Abstract
Background
Advanced stage non-small cell lung cancer (NSCLC) is a heterogenous disease, yet, with the exception of targeted therapies, most guidelines recommended uniform treatment irrespective of tumor burden or sites of metastases and this may explain, in part, the wide range of responses to same lines of therapy.
Aim of work
In this work we tried to explore the effect of metastatic sites in on overall survival (OS), in an unselected group of Non-small cell lung cancer patients who received different treatments line.
Methods
A retrospective analysis was performed on patients with stage IV NSCLC who received systemic treatment at UAB Cancer Center (NCI designated comprehensive cancer center) between 2002 to 2012. The details of sites of metastases, systemic therapy and overall survival were recorded for each patient.
Result
In 409 patients who received systemic treatment, there was statistically significant lower OS in those presenting with liver metastases (p<0.001), adrenal metastases (p=0.011) and metastases to abdominal lymph nodes (p=0.014). There was no statistically significance difference in OS in patient presenting with pleural metastases or effusion (p=0.908), metastases to heart or pericardium (p=0.654), metastases to bone (p=0.281), brain (p=0.717) or skin and subcutaneous tissue (p=0.642).
Conclusion
Intra-abdominal metastases confer a particularly poor prognosis in stage IV NSCLC treated with systemic therapy and may identify patients in whom aggressive treatment beyond first line therapy is not appropriate.
Keywords: Lung cancer, metastases, prognosis
Introduction
Lung cancer is the most common cancer type with 1.8 million new cases diagnosed annually, accounting for 12.9% of newly diagnosed cancer cases. It is also the first cause of worldwide cancer death with estimated 1.59 million death annually that accounted for 19.4% of total cancer death (Jemal et al., 2011; Ferlay, 2013).
In the United States, lung cancer is the second most common cancer in both males and females after prostate cancer and breast cancer with 115,610 and 105,590 new cases, respectively each year. It is also the first cause of cancer death in both males and females with estimated 158040 deaths each year (Siegel et al., 2016).
The WHO classified lung cancer into two main categories Non-small cell lung cancer (NSCLC), which accounts for 85% of totally diagnosed lung cancers and small cell lung cancer which accounts for 15% of totally diagnosed lung cancers. NSCLC is further subdivided into adenocarcinoma, squamous cell carcinoma and large cell carcinoma (Beasley et al., 2005; Travis et al., 2015).
Unfortunately more than half (57%) of patients’ diagnosed NSCLC presented with metastatic disease and nearly one half of patients with early stage lung cancer will progress to metastatic disease within five years with overall survival rate for all stages of 17% and 5-year overall survival for metastatic disease of 4% and 90% of lung cancer death is due to development of metastases (Goldstraw et al., 2016). The mechanism of metastases include: detachment of tumor cells from the extracellular matrix with several proteolytic enzymes then invasion of neighboring tissues and basement membrane then intravasation into the blood stream or lymphatic vessels, by attachment to the endothelial cells with adhesion molecules, infiltration of the vessels, survival and transport through the blood stream and finally arrest and extravasation at a distant site and formation of a metastatic lesion (Mehlen and Puisieux, 2006; Perlikos et al., 2013; Popper, 2016).
The most frequent metastatic sites for NSCLC are the nervous system, bone, liver, respiratory system, and adrenal glands (Nakazawa et al., 2012; Riihimaki et al., 2014; Tamura et al., 2015).
The availability of multiple lines of chemotherapy, targeted therapy and immunotherapy for metastatic NSCLC, reveals a wide range of responses, demonstrating the heterogenous nature of advanced NSCLC
In this work we tried to explore the effect of different metastatic sites in unselected group of Non-small cell lung cancer patients who received different lines of treatment, on overall survival at one of NCI designated comprehensive cancer center and one of NCCN member institute.
Materials and Methods
A retrospective analysis was performed on patients with stage IV NSCLC who received systemic treatment at University of Alabama at Birmingham (UAB) from 2002 to 2012. The details of site of metastases, systemic therapy and overall survival were recorded for each patient.
We excluded patients originally treated with adjuvant treatment who did not receive systemic treatment upon progression and patients who did not receive any active treatment.
Statistical analysis was performed using SPSS version 23 and calculated using log-rank testing.
Results
Four hundred and nine patients received systemic treatment were identified. There were 232 patients <65 years old (56.7% of total study population) and 177 patients > 65 years old (43.3% of total study population). As regard to gender, 230 patients were males (56.2%) and 179 were females (43.8%). Most of patients identified had adenocarcinoma (50.9%), followed by non-small cell lung cancer not otherwise specified (28.6%), followed by squamous cell carcinoma (17.1%) (Table 1).
Table 1.
Demographic and Pathological Characters of the Patients
| Number | Percent | |
|---|---|---|
| Age | ||
| >65 | 177 | 43.3 |
| <65 | 232 | 56.7 |
| Gender | ||
| Female | 179 | 43.8 |
| Male | 230 | 56.2 |
| Histology | ||
| Adenocarcinoma | 208 | 50.9 |
| NSCLC NOS | 117 | 28.6 |
| SCC | 70 | 17.1 |
| Large cell carcinoma | 7 | 1.7 |
| Adenosqamous carcinoma | 7 | 1.7 |
Pleural effusion or deposits were represented in 22.5% of patients, metastases to bone in 28.6%, to brain in 22.7%, to liver in 14.4%, to adrenals in 13%, to abdominal lymph nodes in 5.4%, to subcutaneous tissue or muscle in 5.6%, to other abdominal viscera in 1.2% and cardiac involvement in 2% of patients. Single organ metastases were represented in 75.3% while multiple organ metastases were represented in 24.5% of patients. (Figure 1 and 2).
Figure 1.
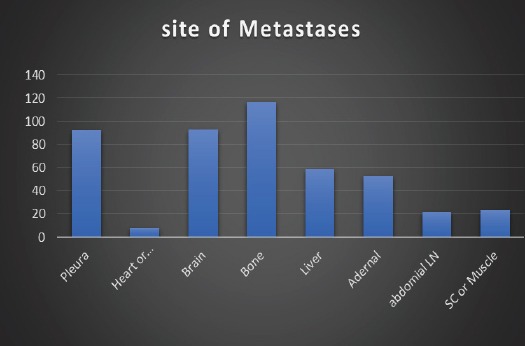
Site of Metastases in Study Population
Figure 2.
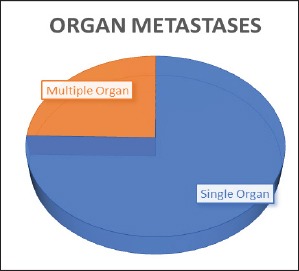
Organ Metasets in Study Population
Regarding site of metastases, there was a statistically significant lower OS in patients presenting with liver metastases (p<0.001), adrenal metastases (p=0.011) and metastases to abdominal lymph nodes (p=0.014). However, there was no statistically significance difference in OS in patients presenting with pleural metastases or effusion (p=0.908), metastases to heart or pericardium (p=0.654), metastases to bone (p=0.281), brain (p=0.717) or skin and subcutaneous tissue (p=0.642) (Table 2).
Table 2.
Log Rank Test of Site of Metastases
| Medians for Survival Time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No metastases | Yes | ||||||||||
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | ||||||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | Chi-Square | df | Sig. | |||||
| Pleura | 13.839 | 0.714 | 12.439 | 15.240 | 12.990 | 2.678 | 7.741 | 18.240 | 0.013 | 1 | 0.908 |
| Heart or Pericardium | 13.793 | 0.718 | 12.387 | 15.200 | 14.849 | 3.942 | 7.122 | 22.576 | 0.201 | 1 | 0.654 |
| Brain | 13.793 | 0.725 | 12.372 | 15.215 | 14.092 | 1.670 | 10.819 | 17.364 | 0.132 | 1 | 0.716 |
| Bone | 14.161 | 1.098 | 12.009 | 16.312 | 12.416 | 1.212 | 10.041 | 14.792 | 1.163 | 1 | 0.281 |
| liver | 15.285 | 1.184 | 12.964 | 17.606 | 10.259 | .881 | 8.532 | 11.986 | 16.944 | 1 | 0.000 |
| Adrenals | 14.849 | 0.989 | 12.911 | 16.787 | 10.259 | 1.003 | 8.294 | 12.224 | 6.468 | 1 | 0.011 |
| abdominal LN | 14.000 | 0.681 | 12.665 | 15.335 | 9.203 | 2.643 | 4.022 | 14.384 | 6.093 | 1 | 0.014 |
| Skin or SC | 13.839 | 0.702 | 12.463 | 15.215 | 12.416 | 2.529 | 7.459 | 17.374 | 0.216 | 1 | 0.642 |
For abdominal metastases the presence of one or two or three abdominal metastases were associated with inferior survival compare to absent of metastatic site in the abdomen (Table 3) (Figure 3).
Table 3.
Log Rank Test for Abdominal Metastatic Sites
| Number of Abdominal Metastases | Median | |||
|---|---|---|---|---|
| Estimate | Std. Error | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | |||
| Three Metastases | 5.669 | |||
| one or two Metastases | 10.351 | 0.489 | 9.392 | 11.310 |
| No Metastases | 16.525 | 1.280 | 14.015 | 19.034 |
| Overall | 13.793 | 0.675 | 12.470 | 15.117 |
Figure 3.
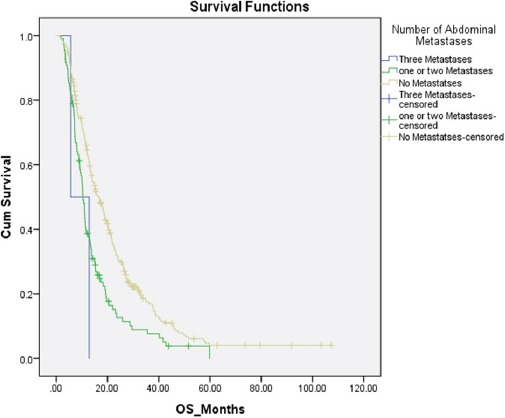
Kaplan Marie Survival Curve for Abdominal Metastases
Discussion
Although advanced stage NSCLC is a heterogenous disease, most treatment guidelines recommend standardized treatments irrespective of tumor burden or site of metastases (Novello et al., 2016; Hanna et al., 2017; Members, 2017).
Many authors report inferior survival with specific sites of metastases. In one abstract reported by Naito and his colleagues’ pleural effusion found to be a poor prognostic factor for survival (Naito et al., 1997).
In another Japanese data reported by Sugiura et al., (1997) on 197 patients with advanced stage NSCLC, pleural effusion was selected as a poor prognostic factor in the multivariate analysis.
Hilsenbeck and colleagues sought to determine the prognostic significance of stage, race, gender, age, and treatment in each histologic subtype in lung cancer. The presence of liver metastases, was an important prognostic factor in all subtypes except large cell carcinoma in univariate but not in multivariate analysis (Hilsenbeck et al., 1993).
In a paper by Maeda and his colleague, in 261 patient with advanced NSCLC who had been enrolled in clinical trials conducted by the Okayama Lung Cancer Study Group between 1978 and 1992, the presence of lung, liver and bone metastases all were associated with poor survival (Maeda et al., 2000).
In the present study, a statistically significant lower OS was seen in patients presenting with liver (p<0.001), adrenal (p=0.011) and abdominal lymph node metastases (p=0.014). However, there was, however, no statistically significant difference in OS in patient presenting with pleural metastases or effusion (p=0.908), metastases to heart or pericardium (p=0.654), metastases to bone (p=0.281), brain (p=0.717) or skin and subcutaneous tissue (p=0.642)
Our findings support previously reported findings that certain sites of metastatic disease confer a poor prognosis on patients diagnosed with NSCLC and identifies abdominal lymph nodes metastases as a poor prognostic factor. These sites of metastases may help identify patients for whom aggressive salvage therapy may be inappropriate, particularly if the patient failed to respond to first line therapy. In addition, our data suggests that these sites of disease should be considered in the stratification of patients participating in randomized clinical trials and the failure to do so may distorted the validity of a study.
In conclusion, intra-abdominal metastases confer a poor prognosis in stage IV NSCLC treated with systemic therapy. This is highly relevant in both clinical practice and in the conduct of clinical trials and should be considered in the selection of therapies and design of randomized clinical trials.
References
- 1.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–7. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw PCJ, Chansky K, Giroux DJ, et al. The IASLC Lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Hanna N, Johnson D, Temin S, et al. Systemic therapy for stage IV non–small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35:3484–515. doi: 10.1200/JCO.2017.74.6065. [DOI] [PubMed] [Google Scholar]
- 5.Hilsenbeck SG, Raub WA, Jr, Sridhar KS. Prognostic factors in lung cancer based on multivariate analysis. Am J Clin Oncol. 1993;16:301–9. doi: 10.1097/00000421-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 7.Maeda T, Ueoka H, Tabata M, et al. Prognostic factors in advanced non-small cell lung cancer: Elevated serum levels of neuron specific enolase indicate poor prognosis. Jpn J Clin Oncol. 2000;30:534–41. doi: 10.1093/jjco/hyd139. [DOI] [PubMed] [Google Scholar]
- 8.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–58. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 9.Naito T, Satoh H, Ishikawa H, et al. Pleural effusion as a significant prognostic factor in non-small cell lung cancer. Anticancer Res. 1997;17:4743–6. [PubMed] [Google Scholar]
- 10.Nakazawa K, Kurishima K, Tamura T, et al. Specific organ metastases and survival in small cell lung cancer. Oncol Lett. 2012;4:617–20. doi: 10.3892/ol.2012.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:1–27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 12.Perlikos F, Harrington KJ, Syrigos KN. Key molecular mechanisms in lung cancer invasion and metastasis: A comprehensive review. Crit Rev Oncol Hematol. 2013;87:1–11. doi: 10.1016/j.critrevonc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35:75–91. doi: 10.1007/s10555-016-9618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riihimaki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 16.Sugiura S, Ando Y, Minami H, et al. Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clin Cancer Res. 1997;3:47–50. [PubMed] [Google Scholar]
- 17.Tamura T, Kurishima K, Nakazawa K, et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 2015;3:217–21. doi: 10.3892/mco.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]


