Abstract
The discovery of the antidepressant effects of ketamine has opened a breakthrough opportunity to develop a truly novel class of safe, effective, and rapid-acting antidepressants (RAADs). In addition, the rapid and robust biological and behavioral effects of ketamine offered a unique opportunity to utilize the drug as a tool to thoroughly investigate the neurobiology of stress and depression in animals, and to develop sensitive and reproducible biomarkers in humans. The ketamine literature over the past two decades has considerably enriched our understanding of the mechanisms underlying chronic stress, depression, and RAADs. However, considering the complexity of the pharmacokinetics and in vivo pharmacodynamics of ketamine, several questions remain unanswered and, at times, even answered questions continue to be considered controversial or at least not fully understood. The current perspective paper will summarize our understanding of the neurobiology of depression, and the mechanisms of action of ketamine and other RAADs. The review will focus on the role of glutamate neurotransmission – reviewing the history of the “glutamate inhibition” and “glutamate activation” hypotheses, proposing a synaptic connectivity model of chronic stress pathology, and describing the mechanism of action of ketamine. It will also summarize the clinical efficacy findings of putative RAADs, present relevant human biomarker findings, and discuss current challenges and future directions.
Keywords: depression, chronic stress, ketamine, rapid-acting antidepressants, glutamate neurotransmission, prefrontal cortex, nucleus accumbens
1. Introduction
Serendipity, combined with astute clinical observations, has dominated the path to drug discovery in psychiatry (Klein, 2008). In 1951, the first antipsychotic drug was discovered unexpectedly, as chlorpromazine was being developed for potentiating anesthesia. The first tricyclic antidepressant, imipramine, was synthesized in 1899 and decades later failed as antipsychotic compound (Ban, 2006). Yet, one case report in mid-1950s showing imipramine’s antidepressant effect in a female with severe depression has led to further investigation and eventual discovery of the monoaminergic class of antidepressants. Similarly, the first benzodiazepine was lingering on a laboratory shelf for years until it was accidentally discovered during a “spring-cleaning” in 1957 and subsequently demonstrated strong anxiolytic effects (Ban, 2006).
Another unanticipated observation in the 1950s was the report that the anti-tuberculosis d-cycloserine, an N-methyl-D-aspartate receptor (NMDAR) modulator, may possess antidepressant properties (Crane, 1959). Yet, this fortuitous observation has gained little to no attention for more than four decades, until it was discovered in the late 1990s that a single subanesthetic dose of the NMDAR antagonist ketamine induces rapid and sustained antidepressant effects in severely depressed patients (Berman, et al., 2000). At the time, in the context of accumulating evidence proposing NMDAR modulation as a target for antidepressants, and relating depression to excess glutamate neurotransmission and excitotoxicity, the ketamine findings have generated considerable interest in the field to target glutamate neurotransmission for the development of novel rapid-acting antidepressants (RAADs) (Berman, et al., 2000; McEwen, 1999; Skolnick, et al., 1996; Zarate, et al., 2006). Early attempts have primarily focused on investigating glutamate release inhibitors and NMDAR antagonists, both of which were thought to inhibit glutamate transmission and offset the depression-related excitotoxicity. Unfortunately, the glutamate release inhibition approach has had limited success in human studies over the past 2 decades, with pilot or inconsistent findings of antidepressant properties following sustained treatment and no evidence of RAAD effects (Mathew, Gueorguieva, Brandt, Fava, & Sanacora, 2017; Solmi, et al., 2016). Conversely, the NMDAR antagonism approach has shown promise (Abdallah, Averill, & Krystal, 2015; Bobo, et al., 2016). Yet, it is becoming increasingly apparent that the NMDAR agents with RAAD properties are putatively exerting their effects through glutamate neurotransmission activation, rather than inhibition (Aleksandrova, Wang, & Phillips, 2017; Murrough, Abdallah, & Mathew, 2017).
In this perspective paper, we will (1) review the history of the “glutamate inhibition” and “glutamate activation” hypotheses, (2) propose a synaptic connectivity model of chronic stress pathology, (3) describe the mechanism of action of ketamine, (4) summarize the clinical efficacy findings of putative RAADs, (5) present relevant human biomarker findings, and (6) discuss current challenges and future directions.
2. Glutamate Inhibition or Activation? A Historical Perspective
Early in the 1990s, a number of NMDAR antagonists have demonstrated antidepressant-like effects in rodents (Trullas & Skolnick, 1990). Follow-up studies have later shown that chronic, but not acute, administration of several traditional antidepressants (i.e., slow-acting antidepressants; SAADs) alter NMDAR binding, leading to the hypothesis that downregulation of NMDAR function may be a common pathway across antidepressants (Skolnick, et al., 1996). During the same period, grey matter structural deficits were demonstrated in stress-related disorders in humans (Bremner, et al., 1995; Sheline, Wang, Gado, Csernansky, & Vannier, 1996), and were thought to parallel the dendritic atrophy observed following chronic stress in rodents (McEwen, 1999). Interestingly, inhibiting NMDARs or glutamate release blocked the effects of chronic stress on dendritic atrophy (McEwen, 1999). Together, these early data supported a model in which downregulation of excess glutamate may exert antidepressant effects, and raised the question whether the RAAD effects of ketamine are due to glutamate neurotransmission inhibition by blocking NMDARs.
In contrast to the glutamate inhibition model, it has been previously shown that subanesthetic doses of ketamine transiently activate rather than inhibit glutamate neurotransmission (Moghaddam, Adams, Verma, & Daly, 1997). Moreover, the 1990s also witnessed the rise of the neurotrophic hypothesis of depression (Duman, Heninger, & Nestler, 1997), which associated chronic stress and depression with a deficit in brain derived neurotrophic factor (BDNF) and demonstrated that traditional antidepressants increase brain BDNF expression (Nibuya, Morinobu, & Duman, 1995). Interestingly, acute glutamate neurotransmission activation – rather than inhibition – was initially associated with upregulation of BDNF and other neurotrophics (Gall & Isackson, 1989; Patterson, Grover, Schwartzkroin, & Bothwell, 1992; Zafra, Castren, Thoenen, & Lindholm, 1991). In addition, it was found that glutamate transmission activation, using α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) potentiators, increases brain BDNF and exhibits RAAD properties in rodent models (Lauterborn, Lynch, Vanderklish, Arai, & Gall, 2000; X. Li, et al., 2001). Hence, by early 2000s, convergent evidence strongly supported the role of neuronal plasticity in the pathophysiology of depression and in the mechanisms of antidepressant action (D'Sa & Duman, 2002; Manji, Drevets, & Charney, 2001; McEwen, 2004). In addition, it became evident that targeting glutamate neurotransmission offers a novel approach for discovery of new antidepressants (Javitt, 2004; Krystal, et al., 2002). However, it was not fully clear whether these novel antidepressants should activate and/or inhibit glutamate neurotransmission, with the latter possibility gaining the most attention considering the evidence available at the time.
3. Synaptic Model of Chronic Stress Pathology (CSP)
The synaptic CSP model proposes that trauma and repeated stressors lead to wide spread neuronal remodeling consistent with both reduced and increased synaptic connectivity, depending on the brain region. The chronic stress induced reduction in synaptic connectivity has been mostly studied in the prefrontal cortex (PFC) and the hippocampus. Conversely, the CSP-related increases in synaptic connectivity were most commonly shown in the nucleus accumbens (NAc) and certain nuclei within the amygdala.
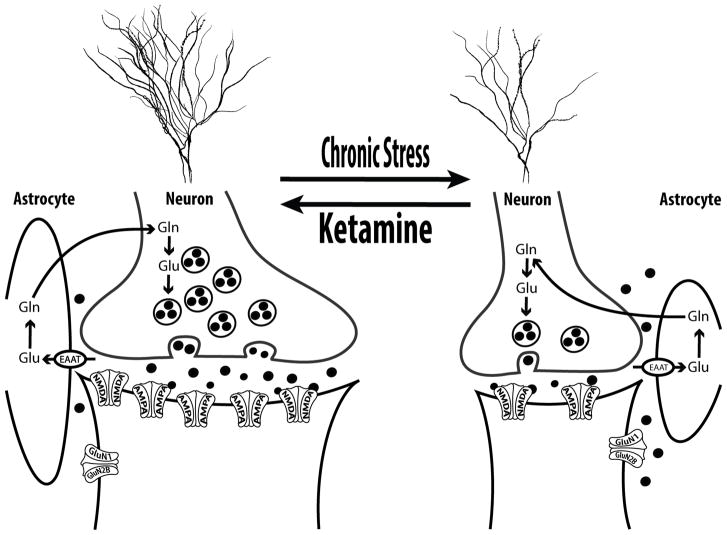
In the PFC (Fig. 1), it was shown that prolonged stress precipitates neuronal synaptic hypoconnectivity, as evident by reduced dendritic length and arborization, and by reduction in synaptic density and strengths (Duman & Aghajanian, 2012). Glial cells, which play a critical role in regulating glutamate neurotransmission and preventing excitotoxicity, were also found to be deficient following chronic stress (Sanacora & Banasr, 2013). While the mechanisms underlying the CSP-related hypoconnectivity are not fully known, accumulating evidence implicates glucocorticoid signaling and dysregulation in glutamate neurotransmission (Popoli, Yan, McEwen, & Sanacora, 2012; Sanacora, Treccani, & Popoli, 2012). In particular, trauma- and stress-related dysregulation of glucocorticoid signaling and glutamate release, combined with glial deficit and reduced glutamate uptake, are believed to paradoxically maintain high levels of extracellular glutamate despite the reduction of resting prefrontal synaptic glutamate neurotransmission following chronic stress.
Figure 1. Chronic Stress Pathology (CSP) in the Prefrontal Cortex (PFC).
The synaptic CSP model proposes that synaptic dysconnectivity may be a common pathological pathway across psychiatric disorders with chronic stress component – as a predisposition, a trigger, or an outcome. In the PFC, chronic stress is believed to induce glial deficit, leading to reduced glutamate reuptake capacity and increased extrasynaptic glutamate levels and excitotoxicity. Subsequently, neuronal atrophy develops, resulting in overall reduction in glutamate neurotransmission, which reflects reduced dendritic length and branching, and reduction of spines and synapses density. In the remaining PFC synapses, the neurotransmission strength is also affected by reduced postsynaptic glutamate N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptors. Ketamine reverses this PFC CSP within 24h of injection. It is thought that ketamine induces a transient (minutes-to-hours) postsynaptic glutamate activation, which leads to upregulation of neurotrophic signaling, increased protein synthesis, and sustained (days-to-weeks) restoration of synaptic connectivity. Abbreviations: EAAT = excitatory aminoacid transporter; Gln = glutamine; GluN1 = NMDA subtype 1; GluN2B = NMDA subtype 2B; Glu = glutamate. The figure was adapted with permission from the Emerge Research Program (emerge.care).
In this model, acute stress precipitates a prefrontal glutamate surge associated with transient (minutes-to-hours) increase in extracellular glutamate (Moghaddam, 1993), but sustained (days-to-weeks) increase in NMDARs, AMPARs, and synaptic strength (Yuen, et al., 2009; Yuen, et al., 2011). In contrast, chronic stress leads to a sustained increase in extracellular glutamate (S. X. Li, et al., 2017), combined with reduced resting prefrontal glutamate transmission (Banasr, et al., 2010), and reduction in NMDARs, AMPARs, and synaptic strength (Yuen, et al., 2012). Here, the distinction between “acute” and “chronic” stress is critical, with the timing (i.e., acute vs. chronic) pertains mostly to the length of the stress response – rather than the duration of the stressor. For example, a single severe traumatic event may induce a chronic sustained threat response. Conversely, repeated escapable and/or predictable mild stressors will result in appropriate adaptation with only acute transient stress responses.
In the NAc, a number of chronic stress paradigms were found to increase synaptic connectivity, as evident by increased dendritic length and arborization, as well as increased synaptic density and strength (Campioni, Xu, & McGehee, 2009; Christoffel, et al., 2011; Christoffel, et al., 2012; Coplan, et al., 2018; Muhammad, Carroll, & Kolb, 2012; Warren, et al., 2014). While the prefrontal hypoconnectivity was associated with glutamate dysregulation and excitotoxicity, the stress-induced NAc synaptic hyperconnectivity is related to monoamine dysregulation. In particular, chronic stress leads to phasic activation of the dopaminergic neurons from the ventral tegmental area (Chaudhury, et al., 2013), which precipitates the co-release of dopamine and BDNF in the NAc (Walsh, et al., 2014). Subsequently, the BDNF upregulation and the induction of its high affinity receptor TrkB lead to the CSP-related NAc neuronal hypertrophy (Wook Koo, et al., 2016).
In preclinical studies, depressive-like behaviors were directly associated with these synaptic alterations in the PFC and NAc (Duman, Aghajanian, Sanacora, & Krystal, 2016; Krishnan & Nestler, 2008; Russo & Nestler, 2013). Reversal of the synaptic impairment induces antidepressant effects. Moreover, both SAADs and RAADs are known to increase PFC, but reduce NAc, synaptic connectivity (Hare, Ghosal, & Duman, 2017; Melo, et al., 2015; Yao, Skiteva, Zhang, Svenningsson, & Chergui, 2017). Notably, the CSP-related microstructural synaptic alterations are evident at the macrostructural level as assessed by magnetic resonance imaging (MRI) (Kassem, et al., 2013). Thus, providing support for the synaptic CSP model, human MRI studies have shown increased NAc, but reduced hippocampal and PFC volumes in major depression (C. G. Abdallah, A. Jackowski, et al., 2017; Kempton, et al., 2011). Here, it is important to highlight that the PFC and hippocampal gray matter deficits were absent in several human depression studies. These gray matter deficits are most evident in patients with amino acid neurotransmitters (i.e., glutamate & GABA) reduction and in individuals who are treatment resistant to SAADs, which are primarily monoaminergic drugs [reviewed in (C. G. Abdallah, A. Jackowski, et al., 2017; Abdallah, Jackowski, et al., 2015)]. Therefore, it was proposed that the synaptic PFC/hippocampus hypoconnectivity and NAc hyperconnectivity reflect two pathways that may independently precipitate clinical depression (C. G. Abdallah, A. Jackowski, et al., 2017). In this Dual Pathology model, patients with underlying amino acid-based pathology (ABP), leading to excitotoxicity and synaptic loss, would show PFC/hippocampus gray matter deficit, present with amino acid impairment, and be treatment resistant to monoaminergic antidepressants. Conversely, patients with monoamine-based pathology (MBP), leading to localized increase in BDNF and synaptic gain, would show NAc gray matter hypertrophy, lack of amino acid impairment, and effectively respond to monoaminergic antidepressants (C. G. Abdallah, A. Jackowski, et al., 2017).
Finally, although the synaptic CSP model has been typically studied and interpreted within the context of major depression, CSP appears to be a common pathway across numerous psychiatric disorders (Adams, et al., 2018; Daskalakis & Binder, 2015; Goddard, 2017; Kwako & Koob, 2017; L. Y. Maeng & Milad, 2017; Patriquin & Mathew, 2017; Prescot, et al., 2018). Hence, the evidence of synaptic loss and dysconnectivity is not limited to major depression, but rather common to several stress-related disorders – e.g., posttraumatic stress disorder (PTSD), generalized anxiety disorder (GAD), obsessive compulsive disorder (OCD), and bipolar depression (Abdallah, et al., 2013; C. G. Abdallah, K. M. Wrocklage, et al., 2017; Akiki, et al., 2017; Anticevic, et al., 2013; Anticevic, et al., 2014; Averill, Abdallah, et al., 2017; Haukvik, et al., 2015; Kwon, et al., 2003; Pietrzak, et al., 2015; Wrocklage, et al., 2017). In addition, antidepressants – known to reverse the CSP in animals – have shown efficacy in alleviating symptoms of PTSD, GAD, OCD, bipolar depression, and other disorders with a considerable chronic stress component. Here, we note that synaptic dysconnectivity could be a predisposing factor, an outcome, or a perpetuator of the psychopathology (Averill, Purohit, et al., 2017; Matosin, Cruceanu, & Binder, 2017; Sheth, McGlade, & Yurgelun-Todd, 2017; Syed & Nemeroff, 2017). Furthermore, while synaptic loss appears to be common across stress-related disorders, the location and pattern of the synaptic dysconnectivity, combined with individual characteristics (e.g., genes & environment), may be the mechanism through which CSP is associated with distinct clinical presentations and psychopathologies (Abdallah, Southwick, & Krystal, 2017; Averill, Purohit, et al., 2017; Krystal, et al., 2017). Together, the presented synaptic model proposes that CSP is common across many psychiatric disorders and that targeting synaptic connectivity may be a convergent pathway across antidepressants.
4. Mechanism of Action of Ketamine and RAADs
The discovery of the robust RAAD effects of ketamine offered a unique opportunity to better understand the neurobiology of depression and to unravel the processes involved in reversing CSP. To date, two ketamine-induced glutamate neurotransmission changes appear to be critical to its RAAD effects: (1) a transient activation of glutamate neurotransmission in the PFC (often referred to as glutamate “surge” or “burst”) and (2) a sustained increase in PFC synaptic connectivity (Fig. 2). It is believed that acute administration of subanesthetic doses of ketamine induces a transient surge in prefrontal glutamate neurotransmission, which in turn induces a number of intracellular processes ultimately leading to sustained increase in prefrontal synaptic connectivity within 24h of treatment (Abdallah, et al., 2016; Duman, et al., 2016).
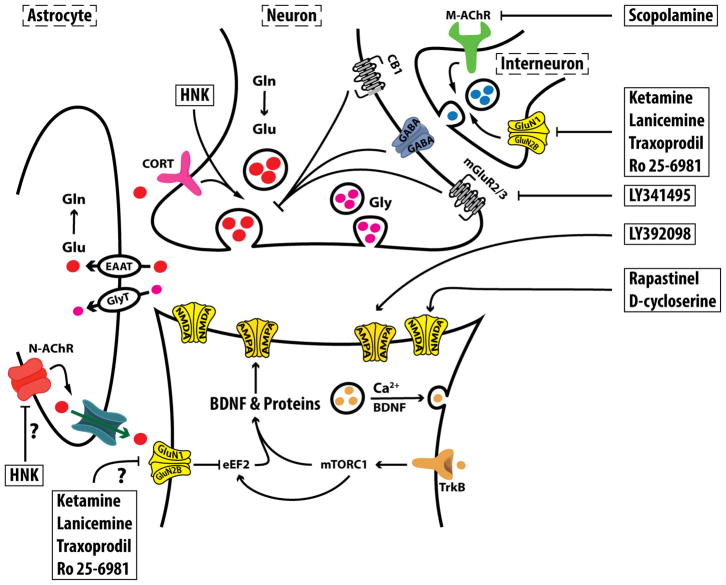
Figure 2. Molecular Targets of Rapid-acting Antidepressants (RAADs).
It is believed that RAADs exert their effects by inducing a transient (minutes-to-hours) postsynaptic glutamate activation, which ultimately leads to sustained (days-to-weeks) increase in synaptic formation and strength in the prefrontal cortex. It remains to be determined in future studies whether inhibition of extrasynaptic N-methyl-D-aspartate (NMDA) receptors would be sufficient to exert RAAD effects. The figure depicts the potential targets of agents suspected to have RAAD properties. Abbreviations: AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; BDNF = brain derived neurotrophic factor; CB1 = cannabinoid receptor; EAAT = excitatory aminoacid transporter; eEF2 = eukaryotic elongation factor 2; Gln = glutamine; GluN1 = NMDA subtype 1; GluN2B = NMDA subtype 2B; Glu = glutamate; Gly = Glycine; GlyT = Glycine transporter; HNK = hydroxynorketamine; M-AChR = muscarinic acetylcholine receptor; mGluR2/3 = metabotropic glutamate receptor subtype 2 and 3; mTORC1 = mechanistic target of rapamycin complex 1; N-AChR = nicotinic AChR; TrkB = tyrosine kinase B receptor. The figure was adapted with permission from the Emerge Research Program (emerge.care).
Transient PFC glutamate neurotransmission activation leads to activity-dependent release of BNDF, activates the mechanistic target of rapamycin complex 1 (mTORC1) signaling, and increases protein synthesis and synaptic strength (Lepack, Bang, Lee, Dwyer, & Duman, 2016; Lepack, Fuchikami, Dwyer, Banasr, & Duman, 2015; Liu, et al., 2012). The preclinical evidence of ketamine-induced glutamate release has long been demonstrated using microdialysis (Moghaddam, et al., 1997) and, more recently, evidence of transient glutamate transmission activation was demonstrated using ex vivo carbon-13 magnetic resonance spectroscopy (13C MRS) (Chowdhury, et al., 2012; Chowdhury, et al., 2016; Rothman, De Feyter, de Graaf, Mason, & Behar, 2011). Here, it is important to distinguish between presynaptic glutamate release and postsynaptic activation. The latter – i.e., postsynaptic glutamate activation – is required for the induction of BDNF and its high affinity receptor TrkB, the increase in synaptic strength, and the RAAD effects. Hence, mechanisms independent of evoked presynaptic release of glutamate have been proposed for ketamine (Autry, et al., 2011; Miller, Moran, & Hall, 2016) and agents directly targeting postsynaptic glutamate activation may possess RAAD properties (e.g., AMPAR potentiators or NMDAR partial agonists; Fig. 2).
The role of the transient postsynaptic glutamate activation in the RAAD effects of ketamine has been abundantly shown in preclinical studies. First line of evidence stems from a consistent observation that the inhibition of postsynaptic glutamate activation – using AMPAR antagonists – blocks the synaptic remodeling and the RAAD effects of ketamine (N. Li, et al., 2010; S. Maeng, et al., 2008). Here, we note that even selective AMPAR antagonists will presumably block overall postsynaptic glutamate activation through inhibition of both AMPAR and NMDAR, considering that AMPAR activation leading to membrane depolarization is required for NMDAR signaling. In this context, most of the evidence relating AMPAR blockade to the inhibition of RAADs would be considered an indication of the need for postsynaptic glutamate activation rather than specific AMPAR activation. For example, NMDAR potentiators – without AMPAR modulating properties – may possess RAAD effects by inducing transient postsynaptic glutamate activity (e.g., NMDAR partial agonists). Furthermore, the RAAD effects of these NMDAR potentiators will still be blocked by pretreatment with AMPAR antagonists, because the latter also inhibits NMDAR signaling. Another line of evidence underscoring the role of transient glutamate activation is that postsynaptic depolarization and activation of L-type voltage-dependent calcium channels (VDCC) are necessary for the synaptic changes and the RAAD effects of ketamine (Jourdi, et al., 2009; Lepack, et al., 2015) and other RAADs (Ghosal, et al., 2018).
While the mechanisms through which ketamine induces a transient postsynaptic glutamate activation are not fully known, a leading hypothesis is that subanesthetic doses of ketamine preferentially inhibits NMDARs on a subpopulation of interneurons precipitating pyramidal neurons disinhibition and paradoxical surge in glutamate release (Homayoun & Moghaddam, 2007). However, alternative hypotheses have also been proposed. One study hypothesized that blockade of at rest NMDAR signaling – i.e., without evoked glutamate release – would be sufficient to increase eukaryotic elongation factor 2 (eEF2) signaling and BDNF translation, leading to increased protein synthesis and synaptic connectivity (Autry, et al., 2011). Yet, using AMPAR modulation, the same study has also demonstrated that postsynaptic glutamate activation is necessary for the RAAD effects of ketamine (Autry, et al., 2011). A main metabolite of ketamine is (2S,6S;2R,6R)-hydroxynorketamine (HNK). Following injection, ketamine rapidly reaches the brain within 1 minute and it maintains a brain/plasma concentration ratio equal 6.5 for 10 minutes (Cohen, Chan, Way, & Trevor, 1973). In rodents, the plasma concentrations of ketamine and norketamine, both potent NMDAR antagonists, peak at 10 minutes post-injection and decrease exponentially thereafter, while the HNK concentration peaks at 30 minutes (Can, et al., 2016; Moaddel, et al., 2015; Paul, et al., 2014). Recently, it was found that HNK possesses RAAD properties without blockade of NMDARs (Zanos, et al., 2016). However, the same study has also demonstrated that postsynaptic glutamate activation is necessary for the RAAD effects of ketamine (Zanos, et al., 2016), and preliminary evidence has since shown that (2R,6R)-HNK also induces a glutamate release surge (Pham, et al., 2017; Schwarcz, Wu, Zanos, & Gould, 2017). Together, the data highlights the ability of ketamine to induce a surge in glutamate transmission and that the transient postsynaptic activation is responsible for its RAAD effects (Fig. 2).
The role of transient postsynaptic glutamate activation is not limited to the neurobiology of ketamine. In fact, the mechanisms of several other RAADs have been related to transient glutamate activation. Scopolamine, a muscarinic cholinergic receptor (M-AChR) antagonist, was shown to increase glutamate release and postsynaptic activation, leading to increased PFC BDNF and synaptic connectivity, and to RAAD effects. The molecular and behavioral effects of scopolamine are blocked by inhibiting postsynaptic glutamate activation (Chowdhury, et al., 2016; Ghosal, et al., 2018; Voleti, et al., 2013; Wohleb, et al., 2016). Similarly, rapastinel (also known as GLYX-13) – a drug with presumable NMDAR partial agonist properties – was shown to increase PFC BDNF and synaptic connectivity, and to exert RAAD effects, all of which were dependent on postsynaptic glutamate activation as evident by manipulation of AMPAR and VDCC (Lepack, et al., 2016; Liu, et al., 2017). The transient glutamate activation was also related to many other putative RAADs, including the selective NMDAR subtype 2B (GluN2B) antagonists Ro 25-6981 and traxoprodil (also known as CP-101,606), the mGluR2/3 antagonist LY341495, and the AMPAR potentiator LY392098 [Positive allosteric modulators (PAM) or ampakines] (Chowdhury, et al., 2016; Karasawa, Shimazaki, Kawashima, & Chaki, 2005; X. Li, et al., 2001; Tang, et al., 2018). Here, it is important to note that the reviewed literature is specific to transient glutamate effects, and may not necessarily translate to the effects of sustained increase in glutamate activation. Indeed, comparable to CSP, it is a concern that frequent daily administration of ketamine or chronic activation of glutamate may lead to excitotoxicity and synaptic dysconnectivity. Consistent with this concern, extensive preclinical literature relates repeated ketamine administration to neurotoxicity and behavioral abnormalities [e.g., (Schobel, et al., 2013)]. Similarly, the substance abuse literature of daily use of ketamine underscores its detrimental effects on cognition and mood (Morgan, Curran, & Independent Scientific Committee on, 2012). Future studies should investigate whether infrequent glutamate activation, such as twice per week administration of ketamine, would provide optimal balance for maintaining the beneficial synaptic connectivity changes.
While there is evidence to support the SAAD properties of glutamate release inhibitors [e.g., lamotrigine (Solmi, et al., 2016)], these medications do not typically induce RAAD effects. Moreover, the ultimate effects of the chronic administration of these glutamate modulators may still be increasing glutamate neurotransmission and BDNF, and subsequent normalization of synaptic connectivity. For example, chronic treatment with the glutamate release inhibitor lamotrigine was shown to reverse the pathology of chronic stress and to increase prefrontal and hippocampal BDNF (N. Li, et al., 2011). In addition, chronic riluzole treatment – an agent believed to inhibit the calcium-dependent glutamate release and increase astrocytic glutamate re-uptake – was shown to reverse CSP and increase overall PFC glutamate neurotransmission activation, rather than decreasing it (Banasr, et al., 2010; Chowdhury, et al., 2008). Finally, in contrast to synaptic NMDARs where activation would lead to increased synaptic formation and strength (i.e., synaptogenesis), the activation of extrasynaptic NMDARs is thought to promote synaptic death (Hardingham & Bading, 2010). Extrasynaptic NMDARs are activated by excessive extracellular levels of glutamate, which causes overstimulation of NMDARs. This leads to an increased calcium influx, activates toxic metabolic processes and triggers cell death (Deutschenbaur, et al., 2016; Paoletti, Bellone, & Zhou, 2013). Therefore, selective blockade of extrasynaptic NMDARs may induce synaptogenesis and exert antidepressant effects. A recent study has shown that targeting the extrasynaptic NMDARs would exert RAAD effects in rodents (S. X. Li, et al., 2017). Another study has shown that ketamine blockade of the lateral habenula bursting activities precipitates RAAD effects (Yang, et al., 2018). However, a major limitation of these studies is that the behavioral effects were tested immediately after ketamine administration, rather than 24h later to confirm the presence of RAAD effects in the absence of ketamine intoxication (S. X. Li, et al., 2017; Yang, et al., 2018). Additionally, the selective NMDAR modulation approaches used may have inadvertently induced a paradoxical glutamate surge in the PFC, similar to the in vivo effects of ketamine and many other NMDAR modulators. Future studies would be necessary to demonstrate the RAAD effects at 24h post administration, and to determine whether selective blockade of extrasynaptic NMDAR signaling is sufficient to exert RAAD effects without the need for postsynaptic glutamate neurotransmission activation.
5. Clinical Efficacy of RAADs
Following the regimen used in the first study (Berman, et al., 2000), clinical trials have mostly administered 0.5 mg/kg intravenous (i.v.) ketamine infused over 40 minutes [reviewed in (Abdallah, Averill, et al., 2015)]. To date, there is well replicated evidence showing the RAAD effects of a single ketamine infusion in MDD (McGirr, et al., 2015). Concerns regarding the efficacy of the treatment blinding were partially addressed using active placebo (Murrough, et al., 2013). A major limitation of the single infusion treatment is that patients often relapse within 1–2 weeks. However, repeated administration of ketamine (e.g., twice per week) appears to maintain the RAAD effects (Singh, Fedgchin, Daly, De Boer, et al., 2016). While the need for intravenous administration could be a limiting factor, pilot evidence suggests that intranasal (i.n.) administration of ketamine may exert RAAD effects (Canuso, et al., 2018; Daly, et al., 2017; Lapidus, et al., 2014). The psychotomimetic effects of ketamine could be considered a limitation, although these adverse events are transient (1–2h) and typically well tolerated. The main remaining limitations of ketamine treatment are its addiction liability and the scarcity of data regarding the safety of chronic treatment (Kokkinou, Ashok, & Howes, 2018; Sanacora, Frye, et al., 2017). The latter is particularly important considering the association of heavy daily use of ketamine with ulcerative cystitis, hepatotoxicity, and neurotoxicity (Cottrell, et al., 2008; Morgan, et al., 2012; Noppers, et al., 2011; Shahani, Streutker, Dickson, & Stewart, 2007).
Other putative RAADs with published clinical trials in MDD include: (1) Scopolamine (3 i.v. infusions separated by 3–4 days) has shown efficacy compared to placebo in small clinical trials (Drevets, Zarate, & Furey, 2013); (2) Traxoprodil showed efficacy at day 5 following single infusion in a proof of concept study (S. H. Preskorn, et al., 2008), yet its development was stopped due to incidence of QT prolongation (Machado-Vieira, Henter, & Zarate, 2017); (3) Esketamine, the S enantiomer of ketamine, appears to have RAAD properties following i.v. or i.n. administration in early studies (Canuso, et al., 2018; Daly, et al., 2017; Singh, Fedgchin, Daly, Xi, et al., 2016); (4) Low doses of d-cycloserine, with NMDAR partial agonist effects, were reported to exert RAAD effects in retrospective investigations (Kim, Kushner, Yoon, Anker, & Grant, 2016); (5) Rapastinel (i.v.) has shown efficacy in a proof of concept study (S. Preskorn, et al., 2015); (6) Lanicemine, a low-trapping NMDAR antagonist, has shown efficacy in one phase II study but failed in a second larger clinical trial that may have been complicated by high placebo response rates (Sanacora, Johnson, et al., 2017; Sanacora, et al., 2013; Zarate, et al., 2013). Together, these clinical trials provide a clear evidence on the prospect of RAADs. However, additional confirmatory clinical trials are still needed to determine the efficacy of these putative RAADs.
As described earlier, the synaptic CSP model would predict that ketamine may have therapeutic effects in many psychiatric disorders with considerable chronic stress component. In fact, pilot trials to date support this hypothesis. Accumulating evidence suggests that ketamine may have independent rapid anti-suicidal effects in depressed patients (Canuso, et al., 2018; Grunebaum, et al., 2017; Wilkinson, et al., 2018). Moreover, pilot evidence suggests potential therapeutic effects of ketamine in treating bipolar depression, PTSD, OCD, GAD, social anxiety disorder (SAD), and substance/alcohol use disorders [(Albott, et al., 2018; Diazgranados, et al., 2010; Feder, et al., 2014; Glue, et al., 2017; Ivan Ezquerra-Romano, Lawn, Krupitsky, & Morgan, 2018; Rodriguez, et al., 2013; Taylor, et al., 2018; Zarate, et al., 2012), but also see (Bloch, et al., 2012)]
6. Clinical Biomarkers of RAADs
To better understand the neurobiology of depression and RAADs, numerous clinical biomarker studies over the past decade capitalized on the RAAD effects of ketamine, its potent effects on prefrontal glutamate neurotransmission, and its robust neuronal remodeling 24h post infusion. Here, we will briefly review biomarker studies of relevance to the ketamine induced acute glutamate surge (i.e., during infusion) and sustained neuronal remodeling (i.e., 24h post treatment).
Several lines of evidence have supported the presence of a ketamine induced prefrontal glutamate surge in humans and have associated this surge with the psychotomimetic effects of the drug. Early studies have shown that glutamate release inhibitors would reduce the psychotomimetic effects of ketamine. Later neuroimaging studies have either shown ketamine induced increases in PFC glucose metabolism or blood flow, PFC blood oxygen level dependent (BOLD) signal, or PFC total glutamate level (Anand, et al., 2000; Breier, Malhotra, Pinals, Weisenfeld, & Pickar, 1997; Deakin, et al., 2008; Javitt, et al., 2017; Krystal, et al., 2005; Krystal, et al., 2010; Milak, et al., 2016; Rowland, et al., 2005; Stone, et al., 2012; Vollenweider, Leenders, Oye, Hell, & Angst, 1997; Vollenweider, Leenders, Scharfetter, et al., 1997). While collectively these studies provide convincing evidence of an acute glutamate surge, most of these studies were in healthy subjects which limits their ability to associate this surge to the RAAD effects. Another main limitation is that these approaches do not distinguish between presynaptic glutamate release and postsynaptic activation. As reviewed earlier, the postsynaptic glutamate activation appears to be the critical process for the RAAD effects. Moreover, recent data using ex vivo (in rats) and in vivo (in humans) 13C MRS suggests that the psychotomimetic effects of ketamine may be due to the decoupling between presynaptic glutamate release and postsynaptic activation (i.e., disruption in communication fidelity across synapses), as evident by increased glutamate cycling combined with inefficient increase in neuroenergetics that are primarily due to postsynaptic activation (i.e., reduction of energy per cycle) [(Chowdhury, et al., 2016) & (Abdallah et al. under review)]. If these pilot data were confirmed in future human studies, it will offer a mechanism through which novel drugs may induce RAAD effects without psychotomimetic symptoms, provided that these new agents equally increase presynaptic release and postsynaptic activation.
Further supporting the presence of ketamine induced glutamate surge, recent studies have shown alterations in the binding of metabotropic glutamate receptors subtype 5 (mGluR5) during infusion of ketamine in healthy and depressed subjects (Davis, Holmes, Pietrzak, & Esterlis, 2017; DeLorenzo, et al., 2015; Esterlis, et al., 2017). Yet, perhaps the most studied approach has been the use of resting state functional MRI. In particular, PFC global brain connectivity (GBC) reduction has been observed in several psychiatric disorders with a strong chronic stress component (Anticevic, et al., 2013; Anticevic, et al., 2015; Anticevic, et al., 2014; Cole, Anticevic, Repovs, & Barch, 2011). This observation has led to the hypothesis that GBC may reflect an underlying CSP of reduced PFC synaptic connectivity. Supporting this hypothesis, several studies have demonstrated reduced PFC global connectivity in MDD (C. G. Abdallah, C. L. Averill, et al., 2017; C. G. Abdallah, L. A. Averill, et al., 2017; Murrough, et al., 2016; Scheinost, et al., 2017; Wang, et al., 2014). In addition, human mechanistic studies have provided evidence directly linking glutamate neurotransmission to PFC GBC (C. G. Abdallah, C. L. Averill, et al., 2017). Moreover, subanesthetic doses of ketamine have been shown to increase PFC GBC during infusion in healthy individuals, which parallel the hypothesized glutamate surge (C. G. Abdallah, C. L. Averill, et al., 2017; Anticevic, et al., 2015; Driesen, McCarthy, Bhagwagar, Bloch, Calhoun, D'Souza, Gueorguieva, He, Ramachandran, et al., 2013; Driesen, McCarthy, Bhagwagar, Bloch, Calhoun, D'Souza, Gueorguieva, He, Leung, et al., 2013). Consistent with the role of PFC synaptic connectivity in the mechanisms of RAADs, ketamine was found to rapidly normalize PFC GBC abnormalities in MDD patients within 24h of treatment. These PFC GBC increases were also associated with treatment response (C. G. Abdallah, L. A. Averill, et al., 2017). More recently, in a randomize placebo controlled design, it was shown that ketamine increases PFC GBC in MDD during infusion and at 24h post-treatment. In addition, the amount of PFC GBC increases during ketamine infusion predicted treatment response at 24h post-treatment (C.G. Abdallah, et al., 2017). Finally, similar to the preclinical data of ketamine induced increases in hippocampal synaptic connectivity along with reduction in NAc synaptic connectivity (Melo, et al., 2015; Reus, et al., 2013; Yao, et al., 2017), recent pilot human evidence using structural MRI have shown that ketamine significantly increases hippocampal and reduces NAc volumes in MDD patients within 24h of treatment, particularly in individuals who responded to treatment (C. G. Abdallah, A. Jackowski, et al., 2017).
7. Current Challenges & Future Directions
The ketamine findings have generated considerable excitement about the promise of a truly novel class of robust and effective RAADs. This excitement was translated into a sizable investment from academia, the pharmaceutical industry, and funding agencies. Hundreds of papers over the past decade have investigated the mechanisms of ketamine and/or its potential therapeutic utility. However, while preclinical data have extensively investigated ketamine’s targets and putative mechanisms, the clinical mechanistic evidence remains lagging.
In particular, to date, we do not have a well-established reproducible biomarker of target engagement (i.e., transient postsynaptic glutamate activation) or target validation (i.e., sustained synaptic remodeling) for the development of RAADs. This issue may have been less problematic for the development of monoaminergic drugs. Following the identification of the in vitro pharmacodynamics of tricyclic antidepressants, the field over the next half a century successfully produced several SAADs, which primarily shared the common in vitro detectable pharmacodynamics of serotonin re-uptake inhibition (SRI). In addition, SRI largely has (1) linear dose response, (2) broad therapeutic window, and (3) relatively stable in vivo pharmacodynamics across administration regimens. In contrast, the development of RAADs has proven more complex; at least two NMDAR antagonists (memantine & CERC-301) and two ampakines (S47445 & ORG 26576) failed in clinical trials and other similar agents have shown promise only in preclinical studies or proof of concept trials. Several challenges may have contributed to the complexity of developing RAADs: (1) While it is evident that blocking NMDAR may induce RAAD effects, only focusing on the in vitro NMDAR antagonism properties appears to have low predictability for the development of new RAADs. (2) Determining the in vivo effects of novel RAAD agents on transient glutamate neurotransmission and sustained synaptic connectivity is critical. However, at this stage, this is only possible in animal studies. The presence of an inverted U-shaped relationship between dose and response further complicates the translatability of preclinical findings. Together, these challenges underscore the need for robust and reproducible biomarkers of prefrontal glutamate activation and synaptic connectivity in humans in vivo. The successful development of these biomarkers would be essential to optimize administration regimens of new RAAD compounds prior to testing them in large expensive trials. Capitalizing on the extensive preclinical and clinical ketamine data, and the swiftness and robustness of its synaptic remodeling and behavioral (psychotomimetic and antidepressant) effects, future studies have a unique opportunity to use ketamine as a tool to establish these synaptic biomarkers that are not only relevant to depression, but also to normal brain function and most neuropsychiatric disorders.
Another opportunity for future studies is that both the chronic stress related synaptic hypoconnectivity and the ketamine induced synaptic hyperconnectivity are reversible within 2 weeks of the intervention, raising mechanistic questions about this individualized homeostatic stable equilibrium of overall synaptic strength. Future studies can capitalize on this notable reversibility to determine the mechanisms underlying this putative homeostatic stable equilibrium of synaptic strength within the context of chronic stress and ketamine treatment. Successful unraveling of these mechanisms may provide information that goes beyond the progress and treatment of depression, and begins to examine the etiology and perhaps cure of depression.
In summary, elegant ketamine studies over the past decade have significantly improved our understanding of the pathophysiology of chronic stress and depression, while unraveling several mechanisms through which transient prefrontal glutamate activation produces rapid restoration of synaptic connectivity along with RAAD effects. Although there is evidence associating glutamate inhibition with antidepressant properties, these data have been primarily limited to SAAD effects. So, is it glutamate inhibition or activation? Within the PFC, chronic stress and depression seem to be associated with high extrasynaptic glutamate level, but overall reduced glutamate neurotransmission as evident by reduction in synaptic connectivity, glutamate cycling, and neuroenergetics. As for the neurobiology of ketamine and other RAADs, it is increasingly evident that transient PFC glutamate postsynaptic activation is a primary underlying mechanism. Yet, it remains to be seen in future studies whether selective inhibition of extrasynaptic NMDARs would be sufficient to induce sustained synaptic remodeling and robust RAAD effects.
Abbreviations
- 13C MRS
carbon-13 magnetic resonance spectroscopy
- ABP
aminoacid-based pathology
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- BDNF
brain derived neurotrophic factor
- BOLD
blood oxygen level dependent
- CSP
chronic stress pathology
- GAD
generalized anxiety disorder
- GBC
global brain connectivity
- GluR2B
N-methyl-D-aspartate receptor subtype 2B
- HNK
hydroxynorketamine
- i.n.
intranasal
- i.v.
intravenous
- M-AChR
muscarinic cholinergic receptor
- MBP
monoamine-based pathology
- mGluR5
metabotropic glutamate receptor subtype 5
- MRI
magnetic resonance imaging
- mTROC1
mechanistic target of rapamycin complex 1
- NAc
nucleus accumbens
- NMDAR
N-methyl-D-aspartate receptor
- OCD
obsessive compulsive disorder
- PFC
prefrontal cortex
- PTSD
posttraumatic stress disorder
- RAAD
rapid-acting antidepressant
- SAAD
slow-acting antidepressant
- SAD
social anxiety disorder
- SRI
serotonin re-uptake inhibition
- VDCC
L-type voltage-dependent calcium channels
Footnotes
Conflict of Interest statement
CGA has served as a consultant and/or on advisory boards for Genentech and Janssen, and editor of Chronic Stress for Sage Publications, Inc.;
GS reports personal consulting fees from Alkermes, Allergan, Biohaven Pharmaceuticals, Eli Lilly and Co., Genetech, Janssen Pharmaceuticals, Lundbeck Research USA, Merck & Co., Naurex, Navitor Pharmaceuticals, Noven Pharmaceuticals, Teva Pharmaceuticals Industries, Taisho Pharmaceutical Co., Takeda Pharmaceutical Co, Sage Pharmaceuticals Inc., Sevier, Valeant Pharmaceuticals, and Vistagen Therapeutics Inc.; grants and research contracts from Eli Lilly and Co., Janssen Pharmaceuticals, Merck & Co., and Sevier and support from Sanofi-Aventis, in the form of free medication for an NIH sponsored study over the last 36 mos. In addition, Dr. Sanacora is a stockholder and holds stock options in Biohaven Pharmaceuticals; and has a patent for Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued Jul 15, 2014) with royalties paid from Biohaven Pharmaceuticals; GFM is a consultant for Sumitomo Dainippon Pharma Co. Ltd and UCB Pharma SA, and serves on the Scientific Advisory Board of Elucidata Inc.;
RSD reports no competing interests;
JHK is a consultant for AbbVie, Inc., Amgen, Astellas Pharma Global Development, Inc., AstraZeneca Pharmaceuticals, Biomedisyn Corporation, Bristol-Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Inc., Neurovance, Inc., FORUM Pharmaceuticals, Janssen Research & Development, Lundbeck Research USA, Novartis Pharma AG, Otsuka America Pharmaceutical, Inc., Sage Therapeutics, Inc., Sunovion Pharmaceuticals, Inc., and Takeda Industries; is on the Scientific Advisory Board for Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Inc., Naurex, Inc., and Pfizer; is a stockholder in Biohaven Pharmaceuticals; holds stock options in Mnemosyne Pharmaceuticals, Inc.; holds patents for Dopamine and Noradrenergic Reuptake Inhibitors in Treatment of Schizophrenia, U.S. Patent No. 5,447,948 (issued Sep 5, 1995), and Glutamate Modulating Agents in the Treatment of Mental Disorders, U.S. Patent No. 8,778,979 (issued Jul 15, 2014); and filed a patent for Intranasal Administration of Ketamine to Treat Depression. U.S. Application No. 14/197,767 (filed on Mar 5, 2014); U.S. application or Patent Cooperation Treaty international application No. 14/306,382 (filed on Jun 17, 2014);
References
- Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH. Ketamine's Mechanism of Action: A Path to Rapid-Acting Antidepressants. Depress Anxiety. 2016;33:689–697. doi: 10.1002/da.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill CL, Salas R, Averill LA, Baldwin PR, Krystal JH, Mathew SJ, Mathalon DH. Prefrontal Connectivity and Glutamate Transmission: Relevance to Depression Pathophysiology and Ketamine Treatment. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:566–574. doi: 10.1016/j.bpsc.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY, Iosifescu DV, Charney DS, Murrough JW. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacology. 2017;42:1210–1219. doi: 10.1038/npp.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Krystal JH. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann N Y Acad Sci. 2015;1344:66–77. doi: 10.1111/nyas.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Coplan JD, Jackowski A, Sato JR, Mao X, Shungu DC, Mathew SJ. A pilot study of hippocampal volume and N-acetylaspartate (NAA) as response biomarkers in riluzole-treated patients with GAD. Eur Neuropsychopharmacol. 2013;23:276–284. doi: 10.1016/j.euroneuro.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Dutta A, Averill CL, McKie S, Averill LA, Deakin JF. Ketamine, but not the NMDA Receptor Antagonist Lanicemine, Increases Prefrontal Connectivity in Depressed Patients. Neuropsychopharmacology. 2017;43:S111. doi: 10.1177/2470547018796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Jackowski A, Salas R, Gupta S, Sato JR, Mao X, Coplan JD, Shungu DC, Mathew SJ. The Nucleus Accumbens and Ketamine Treatment in Major Depressive Disorder. Neuropsychopharmacology. 2017;42:1739–1746. doi: 10.1038/npp.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Jackowski A, Sato JR, Mao X, Kang G, Cheema R, Coplan JD, Mathew SJ, Shungu DC. Prefrontal cortical GABA abnormalities are associated with reduced hippocampal volume in major depressive disorder. Eur Neuropsychopharmacol. 2015;25:1082–1090. doi: 10.1016/j.euroneuro.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Southwick SM, Krystal JH. Neurobiology of posttraumatic stress disorder (PTSD): A path from novel pathophysiology to innovative therapeutics. Neurosci Lett. 2017;649:130–132. doi: 10.1016/j.neulet.2017.04.046. [DOI] [PubMed] [Google Scholar]
- Abdallah CG, Wrocklage KM, Averill CL, Akiki T, Schweinsburg B, Roy A, Martini B, Southwick SM, Krystal JH, Scott JC. Anterior hippocampal dysconnectivity in posttraumatic stress disorder: a dimensional and multimodal approach. Transl Psychiatry. 2017;7:e1045. doi: 10.1038/tp.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams TG, Kelmendi B, Brake CA, Gruner P, Badour CL, Pittenger C. The Role of Stress in the Pathogenesis and Maintenance of Obsessive-Compulsive Disorder. Chronic Stress. 2018;2:2470547018758043. doi: 10.1177/2470547018758043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki TJ, Averill CL, Wrocklage KM, Schweinsburg B, Scott JC, Martini B, Averill LA, Southwick SM, Krystal JH, Abdallah CG. The Association of PTSD Symptom Severity with Localized Hippocampus and Amygdala Abnormalities. Chronic Stress. 2017;1:2470547017724069. doi: 10.1177/2470547017724069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albott CS, Lim KO, Forbes MK, Erbes C, Tye SJ, Grabowski JG, Thuras P, Batres YCTM, Wels J, Shiroma PR. Efficacy, Safety, and Durability of Repeated Ketamine Infusions for Comorbid Posttraumatic Stress Disorder and Treatment-Resistant Depression. J Clin Psychiatry. 2018;79 doi: 10.4088/JCP.17m11634. [DOI] [PubMed] [Google Scholar]
- Aleksandrova LR, Wang YT, Phillips AG. Hydroxynorketamine: Implications for the NMDA Receptor Hypothesis of Ketamine’s Antidepressant Action. Chronic Stress. 2017;1:2470547017743511. doi: 10.1177/2470547017743511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, Krystal JH, Pearlson GD, Glahn DC. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2013;73:565–573. doi: 10.1016/j.biopsych.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y, Repovs G, Murray JD, Driesen NR, Morgan PT, Xu K, Wang F, Krystal JH. N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry. 2015;77:569–580. doi: 10.1016/j.biopsych.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, Bloch MH, Li CS, Pittenger C. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:595–605. doi: 10.1016/j.biopsych.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill LA, Abdallah CG, Pietrzak RH, Averill CL, Southwick SM, Krystal JH, Harpaz-Rotem I. Combat Exposure Severity is Associated with Reduced Cortical Thickness in Combat Veterans: A Preliminary Report. Chronic Stress. 2017;1:2470547017724714. doi: 10.1177/2470547017724714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, Abdallah CG. Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neurosci Lett. 2017;649:147–155. doi: 10.1016/j.neulet.2016.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban TA. The role of serendipity in drug discovery. Dialogues in clinical neuroscience. 2006;8:335–344. doi: 10.31887/DCNS.2006.8.3/tban. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Molecular Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Wasylink S, Landeros-Weisenberger A, Panza KE, Billingslea E, Leckman JF, Krystal JH, Bhagwagar Z, Sanacora G, Pittenger C. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72:964–970. doi: 10.1016/j.biopsych.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobo WV, Vande Voort JL, Croarkin PE, Leung JG, Tye SJ, Frye MA. Ketamine for Treatment-Resistant Unipolar and Bipolar Major Depression: Critical Review and Implications for Clinical Practice. Depress Anxiety. 2016;33:698–710. doi: 10.1002/da.22505. [DOI] [PubMed] [Google Scholar]
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. The American journal of psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. Journal of neurophysiology. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, Cheer JF, Frost DO, Huang XP, Gould TD. Effects of Ketamine and Ketamine Metabolites on Evoked Striatal Dopamine Release, Dopamine Receptors, and Monoamine Transporters. J Pharmacol Exp Ther. 2016;359:159–170. doi: 10.1124/jpet.116.235838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, Pinter C, Hough D, Sanacora G, Manji H, Drevets WC. Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of Symptoms of Depression and Suicidality in Patients at Imminent Risk for Suicide: Results of a Double-Blind, Randomized, Placebo-Controlled Study. Am J Psychiatry. 2018 doi: 10.1176/appi.ajp.2018.17060720. appiajp201817060720. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Banasr M, de Graaf RA, Rothman DL, Behar KL, Sanacora G. Chronic riluzole treatment increases glucose metabolism in rat prefrontal cortex and hippocampus. J Cereb Blood Flow Metab. 2008;28:1892–1897. doi: 10.1038/jcbfm.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. 1H-[13C]-nuclear magnetic resonance spectroscopy measures of ketamine's effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71:1022–1025. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, Bristow L, Schaeffer E, Duman RS, Rothman DL, Behar KL, Sanacora G. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Heshmati M, Graham A, Birnbaum S, Neve RL, Hodes GE, Russo SJ. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37:2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ML, Chan SL, Way WL, Trevor AJ. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 1973;39:370–376. doi: 10.1097/00000542-197310000-00003. [DOI] [PubMed] [Google Scholar]
- Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Lu D, El Sehamy AM, Tang C, Jackowski AP, Abdallah CG, Nemeroff CB, Owens MJ, Mathew SJ, Gorman JM. Early Life Stress Associated with Increased Striatal N-acetyl-aspartate (NAA): Cerebrospinal Fluid (CSF) Corticotropin-Releasing Factor (CRF) Concentrations, Hippocampal Volume, Body Mass and Behavioral Correlates. Chronic Stress. 2018;2 doi: 10.1177/2470547018768450. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell A, Warren K, Ayres R, Weinstock P, Kumar V, Gillatt D. The destruction of the lower urinary tract by ketamine abuse: a new syndrome? BJU international. 2008;102:1178–1179. doi: 10.1111/j.1464-410X.2008.08146_2.x. author reply 1179. [DOI] [PubMed] [Google Scholar]
- Crane GE. Cyloserine as an antidepressant agent. The American journal of psychiatry. 1959;115:1025–1026. doi: 10.1176/ajp.115.11.1025. [DOI] [PubMed] [Google Scholar]
- D'Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC. Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2017 doi: 10.1001/jamapsychiatry.2017.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Binder EB. Schizophrenia in the spectrum of gene-stress interactions: the FKBP5 example. Schizophr Bull. 2015;41:323–329. doi: 10.1093/schbul/sbu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MT, Holmes SE, Pietrzak RH, Esterlis I. Neurobiology of Chronic Stress-Related Psychiatric Disorders: Evidence from Molecular Imaging Studies. Chronic Stress. 2017;1:2470547017710916. doi: 10.1177/2470547017710916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- DeLorenzo C, DellaGioia N, Bloch M, Sanacora G, Nabulsi N, Abdallah C, Yang J, Wen R, Mann JJ, Krystal JH, Parsey RV, Carson RE, Esterlis I. In Vivo Ketamine-Induced Changes in [(11)C]ABP688 Binding to Metabotropic Glutamate Receptor Subtype 5. Biol Psychiatry. 2015;77:266–275. doi: 10.1016/j.biopsych.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschenbaur L, Beck J, Kiyhankhadiv A, Muhlhauser M, Borgwardt S, Walter M, Hasler G, Sollberger D, Lang UE. Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:325–333. doi: 10.1016/j.pnpbp.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Zarate CA, Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73:1156–1163. doi: 10.1016/j.biopsych.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D'Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, Anticevic A, Morgan PT, Krystal JH. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18:1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D'Souza DC, Gueorguieva R, He G, Leung HC, Ramani R, Anticevic A, Suckow RF, Morgan PT, Krystal JH. The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology. 2013;38:2613–2622. doi: 10.1038/npp.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, Yang J, Pittenger C, Sanacora G, Krystal JH, Parsey RV, Carson RE, DeLorenzo C. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [(11)C]ABP688 and PET imaging study in depression. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, Aan Het Rot M, Lapidus KA, Wan LB, Iosifescu D, Charney DS. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71:681–688. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- Gall CM, Isackson PJ. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989;245:758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, Duman RS. Activity-Dependent Brain-Derived Neurotrophic Factor Release Is Required for the Rapid Antidepressant Actions of Scopolamine. Biol Psychiatry. 2018;83:29–37. doi: 10.1016/j.biopsych.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glue P, Medlicott NJ, Harland S, Neehoff S, Anderson-Fahey B, Le Nedelec M, Gray A, McNaughton N. Ketamine's dose-related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. J Psychopharmacol. 2017;31:1302–1305. doi: 10.1177/0269881117705089. [DOI] [PubMed] [Google Scholar]
- Goddard AW. The Neurobiology of Panic: A Chronic Stress Disorder. Chronic Stress. 2017;1:2470547017736038. doi: 10.1177/2470547017736038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ. Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.17060647. appiajp201717060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare B, Ghosal S, Duman R. Rapid acting antidepressants in chronic stress models: molecular and cellular mechanisms. Chronic Stress. 2017;1:1–16. doi: 10.1177/2470547017697317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukvik UK, Westlye LT, Morch-Johnsen L, Jorgensen KN, Lange EH, Dale AM, Melle I, Andreassen OA, Agartz I. In vivo hippocampal subfield volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2015;77:581–588. doi: 10.1016/j.biopsych.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan Ezquerra-Romano I, Lawn W, Krupitsky E, Morgan CJA. Ketamine for the treatment of addiction: Evidence and potential mechanisms. Neuropharmacology. 2018 doi: 10.1016/j.neuropharm.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9:984–997. 979. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Carter CS, Krystal JH, Kantrowitz JT, Girgis RR, Kegeles LS, Ragland JD, Maddock RJ, Lesh TA, Tanase C, Corlett PR, Rothman DL, Mason G, Qiu M, Robinson J, Potter WZ, Carlson M, Wall MM, Choo TH, Grinband J, Lieberman JA. Utility of Imaging-Based Biomarkers for Glutamate-Targeted Drug Development in Psychotic Disorders: A Randomized Clinical Trial. JAMA Psychiatry. 2017 doi: 10.1001/jamapsychiatry.2017.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci. 2009;29:8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005;1042:92–98. doi: 10.1016/j.brainres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC, Hatton SN, Bennett MR. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol. 2013;47:645–661. doi: 10.1007/s12035-012-8365-7. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Archives of General Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kim SW, Kushner MG, Yoon G, Anker J, Grant JE. Low-Dose d-Cycloserine for Depression? J Clin Psychiatry. 2016;77:e1007. doi: 10.4088/JCP.15lr10272. [DOI] [PubMed] [Google Scholar]
- Klein DF. The loss of serendipity in psychopharmacology. JAMA. 2008;299:1063–1065. doi: 10.1001/jama.299.9.1063. [DOI] [PubMed] [Google Scholar]
- Kokkinou M, Ashok AH, Howes OD. The effects of ketamine on dopaminergic function: meta-analysis and review of the implications for neuropsychiatric disorders. Mol Psychiatry. 2018;23:59–69. doi: 10.1038/mp.2017.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Abdallah CG, Averill LA, Kelmendi B, Harpaz-Rotem I, Sanacora G, Southwick SM, Duman RS. Synaptic Loss and the Pathophysiology of PTSD: Implications for Ketamine as a Prototype Novel Therapeutic. Curr Psychiatry Rep. 2017;19:74. doi: 10.1007/s11920-017-0829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Abi-Saab W, Perry E, D'Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology. 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Mathew SJ, D'Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010;24:669–693. doi: 10.2165/11533230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(Suppl 1):S71–80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Koob GF. Neuroclinical Framework for the Role of Stress in Addiction. Chronic Stress. 2017;1:2470547017698140. doi: 10.1177/2470547017698140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, Shin YW, Kim CW, Kim YI, Youn T, Han MH, Chang KH, Kim JJ. Similarity and disparity of obsessive-compulsive disorder and schizophrenia in MR volumetric abnormalities of the hippocampus-amygdala complex. J Neurol Neurosurg Psychiatry. 2003;74:962–964. doi: 10.1136/jnnp.74.7.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76:970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242–252. doi: 10.1016/j.neuropharm.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, He X, Zhang Y, Qi X, Li H, Zhu X, He S. Brain-derived neurotrophic factor signalling mediates antidepressant effects of lamotrigine. Int J Neuropsychopharmacol. 2011;14:1091–1098. doi: 10.1017/S1461145710001082. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Han Y, Xu LZ, Yuan K, Zhang RX, Sun CY, Xu DF, Yuan M, Deng JH, Meng SQ, Gao XJ, Wen Q, Liu LJ, Zhu WL, Xue YX, Zhao M, Shi J, Lu L. Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, Burgdorf J, Moskal J, Taylor J, Aghajanian G, Duman RS. GLYX-13 Produces Rapid Antidepressant Responses with Key Synaptic and Behavioral Effects Distinct from Ketamine. Neuropsychopharmacology. 2017;42:1231–1242. doi: 10.1038/npp.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Henter ID, Zarate CA., Jr New targets for rapid antidepressant action. Progress in neurobiology. 2017;152:21–37. doi: 10.1016/j.pneurobio.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Milad MR. Post-Traumatic Stress Disorder: The Relationship Between the Fear Response and Chronic Stress. Chronic Stress. 2017;1:2470547017713297. doi: 10.1177/2470547017713297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Gueorguieva R, Brandt C, Fava M, Sanacora G. A Randomized, Double-Blind, Placebo-Controlled, Sequential Parallel Comparison Design Trial of Adjunctive Riluzole for Treatment-Resistant Major Depressive Disorder. Neuropsychopharmacology. 2017;42:2567–2574. doi: 10.1038/npp.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matosin N, Cruceanu C, Binder EB. Preclinical and Clinical Evidence of DNA Methylation Changes in Response to Trauma and Chronic Stress. Chronic Stress. 2017;1:2470547017710764. doi: 10.1177/2470547017710764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45:693–704. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- Melo A, Kokras N, Dalla C, Ferreira C, Ventura-Silva AP, Sousa N, Pego JM. The positive effect on ketamine as a priming adjuvant in antidepressant treatment. Transl Psychiatry. 2015;5:e573. doi: 10.1038/tp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Proper CJ, Mulhern ST, Parter AL, Kegeles LS, Ogden RT, Mao X, Rodriguez CI, Oquendo MA, Suckow RF, Cooper TB, Keilp JG, Shungu DC, Mann JJ. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol Psychiatry. 2016;21:320–327. doi: 10.1038/mp.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology. 2016;100:17–26. doi: 10.1016/j.neuropharm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Sanghvi M, Dossou KS, Ramamoorthy A, Green C, Bupp J, Swezey R, O'Loughlin K, Wainer IW. The distribution and clearance of (2S,6S)-hydroxynorketamine, an active ketamine metabolite, in Wistar rats. Pharmacol Res Perspect. 2015;3:e00157. doi: 10.1002/prp2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV Independent Scientific Committee on D. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103–109. doi: 10.1016/j.neuroscience.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Abdallah CG, Anticevic A, Collins KA, Geha P, Averill LA, Schwartz J, DeWilde KE, Averill C, Jia-Wei Yang G, Wong E, Tang CY, Krystal JH, Iosifescu DV, Charney DS. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum Brain Mapp. 2016;37:3214–3223. doi: 10.1002/hbm.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov. 2017;16:472–486. doi: 10.1038/nrd.2017.16. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppers IM, Niesters M, Aarts LP, Bauer MC, Drewes AM, Dahan A, Sarton EY. Drug-induced liver injury following a repeated course of ketamine treatment for chronic pain in CRPS type 1 patients: a report of 3 cases. Pain. 2011;152:2173–2178. doi: 10.1016/j.pain.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Patriquin MA, Mathew SJ. The Neurobiological Mechanisms of Generalized Anxiety Disorder and Chronic Stress. Chronic Stress. 2017;1:2470547017703993. doi: 10.1177/2470547017703993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Paul RK, Singh NS, Khadeer M, Moaddel R, Sanghvi M, Green CE, O'Loughlin K, Torjman MC, Bernier M, Wainer IW. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology. 2014;121:149–159. doi: 10.1097/ALN.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, Landry DW, Brachman RA, Denny CA, Gardier AM. Common Neurotransmission Recruited in (R,S)-Ketamine and (2R,6R)-Hydroxynorketamine-Induced Sustained Antidepressant-like Effects. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Averill LA, Abdallah CG, Neumeister A, Krystal JH, Levy I, Harpaz-Rotem I. Amygdala-hippocampal volume and the phenotypic heterogeneity of posttraumatic stress disorder: a cross-sectional study. JAMA Psychiatry. 2015;72:396–398. doi: 10.1001/jamapsychiatry.2014.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot A, Sheth C, Legarreta M, Renshaw PF, McGlade E, Yurgelun-Todd D. Altered Cortical Gamma-Amino Butyric Acid in Female Veterans With Suicidal Behavior: Sex Differences and Clinical Correlates. Chronic Stress. 2018;2:2470547018768771. doi: 10.1177/2470547018768771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM, Group GCS. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21:140–149. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Reus GZ, Abelaira HM, dos Santos MA, Carlessi AS, Tomaz DB, Neotti MV, Liranco JL, Gubert C, Barth M, Kapczinski F, Quevedo J. Ketamine and imipramine in the nucleus accumbens regulate histone deacetylation induced by maternal deprivation and are critical for associated behaviors. Behav Brain Res. 2013;256:451–456. doi: 10.1016/j.bbr.2013.08.041. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, Flood P, Simpson HB. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38:2475–2483. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Banasr M. From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry. 2013;73:1172–1179. doi: 10.1016/j.biopsych.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB American Psychiatric Association Council of Research Task Force on Novel B & Treatments. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry. 2017;74:399–405. doi: 10.1001/jamapsychiatry.2017.0080. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, Burke MA, Zajecka JM, Barra L, Su HL, Posener JA, Bui KH, Quirk MC, Piser TM, Mathew SJ, Pathak S. Adjunctive Lanicemine (AZD6765) in Patients with Major Depressive Disorder and History of Inadequate Response to Antidepressants: A Randomized, Placebo-Controlled Study. Neuropsychopharmacology. 2017;42:844–853. doi: 10.1038/npp.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, Quirk MC. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Holmes SE, DellaGioia N, Schleifer C, Matuskey D, Abdallah CG, Hampson M, Krystal JH, Anticevic A, Esterlis I. Multimodal Investigation of Network Level Effects Using Intrinsic Functional Connectivity, Anatomical Covariance, and Structure-to-Function Correlations in Unmedicated Major Depressive Disorder. Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Wu HQ, Zanos P, Gould T. Endogenous Kynurenic Acid Mediates Ketamine- and (2R,6R)-Hydroxynorketamine-Induced Increases in Extracellular Glutamate and Antidepressant Actions. Neuropsychopharmacology. 2017;43:S111. [Google Scholar]
- Shahani R, Streutker C, Dickson B, Stewart RJ. Ketamine-associated ulcerative cystitis: a new clinical entity. Urology. 2007;69:810–812. doi: 10.1016/j.urology.2007.01.038. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth C, McGlade E, Yurgelun-Todd D. Chronic Stress in Adolescents and Its Neurobiological and Psychopathological Consequences: An RDoC Perspective. Chronic Stress. 2017;1:2470547017715645. doi: 10.1177/2470547017715645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L. Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study. Biol Psychiatry. 2016;80:424–431. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am J Psychiatry. 2016;173:816–826. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- Solmi M, Veronese N, Zaninotto L, van der Loos ML, Gao K, Schaffer A, Reis C, Normann C, Anghelescu IG, Correll CU. Lamotrigine compared to placebo and other agents with antidepressant activity in patients with unipolar and bipolar depression: a comprehensive meta-analysis of efficacy and safety outcomes in short-term trials. CNS Spectr. 2016;21:403–418. doi: 10.1017/S1092852916000523. [DOI] [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–665. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SA, Nemeroff CB. Early Life Stress, Mood, and Anxiety Disorders. Chronic Stress. 2017;1:2470547017694461. doi: 10.1177/2470547017694461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Kukral D, Li YW, Fronheiser M, Malone H, Pena A, Pieschl R, Sidik K, Tobon G, Chow PL, Bristow LJ, Hayes W, Luo F. Mapping the central effects of (+/−)-ketamine and traxoprodil using pharmacological magnetic resonance imaging in awake rats. J Psychopharmacol. 2018 doi: 10.1177/0269881117746901. 269881117746901. [DOI] [PubMed] [Google Scholar]
- Taylor JH, Landeros-Weisenberger A, Coughlin C, Mulqueen J, Johnson JA, Gabriel D, Reed MO, Jakubovski E, Bloch MH. Ketamine for Social Anxiety Disorder: A Randomized, Placebo-Controlled Crossover Trial. Neuropsychopharmacology. 2018;43:325–333. doi: 10.1038/npp.2017.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, Sanacora G, Eid T, Aghajanian G, Duman RS. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74:742–749. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 1997;7:25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, Angst J. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG) European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 1997;7:9–24. doi: 10.1016/s0924-977x(96)00039-9. [DOI] [PubMed] [Google Scholar]
- Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, Burnham VL, Mazei-Robison MS, Ferguson D, Golden SA, Koo JW, Chaudhury D, Christoffel DJ, Pomeranz L, Friedman JM, Russo SJ, Nestler EJ, Han MH. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dai Z, Peng H, Tan L, Ding Y, He Z, Zhang Y, Xia M, Li Z, Li W, Cai Y, Lu S, Liao M, Zhang L, Wu W, He Y, Li L. Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum Brain Mapp. 2014;35:1154–1166. doi: 10.1002/hbm.22241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, Parise EM, Bolanos-Guzman CA. Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Dev Neurosci. 2014;36:250–260. doi: 10.1159/000362875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA, Jr, Sanacora G. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am J Psychiatry. 2018;175:150–158. doi: 10.1176/appi.ajp.2017.17040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, Duman RS. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. The Journal of clinical investigation. 2016;126:2482–2494. doi: 10.1172/JCI85033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wook Koo J, Labonte B, Engmann O, Calipari ES, Juarez B, Lorsch Z, Walsh JJ, Friedman AK, Yorgason JT, Han MH, Nestler EJ. Essential Role of Mesolimbic Brain-Derived Neurotrophic Factor in Chronic Social Stress-Induced Depressive Behaviors. Biol Psychiatry. 2016;80:469–478. doi: 10.1016/j.biopsych.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrocklage KM, Averill LA, Cobb Scott J, Averill CL, Schweinsburg B, Trejo M, Roy A, Weisser V, Kelly C, Martini B, Harpaz-Rotem I, Southwick SM, Krystal JH, Abdallah CG. Cortical thickness reduction in combat exposed U.S. veterans with and without PTSD. Eur Neuropsychopharmacol. 2017;27:515–525. doi: 10.1016/j.euroneuro.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K. Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.239. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Molecular Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Castren E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci U S A. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Jr, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]