Abstract
Background
Obstetric hemorrhage and access to peripartum blood transfusion remains a global health challenge. The rates of peripartum transfusion in South Africa exceed those in high-income countries despite comparable rates of obstetric hemorrhage. We sought to evaluate factors associated with peripartum transfusion.
Study Design and Methods
A case-control study was conducted at four large South African hospitals. Transfused peripartum women (cases) and non-transfused controls were stratum-matched 1:2 by hospital and delivery date. Data on obstetric, transfusion and HIV history were abstracted from medical records. Blood was obtained for laboratory evaluation. We calculated unadjusted and adjusted odds ratios (ORs) for transfusion using logistic regression.
Results
A total of 1,200 transfused cases and 2,434 controls were evaluated. Antepartum hemorrhage (OR=197.95, 95% CI 104.27–375.78), hemorrhage with vaginal delivery (OR=136.46, 95% CI 75.87–245.18), prenatal anemia (OR=22.76, 95% CI 12.34–41.93 for prenatal hemoglobin < 7g/dl) and failed access to prenatal care (OR=6.71, 95% CI 4.32–10.42) were the major risk factors for transfusion. Platelet count (ORs 4.10, 2.66 and 1.77 for ≤50,000, 51–100,000 and 101–150,000 cells per mm3, respectively), HIV infection (OR 1.29, 95% CI 1.02–1.62) and admitting hospital (two-fold variation) were also associated with transfusion. Mode of delivery, race, age category, gravidity, parity, gestational age, and birth weight were not independently associated with transfusion.
Conclusion
Major risk factors of peripartum transfusion in South Africa, namely prenatal anemia and access to prenatal care, may be amenable to intervention. HIV infection and moderately low platelet count are novel risk factors which merit further investigation.
Keywords: Blood transfusion, HIV, Africa, peripartum period, postpartum hemorrhage
INTRODUCTION
Blood transfusion is critical to the management of obstetric hemorrhage (OH) and other obstetric emergencies. Globally, OH is a leading contributor to obstetric morbidity and mortality1–3. A lack of early recognition of risk factors for recurrent OH,3 coupled with the failure to provide effective peripartum care contribute to adverse maternal outcomes2. Consequently, both OH and high rates of blood transfusion administered for OH are primarily encountered in resource poor countries including South Africa4,5.
Although both the International Confederation of Midwives (ICM) and the International Federation of Gynecology and Obstetrics (FIGO) recommend that there be “blood transfusion facilities in all centers that provide comprehensive health care”2, little research has been conducted on peripartum blood utilization and its predisposing risk factors. In one review of the unavailability of transfusion and its contribution to maternal mortality in sub-Saharan Africa1, only two articles from South Africa6,7 were cited, neither of which was within the preceding decade nor -specifically- related to blood utilization. Obstetric transfusion also accounts for a substantial proportion of blood utilization in sub-Saharan Africa, placing a burden on blood collection organizations that struggle to meet demand. A better understanding of blood utilization and antecedent risk factors could identify deficiencies in transfusion practice and reduce preventable and inappropriate transfusions. There is strong evidence that restrictive transfusion practice confers comparable or even superior clinical outcomes to those of liberal transfusion8,9.
Research on transfusion in HIV positive obstetric patient is particularly relevant, given the association of HIV with anemia and other complications, but such data are limited10. The few published studies evaluating the association between HIV and increased blood use have yielded somewhat different results10–12. While HIV may be a driver of transfusion11,12, the strength of that association dissipates after controlling for potential confounders10. There still remains uncertainty as to why HIV would increase transfusion incidence, if indeed an independent association holds true.
Because of their relevance to obstetric practice, HIV treatment and blood demand, we therefore sought to characterize risk factors for peripartum blood transfusion in a large study of peripartum South African women with very high HIV prevalence.
MATERIALS AND METHODS
Study Design
We hypothesized that HIV status and prenatal anemia were independent predictors of peripartum transfusion even after controlling for potential confounders such as parity, mode of delivery, and advanced maternal age. A case control study was conducted over a 19 month period (31st March 2014 to 31st October 2015) at four major hospitals in South Africa: Chris-Hani Baragwanath Hospital (CHB) in Johannesburg, King Edward VIII Hospital (KEH) in Durban, Mowbray Maternity Hospital (MMH) and Groote Schuur Hospital (GSH) in Cape Town. These obstetric services serve a generally urban, low-income, Black-African and Colored (denotes a specific mixed-race population group) population with high HIV prevalence. CHB and KEH each have both second-tier and tertiary obstetric services while MMH and GSH are linked by referral in a single secondary and tertiary system, respectively. This affords broad representation both by population as well as obstetric pathology, with management of uncomplicated deliveries as well as complex referrals from primary-level hospitals and midwife obstetric units.
The study received ethical approval from the committees representing each of the participating hospitals in addition to University of California San Francisco, the South African National Blood Service (SANBS) and the data-coordinating center (RTI International, Rockville, MD). Written informed consent was elicited from all enrolled participants; deferred consent was allowed for patients who were not able to provide consent at time of transfusion. Once stable, the patients were approached and enrolled under full informed consent. In the event that they declined, any blood samples or associated data were discarded.
Study Population
All peripartum obstetric patients with an index hospitalization at a participating hospital during the enrollment period were eligible for enrollment in the study. The study population was restricted to women with pregnancies of at least 26 weeks gestation; in South Africa, delivery in obstetric patients less than 24 to 26 weeks is managed separately by gynecology as abortion/miscarriage. Stillborn and early neonatal deaths were included as were both normal vaginal delivery (NVD) and births by cesarean section (C/S). Eligible participants were identified through daily review of ward admission logs and maternity, delivery and operating room registers; this approach was complemented by direct communication with the blood bank and ward staff. We excluded patients who were transferred or discharged before consent and enrollment could be performed. We also excluded minors (aged <18 years old) because South African law requires parental/guardian consent for participation in research and it was not feasible to obtain it. From previous data, minors constituted about 2.5% of those patients who sustained OH and/or were transfused12.
Cases (target =1200) included women who were transfused (allogeneic red cells, platelets or plasma) in the peripartum period, namely 48hrs before or after delivery. Controls comprised a random sample of non-transfused deliveries (target=2400), which were stratum-matched by hospital and date of delivery i.e. women who were admitted during the peripartum period, being of at least 26 weeks gestation at time of delivery and not transfused during the peripartum period. This sample size and the 2:1 ratio of controls to cases was chosen to achieve 80 percent power to detect an odds ratio of 1.25 for the association between HIV infection and transfusion, our primary hypothesis.
Data collection
Demographic information, medical and obstetric history, HIV status and treatment, obstetric hemorrhage and transfusion data, were abstracted from the medical records. Machine-readable paper forms [available on request] allowed automated data entry; the electronic data were subsequently transferred to the data-coordinating center for cleaning and analysis.
According to the protocol, complete blood counts (CBC) and coagulation studies (international normalized ratio (INR), prothrombin time [PT], activated partial thromboplastin time [APTT], D-dimers) were to be performed on all cases and 50% of controls; CD4 and HIV viral load were obtained in those patients who were HIV positive. We used a planned missing data design to randomly sample half of the non-transfused women to obtain blood samples 13. Laboratory procedures differed between cases and controls because of logistics and timing of sample collection. For cases, additional tubes of blood were collected by the clinical staff prior to transfusion, under a waiver of consent because the laboratory measures of interest (e.g. PT and APTT are not valid if drawn post-transfusion. If the patient later agreed to participate in the study, the tubes were relayed to the laboratory for testing; if not, they were destroyed. For controls, a single EDTA tube was collected at time of enrollment, which was usually the day after delivery.
Definitions
Notable definitions include “OH”, which was defined as any obstetric-related hemorrhage occurring in the peripartum period; the World Health Organization (WHO) definition of peripartum hemorrhage was applied i.e. ≥500 mL blood loss for vaginal delivery or ≥1000 mL blood loss for Caesarean section. We also used a convenience definition for OH, namely whether OH was recorded in the medical record, regardless of estimated blood loss. “Booked” and “unbooked” refer to patients who had accessed or not accessed prenatal care, respectively. The prenatal hemoglobin value was the lowest recorded measured hemoglobin during prenatal care; because these data were available only from booked women the two variables were considered together. The pre-transfusion hemoglobin refers to the last hemoglobin that was obtained prior to the first blood product being transfused.
At the time of the study the national policy regarding the prevention of mother to child transmission (PMTCT) of HIV was to administer three-drug antiretroviral therapy (ART) to all HIV positive pregnant patients independent of CD4 count. If a patient was diagnosed in labor a modified ART drug regimen was administered with follow-up after delivery.
Statistical Analysis
Cases and controls were compared using counts and percentages for categorical data and means and distributions for continuous variables. For categorical data, counts and percentages for single variables and combinations of variables were produced, using Chi-squared tests of significance. For the continuous data, distributions were examined individually and stratified by covariates, using T-tests to test differences between means.
Because platelet count was intended to be collected from only one half of the controls13 but was also missing on a smaller proportion of controls and cases, we sought to impute the data using multiple imputation by chained equations methodology implemented in the “mice” package14 within the R language and environment for statistical computing (R Core Team 2016, R version 3.3.2 (2016-10-31) – “Sincere Pumpkin Patch” on platform x86_64-w64-mingw32/x64 (64-bit) (R Core Team 2016) running under Windows 7 x64 (build 7601) Service Pack 1). We used the mice defaults except for the number of imputations (B=50)15, a monotone visit sequence for imputing the variables16 and recursive partitioning as the imputation model for all variables17. Several different graphical diagnostics were used to check whether the distribution of the imputed data was similar to that of the observed data18. The mice package was used to analyze the imputed data; this enables repeated imputation inferences as described in Rubin19. This methodology accounts for the uncertainty associated with the imputed data and incorporates this uncertainty into the variance estimates.
Multiple logistic regression was used to model the relationship between case versus control status and the predictor variables, controlling for potential confounding variables. A larger set of variables was initially considered. The model was refined using Bayesian Information Criterion to identify the best model, and the final model was based on all the statistically significant variables. Once the final set of variables was identified, two-way interactions were investigated but none of them were included in the final model.
RESULTS
Of 1,534 potentially eligible cases, 316 (20.6%) were deemed ineligible (transfusion was not administered; age less than 18), 11 (0.7%) refused, 1,206 consented and 1,200 had complete clinical data. Of 2,509 potentially eligible controls, 28 (1.1%) were deemed ineligible, 44 (1.8%) refused, 2,437 consented and 2,434 had complete clinical data. Thus the final dataset included 1,200 cases and 2,434 controls. During the period in which cases and controls were enrolled, 61,405 births were reported at the participating hospitals including 34,188 vaginal deliveries and 27,217 (44%) cesarean sections. Overall, 1,097 (30.6%) of combined cases and controls were HIV positive, of whom 108 (9.8%) had CD4 counts <200 cells per mm3. Of those women who were HIV positive 992 (90.4%) reported being on ART prior to delivery or prevention of mother to child transmission (PMTCT) regimen during delivery.
Transfused cases and controls were evenly distributed by hospital, consistent with our matching criteria (Table 1), and did not differ by age or race: most patients (84.3%) were aged 18 to 34 years and of Black race/ethnicity (82.5%). Cases were more likely than controls to have had no prenatal care (10.2% vs. 2.7%; p<0.0001) while rates of Caesarean delivery did not differ significantly from those of controls (Table 2). Cases were much more likely than controls to have had OH either by clinical mention of the diagnosis (67.4% vs. 4.3%; p< 0.0001) or by application of the WHO definition based upon estimated blood loss (43.6% vs. 4.5%; p< 0.0001). Nadir prenatal hemoglobin values were lower among cases than controls, with 53.2% of cases and 79.2% of controls having hemoglobin values of 10g/dL or higher (p<0.0001). Neither gravidity nor parity differed between cases and controls. Cases were more likely than controls to be HIV positive (35.4 vs. 28.3%; p<0.0001), to have missing CD4 lymphocyte counts, and lower CD4 lymphocyte counts (p<0.0001). Transfused cases were more likely than controls to have pregnancies with shorter gestational age (p<0.0001) and lower birth weights (p<0.0001). Two (0.17%) deaths were recorded in the transfused group (0.04%) and one death was reported in the control (non-transfused group); mortality status was missing in 155 subjects (4.27%).
Table 1.
Demographic and clinical characteristics of cases and controls.
| Variable | Cases | Controls | p value | ||
|---|---|---|---|---|---|
| N | Percent | N | Percent | ||
| All subjects | 1,200 | 100.0 | 2,434 | 100.0 | |
| Hospital | 674 | 56.2 | 1,352 | 55.6 | 0.98 |
| Chris Hani- Baragwanath Hospital | |||||
| King Edward VIII Hospital | 186 | 15.5 | 378 | 15.5 | |
| Groote Schuur Hospital | 128 | 10.7 | 261 | 10.7 | |
| Mowbray Maternity Hospital | 212 | 17.7 | 443 | 18.2 | |
| Age Category | 9 | 0.8 | 17 | 0.7 | 0.67 |
| Missing | |||||
| 18–19 | 99 | 8.3 | 167 | 6.9 | |
| 20–24 | 327 | 27.3 | 646 | 26.6 | |
| 25–29 | 333 | 27.8 | 678 | 27.8 | |
| 30–34 | 252 | 21.0 | 541 | 22.2 | |
| 35–39 | 127 | 10.6 | 268 | 11.0 | |
| 40+ | 53 | 4.4 | 117 | 4.8 | |
| Race | 9 | 0.8 | 23 | 0.9 | 0.95 |
| Missing | |||||
| Black | 987 | 82.3 | 1,980 | 81.3 | |
| White | 4 | 0.3 | 9 | 0.4 | |
| Colored | 189 | 15.8 | 399 | 16.4 | |
| Asian | 10 | 0.8 | 19 | 0.8 | |
| Other | 1 | 0.1 | 4 | 0.2 | |
| Prenatal Care (booking status) | 1,078 | 89.8 | 2,368 | 97.3 | <0.0001 |
| Booked | |||||
| Unbooked | 122 | 10.2 | 66 | 2.7 | |
| Type of Delivery | 1 | 0.1 | 8 | 0.3 | 0.11 |
| Missing | |||||
| C-Section | 557 | 46.4 | 1,195 | 49.1 | |
| Vaginal | 642 | 53.5 | 1,231 | 50.6 | |
Table 2.
Potential risk factors for transfusion, by case versus control status, with p values derived from chi squared tests.
| Variable | Cases | Controls | p value | ||
|---|---|---|---|---|---|
| N | Percent | N | Percent | ||
| WHO definition hemorrhage | 436 | 43.6 | 93 | 4.5 | <0.0001 |
| Yes | |||||
| No | 563 | 56.4 | 1,981 | 95.5 | |
| Reported Hemorrhage | 85 | 7.1 | 167 | 6.9 | <0.0001 |
| Missing | |||||
| Yes | 751 | 62.6 | 98 | 4.0 | |
| No | 364 | 30.3 | 2,169 | 89.1 | |
| Prenatal Care and Hemoglobin | 122 | 10.2 | 66 | 2.7 | <0.0001 |
| Unbooked | |||||
| Booked and | 56 | 4.7 | 17 | 0.7 | |
| Hemoglobin 0 to < 7g/dL | |||||
| Hemoglobin 7 to < 8 g/dL | 67 | 5.6 | 46 | 1.9 | |
| Hemoglobin 8 to < 9 g/dL | 110 | 9.2 | 93 | 3.8 | |
| Hemoglobin 9 to < 10 g/dL | 142 | 11.8 | 172 | 7.1 | |
| Hemoglobin ≥10 g/dL | 638 | 53.2 | 1,927 | 79.2 | |
| Hemoglobin Unknown | 65 | 5.4 | 113 | 4.6 | |
| Gravidity - includes current pregnancy | 5 | 0.4 | 11 | 0.5 | 0.46 |
| Missing | |||||
| 1 | 297 | 24.8 | 632 | 26.0 | |
| 2 | 369 | 30.8 | 786 | 32.3 | |
| 3 | 298 | 24.8 | 564 | 23.2 | |
| 4+ | 231 | 19.3 | 441 | 18.1 | |
| Parity | 11 | 0.9 | 12 | 0.5 | 0.12 |
| Missing | |||||
| 1 | 396 | 33.0 | 776 | 31.9 | |
| 2 | 353 | 29.4 | 814 | 33.4 | |
| 3 | 277 | 23.1 | 532 | 21.9 | |
| 4+ | 163 | 13.6 | 300 | 12.3 | |
| HIV Status | 24 | 2.0 | 26 | 1.1 | <0.0001 |
| Missing | |||||
| Negative | 760 | 63.3 | 1,727 | 71.0 | |
| Positive | 416 | 34.7 | 681 | 28.0 | |
| CD4 lymphocyte count (cells per mm3) | 24 | 2.0 | 26 | 1.1 | <0.0001 |
| Missing | |||||
| HIV − | 760 | 63.3 | 1,727 | 71.0 | |
| HIV +: CD4 Missing | 182 | 15.2 | 205 | 8.4 | |
| HIV +: CD4 < 200 | 47 | 3.9 | 61 | 2.5 | |
| HIV +: CD4 200 to < 350 | 63 | 5.3 | 121 | 5.0 | |
| HIV +: CD4 350+ | 124 | 10.3 | 294 | 12.1 | |
| Birth Weight | 27 | 2.3 | 21 | 0.9 | <0.0001 |
| Missing | |||||
| <= 1,820 | 178 | 14.8 | 182 | 7.5 | |
| 1,821 – 2,400 | 162 | 13.5 | 198 | 8.1 | |
| 2,401 – 2,675 | 132 | 11.0 | 228 | 9.4 | |
| 2,676 – 2,860 | 102 | 8.5 | 265 | 10.9 | |
| 2,861 – 3,025 | 84 | 7.0 | 264 | 10.8 | |
| 3,026 – 3,165 | 116 | 9.7 | 243 | 10.0 | |
| 3,166 – 3,300 | 102 | 8.5 | 258 | 10.6 | |
| 3,301 – 3,475 | 100 | 8.3 | 263 | 10.8 | |
| 3,476 – 3,700 | 101 | 8.4 | 256 | 10.5 | |
| >= 3,701 | 96 | 8.0 | 256 | 10.5 | |
| Gestational Age | 24 | 2.0 | 34 | 1.4 | <0.0001 |
| Missing | |||||
| <= 33 | 213 | 17.8 | 196 | 8.1 | |
| 34 – 36 | 206 | 17.2 | 304 | 12.5 | |
| 37 | 123 | 10.3 | 255 | 10.5 | |
| 38 | 198 | 16.5 | 517 | 21.2 | |
| 39 | 180 | 15.0 | 444 | 18.2 | |
| 40 | 176 | 14.7 | 429 | 17.6 | |
| > 40 | 80 | 6.7 | 255 | 10.5 | |
Hemorrhage cause was missing about 7 percent of its values; gestational age category, HIV/CD4 category, HIV status, birth weight category, race, age category, parity category, gravidity category, and delivery type were all missing less than 2 percent of their values; and booking status, patient transfused, hemoglobin category, and HIV treatment were missing no values.
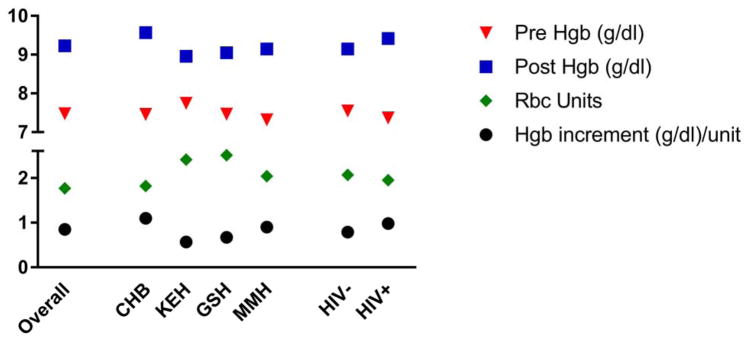
Among transfused cases, the mean pre-transfusion hemoglobin recorded in medical records was 7.5 g/dL (Standard deviation [SD] 1.75g/dL) ranging from 7.3 g/dL (MMH) to 7.8 g/dL (KEH), and mean post-transfusion hemoglobin was 9.2 g/dL (SD 1.74g/dL) ranging from 9.0g/dL (KEH) to 9.6g/dL (CHB) (Figure 1). The median interval between the pre-transfusion hemoglobin and the first transfusion was less than 1 day. The mean number of RBCs administered per transfusion was 2.0 units (SD 1.25), ranging from 1.8 (CHB) to 2.5 units (GSH); 121 (14.3%) women with OH versus 10 (0.4%) women without OH versus were transfused with ≥4 units of RBCs. Most (55%) of women with OH received only 1 or 2 units of RBCs. The hemoglobin increment per RBC unit may be taken as a surrogate for transfusion in the setting of bleeding (low values) versus stable anemia or over-transfusion (high values). The mean hemoglobin increment per RBC unit was 0.85 g/dL per Unit (SD 1.29) varying from 0.57 (KEH) to 1.10 (CHB) g/dL per Unit. Mean pre-transfusion hemoglobin was 7.4 g/dL in HIV positive- and 7.6 g/dL in HIV negative patients (p = 0.0982) and the corrected hemoglobin increment was 0.98 in HIV positive versus 0.79 in HIV negative patients (p= 0.1362)(Figure 1).
Figure 1.
Pre- and post- transfusion hemoglobin, number of units of RBCS, and hemoglobin increment divided by number of RBC units by hospital and HIV status. The Y axis indicates the mean hemoglobin (g/dL) for pre- and post- transfusion hemoglobin, the number of RBC units transfused and the hemoglobin increment divided by the number of RBC units transfused. The X-axis indicates participant subgroups: overall includes all transfused women, followed by hospital (CHB = Chris-Hani Baragwanath, KEH = King Edward VIII, GSH = Groote Schuur Hospital and MMH = Mowbray Maternity Hospital) and positive versus negative HIV status. Abbreviations: Hgb: hemoglobin Tx: Transfusion. The comparison of hemoglobin increment did not differ by HIV status (p=0.24).
Among transfused cases only, vital signs included a mean temperature of 36.5°C, mean heart rate of 98.9 bpm, mean arterial pressure of 91.4 and respirations of 20.3. Vital signs did not differ by HIV status with the exception of a lower mean arterial pressure (89.1 mmHg versus 92.6 mmHg; p = 0.0007) in HIV positive patients. There was a low rate of successful blood specimen collection among cases (17% of cases, range by hospital 6%–36%) but the target of sampling 50% of controls was almost achieved. Complete blood counts were available from 714 cases and 982 controls. Cases and controls differed with regard to mean hemoglobin values (7.9 vs. 10.7 g/dL; p < 0.0001), mean white blood cell count (13.7 versus 12.9 x 103 cells per μL; p = 0.0015) and mean platelet count (202.7 versus 247.4 x 103 cells per μL; p < 0.0001). Coagulation tests were available from 176 cases and 979 controls. The difference in INR (1.1 versus 0.97; p= 0.0144) was statistically, but not likely clinically, significant and only 5 cases and 4 controls had INR > 1.8. Similarly there was a small difference in D dimers between cases and controls (2.5 versus 1.6 μg/L; p=0.0126) but there was no difference in activated PTT (30.3 versus 31.0 seconds). Tranexamic acid was used in only three subjects in our study.
In the multivariate logistic regression model (Table 3), the strongest risk factors for transfusion were several subtypes of OH, most notably antepartum hemorrhage including placenta praevia and abruptio placenta (OR 198) and hemorrhage associated with vaginal delivery including retained placenta, uterine atony and vaginal lacerations (OR 136). Other risk factors for transfusion included prenatal anemia (ORs = 23, 9.48, 6.12 and 3.25 for prenatal hemoglobin less than 7, 7–7.9, 8–8.9 and 9–9.9 g/dl, respectively), whether the patient accessed prenatal care (OR = 6.71 for “unbooked” patients) and platelet count (ORs = 4.10, 2.66, 1.77, and 1.06 for less than 50,000 platelets per μL, 51,000 to 100,000 platelets per μL, 101,000 to 150,000 platelets per μL, and 151,000 to 200,000 platelets per μL, respectively, as compared to women with a count of ≥201,000 platelets per μL). The admitting hospital was also shown to be associated with transfusion rate, with the three other hospitals having approximately half the odds of CHB. Finally, HIV infection was shown to be a weaker but independent risk factor for transfusion (OR 1.29). Mode of delivery (Cesarean vs. vaginal), race, age category, gravidity, parity, gestational age, and birth weight did not contribute significantly in the transfusion model.
Table 3.
Independent risk factors for blood transfusion with odds ratios derived from the final logistic regression model. Definitions of the variables are given in the footnote.
| Variable Category | Odds Ratio | Lower 95% Confidence Limit | Upper 95% Confidence Limit |
|---|---|---|---|
| No Hemorrhage | 1.00 | --- | --- |
| Hemorrhage Cause: APH | 197.95 | 104.27 | 375.78 |
| Hemorrhage Cause: C-Section | 40.13 | 24.95 | 64.52 |
| Hemorrhage Cause: NVD | 136.46 | 75.87 | 245.18 |
| Hemorrhage Cause: Other | 27.19 | 18.84 | 39.25 |
| Unbooked | 6.71 | 4.32 | 10.42 |
| Booked and | |||
| Hemoglobin <7g/dL | 22.76 | 12.34 | 41.93 |
| Hemoglobin 7 to 7.9g/dL | 9.48 | 5.91 | 15.18 |
| Hemoglobin 8 to 8.9g/dL | 6.12 | 4.19 | 8.94 |
| Hemoglobin 9 to 9.9g/dL | 3.25 | 2.32 | 4.55 |
| Hemoglobin >=10 g/dL | 1.00 | --- | --- |
| Hemoglobin unknown | 2.03 | 1.26 | 3.25 |
| Platelet Count <= 50 | 4.10 | 1.42 | 11.92 |
| Platelet Count 51–100 | 2.66 | 1.54 | 4.59 |
| Platelet Count 101–150 | 1.77 | 1.16 | 2.71 |
| Platelet Count 151–200 | 1.06 | 0.78 | 1.44 |
| Platelet Count >= 201 | 1.00 | --- | --- |
| Chris Hani Baragwanath Hospital | 1.00 | --- | --- |
| Groote Schuur Hospital | 0.53 | 0.36 | 0.78 |
| King Edward Hospital | 0.51 | 0.37 | 0.70 |
| Mowbray Maternity Hospital | 0.53 | 0.39 | 0.72 |
| HIV Negative | 1.00 | --- | --- |
| HIV Positive | 1.29 | 1.02 | 1.62 |
APH (antepartum hemorrhage) included placenta previa and abruptio placenta; C-section included hemorrhage in association with cesarean section; NVD included hemorrhage associated with vaginal delivery, namely retained placenta, uterine atony and vaginal lacerations; OTHER included other rare causes of hemorrhage.
DISCUSSION
In addition to recognized causes of OH, the study reveals several potentially modifiable risk factors for peripartum blood transfusion in South Africa. Prenatal anemia, access to prenatal care and the hospital of birth can potentially be addressed by improved prenatal care and or implementation of transfusion protocols to standardize practice. We also identified novel biological risk factors for transfusion including moderately low platelet count and HIV infection that persisted even after controlling for potential confounding. Finally, HIV prevalence was very high, with prevalence of almost 35% among cases and 28% among controls; however, a high rate of ART coverage was observed, consistent with the national rollout of ART.
Antepartum hemorrhage due to abruptio placenta or placenta praevia (OR = 198) was somewhat more strongly associated with transfusion than were complications associated with vaginal delivery including retained placenta, uterine atony and vaginal lacerations (OR = 136), although confidence intervals overlapped. In contrast, bleeding in association with cesarean section carried comparatively lower odds of transfusion (OR=40). These data may be helpful to obstetricians in determining when to order blood preemptively. Regarding practice differences, the association between transfusion risk and one of the four hospitals, as well as a higher hemoglobin increment per unit transfused at the same hospital, suggest some degree of inappropriate transfusion. Redoubled efforts to implement existing South African guidelines for obstetric transfusion could reduce unnecessary transfusion.
Women with a prenatal hemoglobin value less than 7g/dL had estimated odds of transfusion approximately 23 times higher than those with hemoglobin values of ≥10g/dL, and intermediate hemoglobin values carried intermediate levels of risk consistent with a “dose response”. Among women with OH, 14% received four or more RBC units consistent with severe hemorrhage but the majority received only 1 or 2 RBC units consistent with an interplay between OH and anemia as predisposing to transfusion as indicated by our multivariable model. This finding suggests that anemic women are less able to tolerate even small to moderate hemorrhage during delivery and argues strongly that better interventions to diagnose and correct prenatal anemia need to be developed and tested. Physiological anemia results from the relative increase in the plasma volume (50%) vs. red cell mass (25%) in pregnancy.20,21 Iron deficiency anemia is the most common cause of prenatal anemia in South Africa.22 While gastrointestinal iron absorption increases with gestational age, dietary intake is insufficient in 20% of pregnancies to prevent iron deficiency anemia in the absence of supplementation.21 In one study, the prevalence of prenatal anemia at a regional hospital in South Africa was 42%; a significant difference in prevalence was noted between HIV-positive and HIV-negative pregnant women (71.3% vs. 28.7% respectively; p<0.0001).23
Low gestational age has been shown to be a risk factor for peripartum transfusion24. In our study, low gestational age and lower birth weights were associated with peripartum transfusion in bivariate analysis but not the final multivariable model. Gestational age and birth weight are related indicators for diverse maternal (e.g. anemia, hypertension), fetal (e.g. genetic or developmental anomalies, intrauterine growth retardation) and placental (e.g. placenta previa, abruptio, placental insufficiency) pathologies.25 We believe that underlying reasons for low birthweight and prematurity (e.g. abruptio and placenta previa) may contribute to peripartum hemorrhage and transfusion.
Although the association between HIV status was modest as compared to the other major drivers of peripartum transfusion, HIV positive women were still almost 30% more likely to be transfused in the peripartum period than HIV negative women. The reasons for this independent risk are not clear. HIV-related anemia, altered transfusion practice in HIV patients and HIV-related coagulopathy were considered as possible contributors. Residual confounding between HIV related anemia and transfusion is possible, but we controlled for prenatal hemoglobin in the multivariable model. Whether physicians’ transfusion practice differs for HIV positive patients is difficult to determine. Although we found no difference in pre-transfusion hemoglobin or hemoglobin increment according to HIV status, acuity of illness may have been greater in the HIV patients as indicated by a higher temperature and lower mean arterial pressure in transfused HIV positive- compared to transfused HIV negative patients. A possible contribution of HIV-related coagulopathy towards higher transfusion risk could not be ruled out by the current study but we saw no difference in PT, APTT nor d-dimers between HIV positive and negative transfused patients. We cannot rule out a bias toward the null because these laboratory results were available in only a subset of patients.
Our finding that moderate decreases in platelet count increased the odds of transfusion is novel. Women with platelet counts of 51,000–100,000 platelets /μL and 101,000– 150,000 platelets/ μL had three and two times the odds of transfusion than women with counts ≥ 200,000 platelets/ μL. Prevailing wisdom derived from oncology studies suggests that only very low platelet counts (<20,000 platelets/ μL) are associated with bleeding, but higher platelet counts may be needed in the setting of hemorrhage.26 Other potential explanations for the apparent association includes physician practice or a dilutional effect on platelet count due to fluid replacement given before transfusion. Another potential explanation is that the lower platelet count is an effect of rather than the cause of bleeding.
There were limitations to the study. Foremost was the low compliance with collection of pre-transfusion blood samples from cases (range by hospital ~6–36%), stemming from our reliance on housestaff to collect the blood samples. The low compliance raises the possibility of selection bias in that patients for whom blood samples were available may have been sicker or healthier than those without data. Second, risk factor data were limited by the chart abstraction process and the accuracy of source data and as a result may have been missing or inaccurate. Finally, our study was restricted to four obstetric services. Although the participating sites are major obstetric services, the findings may not be generalizable to all deliveries. Specifically, the high rate of cesearean sections at these secondary and/or tertiary referral centers probably does not represent all deliveries. Conversely, low risk births were more likely to deliver at midwife clinics and data from rural hospitals were not captured and remain an important, yet neglected, area of study. However we did represent the geographic and socioeconomic characteristics of most South African births.
In conclusion, blood transfusion is integral to obstetric resuscitation and progress to attainment of the sustainable development goal 3 to reduce, by 2030, the global maternal mortality ratio to less than 70 per 100,000 live births27. Blood transfusion is also a limited resource in much of Africa where transfusion demand often cannot be met28. Our findings suggest that minimization of obstetric RBC transfusions in South Africa may require different approaches than those being adopted in high-income countries 29–32. Strategies to mitigate the morbidity of specific causes of OH have have already been adopted within the obstetric community. There may still be scope for improvement with other measures (e.g. tranexamic acid) that have shown to be beneficial in management of OH33. The earlier diagnosis and better treatment of prenatal anemia, possibly including intravenous iron therapy, is an intervention that could proably have substantial impact on transfusion incidence. Regarding clinical practice, clinical trials of transfusion thesholds are needed in the African obstetric population to develop evidence based guidlines similar to that which have been used to direct practice in other patient groups31. Finally, the wider rollout of antiretroviral therapy in South Africa may obviate HIV infection’s influence on transfusion risk and additional research is needed to confirm our finding that moderately low platelet count impact transfusion risk.
Acknowledgments
The authors wish to thank the research nurses Srs. Hawa Khan, Ntombifuthi Makhanya, Ellen Makhale, Lily Ntombemhle Shabangu and Susan Maimela for their invaluable contribution to the study. We are also grateful to the medical and nursing personnel at Chris-Hani Baragwanath Hospital, King Edward VIII Hospital, Mowbray Maternity Hospital and Groote Schuur Hospital for their support. The Recipient Epidemiology and Donor Evaluation Study-III, International Program in South Africa, is the responsibility of the following: University of California, San Francisco, South African National Blood Service, RTI international, USA and the National Heart, Lung, and Blood Institute, NIH, USA
Source(s) of support: The work was supported by contracts from the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (Recipient Epidemiology and Donor Evaluation Study-III contracts HHSN268201100009I, HHSN268201100002I: HHSN26800002 and HHSN26800003).
Funding: National Heart, Lung and Blood Institute, National Institutes of Health, USA
Footnotes
Conflict of Interest: The authors have no disclosures or conflicts of interest related to this study.
Contribution to authorship
EMB, RC and ELM conceived the study and developed the methodology in communication with the clinical investigators (JH, SF, JA and RGT). CI together with SN and RC oversaw the study in South Africa. Each of the clinical investigators (JH, SF, JA and RGT) was responsible for activities at their individual hospital sites. They were also actively involved in interpretation of results and manuscript preparation. GRMB was involved in coordination of the Cape Town sites; he also contributed to manuscript preparation. LC was responsible for training and logistical coordination. DC was primarily responsible for data analysis and statistical support. EMB and ELM were primarily responsible for a first draft manuscript. All authors contributed to subsequent drafting and revising the final manuscript.
References
- 1.Bates I, Chapotera GK, McKew S, van den Broek N. Maternal mortality in sub-Saharan Africa: the contribution of ineffective blood transfusion services. BJOG. 2008;115:1331–9. doi: 10.1111/j.1471-0528.2008.01866.x. [DOI] [PubMed] [Google Scholar]
- 2.Lalonde A, Daviss BA, Acosta A, Herschderfer K. Postpartum hemorrhage today: ICM/FIGO initiative 2004–2006. Int J Gynaecol Obstet. 2006;94:243–53. doi: 10.1016/j.ijgo.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Homer C, Clements V, McDonnell N, Peek M, Sullivan E. Maternal mortality: what can we learn from stories of postpartum haemorrhage? Women and birth : journal of the Australian College of Midwives. 2009;22:97–104. doi: 10.1016/j.wombi.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi MN, Welz T, Ronsmans C. Severe acute maternal morbidity in rural South Africa. Int J Gynaecol Obstet. 2004;87:180–7. doi: 10.1016/j.ijgo.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Fawcus SR, van Coeverden de Groot HA, Isaacs S. A 50-year audit of maternal mortality in the Peninsula Maternal and Neonatal Service, Cape Town (1953–2002) BJOG. 2005;112:1257–63. doi: 10.1111/j.1471-0528.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 6.Cochet L, Pattinson RC, Macdonald AP. Severe acute maternal morbidity and maternal death audit--a rapid diagnostic tool for evaluating maternal care. S Afr Med J. 2003;93:700–2. [PubMed] [Google Scholar]
- 7.Mantel GD, Buchmann E, Rees H, Pattinson RC. Severe acute maternal morbidity: a pilot study of a definition for a near-miss. Br J Obstet Gynaecol. 1998;105:985–90. doi: 10.1111/j.1471-0528.1998.tb10262.x. [DOI] [PubMed] [Google Scholar]
- 8.Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2016;10:CD002042. doi: 10.1002/14651858.CD002042.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prick BW, Jansen AJ, Steegers EA, Hop WC, Essink-Bot ML, Uyl-de Groot CA, Akerboom BM, van Alphen M, Bloemenkamp KW, Boers KE, Bremer HA, Kwee A, van Loon AJ, Metz GC, Papatsonis DN, van der Post JA, Porath MM, Rijnders RJ, Roumen FJ, Scheepers HC, Schippers DH, Schuitemaker NW, Stigter RH, Woiski MD, Mol BW, van Rhenen DJ, Duvekot JJ. Transfusion policy after severe postpartum haemorrhage: a randomised non-inferiority trial. BJOG. 2014;121:1005–14. doi: 10.1111/1471-0528.12531. [DOI] [PubMed] [Google Scholar]
- 10.van den Berg K, Murphy EL, Pretorius L, Louw VJ. The impact of HIV-associated anaemia on the incidence of red blood cell transfusion: implications for blood services in HIV-endemic countries. Transfus Apher Sci. 2014;51:10–8. doi: 10.1016/j.transci.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ntusi NB, Sonderup MW. HIV/AIDS affects blood and blood product use at Groote Schuur Hospital, Cape Town. S Afr Med J. 2011;101:463–6. [PubMed] [Google Scholar]
- 12.Bloch EM, Crookes RL, Hull J, Fawcus S, Gangaram R, Anthony J, Ingram C, Ngcobo S, Croxford J, Creel DV, Murphy EL International Component of the NRE, Donor Evaluation S III. The impact of human immunodeficiency virus infection on obstetric hemorrhage and blood transfusion in South Africa. Transfusion. 2015;55:1675–84. doi: 10.1111/trf.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghunathan TE, Grizzle JE. A Split Questionnaire Survey Design. Journal of the American Statistical Association. 1995;90:54–63. [Google Scholar]
- 14.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45:1–67. [Google Scholar]
- 15.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? - Some practical clarifications of multiple imputation theory. Prevention Science. 2007;8:206–13. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- 16.Rubin DBSJL. Efficiently creating multiple imputations for incomplete multivariate normal data. Proceedings of the Statistical Computing Section of the American Statistical Association; Alexandria, VA. 1990; pp. 83–8. [Google Scholar]
- 17.Doove LL, Van Buuren S, Dusseldorp E. Recursive partitioning for missing data imputation in the presence of interaction effects. Computational Statistics & Data Analysis. 2014;72:92–104. [Google Scholar]
- 18.van Burren S. Flexible Imputation of Missing Data. Boca Raton, FL: Chapman & Hall/CRC; 2012. [Google Scholar]
- 19.Rubin DB. Multiple Imputation for Nonresponse in Surveys. USA: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 20.Madhavan Nair K, Bhaskaram P, Balakrishna N, Ravinder P, Sesikeran B. Response of hemoglobin, serum ferritin, and serum transferrin receptor during iron supplementation in pregnancy: a prospective study. Nutrition. 2004;20:896–9. doi: 10.1016/j.nut.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Milman N, Bergholt T, Byg KE, Eriksen L, Graudal N. Iron status and iron balance during pregnancy. A critical reappraisal of iron supplementation. Acta Obstet Gynecol Scand. 1999;78:749–57. [PubMed] [Google Scholar]
- 22.Krafft A, Murray-Kolb L, Milman N. Anemia and iron deficiency in pregnancy. J Pregnancy. 2012;2012:241869. doi: 10.1155/2012/241869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tunkyi K, Moodley J. Prevalence of anaemia in pregnancy in a regional health facility in South Africa. S Afr Med J. 2015;106:101–4. doi: 10.7196/SAMJ.2016.v106i1.9860. [DOI] [PubMed] [Google Scholar]
- 24.Shehata N, Chasse M, Colas JA, Murphy M, Forster AJ, Malinowski AK, Ducharme R, Fergusson DA, Tinmouth A, Wilson K. Risks and trends of red blood cell transfusion in obstetric patients: a retrospective study of 45,213 deliveries using administrative data. Transfusion. 2017;57:2197–205. doi: 10.1111/trf.14184. [DOI] [PubMed] [Google Scholar]
- 25.Valero De Bernabe J, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martinez D, Dominguez-Rojas V. Risk factors for low birth weight: a review. Eur J Obstet Gynecol Reprod Biol. 2004;116:3–15. doi: 10.1016/j.ejogrb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Slichter SJ. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transfus Med Rev. 2004;18:153–67. doi: 10.1016/j.tmrv.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 27.UN. Millenium Project: Goals, targets and indicators [monograph on the internet] 2006 Available from: http://www.unmillenniumproject.org/goals/gti.htm - goal5.
- 28.Bloch EM, Vermeulen M, Murphy E. Blood transfusion safety in Africa: a literature review of infectious disease and organizational challenges. Transfus Med Rev. 2012;26:164–80. doi: 10.1016/j.tmrv.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiegmann TL, Mintz PD. The growing role of AABB clinical practice guidelines in improving patient care. Transfusion. 2015;55:935–6. doi: 10.1111/trf.13093. [DOI] [PubMed] [Google Scholar]
- 30.Berger MD, Gerber B, Arn K, Senn O, Schanz U, Stussi G. Significant reduction of red blood cell transfusion requirements by changing from a double-unit to a single-unit transfusion policy in patients receiving intensive chemotherapy or stem cell transplantation. Haematologica. 2012;97:116–22. doi: 10.3324/haematol.2011.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, Gernsheimer T, Holcomb JB, Kaplan LJ, Katz LM, Peterson N, Ramsey G, Rao SV, Roback JD, Shander A, Tobian AA. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025–35. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 32.Shander A, Isbister J, Gombotz H. Patient blood management: the global view. Transfusion. 2016;56(Suppl 1):S94–102. doi: 10.1111/trf.13529. [DOI] [PubMed] [Google Scholar]
- 33.Collaborators WT. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–16. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]