Not reported | RCTs [78] |
| Resveratrol | >150 mg/day | Antioxidant, vasoprotective (both cerebral and peripheral) and insulin-sensitizing activity | Unclear | Rare gastrointestinal side effects | Open-label clinical studies [75,76] |
| Silymarin | 150–450 mg/day | Direct scavenger activity, mitochondrial function optimization, activation of protective molecules such as HSPs, thioredoxin and sirtuins, inhibition of NF-kB activity, proinflammatory cytokine synthesis reduction (IL-1, IL-6, TNF-α), modulation of caspase release and TNF-α effect, inhibition of the conversion of stellate cells into fibroblasts, downregulation of the expression of profibrotic genes (procollagen III, TGF-β), partial activation of estrogen receptors, insulin-sensitizing action, PPAR-agonist action, increased expression of GLUT4 on the cell surface, inhibition of HMG-CoA reductase, upregulation of the bile salt export pump | Transaminase normalization, reduction of gamma-glutamyl transferase levels and degree of ultrasound-related liver steatosis, improvement of fasting glucose, basal insulinemia and insulin resistance | Mild gastrointestinal side effects | Meta-analysis of RCTs [29,30,31] |
| Vitamin D | 2000–50,000 UI/day | Upregulation of the translocation of GLUT4, modulation of transcription of insulin gene, inhibition of NF-kB, release of proinflammatory cytokines and proliferation of hepatic stellate cells | Improvement of insulin sensitivity, hepatic and adipose inflammation | Not reported | RCTs [54,55] |
| Vitamin E | 800 UI/day | Antioxidant | Improvement of arterial stiffness and reduction of risk of myocardial infarction | At 400 UI/day: increases risk of mortality (?) | Meta-analysis of RCTs [33,34,35] |
AMPK = Adenosin-Monophosphate-Kinase-alpha, CFU = colony forming units, eNOS = endothelial nitric oxide synthase, GLUT4 = glucose transporter type 4, HMG-CoA = Hydroxy-Methyl-Glutaryl Coenzyme A, hs-CRP = high sensible C-reactive protein, IL = Interleukin, NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells, NPC1L1 = Niemann-Pick C1-Like 1) Nrf2 = nuclear factor erythroid-2-related factor-2, PCSK9 = proprotein convertase subtilisin/kexin type 9, PPAR = peroxisome proliferator-activated receptor, TGF-β = transforming growth factor beta, TNF = tumor necrosis factor, RCTs = Randomized clinical trials.
Many other nutraceuticals could exert some positive effects on NAFLD. For instance, L-carnitine might be an interesting option as adjuvant in people with NAFLD for its role in a number of intracellular and metabolic functions [85]: in preliminary clinical studies, it seems to improve insulin resistance and inflammatory biomarkers, even if data from an RCT of supplementation with 500 mg/twice daily of this molecule for 1 year for the treatment for NAFLD showed no significant changes in liver function tests and ultrasound grade [86].
The role of flaxseed in NAFLD was recently assessed in a RCT. In a total of 50 people with NAFLD (confirmed by fibroscan examination) the supplementation with 30 g/day of brown milled flaxseed for 12 weeks was associated to an improvement in BMI, waist circumference, serum transaminases, hs-CRP, TNF-alpha, glucose and insulin concentrations compared to control, while HOMA-IR, hepatic fibrosis and steatosis scores were improved in both groups even if significantly greater in the flaxseed group compared to control [87].
Phyllanthus urinaria has been shown to reduce hepatic steatosis and necroinflammation in vitro and in vivo [88]. In a RCT of 60 patients with histology-confirmed NASH treated with 1 g of P. urinaria three times daily or placebo for 24 weeks, the NAFLD activity score, steatosis percentage, and steatosis grade assessed by biopsy were significantly reduced from baseline in the intervention group, while changes were not significant in the control group [89]. The bioactive compound responsible for this effect is not fully clarified.
Anthocyanins, polyphenols other than resveratrol and betaine, are compounds with preliminary data regarding, in particular, the improvement of hyperlipidaemia and the reduction of oxidative stress and vascular inflammation: however, studies on people with liver steatosis are still lacking [90]. Again, clinical data for garlic, Chlorella vulgaris, Myrica (bayberry) and green tea are few and in general concern short-term RCTs [91].
Finally, many traditional Chinese herbal formulas are reported in literature to have significant anti-NAFLD effects: the association of Artemisia capillaris (Thunb), Gardenia jasminoides (Ellis), and Rheum palmatum (L) can reduce the accumulation of hepatic fat, increase endothelial progenitor cell proliferation, enhance adiponectin secretion, and increase PPAR-γ expression [92].
In conclusion, a relatively large number of dietary supplements and herbal extracts seems to improve NAFLD and related parameters. However, long-term double-blind randomised clinical trials are still needed to understand if the observed results are confirmed and maintained in time. Furthermore, the molecular targets and the signalling transduction pathways of many of these nutraceuticals should still be more deeply investigated. Particularly, in plant extracts, the eventual additive or synergistic effect of each single bioactive compounds must be clarified.
Author Contributions
A.F.G.C. conceived the review, A.C. analysed and selected the clinical literature on the subject, while S.B. revised the final version of the paper. All the authors coworked in writing the main structure of the review.
Funding
This research received no external funding.
Conflicts of Interest
None of the authors has a direct or indirect conflict of interest in the publication of this paper.
References
- 1.Leung P.S. The Gastrointestinal System: Gastrointestinal, Nutritional and Hepatobiliary Physiology. Springer; New York, NY, USA: 2016. pp. 24–36. [Google Scholar]
- 2.Araújo A.R., Rosso N., Bedogni G., Tiribelli C., Bellentani S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018;38(Suppl. 1):47–51. doi: 10.1111/liv.13643. [DOI] [PubMed] [Google Scholar]
- 3.Townsend S.A., Newsome P.N. Non-alcoholic fatty liver disease in 2016. Br. Med. Bull. 2016;119:43–56. doi: 10.1093/bmb/ldw031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedogni G., Bellentani S., Miglioli L., Masutti F., Passalacqua M., Castiglione A., Tiribelli C. The Fatty Liver Index: Asimple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asgari-Taee F., Zerafati-Shoae N., Dehghani M., Sadeghi M., Baradaran H.R., Jazayeri S. Association of sugar sweetened beverages consumption with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Nutr. 2018 doi: 10.1007/s00394-018-1711-4. [DOI] [PubMed] [Google Scholar]
- 6.Wijarnpreecha K., Thongprayoon C., Edmonds P.J., Cheungpasitporn W. Associations of sugar- and artificially sweetened soda with nonalcoholic fatty liver disease: A systematic review and meta-analysis. QJM. 2016;109:461–466. doi: 10.1093/qjmed/hcv172. [DOI] [PubMed] [Google Scholar]
- 7.Wijarnpreecha K., Thongprayoon C., Panjawatanan P., Ungprasert P. Insomnia and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. J. Postgrad. Med. 2017;63:226–231. doi: 10.4103/jpgm.JPGM_140_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trovato F.M., Martines G.F., Brischetto D., Catalano D., Musumeci G., Trovato G.M. Fatty liver disease and lifestyle in youngsters: Diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36:427–433. doi: 10.1111/liv.12957. [DOI] [PubMed] [Google Scholar]
- 9.He W., An X., Li L., Shao X., Li Q., Yao Q., Zhang J.A. Relationship between hypothyroidism and Non-Alcoholic Fatty Liver Disease: A systematic review and meta-analysis. Front Endocrinol. 2017;8:335. doi: 10.3389/fendo.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villela-Nogueira C.A., Leite N.C., Cardoso C.R., Salles G.F. NAFLD and increased aortic stiffness: parallel or common physiopathological mechanisms? Int. J. Mol. Sci. 2016;17:460. doi: 10.3390/ijms17040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calzadilla Bertot L., Adams L.A. The natural course of Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016;17:774. doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A., Zaza G., Byrne C.D., Lonardo A., Zoppini G., Bonora E., Targher G. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: A systematic review and meta-analysis. Metabolism. 2018;79:64–76. doi: 10.1016/j.metabol.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Mahfood Haddad T., Hamdeh S., Kanmanthareddy A., Alla V.M. Nonalcoholic Fatty Liver Disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2017;11(Suppl. 1):S209–S216. doi: 10.1016/j.dsx.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Zelber-Sagi S., Godos J., Salomone F. Lifestyle changes for the treatment of nonalcoholic fatty liver disease: A review of observational studies and intervention trials. Therap. Adv. Gastroenterol. 2016;9:392–407. doi: 10.1177/1756283X16638830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.T., Corrà U., Cosyns B., Deaton C., et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Atherosclerosis. 2016;252:207–274. doi: 10.1016/j.atherosclerosis.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Ma J., Hennein R., Liu C., Long M.T., Hoffmann U., Jacques P.F., Lichtenstein A.H., Hu F.B., Levy D. Improved diet quality associates with reduction in liver fat-particularly in individuals with high genetic risk scores for Nonalcoholic Fatty Liver Disease. Gastroenterology. 2018;155:107–117. doi: 10.1053/j.gastro.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trovato F.M., Catalano D., Martines G.F., Pace P., Trovato G.M. Mediterranean diet and non-alcoholic fatty liver disease: The need of extended and comprehensive interventions. Clin. Nutr. 2015;34:86–88. doi: 10.1016/j.clnu.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Shen H., Rodriguez A.C., Shiani A., Lipka S., Shahzad G., Kumar A., Mustacchia P. Association between caffeine consumption and nonalcoholic fatty liver disease: A systemic review and meta-analysis. Therap. Adv. Gastroenterol. 2016;9:113–120. doi: 10.1177/1756283X15593700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu S., Cai X., Sun Z., Li L., Zügel M., Steinacker J.M., Schumann U. Association between physical activity and risk of nonalcoholic fatty liver disease: A meta-analysis. Therap. Adv. Gastroenterol. 2017;10:701–713. doi: 10.1177/1756283X17725977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orci L.A., Gariani K., Oldani G., Delaune V., Morel P., Toso C. Exercise-based Interventions for Nonalcoholic Fatty Liver Disease: A Meta-analysis and Meta-regression. Clin. Gastroenterol. Hepatol. 2016;14:1398–1411. doi: 10.1016/j.cgh.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Kim N.C., Graf T.N., Sparacino C.M., Wani M.C., Wall M.E. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum) Org. Biomol. Chem. 2003;1:1684–1689. doi: 10.1039/b300099k. [DOI] [PubMed] [Google Scholar]
- 22.Loguercio C., Festi D. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011;17:2288–2301. doi: 10.3748/wjg.v17.i18.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barzaghi N., Crema F., Gatti G., Pifferi G., Perucca E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur. J. Drug. Metab. Pharmacokinet. 1990;15:333–338. doi: 10.1007/BF03190223. [DOI] [PubMed] [Google Scholar]
- 24.Abenavoli L., Capasso R., Milic N., Capasso F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010;24:1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- 25.Federico A., Dallio M., Loguercio C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules. 2017;22:191. doi: 10.3390/molecules22020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surai P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants. 2015;4:204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loguercio C., Andreone P., Brisc C., Brisc M.C., Bugianesi E., Chiaramonte M., Cursaro C., Danila M., de Sio I., Floreani A., et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Free Radic Biol. Med. 2012;52:1658–1665. doi: 10.1016/j.freeradbiomed.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Zhong S., Fan Y., Yan Q., Fan X., Wu B., Han Y., Zhang Y., Chen Y., Zhang H., Niu J. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease: A meta-analysis (PRISMA) of randomized control trials. Medicine. 2017;96:e9061. doi: 10.1097/MD.0000000000009061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salomone F., Godos J., Zelber-Sagi S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2016;36:5–20. doi: 10.1111/liv.12975. [DOI] [PubMed] [Google Scholar]
- 30.Saller R., Brignoli R., Melzer J., Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch. Komplementmed. 2008;15:9–20. doi: 10.1159/000113648. [DOI] [PubMed] [Google Scholar]
- 31.Mayo Clinic–Drug Supplements. [(accessed on 23 August 2018)]; Available online: http://www.mayoclinic.org/drugs-supplements/milk-thistle/evidence/hrb-20059806.
- 32.Voroneanu L., Nistor I., Dumea R., Apetrii M., Covic A. Silymarin in Type 2 Diabetes Mellitus: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Res. 2016;2016:5147468. doi: 10.1155/2016/5147468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J., Cordero P., Nguyen V., Oben J.A. The role of vitamins in the pathogenesis of Non-alcoholic Fatty Liver Disease. Integr. Med. Insights. 2016;11:19–25. doi: 10.4137/IMI.S31451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loffredo L., Perri L., Di Castelnuovo A., Iacoviello L., De Gaetano G., Violi F. Supplementation with vitamin E alone is associated with reduced myocardial infarction: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2015;25:354–363. doi: 10.1016/j.numecd.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Joris P.J., Mensink R.P. Effects of supplementation with the fat-soluble vitamins E and D on fasting flow-mediated vasodilation in adults: A meta-analysis of randomized controlled trials. Nutrients. 2015;7:1728–1743. doi: 10.3390/nu7031728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuzawa Y., Kwon T.G., Lennon R.J., Lerman L.O., Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J. Am. Heart Assoc. 2015;4:e002270. doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jablonski N.G., Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int. J. Paleopathol. 2018;29 doi: 10.1016/j.ijpp.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Keane J.T., Elangovan H., Stokes R.A., Gunton J.E. Vitamin D and the liver-correlation or cause? Nutrients. 2018;10:496. doi: 10.3390/nu10040496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaruvongvanich V., Ahuja W., Sanguankeo A., Wijarnpreecha K., Upala S. Vitamin D and histologic severity of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Dig Liver Dis. 2017;9:618–622. doi: 10.1016/j.dld.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Barchetta I., Del Ben M., Angelico F., Di Martino M., Fraioli A., La Torre G., Saulle R., Perri L., Morini S., Tiberti C., et al. No effects of oral vitamin d supplementation on non-alcoholic fatty liver disease in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. BMC Med. 2016;14:92. doi: 10.1186/s12916-016-0638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim H.S., Kim T.H., Lee H.H., Kim S.K., Lee B., Park Y.H. Relationship between serum 25-hydroxy-vitamin d concentration and risk of metabolic syndrome in patients with fatty liver. J. Bone Metab. 2017;24:223–228. doi: 10.11005/jbm.2017.24.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen E.Q., Shi Y., Tang H. New insight of vitamin D in chronic liver diseases. Hepatobiliary Pancreat. Dis. Int. 2014;13:580–585. doi: 10.1016/S1499-3872(14)60295-2. [DOI] [PubMed] [Google Scholar]
- 43.Elangovan H., Chahal S., Gunton J.E. Vitamin D in liver disease: Current evidence and potential directions. Biochim. Biophys. Acta. 2017;1863:907–916. doi: 10.1016/j.bbadis.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Al Nozha O.M. Vitamin D and extra-skeletal health: Causality or consequence. Int. J. Health Sci. 2016;10:443. [PMC free article] [PubMed] [Google Scholar]
- 45.Wimalawansa S.J. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J. Steroid. Biochem. Mol. Biol. 2018;175:177–189. doi: 10.1016/j.jsbmb.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Benetti E., Mastrocola R., Chiazza F., Nigro D., D’Antona G., Bordano V., Fantozzi R., Aragno M., Collino M., Minetto M.A. Effects of vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PLoS ONE. 2018;13:e0189707. doi: 10.1371/journal.pone.0189707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alkharfy K.M., Al-Daghri N.M., Yakout S.M., Hussain T., Mohammed A.K., Krishnaswamy S. Influence of vitamin D treatment on transcriptional regulation of insulin-sensitive genes. Metab. Syndr. Relat. Disord. 2013;11:283–288. doi: 10.1089/met.2012.0068. [DOI] [PubMed] [Google Scholar]
- 48.Riachy R., Vandewalle B., Kerr Conte J., Moerman E., Sacchetti P., Lukowiak B., Gmyr V., Bouckenooghe T., Dubois M., Pattou F. 1,25-dihydroxyvitamin D3 protects RINm5F and human islet cells against cytokine-induced apoptosis: Implication of the antiapoptotic protein A20. Endocrinology. 2002;143:4809–4819. doi: 10.1210/en.2002-220449. [DOI] [PubMed] [Google Scholar]
- 49.Pittas A.G., Joseph N.A., Greenberg A.S. Adipocytokines and insulin resistance. J. Clin. Endocrinol. Metab. 2004;89:447–452. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- 50.D’Ambrosio D., Cippitelli M., Cocciolo M.G., Mazzeo D., Di Lucia P., Lang R., Sinigaglia F., Panina-Bordignon P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J. Clin. Investig. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abramovitch S., Dahan-Bachar L., Sharvit E., Weisman Y., Ben Tov A., Brazowski E., Reif S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728–1737. doi: 10.1136/gut.2010.234666. [DOI] [PubMed] [Google Scholar]
- 52.Potter J.J., Liu X., Koteish A., Mezey E. 1,25-dihydroxyvitamin D3 and its nuclear receptor repress human α1 (I) collagen expression and type I collagen formation. Liver Int. 2013;33:677–686. doi: 10.1111/liv.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eliades M., Spyrou E. Vitamin D: A new player in non-alcoholic fatty liver disease? World J. Gastroenterol. 2015;21:1718–1727. doi: 10.3748/wjg.v21.i6.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lorvand Amiri H., Agah S., Tolouei Azar J., Hosseini S., Shidfar F., Mousavi S.N. Effect of daily calcitriol supplementation with and without calcium on disease regression in non-alcoholic fatty liver patients following an energy-restricted diet: Randomized, controlled, double-blind trial. Clin. Nutr. 2017;36:1490–1497. doi: 10.1016/j.clnu.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 55.Lorvand Amiri H., Agah S., Mousavi S.N., Hosseini A.F., Shidfar F. Regression of Non-Alcoholic Fatty Liver by vitamin D supplement: A double-blind randomized controlled clinical trial. Arch. Iran. Med. 2016;19:631–638. [PubMed] [Google Scholar]
- 56.Chen N., Wan Z., Han S.F., Li B.Y., Zhang Z.L., Qin L.Q. Effect of vitamin D supplementation on the level of circulating high-sensitivity C-reactive protein: A meta-analysis of randomized controlled trials. Nutrients. 2014;6:2206–2216. doi: 10.3390/nu6062206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cicero A.F., Reggi A., Parini A., Borghi C. Application of polyunsaturated fatty acids in internal medicine: Beyond the established cardiovascular effects. Arch. Med. Sci. 2012;8:784–793. doi: 10.5114/aoms.2012.31613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He X.X., Wu X.L., Chen R.P., Chen C., Liu X.G., Wu B.J., Huang Z.M. Effectiveness of omega-3 polyunsaturated fatty acids in Non-Alcoholic Fatty Liver Disease: A meta-analysis of randomized controlled trials. PLoS ONE. 2016;11:e0162368. doi: 10.1371/journal.pone.0162368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cicero A.F., Morbini M., Borghi C. Do we need “new” new omega 3 polyunsaturated fatty acids formulation? Exp. Opin. Pharmacother. 2014;4:1–4. doi: 10.1517/14656566.2015.991308. [DOI] [PubMed] [Google Scholar]
- 60.Chen L.H., Wang Y.F., Xu Q.H., Chen S.S. Omega-3 fatty acids as a treatment for non-alcoholic fatty liver disease in children: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2018;37:516–521. doi: 10.1016/j.clnu.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 61.Ambati R.R., Phang S.M., Ravi S., Aswathanarayana R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen G., Ni Y., Nagata N., Xu L., Ota T. Micronutrient antioxidants and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2016;17:1379. doi: 10.3390/ijms17091379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hernández-Camacho J.D., Bernier M., López-Lluch G., Navas P. Coenzyme Q(10) Supplementation in aging and disease. Front. Physiol. 2018;9:44. doi: 10.3389/fphys.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farsi F., Mohammadshahi M., Alavinejad P., Rezazadeh A., Zarei M., Engali K.A. Functions of Coenzyme Q10 Supplementation on Liver Enzymes, Markers of systemic inflammation, and adipokines in patients affected by Nonalcoholic Fatty Liver Disease: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. J. Am. Coll. Nutr. 2016;35:346–353. doi: 10.1080/07315724.2015.1021057. [DOI] [PubMed] [Google Scholar]
- 65.Sharifi N., Tabrizi R., Moosazadeh M., Mirhosseini N., Lankarani K.B., Akbari M., Chamani M., Kolahdooz F., Asemi Z. The effects of coenzyme Q10 supplementation on lipid profiles among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Curr. Pharm. Des. 2018 doi: 10.2174/1381612824666180406104516. [DOI] [PubMed] [Google Scholar]
- 66.Gutierrez-Mariscal F.M., Yubero-Serrano E.M., Villalba J.M., Lopez-Miranda J. Coenzyme Q(10): From bench to clinic in aging diseases, a translational review. Crit. Rev. Food Sci. Nutr. 2018:1–18. doi: 10.1080/10408398.2018.1442316. [DOI] [PubMed] [Google Scholar]
- 67.Caliceti C., Franco P., Spinozzi S., Roda A., Cicero A.F. Berberine: New insights from pharmacological aspects to clinical evidences in the management of metabolic disorders. Curr. Med. Chem. 2016;23:1460–1476. doi: 10.2174/0929867323666160411143314. [DOI] [PubMed] [Google Scholar]
- 68.Cicero A.F., Baggioni A. Berberine and its role in chronic disease. Adv. Exp. Med. Biol. 2016;928:27–45. doi: 10.1007/978-319-41334-1_2. [DOI] [PubMed] [Google Scholar]
- 69.Wei X., Wang C., Hao S., Song H., Yang L. The therapeutic effect of berberine in the treatment of Nonalcoholic Fatty Liver Disease: A meta-analysis. Evid. Based Complement. Alternat. Med. 2016;2016:3593951. doi: 10.1155/2016/3593951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panahi Y., Kianpour P., Mohtashami R., Jafari R., Simental-Mendía L.E., Sahebkar A. curcumin lowers serum lipids and uric acid in subjects with Nonalcoholic Fatty Liver Disease: A randomized controlled trial. J. Cardiovasc. Pharmacol. 2016;68:223–229. doi: 10.1097/FJC.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 71.Selmanovic S., Beganlic A., Salihefendic N., Ljuca F., Softic A., Smajic E. Therapeutic effects of curcumin on ultrasonic morphological characteristics of liver in patients with metabolic syndrome. Acta Inform. Med. 2017;25:169–174. doi: 10.5455/aim.2017.25.169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu R.W., Carey E.J., Lindor K.D., Tabibian J.H. Curcumin in hepatobiliary disease: pharmacotherapeutic properties and emerging potential clinical applications. Ann. Hepatol. 2017;16:835–841. doi: 10.5604/01.3001.0010.5273. [DOI] [PubMed] [Google Scholar]
- 73.Fogacci F., Tocci G., Presta V., Fratter A., Borghi C., Cicero A.F. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit. Rev. Food Sci. Nutr. 2018:1–14. doi: 10.1080/10408398.2017.1422480. [DOI] [PubMed] [Google Scholar]
- 74.Cicero A.F., Fogacci F., Banach M. Botanicals and phytochemicals active on cognitive decline: The clinical evidence. Pharmacol. Res. 2018;130:204–212. doi: 10.1016/j.phrs.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 75.Elgebaly A., Radwan I.A., AboElnas M.M., Ibrahim H.H., Eltoomy M.F., Atta A.A., Mesalam H.A., Sayed A.A., Othman A.A. Resveratrol supplementation in patients with Non-Alcoholic Fatty Liver Disease: Systematic review and meta-analysis. J. Gastrointestin. Liver Dis. 2017;26:59–67. doi: 10.15403/jgld.2014.1121.261.ely. [DOI] [PubMed] [Google Scholar]
- 76.Fogacci F., Banach M., Cicero A.F. Resveratrol effect on NAFLD patients: It is a matter of dose and treatment length. Diab. Obes. Metab. 2018;20:1798–1799. doi: 10.1111/dom.13324. [DOI] [PubMed] [Google Scholar]
- 77.Peng H., He Y., Zheng G., Zhang W., Yao Z., Xie W. Meta-analysis of traditional herbal medicine in the treatment of nonalcoholic fatty liver disease. Cell. Mol. Biol. 2016;62:88–95. [PubMed] [Google Scholar]
- 78.Lavekar A.S., Raje D.V., Manohar T., Lavekar A.A. Role of probiotics in the treatment of Nonalcoholic Fatty Liver Disease: A Meta-analysis. Euroasian J. Hepatogastroenterol. 2017;7:130–137. doi: 10.5005/jp-journals-10018-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sáez-Lara M.J., Robles-Sanchez C., Ruiz-Ojeda F.J., Plaza-Diaz J., Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A review of human clinical trials. Int. J. Mol. Sci. 2016;17:928. doi: 10.3390/ijms17060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aller R., De Luis D.A., Izaola O., Conde R., Gonzalez Sagrado M., Primo D., De La Fuente B., Gonzalez J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011;15:1090–1095. [PubMed] [Google Scholar]
- 81.Vajro P., Mandato C., Licenziati M.R., Franzese A., Vitale D.F., Lenta S., Caropreso M., Vallone G., Meli R. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J. Gastroenterol. Nutr. 2011;52:740–743. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 82.Alisi A., Bedogni G., Baviera G., Giorgio V., Porro E., Paris C., Giammaria P., Reali L., Anania F., Nobili V. Randomised clinical trial: The beneficial effects of VLS‰3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2014;39:1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nabavi S., Rafraf M., Somi M.H., Homayouni-Rad A., Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J. Dairy Sci. 2014;97:7386–7393. doi: 10.3168/jds.2014-8500. [DOI] [PubMed] [Google Scholar]
- 84.Yan H.M., Xia M.F., Wang Y. Efficacy of berberine in patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE. 2015;10:e0134172. doi: 10.1371/journal.pone.0134172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malaguarnera M., Gargante M.P., Russo C., Antic T., Vacante M., Malaguarnera M., Avitabile T., Li Volti G., Galvano F. L-carnitine supplementation to diet: A new tool in treatment of non alcoholic steatohepatitis—A randomized and controlled clinical trial. Am. J. Gastroenterol. 2010;105:1338–1345. doi: 10.1038/ajg.2009.719. [DOI] [PubMed] [Google Scholar]
- 86.Somi M.H., Fatahi E., Panahi J., Havasian M.R., Judaki A. Data from a randomized and controlled trial of L carnitine prescription for the treatment for non-alcoholic fatty liver disease. Bioinformation. 2014;10:575–579. doi: 10.6026/97320630010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yari Z., Rahimlou M., Eslamparast T., Ebrahimi-Daryani N., Poustchi H., Hekmatdoost A. Flaxseed supplementation in non-alcoholic fatty liver disease: A pilot randomized, open labeled, controlled study. Int. J. Food Sci. Nutr. 2016;67:461–469. doi: 10.3109/09637486.2016.1161011. [DOI] [PubMed] [Google Scholar]
- 88.Shen B., Yu J., Wang S., Chu E.S., Wong V., Zhou X., Lin G., Sung J.J., Chan H.L. Phyllanthus urinaria ameliorates the severity of nutritional steatohepatitis both in vitro and in vivo. Hepatology. 2008;47:473–483. doi: 10.1002/hep.22039. [DOI] [PubMed] [Google Scholar]
- 89.Wong V.W., Wong G.L., Chan A.W., Chu W.C., Choi P.C., Chim A.M., Yiu K.K., Yu J., Chan F.K., Chan H.L. Treatment of non-alcoholic steatohepatitis with Phyllanthus urinaria: A randomized trial. J. Gastroenterol. Hepatol. 2013;28:57–62. doi: 10.1111/j.1440-1746.2012.07286.x. [DOI] [PubMed] [Google Scholar]
- 90.Del Ben M., Polimeni L., Baratta F., Pastori D., Angelico F. The role of nutraceuticals for the treatment of non-alcoholic fatty liver disease. Br. J. Clin. Pharmacol. 2017;83:88–95. doi: 10.1111/bcp.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bagherniya M., Nobili V., Blesso C.N., Sahebkar A. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: A clinical review. Pharmacol. Res. 2018;130:213–240. doi: 10.1016/j.phrs.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 92.Lee T.Y., Chang H.H., Lo W.C., Lin H.C. Alleviation of hepatic oxidative stress by Chinese herbal medicine Yin-Chen-Hao-Tang in obese mice with steatosis. Int. J. Mol. Med. 2010;25:837–844. doi: 10.3892/ijmm_00000412. [DOI] [PubMed] [Google Scholar]