Abstract
Pathological erythropoiesis with consequent anemia is a leading cause of symptomatic morbidity in internal medicine. The etiologies of anemia are complex and include reactive as well as neoplastic conditions. Clonal expansion of erythroid cells in the bone marrow may result in peripheral erythrocytosis and polycythemia but can also result in anemia when clonal cells are dysplastic and have a maturation arrest that leads to apoptosis and hinders migration, a constellation typically seen in the myelodysplastic syndromes. Rarely, clonal expansion of immature erythroid blasts results in a clinical picture resembling erythroid leukemia. Although several mechanisms underlying normal and abnormal erythropoiesis and the pathogenesis of related disorders have been deciphered in recent years, little is known about specific markers and targets through which prognosis and therapy could be improved in anemic or polycythemic patients. In order to discuss new markers, targets and novel therapeutic approaches in erythroid disorders and the related pathologies, a workshop was organized in Vienna in April 2017. The outcomes of this workshop are summarized in this review, which includes a discussion of new diagnostic and prognostic markers, the updated WHO classification, and an overview of new drugs used to stimulate or to interfere with erythropoiesis in various neoplastic and reactive conditions. The use and usefulness of established and novel erythropoiesis-stimulating agents for various indications, including myelodysplastic syndromes and other neoplasms, are also discussed.
Introduction
Erythropoiesis is one of the important physiological supply functions of the bone marrow. In healthy adults, about 200×109 red cells are produced per day in the bone marrow and are released into the peripheral blood.1 Depending on demand, red cell production can be adjusted and upregulated substantially. A complex network of oxygen sensors, cytokines, such as erythropoietin, and other factors, including regulators of iron metabolism, are involved in the control of steady-state and stress-induced erythropoiesis, thereby ensuring appropriate oxygen supply to the peripheral tissues.2–5 This regulatory network can adjust itself to physiological requirements such as the oxygen concentration (altitude) or pregnancy as well as to pathological conditions such as blood loss.2–5 However, in some pathological conditions this regulatory network is overwhelmed or is not functional, resulting in polycythemia or anemia.
In the elderly, the bone marrow and other organs undergo aging. As a result, erythropoietin synthesis and red cell production may decline.6–9 However, even in very old individuals, red cell production and erythropoietin synthesis are usually adequate to keep hemoglobin levels within a reasonable range unless certain co-morbidities that lead to insufficient production of red cells have been acquired.6–9 Therefore, such other etiologies must be ruled out when hemoglobin levels drop in older individuals.7–9
Anemia is a major cause of symptomatic morbidity in daily medical practice. The etiologies contributing to anemic states are complex.6–14 Underlying disorders include trauma or coagulopathies with consequent bleeding, immunological and other inflammatory reactions as well as clonal (neoplastic) conditions. Clonal expansion of erythroid progenitor cells in the bone marrow may result in central and peripheral expansion of erythropoiesis, and thus in the clinical picture of polycythemia vera (PV), but it may also result in clonal anemic states such as the myelodysplastic syndromes (MDS) or even erythroid leukemia, characterized by a major or complete block of differentiation in early erythropoiesis.15–18 Anemic MDS are characterized by red cell dysplasia, ineffective erythropoiesis, apoptosis of late erythroid precursor cells, and the paradoxical combination of erythroid bone marrow hyperplasia and peripheral anemia.
Although several mechanisms underlying normal and pathological red cell production in the bone marrow have been identified in recent years, little is known about disease-related markers and targets through which prognostication and therapy may be improved in anemic or polycythemic patients. In order to discuss novel markers, targets and mechanisms as well as new therapeutic approaches and strategies in various erythroid disorders, a workshop was organized in Vienna in April 2017 (April 28–29). The discussion in this meeting focused on pathological (neoplastic) erythropoiesis in adults. The outcomes of this workshop are summarized in this review.
Erythropoiesis
Molecular mechanisms controlling erythropoiesis in health and disease
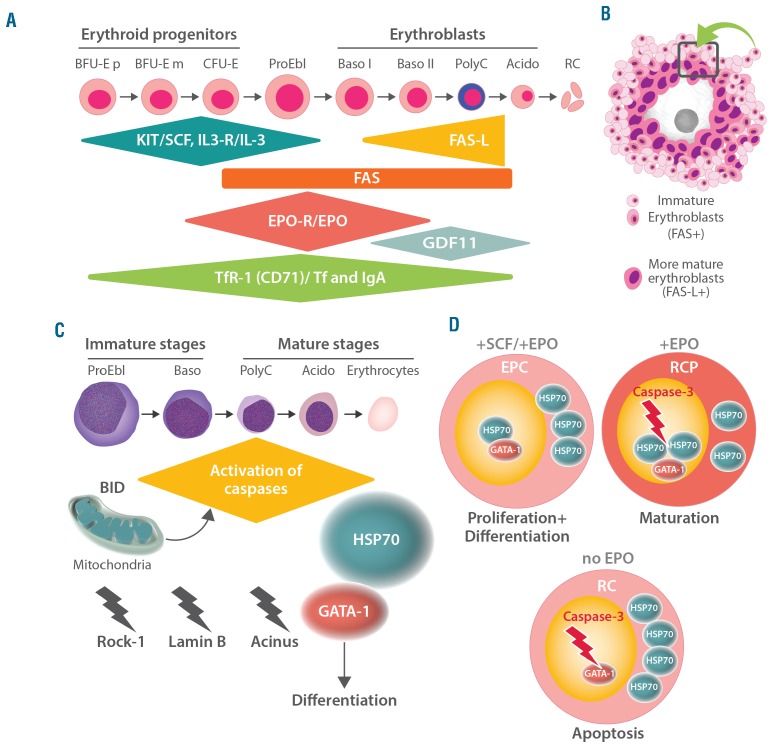
A network of interconnected physiological communication networks and pathways are responsible for the pro duction, distribution and turnover of red blood cells in healthy individuals (Figure 1).2–5,19–26 These networks keep the hemoglobin concentrations at a remarkably stable level throughout lifetime. Erythropoiesis starts in the bone marrow with lineage commitment of pluripotent myeloid progenitor cells and differentiation of these cells into immature erythroid progenitors that retain a certain proliferative capacity. Subsequently, these progenitor cells undergo further differentiation and maturation. A complex network of transcription factors and epigenetic regulators orchestrates this process.22–31 GATA-1 is the main regulator of lineage commitment, differentiation and survival of erythroid progenitors (Figure 1).20,22,27–30 In particular, GATA-1 triggers erythropoiesis by regulating the transcription of several erythroid differentiation-related genes, including genes involved in heme and/or globin synthesis, glycophorins, anti-apoptotic genes of the BH-3 family, genes involved in cell cycle regulation, and the gene for the erythropoietin receptor (EPOR).20,22,28 Major molecular players in these networks include, among others, classical hormones (thyroid hormones, androgens, corticosteroids, activin/inhibin and others), vitamins (e.g. vitamin B12 and folic acid), iron, regulators of iron metabolism such as the transferrin receptors-1 and -2, and early acting hematopoietic growth factors such as stem cell factor and interleukin-3 (Figure 1A).20–33
Figure 1.
Major pathways and molecules involved in the regulation of erythropoiesis. (A) Development of erythropoietic progenitor cells and erythroblasts. Early stages of erythropoietic development include primitive (p) and more mature (m) burst-forming unit erythroid cells (BFU-E) and colony-forming unit erythroid cells (CFU-E). The differentiation and maturation of these cells are regulated by broadly acting hematopoietic cytokines, including stem cell factor (SCF) and interleukin-3 (IL-3) and their receptors (R), the SCF receptor KIT and IL-3-R. Later stages are primarily regulated by erythropoietin (EPO) and EPO-R and are dependent on iron-metabolism and the interaction between the death receptor FAS and its ligand (FAS-L). Transferrin (Tf) and Tf-receptor-1 (TfR-1) are additional major regulators of erythropoiesis. Moreover, growth differentiating factor 11 (GDF11) and polymeric immunoglobulin A (IgA) are considered to be involved in the regulation of certain stages of erythropoiesis. Later stages of erythropoiesis include proerythroblasts (ProEbl), basophilic (Baso) erythroblasts (type I and II), polychromatic (PolyC) erythroblasts and acidophilic (Acido) erythroblasts, also called late erythroblasts (Barbara Bain, personal communication to MCB). FAS-L and GDF11 are involved in the final maturation stage that leads to the generation of red cells (RC). (B) Erythroid blood island: macrophage surrounded by immature erythroblasts expressing FAS and more mature erythroblasts expressing FAS-L. The cell unit (island) acts in concert to promote erythroid differentiation and red cell production and maturation (rectangle). (C) Caspase activation during terminal erythroid differentiation: BID-dependent activation of caspase occurs in mitochondria. Various caspase targets are affected, including Rock-1, Lamin B and Acinus. However, GATA-1 is protected from caspase cleavage by heat shock protein 70 (HSP70). (D) Model of terminal erythroid differentiation and apoptosis regulated by the nuclear localization of HSP70. SCF and EPO trigger the proliferation and differentiation of erythroid progenitor cells (EPC). In RC precursors (CFU-E through erythroblasts) EPO induces maturation as HSP70 translocates into the nucleus to protect GATA-1 from caspase-induced degradation. In the absence of EPO, caspase-3 induces the cleavage of GATA-1 as HSP70 cannot translocate to the nucleus, and, as a result, apoptosis occurs.
The main cytokine regulator of red cell production is erythropoietin, which acts at the level of late erythroid progenitors through a homodimeric receptor that triggers JAK2 kinase activity and subsequently STAT5 activation (Online Supplementary Figure S1). Erythropoietin acts mainly on myeloid precursor cells to ensure survival, thereby allowing the erythroid differentiation program, induced mainly by GATA-1, to occur (Figure 1A).2–5 It has been suggested that erythroid precursors in the bone marrow exhibit differential sensitivity against erythropoietin. The less sensitive cells undergo apoptosis upon caspase activation when the erythropoietin level is low, whereas at higher erythropoietin levels, most cells will survive and differentiate (Figure 1). In addition, in the bone marrow, within erythroid blood islands, late erythroid precursor cells express FAS ligand which may interact with early erythroid precursors that express FAS, resulting in caspase activation, which in turn triggers apoptosis and maturation arrest (Figure 1). In the case of a substantial need to produce more erythroid cells (e.g. after blood loss by bleeding or hemolysis), erythropoietin counteracts this activation allowing the cells to survive and differentiate, even if late erythroid precursors are abundant. In erythropoiesis, caspases, upon activation, cleave not only their main natural targets in the nucleus, but also GATA-1, which amplifies cell death and blocks erythroid differentiation (Figure 1).30 Erythropoietin synthesis is regulated in peritubular cells of the kidney by the transcription factor hypoxia-inducible factor-α, the main regulator of transcription of the gene encoding for erythropoietin (EPO). The stability of hypoxia-inducible factor-α depends on the prolyl-hydroxylase enzyme and the concentration of oxygen.32 To ensure a high level of proliferation and hemoglobin synthesis, iron absorption and the availability of iron for erythroid cells are tightly regulated by a number of modulators of iron metabolism and iron uptake. The latter include transferrin and its receptors (TfR-1 and TfR-2), as well as hepcidin and its target ferroportin, which allow iron export from various cell types, including macrophages and enterocytes, and thereby make iron available to erythroid progenitor cells.33 Erythroferone, which is synthesized by early and late erythroid progenitor cells, has recently been described as a major regulator of hepcidin synthesis in the liver.34 In addition to its role in iron uptake, transferrin receptor-1 is also able to trigger the MAP kinase and phosphoinositide 3 kinase/AKT pathways induced by erythropoietin and may thus participate in the regulation of erythropoiesis.35
In the model of regulation of red cell production by the modulation of erythropoietin sensitivity and rate of apoptosis of erythroid progenitor and precursor cells, caspases are considered to be key enzymes (Figure 1).27–31 To add to the complexity of the system, it has been shown that caspases are also activated during erythroid differentiation and that this activation is absolutely necessary to prepare the cells for enucleation and thus formation of mature red cells (Figure 1C,D). In this process, GATA-1 is not cleaved by caspases, ensuring that erythroid maturation is preserved. To explain this apparent paradox it has been shown that the chaperone heat shock protein 70 (HSP70) plays an essential role in protecting GATA-1 from caspase cleavage (Figure 1C,D).25–31 Indeed, during erythroid differentiation HSP70 enters the nucleus at the time of caspase activation, and, at this level, interacts with GATA-1 to protect it against cleavage. By contrast, during apoptosis induced by erythropoietin deprivation, HSP70 is exported from the nucleus and allows GATA-1 cleavage in erythroid progenitor cells (Figure 1D).25–31 This model, in which the fate of erythroid precursors is determined by the localization of HSP70 in the nucleus, has been shown to be altered in ineffective erythropoiesis in various anemic conditions such as b-thalassemia, MDS,36 and congenital erythroblastopenia,37 and may provide a new conceptual basis to improve anemia in these diseases.31
Apart from these established regulators of erythropoiesis, several novel molecular players regulating erythropoiesis were discussed in the workshop. Among these are members of the BCL-2 family such as BID which may play a critical role in the regulation of mitochondrial depolarization and caspase activation during erythroid differentiation and apoptosis (Figure 1C), various transcription factors, including STAT5A and STAT5B (Table 1), cytokines such as growth differentiating factor 11 (GDF11), and regulators of iron uptake and iron metabolism.33–48 GDF11, a ligand of activin receptor IIA (ActRIIA) that accumulates in erythroblasts in patients with b-thalassemia, has been implicated in the pathogenesis of anemia in these patients.42
Table 1.
Selected regulators of erythropoiesis.
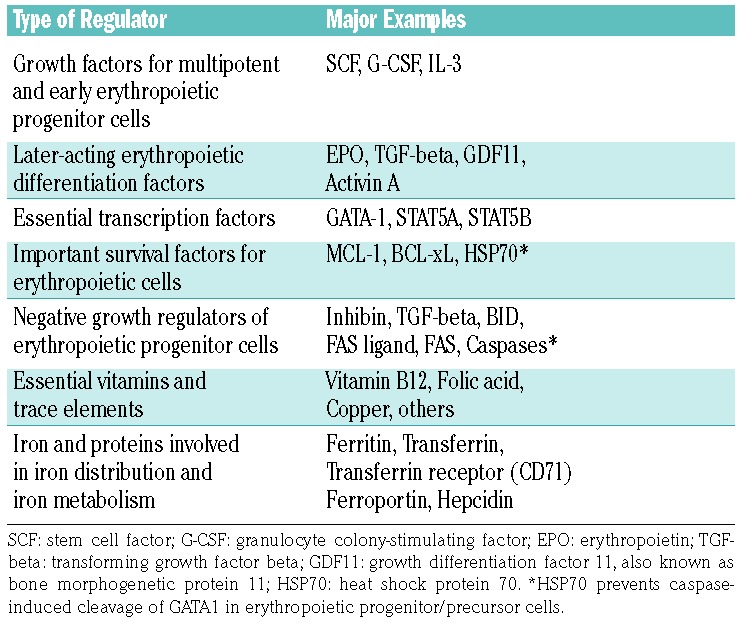
It has also been described that polymeric immunoglobulin A (IgA) produced in the bone marrow may bind to the transferrin receptor-1 to sensitize erythroid cells to erythropoietin (Figure 1A).35 Consistent with this role as a regulator of erythropoiesis, the synthesis of polymeric IgA is increased during hypoxia. In pathological conditions, patients with IgA deficiency have higher levels of erythropoietin and, conversely, patients with unexplained polycythemia associated with an excess of polymeric IgA synthesis have recently been reported.35 Finally, a number of regulators of red cell membrane stability have recently been identified. One of these regulators may be CDK6, a cell cycle regulator that has recently been shown to serve as a membrane stabilizer of red cells in mice.49
Diagnostic evaluation of erythropoiesis: established and novel markers
In most anemic patients the underlying etiology can be identified rapidly through the information gained from a thorough case history and detailed laboratory investigations. A number of different etiologies can underlie anemia, including iron deficiency, chronic inflammation, hemolysis, renal disorders or vitamin B12 deficiency. In each case, it is important to follow the principle diagnostic algorithms, to establish the correct diagnosis and to treat the underlying disorder (for example a gastrointestinal disease). In most of these cases, investigation of the bone marrow is not required. In other patients, however, the etiology remains uncertain after initial studies, so that detailed investigations of the bone marrow have to be performed. These investigations include a thorough cytological, histological and immunohistochemical examination of the bone marrow, multi-parameter flow cytometry studies, a detailed examination of chromosomes (metaphases by conventional karyotyping and interphases by fluorescence in situ hybridization) and molecular studies.9–11,50–52 Depending on blood counts and other parameters, fluorescence in situ hybridization studies are applied to screen for the presence of MDS-related abnormalities. Our faculty recommends that in each case morphological and immunophenotypic studies, including immunohistochemistry and flow cytometry, should be employed to define the percentage and stage of maturation of erythroid cells in bone marrow samples.52
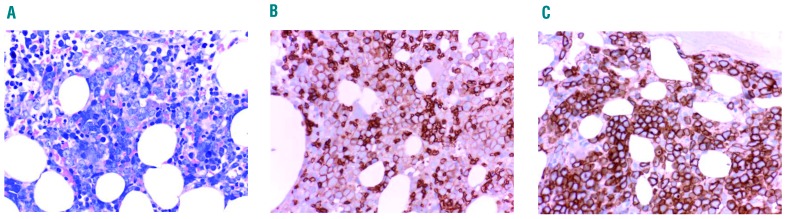
For routine diagnostics, recommended immunohistochemical markers are glycophorin A, CD71 and E-cadherin (Table 2). Additional immunohistochemical markers include glycophorin C and hemoglobin A. E-cadherin is of special value for the detection of immature erythropoietic progenitor cells in patients with MDS and those with erythroleukemia (Figure 2). In contrast, hemoglobin A is preferentially expressed in more mature erythroblasts, and may therefore be helpful in distinguishing between more mature and more immature cells.
Table 2.
Markers used to identify and quantify erythropoietic cells in the bone marrow.
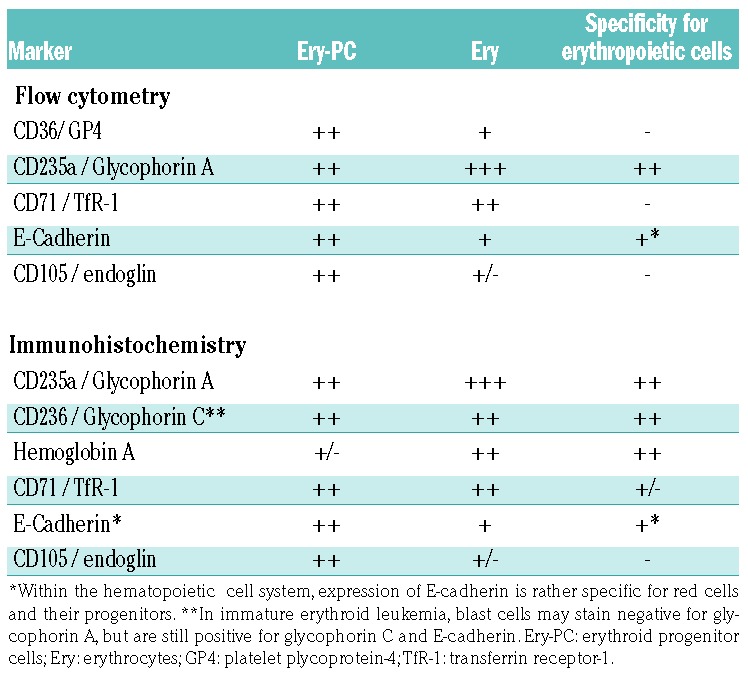
Figure 2.
Immunophenotypic visualization of leukemic erythroblasts. Bone marrow sections of a patient with erythroid leukemia (acute erythroleukemia) stained with (A) Wright-Giemsa solution and antibodies against (B) glycophorin A and (C) E-cadherin. Note that virtually all leukemic erythroblasts co-express abundant amounts of glycophorin A and E-cadherin, thereby confirming the immature stage of maturation of leukemic (erythroid) cells.
In flow cytometry studies, recommended markers are glycophorin A, CD71, and CD105 (Table 2).53–56 Among these, CD105 is of special value for the detection of immature erythropoietic progenitors in the bone marrow of patients with MDS. E-cadherin is also expressed specifically on the surface of erythropoietic progenitor cells in human bone marrow.57 However, E-cadherin is not used routinely as an erythroid marker in flow cytometry studies. Our faculty is of the opinion that CD105 and E-cadherin should be validated further as routine diagnostic markers in erythroid disorders of the bone marrow. Another interesting aspect is that several cell surface antigens appear to be downregulated on erythroid progenitor cells in MDS patients.58 Among these antigens, the coxsackie-adenovirus receptor (CAR) is a rather specific antigen: in fact, a decrease or lack of this receptor on erythroid progenitor cells is typically seen in patients with MDS and in other bone marrow neoplasms accompanied by marked dysplasia.58 Immunophenotypic studies are also of great importance to identify immature stages of erythropoiesis in patients with acute (myeloid/erythroid) leukemia in whom morphological and molecular studies alone are insufficient to establish a correct diagnosis (Figure 2 and Online Supplementary Figure S2). In these cases the use of immunohistochemical studies and multiparameter flow cytometry is essential.
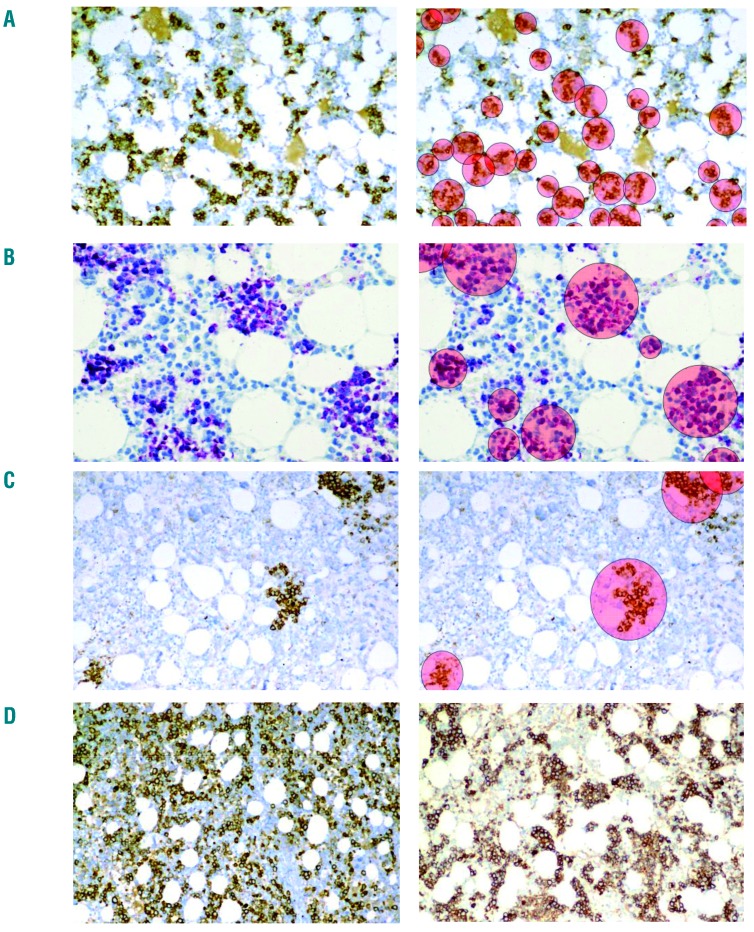
Another important aspect is that the physiological development and maturation of red cells in the bone marrow is regulated by the supporting microenvironment (consisting of macrophages and stromal cells) in so-called erythroid islands.59 These islands, also known as erythroblastic islands, are considered to represent functional units and are detectable by conventional stains and specific immunological stains (see above) on bone marrow biopsies. With age, the number of erythroid islands in the bone marrow decreases, while their size increases, which may suggest that the decrease in stem cell numbers and erythroid progenitors during aging might be compensated by an increased proliferation of local erythroid progenitors (Figure 3).60 In patients with MDS, impaired formation of erythroid islands as well as structural abnormalities within these islands have also been described (Figure 3).60 In low-risk MDS, abnormal formation of erythroid islands may be the only histopathological change found in the bone marrow. Moreover, it has been described that erythroid island density correlates inversely with overall survival in patients with MDS.60 However, alterations of island con figuration and size (e.g. increased size) can also be detected when an increase in red cell production is required in pathological conditions, such as in hemolytic anemia or acute blood loss. In patients with aplastic anemia and other bone marrow failure syndromes, only a few or no erythroid islands are found in pathological examinations, which we consider to be relevant diagnostically.
Figure 3.
Structure and size of erythroid islands in the bone marrow. (A) Erythroid islands in the bone marrow of a 16-year old healthy male visualized by staining for CD71. (B) Erythroid islands in the bone marrow of an 82-year old female without bone marrow neoplasm. Erythroid islands were visualized by staining against hemoglobin A. Note the decreased number and increased size of erythroid islands in the bone marrow of the older healthy control. (C) Erythroid islands in the bone marrow of a 73-year old male patient with myelodysplastic syndrome with excess of blasts (5–9% of marrow cells, MDS-EB-1) visualized by staining for CD71. In the right images of 3A, 3B and 3C, erythroid islands are evidenced by pink circles. Note the decreased number of erythroid islands in this patient. (D, left panel) Bone marrow section of a 78-year old male with myelodysplastic syndrome with excess of blasts (10–19% of marrow cells, MDS-EB-2) stained for CD71. In this patient, numerous confluent, partially disrupted and poorly separable erythroblastic islands are seen. (D, right panel) Bone marrow section of an adult patient with hemolytic anemia. Note that erythroid islands are increased, but are clearly separable and have a regular shape (contrasting with MDS). Original magnifications: A, C, D: × 125; B: × 250.
There are a number of established peripheral parameters through which red cell production can be assessed quantitatively. The most widely applied approach is to measure the numbers of reticulocytes in the peripheral blood. Another approach is to quantify the numbers of circulating erythroid progenitor cells, including multi-lineage colony-forming progenitors (CFU-GEMM) and burst-forming units (BFU-E). With this approach, clonal cytopenias (MDS) and aplastic anemia (in both conditions, CFU-GEMM and BFU-E are markedly reduced or absent) are separable from ‘reactive’ and ‘deficiency’ anemias in which BFU-E and CFU-GEMM are usually within normal ranges.61–63 Even in MDS patients with 5q-, who may develop thrombocytosis, erythropoiesis and BFU-E growth are usually suppressed. Factor (erythropoietin)-independent and erythropoietin-hyperresponsive growth of BFU-E is indicative of (almost diagnostic for) the presence of PV or a related myeloproliferative neoplasm (MPN).64,65 However, the colony-assay is rather tricky and its success depends on the experience of the team performing the test. Thus, although this assay is of practical value (and was previously used as a criterion for PV), it is nowadays only employed routinely in a few specialized centers.
Anemia
Classification of anemia: novel aspects and etiologies
In general, anemia can be classified based on the regenerative capacity of the bone marrow (hypo- or hyper-regenerative), the red cell volume (micro-, normo- or macrocytic), the etiology (bleeding, deficiency-induced, hemolytic, renal, inflammation-related, neoplastic, aplastic, others) and the dynamics with which anemia develops (acute, chronic).9–11 For each specific form of anemia, an extensive amount of published data has accumulated during the past few decades. A detailed description is beyond the scope of this article. There are also special variants of anemia that develop typically in the context of certain physiological conditions such as pregnancy or advanced age.8,9,66,67 In these cases, the primary question is whether anemia is indicative of an undetected pathology and whether and when (at what thresholds) anemia can indeed be diagnosed. For example, in pregnant women, a hemoglobin level of 11.0 g/dL is still considered to be within the normal range by the World Health Organization (WHO). In elderly individuals, however, any decrease of hemoglobin below ‘normal’ is considered an anemia.8,9 On the other hand, there is an ongoing discussion about the definition of anemia in the elderly and related hemoglobin thresholds.9,68–70 When no evidence of an underlying condition is found after a thorough investigation of all relevant organs and potential etiologies, the condition is termed cytopenia (anemia) of unknown significance (ICUS-A).50,52 Thus, the diagnosis of ICUS-A implies that a detailed investigation of the bone marrow, with histological, morphological (cytological), immunophenotypic, cytogenetic and molecular studies, was performed without conclusive evidence of the presence of MDS or any other underlying bone marrow neoplasm.50,52 In this regard it is worth noting that next-generation sequencing may sometimes reveal more or less specific mutations which may lead to re-classification of ICUS-A cases into either clonal cytopenia of undetermined significance or low-risk MDS, respectively.50–52 Other differential diagnoses to ICUS-A include anemia of chronic disease (inflammation-associated anemia), hemodilution, renal anemia, copper deficiency and vitamin B12 deficiency.9–11 In some elderly patients with ICUS-A, inadequately low levels of erythropoietin are found even though the excretory function of the kidney is normal.71,72 In such cases, the aged kidney may be responsible for low erythropoietin production. Another equally important contributor to low red cell and erythropoietin production in elderly (otherwise healthy) people may be an age-related decrease in the production of hypophyseal and other essential hormones, resulting in a decreased supply of testosterone.73 However, unless an additional pathology (co-morbidity) is also present, these individuals have only mild anemia and are free of symptoms. As mentioned, it remains uncertain as to whether all these elderly individuals should indeed be diagnosed as having overt anemia.
Refractory anemias: the updated World Health Organization classification and novel molecular markers and targets used to grade and classify the disease
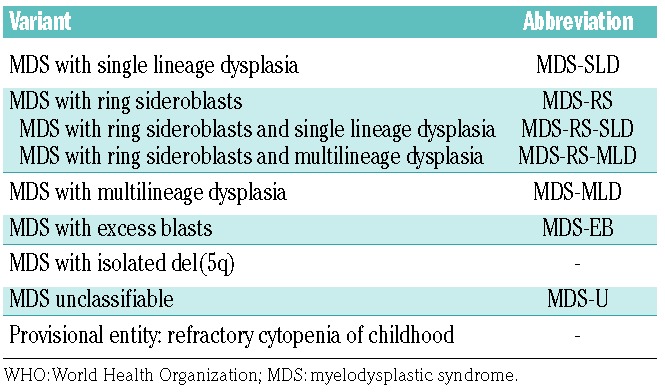
In the current WHO proposal (2017) the term refractory anemia has been removed and replaced by the term MDS with an additional explanatory appendix to define the number of lineage(s) involved with dysplasia (example: MDS with single lineage dysplasia).74–76 It is important to note that MDS with single lineage dysplasia can be separated into anemic, neutropenic and thrombocytopenic types. Table 3 shows the current WHO proposal to classify MDS. Another important aspect is that only a few cytogenetic and molecular markers are used to define the subtype of MDS. For example, the 5q- syndrome is recognized as a separate entity by the WHO (MDS with isolated 5q-) and is associated with particular sensitivity to lenalidomide. It has also been described that the SF3B1 mutation is highly indicative of the presence of ring sidero blasts.74–76 Therefore, this mutation, when present, serves as a diagnostic feature. In particular, MDS variants with ring sideroblasts can be diagnosed when ring sideroblasts account for either ≥15% of all red cells (old definition) or ≥5% in the presence of a SF3B1 mutation (new adjunct).74–76 For the morphologist, this definition implies that more red cells have to be counted in the iron stain (at least 100 red cells) to arrive at a correct diagnosis.52 This individualization might be of importance because patients with this disorder usually do not respond to erythropoietin but may benefit from specific treatment, such as a GDF11 inhibitor. Another relevant change in the current WHO classification (update 2017) relates to the definition of erythroleukemia. Details are discussed below under the section ‘Red cell neoplasms’. Based on the changed definition, a new subset of poor-risk patients with MDS, formerly diagnosed as having erythroid leukemia or M6 acute myeloid leukemia (AML) according to the French-American-British classification, will require attention.74–76 In fact, these patients often behave like patients with AML, are usually resistant to cytoreductive agents and chemotherapy, and are characterized by predominant erythropoiesis (>50% of bone marrow cells). The poor prognosis of these patients is also reflected by their poor-risk cytogenetics, often in the form of a complex karyotype. If possible, debulking with poly- chemotherapy (regimens used for AML) or hypomethylating agents should be considered, and in young and fit patients, intensive post-debulking therapy, preferably in the form of hematopoietic stem cell transplantation, (HSCT) is often recommended.77–79
Table 3.
Updated WHO classification of myelodysplastic syndromes, 2017.
Treatment of anemias: novel agents and concepts
During the workshop, standard treatments and novel therapeutic approaches for various forms of anemias were discussed. With regard to erythropoietin, standard indications remain renal anemia and anemic patients with MDS in whom a relative erythropoietin deficiency has been documented and a response to erythropoietin is seen.80–84 For other similar indications, such as anemia with low endogenous erythropoietin levels accompanying myelomas, lymphomas or chronic leukemias, treatment with erythropoiesis-stimulating agents (ESA) may also be useful.85,86 Even in some form of ICUS (elderly ICUS-A patients) or anemia of chronic disease, the use of erythropoietin may be justified.87,88 However, our faculty is of the opinion that in all these indications, ‘inadequate’ (insufficient) production of erythropoietin should be documented before starting erythropoietin therapy. Moreover, we are of the opinion that erythropoietin therapy is no longer justified for patients with solid tumors or other malignant disorders after chemotherapy (in certain malignancies tumor cells may express erythropoietin receptor) and should no longer be considered for patients with elevated endogenous erythropoietin levels (especially levels >500 U/L). However, there are other potential indications for erythropoietin and other ESA, such as trauma or certain neurological disorders.89–91 Although beneficial effects of erythropoietin for these indications have been documented, the mechanisms contributing to these beneficial effects have not been elucidated so far.
With regard to the type of erythropoietin preparation or erythropoietin-like ESA, no major differences in responses have been reported. In fact, several meta-analyses of patients with MDS and other indications have shown that short-acting and long-acting erythropoietin-based drugs all exert very similar effects.92–94 However, erythropoietin therapy should always be administered with caution, especially when hemoglobin levels rise. In fact, it has been described that hemoglobin levels above 12 g/dL can even be associated with complications and a poor outcome compared to hemoglobin levels below 12 g/dL, especially in patients with chronic kidney disease.95 The target hemoglobin level should, therefore, be around 11 g/dL independently of the ESA applied, underlying disease, or age. For some indications, the use of additional drugs, together with erythropoietin/ESA, may be useful. For example, in patients with chronic kidney disease or chronic inflammation, addition of iron may lead to a more rapid correction of anemia.96–98 In MDS, the addition of granulocyte or granulocyte-macrophage colony-stimulating factor may work and increase the rate of responding patients.83,84 In other patients, low erythropoietin synthesis may be accompanied by other insufficiencies that need to be corrected, such as a lack of vitamin B12, iron deficiency or folate deficiency.
There are also other (in part novel) emerging agents that may be useful in patients with anemias. These drugs include, among others, hypoxia-inducible factor stabilizing compounds, prolyl hydroxylase inhibitors, and activin/GDF11-trapping agents such as sotatercept or luspatercept.97–105 The latter may even work in bone marrow failure syndromes. In addition, GDF11 blockers are able to counteract anemia in patients with b-thalassemia.42,101
However, in advanced bone marrow neoplasms, such as MDS, or in aplastic anemia, the effectiveness of broadly acting growth factors, ESA and other drugs (mentioned above) is limited. In these patients, the use of disease-modifying agents, such as demethylating agents and/or chemotherapy in MDS or immunosuppressive therapy in aplastic anemia may lead to an improvement or even correction of the anemia. In low-risk MDS patients with isolated 5q-, lenalidomide therapy usually works well and often leads to transfusion independence or even to a cytogenetic response. In patients with paroxysmal nocturnal hemoglobinuria the use of complement-targeting drugs has revolutionized the treatment of anemia. Finally, several new agents are currently being tested in patients with hemolytic anemia, thalassemia, or anemia of chronic inflammation. A detailed description of these agents and of new treatment approaches is beyond the scope of this article - we refer the interested reader to the available literature.
Red cell neoplasms
Red cell neoplasms: classification and criteria
Red cell neoplasms can be classified according to their etiology (de novo or secondary, i.e. following MDS or a mutagenic event), WHO criteria and the presence or absence of certain molecular markers. Erythroid neoplasms include PV (JAK2-mutated or wild-type JAK2), MDS with a prominent erythroid compartment (previously AML M6) and (pure) erythroid leukemia (Table 4). Based on the 2017 update of the WHO classification, erythroid neoplasms have been reclassified.74,75,106 In the previous definition provided by the French-American-British group and later by the WHO, a blast cell percentage of ≥20% in the non-erythroid compartment together with erythroid predominance (≥50% of nucleated bone marrow cells) was indicative of AML (AML M6). In the 2017 update of the WHO classification, these cases are reclassified as MDS (usually MDS with excess blasts) unless the total blast cell percentage (without subtracting erythroid cells) is ≥20%.74–76 In the case of a total blast cell percentage of ≥20% and erythroid predominance, the final diagnosis is AML [AML not otherwise specified (NOS), acute erythroid leukemia of erythroid/myeloid type] unless molecular or cytogenetic markers identify another AML subtype.74–76 In those with >80% immature erythroid precursors, ≥30% proerythroblasts and a total blast cell percentage <20%, the final diagnosis is acute erythroid leukemia (AML, pure erythroid type). In an updated revision, AML NOS, acute erythroid leukemia (erythroid/myeloid type) was replaced by AML NOS (erythroid subtype).106 Overall, the erythroid leukemias appear to comprise a heterogeneous group of malignancies. For example, erythroid leukemias can occur as primary (de novo) leukemia or as a secondary form of leukemia, for instance, following MDS or MPN (Online Supplementary Table S1). In rare cases, even the blast phase of Philadelphia chromosome-positive chronic myeloid leukemia may have a clinical and pathological picture indistinguishable from that of erythroleukemia (Online Supplementary Figure S2). It is also important to note that erythroleukemia can develop as an acute and rapidly progressive disease or a more chronic form of leukemia. The acute forms of erythroid leukemias represent a diagnostic challenge and may be overlooked. Rarely, acute erythroleukemia presents as a large-cell anaplastic neoplasm mimicking a histiocytic malignancy or even small-cell carcinoma; such cases can also easily be overlooked unless appropriate phenotypic studies with antibodies against glycophorin A/C or CD71 are conducted (H-PH, unpublished observation). The chronic form of erythroleukemia must be distinguished from PV and reactive erythrocytosis (polyglobulia), for example, in cases with an erythropoietin-producing tumor.
Table 4.
Overview of red cell neoplasms.
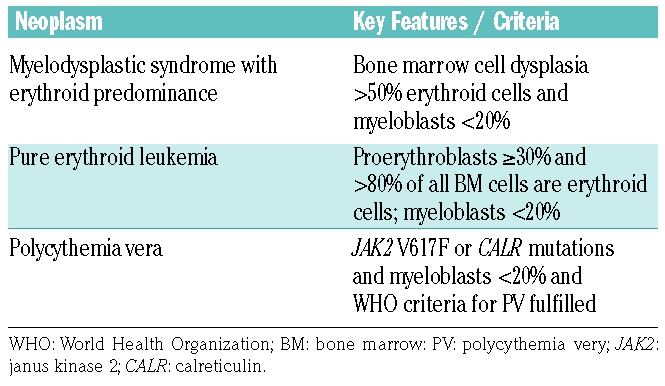
The diagnostic criteria for PV have also changed. Whereas expression of the JAK2 V617F mutation is still considered a major criterion of PV the threshold levels of hemoglobin were changed: in the 2008 WHO definition, cut-offs were 16.5 g/dL for females and 18.5 g/dL for males, while in the 2017 revision, hemoglobin cut-offs were 16.0 g/dL for females and 16.5 g/dL for males.74,75 Although rather specific for MPN and often found in PV patients, CALR mutations are not yet regarded as diagnostic criteria of MPN/PV. A summary of erythroid neoplasms is provided in Table 4.
Molecular mechanisms regulating red cell neoplasms
In many instances, the molecular mechanisms underlying red cell expansion in MPN or erythroid leukemias remain unknown. In the classical MPN, including PV, the JAK2 point mutation V617F and CALR mutations are considered to act as major disease drivers. One critical aspect is that these mutant forms initiate complex networks of signaling cascades that drive the affected cell and trigger growth factor independence. A detailed description of these networks is beyond the scope of this review. Some of the most important networks are shown in Online Supplementary Figure S1. The faculty also discussed novel preclinical models of red cell neoplasms. Based on recent molecular insights into the etiology of PV and erythroid leukemias, several mouse models have been established. In the field of PV/MPN these models are primarily based on the JAK2 mutation V617F and CALR mutations.107–110 Indeed, mice expressing a mutated and thus hyperactive Jak2 may develop a MPN-like condition over time.107–110 However, additional factors (mutations or signaling molecules) are required to convert the condition into a full- blown malignancy. These additional anomalies are indeed found in patients with MPN/PV or secondary AML and are, therefore, of clinical significance.111–116 They include mutations in TP53 and in various driver genes.112–116 Several of these changes lead to hyperactive signaling in clinically relevant pro-oncogenic signaling networks. For example, molecular changes that lead to an increased production and accumulation of tyrosine-phosphorylated STAT5 (a critical JAK2-downstream transcription factor) can transform an indolent MPN-like condition into a highly fatal disease with major thromboembolism in mice (RM and PV, unpublished observation).
In contrast to PV and other MPN, very little is known about the molecular mechanisms underlying the evolution and progression of erythroid-rich MDS and erythroid leukemia. In fact, despite a growing list of mutations associated with erythroid leukemia, their role in the initiation and/or maintenance of the erythroid phenotype remains largely unknown.115–118 Whereas earlier studies showed that particular viruses, such as the avian erythroblastosis virus and Friend spleen focus-forming virus, can induce neoplasms resembling erythroid leukemia in various animal models, no evidence of a viral etiology for the human disease has been identified so far. All in all, the faculty concluded that more research is needed to decipher molecular players and targets in these highly fatal neoplasms.
Therapy of red cell neoplasms
Despite novel treatment options, early and advanced erythroid neoplasms are still regarded as incurable malignancies. Most PV patients can be managed quite well with phlebotomy. If phlebotomy alone is not sufficient to bring erythrocyte counts under control (target hematocrit: <45%) or thromboembolic events occur, additional cytoreductive therapy is recommended. Depending on age, risk factors, and expected adverse side effects, interferon-α or hydroxyurea can be prescribed. In highly symptomatic cases, ruxolitinib may be considered. For patients whose disease transforms into secondary AML, poly-chemotherapy or, in some cases, hypomethylating agents, plus HSCT should be considered. The same holds true for patients with advanced MDS and prominent erythropoiesis (previously AML M6) and cases with full-blown erythroid leukemia (fulfilling 2017 WHO criteria).77,78 In these patients, hypomethylating agents (5- azacytidine or decitabine) or polychemotherapy (AML regimens) should be considered for debulking prior to HSCT.
Unfortunately, however, HSCT can only be offered to a restricted group of young and fit patients. In all patients, the risk of HSCT-related mortality and morbidity must be weighed against the potential benefit. The probability of long-term disease-free survival after HSCT is probably in the same range as that seen in secondary AML following MDS. In some of these patients, hypomethylating agents may exert clinically meaningful anti-neoplastic effects.79 Especially in older patients or those who cannot tolerate intensive chemotherapy, treatment with hypomethylating agents is often recommended as first-line therapy. Hypomethylating agents can also be considered for patients who are refractory to or relapse after poly-chemotherapy. When hypomethylating agents fail and the patient is fit, polychemotherapy or experimental drugs should be considered. Hydroxyurea is the palliative drug of choice in multi-resistant patients and those who are old and refuse intensive or experimental therapy.
Concluding remarks
Red cell production, survival and turnover in health and disease, and mechanisms regulating these processes, have attracted the attention of physicians and scientists in the past five decades and many different mechanisms and molecules involved in the regulation of red cell production and survival have been discovered. In addition, a number of molecular and immunological markers and targets have been identified in red cells and their progenitors. Several of these novel antigens may serve as diagnostic markers or as therapeutic targets. Resulting new diagnostic strategies and novel treatment concepts should advance the field and lead to more precise diagnosis, better therapies and improved prognosis in reactive and clonal erythroid disorders.
Supplementary Material
Acknowledgments
We would like to thank Heidi A. Neubauer, Julia Neusiedler-Nicolas, Sophia-Marie Rammler and Emir Hadzijusufovic for their excellent technical assistance. This study was supported by the Austrian Science Fonds, SFB grants F4701-B20, F4704-B20, F4705-B20 and F4706-B20.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/10/1593
References
- 1.Palis J. Primitive and definitive erythropoiesis in mammals. Front Physiol. 2014; 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hattangadi SM, Wong P, Zhang L, Flygare J, Lodish HF. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011; 118(24):6258–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhrt D, Wojchowski DM. Emerging EPO and EPO receptor regulators and signal transducers. Blood. 2015; 125(23):3536–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang R, Ghaffari S. Advances in understanding the mechanisms of erythropoiesis in homeostasis and disease. Br J Haematol. 2016; 174(5):661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oburoglu L, Romano M, Taylor N, Kinet S. Metabolic regulation of hematopoietic stem cell commitment and erythroid differentiation. Curr Opin Hematol. 2016; 23(3):198–205. [DOI] [PubMed] [Google Scholar]
- 6.Carmel R. Anemia and aging: an overview of clinical, diagnostic and biological issues. Blood Rev. 2001; 15(1):9–18. [DOI] [PubMed] [Google Scholar]
- 7.Steensma DP, Tefferi A. Anemia in the elderly: how should we define it, when does it matter, and what can be done? Mayo Clin Proc. 2007;82(8):958–966. [DOI] [PubMed] [Google Scholar]
- 8.Halawi R, Moukhadder H, Taher A. Anemia in the elderly: a consequence of aging? Expert Rev Hematol. 2017; 10(4):327–335. [DOI] [PubMed] [Google Scholar]
- 9.Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications and management. Blood. 2018;131(5):505–514. [DOI] [PubMed] [Google Scholar]
- 10.Guidi GC, Lechi Santonastaso C. Advancements in anemias related to chronic conditions. Clin Chem Lab Med. 2010; 48(9): 1217–1226. [DOI] [PubMed] [Google Scholar]
- 11.Valent P. Low blood counts: immune mediated, idiopathic, or myelodysplasia. Hematology Am Soc Hematol Educ Program. 2012; 2012:485–491. [DOI] [PubMed] [Google Scholar]
- 12.Guillaud C, Loustau V, Michel M. Hemolytic anemia in adults: main causes and diagnostic procedures. Expert Rev Hematol. 2012;5(2): 229–241. [DOI] [PubMed] [Google Scholar]
- 13.Camaschella C, Nai A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br J Haematol. 2016; 172(4):512–523. [DOI] [PubMed] [Google Scholar]
- 14.Shimamura A. Aplastic anemia and clonal evolution: germ line and somatic genetics. Hematology Am Soc Hematol Educ Program. 2016; 2016(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasserjian RP, Howard J, Wood A, Henry K, Bain B. Acute erythremic myelosis (true erythroleukaemia): a variant of AML FAB-M6. J Clin Pathol. 2001; 54(3):205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prchal JT. Polycythemia vera and other primary polycythemias. Curr Opin Hematol. 2005; 12(2):112–116. [DOI] [PubMed] [Google Scholar]
- 17.Wang SA, Hasserjian RP. Erythroid proliferations in myeloid neoplasms. Hum Pathol. 2012; 43(2):153–164. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Wang SA, Medeiros LJ, Khoury JD. Pure erythroid leukemia. Am J Hematol. 2017; 92(3):292–296. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995; 83(1):59–67. [DOI] [PubMed] [Google Scholar]
- 20.Cantor AB, Orkin SH. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene. 2002; 21(21): 3368–3376. [DOI] [PubMed] [Google Scholar]
- 21.Donovan A, Andrews NC. The molecular regulation of iron metabolism. Hematol J. 2004; 5(5):373–380. [DOI] [PubMed] [Google Scholar]
- 22.Kim SI, Bresnick EH. Transcriptional control of erythropoiesis: emerging mechanisms and principles. Oncogene. 2007;26(47):6777–6794. [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda). 2009; 24:97–106. [DOI] [PubMed] [Google Scholar]
- 24.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013; 27(1):41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermine O, Arlet JB, Ribeil JA, Guillerm F, Vandekerkhove J, Courtois G. HSP70, an erythropoiesis regulator that determines the fate of erythroblasts between death and differentiation. Transfus Clin Biol. 2013; 20(2): 144–147. [DOI] [PubMed] [Google Scholar]
- 26.Kautz L, Nemeth E. Molecular liaisons between erythropoiesis and iron metabolism. Blood. 2014;124(4):479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon MC, Pevny L, Wiles MV, Keller G, Costantini F, Orkin SH. Rescue of erythroid development in gene targeted GATA-1-mouse embryonic stem cells. Nat Genet. 1992;1(2):92–98. [DOI] [PubMed] [Google Scholar]
- 28.Simon MC. Transcription factor GATA-1 and erythroid development. Proc Soc Exp Biol Med. 1993; 202(2):115–121. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko H, Shimizu R, Yamamoto M. GATA factor switching during erythroid differentiation. Curr Opin Hematol. 2010;17(3):163–168. [DOI] [PubMed] [Google Scholar]
- 30.Ribeil JA, Zermati Y, Vandekerckhove J, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007; 445(7123):102–105. [DOI] [PubMed] [Google Scholar]
- 31.Arlet JB, Ribeil JA, Guillem F, et al. HSP70 sequestration by free b-globin promotes ineffective erythropoiesis in b-thalassaemia. Nature. 2014; 514(7521):242–246 [DOI] [PubMed] [Google Scholar]
- 32.Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. Br J Haematol. 2008; 141(3):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017; 168(3):344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coulon S, Dussiot M, Grapton D, et al. Polymeric IgA1 controls erythroblast proliferation and accelerates erythropoiesis recovery in anemia. Nat Med. 2011;17(11):1456–1465. [DOI] [PubMed] [Google Scholar]
- 36.Frisan E, Vandekerckhove J, de Thonel A, et al. Defective nuclear localization of Hsp70 is associated with dyserythropoiesis and GATA-1 cleavage in myelodysplastic syndromes. Blood. 2012; 119(6):1532–1542. [DOI] [PubMed] [Google Scholar]
- 37.Gastou M, Rio S, Dussiot M, et al. The severe phenotype of Diamond-Blackfan anemia is modulated by heat shock protein 70. Blood Adv. 2017; 1(22):1959–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maguer-Satta V, Bartholin L, Jeanpierre S, et al. Regulation of human erythropoiesis by activin A, BMP2, and BMP4, members of the TGFbeta family. Exp Cell Res. 2003; 282(2):110–120. [DOI] [PubMed] [Google Scholar]
- 39.Socolovsky M, Murrell M, Liu Y, Pop R, Porpiglia E, Levchenko A. Negative autoregulation by FAS mediates robust fetal erythropoiesis. PLoS Biol. 2007; 5(10):e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Pop R, Sadegh C, Brugnara C, Haase VH, Socolovsky M. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood. 2006; 108(1):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, Huang Z, Xue H, et al. MicroRNA miR-24 inhibits erythropoiesis by targeting activin type I receptor ALK4. Blood. 2008; 111(2):588–595. [DOI] [PubMed] [Google Scholar]
- 42.Dussiot M, Maciel TT, Fricot A, et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in b-thalassemia. Nat Med. 2014; 20(4):398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koulnis M, Porpiglia E, Hidalgo D, Socolovsky M. Erythropoiesis: from molecular pathways to system properties. Adv Exp Med Biol. 2014; 844:37–58. [DOI] [PubMed] [Google Scholar]
- 44.Vlahakos DV, Marathias KP, Madias NE. The role of the renin-angiotensin system in the regulation of erythropoiesis. Am J Kidney Dis. 2010; 56(3):558–565. [DOI] [PubMed] [Google Scholar]
- 45.Kim YC, Mungunsukh O, Day RM. Erythropoietin regulation by angiotensin II. Vitam Horm. 2017; 105:57–77. [DOI] [PubMed] [Google Scholar]
- 46.Lee PL, Beutler E. Regulation of hepcidin and iron-overload disease. Annu Rev Pathol. 2009; 4:489–515. [DOI] [PubMed] [Google Scholar]
- 47.Silva B, Faustino P. An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim Biophys Acta. 2015; 1852(7):1347–1359. [DOI] [PubMed] [Google Scholar]
- 48.Wang CY, Babitt JL. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol. 2016; 23(3):189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uras IZ, Scheicher RM, Kollmann K, et al. Cdk6 contributes to cytoskeletal stability in erythroid cells. Haematologica. 2017; 102(6):995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valent P, Horny HP, Bennett JM, et al. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: consensus statements and report from a working conference. Leuk Res. 2007; 31(6):727–736. [DOI] [PubMed] [Google Scholar]
- 51.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015; 126(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valent P, Orazi A, Steensma DP, et al. Proposed minimal diagnostic criteria for myelodysplastic syndromes (MDS) and potential pre-MDS conditions. Oncotarget 2017:8(43):73483–73500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malcovati L, Della Porta MG, Lunghi M, et al. Flow cytometry evaluation of erythroid and myeloid dysplasia in patients with myelodysplastic syndrome. Leukemia. 2005;19(5):776–783. [DOI] [PubMed] [Google Scholar]
- 54.Della Porta MG, Malcovati L, Invernizzi R, et al. Flow cytometry evaluation of erythroid dysplasia in patients with myelodysplastic syndrome. Leukemia. 2006; 20(4):549–555. [DOI] [PubMed] [Google Scholar]
- 55.Xu F, Wu L, He Q, Zhang Z, Chang C, Li X. Immunophenotypic analysis of erythroid dysplasia and its diagnostic application in myelodysplastic syndromes. Intern Med J. 2012; 42(4):401–411. [DOI] [PubMed] [Google Scholar]
- 56.Eidenschink Brodersen L, Menssen AJ, et al. Assessment of erythroid dysplasia by “difference from normal” in routine clinical flow cytometry workup. Cytometry B Clin Cytom. 2015; 88(2):125–135. [DOI] [PubMed] [Google Scholar]
- 57.Bühring HJ, Müller T, Herbst R, et al. The adhesion molecule E-cadherin and a surface antigen recognized by the antibody 9C4 are selectively expressed on erythroid cells of defined maturational stages. Leukemia. 1996; 10(1):106–116. [PubMed] [Google Scholar]
- 58.Bauer K, Machherndl-Spandl S, Suessner S, et al. Identification of CAR as a novel mediator of erythroid differentiation and migration that is specifically downregulated in erythropoietic progenitor cells in patients with MDS. Blood. 2014; 124(21):1570.25006130 [Google Scholar]
- 59.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008; 112(3):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buesche G, Teoman H, Giagounidis A, et al. Impaired formation of erythroblastic islands is associated with erythroid failure and poor prognosis in a significant proportion of patients with myelodysplastic syndromes. Haematologica. 2016; 101(5):e177–e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moriyama Y, Sato M, Kinoshita Y. Studies on hematopoietic stem cells: XI. Lack of erythroid burst-forming units (BFU-E) in patients with aplastic anemia. Am J Hematol. 1979; 6(1):11–16. [DOI] [PubMed] [Google Scholar]
- 62.Amato D, Khan NR. Erythroid burst formation in cultures of bone marrow and peripheral blood from patients with refractory anemia. Acta Haematol. 1983; 70(1):1–10. [DOI] [PubMed] [Google Scholar]
- 63.Geissler K, Hinterberger W, Jäger U, et al. Deficiency of pluripotent hemopoietic progenitor cells in myelodysplastic syndromes. Blut. 1988; 57(1):45–49. [DOI] [PubMed] [Google Scholar]
- 64.Manor D, Rachmilewitz EA, Fibach E. Improved method for diagnosis of polycythemia vera based on flow cytometric analysis of autonomous growth of erythroid precursors in liquid culture. Am J Hematol. 1997;54(1):47–52. [DOI] [PubMed] [Google Scholar]
- 65.Geissler K, Ohler L, Födinger M, et al. Interleukin-10 inhibits erythropoietin-independent growth of erythroid bursts in patients with polycythemia vera. Blood. 1998; 92(6):1967–1972. [PubMed] [Google Scholar]
- 66.Sifakis S, Pharmakides G. Anemia in pregnancy. Ann N Y Acad Sci. 2000; 900:125–136. [DOI] [PubMed] [Google Scholar]
- 67.Milman N. Postpartum anemia I: definition, prevalence, causes, and consequences. Ann Hematol. 2011; 90(11):1247–1253. [DOI] [PubMed] [Google Scholar]
- 68.Zakai NA, Katz R, Hirsch C, Shlipak MG, Chaves PH, Newman AB, Cushman M. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005;165(19):2214–2220. [DOI] [PubMed] [Google Scholar]
- 69.Chaves PH, Xue QL, Guralnik JM, Ferrucci L, Volpato S, Fried LP. What constitutes normal hemoglobin concentration in community-dwelling disabled older women? J Am Geriatr Soc. 2004; 52(11):1811–1816. [DOI] [PubMed] [Google Scholar]
- 70.Steensma DP, Tefferi A. Anemia in the elderly: how should we define it, when does it matter, and what can be done? Mayo Clin Proc. 2007; 82(8):958–966. [DOI] [PubMed] [Google Scholar]
- 71.Valent P, Horny HP. Minimal diagnostic criteria for myelodysplastic syndromes and separation from ICUS and IDUS: update and open questions. Eur J Clin Invest. 2009;39 (7):548–553. [DOI] [PubMed] [Google Scholar]
- 72.Valent P. Anemia of the elderly (AOE): does it exist and does it matter in clinical practice? Eur J Clin Invest. 2008; 38(10):782–783. [DOI] [PubMed] [Google Scholar]
- 73.Bachman E, Travison TG, Basaria S, et al. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci. 2014; 69(6):725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arber DA, Hasserjian RP. Reclassifying myelodysplastic syndromes: what’s where in the new WHO and why. Hematology Am Soc Hematol Educ Program. 2015; 2015:294–298. [DOI] [PubMed] [Google Scholar]
- 75.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127(20):2391–405. [DOI] [PubMed] [Google Scholar]
- 76.Strupp C, Nachtkamp K, Hildebrandt B, et al. New proposals of the WHO working group (2016) for the diagnosis of myelodysplastic syndromes (MDS): characteristics of refined MDS types. Leuk Res. 2017; 57:78–84. [DOI] [PubMed] [Google Scholar]
- 77.Fouillard L, Labopin M, Gorin NC, et al. ; Acute Leukemia Working Party of the EBMT. Hematopoietic stem cell transplantation for de novo erythroleukemia: a study of the European Group for Blood and Marrow Transplantation (EBMT). Blood. 2002;100(9):3135–3140. [DOI] [PubMed] [Google Scholar]
- 78.Ishiyama K, Yamaguchi T, Eto T, et al. Acute megakaryoblastic leukemia, unlike acute erythroid leukemia, predicts an unfavorable outcome after allogeneic HSCT. Leuk Res. 2016; 47:47–53. [DOI] [PubMed] [Google Scholar]
- 79.Almeida AM, Prebet T, Itzykson R, et al. Clinical outcomes of 217 patients with acute erythroleukemia according to treatment type and line: a retrospective multinational study. Int J Mol Sci. 2017; 18(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmid H, Schiffl H. Erythropoiesis stimulating agents and anaemia of end-stage renal disease. Cardiovasc Hematol Agents Med Chem. 2010;8(3):164–172. [DOI] [PubMed] [Google Scholar]
- 81.Ramanath V, Gupta D, Jain J, Chaudhary K, Nistala R. Anemia and chronic kidney disease: making sense of the recent trials. Rev Recent Clin Trials. 2012;7(3):187–196. [DOI] [PubMed] [Google Scholar]
- 82.Mimura I, Tanaka T, Nangaku M. How the target hemoglobin of renal anemia should be. Nephron. 2015; 131(3):202–209. [DOI] [PubMed] [Google Scholar]
- 83.Hellström-Lindberg E, Gulbrandsen N, Lindberg G, et al. ; Scandinavian MDS Group. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003;120 (6):1037–1046. [DOI] [PubMed] [Google Scholar]
- 84.Hellström-Lindberg E, van de Loosdrecht A. Erythropoiesis stimulating agents and other growth factors in low-risk MDS. Best Pract Res Clin Haematol. 2013; 26(4):401–410. [DOI] [PubMed] [Google Scholar]
- 85.Ludwig H, Fritz E, Kotzmann H, Höcker P, Gisslinger H, Barnas U. Erythropoietin treatment of anemia associated with multiple myeloma. N Engl J Med. 1990; 322(24):1693–1699. [DOI] [PubMed] [Google Scholar]
- 86.San Miguel JF, García-Sanz R. Recombinant human erythropoietin in the anaemia of multiple myeloma and non-Hodgkin’s lymphoma. Med Oncol. 1998; 15:S29–S34. [PubMed] [Google Scholar]
- 87.Agnihotri P, Telfer M, Butt Z, et al. Chronic anemia and fatigue in elderly patients: results of a randomized, double-blind, placebo-controlled, crossover exploratory study with epoetin alfa. J Am Geriatr Soc. 2007; 55(10):1557–1565. [DOI] [PubMed] [Google Scholar]
- 88.Agarwal N, Prchal JT. Erythropoietic agents and the elderly. Semin Hematol. 2008;45(4): 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hasselblatt M, Ehrenreich H, Sirén AL. The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J Neurosurg Anesthesiol. 2006;18(2):132–138. [DOI] [PubMed] [Google Scholar]
- 90.Westenbrink BD, Voors AA, Ruifrok WP, van Gilst WH, van Veldhuisen DJ. Therapeutic potential of erythropoietin in cardiovascular disease: erythropoiesis and beyond. Curr Heart Fail Rep. 2007; 4(3):127–133. [DOI] [PubMed] [Google Scholar]
- 91.Brines M. The therapeutic potential of erythropoiesis-stimulating agents for tissue protection: a tale of two receptors. Blood Purif. 2010;29(2):86–92. [DOI] [PubMed] [Google Scholar]
- 92.Wilhelm-Leen ER, Winkelmayer WC. Mortality risk of darbepoetin alfa versus epoetin alfa in patients with CKD: systematic review and meta-analysis. Am J Kidney Dis. 2015;66(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pérez-Ruixo JJ, Cucala-Ramos M, García-Gonzalo E, Del Val Romero B, Valveny N. Between subjects variability in haemoglobin and dose are not associated with the erythropoiesis-stimulating agent used to treat anaemia in dialysis: a meta-analysis. Br J Clin Pharmacol. 2013; 75(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park S, Fenaux P, Greenberg P, et al. Efficacy and safety of darbepoetin alpha in patients with myelodysplastic syndromes: a systematic review and meta-analysis. Br J Haematol. 2016;174(5):730–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pfeffer MA, Burdmann EA, Chen CY, et al. ; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–2032. [DOI] [PubMed] [Google Scholar]
- 96.Shirazian S, Grant C, Miller I, Fishbane S. How can erythropoeitin-stimulating agent use be reduced in chronic dialysis patients?: The use of iron supplementation to reduce ESA dosing in hemodialysis. Semin Dial. 2013; 26(5):534–536. [DOI] [PubMed] [Google Scholar]
- 97.Del Vecchio L, Locatelli F. New treatment approaches in chronic kidney disease-associated anaemia. Expert Opin Biol Ther. 2014;14(5):687–696. [DOI] [PubMed] [Google Scholar]
- 98.Bonomini M, Del Vecchio L, Sirolli V, Locatelli F. New treatment approaches for the anemia of CKD. Am J Kidney Dis. 2016; 67(1):133–142. [DOI] [PubMed] [Google Scholar]
- 99.Macdougall IC, Ashenden M. Current and upcoming erythropoiesis-stimulating agents, iron products, and other novel anemia medications. Adv Chronic Kidney Dis. 2009; 16(2):117–130. [DOI] [PubMed] [Google Scholar]
- 100.Raje N, Vallet S. Sotatercept, a soluble activin receptor type 2A IgG-Fc fusion protein for the treatment of anemia and bone loss. Curr Opin Mol Ther. 2010; 12(5):586–597 [PubMed] [Google Scholar]
- 101.Arlet JB, Dussiot M, Moura IC, Hermine O, Courtois G. Novel players in b-thalassemia dyserythropoiesis and new therapeutic strategies. Curr Opin Hematol. 2016;23 (3):181–188. [DOI] [PubMed] [Google Scholar]
- 102.Jelkmann W. The ESA scenario gets complex: from biosimilar epoetins to activin traps. Nephrol Dial Transplant. 2015; 30(4): 553–559. [DOI] [PubMed] [Google Scholar]
- 103.Liu J, Sun B, Yin H, Liu S. Hepcidin: A promising therapeutic target for iron disorders: a systematic review. Medicine (Baltimore). 2016; 95(14):e3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sugahara M, Tanaka T, Nangaku M. Prolyl hydroxylase domain inhibitors as a novel therapeutic approach against anemia in chronic kidney disease. Kidney Int. 2017; 92(2):306–312. [DOI] [PubMed] [Google Scholar]
- 105.Almeida A, Fenaux P, List AF, Raza A, Platzbecker U, Santini V. Recent advances in the treatment of lower-risk non-del(5q) myelodysplastic syndromes (MDS). Leuk Res. 2017; 52:50–57 [DOI] [PubMed] [Google Scholar]
- 106.Arber DA. Revisiting erythroleukemia. Curr Opin Hematol. 2017; 24(2):146–151. [DOI] [PubMed] [Google Scholar]
- 107.Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006; 108(5):1652–1660. [DOI] [PubMed] [Google Scholar]
- 108.Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. 2010; 115(17):3589–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marty C, Pecquet C, Nivarthi H, et al. Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood. 2016; 127(10):1317–1324. [DOI] [PubMed] [Google Scholar]
- 110.Shide K, Kameda T, Yamaji T, et al. Calreticulin mutant mice develop essential thrombocythemia that is ameliorated by the JAK inhibitor ruxolitinib. Leukemia. 2017; 31(5):1136–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rumi E, Harutyunyan A, Elena C, et al. Identification of genomic aberrations associated with disease transformation by means of high-resolution SNP array analysis in patients with myeloproliferative neoplasm. Am J Hematol. 2011; 86(12):974–979. [DOI] [PubMed] [Google Scholar]
- 112.Cerquozzi S, Tefferi A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J. 2015; 5:e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alvarez-Larrán A, Senín A, Fernández-Rodríguez C, et al. Impact of genotype on leukaemic transformation in polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2017; 178(5):764–771. [DOI] [PubMed] [Google Scholar]
- 114.Hidalgo López JE, Carballo-Zarate A, et al. Bone marrow findings in blast phase of polycythemia vera. Ann Hematol. 2018; 97(3):425–434. [DOI] [PubMed] [Google Scholar]
- 115.Grossmann V, Bacher U, Haferlach C, et al. Acute erythroid leukemia (AEL) can be separated into distinct prognostic subsets based on cytogenetic and molecular genetic characteristics. Leukemia. 2013; 27(9):1940–1943. [DOI] [PubMed] [Google Scholar]
- 116.Cervera N, Carbuccia N, Garnier S, et al. Molecular characterization of acute erythroid leukemia (M6-AML) using targeted next-generation sequencing. Leukemia. 2016; 30(4):966–970. [DOI] [PubMed] [Google Scholar]
- 117.Ping N, Sun A, Song Y, et al. Exome sequencing identifies highly recurrent somatic GATA2 and CEBPA mutations in acute erythroid leukemia. Leukemia. 2017; 31(1):195–202. [DOI] [PubMed] [Google Scholar]
- 118.Montalban-Bravo G, Benton CB, Wang SA, et al. More than 1 TP53 abnormality is a dominant characteristic of pure erythroid leukemia. Blood. 2017; 129(18):2584–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.