Abstract
Diverticular disease is common and morbid. Treatments are limited due to poor understanding of its pathophysiology. To elucidate its etiology, we performed a genome-wide association study of diverticular disease (27,444 cases; 382,284 controls) in the UK Biobank and tested for replication in the Michigan Genomics Initiative (2,572 cases; 28,649 controls). We identified 42 loci associated with diverticular disease, 39 of them novel. Using DEPICT, we show that genes in these associated regions are significantly enriched for expression in mesenchymal stem cells and multiple connective tissue cell types and are co-expressed with genes that play a role in vascular and mesenchymal biology. Genes in these associated loci play roles in immunity, extracellular matrix biology, cell adhesion, membrane transport, and intestinal motility. Phenome-wide association analysis of the 42 variants shows a common etiology of diverticular disease with obesity and hernia. These analyses establish the genomic landscape of diverticular disease.
Keywords: diverticular disease, diverticulosis, diverticulitis, genomics, genome-wide association study
Diverticulosis is an outpouching of the gastrointestinal tract present in the majority of older adults in Western countries1. Most patients are asymptomatic, but hundreds of thousands develop diverticular disease. Diverticulosis, the precursor lesion, is highly prevalent in the United States (US), Europe, and Canada; >50% of adults over age 60 have diverticulosis, and 10–25% will become symptomatic2. It is less common in other populations and demonstrates anatomic variation; diverticulosis is predominantly (90%) in the sigmoid colon in Western populations, but for unknown reasons is right-sided (70%) in Asia3. The low fiber Western diet has traditionally been implicated in diverticulosis, but this correlation has been questioned4–6. Diverticulitis (inflammation and infection of diverticula) causes >200,000 hospital admissions in the US annually7. The incidence is increasing; US inpatient admissions increased 26% from 1998–2005, most rapidly among patients <45 years old8. Complications including fistula, stricture, abscess, and intestinal perforation necessitate tens of thousands of surgical interventions annually9. Inpatient mortality is 1.5–3.0%10. Progression from diverticulosis to diverticular disease is poorly understood. Observational studies have correlated age, obesity, decreased physical activity, ultraviolet radiation, and diet with diverticular diseaes4,11–15. Incidence is higher in males <50, but females predominate in older ages16. Diverticular disease is associated with connective tissue disorders: Ehlers-Danlos Syndrome (collagen mutations)17, Williams Syndrome (elastin mutations)18, and polycystic kidney disease19. In the general population, twin studies estimate heritability at 40–53%20,21 indicating a strong genetic component. To date, only one genome-wide association study (GWAS) has been performed, identifying three associated loci22.
Here we report the to-date largest GWAS of diverticular disease. We examined associations of ~30 million single nucleotide polymorphisms (SNPs) with diverticular disease (ICD-10 code K57; N=27,244) in the European component of the United Kingdom Biobank (UKBB) population vs 382,284 control individuals23. K57 is a root code including diverticulitis and diverticular hemorrhage (Supplementary Table 1). It has been validated as a diagnostic code for diverticular disease, with a positive predictive value of 0.9824. Analyses were adjusted for age, sex, and principal components and relatedness using mixed linear modeling25. We tested the top 154 independently-associated SNPs (p< 1×10−5) in 31,221 unrelated European-ancestry individuals enrolled in the Michigan Genomics Initiative (MGI)26 adjusted for gender, age, and principal components25. Cases of diverticular disease in MGI were identified using ICD-9 billing codes for diverticulosis (code 562.10, 562.12, N=1,854) or diverticulitis (code 562.11, 562.13 N=718).
We identified 40 independent loci with genome-wide significant (p < 5 × 10–8) associations for diverticular disease and 112 more loci with suggestive associations (p < 1 × 10–5, Supplementary Table 2, Supplementary Figures 1 and 2) in UKBB. In MGI, 8/154 variants replicated with a consistent direction of effect at an MGI false discovery rate (FDR) < 10%. All loci associated with UKBB-genome-wide significant SNPs (N=40) and two MGI-replicated/UKBB-suggestive-SNPs were carried forward for analysis (Figure 1). Of these 42 loci of interest, 39 represent novel associations (Table 1). Supplementary Table 2 is a full list of associated variants. The 42 loci mapped to 99 genes within a distance of 500kb and R2 >0.5 (Supplementary Table 3). Regional association plots better defined the associated signal (Supplementary Figure 3).
Figure 1:
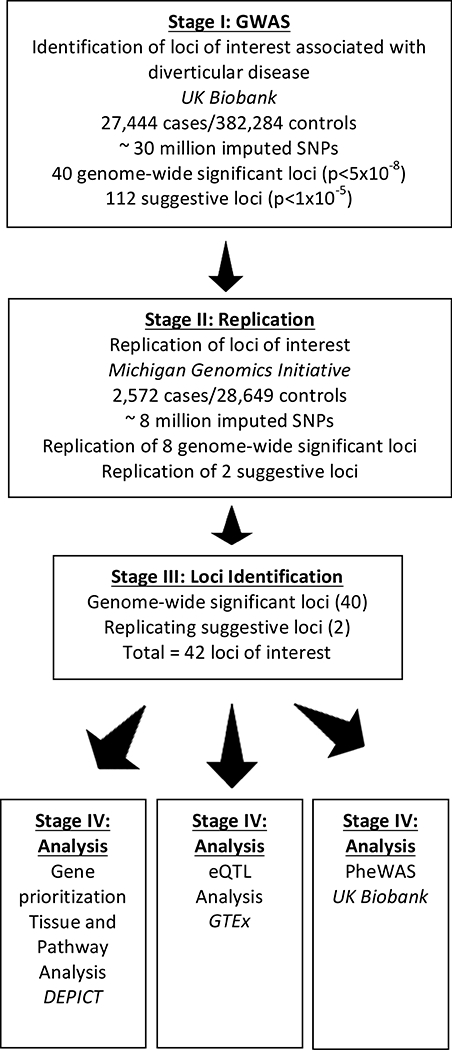
Study Design. Graphic representation of study design. GWAS: Genome-wide association study. SNPs: single nucleotide polymorphisms. PheWAS: Phenome-wide association study. GTEx: Genotype-Tissue Expression project. DEPICT: Data-driven Expression-Prioritized Integration for Complex Traits. eQTL: Expression Quantitative Trait Loci
Table One:
Loci of interest including genome-wide significant variants (p<5×10−8) from UKBB and highly significant SNPs (p<5×10−5) with replication in MGI. Bold gene symbols indicate replication in MGI at FDR <0.1 following Benjamin-Hochberg correction. X–chromosome, DD – diverticular disease, FDR – false discovery rate, GWAS – genome wide association study, EAF – estimated allele frequency. At each locus, a superscript1 or 2 indicates an eQTL and eGene for GTEx v7 sigmoid colon or transverse colon, respectively.
|
UK Biobank (27,444 cases/382,284 controls) |
Michigan Genomics Initiative (2,572 cases/28,649 controls) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Locus | SNP | Chr | Position | Nearest Gene | Other Nearby Genes | EA | EAF | DD p- value | p- diverticulosis | p- diverticulitis |
| 1 | rs6734367 | 2 | 143556678 | ARHGAP15* | G | 0.82 | 4.29E-44 | 0.0038 | 0.07 | |
| 2 | rs4333882 | 1 | 234217153 | SLC35F3 | G | 0.19 | 4.44E-22 | 0.0007 | 0.14 | |
| 3 | rs7609897 | 3 | 15461174 | COLQ*/METTL6 | EAF1 | T | 0.22 | 2.72E-18 | 0.10 | 0.0096 |
| 4 | rs70862491 | 10 | 25522506 | GPR1581 | C | 0.46 | 5.37E-16 | 0.014 | 0.0004 | |
| 5 | rs1802575 | 2 | 55866069 | EFEMP1 | C | 0.13 | 7.71E-16 | 0.08 | 0.07 | |
| 6 | rs11667256 | 19 | 38245164 | PPP1R14A | SPINT2 | T | 0.52 | 1.24E-14 | 0.025 | 0.062 |
| 7 | rs962369 | 11 | 27712873 | BDNF |
BDNF-AS1, KIF18A, METTL15, ENSG00000255496 |
C | 0.31 | 2.16E-14 | 0.18 | 0.74 |
| 8 | rs69493912 | 7 | 102806416 |
FAM185A, PMPCB, LRRC17 |
NAPEPLD, S100A11P1, UPK3BL12, DNACJ2, DPY19L2P2, ARMC10, FBXL13, ENSG00000230257 |
T | 0.34 | 3.74E-14 | 0.17 | 0.45 |
| 9 | rs61823192 | 1 | 219121228 | LYPLAL1 | ENSG00000223842 | T | 0.03 | 1.15E-13 | 0.16 | 0.021 |
| 10 | rs9520344 | 13 | 107250422 | FAM155A* | A | 0.24 | 5.23E-12 | 0.08 | 0.34 | |
| rs11619840 | 107566610 | A | 0.19 | 1.70E-09 | 0.0042 | 0.021 | ||||
| 11 | rs7098322 | 10 | 99631412 | SLC25A28 |
ENTPD7, CUTC, COX15 |
T | 0.87 | 9.94E-12 | 0.67 | 0.33 |
| 12 | rs10472291 | 5 | 37772678 | WDR70 | A | 0.33 | 1.01E-11 | 0.21 | 0.89 | |
| 13 | rs5820941,2 | 9 | 136145484 | ABO1,2 | T | 0.32 | 1.55E-11 | 0.12 | 0.0008 | |
| 14 | rs75434097 | 21 | 45999606 | COL6A1 | PCBP3 | A | 0.15 | 4.90E-11 | 0.42 | 0.98 |
| 15 | rs2280028 | 16 | 86199807 | LINC01082 | A | 0.14 | 7.05E-11 | 0.73 | 0.10 | |
| 16 | rs98561182 | 3 | 151360428 | P2RY122, P2RY14 |
GPR87, IGSF10, P2RY13, GPR171, MED12L |
G | 0.17 | 8.80E-11 | 0.15 | 0.82 |
| 17 | rs714724331 | 15 | 40357408 | DISP21 | IVD, C15orf23 | C | 0.17 | 8.90E-11 | 0.74 | 0.89 |
| 18 | rs2131755 | 16 | 84823772 | CRISPLD2 | G | 0.41 | 1.50E-10 | 0.06 | 0.59 | |
| 19 | rs4839715 | 6 | 97917413 | ENSG00000224849 | A | 0.37 | 1.62E-10 | 0.87 | 0.26 | |
| 20 | rs148376933 | 2 | 98612512 | UNC50 | 1.88E-10 | 0.91 | 0.49 | |||
| 21 | rs13813351,2 | 8 | 119415408 | NOV1,2 | ENPP2 | T | 0.24 | 1.91E-10 | 0.69 | 0.64 |
| 22 | rs618148831 | 1 | 151998153 | S100A101 |
S100A11, TCHH, THEM4 |
A | 0.30 | 2.05E-10 | 0.46 | 0.60 |
| 23 | rs8074740 | 17 | 44235410 | UBTF |
ASB16, TMUB2, ATXN7L3, ASB16-AS1, C17orf53 |
A | 0.32 | 2.34E-10 | 0.88 | 0.08 |
| 24 | rs3113037 | 7 | 96449252 | SHFM1 | CLSTN2 | T | 0.23 | 2.52E-10 | 0.0039 | 0.0033 |
| 25 | rs12293535 | 11 | 14993308 | CALCA | CALCB | A | 0.28 | 6.20E-10 | 0.37 | 0.47 |
| 26 | rs875107 | 11 | 70159268 | FADD | ANO1 | A | 0.52 | 2.33E-09 | 0.0004 | 0.10 |
| rs72945112 | 70247466 | T | 0.15 | 6.30E-06 | 0.0016 | 0.80 | ||||
| 27 | rs3823878 | 7 | 74028915 | ELN | LIMK1 | A | 0.06 | 2.63E-09 | 0.0018 | 0.78 |
| 28 | rs10471645 | 5 | 64999536 | CWC27 | C | 0.83 | 3.03E-09 | 0.037 | 0.0091 | |
| 29 | rs1888693 | 10 | 18151515 | CACNB2 | A | 0.34 | 3.58E-09 | 0.10 | 0.50 | |
| 30 | rs4871180 | 8 | 121246834 | HAS2 | T | 0.25 | 4.15E-09 | 0.77 | 0.77 | |
| 31 | rs2049865 | 8 | 115576319 | TRPS1 | A | 0.58 | 5.54E-09 | 0.59 | 0.52 | |
| 32 | rs1544387 | 4 | 94852434 | BMPR1B | T | 0.58 | 5.74E-09 | 0.023 | 0.0005 | |
| 33 | rs11934833 | 4 | 156636431 | ENSG00000251283 |
ENSG00000249479 ENS00000251511 |
G | 0.30 | 6.21E-09 | 0.0053 | 0.30 |
| 34 | rs2784255 | 1 | 220893031 | HLX | C | 0.48 | 1.06E-08 | 0.99 | 0.61 | |
| 35 | rs10120333 | 9 | 76125437 | PCSK5 | G | 0.53 | 1.54E-08 | 0.92 | 0.39 | |
| 36 | rs129422671,2 | 17 | 7469318 | ZBTB4 | CHRNB11,2, POLR2A | T | 0.64 | 2.55E-08 | 0.042 | 0.73 |
| 37 | rs62126581 | 2 | 18806974 | NT5C1B | A | 0.17 | 3.77E-08 | 0.62 | 0.23 | |
| 38 | rs115490395 | 1 | 110120397 | UBL4B | A | 0.01 | 4.39E-08 | 0.42 | 0.81 | |
| 39 | rs2470653 | 3 | 5804815 | EDEM1 | A | 0.23 | 4.51E-08 | 1.00 | 0.20 | |
| 40 | rs10173528 | 2 | 28065525 | RBKS |
GPN1, BRE, SUPT7L, MRPL33 |
T | 0.61 | 4.73E-08 | 0.82 | 0.94 |
| 47 | rs2056544 | 15 | 76533662 | ISL2, ETFA |
RCN2, TMEM266, SCAPER |
A | 0.57 | 1.01E-07 | 0.99 | 0.08 |
| rs10519134 | 76286749 | A | 0.11 | 7.12E-06 | 0.0036 | 0.66 | ||||
| 68 | rs1386991,2 | 22 | 38733703 | GTPBP1 |
AL021707.21,2, CBY11, FAM227A2 |
A | 0.25 | 8.02E-07 | 0.0039 | 0.25 |
Tissue expression and pathway enrichment analyses were performed using Data-Driven Expression Prioritized Integration for Complex Traits (DEPICT) 27. Mesenchymal stem cells and four related connective tissue cell types were enriched (FDR < 0.20). Digestive, connective, and urogenital tissues (Figure 2AB, Supplementary Table 4) were enriched (FDR < 0.20). 95 of 14,462 independent reconstituted DEPICT gene sets (FDR <0.20 and kappa of 0.5) were enriched for the 99 genes, including pathways involved in vascular biology, mesenchymal development/derivatives, and embryogenesis (Supplementary Table 5).
Figure 2A/B:
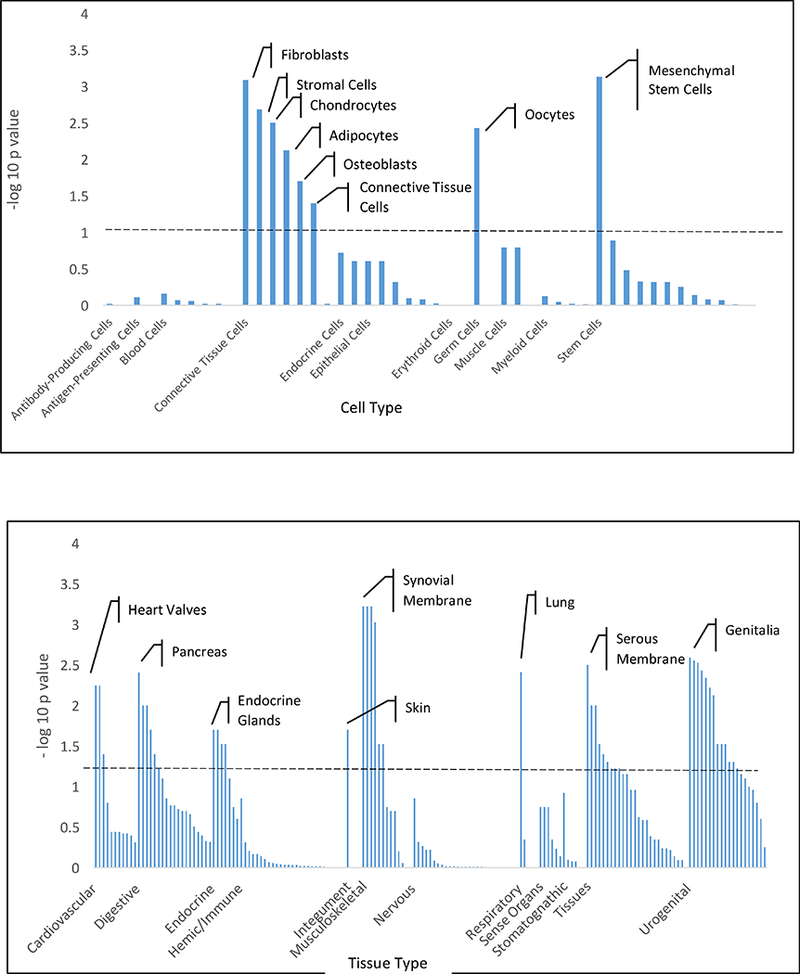
Tissue and cell type enrichment analysis. Plots showing the enrichment of loci associated with diverticular disease (p < 1 × 10−5 in the UKBB; N=27,444 cases/382,284 controls) in specific cell types (A) and tissues (B). Enrichments are grouped according to system or cell type and significance; annotations above the dashed line have FDR ≤ 0.20. Data corrected for multiple comparisons using Benjamini-Hochberg method. Top tissue in each category labelled.
Of the 42 SNPs carried forward for analysis, 7 were expression quantitative trait loci (eQTLs) in sigmoid colon, and 6 were eQTLs in transverse colon, according to GTEx28 (Table 1). The most significant eQTL-SNP was rs7086249 (NM_020752.2:c.1405–28470T>C) regulating GPR158, which encodes orphan G-protein coupled receptor, in the sigmoid colon. Mechanistic studies in fresh tissues are needed. Power to detect eQTLs is limited; post-mortem interval strongly influences colonic RNA quality29. 31/42 SNPs, including the 8 confirmed variants, were intronic; the remainder were intergenic.
We performed Phenome-wide association study (PheWAS) analysis for the 42 loci of interest. PheWAS can be used to agnostically assess whether phenotypes are associated with a genetic variant. Here 42 SNPs were tested for association with 780 UKBB traits30 (Supplementary Table 6). Traits were hierarchically clustered before filtering those without significant association. Twenty-three loci correlated with morphometric traits (ABO, BDNF, CALCA/CALCB, COL6A1, CRISPLD2, CWC27, DISP2, EFEMP1, ENSG00000224849, ENSG00000251283, FADD/ANO1, FAM185A, GTPBP1, HLX, LYPLAL1, NOV, NT5C1B, RBKS, PCSK5, S100A10, TRPS1, UBTF, and ZBTB4). Fourteen loci associated with hematologic variables (ABO, ARHGAP15, BDNF, CRISPLD2, DISP2, ENSG00000224849, GTPBP1, HLX, PPP1R14A/SPINT2, RBKS, SLC25A28, TRPS1, UBTF, and ZBTB4). LYPLAL1, GTPBP1, ELN, EFEMP1, and CRISPLD2 associated with hernias. EFEMP1 and CRISPLD2 also associated with female genital prolapse EFEMP1 has been previously associated with hernia31. SHFM1, UBTF, HLX, ABO, and UNC50 associated with connective tissue traits, such as osteoarthritis and soft tissue inflammation. Eight loci (ABO, CACNB2, ENSG00000224849, FADD/ANO1, NOV, NT5C1B, RBKS, and ZBTB4) associated with vascular traits including venous thrombosis, pulmonary embolism, hypertension, and heart failure. Nineteen loci (ABO, ARHGAP15, COLQ/METTL6, CRISPLD2, EFEMP1, ELN, ENSG00000224849, ENSG00000251283, FADD/ANO1, FAM155A, FAM185A, GPR158, GTPBP1, P2RY12/P2RY14, PPP1R14A/SPINT2, RBKS, SLC25A28, SLC35F3, and UNC50) associated with gastrointestinal disease, but not with the common bowel conditions inflammatory bowel disease, polyps, and cancer. An edited heatmap (Figure 3) highlights these effects.
Figure 3:
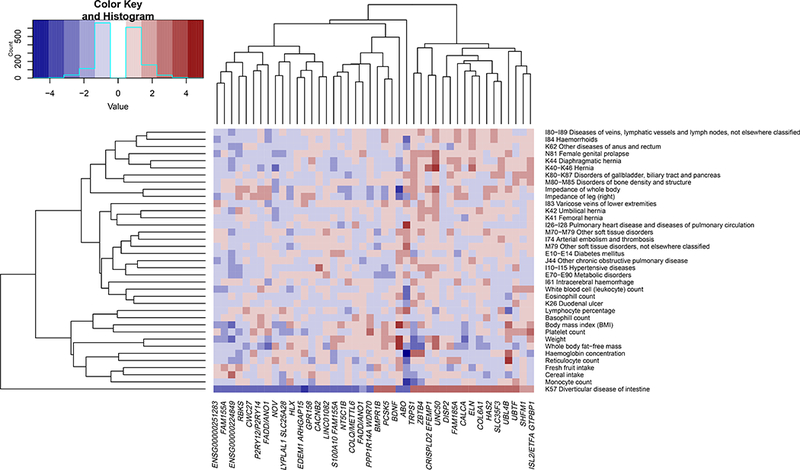
Phenome-wide association matrix. Filtered association matrix highlighting vascular, gastrointestinal, connective tissue, hematologic, morphometric, and dietary traits associated with loci of interest in the UKBB (27,444 cases/382,284 controls) Data controlled for multiple comparisons using Benjamini-Hochberg method, filtered at FDR<5%, and clustered at h=0.2.
One prior GWAS identified risk-loci near ARHGAP15, FAM155A and COLQ22. These associations were confirmed, supporting the validity of our approach. ARHGAP15 encodes a GTPase-activating protein acting on Rac32 and negatively regulates neutrophils33. The function of the gene product of FAM155A is unknown. COLQ encodes a critical protein for acetylcholine-mediated signaling34. CALCB, identified in our study, was identified but not validated in the prior GWAS. TNFSF15 has been associated with diverticular disease35, but this was not found in our study. Despite clinical association17, Ehlers-Danlos genes were not identified.
The 8 replicated loci were associated with 21 genes (Supplementary Table 3). Some contribute to cytoskeletal and extracellular matrix (ECM) dynamics (ELN, SHFM1, BMPR1B, LIMK1, and CLSTN2). BMPR1B and SHFM1 are implicated in bone and cartilage synthesis36,37. LIMK1 stabilizes the cytoskeleton by inhibiting actin de-polymerization38. CLSTN2 encodes an atypical cadherin involved in cell adhesion39. ELN encodes elastin, which is altered in diverticular colons40. Diverticular disease is common in Williams Syndrome, a congenital disorder caused by ELN hemizygosity18. ANO1 encodes a chloride channel in the intestinal pacemaker cells of Cajal41. These cells are reduced in diverticular disease42. ANO1 is critical for intestinal contractility43. Altered intestinal motility is implicated in diverticular disease44. Diverticular colons demonstrate abnormal smooth muscle morphology45 and altered contractile force46. ARHGAP15, GPR158, and GTPBP1 are involved in GTP-signaling. Many identified genes have unknown function or unclear functional link to diverticular disease. Functional characterization should be prioritized to confirm these gene-variant associations. In the absence of strong molecular evidence to the contrary, systematic studies indicate that the closest gene is the best candidate for SNP effect47. All replicated SNPs were located in introns, supporting a molecular mechanism at the RNA-expression level in the surrounding gene. Therefore, expression levels of these genes is the most plausible avenue for further molecular phenotyping48.
Among our other 99 identified genes, many have roles in the ECM, motility, and membrane transport (Figure 4), COL6A1, CRISPLD2, EFEMP1, HAS2, NOV, and TCHH have known roles in the ECM49–53. Enrichment in mesenchymal stem cells, connective tissues, and mesenchymal development pathways, suggest a role for connective tissue biology. PPP1R14A and CHRNB1 effect smooth muscle motility54,55. Others are involved in transport of copper (CUTC), sodium (SPINT2) and calcium (CALCA, CALCB, CACNB2). SPINT2 mutations result in congenital sodium diarrhea57. Altered absorption or motility could produce constipation, which is clinically associated with diverticular disease. Vascular biology identified by pathway analysis/PheWAS may be relevant as diverticula tend to occur adjacent to penetrating arteries.
Figure 4:
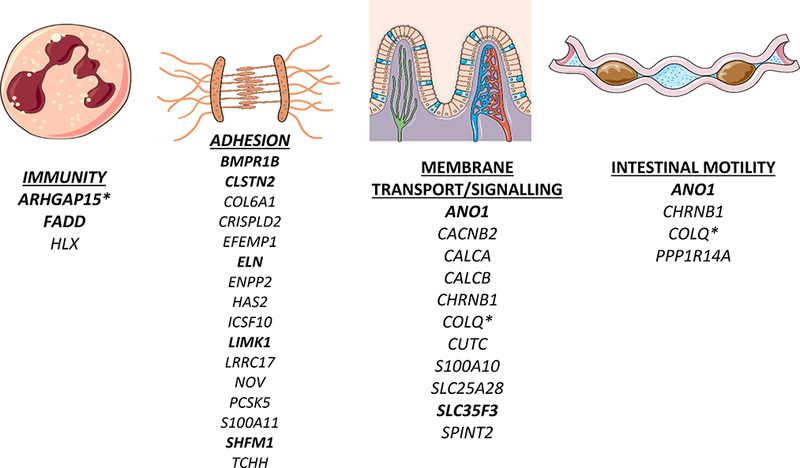
Plausible biological pathways underlying risk loci associated with diverticular disease. Bold gene symbols indicate replication in MGI. * indicates prior identification in GWAS
This study is limited in that it detects diverticular disease via inpatient coding, and does not identify asymptomatic diverticulosis. Given the epidemiology of diverticulosis, the majority of participants likely harbor the precursor lesion and the variants identified only associate with diverticular disease. However, this is the clinically relevant outcome. Given the high reliability of diverticular disease codes24 and the derivation of cases from inpatient hospital admissions, it is likely that most patients suffered severe diverticular disease. However, patients might be erroneously identified if diverticulosis was noted incidentally. Conversely, patients with mild diverticular disease treated as outpatients may be falsely identified as controls. The de-identified nature of the data precludes coding confirmation. Another limitation is ICD9 versus ICD10 coding between populations. We chose grouped, rather than individual codes to mitigate this difference. Additionally, the UKBB entry age of 40–69 prohibits comparison of older/younger patients. Finally, some conditions in our PheWas could be a consequence of diverticular disease rather than sharing a common etiology.
In summary, the biologic basis for both the development of colonic diverticula in the majority of older adults and the triggers that produce diverticulitis in some patients are unknown. We report the largest GWAS thus far for diverticular disease and identify 39 novel loci as contributing to the pathophysiology of these diseases. This work defines the landscape for future functional studies and identifies possible targets for therapeutic development.
Online Methods
UK Biobank
The UK Biobank (UKBB) contains genotypes, clinical and demographic data on over 400,000 individuals aged 40–69 at time of study recruitment. The UKBB protocols were approved by the National Research Ethics Service Committee. Participants signed written informed consent, specifically applicable to health-related research. All ethical regulations were followed. The analyses used in this paper were carried out by Canela-Xandri et al. under UK Biobank Resource project 78830. Diverticular disease was recorded under the International Classification of Disease (ICD) 10 code K57 (N=27,244). Participant genotyping, data collection, and quality control has been described in detail23. In brief, participants were genotyped on one of two purpose-designed arrays (UK BiLEVE Axiom Array (N=50,520) and UK Biobank Axiom Array (N=438,692)) with 95% maker overlap. The Haplotype Reference Consortium was used as a reference panel to phase and impute the data. Following quality control, over 30 million variants in 408,455 white British individuals (http://geneatlas.roslin.ed.ac.uk/) were tested for association with K57 controlling for age, gender, principal components and relatedness using mixed linear modeling.
Michigan Genomics Initiative
The Michigan Genomics Initiative (MGI) is an institutional repository of DNA linked to participants’ medical profile via the electronic medical record at the University of Michigan. The MGI is approved for this research use by the Institutional Review Board of the University of Michigan. All relevant ethical regulations were followed. Diverticular disease was derived from ICD-9 codes (562.11 and 562.13 for diverticulitis and 562.10 and 562.12 for diverticulosis). Following informed consent, individuals (N=35,888) were genotyped using the Illumina HumanCoreExome Array.
Genotype analysis was performed with Illumina GenomeStudio (module 1.9.4, algorithm GenTrain 2.0). After initial clustering, we re-defined variant cluster boundaries using individuals with call rate >99% and genotyped the remaining samples. Samples were excluded if they met any of the following criteria: (1) call rate <99%, (2) estimated contamination >2.5% (BAF Regress)57, (3) large chromosomal copy number variants, (4) lower call rate of a technical duplicate pair and twins, or (5) whose inferred sex contradicted the reported sex.
Variant-quality control was performed in the following manner: we excluded variants if: (1) their probes could not be perfectly mapped or mapped perfectly to multiple positions (2) they showed deviations from Hardy Weinberg equilibrium (p-value< 0.0001), (3) had a call rate < 99%, or (4) another variant with higher call rate assayed the same variant (PLINK (v1.90)58).
Phasing was carried out using SHAPEIT2 (v2. r837)59 on autosomal chromosomes. Genotypes of the Haplotype Reference Consortium (chromosome 1–22: HRC release 1; chromosome X: HRC release 1.1) were imputed into the phased MGI data using Minimac3 (v1.0.13)60. Excluding variants with low imputation quality (R2 <0.3) resulted in dense mapping at 39,127,678 million quality-imputed genetic markers.
We estimated pairwise kinship using the software KING (v1.4.2)61. We excluded any 1st- or 2nd-degree relative pairs within the cohort. In addition, we used principal component analysis to identify ethnically homogeneous groups using individuals from the Human Genome Diversity Project64. We included only European samples.
Locus Identification
197 independent loci were identified for all imputed UKBB variants associated with diverticular disease at p <1×10–5 using criteria of R2 < 0.1 within a distance of 500kb using PLINK version 1.90b4.662 within the DEPICT program27. DEPICT then assigned each SNP to genes in the specified region or genes containing variants in linkage disequilibrium with the SNP. SNPs were then queried for replication in MGI using a nominal one sided FDR of 10% by Benjamini-Hochberg63.
SNP Annotation
Effect allele and allele frequencies were annotated using ANNOVAR chromosome 1–22 imputation data, build 3764.
Tissue and Pathway Analysis
Tissue and pathway enrichment was carried out against 14,462 reconstituted gene sets in DEPICT27 (version 1, release 194) for the 192 loci associated with diverticular disease at a nominal p-value below 1×10–5. Pathways were culled using a kappa statistic of 0.565. Tissue and cell type enrichment was similarly determined in DEPICT by analyzing gene expression enrichment of genes at our 192 loci of interest in 209 MeSH-defined tissue and cell types. FDR of <0.20 was set as a threshold for significance for both pathway and tissue analysis.
Colon eQTLs
Lists of eQTLs in sigmoid or transverse colon were obtained from GTEx version 7. The GTEx project has been described in detail elsewhere28. Briefly it is a gene expression resource created from RNA Sequencing (RNA-Seq) results obtained from post-mortem donors. Gene expression levels and individual variants were correlated to enable discovery of 697,430 gene-variant associations in sigmoid colon, and 832,983 gene-variant associations in transverse colon. In both cases, a false discovery rate below 5% was used.
PheWAS
Phenome-wide association study was carried out for all lead SNPs in our loci of interest. SNPs were queried against 778 traits ascertained for UKBB participants and reported in the Roslin Gene Atlas, including morphometric data, hematologic lab values, ICD-10 clinical diagnoses, and self-reported conditions. First, traits were hierarchically clustered using inverse-absolute Pearson correlation among the Z-scores as a distance metric. The resultant hierarchical clustering/tree was pruned at a height corresponding h=0.2, leaving a total of 97 largely independent traits. Then, the pruned matrix of trait-genotype associations was filtered at an FDR of 0.05 by Benjamini-Hochberg63. This filtered association matrix was used in further analysis and reporting.
Statistics
All p-values described in the manuscript are two-sided. Multiple comparison corrections were made using the method of Benjamini-Hochberg63 at multiple points during the study, as detailed above.
Data Availability Statement
The UK BioBank genomic and phenotypic data supporting this publication are publicly available from the Roslin Institute, University of Edinburgh (http://geneatlas.roslin.ed.ac.uk/). The Michigan Genomics Initiative (MGI) genomic and phenotypic data are not publicly available due to restrictions on participant privacy. MGI data can be made available on reasonable request to the corresponding author with permission of the University of Michigan Institutional Review Board. Detailed information on software, study design, and data availability can be found in the Life Sciences Reporting Summary associated with this manuscript.
Supplementary Material
Acknowledgements:
The authors acknowledge the University of Michigan Medical School Central Biorepository/Michigan Genomics Initiative for providing biospecimen storage, management, and distribution services in support of the research reported in this publication.Graphics in Figure 4 obtained courtesy of Servier Medical Art, Les Laboratoires Servier (https://smart.servier.com). Used under Creative Commons License.
Footnotes
LHM is supported by the University of Michigan Department of Surgery.
EKS, SKH, XD, and YC are supported by RO1 DK106621, RO1 DK107904, The University of Michigan Biological Sciences Scholars Program, and The University of Michigan Department of Internal Medicine. All grants made to EKS.
THP is supported by Lundbeck Foundation and Benzon Foundation.
Conflict of Interest Statement: No, I declare that the authors have no competing financial or non-financial interests as defined by Nature Research.
References
- 1.Painter NS & Burkitt DP Diverticular disease the colon, a 20th century problem. Clin Gastroenterol 4, 3–21 (1975). [PubMed] [Google Scholar]
- 2.Weizman AV & Nguyen GC Diverticular disease: epidemiology and management. Can J Gastroenterol. 25, 385–389 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugihara K et al. Diverticular disease of the colon in Japan: A review of 615 cases. Dis Colon Rectum 27, 531–7 (1984). [DOI] [PubMed] [Google Scholar]
- 4.Pan G, et al. Diverticular disease of the colon in China. Chin Med J 97, 391–4. (1984). [PubMed] [Google Scholar]
- 5.Alatise OI et al. Spectrum of colonoscopy findings in Ile-Ife Nigeria. Niger Postgrad Med J. 19, 219–24 (2012). [PubMed] [Google Scholar]
- 6.Peery AF et al. A high-fiber diet does not protect against asymptomatic diverticulosis. Gastroenterology 142, 266–72 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peery AF et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 143, 1179–1187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etzioni DA, Mack TM, Beart RW Jr, & Kaiser AM Diverticulitis in the United States: 1998–2005: changing patterns of disease and treatment. Ann Surg. 249, 210–7 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Ricciardi R,et al. Is the decline in the surgical treatment for diverticulitis associated with an increase in complicated diverticulitis? Dis Colon Rectum 52, 1558–63 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Delvaux M Diverticular disease of the colon in Europe: epidemiology, impact on citizen health and prevention. Aliment Pharmacol Ther. 18, 71–74. (2003). [DOI] [PubMed] [Google Scholar]
- 11.Strate LL, Liu YL, Aldoori WH & Giovannucci EL Physical activity decreases diverticular complications. Am J Gastroenterol 104, 1221 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strate LL, Liu YL, Aldoori WH, Syngal S, & Giovannucci EL Obesity increases the risks of diverticulitis and diverticular bleeding. Gastroenterology 136, 115–22 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maguire LH, Song M, Strate LL, Giovannucci EL & Chan AT Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin Gastroenterol Hepatol. 11, 1631–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maguire LH, Song M, Strate LL, Giovannucci EL & Chan AT Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg 1, 74–77 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strate LL, Liu YL, Syngal S, Aldoori WH, & Giovannucci EL Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA 300, 907–14 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warner E, et al. Fourteen-year study of hospital admissions for diverticular disease in Ontario. Can J Gastroenterol. 21: 97–9 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leganger J, et al. Association between diverticular disease and Ehlers-Danlos syndrome: a 13-year nationwide population-based cohort study. Int J Colorectal Dis. 12, 1863–1867 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Cherniske EM et al. 2004. Multisystem study of 20 older adults with Williams syndrome. Am J Med Genet 131A, 255–264 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Lederman ED, McCoy G, Conti DJ, & Lee EC Diverticulitis and polycystic kidney disease. Am Surg. 66, 200–203 (2000). [PubMed] [Google Scholar]
- 20.Granlund J, et al. The genetic influence on diverticular disease–a twin study. Aliment Pharm Ther 35, 1103–7 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Strate LL, et al. Heritability and familial aggregation of diverticular disease: a population-based study of twins and siblings. Gastroenterology 144, 736–42 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Sigurdsson S, et al. Sequence variants in ARHGAP15, COLQ and FAM155A associate with diverticular disease and diverticulitis. Nat Commun 8, 15789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sudlow C, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12: e1001779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erichsen R, Strate L, Sørensen HT & Baron JA Positive predictive values of the International Classification of Disease, 10th edition diagnoses codes for diverticular disease in the Danish National Registry of Patients. Clin Exp Gastroenterol 3, 139–42 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price AL, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38, 904–9 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Dey R, Schmidt EM, Abecasis GR, & Lee S A Fast and Accurate Algorithm to Test for Binary Phenotypes and Its Application to PheWAS. Am J Hum Genet 101, 37–49. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pers TH, et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat Commun 6, 5890 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consortium GTEx. Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira PG et al. The effects of death and post-mortem cold ischemia on human tissue transcriptomes. Nat Commun. 9, 490 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canela-Xandri O, Rawlik K, Tenesa A. An atlas of genetic associations in UK Biobank. bioRxiv 176834; doi: 10.1101/176834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorgenson E et al. A genome-wide association study identifies four novel susceptibility loci underlying inguinal hernia. Nat Commun 6, 10130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seoh ML, Ng CH, Yong J, Lim L & Leung T ArhGAP15, a novel human RacGAP protein with GTPase binding property. FEBS letters 539, 131–7 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Costa C, et al. The RacGAP ArhGAP15 is a master negative regulator of neutrophil functions. Blood 118, 1099–108 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Arredondo et al. COOH-terminal collagen Q (COLQ) mutants causing human deficiency of endplate acetylcholinesterase impair the interaction of ColQ with proteins of the basal lamina. Hum Genet 133, 599–616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connelly TM, Berg AS, Hegarty JP, Deiling S, Brinton D, Poritz LS, Koltun WA.The TNFSF15 gene single nucleotide polymorphism rs7848647 is associated with surgical diverticulitis. Ann Surg. 259, 1132–7 (2014) [DOI] [PubMed] [Google Scholar]
- 36.Racacho L et al. Two novel disease-causing variants in BMPR1B are associated with brachydactyly type A1. Eur J Hum Genet 23, 1640–5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen MB, et al. Phenotypic subregions within the split-hand/foot malformation 1 locus. Hum Genet 135, 345–57 (2016) [DOI] [PubMed] [Google Scholar]
- 38.Mashiach-Farkash E et al. Computer-based identification of a novel LIMK1/2 inhibitor that synergizes with salirasib to destabilize the actin cytoskeleton. Oncotarget 6, 629–39 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortiz-Medina H, Emond MR, & Jontes JD Zebrafish calsyntenins mediate homophilic adhesion through their amino-terminal cadherin repeats. Neuroscience. 286 87–96 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteway J & Morson BC Elastosis in diverticular disease of the sigmoid colon. Gut 26, 258–66 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez-Pinilla PJ, et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296, G1370–81 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bassotti G, et al. Interstitial cells of Cajal, enteric nerves, and glial cells in colonic diverticular disease. J Clin Pathol 58, 973–7 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cobine CA, et al. ANO1 in intramuscular interstitial cells of Cajal plays a key role in the generation of slow waves and tone in the internal anal sphincter. J Physiol 595, 2021–2041 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeyarajah S & Papagrigoriadis S Review article: the pathogenesis of diverticular disease--current perspectives on motility and neurotransmitters. Aliment Pharmacol Ther 33, 789–800 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Hughes LE Postmortem survey of diverticular disease of the colon. II. The muscular abnormality of the sigmoid colon. Gut 10, 344–51 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maselli MA, et al. Colonic smooth muscle responses in patients with diverticular disease of the colon: effect of the NK2 receptor antagonist SR48968. Dig Liver Dis 36, 348–54 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Stacey D et al. ProGeM: A framework for the prioritisation of candidate causal genes at molecular quantitative trait loci. bioRxiv 230094. doi: 10.1101/230094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richards S et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 17, 405–424. (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Himes BE et al. RNA-Seq transcriptome profiling identifies CRISPLD2 as a glucocorticoid responsive gene that modulates cytokine function in airway smooth muscle cells. PLoS One 6, e99625 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klenotic PA, Munier FL, Marmorstein LY & Anand-Apte B Tissue inhibitor of metalloproteinases-3 (TIMP-3) is a binding partner of epithelial growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1). Implications for macular degenerations. J Biol Chem 29, 30469–73 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Qu C, et al. Extensive CD44-dependent hyaluronan coats on human bone marrow-derived mesenchymal stem cells produced by hyaluronan synthases HAS1, HAS2 and HAS3. Int J Biochem Cell Biol 48, 45–54 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Yeger H & Perbal B CCN family of proteins: critical modulators of the tumor cell microenvironment. J Cell Commun Signal 10, 229–240 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lettmann S, et al. Col6a1 null mice as a model to study skin phenotypes in patients with collagen VI related myopathies: expression of classical and novel collagen VI variants during wound healing. PLoS One 8, e105686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mori D, et al. Synchronous phosphorylation of CPI-17 and MYPT1 is essential for inducing Ca(2+) sensitization in intestinal smooth muscle. Neurogastroenterol Motil 23, 1111–22 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Akk G, et al. Energetic contributions to channel gating of residues in the muscle nicotinic receptor β1 subunit. PLoS One 8, e78539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janecke AR, Heinz-Erian P & Müller T Congenital Sodium Diarrhea: A Form of Intractable Diarrhea, With a Link to Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr 63, 170–6 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Jun G et al. Detecting and estimating contamination of human DNA samples in sequencing and array-based genotype data. Am J Hum Genet 91, 839–848, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang CC et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Connell J et al. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet 10, e1004234 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das S et al. Next-generation genotype imputation service and methods. Nat Genet 48, 1284–1287, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manichaikul A et al. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Purcell S, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. American Journal of Human Genetics 81, 559–571 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benjamini Y & Hochberg Y Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society 1, 289–300 (1995). [Google Scholar]
- 64.Wang K, Li M & Hakonarson H ANNOVAR: Functional annotation of genetic variants from next-generation sequencing data. Nucleic Acids Research 38, e164 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang DW, Sherman BT & Lempicki RA Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc 4, 44–57 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The UK BioBank genomic and phenotypic data supporting this publication are publicly available from the Roslin Institute, University of Edinburgh (http://geneatlas.roslin.ed.ac.uk/). The Michigan Genomics Initiative (MGI) genomic and phenotypic data are not publicly available due to restrictions on participant privacy. MGI data can be made available on reasonable request to the corresponding author with permission of the University of Michigan Institutional Review Board. Detailed information on software, study design, and data availability can be found in the Life Sciences Reporting Summary associated with this manuscript.


