Abstract
BACKGROUND
Whether weight gain after smoking cessation attenuates the health benefits of quitting is unclear.
METHODS
In three cohort studies involving men and women in the United States, we identified those who had reported quitting smoking and we prospectively assessed changes in smoking status and body weight. We estimated risks of type 2 diabetes, death from cardiovascular disease, and death from any cause among those who had reported quitting smoking, according to weight changes after smoking cessation.
RESULTS
The risk of type 2 diabetes was higher among recent quitters (2 to 6 years since smoking cessation) than among current smokers (hazard ratio, 1.22; 95% confidence interval [CI], 1.12 to 1.32). The risk peaked 5 to 7 years after quitting and then gradually decreased. The temporary increase in the risk of type 2 diabetes was directly proportional to weight gain, and the risk was not increased among quitters without weight gain (P<0.001 for interaction). In contrast, quitters did not have a temporary increase in mortality, regardless of weight change after quitting. As compared with current smokers, the hazard ratios for death from cardiovascular disease were 0.69 (95% CI, 0.54 to 0.88) among recent quitters without weight gain, 0.47 (95% CI, 0.35 to 0.63) among those with weight gain of 0.1 to 5.0 kg, 0.25 (95% CI, 0.15 to 0.42) among those with weight gain of 5.1 to 10.0 kg, 0.33 (95% CI, 0.18 to 0.60) among those with weight gain of more than 10.0 kg, and 0.50 (95% CI, 0.46 to 0.55) among longer-term quitters (>6 years since smoking cessation). Similar associations were observed for death from any cause.
CONCLUSIONS
Smoking cessation that was accompanied by substantial weight gain was associated with an increased short-term risk of type 2 diabetes but did not mitigate the benefits of quitting smoking on reducing cardiovascular and all-cause mortality. (Funded by the National Institutes of Health.)
SMOKING CSSSATION REDUCES THE RISK of major chronic diseases and extends life expectancy,1 but considerable weight gain may occur in quitters after cessation.2 Such weight gain is probably due to increased appetite and reduced energy expenditure3 and may discourage quitting attempts and potentially attenuate the health benefits of smoking cessation through increasing the risk of cardiometabolic disease and premature death.4 Despite this concern, evidence regarding the health consequences of weight gain after smoking cessation is equivocal.5–9 The inconsistent findings may be due to the fact that previous observational studies largely relied on the “point prevalence”10 of smoking cessation (i.e., assessment of smoking status at one particular follow-up period only), and possible changes in smoking behavior during follow-up were usually unknown. It has been reported that more than 30% of quitters who were abstinent for 1 year eventually had a relapse within the next decade.11
With longitudinal, repeated assessments of smoking status and body weight in large cohort studies of men and women in the United States, the current investigation aimed to evaluate the risk trajectories of disease and death among those who reported quitting smoking, according to body-weight changes after smoking cessation.
METHODS
STUDY POPULATION AND STUDY OVERSIGHT
We used data collected from three cohorts — the Nurses’ Health Study (NHS), the Nurses’ Health Study II (NHS II), and the Health Professionals Follow-up Study (HPFS) (Text 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org) — with follow-up by means of questionnaires eliciting medical and lifestyle information that were mailed to participants every 2 years.12,13 The study baseline was set at 1984 for the NHS or the first follow-up cycle since recruitment for the NHS II (1991) and the HPFS (1988) for identifying participants who reported quitting smoking.
In analyses of the risk of type 2 diabetes, participants with prevalent diabetes, cardiovascular disease, or cancer at baseline were excluded; and in analyses of mortality, participants with prevalent cardiovascular disease and cancer at baseline were excluded. The exclusion of prevalent chronic diseases was critical for reducing the probability that participants had quit smoking owing to existing diseases. For all analyses, we also excluded participants with missing information on smoking status in two or more consecutive cycles and those who completed the baseline questionnaire only. In diabetes analyses, participants with a missing date of diagnosis were also excluded. After the exclusions, 162,807 participants were included in the diabetes analyses and 170,723 were included in the mortality analyses.
This study was approved by the institutional review board at Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health. The return of a completed questionnaire was considered to indicate informed consent. The last author had full access to all the data in the study and vouches for the completeness and accuracy of the data and data analysis.
ASSESSMENT OF SMOKING STATUS AND WEIGHT CHANGE
In each 2-year survey cycle, we identified participants who had reported that they were smokers in the previous cycle but were past smokers in the current cycle, assuming the beginning of the previous cycle as the onset of quitting. We calculated the duration of smoking cessation from the onset of quitting to the relapse of smoking, occurrence of study outcomes, or the end of follow-up (Table S1 in the Supplementary Appendix). Quitters were mutually exclusively defined as transient quitters (participants who reported being past smokers in the current cycle but being current smokers in previous and next cycles), recent quitters (2 to 6 consecutive years since smoking cessation), and long-term quitters (>6 consecutive years since smoking cessation). We replaced missing information on smoking status with assessments in the previous cycle only (4.4% of participants for diabetes analyses and 4.6% for mortality analyses). In these cohorts, participant-reported smoking status and body weight have been shown to be highly accurate.14,15
Because the biennial weight-change trajectory overlapped with that of persons who had never smoked after 6 years of cessation (Text 2 in the Supplementary Appendix), we focused on weight change within the first 6 years after quitting, which was consistent with a previous study.16 Missing body-weight estimates were replaced with last available values (9.7% of participants for diabetes analyses and 9.6% for mortality analyses). We defined the “no weight gain” group as those who gained no weight or lost weight, and then we applied cutoff points of 0.1 to 5.0 kg, 5.1 to 10.0 kg, and more than 10.0 kg to define other groups.4 In a sensitivity analysis, we grouped participants according to quartiles of weight gain.
ASSESSMENT OF PHYSICAL ACTIVITY AND DIET
Physical activities were assessed with the use of a validated questionnaire regarding time spent on up to 10 recreational activities and were quantified as metabolic equivalent tasks (METs) in hours per week.17 In 1984, 1986, and every 4 years thereafter, a validated food-frequency questionnaire was administered in the NHS to assess diet. Diet has been assessed every 4 years in the NHS II and the HPFS with the use of similar food-frequency questionnaires since 1991 and 1986, respectively. Overall diet quality was assessed according to the Alternative Healthy Eating Index (AHEI) score (range, 0 to 110, with higher scores indicating a healthier diet).18
ASSESSMENT OF OUTCOMES
Once participants reported a physician diagnosis of diabetes in questionnaires that were completed every 2 years, they were mailed a validated supplementary questionnaire19 to confirm the diagnosis (Text 3 in the Supplementary Appendix). We identified deaths through searches of the National Death Index, reports by the next of kin, or postal authorities.20 Deaths from cardiovascular disease were determined by study physicians’ review of medical records and death certificates with diagnostic codes of the International Classification of Diseases, 9th Revision.
STATISTICAL ANALYSIS
Because of the same study design and relatively homogenous populations in the cohorts, data from the three cohorts were pooled to maximize statistical power. Person-time for each participant was counted from the return of the baseline questionnaire to the occurrence of study outcomes, last return of a valid follow-up questionnaire, or the end of follow-up (June 2012 for the NHS, January 2012 for the HPFS, and June 2013 for the NHS II), whichever happened first. For the diabetes analyses, follow-up was further censored on the incidence of cancer or cardiovascular disease, including myocardial infarction, stroke, and coronary-artery bypass surgery, because these conditions are likely to lead to changes in both smoking status and body weight.
We used proportional-hazards regression to examine the association of smoking cessation with the incidence of type 2 diabetes, death from cardiovascular disease, and death from any cause. We used missing indicators for missing values of categorical variables, including physical activity, AHEI score, and intakes of total energy and alcohol (missing in 3.0 to 17.4% of participants). In a sensitivity analysis, we used a multiple-imputation procedure to impute values for missing data on these variables before making categories (Text 4 in the Supplementary Appendix). Participants with missing data on multivitamin use (15.2% of participants) were assumed to be nonusers. To minimize reverse-causation bias by the “ill quitter effect” in the mortality analyses, we stopped updating smoking status on the development of cancer, nonfatal vascular diseases, chronic obstructive pulmonary disease, or type 2 diabetes. We did not find evidence suggesting potential violation of the proportional-hazards assumption for any exposure-outcome associations (Text 5 in the Supplementary Appendix).
A cubic spline regression model was fitted to delineate the association with duration of smoking cessation. Interactions with weight change were tested by a likelihood-ratio test comparing models with and without product terms between duration-of-smoking-cessation spline variables and weight change. The estimated coefficients from the cubic spline regression were used to project the associations in three-dimensional figures (R rgl package).21 In secondary analyses among quitters, we modeled the association of diet- quality and physical-activity changes after quitting in relation to weight changes using a multivariable-adjusted, mixed-effects model (Text 6 in the Supplementary Appendix). In addition, by including persons who reported quitting since 1978 in the NHS, we modeled the long-term trajectory of the risk of type 2 diabetes among quitters who maintained cessation for up to 30 years. Lastly, to assess potential mediation effects of weight change, data were divided into 6-year intervals, and hazard ratios were compared in models with and without adjustment for weight change (Text 7 in the Supplementary Appendix).22
AII P values presented were two-sided, with statistical significance determined by a false discovery rate of less than 0.05 (Text 8 in the Supplementary Appendix).23 Data were analyzed with the use of SAS software, version 9.4 (SAS Institute), and R software, version 3.3.2 (R Foundation).
RESULTS
CHARACTERISTICS OF THE STUDY PARTICIPANTS
The proportion of past smokers steadily increased in all cohorts during follow-up (Fig. S1 in the Supplementary Appendix). Table 1 presents participants’ characteristics according to personyears by smoking status for diabetes analyses. Participants’ characteristics for mortality analyses are presented in Table S2 in the Supplementary Appendix.
Table 1.
Age-Standardized Characteristics of Participants in the Nurses’ Health Study, the Nurses’ Health Study II, and the Health Professionals Follow-up Study.*
| Characteristic | Current Smokers |
Recent Quitters According to Weight Gain within 6 Years after Quitting |
Long-Term Quitters |
Transient Quitters |
Never Smoked |
|||
|---|---|---|---|---|---|---|---|---|
| ≤0 kg | 0.1–5.0 kg | 5.1–10.0 kg | >10.0 kg | |||||
| Person-yr† | 395,872 | 37,444 | 52,147 | 29,767 | 19,424 | 185,838 | 12,853 | 2,451,805 |
| Age (yr) | 53.3±10.4 | 56.1±11.3 | 54.5±11.0 | 54.2±10.6 | 52.2±10.5 | 62.3±10.5 | 51.5±10.9 | 52.5±11.9 |
| Baseline body-mass index‡ | 24.1±4.3 | 24.6±4.7 | 23.4±3.8 | 24.4±4.2 | 26.4±5.5 | 24.3±4.6 | 24.2±4.4 | 24.5±4.6 |
| Race (%)§ | ||||||||
| White | 97.6 | 97.0 | 97.3 | 97.5 | 97.6 | 97.4 | 97.4 | 95.8 |
| Black | 0.8 | 1.1 | 0.9 | 0.8 | 0.9 | 0.9 | 0.9 | 1.4 |
| Asian | 0.8 | 1.0 | 0.9 | 0.8 | 0.7 | 0.9 | 0.8 | 1.5 |
| Other | 0.8 | 0.9 | 0.9 | 0.9 | 0.8 | 0.8 | 0.9 | 1.3 |
| Participant-reported hypertension (%) | 20.5 | 24.8 | 21.2 | 24.4 | 30.4 | 28.3 | 21.6 | 23.8 |
| Participant-reported hypercholesterolemia (%) | 28.2 | 31.1 | 30.4 | 34.9 | 37.6 | 39.9 | 30.4 | 32.3 |
| Family history of myocardial infarction (%) | 30.1 | 29.5 | 29.2 | 30.6 | 31.8 | 30.6 | 31.5 | 31.7 |
| Family history of diabetes (%) | 28.2 | 27.1 | 26.7 | 30.0 | 32.1 | 28.2 | 28.2 | 28.9 |
| Multivitamin use (%) | 37.8 | 42.1 | 43.0 | 41.6 | 41.1 | 50.0 | 42.9 | 46.7 |
| Physical activity (MET-hr/wk) | ||||||||
| Median | 8.4 | 12.7 | 12.8 | 10.2 | 8.1 | 13.6 | 10.6 | 13.5 |
| Interquartile range | 2.7–21.5 | 4.0–28.8 | 4.4–28.3 | 3.4–23.5 | 2.7–19.6 | 4.3–29.6 | 3.5–25.2 | 4.8–29.3 |
| Alternative Healthy Eating Index scored¶ | 47.6±9.7 | 50.9±9.9 | 50.5±9.9 | 49.3±9.6 | 48.8±9.4 | 52.0±9.6 | 49.3±9.7 | 50.8±10.2 |
| Alcohol consumption (g/day) | ||||||||
| Median | 3.0 | 3.5 | 3.9 | 2.9 | 2.0 | 3.6 | 3.5 | 1.1 |
| Interquartile range | 0.6–11.0 | 0.8–10.7 | 0.9–11.1 | 0.6–9.4 | 0.4–6.8 | 0.9–10.1 | 0.9–10.2 | 0–4.3 |
| Total energy intake (kcal/day) | 1756±564 | 1747±547 | 1761±551 | 1767±548 | 1783±571 | 1780±535 | 1764±561 | 1814±561 |
Plus-minus values are means ±SD. All values are standardized to the age distribution of the study population. Recent quitters were defined as participants who had stopped smoking for 2 to 6 consecutive years. Long-term quitters were defined as participants who had stopped smoking for more than 6 consecutive years. Transient quitters were defined as participants who reported being past smokers in the current 2-year survey cycle but being current smokers in the previous and next cycles. MET denotes metabolic equivalent tasks.
Person-years are based on the analyses for type 2 diabetes.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Race was reported by the participants. Percentages may not total 100 because of rounding.
Scores on the Alternative Healthy Eating Index range from 0 to 110, with higher scores indicating a healthier diet.
SMOKING CESSATION, WEIGHT CHANGE, AND INCIDENCE OF TYPE 2 DIABETES
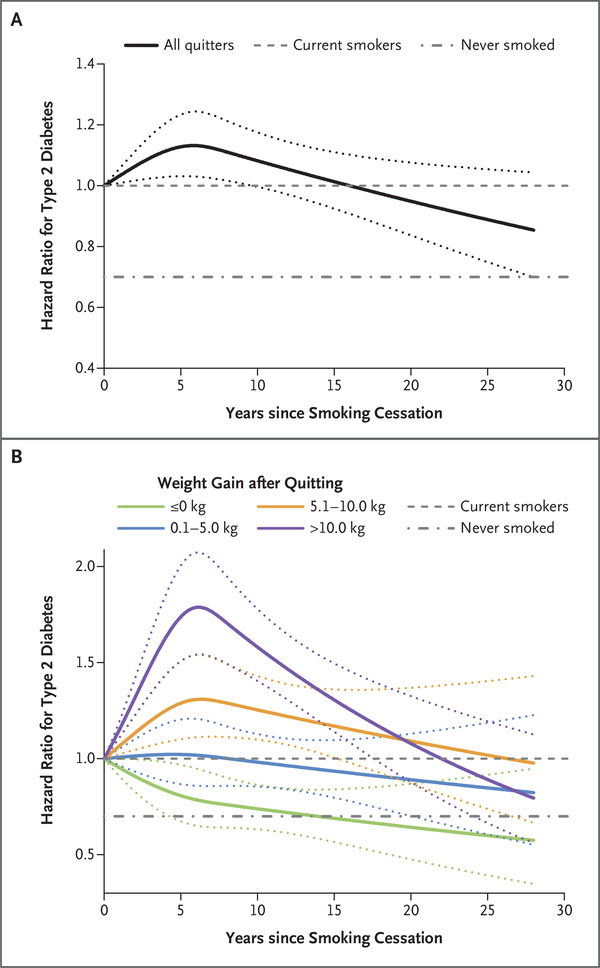
During a mean of 19.6 years of follow-up, 12,384 cases of type 2 diabetes were identified and confirmed. Overall, recent quitters had a higher risk of type 2 diabetes than current smokers (Table 2). In cubic spline analyses, the risk of type 2 diabetes peaked at 5 to 7 years since quitting and gradually decreased thereafter (Fig. 1A). When the analysis was restricted to the NHS participants with the longest follow-up duration since 1978, the risk of type 2 diabetes dropped to that among participants who had never smoked after 30 years of cessation (Fig. S2 in the Supplementary Appendix).
Table 2.
Pooled Hazard Ratios for Association between Smoking Cessation and the Incidence of Type 2 Diabetes, Death from Cardiovascular Disease, and Death from Any Cause.*
| Variable | Cases/Person-yr | Hazard Ratio (95% CI) | ||
|---|---|---|---|---|
| Adjusted for Age |
Adjusted for Baseline BMI |
Adjusted for Multiple Variables |
||
| Type 2 diabetes | ||||
| Current smokers | 1,547/395,872 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Recent quitters† | 836/148,082 | 1.35 (1.24–1.47)‡ | 1.25 (1.14–1.36)‡ | 1.22 (1.12–1.32)‡ |
| No weight gain | 204/37,444 | 1.27 (1.10–1.48) ‡ | 1.09 (0.94–1.27) | 1.08 (0.93–1.26) |
| Weight gain of 0.1–5.0 kg | 206/52,147 | 0.96 (0.83–1.11) | 1.14 (0.99–1.32) | 1.15 (0.99–1.33) |
| Weight gain of 5.1–10.0 kg | 188/29,767 | 1.52 (1.30–1.77)‡ | 1.44 (1.23–1.68)‡ | 1.36 (1.16–1.58)‡ |
| Weight gain of >10.0 kg | 196/19,424 | 2.58 (2.22–2.99)‡ | 1.66 (1.43–1.94)‡ | 1.59 (1.36–1.85)‡ |
| Long-term quitters | 1,168/185,838 | 1.15 (1.07–1.25)‡ | 1.03 (0.96–1.12) | 1.02 (0.94–1.10) |
| Transient quitters | 54/12,853 | 1.12 (0.85–1.47) | 1.09 (0.83–1.44) | 1.09 (0.83–1.44) |
| Never smoked | 8,779/2,451,805 | 0.91 (0.86–0.96)‡ | 0.77 (0.72–0.81)‡ | 0.72 (0.68–0.76)‡ |
| Death from cardiovascular disease§ | ||||
| Current smokers | 1,488/524,182 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Recent quitters† | 167/154,259 | 0.44 (0.37–0.51)‡ | 0.44 (0.37–0.51)‡ | 0.48 (0.41–0.56)‡ |
| No weight gain | 68/39,637 | 0.63 (0.49–0.80)‡ | 0.63 (0.49–0.80)‡ | 0.69 (0.54–0.88)‡ |
| Weight gain of 0.1–5.0 kg | 46/53,969 | 0.39 (0.29–0.52)‡ | 0.41 (0.31–0.55)‡ | 0.47 (0.35–0.63)‡ |
| Weight gain of 5.1–10.0 kg | 14/30,926 | 0.23 (0.14–0.39)‡ | 0.23 (0.14–0.40)‡ | 0.25 (0.15–0.42)‡ |
| Weight gain of >10.0 kg | 11/20,670 | 0.36 (0.20–0.65)‡ | 0.32 (0.18–0.59)‡ | 0.33 (0.18–0.60)‡ |
| Long-term quitters | 656/256,194 | 0.46 (0.42–0.51)‡ | 0.46 (0.42–0.50)‡ | 0.50 (0.46–0.55)‡ |
| Never smoked | 3,181/3,003,966 | 0.34 (0.32–0.36)‡ | 0.31 (0.29–0.33)‡ | 0.34 (0.32–0.37)‡ |
| Death from any cause§ | ||||
| Current smokers | 6,537/519,569 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Recent quitters† | 880/153,642 | 0.53 (0.49–0.57)‡ | 0.53 (0.49–0.57)‡ | 0.58 (0.54–0.62)‡ |
| No weight gain | 360/39,386 | 0.75 (0.67–0.83)‡ | 0.74 (0.67–0.83)‡ | 0.81 (0.73–0.90)‡ |
| Weight gain of 0.1–5.0 kg | 236/53,815 | 0.44 (0.39–0.51)‡ | 0.46 (0.40–0.52)‡ | 0.52 (0.46–0.59)‡ |
| Weight gain of 5.1–10.0 kg | 115/30,826 | 0.42 (0.35–0.50)‡ | 0.42 (0.35–0.51)‡ | 0.46 (0.38–0.55)‡ |
| Weight gain of >10.0 kg | 76/20,616 | 0.51 (0.40–0.64)‡ | 0.48 (0.38–0.61)‡ | 0.50 (0.40–0.63)‡ |
| Long-term quitters | 3,252/253,822 | 0.50 (0.48–0.53)‡ | 0.50 (0.48–0.52)‡ | 0.57 (0.54–0.59)‡ |
| Never smoked | 13,198/2,994,849 | 0.32 (0.31–0.33)‡ | 0.31 (0.30–0.32)‡ | 0.35 (0.34–0.37)‡ |
Hazard ratios and 95% confidence intervals (CIs) were estimated with the use of a Cox proportional-hazards model. Multivariate analyses were adjusted for age (in months, continuous), cohort (Nurses’ Health Study, Nurses’ Health Study II, or Health Professionals Follow-up Study), sex (male or female), race (white, black, Asian, or other), physical activity (in quintiles), baseline body-mass index (BMI, in continuous and quadratic terms), alcohol intake (0, <5.0, 5.0 to 9.9, 10.0 to 14.9, 15.0 to 29.9, or >30.0 g per day), hypertension (yes or no), hypercholesterolemia (yes or no), family history of diabetes (yes or no), multivitamin use (yes or no), Alternative Healthy Eating Index score (in quintiles), and total energy intake (in quintiles). Hazard ratios for death from cardiovascular disease and death from any cause were also adjusted for family history of myocardial infarction (yes or no).
In analyses involving recent quitters with stratification according to weight change since quitting, 0.3% of the cases (42 cases) and 0.3% of the person-time in the analysis of type 2 diabetes, 0.1% of the cases (28 cases) and 0.2% of the person-time in the analysis of death from cardiovascular disease, and 0.4% of the cases (93 cases) and 0.3% of the person-time in the analysis of death from any cause with missing data regarding weight change were removed from the stratified analyses, and therefore the total number of cases and total person-time across weight-change categories were smaller than those in the analyses involving total recent quitters, without consideration of subsequent weight change.
The false discovery rate was less than 0.05.
There were no cases among transient quitters for the analyses of death from cardiovascular disease and death from any cause.
Figure 1. Association between Duration of Smoking Cessation and Risk of Type 2 Diabetes.
Panel A shows the risk of type 2 diabetes according to years since smoking cessation, as compared with the risk among current smokers. P<0.001 for nonlinearity. “All quitters” indicates all persons who had reported quitting smoking. Panel B shows the risk of type 2 diabetes according to years since smoking cessation and weight change within 6 years after quitting, as compared with the risk among current smokers. P values for nonlinearity were 0.33 for no weight gain, 0.59 for weight gain of 0.1 to 5.0 kg, 0.01 for weight gain of 5.1 to 10.0 kg, and less than 0.001 for weight gain of more than 10.0 kg. Multivariate analyses were adjusted for age (in months, continuous), cohort (Nurses’ Health Study [NHS], Nurses’ Health Study II [NHS II], or Health Professionals Follow-up Study [HPFS]), sex (male or female), race (white, black, Asian, or other), physical activity (in quintiles), baseline body-mass index (BMI, in continuous and quadratic terms), alcohol intake (0, <5.0, 5.0 to 9.9, 10.0 to 14.9, 15.0 to 29.9, or >30.0 g per day), history of hypertension (yes or no), history of hypercholesterolemia (yes or no), family history of diabetes (yes or no), multivitamin use (yes or no), Alternative Healthy Eating Index score (in quintiles), and total energy intake (in quintiles). Dotted lines represent 95% confidence intervals.
Weight gain modified the association between smoking cessation and risk of type 2 diabetes (P<0.001 for interaction). The hazard ratios as compared with current smokers were 1.08 (95% confidence interval [CI], 0.93 to 1.26) among recent quitters without weight gain, 1.15 (95% CI, 0.99 to 1.33) among those with weight gain of 0.1 to 5.0 kg, 1.36 (95% CI, 1.16 to 1.58) among those with weight gain of 5.1 to 10.0 kg, and 1.59 (95% CI, 1.36 to 1.85) among those with weight gain of more than 10.0 kg. The risk of type 2 diabetes among recent quitters without weight gain more quickly approached the risk among persons who had never smoked than did the risk among quitters who gained weight (Fig. 1B). In the three-dimensional illustration of the trajectory of the risk of type 2 diabetes according to years since smoking cessation and weight change (Fig. S3A in the Supplementary Appendix), the peak of risk was observed among quitters with the most weight gain at 5 to 7 years of cessation. In the mediation analysis, weight change within 6 years after cessation explained 68.4% (95% CI, 8.3 to 98.1) of the elevated risk of type 2 diabetes among those who reported quitting smoking.
SMOKING CESSATION, WEIGHT CHANGE, AND MORTALITY
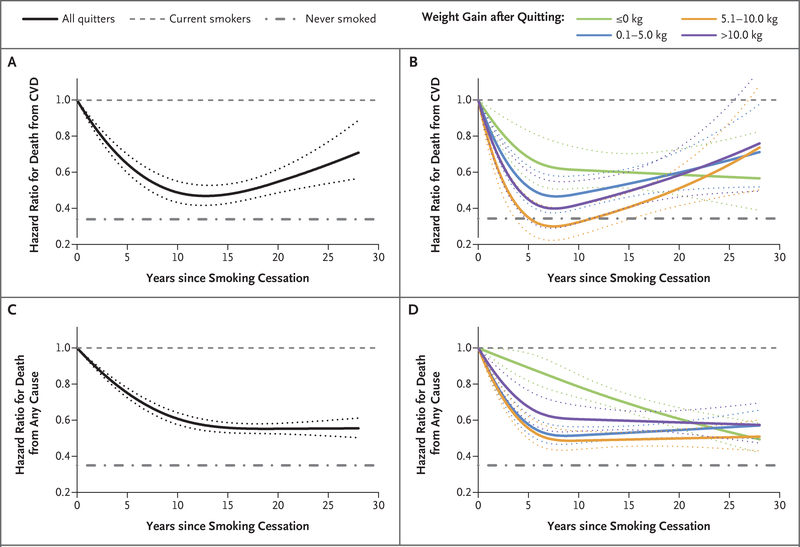
For the mortality analyses, we documented a total of 23,867 deaths, of which 5492 were due to cardiovascular disease. As compared with current smokers, hazard ratios for death from cardiovascular disease were 0.69 (95% CI, 0.54 to 0.88) among recent quitters without weight gain, 0.47 (95% CI, 0.35 to 0.63) among those with weight gain of 0.1 to 5.0 kg, 0.25 (95% CI, 0.15 to 0.42) among those with weight gain of 5.1 to 10.0 kg, 0.33 (95% CI, 0.18 to 0.60) among those with weight gain of more than 10.0 kg, and 0.50 (95% CI, 0.46 to 0.55) among longerterm quitters (Table 2). The corresponding hazard ratios for death from any cause were 0.81 (95% CI, 0.73 to 0.90), 0.52 (95% CI, 0.46 to 0.59), 0.46 (95% CI, 0.38 to 0.55), 0.50 (95% CI, 0.40 to 0.63), and 0.57 (95% CI, 0.54 to 0.59). Cubic spline analyses showed that cardiovascular mortality decreased substantially after quitting, reached a nadir at 10 to 15 years, and then rose slowly but never reached the level of current smokers (Fig. 2A). In stratified analyses, such a pattern of associations was observed in all weight- gain groups, but among those without weight gain, cardiovascular mortality decreased after quitting for 5 to 10 years and plateaued thereafter without the upward trend (Fig. 2B). In the three-dimensional illustration, we found that in a small group of quitters (0.13% of total personyears) with weight gain of at least 18.0 kg at 6 years since smoking cessation, the cardiovascular mortality gradually approached the level of current smokers during an extended period of follow-up (Fig. S3B in the Supplementary Appendix).
Figure 2. Association between Duration of Smoking Cessation and Risk of Death from Cardiovascular Disease (CVD) and Death from Any Cause.
Panel A shows the risk of death from CVD according to years since smoking cessation, as compared with the risk among current smokers. P<0.001 for nonlinearity. Panel B shows the risk of death from CVD according to years since smoking cessation and weight change within 6 years after quitting, as compared with the risk among current smokers. P values for nonlinearity were 0.01 for no weight gain and less than 0.001 for weight gain of 0.1 to 5.0 kg, 5.1 to 10.0 kg, and more than 10.0 kg. Panel C shows the risk of death from any cause according to years since smoking cessation, as compared with the risk among current smokers. P<0.001 for nonlinearity. Panel D shows the risk of death from any cause according to years since smoking cessation and weight change within 6 years after quitting, as compared with the risk among current smokers. P values for nonlinearity were 0.95 for no weight gain and less than 0.001 for weight gain of 0.1 to 5.0 kg, 5.1 to 10.0 kg, and more than 10.0 kg. Multivariate analyses were adjusted for age (in months, continuous), cohort (NHS, NHS II, or HPFS), sex (male or female), race (white, black, Asian, or other), physical activity (in quintiles), baseline BMI (in continuous and quadratic terms), alcohol intake (0, <5.0, 5.0 to 9.9, 10.0 to 14.9, 15.0 to 29.9, or >30.0 g per day), history of hypertension (yes or no), history of hypercholesterolemia (yes or no), family history of diabetes (yes or no), family history of myocardial infarction (yes or no), multivitamin use (yes or no), Alternative Healthy Eating Index score (in quintiles), and total energy intake (in quintiles). Dotted lines represent 95% confidence intervals.
All-cause mortality showed a monotonic reduction at 5 to 7 years of cessation and plateaued afterward (Fig. 2C). This pattern was observed in all weight-change groups, except that quitters without weight gain had a linear risk reduction after quitting (Fig. 2D). The three-dimensional illustration of all-cause mortality corroborated findings in the stratified analyses (Fig. S3C in the Supplementary Appendix).
SECONDARY AND SENSITIVITY ANALYSES
Among quitters, each increase of 10 MET-hours per week in physical activities was associated with 0.13 kg (95% CI, 0.11 to 0.15) less weight gain, and each 10-point increase in the AHEI score was associated with 0.26 kg (95% CI, 0.21 to 0.32) less weight gain. (For details, see Table S3 in the Supplementary Appendix.)
Results were similar when we did not carry forward valid smoking status to replace missing values (Table S4 in the Supplementary Appendix), when the start of cessation was defined as the beginning of the cycle that participants first reported being past smokers (Table S5 in the Supplementary Appendix), when recent quitters were defined as quitting for 2 to 8 consecutive years rather than 2 to 6 years (Table S6 in the Supplementary Appendix), when the model used quartiles of weight change after cessation (Table S7 in the Supplementary Appendix), or when we used imputed values for missing covariates in the analyses (Table S8 in the Supplementary Appendix).
DISCUSSION
In three cohorts of U.S. men and women, we observed a temporary elevation in the risk of type 2 diabetes after smoking cessation, primarily among quitters who gained weight. However, both cardiovascular and all-cause mortality decreased on smoking cessation, and the reduction was largely sustained during extended duration of smoking cessation, even among those who gained weight.
Our observation of the short-term elevation in the risk of type 2 diabetes after smoking cessation was consistent with previous studies, which showed that former smokers who had quit recently had a significantly higher risk of type 2 diabetes than persons who had never smoked, although those who had sustained smoking cessation over a longer period did not.5,6,24–27 Although the weight gain that is commonly observed after smoking cessation may explain such an elevated risk of diabetes, findings from previous studies did not support such a hypothesis,5,6 probably owing to the lack of long-term, repeated assessments of subsequent changes in smoking status and body weight. Indeed, because quitters are likely to have a relapse,11 their subsequent disease risk may vary according to the duration of cessation and concurrent weight change. In our analyses, we estimated that weight change within 6 years after quitting explained 68.4% of the increased risk of type 2 diabetes.
As compared with a short-term increase in the risk of type 2 diabetes, cardiovascular mortality decreased substantially on cessation in all weightchange groups. It is known that most excessive cardiovascular risk is eliminated within the first few years of cessation.28,29 Two investigations explicitly examined the associations of smoking cessation with cardiovascular disease risk according to weight change, but results were mixed, probably because of small numbers of cases among quitters in these cohorts.7,8 In a Korean cohort of men, a lower risk of cardiovascular disease was observed among those who reported quitting smoking and had either stable or increased body weight but not among those who lost weight.9 In addition to small sample sizes, the discrepant findings in these studies can also potentially be ascribed to the important methodologic issue of the “ill quitter effect” — that is, smokers with preexisting diseases are more likely to quit but have a much higher risk of premature death,30 which was not explicitly addressed in these studies.
Our findings of a substantial reduction in allcause mortality on cessation were consistent with previous studies.1,31 We also found that the reduction in total mortality reached a nadir after 10 to 15 years of quitting and plateaued afterward. Further analyses showed that such an L-shape relationship was largely driven by quitters with weight gain; among quitters without weight gain, the mortality reduction was quite linear over time.
Overall, our data showed that subsequent weight gain, on average, did not attenuate the apparent benefits of smoking cessation on reducing cardiovascular mortality or extending longevity. However, preventing excessive weight gain may maximize the health benefits of smoking cessation through reducing the short-term risk of diabetes and further lowering the long-term risk of death.
Our analyses provided further evidence suggesting that improving diet quality and increasing physical activities may help quitters to achieve their weight-maintenance goals. Several long-term (≥1 year) smoking-cessation trials and observational studies have shown the beneficial effects of increasing physical activities on mitigating weight gain after quitting.32–34 Exercise might also improve short-term smoking abstinence.35 Healthy dietary patterns (e.g., a diet with a high AHEI score, the Mediterranean diet, and the Dietary Approaches to Stop Hypertension diet), which typically consist of foods that increase satiety, such as whole fruits, vegetables, and whole grains,36 may attenuate the increased appetite after smoking cessation. It has been shown that smokers with higher consumption of fruits and vegetables gained less weight after quitting than those with low consumption,37 although further studies, especially clinical trials, are needed to further elucidate the role of diet quality in weight management among quitters. Meanwhile, evidence from the PREDIMED (Prevención con Dieta Mediterránea) study suggested that improved diet quality could convey similar health benefits on reducing cardiovascular disease risk among persons who have ever smoked and those who never smoked.38 Taken together, although lifestyle modification after smoking cessation could be challenging to some quitters, existing evidence suggests that lifestyle changes, in particular increasing physical activities,32 are an effective strategy for weight management among quitters and do not appear to increase the possibility of relapse among quitters.
Because the exact date of smoking cessation was not ascertained, the assessments of duration of smoking cessation and related weight-change estimates were subject to misclassifications. However, given the prospective study design, such measurement errors were unrelated to the ascertainment of outcomes and thus were more likely to bias the true associations toward the null. In addition, if quitters who had a relapse reported continued cessation or if quitters underreported their weight gain owing to social-desirability bias, true associations would have been likely to be attenuated toward the null because these persons still had an elevated disease risk. Carrying forward the last available values to replace missing data on smoking status and body weight may have introduced misclassifications. However, the proportions of missing values for smoking status and weight were low, and we observed similar associations in analyses restricted to participants without missing values for these variables, which suggests that any effect of misclassification was likely to be small. Lastly, because our participants were mostly white health professionals without major chronic diseases at baseline, the generalizability of our findings to other groups may be limited.
In conclusion, our findings suggest that a temporary increase in the risk of type 2 diabetes due to weight gain after smoking cessation did not attenuate the benefits of smoking cessation on reducing total and cardiovascular mortality.
Supplementary Material
Acknowledgments
Supported by research grants (CA186107, CA176726, CA167552, HL034594, HL035464, and DK112940) from the National Institutes of Health (NIH). Dr. Sun was supported by NIH grants ES021372 and ES022981.
We thank the participants of the Health Professionals Followup Study, the Nurses’ Health Study, and the Nurses’ Health Study II for their contributions and long-term commitment to scientific research.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 2013;368:341–50. [DOI] [PubMed] [Google Scholar]
- 2.Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 2012;345:e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris KK, Zopey M, Friedman TC. Metabolic effects of smoking cessation. Nat Rev Endocrinol 2016;12:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 2017;318:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med 2010;152:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hur NW, Kim HC, Nam CM, Jee SH, Lee HC, Suh I. Smoking cessation and risk of type 2 diabetes mellitus: Korea Medical Insurance Corporation Study. Eur J Cardiovasc Prev Rehabil 2007;14:244–9. [DOI] [PubMed] [Google Scholar]
- 7.Luo J, Rossouw J, Margolis KL. Smoking cessation, weight change, and coronary heart disease among postmenopausal women with and without diabetes. JAMA 2013;310:94–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clair C, Rigotti NA, Porneala B, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA 2013;309:1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K, Park SM, Lee K. Weight gain after smoking cessation does not modify its protective effect on myocardial infarction and stroke: evidence from a cohort study of men. Eur Heart J 2018;39:1523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klesges RC, Winders SE, Meyers AW, et al. How much weight gain occurs following smoking cessation? A comparison of weight gain using both continuous and point prevalence abstinence. J Consult Clin Psychol 1997;65:286–91. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins J, Hollingworth W, Campbell R. Long-term smoking relapse: a study using the British Household Panel Survey. Nicotine Tob Res 2010;12:1228–35. [DOI] [PubMed] [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–8. [DOI] [PubMed] [Google Scholar]
- 13.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6: 49–62. [DOI] [PubMed] [Google Scholar]
- 14.Al-Delaimy WK, Stampfer MJ, Manson JE, Willett WC. Toenail nicotine levels as predictors of coronary heart disease among women. Am J Epidemiol 2008;167:1342–8. [DOI] [PubMed] [Google Scholar]
- 15.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19:570–2. [PubMed] [Google Scholar]
- 16.O’Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol 1998;148:821–30. [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, Townsend MK, Okereke OI, Franco OH, Hu FB, Grodstein F. Physical activity at midlife in relation to successful survival in women at age 70 years or older. Arch Intern Med 2010;170:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manson JE, Colditz GA, Stampfer MJ, et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med 1991;151:1141–7. [PubMed] [Google Scholar]
- 20.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 1994;140:1016–9. [DOI] [PubMed] [Google Scholar]
- 21.Adler D, Murdoch D. rgl: 3D visualization using OpenGL. R package, version 0.99.16. 2018. (https://cran.r-project.org/package=rgl).
- 22.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 1997;16:1515–27. [DOI] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc [B] 1995;57:289–300. [Google Scholar]
- 24.Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015;3:958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham NM, Nguyen CT, Binns CW, Lee AH. Non-linear association between smoking cessation and incident type 2 diabetes. Lancet Diabetes Endocrinol 2015;3:932. [DOI] [PubMed] [Google Scholar]
- 26.Oba S, Noda M, Waki K, et al. Smoking cessation increases short-term risk of type 2 diabetes irrespective of weight gain: the Japan Public Health Center-Based Prospective Study. PLoS One 2012; 7(2):e17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, Rossouw J, Tong E, et al. Smoking and diabetes: does the increased risk ever go away? Am J Epidemiol 2013;178: 937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Office on Smoking and Health. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2010. [PubMed] [Google Scholar]
- 29.Office on Smoking and Health. The health consequences of smoking — 50 years of progress: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2014:1–36. [PubMed] [Google Scholar]
- 30.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA 2008;299:2037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirie K, Peto R, Reeves GK, Green J, Beral V. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013;381:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev 2012;1:CD006219. [DOI] [PubMed] [Google Scholar]
- 33.Gennuso KP, Thraen-Borowski KM, Schlam TR, et al. Smokers’ physical activity and weight gain one year after a successful versus unsuccessful quit attempt. Prev Med 2014;67:189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawachi I, Troisi RJ, Rotnitzky AG, Coakley EH, Colditz GA. Can physical activity minimize weight gain in women after smoking cessation? Am J Public Health 1996;86:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prochaska JJ, Hall SM, Humfleet G, et al. Physical activity as a strategy for maintaining tobacco abstinence: a randomized trial. Prev Med 2008;47:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fung TT, Pan A, Hou T, et al. Longterm change in diet quality is associated with body weight change in men and women. J Nutr 2015;145:1850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vergnaud AC, Norat T, Romaguera D, et al. Fruit and vegetable consumption and prospective weight change in participants of the European Prospective Investigation into Cancer and Nutrition-Physical Activity, Nutrition, Alcohol, Cessation of Smoking, Eating Out of Home, and Obesity study. Am J Clin Nutr 2012;95:184–93. [DOI] [PubMed] [Google Scholar]
- 38.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with olive oil or nuts. N Engl J Med 2018;378(25):e34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.