Abstract
The development of realistic in vitro blood–brain barrier (BBB) models that recapitulate the physiological parameters and molecular aspect of the neurovascular unit (NVU) is of fundamental importance not only in CNS drug discovery but also in translational research. Successful modeling of the NVU would provide an invaluable tool to aid in dissecting out the pathological factors, mechanism of action (and corresponding targets) prodromal to the onset of CNS disorders. The field of BBB in vitro modeling has seen many radical changes in the last few years with the introduction on novel technologies and methods to improve over existing models and develop new ones. Therefore, the goal of this review is to provide the readers with updated technical and operational details concerning current BBB platforms with special focus on stem cell technology used to establish a functional BBB model in vitro. Furthermore, we provide a detailed update on rapidly advancing 3D printing technologies used for engineering BBB models which use is now fast expanding among researchers.
Keywords: Blood–brain barrier, endothelium, alternative, drug discovery, bio-printing, cell line, stem cells cell lines, microfluidic, drug development
Introduction
The blood–brain barrier (BBB) is a dynamic and complex interface between the blood and the central nervous system (CNS), transporting nutrients essential for the normal metabolism of brain cells and providing protection against many toxic compounds and pathogens.1 The BBB (see also Figure 1) is part of the neurovascular unit (NVU), consisting of, pericytes, glial cells, neuronal cells, endothelial cells (ECs) forming the capillary and extracellular matrix (ECM) proteins.1,2 The brain capillaries comprised tightly linked ECs surrounded by pericytes and a basement membrane, 30 to 40 nm thick, including collagen IV, laminin glycoproteins, proteoglycans, and fibronectin.3 The BBB plays a significant role in the regulation of the passage of ions, nutrients, and other substances from the blood into the brain.4 According to the result of recent research, 98% of small-molecule and 100% of large-molecule drugs cannot cross the BBB.2 Thus, there is a need to develop novel tools to aid the process of CNS drug discovery that are scalable, cost effective and translationally/clinically relevant.5
Figure 1.
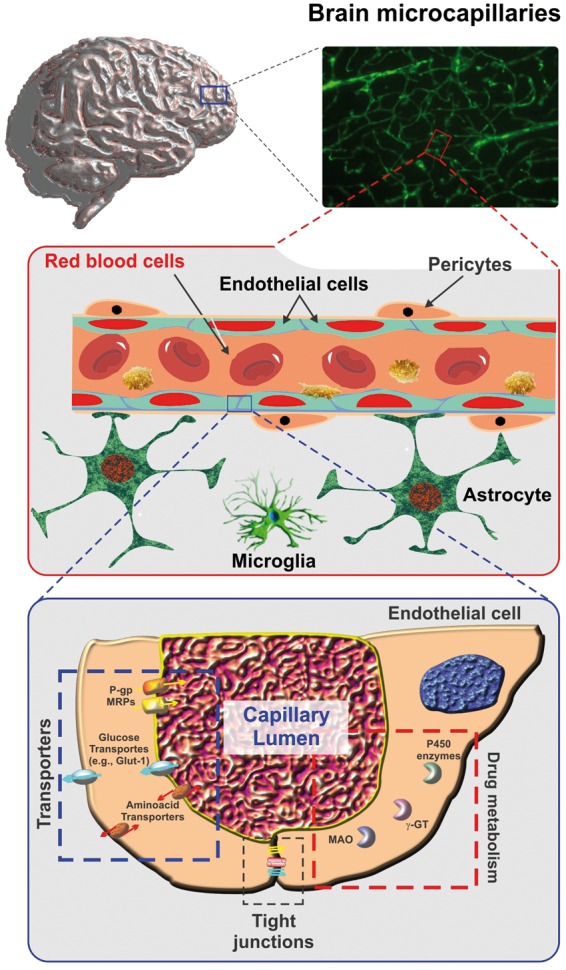
Schematic representation of brain microcapillary. A cross-section of a brain microcapillary segment. The passage of substances across the BBB endothelium is controlled by a multimodal barrier including tight junctions (gating barrier to paracellular diffusion of polar molecules); efflux transporters (P-gp, MRPs, etc.) with affinity for lipophilic substances; cytochrome P450 enzymes, MAO, etc. (metabolic/enzymatic barrier). Note also how endothelial cells and pericytes are tightly ensheathed by the astrocytic end-feet.
Major brain disorders where the development of realistic in vitro BBB/NVU models would be extremely beneficial nowadays includes: Traumatic brain injury (TBI), brain tumors, Alzheimer's disease (AD), etc. TBI is a disruption in the normal function of the brain due to an injury (by an external force) following a trauma, which is more likely to cause severe physical, emotional and cognitive impairment.6,7 In order to protect the intact neural tissue from the destructive immuno-response, a physical barrier termed the glial scar is formed around the injured area to prevent the spread of the inflammation to neighboring neurons and the surrounding area.8 Moreover, a severe local inflammation occurs, expanding the site of injury to include neighboring neurons and exacerbating the damage. The glial scar encloses an area containing inhibitory molecules that prevents the regrowth of neurons and inhibits the repair of the BBB.6 Thus, due to the limited capacity of the injured brain to repair the damaged neurons endogenously and generation of sufficient mature neurons capable of integrating into injured brain neural circuitry, pharmaceutical solutions may be a promising therapy for TBI.9 On the other hand, due to specific structural and biochemical properties of BBB, preventing most of the pharmaceutical solutions, BBB represents a critical hurdle in the treatment of TBI.10 In fact, TBI is still one of the major brain disorders that lack defined pharmacotherapy and treatment options.4,11 This is clearly a case where an in vitro BBB model could provide critical cues to help developing effective therapeutic treatments aimed at the CNS and/or the repair of the BBB.
Nowadays, brain cancer also remains a major public health problem and it is one of the leading causes of mortality.12 Despite many approaches to treat brain tumors such as surgical treatment, chemotherapy, and radiotherapy, only a few successes in survival time of brain tumor patients have been achieved.13 Biological therapy has been noted as a novel strategy for various tumor diseases, especially for the relapsed patients. However, there is a major challenge in cancer biological therapy lying in the inefficient delivery of therapeutic agents and drugs to the tumor sites due to the barrier posed by the BBB, which prevents most therapeutic agents from reaching the tumors.13 AD is growing brain disorder that has been on the fast raise in recent time. AD is a progressive neurodegenerative disorder characterized by memory deficits, cognitive impairment, personality changes related to the degeneration of multiple neuronal types, from a pathological perspective, by the presence of neuritic plaques and neurofibrillary tangles.14 Recent work indicated that in AD reduced levels of the neurotrophic brain-derived neurotrophic factor (BDNF) could be attributed to the neurodegeneration15 and consequently, the administration of BDNF causes the slow process of neural degradation.16,17 However, due to the difficulty of crossing the BBB, the treatment with BDNF was delivered by intracranial injection which might not be practical for neurodegenerative patients. There are also more promising therapeutic options that might benefit from a better understanding of the BBB pathophysiology in AD such as the delivery of antibodies against Aβ, which have been shown to reduce amyloid-β peptides plaques in AD.18,19 Overall, due to huge limitations in crossing the BBB for treatment of brain disorders, there is a huge interest in the development of effective solutions to overcome this barrier.10 In this specific case, in vitro experimentation using reliable and translationally relevant in vitro BBB models could provide the ideal platform to evaluate new drugs and drug delivery systems to the CNS target.
In this review manuscript, we provide technical and operational details to help the user determine the best approach/model to use based on the prefixed research goals. First, we detailed the various types of cells (with a special emphasis on stem cells) that can be used to establish an in vitro BBB model, and then we cover the technical details (plus advantages and drawbacks) of current in vitro BBB platforms including microfluidic systems. Finally, we expanded over recently developed 3D printing technologies for in vitro BBB modeling/engineering. This is a fast-developing technology that is rapidly spreading among researchers.
BBB in vitro models
The development of in vitro BBB models has been driven by the need to develop a fast, reliable and cost effective tool to help dissecting out and study the complexities (both biological and structural) of the BBB as well as for the screening of putative CNS drugs and develop strategic solutions to bypass the intrinsic resistance of the BBB.5,20–23 Different approaches have been used to mimic the BBB in vitro. This includes static and dynamic (flow-capable) platforms, as well as the use of different cell types such primary cells, immortalized cell line and more recently, stem cells. In addition to using different cell typologies, cell cultures for BBB modeling have grown in structural complexity ranging from basic monocultures to multiple culture systems such as co-culture and try-culture settings.5,10
Cell types used in in vitro BBB models
The type of cells used to develop a BBB in vitro plays a critical role in determining the usability, cost effectiveness and translational relevance of the model since the more closely the cellular milieu can mimic the physiological characteristics and responses of the BBB in situ, the more reliable the model is expected to be. Also, availability and expandability of these cell types can significantly impact the cost to operate and run the platform. Generally, among the various cell types (especially when considering the brain microvascular endothelium), primary cells provide at large the closest approximation to their in-situ counterpart proven the limited number of passages in culture before use and purity of the original batch.24 The drawback of using primary cells generally come in the form of availability and cost effectiveness. Specifically, for human, primary cells availability is limited and generally depends on being able to rely on a clinical counterpart to obtain the brain tissue from which cells can be isolated. The process is also time consuming and the yield is generally very low.24 Cells will require to be cultured for a prolonged period of time and will need to be purified to eliminate contaminants (e.g. fibroblasts, pericytes, etc.). The isolation and purification process is rather complex and requires specific technical skills. Cells isolated from human brain tissue resection are likely to be associated with a brain disorders that required the surgical removal of the tissue in the first place (e.g. temporal lobectomies for patients with drug refractory epilepsy or brain tumors for example), thus they are likely to carry pathological properties of the patient (e.g. drug resistance) and to retain them to some extent in vitro.25,26 This is certainly a unique advantage (cells are somewhat patient specific) not offered by cell lines or animal-derived primary cells but limited availability of these cells is major obstacle for many laboratories. Cell viability is also another issue since primary cells tend to differentiate quite rapidly in culture limiting the amount of useful passages. On the other hand, commercial sources of primary human BBB ECs come with a high price tag and generally these cells are fetal primary cells and may not display the full range of differentiation of a mature BBB endothelium. Properties that also carry in vitro cell viability is also a critical factor especially for human cells. Primary-derived BBB ECs are commercially available (generally less expensive of primary human cells) but can be also isolated from brain tissue of the source animal (generally rodents). The drawback is that like primary human cells, animal-derived primary cells will also tend to differentiate quite rapidly in vitro and may respond differently to specific testing with regard to pharmacoresistance.
Herein, we provide a short description of commonly used endothelial cell types for BBB modeling (a detail description of commonly used cell types is already available elsewhere2), thus we focus primarily on stem cells typologies used/usable in BBB/NVU in vitro modeling.
Brain endothelial cells
The morphological and functional characteristics of brain endothelial cells (BECs) as a major cellular constituent of the brain differ from ECs in peripheral vessels.10 Peripheral capillaries have pores between the cells that are normally 6–7 nm in size.20 Brain capillaries are 50–100 folds tighter than peripheral capillaries and thus have lower paracellular permeability to hydrophilic solutes. In the last decades, most of the current successful BBB in vitro models were developed based on primary BECs cultures due to their high TEER values and a low cellular passage.10 However, limited availability, high costs, time-consuming preparations (including the necessity for special skills required for the cellular isolation) and cultures being susceptible to internal and external contamination, primary cells might not be an encompassing convenient choice for every in vitro study/testing.27,28 Especially those that requires scalability to be cost effective (e.g. large volumes of pharmacological testing). On the other hand, immortalized cell lines remain viable over many passages with a higher experimental reproducibility between tests compared with primary cells.29 Thus, making these cells relatively reliable, easily accessible and affordable considering that culture preparation time and costs are reduced.
Cell lines can originate from different species including rodents (such as, bEnd.3, bEnd.5 and RBE4), humans (such as HCMEC/D3, TY10, and BB1929) and porcine-derived immortalized brain microvascular ECs. A comprehensive review detailing their characteristics has been recently published by Rahman et al.30 However, there are several drawbacks to be taken into consideration when using immortalized cell lines such as altered expression of characteristic BBB endothelial features including tight junctional (TJ) proteins, efflux transporters, and altered physiological behaviors including unresponsiveness to glia or pericytes stimuli29 when used in co-culture settings (demonstrated by a low trans-endothelial electrical resistance –TEER).31 Despite these limitations, from a practical stand point, immortalized cell lines remain viable alternative in BBB modeling10 especially for the development of cost-effective high-throughputs screening (HTS) platforms. Primary BBB ECs (very low passage) are more indicated for basic and translational in vitro studies since they are more likely to retain most of the phenotypic and pathological properties (e.g. drug resistance25,32) than immortalized cell lines but at a much higher cost for the end user. A comprehensive list (and corresponding references) of BECs-based BBB models available today is reported in the Supplementary Table 1. Additional detailed information on commonly used brain endothelial cell culture models can be found elsewhere.2,33
Stem cells
More than 10 years ago in vitro BBB models were generated from both primary and immortalized BECs.34 However, due to several limitations inherent to obtaining viable primary cells, and immortalized cell lines closely resembling the functional and physiological characteristic of the BBB cells in vivo, new in vitro BBB models based on stem cells technology have been generated. Stem cells are a promising source of cells for the generation of in vitro human BBB models because these cells have the capacity to differentiate into BECs, they can give rise to a significant number of BBB cells, home to the brain and be used to model BBB pathologies.20 Herein, we review several types of stem cells used in BBB modeling.
Embryonic stem cells
Embryonic stem cells (ESCs) derived from human or mice fetal brains have been used as a source for cell transplantation in different animal models to treat TBI.9 In fact, ESCs are an alternative pluripotent stem cell therapy option due to their ability to differentiate into all kind of brain cells in addition to their indefinite self-renewal abilities in vitro.35 Although ESCs offer new means of treatments, it still raises some complex ethical restrictions since it involves the destruction of human embryos.9
Neural stem cells
Neural stem cells (NSCs) are multipotent stem cells in adult brains that, unlike ESCs, have a decreased potential of self-renewal and normally, for the purpose of repair, differentiate into only one cell lineage of the tissue.9 NSCs can differentiate into neuronal cells and hence have huge potential for the generation of in vitro human BBB models featuring a more complex NVU system encompassing both vascular and brain tissue. NSCs are also being investigated for a possible therapeutic option in TBI.9,20 Although the experimental results about NSCs are promising, it is difficult to obtain NSCs from human brains because of the inevitable potential of immunological incompatibility in allogeneic transplantation as well as practical and ethical problems.
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are potential stem cells that can be used as a replacement therapy for human cellular models. The two main advantages of iPSCs are the avoidance of the use of ESCs for ethical reasons and the ability to be generated from the patients themselves, allowing iPSCs to be transplanted without any immunological rejection.36 On the other hand, iPSCs technology has some major limitations.35 In fact, the risk of tumor formation is present due to the use of viral infections and a low efficiency of reprogramming during the production of these cells. In BBB modeling, iPSCs are becoming of more streamlined use.37,38 For example, Destefano et al.39 used the monolayers of human brain microvascular ECs derived from iPSCs to evaluate the role of shear stress in modulating the morphology, motility, proliferation, apoptosis, and protein and gene expression, of confluent monolayers of human brain microvascular ECs.39 The advantage of using patient-derived iPSCs can allow for developing BBB in vitro models that are patient specific. Unfortunately, another not yet overcome limitation of the model is the narrow experimental window provided by iPSC-derived cells which generally tend to de-differentiate quite rapidly (days after reaching full differentiation) under in vitro culture conditions.
Mesenchymal stem cells in BBB modeling
The use of mesenchymal stem cells (MSCs) offers huge potential for application in the treatment of brain diseases. Not only are MSCs easily isolated, but also, they can be easily expanded from tissues without ethical concerns. They also have immunosuppressive properties that cause a reduction in inflammation in injured tissue. MSCs have also the ability of secreting growth factors that facilitate the regrowth of neurons in the brain tissue. Besides, MSCs do not organize tumors as other primitive stem cells such as ESCs.4 The promising abilities of MSCs present them as an attractive platform for application in the fields of stem cell tissue engineering, gene therapy, and cancer biology.
Pericytes as a key component of the NVU, wrapping around capillaries and playing crucial roles in BBB formation and regulation, are also required for the maintenance of the BBB in adulthood.21,40 Recent work has shown strong similarities between MSCs and pericytes.41 Similar multipotential stem cell activity has been exhibited in CNS microvascular cells to that seen in MSCs and also pericytes and MSCs express many of the same cell surface markers.40 Tian et al.40 describe a BBB in vitro model using brain capillary ECs (mouse bEND.3 cells) co-cultured with MSCs to investigate the contribution of MSCs to BBB structure and function as a potential substitute for pericytes.40
Therefore, these phenotypic similarities may result in functional equivalence.42 So, due to time-consuming and technically challenging to extract and culture primary pericytes from brain tissue for BBB studies, MSCs can play a role as a substitute of pericytes.
In addition, several studies suggest that MSCs may possess leukocyte-like, active homing mechanisms involving adhesion molecules, chemokines, and proteases which enable MSC/EC interactions and transmigration that enable them to interact with and migrate across the BBB under injury or inflammation.43,44
Co-culture
The simplest and most feasible in vitro BBB model consists of a monolayer of BECs seeded on a semi-permeable support under static culture conditions. However, due to mentioned drawbacks of BECs such as lack of barriergenic modulatory stimuli afforded by neighboring cell signaling (astrocytes and pericytes) and shear stress, recently developed multicellular BBB models have incorporated BECs with NSCs, astrocytes, pericytes and MSCs.5,45,46 In fact, the shift to multicellular BBB models with one or more additional NVU cell types has greatly expanded their potential beyond drug permeability screening.21 From in vitro co-culture models, generally, co-cultures of BECs with astrocytes and pericytes are widely used since they play a crucial role in the development of the paracellular tightness of the BBB and modulating BECs’ functions.5,10
Monoculture on plastic surfaces
The first studies on intracellular signaling were performed mainly on monocultures, using Petri dishes. In this method, BECs can be used in large quantities for biochemical and physiological studies. Moreover, not only do the optical characteristics of the plastic Petri dishes make it possible to see and locate cells easily, but also experimental costs are very low.10 This approach poses several limitations so that although Petri dish cultures may be useful to assess the cytotoxicity of a drug candidate, they are not fit for the study of drug transport through the BBB and are also too simple to answer complex research questions.2 Moreover, interactions with plastic surfaces prevail over interactions between cells or between cells and the ECM.47 The stiffness of plastic dishes is not physiological and many cells isolated from organs or tumors become flat when cultured in 2D, altering their proliferation rate and differentiation status.48
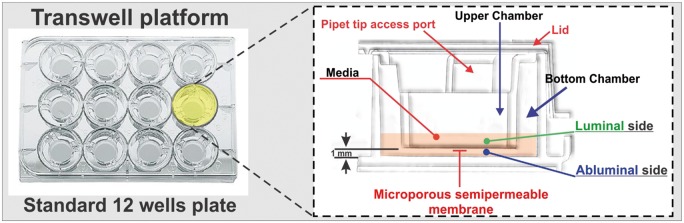
Transwell
To enable drug transportation studies, advances in the culture setup have been made, resulting in cell culture on a filter membrane suspended in a well, so-called Transwell system.28 Transwell system is essentially a side by side vertical diffusion system which comprises a microporous semipermeable membrane (on which cell can be seeded submerged in feeding medium49) that separates the vascular and parenchymal side compartments (see Figure 2). This apparatus is indicated for the study of permeability of drugs across BBB and allows co-culture of BECs and other cells that are associated with the NVU.21,50 Ease of establishing cultures, moderate scalability, and low cost make this apparatus desirable to be used in various research settings including basic and translational (moderately) studies as well as HTS screening tools.51,52 Transwell systems are ideal for linear kinetic studies of transport due to the fixed volumes of each compartment; the cells can be detached and harvested for further study (proteomic and genomic analyses), and are available in a range of pore sizes and with different membranes to satisfy diverse experimental requirements. However, there are substantial limitations inherent to these platforms that need to be taken into consideration. For example, the lack of a three-dimensional structure present in vivo; lack of endothelial exposure to physiological shear stress which limits the differentiation of the endothelium into a BBB phenotype (or maintenance of BBB properties in fully differentiated cells).52 The result is that the cells may present with reduced polarized transport, limited expression of specific efflux systems and (in most cases) relative low trans endothelial electrical resistance (TEER) when compared to the BBB in vivo (e.g. isolated brain microcapillaries) and corresponding high paracellular permeability to hydrophilic substances. These drawbacks may limit the reliability of their predictive value for human responses.21,53
Figure 2.
Schematic representation of a typical transwell apparatus. The transwell is a vertical side-by-side diffusion system across a semipermeable microporous membrane. Endothelial cells are seeded on the luminal side of the membrane, while astrocytes and/or pericytes can be seeded in juxtaposition on the abluminal side.
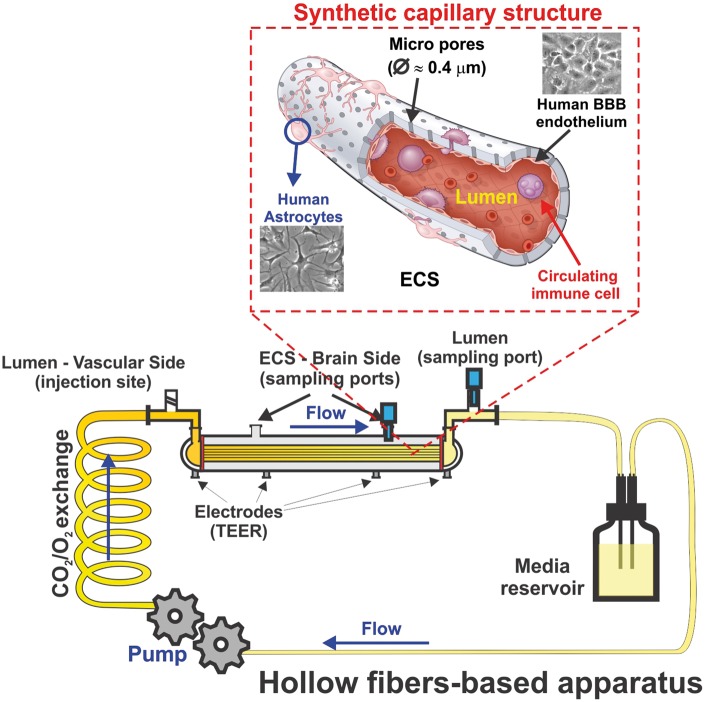
Dynamic in vitro model
One of the 3D models is the dynamic in vitro (DIV) model model of the BBB which allows the use of co-cultures and creates intraluminal flow through artificial capillary-like structural supports.49,51 In this system (see Figure 3), BECs are cultured in the lumen of hollow fibers inside a sealed chamber and are exposed to flow, while the NVU is seeded in the extraluminal compartment. Intraluminal flow is generated by a variable-speed pulsatile pump that can be regulated to produce desirable intraluminal pressure physiologically comparable to that observed in capillaries in vivo.49 Low permeability to intraluminal polar molecules, high TEER, negligible extravasation of proteins, expression of specialized transporters, ion channels, and efflux systems are several significant advantages of DIV models. Nonetheless, several disadvantages also exist because this system is not intended to be used in HTS studies, it requires more time and technical skills to be established, and a high cell load is required for the initial setup of the system.49 Moreover, its design does not allow for visualization of the intraluminal compartment to assess morphological and phenotypic changes of the vascular endothelium. Scarce adoption of this system among researchers also limited (due to cost constrains) the availability of wide setup options for the end user.
Figure 3.
Schematic illustration of the DIV-BBB model. In this system, BBB endothelial cells are cultured inside hollow fiber structures (lumen) coated with fibronectin or ECM matrices. Astrocytes can be seeded on the abluminal surface of the same hollow fibers are in juxtaposition to ECs once the abluminal surface is properly coated. The bundle of hollow fibers is suspended inside a sealed chamber and in continuity with gas-permeable silicon tubing circulating media throughout the system. Access to the luminal (vascular) and abluminal (parenchymal) compartments is granted through inlet and outlet ports positioned on the opposite sides of the module and two additional ports on top of the longitudinal section, respectively. TEER is measured in real time through a set of electrodes embedded in the module’s scaffold. The electrodes are in contact with either the luminal or the abluminal chambers.
Microfluidic platforms
Microfluidic-on-chips as a type of 3D models and novel class of microengineered laboratory models combine several advantages of current in vivo and in vitro models2,54 (see also Figure 4). In a typical tissue-on-a-chip embodiment, microfluidic channels are fabricated using soft lithography techniques by molding an elastomeric material, polydimethylsiloxane (PDMS), against a photo-defined master mold and a porous cell culture substrate is then sandwiched and sealed between the channel networks.55 This system improves BBB modeling by having more realistic dimensions and geometries, and by exposing the endothelium to physiological fluid flow, and thus enables the real-time study of cells in a 3D engineered physiological microenvironment.49,56–58 Flexibility in the design, immediate permeability measurements, rapid and low-cost fabrication are other advantages of this model. On the other hand, there are several challenges limiting the usability of these platforms.2,10 The lack of standardized parameters and quantification of critical experimental factors such as luminal shear stress, TEER, selective permeability to well established paracellular markers, etc. makes comparing the characteristics (from a biological perspective) of the established BBB models on different platforms quite difficult.2 Additional drawbacks include limited scalability and the requirement of specialized equipment and expertise for their construction21 which are not easily transferable. This severely limits the use and validation of these systems across the scientific community. Despite these limitations, microfluidic-on-chips provide an extra tool to conduct research complementary to classic in vitro cultures and in vivo animal studies (see also Table 1 and Supplementary Table 1).
Figure 4.
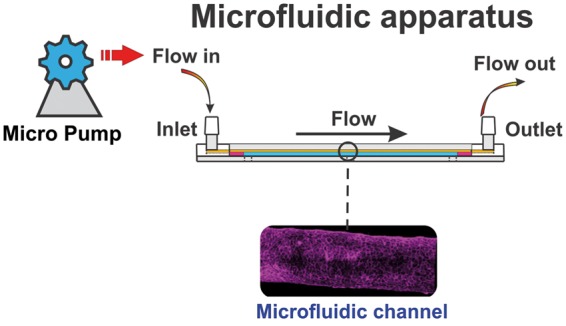
Schematic illustration of a microfluidic system. Note the microengineered microcapillary within the cylindrical environmental chamber is viable for imaging.97
Table 1.
Advantages and disadvantages of various in vitro BBB models.
| In vitro BBB models | Advantages | Drawbacks | |
|---|---|---|---|
| 2D | Petri dishes | • Very low-cost fabrication • Large quantities • Simple fabrication • Control over microenvironment optically | • No shear stress • Limited to monolayers • Cell dedifferentiate quite rapidly |
| Transwells | • Very low-cost fabrication • Allows co-culture • Simple fabrication • Moderate scalability • Highly convenient for high-throughput screens | • No shear stress • Limited cell differentiation • Permeability to polar molecules is not stringent • Ideal for linear kinetic studies | |
| 3D | Dynamic | • Low-cost fabrication • High TEER • Allows co-culture • Complex fabrication • Enables the effect of sheer stress • Allows for hemodynamic studies | • Setup require high cell numbers • Time consuming • Technically challenging • Not ideal for high throughput screening and linear kinetic studies • Not permissive for visual microscopy |
| Microfluidics | • Low-cost fabrication • Flexibility in the design • Requires less cell number • Realistic microenvironment • Control over microenvironment • Resembles more closely the actual in vivo brain anatomy • Consider the effect of sheer stress • Immediate permeability measurements • Improvement in paracellular barrier functions • Allows for cell inspection via visual microscopy | • Moderate TEER • Limited scalability • Complex fabrication • Lack of standardized quantification of parameters • Not ideal linear kinetic studies | |
| Microfluidics (fabricated via 3D printing) | • Low cost fabrication • Moderate fabrication • Flexibility in the design • Requires less cell number • Realistic microenvironment • Visualization of cells is possible • Precise control over microenvironment • Resembles more closely the actual in vivo brain anatomy • Consider the effect of sheer stress • Immediate permeability measurements • Improvement in paracellular barrier functions | • Lack of high-throughput • Complex process technically • Not ideal linear kinetic studies | |
BBB: blood–brain barrier.
Microfluidics via 3D printing
In recent years, in the field of biomedical research, microfluidics have emerged as a promising alternative due to their high throughput, automation capabilities, and their low-cost in fabrication and operation.59 However, current microfluidic devices have relied on multi-step lithographic processes which are time-consuming and complex. In order to solve this critical issue, currently, 3D printing (additive manufacturing) is becoming an alternative approach to microfluidic fabrication with complex architectures, avoiding multi-step processing with wide range of materials.60–62 In fact, 3D printing as a digital fabrication technology, is a process of adding materials to fabricate objects from 3D model data, layer by layer, enabling precise construction of complex objects directly from a computer-aided design (CAD) software.63 Indeed, 3D printed microfluidic technology provides researchers with several advantages over traditional fabrication techniques including the ability to build channels with unprecedented shape and complexity, uniform and reproducible manufacture, minimal operating cost and time (reduced from weeks to a few hours), product complexity, reduction of user error, precisely controlled size, interconnectivity and geometry, flexibility and throughput.60,64–66
The similar basic procedure is used in most 3D printing processes for manufacturing solid products from digital designs [15]. Briefly, the intended product is digitally rendered in 3D with CAD software, and then 3D designs are converted to the stereolithography (SLA) file format (STL), describing the external surface of a 3D model.64 The data are then further sliced into a build file of 2D layers and sent to the 3D printing machine.63 Raw materials, processed into filaments, granules, or binder solutions, are added and solidified automatically, in a layer-by-layer manner to produce the desired product.64 After printing, products may require polishing, drying, sintering, or other post-processing steps. Unprinted materials will be also harvested and recycled for continued use in the printing process.67
Materials used in 3D printing approach
In 3D printing, the material selection is one of the most critical steps.68 Wide range of materials are used in 3D printing methods such as thermoplastic polymer materials (including acrylonitrile butadiene styrene (ABS), polylactic acid (PLA), polyamide (PA) and polycarbonate (PC)), natural polymers and biocompatible synthetic polymers (such as gelatin, sodium alginate, chitosan and acrylates-based polymers) and thermosetting polymer materials (resins).62,69–73 Moreover, 3D printing method allows for fabricating polymer composites (by combining various polymers to achieve a system with excellent functionality and high mechanical performance) to overcome the lack of strength of pure polymer products.74,75
3D Printing techniques
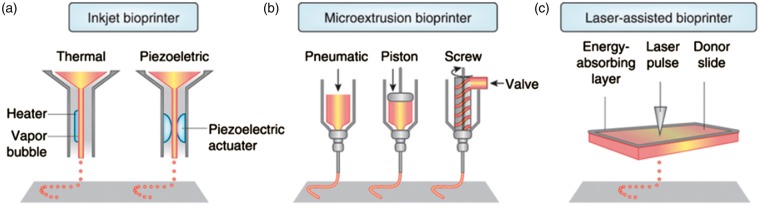
Various printing techniques have been utilized for microfluidic applications. The main 3D set up processes for microfluidic systems are 3D printed transfer molding (PTM), fused deposition modelling, SLA, direct ink writing, and selective laser sintering (SLS).67,76,77 The selection of fabrication technique depends on the requirements of processing speed, available instruments, costs, starting materials, and performance requirements of final products.63 On following, the main 3D set up processes are described briefly (see also Figure 5), and the advantages and drawbacks of each technique are also summarized in Table 2.
Figure 5.
Schematic overview of inkjet, microextrusion and laser-assisted processes in used in bioprinters. (a) Printheads are electrically heated to produce air-pressure pulses forcing droplets from the nozzle in thermal inkjet printers. (b) Microextrusion printers use instead either pneumatic or mechanical dispensing systems (screw or a piston) to extrude biomaterial and/or cells in continuous beads. (c) Finally, laser-assisted bioprinters (LAB) use lasers beams focused on a film of shock-absorbing substrate (donor support), thus generating a pulse that propels cell-containing biomaterials on the collecting substrate. Reproduced with permission from Murphy et al.68 Nature Biotechnology 2014; 32: 773.
Table 2.
Advantages and drawbacks of current 3D printing techniques.
| Technique | Working principle | Advantages | Drawbacks |
|---|---|---|---|
| 3D printed transfer molding (PTM) | Molding | • Fast • Cost-effective • Complex geometric characteristics | • Low resolutions • Limited geometric versatility • Rough surface topologies |
| Fused deposition modeling (FDM) | Extrusion and deposition | • Fast • Low cost • capability • Simplicity • Acceptable strength • Multi material multi-functionality | • Nozzle clogging • Limitation in usable material |
| 3D plotting/direct-writting | Pressurized syringe extrusion and heat or UV-assisted curing | • Material flexibility • High resolution | • Slow • Low mechanical strength |
| Stereolithography (SLA) | Laser scanning and UV-induced curing | • Nozzle-free • High resolution | • High cost • Cytotoxicity • Material Limitation |
| Selective laser sintering (SLS) | Laser scanning and heat-induced sintering | • Acceptable strength • Easy removal of support powder | • High cost • Powdery surface • Limitation in usable material |
BBB: blood–brain barrier.
3D PTM
PTM is one of the earliest examples of 3D printing technologies for microfluidic applications. The process involves shaping of elastomeric polymers such as PDMS using a 3D printed mold.78–80 In fact, in the conventional methods, substrates with positive surface relief structures are required to fabricate a mold, to be used to shape the PDMS, the most common polymer used in the fabrication of microfluidic systems. Fabricating these mold microstructures requires multiple lithography steps, microfabrication tools, cleanroom access, extensive training, and expensive reagents.66 However, 3D printing, as a promising method of fabricating molds, is fast and cost-effective, since each mold can be used for the fabrication of multiple microfluidic devices. Moreover, PTM allows researchers to achieve geometric characteristics which were difficult to be obtain by conventional methods.67 Despite these advantages, one disadvantage of this method is the overall limited geometric versatility. Moreover, the low resolutions and rough surface topologies of the fabricated devices could constitute an additional disadvantage of this method when considering the need for a smooth surface to allow for cell adhesion.
Fused deposition modeling
In fused deposition modeling (FDM) printers, as the most commonly used printers, filaments melt into a semi-liquid state at a nozzle and are extruded layer by layer onto the built platform where layers are fused together and then solidify into final parts.63 FDM provides several advantages, including low cost, high speed, simplicity and multi-functionality (deposition of diverse materials simultaneously). FDM method has also several drawbacks. One of the common drawbacks is that the composite materials have to be in a filament form to enable the extrusion process. Nonetheless, it is so difficult to homogeneously disperse materials and remove the void formed during the manufacturing of composite filaments. Another disadvantage of FDM technique is that the material should be thermoplastic polymers with high enough melt viscosity to provide structural support and low enough melt viscosity to enable extrusion. This overall limits the adoptability of a wide range of construction material.
3D plotting/direct-writing
3D plotting is based on extruding a viscous material from a pressurized syringe to create a 3D shape. During the process, the syringe head move spatially in the three dimensions, while the platform where the extruded materials are joint together layer by layer is kept stationary.63 Then induction by heat or UV light or dispensing two reactive components using mixing nozzles can be utilized in order to conduct curing reactions.81 The main advantage of this 3D plotting method technique is the flexibility of being able to use diverse materials such as pastes, solutions, and hydrogels. Ability to print parts with high resolution is also another advantage of this method. However, drawbacks of 3D plotting include low speed and therefore a longer manufacturing process time and low mechanical strength of the final products which may hinder the viability of the platform during pronged or sustained use.
SLA
SLA is a photopolymerisation process, involving the curing of liquid polymers using an UV-laser to solidify the material layer-by-layer.82 In SLA, UV-laser is controlled in a desired path to shoot in the resin reservoir, and the photocurable polymer will polymerize into a 2D patterned layer. After each layer is cured, the platform lowers and another layer of uncured polymer is ready to be patterned.83 The main advantage of SLA printing technology is the ability to print parts with high resolution. Moreover, the problem of nozzle clogging can be avoided since SLA is a nozzle-free technique.63 The high cost of this system and possible cytotoxicity of residual photoinitiators and uncured polymer remains a main concern for industrial application and use in biological settings.
SLS
In SLS, a laser beam with a controlled path scans the powders and sinter them by heating. Neighboring powders are fused together through molecular diffusion under high-power lasers and then processing of the next layer starts. Unbounded powder should be also removed to get the final products.84 Although this technique can deliver manufactured products with acceptable structural strength, the main limitation of the SLS method is the complex consolidation behavior and molecular diffusion process during sintering which limits the choice of materials to be used in SLS process.85 High cost and powdery surface can also be considered as additional drawbacks of this technique.
In recent years, several new techniques have been developed for 3D printing, including: (1) Poly jet, which works by polymerization of deposited droplets of photopolymer ink; (2) digital light processing (DLP) works based on selective polymerization of an entire surface of photopolymer by a projector light; (3) liquid deposition modeling (LDM), consisting in the additive deposition of material layers directly from a solution in a volatile solvent, and (4) fiber encapsulation additive manufacturing (FEAM), whereas fibers are directly encapsulated within an extruded polymer matrix.86–89 Although these methods have more material selections or less processing time, only a few studies have been conducted to assess the viability of these techniques to manufacture microfluidic systems, due to their high cost and complexity compared to traditional 3D printing methods.
Recently, Marino et al.90 developed a 3D printing technique which allowed them to faithfully reproduce the microcapillaries of the neurovascular system. The novelty of their work mainly includes the fabrication of a reliable platform to conduct high throughput quantitative screening of drug delivery to the brain. The bio-hybrid BBB developed in their laboratories allows to carry out high-throughput screening of drugs, assess toxicity of nanoparticles and evaluate their ability to cross the BBB. Moreover, this model provides a strictly controlled quasi-physiological environment allowing for easy monitoring of experimental variables such as drug concentration, blood speed, pH, and temperature.
The model consists of a microfluidic system of 50 parallel microtubes with a diameter of 10 µm connected by junctions and featuring pores of 1 µm diameter uniformly distributed on the cylindrical walls to enable transport toward the external environment.91,92 The porous microtubes include both artificial and biological components. The artificial structure including 3D porous microtubes is fabricated using two-photon lithography (SLA) and provides the scaffolding upon which vascular ECs can be grown to create the luminal layer of the blood vessel. The biological portion built by BECs covers the 3D printed structure resulting in a biohybrid system which mimics the natural model.90
Despite the potential advantages provided by 3D printing fabrication processes in BBB modeling, the technology is not yet mature with several limitations that still hinder its wide spread adoption. For example, the lack of high-throughput 3D-bioprinted tissue models for research makes this technology not yet suitable for drug discovery and toxicology studies. Complexity of the tissue to be reproduced increases exponentially the complexity of the technical challenges that needs to be overcome. These include conjugating multiple elements such as fabrication materials, cell types, cell distribution as well as loading of the necessary biological factors to maintain cell viability, and construction of the tissue scaffold itself. Needless to say, that to advance this developing technology any further is a task that will require the integration of multiple fields of research including engineering, biomaterials science, cell biology, physics and medicine.
Assays and technologies used in in vitro BBB models
There are numerous assays and technologies have been used in mentioned models to assess BBB properties such as tightness, integrity and permeability. Transepithelial/transendothelial electrical resistance (TEER) is a widely accepted quantitative technique to measure the integrity of tight junction dynamics in cell culture models of endothelial and epithelial monolayers.93 TEER measurement can be performed in real time without cell damage and generally are based on measuring ohmic resistance or measuring impedance across a wide spectrum of frequencies. TEER values as strong indicators of the integrity of the cellular barriers are evaluated for transport of drugs or chemicals. BBB permeability can be determined by measuring sodium fluorescein (NaF)94 or dextran uptake.95 In order to better assess the functional permeability data, it is significant to obtain expression changes in permeability-related genes.96 Expression analysis using the same cells increases the confidence in the functional data as it permits comparison between barrier integrity and gene expression of barrier-related proteins. RT-PCR (using RT-PCR kit) and Western blotting (using BCA Protein Assay Kit) are of two common techniques for expression analysis of various tight junction proteins such as ZO-1, Occludin and NQO-1 at the mRNA level. To determine specific protein localization and patterning, immunocytochemistry analysis is suggested to localize of various proteins.96 Moreover, there are specific tools such as visual microscopy and auto sampling that can be used in conjunction with these platforms. The advantages and limitations of these BBB models are also reported in Table 1.
Conclusion
Over the course of the last two decades, further understanding of the processes regulating barriergenesis and barrier functions has provided the foundation for biotechnological advancements allowing the development of more sophisticated and realistic in vitro BBB models. Among these promising new technologies, modulation of stem cells differentiation for the purpose of deriving relevant NVU cell types is a major breakthrough. Although we are still far from mastering this technology, stem cells could indeed deliver a breakthrough in BBB modeling allowing for the development of the desired cell cultures in situ within the platform itself. The most immediate impact would be a reduction of the setup cost and dedifferentiation issues that originate from having to passage the primary cells multiple times. It will also afford the possibility to develop patient-specific models.
In terms of platform development, microfluidic systems are enjoying a rapid development and awareness of this technology among the various laboratories is spreading rapidly. Although the availability of microfluidic platforms is still confined within the laboratories/research groups who develop the system, the potential for a wide adoption among the scientific community and perhaps industry is increasing. Further boost to the technology could come through the use of 3D bioprinting technologies (also rapidly advancing but not mature yet) where the intrinsic components and structure(s) of the targeted tissue can be rapidly reconstructed in vitro and with high precision.
Supplemental Material
Supplemental material for In-vitro blood–brain barrier modeling: A review of modern and fast-advancing technologies by Farzane Sivandzade and Luca Cucullo in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health/ National Institute on Drug Abuse 2R01-DA029121-01A1 and ARDF to Dr. Luca Cucullo.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Authors’ contributions
FS conceived the study and prepared the drafting of the manuscript. LC assisted with the drafting of the manuscript and preparation of the figures. LC also oversaw the all study and provided funding. All authors reviewed the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Abbott NJ. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 2013; 36: 437–449. [DOI] [PubMed] [Google Scholar]
- 2.Helms HC, Abbott NJ, Burek M, et al. In vitro models of the blood–brain barrier: an overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab 2016; 36: 862–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serlin Y, Shelef I, Knyazer B, et al. Anatomy and physiology of the blood–brain barrier. Sem Cell Develop Biol 2015; 38: 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aleynik A, Gernavage KM, Mourad YS, et al. Stem cell delivery of therapies for brain disorders. Clini Transl Med 2014; 3: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaisar MA, Sajja RK, Prasad S, et al. New experimental models of the blood-brain barrier for CNS drug discovery. Exp Opin Drug Discov 2017; 12: 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasan A, Deeb G, Rahal R, et al. Mesenchymal stem cells in the treatment of traumatic brain injury. Front Neurol 2017; 8: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menon D, Schwab K, Wright D, et al. Demographics and clinical assessment working group of the international and interagency initiative toward common data elements for research on traumatic brain injury and psychological health. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 2010; 91: 1637–1640.21044706 [Google Scholar]
- 8.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci 2009; 10: 235. [DOI] [PubMed] [Google Scholar]
- 9.Dekmak A, Mantash S, Shaito A, et al. Stem cells and combination therapy for the treatment of traumatic brain injury. Behav Brain Res 2018; 340: 49–62. [DOI] [PubMed] [Google Scholar]
- 10.Gomes MJ, Mendes B, Martins S, et al. Cell-based in vitro models for studying blood–brain barrier (BBB) permeability. Concepts Models Drug Permeabil Stud 2015; 1: 169–188. [Google Scholar]
- 11.Agoston DV. Bench-to-bedside and bedside back to the bench; seeking a better understanding of the acute pathophysiological process in severe traumatic brain injury. Front Neurol 2015; 6: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Fan D, Xiong D. Mesenchymal stem cells as delivery vectors for anti-tumor therapy. Stem Cell Invest 2015; 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Chen R, Liu M, et al. Remodeling the blood–brain barrier microenvironment by natural products for brain tumor therapy. Acta Pharm Sin B 2017; 7: 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang B, Tabata Y, Gao J-Q. Mesenchymal stem cells as therapeutic agents and potential targeted gene delivery vehicle for brain diseases. J Controll Rel 2012; 162: 464–473. [DOI] [PubMed] [Google Scholar]
- 15.Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Discov Med 2009; 6: 139–143. [PubMed] [Google Scholar]
- 16.Aliaga E, Silhol M, Bonneau N, et al. Dual response of BDNF to sublethal concentrations of β-amyloid peptides in cultured cortical neurons. Neurobiol Dis 2010; 37: 208–217. [DOI] [PubMed] [Google Scholar]
- 17.Tapia-Arancibia L, Aliaga E, Silhol M, et al. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev 2008; 59: 201–220. [DOI] [PubMed] [Google Scholar]
- 18.Jordao JF, Ayala-Grosso CA, Markham K, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS One 2010; 5: e10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordao JF, Thevenot E, Markham-Coultes K, et al. Amyloid-beta plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol 2013; 248: 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aday S, Cecchelli R, Hallier-Vanuxeem D, et al. Stem cell-based human blood–brain barrier models for drug discovery and delivery. Trends Biotechnol 2016; 34: 382–393. [DOI] [PubMed] [Google Scholar]
- 21.Gastfriend BD, Palecek SP, Shusta EV. Modeling the blood-brain barrier: beyond the endothelial cells. Curr Opin Biomed Eng 2018; 5: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippmann ES, Azarin SM, Kay JE, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol 2012; 30: 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cecchelli R, Aday S, Sevin E, et al. A stable and reproducible human blood-brain barrier model derived from hematopoietic stem cells. PLoS One 2014; 9: e99733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marroni M, Kight KM, Hossain M, et al. Dynamic in vitro model of the blood-brain barrier. Gene profiling using cDNA microarray analysis. Meth Mol Med 2003; 89: 419–434. [DOI] [PubMed] [Google Scholar]
- 25.Cucullo L, Hossain M, Rapp E, et al. Development of a humanized in vitro blood-brain barrier model to screen for brain penetration of antiepileptic drugs. Epilepsia 2007; 48: 505–516. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh C, Hossain M, Solanki J, et al. Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells. Epilepsia. 2017; 58: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelm I, Krizbai InA. In vitro models of the blood–brain barrier for the study of drug delivery to the brain. Mol Pharm 2014; 11: 1949–1963. [DOI] [PubMed] [Google Scholar]
- 28.Abbott NJ, Dolman DE, Yusof SR, et al. In vitro models of CNS barriers. Drug Deliv Brain 2014; 1: 163–197. [Google Scholar]
- 29.Eigenmann DE, Xue G, Kim KS, et al. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013; 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman NA, Rasil A, Meyding-Lamade U, et al. Immortalized endothelial cell lines for in vitro blood-brain barrier models: a systematic review. Brain Res 2016; 1642: 532–545. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, McGoron AJ, Crumpler ET, et al. Co-culture based blood-brain barrier in vitro model, a tissue engineering approach using immortalized cell lines for drug transport study. Appl Biochem Biotechnol 2011; 163: 278–295. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh C, Gonzalez-Martinez J, Hossain M, et al. Pattern of P450 expression at the human blood-brain barrier: roles of epileptic condition and laminar flow. Epilepsia 2010; 51: 1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson HK and Shusta EV. Human-based in vitro brain endothelial cell models. In: Di L and Kerns EH (eds) Blood-brain barrier in drug discovery: optimizing brain exposure of CNS drugs and minimizing brain side effects for peripheral drugs. Hoboken, NJ: John Wiley & Sons, Inc., 2015, pp.238–273.
- 34.Sano Y, Shimizu F, Abe M, et al. Establishment of a new conditionally immortalized human brain microvascular endothelial cell line retaining an in vivo blood–brain barrier function. J Cell Physiol 2010; 225: 519–528. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature 2010; 465: 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barkho BZ, Zhao X. Adult neural stem cells: response to stroke injury and potential for therapeutic applications. Curr Stem Cell Res Ther 2011; 6: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lian X, Bao X, Al-Ahmad A, et al. Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Rep 2014; 3: 804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel R, Page S, Al-Ahmad AJ. Isogenic blood-brain barrier models based on patient-derived stem cells display inter-individual differences in cell maturation and functionality. J Neurochem 2017; 142: 74–88. [DOI] [PubMed] [Google Scholar]
- 39.DeStefano JG, Xu ZS, Williams AJ, et al. Effect of shear stress on iPSC-derived human brain microvascular endothelial cells (dhBMECs). Fluids Barriers CNS 2017; 14: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian X, Brookes O, Battaglia G. Pericytes from Mesenchymal Stem Cells as a model for the blood-brain barrier. Sci Rep 2017; 7: 39676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3: 301–313. [DOI] [PubMed] [Google Scholar]
- 42.Caplan AI. All MSCs are pericytes? Cell Stem Cell 2008; 3: 229–230. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Eckert MA, Riazifar H, et al. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int 2013; 2013: 435093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menon LG, Shi VJ and Carroll RS. Mesenchymal stromal cells as a drug delivery system. 2009; Cambridge, MA: Harvard Stem Cell Institute. [PubMed]
- 45.Shayan G, Choi YS, Shusta EV, et al. Murine in vitro model of the blood–brain barrier for evaluating drug transport. Eur J Pharm Sci 2011; 42: 148–155. [DOI] [PubMed] [Google Scholar]
- 46.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 2010; 468: 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilhelm I, Fazakas C, Krizbai IA. In vitro models of the blood-brain barrier. Acta Neurobiol Exp 2011; 71: 113–128. [DOI] [PubMed] [Google Scholar]
- 48.Paşca SP. The rise of three-dimensional human brain cultures. Nature 2018; 553: 437. [DOI] [PubMed] [Google Scholar]
- 49.Naik P, Cucullo L. In vitro blood–brain barrier models: current and perspective technologies. J Pharm Sci 2012; 101: 1337–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatherell K, Couraud P-O, Romero IA, et al. Development of a three-dimensional, all-human in vitro model of the blood–brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Meth 2011; 199: 223–229. [DOI] [PubMed] [Google Scholar]
- 51.Cucullo L, Couraud P-O, Weksler B, et al. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J Cereb Blood Flow Metab 2008; 28: 312–328. [DOI] [PubMed] [Google Scholar]
- 52.Cucullo L, Hossain M, Puvenna V, et al. The role of shear stress in blood-brain barrier endothelial physiology. BMC Neurosci 2011; 12: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Meer AD, van den Berg A. Organs-on-chips: breaking the in vitro impasse. Integrat Biol 2012; 4: 461–470. [DOI] [PubMed] [Google Scholar]
- 54.Yeon JH, Na D, Choi K, et al. Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed Microdev 2012; 14: 1141–1148. [DOI] [PubMed] [Google Scholar]
- 55.Esch MB, Sung JH, Yang J, et al. On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’devices. Biomed Microdev 2012; 14: 895–906. [DOI] [PubMed] [Google Scholar]
- 56.Griep L, Wolbers F, De Wagenaar B, et al. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdev 2013; 15: 145–150. [DOI] [PubMed] [Google Scholar]
- 57.Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012; 12: 1784–1792. [DOI] [PubMed] [Google Scholar]
- 58.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol 2014; 32: 760. [DOI] [PubMed] [Google Scholar]
- 59.Villegas M, Cetinic Z, Shakeri A, et al. Fabricating smooth PDMS microfluidic channels from low-resolution 3D printed molds using an omniphobic lubricant-infused coating. Analyt Chim Acta 2018; 1000: 248–255. [DOI] [PubMed] [Google Scholar]
- 60.Hampson S, Rowe W, Christie SD, et al. 3D printed microfluidic device with integrated optical sensing for particle analysis. Sensors Actuat B 2018; 256: 1030–1037. [Google Scholar]
- 61.Gaal G, Mendes M, de Almeida TP, et al. Simplified fabrication of integrated microfluidic devices using fused deposition modeling 3D printing. Sensors Actuat B 2017; 242: 35–40. [Google Scholar]
- 62.Lee J-Y, An J, Chua CK. Fundamentals and applications of 3D printing for novel materials. Appl Mater Today 2017; 7: 120–133. [Google Scholar]
- 63.Wang X, Jiang M, Zhou Z, et al. 3D printing of polymer matrix composites: a review and prospective. Compos Part B 2017; 110: 442–458. [Google Scholar]
- 64.Norman J, Madurawe RD, Moore CM, et al. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev 2017; 108: 39–50. [DOI] [PubMed] [Google Scholar]
- 65.Lewis PL, Green RM, Shah RN. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomater 2018; 69: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwang Y, Paydar OH, Candler RN. 3D printed molds for non-planar PDMS microfluidic channels. Sensors Actuat A 2015; 226: 137–142. [Google Scholar]
- 67.Sochol RD, Sweet E, Glick CC, et al. 3D printed microfluidics and microelectronics. Microelectron Eng 2018; 189: 52–68. [Google Scholar]
- 68.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014; 32: 773. [DOI] [PubMed] [Google Scholar]
- 69.Tymrak B, Kreiger M, Pearce JM. Mechanical properties of components fabricated with open-source 3-D printers under realistic environmental conditions. Mater Des 2014; 58: 242–246. [Google Scholar]
- 70.Tran P, Ngo TD, Ghazlan A, et al. Bimaterial 3D printing and numerical analysis of bio-inspired composite structures under in-plane and transverse loadings. Compos Part B 2017; 108: 210–223. [Google Scholar]
- 71. Melnikova R, Ehrmann A and Finsterbusch K. 3D printing of textile-based structures by fused deposition modelling (FDM) with different polymer materials. In: IOP conference series: materials science and engineering, 27 29 May, Ningbo, China, 2014. Bristol, UK: IOP Publishing, 2014, p.012018.
- 72.Kim K, Zhu W, Qu X, et al. 3D optical printing of piezoelectric nanoparticle–polymer composite materials. ACS Nano 2014; 8: 9799–9806. [DOI] [PubMed] [Google Scholar]
- 73.Tayebi L, Rasoulianboroujeni M, Cui Z, et al. 3D-printed thick structured gelatin membrane for engineering of heterogeneous tissues. Mater Lett 2018; 217: 39–43. [Google Scholar]
- 74.Malhotra SK, Goda K and Sreekala MS. Part one introduction to polymer composites. In: Thomas S, Joseph K, Malhotra SK, Goda K, and Sreekala MS (eds) Polymer Composites: Volume 1, First Edition. Weinheim, Germany: Wiley-VCH, 2012, pp.1–2.
- 75.Huang SH, Liu P, Mokasdar A, et al. Additive manufacturing and its societal impact: a literature review. Int J Adv Manuf Technol 2013; 67: 1191–1203. [Google Scholar]
- 76.Ho CMB, Ng SH, Li KHH, et al. 3D printed microfluidics for biological applications. Lab Chip 2015; 15: 3627–3637. [DOI] [PubMed] [Google Scholar]
- 77.Li J, Rossignol F, Macdonald J. Inkjet printing for biosensor fabrication: combining chemistry and technology for advanced manufacturing. Lab Chip 2015; 15: 2538–2558. [DOI] [PubMed] [Google Scholar]
- 78.Bonyár A, Sántha H, Varga M, et al. Characterization of rapid PDMS casting technique utilizing molding forms fabricated by 3D rapid prototyping technology (RPT). Int J Mater Form 2014; 7: 189–196. [Google Scholar]
- 79.Comina G, Suska A, Filippini D. 3D printed unibody lab-on-a-chip: features survey and check-valves integration. Micromachines 2015; 6: 437–451. [Google Scholar]
- 80.Thomas MS, Millare B, Clift JM, et al. Print-and-peel fabrication for microfluidics: what’s in it for biomedical applications? Ann Biomed Engi 2010; 38: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Billiet T, Vandenhaute M, Schelfhout J, et al. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012; 33: 6020–6041. [DOI] [PubMed] [Google Scholar]
- 82.Capel AJ, Edmondson S, Christie SD, et al. Design and additive manufacture for flow chemistry. Lab Chip 2013; 13: 4583–4590. [DOI] [PubMed] [Google Scholar]
- 83.Melchels FP, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010; 31: 6121–6130. [DOI] [PubMed] [Google Scholar]
- 84.Gu D, Meiners W, Wissenbach K, et al. Laser additive manufacturing of metallic components: materials, processes and mechanisms. Int Mater Rev 2012; 57: 133–164. [Google Scholar]
- 85.Goodridge R, Shofner M, Hague R, et al. Processing of a polyamide-12/carbon nanofibre composite by laser sintering. Polym Test 2011; 30: 94–100. [Google Scholar]
- 86.Ge Q, Dunn CK, Qi HJ, et al. Active origami by 4D printing. Smart Mater Struct 2014; 23: 094007. [Google Scholar]
- 87.Cooperstein I, Layani M, Magdassi S. 3D printing of porous structures by UV-curable O/W emulsion for fabrication of conductive objects. J Mater ChemC 2015; 3: 2040–2044. [Google Scholar]
- 88.Postiglione G, Natale G, Griffini G, et al. Conductive 3D microstructures by direct 3D printing of polymer/carbon nanotube nanocomposites via liquid deposition modeling. Compos Part A 2015; 76: 110–114. [Google Scholar]
- 89.Saari M, Cox B, Richer E, et al. Fiber encapsulation additive manufacturing: an enabling technology for 3D printing of electromechanical devices and robotic components. 3D Print Add Manuf 2015; 2: 32–39. [Google Scholar]
- 90.Marino A, Tricinci O, Battaglini M, et al. A 3D realscale, biomimetic, and biohybrid model of the bloodbrain barrier fabricated through two-photon lithography. Small 2018; 14. DOI: 10.1002/smll.201702959. [DOI] [PMC free article] [PubMed]
- 91.Stefanovic B, Hutchinson E, Yakovleva V, et al. Functional reactivity of cerebral capillaries. J Cereb Blood Flow Metab 2008; 28: 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wong A, Ye M, Levy A, et al. The blood-brain barrier: an engineering perspective. Front Neuroeng 2013; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom 2015; 20: 107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chai Q, He WQ, Zhou M, et al. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J Virol 2014; 88: 4698–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prasad S, Sajja RK, Kaisar MA, et al. Role of Nrf2 and protective effects of Metformin against tobacco smoke-induced cerebrovascular toxicity. Redox Biol 2017; 12: 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Czupalla CJ, Liebner S, Devraj K. In vitro models of the blood-brain barrier. Methods Mol Biol 2014; 1135: 415–437. [DOI] [PubMed] [Google Scholar]
- 97.Brown JA, Pensabene V, Markov DA, et al. Recreating blood-brain barrier physiology and structure on chip: a novel neurovascular microfluidic bioreactor. Biomicrofluidics 2015; 9: 054124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for In-vitro blood–brain barrier modeling: A review of modern and fast-advancing technologies by Farzane Sivandzade and Luca Cucullo in Journal of Cerebral Blood Flow & Metabolism