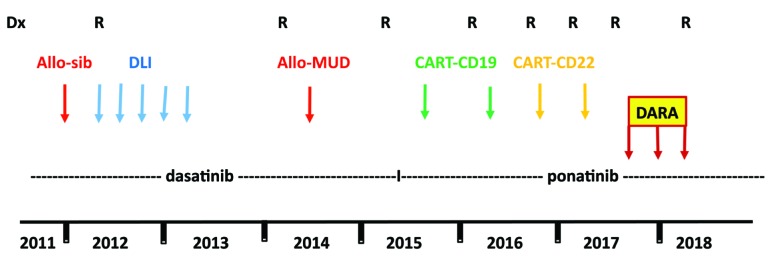
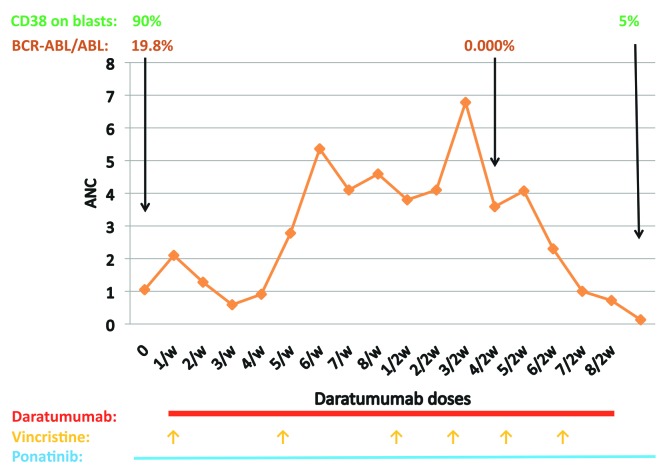
Immunotherapy, or antibody conjugates, are commonly being used for relapsed/refractory B-ALL. The main strategies currently in practice are specific antibodies such as blinatumumab (anti-CD19 and CD3) and inotuzumab ozogamicin (anti-CD22) and, more recently, CAR T cells. Daratumumab is a monoclonal antibody that binds to CD38 and preclinical data have recently reported efficacy of daratumumab in T-cell ALL1 but no data have been published regarding CD38-positive BALL, preclinical or clinical. Recently, we had an experience with treating a patient with CD38-positive, Ph-positive relapsed B-lineage ALL with daratumumab, resulting in a good response and no toxicity. A 14-year-old boy was diagnosed on 8/2011 as suffering from Ph-positive (p190) ALL. The immumophenotype of his blasts at diagnosis was positive for CD19, CD10, CD22, CD34 and HLA-DR, and by karyotyping all the examined cells contained t(9;22)(q34,q11.2), but some of them also presented with del20(q11.2) or del 20. He did not achieve remission post conventional induction therapy plus dasatininb but went into remission following reinduction/consolidation (including high dose cytarabine, etoposide, dexamethasone and high dose methotrexate) and underwent hematopoietic cell transplant from his sibling on 12/2011. Four months later he became a mixed chimera and FISH for BCR-ABL turned again to be positive. No BCR-ABL mutation was detected. Dasatinib was restarted, in addition to recurrent monthly donor lymphocyte infusion (DLI) treatments, for about a year. On 3/2014 he relapsed again. Conventional chemotherapy and dasatinib were restarted and he underwent a second allogeneic transplant from an unrelated (9/10) donor. Six months later (2/2015) he relapsed with a new BCR-ABL mutation (V299L). Ponatinib with chemotherapy were started and he achieved a short morphological remission. At this point, he was treated twice with anti-CD19 CAR-T cells at the Fred Hutchinson Cancer Research Center in Seattle (clinicaltrials.gov identifier 02028455) and later on, twice, with an anti-CD22 CAR-T cells, at the NIH (clinicaltrials.gov identifier 02315612), with a conditioning regimen, before every treatment, which included fludarabine and cyclophosphamide. Approximately 3 months after every treatment, he relapsed. Since 2015 he has received ponatinib continually, but at no time did this prevent a relapse (Figure 1). Vincristine was given a few times around relapses but without an overt response. In 08/2017, in relapse, the BCR-ABL/ABL according to the international score (IS) was 19.8% and his lymphoblasts were highly positive for CD38 (90%) by immunophenotyping. As the expression of CD19 and CD22 were zero, following his CAR-T treatments, the use of blinatumumab or inotuzumab was not an option. He was then treated with daratumumab, given on a compassionate basis, at a dosing (16 mg/kg) and schedule (once weekly for 8 weeks and then once every 2 weeks for 8 doses) as typically used in multiple myeloma, where extensive safety data are known. Vincristine was administered every 4 weeks and ponatinib was continued. Steroids were given only as premedication treatment before the administration of daratumumab. After a short while he went into a morphological remission, and in 12/2017 he became BCR-ABL-negative (BCR-ABL/ABL of 0.000). Six months after beginning daratumumab he relapsed again (Figure 2) and is now receiving bortezomib and gemtuzumab ozogamicin. Tumor lysis syndrome or other significant adverse events were not noted while he was on daratumumab. In the last relapse, the CD38 on the lymphoblasts went down from 90% to 5% and has remained low for at least 10 weeks after the last dose of daratumumab. It is unknown whether this reduction represents a downregulation of CD38 on the blasts or, as was reported in multiple myeloma patients,2 it is a result of long-term saturation of the CD38 by daratumumab. This patient demonstrates the potential efficacy of daratumumab for the treatment of CD38-positive ALL patients. The complete lack of expression of CD19 and CD22 lent further support for considering this strategy. Although reporting only a single patient, this therapy was based on a sound rationale given his immunophenotypic expression profile. This multiply relapsed Ph+ALL patient achieved a rapid response with virtually no toxicity. While intriguing, this clearly must be confirmed in a larger setting.
Figure 1.
The entire course of the disease.
Figure 2.
The course of daratumumab.
Supplementary Material
Acknowledgements
The authors would like to thank the hematology and transfusion medicine teams at Children’s Healthcare of Atlanta for their constructive review of the manuscript. No funding was used to conduct this study.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Bride KL, Vincent TL, Im SY, et al. Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia. Blood. 2018; 131(9):995–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oberle A, Brandt A, Alawi M, et al. Long-term CD38 saturation by daratumumab interferes with diagnostic myeloma cell detection. Haematologica. 2017; 102(9):e368–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.