Abstract
Cancer cells must adapt their metabolism in order to meet the energy requirements for cell proliferation, survival in nutrient-deprived environments, and dissemination. In particular, FA metabolism is emerging as a critical process for tumors. FA metabolism can be modulated through intrinsic changes in gene expression or signaling between tumor cells and also in response to signals from the surrounding microenvironment. Among these signals, extracellular vesicles (EVs) could play an important role in FA metabolism remodeling. In this review, we will present the role of EVs in tumor progression and especially in metabolic reprogramming. Particular attention will be granted to adipocytes. These cells, which are specialized in storing and releasing FAs, are able to shift tumor metabolism toward the use of FAs and, subsequently, increase tumor aggressiveness. Recent work demonstrates the involvement of EVs in this metabolic symbiosis.
Keywords: adipocytes, cancer, fatty acid metabolism, fatty acid oxidation, fatty acid synthesis, tumor microenvironment • exosomes • microvesicles • obesity • biomarker
Tumor cell traits were first limited to six hallmarks: self-supporting proliferation, insensitivity to growth suppressors, evading cell death, infinite replicative potential, supporting angiogenesis, and invasion/metastasis (1). However, a decade later, four other characteristics were advanced: genome instability, immune evasion, inflammation, and altered cell metabolism (2). The latter hallmark refers to the ability of cancer cells to reprogram their metabolism to fuel cell growth and promote local and distant dissemination. Moreover, the tumor microenvironment is often nutrient- and oxygen-deprived, driving cells to use alternative metabolic pathways in order to survive. Although the importance of glycolysis for tumor cells has long been recognized, the key role of lipid metabolism has only emerged more recently (3). Lipid metabolism reprogramming can be intrinsic to tumors or in response to external stimuli from the tumor microenvironment (3, 4). In particular, adipocytes, cells specialized in maintaining energy homeostasis through storing and releasing lipids, are emerging as key regulators of tumor lipid metabolism (5).
Cells can communicate through direct contact, the secretion of soluble factors, and via extracellular vesicles (EVs). The term EV subsumes three types of vesicles: microvesicles (also named microparticles or ectosomes), exosomes, and apoptotic bodies (6), the last being produced by membrane blebbing during apoptosis. Most EV studies concern vesicles secreted by living cells (i.e., exosomes and microvesicles). Therefore, the present review will focus only on these types of EVs. Microvesicles and exosomes can be distinguished by their mode of biogenesis. Indeed, microvesicles bud directly from the plasma membrane, whereas exosomes are formed in multivesicular bodies that fuse with the plasma membrane to release the contained vesicles. Moreover, microvesicles are highly heterogeneous in size and density (100–1,000 nm in diameter with varying densities), whereas exosomes range from 50 to 150 nm in diameter and from 1.13 to 1.19 in density (7). However, discriminating between these two populations of EVs proves extremely challenging experimentally. Therefore, the International Society of Extracellular Vesicles suggests using the term “EVs” to subsume all secreted vesicles isolated from the extracellular medium (8, 9). For clarity, in this review, the term “exosome” (used in many of the cited publications) will therefore be replaced by the term EV to be in accordance with these new guidelines. Consequently, the term EV will refer, here, to both exosomes and microvesicles.
EVs are composed of a lipid bilayer encapsulating a large variety of molecules, referred to as cargo, which includes proteins, lipids, and nucleic acids. This cargo is highly variable and depends on the cell type of origin and the cell state and its environment (7, 10). EVs can be internalized by other cells, allowing the horizontal transfer of cargo, or EVs can interact with cells through the receptors or ligands carried at their surface, inducing signaling cascades (6). EVs mediate communication between neighboring cells, but can also act at a distance as they circulate in body fluids (6).
EVs play a particularly important role in cancer, a disease in which they mediate communication not only between tumor cells themselves but also with their micro- and macroenvironment (11). Most studies involving EVs focus on protein and RNA content. However, increasing evidence demonstrates that EV cargo is more complex and includes metabolites, such as organic acids and their derivatives, nucleotides, sugars, and amines (12, 13). Moreover, EVs can influence the metabolism of recipient cells. We will discuss how EVs, within tumors and their surrounding environment, can affect glycolysis, oxidative phosphorylation, and lipid metabolism, the latter being the main subject of this review. Particular emphasis will be placed on the role of adipocyte vesicles in tumor lipid metabolism.
EVs AS KEY ACTORS IN TUMOR INITIATION AND PROGRESSION
The communication between the cells found within tumors and their surrounding stroma can be mediated by soluble factors such as cytokines, chemokines, growth factors, and matrix remodeling enzymes (14). Nevertheless, the importance of EVs in this dialog is now also clearly recognized (11).
Tumor-derived EVs as key players in communication between tumor cells
Tumors are highly heterogeneous tissues in which cancer cells present different phenotypes with variable levels of aggressiveness (15). The importance of EVs in the communication between these cells is now clearly recognized. Briefly, tumor EVs can transfer molecules, such as EGFRvIII (16) or KRAS (17), that promote oncogenic signaling, such as MAPK, PTEN, GSK3β, NOTCH, or AKT pathways, consequently promoting tumor initiation, progression, and/or drug resistance (18). Indeed, studies have proven the involvement of EVs in transfer of metastatic potential between tumor cells in vitro (19–21) and in vivo (22). Moreover, EVs can mediate drug resistance through the sequestration, transport, and/or expulsion of chemotherapeutic drugs from tumor cells [for recent reviews concerning this point, see (23, 24)].
Role of EVs in the dialog between tumor and stromal cells
Tumor micro- and macroenvironment.
Tumors are complex tissue diseases whose development and progression depend on a dynamic interaction with their microenvironment. Indeed, cancer cells profoundly modify the surrounding stroma, which, in turn, promotes tumor angiogenesis, growth, and dissemination, and influences the outcome of therapy (25). The tumor microenvironment is composed of noncancer cells (mainly fibroblasts, bone marrow-derived inflammatory cells, and immune and endothelial cells) and the extracellular matrix, which contains many distinct components including proteins, glycoproteins, and proteoglycans (26). The importance of cancer-associated fibroblasts (CAFs) and tumor-associated macrophages in tumor progression has long been described [for recent reviews, see (27, 28)]. In addition, other immune cells, vascular endothelial cells, pericytes, and bone-marrow mesenchymal stromal cells also contribute to the deleterious impact of the stroma on tumor behavior (14). Lastly, adipocytes also influence tumor progression, and this topic will be addressed in the last section of this review. As tumors progress to advanced stages, changes within the systemic tumor environment, or macroenvironment, favor dissemination to distant sites. These changes include modifications in bone marrow-derived cell mobilization and the preparation of pre-metastatic niches to which tumor cells will home (29, 30).
Tumor-derived EVs as key players in communication with their micro- and macroenvironment.
In addition to influencing other cancer cells, tumor-derived EVs also affect the tumor stroma (11). For example, cancer cell-derived EVs exhibit transforming growth factor β (TGFβ) at their surface, which activates fibroblasts (31). Cancer cell EVs can also be internalized by endothelial cells, leading to their activation and angiogenesis in squamous cell carcinoma (32), glioma (33), and chronic myeloid leukemia (34). Angiogenesis and cell invasion are also promoted by mesenchymal stem cells (MSCs) upon activation by prostate cancer-derived EVs (35). Tumor-derived EVs also promote tumor progression by exerting immunosuppressive functions (36). For instance, vesicular Fas ligand induces CD8+ T cell apoptosis (37, 38). Moreover, TGFβ and IL10 found within tumor EVs exert immunosuppressive functions (39). Vesicular TGFβ is also involved in inhibition of natural killer cells through downregulation of NKG2D/KLRK1 (killer cell lectin-like receptor subfamily K member 1) (40, 41). However, tumor-derived EVs can also present immunoactivation properties (36). Indeed, EVs can carry tumor antigens, which effectively induce the activation of dendritic cells and, subsequently, an anti-tumor response (42).
Finally, the role of tumor-derived EVs on distant organs was shown for the first time in a melanoma model, where EVs were found to be preferentially addressed to lymph nodes where they induced a permissive environment for metastasis called the pre-metastatic niche (43). Moreover, tumor-derived EVs are taken up by myeloid cells in the bone marrow, inhibiting their differentiation into dendritic cells and leading to the development of a myeloid-derived suppressor cell phenotype, which exerts tumor promoting properties by inhibiting T cell proliferation (44). Moreover, it was demonstrated that melanoma EVs “educate” bone marrow progenitors, leading to their recruitment and the development of a favorable pre-metastatic niche (30). Cancer EVs also control organotropism toward specific metastatic sites, which they target due to the distinct integrin combinations that they transport (45).
Role of stromal-derived EVs.
The role of immune cell EVs on tumors has been a well-studied field for some time. Indeed, more than 20 years ago, the pioneering work of Raposo et al. (46) paved the way to understanding how EVs could modulate the immune response to tumors. Their work showed that antigen-presenting cells secrete vesicles exhibiting major histocompatibility complexes I and II, and costimulatory molecules at their surface, which can prime naïve T cells (46), and similar processes have since been observed in many models [for review, see (47)]. On the other hand, after immune cell activation by cancer cells, the EVs that are then secreted by these cells can promote tumor progression. For instance, regulatory T lymphocyte vesicles are involved in immunosuppression (48, 49). Moreover, tumor-associated macrophage-derived vesicles were found to affect different aspects of tumor progression, including invasion (50) and resistance to drugs (51).
The effects of other stromal cell-derived EVs have only emerged more recently. CAF-derived EVs can promote cancer cell motility through two independent mechanisms involving WNT (52) or NOTCH (53) signaling in cancer cells. They also convey RNAs, which prompt STAT1 signaling that leads to radio- and chemoresistance in a breast cancer model (54). Moreover, bone marrow MSCs secrete EVs that influence tumor cell behavior and modulate other stromal cells. Indeed, MSCs activated by multiple myeloma cells secrete EVs that promote cancer cell proliferation (55). On the other hand, in a breast cancer model, these EVs induce a quiescent phenotype leading to resistance to therapy (56). MSC-derived EVs also induce the switching of activated T cells to regulatory T lymphocytes (57) and reduce the proliferation of B and natural killer cells (58), suggesting that MSC-derived EVs could also favor tumor escape from immune surveillance.
EVs AND METABOLISM
Metabolic remodeling in tumor cells, emerging role of FA metabolism
Cancer cells must display a high metabolic plasticity to generate the energy and to meet the biosynthetic demand required to support their proliferation and dissemination in a poorly oxygenated and nutrient-deprived microenvironment (59, 60). Cancer cells can metabolically adapt due to intrinsic alterations. Indeed, many oncogene signaling pathways modify gene expression, leading to the upregulation of enzymes involved in glycolysis, the pentose phosphate pathway, or FA synthesis (61, 62). Cancer cell metabolic remodeling can also take place in response to a metabolic cooperation with other tumor cells. Indeed, cancer cells in hypoxic regions use glucose to produce lactate, which then fuels the oxidative metabolism in well-oxygenated regions (63). Finally, a metabolic symbiosis also exists between tumors and their surrounding stroma. For example, CAFs display increased anaerobic glycolysis in response to tumor cell signals leading to lactate release, which is then used by tumor cells (known as the “reverse Warburg effect”) (64, 65).
The crucial role of lipid metabolism in tumor progression is becoming increasingly evident, and the microenvironment can influence this process. First, the glucose and glutamine provided by stromal cells could be substrates for de novo FA synthesis (or lipogenesis), a metabolic pathway increased in the vast majority of tumors (66). Indeed, they both feed the tricarboxylic acid (TCA) cycle, generating citrate that is cleaved into acetyl-CoA. Thereafter, de novo lipogenesis is mediated first by acetyl-CoA carboxylase, which converts acetyl-CoA to malonyl-CoA, and second by the multifunctional enzyme, FASN, which catalyzes the reactions to form palmitoyl-CoA. The newly synthetized lipids can be used for membrane synthesis during cancer cell growth, for ATP synthesis, or as signaling molecules for cell proliferation, survival, and dissemination [for review, see (66)]. Moreover, in contrast to normal cells, cancer cells can simultaneously synthesize and utilize FAs through FA oxidation (FAO) (67). The key role of FAO in cancer cell metabolism has recently emerged (68, 69). This catabolic process is upregulated in numerous tumors. Particularly, the expression of carnitine palmitoyltransferase 1 (CPT1), the rate-limiting enzyme involved in FA transport from the cytosol to the mitochondria, is increased in tumor cells and, in many models, CPT1 inhibition has an antitumor effect (69). Increased FAO in tumors drives cell proliferation (70) and survival [by preventing loss of attachment-induced anoikis (71)], both of which depend on ATP production. FAO also produces NADPH that allows cancer cells to counteract oxidative stress (72) and is an important coenzyme for the synthesis of building blocks to support tumor growth (73). More recently, the role of FAO in metastasis has been revealed (74).
Inside tumor cells, FAs are not always directly transferred to mitochondria for immediate FAO, but can be stored as triglycerides (TGs). In this case, cancer cells can later mobilize these lipids through lipophagy (75, 76) or lipolysis (77–79) and, in aggressive cancer cells, the lipolytic enzymes, adipose TG lipase (ATGL), hormone-sensitive lipase (HSL), (78) and monoacylglycerol lipase (MAGL) (79), are upregulated, leading to increased FA mobilization and, consequently, increasing FAO.
Very interestingly, in addition to de novo lipogenesis, adipocytes also play a crucial role in supplying cancer cells with exogenous FAs. Indeed, these lipid-filled cells are key regulators of tumor FA metabolism, supplying tumors with FAs and increasing FAO. This novel example of metabolic symbiosis will be developed in the last section.
Role of EVs in tumor metabolism remodeling
Proteins implicated in metabolism are among the most frequently identified proteins in EVs. Indeed, the top three proteins identified in the EVpedia database (80) are enzymes involved in glycolysis, underlining a probable role of EVs in metabolism. EVs also contain miRNAs that are known to target proteins implicated in metabolism (81), and recent studies point out the presence of metabolites in EVs (13, 82, 83). Moreover, enzymes carried by hepatocyte vesicles can modify serum metabolome, especially metabolites involved in oxidative stress (84, 85). Collectively, these studies suggest that regulation of cell metabolism by EVs could be multifactorial. Here, we will present the roles of EVs in tumor metabolism and, especially, FA metabolism. The role of these vesicles in the metabolic cross-talk that takes place between adipocytes and tumor cells will be addressed in the following section.
EVs and metabolism (excluding FA-related pathways).
Among the metabolic routes affected by EVs are sugar-related pathways. Indeed, in a study aiming to compare the exoproteome of a nonaggressive versus an aggressive hepatocellular carcinoma cell line, aggressive cell-derived EVs are enriched in sugar-related metabolic pathways, namely, glycolysis, gluconeogenesis, and pentose phosphate pathways (86). Hence, transfer of glycolytic enzymes between cancer cells may impact the metabolism of recipient cells. Indeed, it has been shown that glycolytic enzymes found within prostate-derived EVs are functional inside the vesicles and can generate ATP upon incubation with their substrates (87) and that this process is required for EV uptake (88). As glycolytic enzymes were found to be more abundant in the EVs secreted by aggressive cancer cells, these vesicles may be more efficiently taken up by recipient cells, leading to an increased transfer of material (86). Nevertheless, the presence of glycolytic enzymes in EVs is not necessarily associated with a functional transfer. Indeed, in a proteomic study performed on adipocyte EVs, we have identified a large number of proteins involved in glycolysis. Nevertheless, when tumor cells were treated with these vesicles, glucose oxidation and lactic acid release were unchanged (89).
Cell metabolism can be regulated not only through EV-mediated protein transfer but also through the transfer of other types of molecules. In a breast cancer model, Fong et al. (81) have shown that, through miR-122, cancer cell-derived EVs decrease the glycolytic flux in normal cells, increasing nutrient availability for tumor cells at pre-metastatic sites. Mechanistically, miR-122 acts by downregulating pyruvate kinase, which catalyzes the last step of glycolysis.
In a recent study, Zhao et al. (82) found that EVs secreted by CAFs promote prostate and pancreatic cancer cell proliferation, block mitochondrial oxidative phosphorylation, and promote glycolysis and glutamine-dependent reductive carboxylation. Metabolomic analysis revealed the presence of TCA-cycle intermediates, lipids, and amino acids in these vesicles, which may act directly or indirectly on the observed phenotype (82, 90).
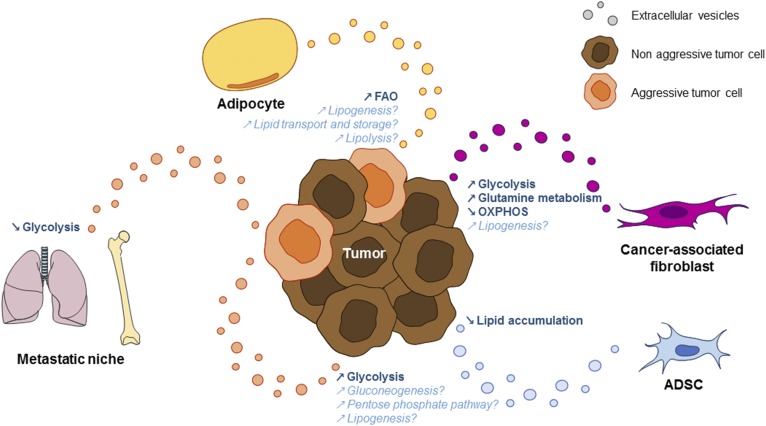
Thus, both tumor-derived and stromal-derived EVs affect many tumor metabolic pathways, ultimately promoting tumor progression (Fig. 1).
Fig. 1.
EVs mediate a metabolic cross-talk between tumor cells and with the stroma. EVs secreted by aggressive tumor cells can transfer molecules involved in sugar-related pathways and lipid synthesis to more indolent tumor cells. Tumor-derived EVs can also decrease glycolysis within the pre-metastatic niche to increase nutrient availability to metastatic cells. EVs from CAFs increase glycolysis and glutamine-dependent reductive carboxylation, but decrease oxidative phosphorylation in tumors. They also transport metabolites required for lipid synthesis, such as acetate. ADSCs secrete EVs containing miR-126, which decreases lipid accumulation in mammary epithelial cells. Finally, adipocyte EVs transport proteins implicated at all stages of lipid metabolism, including lipogenesis, lipid transport and storage, lipolysis, and FAO. These EVs are able to increase FAO in tumor cells, which consequently promotes tumor aggressiveness. Pathways known to be impacted by the indicated EVs are shown in bold. Pathways that could be affected by the molecules transported by the indicated EVs are shown in italic. This figure summarizes most of the citations from (80) to (119).
EVs and metabolism: FA-related pathways.
LIPOGENESIS.
One of the most frequently identified proteins in EVs is FASN, which is a key enzyme in the de novo synthesis of FAs (80). Not only the protein but also FASN mRNA have been identified in prostate cancer cell EVs (91), highlighting a possible role of EVs in lipogenesis in cancer. In accordance, in a study aiming to mimic the hypoxic conditions found in the adipose tissue (AT) of obese patients, Sano et al. (92) observed that hypoxic adipocyte-derived vesicles are enriched in enzymes involved in FA synthesis, namely, acetyl-CoA carboxylase and FASN. Using EVs secreted by HEK293T cells expressing a tagged FASN, the authors demonstrated that FASN can be transferred through EVs. Consistent with EV-induced lipogenesis, incubation of hypoxic adipocyte-derived EVs promoted an increase in lipid accumulation during the differentiation of recipient adipocytes (92). However, no studies have focused on this process in cancer cells.
The effect of EVs on lipogenesis is probably multifactorial and may not only depend on the protein compartment. Indeed, in a metabolomic analysis of CAF-derived vesicles, it was found that EVs transport metabolites required for lipid synthesis, such as acetate (82). These data suggest that EVs can supply recipient cells with lipogenic substrates, a feature highly relevant in cancer where tumor cells need these building blocks to proliferate. However, the role of EVs on lipogenesis is dual and surely depends on the cell of origin and the growth conditions. For example, miR-126 is present in the vesicles secreted by AT-derived stem cells (ADSCs) and is decreased when ADSCs are isolated from obese individuals (93). Interestingly, miR-126 was found to decrease lipid accumulation in mammary epithelial cells, while its inhibition led to an increased number of intracellular lipid droplets (LDs) concomitantly with a change in FASN level (94).
FA TRANSPORT AND STORAGE.
EVs are emerging as a novel mechanism to allow FA transport from cell to cell and across cell membranes. Usually albumin is required to transport FAs through the circulation and, upon internalization, other intracellular carriers, such as FA binding proteins (FABPs), are required. However, a number of studies now show that EVs also transport FAs (95). Different FA forms are transported by EVs, although they are enriched in saturated FAs as opposed to monounsaturated and polyunsaturated FAs. EV FAs can be generated from phospholipids through phospholipase activities within the vesicles themselves (96). However, they also originate directly from parental cells, as the quantity of FAs found within EVs is greater than the amount that could be generated from their own phospholipids (96). Tri- and diglycerides have also been observed in EVs secreted by prostate cancer cells, and their presence is regulated by hypoxia. Indeed, after exposure to 1% O2, EVs were significantly enriched in TGs and the nature of the FAs composing the tri- and diglycerides was modified. For instance, myristic and palmitoleic chains are respectively increased and decreased under hypoxia (97).
Moreover, FABPs, key extracellular and intracellular FA transporters, are abundant in EVs released by many cell types [EVpedia database (80)]. Another membrane-associated FA transporter, CD36, has been identified in the EVs secreted by macrophages (98) and is involved in the control of EV uptake (99). Once internalized, FAs are converted to fatty acyl-CoAs that are transported by acyl-CoA binding proteins, which have been identified in hepatocellular carcinoma EVs (100, 101). However, the functions of these vesicular transporters in tumor cells remain to be studied.
Once inside cells, FAs must be stored in LDs to avoid lipotoxicity (102). For this, in the endoplasmic reticulum, fatty acyl-CoA is used to generate diacylglycerols by glycerolipid-synthesis enzymes [such as 1-acylglycerol-3-phosphate acyltransferase, which has been identified in hepatocyte- and hepatocellular carcinoma-derived EVs (101)]. These diacylglycerols are then converted to triacylglycerols by diacylglycerol O-acyltransferases (DGATs). EVs from many different cancer models contain DGAT1 protein (103) and, in colon cancer and glioblastoma EVs, DGAT1 mRNA has been identified (104, 105). After synthesis, neutral lipids then amass to form LDs that are coated with structural proteins named perilipins, which play important roles in protecting stored lipids from cytosolic lipases. Perilipin-2 has been identified in the EVs secreted by numerous cancer cells (brain, colon, kidney, lung, and ovarian cancer cells and melanoma cells) (103). Caveolins are also found within LDs (106) and their presence on these organelles affects the phospholipid and protein surface composition of LDs, as well as LD size (107). Interestingly, caveolins are abundant in many types of EVs, especially those secreted by cancer cells (19, 103). Caveolin-1 has now been recognized as a tumor promoter (108) and interestingly, in melanoma patients, plasma levels of EVs transporting caveolin-1 are much higher than in healthy individuals (109).
TG LIPOLYSIS.
To be used by cancer cells, stored lipids must be mobilized. This process is often regulated by cytoplasmic lipases through lipolysis. Briefly, multiple steps of hydrolysis breakdown TGs by removing FA. First, ATGL converts triacylglycerol to diacylglycerol, and then HSL carries out the next step of hydrolysis to form monoacylglycerol. Finally, MAGL converts monoacylglycerol to glycerol, thus releasing the third FA (110). Both HSL and MAGL proteins are found in the EVs secreted by different tumor cell types (103). Although mRNAs of all three of these lipases have also been identified in the EVs from colon cancer cells (104), data are lacking to show the functional role of these vesicular molecules. An important regulator of lipolysis is protein kinase A (PKA) (or cAMP-dependent protein kinase). PKA phosphorylates perilipin-1, releasing ATGL’s coactivator, comparative gene identification 58 (CGI-58 or ABHD5), which rapidly disperses into the cytoplasm, enabling ATGL activation (111). PKA also mediates translocation of HSL to LDs. Moreover, PKA phosphorylates and subsequently activates HSL and also phosphorylates perilipin-1, leading to conformational changes in order to act as a scaffold to HSL on the LD surface (112, 113). PKA has been identified in tumor cell EVs (103, 114, 115) as well as CGI-58 mRNA (104), but their role in tumor EV-mediated lipolysis has not been studied. However, it has been shown that cancer EV-generated adenosine can induce a cAMP response in T cells (116). Thus, one can postulate that such a response in other cell types could lead to PKA activation and subsequent lipolysis.
FAO.
The role of EVs on lipid metabolism is complex as they are implicated in lipid synthesis and storage but also in FA mobilization and use as an energy source by FAO. FAO requires the transport of FAs into the mitochondria. This process is catalyzed by CPT1, which transfers the acyl group of a fatty acyl-CoA from CoA to carnitine. Hence, carnitine is a key metabolite required for FAO. Interestingly, a recent study showed that EVs from prostate cancer patients are enriched in carnitine, suggesting that FA transport to mitochondria is increased in prostate cancer (13). Once FAs enter the mitochondria, FAO ensues in successive cycles that each cleave two carbons from the acyl-CoA chain and form one acetyl-CoA. Each cycle also produces FADH2 and NADH, which are used by the electron transport chain to produce ATP. The four main enzymes involved in FAO are (in order) acyl-CoA dehydrogenase, enoyl-CoA hydratase, hydroxyacyl-CoA dehydrogenase, and ketoacyl-CoA thiolase. These enzymes have been found in the EVs secreted by tumor cells in a number of models, as well as in the EVs isolated from pleural effusions of lung cancer patients (103, 115, 117). FAO can be modulated by PPARs (118, 119) and, interestingly, both protein and mRNA from different PPAR isoforms have been identified in the EVs from tumor cell lines (103–105). Finally, FAO can be modulated directly through an increase in substrate availability and, as discussed above, EVs can carry TGs and FAs. Taken together, these data suggest that the impact of EVs on FAO is probably multifactorial and regulated by the transport of the metabolites, substrates, and enzymes required for this process.
These studies demonstrate that EVs are enriched in molecules involved in FA transport and storage, as well as lipolysis and FAO. Although, their function within cancer cells is not always fully elucidated, these data suggest an important role of EVs in tumor lipid metabolism (Fig. 1).
EVs MEDIATE A METABOLIC SYMBIOSIS BETWEEN ADIPOCYTES AND TUMORS
Adipocytes as key players in the tumor microenvironment
Among the cell types found within the tumor microenvironment, adipocytes were long ignored, although their role in cancer is of major clinical interest due to the established link between obesity and cancer occurrence and progression (120–122). In addition to their most well-known role of storing FAs in the form of TGs and releasing them to supply energy when needed, adipocytes are also powerful endocrine cells. Indeed, they secrete a large panel of bioactive molecules, named adipokines, including hormones, growth factors, chemokines, and pro-inflammatory molecules, whose balance is perturbed in obesity (123, 124). Due to the distribution of AT, many cancer cells will come into contact with adipocytes during local invasion (for example in breast, colon, and prostate cancers, as well as melanoma) but also distant dissemination (to the omental fat for gastric and ovarian cancer, or to the bone marrow for several tumor types such as prostate cancer) (125, 126). Using a coculture system, we have demonstrated that adipocytes cultivated with cancer cells display a modified phenotype, with decreased lipid content and downregulation of adipocyte markers. Moreover, these cells present an activated phenotype, marked by overexpression of proteases (including MMP11) and pro-inflammatory cytokines (IL6, IL1β). We named these activated adipocytes cancer-associated adipocytes (CAAs) (127). After continued exposure to cancer cells, CAAs undergo further delipidation and adopt a fibroblast-like morphology, giving rise to adipocyte-derived fibroblasts, which could contribute to the CAF population (desmoplastic reaction) (128). In turn, CAAs enhance tumor aggressiveness by promoting cancer cell survival and proliferation (129), as well as migration and invasion (127), by secreting extracellular matrix proteins, proteases, and pro-inflammatory cytokines, and by modulating cancer cell metabolism [for recent reviews, see (5, 130, 131)]. We have confirmed the presence of CAAs at the invasive front of human breast tumors, where the two populations are in close contact (127), and similar observations have been made in various cancers, indicating that this is a general phenomenon. Indeed, when AT is in contact with tumors, it disappears and is replaced by a desmoplastic reaction (5, 131).
Among the mechanisms that orchestrate the tumor-promoting effect of adipocytes, modified FA metabolism in both cell types is emerging as a key process. First, as stated before, under the influence of tumor cells, adipocytes undergo massive delipidation and loss of adipocyte markers. This phenotype was originally proposed to be the result of “dedifferentiation” (127), although recent findings from our laboratory, and others, show that tumor cells also induce adipocyte lipolysis (78, 129, 132–134). The released FAs are then transferred to cancer cells and stored as TGs (78, 127, 129, 132). When needed, these FAs are mobilized to fuel FAO, as previously discussed. Moreover, numerous studies show that adipocytes stimulate FAO in ovarian (132), breast (78, 129), and colon (135) cancer and in leukemia (136). In their elegant study, Nieman et al. (132) have shown that adipocytes transfer FAs to ovarian tumor cells, promoting FAO and tumor growth, and propose the importance of subsequent energy production. However, ATP production was not studied in this model. In breast cancer, we have recently shown that adipocytes induce FAO that is uncoupled from ATP production, which supports invasion, but not proliferation, of cancer cells (78). Although the mechanisms linking uncoupled FAO and invasion remain elusive, different hypotheses could be proposed. Accumulation of TCA intermediates (i.e., acetyl-CoA) could contribute to epigenetic changes that modify gene expression (137). In addition, uncoupling protein 2 (UCP2) is overexpressed in breast cancer cells after coculture with adipocytes. As this protein has previously been shown to be implicated in epithelial to mesenchymal transition (138), a similar process could be responsible for the pro-invasive effect of adipocytes. Therefore, although the impact of adipocytes on tumor FAO is clearly demonstrated, the effect of this metabolic remodeling may depend on the cancer model. Finally, we have demonstrated that the lipolytic enzyme, ATGL, is upregulated in response to adipocytes (78). Thus, the lipids transferred from adipocytes at the primary tumor site, which are stored by tumor cells, can be mobilized later by ATGL to fuel FAO and concomitantly increase invasiveness for dissemination toward distant organs (78).
Until recently, most of the studies performed show that the deleterious cross-talk between adipocytes and cancer cells is established when tumor cells invade the surrounding AT, with close proximity between the two cell types. However, adipocytes can also influence tumor cells at a distance and favor the early steps of dissemination by secreting chemokines that promote tumor dissemination toward AT (132, 139, 140). Concerning the effect of adipocytes on distant tumors, the role of exosomes could also be of key importance.
EVs orchestrate a bidirectional metabolic cross-talk between adipocytes and tumor cells
Several novel studies reveal the involvement of EVs in the dialog between tumors and adipocytes. Interestingly, as EVs travel through the circulation, they could allow adipocytes and tumors to communicate not only at proximity but also at a distance.
Tumor-derived EVs could mediate adipocyte delipidation that, as discussed above, could lead to FA transfer to cancer cells. Indeed, pancreatic cancer cells secrete EVs that promote adipocyte lipolysis through adrenomedullin-induced signaling, which consequently leads to phosphorylation and activation of HSL (141). Whether this process is specific to highly cachexic cancer models, such as pancreatic cancer, remains to be determined.
Our laboratory recently revealed that naïve adipocytes, which have never been in contact with tumor cells, secrete EVs that promote melanoma aggressiveness through metabolic reprogramming in favor of FAO (89). Using an untargeted proteomic analysis, we found that adipocyte vesicles are enriched in proteins involved in lipid metabolism, including enzymes catalyzing FAO. This signature is specific to adipocytes (89). Incubation of adipocyte-derived EVs conferred increased migration and invasion to cancer cells. These aggressive traits were associated with increased FAO in a melanoma and also in a prostate cancer model. Incubation with two different inhibitors of FAO, etomoxir and trimetazidine, which respectively inhibit the first and last step of FAO, led to a complete rescue of the phenotype, hence demonstrating that adipocyte-derived EVs promote cancer aggressiveness through FAO. In obesity, AT is under chronic stress (142), such as hypoxia and inflammation, which are known to affect EV composition and secretion (92, 143, 144). Accordingly, we showed that adipocytes from obese individuals secrete significantly more EVs than those from normal weight individuals. Moreover, when used at equal concentrations, the EVs from obese individuals had an amplified effect on tumor cell migration, which remained dependent on FAO. These quantitative and qualitative modifications of adipocyte EVs could work in synergy in obese patients, favoring tumor aggressiveness to an even higher extent (89).
This work reveals, for the first time, the importance of adipocyte EVs in tumor progression. In our study, we also identified proteins implicated in all the other stages of FA metabolism, including FA uptake, transport, and storage as well as lipolysis (Fig. 2). Although the functional implications of these proteins in recipient tumor cells remain to be studied, we postulate that adipocyte EVs induce a global remodeling of FA metabolism, ultimately leading to a switch toward FAO, which is required for increased cell invasion.
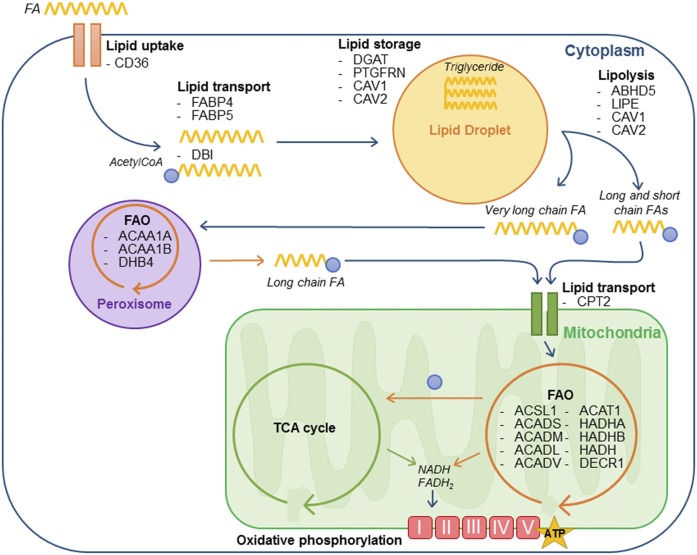
Fig. 2.
Adipocyte EVs transport proteins involved in FA metabolism. Exogenous FAs are taken up by tumor cells, then transported (either as FAs by FABPs or after conversion to fatty acyl-CoAs by acyl-CoA binding proteins) to LDs to be stored as TGs. These lipids then undergo lipolysis to mobilize fatty acyl-CoAs, which can be transferred directly to mitochondria (for long and short chain fatty acyl-CoAs) or be processed first by peroxisomal FAO to shorten chain length before transfer to mitochondria (for very long chain fatty acyl-CoAs). These fatty acyl-CoAs then undergo mitochondrial FAO, producing one acetyl-CoA per cycle that enters the TCA cycle. The NADH and FADH2 produced by both FAO and the TCA cycle then fuel the electron transport chain to produce ATP. The proteins (gene names are indicated) that we identified in adipocyte EVs in our proteomic study (89), and which are implicated in every stage of exogenous FA metabolism, are shown. ABHD5, abhydrolase domain containing 5; ACAA, acetyl-CoA acyltransferase; ACADL, long-chain-specific acyl-CoA dehydrogenase; ACADM, medium-chain-specific acyl-CoA dehydrogenase; ACADS, short-chain-specific acyl-CoA dehydrogenase; ACADV, very long-chain-specific acyl-CoA dehydrogenase; ACSL1, acyl-CoA synthetase long-chain family member 1; CAV, caveolin; CPT2, carnitine palmitoyltransferase 2; DECR1, 2,4-dienoyl-CoA reductase; DHB4/HSD17B4, peroxisomal multifunctional enzyme type 2; HADHA, trifunctional enzyme subunit α HADHB, trifunctional enzyme subunit β LIPE, lipase E (hormone sensitive type); PTGFRN, prostaglandin F2 receptor inhibitor.
CONCLUSIONS AND PERPECTIVES
EVs are key actors in tumor metabolism remodeling due to the proteins and nucleic acids, and also to the metabolic substrates, which they convey. Among the metabolic pathways affected by EVs is FA metabolism. Numerous molecules involved in lipogenesis as well as in FA uptake, transport, storage, mobilization, and utilization by FAO are present in EVs. Nevertheless, whether these molecules are effectively transferred to recipient cells, consequently influencing their metabolism, remains to be studied for most of them. Moreover, the supply of metabolites by EVs in the metabolism of receiving cells deserves further studies. Thus, particular effort should now be focused on deciphering the functional role of EVs in FA metabolism.
Given the importance of lipid metabolism in tumor aggressiveness (3, 145), targeting this EV-mediated dialog could be a novel therapeutic strategy. Moreover, as EVs circulate in body fluids, such as blood, saliva, and urine, metabolic markers present on tumor EVs could serve as a noninvasive tool for diagnosis and prognosis (146). Indeed, studies show that EVs from aggressive cancer cell lines are enriched in FA metabolism proteins such as FASN (147). Interestingly, the FA transporter, FABP5, is significantly increased in urine EVs from prostate cancer patients, when compared with healthy individuals (148). Moreover, EV-associated FABP5 correlated with the Gleason score, a grading system of prostate cancer aggressiveness (148). Although further studies are needed to fully elucidate the potential of metabolic biomarkers on tumor EVs, these data open new and encouraging perspectives for the use of EVs as diagnostic and prognostic tools.
Adipocytes are cells that are specialized in maintaining energy homeostasis through storing and releasing FAs. In a tumor context, these cells undergo massive delipidation, which can supply tumor cells with FAs to fuel FAO. Interestingly, adipocyte EVs are highly enriched in proteins involved in FA metabolism, including FAO. Therefore, adipocytes provide tumors with both the substrate and the machinery needed for optimal FAO. Accordingly, we have shown that adipocyte EVs promote FAO and, consequently, tumor aggressiveness. Interestingly, the effect of adipocyte EVs is further amplified by obesity and, again, depends entirely on FAO (89). These data suggest that targeting FAO could be particularly relevant for obese cancer patients and, in the long term, targeting this EV-mediated dialog could lead to the development of new therapeutic strategies.
Footnotes
Abbreviations:
- ADSC
- adipose tissue-derived stem cell
- AT
- adipose tissue
- ATGL
- adipose triglyceride lipase
- CAA
- cancer-associated adipocyte
- CAF
- cancer-associated fibroblast
- CPT1
- carnitine palmitoyltransferase 1
- DGAT
- diacylglycerol O-acyltransferase
- EV
- extracellular vesicle
- FABP
- fatty acid binding protein
- FAO
- FA oxidation
- HSL
- hormone-sensitive lipase
- LD
- lipid droplet
- MAGL
- monoacylglycerol lipase
- MSC
- mesenchymal stem cell
- PKA
- protein kinase A
- TCA
- tricarboxylic acid
- TG
- triglyceride
- TGFβ
- transforming growth factor β
This work was supported by Société Française de Dermatologie et de Pathologie Sexuellement Transmissible Grant 140372, Fondation ARC pour la Recherche sur le Cancer Grant PGA1*20160203841 and Ligue Contre le Cancer Grant 42637 (PhD fellowships to I.L. and E.C.), Fondation de France Grant 00081132 (C.A.), and Institut National Du Cancer Grant PLBIO 2016-176. The team was funded by Fondation ARC pour la Recherche sur le Cancer.
REFERENCES
- 1.Hanahan D., and Weinberg R. A.. 2000. The hallmarks of cancer. Cell. 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., and Weinberg R. A.. 2011. Hallmarks of cancer: the next generation. Cell. 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 3.Liu Q., Luo Q., Halim A., and Song G.. 2017. Targeting lipid metabolism of cancer cells: a promising therapeutic strategy for cancer. Cancer Lett. 401: 39–45. [DOI] [PubMed] [Google Scholar]
- 4.Ray U., and Roy S. S.. 2018. Aberrant lipid metabolism in cancer cells - the role of oncolipid-activated signaling. FEBS J. 285: 432–443. [DOI] [PubMed] [Google Scholar]
- 5.Duong M. N., Geneste A., Fallone F., Li X., Dumontet C., and Muller C.. 2017. The fat and the bad: mature adipocytes, key actors in tumor progression and resistance. Oncotarget. 8: 57622–57641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tkach M., and Thery C.. 2016. Communication by extracellular vesicles: where we are and where we need to go. Cell. 164: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 7.Colombo M., Raposo G., and Thery C.. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30: 255–289. [DOI] [PubMed] [Google Scholar]
- 8.Gould S. J., and Raposo G.. 2013. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles. 2: 20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lötvall J., Hill A. F., Hochberg F., Buzás E. I., Di Vizio D., Gardiner C., Gho Y. S., Kurochkin I. V., Mathivanan S., Quesenberry P., et al. 2014. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 3: 26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowal J., Tkach M., and Thery C.. 2014. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 29: 116–125. [DOI] [PubMed] [Google Scholar]
- 11.Wendler F., Favicchio R., Simon T., Alifrangis C., Stebbing J., and Giamas G.. 2017. Extracellular vesicles swarm the cancer microenvironment: from tumor-stroma communication to drug intervention. Oncogene. 36: 877–884. [DOI] [PubMed] [Google Scholar]
- 12.Yáñez-Mó M., Siljander P. R., Andreu Z., Zavec A. B., Borràs F. E., Buzas E. I., Buzas K., Casal E., Cappello F., Carvalho J., et al. 2015. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 4: 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puhka M., Takatalo M., Nordberg M. E., Valkonen S., Nandania J., Aatonen M., Yliperttula M., Laitinen S., Velagapudi V., Mirtti T., et al. 2017. Metabolomic profiling of extracellular vesicles and alternative normalization methods reveal enriched metabolites and strategies to study prostate cancer-related changes. Theranostics. 7: 3824–3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quail D. F., and Joyce J. A.. 2013. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19: 1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meacham C. E., and Morrison S. J.. 2013. Tumour heterogeneity and cancer cell plasticity. Nature. 501: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., and Rak J.. 2008. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10: 619–624. [DOI] [PubMed] [Google Scholar]
- 17.Demory Beckler M., Higginbotham J. N., Franklin J. L., Ham A. J., Halvey P. J., Imasuen I. E., Whitwell C., Li M., Liebler D. C., and Coffey R. J.. 2013. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol. Cell. Proteomics. 12: 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu K., Xing F., Wu S. Y., and Watabe K.. 2017. Extracellular vesicles as emerging targets in cancer: Recent development from bench to bedside. Biochim. Biophys. Acta. 1868: 538–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazar I., Clement E., Ducoux-Petit M., Denat L., Soldan V., Dauvillier S., Balor S., Burlet-Schiltz O., Larue L., Muller C., et al. 2015. Proteome characterization of melanoma exosomes reveals a specific signature for metastatic cell lines. Pigment Cell Melanoma Res. 28: 464–475. [DOI] [PubMed] [Google Scholar]
- 20.Higginbotham J. N., Demory Beckler M., Gephart J. D., Franklin J. L., Bogatcheva G., Kremers G. J., Piston D. W., Ayers G. D., McConnell R. E., Tyska M. J., et al. 2011. Amphiregulin exosomes increase cancer cell invasion. Curr. Biol. 21: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangoda L., Liem M., Ang C. S., Keerthikumar S., Adda C. G., Parker B. S., and Mathivanan S.. 2017. Proteomic profiling of exosomes secreted by breast cancer cells with varying metastatic potential. Proteomics. 17: 201600370. [DOI] [PubMed] [Google Scholar]
- 22.Zomer A., Maynard C., Verweij F. J., Kamermans A., Schafer R., Beerling E., Schiffelers R. M., de Wit E., Berenguer J., Ellenbroek S. I. J., et al. 2015. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 161: 1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel P., Fabbri M., and Carter D. R. F.. 2017. Mechanisms of drug resistance in cancer: the role of extracellular vesicles. Proteomics. 17: 201600375. [DOI] [PubMed] [Google Scholar]
- 24.Bach D. H., Hong J. Y., Park H. J., and Lee S. K.. 2017. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int. J. Cancer. 141: 220–230. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D., and Coussens L. M.. 2012. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 21: 309–322. [DOI] [PubMed] [Google Scholar]
- 26.Lu P., Weaver V. M., and Werb Z.. 2012. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalluri R. 2016. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 16: 582–598. [DOI] [PubMed] [Google Scholar]
- 28.Qian B. Z., and Pollard J. W.. 2010. Macrophage diversity enhances tumor progression and metastasis. Cell. 141: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutkowski M. R., Svoronos N., Perales-Puchalt A., and Conejo-Garcia J. R.. 2015. The tumor macroenvironment: cancer-promoting networks beyond tumor beds. Adv. Cancer Res. 128: 235–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peinado H., Aleckovic M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., Garcia-Santos G., Ghajar C., et al. 2012. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18: 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webber J., Steadman R., Mason M. D., Tabi Z., and Clayton A.. 2010. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 70: 9621–9630. [DOI] [PubMed] [Google Scholar]
- 32.de Andrade A., de Oliveira C. E., Dourado M. R., Macedo C. C. S., Winck F. V., Paes Leme A. F., Salo T., Coletta R. D., de Almeida Freitas R., et al. Extracellular vesicles from oral squamous carcinoma cells display pro- and antiangiogenic properties. Oral Dis. Epub ahead of print. September 8, 2017; doi:10.1111/odi.12765. [DOI] [PubMed] [Google Scholar]
- 33.Kucharzewska P., Christianson H. C., Welch J. E., Svensson K. J., Fredlund E., Ringner M., Morgelin M., Bourseau-Guilmain E., Bengzon J., and Belting M.. 2013. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA. 110: 7312–7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mineo M., Garfield S. H., Taverna S., Flugy A., De Leo G., Alessandro R., and Kohn E. C.. 2012. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis. 15: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowdhury R., Webber J. P., Gurney M., Mason M. D., Tabi Z., and Clayton A.. 2015. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget. 6: 715–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robbins P. D., and Morelli A. E.. 2014. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whiteside T. L. 2013. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem. Soc. Trans. 41: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L., Wu X., Wang D., Luo C., and Chen L.. 2013. Renal carcinoma cell-derived exosomes induce human immortalized line of Jurkat T lymphocyte apoptosis in vitro. Urol. Int. 91: 363–369. [DOI] [PubMed] [Google Scholar]
- 39.Rong L., Li R., Li S., and Luo R.. 2016. Immunosuppression of breast cancer cells mediated by transforming growth factor-beta in exosomes from cancer cells. Oncol. Lett. 11: 500–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clayton A., Mitchell J. P., Court J., Linnane S., Mason M. D., and Tabi Z.. 2008. Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 180: 7249–7258. [DOI] [PubMed] [Google Scholar]
- 41.Szczepanski M. J., Szajnik M., Welsh A., Whiteside T. L., and Boyiadzis M.. 2011. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 96: 1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfers J., Lozier A., Raposo G., Regnault A., Thery C., Masurier C., Flament C., Pouzieux S., Faure F., Tursz T., et al. 2001. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 7: 297–303. [DOI] [PubMed] [Google Scholar]
- 43.Hood J. L., San R. S., and Wickline S. A.. 2011. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71: 3792–3801. [DOI] [PubMed] [Google Scholar]
- 44.Valenti R., Huber V., Filipazzi P., Pilla L., Sovena G., Villa A., Corbelli A., Fais S., Parmiani G., and Rivoltini L.. 2006. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 66: 9290–9298. [DOI] [PubMed] [Google Scholar]
- 45.Hoshino A., Costa-Silva B., Shen T. L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. 2015. Tumour exosome integrins determine organotropic metastasis. Nature. 527: 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raposo G., Nijman H. W., Stoorvogel W., Liejendekker R., Harding C. V., Melief C. J., and Geuze H. J.. 1996. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183: 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen C., Seeger R. C., Fabbri M., Wang L., Wayne A. S., and Jong A. Y.. 2017. Biological roles and potential applications of immune cell-derived extracellular vesicles. J. Extracell. Vesicles. 6: 1400370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smyth L. A., Ratnasothy K., Tsang J. Y., Boardman D., Warley A., Lechler R., and Lombardi G.. 2013. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur. J. Immunol. 43: 2430–2440. [DOI] [PubMed] [Google Scholar]
- 49.Okoye I. S., Coomes S. M., Pelly V. S., Czieso S., Papayannopoulos V., Tolmachova T., Seabra M. C., and Wilson M. S.. 2014. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 41: 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang M., Chen J., Su F., Yu B., Lin L., Liu Y., Huang J. D., and Song E.. 2011. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer. 10: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng P., Chen L., Yuan X., Luo Q., Liu Y., Xie G., Ma Y., and Shen L.. 2017. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J. Exp. Clin. Cancer Res. 36: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luga V., Zhang L., Viloria-Petit A. M., Ogunjimi A. A., Inanlou M. R., Chiu E., Buchanan M., Hosein A. N., Basik M., and Wrana J. L.. 2012. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 151: 1542–1556. [DOI] [PubMed] [Google Scholar]
- 53.Shimoda M., Principe S., Jackson H. W., Luga V., Fang H., Molyneux S. D., Shao Y. W., Aiken A., Waterhouse P. D., Karamboulas C., et al. 2014. Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nat. Cell Biol. 16: 889–901. [DOI] [PubMed] [Google Scholar]
- 54.Boelens M. C., Wu T. J., Nabet B. Y., Xu B., Qiu Y., Yoon T., Azzam D. J., Twyman-Saint Victor C., Wiemann B. Z., Ishwaran H., et al. 2014. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 159: 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roccaro A. M., Sacco A., Maiso P., Azab A. K., Tai Y. T., Reagan M., Azab F., Flores L. M., Campigotto F., Weller E., et al. 2013. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J. Clin. Invest. 123: 1542–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bliss S. A., Sinha G., Sandiford O. A., Williams L. M., Engelberth D. J., Guiro K., Isenalumhe L. L., Greco S. J., Ayer S., Bryan M., et al. 2016. Mesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrow. Cancer Res. 76: 5832–5844. [DOI] [PubMed] [Google Scholar]
- 57.Zhang B., Yin Y., Lai R. C., Tan S. S., Choo A. B., and Lim S. K.. 2014. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 23: 1233–1244. [DOI] [PubMed] [Google Scholar]
- 58.Di Trapani M., Bassi G., Midolo M., Gatti A., Kamga P. T., Cassaro A., Carusone R., Adamo A., and Krampera M.. 2016. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 6: 24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeBerardinis R. J., and Chandel N. S.. 2016. Fundamentals of cancer metabolism. Sci. Adv. 2: e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lehuédé C., Dupuy F., Rabinovitch R., Jones R. G., and Siegel P. M.. 2016. Metabolic plasticity as a determinant of tumor growth and metastasis. Cancer Res. 76: 5201–5208. [DOI] [PubMed] [Google Scholar]
- 61.Cairns R. A., Harris I. S., and Mak T. W.. 2011. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 11: 85–95. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida G. J. 2015. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J. Exp. Clin. Cancer Res. 34: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sonveaux P., Vegran F., Schroeder T., Wergin M. C., Verrax J., Rabbani Z. N., De Saedeleer C. J., Kennedy K. M., Diepart C., Jordan B. F., et al. 2008. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118: 3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavlides S., Whitaker-Menezes D., Castello-Cros R., Flomenberg N., Witkiewicz A. K., Frank P. G., Casimiro M. C., Wang C., Fortina P., Addya S., et al. 2009. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 8: 3984–4001. [DOI] [PubMed] [Google Scholar]
- 65.Martinez-Outschoorn U. E., Lisanti M. P., and Sotgia F.. 2014. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin. Cancer Biol. 25: 47–60. [DOI] [PubMed] [Google Scholar]
- 66.Röhrig F., and Schulze A.. 2016. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer. 16: 732–749. [DOI] [PubMed] [Google Scholar]
- 67.Menendez J. A. 2010. Fine-tuning the lipogenic/lipolytic balance to optimize the metabolic requirements of cancer cell growth: molecular mechanisms and therapeutic perspectives. Biochim. Biophys. Acta. 1801: 381–391. [DOI] [PubMed] [Google Scholar]
- 68.Carracedo A., Cantley L. C., and Pandolfi P. P.. 2013. Cancer metabolism: fatty acid oxidation in the limelight. Nat. Rev. Cancer. 13: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qu Q., Zeng F., Liu X., Wang Q. J., and Deng F.. 2016. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 7: e2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zaugg K., Yao Y., Reilly P. T., Kannan K., Kiarash R., Mason J., Huang P., Sawyer S. K., Fuerth B., Faubert B., et al. 2011. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 25: 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schafer Z. T., Grassian A. R., Song L., Jiang Z., Gerhart-Hines Z., Irie H. Y., Gao S., Puigserver P., and Brugge J. S.. 2009. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 461: 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pike L. S., Smift A. L., Croteau N. J., Ferrick D. A., and Wu M.. 2011. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim. Biophys. Acta. 1807: 726–734. [DOI] [PubMed] [Google Scholar]
- 73.Chiarugi A., Dolle C., Felici R., and Ziegler M.. 2012. The NAD metabolome–a key determinant of cancer cell biology. Nat. Rev. Cancer. 12: 741–752. [DOI] [PubMed] [Google Scholar]
- 74.Rodrigues M. F., Obre E., de Melo F. H., Santos G. C. Jr., Galina A., Jasiulionis M. G., Rossignol R., Rumjanek F. D., and Amoedo N. D.. 2016. Enhanced OXPHOS, glutaminolysis and beta-oxidation constitute the metastatic phenotype of melanoma cells. Biochem. J. 473: 703–715. [DOI] [PubMed] [Google Scholar]
- 75.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A. M., and Czaja M. J.. 2009. Autophagy regulates lipid metabolism. Nature. 458: 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zechner R., Madeo F., and Kratky D.. 2017. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat. Rev. Mol. Cell Biol. 18: 671–684. [DOI] [PubMed] [Google Scholar]
- 77.Zaidi N., Lupien L., Kuemmerle N. B., Kinlaw W. B., Swinnen J. V., and Smans K.. 2013. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog. Lipid Res. 52: 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y. Y., Attane C., Milhas D., Dirat B., Dauvillier S., Guerard A., Gilhodes J., Lazar I., Alet N., Laurent V., et al. 2017. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2: e87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nomura D. K., Long J. Z., Niessen S., Hoover H. S., Ng S. W., and Cravatt B. F.. 2010. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 140: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim D. K., Lee J., Kim S. R., Choi D. S., Yoon Y. J., Kim J. H., Go G., Nhung D., Hong K., Jang S. C., et al. 2015. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics. 31: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fong M. Y., Zhou W., Liu L., Alontaga A. Y., Chandra M., Ashby J., Chow A., O’Connor S. T., Li S., Chin A. R., et al. 2015. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao H., Yang L., Baddour J., Achreja A., Bernard V., Moss T., Marini J. C., Tudawe T., Seviour E. G., San Lucas F. A., et al. 2016. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife. 5: e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vallabhaneni K. C., Penfornis P., Dhule S., Guillonneau F., Adams K. V., Mo Y. Y., Xu R., Liu Y., Watabe K., Vemuri M. C., et al. 2015. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 6: 4953–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Royo F., Moreno L., Mleczko J., Palomo L., Gonzalez E., Cabrera D., Cogolludo A., Vizcaino F. P., van-Liempd S., and Falcon-Perez J. M.. 2017. Hepatocyte-secreted extracellular vesicles modify blood metabolome and endothelial function by an arginase-dependent mechanism. Sci. Rep. 7: 42798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Royo F., Palomo L., Mleczko J., Gonzalez E., Alonso C., Martinez I., Perez-Cormenzana M., Castro A., and Falcon-Perez J. M.. 2017. Metabolically active extracellular vesicles released from hepatocytes under drug-induced liver-damaging conditions modify serum metabolome and might affect different pathophysiological processes. Eur. J. Pharm. Sci. 98: 51–57. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J., Lu S., Zhou Y., Meng K., Chen Z., Cui Y., Shi Y., Wang T., and He Q. Y.. 2017. Motile hepatocellular carcinoma cells preferentially secret sugar metabolism regulatory proteins via exosomes. Proteomics. 17: 201700103. [DOI] [PubMed] [Google Scholar]
- 87.Ronquist K. G., Ek B., Stavreus-Evers A., Larsson A., and Ronquist G.. 2013. Human prostasomes express glycolytic enzymes with capacity for ATP production. Am. J. Physiol. Endocrinol. Metab. 304: E576–E582. [DOI] [PubMed] [Google Scholar]
- 88.Ronquist K. G., Sanchez C., Dubois L., Chioureas D., Fonseca P., Larsson A., Ullen A., Yachnin J., Ronquist G., and Panaretakis T.. 2016. Energy-requiring uptake of prostasomes and PC3 cell-derived exosomes into non-malignant and malignant cells. J. Extracell. Vesicles. 5: 29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lazar I., Clement E., Dauvillier S., Milhas D., Ducoux-Petit M., LeGonidec S., Moro C., Soldan V., Dalle S., Balor S., et al. 2016. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Cancer Res. 76: 4051–4057. [DOI] [PubMed] [Google Scholar]
- 90.Achreja A., Zhao H., Yang L., Yun T. H., Marini J., and Nagrath D.. 2017. Exo-MFA - A 13C metabolic flux analysis framework to dissect tumor microenvironment-secreted exosome contributions towards cancer cell metabolism. Metab. Eng. 43: 156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lázaro-Ibáñez E., Lunavat T. R., Jang S. C., Escobedo-Lucea C., Oliver-De La Cruz J., Siljander P., Lötvall J., and Yliperttula M.. 2017. Distinct prostate cancer-related mRNA cargo in extracellular vesicle subsets from prostate cell lines. BMC Cancer. 17: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sano S., Izumi Y., Yamaguchi T., Yamazaki T., Tanaka M., Shiota M., Osada-Oka M., Nakamura Y., Wei M., Wanibuchi H., et al. 2014. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 445: 327–333. [DOI] [PubMed] [Google Scholar]
- 93.Togliatto G., Dentelli P., Gili M., Gallo S., Deregibus C., Biglieri E., Iavello A., Santini E., Rossi C., Solini A., et al. 2016. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: impact on clinical applications. Int. J. Obes. (Lond). 40: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chu M., Zhao Y., Feng Y., Zhang H., Liu J., Cheng M., Li L., Shen W., Cao H., Li Q., et al. 2017. MicroRNA-126 participates in lipid metabolism in mammary epithelial cells. Mol. Cell. Endocrinol. 454: 77–86. [DOI] [PubMed] [Google Scholar]
- 95.Record M., Carayon K., Poirot M., and Silvente-Poirot S.. 2014. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta. 1841: 108–120. [DOI] [PubMed] [Google Scholar]
- 96.Subra C., Grand D., Laulagnier K., Stella A., Lambeau G., Paillasse M., De Medina P., Monsarrat B., Perret B., Silvente-Poirot S., et al. 2010. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 51: 2105–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schlaepfer I. R., Nambiar D. K., Ramteke A., Kumar R., Dhar D., Agarwal C., Bergman B., Graner M., Maroni P., Singh R. P., et al. 2015. Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation. Oncotarget. 6: 22836–22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hassani K., and Olivier M.. 2013. Immunomodulatory impact of leishmania-induced macrophage exosomes: a comparative proteomic and functional analysis. PLoS Negl. Trop. Dis. 7: e2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Record M., Poirot M., and Silvente-Poirot S.. 2014. Emerging concepts on the role of exosomes in lipid metabolic diseases. Biochimie. 96: 67–74. [DOI] [PubMed] [Google Scholar]
- 100.Buschow S. I., van Balkom B. W., Aalberts M., Heck A. J., Wauben M., and Stoorvogel W.. 2010. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 88: 851–856. [DOI] [PubMed] [Google Scholar]
- 101.He M., Qin H., Poon T. C., Sze S. C., Ding X., Co N. N., Ngai S. M., Chan T. F., and Wong N.. 2015. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 36: 1008–1018. [DOI] [PubMed] [Google Scholar]
- 102.Pol A., Gross S. P., and Parton R. G.. 2014. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J. Cell Biol. 204: 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hurwitz S. N., Rider M. A., Bundy J. L., Liu X., Singh R. K., and Meckes D. G. Jr.. 2016. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget. 7: 86999–87015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hong B. S., Cho J. H., Kim H., Choi E. J., Rho S., Kim J., Kim J. H., Choi D. S., Kim Y. K., Hwang D., et al. 2009. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 10: 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skog J., Wurdinger T., van Rijn S., Meijer D. H., Gainche L., Sena-Esteves M., Curry W. T. Jr., Carter B. S., Krichevsky A. M., and Breakefield X. O.. 2008. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10: 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fujimoto T., Kogo H., Ishiguro K., Tauchi K., and Nomura R.. 2001. Caveolin-2 is targeted to lipid droplets, a new “membrane domain” in the cell. J. Cell Biol. 152: 1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blouin C. M., Le Lay S., Eberl A., Kofeler H. C., Guerrera I. C., Klein C., Le Liepvre X., Lasnier F., Bourron O., Gautier J. F., et al. 2010. Lipid droplet analysis in caveolin-deficient adipocytes: alterations in surface phospholipid composition and maturation defects. J. Lipid Res. 51: 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Senetta R., Stella G., Pozzi E., Sturli N., Massi D., and Cassoni P.. 2013. Caveolin-1 as a promoter of tumour spreading: when, how, where and why. J. Cell. Mol. Med. 17: 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Logozzi M., De Milito A., Lugini L., Borghi M., Calabro L., Spada M., Perdicchio M., Marino M. L., Federici C., Iessi E., et al. 2009. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 4: e5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Frühbeck G., Méndez-Giménez L., Fernández-Formoso J. A., Fernández S., and Rodríguez A.. 2014. Regulation of adipocyte lipolysis. Nutr. Res. Rev. 27: 63–93. [DOI] [PubMed] [Google Scholar]
- 111.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., and Zechner R.. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 3: 309–319. [DOI] [PubMed] [Google Scholar]
- 112.Miyoshi H., Souza S. C., Zhang H. H., Strissel K. J., Christoffolete M. A., Kovsan J., Rudich A., Kraemer F. B., Bianco A. C., Obin M. S., et al. 2006. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J. Biol. Chem. 281: 15837–15844. [DOI] [PubMed] [Google Scholar]
- 113.Morigny P., Houssier M., Mouisel E., and Langin D.. 2016. Adipocyte lipolysis and insulin resistance. Biochimie. 125: 259–266. [DOI] [PubMed] [Google Scholar]
- 114.Inder K. L., Zheng Y. Z., Davis M. J., Moon H., Loo D., Nguyen H., Clements J. A., Parton R. G., Foster L. J., and Hill M. M.. 2012. Expression of PTRF in PC-3 Cells modulates cholesterol dynamics and the actin cytoskeleton impacting secretion pathways. Mol. Cell. Proteomics. 11: 012245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liang B., Peng P., Chen S., Li L., Zhang M., Cao D., Yang J., Li H., Gui T., Li X., et al. 2013. Characterization and proteomic analysis of ovarian cancer-derived exosomes. J. Proteomics. 80: 171–182. [DOI] [PubMed] [Google Scholar]
- 116.Clayton A., Al-Taei S., Webber J., Mason M. D., and Tabi Z.. 2011. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 187: 676–683. [DOI] [PubMed] [Google Scholar]
- 117.Park J. O., Choi D. Y., Choi D. S., Kim H. J., Kang J. W., Jung J. H., Lee J. H., Kim J., Freeman M. R., Lee K. Y., et al. 2013. Identification and characterization of proteins isolated from microvesicles derived from human lung cancer pleural effusions. Proteomics. 13: 2125–2134. [DOI] [PubMed] [Google Scholar]
- 118.Smith S. A. 2002. Peroxisome proliferator-activated receptors and the regulation of mammalian lipid metabolism. Biochem. Soc. Trans. 30: 1086–1090. [DOI] [PubMed] [Google Scholar]
- 119.Huss J. M., and Kelly D. P.. 2004. Nuclear receptor signaling and cardiac energetics. Circ. Res. 95: 568–578. [DOI] [PubMed] [Google Scholar]
- 120.Calle E. E., and Kaaks R.. 2004. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 4: 579–591. [DOI] [PubMed] [Google Scholar]
- 121.Renehan A. G., Zwahlen M., and Egger M.. 2015. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat. Rev. Cancer. 15: 484–498. [DOI] [PubMed] [Google Scholar]
- 122.Calle E. E., Rodriguez C., Walker-Thurmond K., and Thun M. J.. 2003. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl. J. Med. 348: 1625–1638. [DOI] [PubMed] [Google Scholar]
- 123.Lafontan M. 2012. Historical perspectives in fat cell biology: the fat cell as a model for the investigation of hormonal and metabolic pathways. Am. J. Physiol. Cell Physiol. 302: C327–C359. [DOI] [PubMed] [Google Scholar]
- 124.Ouchi N., Parker J. L., Lugus J. J., and Walsh K.. 2011. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muller C., Nieto L., and Valet P.. 2013. Unraveling the local influence of tumor-surrounding adipose tissue on tumor progression: cellular and molecular actors involved. In Adipose Tissue and Cancer. M. G. Kolonin, editor. Springer, New York. 121–146. [Google Scholar]
- 126.Morris E. V., and Edwards C. M.. 2016. Bone marrow adipose tissue: a new player in cancer metastasis to bone. Front. Endocrinol. (Lausanne). 7: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dirat B., Bochet L., Dabek M., Daviaud D., Dauvillier S., Majed B., Wang Y. Y., Meulle A., Salles B., Le Gonidec S., et al. 2011. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 71: 2455–2465. [DOI] [PubMed] [Google Scholar]
- 128.Bochet L., Lehuede C., Dauvillier S., Wang Y. Y., Dirat B., Laurent V., Dray C., Guiet R., Maridonneau-Parini I., Le Gonidec S., et al. 2013. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 73: 5657–5668. [DOI] [PubMed] [Google Scholar]
- 129.Balaban S., Shearer R. F., Lee L. S., van Geldermalsen M., Schreuder M., Shtein H. C., Cairns R., Thomas K. C., Fazakerley D. J., Grewal T., et al. 2017. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clement E., Lazar I., Muller C., and Nieto L.. 2017. Obesity and melanoma: could fat be fueling malignancy? Pigment Cell Melanoma Res. 30: 294–306. [DOI] [PubMed] [Google Scholar]
- 131.Nieman K. M., Romero I. L., Van Houten B., and Lengyel E.. 2013. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta. 1831: 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nieman K. M., Kenny H. A., Penicka C. V., Ladanyi A., Buell-Gutbrod R., Zillhardt M. R., Romero I. L., Carey M. S., Mills G. B., Hotamisligil G. S., et al. 2011. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17: 1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Diedrich J. D., Rajagurubandara E., Herroon M. K., Mahapatra G., Huttemann M., and Podgorski I.. 2016. Bone marrow adipocytes promote the Warburg phenotype in metastatic prostate tumors via HIF-1alpha activation. Oncotarget. 7: 64854–64877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kwan H. Y., Fu X., Liu B., Chao X., Chan C. L., Cao H., Su T., Tse A. K., Fong W. F., and Yu Z. L.. 2014. Subcutaneous adipocytes promote melanoma cell growth by activating the Akt signaling pathway: role of palmitic acid. J. Biol. Chem. 289: 30525–30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wen Y. A., Xing X., Harris J. W., Zaytseva Y. Y., Mitov M. I., Napier D. L., Weiss H. L., Mark Evers B., and Gao T.. 2017. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 8: e2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tabe Y., Yamamoto S., Saitoh K., Sekihara K., Monma N., Ikeo K., Mogushi K., Shikami M., Ruvolo V., Ishizawa J., et al. 2017. Bone marrow adipocytes facilitate fatty acid oxidation activating AMPK and a transcriptional network supporting survival of acute monocytic leukemia cells. Cancer Res. 77: 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McDonnell E., Crown S. B., Fox D. B., Kitir B., Ilkayeva O. R., Olsen C. A., Grimsrud P. A., and Hirschey M. D.. 2016. Lipids reprogram metabolism to become a major carbon source for histone acetylation. Cell Reports. 17: 1463–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang L., Qiu W., Zhou Y., Wen P., Fang L., Cao H., Zen K., He W., Zhang C., Dai C., et al. 2013. A microRNA-30e/mitochondrial uncoupling protein 2 axis mediates TGF-beta1-induced tubular epithelial cell extracellular matrix production and kidney fibrosis. Kidney Int. 84: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Laurent V., Guerard A., Mazerolles C., Le Gonidec S., Toulet A., Nieto L., Zaidi F., Majed B., Garandeau D., Socrier Y., et al. 2016. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 7: 10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.D’Esposito V., Liguoro D., Ambrosio M. R., Collina F., Cantile M., Spinelli R., Raciti G. A., Miele C., Valentino R., Campiglia P., et al. 2016. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget. 7: 24495–24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sagar G., Sah R. P., Javeed N., Dutta S. K., Smyrk T. C., Lau J. S., Giorgadze N., Tchkonia T., Kirkland J. L., Chari S. T., et al. 2016. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut. 65: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Balistreri C. R., Caruso C., and Candore G.. 2010. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010: 802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.King H. W., Michael M. Z., and Gleadle J. M.. 2012. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 12: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Jong O. G., Verhaar M. C., Chen Y., Vader P., Gremmels H., Posthuma G., Schiffelers R. M., Gucek M., and van Balkom B. W.. 2012. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles. 1: 18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Beloribi-Djefaflia S., Vasseur S., and Guillaumond F.. 2016. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 5: e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Whiteside T. L. 2017. Extracellular vesicles isolation and their biomarker potential: are we ready for testing? Ann. Transl. Med. 5: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Duijvesz D., Burnum-Johnson K. E., Gritsenko M. A., Hoogland A. M., Vredenbregt-van den Berg M. S., Willemsen R., Luider T., Pasa-Tolic L., and Jenster G.. 2013. Proteomic profiling of exosomes leads to the identification of novel biomarkers for prostate cancer. PLoS One. 8: e82589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fujita K., Kume H., Matsuzaki K., Kawashima A., Ujike T., Nagahara A., Uemura M., Miyagawa Y., Tomonaga T., and Nonomura N.. 2017. Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer. Sci. Rep. 7: 42961. [DOI] [PMC free article] [PubMed] [Google Scholar]